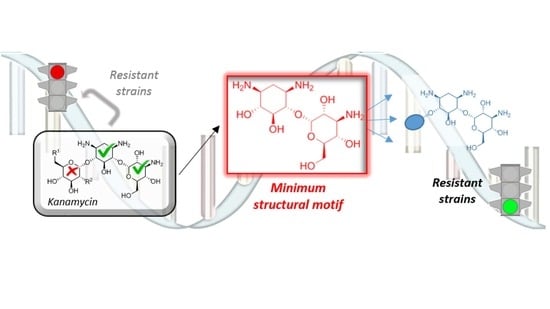
Synthesis of Ring II/III Fragment of Kanamycin: A New Minimum Structural Motif for Aminoglycoside Recognition
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Pseudo-Disaccharide 7
2.2. Antibiotic Activity and Resistance Enzyme Susceptibility of 7
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis of 4-O-(2,6-di-Deoxy-α-d-2,6-di-amine-glucopyranosyl)-2-deoxy-streptamine (Neamine) (4)
3.3. Synthesis of 6-O-[(3-Deoxy-3-amino)-α-d-glucopyranosyl]-2-deoxy-streptamine (7)
3.3.1. Synthesis of 1,3,6′,3″-Tetra-azido-kanamycin A (8)
3.3.2. Synthesis of 4″,6″-O-di-tert-Butyl-silane-1,3,6′,3″-tetra-azido-kanamycin A (9)
3.3.3. Synthesis of 2′,3′-O-(2,3-Butanedione-bis-dimethyl-acetal)-4″,6″-O-di-tert-butyl-silane-1,3,6′,3″-tetra-azido-kanamycin A (10a) and 3′,4′-O-(2,3-Butanedione-bis-dimethyl-acetal)-4″,6″-O-di-tert-butyl-silane-1,3,6′,3″-tetra-azido-kanamycin A (10b)
3.3.4. Synthesis of 5,2′,3″-O-tri-Benzyl-2′,3′-O-(2,3-butanedione-bis-dimethyl-acetal)-4″,6″-O-di-tert-butyl-silane-1,3,6′,3″-tetra-azido-kanamycin A (11)
3.3.5. Synthesis of 5,2′,3″-O-tri-Benzyl-4″,6″-O-di-tert-butyl-silane-1,3,6′,3″-tetra-azido-kanamycin A (12)
3.3.6. Synthesis of 1,3-di-Azido-5-O-benzyl-[(3-deoxy-3-azido-2-O-Benzyl-4,6-O-di-tert-butyl-silane)-α-d-glucopyranosyl]-2-deoxy-streptamine (13)
3.3.7. Synthesis of 6-O-[(3-Deoxy-3-amino)-α-d-glucopyranosyl]-2-deoxy-streptamine (7)
3.4. MIC Determination
3.5. Enzymatic Activity
3.5.1. Enzymatic Activity of ANT-(4′) from S. aureus by HPLC Assay
3.5.2. Enzymatic Activity of APH-(3′) from E. coli by ELISA
3.5.3. Enzymatic Activity of AAC-(6′) from S. aureus by ELISA
3.6. Thermal Shift Assay
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schatz, A.; Waksman, S.A. Effect of streptomycin and other antibiotic substances upon Mycobacterium tuberculosis and related organisms. Proc. Soc. Exp. Biol. Med. 1944, 57, 244–248. [Google Scholar] [CrossRef]
- Santana, A.G.; Zárate, S.G.; Bastida, A.; Revuelta, J. Targeting RNA with Aminoglycosides: Current Improvements in their synthesis and Biological Activitiy. In Frontiers in Anti-Infective Drug Discovery; Atta-Ur-Rahman, F., Choudhray, M.I., Eds.; Bentham E-Books: Sharjah, UAE, 2015; pp. 131–209. [Google Scholar]
- Takahashi, Y.; Igarashi, M. Destination of aminoglycoside antibiotics in the ‘post-antibiotic era’. J. Antibiot. 2018, 71, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Walter, F.; Vicens, Q.; Westhof, E. Aminoglycoside–RNA interactions. Curr. Opin. Chem. Biol. 1999, 3, 694–704. [Google Scholar] [CrossRef]
- Hermann, T. Strategies for the design of drugs targeting RNA and RNA–Protein complexes. Angew. Chem. Int. Edit. 2000, 39, 1891–1905. [Google Scholar] [CrossRef]
- Schroeder, R.; Waldsich, C.; Wank, H. Modulation of RNA function by aminoglycoside antibiotics. Embo. J. 2000, 19, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sucheck, S.J.; Wong, C.H. RNA as a target for small molecules. Curr. Opin. Chem. Biol. 2000, 4, 678–686. [Google Scholar] [CrossRef]
- Chellat, M.F.; Raguz, L.; Riedl, R. Targeting Antibiotic Resistance. Angew. Chem. Int. Edit. 2016, 55, 6600–6626. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Kondo, S. Kanamycin and its derivative, arbekacin: Significance and impact. J. Antibiot. 2018, 71, 417–424. [Google Scholar] [CrossRef]
- Fourmy, D.; Recht, M.I.; Puglisi, J.D. Binding of neomycin-class aminoglycoside antibiotics to the A-site of 16 S rRNA. J. Mol. Biol. 1998, 277, 347–362. [Google Scholar] [CrossRef]
- Rai, R.; McAlexander, I.; Chang, C.W.T. Synthetic glycodiversification. From aminosugars to aminoglycoside antibiotics. A review. Org. Prep. Proced. Int. 2005, 37, 337–375. [Google Scholar] [CrossRef]
- Allam, A.; Maigre, L.; de Sousa, R.A.; Dumont, E.; Vergalli, J.; Pages, J.M.; Artaud, I. New amphiphilic neamine conjugates bearing a metal binding motif active against MDR E-aerogenes Gram-negative bacteria. Eur. J. Med. Chem. 2017, 127, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, L.; Das, I.; Desire, J.; Sautrey, G.; Barros, R.S.V.; El Khoury, M.; Mingeot-Leclercq, M.P.; Decout, J.L. New Broad-Spectrum Antibacterial Amphiphilic Aminoglycosides Active against Resistant Bacteria: From Neamine Derivatives to Smaller Neosamine Analogues. J. Med. Chem. 2016, 59, 9350–9369. [Google Scholar] [CrossRef] [PubMed]
- Garneau-Tsodikova, S.; Labby, K.J. Mechanisms of resistance to aminoglycoside antibiotics: Overview and perspectives. Med. Chem. Comm. 2016, 7, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Zarate, S.G.; Claure, M.L.D.; Benito-Arenas, R.; Revuelta, J.; Santana, A.G.; Bastida, A. Overcoming Aminoglycoside Enzymatic Resistance: Design of Novel Antibiotics and Inhibitors. Molecules 2018, 23, 18. [Google Scholar] [CrossRef] [PubMed]
- Chandrika, N.T.; Garneau-Tsodikova, S. Comprehensive review of chemical strategies for the preparation of new aminoglycosides and their biological activities. Chem. Soc. Rev. 2018, 47, 1189–1249. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Moreno, E.; Gomez-Pinto, I.; Corzana, F.; Santana, A.G.; Revuelta, J.; Bastida, A.; Jimenez-Barbero, J.; Gonzalez, C.; Asensio, J.L. Chemical Interrogation of Drug/RNA Complexes: From Chemical Reactivity to Drug Design. Angew. Chem. Int. Edit. 2013, 52, 3148–3151. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.G.; Zárate, S.G.; Asensio, J.L.; Revuelta, J.; Bastida, A. Selective modification of the 3”-amino group of kanamycin prevents significant loss of activity in resistant bacterial strains. Org. Biomol. Chem. 2016, 14, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.S.; Tolmasky, M.E. Amikacin: Uses, Resistance, and Prospects for Inhibition. Molecules 2017, 22, 23. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, T.; Sati, G.C.; Kondasinghe, N.; Pirrone, M.G.; Kato, T.; Waduge, P.; Kumar, H.S.; Sanchon, A.C.; Dobosz-Bartoszek, M.; Shcherbakov, D.; et al. Design, Multigram Synthesis, and in Vitro and in Vivo Evaluation of Propylamycin: A Semisynthetic 4,5-Deoxystreptamine Class Aminoglycoside for the Treatment of Drug-Resistant Enterobacteriaceae and Other Gram-Negative Pathogens. J. Am. Chem. Soc. 2019, 141, 5051–5061. [Google Scholar] [CrossRef] [PubMed]
- Santana, A.G.; Bastida, A.; del Campo, T.M.; Asensio, J.L.; Revuelta, J. An Efficient and General Route to the Synthesis of Novel Aminoglycosides for RNA Binding. Synlett 2011, 219–222. [Google Scholar] [CrossRef]
- Yu, M.; Pagenkopf, B.L. The regioselective mono-deprotection of 1,3-dioxa-2,2-(di-tert-butyl)-2-silacyclohexanes with BF3 center dot SMe2. J. Org. Chem. 2002, 67, 4553–4558. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.B.; Yuan, M.; Wu, Y.F.; You, X.F.; Ye, X.S. Rational design and synthesis of potent aminoglycoside antibiotics against resistant bacterial strains. Bioorg. Med. Chem. 2011, 19, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Montchamp, J.L.; Tian, F.; Hart, M.E.; Frost, J.W. Butane 2,3-bisacetal protection of vicinal diequatorial diols. J. Org. Chem. 1996, 61, 3897–3899. [Google Scholar] [CrossRef] [PubMed]
- van den Broek, S.; Gruijters, B.W.T.; Rutjes, F.; van Delft, F.L.; Blaauw, R.H. A short and scalable route to orthogonally O-protected 2-deoxystreptamine. J. Org. Chem. 2007, 72, 3577–3580. [Google Scholar] [CrossRef] [PubMed]
- Corzana, F.; Cuesta, I.; Freire, F.; Revuelta, J.; Torrado, M.; Bastida, A.; Jiménez-Barbero, J.; Asensio, J.L. The Pattern of Distribution of Amino Groups Modulates the Structure and Dynamics of Natural Aminoglycosides: Implications for RNA Recognition. J. Am. Chem. Soc. 2007, 129, 2849–2865. [Google Scholar] [CrossRef] [PubMed]
- Matesanz, R.; Diaz, J.F.; Corzana, F.; Santana, A.G.; Bastida, A.; Asensio, J.L. Multiple keys for a single lock: The unusual structural plasticity of the nucleotidyltransferase (4’)/kanamycin complex. Chem. A Eur. J. 2012, 18, 2875–2889. [Google Scholar] [CrossRef] [PubMed]
- Titz, A.; Radic, Z.; Schwardt, O.; Ernst, B. A safe and convenient method for the preparation of triflyl azide, and its use in diazo transfer reactions to primary amines. Tetrahedron Lett. 2006, 47, 2383–2385. [Google Scholar] [CrossRef]
- Lo, M.C.; Aulabaugh, A.; Jin, G.; Cowling, R.; Bard, J.; Malamas, M.; Ellestad, G. Evaluation of fluorescence-based thermal shift assays for hit identification in drug discovery. Anal. Biochem. 2004, 332, 153–159. [Google Scholar] [CrossRef]


| Entry | Strain | MIC (µg mL−1) Compounds | ||
|---|---|---|---|---|
| 1 | 4 | 7 | ||
| 1 | E. coli/ATCC 2592 | 4 | 100 | 50 |
| 2 | E. coli BL21 | 6 | 100 | 50 |
| 3 | B. cereus/ATCC 117781 | 1.5 | 100 | 50 |
| 4 | S. epidermis/ATCC 1228 | >100 | >50 | 50 |
| 5 | E. coli (APH-3″IIa) | >200 | >200 | 100 |
| 6 | E. coli (ANT-4′) | >200 | >200 | 100 |
| 7 | E. coli (AAC-6′) | >200 | >200 | 100 |
| Enzyme | Blank | 1 | 4 | 7 |
|---|---|---|---|---|
| ANT-(4′) | 58 ± 0.1 | 61.5 ± 0.1 | 61 ± 0.1 | 59 ± 0.1 |
| AAC-(6′) | 44 ± 0.09 | 49 ± 0.1 | 48 ± 0.2 | 49 ± 0.1 |
| APH-(3′) | 49 ± 0.09 | 62 ± 0.1 | 56 ± 0.2 | 56 ± 0.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zárate, S.G.; Bastida, A.; Santana, A.G.; Revuelta, J. Synthesis of Ring II/III Fragment of Kanamycin: A New Minimum Structural Motif for Aminoglycoside Recognition. Antibiotics 2019, 8, 109. https://doi.org/10.3390/antibiotics8030109
Zárate SG, Bastida A, Santana AG, Revuelta J. Synthesis of Ring II/III Fragment of Kanamycin: A New Minimum Structural Motif for Aminoglycoside Recognition. Antibiotics. 2019; 8(3):109. https://doi.org/10.3390/antibiotics8030109
Chicago/Turabian StyleZárate, Sandra G., Agatha Bastida, Andrés G. Santana, and Julia Revuelta. 2019. "Synthesis of Ring II/III Fragment of Kanamycin: A New Minimum Structural Motif for Aminoglycoside Recognition" Antibiotics 8, no. 3: 109. https://doi.org/10.3390/antibiotics8030109