Associations between Gastrointestinal Nematode Infection Burden and Lying Behaviour as Measured by Accelerometers in Periparturient Ewes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Data Processing and Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Berckmans, D. Precision livestock farming technologies for welfare management in intensive livestock systems. Sci. Tech. Rev. 2014, 33, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Banhazi, T.M.; Lehr, H.; Black, J.L.; Crabtree, H.; Schofield, P.; Tscharke, M.; Berckmans, D. Precision livestock farming: An international review of scientific and commercial aspects. Int. J. Agric. Biol. 2012, 5, 1–9. [Google Scholar]
- Morgan-Davies, C.; Lambe, N.; Wishart, H.; Waterhouse, T.; Kenyon, F.; McBean, D.; McCracken, D. Impacts of using a precision livestock system targeted approach in mountain sheep flocks. Livest. Sci. 2018, 208, 67–76. [Google Scholar] [CrossRef]
- Charlier, J.; Rinaldi, L.; Musella, V.; Ploeger, H.W.; Chartier, C.; Vineer, H.R.; Hinney, B.; von Samson-Himmelstjerna, G.; Băcescu, B.; Mickiewicz, M.; et al. Initial assessment of the economic burden of major parasitic helminth infections to the ruminant livestock industry in Europe. Prev. Vet. Med. 2020, 182, 105103. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.M.; Vidyashankar, A.N. An inconvenient truth: Global warming and anthelmintic resistance. Vet. Parasitol. 2012, 186, 70–78. [Google Scholar] [CrossRef]
- Williams, E.G.; Brophy, P.M.; Williams, H.W.; Davies, N.; Jones, R.A. Gastrointestinal nematode control practices in ewes: Identification of factors associated with application of control methods known to influence anthelmintic resistance development. Vet. Parasitol. Reg. Stud. Rep. 2021, 24, 100562. [Google Scholar] [CrossRef]
- Kenyon, F.; McBean, D.; Greer, A.W.; Burgess, C.G.S.; Morrison, A.A.; Bartley, D.J.; Devin, L.; Nath, M.; Jackson, F. A comparative study of the effects of four treatment regimes on ivermectin efficacy, body weight pasture contamination in lambs naturally infected with gastrointestinal nematodes in Scotland. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 77–84. [Google Scholar] [CrossRef]
- Charlier, J.; Thamsborg, S.M.; Bartley, D.J.; Skuce, P.J.; Kenyon, F.; Geurden, T.; Hoste, H.; Williams, A.R.; Sotiraki, S.; Höglund, J.; et al. Mind the gaps in research on the control of gastrointestinal nematodes of farmed ruminants and pigs. Transbound Emerg. Dis. 2017, 65, 217–234. [Google Scholar] [CrossRef]
- Cornelius, M.P.; Jacobson, C.; Besier, R.B. Body condition score as a selection tool for targeted selective treatment-based nematode control strategies in Merino ewes. Vet. Parasitol. 2014, 206, 173–181. [Google Scholar] [CrossRef]
- Greer, A.W.; Kenyon, F.; Bartley, D.J.; Jackson, E.B.; Gordon, Y.; Donnan, A.A.; McBean, D.W.; Jackson, F. Development and field evaluation of a decision support model for anthelmintic treatments as part of a targeted selective treatment (TST) regime in lambs. Vet. Parasitol. 2009, 164, 12–20. [Google Scholar] [CrossRef]
- Williams, E.; Brophy, P.; Williams, H.W.; Davies, N.; Jones, R. Reproductive performance of ewes following pre-mating targeted selective treatment against gastrointestinal nematodes. Anim.-Sci. Proc. 2021, 12, 149. [Google Scholar] [CrossRef]
- Höglund, J.; Morrison, D.A.; Charlier, J.; Dimander, S.-O.; Larsson, A. Assessing the feasibility of targeted selective treatment for gastrointestinal nematodes in first-season grazing cattle based on mid-season daily weight gains. Vet. Parasitol. 2009, 164, 80–88. [Google Scholar] [CrossRef]
- Thlama, P.B.; Abdullahi, B.A.; Ahmed, G.M.; Mohammed, A.; Philip, M.H.; Yusuf, J. Point Prevalence and Intensity of Gastrointestinal Parasite Ova/Oocyst and Its Association with Body Condition Score (BCS) of Sheep and Goats in Maiduguri, Nigeria. J. Adv. Parasitol. 2016, 3, 81–88. [Google Scholar] [CrossRef]
- Kyriazakis, I.; Tolkamp, B.J.; Hutchings, M.R. Towards a functional explanation for the occurrence of anorexia during parasitic infections. Anim. Behav. 1998, 56, 265–274. [Google Scholar] [CrossRef]
- Taylor, M.A. Emerging parasitic diseases of sheep. Vet. Parasitol. 2012, 189, 2–7. [Google Scholar] [CrossRef]
- Neethirajan, S.; Kemp, B. Digital livestock farming. Sens. Bio-Sens. Res. 2021, 32, 100408. [Google Scholar] [CrossRef]
- Hutchings, M.R.; Kyriazakis, I.; Anderson, D.H.; Gordon, I.J.; Coop, R.L. Behavioural strategies used by parasitized and non-parasitized sheep to avoid ingestion of gastro-intestinal nematodes associated with faeces. Anim. Sci. 1998, 67, 97–106. [Google Scholar] [CrossRef]
- Falzon, G.; Schneider, D.; Trotter, M.; Lamb, D.W. A relationship between faecal egg counts and the distance travelled by sheep. Small Rumin. 2013, 111, 171–174. [Google Scholar] [CrossRef]
- Hogberg, N.; Hoglund, J.; Carlsson, A.; Saint-Jeveint, M.; Lidfors, L. Validation of accelerometers to automatically record postures and number of steps in growing lambs. Appl. Anim. Behav. Sci. 2020, 229, 105014. [Google Scholar] [CrossRef]
- Ikurior, S.J.; Pomroy, W.E.; Scott, I.; Corner-Thomas, R.; Marquetoux, N.; Leu, S.T. Gastrointestinal nematode infection affects overall activity in young sheep monitored with tri-axial accelerometers. Vet. Parasitol. 2020, 283, 109188. [Google Scholar] [CrossRef]
- Szyszka, O.; Kyriazakis, I. What is the relationship between level of infection and ‘sickness behaviour’ in cattle? Appl. Anim. Behav. Sci. 2013, 147, 1–10. [Google Scholar] [CrossRef]
- Högberg, N.; Lidfors, L.; Hessle, A.; Segerkvist, K.A.; Herlin, A.; Höglund, J. Effects of nematode parasitism on activity patterns in first-season grazing cattle. Vet. Parasitol. 2019, 1, 100011. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.; Davis, C.N.; Jones, D.L.; Davies, E.S.; Vasina, P.; Cutress, D.; Rose, M.T.; Jones, R.H.; Williams, H.W. Lying behaviour of housed and outdoor-managed pregnant sheep. Appl. Anim. Behav. Sci. 2021, 241, 105370. [Google Scholar] [CrossRef]
- Cringoli, G.; Maurelli, M.P.; Levecke, B.; Bosco, A.; Vercruysse, J.; Utzinger, J.; Rinaldi, L. The Mini-FLOTAC technique for the diagnosis of helminth and protozoan infection in humans and animals. Nat. Protoc. 2017, 12, 1723–1732. [Google Scholar] [CrossRef]
- Russel, A. Body condition scoring of sheep. In Pract. 1984, 6, 91. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Echeverri, A.C.; Gonyou, H.W.; Ghent, A.W. Preparturient behavior of confined ewes: Time budgets, frequencies, spatial distribution and sequential analysis. Appl. Anim. Behav. Sci. 1992, 34, 329–344. [Google Scholar] [CrossRef]
- Fogarty, E.S.; Swain, D.L.; Cronin, G.M.; Moraes, L.E.; Trotter, M. Can accelerometer ear tags identify behavioural changes in sheep associated with parturition? Anim. Reprod. Sci. 2020, 216, 106345. [Google Scholar] [CrossRef]
- Ito, K.; Weary, D.M.; von Keyserlingk, M.A.G. Lying behavior: Assessing within- and between-herd variation in free-stall-housed dairy cows. J. Dairy Sci. 2009, 92, 4412–4420. [Google Scholar] [CrossRef]
- Zobel, G.; Weary, D.M.; Leslie, K.; Chapinal, N.; von Keyserlingk, M.A.G. Technical note: Validation of data loggers for recording lying behavior in dairy goats. J. Dairy Sci. 2015, 98, 1082–1089. [Google Scholar] [CrossRef]
- Brooks, M.E.; Kristensen, K.; van Benthem, K.J.; Magnusson, A.; Berg, C.W.; Casper, W.; Nielsen, A.; Skaug, H.J.; Machler, M.; Bolker, B.M. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modelling. R J. 2017, 9, 378–400. [Google Scholar] [CrossRef]
- Hartig, F.; Hartig, M.F. Package ‘DHARMa’; R Development Core Team: Vienna, Austria, 2017. [Google Scholar]
- Hogberg, N.; Hessle, A.; Lidfors, L.; Enweji, N.; Hoglund, J. Nematode parasitism affects lying time and overall activity patterns in lambs following pasture exposure around weaning. Vet. Parasitol. 2021, 296, 109500. [Google Scholar] [CrossRef]
- Grant, E.P.; Wickham, S.L.; Anderson, F.; Barnes, A.L.; Fleming, P.A.; Miller, D.W. Behavioural assessment of sheep is sensitive to level of gastrointestinal parasite infection. Appl. Anim. Behav. Sci. 2020, 223, 104920. [Google Scholar] [CrossRef]
- Burgunder, J.; Petrzelkova, K.J.; Modry, D.; Kato, A.; Macintosh, A.J.J. Fractal measures in activity patterns: Do gastrointestinal parasites affect the complexity of sheep behaviour? Appl. Anim. Behav. Sci. 2018, 205, 44–53. [Google Scholar] [CrossRef]
- Hogberg, N.; Hessle, A.; Lidfors, L.; Baltrusis, P.; Claerebout, E.; Hoglund, J. Subclinical nematode parasitism affects activity and rumination patterns in first-season grazing cattle. Animal 2021, 15, 100237. [Google Scholar] [CrossRef]
- Jensen, D.B.; Hogeveen, H.; De Vries, A. Bayesian integration of sensor information and a multivariate dynamic linear model for prediction of dairy cow mastitis. J. Dairy Sci. 2016, 99, 7344–7361. [Google Scholar] [CrossRef]
- Sepulveda-Varas, P.; Weary, D.M.; von Keyserlingk, M.A.G. Lying behaviour and postpartum health status in grazing dairy cows. J. Dairy Sci. 2014, 97, 6334–6343. [Google Scholar] [CrossRef]
- Reiner, G.; Hubner, K.; Hepp, S. Suffering in diseased pigs as expressed by behavioural, clinical and clinical-chemical traits, in a well defined parasite model. Appl. Anim. Behav. Sci. 2009, 118, 222–231. [Google Scholar] [CrossRef]
- Weary, D.M.; Huzzey, J.M.; von Keyserlingk, M.A.G. Using behavior to predict and identify ill health in animals. J. Anim. Sci. 2009, 87, 770–777. [Google Scholar] [CrossRef]
- Hart, B.L. Biological basis of the behavior of sick animals. Neurosci. Biobehav. Rev. 1988, 12, 123–127. [Google Scholar] [CrossRef]
- Olechnowicz, J.; Jaskowski, J.M. Behaviour of lame cows: A review. Vet. Med. 2011, 56, 581–588. [Google Scholar] [CrossRef]
- Sepulveda-Varas, P.; Proudfoot, K.L.; Weary, D.M.; von Keyserlingk, M.A.G. Changes in behaviour of dairy cows with clinical mastitis. Appl. Anim. Behav. Sci. 2016, 175, 8–13. [Google Scholar] [CrossRef]
- Siivonen, J.; Taponen, S.; Hovinen, M.; Pastell, M.; Lensink, B.J.; Pyorala, S.; Hannien, L. Impact of acute clinical mastitis on cow behaviour. Appl. Anim. Behav. Sci. 2011, 132, 101–106. [Google Scholar] [CrossRef]
- Holman, A.; Thompson, J.; Routly, J.E.; Cameron, J.; Jones, D.N.; Grove-White, D.; Smith, R.F.; Dobson, H. Comparison of oestrus detection methods in dairy cattle. Vet. Rec. 2011, 169, 47. [Google Scholar] [CrossRef]
- Price, E.; Langford, J.; Fawcett, T.W.; Wilson, A.J.; Croft, D.P. Classifying the posture and activity of ewes and lambs using accelerometers and machine learning on a commercial flock. Appl. Anim. Behav. Sci. 2022, 251, 105630. [Google Scholar] [CrossRef]
- Melville, L.A.; Hayward, A.; Morgan, E.R.; Shaw, D.J.; McBean, D.; Andrews, L.; Morrison, A.; Kenyon, F. Precision worm control in grazing lambs by targeting group treatment based on performance of sentinels. Animal 2021, 15, 100176. [Google Scholar] [CrossRef]

| Descriptive Statistics | Mean | S.D | |
|---|---|---|---|
| FEC (EPG) | 241.85 | 283.8 | |
| Number of daily lying bouts (n/d) | 27.93 | 6.71 | |
| Lying bout duration (min/bout) | 29.75 | 7.31 | |
| Daily lying time (min/d) | 792.79 | 112.76 | |
| BCS | 2.85 | 0.51 | |
| Age | 3.13 | 1.05 | |
| Lamb birth weight (kg) | Single (n = 22) | 6.22 | 1.03 |
| Twins (n = 32) | 4.92 | 0.53 | |
| Behavioural Measurement (Unit) | Estimate | S.E | Z | p |
|---|---|---|---|---|
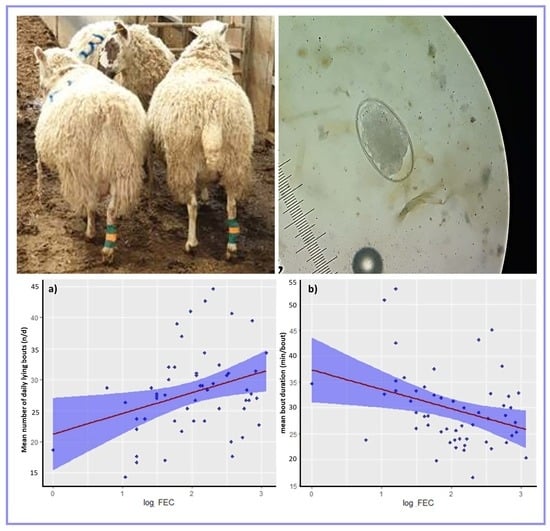
| Mean lying bout duration (min/bout) | −3.745 | 1.451 | −2.582 | 0.010 |
| Mean number of daily lying bouts (n/d) | 3.323 | 1.337 | 2.485 | 0.013 |
| Mean daily lying time (min/d) | 9.763 | 23.673 | 0.412 | 0.680 |
| Dependent Variable | Independent Variable | Estimate | S.E | Z | p |
|---|---|---|---|---|---|
| Mean lying bout duration (min/bout) | Intercept | 37.927 | 9.563 | 3.966 | <0.001 |
| Log FEC | −3.784 | 1.526 | −2.479 | 0.013 | |
| Litter size (Twin) | −0.585 | 2.492 | −0.235 | 0.814 | |
| Litter size (Single) | 0 | - | - | - | |
| BCS | −1.138 | 1.834 | −0.621 | 0.535 | |
| Age | 1.122 | 0.907 | 1.237 | 0.216 | |
| Mean lamb birth weight (kg) | −0.082 | 1.193 | −0.069 | 0.945 | |
| Mean number of daily lying bouts (n/d) | Intercept | 22.631 | 8.545 | 2.648 | 0.008 |
| Log FEC | 3.606 | 1.364 | 2.644 | 0.008 | |
| Litter size (Twin) | 0.203 | 2.226 | 0.091 | 0.927 | |
| Litter size (Single) | 0 | - | - | - | |
| BCS | 2.066 | 1.638 | 1.261 | 0.207 | |
| Age | −1.21 | 0.81 | −1.494 | 0.135 | |
| Mean lamb birth weight (kg) | −0.78 | 1.066 | −0.731 | 0.465 | |
| Mean daily lying time (min/d) | Intercept | 747.646 | 148.199 | 5.045 | <0.001 |
| Log FEC | 9.032 | 23.651 | 0.382 | 0.703 | |
| Litter size (Twin) | 39.287 | 38.613 | 1.017 | 0.309 | |
| Litter size (Single) | 0 | - | - | - | |
| BCS | 41.456 | 28.415 | 1.459 | 0.145 | |
| Age | −24.04 | 14.052 | −1.711 | 0.087 | |
| Mean lamb birth weight (kg) | −7.285 | 18.49 | −0.394 | 0.694 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, E.G.; Davis, C.N.; Williams, M.; Jones, D.L.; Cutress, D.; Williams, H.W.; Brophy, P.M.; Rose, M.T.; Stuart, R.B.; Jones, R.A. Associations between Gastrointestinal Nematode Infection Burden and Lying Behaviour as Measured by Accelerometers in Periparturient Ewes. Animals 2022, 12, 2393. https://doi.org/10.3390/ani12182393
Williams EG, Davis CN, Williams M, Jones DL, Cutress D, Williams HW, Brophy PM, Rose MT, Stuart RB, Jones RA. Associations between Gastrointestinal Nematode Infection Burden and Lying Behaviour as Measured by Accelerometers in Periparturient Ewes. Animals. 2022; 12(18):2393. https://doi.org/10.3390/ani12182393
Chicago/Turabian StyleWilliams, Eiry Gwenllian, Chelsea N. Davis, Manod Williams, Dewi Llyr Jones, David Cutress, Hefin Wyn Williams, Peter M. Brophy, Michael T. Rose, Rebekah B. Stuart, and Rhys Aled Jones. 2022. "Associations between Gastrointestinal Nematode Infection Burden and Lying Behaviour as Measured by Accelerometers in Periparturient Ewes" Animals 12, no. 18: 2393. https://doi.org/10.3390/ani12182393