Ethnobotanical Research and Compilation of the Medicinal Uses in Spain and the Active Principles of Chiliadenus glutinosus (L.) Fourr. for the Scientific Validation of Its Therapeutic Properties
Abstract
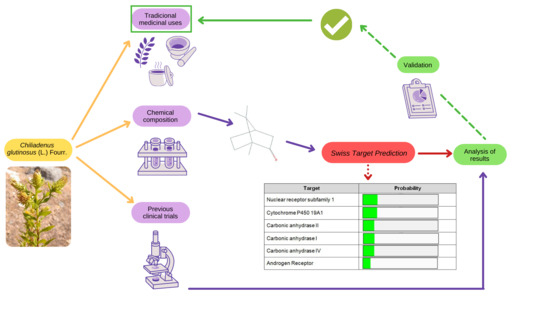
:1. Introduction
2. Results
2.1. Description of Chiliadenus glutinosus (L.) Fourr.
2.2. Compilation of the Medicinal Uses of Chiliadenus glutinosus
2.2.1. Medicinal Uses Applied to the Human Circulatory System
2.2.2. Medicinal Uses Applied to the Human Digestive System
2.2.3. Medicinal Uses Applied to the Human Genitourinary System
2.2.4. Medicinal Uses Applied to the Human Locomotor System
2.2.5. Medicinal Uses Applied to the Human Nervous System
2.2.6. Medicinal Uses Applied to the Human Respiratory System
2.2.7. Medicinal Uses Applied to the Human Integumentary System
2.3. Validation of the Pharmacological Effects of Chiliadenus glutinosus
2.3.1. Phytochemicals from Chiliadenus glutinosus
2.3.2. Experimental Studies on the Biological Activity of Phytochemicals Present in Chiliadenus glutinosus
2.3.3. In Silico Modeling for the Prediction of Bioactive Compound Targets
Camphor
Borneol
Glutinone
Quercetin
Kaempferol
3. Discussion
3.1. Circulatory System
3.2. Digestive System
3.3. Genito-Urinary System
3.4. Locomotor System
3.5. Nervous System
3.6. Respiratory System
3.7. Integumentary System
3.8. Other Biological Activities of Interest Present in the Selected Compounds.
4. Material and Methods
4.1. Sources of Information
4.2. Distribution: Data Collection and Mapping
4.3. Validation Methodology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pardo de Santayana, M.; Morales, R. Consideraciones sobre el género Jasonia (Compositae, Inuleae). Sistemática y usos. Acta Bot. Malacit. 2004, 29, 221–232. [Google Scholar] [CrossRef]
- Gómiz García, F.; Morales Valverde, R. Acerca de Jasonia hesperia Maire & Wilczek (Asteraceae) y las especies norteafricanas de este género. Acta Bot. Malacit. 2006, 31, 81–87. [Google Scholar] [CrossRef]
- Guillén Bas, A.; Ferrer-Gallego, P.P.; Roselló Gimeno, R.; Gómez Navarro, J.; Laguna Lumbreras, E.; Peris, J.B. Jasonia glutinosa subsp. congesta subsp. nov. (Compositae, Inuleae). Fl. Montiber. 2013, 55, 76–80. [Google Scholar] [CrossRef]
- Lanfranco, S.; Lanfranco, E.; Westermeier, R.; Zammit, M.-A.; Mifsud, M.-A.; Xiberras, J. The vascular flora of the Maltese Islands. In Islands and Plants: Preservation and Understanding of Flora on Mediterranean Islands, 1st ed.; Consell Insular de Menorca, Ed.; Institut Menorquí d’Estudis: Menorca, Spain, 2013; pp. 261–268. [Google Scholar]
- Kew Gardens; Missouri Botanical Garden. The Plant List, a Working List of All Plant Species. Available online: http://www.theplantlist.org/ (accessed on 8 May 2020).
- Species 2000; Sistema Integrado de Información Taxonómica. Catalogue of Life: 2019 Annual Checklist. Available online: http://www.catalogueoflife.org/ (accessed on 8 May 2020).
- Muñoz Centeno, L.; Rico, E. Chiliadenus Cass. In Flora Iberica. Plantas Vasculares de la Península Ibérica e Islas Baleares. Vol. XVI (III) Compositae (Partim), 1st ed.; Real Jardín Botánico, Consejo Superior de Investigaciones Científicas: Madrid, Spain, 2019; pp. 2092–2095. [Google Scholar]
- De Laguna, A. Pedacio Dioscorides Anazarbeo, Acerca de la Materia Medicinal y de los Venenos Mortíferos, 1st ed.; Hans de Laet: Amberes, Belgium, 1555. [Google Scholar]
- Clusius, C. Rariorum Aliquot Stirpium per Hispanias Observatarum Historia, 1st ed.; Christophori Plantini: Amberes, Belgium, 1576. [Google Scholar]
- Scalinger Institute. Carolus Clusius (1526–1609): A life. Available online: https://web.archive.org/web/20050105153146/http://ub.leidenuniv.nl/Bc/scaligerinstitute/clusius/biography.html (accessed on 28 July 2020).
- Quer y Martínez, J. Flora Española ó Historia de las Plantas que se Crían en España, 1st ed.; Joachin Ibarra: Madrid, Spain, 1762. [Google Scholar]
- Von Linné, C. Parte Práctica de Botánica del Caballero Carlos Linneo, 1st ed.; Imprenta Real: Madrid, Spain, 1784. [Google Scholar]
- Loscos Bernal, F. Tratado de Plantas de Aragón, 3rd ed.; Establecimiento Tipográfico del Hospicio: Madrid, Spain, 1867. [Google Scholar]
- Laguia Minguillon, M.P. Los botánicos aragoneses del siglo XIX. In La Ciencia y la Técnica en España Entre 1850 y 1936: Comunicaciones; Hormigón Blánquez, M., Ed.; Sociedad Española de Historia de las Ciencias y de las Técnicas: Jaca, Spain, 1984; pp. 227–248. [Google Scholar]
- Gadow, H. Northern Spain, 1st ed.; Adam & Charles Black: London, UK, 1897. [Google Scholar]
- Agencia española de Medicamentos y Productos Sanitarios. Real Farmacopea Española, 3rd ed.; Ministerio de Sanidad y Consumo: Madrid, Spain, 2005.
- Pardo de Santayana, M.; Morales, R.; Aceituno, L.; Molina, M. Inventario Español de Los Conocimientos Tradicionales Relativos a La Biodiversidad; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2014.
- Villar, L.; Palacín, J.M. Estudis etnobotànics al Pirineu Aragonès i les altres terres d’Osca. Semin. Estud. Univ. 1994, 8, 44–49. Available online: http://hdl.handle.net/10261/54157 (accessed on 8 May 2020).
- García-Baquero Moneo, G. Especies vegetales (plantas vasculares) de interés medicinal presentes en La Rioja. In Investigación Humanística y Científica en La Rioja: Homenaje a Julio Luis Fernández Sevilla y Mayela Balmaseda Aróspide; Institutos de Estudios Riojanos: Logroño, Spain, 2000; pp. 369–379. [Google Scholar]
- Tardío, J.; Pascual, H.; Morales, R. Wild food plants traditionally used in the province of Madrid, central Spain. Econ. Bot. 2005, 59, 122–136. [Google Scholar] [CrossRef]
- Benítez, G.; González-Tejero, M.R.; Molero-Mesa, J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): Ethnopharmacological synthesis. J. Ethnopharmacol. 2010, 129, 87–105. [Google Scholar] [CrossRef]
- Menendez-Baceta, G.; Aceituno-Mata, L.; Molina, M.; Reyes-García, V.; Tardío, J.; Pardo-de Santayana, M. Medicinal plants traditionally used in the northwest of the Basque Country (Biscay and Alava), Iberian Peninsula. J. Ethnopharmacol. 2014, 152, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Amengual i Vicens, J.C. Flora Medicinal de les Illes Balears. Ph.D. Thesis, Universitat de les Illes Balears, Palma, Spain, 2017. Available online: http://hdl.handle.net/11201/148295 (accessed on 9 May 2020).
- Villaescusa Castillo, L.; Diaz Lanza, A.M.; Faure, R.; Debrauwer, L.; Elias, R.; Balansard, G. Two sesquiterpenoids, lucinone and glutinone, from Jasonia glutinosa. Phytochemistry 1995, 40, 1193–1195. [Google Scholar] [CrossRef]
- Valero, M.S.; Berzosa, C.; Langa, E.; Gómez-Rincón, C.; López, V. Jasonia glutinosa D.C (“Rock Tea”): Botanical, phytochemical and pharmacological aspects. Bol. Latinoam. Caribb. Plant. Med. Aromat. 2013, 12, 543–557. [Google Scholar]
- Guillén, M.D.; Ibargoitia, M.L. Volatile components obtained from the leaves of Jasonia glutinosa. Food Chem. 1996, 56, 155–158. [Google Scholar] [CrossRef]
- Esri. ArcGIS (10.5.1). Available online: https://desktop.arcgis.com/es/arcmap/10.5/get-started/setup/arcgis-desktop-quick-start-guide.htm (accessed on 8 May 2020).
- Global Biodiversity Information Facility (GBIF). Chiliadenus glutinosus Fourr. Available online: https://www.gbif.org/species/3089839 (accessed on 31 August 2020).
- Muñoz Centeno, L.M. Plantas medicinales españolas: Jasonia glutinosa (L.) DC. (Asteraceae) (Té de roca). Acta Bot. Malacit. 2003, 28, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Uribe-Echebarría, P.M. Jasonia glutinosa (L.) DC. Available online: http://floragon.ipe.csic.es/ficha.php?genero=Jasonia&especie=glutinosa&subespecie=&variedad= (accessed on 16 June 2020).
- Aizpuru, I.; Aseginolaza, C.; Uribe-Echebarría, P.M.; Urrutia, P.; Zorrakin, I. Claves Ilustradas de La Flora Del País Vasco y Territorios Limítrofes, 4th ed.; Servicio Central de Publicaciones del Gobierno Vasco: Vitoria-Gasteiz, Spain, 2015. [Google Scholar]
- Guzmán Tirado, M.A. Aproximación a la Etnobotánica de la Provincia de Jaén. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1997. Available online: https://bibdigital.rjb.csic.es/idurl/1/1526072 (accessed on 10 May 2020).
- Fernández Ocaña, A.M. Estudio Etnobotánico en el Parque Natural de la Sierra de Cazorla, Segura y las Villas. Investigación Química de un Grupo de Especies Interesantes. Ph.D. Thesis, Universidad de Jaén, Jaén, Spain, 2000. Available online: http://hdl.handle.net/10953/326 (accessed on 8 May 2020).
- Akerreta, S.; Calvo, M.I.; Cavero, R.Y. Sabiduría Popular y Plantas Curativas. Recopilación Extraída de un Estudio Etnobotánico en Navarra, 1st ed.; Ediciones i (Integralia La casa natural S.L.): Madrid, Spain, 2013. [Google Scholar]
- Obón de Castro, C.; Rivera Nuñez, D. Las Plantas Medicinales de Nuestra Región, 1st ed.; Editora Regional de Murcia: Murcia, Spain, 1991. [Google Scholar]
- Panareda, J.M.; Masnou, J.; Boccio, M. La distribució de les plantes rupícoles al parc natural del Montseny. Monogr. Montseny 2010, 25, 329–343. [Google Scholar]
- Villar Perez, L. Los Saberes Científico y Popular en Torno a las Plantas del Pirineo Aragonés. Un Ejemplo de Biodiversidad Cultural; Academia de Ciencias Exactas, Físicas, Químicas y Naturales de Zaragoza: Zaragoza, Spain, 2003; Volume 23, pp. 1–41. Available online: http://hdl.handle.net/10261/58274 (accessed on 11 May 2020).
- Akerreta, S.; Cavero, R.Y.; López, V.; Calvo, M.I. Analyzing factors that influence the folk use and phytonomy of 18 medicinal plants in Navarra. J. Ethnobiol. Ethnomed. 2007, 3, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo, M.I.; Akerreta, S.; Cavero, R.Y. Pharmaceutical ethnobotany in the riverside of Navarra (Iberian Peninsula). J. Ethnopharmacol. 2011, 135, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.I.; Akerreta, S.; Cavero, R.Y. The pharmacological validation of medicinal plants used for digestive problems in Navarra, Spain. Eur. J. Integr. Med. 2013, 5, 537–546. [Google Scholar] [CrossRef]
- Alarcón, R.; Pardo-De-Santayana, M.; Priestley, C.; Morales, R.; Heinrich, M. Medicinal and local food plants in the south of Alava (Basque Country, Spain). J. Ethnopharmacol. 2015, 176, 207–224. [Google Scholar] [CrossRef] [Green Version]
- Menendez Baceta, G. Etnobotánica de las Plantas Silvestres Comestibles y Medicinales en Cuatro Comarcas de Araba y Bizkaia. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2015. Available online: http://hdl.handle.net/10486/667855 (accessed on 8 May 2020).
- Las Heras Etayo, N. Estudio Etnobotánico Sobre los Usos Tradicionales de las Especies Vegetales en el Valle del Cidacos, La Rioja; Trabajo Fin de Grado, Universidad de León: León, Spain, 2019. [Google Scholar]
- Museo de Ecología Humana. La Herencia del Conocimiento Tradicional de las Plantas Medicinales: Té de Roca, Sebúlcor, Segovia. Available online: http://museoecologiahumana.org/obras/la-herencia-del-conocimiento-tradicional-de-las-plantas-medicinales/ (accessed on 1 July 2020).
- Fresquet Febrer, J.L.; Blanquer Roselló, G.; Galindo Dobón, M.; Gallego Estrada, F.; García de la Cuadra Arizo, R.; López Bueno, J.A.; Sanjosé, P.A. Inventario de las plantas medicinales de uso popular en la ciudad de Valencia. Med. Cienc. Soc. 2001, 13, 1–25. [Google Scholar]
- Peris Gisbert, J.B. Etnobotánica Farmacológica Valenciana. An. R. Acad. Med. Comunitat Valencia. 2013, 14, 1–22. [Google Scholar]
- Segarra Durá, E. Etnobotánica farmacéutica del Campo de Turia y de los Serranos. Ph.D. Thesis, Universidad de Valencia, Valencia, Spain, 2015. Available online: http://hdl.handle.net/10550/50596 (accessed on 10 May 2020).
- Gómez Gutiérrez, A. Aproximación Etnobotánica de la Comarca Alicantina del Medio-Alto Vinalopó. Trabajo de Fin de Grado, Universitat d’Alacant, Spain. 2014. Available online: http://hdl.handle.net/10045/40164 (accessed on 8 May 2020).
- Rojo, J.; García Carrero, P.; García López, E.; Pérez Badia, R. Estudio Etnobotánico del Municipio de Enguídanos (Cuenca); Instituto de Ciencias Ambientales de Castilla-La Mancha: Cuenca, Spain, 2007. [Google Scholar]
- Esteso Esteso, F. Vegetación y Flora del Campo de Montiel: Interés Farmacéutico; Instituto de Estudios Albacetenses: Albacete, Spain, 1992. [Google Scholar]
- Benítez Cruz, G. Etnobotánica y Etnobiología del Poniente Granadino. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 2009. Available online: http://hdl.handle.net/10481/2163 (accessed on 8 May 2020).
- González-Tejero García, M.R. Investigaciones Etnobotánicas en la Provincia de Granada. PhD Thesis, Universidad de Granada, Granada, Spain, 1989. Available online: http://hdl.handle.net/10481/6493 (accessed on 8 May 2020).
- Pieroni, A.; Price, L.L. Eating and Healing: Tradicional Food as Medicine, 1st ed.; Haworth Press: New York, NY, USA, 2006. [Google Scholar]
- Villar Perez, L.; Palacin Latorre, J.M.; Calvo Eito, C.; Gomez Garcia, D.; Montserrat Marti, G. Plantas Medicinales del Pirineo Aragonés y Demás Tierras Oscenses, 1st ed.; CSIC-Instituto Pirenaico de Ecología (IPE): Zaragoza, Spain, 1987; Available online: http://hdl.handle.net/10261/101858 (accessed on 10 May 2020).
- Cavero, R.Y.; Akerreta, S.; Calvo, M.I. Pharmaceutical ethnobotany in the middle Navarra (Iberian Peninsula). J. Ethnopharmacol. 2011, 137, 844–855. [Google Scholar] [CrossRef]
- Aceituno Mata, L. Estudio Etnobotánico y Agroecológico de la Sierra Norte de Madrid. Ph.D. Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2010. Available online: https://bibdigital.rjb.csic.es/idurl/1/1526028 (accessed on 8 May 2020).
- Verde López, A. Estudio Etnofarmacológico de Tres Áreas de Montaña de Castilla-La Mancha. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2002. Available online: https://bibdigital.rjb.csic.es/idurl/1/1526060 (accessed on 8 May 2020).
- Fajardo, J.; Verde, A.; Rivera, D.; Obón, C. Las Plantas en la Cultura Popular de la Provincia de Albacete, 1st ed.; Instituto de Estudios Albacetenses “Don Juan Manuel”: Albacete, Spain, 2000. [Google Scholar]
- Burguet Zamit, J. Etnobotánica del Municipio de Alcalá de la Selva en la Sierra de Gúdar-Javalambre (Teruel). Trabajo de Fin de Grado, Universitat Politècnica de València, Spain. 2017. Available online: http://hdl.handle.net/10251/87169 (accessed on 8 May 2020).
- Calvo, M.I.; Cavero, R.Y. Medicinal plants used for neurological and mental disorders in Navarra and their validation from official sources. J. Ethnopharmacol. 2015, 169, 263–268. [Google Scholar] [CrossRef]
- Raja, D.; Blanché, C.; Xirau, J.V. Contribution to the knowledge of the pharmaceutical ethnobotany of la Segarra region (Catalonia, Iberian Peninsula). J. Ethnopharmacol. 1997, 57, 149–160. [Google Scholar] [CrossRef]
- PubChem Identifier: CID 2537. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2537#section=2D-Structure (accessed on 17 June 2020).
- PubChem Identifier: CID 64685. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/64685#section=2D-Structure (accessed on 17 June 2020).
- PubChem Identifier: CID 5280343. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280343#section=2D-Structure (accessed on 17 June 2020).
- PubChem Identifier: CID 10071029. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/10071029#section=2D-Structure (accessed on 17 June 2020).
- PubChem Identifier: CID 44584275. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/44584275#section=2D-Structure (accessed on 17 June 2020).
- PubChem Identifier: CID 11196115. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/11196115#section=2D-Structure (accessed on 17 June 2020).
- PubChem Identifier: CID 5280863. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5280863#section=2D-Structure (accessed on 17 June 2020).
- Ehrnhöfer-Ressler, M.M.; Fricke, K.; Pignitter, M.; Walker, J.M.; Walker, J.; Rychlik, M.; Somoza, V. Identification of 1,8-cineole, borneol, camphor, and thujone as anti-inflammatory compounds in a Salvia officinalis L. infusion using human gingival fibroblasts. J. Agric. Food Chem. 2013, 61, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Belz, G.G.; Loew, D. Dose-response related efficacy in orthostatic hypotension of a fixed combination of D-camphor and an extract from fresh Crataegus berries and the contribution of the single components. Phytomedicine 2003, 10, 61–67. [Google Scholar] [CrossRef]
- Schandry, R.; Lindauer, D.; Mauz, M. Blood pressure and cognitive performance after a single administration of a camphor-Crataegus combination in adolescents with low blood pressure. Planta Med. 2018, 84, 1249–1254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, J.; Chen, J.; Liao, S.; Li, L.; Zhu, L.; Chen, L. Composition and biological activities of the essential oil extracted from a novel plant of Cinnamomum camphora Chvar. borneol. J. Med. Plants Res. 2012, 6, 3487–3494. [Google Scholar] [CrossRef]
- Kotaka, T.; Kimura, S.; Kashiwayanagi, M.; Iwamoto, J. Camphor induces cold and warm sensations with increases in skin and muscle blood flow in human. Biol. Pharm. Bull. 2014, 37, 1913–1918. [Google Scholar] [CrossRef] [Green Version]
- Guedes da Silva Almeida, J.R.; Rocha Souza, G.; Cabral Silva, J.; Gomes de Lima Saraiva, S.R.; Gonçalves de Oliveira Júnior, R.; De Siqueira Quintans, J.S.; De Siqueira Barreto, R.S.; Rigoldi Bonjardim, L.; De Sócrates, C.H.C.; Quintans Junior, L.J. Borneol, a bicyclic monoterpene alcohol, reduces nociceptive behavior and inflammatory response in mice. Sci. World J. 2013, 4, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Tabanca, N.; Kirimer, N.; Demirci, B.; Demirci, F.; Can Başer, K.H. Composition and antimicrobial activity of the essential oils of Micromeria cristata subsp. phrygia and the enantiomeric distribution of borneol. J. Agric. Food Chem. 2001, 49, 4300–4303. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Hu, J.; Jia, Q.; Xu, W.; Su, D.; Song, H.; Xu, Z.; Cui, J.; Zhou, M.; et al. A clinical and mechanistic study of topical borneol-induced analgesia. EMBO Mol. Med. 2017, 9, 802–815. [Google Scholar] [CrossRef]
- Chen, Z.; Xu, Q.; Shan, C.; Shi, Y.; Wang, Y.; Chang, R.C.-C.; Zheng, G. Borneol for regulating the permeability of the blood-brain barrier in experimental ischemic stroke: Preclinical evidence and possible mechanism. Oxid. Med. Cell. Longev. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Li, Y.H.; Sun, X.P.; Zhang, Y.Q.; Wang, N.S. The antithrombotic effect of borneol related to its anticoagulant property. Am. J. Chin. Med. 2008, 36, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Álvarez Castro, E.; Orallo Cambeiro, F. Actividad biológica de los flavonoides (II). Acción cardiovascular y sanguínea. Offarm 2003, 22, 102–107. [Google Scholar]
- García-Mateos, R.; Aguilar-Santelises, L.; Soto-Hernández, M.; Nieto-Angel, R.; Kite, G. Total phenolic compounds, flavo-noids and antioxidant activity in the flowers of Crataegus spp. from México. Agrociencia 2012, 46, 651–662. [Google Scholar]
- García-Mateos, R.; Ibarra-Estrada, E.; Nieto-Angel, R. Antioxidant compounds in hawthorn fruits (Crataegus spp.) of Mexico. Rev. Mex. Biodivers. 2013, 84, 1298–1304. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Dabeek, W.M.; Ventura Marra, M. Dietary quercetin and kaempferol: Bioavailability and potential cardiovascular-related bioactivity in humans. Nutrients 2019, 11, 2288. [Google Scholar] [CrossRef] [Green Version]
- Ji, M.; Gong, X.; Li, X.; Wang, C.; Li, M. Advanced research on the antioxidant activity and mechanism of polyphenols from Hippophae species. Molecules 2020, 25, 917. [Google Scholar] [CrossRef] [Green Version]
- Fariña Flores, D. Obtención de Flavonoides de Plantas Superiores. Actividad Biológica. Trabajo de Fin de Grado, Universidad de La Laguna, Santa Cruz de Tenerife, Spain. 2016. Available online: http://riull.ull.es/xmlui/handle/915/2674 (accessed on 8 May 2020).
- Morales, M.A.; Lozoya, X. Calcium-antagonists effects of quercetin on aortic smooth muscle. Planta Med. 1994, 60, 313–317. [Google Scholar] [CrossRef]
- Ventura-Martínez, R.; Ángeles-López, G.E.; Rodríguez, R.; González-Trujano, M.E.; Déciga-Campos, M. Spasmolytic effect of aqueous extract of Tagetes erecta L. flowers is mediated through calcium channel blockade on the guinea-pig ileum. Biomed. Pharmacother. 2018, 103, 1552–1556. [Google Scholar] [CrossRef]
- Janssen, P.K.; Mensink, R.P.; Cox, F.J.J.; Harryvan, J.L.; Hovenier, R.; Hollman, P.C.; Katan, M.B. Effects of the flavo-noids quercetin and apigenin on hemostasis in healthy volunteers: Results from an in vitro and a dietary supplement study. Am. J. Clin. Nutr. 1998, 67, 255–262. [Google Scholar] [CrossRef]
- Hubbard, G.P.; Wolffram, S.; Lovegrove, J.A.; Gibbins, J.M. Ingestion of quercetin inhibits platelet aggregation and essential components of the collagen-stimulated platelet activation pathway in humans. J. Thromb. Haemost. 2004, 2, 2138–2145. [Google Scholar] [CrossRef]
- Nieman, D.C.; Henson, D.A.; Gross, S.J.; Jenkins, D.P.; Davis, J.M.; Murphy, E.A.; Carmichael, M.D.; Dumke, C.L.; Utter, A.C.; Mcanulty, S.R.; et al. Quercetin reduces illness but not immune perturbations after intensive exercise. Med. Sci. Sports Exerc. 2007, 39, 1561–1569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, B.; Wang, C.; Zhu, Q.; Mo, Z. Mechanism of quercetin as an antidiarrheal agent. Di Yi Jun Yi Da Xue Xue Bao 2003, 23, 1029–1031. [Google Scholar]
- Jun, S.P.; Ho, S.R.; Duck, H.K.; Ih, S.C. Enzymatic preparation of kaempferol from green tea seed and its antioxidant activity. J. Agric. Food Chem. 2006, 54, 2951–2956. [Google Scholar] [CrossRef]
- Singh, R.; Singh, B.; Singh, S.; Kumar, N.; Kumar, S.; Arora, S. Anti-free radical activities of kaempferol isolated from Acacia nilotica (L.) Willd. Toxicol. Vitr. 2008, 22, 1965–1970. [Google Scholar] [CrossRef]
- Devi, K.P.; Malar, D.S.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Nabavi, S.M.; Daglia, M. Kaempferol and inflammation: From chemistry to medicine. Pharmacol. Res. 2015, 99, 1–10. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Shah, Z.A.; Saeed, F.; Imran, A.; Arshad, M.U.; Ahmad, B.; Bawazeer, S.; Atif, M.; Peters, D.G.; et al. Chemo-preventive and therapeutic effect of the dietary flavonoid kaempferol: A comprehensive review. Phyther. Res. 2018, 1, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Montaño, J.M.; Burgos-Morón, E.; Pérez-Guerrero, C.; López-Lázaro, M. A Review on the dietary flavonoid kaempferol. Mini Rev. Med. Chem. 2011, 11, 298–344. [Google Scholar] [CrossRef]
- Chen, A.Y.; Chen, Y.C. A Review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Salehi, B.; Sharifi-rad, J.; Gondal, T.A.; Arshad, M.U.; Khan, H.; Guerreiro, S.G. Kaempferol: A key emphasis to its anticancer potential. Molecules 2019, 24, 2277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.K.; Chin, K.Y.; Ima-Nirwana, S. The osteoprotective effects of kaempferol: The evidence from in vivo and in vitro studies. Drug Des. Dev. Ther. 2019, 13, 3497–3514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorginzadeh, M.; Vahdat, M. Smooth muscle relaxant activity of Crocus sativus (saffron) and its constituents: Possible mechanisms. Avicenna J. Phytomed. 2015, 5, 365–375. [Google Scholar]
- Swiss Institute of Bioinformatics (SIB). Swiss Target Prediction. Available online: http://swisstargetprediction.ch/ (accessed on 31 August 2020).
- Aguilera Carbonell, L.M.; Casas Úbeda, J.M.; Cerezo Gallego, J.M.; Chaves González, F.; Garrido Garrido, J.L.; Javaloyes Tarí, E.; Majadas García, A.; Martín Peña, A.; Romero Sánchez, J.; Vives Boix, F.; et al. La Enciclopedia Del Estudiante—09 Ciencias de La Vida, 1st ed.; Santilla Educación, S.L.: Madrid, Spain, 2005. [Google Scholar]
- Sepúlveda Saavedra, J. Texto Atlas Histología Biología Celular y Tisular, 2nd ed.; McGraw-Hill Interamericana de España: Nuevo León, Mexico, 2015. [Google Scholar]
- Junta de Castilla y León—Consejería de Salud. Nuestro Aparato Respiratorio: ¿Cómo es y Cómo Funciona? Available online: https://www.saludcastillayleon.es/AulaPacientes/es/guia-asma/aparato-respiratorio-funciona (accessed on 1 July 2020).
- Página web Concepto Definición. Sistema Genitourinario. Available online: https://conceptodefinicion.de/sistema-genitourinario/ (accessed on 1 July 2020).
- González Romero, M.A.; Villaescusa Castillo, L.; Díaz Lanza, A.M.; Arribas Bricio, J.M.; Soria Monzón, C.A.; Sanz Perucha, J. Volatile composition of Jasonia glutinosa D. C. Z. Nat. 2003, 58, 804–806. [Google Scholar] [CrossRef]
- Knekt, P.; Isotupa, S.; Rissanen, H.; Heliövaara, M.; Järvinen, R.; Häkkinen, S.; Aromaa, A.; Reunanen, A. Quercetin intake and the incidence of cerebrovascular disease. Eur. J. Clin. Nutr. 2000, 54, 415–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective effects of Gynostemma pentaphyllum (var. Ginpent) against lipopolysaccharide-induced inflammation and motor alteration in mice. Molecules 2021, 26, 570. [Google Scholar] [CrossRef]
- Álvarez Leyva, C.A. Valoración de la Actividad Anticonvulsionante del Borneol en Rata Wistar. Tesis Profesional, Universidad de Guadalajara, Guadalajara, Mexico, 1992. Available online: http://repositorio.cucba.udg.mx:8080/xmlui/handle/123456789/3028 (accessed on 8 May 2020).
- Eryiğit, T.; Okut, N.; Ekici, K.; Yildirim, B. Chemical composition and antibacterial activities of Juniperus horizontalis essential oil. Can. J. Plant Sci. 2014, 94, 323–327. [Google Scholar] [CrossRef]
- Petrović, A.; Milutinović, M.M.; Petri, E.T.; Živanović, M.; Milivojević, N.; Puchta, R.; Scheurer, A.; Korzekwa, J.; Klisurić, O.R.; Bogojeski, J. Synthesis of camphor-derived bis(pyrazolylpyridine) rhodium(III) complexes: Structure-reactivity relation-ships and biological activity. Inorg. Chem. 2019, 58, 307–319. [Google Scholar] [CrossRef]
- Lax Vivancos, V. Estudio de la Variabilidad Química, Propiedades Antioxidantes y Biocidas de Poblaciones Espontáneas de Rosmarinus officinalis L. en la Región de Murcia. Ph.D. Thesis, Universidad de Murcia, Murcia, Spain, 2014. Available online: http://hdl.handle.net/10803/284820 (accessed on 10 May 2020).
- Armaka, M.; Papanikolaou, E.; Sivropoulou, A.; Arsenakis, M. Antiviral properties of isoborneol, a potent inhibitor of herpes simplex virus type 1. Antivir. Res. 1999, 43, 79–92. [Google Scholar] [CrossRef]
- Sánchez Gallego, J.I. Efecto de la Quercetina y la Rutina Frente al Daño Oxidativo Inducido en Eritrocitos con Distintos Con-Tenidos de Colesterol. Ph.D. Thesis, Universidad de Salamanca, Salamanca, Spain, 2009. Available online: http://hdl.handle.net/10366/76297 (accessed on 9 May 2020).
- Álvarez Castro, E.; Orallo Cambeiro, F. Actividad biológica de los flavonoides (I). Acción frente al cáncer. Offarm 2003, 22, 130–135. [Google Scholar]
- PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 29 November 2020).



| Remedy or Use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| Raise blood pressure | Infusion | Internal | Catalonia [17] |
| Lower blood pressure | Andalusia [17,32] | ||
| Give fluidity to the blood | Aragon [17] Castilla-La Mancha [17] Andalusia [33] | ||
| Purify the blood | Navarre [34] | ||
| Anemia | Region of Murcia [35] | ||
| Improve circulation | Region of Murcia [35] Andalusia [33] | ||
| Weak heart | Castilla-La Mancha [17] | ||
| Heart ailments | Andalusia [33] | ||
| Varicose veins | Castilla-La Mancha [17] | ||
| Relieve leg swelling | Infusion | Valencian Community [1] | |
| Decoction | Region of Murcia [17,35] |
| Remedy or Use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| Digestive | Macerate in anise | Internal | Navarre [34] |
| Catalonia [1] | |||
| Infusion | Catalonia [36] Aragon [18,37] Navarre [38,39,40] Basque Country [22,41,42] La Rioja [43] Castile and Leon [44] Valencian Community [45,46,47,48] Castilla-La Mancha [49,50] Andalusia [33,51,52] Cantabria [53] | ||
| Diarrhea | Infusion | Aragon [54] Navarre [40] Basque Country [22,41,42] Valencian Community [1,45,46,47] Andalusia [17] | |
| Appendicitis | Aragon [54] | ||
| Stomach ache | Navarre [34,55] Basque Country [22,41,42] Valencian Community [45,46,47] Community of Madrid [56] Castilla-La Mancha [57] | ||
| Stomach ulcers | Region of Murcia [35] Castilla-La Mancha [58] Andalusia [17,33] | ||
| Gasses | Valencian Community [1,46] Andalusia [33] | ||
| Indigestion or heaviness | Region of Murcia [35] | ||
| Stimulate the appetite | Valencian Community [47] Region of Murcia [35] | ||
| Digestive problems or indisposition (in general) | Aragon [54,59] Valencian Community [47,48] Region of Murcia [35] Balearic Islands [23] Castilla-La Mancha [49,57,58] Andalusia [21,32] | ||
| Help to vomit | Infusion (high doses) | Valencian Community [1] |
| Remedy or use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| Diuretic | Infusion | Internal | Region of Murcia [17] Castilla-La Mancha [17,58] Cantabria [53] |
| Kidney pain | Region of Murcia [17] Castilla-La Mancha [17,58] | ||
| Kidney stones | Region of Murcia [17] Castilla-La Mancha [17,58] | ||
| Kidney diseases (in general) | Region of Murcia [35] | ||
| Improve the kidney | Andalusia [33] |
| Remedy or Use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| Rheumatism/bone pain | Cocimiento | External (baths) | Andalusia [33] |
| Macerated in alcohol | External (rubbing) | Region of Murcia [35] | |
| Fatigue | Infusion | Internal | Castilla-La Mancha [57,58] |
| Remedy or Use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| The nerves | Infusion | Internal | Catalonia [1] Valencian Community [45] Castilla-La Mancha [1] |
| Lift mood | Navarre [34,55] | ||
| Clear the mind | Navarre [34,55] | ||
| Headache | Navarre [60] Castilla-La Mancha [49] Andalusia [32] |
| Remedy or Use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| Colds and flu | Infusion/decoction | Internal | Catalonia [17,36,61] Aragon [54] Navarre [34,39] Valencian Community [45,47] Region of Murcia [17] Castilla-La Mancha [50,58] Andalusia [32,33] Cantabria [53] |
| Sore throats | Catalonia [17] Region of Murcia [17] Andalusia [33] | ||
| Bronchial ailments (bronchitis) | Decoction | Andalusia [33] | |
| Infusion | Castilla-La Mancha [17] | ||
| Asthmatic processes | Infusion | Castilla-La Mancha [17] | |
| Asthmatic processes (bronchodilator) | Decoction vapors | Castilla-La Mancha [17,57,58] | |
| Respiratory infections (in general) | Infusion/decoction | Balearic Islands [23] |
| Remedy or Use | Form of Employment | Administration | Autonomous Community |
|---|---|---|---|
| Skin pimples | Poultice (fresh leaves) | External | Navarre [34] |
| Disinfect wounds/bruises | Washings (infusion) | Region of Murcia [35] | |
| Washings (decoction) | |||
| Ointment | Castilla-La Mancha [17] | ||
| Insect bites | Decoction | Castilla-La Mancha [57] | |
| Skin burns | Plaster | Castilla-La Mancha [57] | |
| Decoction | Castilla-La Mancha [57] | ||
| Anti-inflammatory for wounds | Infusion/Decoction | Andalusia [32] |
| Chemical Group | Phytochemical | Biological Activity |
|---|---|---|
| Monoterpenes | Camphor | Anti-inflammatory [69] Antihypotensive [70,71] Antioxidant [72] Antibacterial [72] Antiparasitic [25] Topical vasodilator [73] |
| Borneol | Anti-inflammatory [69,74] Antioxidant [25,72] Antibacterial [72,75] Antifungal [72,75] Antinociceptive [74] Topical analgesic [76] Neuroprotective [72] Antispasmodic [77] Choleretic [75] Tranquilizer [75] Anticoagulant [78] Antiparasitic [25] | |
| Sesquiterpenes | Lucinone | Anti-inflammatory [25] |
| Glutinone | Anti-inflammatory [25] | |
| Kutdtriol | Anti-inflammatory [25] Antiparasitic [25] | |
| Flavonols | Quercetin | Antioxidant [79,80,81,82,83,84] Anti-inflammatory [82,83,85] Antiviral [85] Spasmolytic [25,86,87] Antithrombotic [88,89] Antipathogenic [85,90] Antidiabetic [91] Antidiarrheal [92] Antihypertensive [86] |
| Kaempferol | Antioxidant [25,80,83,93,94] Anti-inflammatory [83,95,96] Spasmolytic [25] Antidiabetic [91] Anticancer [96,97,98,99] Cardioprotective [83,97] Osteoprotective [100] Relaxing [101] |
| Target | Common Name | Uniprot ID | ChEMBL ID | Target Class | Probability |
|---|---|---|---|---|---|
| Nuclear receptor subfamily 1 group I member 3 (by homology) | NR1I3 | Q14994 | CHEMBL5503 | Nuclear receptor | 0.2 |
| Cytochrome P450 19A1 | CYP19A1 | P11511 | CHEMBL1978 | Cytochrome P450 | 0.19 |
| Carbonic anhydrase II | CA2 | P00918 | CHEMBL205 | Lyase | 0.15 |
| Carbonic anhydrase I | CA1 | P00915 | CHEMBL261 | Lyase | 0.15 |
| Carbonic anhydrase IV | CA4 | P22748 | CHEMBL3729 | Lyase | 0.15 |
| Androgen Receptor | AR | P10275 | CHEMBL1871 | Nuclear receptor | 0.10 |
| Target | Common Name | Uniprot ID | ChEMBL ID | Target Class | Probability |
|---|---|---|---|---|---|
| Carbonic anhydrase II | CA2 | P00918 | CHEMBL205 | Lyase | 0.36 |
| Carbonic anhydrase I | CA1 | P00915 | CHEMBL261 | Lyase | 0.36 |
| Carbonic anhydrase IV | CA4 | P22748 | CHEMBL3729 | Lyase | 0.36 |
| Transient receptor potential cation channel subfamily M member 8 | TRPM8 | Q7Z2W7 | CHEMBL1075319 | Voltage-gated ion channel | 0.31 |
| Target | Common Name | Uniprot ID | ChEMBL ID | Target Class | Probability |
|---|---|---|---|---|---|
| Cyclooxygenase-1 | PTGS1 | P23219 | CHEMBL221 | Oxidoreductase | 1 |
| Cytochrome P450 19A1 | CYP19A1 | P11511 | CHEMBL1978 | Cytochrome P450 | 0.54 |
| Cathepsin D | CTSD | P07339 | CHEMBL2581 | Protease | 0.23 |
| Target | Common Name | Uniprot ID | ChEMBL ID | Target Class | Probability |
|---|---|---|---|---|---|
| NADPH oxidase 4 | NOX4 | Q9NPH5 | CHEMBL1250375 | Enzyme | 1 |
| Vasopressin V2 receptor | AVPR2 | P30518 | CHEMBL1790 | Family A G protein-coupled receptor | 1 |
| Aldose reductase | AKR1B1 | P15121 | CHEMBL1900 | Enzyme | 1 |
| Xanthine dehydrogenase | XDH | P47989 | CHEMBL1929 | Oxidoreductase | 1 |
| Monoamine oxidase A | MAOA | P21397 | CHEMBL1951 | Oxidoreductase | 1 |
| Insulin-like growth factor I receptor | IGF1R | P08069 | CHEMBL1957 | Kinase | 1 |
| Target | Common Name | Uniprot ID | ChEMBL ID | Target Class | Probability |
|---|---|---|---|---|---|
| NADPH oxidase 4 | NOX4 | Q9NPH5 | CHEMBL1250375 | Enzyme | 1 |
| Aldose reductase (by homology) | AKR1B1 | P15121 | CHEMBL1900 | Enzyme | 1 |
| Xanthine dehydrogenase | XDH | P47989 | CHEMBL1929 | Oxidoreductase | 1 |
| Tyrosinase | TYR | P14679 | CHEMBL1973 | Oxidoreductase | 1 |
| Tyrosine-protein kinase receptor FLT3 | FLT3 | P36888 | CHEMBL1974 | Kinase | 1 |
| Carbonic anhydrase II | CA2 | P00918 | CHEMBL205 | Lyase | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Las Heras Etayo, N.; Llamas, F.; Acedo, C. Ethnobotanical Research and Compilation of the Medicinal Uses in Spain and the Active Principles of Chiliadenus glutinosus (L.) Fourr. for the Scientific Validation of Its Therapeutic Properties. Plants 2021, 10, 584. https://doi.org/10.3390/plants10030584
Las Heras Etayo N, Llamas F, Acedo C. Ethnobotanical Research and Compilation of the Medicinal Uses in Spain and the Active Principles of Chiliadenus glutinosus (L.) Fourr. for the Scientific Validation of Its Therapeutic Properties. Plants. 2021; 10(3):584. https://doi.org/10.3390/plants10030584
Chicago/Turabian StyleLas Heras Etayo, Nadia, Félix Llamas, and Carmen Acedo. 2021. "Ethnobotanical Research and Compilation of the Medicinal Uses in Spain and the Active Principles of Chiliadenus glutinosus (L.) Fourr. for the Scientific Validation of Its Therapeutic Properties" Plants 10, no. 3: 584. https://doi.org/10.3390/plants10030584