Metal Concentration in Muscle and Digestive Gland of Common Octopus (Octopus vulgaris) from Two Coastal Site in Southern Tyrrhenian Sea (Italy)
Abstract
:1. Introduction
2. Results and Discussion
2.1. Metal Concentration versus Sampling Sites and Tissue Type
2.2. Metal Concentration versus Biological Parameters
2.3. Concern for Public Health
3. Materials and Methods
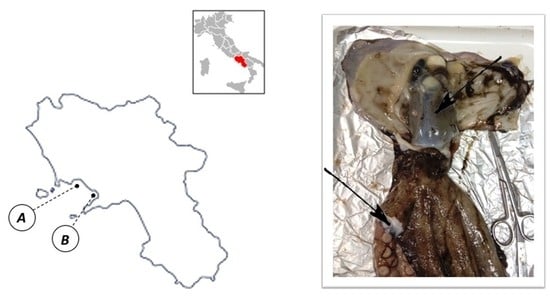
3.1. Sampling
3.2. Chemical and Instrumental Analysis
3.3. Quality Assurance
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cirillo, T.; Fasano, E.; Viscardi, V.; Arnese, A.; Amodio-Cocchieri, R. Survey of lead, cadmium, mercury and arsenic in seafood purchased in Campania, Italy. Food Addit. Contam. B 2010, 3, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations. The State of World Fisheries and Aquaculture. 2018. Available online: http://www.fao.org/3/i9540en/i9540en.pdf (accessed on 28 June 2019).
- ISMEA. Rete di Rilevazione dei Mercati Ittici. 2016. Available online: http://archivio.ismea.it/ReportIsmea/Filiera_pesca_2016.pdf (accessed on 23 September 2016).
- Has-Schön, E.; Bogut, I.; Strelec, I. Heavy metal profile in five fish species included in human diet, domiciled in the end flow of River Neretva (Croatia). Arch. Environ. Contam. Toxicol. 2006, 50, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Mustafa Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef]
- Bustamante, P.; Cosson, R.P.; Gallien, I.; Caurant, F.; Miramand, P. Cadmium detoxification processes in the digestive gland of cephalopods in relation to accumulated cadmium concentrations. Mar. Environ. Res. 2002, 53, 227–241. [Google Scholar] [Green Version]
- Maselli, V.; Polese, G.; Rippa, D.; Ligrone, R.; Kumar Rastogi, R.; Fulgione, D. Frogs, sentinels of DNA damage induced by pollution in Naples and the neighbouring provinces. Ecotoxicol Environ. Saf. 2010, 73, 1525–1529. [Google Scholar] [CrossRef] [PubMed]
- Commission regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union L 2006, 364, 324–365.
- Joint FAO/WHO Expert Committee on Food Additives (JECFA), Safety Evaluation of Certain Food Additives and Contaminants. WHO Food Addit. Ser. 2011, 966, 1–136.
- Carvalho, M.L.; Santiago, S.; Nunes, M.L. Assessment of the essential element and heavy metal content of edible fish muscle. Anal. Bioanal. Chem. 2005, 382, 426–432. [Google Scholar]
- Raimundo, J.; Vale, C.; Canário, J.; Branco, V.; Moura, I. Relations between mercury, methyl-mercury and selenium in tissues of Octopus vulgaris from the Portuguese Coast. Environ. Pollut. 2010, 158, 2094–2100. [Google Scholar]
- Seixas, S.; Bustamante, P.; Pierce, G. Accumulation of mercury in the tissues of the common octopus Octopus vulgaris (L.) in two localities on the Portuguese coast. Sci. Total Environ. 2005, 340, 113–122. [Google Scholar] [CrossRef]
- Raimundo, J.; Caetano, M.; Vale, C. Geographical variation and partition of metals in tissues of Octopus vulgaris along the Portuguese coast. Sci. Total Environ. 2004, 325, 71–81. [Google Scholar]
- Napoleão, P.; Pinheiro, T.; Reis, C.S. Elemental characterization of tissues of Octopus vulgaris along the Portuguese coast. Sci. Total Environ. 2001, 345, 41–49. [Google Scholar]
- Storelli, M.M.; Garofalo, R.; Giungato, D.; Giacominelli-Stuffler, R. Intake of essential and non-essential elements from consumption of octopus, cuttlefish and squid. Food Addit. Contam. B 2010, 3, 14–18. [Google Scholar]
- Pereira, P.; Raimundo, J.; Vale, C.; Kadar, E. Metal concentrations in digestive gland and mantle of Sepia officinalis from two coastal lagoons of Portugal. Sci. Total Environ. 2009, 407, 1080–1088. [Google Scholar]
- Piras, P.; Chessa, G.; Cossu, M.; Rubattu, F.; Fiori, G. Variability of cadmium accumulation in cephalopods (Octopus vulgaris, Sepia officinalis, Loligo vulgaris and Todarodes sagittatus) collected in Sardinia in 2008–2012. Ital. J. Food Saf. 2013, 2, 24. [Google Scholar]
- Bustamante, P.; Cherel, Y.; Caurant, F.; Miramand, P. Cadmium, copper and zinc in octopuses from Kerguelen Islands, Southern Indian Ocean. Polar Bio. 1998, 19, 264–271. [Google Scholar]
- Ariano, A.; Lo Voi, A.; D’Ambola, M.; Marrone, R.; Cacace, D.; Severino, L. Levels of Cadmium in White and Brown Meat of Warty Crab (Eriphia verrucosa). J. Food Prot. 2015, 78, 2253–2256. [Google Scholar] [Green Version]
- Nthunya, L.N.; Masheane, M.L.; Malinga, S.P.; Nxumalo, E.E.; Mamba, B.B.; Mhlanga, S.D. Determination of toxic metals in drinking water sources in the Chief Albert Luthuli Local Municipality in Mpumalanga, South Africa. Phys. Chem. Earth. 2017, 100, 94–100. [Google Scholar] [CrossRef]
- Nthunya, L.N.; Maifadi, S.; Mamba, B.B.; Verliefde, A.R.; Mhlanga, S.D. Spectroscopic Determination of Water Salinity in Brackish Surface Water in Nandoni Dam, at Vhembe District, Limpopo Province, South Africa. Water 2018, 10, 990. [Google Scholar] [CrossRef]
- Russo, R.; Voi, A.L.; Simone, A.D.; Serpe, F.P.; Anastasio, A.; Pepe, T.; Severino, L. Heavy metals in canned tuna from Italian markets. J. Food Prot. 2013, 76, 355–359. [Google Scholar]
- Roldán-Wong, N.T.; Kidd, K.A.; Ceballos-Vázquez, B.P.; Arellano-Martínez, M. Is There a Risk to Humans from Consuming Octopus Species from Sites with High Environmental Levels of Metals? Bull. Environ. Contam. Toxicol. 2018, 101, 796–802. [Google Scholar] [CrossRef] [PubMed]
- Sangiuliano, D.; Rubio, C.; Gutiérrez, A.J.; González-Weller, D.; Revert, C.; Hardisson, A.; Zanardi, E.; Paz, S. Metal Concentrations in Samples of Frozen Cephalopods (Cuttlefish, Octopus, Squid, and Shortfin Squid): An Evaluation of Dietary Intake. J. Food Prot. 2017, 80, 1867–1871. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |



| Source of Variation | Dependent Variable | df | Mean Square | F |
|---|---|---|---|---|
| Site | Pb | 1 | 7.292 | 10.08 ** |
| Cd | 1 | 0.521 | 0.06 | |
| Hg | 1 | 0.012 | 1.15 | |
| Organ | Pb | 1 | 13.583 | 18.77 *** |
| Cd | 1 | 148.532 | 16.08 *** | |
| Hg | 1 | 0.081 | 7.68 |
| Sites | Geographic Coordinates | n | Weight Range (g) | Total Lenght Range (cm) | Sex |
|---|---|---|---|---|---|
| Napoli | 40° 49′ 39″ N; 14°14′ 47″ E | 19 | 845 ± 143 | 71.7 ± 18.3 | 15 ♀ 4 ♂ |
| Castellammare di Stabia | 40° 41′ 46″ N; 14°27′ 53″ E | 19 | 740 ± 229 | 67.6 ± 21.5 | 8 ♀ 11 ♂ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ariano, A.; Marrone, R.; Andreini, R.; Smaldone, G.; Velotto, S.; Montagnaro, S.; Anastasio, A.; Severino, L. Metal Concentration in Muscle and Digestive Gland of Common Octopus (Octopus vulgaris) from Two Coastal Site in Southern Tyrrhenian Sea (Italy). Molecules 2019, 24, 2401. https://doi.org/10.3390/molecules24132401
Ariano A, Marrone R, Andreini R, Smaldone G, Velotto S, Montagnaro S, Anastasio A, Severino L. Metal Concentration in Muscle and Digestive Gland of Common Octopus (Octopus vulgaris) from Two Coastal Site in Southern Tyrrhenian Sea (Italy). Molecules. 2019; 24(13):2401. https://doi.org/10.3390/molecules24132401
Chicago/Turabian StyleAriano, Andrea, Raffaele Marrone, Rebecca Andreini, Giorgio Smaldone, Salvatore Velotto, Serena Montagnaro, Aniello Anastasio, and Lorella Severino. 2019. "Metal Concentration in Muscle and Digestive Gland of Common Octopus (Octopus vulgaris) from Two Coastal Site in Southern Tyrrhenian Sea (Italy)" Molecules 24, no. 13: 2401. https://doi.org/10.3390/molecules24132401