Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products
Abstract
:1. Introduction
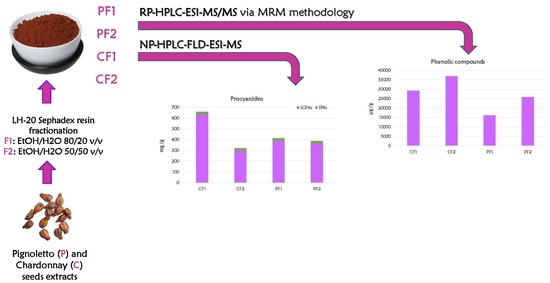
2. Results
2.1. Separation and Identification of Oligomeric Proantocyanidins
2.2. Quantification of Oligomeric Proantocyanidins
2.3. Identification and Quantification of Other Phenolic Compounds
3. Materials and Methods
3.1. Extraction and Purification of Phenolic Compounds
3.2. HPLC-FLD-ESI-MS Analyses of Oligomeric Proanthocyanidins
3.3. HPLC-QqQ-ESI-MS Analyses of Other Phenolic Compounds
3.4. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, L.L.; Zhu, M.T.; Shi, T.; Guo, C.; Huang, Y.S.; Chen, Y.; Xie, M.Y. Recovery of dietary fibre and polyphenol from grape juice pomace and evaluation of their functional properties and polyphenol compositions. Food Funct. 2017, 8, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Medouni-Adrar, S.; Boulekbache-Makhlouf, L.; Cadot, Y.; Medouni-Haroune, L.; Dahmoune, F.; Makhoukhe, A.; Madani, K. Optimization of the recovery of phenolic compounds from Algerian grape by-products. Ind. Crop. Prod. 2015, 77, 123–132. [Google Scholar] [CrossRef]
- Mateo, J.J.; Maicas, S. Valorization of winery and oil mill wastes by microbial technologies. Food Res. Int. 2015, 73, 13–25. [Google Scholar] [CrossRef]
- Maier, T.; Schieber, A.; Kammerer, D.R.; Carle, R. Residues of grape (Vitis vinifera L.) seed oil production as a valuable source of phenolic antioxidants. Food Chem. 2009, 112, 551–559. [Google Scholar] [CrossRef]
- Spranger, I.; Sun, B.; Mateus, A.M.; de Freitas, V.; Ricardo-da-Silva, J.M. Chemical characterization and antioxidant activities of oligomeric and polymeric procyanidin fractions from grape seeds. Food Chem. 2008, 108, 519–532. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Y.; Zhao, C.N.; Liu, Q.; Feng, X.L.; Xu, X.Y.; Cao, S.Y.; Meng, X.; Li, S.; Gan, R.Y.; Li, H.B. Potential of Grape Wastes as a Natural Source of Bioactive Compounds. Molecules 2018, 23, 2598. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Li, L.; Cui, Y.; Luo, L.; Li, Y.; Zhou, P.; Sun, B. Preparative high-speed counter-current chromatography separation of grape seed proanthocyanidins according to degree of polymerization. Food Chem. 2017, 219, 399–407. [Google Scholar] [CrossRef]
- Jara-Palacios, M.J.; Hernanz, D.; Escudero-Gilete, M.L.; Heredia, F.J. The use of grape seed byproducts rich in flavonoids to improve the antioxidant potential of red wines. Molecules 2016, 21, 1526. [Google Scholar] [CrossRef]
- Lucarini, M.; Durazzo, A.; Romani, A.; Campo, M.; Lombardi-Boccia, G.; Cecchini, F. Bio-based compounds from grape seeds: A biorefinery approach. Molecules 2018, 23, 1888. [Google Scholar] [CrossRef]
- Bagchi, D.; Bagchi, M.; Stohs, S.J.; Das, D.K.; Ray, S.D.; Kuszynski, C.A.; Joshi, S.S.; Pruess, H.G. Free radicals and grape seed proanthocyanidin extract: Importance in human health and disease prevention. Toxicology 2000, 148, 187–197. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Vinson, J.A.; Dabbagh, Y.A.; Sherry, M.M.; Jang, J. Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J. Agric. Food Chem. 1995, 43, 2800–2802. [Google Scholar]
- Bomser, J.; Singletary, K.; Wallig, M.; Smith, M. Inhibition of TPA-induced tumor promotion in CD-1 mouse epidermis by a polyphenolic fraction from grape seeds. Cancer Lett. 1999, 135, 151–157. [Google Scholar] [CrossRef]
- Ye, X.; Krohn, R.; Liu, W.; Joshi, S.; Kuszynski, C.; McGinn, T.; Bagchi, M.; Preuss, H.; Stohs, S.; Bagchi, D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol. Cell. Biochem. 1999, 196, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-Y.; Li, W.-G.; Wu, Y.-J.; Zheng, T.-Z.; Li, W.; Qu, S.-Y.; Liu, N.-F. Proanthocyanidin from grape seeds potentiates anti-tumor activity of doxorubicin via immunomodulatory mechanism. Int. Immunopharmacol. 2005, 5, 1247–1257. [Google Scholar] [CrossRef] [PubMed]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Li, W.-G.; Zhang, X.-Y.; Wu, Y.-J.; Tian, X. Anti-inflammatory effect and mechanism of proanthocyanidins from grape seeds. Acta Pharmacol. Sin. 2001, 22, 1117–1120. [Google Scholar]
- Saito, M.; Hosoyama, H.; Ariga, T.; Kataoka, S.; Yamaji, N. Antiulcer activity of grape seed extract and procyanidins. J. Agric. Food Chem. 1998, 46, 1460–1464. [Google Scholar] [CrossRef]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Tokutake, S. Procyanidin-rich extract from grape seeds prevents cataract formation in hereditary cataractous (ICR/f) rats. J. Agric. Food Chem. 2002, 50, 4983–4988. [Google Scholar] [CrossRef]
- Brown, R.H.; Mueller-Harvey, I.; Zeller, W.E.; Reinhardt, L.; Stringano, E.; Gea, A.; Drake, C.; Ropiak, H.M.; Fryganas, C.; Ramsay, A.; et al. Facile Purification of Milligram to Gram Quantities of Condensed Tannins According to Mean Degree of Polymerization and Flavan-3-ol Subunit Composition. J. Agric. Food Chem. 2017, 65, 8072–8082. [Google Scholar] [CrossRef]
- Leppä, M.M.; Karonen, M.; Tähtinen, P.; Engström, M.T.; Salminen, J.P. Isolation of chemically well-defined semipreparative liquid chromatography fractions from complex mixtures of proanthocyanidin oligomers and polymers. J. Chromatogr. A 2018, 1576, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Amarowicz, R.; Shahidi, F. A rapid chromatographic method for separation of individual catechins fromgreen tea. Food Res. Int. 1996, 29, 71–76. [Google Scholar] [CrossRef]
- Li, K.; Zhou, X.; Liu, C.L.; Yang, X.; Han, X.; Shi, X.; Song, X.; Ye, C.; Ko, C.H. Preparative separation of gallocatechin gallate from Camellia ptilophylla using macroporous resins followed by sephadex LH-20 column chromatography. J. Chromatogr. B 2016, 1011, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liimatainen, J.; Puganen, A.; Alakomi, H.L.; Sinkkonen, J.; Yang, B. Sephadex LH-20 fractionation and bioactivities of phenolic compounds from extracts of Finnish berry plants. Food Res. Int. 2018, 113, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Prodanov, M.; Vacas, V.; Hernández, T.; Estrella, I.; Amador, B.; Winterhalter, P. Chemical characterisation of Malvar grape seeds (Vitis vinifera L.) by ultrafiltration and RP-HPLC-PAD-MS. J. Food Comp. Anal. 2013, 31, 284–292. [Google Scholar] [CrossRef]
- Kuhnert, S.; Lehmann, L.; Winterhalter, P. Rapid characterisation of grape seed extracts by a novel HPLC method on a diol stationary phase. J. Funct. Foods 2015, 15, 225–232. [Google Scholar] [CrossRef]
- Ky, I.; Teissedre, P.L. Characterisation of Mediterranean Grape Pomace Seed and Skin Extracts: Polyphenolic Content and Antioxidant Activity. Molecules 2015, 20, 2190–2207. [Google Scholar] [CrossRef] [Green Version]
- Prieur, C.; Rigaud, J.; Cheynier, V.; Moutounet, M. Oligomeric and Polymeric procyanidins from grape seeds. Phytochemistry 1994, 36, 781–784. [Google Scholar] [CrossRef]
- Bautista-Ortín, A.B.; Busse-Valverde, N.; Fernández-Fernández, J.I.; Gómez-Plaza, E.; Gil-Muñoz, R.J. The extraction kinetics of anthocyanins and proanthocyanidins from grape to wine in three different varieties. Int. Sci. Vigne Vin. 2016, 50, 91–100. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.L.; Cabral, L.M.C. Grape by-product extracts against microbial proliferation and lipid oxidation: A review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef]
- Lavelli, V.; Torri, L.; Zeppa, G.; Fiori, L.; Spigno, G. Recovery of Winemaking By-Products for Innovative Food Applications. Ital. J. Food Sci. 2016, 28, 542–564. [Google Scholar]
- Weseler, A.R.; Bast, A. Masquelier’s grape seed extract: From basic flavonoid research to a well-characterized food supplement with health benefits. Nutr. J. 2017, 16, 5. [Google Scholar] [CrossRef] [PubMed]
- Downing, L.E.; Edgar, D.; Ellison, P.A.; Ricketts, M.L. Mechanistic insight into nuclear receptor-mediated regulation of bile acid metabolism and lipid homeostasis by grape seed procyanidin extract (GSPE). Cell Biochem. Funct. 2017, 35, 12–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes, M.A.; Pimentel, F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Cardioprotective properties of grape seed proanthocyanidins: An update. Trends Food Sci. Technol. 2016, 57, 31–39. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, S. Dietary proanthocyanidins inhibit UV radiation-induced skin tumor development through functional activation of the immune system. Mol. Nutr. Food Res. 2016, 60, 1374–1382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [Green Version]
- Bagchi, D.; Swaroop, A.; Preuss, H.G.; Bagchi, M. Free radical scavenging, antioxidant and cancer chemoprevention by grape seed proanthocyanidin: An overview. Mutat. Res. 2014, 768, 69–73. [Google Scholar] [CrossRef]
- Banerji, S.; Banerjee, S. A formulation of grape seed, Indian gooseberry, turmeric and fenugreek helps controlling type 2 diabetes mellitus in advanced-stage patients. Eur. J. Integr. Med. 2016, 8, 645–653. [Google Scholar] [CrossRef]
- Seo, K.H.; Bartley, G.E.; Tam, C.; Kim, H.S.; Kim, D.H.; Chon, J.W.; Kim, H.; Yokoyama, W. Chardonnay Grape Seed Flour Ameliorates Hepatic Steatosis and Insulin Resistance via Altered Hepatic Gene Expression for Oxidative Stress, Inflammation and Lipid and Ceramide Synthesis in Diet-Induced Obese Mice. PLoS ONE 2016, 11, e0167680. [Google Scholar] [CrossRef]
- Aragonès, G.; Suárez, M.; Ardid-Ruiz, A.; Vinaixa, M.; Rodríguez, M.A.; Correig, X.; Arola, L.; Bladé, C. Dietary proanthocyanidins boost hepatic NAD+ metabolism and SIRT1 expression and activity in a dose-dependent manner in healthy rats. Sci. Rep. 2016, 6, 24977. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Xiu, C.; Liu, W.; Tao, Y.; Wang, J.; Qu, Y. Grape seed proanthocyanidin extract protects the retina against early diabetic injury by activating the Nrf2 pathway. Exp. Ther. Med. 2016, 11, 1253–1258. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, Y.; Li, Y. Grape seed proanthocyanidin extracts prevent hyperglycemia-induced monocyte adhesion to aortic endothelial cells and ameliorates vascular inflammation in high-carbohydrate/high-fat diet and streptozotocin-induced diabetic rats. Int. J. Food Sci. Nutr. 2015, 67, 524–534. [Google Scholar] [CrossRef]
- Pons, Z.; Margalef, M.; Bravo, F.I.; Arola-Arnal, A.; Muguerza, B. Chronic administration of grape-seed polyphenols attenuates the development of hypertension and improves other cardiometabolic risk factors associated with the metabolic syndrome in cafeteria diet-fed rats. Br. J. Nutr. 2017, 117, 200–208. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, Y.; Shao, H.; Bi, Q.; Chen, J.; Ye, Z. Grape seed procyanidin B2 inhibits adipogenesis of 3T3-L1 cells by targeting peroxisome proliferator-activated receptor γ with miR-483-5p involved mechanism. Biomed. Pharmacother. 2017, 86, 292–296. [Google Scholar] [CrossRef]
- Pinna, C.; Morazzoni, P.; Sala, A. Proanthocyanidins from Vitis vinifera inhibit oxidative stress-induced vascular impairment in pulmonary arteries from diabetic rats. Phytomedicine 2017, 25, 39–44. [Google Scholar] [CrossRef]
- Serrano, J.; Casanova-Martí, À.; Gual, A.; Pérez-Vendrell, A.M.; Blay, M.T.; Terra, X.; Ardévol, A.; Pinent, M. A specific dose of grape seed-derived proanthocyanidins to inhibit body weight gain limits food intake and increases energy expenditure in rats. Eur. J. Nutr. 2017, 56, 1629–1636. [Google Scholar] [CrossRef]
- Heidker, R.M.; Caiozzi, G.C.; Ricketts, M.L. Grape Seed Procyanidins and Cholestyramine Differentially Alter Bile Acid and Cholesterol Homeostatic Gene Expression in Mouse Intestine and Liver. PLoS ONE 2016, 11, e0154305. [Google Scholar] [CrossRef]
- Garcia-Jares, C.; Vazquez, A.; Lamas, J.P.; Pajaro, M.; Alvarez-Casas, M.; Lores, M. Antioxidant White Grape Seed Phenolics: Pressurized Liquid Extracts from Different Varieties. Antioxidants 2015, 4, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Flamini, R. Recent Applications of Mass Spectrometry in the Study of Grape and Wine Polyphenols. ISRN Spectrosc. 2013, 2013, 813563. [Google Scholar] [CrossRef]
- Perestrelo, R.; Lu, Y.; Santos, S.A.O.; Silvestre, A.J.D.; Neto, C.P.; Câmara, J.S.; Rocha, M.S. Phenolic profile of Sercial and Tinta Negra Vitis vinifera L. grape skins by HPLC–DAD–ESI-MSn. Novel phenolic compounds in Vitis vinifera L. grape. Food Chem. 2012, 135, 94–104. [Google Scholar] [CrossRef]
- Robbins, R.J.; Leonczak, J.; Johnson, J.C.; Li, J.; Kwik-Uribe, C.; Prior, R.L.; Gu, L. Method performance and multi-laboratory assessment of a normal phase high pressure liquid chromatography–fluorescence detection method for the quantitation of flavanols and procyanidins in cocoa and chocolate containing samples. J. Chromatogr. A 2009, 1216, 4831–4840. [Google Scholar] [CrossRef]
- Gómez-Caravaca, A.M.; Verardo, V.; Berardinelli, A.; Marconi, E.; Caboni, M.F. A chemometric approach to determine the phenolic compounds indifferent barley samples by two different stationary phases: A comparison between C18 and pentafluorophenyl core shell columns. J. Chromatogr. A 2014, 1355, 134–142. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |

| Compounds | Retention Time (min) | [M-H]− | In Source Fragment (m/z) |
|---|---|---|---|
| Monomers (DP1) | 6.7 | 289 | 245 |
| Dimers (DP2) | 18.2 | 577 | 425, 289 |
| Galloylated dimers (DP2-G) | 25 | 729 | 303 |
| Trimers (DP3) | 28.1 | 865 | 739, 713 |
| Galloylated trimers (DP3-G) | 30.4 | 881, 1017 | 593, 303 |
| Tetramers (DP4) | 35.3 | 1153 c | 865 |
| Galloylated tetramers (DP4-G) | 36.8 | 1305 | - |
| Pentamers (DP5) | 41.2 | 1441, 797, 873 | - |
| Hexamers (DP6) | 45.2 | - | - |
| Heptamers (DP7) | 49 | - | - |
| Octamers (DP8) | 52.2 | - | - |
| Nonamers (DP9) | 55.0 | - | - |
| Decamers (DP10) | 57.8 | - | - |
| Undecamers (DP11) | 60.1 | - | - |
| Dodecamers (DP12) | 62.0 | - | - |
| Polymers | 65.7 | - | - |
| Compounds | CF1 | CF2 | PF1 | PF2 |
|---|---|---|---|---|
| PAs (mg/g) | ||||
| Monomers (DP1) | 456.1 ± 3.4 a | 185.2 ± 2.9 d | 252.6 ± 3.0 b | 222.5 ± 8.3 c |
| Dimers (DP2) | 55.4 ± 0.1 a | 29.1 ± 0.02 d | 42.9 ± 0.2 b | 38.4 ± 0.01 c |
| Galloylated dimers (DP2-G) | 9.8 ± 0.1 a | 8.3 ± 0.3 b | 9.6 ± 0.1 a | 10.3 ± 0.1 a |
| Trimers (DP3) | 22.8 ± 0.01 a | 11.4 ± 0.1 c | 15.2 ± 0.1 b | 14.7 ± 1.5 b |
| Galloylated trimers (DP3-G) | 8.4 ± 0.01 a | 6.3 ± 0.02 b | 8.7 ± 0.3 a | 8.8 ± 0.0 a |
| Tetramers (DP4) | 14.6 ± 0.1 a | 8.3 ± 0.1 c | 9.9 ± 0.1 b | 9.2 ± 0.5 b,c |
| Galloylated tetramers (DP4-G) | 5.6 ± 0.1 a | 4.1 ± 0.0 c | 5.2 ± 0.1 b | 5.2 ± 0.2 b |
| Pentamers (DP5) | 11.7 ± 0.1 a | 6.4 ± 0.1 c | 8.0 ± 0.1 b | 7.9 ± 0.3 b |
| Hexamers (DP6) | 6.4 ± 0.1 a | 3.9 ± 0.0 d | 4.3 ± 0.02 c | 4.5 ± 0.01 b |
| Heptamers (DP7) | 6.5 ± 0.1 a | 4.5 ± 0.0 c | 4.6 ± 0.0 c | 4.9 ± 0.0 b |
| Octamers (DP8) | 4.2 ± 0.2 a | 3.2 ± 0.0 b | 3.2 ± 0.1 b | 3.4 ± 0.1 b |
| Nonamers (DP9) | 2.3 ± 0.02 a | 2.0 ± 0.02 b,c | 2.0 ± 0.02 c | 2.1 ± 0.03 b |
| Decamers (DP10) | 3.2 ± 0.01 a | 3.0 ± 0.02 b,c | 2.9 ± 0.02 c | 3.1 ± 0.02 a,b |
| Undecamers (DP11) | 3.0 ± 0.0 a | 2.9 ± 0.0 b | 2.8 ± 0.0 c | 3.0 ± 0.0 a,b |
| Dodecamers (DP12) | 2.9 ± 0.01 a | 2.8 ± 0.01 b | 2.7 ± 0.01 c | 2.9 ± 0.02 a |
| Polymers | 21.0 ± 0.2 a,b | 19.9 ± 0.7 b | 15.8 ± 0.2 c | 22.0 ± 0.6 a |
| SGPAs * | 23.8 ± 0.1 a | 18.7 ± 0.4 b | 23.5 ± 0.5 a | 24.3 ± 0.1 a |
| SPAs * | 634.0 ± 3.1 a | 301.4 ± 3.2 d | 390.2 ± 2.5 b | 362.9 ± 9.7 c |
| Compound | Retention Time (min) | [M-H]− | Product Ions | Quantification Transition (m/z) | Fragmentor (V) | CE (V) |
|---|---|---|---|---|---|---|
| Gallic acid | 1.53 | 169 | 125 | 169 → 125 | 108 | 12 |
| Protocatechuic aldehyde | 5.03 | 137 | 108 | 137 → 108 | 98 | 12 |
| Dihydrofisetin glucoside | 9.05 | 449 | 287, 259 | 449 → 287 | 131 | 16 |
| Ellagic acid | 12.25 | 301 | 284, 257 | 301→ 284 | 169 | 28 |
| Ellagic acid hexoside 1 | 12.45 | 463 | 301, 169 | 463 → 301 | 169 | 28 |
| Ellagic acid hexoside 2 | 12.82 | 463 | 301, 169 | 463 → 301 | 169 | 28 |
| Kaempferol-glucoside | 12.95 | 447 | 285 | 447 → 285 | 131 | 16 |
| Quercetin-pentoside 1 | 14.24 | 433 | 301, 179, 151 | 433 → 151 | 131 | 16 |
| Quercetin-pentoside 2 | 14.46 | 433 | 301, 179, 151 | 433 → 151 | 131 | 16 |
| Quercetin | 17.81 | 301 | 179, 151 | 301 → 151 | 131 | 16 |
| Compounds | CF1 | CF2 | PF1 | PF2 |
|---|---|---|---|---|
| Phenolic Compounds (µg/g) | ||||
| Gallic acid | 1788.7 ± 52.3 a | 1516.1 ±24.7 b | 832.4 ±10.8 c | 1490.6 ±19.9 b |
| Protocatechuic aldehyde | 108.8 ± 6.4 a | 62.0 ±2.9 b | 26.2 ±0.9 c | 58.0 ±1.5 b |
| Dihydrofisetin glucoside | 207.1 ± 2.2 a | 147.5 ±1.0 c | 152.2 ±1.2 b | 65.4 ±0.8 d |
| Ellagic acid | 1102.4 ± 6.9 b | 1401.7 ± 12.7 a | < LOQ | < LOQ |
| Ellagic acid hexoside 1 | 22473.6 ± 24.3 b | 28726.7 ± 34.6 a | 12570.4 ± 21.8 d | 19509.3 ± 25.1 c |
| Ellagic acid hexoside 2 | 3371.9 ± 8.9 c | 4719.2 ± 10.5 a | 2426.6 ± 6.1 d | 4407.6 ± 4.8 b |
| Kaempferol-glucoside | 55.5 ± 0.5 c | 77.5 ± 0.8 a | 35.2 ± 0.3 d | 66.3 ± 0.6 b |
| Quercetin-pentoside 1 | 5.6 ± 0.2 b | 13.3 ± 0.5 a | 1.6 ± 0.2 c | 12.9 ± 0.3 a |
| Quercetin-pentoside 2 | 4.0 ± 0.2 c | 11.8 ± 0.6 b | 2.0 ± 0.1 d | 20.7 ± 0.5 a |
| Quercetin | 85.0 ± 1.3 c | 177.5 ± 2.5 a | 10.3 ± 0.2 d | 98.8 ± 1.6 b |
| Total | 29202.5 ± 72.5 b | 36853.3 ± 69.8 a | 16056.8 ± 21.5 d | 25729.6 ± 20.7 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasini, F.; Chinnici, F.; Caboni, M.F.; Verardo, V. Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products. Molecules 2019, 24, 677. https://doi.org/10.3390/molecules24040677
Pasini F, Chinnici F, Caboni MF, Verardo V. Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products. Molecules. 2019; 24(4):677. https://doi.org/10.3390/molecules24040677
Chicago/Turabian StylePasini, Federica, Fabio Chinnici, Maria Fiorenza Caboni, and Vito Verardo. 2019. "Recovery of Oligomeric Proanthocyanidins and Other Phenolic Compounds with Established Bioactivity from Grape Seed By-Products" Molecules 24, no. 4: 677. https://doi.org/10.3390/molecules24040677