Pharmacokinetic Evaluation of Clozapine in Concomitant Use of Radix Rehmanniae, Fructus Schisandrae, Radix Bupleuri, or Fructus Gardeniae in Rats
Abstract
:1. Introduction
2. Results
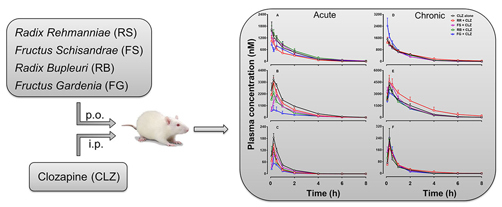
2.1. Effects of Herbal Medicine on CLZ Pharmacokinetics in Rats
2.2. Biodistribution of CLZ in Various Organs
2.3. Safety Evaluation of Chronic Administration of Herbs on Rat Liver and Renal Functions
3. Discussion
4. Experimental Section
4.1. Drugs and Reagents
4.2. Herbal Extraction Preparation
4.3. Rat Pharmacokinetic Studies
4.4. Biochemical Assay of AST, ALT, CR, and BUN Levels in Chronic Treated Rats
4.5. The Measurement of CLZ, norCLZ, and CLZ N-oxide
4.6. Data Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Asenjo Lobos, C.; Komossa, K.; Rummel-Kluge, C.; Hunger, H.; Schmid, F.; Schwarz, S.; Leucht, S. Clozapine versus other atypical antipsychotics for schizophrenia. Cochrane Database Syst. Rev. 2010, 11, CD006633. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Detraux, J.; van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat. Rev. Endocrinol. 2012, 8, 114–126. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.J.; Tan, Q.R.; Tong, Y.; Wang, X.Y.; Wang, H.H.; Ho, L.M.; Wong, H.K.; Feng, Y.B.; Wang, D.; Ng, R.; et al. An epidemiological study of concomitant use of Chinese medicine and antipsychotics in schizophrenic patients: Implication for herb-drug interaction. PLoS ONE 2011, 6, e17239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China, 2010 ed.; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Zhang, R.X.; Li, M.X.; Jia, Z.P. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Zhang, J.; Zhang, S.; Zhou, H.H.; Toma, D.; Ren, S.; Huang, L.; Yaramus, M.; Baum, A.; Venkataramanan, R.; et al. Traditional Chinese medicines Wu Wei Zi (Schisandra chinensis Baill) and Gan Cao (Glycyrrhiza uralensis Fisch) activate pregnane X receptor and increase warfarin clearance in rats. J. Pharmacol. Exp. Ther. 2006, 316, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Ashour, M.L.; Wink, M. Genus Bupleurum: A review of its phytochemistry, pharmacology and modes of action. J. Pharm. Pharmacol. 2011, 63, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Chen, Y.F.; Li, F.; Zhang, H.Y. Fructus Gardenia (Gardenia jasminoides J. Ellis) phytochemistry, pharmacology of cardiovascular, and safety with the perspective of new drugs development. J. Asian Nat. Prod. Res. 2013, 15, 94–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tian, D.D.; Zheng, B.; Wang, D.; Tan, Q.R.; Wang, C.Y.; Zhang, Z.J. Peony-Glycyrrhiza Decoction, an Herbal Preparation, Inhibits Clozapine Metabolism via Cytochrome P450s, but Not Flavin-Containing Monooxygenase in in Vitro Models. Drug Metab. Dispos. 2015, 43, 1147–1153. [Google Scholar] [CrossRef] [PubMed]
- Chetty, M.; Murray, M. CYP-mediated clozapine interactions: How predictable are they? Curr. Drug Metab. 2007, 8, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Pfuhlmann, B.; Hiemke, C.; Unterecker, S.; Burger, R.; Schmidtke, A.; Riederer, P.; Deckert, J.; Jabs, B. Toxic clozapine serum levels during inflammatory reactions. J. Clin. Psychopharm. 2009, 29, 392–394. [Google Scholar] [CrossRef] [PubMed]
- Leung, J.G.; Nelson, S.; Takala, C.R.; Goren, J.L. Infection and inflammation leading to clozapine toxicity and intensive care: A case series. Ann. Pharmacother. 2014, 48, 801–805. [Google Scholar] [CrossRef] [PubMed]
- Gee, S.; Dixon, T.; Docherty, M.; Shergill, S.S. Optimising plasma levels of clozapine during metabolic interactions: A review and case report with adjunct rifampicin treatment. BMC Psychiatry 2015, 15. [Google Scholar] [CrossRef] [PubMed]
- Van Strater, A.C.; Bogers, J.P. Interaction of St John’s wort (Hypericum perforatum) with clozapine. Int. Clin. Psychopharmacol. 2012, 27, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Gabbay, V.; O’Dowd, M.A.; Mamamtavrishvili, M.; Asnis, G.M. Clozapine and oral contraceptives: A possible drug interaction. J. Clin. Psychopharm. 2002, 22, 621–622. [Google Scholar] [CrossRef]
- Schaber, G.; Wiatr, G.; Wachsmuth, H.; Dachtler, M.; Albert, K.; Gaertner, I.; Breyer-Pfaff, U. Isolation and identification of clozapine metabolites in patient urine. Drug Metab. Dispos. 2001, 29, 923–931. [Google Scholar] [PubMed]
- Casey, D.E. Metabolic issues and cardiovascular disease in patients with psychiatric disorders. Am. J. Med. 2005, 118 (Suppl. 2), 15S–22S. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Huang, Z.; Wu, R.; Zhong, Z.; Wei, Q.; Wang, H.; Diao, F.; Wang, J.; Zheng, L.; Zhao, J.; et al. The comparison of glycometabolism parameters and lipid profiles between drug-naive, first-episode schizophrenia patients and healthy controls. Schizophr. Res. 2013, 150, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.N.; Cho, M.; So, I.; Jeon, J.H. The protective effects of Schisandra chinensis fruit extract and its lignans against cardiovascular disease: A review of the molecular mechanisms. Fitoterapia 2014, 97, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Pan, S.Y.; Zhang, Y.; Wang, X.Y.; Zhu, P.L.; Chu, Z.S.; Yu, Z.L.; Zhou, S.F.; Ko, K.M. Dietary pulp from Fructus Schisandra Chinensis supplementation reduces serum/hepatic lipid and hepatic glucose levels in mice fed a normal or high cholesterol/bile salt diet. Lipids Health Dis. 2014, 13. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, T.F.; Lu, H.J.; Liou, S.S.; Chang, C.J.; Liu, I.M. Vinegar-Baked Radix Bupleuri Regulates Lipid Disorders via a Pathway Dependent on Peroxisome-Proliferator-Activated Receptor-alpha in High-Fat-Diet-Induced Obese Rats. Evid. Based Complement. Altern. 2012, 2012. [Google Scholar] [CrossRef]
- Kojima, K.; Shimada, T.; Nagareda, Y.; Watanabe, M.; Ishizaki, J.; Sai, Y.; Miyamoto, K.; Aburada, M. Preventive effect of geniposide on metabolic disease status in spontaneously obese type 2 diabetic mice and free fatty acid-treated HepG2 cells. Biol. Pharm. Bull. 2011, 34, 1613–1618. [Google Scholar] [CrossRef] [PubMed]
- Or, P.M.; Lam, F.F.; Kwan, Y.W.; Cho, C.H.; Lau, C.P.; Yu, H.; Lin, G.; Lau, C.B.; Fung, K.P.; Leung, P.C.; et al. Effects of Radix Astragali and Radix Rehmanniae, the components of an anti-diabetic foot ulcer herbal formula, on metabolism of model CYP1A2, CYP2C9, CYP2D6, CYP2E1 and CYP3A4 probe substrates in pooled human liver microsomes and specific CYP isoforms. Phytomedicine 2012, 19, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Chen, X.; Wang, Y.; Zhao, R.; Mao, S. Modulatory effects of extracts of vinegar-baked Radix Bupleuri and saikosaponins on the activity of cytochrome P450 enzymes in vitro. Xenobiotica 2014, 44, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.N.; Zhang, Y.; Cui, Y.L.; Yan, K. Evaluation of genipin on human cytochrome P450 isoenzymes and P-glycoprotein in vitro. Fitoterapia 2014, 98, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Su, T.; Mao, C.; Yin, F.; Yu, Z.; Lin, Y.; Song, Y.; Lu, T. Effects of unprocessed versus vinegar-processed Schisandra chinensis on the activity and mRNA expression of CYP1A2, CYP2E1 and CYP3A4 enzymes in rats. J. Ethnopharmacol. 2013, 146, 734–743. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Hao, H.; Wang, Q.; Zheng, C.; Zhou, F.; Liu, Y.; Wang, Y.; Yu, G.; Kang, A.; Peng, Y.; et al. Effects of short-term and long-term pretreatment of Schisandra lignans on regulating hepatic and intestinal CYP3A in rats. Drug Metab. Dispos. 2009, 37, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Ridout, K.K.; Sun, D.; Lazarus, P. Glucuronidation of the second-generation antipsychotic clozapine and its active metabolite N-desmethylclozapine. Potential importance of the UGT1A1 A(TA)7TAA and UGT1A4 L48V polymorphisms. Drug Metab. Dispos. 2012, 22, 561–576. [Google Scholar]
- Lameh, J.; Burstein, E.S.; Taylor, E.; Weiner, D.M.; Vanover, K.E.; Bonhaus, D.W. Pharmacology of N-desmethylclozapine. Pharmacol. Ther. 2007, 115, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Huang, Y.; Tian, Y.; Xu, L.; Liu, G.Q.; Zahng, Z.J. Assessment of the effects of Radix bupleuri and vinegar-baked Radix bupleuri on cytochrome 450 activity by a six-drug cocktail approach. Chin. J. Nat. Med. 2013, 11, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.N.; Wang, C.Y.; Sze, C.W.; Tong, Y.; Tan, Q.R.; Feng, X.J.; Liu, R.M.; Zhang, J.Z.; Zhang, Y.B.; Zhang, Z.J. A randomized, crossover comparison of herbal medicine and bromocriptine against risperidone-induced hyperprolactinemia in patients with schizophrenia. J. Clin. Psychopharm. 2008, 28, 264–370. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tian, D.D.; Zhang, Z.J. In-vitro effects of concomitant use of herbal preparations on cytochrome P450s involved in clozapine metabolism. Molecules 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Aravagiri, M.; Marder, S.R. Simultaneous determination of clozapine and its N-desmethyl and N-oxide metabolites in plasma by liquid chromatography/electrospray tandem mass spectrometry and its application to plasma level monitoring in schizophrenicpatients. J. Pharm. Biomed. Anal. 2001, 26, 301–311. [Google Scholar] [CrossRef]
- Sample Availability: Not available.



| Variable b | CLZ Alone (n = 5) | RR + CLZ (n = 4) | FS + CLZ (n = 6) | RB + CLZ (n = 6) | FG + CLZ (n = 4) |
|---|---|---|---|---|---|
| CLZ | |||||
| Cmax (nM) | 1721 ± 349 | 1118 ± 350 | 1434 ± 232 | 1807 ± 205 | 1344 ± 469 |
| Tmax (h) | 0.20 ± 0.12 | 0.12 ± 0.042 | 0.40 ± 0.14 | 0.28 ± 0.08 | 0.36 ± 0.14 |
| AUC0-C6 (nM∙h) | 2370 ± 526 | 1418 ± 543 | 2640 ± 350 | 2992 ± 509 | 1871 ± 649 |
| t1/2 (h) | 1.16 ± 0.091 | 1.01 ± 0.7 | 2.50 ± 1.06 | 1.17 ± 0.15 | 2.09 ± 0.99 |
| MRT (h) | 1.57 ± 0.053 | 1.45 ± 0.085 | 2.46 ± 0.74 | 1.82 ± 0.30 | 2.68 ± 1.17 |
| CL/F (L/h/kg) | 16.7 ± 4.43 | 30.1 ± 8.06 | 12.9 ± 2.01 | 11.7 ± 1.77 | 55.2 ± 41.8 |
| Vd (L) | 27.1 ± 6.36 | 43.6 ± 10.9 | 39.0 ± 12.5 | 18.3 ± 1.72 | 94.0 ± 50.3 |
| NorCLZ | |||||
| Cmax (nM) | 3644 ± 426 | 3283 ± 461 | 1996 ± 620 | 2364 ± 879 | 768 ± 379 (0.008) * |
| Tmax (h) | 0.27 ± 0.07 | 0.25 ± 0 | 0.67 ± 0.27 | 0.64 ± 0.30 | 1.15 ± 0.95 |
| AUC0-C5 (nM∙h) | 5819 ± 382 | 4238 ± 875 | 2862 ± 878 (0.013) * | 2878 ± 908 (0.014) * | 1463 ± 619 (0.002) * |
| t1/2 (h) | 1.08 ± 0.09 | 1.05 ± 0.10 | 1.20 ± 0.19 | 1.08 ± 0.16 | 1.5 ± 0.38 |
| MRT (h) | 1.66 ± 0.10 | 1.34 ± 0.10 | 1.75 ± 0.39 | 1.97 ± 0.47 | 2.61 ± 0.86 |
| CLZ N-oxide | |||||
| Cmax (nM) | 185 ± 21.3 | 139± 14.0 | 112 ± 28.2 | 134 ± 47.2 | 58.2 ± 42.8 (0.008) * |
| Tmax (h) | 0.25± 0 | 0.25 ± 0 | 0.46 ± 0.12 | 0.64 ± 0.30 | 0.21 ± 0.11 |
| AUC0-C1 (nM∙h) | 159 ± 16.2 | 82.5 ± 10.9 (0.023) * | 130 ± 14.2 | 81.6 ± 23.4 (0.012) * | 43.1 ± 33.1 (0.007) * |
| t1/2 (h) | 0.69 ± 0.12 | 0.39 ± 0.09 | 1.04 ± 0.44 | 0.39 ± 0.04 | 0.59 ± 0.12 |
| MRT (h) | 0.95 ± 0.15 | 0.57 ± 0.13 | 1.43 ± 0.61 | 0.79 ± 0.15 | 0.70 ± 0.21 |
| Variable b | CLZ Alone (n = 5) | RR + CLZ (n = 6) | FS + CLZ (n = 4) | RB + CLZ (n = 4) | FG + CLZ (n = 4) |
|---|---|---|---|---|---|
| CLZ | |||||
| Cmax (nM) | 1430 ± 122 | 1499 ± 280 | 1537 ± 118 | 1330 ± 125 | 2493 ± 472 |
| Tmax (h) | 0.15 ± 0.04 | 0.21 ± 0.07 | 0.08 ± 0 | 0.19 ± 0.10 | 0.12 ± 0.04 |
| AUC0-∞ (nM∙h) | 2039 ± 211 | 2710 ± 545 | 1886 ± 218 | 2190 ± 408 | 2429 ± 138 |
| t1/2 (h) | 1.36 ± 0.23 | 2.40 ± 0.82 | 1.26 ± 0.47 | 2.19 ± 1.10 | 1.25 ± 0.21 |
| MRT (h) | 1.63 ± 0.13 | 2.43 ± 0.59 | 1.31 ± 0.07 | 1.75 ± 0.46 | 1.39 ± 0.21 |
| CL/F (L/h/kg) | 15.5 ± 1.30 | 14.1 ± 3.10 | 17.0 ± 2.19 | 15.4 ± 2.57 | 12.8 ± 0.75 |
| Vd (L) | 26.5 ± 50.2 | 33.0 ± 9.48 | 30.9 ± 11.9 | 38.3 ± 11.8 | 21.5 ± 2.66 |
| NorCLZ | |||||
| Cmax (nM) | 4050 ± 268 | 4677 ± 807 | 4383 ± 444 | 3754 ± 1266 | 3200 ± 528 |
| Tmax (h) | 0.30 ± 0.05 | 0.29 ± 0.04 | 0.25 ± 0 | 0.31 ± 0.06 | 0.31 ± 0.06 |
| AUC0-∞ (nM∙h) | 7192 ± 762 | 11494 ± 2767 | 7347 ± 1237 | 6125 ± 1738 | 6078 ± 911 |
| t1/2 (h) | 2.08 ± 0.49 | 1.72 ± 0.23 | 1.93 ± 0.15 | 1.80 ± 0.16 | 1.39 ± 0.19 |
| MRT (h) | 1.82 ± 0.14 | 2.19 ± 0.43 | 1.48 ± 0.12 | 1.83 ± 0.45 | 1.73 ± 0.06 |
| CLZ N-oxide | |||||
| Cmax (nM) | 214 ± 57.4 | 191 ± 32.7 | 196 ± 22.0 | 237 ± 69.5 | 200 ± 46.9 |
| Tmax (h) | 0.30 ± 0.05 | 0.29 ± 0.04 | 0.25 ± 0 | 0.31 ± 0.06 | 0.25 ± 0 |
| AUC0-∞ (nM∙h) | 146 ± 20.1 | 199 ± 48.5 | 182 ± 7.41 | 172 ± 39.9 | 159 ± 34.0 |
| t1/2 (h) | 0.55 ± 0.05 | 0.87 ± 0.12 | 0.61 ± 0.03 | 0.63 ± 0.12 | 0.44 ± 0.02 |
| MRT (h) | 0.91 ± 0.11 | 1.26 ± 0.22 | 0.82 ± 0.04 | 1.00 ± 0.26 | 0.72 ± 0.02 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, D.-D.; Wang, W.; Wang, H.-N.; Sze, S.C.W.; Zhang, Z.-J. Pharmacokinetic Evaluation of Clozapine in Concomitant Use of Radix Rehmanniae, Fructus Schisandrae, Radix Bupleuri, or Fructus Gardeniae in Rats. Molecules 2016, 21, 696. https://doi.org/10.3390/molecules21060696
Tian D-D, Wang W, Wang H-N, Sze SCW, Zhang Z-J. Pharmacokinetic Evaluation of Clozapine in Concomitant Use of Radix Rehmanniae, Fructus Schisandrae, Radix Bupleuri, or Fructus Gardeniae in Rats. Molecules. 2016; 21(6):696. https://doi.org/10.3390/molecules21060696
Chicago/Turabian StyleTian, Dan-Dan, Wei Wang, Hua-Ning Wang, Stephen Cho Wing Sze, and Zhang-Jin Zhang. 2016. "Pharmacokinetic Evaluation of Clozapine in Concomitant Use of Radix Rehmanniae, Fructus Schisandrae, Radix Bupleuri, or Fructus Gardeniae in Rats" Molecules 21, no. 6: 696. https://doi.org/10.3390/molecules21060696