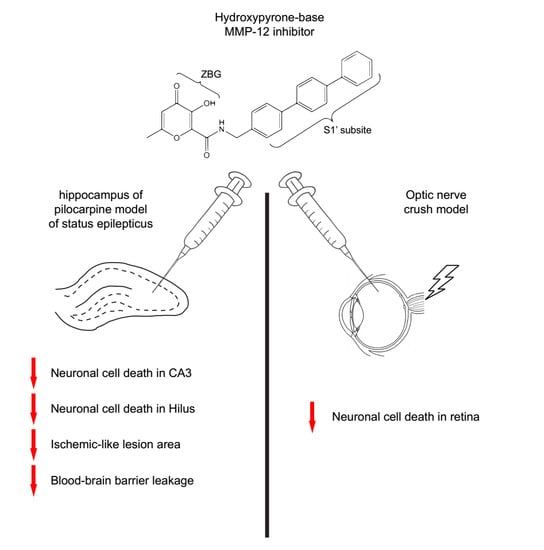
A Hydroxypyrone-Based Inhibitor of Metalloproteinase-12 Displays Neuroprotective Properties in Both Status Epilepticus and Optic Nerve Crush Animal Models
Abstract
:1. Introduction
2. Results
2.1. The Hydroxypyrone-Based MMP-12 Inhibitor Protects Neurons in the CA3 and DH
2.2. Inhibition of MMP-12 Limits the Developement of the Ischemic-Like Lesion Present in the CA3 Stratum Lacunosum-Moleculare
2.3. The Hydroxypyrone-Based MMP-12 Inhibitor Reduces BBB Leakage at the Site of the Ischemic-Like Lesion
2.4. Zonula Occludens-1 (ZO-1) Is Another Marker for the Ischemic-Like Lesion
2.5. The Hydroxypyrone-Based MMP-12 Inhibitor Is also Neuroprotective in a Murine ONC Model
3. Discussion
4. Materials and Methods
4.1. Status Epilepticus Model
4.1.1. Animals
4.1.2. Cannulae Implantation
4.1.3. Status Epilepticus Induction
4.1.4. MMP-12 Inhibitor Injection
4.1.5. Immunohistochemistry
4.1.6. Fluoro-Jade B
4.1.7. Image Analysis
4.2. ONC Model
4.2.1. Animal Handling and Ethics Statement
4.2.2. Animal Surgery: Intraorbital ONC and Ivt Injection
4.2.3. Immunohistochemistry
4.2.4. Image Analysis
4.3. Statistical Analysis
Supplementary Materials
Supplementary File 1Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood-brain barrier |
| Brn3a | Brain-specific hemeobox/POU domain protein 3A |
| CA3 | Cornu Ammonis 3 |
| CNS | Central nervous system |
| DAB | 3,3-Diaminobenzidine tetrahydrochloride |
| DH | Hilus of the dentate gyrus |
| DMSO | Dimethyl sulfoxide |
| FJB | Fluoro-Jade B |
| GFAP | Glial fibrillary acidic protein |
| IgG | Immunoglobulin G |
| Ivt | Intravitreal |
| MMP | Metalloproteinase |
| NeuN | Neuron-specific nuclear protein |
| ONC | Optic nerve crush |
| PBS | Phosphate buffer saline |
| RGC | Retinal ganglion cell |
| SE | Status epilepticus |
| SOD | Superoxide dismutase |
| TLE | Temporal lobe epilepsy |
| TNFα | Tumor necrosis factor α |
| ZO-1 | Zonula occludens-1 |
References
- Fingleton, B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim. Biophys. Acta 2017, 1864, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Gronski, T.J., Jr.; Martin, R.L.; Kobayashi, D.K.; Walsh, B.C.; Holman, M.C.; Huber, M.; Van Wart, H.E.; Shapiro, S.D. Hydrolysis of a broad spectrum of extracellular matrix proteins by human macrophage elastase. J. Biol. Chem. 1997, 272, 12189–12194. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Nalamolu, K.R.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. MMP-12, a promising therapeutic target for neurological diseases. Mol. Neurobiol. 2018, 55, 1405–1409. [Google Scholar] [CrossRef] [PubMed]
- Wells, J.E.; Rice, T.K.; Nuttall, R.K.; Edwards, D.R.; Zekki, H.; Rivest, S.; Yong, V.W. An adverse role for matrix metallloproteinase 12 after spinal cord injury in mice. J. Neurosci. 2003, 23, 10107–10115. [Google Scholar] [CrossRef] [PubMed]
- Vos, C.M.; van Haastert, E.S.; de Groot, C.J.; van der Valk, P.; de Vries, H.E. Matrix metalloproteinase-12 is expressed in phagocytotic macrophages in active multiple sclerosis lesions. J. Neuroimmunol. 2003, 138, 106–114. [Google Scholar] [CrossRef]
- Chelluboina, B.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Vemuganti, R.; Veeravalli, K.K. Matrix metalloproteinase-12 induces blood–brain barrier damage after focal cerebral ischemia. Stroke 2015, 46, 3523–3531. [Google Scholar] [CrossRef] [PubMed]
- Chelluboina, B.; Warhekar, A.; Dillard, M.; Klopfenstein, J.D.; Pinson, D.M.; Wang, D.Z.; Veeravalli, K.K. Post-transcriptional inactivation of matrix metalloproteinase-12 after focal cerebral ischemia attenuates brain damage. Sci. Rep. 2015, 5, 9504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pitkänen, A.; Sutula, T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002, 1, 173–181. [Google Scholar] [CrossRef]
- Vezzani, A.; French, J.; Bartfai, T.; Baram, T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011, 7, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Lucchi, C.; Vinet, J.; Gualtieri, F.; Marinelli, C.; Torsello, A.; Costantino, L.; Biagini, G. Pathophysiology of mesial temporal lobe epilepsy: Is prevention of damage antiepileptogenic? Curr. Med. Chem. 2014, 21, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Bronisz, E.; Kurkowska-Jastrzębska, I. Matrix metalloproteinase 9 in epilepsy: The role of neuroinflammation in seizure development. Mediat. Inflamm. 2016, 2016, 7369020. [Google Scholar] [CrossRef] [PubMed]
- Hoehna, Y.; Uckermann, O.; Luksch, H.; Stefovska, V.; Marzahn, J.; Theil, M.; Gorkiewicz, T.; Gawlak, M.; Wilczynski, G.M.; Kaczmarek, L.; et al. Matrix metalloproteinase 9 regulates cell death following pilocarpine-induced seizures in the developing brain. Neurobiol. Dis. 2012, 48, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Stawarski, M.; Stefaniuk, M.; Wlodarczyk, J. Matrix metalloproteinase-9 involvement in the structural plasticity of dendritic spines. Front. Neuroanat. 2014, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Vinet, J.; Vainchtein, I.D.; Spano, C.; Giordano, C.; Bordini, D.; Curia, G.; Dominici, M.; Bodekke, H.W.; Eggen, B.J.; Biagini, G. Microglia are less pro-inflammatory than myeloid infiltrates in the hippocampus of mice exposed to status epilepticus. Glia 2016, 64, 1350–1362. [Google Scholar] [CrossRef] [PubMed]
- Aerts, J.; Vandenbroucke, R.E.; Dera, R.; Balusu, S.; Van Wonterghem, E.; Moons, L.; Libert, C.; Dehaen, W.; Ackens, L. Synthesis and validation of a hydroxypyrone-based, potent, and specific matrix metalloproteinase-12 inhibitor with anti-inflammatory activity in vitro and in vivo. Mediat. Inflamm. 2015, 2015, 510679. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, C.; Curia, G.; Vinet, J.; Gualtieri, F.; Bresciani, E.; Locatelli, V.; Torsello, A.; Biagini, G. Protective but not anticonvulsant effects of ghrelin and JMV-1843 in the pilocarpine model of status epilepticus. PLoS ONE 2013, 8, e72716. [Google Scholar] [CrossRef] [PubMed]
- Biagini, G.; Baldelli, E.; Longo, D.; Contri, M.B.; Guerrini, U.; Sironi, L.; Gelosa, P.; Zini, I.; Ragsdale, D.S.; Avoli, M. Proepileptic influence of a focal vascular lesion affecting entorhinal cortex-CA3 connections after status epilepticus. J. Neuropathol. Exp. Neurol. 2008, 67, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, F.; Curia, G.; Marinelli, C.; Biagini, G. Increased perivascular laminin predicts damage to astrocytes in CA3 and piriform cortex following chemoconvulsive treatments. Neuroscience 2012, 218, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.; Kaufer, D.; Heinemann, U. Blood-brain barrier breakdown-inducing astrocytic transformation: Novel targets for the prevention of epilepsy. Epilepsy Res. 2009, 85, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, U.; Kaufer, D.; Friedman, A. Blood-brain barrier dysfunction, TGFβ signaling, and astrocyte dysfunction in epilepsy. Glia 2012, 60, 1251–1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Buckwalter, M.; Soreq, H.; Vezzani, A.; Kaufer, D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia 2012, 53, 37–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biagini, G.; Rustichelli, C.; Curia, G.; Vinet, J.; Lucchi, C.; Pugnaghi, M.; Meletti, S. Neurosteroids and epileptogenesis. J. Neuroendocrinol. 2013, 25, 980–990. [Google Scholar] [CrossRef] [PubMed]
- Curia, G.; Longo, D.; Biagini, G.; Jones, R.S.; Avoli, M. The pilocarpine model of temporal lobe epilepsy. J. Neurosci. Methods 2008, 172, 143–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Groef, L.; Dekeyster, E.; Geeraerts, E.; Lefevere, E.; Stalmans, I.; Salinas-Navarro, M.; Moons, L. Differential visual system organization and susceptibility to experimental models of optic neuropathies in three commonly used mouse strains. Exp. Eye Res. 2016, 145, 235–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dekeyster, E.; Geeraerts, E.; Buyens, T.; Van den Haute, C.; Baekelandt, V.; De Groef, L.; Salinas-Navarro, M.; Moons, L. Tackling Glaucoma from within the Brain: An Unfortunate Interplay of BDNF and TrkB. PLoS ONE 2015, 10, e0142067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo-Romero, C.; Avilés-Trigueros, M.; Jiménez-López, M.; Valiente-Soriano, F.J.; Salinas-Navarro, M.; Nadal-Nicolás, F.; Villegas-Pérez, M.P.; Vidal-Sanz, M.; Agudo-Barriuso, M. Axotomy-induced retinal ganglion cell death in adult mice: Quantitative and topographic time course analyses. Exp. Eye Res. 2011, 92, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Salinas-Navarro, M.; Jiménez-López, M.; Valiente-Soriano, F.J.; Alarcón-Martínez, L.; Avilés-Trigueros, M.; Mayor, S.; Holmes, T.; Lund, R.D.; Villegas-Pérez, M.P.; Vidal-Sanz, M. Retinal ganglion cell population in adult albino and pigmented mice: A computerized analysis of the entire population and its spatial distribution. Vis. Res. 2009, 49, 637–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Stefano, M.E.; Herrero, M.T. The multifaceted role of metalloproteinases in physiological and pathological conditions in embryonic and adult brains. Prog. Neurobiol. 2017, 155, 36–56. [Google Scholar] [CrossRef] [PubMed]
- Lorigados Pedre, L.; Morales Chacón, L.M.; Orozco Suárez, S.; Pavón Fuentes, N.; Estupiñán Díaz, B.; Serrano Sánchez, T.; García Maeso, I.; Rocha Arrieta, L. Inflammatory mediators in epilepsy. Curr. Pharm. Des. 2013, 19, 6766–6772. [Google Scholar] [CrossRef] [PubMed]
- Rempe, R.G.; Hartz, A.M.S.; Soldner, E.L.B.; Sokola, B.S.; Alluri, S.R.; Abner, E.L.; Kryscio, R.J.; Pekcec, A.; Schlichtiger, J.; Bauer, B. Matrix Metalloproteinase-Mediated Blood-Brain Barrier Dysfunction in Epilepsy. J. Neurosci. 2018, 38, 4301–4315. [Google Scholar] [CrossRef] [PubMed]
- Gkogkas, C.G.; Khoutorsky, A.; Cao, R.; Jafarnejad, S.M.; Prager-Khoutorsky, M.; Giannakas, N.; Kaminari, A.; Fragkouli, A.; Nader, K.; Price, T.J.; et al. Pharmacogenetic inhibition of eIF4E-dependent Mmp9 mRNA translation reverses fragile X syndrome-like phenotypes. Cell Rep. 2014, 9, 1742–1755. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, E.A.; Otte, W.M.; Wadman, W.J.; Aronica, E.; Kooij, G.; de Vries, H.E.; Dijkhuizen, R.M.; Gorter, J.A. Blood-brain barrier leakage after status epilepticus in rapamycin-treated rats II: Potential mechanisms. Epilepsia 2016, 57, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Fabene, P.F.; Navarro Mora, G.; Martinello, M.; Rossi, B.; Merigo, F.; Ottoboni, L.; Bach, S.; Angiari, S.; Benati, D.; Chakir, A.; et al. A role for leukocyte-endothelial adhesion mechanisms in epilepsy. Nat. Med. 2008, 14, 1377–1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef] [PubMed]
- Riazi, K.; Galic, M.A.; Kuzmiski, J.B.; Ho, W.; Sharkey, K.A.; Pittman, Q.J. Microglial activation and TNFalpha production mediate altered CNS excitability following peripheral inflammation. Proc. Natl. Acad. Sci. USA 2008, 105, 17151–17156. [Google Scholar] [CrossRef] [PubMed]
- Zattoni, M.; Mura, M.L.; Deprez, F.; Schwendener, R.A.; Engelhardt, B.; Frei, K.; Fritschy, J.M. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J. Neurosci. 2011, 31, 4037–4050. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhu, W.; Zhao, H.; Wang, X.; Wang, W.; Li, Z. Kainic acid-activated microglia mediate increased excitability of rat hippocampal neurons in vitro and in vivo: Crucial role of interleukin-1beta. Neuroimmunomodulation 2010, 17, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ko, A.R.; Hyun, H.W.; Kang, T.C. ETB receptor-mediated MMP-9 activation induces vasogenic edema via ZO-1 protein degradation following status epilepticus. Neuroscience 2015, 304, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, S.; Kobayashi, T.; Katoh, M.; Saito, S.; Ikeda, Y.; Kobori, M.; Masuho, Y.; Watanabe, T. Expression and localization of matrix metalloproteinase-12 in the aorta of cholesterol-fed rabbits: Relationship to lesion development. Am. J. Pathol. 1998, 153, 109–119. [Google Scholar] [CrossRef]
- Ito, S.; Kimura, K.; Haneda, M.; Ishida, Y.; Sawada, M.; Isobe, K. Induction of matrix metalloproteinases (MMP3, MMP12 and MMP13) expression in the microglia by amyloid-beta stimulation via the PI3K/Akt pathway. Exp. Gerontol. 2007, 42, 532–537. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Zha, G.B.; Yu, J.; Zhang, H.H.; Yi, S. Differential temporal expression of matrix metalloproteinases following sciatic nerve crush. Neural Regen. Res. 2016, 11, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Svedin, P.; Hagberg, H.; Mallard, C. Expression of MMP-12 after neonatal hypoxic-ischemic brain injury in mice. Dev. Neurosci. 2009, 31, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Van Vliet, E.A.; Aronica, E.; Gorter, J.A. Role of blood–brain barrier in temporal lobe epilepsy and pharmacoresistance. Neuroscience 2014, 277, 455–473. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, R.; Baumgartner, W.; Gerhauser, I.; Seeliger, F.; Haist, V.; Deschl, U.; Alldinger, S. MMP-12, MMP-3, and TIMP-1 are markedly upregulated in chronic demyelinating theiler murine encephalomyelitis. J. Neuropathol. Exp. Neurol. 2006, 65, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Jakobs, T.C. The Time Course of Gene Expression during Reactive Gliosis in the Optic Nerve. PLoS ONE 2013, 8, e67094. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Abdel-Rahman, M.H.; Wang, T.; Pouly, S.; Mahmoud, A.M.; Cebulla, C.M. Retinal MMP-12, MMP-13, TIMP-1, and TIMP-2 expression in murine experimental retinal detachment. Investig. Ophthalmol. Vis. Sci. 2014, 55, 2031–2040. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, J.J.; Peng, Q.; Chen, C.; Humphrey, M.B.; Heinecke, J.; Zhang, S.X. Macrophage metalloelastase (MMP-12) deficiency mitigates retinal inflammation and pathological angiogenesis in ischemic retinopathy. PLoS ONE 2012, 7, e52699. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.; Wang, Z.; Lee, E.; Moreno, S.; Abuelnasr, O.; Baudry, M.; Bi, X. Enhanced expression of matrix metalloproteinase-12 contributes to Npc1 deficiency-induced axonal degeneration. Exp. Neurol. 2015, 269, 67–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Tao, Y.; Tang, J.; Chen, Q.; Yang, Y.; Feng, Z.; Chen, Y.; Yang, L.; Yang, Y.; Zhu, G.; et al. A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood-Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl. Stroke Res. 2015, 6, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Matsye, P.; Zheng, L.; Si, Y.; Kim, S.; Luo, W.; Crossman, D.K.; Bratcher, P.E.; King, P.H. HuR promotes the molecular signature and phenotype of activated microglia: Implications for amyotrophic lateral sclerosis and other neurodegenerative diseases. Glia 2017, 65, 945–963. [Google Scholar] [CrossRef] [PubMed]
- Lucchi, C.; Costa, A.M.; Giordano, C.; Curia, G.; Piat, M.; Leo, G.; Vinet, J.; Brunel, L.; Fehrentz, J.A.; Martinez, J.; et al. Involvement of PPARγ in the anticonvulsant activity of EP-80317, a ghrelin receptor antagonist. Front. Pharmacol. 2017, 8, 676. [Google Scholar] [CrossRef] [PubMed]
- Racine, R.J. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr. Clin. Neurophysiol. 1972, 32, 281–294. [Google Scholar] [CrossRef]
- Lowenstein, D.H. Status epilepticus: An overview of the clinical problem. Epilepsia 1999, 40, S3–S8. [Google Scholar] [CrossRef] [PubMed]
- Giordano, C.; Vinet, J.; Curia, G.; Biagini, G. Repeated 6-Hz corneal stimulation progressively increases FosB/ΔFosB levels in the lateral amygdala and induces seizure generalization to the hippocampus. PLoS ONE 2015, 10, e0141221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, C.; Costa, A.M.; Lucchi, C.; Leo, G.; Brunel, L.; Fehrenz, J.A.; Martinez, J.; Torsello, A.; Biagini, G. Progressive seizure aggravation in the repeated 6-Hz corneal stimulation model is accompanied by marked increase in hippocampal p-ERK1/2 immunoreactivity in neurons. Front. Cell. Neurosci. 2016, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, F.; Marinelli, C.; Longo, D.; Pugnaghi, M.; Nichelli, P.F.; Meletti, S.; Biagini, G. Hypoxia markers are expressed in interneurons exposed to recurrent seizures. Neuromol. Med. 2013, 15, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Templeton, J.P.; Geisert, E.E. A practical approach to optic nerve crush in the mouse. Mol. Vis. 2012, 18, 2147–2152. [Google Scholar] [PubMed]
- Mead, B.; Thompson, A.; Scheven, B.A.; Logan, A.; Berry, M.; Leadbeater, W. Comparative evaluation of methods for estimating retinal ganglion cell loss in retinal sections and wholemounts. PLoS ONE 2014, 9, e110612. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolás, F.M.; Jiménez-López, M.; Sobrado-Calvo, P.; Nieto-López, L.; Cánovas-Martínez, I.; Salinas-Navarro, M.; Vidal-Sanz, M.; Agudo, M. Brn3a as a marker of retinal ganglion cells: Qualitative and quantitative time course studies in naïve and optic nerve-injured retinas. Investig. Ophthalmol. Vis. Sci. 2009, 50, 3860–3868. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, E.; Dekeyster, E.; Gaublomme, D.; Salinas-Navarro, M.; De Groef, L.; Moons, L. A freely available semi-automated method for quantifying retinal ganglion cells in entire retinal flatmounts. Exp. Eye Res. 2016, 147, 105–113. [Google Scholar] [CrossRef] [PubMed]







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vinet, J.; Costa, A.-M.; Salinas-Navarro, M.; Leo, G.; Moons, L.; Arckens, L.; Biagini, G. A Hydroxypyrone-Based Inhibitor of Metalloproteinase-12 Displays Neuroprotective Properties in Both Status Epilepticus and Optic Nerve Crush Animal Models. Int. J. Mol. Sci. 2018, 19, 2178. https://doi.org/10.3390/ijms19082178
Vinet J, Costa A-M, Salinas-Navarro M, Leo G, Moons L, Arckens L, Biagini G. A Hydroxypyrone-Based Inhibitor of Metalloproteinase-12 Displays Neuroprotective Properties in Both Status Epilepticus and Optic Nerve Crush Animal Models. International Journal of Molecular Sciences. 2018; 19(8):2178. https://doi.org/10.3390/ijms19082178
Chicago/Turabian StyleVinet, Jonathan, Anna-Maria Costa, Manuel Salinas-Navarro, Giuseppina Leo, Lieve Moons, Lutgarde Arckens, and Giuseppe Biagini. 2018. "A Hydroxypyrone-Based Inhibitor of Metalloproteinase-12 Displays Neuroprotective Properties in Both Status Epilepticus and Optic Nerve Crush Animal Models" International Journal of Molecular Sciences 19, no. 8: 2178. https://doi.org/10.3390/ijms19082178