Allergens with Protease Activity from House Dust Mites
Abstract
:1. Introduction
Group 1 Allergens
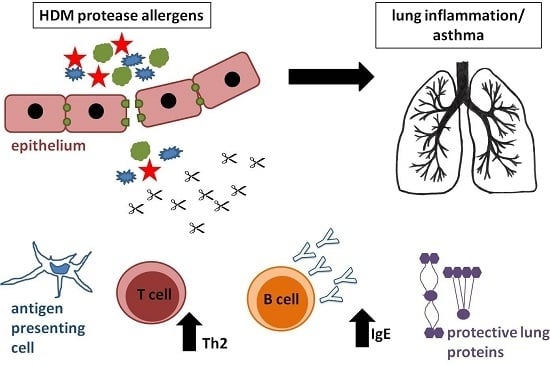
2. Disruption of the Epithelial Barrier
3. Th2-Biased Immune Response
4. Reduced Th1 Polarization
5. Reduced Lung Clearance
6. Excessive IgE Production
Group 3, 6 and 9 Allergens
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Wawrzyniak, M.; Mahony, L.O.; Akdis, M. Role of Regulatory Cells in Oral Tolerance. Allergy Asthma Immunol. Res. 2017, 9, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Romagnani, S. New millennium: The conquest of allergy The role of lymphocytes in allergic disease. J. Allergy Clin. Immunol. 2000, 105, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, T.F.; Joyce, J.; Fitch, F.W.; Gajewskl, T.F.; Joyce, J.; Fitch, F.W. Antiproliferative effect of IFN-gamma in immune regulation. III. Differential selection of TH1 and TH2 murine helper T lymphocyte clones using recombinant IL-2 and Recombinant IFN-γ. J. Immunol. 1989, 143, 15–22. [Google Scholar] [PubMed]
- Platts-Mills, T.A.E.; Chapman, M.D. Dust mites: Immunology, allergic disease, and environmental control. J. Allergy Clin. Immunol. 1987, 80, 755–775. [Google Scholar] [CrossRef]
- Zock, J.; Heinrich, J.; Jarvis, D.; Plana, E.; Sunyer, J. Distribution and determinants of house dust mite allergens in Europe: The European Community Respiratory Health Survey II. J. Allergy Clin. Immunol. 2006, 118, 682–690. [Google Scholar] [CrossRef] [PubMed]
- Calderón, M.A.; Kleine-Tebbe, J.; Linneberg, A.; de Blay, F.; Hernandez Fernandez de Rojas, D.; Virchow, J.C.; Demoly, P. House Dust Mite Respiratory Allergy: An Overview of Current Therapeutic Strategies. J. Allergy Clin. Immunol. 2015, 3, 843–855. [Google Scholar] [CrossRef] [PubMed]
- Wang, J. The Innate Immune Response in House Dust Mite-Induced Allergic Inflammation. Allergy Asthma Immunol. Res. 2013, 5, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Sala-Cunill, A.; Bartra, J.; Dalmau, G.; Tella, R.; Botey, E.; Raga, E.; Valero, A. Prevalence of Asthma and Severity of Allergic Rhinitis Comparing 2 Perennial Allergens: House Dust Mites and Parietaria judaica Pollen. J. Investig. Allergol. Clin. Immunol. 2013, 23, 145–151. [Google Scholar] [PubMed]
- Colloff, M.J. Dust Mites; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Jacquet, A. Interactions of airway epithelium with protease allergens in the allergic response Clinical & Experimental Allergy. Clin. Exp. Allergy 2010, 41, 305–311. [Google Scholar] [PubMed]
- Arlian, L.G.; Platts-Mills, T.A.E. The biology of dust mites and the remediation of mite allergens in allergic disease. J. Allergy Clin. Immunol. 2001, 107, 406–413. [Google Scholar] [CrossRef]
- Thomas, W.R. House dust allergy and immunotherapy House dust allergy and immunotherapy. Hum. Vaccin. Immunother. 2012, 5515, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.L.; Hashim, Z.; Thian, L.; Than, L.; Said, S. Asthma, Airway Symptoms and Rhinitis in Office Workers in Malaysia: Associations with House Dust Mite (HDM) Allergy, Cat Allergy and Levels of House Dust Mite Allergens in Office Dust. PLoS ONE 2015, 10, e0124905. [Google Scholar] [CrossRef] [PubMed]
- Sporik, R.; Holgate, S.T.; Platts-Mills, T.A.E.; Cogswell, J.J. Exposure to house-dust mite allergen (Der p 1) and the development of asthma in childhood. N. Engl. J. Med. 1990, 323, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R. House Dust Mite Allergens: New Discoveries and Relevance to the Allergic Patient. Curr. Allergy Asthma Rep. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Trompette, A.; Divanovic, S.; Visintin, A.; Blanchard, C.; Hegde, R.S.; Madan, R.; Thorne, P.S.; Wills-karp, M.; Gioannini, T.L.; Weiss, J.P.; et al. Allergenicity resulting from functional mimicry of a Toll-like receptor complex protein. Nature 2009, 457, 585–589. [Google Scholar] [CrossRef]
- Weghofer, M.; Grote, M.; Resch, Y.; Kneidinger, M.; Kopec, J.; Wayne, R.; Fernández-caldas, E.; Kabesch, M.; Ferrara, R.; Mari, A.; et al. Identification of Der p 23, a Peritrophin-like Protein, as a New Major Dermatophagoides pteronyssinus Allergen Associated with the Peritrophic Matrix of Mite Fecal Pellets. J. Immunol. 2013, 190, 3059–3067. [Google Scholar] [CrossRef] [PubMed]
- Thomas, W.R. Allergology International Hierarchy and molecular properties of house dust mite allergens. Allergol. Int. 2015, 64, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.D.; Platts-Mills, T.A. Purification and characterization of the major allergen from Dermatophagoides pteronyssinus-antigen P1. J. Immunol. 1980, 125, 587–592. [Google Scholar] [PubMed]
- Tovey, M.E.R.; Chapman, M.D.; Platts-Mills, T.A.E. Mite faeces are a major source of house dust allergens. Nature 1981, 289, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.D.; Platts-Mills, T.A. Measurement of IgG, IgA and IgE antibodies to Dermatophagoides pteronyssinus by antigen-binding assay, using a partially purified fraction of mite extract (F4P1). Clin. Exp. Immunol. 1978, 34, 126–136. [Google Scholar] [PubMed]
- Chua, K.Y.; Stewart, G.A.; Thomas, W.R.; Simpson, R.J.; Dilworth, R.J.; Plozza, T.M.; Turner, K.J. Sequence analysis of cDNA coding for a major house dust mite allergen, Der p 1. J. Exp. Med. 1988, 167, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Vrtala, S.; Huber, H.; Thomas, R.W. Recombinant house dust mite allergens. Methods 2015, 66, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.; Mason, D.E.; Li, J.; Burdick, K.W.; Backes, B.J.; Chen, T.; Shipway, A.; van Heeke, G.; Gough, L.; Ghaemmaghami, A.; et al. Activity Profile of Dust Mite Allergen Extract Using Substrate Libraries and Functional Proteomic Microarrays. Chem. Biol. 2004, 11, 1361–1372. [Google Scholar] [CrossRef] [PubMed]
- Furmonaviciene, R.Ã.; Ghaemmaghami, A.M.Ã.; Boyd, S.E.; Jones, N.S.; Bailey, K.Ã.; Willis, A.C.; Sewell, H.F.Ã.; Mitchell, D.A.; Shakib, F. The protease allergen Der p 1 cleaves cell surface DC-SIGN and DC-SIGNR: Experimental analysis of in silico substrate identification and implications in allergic responses. Clin. Exp. Allergy 2007, 37, 231–242. [Google Scholar] [CrossRef] [PubMed]
- Osinski, T.; Pomés, A.; Majorek, K.A.; Offermann, L.R.; Vailes, L.D.; Martin, D.; Minor, W.; Chruszcz, M. Structural Analysis of Der p 1—Antibody Complexes and Comparison with Complexes of Proteins or Peptides with Monoclonal Antibodies. J. Immunol. 2015, 195, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Glesner, J.; Vailes, L.D.; Schlachter, C.; Minor, W.; Osinski, T.; Chapman, M.D.; Pomés, A. Antigenic Determinants of Der p 1: Specificity and Cross-Reactivity Associated with IgE Antibody Recognition. J. Immunol. 2017, 198, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, Y.; Takai, T.; Kuhara, T.; Kato, T.; Hatanaka, H.; Ichikawa, S.; Tokura, T.; Akiba, H.; Mitsuishi, K.; Okumura, K.; et al. Crucial Commitment of Proteolytic Activity of a Purified Recombinant Major House Dust Mite Allergen Der p 1 to Sensitization toward IgE and IgG Responses. J. Immunol. 2006, 177, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Hammad, H.; Lambrecht, B.N.; Pochard, P.; Gosset, P.; Marquillies, P.; Tonnel, A.; Pestel, J. Monocyte-Derived Dendritic Cells Induce a House Dust Mite-Specific Th2 Allergic Inflammation in the Lung of Humanized SCID Mice: Involvement of CCR7. J. Immunol. 2002, 169, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- RCSB Protein Data Bank. Available online: http://www.rcsb.org/pdb/explore/explore.do?structureId=2AS8 (accessed on 26 June 2017).
- Wan, H.; Winton, H.L.; Soeller, C.; Tovey, E.R.; Gruenert, D.C.; Thompson, P.J.; Stewart, G.A.; Taylor, G.W.; Garrod, D.R.; Cannell, M.B.; et al. Der p 1 facilitates transepithelial allergen delivery by disruption of tight junctions. J. Clin. Investig. 1999, 104, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Roche, N.; Chinet, T.C.; Belouchi, N.; Julie, C.; Huchon, G.J. Dermatophagoides pteronyssinus and bioelectric properties of airway epithelium: Role of cysteine proteases. Eur. Respir. J. 2000, 16, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Herbert, C.A.; King, C.M.; Ring, P.C.; Holgate, S.T.; Stewart, G.A.; Thompson, P.J.; Robinson, C. Augmentation of Permeability in the Bronchial Epithelium by the House Dust Mite Allergen Der p 1. Am. J. Respir. Cell Mol. Biol. 1995, 12, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Henriquez, A.O.; den Beste, K.; Hoddeson, E.; Parkos, A.C.; Nusrat, A.; Wise, S.K. House Dust Mite Der p 1 Effects on Sinonasal Epithelial Tight Junctions. Int. Forum Allergy Rhinol. 2014, 3, 630–635. [Google Scholar] [CrossRef] [PubMed]
- Pichavant, M.; Charbonnier, A.; Taront, S.; Brichet, A.; Wakkaert, B.; Pestel, J.; Tonnel, A.-B.; Gosset, P. Asthmatic bronchial epithelium activated by the proteolytic allergen Der p 1 increases selective dendritic cell recruitment. J. Allergy Clin. Immunol. 2005, 115, 771–778. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Brennan, S.; Thompson, P.J.; Stewart, G.A. Dust Mite Proteolytic Allergens Induce Cytokine Release from Cultured Airway Epithelium. J. Immunol. 1998, 161, 3645–3652. [Google Scholar] [PubMed]
- Adam, E.; Hansen, K.K.; Astudillo, O.F.; Coulon, L.; Duhant, X.; Jaumotte, E.; Hollenberg, M.D.; Jacquet, A. The House Dust Mite Allergen Der p 1, Unlike Der p 3, Stimulates the Expression of Interleukin-8 in Human Airway Epithelial Cells via a Proteinase-activated Receptor-2-independent Mechanism. J. Biol. Chem. 2006, 281, 6910–6923. [Google Scholar] [CrossRef] [PubMed]
- Asokananthan, N.; Graham, P.T.; Stewart, D.J.; Bakker, A.J.; Eidne, K.A.; Thompson, P.J.; Stewart, G.A. House Dust Mite Allergens Induce Proinflammatory Cytokines from Respiratory Epithelial Cells: The Cysteine Protease Allergen, Der p 1, Activates Protease-Activated Receptor (PAR)-2 and Inactivates PAR-1. J. Immunol. 2002, 169, 4572–4578. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Schulz, B.O.; Sewell, H.F.; Shakib, F. Proteolytic Cleavage of CD25, the Alpha Subunit of the Human T Cell Interleukin 2 Receptor, by Der p 1, a Major Mite Allergen with Cysteine Protease Activity. J. Exp. Med. 1988, 187, 271–275. [Google Scholar] [CrossRef]
- Halim, T.Y.F.; Steer, C.A.; Matha, L.; Gold, M.J.; Martinez-gonzalez, I.; Mcnagny, K.M.; Mckenzie, A.N.J.; Takei, F. Group 2 Innate Lymphoid Cells Are Critical for the Initiation of Adaptive T Helper 2 Cell-Mediated Allergic Lung Inflammation. Immunity 2014, 40, 425–435. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Kasran, A.; Wannerdam, P.A.M.; de Bee, M.; Ceuppens, J.L. Accessory signaling by CD40 for T cell activation: Induction of Thl and Th2 cytokines and synergy with interleukin 42 for interferon-γ production. Eur. J. Immunol. 1996, 26, 1621–1627. [Google Scholar] [CrossRef] [PubMed]
- Ghaemmaghami, A.M.; Gough, L.; Sewell, H.F.; Shakib, F. Proteolytic activity of the major dust mite allergen Der p 1 conditions dendritic cells to produce less interleukin-12: Allergen-induced Th2 bias determined at the dendritic cell level. Clin. Exp. Allergy 2002, 32, 1468–1475. [Google Scholar] [CrossRef] [PubMed]
- Hasan, A.A.; Ghaemmaghami, A.M.; Fairclough, L.; Robins, A.; Sewell, H.F.; Shakib, F. Allergen-driven suppression of thiol production by human dendritic cells and the effect of thiols on T cell function. Immunobiology 2009, 214, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Shakib, F.; Schulz, O.; Sewell, H. A mite subversive: Cleavage of CD23 and CD25 by Der p 1 enhances allergenicity. Immunol. Today 1998, 17, 313–316. [Google Scholar] [CrossRef]
- Schulz, O.; Sewell, H.F.; Shakib, F. The interaction between the dust mite antigen Der p 1 and cell-signalling molecules in amplifying allergic disease. Clin. Exp. Allergy 1999, 29, 439–444. [Google Scholar] [CrossRef] [PubMed]
- Wright, J.R. Pulmonary surfactant: A front line of lung host defense. J. Clin. Investig. 2003, 111, 1453–1455. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Kishore, U.; Lim, B.L.; Strong, P.; Reid, K.B.M. Interaction of human lung surfactant proteins A and D with mite (Dermatophagoides pteronyssinus) allergens. Clin. Exp. Immunol. 1996, 106, 367–373. [Google Scholar] [CrossRef] [PubMed]
- Madan, T.; Kishore, U.; Singh, M.; Strong, P.; Clark, H.; Hussain, E.M.; Reid, K.B.M.; Sarma, P.U. Surfactant proteins A and D protect mice against pulmonary hypersensitivity induced by Aspergillus fumigatus antigens and allergens. J. Clin. Investig. 2001, 107, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, Y.; Shieh, C.; Yu, C.; Reid, K.B.M.; Wang, J. Therapeutic effect of surfactant protein D in allergic inflammation of mite-sensitized mice. Clin. Exp. Allergy 2005, 35, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Strong, P.; Townsend, P.; Mackay, R.; Reid, K.B.M.; Clark, H.W. A recombinant fragment of human SP-D reduces allergic responses in mice sensitized to house dust mite allergens. Clin. Exp. Immunol. 2003, 134, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Deb, R.; Shakib, F.; Reid, K.; Clark, H. Major House Dust Mite Allergens Dermatophagoides pteronyssinus 1 and Dermatophagoides farinae 1 Degrade and Inactivate Lung Surfactant Proteins A and D. J. Biol. Chem. 2007, 282, 36808–36819. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.; Kilmon, M.A.; Studer, E.J.; van der Putten, H.; Conrad, D.H. B Cell Activation and Ig, Especially IgE, Production Is Inhibited by High CD23 Levels in Vivo and in Vitro. Cell. Immunol. 1997, 46, 36–46. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Kosco-Vilbois, M.; Richards, M.; Köhler, G.; Lamers, M.C. Negative feedback regulation of IgE synthesis by murine CD23. Nature 1994, 369, 753–756. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, C.R.; Brown, A.P.; Hart, B.J.; Pritchard, D.I. A Major House Dust Mite Allergen Disrupts the Immunoglobulin E Network by Selectively Cleaving CD23: Innate Protection by Antiproteases. J. Exp. Med. 1995, 182, 1537–1544. [Google Scholar] [CrossRef] [PubMed]
- Gough, B.L.; Schulz, O.; Sewell, H.F.; Shakib, F. The Cysteine Protease Activity of the Major Dust Mite Allergen Der p 1 Selectively Enhances the Immunoglobulin E Antibody Response. J. Exp. Med. 1999, 190, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.D.; Wünschmann, S.; Pomés, A. Proteases as Th2 Adjuvants. Curr. Allergy Asthma Rep. 2007, 7, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Bennett, B.J.; Thomas, W.R. Cloning and sequencing of the group 6 allergen of Dermatophagoides pteronyssinus. Clin. Exp. Allergy 1996, 26, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- King, C.; Simpson, R.J.; Moritz, R.L.; Reed, G.E.; Thompson, P.J.; Stewart, G.A. The isolation and characterization of a novel collagenolytic serine protease allergen (Der p 9) from the dust mite Dermatophagoides pteronyssinus. J. Allergy Clin. Immunol. 1996, 98, 739–747. [Google Scholar] [CrossRef]
- Ando, T.; Homma, R.; Ino, Y.; Ito, G.; Miyahara, A.; Yanagihara, T.; Kimura, H.; Ikeda, S.; Yamakawa, H.; Iwaki, M.; et al. Trypsin-like protease of mites: Purification and characterization of trypsin-like protease from mite faecal extract Dermatophagoides farinae. Relationship between trypsin-like protease and Der f III. Clin. Exp. Allergy 1993, 23, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Wan, H.; Winton, H.L.; Soeller, C.; Taylor, G.W.; Gruenert, D.C.; Thompson, P.J.; Cannell, M.B.; Stewart, G.A.; Garrod, D.R.; Robinson, C. The transmembrane protein occludin of epithelial tight junctions is a functional target for serine peptidases from faecal pellets of Dermatophagoides pteronyssinus. Clin. Exp. Allergy 2001, 31, 279–294. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Stacey, M.A.; Schmidt, M.; Mori, L.; Mattoli, S.; Sun, G.; Stacey, M.A.; Schmidt, M.; Mori, L.; Mattoli, S. Interaction of Mite Allergens Der p 3 and Der p 9 with Protease-Activated Receptor-2 Expressed by Lung Epithelial Cells. J. Immunol. 2001, 167, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Roelandt, T.; Heughebaert, C.; Hachem, J. Proteolytically Active Allergens Cause Barrier Breakdown. J. Investig. Dermatol. 2008, 128, 1878–1880. [Google Scholar] [CrossRef] [PubMed]
- Dumez, M.-E.; Teller, N.; Mercier, F.; Tanaka, T.; Vandenberghe, I.; Vandenbranden, M.; Devreese, B.; Luxen, A.; Frére, J.; Matagne, A.; et al. Activation Mechanism of Recombinant Der p 3 Allergen Zymogen. J. Biol. Chem. 2008, 283, 30606–30617. [Google Scholar] [CrossRef] [PubMed]
- Herman, J.; Thelen, N.; Smargiasso, N.; Mailleux, A.; Luxen, A.; Cloes, M.; de Pauw, E.; Chevigné, A.; Galleni, M.; Dumez, M. Der p 1 is the primary activator of Der p 3, Der p 6 and Der p 9 the proteolytic allergens produced by the house dust mite Dermatophagoides pteronyssinus. Biochim. Biophys. Acta J. 2014, 1840, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Dumez, M.; Herman, J.; Campizi, V.; Galleni, M.; Jacquet, A.; Chevigné, A. Orchestration of an uncommon maturation cascade of the house dust mite protease allergen quartet. Front. Immunol. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Posa, D.; Perna, S.; Resch, Y.; Lupinek, C.; Panetta, V.; Hofmaier, S.; Rohrbach, A.; Hatzler, L.; Grabenhenrich, L.; Tsilochristou, O.; et al. Evolution and predictive value of IgE responses toward a comprehensive panel of house dust mite allergens during the first 2 decades of life. J. Allergy Clin. Immunol. 2017, 139, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Matricardi, P.M.; Kleine-Tebbe, J.; Hoffmann, H.J.; Valenta, R.; Hilger, C.; Hofmaier, S.; Aalberse, R.C.; Agache, I.; Asero, R.; Ballmer-Weber, B.; et al. EAACI Molecular Allergology User’s Guide. Pediatr. Allergy Immunol. 2016, 27 (Suppl. S23), 11–16. [Google Scholar] [CrossRef] [PubMed]

| Allergen Group | Identified Allergens | Protein Family | Prevalence |
|---|---|---|---|
| 1 | Der p 1, Der f 1, Der m 1, Der s 1, Eur m 1, Blo t 1, Pso o 1, Sar s 1 | Cysteine protease, papain-like | 80% |
| 3 | Der p 3, Der f 3, Der s 3, Eur m 3, Blo t 3, Sar s 3, Gly d 3, Lep d 3 | Trypsin-like serine protease | 16–100% |
| 6 | Der p 6, Der f 6, Blo t 6 | Chymotrypsin-like serine protease | 40% |
| 9 | Der p 9, Der f 9, Blo t 9 | Collagenolytic-like serine protease | 90% |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reithofer, M.; Jahn-Schmid, B. Allergens with Protease Activity from House Dust Mites. Int. J. Mol. Sci. 2017, 18, 1368. https://doi.org/10.3390/ijms18071368
Reithofer M, Jahn-Schmid B. Allergens with Protease Activity from House Dust Mites. International Journal of Molecular Sciences. 2017; 18(7):1368. https://doi.org/10.3390/ijms18071368
Chicago/Turabian StyleReithofer, Manuel, and Beatrice Jahn-Schmid. 2017. "Allergens with Protease Activity from House Dust Mites" International Journal of Molecular Sciences 18, no. 7: 1368. https://doi.org/10.3390/ijms18071368