Transfection of Antisense Oligonucleotides Mediated by Cationic Vesicles Based on Non-Ionic Surfactant and Polycations Bearing Quaternary Ammonium Moieties
Abstract
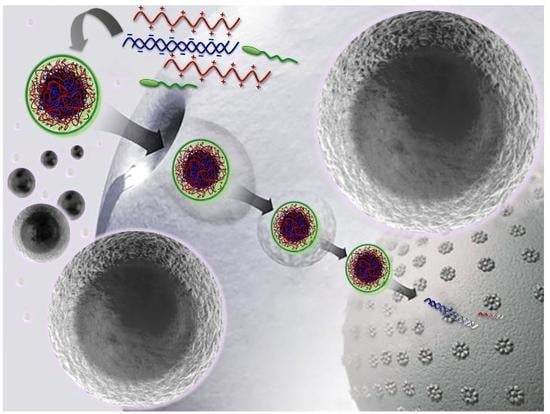
:1. Introduction
2. Results and Discussion
2.1. Size and ζ-Potential Measurements
2.2. SAXS Measurements
2.3. Cytotoxicity Assay
2.4. Transfection
3. Materials and Methods
3.1. Materials
3.2. Preparation of Polyplexes
3.3. Zeta Potential
3.4. Size Measurements
3.5. Small-Angle X-ray Scattering (SAXS) Measurements of Ionene Polyplexes
3.6. Cryo-Scanning Electron Microscopy (cryo-SEM)
3.7. Cytotoxicity Assay
3.8. Gene Transfection and Antisense Technology Studies
3.8.1. In the Absence of FBS
3.8.2. In the Presence of FBS
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stephenson, M.L.; Zamecnik, P.C. Inhibition of Rous sarcoma viral RNA translation by a specific oligodeoxyribonucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 285–288. [Google Scholar] [CrossRef] [PubMed]
- Watts, J.; Deleavey, G.; Damha, M. Chemically modified siRNA: tools and applications. Drug Discov. Today 2008, 13, 842–855. [Google Scholar] [CrossRef] [PubMed]
- Keefe, A.D.; Pai, S.; Ellington, A. Aptamers as therapeutics. Nat. Rev. Drug Discov. 2010, 9, 537–550. [Google Scholar] [CrossRef] [PubMed]
- Mulhbacher, J.; St-Pierre, P.; Lafontaine, D.A. Therapeutic applications of ribozymes and riboswitches. Curr. Opin. Pharmacol. 2010, 10, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Niks, E.H.; Aartsma-Rus, A. Exon-skipping: A first in class strategy for Duchenne muscular dystrophy. Expert Opin. Biol. Ther. 2017, 17, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Chiu, Y.-L. siRNA function in RNAi: A chemical modification analysis. RNA 2003, 9, 1034–1048. [Google Scholar] [CrossRef] [PubMed]
- Terrazas, M.; Alagia, A.; Faustino, I.; Orozco, M.; Eritja, R. Functionalization of the 3′-ends of DNA and RNA strands with N-ethyl-N-coupled nucleosides: A promising approach to avoid 3′-exonuclease-catalyzed hydrolysis of therapeutic oligonucleotides. Chem. Biol. Chem. 2013, 14, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Geary, R.; Tillman, L.; Hardee, G. Routes and formulations for delivery of antisense oligonucleotides. In Antisense Drug Technology; CRC Press: Boca Raton, FL, USA, 2007; pp. 217–236. [Google Scholar]
- Felgner, P.L.; Gadek, T.R.; Holm, M.; Roman, R.; Chan, H.W.; Wenz, M.; Northrop, J.P.; Ringold, G.M.; Danielsen, M. Lipofection: A highly efficient, lipid-mediated DNA-transfection procedure. Proc. Natl. Acad. Sci. USA 1987, 84, 7413–7417. [Google Scholar] [CrossRef] [PubMed]
- Azzam, T.; Domb, A. Current developments in gene transfection agents. Curr. Drug Deliv. 2004, 1, 165–193. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Alagia, A.; Puras, G.; Zárate, J.; Pedraz, J.L.; Eritja, R. Cationic vesicles based on non-ionic surfactant and synthetic aminolipids mediate delivery of antisense oligonucleotides into mammalian cells. Colloids Surf. B Biointerfaces 2014, 119, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Audouy, S.; Molema, G.; de Leij, L.; Hoekstra, D. Serum as a modulator of lipoplex-mediated gene transfection: Dependence of amphiphile, cell type and complex stability. J. Gene Med. 2000, 2, 465–476. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control Release 2006, 114, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Wagner, E. History of polymeric gene delivery systems. Top. Curr. Chem. 2017, 26, 375–414. [Google Scholar] [CrossRef] [PubMed]
- Punyani, S.; Singh, H. Preparation of iodine containing quaternary amine methacrylate copolymers and their contact killing antimicrobial properties. J. Appl. Polym. Sci. 2006, 102, 1038–1044. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Putnam, D.; Shastri, P.; Langer, R.; Izumrudov, V.A. Aliphatic ionenes as gene delivery agents: Elucidation of structure-function relationship through modification of charge density and polymer length. Bioconjug. Chem. 2002, 13, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Laschewsky, A. Recent trends in the synthesis of polyelectrolytes. Curr. Opin. Colloid Interface Sci. 2012, 17, 56–63. [Google Scholar] [CrossRef]
- Williams, S.R.; Long, T.E. Recent advances in the synthesis and structure—Property relationships of ammonium ionenes. Prog. Polym. Sci. 2009, 34, 762–782. [Google Scholar] [CrossRef]
- Bachl, J.; Zanuy, D.; López-Pérez, D.E.; Revilla-López, G.; Cativiela, C.; Alemán, C.; Díaz, D.D. Synergistic computational-experimental approach to improve ionene polymer-based functional hydrogels. Adv. Funct. Mater. 2014, 24, 4893–4904. [Google Scholar] [CrossRef]
- Dragan, E.S.; Mayr, J.; Häring, M.; Cocarta, A.I.; Díaz, D.D. Spectroscopic characterization of azo dyes aggregation induced by DABCO-based ionene polymers and dye removal efficiency as a function of ionene structure. ACS Appl. Mater. Interfaces 2016, 8, 30908–30919. [Google Scholar] [CrossRef] [PubMed]
- Tiffner, M.; Zielke, K.; Mayr, J.; Häring, M.; Díaz, D.D.; Waser, M. Phase-transfer catalysis with ionene polymers. Chem. Sel. 2016, 1, 4030–4033. [Google Scholar] [CrossRef]
- Yudovin-Farber, I.; Yanay, C.; Azzam, T.; Linial, M.; Domb, A.J. Quaternary ammonium polysaccharides for gene delivery. Bioconjugate Chem. 2005, 16, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- San Juan, A.; Letourneur, D.; Izumrudov, V.A. Quaternized poly(4-vinylpyridine)s as model gene delivery polycations: Structure-function study by modification of side chain hydrophobicity and degree of alkylation. Bioconjugate Chem. 2007, 18, 922–928. [Google Scholar] [CrossRef] [PubMed]
- Reineke, T.M.; Davis, M.E. The effects of charge separation in quaternary ammonium, DABCO-containing polymers on in vitro toxicity and gene delivery. Mat. Res. Soc. Symp. Proc. 2002, 724, 209–214. [Google Scholar]
- Wheeler, C.J. Complex Cationic Lipids Having Quaternary Nitrogen Therein. U.S. Patent 6670332 B1, 30 December 2003. [Google Scholar]
- Jorge, A.F.; Dias, R.S.; Pereira, J.C.; Pais, A.A. DNA condensation by pH-responsive polycations. Biomacromolecules 2010, 11, 2399–2406. [Google Scholar] [CrossRef] [PubMed]
- Mezei, A.; Pons, R. Release of DNA and surfactant from gel particles: The receptor solution effect and the dehydration aspects. Colloids Surf. B Biointerfaces 2014, 123, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Leal, C.; Wadsö, L.; Olofsson, G.; Miguel, M.; Wennerström, H. The hydration of a DNA-amphiphile complex. J. Phys. Chem. B 2004, 108, 3044–3050. [Google Scholar] [CrossRef]
- Mezei, A.; Pons, R.; Morán, M.C. The nanostructure of surfactant-DNA complexes with different arrangements. Colloids Surf. B Biointerfaces 2013, 111, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Mandelkern, M.; Elias, J.G.; Eden, D.; Crothers, D.M. The dimensions of DNA in solution. J. Mol. Biol. 1981, 152, 153–161. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxic assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Xiong, F.; Mi, Z.; Gu, N. Cationic liposomes as gene delivery system: Transfection efficiency and new application. Pharmazie 2011, 66, 158–164. [Google Scholar] [PubMed]
- Rehman, Z.; Hoekstra, D.; Zuhorn, I.S. Mechanism of polyplex and lipoplex-mediated delivery of nucleic acids: Real-time visualization of transient membrane destabilization without endosomal lysis. ACS Nano 2013, 7, 3767–3777. [Google Scholar] [CrossRef] [PubMed]
- Grijalvo, S.; Eritja, R. Synthesis and in vitro inhibition properties of oligonucleotide conjugates carrying amphipathic proline-rich peptide derivatives of the sweet arrow peptide (SAP). Mol. Div. 2012, 16, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Fornaguera, C.; Grijalvo, S.; Galán, M.; Fuentes-Paniagua, E.; de la Mata, F.J.; Gómez, R.; Eritja, R.; Calderó, G.; Solans, C. Novel non-viral gene delivery systems composed of carbosilane dendron functionalized nanoparticles prepared from nano-emulsions as non-viral carriers for antisense oligonucleotides. Int. J. Pharm. 2015, 478, 113–123. [Google Scholar] [CrossRef] [PubMed]
- Mayr, J.; Bachl, J.; Schlossmann, J.; Díaz, D.D. Antimicrobial and hemolytic studies of a series of polycations bearing quaternary ammonium moieties: Structural and topological effects. Int. J. Mol. Sci. 2017, 18, 303. [Google Scholar] [CrossRef] [PubMed]
- Metwally, A.A.; Pourzand, C.; Blagbrough, I.S. Efficient gene silencing by self-assempled complexes of siRNA and symmetrical fatty acid amides of spermine. Pharmaceutics 2011, 3, 125–140. [Google Scholar] [CrossRef] [PubMed]





© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayr, J.; Grijalvo, S.; Bachl, J.; Pons, R.; Eritja, R.; Díaz Díaz, D. Transfection of Antisense Oligonucleotides Mediated by Cationic Vesicles Based on Non-Ionic Surfactant and Polycations Bearing Quaternary Ammonium Moieties. Int. J. Mol. Sci. 2017, 18, 1139. https://doi.org/10.3390/ijms18061139
Mayr J, Grijalvo S, Bachl J, Pons R, Eritja R, Díaz Díaz D. Transfection of Antisense Oligonucleotides Mediated by Cationic Vesicles Based on Non-Ionic Surfactant and Polycations Bearing Quaternary Ammonium Moieties. International Journal of Molecular Sciences. 2017; 18(6):1139. https://doi.org/10.3390/ijms18061139
Chicago/Turabian StyleMayr, Judith, Santiago Grijalvo, Jürgen Bachl, Ramon Pons, Ramon Eritja, and David Díaz Díaz. 2017. "Transfection of Antisense Oligonucleotides Mediated by Cationic Vesicles Based on Non-Ionic Surfactant and Polycations Bearing Quaternary Ammonium Moieties" International Journal of Molecular Sciences 18, no. 6: 1139. https://doi.org/10.3390/ijms18061139