Evaluation of Polyethylene Glycol Crosslinked β-CD Polymers for the Removal of Methylene Blue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-CD Polymers
2.3. Structural Analysis
2.3.1. FTIR Spectroscopy
2.3.2. TGA/DTA Analysis
2.3.3. Brunauer–Emmett–Teller Analysis
2.3.4. Scanning Electron Microscopy Analysis
2.3.5. X-ray Diffraction Analysis
2.4. Adsorption Experiments
2.4.1. Preliminary Assessment of Polymers
2.4.2. Experimental Validation of Polymers Towards MB
3. Results
3.1. Preparation of β-CD Polymers
3.2. Structural Characterization
3.2.1. FTIR Spectroscopy
3.2.2. TGA/TDA Analysis
3.2.3. Brunauer–Emmett–Teller Analysis
3.2.4. Scanning Electron Microscopy Analysis
3.2.5. XRD Analysis
3.3. Adsorption Experiments of Polymers Towards Methylene Blue
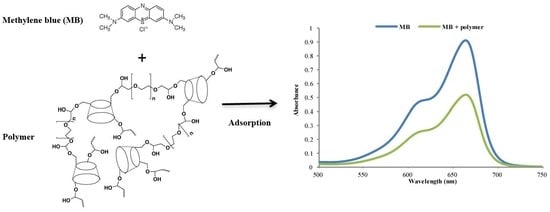
3.3.1. Prior Analysis
3.3.2. Effect of pH
3.3.3. Effect of Adsorbent Mass
3.3.4. Effect of Initial Dye Concentration
3.3.5. Reutilization Studies
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wong, Y.; Yu, J. Laccase-catalyzed decolorization of synthetic dyes. Water Res. 1999, 33, 3512–3520. [Google Scholar] [CrossRef]
- Bumpus, J. Microbial degradation of azo dyes. In Biotransformations; Singh, V.P., Ed.; Elsevier: Amsterdam, The Netherlands, 1995; pp. 157–176. [Google Scholar]
- Rawat, D.; Mishra, V.; Sharma, R.S. Detoxification of azo dyes in the context of environmental process. Chemosphere 2016, 155, 591–605. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, J.; Sikder, J.; Chakraborty, S.; Curcio, S.; Drioli, E. Remediation of textile effluents by membrane based treatment techniques: A state of the art review. J. Environ. Manag. 2015, 147, 55–72. [Google Scholar] [CrossRef]
- Benidris, E.B.; Ghezzar, M.R.; Ma, A.; Ouddane, B.; Addou, A. Water purification by a new hybrid plasma-sensitization coagulation process. Sep. Purif. Technol. 2017, 178, 253–260. [Google Scholar] [CrossRef]
- Chen, S.H.; Yien Ting, A.S. Biodecolorization and biodegradation potential of recalcitrant triphenylmethane dyes by Coriolopsis sp. Isolated from compost. J. Environ. Manag. 2015, 150, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Dinh, D.M.; Hsieh, Y.L. Adsorption and desorption of cationic malachite green dye on cellulose nanofibril aerogels. Carbohydr. Polym. 2017, 173, 286–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doke, S.M.; Yadav, G.D. Novelties of combustion synthesized titania ultrafiltration membrane in efficient removal of methylene blue dye from aqueous effluent. Chemosphere 2014, 117, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Nuengmatcha, P.; Chanthai, S.; Mahachai, R.; Oh, W.C. Sonocatalytic performance of ZnO/graphene/TiOs nanocomposites for degradation of dye pollutants (methylene blue, texbrite BAC-L, texbrite BBU-L and texbrite NFW-L) under ultrasonic irradiation. Dyes Pigment. 2016, 134, 487–497. [Google Scholar] [CrossRef]
- Ebadi, A.; Rafati, A.A. Preparation of silica-mesoporous nanoparticles functionalized with β-cyclodextrin and its application for methylene blue removal. J. Mol. Liq. 2015, 209, 239–245. [Google Scholar] [CrossRef]
- Khan, A.M.; Shafiq, F.; Khan, S.A.; Ali, S.; Ismail, B.; Hakeem, A.S.; Rahdar, A.; Nazar, M.F.; Sayed, M.; Khan, A.R. Surface modification of colloidal particles using cationic surfactant and the resulting adsorption of dyes. J. Mol. Liq. 2019, 274, 673–680. [Google Scholar] [CrossRef]
- Kumar, A.; Jena, H.M. Removal of methylene blue and phenol onto prepared activated carbon from Fox nutshell by chemical activation in batch and fixed bed column. J. Clean. Prod. 2016, 137, 1246–1259. [Google Scholar] [CrossRef]
- Liu, S.; Ding, Y.; Ti, P.; Diao, K.; Tan, X.; Lei, F.; Zhan, Y.; Li, Q.; Huang, Z. Adsorption of the anionic dye Congo red from aqueous solution onto natural zeolites modified with N,N-dimethyl dehydroabietylamine oxide. Chem. Eng. J. 2014, 248, 135–144. [Google Scholar] [CrossRef]
- Jiao, T.; Zhao, H.; Zhou, J.; Zhang, Q.; Luo, X.; Hu, J.; Peng, Q.; Yan, X. Self-assembly reduced graphene oxide nanosheet hydrogel fabrication by anchorage of chitosan/silver and its potential efficient application toward dye degradation of wastewater treatments. ACS Sustain. Chem. Eng. 2015, 3, 3130–3139. [Google Scholar] [CrossRef]
- Yao, X.; Ji, L.; Guo, J.; Ge, S.; Lu, W.; Cai, L.; Wang, Y.; Song, W.; Zhang, H. Magnetic activated biochar nanocomposites derived from wakame and its application in methylene blue adsorption. Bioresour. Technol. 2020, 302, 122842. [Google Scholar] [CrossRef] [PubMed]
- Kono, H.; Ogasawara, K.; Kusumoto, R.; Oshima, K.; Hashimoto, H.; Shimizu, Y. Cationic cellulose hydrogels cross-linked by poly(ethyleneglycol): Preparation, molecular dynamics, and adsorption of anionic dyes. Carbohydr. Polym. 2016, 152, 170–180. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrins chemistry. Chem. Rev. 1998, 98, 1743–1753. [Google Scholar] [CrossRef]
- Ijaz, M.; Matuszczak, D.; Rahmat, A.; Mahmood, A.; Bonengel, S.; Hussain, S.; Huck, C.W. Synthesis and characterization of thiolated β-cyclodextrin as a novel mucoadhesive excipient for intra-oral drug delivery. Carbohydr. Polym. 2015, 132, 187–195. [Google Scholar] [CrossRef]
- Strickley, R.G. Solubilizing excipient in oral and injectable formulation. Pharm. Res. 2004, 21, 201–230. [Google Scholar] [CrossRef]
- Uekama, K.; Hirayama, F.; Arima, H. Recent Aspect of Cyclodextrin-Based Drug Delivery System. J. Incl. Phenom. Macrocycl. Chem. 2006, 56, 3–8. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuro, J.C.; Rial-Otero, R.; Simal Gandara, J. A review on the use of cyclodextrins in foods. Food Hydrocoll. 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Astray, G.; Mejuto, J.C.; Morales, J.; Rial-Otero, R.; Simal Gandara, J. Factors controlling flavors binding constants to cyclodextrins and their applications in foods. Food Res. Int. 2010, 43, 1212–1218. [Google Scholar] [CrossRef]
- Cho, E.; Nazir Tahir, M.; Choi, J.M.; Kim, H.; Yu, J.H.; Jung, S.H. Novel magnetic nanoparticles coated by benzene- and β-cyclodextrin-bearing dextran and the sorption of polycyclic aromatic hydrocarbon. Carbohydr. Polym. 2015, 133, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Renard, E.; Deratani, A.; Volet, G.; Sebille, B. Preparation and characterization of water soluble high molecular weight β-cyclodextrin-epichlorohydrin polymers. Eur. Polym. J. 1997, 33, 49–57. [Google Scholar] [CrossRef]
- Crini, G.; Bertini, S.; Torri, G.; Naggi, A.; Sforzini, D.; Vecchi, C.; Janus, L.; Lekchiri, Y.; Morcellet, M. Sorption of aromatic compounds in water using insoluble cyclodextrin polymers. J. Appl. Polym. Sci. 1998, 68, 1973–1978. [Google Scholar] [CrossRef]
- Crini, G.; Morcellet, M. Synthesis and applications of adsorbents containing cyclodextrins. J. Sep. Sci. 2002, 25, 789–813. [Google Scholar] [CrossRef]
- Mallard-Favier, I.; Baudelet, D.; Fourmentin, S. VOC trapping by new crosslinked cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 2011, 69, 433–437. [Google Scholar] [CrossRef]
- Ghoul, Y.Y.; Martel, B.; Achari, A.E.; Campagne, C.; Razafimahefa, L.; Vroman, I. Improved dyeability of polypropylene fabrics finished with β-cyclodextrin-citric acid polymer. Polym. J. 2010, 42, 804–811. [Google Scholar] [CrossRef] [Green Version]
- Khaoulani, S.; Charker, H.; Cadet, C.; Bychkov, E.; Cherif, L.; Bengueddach, A.; Fourmentin, S. Wastewater treatment by cyclodextrin polymers and noble metal/mesoporous TiO2 photocatalysts. Comptes Rendus Chim. 2015, 18, 23–31. [Google Scholar] [CrossRef]
- Vasconcelos, D.A.; Kubota, T.; Santos, D.C.; Araiyo, M.V.G.; Teixeira, Z.; Gimenez, I.F. Preparation of Aun quantum clusters with catalytic activity in β-cyclodextrin polyurethane nanosponges. Carbohydr. Polym. 2016, 136, 54–62. [Google Scholar] [CrossRef]
- Renard, E.; Barnathan, G.; Deratani, A.; Sebille, B. Polycondensation of cyclodextrins with epichlorohydrin. Influence of reaction conditions on the polymer structure. Macromol. Symp. 1997, 122, 229–234. [Google Scholar] [CrossRef]
- Romo, A.; Peñas, F.J.; Sevillano, X.; Isasi, J.R. Application of factorial experimental design to the study of the suspension polymerization of β-cyclodextrin and epichlorohydrin. J. Appl. Polym. Sci. 2006, 100, 3393–3402. [Google Scholar] [CrossRef]
- Crini, G. Studies on adsorption of dyes on β-cyclodextrin polymer. Bioresour. Technol. 2003, 90, 193–198. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.S.; Shen, X.; Zhang, X.; Sha, B. Comparative study of polymer containing β-cyclodextrin and -COOH for adsorption toward aniline, 1-naphthylamine and methylene blue. J. Hazard. Mater. 2009, 171, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz Ozmen, E.; Sezgin, M.; Yilmaz, A.; Yilmaz, M. Synthesis of β-cyclodextrin and starch based polymers for sorption of azo dyes from aqueous solutions. Bioresour. Technol. 2008, 99, 526–531. [Google Scholar] [CrossRef]
- Yilmaz, E.; Memon, S.; Yilmaz, M. Removal of direct azo dyes and aromatic amines from aqueous solutions using two β-cyclodextrin-based polymer. J. Hazard. Mater. 2010, 174, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Alsbaiee, A.; Smith, B.J.; Xiao, L.; Ling, Y.; Helbling, D.E.; Ditchel, W.R. Rapid removal of organic micropollutants from water by a porous β-cyclodextrin polymer. Nature 2016, 529, 190–194. [Google Scholar] [CrossRef]
- Huang, Z.; Wu, Q.; Liu, S.; Liu, T.; Zhang, B. A novel biodegradable β-cyclodextrin-based hydrogel for the removal of heavy metal ions. Carbohydr. Polym. 2013, 97, 496–501. [Google Scholar] [CrossRef]
- Wester, P.W.; Van der Heijden, C.W.; Bisschop, G.J.; Van Esch, G.J. Carcinogenicity study with epichlorohydrin (CEP) by gavage in rats. Toxicology 1985, 36, 325–339. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Lin, J.-C.; Hai, W.; Tan, H.-W.; Luo, Y.-W.; Xie, X.-L.; Cao, Y.; He, F.-H. A novel crosslinked β-cyclodextrin-based polymer for removing methylene blue from water with high efficiency. Colloids Surf. A 2019, 560, 59–68. [Google Scholar] [CrossRef]
- Yang, W.; He, N.; Fu, J.; Li, Z.; Ji, X. Preparation of porous core-shell poly-L-lactic acid/polyethylene glycol superfine fibres containing drug. J. Nanosc. Nanotechnol. 2015, 15, 9911–9917. [Google Scholar] [CrossRef]
- Verhoef, J.J.F.; Carpenter, J.F.; Anchordoquy, T.J.; Schellekens, H. Potential induction of anti-PEG antibodies and complement activation toward PEGylated therapeutics. Drug Discov. Today 2014, 19, 1945–1952. [Google Scholar] [CrossRef]
- Gao, N.; Zhou, W.; Jiang, X.; Hong, G.; Fu, T.M.; Lieber, C.M. General strategy for biodetection in high ionic strength solutions using transistor-based nanoelectronics sensors. Nano Lett. 2015, 15, 2143–2148. [Google Scholar] [CrossRef] [Green Version]
- Iverson, N.M.; Bisker, G.; Farias, E.; Ivanov, V.; Ahn, J.; Wogan, G.G.; Stranos, M.S. Quantitative tissue spectroscopy of near infrared fluorescent nanosensor implants. J. Biomed. Nanotechnol. 2016, 12, 1035–1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diez-Pascual, A.M.; Diez-Vicente, A.L. Poly (propylene fumarate)/polyethylene glycol-modified graphene oxide nanocomposites for tissue engineering. ACS Appl. Mater. Interfaces 2016, 28, 17902–17914. [Google Scholar] [CrossRef] [PubMed]
- Jafari, A.; Hassanajili, S.; Azarpira, N.; Karimi, M.B.; Geramizadeh, B. Development of thermal-crosslinkable chitosan/maleic terminated polyethylene glycol hydrogels for full thickness wound healing: In vitro and in vivo evaluation. Eur. Polym. J. 2019, 118, 113–127. [Google Scholar] [CrossRef]
- Bratskaya, S.; Privar, Y.; Nesterov, D.; Modin, E.; Kodess, M.; Slobodyuk, A.; Marinin, D.; Pestov, A. Chitosan Gels and Cryogels Cross-Linked with Diglycidyl Ethers of Ethylene Glycol and Polyethylene Glycol in Acidic Media. Biomacromolecules 2019, 20, 1635–1643. [Google Scholar] [CrossRef]
- Nielsen, T.T.; Wintgens, V.; Larsen, K.L.; Amiel, C. Synthesis and characterization of poly(ethylene glycol) based β-cyclodextrin polymers. J. Incl. Phenom. Macrocycl. Chem. 2009, 65, 341–348. [Google Scholar] [CrossRef]
- Cesteros, L.L.; Ramirez, C.C.; Pecina, A.; Katime, I. Poly(ethylene glycol-β-cyclodextrin) gels: Synthesis and properties. J. Appl. Polym. Sci. 2006, 102, 1162–1166. [Google Scholar] [CrossRef]
- Cesteros, L.C.; Gonzales-Teresa, R.; Katime, I. Hydrogels of β-cyclodextrin crosslinked by acylated poly(ethylene glycol): Synthesis and properties. Eur. Polym. J. 2009, 45, 674–679. [Google Scholar] [CrossRef]
- Kono, H.; Onishi, K.; Nakamura, T. Characterization and bisphenol A adsorption capacity of β-cyclodextrin-carboxymethylcellulose-based hydrogel. Carbohydr. Polym. 2013, 98, 784–792. [Google Scholar] [CrossRef]
- Kono, H.; Nakamura, T.; Hashimoto, H.; Shimizu, Y. Characterization molecular dynamics and encapsulation ability of β-cyclodextrin polymers crosslinked by polyethylene glycol. Carbohydr. Polym. 2015, 123, 11–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Kwak, S.Y. Branched polyethylenimine-polyethylene glycol-β-cyclodextrins polymers for efficient removal of bisphenol A and copper from wastewater. J. Appl. Polym. Sci. 2019, 48475, 1–9. [Google Scholar] [CrossRef]
- Luppi, F.; Mai, N.; Kister, G.; Gill, P.P.; Gaulter, S.E.; Stennett, C.; Dossi, E. Chemical modification of β-cyclodextrins: Balancing soft and rigid domains in complex structures. Chem. Eur. J. 2019, 25, 15646–15655. [Google Scholar] [CrossRef] [PubMed]
- Luppi, F.; Cavaye, H.; Dossi, E. Nitrated Cross-linked β-Cyclodextrin Binders Exhibiting Low Glass Transition Temperatures. Propellants Explos. Pyrotech. 2019, 43, 1023–1031. [Google Scholar] [CrossRef] [Green Version]
- Luppi, F.; Kister, G.; Carpenter, M.; Dossi, E. Thermomechanical characterization of cross-linked β-cyclodextrin polyether binders. Polym. Test. 2019, 73, 338–345. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Shuang, S.; Dong, C.; Pan, J. Study on the interaction of methylene blue with cyclodextrin derivatives by absorption and fluorescence spectroscopy. Spectrochim. Acta Part A 2003, 59, 2935–2941. [Google Scholar] [CrossRef]
- Badruddoza, A.Z.M.; Hazel, G.S.S.; Hidajat, K.; Uddin, M.S. Synthesis of carboxymethyl-β-cyclodextrin conjugated magnetic nano-adsorbent for removal of methylene blue. Colloids. Surf. A 2010, 367, 85–95. [Google Scholar] [CrossRef]
- Liu, J.; Liu, G.; Liu, W. Preparation of water soluble β-cyclodextrin/poly(acrylic acid) graphene oxide nanocomposites as new adsorbents to remove cationic dyes from aqueous solution. Chem. Eng. J. 2014, 257, 299–308. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsoprtion of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Mahapatra, K.; Ramteke, D.D.; Paliwal, L.J. Production of activated carbon from sludge of food processing industry under controlled pyrolysis and its application for methylene blue removal. J. Anal. Appl. Pyrolysis 2012, 95, 79–86. [Google Scholar] [CrossRef]
- Komiayama, M.; Hirai, H. Preparation of immobilized β−cyclodextrins by use of alkanediol diglycidyl ethers as crosslinking agents and their guest binding abilities. Polym. J. 1987, 19, 773–775. [Google Scholar] [CrossRef]
- Guo, C.G.; Wang, L.; Li, Y.K.; Wang, C.Q. Supramolecular hydrogels based on low-molecular-weight poly(ethylene glycol) and α-cyclodextrin. J. Appl. Polym. Sci. 2013, 129, 901–907. [Google Scholar] [CrossRef]
- Trotta, F.; Zanetti, M.; Camino, G. Thermal degradation of cyclodextrins. Polym. Degrad. Stab. 2000, 69, 373–379. [Google Scholar] [CrossRef]
- Mane, S.; Ponrathnam, S.; Chavan, N. Effect of chemical crosslinking on properties of polymer microbeads: A review. Can. Chem. Trans. 2016, 3, 473–485. [Google Scholar] [CrossRef]
- Anne, J.M.; Boon, Y.H.; Saad, B.; Miskam, M.; Yusoff, M.M.; Sharhiman, M.S.; Zain, N.N.M.; Lim, V.; Raoov, M. β-cyclodextrin conjugated bifunctional isocyanate linker polymer for enhanced removal of 2,4-dinitrophenol from environmental waters. R. Soc. Open Sci. 2018, 5, 180942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vilar, V.J.P.; Botelho, C.M.S.; Boaventura, R.A.R. Methylene Blue Adsorption by Algal Biomass based Materials: Biosorbents Characterization and Process Behavior. J. Hazard. Mat. 2007, 147, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Salgin, S.; Salgin, U.; Vatansever, O. Synthesis and characterization of β-cyclodextrin nanosponge and its application for the removal of p-nitrophenol from water. Clean Soil Air Water 2017, 45, 1500837. [Google Scholar] [CrossRef]
- Wang, X.S.; Zhou, Y.; Jiang, Y.; Sun, C. The removal of basic dyes from aqueous solution using agricultural by-products. J. Hazard. Mater. 2008, 157, 374–385. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Li, X.; Sun, B.; Jiang, Z.; Wang, C. Water insoluble sericin/β-cyclodextrin/PVA composite electrospun nanofibers as effective adsorbents towards methylene blue. Colloids Surf. B 2015, 136, 375–382. [Google Scholar] [CrossRef]
- Freundlich, H.M.F.Z. Over the adsorption in solution. Phys. Chem. 1906, 57, 385–470. [Google Scholar]
- Langmuir, I. The constitution and fundamental properties of solids and liquids. Part I. Solids. J. Am. Chem. Soc. 1916, 8, 2231–2295. [Google Scholar] [CrossRef] [Green Version]
- Annadurai, A.G.; Ling, L.Y.; Ling, L.Y.; Lee, J.F. Adsorption of reactive dye from an aqueous solution by chitosan: Isotherm, kinetic and thermodynamic analysis. J. Hazard. Mater. 2008, 152, 337–346. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, L.; Zhu, C.S.; Huang, W.Q.; Hue, J.L. Water insoluble β-cyclodextrin polymer crosslinked by citric acid synthesis and adsorption toward phenol and methylene blue. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 195–201. [Google Scholar] [CrossRef]
- Hou, N.; Wang, R.; Wang, F.; Bai, J.; Jiao, T.; Bai, Z.; Zhang, L.; Zhou, J.; Peng, Q. Self-assembled hydrogels constructed via host-guest polymers with highly efficient dye removal capability for wastewater treatment. Colloids Surf. A 2019, 579, 123670. [Google Scholar] [CrossRef]
- Hou, N.; Wang, R.; Geng, R.; Wang, F.; Jiao, T.; Zhang, L.; Zhou, J.; Bai, Z.; Peng, Q. Facile preparation of self-assembled hydrogels constructed from poly-cyclodextrin and polyadamantane as highly selective adsorbents for wastewater treatements. Soft Matter 2019, 15, 6097–6106. [Google Scholar] [CrossRef]
- Chen, S.; Guo, H.; Yang, F.; Di, X. Cyclodextrin grafted thiocalix [4]arene netty polymer based on the click chemistry, preparation and efficient adsorption for organic dyes. J. Polym. Res. 2016, 2, 1–12. [Google Scholar] [CrossRef]
- Morin-Crini, N.; Wintertin, P.; Fourmentin, S.; Wilson, L.D.; Fenyvesi, E.; Crini, G. Water-insoluble β-cyclodextrin-epichlorohydrin polymers for removal of pollutants from aqueous solution by sorption processes using batch studies: A review of inclusion mechanisms. Prog. Polymer. Sci. 2018, 78, 1–23. [Google Scholar] [CrossRef]











| Polymer | Crosslinker | Initial Amount of β-CD (g) | Initial Amount of Crosslinker (g) | Weight of Polymer (g) | Yield (%) |
|---|---|---|---|---|---|
| PEGCD1 | PEDGE 500 | 5 | 22.02 | 5.10 | 18.8 |
| PEGCD2 | PEDGE 640 | 5 | 28.19 | 2.11 | 6.0 |
| EGCD | EDGE | 5 | 8.90 | 5.09 | 36.6 |
| EGCD | PEGCD1 | PEGCD2 | |
|---|---|---|---|
| KL (L/mg) | 2.77 | 8.82 | 1.87 |
| Qmax (mg/g) | 10.68 | 6.37 | 15 |
| RL | 0.003–0.014 | 0.002–0.010 | 0.005–0.020 |
| R2 | 0.93 | 0.96 | 0.99 |
| Adsorbents | Qmax (mg/g) | References |
|---|---|---|
| β-CD polymers containing carboxylic group | 17.7 | [40] |
| β-CD polymers crosslinked by citric acid | 105.0 | [74] |
| β-CD hydrogels | 23.6 | [75] |
| Hydrogels from poly-CD and polyadamantane | 20.2 | [76] |
| CD grafted thiacalix(4)arene polymers | 5.4 | [77] |
| Polymers of CD based on PEDGE (our study) | 15.0 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mallard, I.; Landy, D.; Fourmentin, S. Evaluation of Polyethylene Glycol Crosslinked β-CD Polymers for the Removal of Methylene Blue. Appl. Sci. 2020, 10, 4679. https://doi.org/10.3390/app10134679
Mallard I, Landy D, Fourmentin S. Evaluation of Polyethylene Glycol Crosslinked β-CD Polymers for the Removal of Methylene Blue. Applied Sciences. 2020; 10(13):4679. https://doi.org/10.3390/app10134679
Chicago/Turabian StyleMallard, Isabelle, David Landy, and Sophie Fourmentin. 2020. "Evaluation of Polyethylene Glycol Crosslinked β-CD Polymers for the Removal of Methylene Blue" Applied Sciences 10, no. 13: 4679. https://doi.org/10.3390/app10134679