Mersaquinone, A New Tetracene Derivative from the Marine-Derived Streptomyces sp. EG1 Exhibiting Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General Experimental Procedures
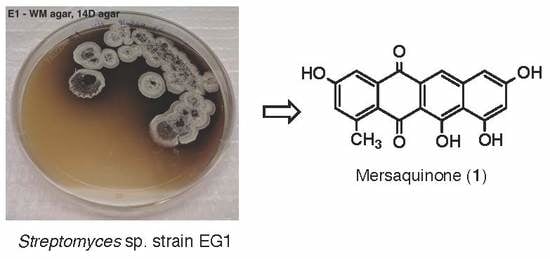
3.2. Isolation and Identification of Streptomyces sp.EG1
3.3. Cultivation, and Extraction of Streptomyces sp. EG1
3.4. Isolation of Compounds
3.5. Spectral Data of Mersaquinone
3.6. Antibacterial Testing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shankar, N.; Soe, P.-M.; Tam, C.C. Prevalence and risk of acquisition of methicillin-resistant Staphylococcus aureus among households: A systematic review. Int. J. Infect. Dis. 2020, 92, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Yokoe, D.S.; Classen, D. Introduction: Improving Patient Safety Through Infection Control: A New Healthcare Imperative. Infect. Control. Hosp. Epidemiology 2008, 29, S3–S11. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.S.; de Lencastre, H.; Garau, J.; Kluytmans, J.; Malhotra-Kumar, S.; Peschel, A.; Harbarth, S. Methicillin-resistant Staphylococcus aureus. Nat. Rev. Dis. Prim. 2018, 4, 1–23. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Sader, H.S.; Shortridge, D.; Flamm, R.K.; Mendes, R.E. Analysis of Oritavancin Activity Against Gram-Positive Clinical Isolates Responsible for Bacterial Endocarditis in United States and European Hospitals (2008–2016). Open Forum Infect. Dis. 2017, 4, 369. [Google Scholar] [CrossRef]
- Stapleton, P.D.; Taylor, P.W. Methicillin Resistance in Staphylococcus Aureus: Mechanisms and Modulation. Sci. Prog. 2002, 85, 57–72. [Google Scholar] [CrossRef]
- Stincone, P.; Brandelli, A. Marine bacteria as source of antimicrobial compounds. Crit. Rev. Biotechnol. 2020, 40, 306–319. [Google Scholar] [CrossRef]
- Petersen, L.-E.; Kellermann, M.Y.; Schupp, P.J. Secondary Metabolites of Marine Microbes: From Natural Products Chemistry to Chemical Ecology. In YOUMARES 9-The Oceans: Our Research, Our Future; Springer: Basel, Switzerland, 2020; pp. 159–180. [Google Scholar]
- Hughes, C.C.; Jensen, P.R.; Fenical, W.; Prieto-Davo, A. ChemInform Abstract: The Marinopyrroles, Antibiotics of an Unprecedented Structure Class from a Marine Streptomyces sp. Chemin 2008, 39, 629–631. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Kirkland, T.N.; Jensen, P.R.; Fenical, W. ChemInform Abstract: Arenimycin, an Antibiotic Effective Against Rifampin- and Methicillin-Resistant Staphylococcus aureus from the Marine Actinomycete Salinispora arenicola. Chemin 2010, 41, 37–39. [Google Scholar] [CrossRef]
- Pereira, F.; Almeida, J.R.; Paulino, M.; Grilo, I.R.; Macedo, H.; Cunha, I.; Sobral, R.G.; Vasconcelos, V.; Gaudêncio, S.P. Antifouling Napyradiomycins from Marine-Derived Actinomycetes Streptomyces aculeolatus. Mar. Drugs 2020, 18, 63. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.; Qian, R.; Xu, Y.; Yi, J.; Gu, Y.; Liu, X.; Yu, H.; Jiao, B.; Lu, X.; Zhang, W. Marine Actinomycetes-derived Natural Products. Curr. Top. Med. Chem. 2019, 19, 2868–2918. [Google Scholar] [CrossRef]
- Blunt, J.; Carroll, A.R.; Copp, B.R.; Davis, R.A.; Keyzers, R.A.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2018, 35, 8–53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenical, W.; Sethna, K.; Lloyd, G. Marine microorganisms as a developing resource for drug discovery. Pharm. News 2002, 9, 489–494. [Google Scholar]
- Wu, J.C.Q. Advances in Antimicrobial Natural Products Derived from Marine Actinomycetes. Mini-Rev. Org. Chem. 2017, 14, 1. [Google Scholar] [CrossRef]
- Goodfellow, M.; Williams, S.T. Ecology of Actinomycetes. Annu. Rev. Microbiol. 1983, 37, 189–216. [Google Scholar] [CrossRef]
- Zhang, L.M.; Xi, L.; Ruan, J.; Huang, Y. Microbacterium marinum sp. nov., isolated from deep-sea water. Syst. Appl. Microbiol. 2012, 35, 81–85. [Google Scholar] [CrossRef]
- Kamjam, M.; Sivalingam, P.; Deng, Z.; Hong, K. Deep Sea Actinomycetes and Their Secondary Metabolites. Front. Microbiol. 2017, 8, 2352. [Google Scholar] [CrossRef] [Green Version]
- Mannino, A.; Balistreri, P.; Deidun, A. The Marine Biodiversity of the Mediterranean Sea in a Changing Climate. The Impact of Biological Invasions. In Mediterranean Identities-Environment, Society, Culture; Fuerst-Bjelis, B., Ed.; IntechOpen: London, UK, 2017. [Google Scholar]
- Coll, M.; Piroddi, C.; Steenbeek, J.; Kaschner, K.; Lasram, F.B.R.; Aguzzi, J.; Ballesteros, E.; Bianchi, C.N.; Corbera, J.; Dailianis, T. The biodiversity of the Mediterranean Sea: Estimates, patterns, and threats. PLoS ONE 2010, 5, e11842. [Google Scholar] [CrossRef] [Green Version]
- El-Gendy, M.; Shaaban, M.; Shaaban, K.A.; El-Bondkly, A.; Laatsch, H. Essramycin: A First Triazolopyrimidine Antibiotic Isolated from Nature. J. Antibiot. 2008, 61, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Rashad, F.M.; Fathy, H.M.; El-Zayat, A.S.; Elghonaimy, A.M. Isolation and characterization of multifunctional Streptomyces species with antimicrobial, nematicidal and phytohormone activities from marine environments in Egypt. Microbiol. Res. 2015, 175, 34–47. [Google Scholar] [CrossRef]
- Abdelfattah, M.; Elmallah, M.I.Y.; Faraag, A.H.I.; Hebishy, A.M.S.; Ali, N.H. Heliomycin and tetracinomycin D: Anthraquinone derivatives with histone deacetylase inhibitory activity from marine sponge-associated Streptomyces sp. Biotech 2018, 8, 282. [Google Scholar] [CrossRef]
- Gorajana, A.; Venkatesan, M.; Vinjamuri, S.; Kurada, B.V.; Peela, S.; Jangam, P.; Poluri, E.; Zeeck, A. Resistoflavine, cytotoxic compound from a marine actinomycete, Streptomyces chibaensis AUBN1/7. Microbiol. Res. 2007, 162, 322–327. [Google Scholar] [CrossRef] [PubMed]
- Kock, I.; Maskey, R.P.; Biabani, M.A.F.; Helmke, E.; Laatsch, H. 1-Hydroxy-1-norresistomycin and Resistoflavin Methyl Ether: New Antibiotics from Marine-derived Streptomycetes. J. Antibiot. 2005, 58, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Thomson, R. Naturally Occurring Quinones; Elsevier: Amsterdam, The Netherlands, 2012; p. 745. [Google Scholar]
- Fukumoto, A.; Kim, Y.-P.; Matsumoto, A.; Takahashi, Y.; Suzuki, M.; Onodera, H.; Tomoda, H.; Matsui, H.; Hanaki, H.; Iwatsuki, M. Naphthacemycins, novel circumventors of β-lactam resistance in MRSA, produced by Streptomyces sp. KB-3346-5. I. The taxonomy of the producing strain, and the fermentation, isolation and antibacterial activities. J. Antibiot. 2017, 70, 562–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, B.; Tan, Y.; Gan, M.; Zhou, H.-X.; Wang, Y.-G.; Ping, Y.-H.; Li, B.; Yang, Z.-Y.; Xiao, C.-L. [Identification of tetracenomycin X from a marine-derived Saccharothrix sp. guided by genes sequence analysis]. Acta Pharm. Sin. 2014, 49, 230–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haste, N.M.; Farnaes, L.; Perera, V.R.; Fenical, W.; Nizet, V.; Hensler, M.E. Bactericidal Kinetics of Marine-Derived Napyradiomycins against Contemporary Methicillin-Resistant Staphylococcus aureus. Mar. Drugs 2011, 9, 680–689. [Google Scholar] [CrossRef]
- Balachandran, C.; Duraipandiyan, V.; Arun, Y.; Sangeetha, B.; Emi, N.; Al-Dhabi, N.A.; Ignacimuthu, S.; Inaguma, Y.; Okamoto, A.; Perumal, P.T. Isolation and characterization of 2-hydroxy-9,10-anthraquinone from Streptomyces olivochromogenes (ERINLG-261) with antimicrobial and antiproliferative properties. Revista Brasileira de Farmacognosia 2016, 26, 285–295. [Google Scholar] [CrossRef] [Green Version]
- Abdelfattah, M.; Elmallah, M.I.Y.; Hawas, U.; El-Kassem, L.A.; Eid, M.A.G. Isolation and characterization of marine-derived actinomycetes with cytotoxic activity from the Red Sea coast. Asian Pac. J. Trop. Biomed. 2016, 6, 651–657. [Google Scholar] [CrossRef] [Green Version]
- Al-Dhabi, N.A.; Esmail, G.A.; Duraipandiyan, V.; Arasu, M.V.; Salem-Bekhit, M.M. Isolation, identification and screening of antimicrobial thermophilic Streptomyces sp. Al-Dhabi-1 isolated from Tharban hot spring, Saudi Arabia. Extremophiles 2016, 20, 79–90. [Google Scholar] [CrossRef]
- Jorgensen, J.H.; Hindler, J.F.; Reller, L.B.; Weinstein, M.P. New Consensus Guidelines from the Clinical and Laboratory Standards Institute for Antimicrobial Susceptibility Testing of Infrequently Isolated or Fastidious Bacteria. Clin. Infect. Dis. 2007, 44, 280–286. [Google Scholar] [CrossRef] [Green Version]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.C.; Cullum, R.; Hebishy, A.M.S.; Mohamed, H.A.; Faraag, A.H.I.; Salah, N.M.; Abdelfattah, M.S.; Fenical, W. Mersaquinone, A New Tetracene Derivative from the Marine-Derived Streptomyces sp. EG1 Exhibiting Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics 2020, 9, 252. https://doi.org/10.3390/antibiotics9050252
Kim MC, Cullum R, Hebishy AMS, Mohamed HA, Faraag AHI, Salah NM, Abdelfattah MS, Fenical W. Mersaquinone, A New Tetracene Derivative from the Marine-Derived Streptomyces sp. EG1 Exhibiting Activity against Methicillin-Resistant Staphylococcus aureus (MRSA). Antibiotics. 2020; 9(5):252. https://doi.org/10.3390/antibiotics9050252
Chicago/Turabian StyleKim, Min Cheol, Reiko Cullum, Ali M. S. Hebishy, Hala A. Mohamed, Ahmed H. I. Faraag, Nehad M. Salah, Mohamed S. Abdelfattah, and William Fenical. 2020. "Mersaquinone, A New Tetracene Derivative from the Marine-Derived Streptomyces sp. EG1 Exhibiting Activity against Methicillin-Resistant Staphylococcus aureus (MRSA)" Antibiotics 9, no. 5: 252. https://doi.org/10.3390/antibiotics9050252