Heparin Thromboprophylaxis in Simultaneous Pancreas-Kidney Transplantation: A Systematic Review and Meta-Analysis of Observational Studies
- 1Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
- 2Department of Microbiology and Immunology, Schulich School of Medicine and Dentistry, Western University, London, ON, Canada
- 3Matthew Mailing Center for Translational Transplant Studies, London, ON, Canada
- 4Multi-Organ Transplant Program, London Health Sciences Center, London, ON, Canada
- 5Department of Surgery, Division of Urology, London Health Sciences Center, London, ON, Canada
Thrombosis is a leading causes of pancreas graft loss after simultaneous pancreas kidney (SPK), pancreas after kidney (PAK), and pancreas transplant alone (PTA). There remains no standardized thromboprophylaxis protocol. The aim of this systematic review and meta-analysis is to evaluate the impact of heparin thromboprophylaxis on the incidence of pancreas thrombosis, pancreas graft loss, bleeding, and secondary outcomes in SPK, PAK, and PTA. Following PRISMA guidelines, we systematically searched BIOSIS®, PubMed®, Cochrane Library®, EMBASE®, MEDLINE®, and Web of Science® on April 21, 2021. Primary peer-reviewed studies that met inclusion criteria were included. Two methods of quantitative synthesis were performed to account for comparative and non-comparative studies. We included 11 studies, comprising of 1,122 patients in the heparin group and 236 patients in the no-heparin group. When compared to the no-heparin control, prophylactic heparinization significantly decreased the risk of early pancreas thrombosis and pancreas loss for SPK, PAK and PTA without increasing the incidence of bleeding or acute return to the operating room. Heparin thromboprophylaxis yields an approximate two-fold reduction in both pancreas thrombosis and pancreas loss for SPK, PAK and PTA. We report the dosage, frequency, and duration of heparin administration to consolidate the available evidence.
Introduction
Pancreas transplantation remains the only curative procedure for type 1 diabetes mellitus (T1DM) patients, resulting in long-term control of HbA1c without the risk of serious hypoglycemic events. (1) The first pancreas transplant was performed in 1966 at the University of Minnesota, and since then advancements in immunosuppression, surgical techniques, and surgeon experience have resulted in good overall outcomes for patients (2).
Pancreas transplantation most often occurs simultaneously with kidney transplantation in uremic patients. Simultaneous pancreas-kidney (SPK) transplantation comprises the vast majority of all pancreas transplants, with pancreas after kidney (PAK) being the second most common and pancreas transplant alone (PTA) being the least common. (3, 4) Although T1DM remains the most important indication for pancreas transplantation, there have been a growing number of transplants done in T2DM patients. (5) Other, less frequent indications include transplantation for chronic pancreatitis and after pancreatomy due to malignancy (6).
A common but important complication in pancreas transplantation is thrombosis, which has been reported to have an incidence from 4%–20% (7–9). Thrombosis has been reported as one of the leading causes of pancreas graft loss and technical failure. (10) Despite this, the role of prophylactic anticoagulation remains controversial, with some groups reporting favorable outcomes following anticoagulation and other groups reporting no benefit. (11, 12) There remains no standardized thromboprophylaxis protocol in pancreas transplantation, and transplant centers have different internal protocols and practices. The purpose of this systematic review and meta-analysis is to evaluate the available literature to explore the impact of thromboprophylaxis on the incidence of thrombosis, graft loss, bleeding, and secondary outcomes in SPK, PAK, and PTA.
Methods
Data Sources and Search Strategy
The Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guideline was followed to construct this review. We searched the following six databases: BIOSIS®, PubMed®, Cochrane Library®, EMBASE®, MEDLINE®, and Web of Science®. The search strategy is provided in Supplementary Appendix SA1 (Supplemental Digital Content). The search end date was April 21st, 2021. This systematic review and meta-analysis was registered on PROSPERO (CRD42021260585) and may be accessed at: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=260585.
Inclusion and Exclusion Criteria
The objective of this review was to assess the outcomes related to prophylactic heparin in SPK, PAK and PTA. We therefore included prospective and retrospective studies written in English and published in peer-reviewed journals in this study. Studies that explored heparin thromboprophylaxis in the intraoperative and/or post-operative period after SPK, PAK or PTA where included. Studies that reported outcomes including incidence of pancreas thrombosis in the early post-transplant period, pancreas graft loss, bleeding episodes, acute return to the operating room (OR), and units of packed red blood cells (pRBC) transfused were included. Given that the inclusion criteria involved heparin as the intervention and thrombosis as the primary outcome, all the included papers reported thromboses that were relevant to the intervention. Early pancreas thrombosis was defined as mention of “early” thrombosis or thrombosis that occurred within 30 days post-transplant. Reviews, editorials, case-reports, conference proceedings, and animal studies were excluded. Studies involving other types of solid organ transplants, where they focused on an intervention other than prophylactic anticoagulation, or where anticoagulation was used to treat diagnosed thrombotic events, were also excluded.
Study Selection
Studies underwent screening of study titles and abstracts by two reviewers (E.A.L and K.F.) using Rayyan (Rayyan Systems, Boston, United States). The included studies then underwent full-text screening by the same two reviewers. Screening conflicts were reviewed and resolved during meetings with all the reviewers.
Data Extraction and Quality Assessment
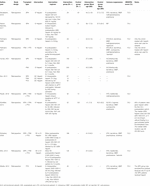
The following data were extracted from the selected articles: title, author(s), journal, study type, transplant type(s), number of patients, mean age, mean BMI, and sex proportion. Recipient specific factors included: mean time of diabetes diagnosis, and mean time on dialysis. Operative factors included: type of anticoagulation, timing of administration (intra/postoperative), dose, and frequency. Donor specific factors included: warm and cold ischemia time. Outcomes included: pancreas thrombosis in the early post-transplant period, pancreas graft loss due to thrombosis, postoperative bleeding incidence, acute return to the operating room, and units of pRBC used. Pancreas thrombosis was defined as any instance where “pancreas thrombosis” was mentioned. Pancreas loss was defined as mention of “pancreas loss” or “pancreatectomy.” Bleeding was defined as mention of “bleed,” “hemorrhage,” or “hematoma”. Acute return to the OR was defined as mention of “re-exploration,” “relaparotomy,” or “thrombectomy.” Data were extracted by two reviewers (E.A.L and K.F) and verified for accuracy and completeness by a different reviewer (M.Y.Z). The Methodological Index for Non-Randomized Studies (MINORS) was used to assess risk of bias of manuscripts (Table 1). (13) The MINORS score examines 12 methodological parameters for non-randomized studies and an additional four parameters for comparative studies. Studies that scored 60% or higher were considered high quality and included.
Statistical Analysis
Two methods of data synthesis were performed to account for differences in study methodology. First, we conducted meta-analysis of the comparative studies involving a no-heparin control using Review Manager (RevMan, Version 5.4, The Nordic142 Cochrane Center, Copenhagen, Denmark). Study heterogeneity was examined using the I2 statistic. An I2 < 50% suggested low study heterogeneity and a fixed-effect model was used, whereas an I2 > 50% suggested high study heterogeneity and a random-effects model was used. Results were visualized as forest plots. Publication bias was assessed using funnel plots for each outcome.
Second, we pooled the populations from the comparative and non-comparative studies to allow for comparison of demographic, intraoperative, and postoperative characteristics between the heparin and no-heparin groups. Statistical analysis was performed using R (Version 4.1.2., Boston, United States) and GraphPad QuickCalcs (GraphPad Software, Inc., California, United States). Two-tailed Fisher’s exact tests were conducted for the categorical variables and results were reported as incidence (percentage of total). Unpaired t-tests were conducted for the continuous variables and results were reported as mean (SD). Cohen’s kappa coefficient was obtained to assess inter-rater reliability. A p-value ≤0.05 was considered statistically significant.
Results
Search Results
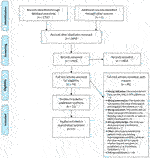
The study inclusion process is summarized in the PRISMA flow diagram (Figure 1). After removal of duplicates, 1,456 studies underwent Level 1 abstract and title screening, of which 51 studies were eligible for Level 2 full-text screening. Eleven studies were ultimately included for quantitative synthesis (11, 12–22). All 11 studies were retrospective. Level 1 screening had moderate agreement with a Cohen’s kappa of 0.56 and Level 2 screening had substantial agreement with a Cohen’s kappa of 0.67.
Study Characteristics
Characteristics of the included studies are summarized in Table 1. These included studies comprised of a total of 1,358 patients, with 1,122 patients in the intervention group and 236 patients in the control group. Mean recipient age was 40.4 (7.85) for the heparin group and 39.7 (6.90) for the control group, with no significant difference detected (p = 0.96). There were three studies involving only SPK transplant patients, (11, 12, 17) one study with both PAK and PTA patients; (16) one study with both SPK and PAK patients; (22) and five studies combining SPK, PAK, and PAK patients; (14, 18–21). There was also one study involving both SPK and PTA patients. However, only the PTA data was suitable for quantitative synthesis (Table 1) (15).
Study Quality and Publication Bias
Methodological quality of the included studies is summarized in Table 1 and in Supplementary Appendix SA2 (see Supplemental Digital Content). All studies lacked prospective data collection and prospective calculation of the study size. However, all studies possessed a clearly stated aim, with appropriate study endpoints. There were five comparative studies involving a no-heparin control, and these studies had baseline equivalence between groups and adequate statistical analysis.
Funnel plots were assessed for publication bias, with one funnel plot for each outcome of interest. We did not observe any overt asymmetry or pattern in the funnel plots for the incidence of thrombosis, graft loss, bleeding, acute return to the OR, or mean units of pRBCs transfused (see Supplemental Digital Content, Supplementary Appendix SA3).
Analysis of Comparative Studies
Heparin Reduced the Incidence of Pancreas Thrombosis
There were four studies available for analysis, comprising of 240 patients in the heparin treatment group and 194 patients in the control group. (11, 12, 18, 20) Overall, there was a significantly lower incidence of pancreas graft thrombosis in the heparin group compared to the no-heparin group, with a risk difference of −0.15 (95% CI = −0.30, −0.00; p = 0.05) (Figure 2A). Subgroup analysis revealed no significant difference in pancreas thrombosis between treatment and control groups when looking at the studies that mixed their SPK, PAK, and PTA patients together (Risk difference = −0.23; 95% CI = −0.75, 0.29; p = 0.38). However, there was a significantly lower incidence of early pancreas thrombosis in the heparin group compared to the control group when looking at the studies that only included SPK patients (Risk difference = −0.14; 95% CI = −0.26, −0.01; p = 0.03). In fact, the total incidence of pancreas thrombosis in the heparin group was less than half of that in the no-heparin group.

FIGURE 2. Forest plots examining the impact of heparin on the (A) incidence of pancreas thrombosis, (B) incidence of pancreas loss due to thrombosis, (C) incidence of postoperative bleeding, (D) incidence of acute return to the OR, (E) mean units of pRBCs transfused.
Heparin Reduced the Incidence of Pancreas Loss
Three studies were available for analysis, consisting of 113 patients in the heparin group and 143 patients in the no-heparin control group. (12, 18, 20) When assessing the overall effect, there was a significantly lower incidence of pancreas loss due to graft thrombosis in the heparin group compared to the control group (Risk difference = −0.10; 95% CI = −0.18, −0.02; p = 0.02) (Figure 2B). When examining the subgroup consisting of SPK, PAK, and PTA patients, there was no significant difference between groups in incidence of pancreas loss (Risk difference = −0.06; 95% CI = −0.15, 0.04; p = 0.24). For the SPK subgroup, there was a significantly lower incidence of pancreas loss in the heparin group (Risk difference = −0.21; 95% CI = −0.36, −0.07; p = 0.005).
Heparin did Not Impact Postoperative Incidence of Bleeds, Incidence of Acute Return to the OR, or Units of pRBC Used
Four studies were available for analysis of the incidence of bleeding, consisting of 240 patients in the heparin group and 194 patients in the no-heparin control group. (11, 12, 18, 20) Overall, there was no significant difference in the incidence of bleeding between groups (Risk difference = −0.03; 95% CI = −0.10, 0.04; p = 0.41) (Figure 2C). Subgroup analysis also revealed no differences in the incidence of bleeds in neither the combined transplant subgroup (Risk difference = −0.02; 95% CI = −0.11, 0.08; p = 0.74), nor the SPK subgroup (Risk difference = −0.04; 95% CI = −0.14, 0.06; p = 0.44).
Three studies were available for analysis of the incidence of acute return to the OR and the mean number of packed RBCs used. (12, 18, 20) There were 113 patients in the heparin group and 143 patients in the control group (Figures 2D,E). Overall, there was no significant difference in the incidence of acute return to the OR between the heparin and no-heparin groups (Risk difference = −0.10; 95% CI = −0.32, 0.12; p = 0.39). There were no significant differences when examining the combined SPK, PAK, PTA subgroup (Risk difference = −0.01; 95% CI = −0.24, 0.22; p = 0.90). There was a significantly lower risk of acute return to the OR in the SPK subgroup (Risk difference = −0.21; 95% CI = −0.36, −0.07; p = 0.005).
Furthermore, there was overall no significant difference in units of pRBCs used between the heparin and no-heparin groups (Mean difference = 0.48; 95% CI = -0.11,1.08; p = 0.11). Subgroup analysis also revealed no significant differences in the combined SPK, PAK, PTA subgroup (Mean difference = 0.63; 95% CI = 0.01, 1.26; p = 0.05) and the SPK subgroup (Mean difference = −0.80; 95% CI = −2.64, 1.04; p = 0.39).
Analysis of Both Comparative and Non-Comparative Studies
SPK Only
There were studies that met inclusion criteria that lacked a no-heparin control. To incorporate these studies, we grouped their patient cohorts with the heparin cohorts from the comparative studies. The no-heparin group derived from the comparative studies was used as the control. When examining the SPK subgroup, there were five studies available for analysis, (11, 12, 17, 19, 22) with two of these studies being comparative studies. (11, 12) For the study by Fertmann et. al. 2006, only the cohort treated with heparin alone was used for analysis (Table 1). Donor mean age, donor mean BMI, mean cold ischemia time, and mean warm ischemia time were significantly higher in the heparin group compared to the control group (Table 2). The rate of thrombosis and pancreas loss due to thrombosis were significantly lower in the heparin group compared to the no-heparin control group. There was no significant difference in the incidence of bleeding, acute return to the OR, or units of pRBCs transfused between groups.
Analysis of all Included Studies: SPK, PAK and PTA
Eleven studies were available for analysis (11, 12, 14–22), of which four were comparative studies. (11, 12, 18, 20) For the study by Fertmann et. al. 2011, only the cohort treated with heparin alone was used for analysis (Table 1). Both Fertmann studies were included because two different patient cohorts were used for each study. Fertmann 2006 included only SPK patients, whereas Fertmann 2011 included only PAK and PTA patients. The combined cohort consisted of SPK, PAK, and PTA patients. There were significantly more male patients in the no-heparin group compared to the heparin group (Table 3). The rate of thrombosis and pancreas loss due to thrombosis were significantly lower in the heparin group. Furthermore, there was no significant difference in the incidence of bleeding or return to the OR but there was a significantly higher mean number of units of pRBC transfused in the heparin group compared to the no-heparin group.
Analysis of Contemporary Studies: SPK, PAK and PTA
Lastly, we explored the effect of heparin when including only contemporary works published within the past 10 years. The analysis of comparative studies conducted in the previous section involved only contemporary studies from 2013 and onward. When combining both the comparative and non-comparative studies, seven papers were available for analysis, which included studies from 2012 and onward (11, 12, 14–20). Four of these studies were comparative studies. Analysis of these contemporary works revealed similar trends to that of the analysis of all included studies (Table 4).
Discussion
Graft thrombosis remains a leading cause of pancreas graft loss after SPK, PAK, and PTA. Despite this, the evidence for heparin thromboprophylaxis is mixed and there is great variability in the regimens used. We systematically reviewed the available literature to investigate the effect of prophylactic heparin on pancreas graft thrombosis and loss, as well as on other postoperative complications. We conducted two methods of quantitative synthesis: the first involving analysis of the included comparative studies, and the second involving analysis of both comparative and non-comparative studies. The first method of analysis revealed that heparin significantly reduced the overall incidence of early pancreas thrombosis and pancreas loss, without impacting the incidence of acute return to the OR, bleeding, or units of pRBC transfused. The second method of analysis revealed similar findings for SPK, PAK, and PTA, with an increase in the mean units of pRBCs transfused in the heparin group compared to the no-heparin group.
Analysis of comparative studies revealed greater overall effects and lower study heterogeneity in the SPK subgroup compared to the combined SPK, PAK, and PTA subgroup. This was evident for the pancreas thrombosis outcome. Previous studies report differential rates of pancreas thrombosis and pancreas loss for SPK, PAK and PTA, (16, 23) which when combined as a subgroup, may offset any true trends. Even so, we found that the overall incidence of pancreas thrombosis was over two times lower in the heparin group, while the overall incidence of pancreas loss was over three times lower in the heparin group. Because SPK requires greater time and technical involvement, the impact of heparin may be especially evident in this subgroup. With pancreas thrombosis being a common cause of pancreas loss, (8) heparin has an appreciable effect on improving graft survival, particularly after SPK transplantation.
Analysis of both comparative and non-comparative SPK studies showed a significantly higher mean donor age, mean donor BMI, mean cold ischemia time, and mean warm ischemia time in the heparin group. These variables have been previously shown to be associated with graft thrombosis. (24) Heparin remained effective in reducing the incidence of thrombosis and pancreas loss despite this group possessing factors associated with pancreas thrombosis. For this review, caution is warranted when interpreting these demographic differences because for three variables, there was only one study in the no-heparin group. The observed statistical significance may then be a by-product of the heterogeneity in the reporting of demographic data between the intervention group and control group. Furthermore, some pancreas transplant centers have avoided the use of systemic anticoagulation to prevent bleeding complications. (25, 26) However, a number of transplant groups note that graft thrombosis and loss are more detrimental than bleeds that can be controlled by transfusion or laparotomy. (27, 28) Importantly, our analysis shows that in SPK transplants, the beneficial effects of heparin did not increase the risk of acute bleeding requiring laparotomy or use of blood transfusions.
When examining all the included comparative and non-comparative studies, we confirm that heparin is associated with a significant reduction in thrombosis and pancreas loss for SPK, PAK, and PTA. In this combined analysis, patients treated with heparin were transfused with a mean of 0.62 units of additional pRBC compared to the control—which although is statistically significant—is not clinically significant. We may attribute this statistical difference to the large sample sizes and relatively small standard deviations for both groups. By incorporating all the included studies, we also balance the demographic differences between the heparin group and no-heparin group, without observing any changes in the efficacy of heparin in mitigating early post-transplant thrombosis and pancreas loss.
This analysis is limited by the lack of prospective, randomized controlled studies, as well as by the inclusion of only English studies. The available literature presents with higher degrees of confounding and greater variability in reporting, which may limit the conclusions drawn. A limitation with retrospective data is that we lack insight on the decision-making process behind which patients were heparinized. Even in comparative studies, there exists patient-specific factors—such as a history of a clotting disorder or a prolonged cold ischemia time—which may influence the decision for heparin thromboprophylaxis. (15) Changes in personnel, surgical technique, and postoperative management in institutions over time may also impact patient outcomes. Of the included studies, there is also great variability in the timing and dosage of heparin administered. This may be the result of evolving institutional practices and a lack of consistent evidence on prophylactic heparin usage. Additionally, there are technical factors that may influence the risk of thrombosis, such as the type of exocrine drainage. Well-established evidence indicates that bladder drainage may confer long-term urologic and metabolic complications, with contemporary practice utilizing enteric drainage, or occasionally portal-enteric drainage. (29) We controlled for these institutional and technical factors by analyzing only the included contemporary studies. Given that the impact of heparin on post-operative outcomes remained the same even with this contemporary analysis, the effects of heparin may be robust enough to withstand evolving institutional and surgical practices.
This review highlights the gaps in the literature, while providing a synthesis of the data that is available to us at this current time. To our knowledge, this is the first systematic review in existence that explores the impact of heparin thromboprophylaxis in SPK, PAK, and PTA. The pooled sample sizes for the heparin and no-heparin groups are sufficiently large such that the assumption of normal sampling distribution may be fulfilled by the Central Limit Theorem. (30) The large sample sizes help account for the probability of error from the above limitations. Future research that prospectively compares the impact of heparin to that of a no-heparin control is warranted. Given that all the comparative studies in this review were published within the last 10 years, this suggests an influx of higher quality studies exploring this subject in recent times. We foresee more high-quality studies will become published in the near future, which will warrant additional meta-analyses.
Based on the findings of this study, we present a flow diagram outlining the available treatment regimens (Figure 3). In the absence of contraindications to heparin thromboprophylaxis, intraoperative intravenous heparin 30–70 IU/kg was used. (15, 19) During the postoperative period, either subcutaneous heparin 3,000–5000 IU 1–2 times per day (14, 19, 21) or intravenous heparin infusion 200–1000 IU/h for 1–14 days (12, 14–17, 20–22) were reported. Subsequent maintenance with aspirin 81–325 mg daily was then used (11, 12, 20).
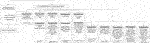
FIGURE 3. Flow diagram of the prophylactic heparin regimens presented in this review. ASA, acetylsalicylic acid; IU, international unit; IV, intravenous; SC, subcutaneous.
Conclusion
We demonstrate that prophylactic heparinization produces over a two-fold reduction in early pancreas thrombosis and pancreas loss for SPK, PAK and PTA, without increasing the incidence of bleeding or acute return to the OR. With early postoperative complications, such as pancreas graft thrombosis, persisting as a leading cause for graft loss, heparin thromboprophylaxis holds promise for enhancing graft survival without imposing additional postoperative complications. This meta-analysis culminates 2 decades of available evidence to highlight the efficacy of heparin thromboprophylaxis for improving graft survival for SPK, PAK, and PTA patients.
Author Contributions
EL: Participated in research design, search strategy formation, literature screening, data extraction, statistical analysis, manuscript writing. KF: Participated in research design, search strategy formation, literature screening, data extraction, manuscript writing. MZ: Participated in research design, data extraction, manuscript writing. JO: Participated in research design, literature screening, PL: Participated in research design, manuscript writing. AS: Participated in research design, search strategy formation, literature screening, data extraction, statistical analysis, manuscript writing.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontierspartnerships.org/articles/10.3389/ti.2023.10442/full#supplementary-material
Abbreviations
IU, International unit; PAK, Pancreas after kidney transplant; PTA, Pancreas transplant alone; SPK, Simultaneous pancreas-kidney transplant.
References
1. Gruessner, RWG, and Gruessner, AC. The Current State of Pancreas Transplantation. Nat Rev Endocrinol (2013) 9(9):555–62. doi:10.1038/nrendo.2013.138
2. Kelly, WD, Lillehei, RC, Merkel, FK, Idezuki, Y, and Goetz, FC. Allotransplantation of the Pancreas and Duodenum along with the Kidney in Diabetic Nephropathy. Surgery (1967) 61(6):145–37. doi:10.1097/00007890-196801000-00034
3. Kandaswamy, R, Stock, PG, Miller, J, Skeans, MA, White, J, Wainright, J, et al. OPTN/SRTR 2019 Annual Data Report: Pancreas. Am J Transpl Off J Am Soc Transpl Am Soc Transpl Surg (2021) 21(2):138–207. doi:10.1111/ajt.16496
4. Jiang, AT, Bhsc, , Rowe, N, Sener, A, and Luke, P. Simultaneous Pancreas-Kidney Transplantation: The Role in the Treatment of Type 1 Diabetes and End-Stage Renal Disease. Can Urol Assoc J (2014) 8(3-4):135–8. doi:10.5489/cuaj.1597
5. Al-Qaoud, TM, Odorico, JS, and Redfield, RR. Pancreas Transplantation in Type 2 Diabetes: Expanding the Criteria. Curr Opin Organ Transpl (2018) 23(4):454–60. doi:10.1097/MOT.0000000000000553
6. Cerise, A, Nagaraju, S, Powelson, JA, Lutz, A, and Fridell, JA. Pancreas Transplantation Following Total Pancreatectomy for Chronic Pancreatitis. Clin Transpl (2019) 33(12):e13731. doi:10.1111/ctr.13731
7. Troppmann, C. Complications after Pancreas Transplantation. Curr Opin Organ Transpl (2010) 15(1):112–8. doi:10.1097/MOT.0b013e3283355349
8. Farney, AC, Rogers, J, and Stratta, RJ. Pancreas Graft Thrombosis: Causes, Prevention, Diagnosis, and Intervention. Curr Opin Organ Transpl (2012) 17(1):87–92. doi:10.1097/MOT.0b013e32834ee717
9. Delis, S, Dervenis, C, Bramis, J, Burke, GW, Miller, J, Ciancio, G, et al. Vascular Complications of Pancreas Transplantation. Pancreas (2004) 28(4):413–20. doi:10.1097/00006676-200405000-00010
10. Blundell, J, Shahrestani, S, Lendzion, R, Pleass, HJ, and Hawthorne, WJ. Risk Factors for Early Pancreatic Allograft Thrombosis Following Simultaneous Pancreas-Kidney Transplantation: A Systematic Review. Clin Appl Thromb Off J Int Acad Clin Appl Thromb (2020) 26:1076029620942589. doi:10.1177/1076029620942589
11. Arjona-Sánchez, A, Rodríguez-Ortiz, L, Sánchez-Hidalgo, JM, ArjonA-Sanchez, A, Salamanca-Bustos, JJ, Rodriguez-Benot, A, et al. Intraoperative Heparinization during Simultaneous Pancreas-Kidney Transplantation: Is it Really Necessary? Transpl Proc (2018) 50(2):673–5. doi:10.1016/j.transproceed.2017.09.055
12. Aboalsamh, G, Anderson, P, Al-Abbassi, A, McAlister, V, Luke, PP, and Sener, A. Heparin Infusion in Simultaneous Pancreas and Kidney Transplantation Reduces Graft Thrombosis and Improves Graft Survival. Clin Transpl (2016) 30(9):1002–9. doi:10.1111/ctr.12780
13. Slim, K, Nini, E, Forestier, D, Kwiatkowski, F, Panis, Y, and Chipponi, J. Methodological index for Non-randomized Studies (Minors): Development and Validation of a New Instrument. ANZ J Surg (2003) 73(9):712–6. doi:10.1046/j.1445-2197.2003.02748.x
14. Shin, S, Han, DJ, Kim, YH, Choi, BH, Jung, JH, et al. Long-term Effects of Delayed Graft Function on Pancreas Graft Survival after Pancreas Transplantation. Transplantation (2014) 98(12):1316–22. doi:10.1097/TP.0000000000000214
15. Stratta, RJ, Farney, AC, Orlando, G, Farooq, U, Al-Shraideh, Y, and Rogers, J. Similar Results with Solitary Pancreas Transplantation Compared with Simultaneous Pancreas-Kidney Transplantation in the New Millennium. Transpl Proc (2014) 46(6):1924–7. doi:10.1016/j.transproceed.2014.05.079
16. Fertmann, JM, Arbogast, HP, Illner, W-D, Tarabichi, A, Dieterle, C, Land, W, et al. Antithrombin Therapy in Pancreas Retransplantation and Pancreas-After-Kidney/pancreas-Transplantation-Alone Patients. Clin Transpl (2011) 25(5):E499–508. doi:10.1111/j.1399-0012.2011.01472.x
17. Fertmann, JM, Wimmer, CD, Arbogast, HP, Illner, WD, Tarabichi, A, Calasan, I, et al. Single-shot Antithrombin in Human Pancreas-Kidney Transplantation: Reduction of Reperfusion Pancreatitis and Prevention of Graft Thrombosis. Transpl Int Off J Eur Soc Organ Transpl (2006) 19(6):458–65. doi:10.1111/j.1432-2277.2006.00325.x
18. Raveh, Y, Ciancio, G, Burke, GW, Figueiro, J, Chen, L, Morsi, M, et al. Susceptibility-directed Anticoagulation after Pancreas Transplantation: A Single-center Retrospective Study. Clin Transpl (2019) 33(7):e13619. doi:10.1111/ctr.13619
19. Kim, YH, Park, JB, Lee, SS, Byun, JH, Kim, S-C, and Han, D-J. How to Avoid Graft Thrombosis Requiring Graftectomy: Immediate Posttransplant CT Angiography in Pancreas Transplantation. Transplantation (2012) 94(9):925–30. doi:10.1097/TP.0b013e3182692b4d
20. Scheffert, JL, Taber, DJ, Pilch, NA, Chavin, KD, Baliga, PK, and Bratton, CF. Clinical Outcomes Associated with the Early Postoperative Use of Heparin in Pancreas Transplantation. Transplantation (2014) 97(6):681–5. doi:10.1097/01.TP.0000437790.26255.5d
21. Schenker, P, Vonend, O, Ertas, N, Wunsch, A, Schaeffer, M, Rump, LC, et al. Incidence of Pancreas Graft Thrombosis Using Low-Molecular-Weight Heparin. Clin Transpl (2009) 23(3):407–14. doi:10.1111/j.1399-0012.2008.00911.x
22. Humar, A, Ramcharan, T, Kandaswamy, R, MatAs, A, Gruessner, AC, et al. Pancreas after Kidney Transplants. Am J Surg (2001) 182(2):155–61. doi:10.1016/s0002-9610(01)00676-6
23. Troppmann, C, Gruessner, AC, Benedetti, E, Papalois, BE, Dunn, DL, Najarian, JS, et al. Vascular Graft Thrombosis after Pancreatic Transplantation: Univariate and Multivariate Operative and Nonoperative Risk Factor Analysis. J Am Coll Surg (1996) 182(4):285–316.
24. Stratta, RJ, Fridell, JA, Gruessner, AC, Odorico, JS, and Gruessner, RWG. Pancreas Transplantation: a Decade of Decline. Curr Opin Organ Transpl (2016) 21(4):386–92. doi:10.1097/MOT.0000000000000319
25. Okabe, Y, Kitada, H, Miura, Y, Nishiki, T, Kurihara, K, Kawanami, S, et al. Pancreas Transplantation: a Single-Institution Experience in Japan. Surg Today (2013) 43(12):1406–11. doi:10.1007/s00595-013-0516-6
26. Sollinger, HW, Odorico, JS, Becker, YT, D’Alessandro, AM, and Pirsch, JD. One Thousand Simultaneous Pancreas-Kidney Transplants at a Single Center with 22-Year Follow-Up. Ann Surg (2009) 250(4):618–30. doi:10.1097/SLA.0b013e3181b76d2b
27. Gruessner, AC, Sutherland, DER, Dunn, DL, Najarian, JS, HumAr, A, Kandaswamy, R, et al. Pancreas after Kidney Transplants in Posturemic Patients with Type I Diabetes Mellitus. J Am Soc Nephrol (2001) 12(11):2490–9. doi:10.1681/ASN.V12112490
28. Humar, A, Kandaswamy, R, Granger, D, Gruessner, RW, Gruessner, AC, and Sutherland, DE. Decreased Surgical Risks of Pancreas Transplantation in the Modern Era. Ann Surg (2000) 231(2):269–75. doi:10.1097/00000658-200002000-00017
29. El-Hennawy, H, Stratta, RJ, and Smith, F. Exocrine Drainage in Vascularized Pancreas Transplantation in the New Millennium. World J Transpl (2016) 6(2):255–71. doi:10.5500/wjt.v6.i2.255
Keywords: pancreas transplantation, meta-analysis, heparin, simultaneous pancreas-kidney transplantation, systematic review, thrombosis, thromboprophylaxis
Citation: Ai Li E, Farrokhi K, Zhang MY, Offerni J, Luke PP and Sener A (2023) Heparin Thromboprophylaxis in Simultaneous Pancreas-Kidney Transplantation: A Systematic Review and Meta-Analysis of Observational Studies. Transpl Int 36:10442. doi: 10.3389/ti.2023.10442
Received: 18 February 2022; Accepted: 18 January 2023;
Published: 01 February 2023.
Copyright © 2023 Ai Li, Farrokhi, Zhang, Offerni, Luke and Sener. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alp Sener, Alp.Sener@lhsc.on.ca
 Erica Ai Li
Erica Ai Li Kaveh Farrokhi1,
Kaveh Farrokhi1,