Reactions to environmental allergens in cats with feline lower airway disease
- 1LMU Small Animal Clinic, Ludwig Maximilian University of Munich, Munich, Germany
- 2Statistical Consulting Unit StaBLab, Department of Statistics, Ludwig Maximilian University of Munich, Munich, Germany
- 3Vet Med Labor GmbH Division of IDEXX Laboratories, Kornwestheim, Germany
Objectives: Aeroallergens have been discussed as potential triggers for feline asthma (FA), which can be induced experimentally by allergen sensitization. To date, only few studies have investigated reactions to environmental allergens in cats with naturally occurring feline lower airway disease (FLAD). The aim of the study was to compare results of intradermal testing (IDT) and serum allergen-specific immunoglobulin E-(IgE) testing (SAT) in cats with FLAD, and to investigate possible associations with allergen exposure.
Material and methods: Eight cats with eosinophilic airway inflammation (EI), ten cats with mixed inflammation (MI), six with neutrophilic inflammation (NI), and 24 healthy cats (HC) were included. Cats diagnosed with FLAD were assigned to the different inflammatory groups based on bronchoalveolar lavage fluid (BLAF) cytology. SAT was performed in all cats; IDT was only carried out in cats with FLAD. Information about the cats' environment and potential allergen exposure was obtained using an owner questionnaire.
Results: In comparison to 83% of HC with positive reactions on SAT only 52% of cats with FLAD had positive responses (p = 0.051). Significantly more positive reactions per cat were detected on IDT than on SAT (p = 0.001). No significant difference was found for positive reactions per cat on SAT when compared between HC, NI, EI, and MI (p = 0.377). Only “slight” agreement was found for most allergens when reactions obtained in both tests in cats with FLAD were compared, except for “moderate” agreement for English plantain (k = 0.504) and Alternaria alternata (k = 0.488). Overall, no clear association between the cats' environment and allergen reactions were detected.
Conclusions and clinical importance: Interpretation of allergy test results in cats with FLAD should be done in the context of clinical signs and individual factors.
1 Introduction
Feline lower airway disease (FLAD) is a common inflammatory condition in cats. Recent studies support the idea that feline asthma (FA) and feline chronic bronchitis (CB) are the two most common inflammatory diseases falling under this term (1–4). However, a clear discrimination between these two phenotypically similar syndromes and their potential etiologies could not be established (5, 6). That raises the question whether FA and CB are two different disease entities or if they are just arising from the same underlying cause with variations in their inflammatory profile (6, 7). To date, the only attempt to differentiate between FA and CB is based on cell differentiation of bronchoalveolar lavage fluid (BALF) cytology, suggesting an eosinophilic inflammation (EI) representing FA, and a sterile neutrophilic inflammation (NI) typical for CB (2, 8). Recently, a subdivision of so-called “mixed inflammation” (MI) has been introduced for further categorization (9–13). It is assumed that MI is a consequence of chronic EI, resulting in damage of airway epithelium, and therefore leading to immigration of neutrophils (1, 5, 14). According to recent definitions, FA is assigned to the feline atopic syndrome comprising allergic diseases of the skin, respiratory and gastrointestinal tract in cats (15).
In multiple studies FA could be induced experimentally via parenteral sensitization and aerosol challenge using common environmental allergens. Therefore, it is highly suggestive that an allergic reaction is an underlying major cause for the inflammation seen with FA (16–20). Currently, standard therapy consists of inhaled or systemically administered glucocorticoids, often in combination with bronchodilators (1, 8, 14, 21). Even though it could be demonstrated that clinical signs improve significantly with this therapy (21, 22), subclinical inflammation may continue to damage the lower airways in some cats (23), and side effects associated with long-term use of corticosteroids can pose a risk for patients (24, 25). Therefore, it has been suggested to utilize allergen immunotherapy (AIT) as a causative treatment of a possible allergic etiology, which has been successfully performed in experimental studies using FA models (17, 26, 27). For successful AIT, it is essential to identify patient-relevant allergens which can be supported by serum or skin allergy tests (20). In one study using an experimental model of cats sensitized to Bermuda grass allergen or house dust mite allergen (HDMA), IDT revealed a greater sensitivity compared to serum allergen-specific immunoglobulin E-(IgE) testing (SAT), although SAT showed higher specificity (18). In naturally occurring FA, IDT and SAT results have been compared in a pilot study which showed significantly more individual positive reactions on IDT and SAT in cats with FLAD compared to healthy cats (HC) (28). Recent studies identified responses to house dust mites and storage mites (SM) as common reactions on SAT in cats with FLAD; however, IDT was not performed in these investigations and environmental factors were not specifically assessed (9, 29).
Therefore, the aim of the study was to prospectively investigate agreement between IDT and SAT results in cats with FLAD and to assess possible correlations with environmental factors.
2 Materials and methods
The prospective case-controlled study was approved by the Ethics Committee of the Centre for Clinical Veterinary Medicine of the Ludwig Maximilian University of Munich, Germany (No. 239-16-11-2020) and was conducted between December 2020 and June 2022.
2.1 Study population
All cats with FLAD were privately owned patients of the Small Animal Clinic of the Ludwig Maximilian University of Munich, Germany, presenting with respiratory complaints. HC were presented for health care check-up, vaccination titer check or dental care. Inclusion criteria were clinical signs indicative of FLAD (chronic cough, wheezing, episodes of tachypnea or respiratory distress), radiographic evidence of bronchial or bronchointerstitial lung pattern, elevated total cell count in BALF >400 cells/μl (30, 31), and cytological evidence of sterile airway inflammation. For BALF cytology, 200 cells of a cytospin sample were assessed by the same board-certified clinical pathologist. Inflammation was categorized as eosinophilic (eosinophils >20% with neutrophils <14%, or eosinophils >50%), mixed (eosinophils 20%-50% and neutrophils >14%), or neutrophilic (neutrophils >14% and eosinophils <20%) inflammation as previously described (6). In addition, aerobic bacterial cultures and Mycoplasma spp.-PCR were performed on all BALF-samples. Cats with positive Mycoplasma spp.-PCR were not excluded, as Mycoplasma spp. has been described as a commensal organism in the lower airways of cats (32). Additionally, cats with a positive Mycoplasma spp. PCR received oral Doxycycline for 3 weeks, yet symptoms persistet. Deworming status of the cats included in this study could not be determined. In all outdoor cats, Baermann fecal analysis was performed to rule out lungworm disease. Exclusion criteria were the administration of glucocorticoids within 4 weeks or of antihistamines within 2 weeks prior to examination, detection of respiratory disease other than FLAD, presence of severe systemic disease, or unstable patients exhibiting respiratory distress at presentation. Inclusion criteria for HC were an unremarkable clinical examination and the absence of a history of respiratory or dermatological diseases.
2.2 Sample collection
2.2.1 Diagnostic workup
All owners of cats with FLAD and HC filled out a questionnaire on environmental factors of their cats (Supplementary material). In cats suspicious for FLAD, a thorough clinical examination and thoracic radiographs (ventrodorsal and laterolateral right-sided) were carried out. Blood samples were collected for a complete blood cell count and serum biochemistry analysis. In cats with outdoor access, Baerman analysis from a three-day fecal collection was carried out to exclude lung worm infection. As Dirofilaria immitis is not endemic in Germany, and none of the patients originated from or had traveled to an endemic area, heart worm testing was not performed. Prior to anesthesia, cats received 0.01 mg/kg terbutaline subcutaneously (Bricanyl®, AstraZeneca, Wedel, Germany) to minimize the risk of bronchoconstriction, and 0.2mg/kg Butorphanol (Butorgesic®, cp pharma, Burgdorf, Germany) was given intravenously for mild sedation. Anesthesia was induced and maintained intravenously with Alfaxan (Alfaxalon®, Jurox, Rutherford, Australia). Blind bronchoalveolar lavage was performed in cats with FLAD according to a previously published protocol (32). Each BALF was submitted for aerobic bacterial culture (Institute for Infectious Diseases and Zoonoses of the Ludwig Maximilian University of Munich, Germany) and Mycoplasma-spp.-PCR (Synlab Laboratory, Augsburg, Germany). Total cell count of BALF was determined directly after sample collection, and stained native and cytocentrifuged BALF-smears were evaluated cytologically by a board-certified clinical pathologist (JP). For this purpose, two direct smears and two cytospin preparations were stained with modified Wright's stain. Multiple microscopic fields were examined to obtain a total of 200 cell differential count.
2.2.2 Intradermal testing
During anesthesia for the diagnostic workup, IDT was performed according to a published protocol (33). For this procedure the lateral aspect of the left thorax was carefully clipped, and injection sites were marked with a water-soluble pen. In total, 39 injections were administered intradermally with a volume of 0.08 ml each, including a negative (saline) and a positive control (histamine phosphate) (Table 1). After 15 and 25 min, injection sites were evaluated for diameter of the wheal, turgidity, and erythema in comparison to positive and negative control. The local reactions were graded from 0 (negative control) to 4 (positive control) and results were recorded. Evaluation was carried out by a board-certified dermatologist as well as dermatology residents, who were familiar and experienced with the procedure.
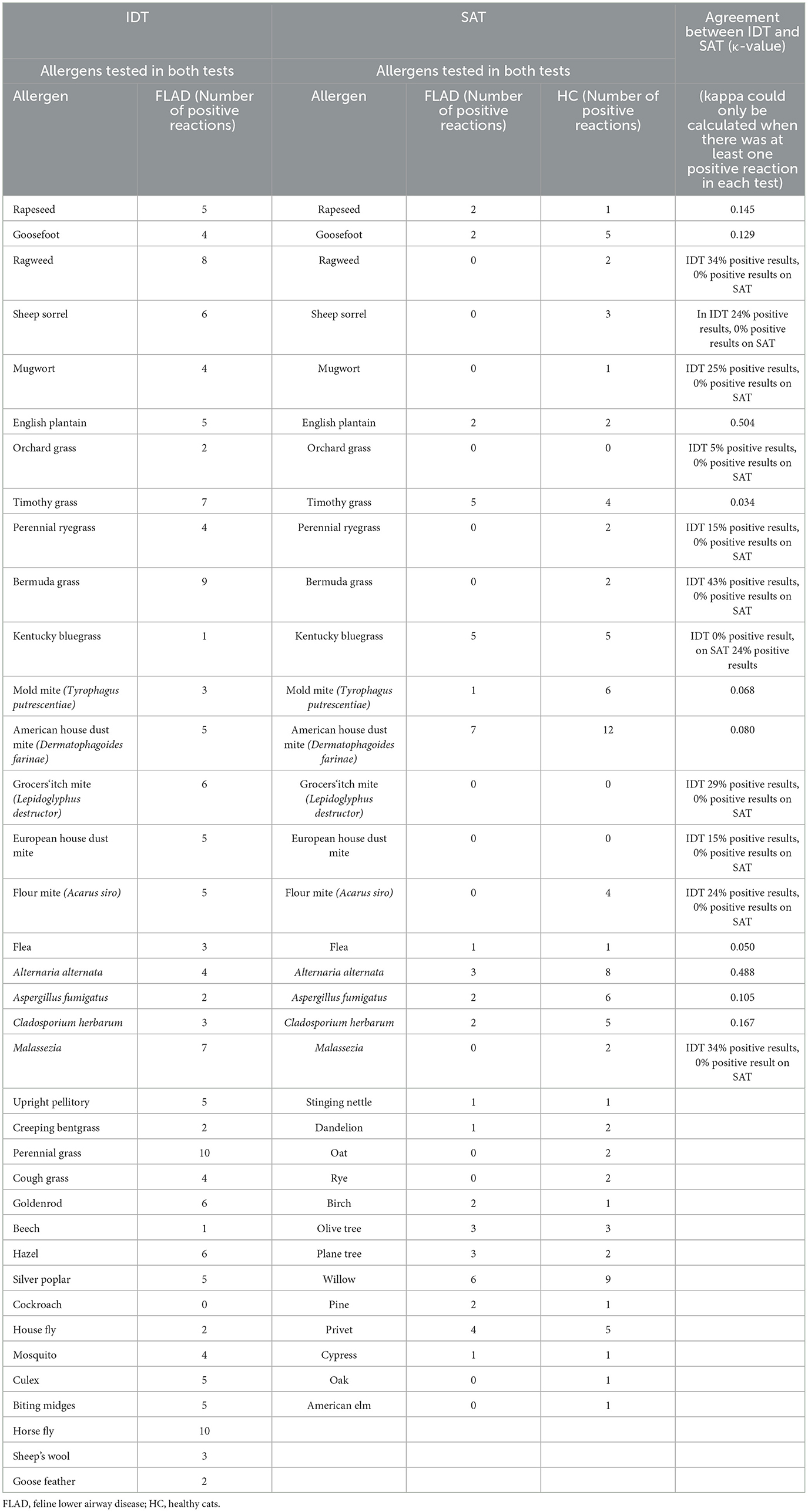
Table 1. Allergens, number of reactions to allergens in cats with FLAD and HC and agreement between tests.
2.2.3 Serum allergen-specific immunoglobulin E-(IgE) testing
In HC, left over material was used for analysis. Serum had been collected for general check-up, vaccination titer check or blood work collected prior to anesthesia for dental care. All serum samples were stored at −80°C immediately after centrifugation. The samples were then sent in groups to the laboratory (Nextmune S.L.U., Madrid, Spain) for specific IgE-antibody detection for 34 different allergens (Table 1). On the way to the external laboratory, one batch of serum samples was lost in mail transit, which reduced the total number of SATs carried out to 21 instead of 24 in the group of cats with FLAD. All sera were tested against cross-reactive carbohydrate determinants (CCDs) using indirect ELISA (CCD-screening). Sera then were diluted 1/6 in buffer solution containing 1% bovine serum albumin and 0.1% Tween 20. Samples with positive specific IgE against CCDs were processed after adding a CCD-blocker before dilution. For blocking, serum was incubated for 1 h at room temperature with a CCD-blocker. After that, samples were added to 96-well plates coated with allergens in duplicates. The plates were incubated at 4 °C overnight. Next, plates were washed four times with washing buffer, and monoclonal antibody was added, followed by incubation at 4 °C for 2 h. Thereafter, plates were washed six times with washing buffer, and p-nitrophenyl phosphate substrate (Moss, MD, USA) was added to the wells. After 30 min incubation time at room temperature, 1 N sodium hydroxide (NaOH) was added to stop the reaction. Absorbances were read at 405 nm using a spectrophotometer. Positivity was defined according to a protocol established by Nextmune expressing results in ELISA absorbance units.
2.3 Statistical analysis
All analyses were performed using commercially available software (IBM®, SPSS Statistics version 28.0.1.0). Nonparametric tests were used, as data was not normally distributed. Mann-Whitney U test was used for age comparison between cats with FLAD and HC. In addition, it was used to compare positive reactions per cat in IDT and SAT with allergens appearing in both tests (n = 21) as well as positive reactions in indoor- and outdoor cats. Comparison of more than two groups was performed with Kruskal–Wallis test, which was applied for positive reactions on SAT and IDT between the different groups, and for positive reactions between specific allergen groups. When significance was demonstrated, a post-hoc Bonferroni correction was performed. Fisher's-exact test was applied for evaluation of an association of SM reactions and dry food diet. In addition, it was used to compare the number of cats with at least one reaction on SAT between groups. Agreement of IDT and SAT results for allergens appearing in both tests was evaluated using Cohen's kappa. “Poor” to “almost perfect” agreement was reported as previously defined (Table 2) (34). Eleven allergens were compared only descriptively as kappa was by definition zero given the fact that one test showed no positive reactions for a specific allergen. Seasonality was assessed descriptively. For all tests P < 0.05 was considered significant.
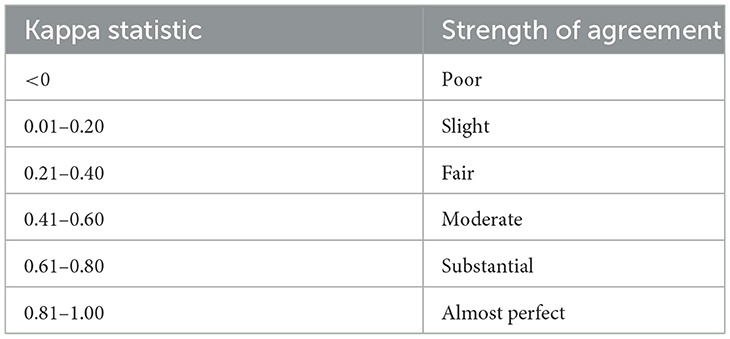
Table 2. Standard interpretations of Cohen's Kappa (34).
3 Results
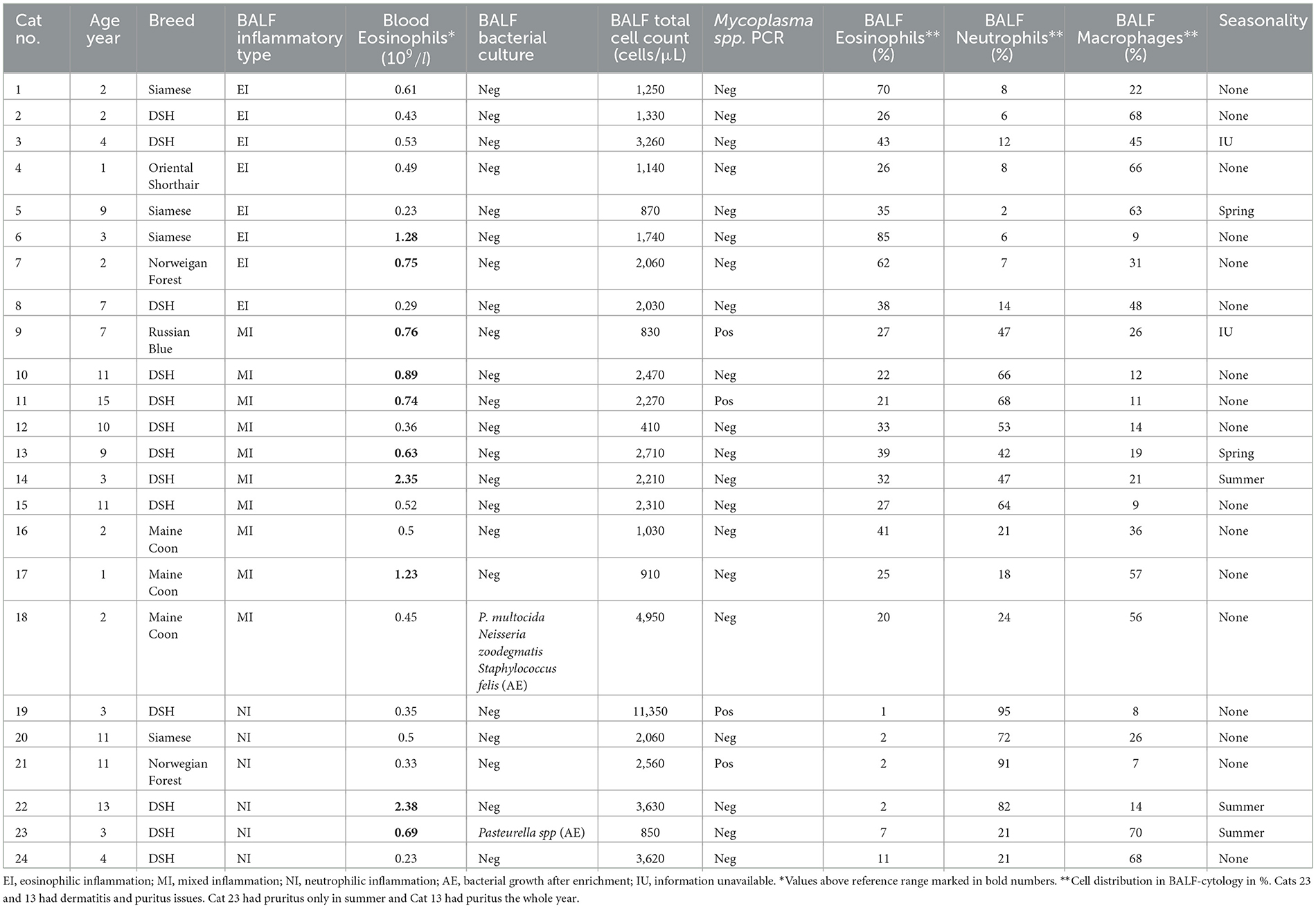
Twenty-four client-owned cats diagnosed with FLAD and 24 HC who served as a control group were included. Four cats suspected of having FLAD were previously excluded due to physiological BALF cytology. Of the remaining 24 cats with FLAD, eight showed EI, ten MI, and six NI on BALF cytology (Table 3). Four cats had positive PCR results for Mycoplasma spp. and two cats had low levels of bacterial growth after enrichment on BALF culture: growth of Pasteurella multocida, Neisseria zoodegmatis, and Staphylococcus felis in one cat, and Pasteurella spp. in another patient (Table 3).
Of the 24 cats with FLAD ten were female spayed (42%), and 14 male neutered (58%). HC consisted of ten female spayed (42%) and one intact female (4%), and twelve male neutered (50%), and one intact male (4%). Breeds of FLAD cats included Domestic Shorthair (n = 13), Siamese (n = 4), Maine Coon (n = 3), Norwegian Forest (n = 2), Russian Blue (n = 1), and Oriental Shorthair (n = 1) (Table 3). Breeds of HC were Domestic Shorthair (n = 20), Siberian (n = 1), Russian Blue (n = 1), Norwegian Forest (n = 1) and British Shorthair (n = 1). Median age did not differ significantly between cats with EI (2.5, range: 1–9 years), MI (8.0, range: 1–15 years), NI (7.5, range: 3–13 years) and HC (3.0, range: 1–13 years) (p = 0.118). In both groups most cats were kept indoors (63% with FLAD and 83% HC) and lived in an urban environment (79% FLAD and 88% HC). Two cats with FLAD showed additional signs of dermatitis and pruritus, one of them with more severe dermatological and respiratory signs during summer (Table 3). The duration of time since first onset of clinical signs ranged from 3 months to 6 years (median 1.5 years), with cough n = 22 (92%) being the most predominant clinical sign besides tachypnea n = 3 (13%), episodes of respiratory distress n = 4 (17%) and wheezing n = 2 (8%). Blood eosinophilia was present in ten cats with FLAD (42%) (Table 3).
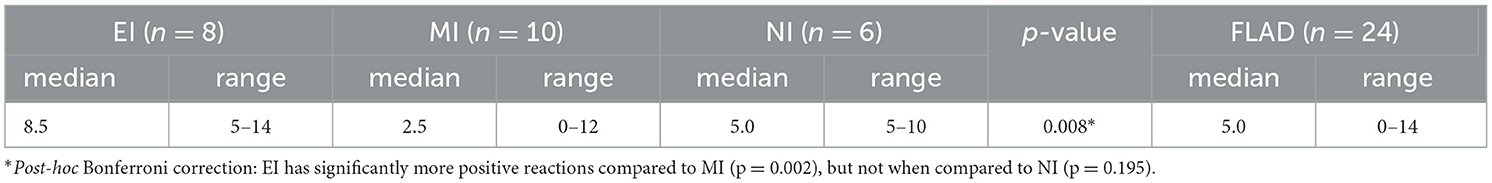
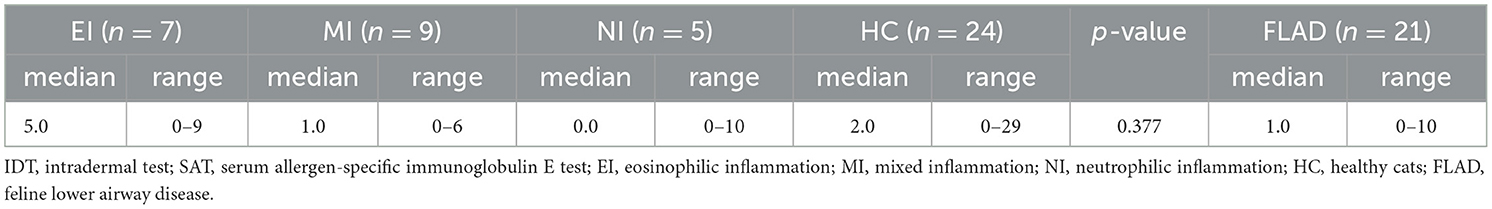
More HC 20/24 (83%) showed at least one positive allergen reaction on SAT compared to cats with FLAD 11/21 (52%) (p = 0.051). On IDT 22/24 (91%) cats with FLAD showed positive allergen reactions. When results of IDT and SAT for cats with FLAD were compared, significantly more positive reactions per cat were found on IDT (p = 0.001). No significant difference could be detected for median positive reactions per cat on SAT between cats with FLAD and HC (p = 0.185), nor when compared between NI, EI, MI, and HC (p = 0.377) (Tables 4A, B).
When agreement between IDT and SAT results was assessed for the ten allergens evaluated in both tests, “moderate agreement” was observed for the allergens Alternaria alternata (κ = 0.488) and English plantain (κ = 0.504). For the other eight allergens, agreement was only “slight” (Table 1).
On SAT the most common allergen reaction in cats with FLAD (n = 7) and HC (n = 12) was American house dust mite (Dermatophagoides farinae), followed by fungi in HC with Alternaria alternata (n = 8) and Aspergillus fumigatus (n = 6) (Table 1).
When median positive reactions per cat were compared for the three inflammatory types regarding different allergen groups on IDT, reactions to tree pollen were significantly more common in cats with EI than in those with NI and MI. In addition, cats with EI and those with NI reacted significantly more commonly against allergens of the insect group than cats with MI (Table 5A). There were no significant differences for the comparison of median positive reactions per cat in allergen groups tested on SAT (Table 5B). Total positive reactions for all groups of cats are listed in Tables 5A, B.
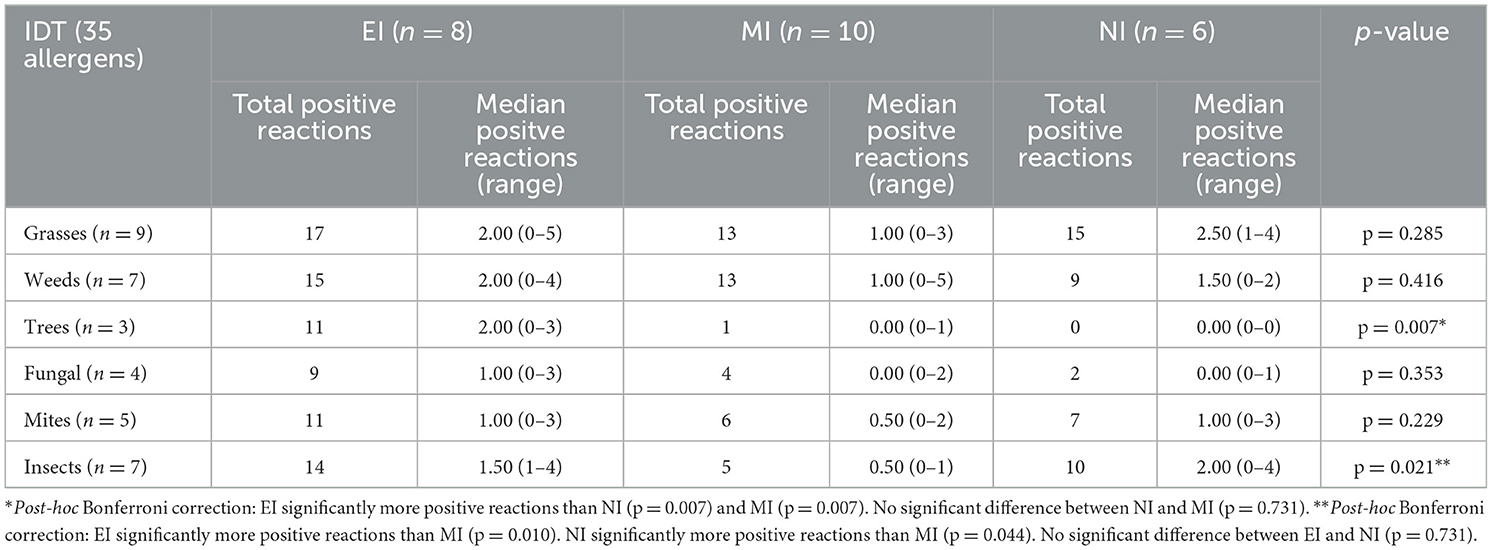
Table 5A. Total positive reactions in cats (and median positive reactions per cat) with different type of inflammation on IDT, reactions to groups of allergens.
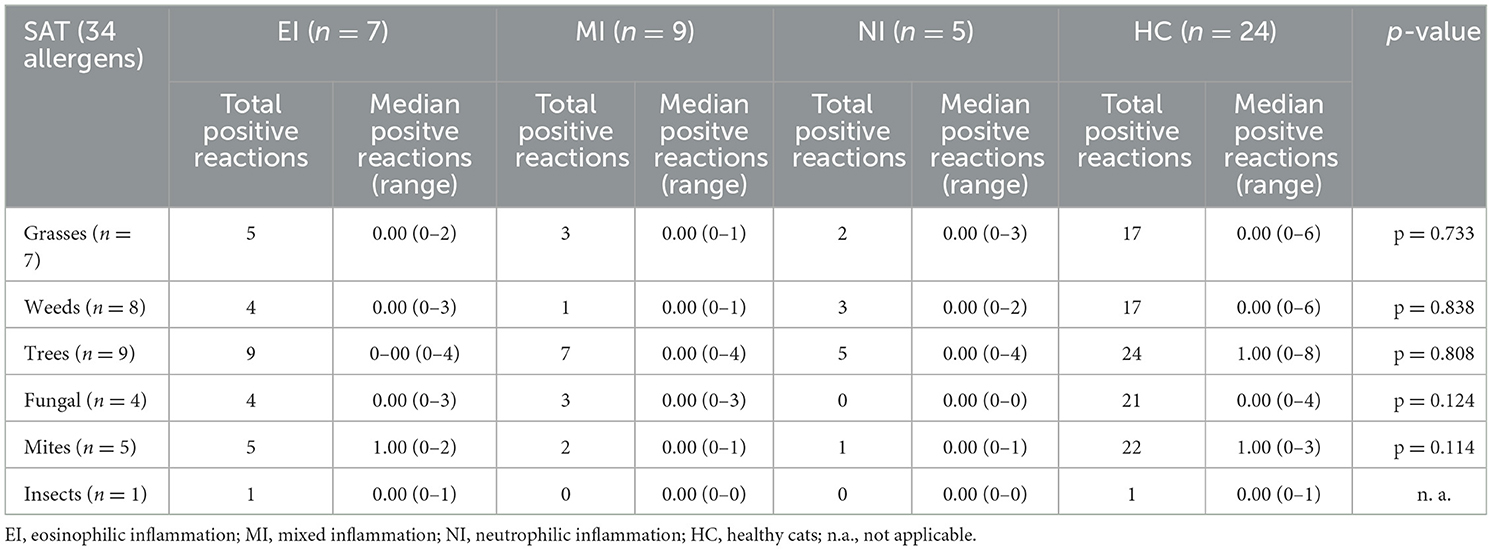
Table 5B. Total positive reactions in cats (and median positive reactions per cat) with different type of inflammation on SAT, reactions to groups of allergens.
Two cats have had clinical signs of FLAD for <1 year at the time of presentation; therefore, seasonality could be assessed only in the remaining 22 cats. All 22 cats showed clinical signs throughout the year. While in 17 cats the severity of clinical signs did not change throughout the year, five cats had seasonal changes of severity. Of those five, two cats showed more signs in spring and three cats in summer.
Significantly more cats with FLAD (12/24, 50%) lived in a household with smoke exposure compared to HC (4/24, 17%) (p = 0.030). No difference was found regarding this factor when cats with EI, MI and NI were compared (p = 0.641).
In the FLAD group, no difference could be detected between cats eating mainly dry food and those eating moist food in reactions to SM (Tyrophagus putrescentiae, Acarus siro, Lepidoglyphus destructor) on IDT (p = 0.629). No calculation was done on SAT as only one cat showed a reaction to one of the mite allergens.
There was no difference in median positive reactions per cat for “outdoor-allergens” such as weed, grass, and tree pollen in the FLAD group between cats living exclusively indoors (median: 0.00 on SAT, median: 4.00 on IDT) and cats with outdoor access (median: 0.00 on SAT, median: 3.5 on IDT) on SAT (p = 0.630) or IDT (p = 0.252), respectively.
4 Discussion
Experimentally induced FA has been shown to elicit positive allergy test results (16, 20). In addition, application of AIT resulted in resolution of induced clinical signs and airway inflammation, strongly supporting the hypothesis that aeroallergens can trigger an allergic reaction causing clinical signs of FLAD (17, 26). To date, only few studies have been investigating the role of environmental allergens in cats with naturally occurring inflammatory airway disease.
In the present study HC showed more positive reactions per cat than cats with FLAD on SAT, questioning the diagnostic accuracy of allergy testing in cats with FLAD. In contrast to this finding, two other studies detected significantly more positive reactions in cats with naturally occurring FLAD compared to HC (9, 28). One of these studies that compared results from SAT and IDT in ten HC and ten cats with not further classified FLAD detected more positive reactions in both tests in affected cats compared to HC (28). Similarly, the second study found that 15 cats with EI and MI had more positive allergen reactions on SAT compared to nine HC (9). However, both studies included only small patient groups which might have influenced the statistical power. Another explanation for different findings in the present study could be the measurement methods used for SAT. In the present study, a monoclonal antibody methodology was used where antibodies may falsely bind irrelevant IgG antibodies (35). For higher specificity, the alpha chain of the high-affinity mast cell receptor for IgE (FcεR1α) is recommended as binding site for antibodies used for testing (18, 35). However, only Moriello and coworkers (28) used the FcεR1α-receptor measurement in their study. Buller and coworkers (9) performed SAT using polyclonal antibody measurement which, compared to the FcεR1α-receptor measurement, is more likely to cause false positive results (35). Another possible explanation could be a differing deworming status for cats in different studies. Referring to a study investigating pruritic cats, non-dewormed cats showed significantly more positive reactions for allergen-specific IgE than dewormed cats. The authors assumed that the presence of intestinal parasites could lead to production of IgE cross-reacting against environmental allergens (36). Deworming status was unknown for most cats in the present study as well as in the study performed by Moriello et al. (28), making it impossible to compare allergy test results regarding that point. In the study performed by Buller and coworkers on the other hand, all cats were regularly dewormed. As most cats with FLAD and HC in the present study were indoor only cats, it is unlikely that infestation with parasites could have explained the higher number of positive reactions on SAT in HC compared to cats with FLAD. Moreover, a functional heterogeneity due to different glycosylation of allergen-specific IgE could be the cause for positive reactions in healthy animals leading to the assumption that “pathogenic” and “non-pathogenic” IgE should be considered (37, 38). However, the results of the present study are consistent with findings in cats with feline atopic skin syndrome (FASS), in which no difference in allergen-specific IgE could be detected in HC compared to cats with FASS (37–40). These findings emphasize that, considering AIT as a potential treatment of FA, SAT should only be used as a guidance for allergen selection and results should always be evaluated in context with clinical signs and environmental factors, as previously recommended for treatment of FASS (33, 41, 42).
Due to ethical reasons, we could not perform IDT in HC, therefore it is not known whether more HC than cats with FLAD would have shown positive reactions on IDT as it was the case on SAT. In one study on cats with FASS it could be seen that more cats with FASS showed positive reactions on IDT than HC (43). This is in concordance with the study from Moriello et al. (28). This leads to the assumption that in our study more cats with FLAD than HC would have shown positive reactions on IDT. These findings could be explained with the high sensitivity of the test. Furthermore, IDT rather faces problems of subtle wheal formation, making it hard to read compared to dogs (44), meaning that rather some relevant allergen reactions might be missed than false positive reactions generated in HC.
Significantly more positive reactions per cat were seen on IDT compared to SAT in the present study. Previously, in a study evaluating allergy test results in cats with induced FA sensitized with bermuda grass allergen or HDMA, IDT showed higher sensitivity compared to SAT (18). In the same study, good agreement was found between allergens tested in both tests; however, because of the experimental study design allergens were known in that investigation limiting the ability to compare the results with patients suffering from naturally acquired disease.
When positive results of allergens evaluated in both tests were compared with cohen's kappa, only Alternaria alternata and English Plantain showed “moderate agreement.” This is consistent with results from studies on FASS (37, 45). In one study eight cats diagnosed with FASS had positive IDT results but tested negative on SAT (45). In another study comparing allergy test results for HDMA in cats with FASS, only weak correlation was found between results in both tests (37). One reason for the discrepancies between both tests could be different detection methods for allergen-specific IgE. While free circulating IgE is detected in serological assays, IDT reveals IgE bound to the FcεR1α-receptor on dermal mast cells (40). In contrast to IgE circulating in blood, dermal mast cell-bound IgE shows a significantly longer half-life, resulting in positive reactions lasting longer on IDT compared to a shorter period of detection in SAT (46). Furthermore, it can be assumed that irregular allergen exposure could also lead to the absence of circulating IgE. This might explain a higher sensitivity of IDT compared to SAT, therefore potentially “missing” some relevant allergens when using SAT.
Ten cats with FLAD in the present study showed reactions to SM on IDT, but no association with dry food diet could be found in these patients. A previous study also investigated IDT results in cats with naturally occurring FLAD in the context with environmental factors, using information gained by an owner questionnaire. Three of the six cats that showed reactions to SM reached remission of clinical signs when dry food was changed to moist food diet (47). This was also mentioned in an abstract reporting that removal of dry food led to clinical remission in 2/5 cats with naturally occurring FLAD and positive reactions to SM (48). However, in dry dog food, contamination with SM is usually very low or undetectable and therefore with the correct storage conditions quite unlikely (49). It can be assumed that these findings account for dry cat food as well, although no specific studies on SM contamination of dry cat food exist so far. In the present study, however, cats that showed reactions to SM were not changed to moist food to assess a potential clinical improvement related to a dietary change.
No difference could be detected when indoor cats and cats with outdoor access were compared in the present study regarding positive reactions per cat on environmental allergens in both tests. This finding stands in contrast to results from a study on pruritic cats, suggesting that outdoor cats are consistently challenged by environmental allergens, endo-, and ectoparasites and therefore tend to have more positive reactions on SAT. In that study, outdoor cats showed more positive reactions to food and environmental allergens compared to indoor cats (36).
Seasonality has scarcely been assessed in cats with naturally occurring FLAD. One study mentioned more intense signs and episodes of respiratory distress in five cats during summer, in two during autumn and in two during winter. The remaining 16 cats did not show any seasonal changes (12). In the present investigation, only five cats revealed stronger clinical signs during spring and summer. It can be assumed that seasonality is not common in cats with FLAD.
On SAT the most common positive reactions in cats with FLAD were seen for American house dust mite (Dermatophagoides farinae). This is not surprising, as HDMA has been shown to be one of the most prevalent allergens cats are sensitized to in several studies on FASS (37, 45). In addition, cats with FA were commonly sensitized to this allergen on SAT in previous studies (9, 29). One study compared SAT results in cats with FA to those in HC and interestingly, reactions to HDMA were only found in cats with FLAD (9). In contrast to that, in the present study house dust mite was shown to be one of the most common reactions on SAT in HC as well as in affected cats. However, in the previous study only nine cats served as a control group and just one of those showed positive reactions on SAT, which potentially underestimates the most common allergen reactions in HC (9).
While in the present study house dust mite was not one of the most common allergen reactions on IDT in cats with FLAD, in two other studies using IDT for allergen testing, reactions to American house dust mite in 8/15 cats showing positive reactions (47) and 4/9 cats showing positive reactions to this allergen (48), represented the most common positive skin reactions in cats with FLAD (47, 48).
To date, no studies have been investigating allergy test results in the context of different types of airway inflammation. In the present study, for most allergen groups no significant differences could be detected between median positive reactions per cat comparing EI, MI, NI and HC. However, cats with EI showed significantly more positive allergen reactions compared to those with MI for tree pollen and insects, and cats with NI had significantly more positive reactions to insects compared to cats with MI. These findings stand in contrast to the assumption that EI is a consequence of an allergic reaction. Similar to human asthma, FLAD might also have a non-allergic non-IgE-mediated etiology in cats, therefore overestimating the role of allergens in cats with airway disease and potentially explaining the overall low rate of positive reactions in SAT in cats with FLAD in the present study.
In general, categorization into different inflammatory types based on BALF cytology alone remains questionable, since definitions and cytology cut-off values for the different groups vary in many studies (6, 9, 10, 12). Therefore, it would have had an impact on the results of this study, if a different classification scheme had been chosen, emphasizing the problem of non-existing standardized cutoff-values. In addition to that, it has been shown that BALF samples from different lung segments of cats revealed different predominance of inflammatory cells in BALF cytology (30), raising the question whether FA and CB represent different conditions with different etiologies at all. It seems not unlikely that NI can develop as a consequence of an allergic reaction to environmental allergens as well since in one study NI could be induced experimentally by allergen exposure in research cats (2). Likewise, horses with severe equine asthma commonly express a neutrophilic inflammatory response associated with exposure to environmental allergens (50, 51). In humans, allergen-induced severe asthma is commonly associated with a neutrophilic inflammatory pattern as well (52).
CB in cats as a non-septic inflammatory condition of the lower airways with involvement of non-degenerated neutrophils is thought to arise secondarily to insults of the airways induced by multiple possible irritants, one of them being cigarette smoke in humans (1, 53). It could be shown that cigarette smoke can generate various lesions in the epithelial cells of human airways (54). In the present study, significantly more cats with FLAD lived in a smoker-household compared to HC. Nonetheless, cats with NI were not overrepresented in the group of cats from smoker households. However, to investigate the potential role of smoke exposure in the pathogenesis of FLAD, a larger sample size of cats with FLAD and HC would be needed to evaluate this factor.
There are several limitations of this study. One of them was the evaluation of the cats' environment dependent on their owners' subjectivity in the questionnaire. In addition, the impact of seasonality on clinical signs was also assessed by the cat owners only. Allergens tested in this study might not have reflected all relevant allergens present in the cats' environment, but to date, data on relevant allergens inducing sensitization in cats are limited (55). Therefore, some relevant allergens including food allergens might not have been tested in the study population. To evaluate potential differences in allergen profiles between the three types of airway inflammation, studies with distinctively larger groups are warranted. Since most cats included in this study showed perennial signs and were therefore more likely sensitized against perennial allergens such as HDMA, it would be interesting to collect dust samples in the cats' environment for investigation of mite exposure, since HDMA seems to be a relevant allergen in cats with FLAD (56).
5 Conclusions and relevance
Cats with FLAD do not show more positive reactions to environmental allergens on SAT than HC. In addition, correlation for most allergens is weak between SAT and IDT. Therefore, positive reactions to environmental allergens primarily demonstrate exposure to allergens but need to be interpreted with some caution in patients with FLAD. For selection of allergens for AIT, interpretation of results should always be performed in the context of clinical signs and allergen exposure for the individual cat.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal studies were approved by the Ethics Committee of the Centre for Clinical Veterinary Medicine of the Ludwig Maximilian University of Munich, Germany (No. 239-16-11-2020). The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent was obtained from the owners for the participation of their animals in this study.
Author contributions
BH: Writing—original draft. RM: Conceptualization, Data curation, Project administration, Supervision, Writing—review & editing. JG: Data curation, Writing—review & editing. TW: Conceptualization, Writing—review & editing. TB: Conceptualization, Writing—review & editing. JP: Conceptualization, Methodology, Writing—review & editing. BS: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Supervision, Validation, Writing—review & editing.
Funding
The author(s) declare that no financial support was received for the research, authorship, and/or publication of this article.
Acknowledgments
All materials used, such as equipment and consumables, were provided by the Small Animal Clinic of the Ludwig Maximilian University of Munich, Germany. Furthermore, we would like to thank Artu Biologicals for supplying the allergens for skin testing and Nextmune for running the serum allergen-specific IgE tests at no charge. Statistical analysis was performed with support of StaBLab Ludwig Maximilian University of Munich, Germany.
Conflict of interest
JP is employed by the Vet Med Labor GmbH Division of IDEXX Laboratories, Kornwestheim, Germany.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2023.1267496/full#supplementary-material
Abbreviations
AIT, allergen immunotherapy; BALF, bronchoalveolar lavage fluid; CB, chronic bronchitis; CCD, cross-reactive carbohydrate determinant; EI, eosinophilic inflammation; FA, feline asthma; FASS, feline atopic skin syndrome; FLAD, feline lower airway disease; HDMA, house dust mite allergen; HC, healthy cat; IgE, immunoglobulin E; MI, mixed inflammation; NI, neutrophilic inflammation; SAT, serum allergen-specific IgE testing; SM, storage mite.
References
1. Reinero CR. Advances in the understanding of pathogenesis, and diagnostics and therapeutics for feline allergic asthma. Vet J. (2011) 190:28–33. doi: 10.1016/j.tvjl.2010.09.022
2. Nafe LA, DeClue AE, Lee-Fowler TM, Eberhardt JM, Reinero CR. Evaluation of biomarkers in bronchoalveolar lavage fluid for discrimination between asthma and chronic bronchitis in cats. Am J Vet Res. (2010) 71:583–91. doi: 10.2460/ajvr.71.5.583
3. Adamama-Moraitou KK, Patsikas MN, Koutinas AF. Feline lower airway disease: a retrospective study of 22 naturally occurring cases from Greece. J Feline Med Surg. (2004) 6:227–33. doi: 10.1016/j.jfms.2003.09.004
4. Corcoran BM, Foster DJ, Fuentes VL. Feline asthma syndrome: a retrospective study of the clinical presentation in 29 cats. J Small Anim Pract. (1995) 36:481–8. doi: 10.1111/j.1748-5827.1995.tb02787.x
5. Grotheer M, Hirschberger J, Hartmann K, Castelletti N, Schulz B. Comparison of signalment, clinical, laboratory and radiographic parameters in cats with feline asthma and chronic bronchitis. J Feline Med Surg. (2020) 22:649–55. doi: 10.1177/1098612X19872428
6. Lee EA, Johnson LR, Johnson EG, Vernau W. Clinical features and radiographic findings in cats with eosinophilic, neutrophilic, and mixed airway inflammation (2011-2018). J Vet Intern Med. (2020) 34:1291–9. doi: 10.1111/jvim.15772
7. Kirschvink N, Leemans J, Delvaux F, Snaps F, Jaspart S, Evrard B, et al. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis. J Feline Med Surg. (2006) 8:45–54. doi: 10.1016/j.jfms.2005.07.001
8. Venema CM, Patterson CC. Feline asthma: what's new and where might clinical practice be heading? J Feline Med Surg. (2010) 12:681–92. doi: 10.1016/j.jfms.2010.07.012
9. Buller MC, Johnson LR, Outerbridge CA, Vernau W, White SD. Serum immunoglobulin E responses to aeroallergens in cats with naturally occurring airway eosinophilia compared to unaffected control cats. J Vet Intern Med. (2020) 34:2671–6. doi: 10.1111/jvim.15951
10. Johnson LR, Vernau W. Bronchoscopic findings in 48 cats with spontaneous lower respiratory tract disease (2002-2009). J Vet Intern Med. (2011) 25:236–43. doi: 10.1111/j.1939-1676.2011.00688.x
11. Foster SF, Martin P, Braddock JA, Malik R, A. retrospective analysis of feline bronchoalveolar lavage cytology and microbiology (1995-2000). J Feline Med Surg. (2004) 6:189–98. doi: 10.1016/j.jfms.2003.12.001
12. Foster SF, Allan GS, Martin P, Robertson ID, Malik R. Twenty-five cases of feline bronchial disease (1995-2000). J Feline Med Surg. (2004) 6:181–8. doi: 10.1016/j.jfms.2003.12.008
13. Norris CR, Griffey SM, Samii VF, Christopher MM, Mellema MS. Thoracic radiography, bronchoalveolar lavage cytopathology, and pulmonary parenchymal histopathology: a comparison of diagnostic results in 11 cats. J Am Anim Hosp Assoc. (2002) 38:337–45. doi: 10.5326/0380337
14. Moise NS, Wiedenkeller D, Yeager AE, Blue JT, Scarlett J. Clinical, radiographic, and bronchial cytologic features of cats with bronchial disease: 65 cases (1980-1986). J Am Vet Med Assoc. (1989) 194:1467–73.
15. Mueller RS, Nuttall T, Prost C, Schulz B, Bizikova P. Treatment of the feline atopic syndrome - a systematic review. Vet Dermatol. (2021) 32:43–e8. doi: 10.1111/vde.12933
16. Norris Reinero CR, Decile KC, Berghaus RD, Williams KJ, Leutenegger CM, Walby WF, et al. An experimental model of allergic asthma in cats sensitized to house dust mite or bermuda grass allergen. Int Arch Allergy Immunol. (2004) 135:117–31. doi: 10.1159/000080654
17. Reinero CR, Byerly JR, Berghaus RD, Berghaus LJ, Schelegle ES, Hyde DM, et al. Rush immunotherapy in an experimental model of feline allergic asthma. Vet Immunol Immunopathol. (2006) 110:141–53. doi: 10.1016/j.vetimm.2005.09.013
18. Lee-Fowler TM, Cohn LA, DeClue AE, Spinka CM, Ellebracht RD, Reinero CR. Comparison of intradermal skin testing (IDST) and serum allergen-specific IgE determination in an experimental model of feline asthma. Vet Immunol Immunopathol. (2009) 132:46–52. doi: 10.1016/j.vetimm.2009.09.014
19. Kirschvink N, Kersnak E, Leemans J, Delvaux F, Clercx C, Snaps F, et al. Effects of age and allergen-induced airway inflammation in cats: radiographic and cytologic correlation. Vet J. (2007) 174:644–51. doi: 10.1016/j.tvjl.2006.11.010
20. Chang CH, Lee-Fowler TM, DeClue AE, Cohn LA, Robinson KL, Reinero CR. The impact of oral versus inhaled glucocorticoids on allergen specific IgE testing in experimentally asthmatic cats. Vet Immunol Immunopathol. (2011) 144:437–41. doi: 10.1016/j.vetimm.2011.09.003
21. Cohn LA, DeClue AE, Cohen RL, Reinero CR. Effects of fluticasone propionate dosage in an experimental model of feline asthma. J Feline Med Surg. (2010) 12:91–6. doi: 10.1016/j.jfms.2009.05.024
22. Reinero CR, Decile KC, Byerly JR, Berghaus RD, Walby WE, Berghaus LJ, et al. Effects of drug treatment on inflammation and hyperreactivity of airways and on immune variables in cats with experimentally induced asthma. Am J Vet Res. (2005) 66:1121–7. doi: 10.2460/ajvr.2005.66.1121
23. Cocayne CG, Reinero CR, DeClue AE. Subclinical airway inflammation despite high-dose oral corticosteroid therapy in cats with lower airway disease. J Feline Med Surg. (2011) 13:558–63. doi: 10.1016/j.jfms.2011.04.001
24. Nerhagen S, Moberg HL, Boge GS, Glanemann B. Prednisolone-induced diabetes mellitus in the cat: a historical cohort. J Feline Med Surg. (2021) 23:175–80. doi: 10.1177/1098612X20943522
25. Todd GR, Acerini CL, Buck JJ, Murphy NP, Ross-Russell R, Warner JT, et al. Acute adrenal crisis in asthmatics treated with high-dose fluticasone propionate. Eur Respir J. (2002) 19:1207–9. doi: 10.1183/09031936.02.00274402
26. Lee-Fowler TM, Cohn LA, DeClue AE, Spinka CM, Reinero CR. Evaluation of subcutaneous versus mucosal (intranasal) allergen-specific rush immunotherapy in experimental feline asthma. Vet Immunol Immunopathol. (2009) 129:49–56. doi: 10.1016/j.vetimm.2008.12.008
27. Reinero CR, Cohn LA, Delgado C, Spinka CM, Schooley EK, DeClue AE. Adjuvanted rush immunotherapy using CpG oligodeoxynucleotides in experimental feline allergic asthma. Vet Immunol Immunopathol. (2008) 121:241–50. doi: 10.1016/j.vetimm.2007.09.013
28. Moriello KA, Stepien RL, Henik RA, Wenholz LJ. Pilot study: prevalence of positive aeroallergen reactions in 10 cats with small-airway disease without concurrent skin disease. Vet Dermatol. (2007) 18:94–100. doi: 10.1111/j.1365-3164.2007.00573.x
29. van Eeden ME, Vientos-Plotts AI, Cohn LA, Reinero CR. Serum allergen-specific IgE reactivity: is there an association with clinical severity and airway eosinophilia in asthmatic cats? J Feline Med Surg. (2020) 22:1129–36. doi: 10.1177/1098612X20907178
30. Ybarra WL, Johnson LR, Drazenovich TL, Johnson EG, Vernau W. Interpretation of multisegment bronchoalveolar lavage in cats (1/2001-1/2011). J Vet Intern Med. (2012) 26:1281–7. doi: 10.1111/j.1939-1676.2012.01016.x
31. Hawkins EC, DeNicola DB, Kuehn NF. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat. State of the art J Vet Intern Med. (1990) 4:267–74. doi: 10.1111/j.1939-1676.1990.tb03120.x
32. Schulz BS, Richter P, Weber K, Mueller RS, Wess G, Zenker I, et al. Detection of feline Mycoplasma species in cats with feline asthma and chronic bronchitis. J Feline Med Surg. (2014) 16:943–9. doi: 10.1177/1098612X14524969
33. Santoro D, Pucheu-Haston CM, Prost C, Mueller RS, Jackson H. Clinical signs and diagnosis of feline atopic syndrome: detailed guidelines for a correct diagnosis. Vet Dermatol. (2021) 32:26–e6. doi: 10.1111/vde.12935
34. Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. (1977) 33:159–74. doi: 10.2307/2529310
35. Wassom, G. In vitro measurement of canine and feline IgE: a review of FcεR1α-based assays for detection of allergen-reactive IgE. Vet Dermatol. (1998) 9:173–8. doi: 10.1046/j.1365-3164.1998.00121.x
36. Belova S, Wilhelm S, Linek M, Beco L, Fontaine J, Bergvall K, et al. Factors affecting allergen-specific IgE serum levels in cats. Can J Vet Res. (2012) 76:45–51.
37. Gilbert S, Halliwell RE. Feline immunoglobulin E: induction of antigen-specific antibody in normal cats and levels in spontaneously allergic cats. Vet Immunol Immunopathol. (1998) 63:235–52. doi: 10.1016/S0165-2427(98)00100-7
38. Halliwell G, Mei L. Induced and spontaneous IgE antibodies to Dermatophagoides farinae in dogs and cats: evidence of functional heterogeneity of IgE. Vet Dermatol. (1998) 9:179–84. doi: 10.1046/j.1365-3164.1998.00112.x
39. Diesel A, DeBoer DJ. Serum allergen-specific immunoglobulin E in atopic and healthy cats: comparison of a rapid screening immunoassay and complete-panel analysis. Vet Dermatol. (2011) 22:39–45. doi: 10.1111/j.1365-3164.2010.00908.x
40. Taglinger K, Helps CR, Day MJ, Foster AP. Measurement of serum immunoglobulin E (IgE) specific for house dust mite antigens in normal cats and cats with allergic skin disease. Vet Immunol Immunopathol. (2005) 105:85–93. doi: 10.1016/j.vetimm.2004.12.017
41. Bell A, Jones B. Allergy and immunoglobulin E- an evolving understanding of the relationship. Vet Ireland J. (2021) 11:723–9.
42. Trimmer AM, Griffin CE, Boord MJ, Rosenkrantz WS. Rush allergen specific immunotherapy protocol in feline atopic dermatitis: a pilot study of four cats. Vet Dermatol. (2005) 16:324–9. doi: 10.1111/j.1365-3164.2005.00462.x
43. Schleifer SG, Willemse T. Evaluation of skin test reactivity to environmental allergens in healthy cats and cats with atopic dermatitis. Am J Vet Res. (2003) 64:773–8. doi: 10.2460/ajvr.2003.64.773
44. Scholz FM, Burrows AK, Griffin CE, Muse R. Determination of threshold concentrations of plant pollens in intradermal testing using fluorescein in clinically healthy nonallergic cats. Vet Dermatol. (2017) 28:351–e78. doi: 10.1111/vde.12320
45. Foster AP, O'Dair H. Allergy testing for skin disease in the cat in vivo vs in vitro tests. Vet Dermatol. (1993) 4:111–5. doi: 10.1111/j.1365-3164.1993.tb00203.x
46. Shade KT, Conroy ME, Anthony RM. IgE Glycosylation in Health and Disease. Curr Top Microbiol Immunol. (2019) 423:77–93. doi: 10.1007/82_2019_151
47. Prost C. Treatment of feline asthma with allergen avoidance and specific immunotherapy: experience with 20 cats. Revue Francaise D Allergologie Et D Immunologie Clinique. (2008) 48:409–13. doi: 10.1016/j.allerg.2008.07.003
48. Prost C. Immunotherapy treatment in nine cats with feline asthma syndrome. Vet Dermatol. (2000) 11:14–40.
49. Olivry T, Mueller RS. Critically appraised topic on adverse food reactions of companion animals (8): storage mites in commercial pet foods. BMC Vet Res. (2019) 15:385. doi: 10.1186/s12917-019-2102-7
50. Davis KU, Sheats MK. The role of neutrophils in the pathophysiology of asthma in humans and horses. Inflammation. (2021) 44:450–65. doi: 10.1007/s10753-020-01362-2
51. Pirie RS. Recurrent airway obstruction: a review. Equine Vet J. (2014) 46:276–88. doi: 10.1111/evj.12204
52. Hosoki K, Itazawa T, Boldogh I, Sur S. Neutrophil recruitment by allergens contribute to allergic sensitization and allergic inflammation. Curr Opin Allergy Clin Immunol. (2016) 16:45–50. doi: 10.1097/ACI.0000000000000231
53. Lin CH, Lo PY, Wu HD, Chang C, Wang LC. Association between indoor air pollution and respiratory disease in companion dogs and cats. J Vet Intern Med. (2018) 32:1259–67. doi: 10.1111/jvim.15143
54. Dye JA, Adler KB. Effects of cigarette smoke on epithelial cells of the respiratory tract. Thorax. (1994) 49:825–34. doi: 10.1136/thx.49.8.825
55. Mueller RS, Janda J, Jensen-Jarolim E, Rhyner C, Marti E. Allergens in veterinary medicine. Allergy. (2016) 71:27–35. doi: 10.1111/all.12726
Keywords: immunoglobulin E, intradermal test, feline asthma, chronic bronchitis, feline atopic syndrome
Citation: Hartung BF, Mueller RS, Gauss J, Weitzer T, Boehm TMSA, Palić J and Schulz B (2023) Reactions to environmental allergens in cats with feline lower airway disease. Front. Vet. Sci. 10:1267496. doi: 10.3389/fvets.2023.1267496
Received: 26 July 2023; Accepted: 14 November 2023;
Published: 07 December 2023.
Edited by:
Micaela Sgorbini, University of Pisa, ItalyReviewed by:
Chung-Hui Lin, National Taiwan University, TaiwanSanna Viitanen, University of Helsinki, Finland
Copyright © 2023 Hartung, Mueller, Gauss, Weitzer, Boehm, Palić and Schulz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Birte F. Hartung, b.hartung@medizinische-kleintierklinik.de
 Birte F. Hartung
Birte F. Hartung Ralf S. Mueller
Ralf S. Mueller Jana Gauss
Jana Gauss Tamara Weitzer1
Tamara Weitzer1