Effects of nano-selenium on cecum microbial community and metabolomics in chickens challenged with Ochratoxin A
- 1College of Life Science and Resources and Environment, Yichun University, Yichun, China
- 2Engineering Technology Research Center of Jiangxi Universities and Colleges for Selenium Agriculture, Yichun University, Yichun, China
Introduction: Ochratoxin A (OTA) is a widely distributed mycotoxin. Nano-selenium (Nano-Se) is an emerging form of selenium known for its superior bioavailability, remarkable catalytic efficiency, and robust adsorbing capacity. Despite these characteristics, its impact on the microbial community and metabolomics in the cecum of chickens exposed to OTA has been infrequently investigated. This research examined the microbiota and metabolomic alterations linked to OTA in chickens, with or without Nano-Se present.
Methods: A cohort of 80 healthy chickens at the age of 1 day was randomly distributed into four groups of equal numbers, namely the Se cohort (1 mg/kg Nano-Se), the OTA cohort (50 μg/kg OTA), the OTA-Se cohort (50 μg/kg OTA + 1 mg/kg Nano-Se), and the control group. Each chicken group’s caecal microbiome and metabolome were characterized using 16S rRNA sequencing and Liquid chromatography coupled with mass spectrometry (LC–MS) analyses.
Results and discussion: Our results showed that the on day 21, the final body weight was significantly reduced in response to OTA treatments (p < 0.05), the average daily gain in the OTA group was found to be inferior to the other groups (p < 0.01). In addition, Nano-Se supplementation could reduce the jejunum and liver pathological injuries caused by OTA exposure. The 16S rRNA sequencing suggest that Nano-Se supplementation in OTA-exposed chickens mitigated gut microbiota imbalances by promoting beneficial microbiota and suppressing detrimental bacteria. Moreover, untargeted metabolomics revealed a significant difference in caecal metabolites by Nano-Se pretreatment. Collectively, the dataset outcomes highlighted that Nano-Se augmentation regulates intestinal microbiota and associated metabolite profiles, thus influencing critical metabolic pathways, and points to a possible food-additive product.
1. Background
Ochratoxin A (OTA) is produced by various species of Penicillium and Aspergillus fungi (1). OTA is frequently found in various food sources such as coffee, beer, wine, fruits, vegetables, eggs, meat, animal feeds, and grains (2, 3). OTA has a broad distribution and high toxicity, leading to substantial financial losses within livestock and poultry industries. This situation poses challenges in meeting globalized meat and dairy product requirements and compromises food safety. Selenium (Se), an essential mineral for animal health, has garnered considerable interest in recent years (4–6). Organic forms of Se (such as selenomethionine, selenocysteine, and Se-enriched yeast), inorganic forms (such as sodium selenite), and nano forms are currently utilized as poultry feed additives (7). In previous studies, nano-Se has demonstrated superior bioavailability to its inorganic counterparts (8, 9). The intestinal microbiota, an essential constituent of the intestinal barrier, performs a critical function in preserving intestinal homeostasis and safeguarding intestinal tract health (10). The level of diversity exhibited by the gut microbiota indicates the intricate array of species present within it. Various external variables, including dietary intake, medications, and toxins derived from environmental sources, can affect gut microbiota composition. Research has demonstrated that mycotoxins, including but not limited to fumonisin B1, Zearalenone, aflatoxin B1, and Ochratoxin A, can potentially disrupt the balance of gut microbiota by reducing the population of beneficial bacteria and promoting the growth of pathogenic bacteria (11–13). Metabolomics, which analyzes and identifies metabolites in cells and tissues, is essential for disease diagnosis, drug development, and toxicology analysis (14–16). However, limited research exists on the mechanisms of action of mycotoxins like OTA based on metabolomics. This study investigated the impact of Nano-Se supplementation in diet on gut microbiota in OTA-challenged chickens using 16S rRNA analysis. LC–MS provides comprehensive coverage of substances and is considered the most stable and appropriate technique for studying metabolism. The cecal content was examined using an LC–MS metabolomics research strategy. The study screened endogenous metabolites between groups based on bioinformatics and analyzed metabolic pathways to identify potential biomarkers. This approach helped us understand the mechanism of Nano-Se action in OTA-exposed chickens.
2. Methodology
2.1. Materials
The OTA benchmarks utilized were obtained from Pribolab (Immunos, Singapore) and had a purity level exceeding 98%. The specimen of Nano-Se used in this study had a diameter ranging from 30 to 60 nm and a Se purity of 99.5%. The source for Nano-Se was the Bosar Biology cohort (Guangzhou, China). OTA crystals of high purity were solubilized in absolute ethanol at 1 mg per 10 mL. A suspension was created by mixing the resultant solution with 90 mL of sterile sunflower oil. Analytical-grade reagents were used in the experiment.
2.2. Animals
A cohort of 80 neonatal broiler chicks was procured from a commercial hatchery and allowed 3 days for acclimation after transportation to a novel environment. The chickens were provided with unrestricted access to both food and water. The formulation and nutritional content of the basal diet were designed in accordance with the NRC Nutrient Requirement (2012), as detailed in Table 1. The diet for each cohort was prepared concurrently and preserved in airtight containers prior to feeding, and the experiment lasted for 21 days.
2.3. Experimental design and treatment
The chicks were divided randomly into four equal groups, including the Se group (1 mg/kg Nano-Se in diet), the OTA group (50 μg/kg OTA by body weight), the OTA-Se group (50 μg/kg OTA + 1 mg/kg Nano-Se), and a control group. The dosages were recalibrated in alignment with the weight variations in the broilers (17, 18). Upon the experiment’s conclusion, all chickens were euthanized via cervical vessel bisection. Six broiler chickens from each group had their cecal contents frozen using sterile methods, and their liver and jejunum were fixed in 4% (w/v) paraformaldehyde. These tissues were subsequently processed through dehydration, infiltration, embedding, slicing, and staining with hematoxylin and eosin (H&E) in adherence to standard histopathological examination procedures.
2.4. 16S rRNA sequencing and bioinformatics analyses
QIAamp DNA® Stool Mini Kit (Qiagen™, Germany) extracted enteric bacterial DNA from stored cecal contents. The 16S rDNA’s V3-V4 region was subjected to PCR amplification through primers 338F (ACTCCTACGGGAGGCAGCA) and 806R (GGACTACHVGGGTWTCTAAT). Amplicons were then evaluated through 2% gel electrophoresis. After that, AxyPrep DNA Gel Extraction® (Axygen™, United States) retrieved tested amplicons from such gel. The detection and quantification of PCR products were carried out using a QuantiFluor™-ST micro fluorometer (Promega, Madison, WI, United States). Subsequently, MiSeq libraries were prepared for pyrosequencing upon MiSeq® (Illumina™, United States). Sequences that exhibited a similarity of at least 97% were grouped into a single operational taxonomic unit (OTU) after being subjected to cleaning. The bioinformatics analyses were done through Majorbio1 (Shanghai, China). The platform was accessed on October 25, 2022. The study employed Partial Least Squares Discriminant Analysis (PLS-DA), demonstrating β-diversity analyses at the OUT level, utilizing weighted UniFrac indices and Bray-Curtis. Kruskal-Wallis rank sum test probed variations with statistical significance relating to abundance within phylum/genus levels.
2.5. Metabolome specimen processing
A 50 mg specimen of cecal content was precisely weighed and combined with 6 mm grinding beads, 400 mL extracting solution consisting of a 4:1 ratio of methanol to water (v:v), and a standard solution carrying 0.02 mg mL−1 standard (L-2-chlorophenylalanine). The resulting mixture was placed in a 2 mL centrifuging-tube. Following 6 min of tissue-grinding (−10°C/50 Hz), ultrasonic collecting was conducted (30 min/5°C/40 kHz). Subsequently, the specimen was subjected to a temperature of −20°C for 30 min and underwent centrifugation for 15 min at 9,400×g at 4°C. A volume of 20 μL (supernatant) from each specimen was transferred using a pipette into a specimen vial that contained an inner cannula for computerized assessment. One quality control (QC) specimen injection was interspersed after every six specimens. The specimens underwent analysis via LC–MS, with six biological replicates per group.
2.6. LC–MS detection of metabolites
LC–MS analysis was conducted using the UHPLC-Q Exactive® platform (ThermoFisher-Scientific™, China). Chromatography parameters were set as follows: the column used was HSS T3 with dimensions of 100 mm × 2.1 mm inner diameter and a particle size of 1.8 μm. Mobile phase A consisted of 5% acetonitrile and 95% water with 0.1% formic acid, and mobile phase B contained 47.5% isopropyl alcohol, 47.5% acetonitrile, and 5% water with 0.1% formic acid. The volumetric flow rate was 0.40 mL/min, the injection volume was 2 μL, and the column temperature was 40°C. MS technique utilized electrospray ionization to obtain mass spectrum signals for specimens. The mass scanning range was set to 70–1,050 m/z, and both negative and positive ion scanning modes were employed. The experimental conditions encompassed the following operational parameters: a negative ion voltage of 2,800 V, positive ion voltage of 3,500 V, sheath gas pressure at 40 psi, auxiliary heating gas pressure at 10 psi, ion source heating temperature at 400°C, cyclic collision energy of 20-40-60 V, a primary mass spectrometry(MS1) resolution of 70,000, together with secondary mass spectrometry (MS2) resolution of 17,500.
2.7. Data preprocessing and database searching
Progenesis QI software processed raw data, standardizing specimen response intensities from mass spectrum peaks to minimize errors arising from specimen preparation and instrument instability. Specimens with a relative standard deviation (RSD) > 30% were excluded from assessments. The eventual data matrix for further investigation was obtained by performing a logarithmic transformation on the variables. Metabolomic data were acquired by integrating MS1 and MS2 spectra through publicly available metabolic database datasets, namely HMDB2 and Metlin.3
2.8. Orthogonal projections to latent structures discriminant analysis (OPLS-DA)
OPLS-DA gained insight into metabolic variations both within and between specimen groups. Metabolites that exhibited significant differences were chosen depending upon their Variable Important in Projection (VIP) scores and p-values obtained from Student’s t-test. Metabolites exhibiting a VIP score of ≥1 and p < 0.05 were assumed to have statistical significance. Subsequently, differential metabolites were identified and classified through the Kyoto Encyclopedia of Genes and Genomes (KEGG)4 to determine the impacted metabolic pathways. KEGG pathways were deemed to be significantly enriched if they had p < 0.05.
2.9. Statistical analyses
Experimental results were reported in the form of mean values along with their corresponding standard deviations. The anticoccidial effects were analyzed using the One-way ANOVA test. The statistical methods for T-/Wilcoxon rank sum−/Tukey-tests evaluated disparities across all four treatments. p < 0.05 was deemed to confer statistical significance.
3. Results
3.1. Growth performance
Table 2 presents the impact of OTA and Nano-Se supplementation on the growth performance of broilers. There were no statistically significant differences (p > 0.10) observed in the initial body weight (IBW) among the groups. However, on day 21, the final body weight (FBW) was significantly reduced in response to OTA treatments (p < 0.05). Additionally, the average daily gain (ADG) in the OTA group was found to be inferior to the other groups (p < 0.01). However, the average daily feed intake (ADFI) remained consistent across all four groups. Pertaining to the feed conversion ratio (FCR), a decrease in FCR was noted with the addition of Nano-Se from day 1 to 21 (p < 0.05).
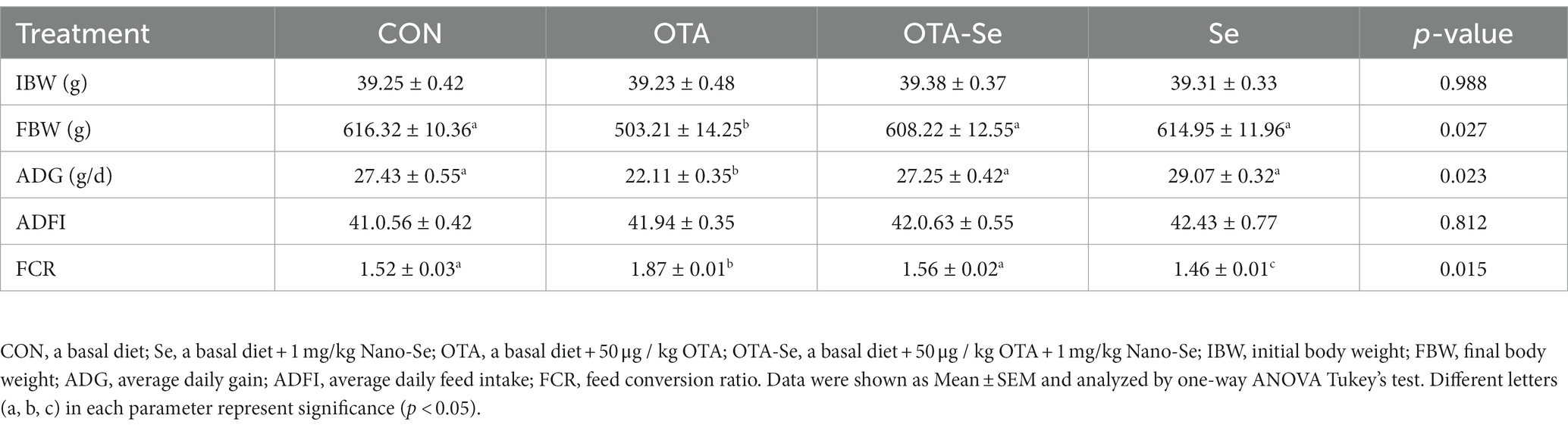
Table 2. The effect of OTA and Nano-Se supplementation on growth performance of broilers from 1 to 21 d.
3.2. Histopathological changes in the intestinal and liver
The jejunum’s morphological features were normal in both the control and the Se treatment groups, exhibiting a complete intestinal mucous membrane structure. The intestinal villi were clear and well-aligned, without significant abnormal changes (Figures 1A,D). Conversely, the jejunum of broilers in the OTA group was notably distended, with extensive detachment of the intestinal mucosa and observable structural damage (Figure 1B). Following pretreatment with Nano-Se, the detachment of the intestinal mucosa in the broiler jejunum was mitigated, and no discernible changes were seen in the lamina propria (Figure 1C).
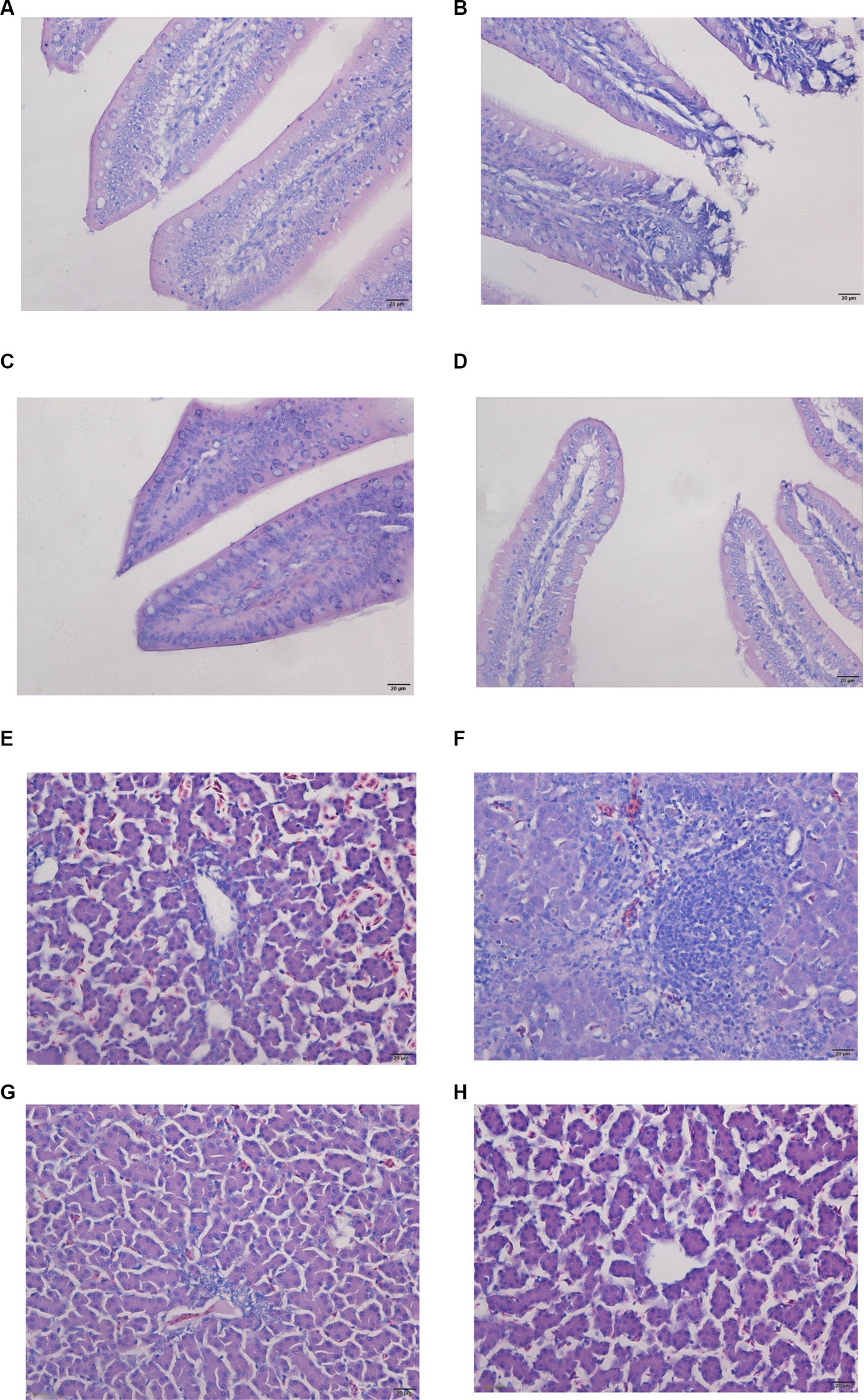
Figure 1. Pathological changes in the jejunum and liver were detected by tissue section H&E staining. Images were taken at magnifications of 400×. (A,E) Control group; (B,F) OTA group; (C,G) OTA-Se group; (D,H) Se group.
HE staining further clarified the condition of the liver tissue. In the chickens from the control and Se groups, no signs of inflammation, congestion, bleeding, necrosis, or exudation were observed (Figures 1E,H). In the OTA group, the liver displayed signs of inflammation, most notably due to OTA-triggered extensive infiltration of inflammatory cells, particularly a high concentration of lymphocytes and eosinophils (Figure 1F). Minimal pathomorphological alterations were detected in the OTA-Se group (Figure 1G). Collectively, these findings indicate that Nano-Se supplementation provided a protective effect against OTA-induced hepatic injury in chickens.
3.3. DNA sequence data
High-throughput sequencing analysis for chicken cecal contents was conducted using the Illumina MiSeq platform. After QC, 640,107 valid sequences were obtained, averaging 40,006 sequences per specimen. Among these high-quality sequences, approximately 99.95% were longer than 400 bp, with the majority ranging between 401 and 420 bp (Figure 2A).

Figure 2. (A) Fragment length distribution of sequences from each sample after merging and trimming. (B) The rarefaction curves plotted to visualize the OTUs of each group. The red line represents the control group, the blue line the OTA group, the green line the OTA-Se group, and the purple line the Se group. (C) Rank abundance curves of gut microbiota at the OUT level in the four groups.
Based on a 97% similarity threshold, all effective reads were grouped into OTUs. A plateau was reached by the rarefaction curves for the four groups, suggesting that sequencing depth employed within such an investigation sufficed for assessing specimen-derived microbial population makeups and accurately depicting faecal microbial colonies (Figure 2B). The microbiota composition’s richness and evenness were comparable among the four groups by the rank abundance analysis within the OTU level (Figure 2C). The adequacy of sampling efforts was supported by both rarefaction and rank abundance curves for each specimen.
3.4. Effects of Ochratoxin A and Nano-Se on the gut microbial composition
Alpha diversity measures the number of species and their diversity within a particular specimen. Alpha diversity can be evaluated using different indices. The indices mentioned above comprise ACE, Chao1, Simpson, and Shannon. The ACE and Chao1 indices quantify the abundance of species, which refers to overall species quantities present within a given specimen. On the other hand, the Simpson and Shannon indices assess species’ diversity, considering the number of species and their relative abundance within the specimen. The alpha diversity analysis indicated that there were no statistically significant variations within the Simpson index, Shannon index, Chao1 index, and ACE index across all four therapeutic cohorts (p > 0.05) (Table 3).
Beta diversity assessed variations within species complexity within specimens, as demonstrated by a weighted Unifrac index. Regarding β-diversity, PLS-DA revealed that the similarity in species diversity significantly differed when chickens were given OTA and Nano-Se (Figure 3A). A distinct separation between the groups was observed within the chicken’s cecal flora, indicating that the structure of the chicken’s cecal flora had been altered.
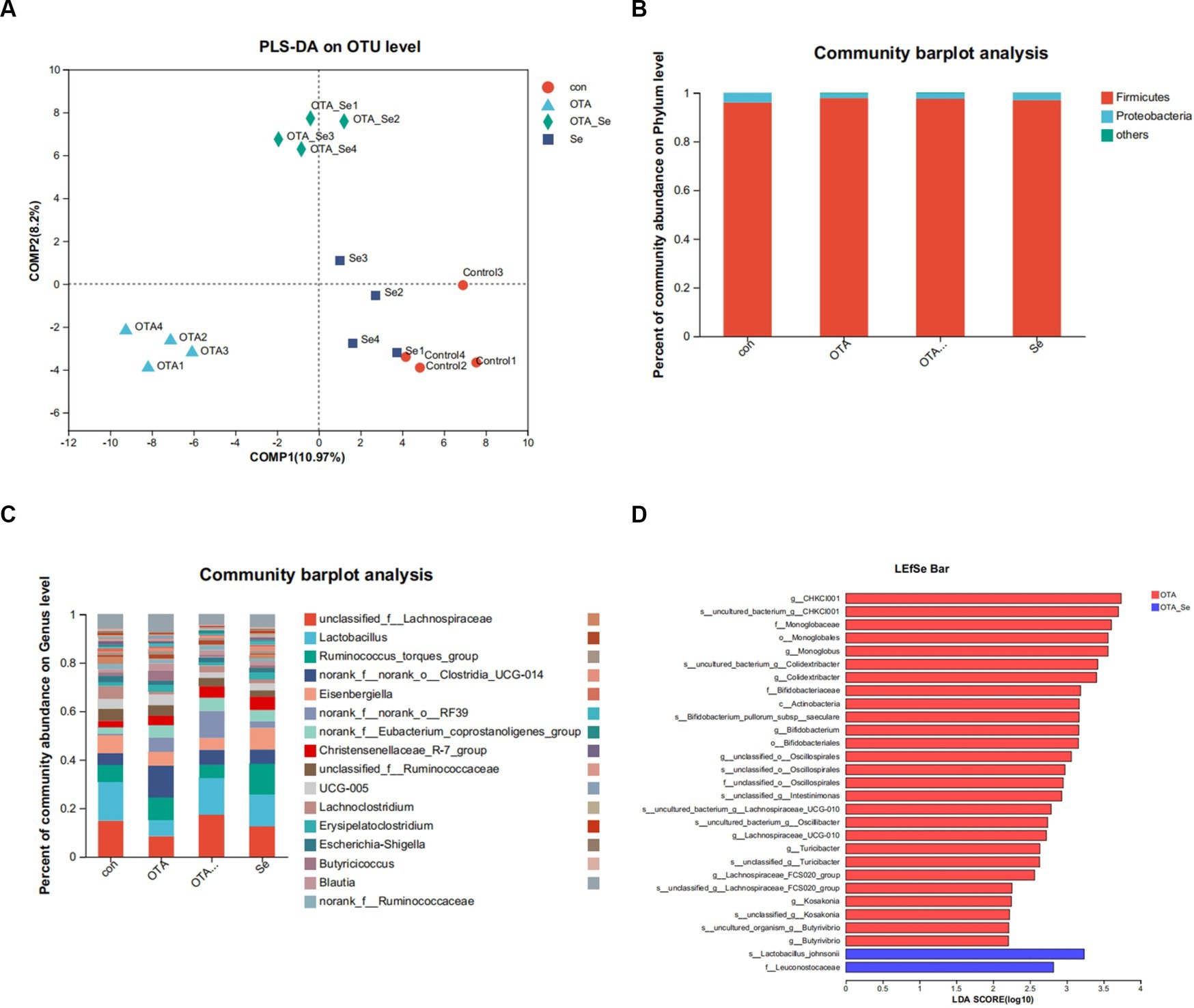
Figure 3. (A) The impact of OTA and Nano-Se on the structure of the microbial community in the cecum of chickens evaluated through PLS-DA. Each data point represents an individual sample, and data points that share the same colour belong to the same group. A superior classification model is suggested when the intra-group distance is minimized and the inter-group distance is maximized. (B) Relative abundance of phyla in gut microbiota. (C) Relative abundance of genera in gut microbiota. (D) LEfSe bar based on phylum to genus level (LDA > 2). p < 0.05.
As illustrated in Figure 3B, the primary phylum of broiler gut microbiota was Firmicutes, followed by Proteobacteria. Relative abundance for Firmicutes within the OTA cohort was higher than the control cohort and similar to the OTA-Se group.
The predominant genera within the gut included Lactobacillus, norank_f__norank_o__Clostridia_UCG-014, unclassified_f__Lachnospiraceae, and Ruminococcus_torques_group (Figure 3C). Among these genera, the relative abundance of Lachnospiraceae was highest within the OTA-Se group. Conversely, the relative abundance of Clostridia was highest within the OTA group.
A supervised specimens comparison was conducted using LEfSe and logarithmic linear discriminant analysis (LDA). The LEfSe results, displayed in Figure 3D, revealed that compared to the control group, the dominant bacteria within the OTA-Se cohort were s__Lactobacillus_johnsonii and f__Leuconostocaceae at phylum to genus levels.
3.5. Metabolome analysis
Quality control specimens assessed consistency/reproducibility for investigational methods, with arbitrary injections performed during specimen injection. More than 70% of RSD values for the QC specimens were less than 30%, suggesting datasets collected through such investigational methods exhibited stability.
To explore the changes in gut metabolites induced by dietary OTA and Nano-Se supplementation, an untargeted metabolomics approach was applied to analyze the metabolite profiles of the cecal content. OPLS-DA was utilized to discern distinct metabolic patterns, as illustrated in Figures 4A,C. This analysis revealed separate metabolic profiles between the OTA and Control groups and between the OTA-Se and OTA cohorts. OPLS-DA allowed for filtering irrelevant variations, enhancing the ability to identify substantial differential metabolites among the different groups. The closeness of duplicate points for the two sample groups indicates good data repeatability.

Figure 4. OPLS-DA model plot for the comparison group Control vs OTA: (A) score diagram; (B) model validation diagram; OPLS-DA model plot for the comparison group OTA vs OTA-Se; (C) score diagram; (D) model validation diagram.
OPLS-DA model validation was carried out within this investigation to avoid overfitting. Clear separations between OTA (blue dots) and the Control control (green dots) groups (R2 = 0.9956, Q2 = −0.0026, Figure 4B) and between OTA (blue dots) and OTA-Se (yellow dots) groups (R2 = 0.9967, Q2 = 0.0661, Figure 4D) were demonstrated by the OPLS-DA models. The higher the R2 and Q2 values, the greater the theoretical reliability for this model. The acquired data suggest that both models demonstrate favourable stability and no overfitting. The material is confirmed to be sufficiently reproducible and appropriate for subsequent qualitative and quantitative assay validations, as demonstrated by these results.
3.6. Differential metabolite analysis
Metabolites were selected differentially by utilizing VIP value (VIP > 1) from the OPLS-DA model together with p value (p < 0.05) from stand-alone specimen t-test from all identified metabolites. The chosen differential metabolites served as marker variables to distinguish across cohorts. Compared to the control cohort, the OTA cohort exhibited an initial identification of 515 differential metabolites, with 259 within positive mode and 256 within negative ion mode. Among these metabolites, 276 were up-regulated, while 239 were downregulated (Figure 5A). Upon comparison for OTA and OTA-Se groups, a collective of 514 distinct metabolites (277 within positive mode and 237 within negative ion mode) were detected through both modes of analysis (Figure 5B). Among the recognized metabolites, 214 displayed a significant decrease, while 300 exhibited an increase.
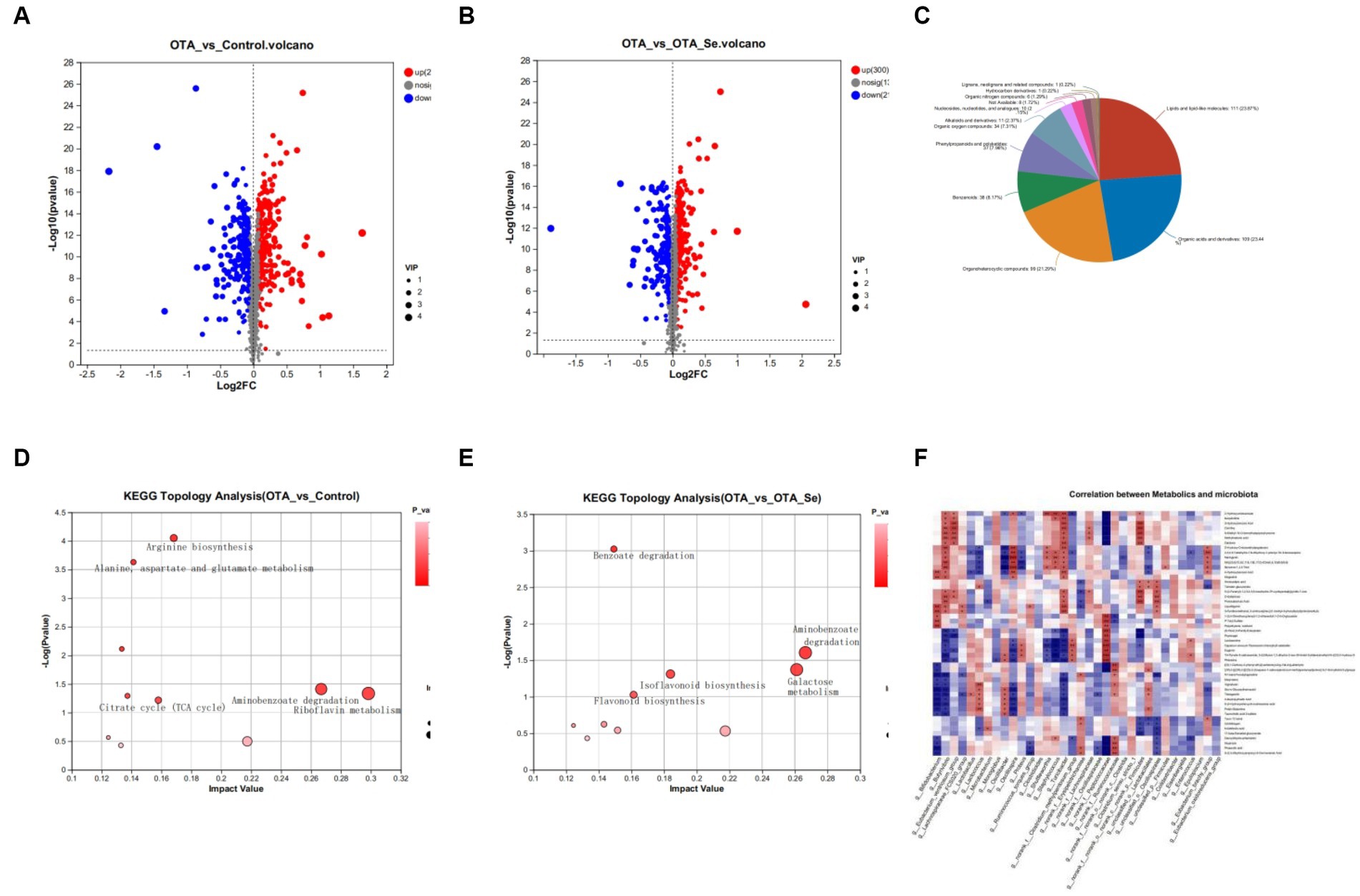
Figure 5. (A) Differential metabolite expression analysis to compare the control group with the OTA group. (B) The volcano plots exhibited distinct metabolites in the OTA-Se group compared to the OTA group. The red colour is indicative of an elevation in the content of metabolites, while blue signifies a reduction in metabolite content. The grey colour represents the absence of any noteworthy difference. (C) Classification of differential metabolites in the HMDB database. (D) Bubble diagram showing the KEGG enrichment analysis. Con vs OTA. (E) The KEGG enrichment analysis OTA vs OTA-Se. (F) Spearman’s rank correlation between cecum metabolites and associated gut microbiota in OTA and OTA-Se group. Connections depicted in red signify a direct positive correlation, whereas those in blue represent an inverse negative correlation. The statistical significance of the data is calculated using a two-tailed Wilcoxon rank-sum test to determine the p-values. *p < 0.05, **p < 0.01.
The differential metabolites identified in this study were annotated within HMDB to be classified. The results showed that lipids/lipid-like molecules constituted 23.87% of differentially aggregated metabolites within OTA and OTA-Se cohorts. Organic acids/derivatives were the second most abundant cohort at 23.44%, followed by organoheterocyclic compounds at 21.29%, benzenoids at 8.17%, phenylpropanoids, and polyketides at 7.96%, organic oxygen compounds at 7.31%, alkaloids and derivatives at 2.37%, nucleosides, nucleotides, and analogues at 2.15%, organic nitrogen compounds at 1.29%, and the remaining compounds at 0.44% (Figure 5C).
Further analysis of identified metabolites was conducted using the KEGG analysis to investigate potential pathways impacted by OTA and Nano-Se interventions. The top 10 KEGG metabolic pathways with significant enrichment for different metabolites were found using the KEGG database. Compared to the control cohort, pathways with the highest impact values within the OTA cohort included Riboflavin metabolism, Aminobenzoate degradation, Limonene and pinene degradation, Arginine biosynthesis, and Alanine, aspartate, and glutamate metabolism. Among the metabolites implicated within these pathways, Riboflavin, Benzoate, Oxoglutaric acid, and L-Glutamine were notably decreased within the OTA cohort compared to the control group. Conversely, Benzoic Acid, Gallic Acid, and Citrulline increased within the OTA cohort relative to the control cohort (Figure 5D).
When comparing the OTA cohort with the OTA-Se cohort, the distinct metabolic pathways within intestinal contents included Aminobenzoate degradation, Galactose metabolism, Isoflavonoid biosynthesis, and Flavonoid biosynthesis. Among the metabolites participating in these metabolic pathways, Protocatechuic Acid, 4-Hydroxybenzoic Acid, D-Galactose, Daidzein, Liquiritigenin, and Naringenin were highly raised within the OTA-Se cohort in comparison with the OTA group. Conversely, Galactonic acid and Myo-Inositol were reduced within the OTA-Se cohort compared to the OTA cohort (Figure 5E).
The correlation between different microbial genera and the modified cecal metabolites was determined using Spearman’s correlation coefficient values in a correlation analysis (Figure 5F). The present study has revealed associations having considerable statistical significance (p < 0.05, r > 0.70) across particular bacteria present within the cecum and distinctive metabolites in comparing OTA vs OTA-Se. Two distinct metabolites, Daidzein and Liquiritigenin, involved within the Isoflavonoid biosynthesis pathway, exhibited significant positive correlations with Firmicutes, Turicibacter, and Butyrivibrio. Furthermore, Protocatechuic Acid, present within Aminobenzoate degradation and Benzoate degradation pathways, demonstrated a positive correlation with Oscillospirales, Firmicutes, and Butyrivibrio while revealing a negative correlation with Erysipelotrichaceae. Although the direct metabolism of these metabolic products by gut microbiota remains conclusively determined, the results highlight a close interplay between gut microbial species and metabolites, suggesting that Nano-Se supplementation may have induced notable alterations in gut microbiota, ultimately leading to significant shifts in host metabolite abundance.
4. Discussion
OTA (C20H18ClNO6; molecular weight:403.8) is a predominant mycotoxin originating from various Penicillium and Aspergillus species. Its pervasive presence and contamination of crops and foodstuffs pose significant health threats to humans and animals (2). Se, an essential trace element for mammals, including humans, exhibits a strong correlation between its physiological functions and disease prevalence (19). Previous research has highlighted Se′s crucial role in diminishing the damage inflicted on animals by mycotoxins (20–22). In the current investigation, chickens fed with OTA-contaminated feed displayed a marked decrease in FBW, ADG and a notable elevation in FCR findings congruent with prior studies (23, 24). Additionally, existing literature has demonstrated that even minor exposure to OTA in animals can provoke pathological and functional alterations in the liver and intestines (25–30). Our findings align with these earlier studies, indicating that Nano-Se supplementation can protect chickens against OTA-induced harm. Gut microbiota, recognized as a critical and responsive indicator of gastrointestinal health, is inevitably influenced by environmental contaminants, including mycotoxins such as OTA. In recent years, a growing body of evidence has demonstrated that OTA has a detrimental effect on the integrity of the intestinal barrier, induces oxidative stress and inflammation, and lowers the abundance of beneficial microorganisms (31–35). OTA induces alterations within gut microbiota composition, affecting the phylum, genus, and species levels (36). Literature has documented a considerable increase in Bacteroides enrichment in mice exposed to OTA (37). Research on broilers and ducks has indicated that OTA lowers abundance/diversity for cecal microbiota, triggering intestinal tight junction damage (38).
Recently, the scientific community has exhibited increased curiosity in understanding the modulatory effects of Se on the intestinal microbiota. When exposed to selenium-enriched yeast, broilers subjected to OTA experienced alterations in the diversity of their caecal microbiota (30). Zhang et al., while analyzing variations in Se distribution in the Enshi region of Hubei, China, noted a heightened prevalence of Bacteroidetes in areas with higher Se concentrations as opposed to regions with lower Se concentrations (39). Their findings revealed that dietary Se supplementation can influence the gut’s immunological reactions and barrier functionality. This is linked to the alteration of gut microbiota via faecal microbiota transplantation procedures (40). Furthermore, dietary Se supplementation noticeably enhanced mice microbiota diversity (41). Yeast Se and selenium-enriched yeast culture supplementation contributed to intestinal homeostasis by elevating the relative abundance of anti-inflammatory-associated microbiota, thereby mitigating damage caused by S. enteritidis infection (42). The research findings indicate that adding dietary Se Nanoparticles (SeNPs) can effectively modulate the gut microbiota and its metabolic processes, reducing the severity of acute toxicity caused by diquat (43). Consuming SeNPs exceeding nutritional requirements may enhance gut microbiota composition, thereby protecting against intestinal dysfunctions (44). An intermediate concentration of SeNPs at 0.9 mg/kg demonstrated the highest efficacy in enhancing gut health by promoting beneficial bacteria, including Faecalibacterium and Lactobacillus (45).
The present study determined that the Clostridia populations increased while Lactobacillus and Lachnospiraceae abundance decreased within the OTA group, but these levels normalized following Nano-Se supplementation. Clostridium, including species like Clostridium perfringens, Clostridium botulinum, and Clostridium tetani, can generate exotoxins and are associated with numerous diseases (46). Research has revealed a positive correlation between Clostridium and monocyte chemoattractant protein-1 (MCP-1), a pro-inflammatory cytokine (47). The level of Clostridium bacteria was found to be strongly correlated with the severity of inflammatory bowel disease (IBD) (48) within the gut microbiota of chickens exposed to OTA, oral administration of aflatoxin B1 (AFB1) diminished the alpha diversity of gut microbiota in broilers and augmented the abundance of several detrimental bacteria, including Clostridium (49). In addition, AFB1 significantly increased Bacteroidales of Bacteroidetes and Clostridiales of Firmicutes in rats, while Lactococcus sp., Streptococcus sp., and Lactobacillales from Firmicutes decreased (50). Another study found that Deoxynivalenol (DON)treatment increased the abundance of Clostridiales within gut microbiota (51, 52). In other studies, the OTA cohort had significantly higher levels of Aerococcus, Romboutsia, and Clostridium sensu stricto 1. However, Tibetan kefir reduced the abundance of these three bacteria (53). These findings imply that mycotoxin alters intestinal microbiota makeup and encourages the growth of harmful bacteria, producing toxic effects.
Furthermore, Lactobacillus abundance increased considerably within the OTA-Se cohort compared to other groups. It is well-established that Lactobacillus has pivotal parts in keeping healthy together with preventing and treating diseases (54, 55). With numerous beneficial effects, Lactobacillus produces lactate, which can elevate butyrate production in faeces (56). Lactobacillus johnsonii has exhibited anti-obesity properties by inhibiting gut inflammation and maintaining the integrity of the mucosal barrier (57). The high-energy diet feeding cohort exhibited a greater abundance of Lactobacillaceae species, specifically Lactobacillus reuteri and Lactobacillus johnsonii, within the upper intestine (duodenum and cecum). This colonization led to protective effects on the mucosal lining and reduced inflammation (58). The investigation concerning OTA influence on intestinal microbiota revealed decreased levels of advantageous microorganisms such as bifidobacteria and Lactobacillus. This decrease indicates that OTA altered the balance of microbiota, which could result in compromised immunity (59). Mice administered with OTA through intragastric means exhibited a reduction within the proportionate prevalence of Bifidobacterium spp. and Lactobacillus spp. (60). Furthermore, Zearalenone elicited an increase in Desulfovibrio and a decrease in Lactobacillus abundance within the colon tissue of mice (61). The combination of Bacillus cereus BC7 and Lactobacilli strain positively impacted intestinal inflammatory responses and microbiota disturbances induced by Zearalenone (62, 63). The results indicated Lactobacillus’s increased resistance to OTA and its potential role within the OTA detoxification process.
The bacterial family, Lachnospiraceae, can convert lactate and acetate into butyrate. This conversion can be achieved using different enzymatic pathways, such as butyryl-CoA or acetate CoA transferase pathways or the butyrate kinase pathway (64) in addition to its butyrate production capabilities, Lachnospiraceae is known to participate in the biosynthesis of vitamin B12 and exhibit the potential to inhibit the colonization of Clostridium difficile within the gastrointestinal tract (65). The family Lachnospiraceae has been documented to have crucial functions in acquiring dietary glycans, synthesizing advantageous metabolites, enhancing immunity, and facilitating neurodevelopment in animals (66). Studies have shown that administering DON to pigs at a dose of 2.89 mg/kg resulted in various alterations within the cecal microbiota. This ushered a major decrease within levels of unclassified f_Lachnospiraceae, which was found to have a positive correlation with the average daily feed intake (67). The administration of OTA to mice, followed by the supplementation of Tibetan kefir, resulted in a significant increase in the abundance of bacterial populations that develop short-chain fatty acids (SCFAs), such as Lachnospiraceae, Ruminococcus, and Blautia. SCFAs are synthesized through intestinal microbiota via anaerobic fermentation. These compounds enhance the integrity of the intestinal barrier and suppress inflammatory reactions (68). Given the positive effects of Se on chickens’ gut microbiota composition, the current results suggest that Se could enhance gut health and serve as a candidate additive for promoting intestinal health in OTA-exposed chickens. However, further investigation is required to elucidate the mechanism through which Se promotes the growth of intestinal microorganisms.
Metabolomics represents a novel analytical approach for detecting changes in small endogenous metabolites. External factors or internal disruptions may influence these alterations, and metabolomics can be employed to diagnose and predict the underlying mechanisms of such changes (69, 70). Multiple research investigations have documented the impact of Se consumption on metabolic processes (43, 71, 72). 515 distinct metabolites within the cecum of broiler chickens were identified through metabolomics analysis after an OTA challenge. KEGG analysis revealed that these metabolites are enriched in riboflavin metabolism, aminobenzoate degradation, limonene and pinene degradation, arginine biosynthesis, and alanine, aspartate, and glutamate metabolism.
Riboflavin metabolism is intricately linked to the energy metabolism of mitochondria. Riboflavin is a precursor for two coenzymes, flavin adenine dinucleotide and flavin mononucleotide. These coenzymes are pivotal in several biological redox reactions, including electron transport chain, fatty acid oxidation, amino acid degradation, and tricarboxylic acid cycle (73–76). Numerous studies have demonstrated riboflavin’s ability to preserve the intestinal tract’s structural integrity and promote optimal gastrointestinal performance in various animal species (77). Riboflavin can regulate various pathways that are involved in the maintenance of gastrointestinal function. Riboflavin has been found to increase iron absorption and play a role within the antioxidant system. In vitro and in vivo investigations demonstrated that riboflavin deficiency can lead to oxidative stress within the intestines (78). Furthermore, studies have demonstrated that the level of riboflavin in an organism can affect the composition of its gut microbiome, and a riboflavin deficiency can lead to an imbalance within gut microflora (79–84). Hence, the diminished concentrations of riboflavin indicate that OTA could potentially induce harm to the intestines by altering riboflavin metabolism.
The anti-inflammatory properties of limonene and pinene have been extensively documented in scientific literature. Limonene, a naturally occurring monocyclic terpene hydrocarbon in citrus fruits, exhibits anti-inflammatory characteristics by selectively binding to A2A receptors and reducing inflammation (85). α-pinene, a bicyclic monoterpene hydrocarbon, is commonly present within essential oils of coniferous trees and is a major constituent of volatile organic compounds derived from diverse tree species. This compound exhibits a range of biological activities, including anti-inflammatory properties (86). Studies have demonstrated that α-pinene exhibits anti-inflammatory properties and holds potential as an alternative therapeutic intervention for inflammation (87). Based on the observed properties, it is a reasonable hypothesis that the increased activity of limonene and pinene degradation pathways within the OTA cohort could potentially reduce their concentrations, thereby mitigating the inflammatory reaction induced by OTA. However, additional investigation is required to comprehend the fundamental mechanisms.
L-Arginine is integral to numerous processes, including the ornithine cycle, protein synthesis, nitric oxide generation, oxidative stress management, and immune response regulation. Metabolomic alterations in cells exposed to ZEA revealed a significant enrichment of arginine biosynthesis-related differential metabolites. Subsequent research demonstrated that L-arginine supplementation considerably enhanced cell viability and diminished reactive oxygen species (ROS) production levels in Zearalenone-exposed cells (88). Moreover, OTA notably reduced the levels of differential metabolites, such as L-Glutamine, associated with arginine biosynthesis compared to the control group. L-Glutamine, a nonessential amino acid, is a critical energy source and an active free radical scavenger for enterocyte and lymphocyte cells. The intestine can utilize approximately 30% of total L-Glutamine, emphasizing its importance as a key nutrient for intestinal health (89). L-Glutamine has been shown to stimulate cell proliferation and differentiation, playing a crucial role in supporting the growth and development of the gastrointestinal tract (90). L-Glutamine supplementation has been confirmed to preserve intestinal tissue integrity and bolster the small intestine mucosa barrier (91). Both animal and clinical studies suggest that L-Glutamine offers protection against stress, pathogenic organism invasion, infection, and immunological challenges in vitro and in vivo (92, 93).
Based on these findings, it can be speculated that the downregulation of arginase-related pathways results in decreased arginine metabolism within the examined model. The outcomes indicate that OTA significantly impacts relevant metabolic pathways through its effects on metabolites, ultimately leading to detrimental consequences on nutrient metabolism and intestinal morphology.
KEGG analysis comparing OTA and OTA-Se groups revealed that Nano-Se could influence aminobenzoate and benzoate degradation by elevating 4-Hydroxybenzoic Acid, Protocatechuic Acid levels. This aminobenzoate degradation pathway may promote tryptophan metabolism and benzoate breakdown (94). Protocatechuic acid, a primary metabolite of complex polyphenols, exhibits numerous biological activities, encompassing antioxidant, anti-inflammatory, antibacterial, and antiapoptotic properties (95–97). Prior research has reported protocatechuic acid’s potential to attenuate intestinal damage (98). A study demonstrated that the inclusion of protocatechuic acid within yellow-feathered broilers’ diet significantly improved their growth performance, antioxidant capacity, gut immune function, and gut microbiota structure (99). Research utilizing piglets challenged with LPS revealed that protocatechuic acid mitigated oxidative stress, inflammation, intestinal barrier impairment, and gut flora disruption (100). Furthermore, protocatechuic acid demonstrates promise as a detoxifying agent against Fumonisin B1 and AFB1 (101, 102).
Additionally, upregulation of Daidzein, Liquiritigenin, and Naringenin within the OTA-Se group was observed, enhancing Isoflavonoid biosynthesis and Flavonoid biosynthesis. Flavonoids are recognized for their antioxidant and anti-inflammatory properties (103). Certain flavonoids, such as naringin and luteolin, have been identified to alleviate intestinal inflammation (104, 105). It has been reported that plant-derived flavonoids can modulate intestinal flora composition and maintain intestinal and organism health (106). Daidzein, recognized as a safe and natural alternative estrogen-like compound, has been increasingly investigated in scientific research. Studies conducted in avian science have revealed that daidzein exhibits potential as a dietary supplement for augmenting reproductive organ development, eggshell quality, and laying performance in laying hens during the final stages of the laying cycle (107, 108). The Daidzein and CH mixture significantly enhanced SOD and GSH-px activity in plasma (109). Slackia sp., D-G6, a bacterium capable of detoxifying deoxynivalenol, was discovered within the intestinal tract of chickens. Besides deoxynivalenol detoxification, the D-G6 enzyme also catalyzes the conversion of daidzein to equol. This compound exhibits potent estrogenic effects and effectively mitigates the risk of estrogen-dependent and age-related ailments (110). The flavonoid Liquiritigenin exhibits antiapoptotic, anti-inflammatory, antioxidant, and anti-fibrotic pharmacological properties (111). Naringenin, a naturally occurring flavonoid, exhibits antioxidative, antiproliferative, anti-inflammatory, and antimutagenic characteristics (112). Previous studies have demonstrated that naringenin provides cellular protection against oxidative damage induced by arsenic (113).
The gastrointestinal tract serves as the primary location of exposure to OTA and is pivotal for OTA-induced toxicity. The primary manifestation of OTA’s enteric toxicity is the induction of oxidative stress and inflammatory response, and the impact of OTA intoxication on gene expression of signalling pathway markers related to inflammation and inflammatory cytokines was more pronounced within the gut compared to the kidney of piglets (114). Research has demonstrated that OTA causes oxidative damage to the intestinal tract of broiler chickens, leading to pro-inflammatory cytokines elevation and anti-inflammatory cytokines reduction within the cecal tissues of these birds (40, 115). Studies conducted in vitro and in vivo have shown that OTA exposure leads to an overproduction of free radicals, which disrupts the equilibrium between antioxidant and oxidant systems and impairs the function and structure of cell membranes (11). The findings indicate that subchronic exposure to a low dosage of OTA for 30 days significantly impacts the immune response and antioxidant self-defence mechanisms within the gut and kidney. Furthermore, there have been reports linking disruption of intestinal microbiota to intestinal damage, inflammation, and compromised barrier integrity (116). As a result, the elevation of these anti-inflammatory and antioxidant metabolites(Protocatechuic Acid, Daidzein, Liquiritigenin, and Naringenin) due to Se intervention may bolster the anti-inflammatory and antioxidant capabilities of host tissue. Se supplementation in OTA-exposed chickens can safeguard the intestine by modulating metabolites. Future studies should further verify these findings and delve into the underlying mechanism of Se in enhancing gut health more comprehensively using various experimental models. Moreover, the relationship between gut microbiota and the gut microbiome warrants further exploration.
5. Conclusion
In summary, this investigation probed the protective influence of Nano-Se in OTA-fed chickens. Results from the analysis of 16S rRNA sequencing and untargeted metabolomics indicated that Nano-Se could modulate the intestinal microbiota community and metabolites. Supplementation of Nano-Se has the potential to mitigate alterations in gut microbiota caused by OTA exposure through the promotion of favourable microbial populations and suppression of pathogenic bacteria. Furthermore, Nano-Se treatment also influenced the contents of cecal metabolites. This work offers a novel approach to investigating the protective role of Nano-Se within enterotoxicity induced by OTA in chickens. To the best of our knowledge, this is the first research exploring OTA’s toxic impact on poultry, especially concerning gut microbiota and intestinal metabolic responses. Our results contribute to a deeper understanding of how gut microbiota is involved in OTA-induced intestinal barrier disruptions and suggest potential innovative preventive measures against such dysfunctions. Notably, our investigation was limited to the effects of a single mycotoxin on chickens, even though myriad mycotoxins are prevalent in livestock farming. Therefore, more comprehensive studies are warranted to elucidate the impacts of diverse mycotoxins on gut microbiota and the counteracting capabilities of Nano-Se.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA934281.
Ethics statement
The animal study was approved by the Animal Care and Use Committee of Yichun University, Yichun, China. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
MF and WH were responsible for the study conception and design. MF and BL revised the manuscript. MF, WH, and BL were involved in drafting the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Science and Technology Project of the Jiangxi Provincial Department of Education (GJJ211632) and the Initial Scientific Research Fund of Yichun University (3360119046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
References
1. Liuzzi, VC, Fanelli, F, Tristezza, M, Haidukowski, M, Picardi, E, Manzari, C, et al. Transcriptional analysis of Acinetobacter sp. neg1 capable of degrading Ochratoxin A. Front Microbiol. (2017) 9:2162. doi: 10.3389/fmicb.2016.02162
2. Kőszegi, T, and Poór, M. Ochratoxin A: molecular interactions, mechanisms of toxicity and prevention at the molecular level. Toxins (Basel). (2016) 15:111. doi: 10.3390/toxins8040111
3. Smith, MC, Madec, S, Coton, E, and Hymery, N. Natural co-occurrence of mycotoxins in foods and feeds and their in vitro combined toxicological effects. Toxins (Basel). (2016) 8:94. doi: 10.3390/toxins8040094
4. Dalia, AM, Loh, TC, Sazili, AQ, Jahromi, MF, and Samsudin, AA. Effects of vitamin E, inorganic selenium, bacterial organic selenium, and their combinations on immunity response in broiler chickens. BMC Vet Res. (2018) 14:249. doi: 10.1186/s12917-018-1578-x
5. Li, J, Shen, B, Nie, S, Duan, Z, and Chen, K. A combination of selenium and polysaccharides: promising therapeutic potential. Carbohydr Polym. (2019) 15:163–73. doi: 10.1016/j.carbpol.2018.10.088
6. Xia, IF, Cheung, JS, Wu, M, Wong, KS, Kong, HK, Zheng, XT, et al. Dietary chitosan-selenium nanoparticle (CTS-SeNP) enhance immunity and disease resistance in zebrafish. Fish Shellfish Immunol. (2019) 87:449–59. doi: 10.1016/j.fsi.2019.01.042
7. Marković, R, Ćirić, J, Starčević, M, Šefer, D, and Baltić, MŽ. Effects of selenium source and level in diet on glutathione peroxidase activity, tissue selenium distribution, and growth performance in poultry. Anim Health Res Rev. (2018) 19:166–76. doi: 10.1017/S1466252318000105
8. Dukare, S, Mir, NA, Mandal, AB, Dev, K, Begum, J, Tyagi, PK, et al. Comparative study on the responses of broiler chicken to hot and humid environment supplemented with different dietary levels and sources of selenium. J Therm Biol. (2020) 88:102515. doi: 10.1016/j.jtherbio.2020.102515
9. Sheiha, AM, Abdelnour, SA, Abd El-Hack, ME, Khafaga, AF, Metwally, KA, Ajarem, JS, et al. Effects of dietary biological or chemical-synthesized nano-selenium supplementation on growing rabbits exposed to thermal stress. Animals (Basel). (2020) 10:430. doi: 10.3390/ani10030430
10. Yang, Z, Zhang, C, Wang, J, Celi, P, Ding, X, Bai, S, et al. Characterization for intestinal microbiota of broiler breeders with different egg laying rate. Front Vet Sci. (2020) 7:599337. doi: 10.3389/fvets.2020.599337
11. Liew, WP, and Mohd-Redzwan, S. Mycotoxin: its impact on gut health and microbiota. Front Cell Infect Microbiol. (2018) 26:60. doi: 10.3389/fcimb.2018.00060
12. Elmassry, MM, Zayed, A, and Farag, MA. Gut homeostasis and microbiota under attack: impact for different types of food contaminants on gut health. Crit Rev Food Sci Nutr. (2022) 62:738–63. doi: 10.1080/10408398.2020.1828263
13. Robert, H, Payros, D, Pinton, P, Théodorou, V, Mercier-Bonin, M, and Oswald, IP. Impact of mycotoxins on the intestine: are mucus and microbiota new targets? J Toxicol Environ Health B Crit Rev. (2017) 20:249–75. doi: 10.1080/10937404.2017.1326071
14. Johnson, CH, Ivanisevic, J, and Siuzdak, G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. (2016) 17:451–9. doi: 10.1038/nrm.2016.25
15. Saia, S, Fragasso, M, De Vita, P, and Beleggia, R. Metabolomics provides valuable insight for the study of durum wheat: a review. J Agric Food Chem. (2019) 67:3069–85. doi: 10.1021/acs.jafc.8b07097
16. He, H, An, F, Huang, Q, Kong, Y, He, D, Chen, L, et al. Metabolic effect of AOS-iron in rats with iron deficiency anemia using LC-MS / MS based metabolomics. Food Res Int. (2020) 130:108913. doi: 10.1016/j.foodres.2019.108913
17. Li, K, Cao, Z, Guo, Y, Tong, C, Yang, S, Long, M, et al. Selenium yeast alleviates Ochratoxin A-induced apoptosis and oxidative stress via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in the kidneys of chickens. Oxidative Med Cell Longev. (2020) 18:4048706. doi: 10.1155/2020/4048706
18. Li, H, Zhang, J, Xia, Y, Pan, W, and Zhou, D. Antagonistic effect of nano-selenium on hepatocyte apoptosis induced by DEHP via PI3K/AKT pathway in chicken liver. Ecotoxicol Environ Saf. (2021) 29:112282. doi: 10.1016/j.ecoenv.2021.112282
19. Qiao, L, Dou, X, Yan, S, Zhang, B, and Xu, C. Biogenic selenium nanoparticles synthesized by Lactobacillus casei ATCC 393 alleviate diquat-induced intestinal barrier dysfunction in C57BL / 6 mice through their antioxidant activity. Food Funct. (2020) 11:3020–31. doi: 10.1039/d0fo00132e
20. Liu, Y, Yang, Y, Dong, R, Zhang, Z, Jia, F, Yu, H, et al. Protective effect of selenomethionine on intestinal injury induced by T-2 toxin. Res Vet Sci. (2020) 132:439–47. doi: 10.1016/j.rvsc.2020.07.018
21. Ren, Z, He, H, Fan, Y, Chen, C, Zuo, Z, and Deng, J. Research progress on the toxic antagonism of selenium against mycotoxins. Biol Trace Elem Res. (2019) 190:273–80. doi: 10.1007/s12011-018-1532-6
22. Wang, X, Zuo, Z, Zhao, C, Zhang, Z, Peng, G, Cao, S, et al. Protective role of selenium within activities of antioxidant enzymes in piglet splenic lymphocytes exposed to deoxynivalenol. Environ Toxicol Pharmacol. (2016) 47:53–61. doi: 10.1016/j.etap.2016.09.003
23. Bhatti, SA, Khan, MZ, Saleemi, MK, and Hassan, ZU. Dietary Trichosporon mycotoxinivoron modulates Ochratoxin-A induced altered performance, hepatic and renal antioxidant capacity and tissue injury in broiler chickens. Chem Biol Interact. (2021) 347:109614. doi: 10.1016/j.cbi.2021.109614
24. Qing, H, Huang, S, Zhan, K, Zhao, L, Zhang, J, Ji, C, et al. Combined toxicity evaluation of Ochratoxin A and aflatoxin B1 on kidney and liver injury, immune inflammation, and gut microbiota alteration through pair-feeding pullet model. Front Immunol. (2022) 13:920147. doi: 10.3389/fimmu.2022.920147
25. Yu, Z, Wu, F, Tian, J, Guo, X, and An, R. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by Ochratoxin A in primary chicken hepatocytes. Mol Med Rep. (2018) 18:2551–60. doi: 10.3892/mmr.2018.9285
26. Li, P, Li, K, Zou, C, Tong, C, Sun, L, Cao, Z, et al. Selenium yeast alleviates Ochratoxin A-induced hepatotoxicity via modulation of the PI3K/AKT and Nrf2/Keap1 signaling pathways in chickens. Toxins (Basel). (2020) 12:143. doi: 10.3390/toxins12030143
27. Tong, C, Li, P, Yu, LH, Li, L, Li, K, Chen, Y, et al. Selenium-rich yeast attenuates Ochratoxin A-induced small intestinal injury in broiler chickens by activating the Nrf2 pathway and inhibiting NF-KB activation. J Funct Foods. (2020) 66:103784. doi: 10.1016/j.jff.2020.103784
28. Malekinezhad, P, Ellestad, LE, Afzali, N, Farhangfar, SH, Omidi, A, and Mohammadi, A. Evaluation of berberine efficacy in reducing the effects of aflatoxin B1 and Ochratoxin A added to male broiler rations. Poult Sci. (2021) 100:797–809. doi: 10.1016/j.psj.2020.10.040
29. Zhai, S, Zhu, Y, Feng, P, Li, M, Wang, W, Yang, L, et al. Ochratoxin A: its impact on poultry gut health and microbiota, an overview. Poult Sci. (2021) 100:101037. doi: 10.1016/j.psj.2021.101037
30. Yang, S, Li, L, Yu, L, Sun, L, Li, K, Tong, C, et al. Selenium-enriched yeast reduces caecal pathological injuries and intervenes changes for diversity of caecal microbiota caused by Ochratoxin-A in broilers. Food Chem Toxicol. (2020) 137:111139. doi: 10.1016/j.fct.2020.111139
31. Chen, Y, Zhao, S, Jiao, D, Yao, B, Yang, S, Li, P, et al. Astaxanthin alleviates Ochratoxin A-induced cecum injury and inflammation in mice by regulating the diversity of cecal microbiota and TLR4/MyD88/NF-κB signaling pathway. Oxidative Med Cell Longev. (2021) 5:8894491. doi: 10.1155/2021/8894491
32. Zhang, H, Yan, A, Liu, X, Ma, Y, Zhao, F, Wang, M, et al. Melatonin ameliorates Ochratoxin A induced liver inflammation, oxidative stress and mitophagy in mice involving in intestinal microbiota and restoring the intestinal barrier function. J Hazard Mater. (2021) 5:124489. doi: 10.1016/j.jhazmat.2020.124489
33. Li, L, Chen, Y, Jiao, D, Yang, S, Li, L, and Li, P. Protective effect of astaxanthin on Ochratoxin A-induced kidney injury to mice by regulating oxidative stress-related NRF2/KEAP1 pathway. Molecules. (2020) 25:1386. doi: 10.3390/molecules25061386
34. Zhang, Z, Wang, J, Wang, J, Xie, H, Zhang, Z, Shi, L, et al. Selenomethionine attenuates Ochratoxin A-induced small intestinal injury in rabbits by activating the Nrf2 pathway and inhibiting NF-κB activation. Ecotoxicol Environ Saf. (2023) 256:114837. doi: 10.1016/j.ecoenv.2023.114837
35. Izco, M, Vettorazzi, A, de Toro, M, Sáenz, Y, and Alvarez-Erviti, L. Oral sub-chronic Ochratoxin A exposure induces gut microbiota alterations in mice. Toxins (Basel). (2021) 13:106. doi: 10.3390/toxins13020106
36. Guerre, P. Mycotoxin and gut microbiota. Interactions Toxins (Basel). (2020) 12:769. doi: 10.3390/toxins12120769
37. Wang, W, Zhai, S, Xia, Y, Wang, H, Ruan, D, Zhou, T, et al. Ochratoxin A induces liver inflammation: involvement of intestinal microbiota. Microbiome. (2019) 7:151. doi: 10.1186/s40168-019-0761-z
38. Zhai, SS, Ruan, D, Zhu, YW, Li, MC, Ye, H, Wang, WC, et al. Protective effect of curcumin on Ochratoxin A-induced liver oxidative injury in duck is mediated by modulating lipid metabolism and the intestinal microbiota. Poult Sci. (2020) 99:1124–34. doi: 10.1016/j.psj.2019.10.041
39. Zhang, ZX, Xiang, H, Sun, GG, Yang, YH, Chen, C, and Li, T. Effect of dietary selenium intake on gut microbiota in older population in Enshi region. Genes Environ. (2021) 43:56. doi: 10.1186/s41021-021-00220-3
40. Zhai, QX, Cen, S, Li, P, Tian, FW, Zhao, JX, Zhang, H, et al. Effects of dietary selenium supplementation on intestinal barrier and immune responses associated with its modulation of gut microbiota. Environ Sci Tech Let. (2018) 5:724–30. doi: 10.1021/acs.estlett.8b00563
41. Zhu, H, Lu, C, Gao, F, Qian, Z, Yin, Y, Kan, S, et al. Selenium-enriched Bifidobacterium longum DD98 attenuates irinotecan-induced intestinal and hepatic toxicity in vitro and in vivo. Biomed Pharmacother. (2021) 143:112192. doi: 10.1016/j.biopha.2021.112192
42. Kang, R, Wang, W, Liu, Y, Huang, S, Xu, J, Zhao, L, et al. Dietary selenium sources alleviate immune challenge induced by Salmonella Enteritidis potentially through improving the host immune response and gut microbiota in laying hens. Front Immunol. (2022) 9:928865. doi: 10.3389/fimmu.2022.928865
43. Qiao, L, Dou, X, Song, X, Chang, J, Pi, S, Zhang, X, et al. Protective effect of biogenic selenium nanoparticles against diquat-induced acute toxicity via regulation of gut microbiota and its metabolites. Food Chem Toxicol. (2022) 170:113480. doi: 10.1016/j.fct.2022.113480
44. Qiao, L, Yan, S, Dou, X, Song, X, Chang, J, Pi, S, et al. Biogenic selenium nanoparticles alleviate intestinal epithelial barrier damage through regulating endoplasmic reticulum stress-mediated Mitophagy. Oxidative Med Cell Longev. (2022) 5:3982613. doi: 10.1155/2022/3982613
45. Gangadoo, S, Dinev, I, Chapman, J, Hughes, RJ, Van, TTH, Moore, RJ, et al. Selenium nanoparticles in poultry feed modify gut microbiota and increase abundance of Faecalibacterium prausnitzii. Appl Microbiol Biotechnol. (2018) 102:1455–66. doi: 10.1007/s00253-017-8688-4
46. Heida, FH, van Zoonen, AGJF, Hulscher, JBF, Te Kiefte, BJC, Wessels, R, Kooi, EMW, et al. A necrotizing enterocolitis-associated gut microbiota is present within meconium: results of a prospective study. Clin Infect Dis. (2016) 62:863–70. doi: 10.1093/cid/ciw016
47. Shintouo, CM, Mets, T, Beckwee, D, Bautmans, I, Ghogomu, SM, Souopgui, J, et al. Is inflammageing influenced by the microbiota within aged gut? A systematic review. Exp Gerontol. (2020) 141:111079. doi: 10.1016/j.exger.2020.111079
48. Zhou, Y, Xu, ZZ, He, Y, Yang, Y, Liu, L, Lin, Q, et al. Gut microbiota offers universal biomarkers across ethnicity in inflammatory bowel disease diagnosis and infliximab response prediction. mSystems. (2018) 3:e00188–17. doi: 10.1128/mSystems.00188-17
49. Yang, X, Liu, L, Chen, J, and Xiao, A. Response of intestinal bacterial flora to the long-term feeding of aflatoxin B1 (AFB1) in mice. Toxins (Basel). (2017) 9:317. doi: 10.3390/toxins9100317
50. Wang, J, Tang, L, Glenn, TC, and Wang, JS. Aflatoxin B1 induced compositional changes in gut microbial communities of male F344 rats. Toxicol Sci. (2016) 150:54–63. doi: 10.1093/toxsci/kfv259
51. Lucke, A, Böhm, J, Zebeli, Q, and Metzler-Zebeli, BU. Dietary deoxynivalenol contamination and oral lipopolysaccharide challenge alters the cecal microbiota of broiler chickens. Front Microbiol. (2018) 25:804. doi: 10.3389/fmicb.2018.00804
52. Lin, R, Sun, Y, Mu, P, Zheng, T, Mu, H, Deng, F, et al. Lactobacillus rhamnosus GG supplementation modulates the gut microbiota to promote butyrate production, protecting against deoxynivalenol exposure in nude mice. Biochem Pharmacol. (2020) 175:113868. doi: 10.1016/j.bcp.2020.113868
53. Du, G, Guo, Q, Yan, X, Chen, H, Yuan, Y, and Yue, T. Potential protective mechanism of Tibetan kefir underlying gut-derived liver injury induced by Ochratoxin A. Food Funct. (2022) 13:11690–704. doi: 10.1039/d2fo02360a
54. Hill, C, Guarner, F, Reid, G, Gibson, GR, Merenstein, DJ, Pot, B, et al. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use for term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
55. Marco, ML, Heeney, D, Binda, S, Cifelli, CJ, Cotter, PD, Foligné, B, et al. Health benefits of fermented foods: microbiota and beyond. Curr Opin Biotechnol. (2017) 44:94–102. doi: 10.1016/j.copbio.2016.11.010
56. Belenguer, A, Holtrop, G, Duncan, SH, Anderson, SE, Calder, AG, Flint, HJ, et al. Rates of production and utilization of lactate by microbial communities from the human colon. FEMS Microbiol Ecol. (2011) 77:107–19. doi: 10.1111/j.1574-6941.2011.01086.x
57. Yang, G, Hong, E, Oh, S, and Kim, E. Non-viable Lactobacillus johnsonii JNU3402 protects against diet-induced obesity. Foods. (2020) 9:1494. doi: 10.3390/foods9101494
58. Bolte, LA, Vich Vila, A, Imhann, F, Collij, V, Gacesa, R, Peters, V, et al. Long-term dietary patterns are associated with pro-inflammatory and anti-inflammatory features for gut microbiome. Gut. (2021) 70:1287–98. doi: 10.1136/gutjnl-2020-322670
59. Ouethrani, M, Van de Wiele, T, Verbeke, E, Bruneau, A, Carvalho, M, Rabot, S, et al. Metabolic fate of Ochratoxin A as a coffee contaminant in a dynamic simulator for human colon. Food Chem. (2013) 141:3291–300. doi: 10.1016/j.foodchem.2013.05.157
60. Orsolic, N, Jembrek Jazvinscak, M, and Terzic, S. Honey and quercetin reduce Ochratoxin A-induced DNA damage within liver and the kidney through the modulation of intestinal microflora. Food Agric Immunol. (2017) 28:812–33. doi: 10.1080/09540105.2017.1313819
61. Wang, Y, Zhang, J, Wang, Y, Wang, K, Wei, H, and Shen, L. Isolation and characterization for Bacillus cereus BC7 strain, which is capable of zearalenone removal and intestinal flora modulation in mice. Toxicon. (2018) 1:9–20. doi: 10.1016/j.toxicon.2018.09.005
62. Taranu, I, Marin, DE, Pistol, GC, Motiu, M, and Pelinescu, D. Induction of pro-inflammatory gene expression by Escherichia coli and mycotoxin zearalenone contamination and protection by a Lactobacillus mixture in porcine IPEC-1 cells. Toxicon. (2015) 97:53–63. doi: 10.1016/j.toxicon.2015.01.016
63. Wang, YL, Wang, BJ, Liu, M, Jiang, KY, Wang, MQ, and Wang, L. Aflatoxin B1 (AFB1) induced dysregulation of intestinal microbiota and damage of antioxidant system in pacific white shrimp (Litopenaeus vannamei). Aquaculture. (2018) 495:940–7. doi: 10.1016/j.aquaculture.2018.06.065
64. Flint, HJ, Duncan, SH, Scott, KP, and Louis, P. Links between diet, gut microbiota composition and gut metabolism. Proc Nutr Soc. (2015) 74:13–22. doi: 10.1017/S0029665114001463
65. Reeves, AE, Koenigsknecht, MJ, Bergin, IL, and Young, VB. Suppression of Clostridium difficile within gastrointestinal tracts of germfree mice inoculated with a murine isolate from the family Lachnospiraceae. Infect Immun. (2012) 80:3786–94. doi: 10.1128/IAI.00647-12
66. Oliphant, K, Ali, M, D’Souza, M, Hughes, PD, Sulakhe, D, Wang, AZ, et al. Bacteroidota and Lachnospiraceae integration into the gut microbiome at key time points in early life are linked to infant neurodevelopment. Gut Microbes. (2021) 13:1997560. doi: 10.1080/19490976.2021.1997560
67. Wang, S, Yang, J, Zhang, B, Zhang, L, Wu, K, Yang, A, et al. Potential link between gut microbiota and Deoxynivalenol-induced feed refusal in weaned piglets. J Agric Food Chem. (2019) 67:4976–86. doi: 10.1021/acs.jafc.9b01037
68. Pérez-Reytor, D, Puebla, C, Karahanian, E, and García, K. Use of short-chain fatty acids for the recovery for intestinal epithelial barrier affected by bacterial toxins. Front Physiol. (2021) 24:650313. doi: 10.3389/fphys.2021.650313
69. Von-Hafe, M, Borges-Canha, M, Vale, C, Leite, AR, Sérgio Neves, J, Carvalho, D, et al. Nonalcoholic fatty liver disease and endocrine axes-a scoping review. Metabolites. (2022) 12:298. doi: 10.3390/metabo12040298
70. Vang, S, Cochran, P, Sebastian Domingo, J, Krick, S, and Barnes, JW. The glycobiology of pulmonary arterial hypertension. Meta. (2022) 12:316. doi: 10.3390/metabo12040316
71. Lin, XY, Wang, LM, Zhao, JT, He, L, Cui, L, Gao, Y, et al. Nanosafety evaluation through feces: a comparison between selenium nanoparticles and selenite in rats. Nano Today. (2021) 36:101010. doi: 10.1016/j.nantod.2020.101010
72. Callejón-Leblic, B, Selma-Royo, M, Collado, MC, Gómez-Ariza, JL, Abril, N, and García-Barrera, T. Untargeted gut metabolomics to delve the interplay between selenium supplementation and gut microbiota. J Proteome Res. (2022) 21:758–67. doi: 10.1021/acs.jproteome.1c00411
73. Thakur, K, Tomar, SK, Singh, AK, Mandal, S, and Arora, S. Riboflavin and health: a review of recent human research. Crit Rev Food Sci Nutr. (2017) 257:3650–60. doi: 10.1080/10408398.2016.1145104
74. Saedisomeolia, A, and Ashoori, M. Riboflavin in human health: a review of current evidences. Adv Food Nutr Res. (2018) 83:57–81. doi: 10.1016/bs.afnr.2017.11.002
75. Suwannasom, N, Kao, I, Pruß, A, Georgieva, R, and Baumler, H. Riboflavin: the health benefits of a forgotten natural vitamin. Int J Mol Sci. (2020) 21:950. doi: 10.3390/ijms21030950
76. Olfat, N, Ashoori, M, and Saedisomeolia, A. Riboflavin is an antioxidant: a review update. Br J Nutr. (2022) 128:1887–95. doi: 10.1017/S0007114521005031
77. Yates, CA, Evans, GS, Pearson, T, and Powers, HJ. Absence of luminal riboflavin disturbs early postnatal development for gastrointestinal tract. Dig Dis Sci. (2003) 48:1159–64. doi: 10.1023/a:1023785200638
78. Powers, HJ, Weaver, LT, Austin, S, and Beresford, JK. A proposed intestinal mechanism for the effect of riboflavin deficiency on iron loss within rat. Br J Nutr. (1993) 69:553–61. doi: 10.1079/bjn19930055
79. Lee, ES, Corfe, BM, and Powers, HJ. Riboflavin depletion of intestinal cells in vitro leads to impaired energy generation and enhanced oxidative stress. Eur J Nutr. (2013) 52:1513–21. doi: 10.1007/s00394-012-0458-6
80. Tang, J, Wen, Z, Guo, Z, Huang, W, Guo, Y, and Xie, M. Dietary riboflavin supplementation improve the growth performance and antioxidant status of starter white Pekin ducks fed a corn-soybean meal diets. Livest Sci. (2014) 170:131e6. doi: 10.1016/j.livsci.2014.10.016
81. Biagi, E, Mengucci, C, Barone, M, Picone, G, Lucchi, A, Celi, P, et al. Effects of vitamin B2 supplementation in broilers microbiota and metabolome. Microorganisms. (2020) 28:1134. doi: 10.3390/microorganisms8081134
82. Pan, F, Xu, X, Zhang, LL, Luo, HJ, Chen, Y, Long, L, et al. Dietary riboflavin deficiency induces genomic instability of esophageal squamous cells that is associated with gut microbiota dysbiosis in rats. Food Funct. (2020) 11:10070–83. doi: 10.1039/d0fo01944e
83. Pan, F, Zhang, LL, Luo, HJ, Chen, Y, Long, L, Wang, X, et al. Dietary riboflavin deficiency induces ariboflavinosis and esophageal epithelial atrophy in association with modification of gut microbiota in rats. Eur J Nutr. (2021) 60:807–20. doi: 10.1007/s00394-020-02283-4
84. Zhu, YY, Thakur, K, Feng, JY, Zhang, JG, Hu, F, Cespedes-Acuña, CL, et al. Riboflavin bioenriched soymilk alleviates oxidative stress mediated liver injury, intestinal inflammation, and gut microbiota modification in B2 depletion-repletion mice. J Agric Food Chem. (2022) 70:3818–31. doi: 10.1021/acs.jafc.2c00117
85. Patel, M, Narke, D, Kurade, M, Frey, KM, Rajalingam, S, Siddiquee, A, et al. Limonene-induced activation of A2A adenosine receptors reduces airway inflammation and reactivity in a mouse model of asthma. Purinergic Signal. (2020) 16:415–26. doi: 10.1007/s11302-020-09697-z
86. Bouzenna, H, Hfaiedh, N, Giroux-Metges, MA, Elfeki, A, and Talarmin, H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin within IEC-6 cells. Biomed Pharmacother. (2017) 93:961–8. doi: 10.1016/j.biopha.2017.06.031
87. Kim, DS, Lee, HJ, Jeon, YD, Han, YH, Kee, JY, Kim, HJ, et al. Alpha-Pinene exhibits anti-inflammatory activity through the suppression of MAPKs and the NF-κB pathway in mouse peritoneal macrophages. Am J Chin Med. (2015) 43:731–42. doi: 10.1142/S0192415X15500457
88. Wang, H, Xiao, Y, Xu, C, Cao, Y, Jing, P, Wu, S, et al. Integrated metabolomics and transcriptomics analyses reveal metabolic mechanisms in porcine intestinal epithelial cells under zearalenone stress. J Agric Food Chem. (2022) 70:6561–72. doi: 10.1021/acs.jafc.2c01107
89. Kim, MH, and Kim, H. The roles of glutamine within intestine and its implication in intestinal diseases. Int J Mol Sci. (2017) 18:1051. doi: 10.3390/ijms18051051
90. Xue, GD, Barekatain, R, Wu, SB, Choct, M, and Swick, RA. Dietary L-glutamine supplementation improves growth performance, gut morphology, and serum biochemical indices of broiler chickens during necrotic enteritis challenge. Poult Sci. (2018) 97:1334–41. doi: 10.3382/ps/pex444
91. Xing, S, Zhang, B, Lin, M, Zhou, P, Li, J, Zhang, L, et al. Effects of alanyl-glutamine supplementation on the small intestinal mucosa barrier in weaned piglets. Asian-Australas J Anim Sci. (2017) 30:236–45. doi: 10.5713/ajas.16.0077
92. Rao, RK, Li, L, Baker, RD, Baker, SS, and Gupta, A. Glutathione oxidation and PTPase inhibition by hydrogen peroxide in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol. (2000) 279:G332–40. doi: 10.1152/ajpgi.2000.279.2.G332
93. Wu, QJ, Zheng, XC, Wang, T, and Zhang, TY. Effects of oridonin on immune cells, Th1/Th2 balance and the expression of BLys within spleens of broiler chickens challenged with Salmonella pullorum. Res Vet Sci. (2018) 119:262–7. doi: 10.1016/j.rvsc.2018.07.008
94. Toraya, T, Oka, T, Ando, M, Yamanishi, M, and Nishihara, H. Novel pathway for utilization of cyclopropanecarboxylate by Rhodococcus rhodochrous. Appl Environ Microbiol. (2004) 70:224–8. doi: 10.1128/AEM.70.1.224-228.2004
95. Reis, B, Martins, M, Barreto, B, Milhazes, N, Garrido, EM, Silva, P, et al. Structure-property-activity relationship of phenolic acids and derivatives. Protocatechuic acid alkyl esters. J Agric Food Chem. (2010) 58:6986–93. doi: 10.1021/jf100569j
96. Pacheco-Ordaz, R, Wall-Medrano, A, Goñi, MG, Ramos-Clamont-Montfort, G, Ayala-Zavala, JF, and González-Aguilar, GA. Effect of phenolic compounds on the growth of selected probiotic and pathogenic bacteria. Lett Appl Microbiol. (2018) 66:25–31. doi: 10.1111/lam.12814
97. Adedara, IA, Okpara, ES, Busari, EO, Omole, O, Owumi, SE, and Farombi, EO. Dietary protocatechuic acid abrogates male reproductive dysfunction in streptozotocin-induced diabetic rats via suppression of oxidative damage, inflammation and caspase-3 activity. Eur J Pharmacol. (2019) 15:30–42. doi: 10.1016/j.ejphar.2019.01.033
98. Tan, J, Li, Y, Hou, DX, and Wu, S. The effects and mechanisms of cyanidin-3-glucoside and its phenolic metabolites in maintaining intestinal integrity. Antioxidants (Basel). (2019) 8:479. doi: 10.3390/antiox8100479
99. Wang, Y, Wang, Y, Wang, B, Mei, X, Jiang, S, and Li, W. Protocatechuic acid improved growth performance, meat quality, and intestinal health of Chinese yellow-feathered broilers. Poult Sci. (2019) 98:3138–49. doi: 10.3382/ps/pez124
100. Hu, R, He, Z, Liu, M, Tan, J, Zhang, H, Hou, DX, et al. Dietary protocatechuic acid ameliorates inflammation and up-regulates intestinal tight junction proteins by modulating gut microbiota in LPS-challenged piglets. J Anim Sci Biotechnol. (2020) 9:92. doi: 10.1186/s40104-020-00492-9
101. Wang, F, Chen, Y, Hu, H, Liu, X, Wang, Y, Saleemi, MK, et al. Protocatechuic acid: a novel detoxication agent of fumonisin B1 for poultry industry. Front Vet Sci. (2022) 26:923238. doi: 10.3389/fvets.2022.923238
102. Wu, T, Fan, T, and Xie, Y. Antagonism of cyanamide-3-O-glucoside and protocatechuic acid on aflatoxin B1-induced toxicity in zebrafish larva (Danio rerio). Toxicon. (2022) 216:139–47. doi: 10.1016/j.toxicon.2022.06.009
103. He, L, Zhang, S, Luo, C, Sun, Y, Lu, Q, Huang, L, et al. Functional teas from the stems of Penthorum chinense Pursh.: phenolic constituents, antioxidant and hepatoprotective activity. Plant Foods Hum Nutr. (2019) 74:83–90. doi: 10.1007/s11130-018-0701-2
104. Dou, W, Zhang, J, Sun, A, Zhang, E, Ding, L, Mukherjee, S, et al. Protective effect of naringenin against experimental colitis via suppression of toll-like receptor 4/NF-κB signalling. Br J Nutr. (2013) 110:599–608. doi: 10.1017/S0007114512005594
105. Kim, JS, and Jobin, C. The flavonoid luteolin prevents lipopolysaccharide-induced NF-kappaB signalling and gene expression by blocking IkappaB kinase activity in intestinal epithelial cells and bone-marrow derived dendritic cells. Immunology. (2005) 115:375–87. doi: 10.1111/j.1365-2567.2005.02156.x
106. Fernandes, I, Pérez-Gregorio, R, Soares, S, Mateus, N, and de Freitas, V. Wine flavonoids in health and disease prevention. Molecules. (2017) 22:292. doi: 10.3390/molecules22020292
107. Shi, SR, Gu, H, Chang, LL, Wang, ZY, Tong, HB, and Zou, JM. Safety evaluation of daidzein in laying hens: part I. Effects on laying performance, clinical blood parameters, and organs development. Food Chem Toxicol. (2013) 55:684–8. doi: 10.1016/j.fct.2013.01.009
108. Liu, HY, and Zhang, CQ. Effects of daidzein on messenger ribonucleic acid expression of gonadotropin receptors in chicken ovarian follicles. Poult Sci. (2008) 87:541–5. doi: 10.3382/ps.2007-00274
109. Xiao, YQ, Shao, D, Sheng, ZW, Wang, Q, and Shi, SR. A mixture of daidzein and Chinese herbs increases egg production and eggshell strength as well as blood plasma ca, P, antioxidative enzymes, and luteinizing hormone levels in post-peak, brown laying hens. Poult Sci. (2019) 98:3298–303. doi: 10.3382/ps/pez178
110. Gao, X, Mu, P, Zhu, X, Chen, X, Tang, S, Wu, Y, et al. Dual function of a novel bacterium, Slackia sp. D-G6: detoxifying deoxynivalenol and producing the natural estrogen analogue, equol. Toxins (Basel). (2020) 12:85. doi: 10.3390/toxins12020085
111. Peng, F, Du, Q, Peng, C, Wang, N, Tang, H, Xie, X, et al. A review: the pharmacology of isoliquiritigenin. Phytother Res. (2015) 29:969–77. doi: 10.1002/ptr.5348
112. Yu, J, Wang, L, Walzem, RL, Miller, EG, Pike, LM, and Patil, BS. Antioxidant activity of citrus limonoids, flavonoids, and coumarins. J Agric Food Chem. (2005) 53:2009–14. doi: 10.1021/jf0484632
113. Mershiba, SD, Dassprakash, MV, and Saraswathy, SD. Protective effect of naringenin on hepatic and renal dysfunction and oxidative stress in arsenic intoxicated rats. Mol Biol Rep. (2013) 40:3681–91. doi: 10.1007/s11033-012-2444-8
114. Marin, DE, Pistol, GC, Gras, MA, Palade, ML, and Taranu, I. Comparative effect of Ochratoxin A on inflammation and oxidative stress parameters in gut and kidney of piglets. Regul Toxicol Pharmacol. (2017) 89:224–31. doi: 10.1016/j.yrtph.2017.07.031
115. Rašić, D, Micek, V, Klarić, MS, and Peraica, M. Oxidative stress as a mechanism of combined OTA and CTN toxicity in rat plasma, liver and kidney. Hum Exp Toxicol. (2019) 38:434–45. doi: 10.1177/0960327118819049
Keywords: nano-selenium, cecum microbial community, metabolomics, chickens, Ochratoxin A
Citation: Fang M, Hu W and Liu B (2023) Effects of nano-selenium on cecum microbial community and metabolomics in chickens challenged with Ochratoxin A. Front. Vet. Sci. 10:1228360. doi: 10.3389/fvets.2023.1228360
Edited by:
Yongpeng Guo, Henan Agricultural University, ChinaReviewed by:
Sabreen Ezzat Fadl, Matrouh University, EgyptNing Jiao, Shandong Agricultural University, China
Xu Yang, Henan Agricultural University, China
Copyright © 2023 Fang, Hu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Manxin Fang, manxinfang@jxycu.edu.cn
 Manxin Fang
Manxin Fang Wei Hu
Wei Hu Ben Liu1,2
Ben Liu1,2