A meta-analysis of Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets
- 1College of Animal Science and Technology, Yangzhou University, Yangzhou, China
- 2Institute of Epigenetics and Epigenomics, Yangzhou University, Yangzhou, China
Antibiotics are widely used as growth promoters (AGPs) in livestock production to improve animal performance and health. However, pig producers today face the prohibition of in-feed antimicrobials and have to find safe and effective alternatives. Lactobacillus species are active microorganisms that convey multiple beneficial effects to the host and are one of the most promising AGPs replacements. Here, we aim to comprehensively assess the effects of Lactobacillus spp. supplementation on growth performance and intestinal morphology (villus height [VH], crypt depth [CD], and the V/C ratio) of piglets. Among the 196 identified studies, 20 met the criteria and were included in the meta-analysis. The effects of Lactobacillus-based probiotics supplementation on growth performance and intestinal morphology were analyzed using a random-effects model. And the publication bias was evaluated by funnel plots. Our results revealed that Lactobacillus spp. supplementation significantly improved the growth performance, including average daily feed intake (ADFI), average daily gain (ADG), and the gain-to-feed ratio (G/F) in piglets (P < 0.05). Meanwhile, Lactobacillus spp. remarkably increased VH and the V/C ratio (P < 0.05) in the small intestine, including the duodenum, jejunum, and ileum, which might contribute to an improved digestive capacity of these animals. In conclusion, our findings provide concrete evidence of the growth-promoting effects of Lactobacillus spp. supplementation in piglets and a better understanding of the potential of Lactobacillus-based probiotics as AGPs alternatives in pig production.
Introduction
Antibiotics used as growth promoters at sub-therapeutic doses to animals are an integral part of livestock production. Large-scale addition of antibiotic growth promoters (AGPs) to animal feed can help to improve production efficiency by improving animal performance represented by average daily feed intake (ADFI), average daily gain (ADG), and gain-to-feed ratio (G/F) (1–3). Moreover, AGPs may alter intestinal morphology and promote digestion and absorption of nutrients in the intestine, which also contribute to animal growth and development (4). In pigs, the improved performance attributed to AGPs addition was between 4 to 8% (3, 5). However, the overuse of antibiotics has induced the development of multi-drug-resistant microorganisms in farm animals. It could seriously endanger animal production and public health (6, 7). Due to this concern, antibiotics used as growth promoters in livestock have been banned in the European Union since 2006 (8). As of 2017, the US has banned the use of medically-important antimicrobials for preventative and growth-promotion purposes the in the livestock sector (9). In addition, China prohibited in-feed antimicrobials in animal production in 2020 (10). Several antibiotic alternatives have been developed, studied, and tested in livestock to face the increasing global restrictions on antibiotic usage while maintaining animal health and performance.
Probiotics are live microbial supplements in adequacy or components of bacteria that confer beneficial effects on the intestinal health of the host (11) through occupying binding sites of the intestinal mucosa or competing for nutrients and niches with pathogenic bacteria (12, 13). Numerous studies have revealed that Lactobacillus species improve the growth performance and decrease the diarrhea ratio of piglets by enhancing nutrition digestibility and intestinal barrier function (14–19). Among the tested probiotics, Lactobacillus species are considered one of the most promising replacements and therefore represent a safe opportunity to substitute AGPs in pigs (18, 20). However, the wide variety of Lactobacillus species and different experimental designs make it difficult to comprehensively understand and further evaluate the effects of Lactobacillus species on swine and finally use them to replace AGPs at large in production. In this regard, meta-analysis constitutes a method integrating and analyzing numerous independent studies on the same subjects and makes the most representative conclusions (21). More importantly, in a meta-analysis, as the amount of data used increases, the precision of estimates can be improved on separate studies with different results (22). It justifies our attempt to employ this approach to determine the effects of Lactobacillus-based probiotics in pigs in the background of the emerging antibiotics alternative research. To provide a mechanism for estimating the effect degree, a strict design and clear selection criteria of studies, and the measurement index are necessary (22).
It is worth mentioning that the gut development of piglets is sensitive to alterations of feed components, reflected by their morphological changes (23). Histologically, the porcine intestine follows the general structure throughout its length and is similar to other monogastric animals and humans: the mucosa surface is covered by a monolayer of epithelium including absorptive enterocytes, goblet, and endocrine cells, etc. (23). The surface lining of epithelium quickly renews themselves and contributes to the absorptive surface and capacity of the intestine. It is commonly accepted to determine villus height, crypt depth, and the V/C ratio as “gold standards” of intestinal morphology, while these histological parameters could be used as a tool to evaluate gut function and responses toward feed ingredients (24). In the current study, we therefore performed a set of meta-analyses to delineate the effects of Lactobacillus species on pig growth performance and intestinal morphology.
Materials and methods
Study search and inclusion criteria
The protocols used were following the MOOSE guidelines (25). This study aimed to analyze the effects of Lactobacillus species supplementation in piglets with or without E. coli/LPS challenges on growth performance and intestinal morphological parameters. We have identified studies using Lactobacillus spp. including Lactobacillus delbrueckii, Lactobacillus reuteri, Lactobacillus plantarum and Lactobacillus acidophilus etc. published in English from June 2010 to June 2022. The search strategy consisted of a search of English databases, including PubMed, Google scholar, Cochrane library, semantic scholar Embased and Clinical Trials, and a search of Chinese databases, including VIP, CNKI, and WANFANG Data. The search terms included: (Lactobacillus OR Lactobacilli OR Lactic acid bacteria) AND (piglets OR piggy OR pigling) AND (growth performance OR average daily gain OR average daily feed intake OR feed efficiency) AND (duodenal villus height, crypt depth, and villus height to crypt depth OR jejunal villus height, crypt depth and villus height to crypt depth OR Ileal villus height, crypt depth and villus height to crypt depth) in titles or abstracts.
The eligibility for inclusion of all studies identified from the searches was independently assessed and compared by the authors where the inclusion/exclusion criteria described previously were also considered (26). Manual selections were conducted on all returned publications based on the relevance of the titles and/or abstracts of the publications to Lactobacillus. Prerequisites of the selected publications were a downloadable full text and available data in English regarding Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets.
Exclusion criteria
Studies excluded from this systematic review and meta-analysis were those that met the following criteria. Firstly, non-experimental articles (review articles); Secondly, articles with incorrect or incomplete data; Thirdly, articles without a control group; Finally, non-probiotics added or combined with other drugs and preparations in the experimental groups. A flow diagram was used to summarize the process of the article selection and study inclusion/exclusion for our meta-analysis (Figure 1).
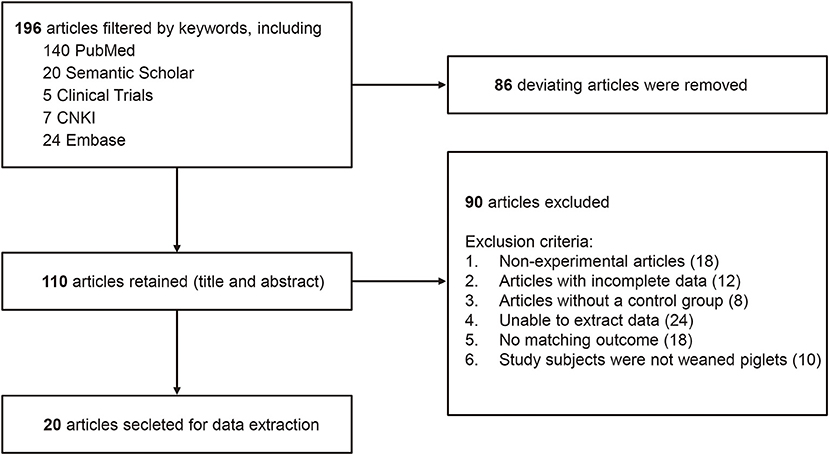
Figure 1. A flow diagram of studies included in the meta-analysis Lactobacillus-based probiotics for growth performance and intestinal physiology in piglets.
Data extraction
Data relating to the effects of Lactobacillus spp. supplementation with or without E. coli/LPS challenges on the growth performance and intestinal physiology in piglets were collected from each selected article using a custom-tailored excel form that included detailed information as follows: animals (breed, sex distribution, and age), sample size, experimental design including the control group set up, target Lactobacillus strains, the amount (dose) of probiotic supplementation, administration methods and duration, authors, When results were available only in graphical format, data were extracted using Review Manager (Version 5.4 provided by Cochrane Training). Graph digitization has been previously shown to be a valid method for extracting study data (27). If the data were unclear or some key data were missing, we attempted to contact the corresponding authors through email to obtain further information. The outcomes were as follows: ADG (average daily gain); ADFI (average daily feed intake); G/F (gain-to-feed ratio); diarrhea rate; Duodenum VH (villus height), CD (crypt depth) and V/C (villus height: crypt depth); Jejunum VH, CD and V/C; and Ileum VH, CD and V/C.
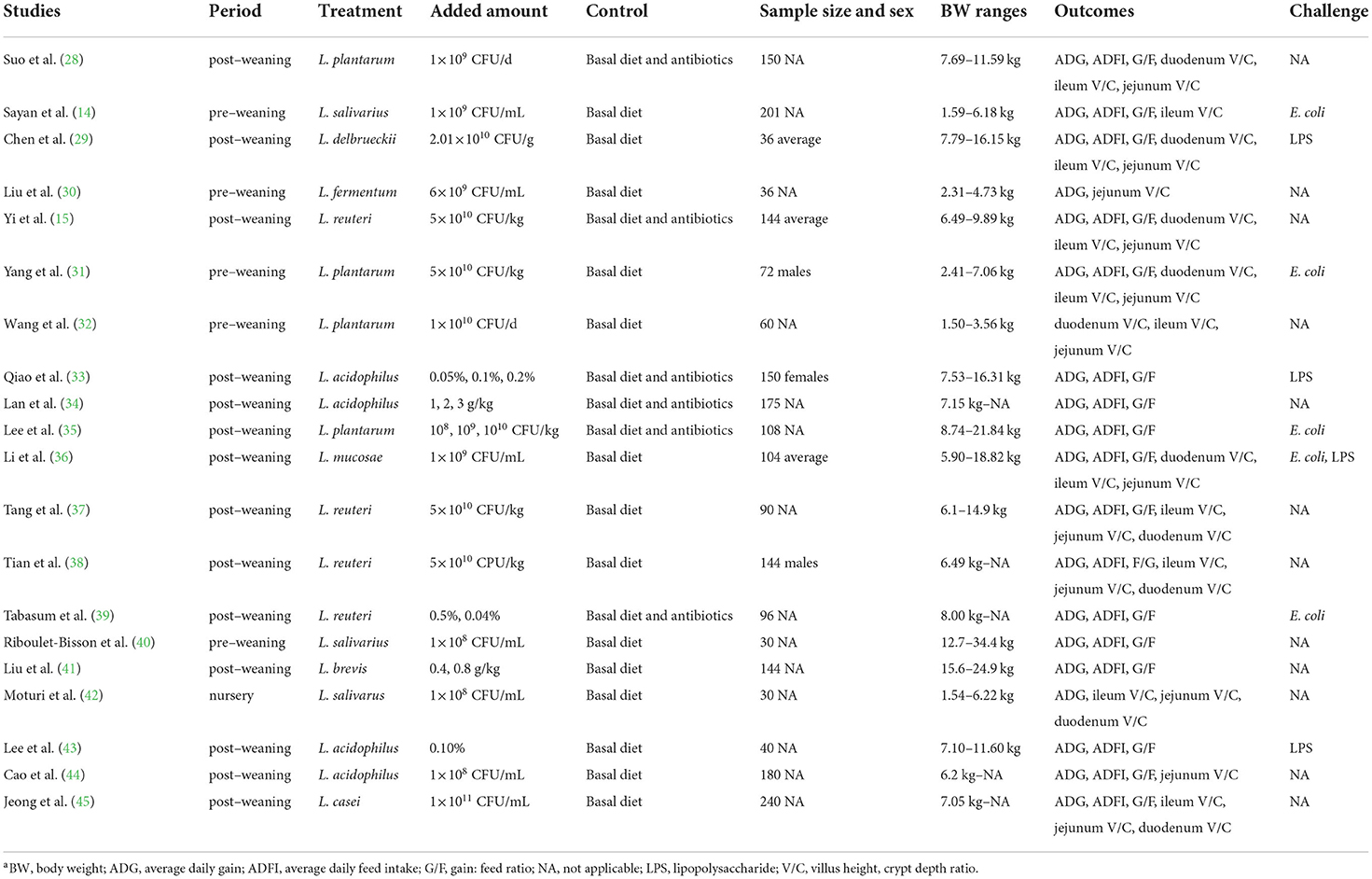
In our initial search, 2123 English language records were hit which, after screening, revealed 196 unique articles. A total of 20 papers that fulfilled the selection criteria were included in the quantitative meta-analysis, including Suo et al. (28); Sayan et al. (14); Chen et al. (29); Liu et al. (30); Yi et al. (15); Yang et al. (31); Wang et al. (32); Qiao et al. (33); Lan et al. (34); Lee et al. (35); Li et al. (36); Tang et al. (37); Tian et al. (38); Sonia et al. (39); Eliette et al. (40); Liu et al. (41); Moturi et al. (42); Lee et al. (43); Cao et al. (44); and Jeong et al. (45). The characteristics of each study are shown in Table 1. The median initial body weight (BW) of the piglet was 6.50 kg (from 1.50 kg to 15.6 kg). In total, 6 studies included used antibiotics as the control group, and 8 studies included had piglets challenged with E. coli or LPS.
Statistical analyses
Considering that there are contrary data within the range of the research purpose and the heterogeneity between different studies may interfere with the analyzed results, we used a random-effects model to compute the 95% confidence interval (95% CI) of the standardized mean difference (SMD). Heterogeneity was assessed using chi-square test and the I2 parameter (30–60% indicating moderate, 50–90% indicating substantial, and 75–100% indicating considerable heterogeneity). For the identification and assessment of reporting bias, we tested funnel plot symmetry by the Begg and Egger method (47). All the above analyses were performed by the Review Manager (Version 5.4 provided by Cochrane Training). P < 0.05 is considered significant.
Results
Forest plots and sensitivity analysis of Lactobacillus species to growth performance in piglets
A meta-analysis was performed to examine the effect of probiotic Lactobacillus spp. on ADG (24 trials, n = 1683 subjects). The summarized results of standardized mean difference (SMD) and 95% confidence interval (CI) for each study are shown in Table 2. There was a significant positive correlation between ADG and the Lactobacillus spp. addition (SMD = 0.65; 95% CI: 0.48~0.82; I2 = 63%; P < 0.001), and Lactobacillus spp. supplementation improved the ADG of piglets. Next, the effects of Lactobacillus spp. supplementation on ADFI in piglets were determined by forest plots and sensitivity analysis (19 trials, n = 1346 subjects). The summarized results of SMD and 95% CI are shown in Table 3. Lactobacillus spp. remarkably increased the ADFI of piglets compared to the control group (SMD = 0.61; 95% CI: 0.35~0.88; P < 0.001), but showed a high heterogeneity (I2 = 82%), indicating a high degree of inter-study variability. Furthermore, we analyzed the effects of probiotic Lactobacillus spp. on the G/F ratio (18 trials, n = 1298 subjects) including forest plots and sensitivity analysis. The summarized results are shown in Table 4, the resulting forest map is shown in Figure 2 and the publication bias analysis of all parameters is shown in Figures 3A–L. We found that G/F was significantly increased after Lactobacillus spp. supplementation (SMD = 0.23; 95% CI: 0.12~0.34; I2 = 0%; P < 0.001).
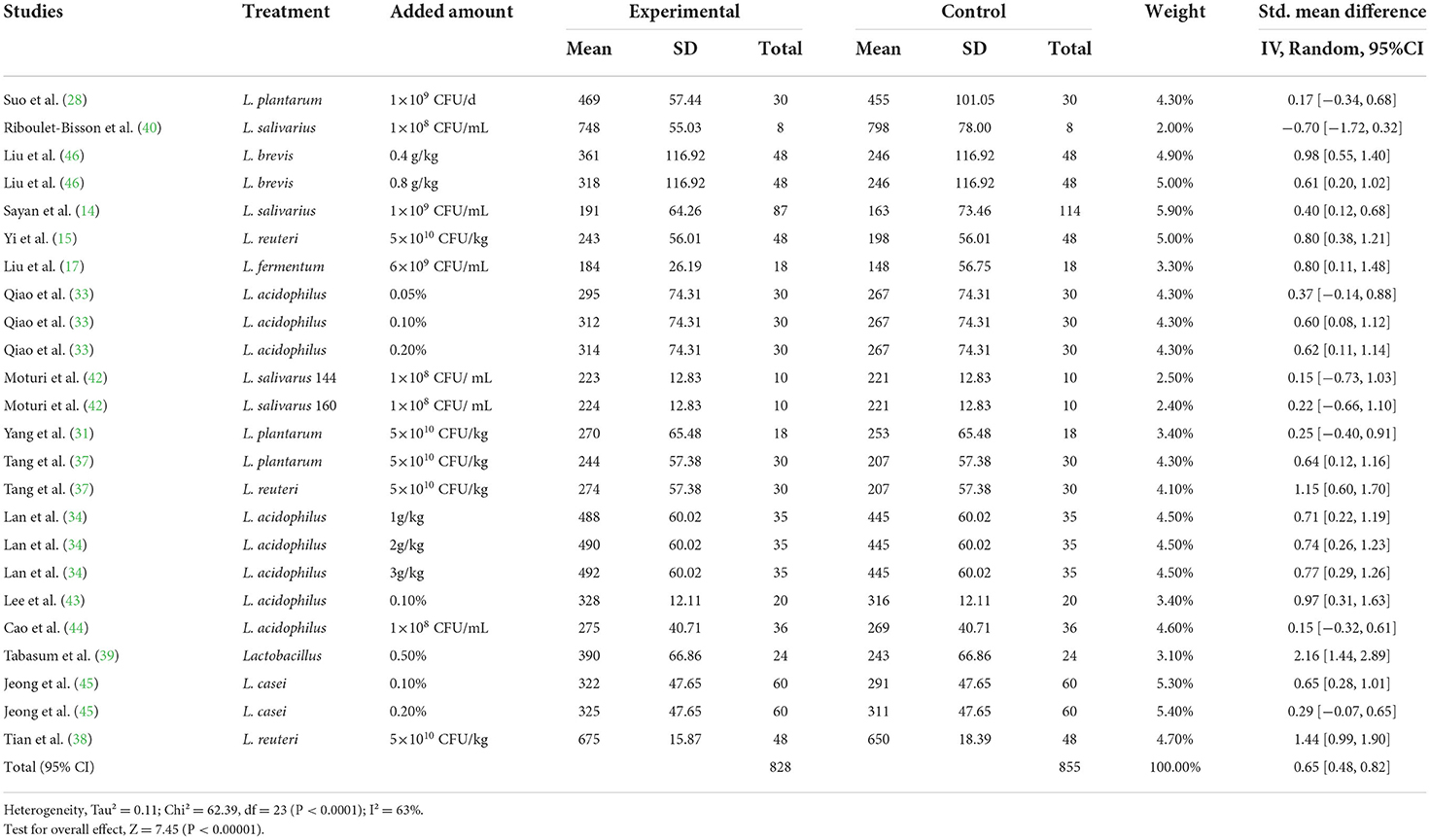
Table 2. Effects of Lactobacillus–based probiotics on the average daily gain (ADG) of pigs from included studies.
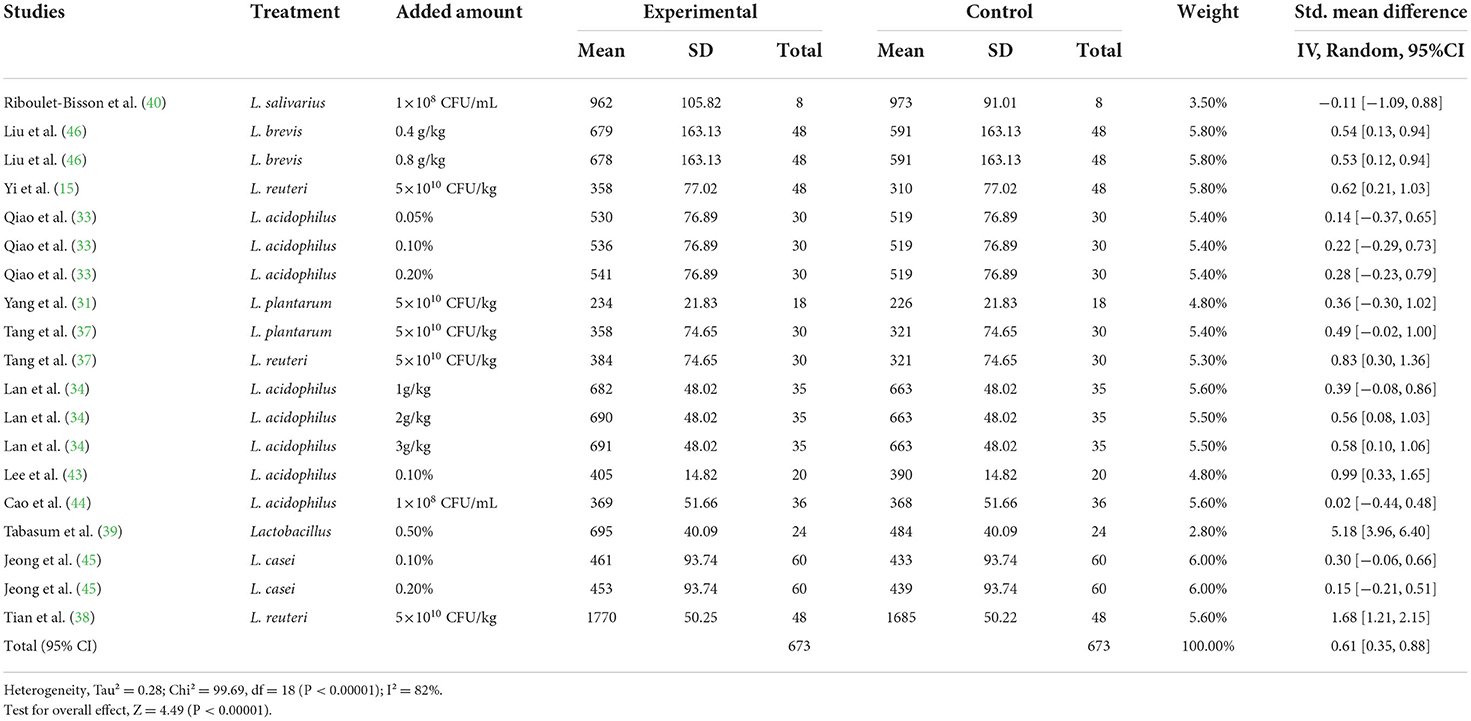
Table 3. Effects of Lactobacillus–based probiotics on the average daily feed intake (ADFI) pigs from included studies.
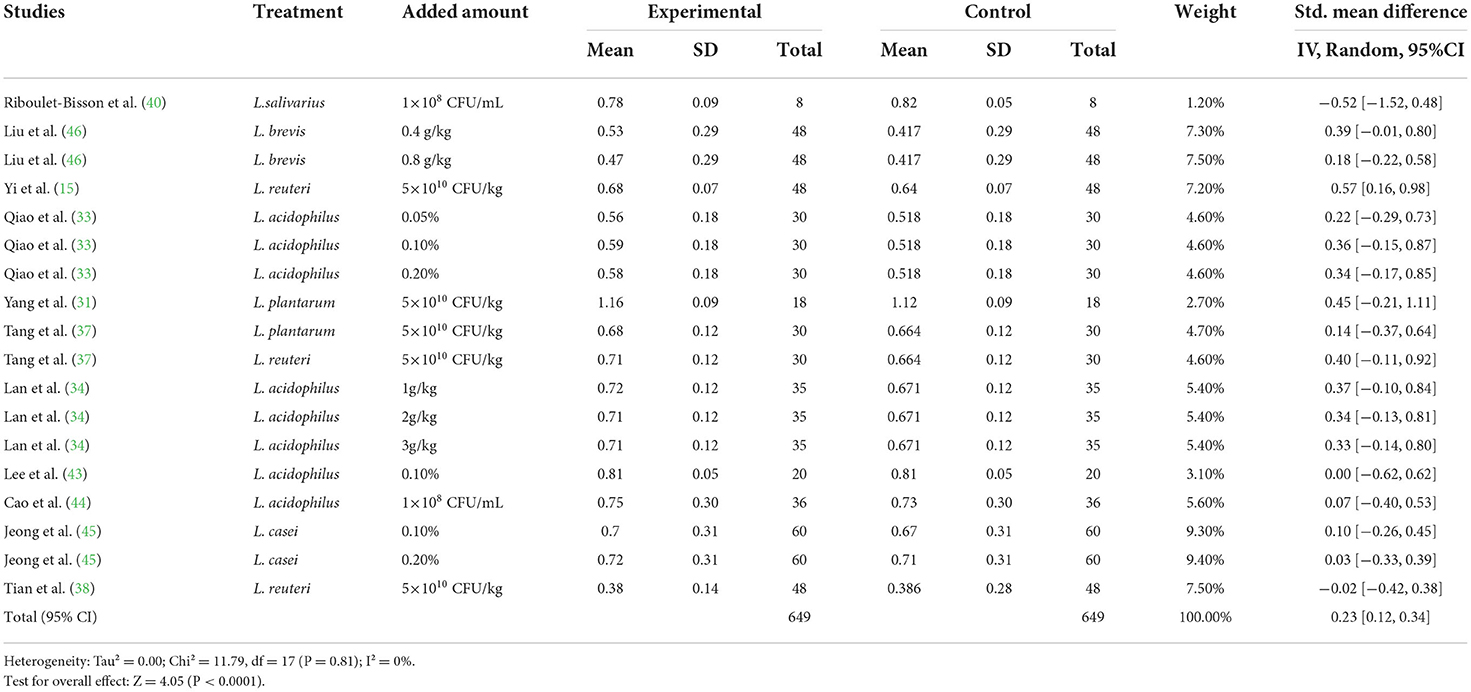
Table 4. Effects of Lactobacillus–based probiotics on the gain to feed (G/F) ratio of pigs from included studies.
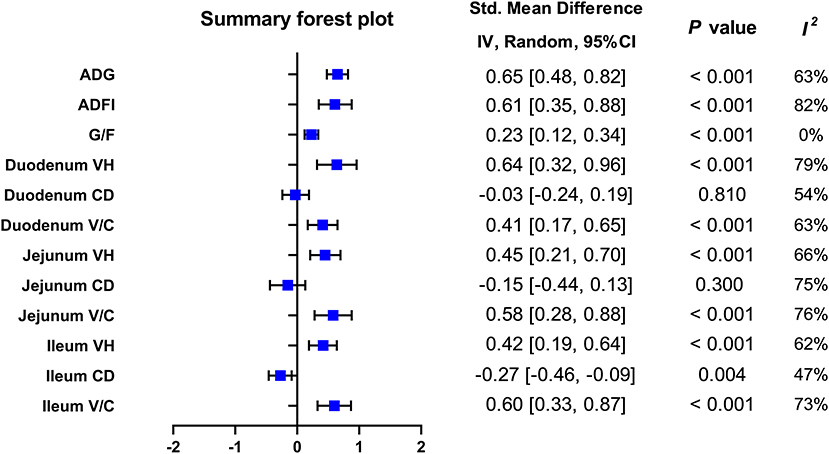
Figure 2. Summary forest plot of included outcomes on pigs. ADG, average daily gain; ADFI, average daily feed intake; CI, confidence interval; G/F, gain: feed ratio; V/C, villus height: crypt depth ratio.
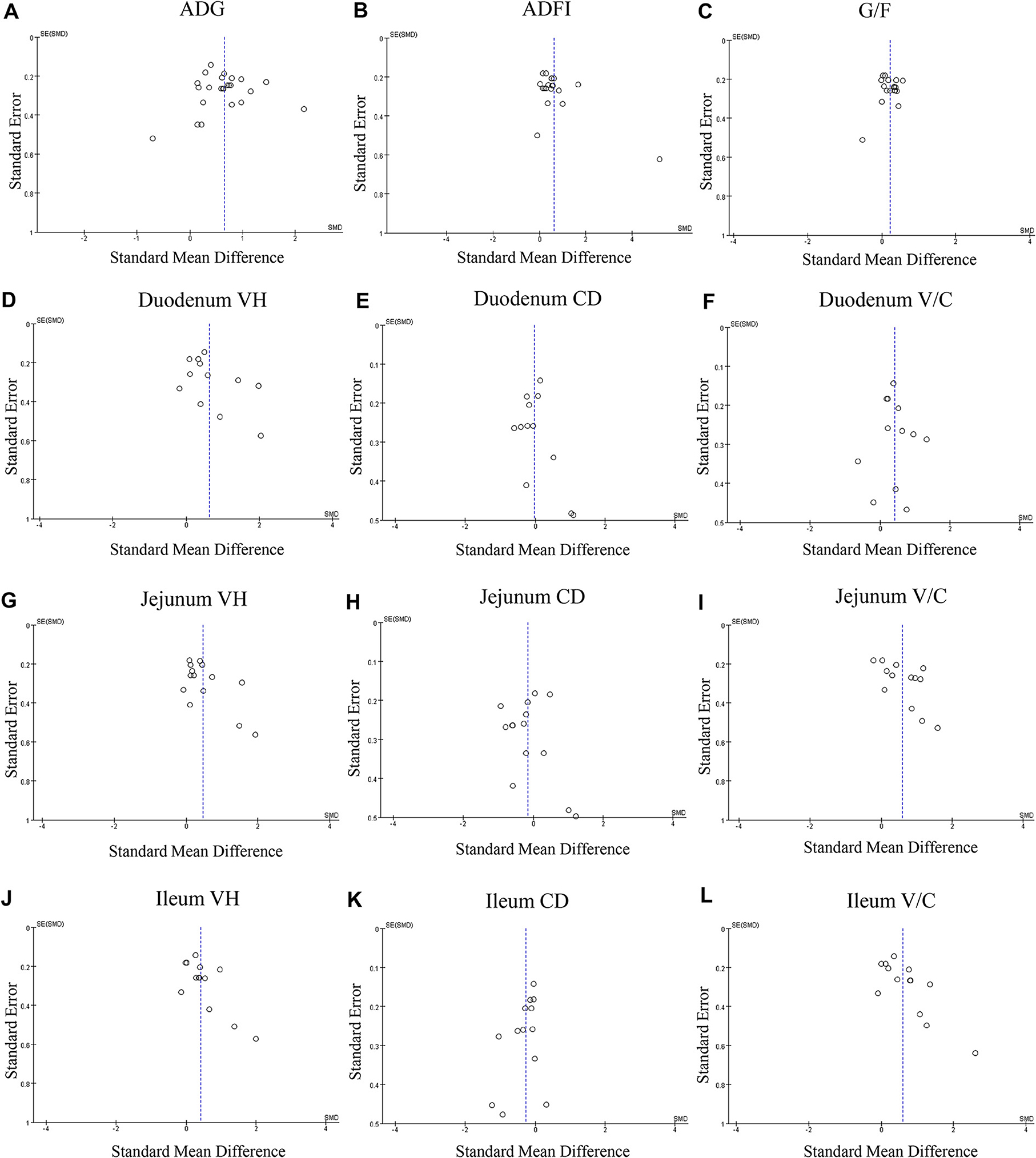
Figure 3. Funnel plots of the publication bias analysis of included outcomes on pigs. (A) Average daily gain, (B) Average daily feed intake. (C) Gain: feed ratio. (D) Duodenal VH. (E) Duodenal CD. (F) duodenal V/C. (G) Jejunal VH. (H) Jejunal CD. (I) Jejunal. (J) Ileal VH. (K) Ileal CD. (L) Ileal V/C. n = 12; CD, crypt depth; SE, standard error; SMD, standard mean difference; V/C, villus height: crypt depth; VH, villus height; V/C, villus height: crypt depth.
Forest plots and sensitivity analysis of Lactobacillus species to the duodenal morphology in piglets
The Lactobacillus-promoted growth performance of piglets identified by our analysis prompted us to further study the effects of Lactobacillus spp. on parameters like villus height (VH), crypt depth (CD), and the V/C ratio, representing changes in the small intestinal morphology of piglets (Table 5, Figure 2). First, we found that porcine duodenal VH (12 trials, n = 877 subjects) was significantly increased by Lactobacillus spp. supplementation compared to the control (SMD = 0.64; 95% CI: 0.32~0.92; I2 = 79%; P < 0.001). In contrast, no significant correlation between the addition of Lactobacillus spp. and the duodenal CD was observed (12 trials, n = 877 subjects; SMD = −0.03; 95% CI:−0.24~0.19; I2 = 54%; P = 0.81). In the duodenum of piglets, the analysis showed a positive correlation between the V/C ratio and Lactobacillus spp. supplementation (SMD = 0.41; 95% CI: 0.17~0.65; I2 = 65%; P = 0.002).
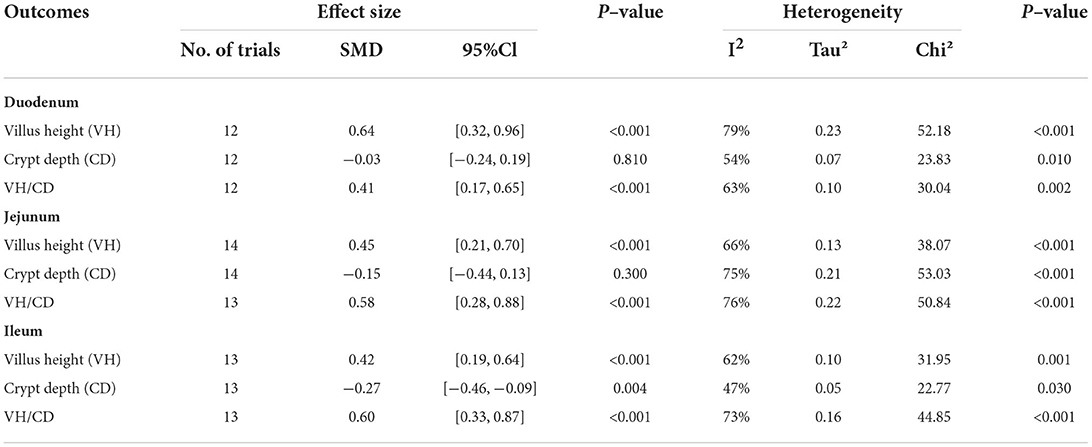
Table 5. Effects of Lactobacillus–based probiotics on small intestinal morphology of pigs from included studies.
Forest plots, sensitivity analysis, and funnel plots of Lactobacillus species to jejunal morphology in piglets
In the porcine jejunum, the effects of probiotics on VH, CD, and the V/C ratio were evaluated (Table 5, Figure 2). In the jejunum of piglets (14 trials, n = 880 subjects), the analysis showed a positive effect with the addition of Lactobacillus spp. on the VH values (SMD = 0.45; 95% CI: 0.21~0.70; I2 = 65%; P = 0.005). In addition, the effect of Lactobacillus spp. addition on the jejunal CD was also examined (14 trials, n = 880 subjects), and Lactobacillus supplementation significantly decreased CD, compared to the control (SMD = −0.15; 95% CI: −0.44~0.13; I2 = 75%; P < 0.001). The changes of the V/C ratio between the control and the addition of Lactobacillus spp. were analyzed (13 trials, n = 844 subjects), and found that it was significantly increased by the addition of Lactobacillus spp. (SMD = 0.58; 95% CI: 0.28~0.88; I2 = 76%; P < 0.001).
Forest plots and sensitivity analysis of Lactobacillus species to ileum morphology in piglets
Finally, we analyzed the effects of probiotic Lactobacillus spp. supplementation on the ileal morphology of piglets (13 trials, n = 973 subjects; Table 5, Figure 2). The ileal VH was strongly positively correlated with Lactobacillus spp. addition (SMD = 0.42; 95% CI: 0.19~0.64; I2 = 62%; P = 0.001). Next, the effects of Lactobacillus spp. addition on the ileal CD were determined (13 trials, n = 973 subjects), Lactobacillus spp. was associated with a significant reduction of the ileal CD (SMD = −0.27; 95% CI: −0.46~-0.09; I2 = 47%; P =0.03). For the ileal V/C ratio (13 trials, n = 973 subjects), the addition of probiotic Lactobacillus spp. significantly increased this value when compared to the control (SMD = 0.60; 95% CI: 0.33~0.87; I2 = 73%; P < 0.001).
Discussion
Antibiotics are widely used as growth promoters in livestock to improve animal growth performance and health. Several hypotheses on the AGPs' mode of action have been proposed, including reducing pathogenic load and toxin production and inhibiting gut disorders while improving intestinal physiology (48, 49). However, due to the side effects (e.g., antibiotic resistance and environmental pollutants of its residues), the use of antibiotics has been restricted worldwide, which results in the urgent need to find alternative routes to manage animal health and maintain production efficiency (50, 51). Lactobacillus species are one of the most commonly used probiotic agents in swine and are considered one such potent AGPs replacement (18, 52, 53). Therefore, this study systematically reviewed and performed a set of meta-analyses to determine the effects of probiotic Lactobacillus spp. on porcine growth performance and intestinal morphology. The main findings were as follows: (1) Lactobacillus spp. supplementation can improve piglets' performance including ADFI, ADG and the G/F ratio, and is superior to antibiotics in growth promotion; (2) Lactobacillus spp. supplementation substantially modified the small intestinal morphology, increased VH and the V/C ratio of piglets in all segments, whereas decreased the jejunal and ileal CD.
The growth performance is an essential indicator of pig health and the economic benchmark of the production system. Comparable to the AGPs, there are also theories on the mechanism of growth improvement of probiotics in animals, which include improving the gut barrier function, nutrient utilization, gut microbiome, intestinal morphology, and immunity (18). In particular, important indexes like average daily gain, average daily feed intake, and the gain-to-feed ratio reflect nutrient uptake and absorptive capacity, where higher values in the Lactobacillus-treated piglets implied improved nutrient utilization (45). Our meta-analysis revealed that ADG and ADFI were increased by 13.8% and 7.02% on average by Lactobacillus addition. It is suggested that Lactobacillus-based feeding could enhance feed palatability (54), which may explain the increased feed intake. A probiotic-improved G/F ratio was reported by us, suggesting that Lactobacillus supplementation brings about a more cost-effective feeding program than the control piglets. Another mechanism by which Lactobacillus spp. enhance animal performance may be via the promotion of beneficial bacteria and inhibition of harmful microorganisms in the intestinal microenvironment (55).
Growing evidence indicates that gut microbiota plays a crucial role in host metabolic health and fitness. A healthy small intestine has a dominance of Lactobacillus spp., which may be disrupted by perturbations like weaning and changes of feed in piglets (56). By adding a Lactobacilli compound (including L. gasseri, L. reuteri, L. acidophilus and L. fermentum), Huang et al. showed that significantly decreased the E. coli and aerobe counts, and increased Lactobacilli and anaerobe counts in the digesta and mucosa, thereby promoting growth performance of pigs (57). Furthermore, Lactobacillus spp. can increase the levels of microbial metabolites such as butyrate to alleviate piglet diarrhea, which directly and indirectly affects growth performance (58). Although the gut microbiome is not analyzed here, we choose to study the effects of Lactobacillus spp. in piglets at the age of weaning. When antibiotics are often used due to sudden changes in diets and gut microbiota dysbiosis, which further impair pig performance and health. It is noteworthy that at weaning, a reduction of intestinal VH or villus atrophy may occur (56).
In this regard, we found that Lactobacillus spp. supplementation significantly increased VH and the V/C ratio in the small intestine of piglets. It may be partly due to the Lactobacillus-increased daily feed intake, resulting in a trophic action on the development of intestinal epithelium (18). As the main digestive and absorptive site, increases in small intestinal VH and the V/C ratio are directly related to the larger surface area and enhanced epithelium turnover and cell mitosis activation (24, 59). It allows for enhanced uptake of dietary substances while excluding noxious agents in the gut lumen (60). Finally, the improved gut morphology can facilitate digestion, and absorption of nutrients, fluid, and electrolytes for piglets (18, 24, 59, 60), promoting growth performance (61). This was supported by studies we have summarized in our meta-analysis and numerous other studies (14, 18). In addition, in piglets challenged with LPS or E. coli, carbohydrate and fatty acid utilization can be compromised due to inflammation, while the probiotic can alter the villus-crypt architecture and influence the associated enzyme activity and nutrient transport receptor expression (62, 63). Similarly, Zhang et al. demonstrated that Lactobacilli supplementation increases digestive enzyme activities and promotes growth performance (12). Furthermore, the addition of Lactobacillus resulted in the enhancement of genes for the metabolism, transport, and catabolism of vitamins, amino acids, lipids, and polyketides, thus the improvement of growth performance (64). In addition, we also reported a location-specific response of intestinal histology driven by Lactobacillus spp. i.e., Lactobacillus supplementation improved all jejunal and ileal histological parameters, likely related to the gradient distribution of microbiota along the pig small intestine (65, 66). It is also suggested that the probiotics, live bacteria, their signaling or metabolites, must have reached the distal part of the intestine in piglets and become effective. However, care must be taken as the meta-analysis approach has limitations. For instance, when the number of included studies is small, the number of trial characteristics is large, and the heterogeneity of data becomes large, which was also seen in our studies. And even if the number of studies is increased, Meta-analysis might not fully explain all but kept a residual heterogeneity (21, 22, 25).
Conclusion
In conclusion, our findings indicate that Lactobacillus spp. supplementation plays a crucial role in improving growth performance of piglets by increasing ADF, ADFI and the G/F ratio, in parallel modifying the intestinal morphology, especially in the jejunum and ileum. It suggests that Lactobacillus spp. can be regarded as a promising alternative to replace AGPs usage in pig production. Based on our analysis, we suggest that future studies focus on documenting the effects of Lactobacillus spp. supplementation on the porcine gut microbiome; evaluating probiotics viability in farm conditions and generating protocols and regulations for the application in the industry.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
PH and H-YL: conceptualization, writing, and supervision. CuZ and JY: formal analysis. CuZ, JY, MZ, ChZ, LY, and ZL: investigation. CuZ, JY, PH, and H-YL: writing the original draft. DC and SC: reviewing, revising, and editing the manuscript. DC, PH, and H-YL: funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Postgraduate Research and Practice Innovation Program of Yangzhou University (X20211025), the Natural Science Foundation of Jiangsu Province (BK20220582), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Pedroso AA, Menten JFM, Lambais MR, Racanicci AMC, Longo FA, Sorbara JOB. Intestinal bacterial community and growth performance of chickens fed diets containing antibiotics. Poult Sci. (2006) 85:747–52. doi: 10.1093/ps/85.4.747
2. Dibner JJ, Richards JD. Antibiotic growth promoters in agriculture: history and mode of action. Poult Sci. (2005) 84:634–43. doi: 10.1093/ps/84.4.634
3. Luecke RW, Thorp F. Jr., Newland HW, Mc MW. The growth promoting effects of various antibiotics on pigs. J Anim Sci. (1951) 10:538–42. doi: 10.2527/jas1951.102538x
4. Rodrigues G, Maximiano MR, Franco OL. Antimicrobial peptides used as growth promoters in livestock production. Appl Microbiol Biot. (2021) 105:7115–21. doi: 10.1007/s00253-021-11540-3
5. Li J. Current status and prospects for in-feed antibiotics in the different stages of pork production - a review. Asian-Australas J Anim Sci. (2017) 30:1667–73. doi: 10.5713/ajas.17.0418
6. Wang S, Zeng X, Yang Q, Qiao S. Antimicrobial peptides as potential alternatives to antibiotics in food animal industry. Int J Mol Sci. (2016) 17:7050603. doi: 10.3390/ijms17050603
7. Liu YY, Wang Y, Walsh TR Yi LX, Zhang R, Spencer J, et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: a microbiological and molecular biological study. Lancet Infect Dis. (2016) 16:161–8. doi: 10.1016/S1473-3099(15)00424-7
8. E.C. (EC). Ban on Antibiotics as Growth Promoters in Animal Feed Enters into Effect. (2005). Available online at: http://europa.eu/rapid/press-release_IP-05-1687_en.html (accessed 13, September 2018).
9. C.f.D.C.a.P. (CDC), Food and Food Animals. (2020). Available online at: https://www.cdc.gov/drugresistance/food.html (accessed 18, June 2020).
10. Ministry of Agriculture and Rural Affairs of the People's Republic of China 2018. Notice of the Ministry of Agriculture and Rural Affairs Office on the Pilot Work on the Reduction of the Use of Veterinary Antimicrobial Drugs. (2018). Available online at: http://www.moa.gov.cn/govpublic/SYJ/201804/t20180420_6140711.htm (accessed May 27, 2019).
11. Salminen S, Collado MC, Endo A, Hill C, Lebeer S, Quigley EMM, et al. The international scientific association of probiotics and prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol. (2021) 18:649–67. doi: 10.1038/s41575-021-00440-6
12. Zhao PY, Kim IH. Effect of direct-fed microbial on growth performance, nutrient digestibility, fecal noxious gas emission, fecal microbial flora and diarrhea score in weanling pigs. Anim Feed Sci Tech. (2015) 200:86–92. doi: 10.1016/j.anifeedsci.2014.12.010
13. Yu Q, Yuan L, Deng J, Yang Q. Lactobacillus protects the integrity of intestinal epithelial barrier damaged by pathogenic bacteria. Front Cell Infect Microbiol. (2015) 5:26. doi: 10.3389/fcimb.2015.00026
14. Sayan H, Assavacheep P, Angkanaporn K, Assavacheep A. Effect of Lactobacillus salivarius on growth performance, diarrhea incidence, fecal bacterial population and intestinal morphology of suckling pigs challenged with F4+ enterotoxigenic escherichia coli. Asian Austral J Anim Sci. (2018) 31:1308–14. doi: 10.5713/ajas.17.0746
15. Yi H, Wang L, Xiong Y, Wen X, Wang Z, Yang X, et al. Effects of Lactobacillus reuteri LR1 on the growth performance, intestinal morphology, and intestinal barrier function in weaned pigs. J Anim Sci. (2018) 96:2342–51. doi: 10.1093/jas/sky129
16. Wang TW, Teng KL, Liu YY, Shi WX, Zhang J, Dong EQ, et al. Lactobacillus plantarum PFM 105 promotes intestinal development through modulation of gut microbiota in weaning piglets. Front Microbiol. (2019) 10:90. doi: 10.3389/fmicb.2019.00090
17. Liu H, Dicksved J, Lundh T, Lindberg JE. Heat shock proteins: intestinal gatekeepers that are influenced by dietary components and the gut microbiota. Pathogens. (2014) 3:187–210. doi: 10.3390/pathogens3010187
18. Dowarah R, Verma AK, Agarwal N. The use of Lactobacillus as an alternative of antibiotic growth promoters in pigs: a review. Anim Nutr. (2017) 3:1–6. doi: 10.1016/j.aninu.2016.11.002
19. Wang Y, Gong L, Wu YP, Cui ZW, Wang YQ, Huang Y, et al. Oral administration of Lactobacillus rhamnosus GG to newborn piglets augments gut barrier function in pre-weaning piglets. J Zhejiang Univ Sci B. (2019) 20:180–92. doi: 10.1631/jzus.B1800022
20. Patil AK, Kumar S, Verma AK, Baghel RPS, editors. Probiotics as feed additives in weaned pigs: a review. Livest Res Int. (2015) 3:31–9.
21. Ones DS, Viswesvaran C, Schmidt FL. Realizing the full potential of psychometric meta-analysis for a cumulative science and practice of human resource management. Hum Resour Manag Rev. (2017) 27:201–15. doi: 10.1016/j.hrmr.2016.09.011
22. Pigott T. Methods of meta-analysis: correcting error and bias in research findings. Eval Program Plann. (2006) 29:236–7. doi: 10.1016/j.evalprogplan.2006.06.002
23. Bailey M. The mucosal immune system: recent developments and future directions in the pig. Dev Comp Immunol. (2009) 33:375–83. doi: 10.1016/j.dci.2008.07.003
24. Rieger J. The intestinal mucosal network in the pig: a histological view on nutrition-microbiota-pathogen-host-interactions. Veterinärmedizin. (2016) 569:641–8.
25. Brooke BS, Schwartz TA, Pawlik TM, MOOSE. reporting guidelines for meta-analyses of observational studies. JAMA Surg. (2021) 156:787–8. doi: 10.1001/jamasurg.2021.0522
26. Xu BC, Fu J, Zhu LY Li Z, Wang YZ, Jin ML. Overall assessment of antimicrobial peptides in piglets: a set of meta-analyses. Animal. (2020) 14:2463–71. doi: 10.1017/S1751731120001640
27. Guyot P, Ades AE, Ouwens MJNM, Welton NJ. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan-Meier survival curves. BMC Med Res Methodol. (2012) 12:9. doi: 10.1186/1471-2288-12-9
28. Suo C, Yin Y, Wang X, Lou X, Song D, Wang X, et al. Effects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet Res. (2012) 8:89. doi: 10.1186/1746-6148-8-89
29. Chen FM, Wang HJ, Chen JY, Liu Y, Wen W, Li YH, et al. Lactobacillus delbrueckii ameliorates intestinal integrity and antioxidant ability in weaned piglets after a lipopolysaccharide challenge. Oxid Med Cell Longev. (2020) 2020:6028606. doi: 10.1155/2020/6028606
30. Liu H, Zhang J, Zhang SH, Yang FJ, Thacker PA, Zhang GL, et al. Oral administration of Lactobacillus fermentum I5007 favors intestinal development and alters the intestinal microbiota in formula-fed piglets. J Agr Food Chem. (2014) 62:860–6. doi: 10.1021/jf403288r
31. Yang KM, Jiang ZY, Zheng CT, Wang L, Yang XF. Effect of Lactobacillus plantarum on diarrhea and intestinal barrier function of young piglets challenged with Enterotoxigenic Escherichia coli K88. J Anim Sci. (2014) 92:1496–503. doi: 10.2527/jas.2013-6619
32. Wang Q, Sun Q, Qi RL, Wang J, Qiu XY, Liu ZH, et al. Effects of Lactobacillus plantarum on the intestinal morphology, intestinal barrier function and microbiota composition of suckling piglets. J Anim Physiol Anim Nutr (Berl). (2019) 103:1908–18. doi: 10.1111/jpn.13198
33. Qiao J, Li H, Wang Z, Wang W. Effects of Lactobacillus acidophilus dietary supplementation on the performance, intestinal barrier function, rectal microflora and serum immune function in weaned piglets challenged with Escherichia coli lipopolysaccharide. Antonie Leeuwenhoek. (2015) 107:883–91. doi: 10.1007/s10482-015-0380-z
34. Lan R, Koo J, Kim I. Effects of Lactobacillus acidophilus supplementation on growth performance, nutrient digestibility, fecal microbial and noxious gas emission in weaning pigs. J Sci Food Agric. (2017) 97:1310–5. doi: 10.1002/jsfa.7866
35. Lee JS, Awji EG, Lee SJ, Tassew DD, Park YB, Park KS, et al. Effect of Lactobacillus plantarum CJLP243 on the growth performance and cytokine response of weaning pigs challenged with Enterotoxigenic Escherichia coli. J Anim Sci. (2012) 90:3709–17. doi: 10.2527/jas.2011-4434
36. Li YS, San Andres JV, Trenhaile-Grannemann MD, van Sambeek DM, Moore KC, Winkel SM, et al. Effects of mannan oligosaccharides and Lactobacillus mucosae on growth performance, immune response, and gut health of weanling pigs challenged with Escherichia coli lipopolysaccharides. J Anim Sci. (2021) 99:skab286. doi: 10.1093/jas/skab286
37. Tang QS, Yi HB, Hong WB, Wu QW, Yang XF, Hu SL, et al. Comparative effects of L. plantarum CGMCC 1258 and L. reuteri LR1 on growth performance, antioxidant function, and intestinal immunity in weaned pigs. Front Vet Sci. (2021) 8:3389. doi: 10.3389/fvets.2021.728849
38. Tian ZM, Cui YY, Lu HJ, Ma XY. Effects of long-term feeding diets supplemented with Lactobacillus reuteri 1 on growth performance, digestive and absorptive function of the small intestine in pigs. J Funct Foods. (2020) 71:104010. doi: 10.1016/j.jff.2020.104010
39. Sonia T, Ji H, Hong-Seok M, Chul-Ju Y. Evaluation of Lactobacillus and Bacillus-based probiotics as alternatives to antibiotics in enteric microbial challenged weaned piglets. Afr J Microbiol Res. (2014) 8:96–104. doi: 10.5897/AJMR2013.6355
40. Riboulet-Bisson E, Sturme MH, Jeffery IB, O'Donnell MM, Neville BA, Forde BM, et al. Effect of Lactobacillus salivarius bacteriocin Abp118 on the mouse and pig intestinal microbiota. PLoS One. (2012) 7:e31113. doi: 10.1371/journal.pone.0031113
41. Liu H, Ji HF, Zhang DY, Wang SX, Wang J, Shan DC, et al. Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest Sci. (2015) 178:251–4.
42. Moturi J, Kim KY, Hosseindoust A, Lee JH, Xuan B, Park J, et al. Effects of Lactobacillus salivarius isolated from feces of fast-growing pigs on intestinal microbiota and morphology of suckling piglets. Sci Rep. (2021) 11:6757. doi: 10.1038/s41598-021-85630-7
43. Lee SI, Kim HS, Koo JM, Kim IH. Lactobacillus acidophilus modulates inflammatory activity by regulating the TLR4 and NF-kappaB expression in porcine peripheral blood mononuclear cells after lipopolysaccharide challenge. Br J Nutr. (2016) 115:567–75. doi: 10.1017/S0007114515004857
44. Cao ST, Wang L, Jiao LF, Lin FH, Xiao K, Hu CH. Effects of diosmectite-Lactobacillus acidophilus on growth performance, intestine microbiota, mucosal architecture of weaned pigs. Anim Feed Sci Tech. (2016) 220:180–6. doi: 10.1016/j.anifeedsci.2016.08.012
45. Jeong YD, Ko HS, Hosseindoust A, Choi YH, Chae BJ Yu DJ, et al. Lactobacillus-based fermentation product and lactose level in the feed for weanling pigs: effects on intestinal morphology, microbiota, gas emission, and targeted intestinal coliforms. Livest Sci. (2019) 227:90–6. doi: 10.1016/j.livsci.2019.06.018
46. Liu H, Ji HF, Zhang DY, Wang SX, Wang J, Shan DC, et al. Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest Sci. (2015) 178:251–4. doi: 10.1016/j.livsci.2015.06.002
47. Lin L, Chu H. Quantifying publication bias in meta-analysis. Biometrics. (2018) 74:785–94. doi: 10.1111/biom.12817
48. Cox Laura M, Yamanishi S, Sohn J, Alekseyenko Alexander V, Leung Jacqueline M, Cho I, et al. Altering the intestinal microbiota during a critical developmental window has lasting metabolic consequences. Cell. (2014) 158:705–21. doi: 10.1016/j.cell.2014.05.052
49. Gaskins HR, Collier CT, Anderson DB. Antibiotics as growth promotants: mode of action. Anim Biotechnol. (2002) 13:29–42. doi: 10.1081/ABIO-120005768
50. Tian M, He XM, Feng YZ, Wang WT, Chen HS, Gong M, et al. Pollution by antibiotics and antimicrobial resistance in livestock and poultry manure in China, and countermeasures. Antibiotics (Basel). (2021) 10:0050539. doi: 10.3390/antibiotics10050539
51. Schulze M, Nitsche-Melkus E, Hensel B, Jung M, Jakop U. Antibiotics and their alternatives in artificial breeding in livestock. Anim Reprod Sci. (2020) 220:106284. doi: 10.1016/j.anireprosci.2020.106284
52. Hou CL, Zeng XF, Yang FJ, Liu H, Qiao SY. Study and use of the probiotic Lactobacillus reuteri in pigs: a review. J Anim Sci Biotechnol. (2015) 6:3. doi: 10.1186/s40104-015-0014-3
53. Pupa P, Apiwatsiri P, Sirichokchatchawan W, Pirarat N, Maison T, Koontanatechanon A, et al. Use of Lactobacillus plantarum (strains 22F and 25F) and Pediococcus acidilactici (strain 72N) as replacements for antibiotic-growth promotants in pigs. Sci Rep. (2021) 11:5. doi: 10.1038/s41598-021-91427-5
54. Cullen JT, Lawlor PG, Cormican P, Gardiner GE. Microbial quality of liquid feed for pigs and its impact on the porcine gut microbiome. Animals (Basel). (2021) 11:2983. doi: 10.3390/ani11102983
55. Dong XL, Zhang NF, Zhou M, Tu Y, Deng KD, Diao QY. Effects of dietary probiotics on growth performance, faecal microbiota and serum profiles in weaned piglets. Anim Prod Sci. (2014) 54:616–21. doi: 10.1071/AN12372
56. Gresse R, Chaucheyras-Durand F, Fleury MA, Van de Wiele T, Forano E, Blanquet-Diot S. Gut microbiota dysbiosis in postweaning piglets: understanding the keys to health. Trends Microbiol. (2017) 25:851–73. doi: 10.1016/j.tim.2017.05.004
57. Huang CH, Qiao SY Li DF, Piao XS, Ren JP. Effects of lactobacilli on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian Austral J Anim. (2004) 17:401–9. doi: 10.5713/ajas.2004.401
58. Yue Y, He ZJ, Zhou YH, Ross RP, Stanton C, Zhao JX, et al. Lactobacillus plantarum relieves diarrhea caused by enterotoxin-producing Escherichia coli through inflammation modulation and gut microbiota regulation. Food Funct. (2020) 11:10362–74. doi: 10.1039/D0FO02670K
59. Liao YL, Jiang RL, Lonnerdal B. Biochemical and molecular impacts of lactoferrin on small intestinal growth and development during early life. Biochem Cell Biol. (2012) 90:476–84. doi: 10.1139/o11-075
60. Kongsted H, Jonach B, Haugegaard S, Angen Ø, Jorsal SE, Kokotovic B, et al. Microbiological, pathological and histological findings in four danish pig herds affected by a new neonatal diarrhoea syndrome. BMC Vet Res. (2013) 9:206. doi: 10.1186/1746-6148-9-206
61. Heo JM, Opapeju FO, Pluske JR, Kim JC, Hampson DJ, Nyachoti CM. Gastrointestinal health and function in weaned pigs: a review of feeding strategies to control post-weaning diarrhoea without using in-feed antimicrobial compounds. J Anim Physiol Anim Nutr. (2013) 97:207–37. doi: 10.1111/j.1439-0396.2012.01284.x
62. Tsukahara T, Inoue R, Nakatani M, Fukuta K, Kishino E, Ito T, et al. Influence of weaning age on the villous height and disaccharidase activities in the porcine small intestine. Anim Sci J. (2016) 87:67–75. doi: 10.1111/asj.12399
63. Yin YB, Guo SG, Wan D, Wu X, Yin YL. Enteroids: promising in vitro models for studies of intestinal physiology and nutrition in farm animals. J Agr Food Chem. (2019) 67:2421–8. doi: 10.1021/acs.jafc.8b06908
64. Li P, Zhou QQ, Gu Q. Complete genome sequence of Lactobacillus plantarum LZ227, a potential probiotic strain producing B-group vitamins. J Biotechnol. (2016) 234:66–70. doi: 10.1016/j.jbiotec.2016.07.020
65. Wylensek D, Hitch TCA, Riedel T, Afrizal A, Kumar N, Wortmann E, et al. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat Commun. (2020) 11:6389. doi: 10.1038/s41467-020-19929-w
Keywords: Lactobacillus, probiotics, piglets, antibiotic alternatives, intestinal morphology, growth performance
Citation: Zhu C, Yao J, Zhu M, Zhu C, Yuan L, Li Z, Cai D, Chen S, Hu P and Liu H-Y (2022) A meta-analysis of Lactobacillus-based probiotics for growth performance and intestinal morphology in piglets. Front. Vet. Sci. 9:1045965. doi: 10.3389/fvets.2022.1045965
Received: 16 September 2022; Accepted: 25 October 2022;
Published: 08 November 2022.
Edited by:
Yimin Jia, Nanjing Agricultural University, ChinaReviewed by:
Han Yunsheng, Feed Research Institute (CAAS), ChinaKris Angkanaporn, Chulalongkorn University, Thailand
Wei Zhong, University of North Carolina at Greensboro, United States
Copyright © 2022 Zhu, Yao, Zhu, Zhu, Yuan, Li, Cai, Chen, Hu and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ping Hu, pinghu@yzu.edu.cn; Hao-Yu Liu, haoyu.liu@yzu.edu.cn
†These authors have contributed equally to this work
 Cuipeng Zhu1†
Cuipeng Zhu1†  Demin Cai
Demin Cai Shihao Chen
Shihao Chen Ping Hu
Ping Hu Hao-Yu Liu
Hao-Yu Liu