The Effects of Prostaglandin E2 Treatment on the Secretory Function of Mare Corpus Luteum Depends on the Site of Application: An in vivo Study
- 1Department Reproductive Immunology and Pathology, Institute of Animal Reproduction and Food Research, Polish Academy of Sciences, Olsztyn, Poland
- 2Faculty of Veterinary Medicine, CIISA - Centre for Interdisciplinary Research in Animal Health, University of Lisbon, Lisbon, Portugal
We examined the effect of prostaglandin (PG) E2 on the secretory function of equine corpus luteum (CL), according to the application site: intra-CL injection vs. an intrauterine (intra-U) administration. Moreover, the effect of intra-CL injection vs. intra-U administration of both luteotropic factors: PGE2 and human chorionic gonadotropin (hCG) as a positive control, on CL function was additionally compared. Mares were assigned to the groups (n = 6 per group): (1) an intra-CL saline injection (control); (2) an intra-CL injection of PGE2 (5 mg/ml); (3) an intra-CL injection of hCG (1,500 IU/ml); (4) an intra-U saline administration (control); (5) an intra-U administration of PGE2 (5 mg/5 ml); (6) an intra-U administration of hCG (1,500 IU/5 ml). Progesterone (P4) and PGE2 concentrations were measured in blood plasma samples collected at −2, −1, and 0 (pre-treatment), and at 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after treatments. Moreover, effects of different doses of PGE2 application on the concentration of total PGF2α (PGF2α and its main metabolite 13,14-dihydro-15-keto-prostaglandin F2α– PGFM) was determined. The time point of PGE2, hCG, or saline administration was defined as hour “0” of the experiment. An intra-CL injection of PGE2 increased P4 and PGE2 concentrations between 3 and 4 h or at 3 and 12 h, respectively (p < 0.05). While intra-U administration of PGE2 elevated P4 concentrations between 8 and 24 h, PGE2 was upregulated at 1 h and between 3 and 4 h (p < 0.05). An intra-CL injection of hCG increased P4 concentrations at 1, 6, and 12 h (p < 0.05), while its intra-U administration enhanced P4 and PGE2 concentrations between 1 and 12 h or at 3 h and between 6 and 10 h, respectively (p < 0.05). An application of PGE2, dependently on the dose, supports equine CL function, regardless of the application site, consequently leading to differences in both P4 and PGE2 concentrations in blood plasma.
Introduction
Corpus luteum (CL) is critical for reproductive cyclicity and pregnancy maintenance, which depends on the supportive action of progesterone (P4) secreted by this transient endocrine gland (1–3). The lifespan of CL is controlled by numerous regulatory factors with luteotropic and luteolytic effects (4) such as cytokines, growth factors, P4, 17β-estradiol (E2), luteinizing hormone (LH), prostaglandin (PG) E2, and PGF2α, respectively (5–7). Some of these factors are widely applied in veterinary practice for estrus synchronization. Mostly, PGF2α is used for the regulation of the estrous cycles in the mare. However, application of PGE2 or LH analogs (human chorionic gonadotropin; hCG and equine chorionic gonadotropin; eCG) are also key areas of veterinarian interests in the control of equine reproduction. In addition, the interesting issues in the veterinary practice are different models of drug administration have been investigated in farm animals (5, 8, 9).
Human chorionic gonadotropin is a glycoprotein purified from the urine of pregnant women (10). This glycoprotein acts as LH, sharing the same receptor (1). The evidence for the presence of the LH/CGR receptor in the reproductive tract of humans and other domestic animals has been previously described (11, 12). Moreover, in mares, the LH receptor is expressed in the endometrium and myometrium during the estrous cycle and anestrus (13). Intramuscular (i.m.) (14), subcutaneous (s.c.) (10), or intravenous (i.v.) (15–17) hCG administration has shown a good efficacy in the induction of ovulation to improve the time of mating in mares. Moreover, in mares at early diestrus, i.m. (18) or i.v. (19) hCG application results in an increase in circulating progestin concentrations. Other studies using hCG found promising results in breeding mares. Intravenous hCG administration has been advocated for use to increase fertility and early equine pregnancy rates (20). In addition, the positive effect of i.v. or i.m. hCG administration on an additional CL formation and an increase in pregnancy rates have been reported in cattle (21–24). Therefore, in our study hCG was used as a control–reference luteotropic factor.
Prostaglandins are key factors in many reproductive processes in mammals, such as luteolysis, fertilization, maternal recognition of pregnancy, and implantation (5). It has been previously demonstrated that PG are produced by the CL in numerous species (25–30). Prostaglandin E2 is known as a luteotropic factor (1, 31, 32). Our preliminary in vitro study confirmed that PGE2 plays a luteotropic role as an auto-paracrine factor stimulating P4 production by equine luteal steroidogenic cells and CL tissues (33, 34). The effects of PGE2 are mediated by four receptor subtypes, which are encoded by different genes: EP1, EP2, EP3, and EP4 (35). The expression of the EP2 and EP4 receptors in the uterus during the estrous cycle and pregnancy has been reported in mares (36). In contrast to PGE2, PGF2α is the main luteolytic agent secreted in pulses from the uterine endometrium of numerous mammals during luteolysis including mares (37–40). Ginther et al. (41) demonstrated that pulses of PGF2α detected before the onset of luteolysis were less frequent per session and less prominent than during and after luteolysis.
According to our in vitro studies, in mare, many factors are involved in the secretion of PG from equine CL such as cytokine (42, 43) and from the endometrium such as P4, E2, oxytocin, LH, or cortisol (44–46) regulating modulating enzymatic cascade of AA metabolism. In the PG production cascade, prostaglandin–endoperoxide synthases (PTGS2) convert arachidonic acid (AA) into PGH2. The conversion of PGH2 into PGF2α and PGE2 is catalyzed by PGF2α synthases (PTGFS) and PGE2 synthases (PTGES), respectively. Prostaglandin H2 is converted to PGI2 by the action of PGI2 synthases (PTGIS) (47). In addition, PGE2 can be converted into PGF2α through PGE2-9-ketoreductase (PGE2-9-K) activation, an enzyme which works also as 20-α-hydroxysteroid dehydrogenase (20α-HSD), converting P4 into inactive 20-α-hydroxyprogesterone (20α-OHP) (4, 48). In mares, the aldo-keto reductase (AKR1C23), which has 20α-HSD activity, converting P4 to its inactive metabolite, was expressed in the CL (30, 48) and placenta during placentitis (49). Moreover, 15-hydroxyprostaglandin dehydrogenase (PGDH), which is involved in the first step of PG inactivation, leading into the generation of 15-keto-metabolites, was expressed in mares in the CL (50), gravity uterus (51), and presented from 150 days of gestation onwards (52). Similar mechanisms that involved the activity of PGE2-9-K were confirmed in the rabbit ovary (53, 54) and bovine placenta (55, 56). Therefore, due to the analysis of the action of PGE2, its conversion into PGF2α should also be considered. The above effect may depend on different interactions between luteotropic PGE2 and luteolytic PGF2α.
Many studies have discussed the benefits and disadvantages of different routes of PGE2 administration and its proper dosages in mares (32, 57, 58). While some studies reported intrafollicular PGE2 administration induced ovulation (58), in other studies intrauterine (intra-U) administration of PGE2 resulted in prolonged CL (32). Moreover, the positive influence of intracervical administration of PGE2 on the preparation of the uterine cervix to parturition in mares has been observed (59).
The area of research seeking the most effective routes and site for administration of luteotropic agents, used for manipulation of the reproductive processes in breeding mares, is still valuable for veterinary practitioners. To the best of our knowledge, no reports have demonstrated so far the action of PGE2 on equine mid-luteal CL (day 10 of the estrous cycle) secretory function according to its application site. Therefore, the objective of this study was to determine the effects of PGE2 on the secretory function of CL, according to the application site: ultrasound-guided intra-CL injection vs. intra-U administration. Moreover, the effect of intra-CL injection vs. intra-U administration of both luteotropic factors, PGE2 and hCG (as a positive control), on CL function was additionally compared. Possibility of the conversion of luteotropic PGE2 into luteolytic PGF2α dependently on the dose was also examined.
Materials and Methods
Animals and Surgical Procedures
Fifty-one clinically healthy, non-pregnant, and normally cycling mixed-breed mares (aged 3–13 years, weighing 400 ± 150 kg) were used. The study was conducted between April and September 2016 in Poland. Mares were housed in private stables and were provided ad libitum access to water and fed hay and cereal grain. Horses deemed otherwise healthy based on a veterinary physical examination. Animal procedures were conducted in accordance with the EU Directive of the European Parliament and the Council on the protection of animals used for scientific purposes (22 September 2010; no 2010/63/EU), the Polish Parliament Act on Animal Protection (21 August 1997, Journal of Laws 1997 No 111 item 724) with further updates—the Polish Parliament Act on the protection of animals used for scientific or educational purposes (15 January 2015, Journal of Laws 2015 item 266). All animal procedures were designed to avoid or minimize discomfort, distress, and pain to the animals. Procedures were reviewed and accepted following the guidelines of the Local Ethics Committee for Experiments on Animals in Olsztyn, Poland (Approval No. 51/2011). Animals had no abnormalities of the reproductive tract detected by ultrasonic imaging. Prior to the experiment, mares received two doses of a PGF2α analog (5 mg dinoprost, Dinolytic; Zoetis, Poland), 12 days apart, for synchronization of estrus. Follicular development was monitored in mares using transrectal palpation and USG at 12-h intervals during the periovulatory period until ovulation and every 2 days until day 10 (day 0 = day of ovulation). Moreover, structural changes of the CL during the entire estrous cycle were evaluated by ultrasonography with a 7.5-MHz linear probe (MyLabOne Vet Ultrasound System; ESOATE Pie Medica, Genoa, Italy), and visible signs of estrus (i.e., vaginal mucus and standing behavior) were assessed. In addition, the stage of the estrous cycle was confirmed by measurement of peripheral concentrations of P4 in blood plasma samples collected from mares. Figure 1 shows the in vivo study design where mares (n = 51) at day 10 of the estrous cycle were enrolled to the following experiments.
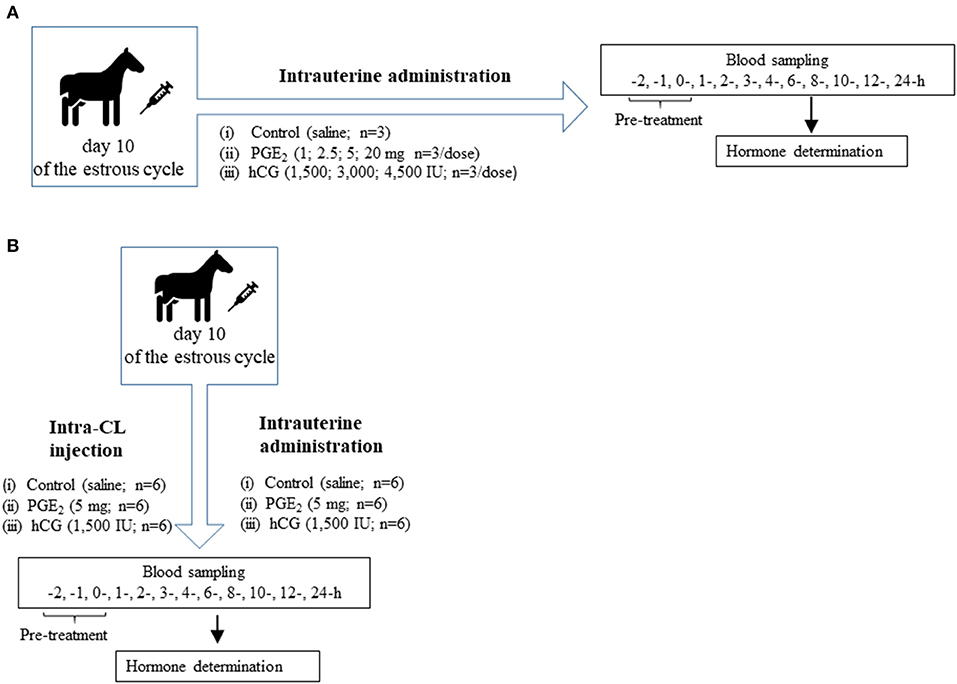
Figure 1. (A) Schematic diagram of experiment 1. Mares on day 10 of the estrous cycle were managed as follows: (1) One intrauterine saline administration (control group; n = 3); (2) One intrauterine administration of prostaglandin (PG) E2 (PGE2; 1 mg/5 ml, 2.5 mg/5 ml, 5 mg/5 ml, 20 mg/5 ml; n = 3 per dose); (3) One intrauterine administration of human chorionic gonadotropin (hCG; positive control; 1,500 IU/5 ml; 3,000 IU/5 ml, 4,500 IU/5ml; n = 3 per dose). After treatment (0 h), blood plasma samples were collected for 24 h throughout the experiment. (B) Schematic diagram of experiment 2. Mares on day 10 of the estrous cycle were managed as follows: (1) One intra-CL saline injection (control; n = 6); (2) One intra-CL injection of PGE2 (5 mg/ml; n = 6); (3) One intra-CL injection of hCG (positive, control; 1,500 IU/ml; n = 6); (4) One intrauterine saline administration (control group; n = 6); (5) One intrauterine administration of PGE2 (5 mg/5 ml; n = 6); (6) One intrauterine administration of hCG (positive control; 1,500 IU/5 ml; n = 6). After treatment (0 h), blood plasma samples were collected for 24 h throughout the experiment.
Intravenous Catheterization
Each mare was sedated with detomidine hydrochloride (Domosedan 0.01 mg/kg i.v.; Orion Pharma Poland Sp, Poland), followed by insertion of a temporary catheter (Intraflon IV cannulae 2.1 × 80 mm 14G, KRUUSE, 121805; KRUSSE Poland) into the jugular vein of mares. Intravenous catheters were flushed with heparinized saline and used for frequent blood sample collections.
An Intra-CL Injection
Caudal epidural anesthesia was achieved with 4 ml procaine hydrochloride (2% Polocainum Hydrochloricum; Biowet Drwalew, Poland). All intra-CL injections were administered under ultrasound guidance (7.5 MHz linear array transducer, MyLab 30 VET Gold Color Doppler Diagnostic Ultrasound System; ESOATE Pie Medica) through a sterile 1.25 × 50 mm (2-in. 18-gauge) ovum pick-up disposable veterinary injection needle (Bovivet, Poznan, Poland). The transducer and needle guide were coated with a sterile lubricant (Medicum, Lodz, Poland), and positioned within the vagina. The convex transducer was placed in the vagina against the vaginal fornix ipsilateral to the target ovary. The needle was then passed through the vaginal wall, and intraluteal treatments, PGE2 (PGE2, P0409; Sigma-Aldrich, Saint Louis, Missouri, USA) or hCG (Chorulon; Intervet International B.V., The Netherlands) dissolved in sterile saline solution (1 ml), were injected directly into the CL.
Intrauterine (Intra-U) Administration
The luteotropic factors were administrated directly into the uterine lumen of mares. The catheter was protected by a sanitary sheath that was broken immediately before the catheter passed through the opening of the cervix. Prostaglandin E2 or hCG dissolved in sterile saline solution was infused into the uterine horn using a 5-ml sterile syringe.
Experimental Design
Experiment 1. Dose-Dependent Effect of Prostaglandin E2 on CL Function, Compared With Human Chorionic Gonadotropin Action
Experiment 1 design is shown in Figure 1A. The dose-dependent effect of PGE2 on blood plasma P4 concentrations in mares on day 10 of the estrous cycle was determined as follows: (1) one intra-U saline administration (control group; n = 3); (2) one intra-U administration of PGE2 (1 mg/5 ml, 2.5 mg/5 ml, 5 mg/5 ml, 20 mg/5 ml; n = 3/per dose); (3) one intra-U administration of hCG (positive control; 1,500 IU/5 ml, 3,000 IU/5 ml, 4,500 IU/5 ml; n = 3/per dose).
Moreover, the possibility of PGE2 conversion into PGF2α, dependently on the dose, was also examined. The concentration of total PGF2α (PGF2α plus its main metabolite 13,14-dihydro-15-keto-prostaglandin F2α– PGFM) in blood plasma of mares on day 10 of the estrous cycle was determined after different doses of PGE2 application (Table 2). In mares, PGF2α in the uterine vein reaches systemic circulation and is metabolized in the lungs much via PGDH, resulting in lower concentrations of PGF2α (38, 60). The half-life of PGF2α in mares is short (94 s); therefore, plasma concentrations of PGFM are used to represent changes in PGF2α output (38, 60). The blood sampling was described in Blood Sampling section.
Experiment 2. The Comparison of Intra-CL Versus Intra-U Application Site of Prostaglandin E2 on CL Function, Compared With Human Chorionic Gonadotropin Action
Experiment 2 design is shown in Figure 1B. To investigate the effect of PGE2 according to the application site on the function of equine CL, mares on day 10 of the estrous cycle were managed as follows: (1) an intra-CL saline injection (control; n = 6); (2) one intra-CL injection of PGE2 (5 mg/ml; n = 6); (3) an intra-CL injection of hCG (1,500 IU/ml; n = 6); (4) one intra-U saline administration (control; n = 6); (5) one intra-U administration of PGE2 (5 mg/5 ml; n = 6); (6) one intra-U administration of hCG (1,500 IU/5 ml; n = 6). Mares (from experiment 1) with intra-U administrations of saline (n = 3), PGE2 (5 mg/5 ml n = 3), and hCG (1,500 IU/5 ml; n = 3) were used in experiment 2, respectively. The blood sampling is described in Blood Sampling section.
Blood Sampling
In mares, blood was aspirated frequently from the jugular vein according to the schedule: at −2, −1, and 0 (pre-treatment), and at 1, 2, 3, 4, 6, 8, 10, 12, and 24 h after injection/administration as shown in Figures 1A,B. The time point of intra-CL injection or intra-U administration of PGE2, hCG or saline was defined as hour “0” of the experiment. Blood was aspirated into sterile 10-ml tubes containing 100 μl of 0.3 M EDTA and 1% acetylsalicylic acid, pH 7.4. After centrifugation (2,000 × g for 10 min at 4°C), plasma was stored at −20°C for determination of P4, PGE2, PGF2α, and PGFM concentrations.
Hormone Determination
Progesterone concentration in blood plasma was measured in duplicates via RIA (P4125 104 I” RIA kit, Immunotech, Czech Republic, IM1188), according to the manufacturer's instructions. The standard curve for P4 ranged from 0.1 to 100 ng/ml. The intra- and inter-assay coefficients of variation (CV) were 6.5 and 8.6%, respectively.
Prostaglandin E2 was determined in blood samples using commercial ELISA kit (Enzyme Immunoassay kit; Enzo Life Science, Farmingdale, New York, USA, #ADI-901-001), according to the manufacturer's instructions. The standard curve for PGE2 ranged from 39.1 to 2,500 pg/ml. The sensitivity of the PGE2 assay was 13.4 pg/ml. The cross-reactivity for various prostaglandins and their metabolites was as follows: PGE2 100%, PGE1 70%, PGE3 16.3%, PGF1α 1.4%, PGF2α 0.7%, and 6-keto-PGF1α 0.6%. The intra- and inter-assay CV were 13.1% and 9.7%, respectively. The intra- and inter-assay CV were 5.8 and 5.1%, respectively.
13,14-Dihydro-15-keto-PGF2α (PGFM) was determined in blood samples using a commercial ELISA kit (PGFM Enzyme Competitive ELISA Kit, Invitrogen, Thermo Fisher Scientific, #EIAPGFM, UK), according to the manufacturer's instructions. The standard curve for PGFM ranged from 50 to 3,200 pg/ml. The sensitivity of the PGFM assay was 20.8 pg/ml. The cross-reactivity for various prostaglandins and their metabolites was as follows: PGFM 100%, PGEM 1.5%, PGF2α 0%, and PGE2 0%. The intra- and inter-assay CV were 7.5 and 9.6%, respectively.
Prostaglandin F2α was determined in blood samples using a commercial PGF2α ELISA kit (ENZO Life Sciences Inc., Farmingdale, NY, USA; ADI-901-069) according to the manufacturer's instructions. The standard curve for PGF2α ranged from 3.05 to 50,000 pg/ml. The sensitivity of the PGF2α assay was 6.71 pg/ml. The cross-reactivity for various prostaglandins and their metabolites was as follows: PGF2α 100%, PGF1α 11.82%, PGD2 3.62%, 6-keto-PGF1α 1.38%, PGI2 1.25%, and PGE2 0.77%. The intra- and inter-assay CV were 6.8 and 9.7%, respectively.
Statistical Analysis
For each statistical analysis, a Gaussian distribution was tested using D'Agostino and Pearson normality test (GraphPad Software version 8.3.0; GraphPad, San Diego, CA, USA). Parametric analyses were performed because normal distribution was assumed. Two-way ANOVA (GraphPad) test was used in experiment 1 (Supplementary Tables 1–3) and in experiment 2 (Supplementary Tables 4, 5). The results were considered significantly different at p < 0.05.
In experiment 1, the differences in P4 concentrations in blood plasma samples between groups treated with different doses of PGE2 or hCG and control group were measured as the area under the curve (AUC), using the total amount of P4 concentrations (mean ± SEM) secreted during the experiments (Table 1). The differences in concentrations of P4 (Figure 2), and total PGF2α concentrations (Table 2) in blood plasma samples in response to treatment with different doses of PGE2 or hCG were analyzed using a repeated measures design approach in which treatments and time of sample collection (h) were fixed effects and all interactions were included (two-way ANOVA test followed by Dunnett's multiple comparison test).
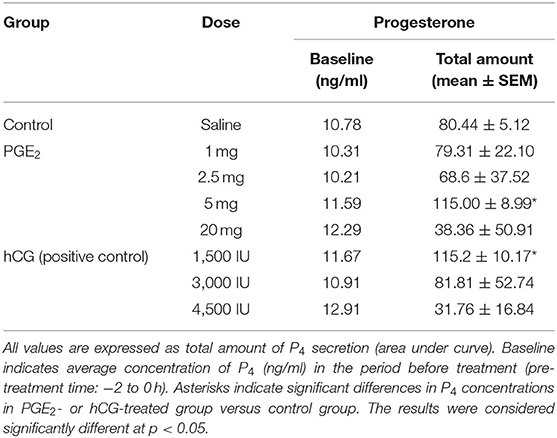
Table 1. The effect of one intrauterine administration of prostaglandin (PG) E2 or human chorionic gonadotropin (hCG; positive control) on progesterone (P4) concentrations in mares' blood plasma samples (n = 3 per dose) at day 10 of the estrous cycle.
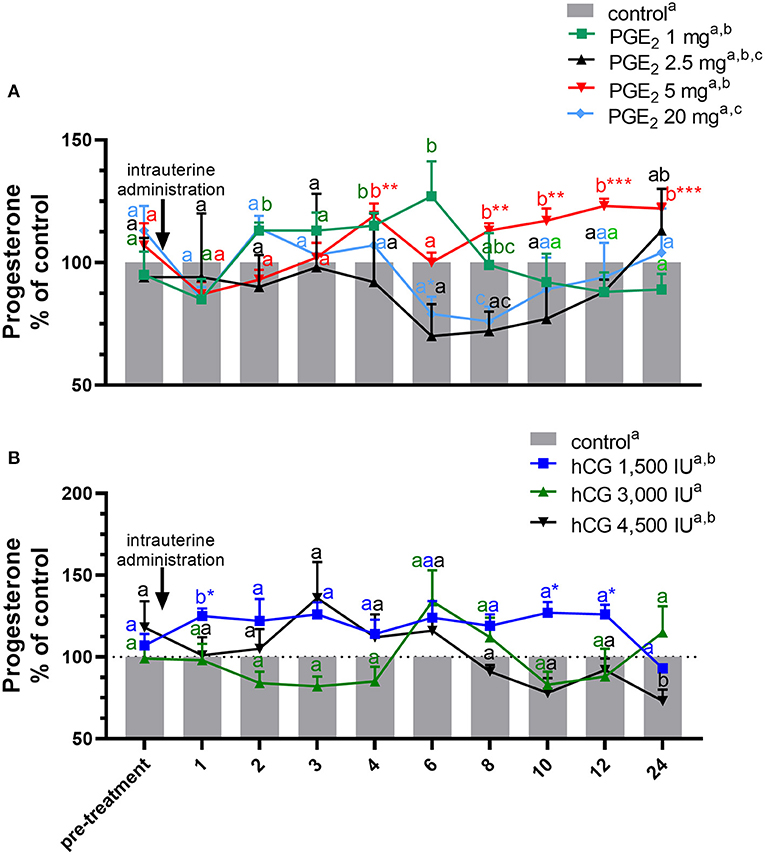
Figure 2. Concentration of progesterone (P4) in the jugular vein blood plasma in mares with one intrauterine administration of (A) four different doses of prostaglandin (PG) E2 (PGE2; 1, 2.5, 5, 20 mg/5 ml, n = 3/dose) and (B) three different doses of human chorionic gonadotropin (hCG; positive control; 1,500, 3,000, 4,500 IU/5 ml, n = 3/dose) on day 10 of the estrous cycle, compared with control groups. All values are presented as % of the control. Different superscript letters a, b, c indicate significant differences in P4 concentrations between PGE2- or hCG-treated group vs. control group at specific time points of blood sample collection. Asterisks indicate significant differences between P4 levels in PGE2- or hCG-treated group vs. average concentration of P4 in blood plasma in the period before treatment (pre-treatment time: −2 to 0 h). The results were considered significantly different at p < 0.05.
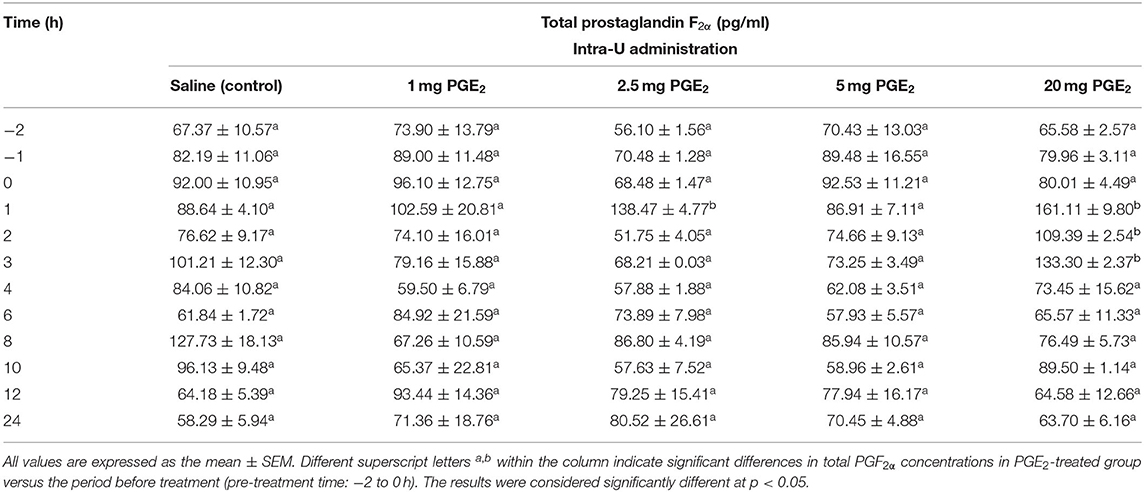
Table 2. The effect of one intrauterine administration of prostaglandin (PG) E2 on total prostaglandin F2α (the sum of PGF2α and PGF2α metabolite 13,14-dihydro-15-keto PGF2α–PGFM) concentrations in mares' blood plasma samples (n = 3 per dose) at day 10 of the estrous cycle.
In experiment 2, data were analyzed by two-way ANOVA (treatments vs. the differences in P4 and PGE2 concentrations) (Figures 3, 4) and blood plasma samples collected after application of PGE2 or hCG were analyzed using a repeated measures design approach in which treatments and time of sample collection (h) were fixed effects and all interactions were included (two-way ANOVA test followed by Dunnett's multiple comparison test). All values are presented as percentage of the control. The differences in P4 (Table 3) and PGE2 (Table 4) concentrations in blood plasma samples between PGE2 or hCG groups were measured as AUC, using the total amount of P4 or PGE2 concentrations secreted during the experiments and were calculated using two-way ANOVA, followed by Dunnett's multiple comparisons test.
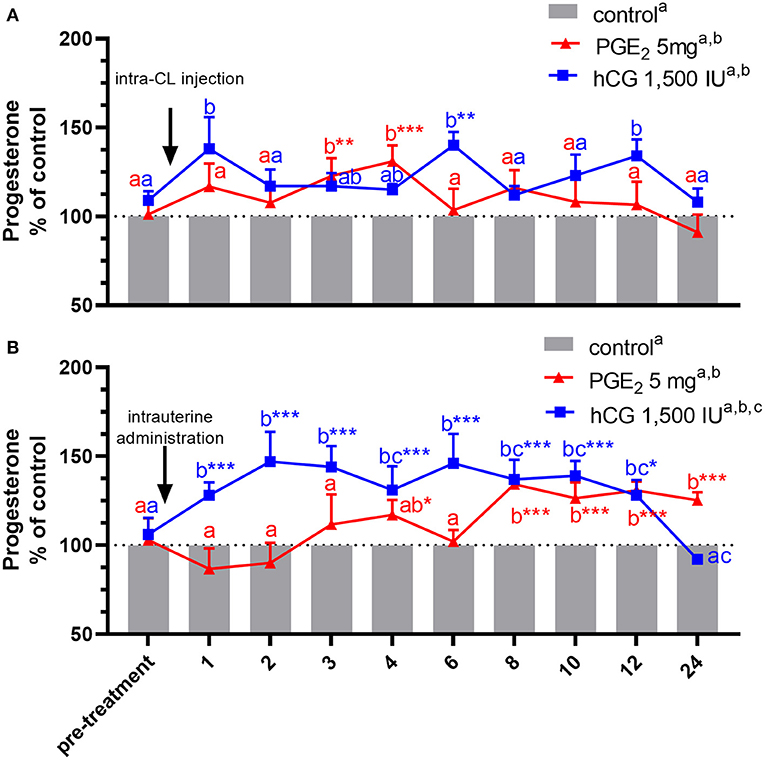
Figure 3. Concentrations of progesterone (P4) in the jugular vein blood plasma in mares with one (A) intra-CL injection of saline (control; gray bar), prostaglandin (PG) E2 (PGE2; 5 mg/ml; red line), or human chorionic gonadotropin (hCG, positive control; 1,500 IU/ml, blue line) or (B) one intrauterine administration of saline (control; gray bar), PGE2 (5 mg/5 ml; red line), or hCG (positive control; 1,500 IU/5 ml, blue line) on day 10 of the estrous cycle. All values were presented as % of the control. Different superscript letters a, b, c indicate significant differences between blood P4 level in PGE2- or hCG-treated groups of mares vs. control group at specific time points of blood sample collection. Asterisks indicate significant differences in blood P4 level within PGE2- or hCG-treated group of mares vs. average concentration of P4 in blood plasma in the period before treatment (pre-treatment time: −2 to 0 h). Average concentrations of P4 in the blood plasma samples of control mares during the period before treatment (pre-treatment time) were (A) 10.92 ng/ml or (B) 11.15 ng/ml, respectively. The results were considered significantly different at p <0.05.
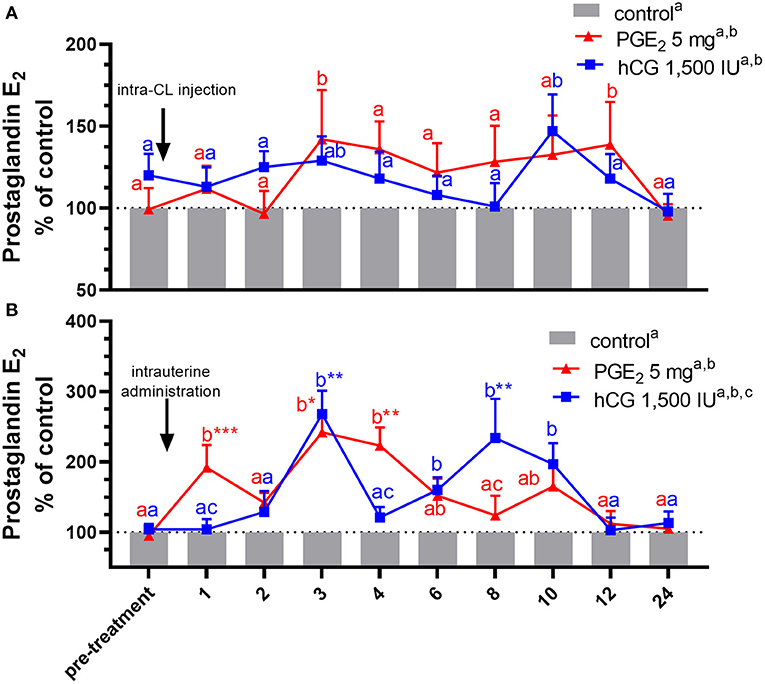
Figure 4. Concentrations of prostaglandin (PG) E2 in the jugular vein blood plasma in mares with one (A) intra-CL injection of saline (control; gray bar), PGE2 (5 mg/ml; red line), or human chorionic gonadotropin (hCG, positive control; 1,500 IU/ml, blue line) or (B) one intrauterine administration of saline (control; gray bar), PGE2 (5 mg/5 ml; red line), or hCG (positive control; 1,500 IU/5ml, blue line) on day 10 of the estrous cycle. All values are presented as % of the control. Different superscript letters a, b, c indicate significant differences between blood PGE2 level in PGE2- or hCG-treated groups of mares vs. control group at specific time points of blood sample collection. Asterisks indicate significant differences in blood PGE2 level within PGE2- or hCG-treated group of mares vs. average concentration of PGE2 in the period before treatment (pre-treatment time: −2 to 0 h). Average concentrations of PGE2 in the blood plasma samples of control mares during the period before treatment (pre-treatment time) were (A) 243.39 ng/ml or (B) 264.18, respectively. The results were considered significantly different at p < 0.05.
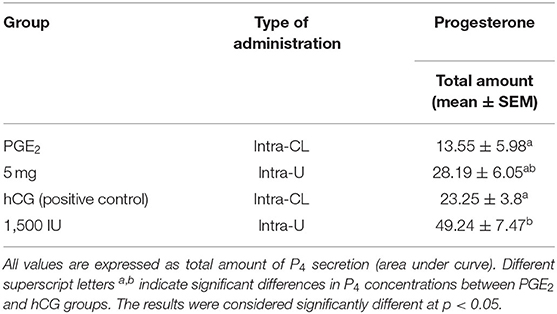
Table 3. The effect of one intra-CL injection or one intrauterine administration of prostaglandin (PG) E2 or human chorionic gonadotropin (hCG; positive control) on progesterone (P4) concentrations in mares' blood plasma samples (n = 6 per group) at day 10 of the estrous cycle.
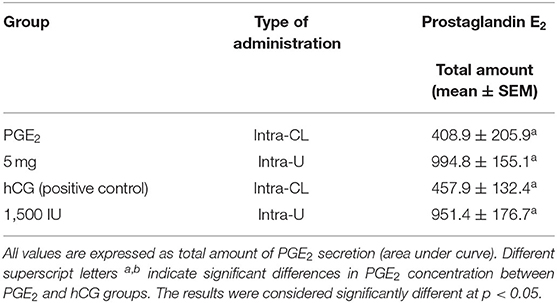
Table 4. The effect of one intra-CL injection or one intrauterine administration of prostaglandin (PG) E2 or human chorionic gonadotropin (hCG; positive control) on PGE2 concentrations in mares' blood plasma samples (n = 6 per group) at day 10 of the estrous cycle.
Results
Experiment 1. Dose-Dependent Effect of Prostaglandin E2 on CL, Compared With Human Chorionic Gonadotropin Action
In mares, only one intra-U administration of PGE2 at the dose of 5 mg/5 ml increased the total amount of P4 concentrations in blood plasma, compared with the control group (p < 0.05; Table 1). An increase in P4 concentrations was observed at 4 h and between 8 and 24 h after intra-U administration of PGE2 at the dose of 5 mg/5 ml, compared with the control group and to its concentrations in the period before treatment (pre-treatment time) (p < 0.05; Figure 2A).
An increase in P4 concentrations in blood plasma was observed at 2 h and between 4 h and 6 h after intra-U administration of PGE2 at the dose of 1 mg/5 ml, compared with the control group (p < 0.05; Figure 2A), while intra-U administration of PGE2 at the dose of 20 mg/5 ml decreased its concentrations at 6 h compared with the pre-treatment time, and at 8 h, compared with the control group (p < 0.05; Figure 2A).
The total amount of P4 concentrations increased in blood plasma only after one intra-U administration of hCG at the dose of 1,500 IU/5 ml, compared with the control group (p < 0.05; Table 1). An increase in P4 concentrations was observed at 1 h after one intra-U administration of hCG at the dose of 1,500 IU/5 ml compared with the control group (p < 0.05; Figure 2B). Moreover, an increase in P4 concentrations was noticed at 1 h and between 10 and 12 h after intra-U hCG administration at the dose of 1,500 IU/5 ml, compared with its concentrations in the pre-treatment time (p < 0.05; Figure 2B).
An intra-U administration of hCG at the dose of 4,500 IU/5 ml decreased P4 concentrations at 24 h, compared with the control group (p < 0.001; Figure 2B).
Concentrations of PGF2α and its metabolite PGFM (total PGF2α) in blood plasma samples increased at 1 h after an intra-U administration of PGE2 at the dose of 2.5 mg/5 ml, compared with its concentrations in the pre-treatment time (p < 0.05; Table 2). Moreover, an increase in total PGF2α concentrations was observed between 1 and 3 h after an intra-U administration of PGE2 at the dose of 20 mg/5 ml, compared with its concentrations in the pre-treatment time (p < 0.05; Table 2).
Experiment 2. Comparison of Intra-CL vs. Intra-U Application of Prostaglandin E2 on Corpus Luteum Function, Compared With Human Chorionic Gonadotropin Action
An increase in P4 concentrations in blood plasma samples was noticed in mares, between 3 and 4 h after receiving one intra-CL injection of PGE2, compared with its concentrations in the pre-treatment time within PGE2-treated group (p < 0.01), and with respect to the control mares (p < 0.01; Figure 3A). At the same time, P4 concentrations increased between 8 and 24 h after intra-U administration of PGE2, compared with P4 concentrations in the pre-treatment time within PGE2-treated group (p < 0.001), as well as compared with the control mares (p < 0.05; Figure 3B).
In mares, an intra-CL injection of hCG elevated P4 levels at 6 h, compared with P4 levels in the pre-treatment time within PGE2-treated group (p < 0.05; Figure 3A). Moreover, one intra-CL injection of hCG (positive control) increased P4 concentrations in blood plasma at 1, 6, and 12 h after its application, compared with the control group (p < 0.001; Figure 3A), while its intra-U administration elevated P4 concentrations between 1 and 12 h, compared with the control group, and to P4 concentrations in the pre-treatment time within this group of mares (p < 0.05; Figure 3B). Total amount of P4 found in mares with intra-U administration of hCG was greater compared with total amount of P4 in mares with its intra-CL injection (p < 0.05; Table 3).
In mares, an intra-CL injection of PGE2 increased PGE2 concentrations in blood plasma at 3 and 12 h after its administration, compared with the control group (p < 0.05; Figure 4A), while an intra-CL injection of hCG (positive control) increased its concentrations at 10 h after injection, compared with the control group (p < 0.05; Figure 4A). Prostaglandin E2 concentrations were elevated after intra-U administration of PGE2 at 1 h and between 3 and 4 h, relative to the control mares (p < 0.001), and to PGE2 levels in the pre-treatment time (p < 0.05; Figure 4B). Moreover, intra-U administration of hCG (positive control) increased PGE2 concentrations at 3 h and between 6 and 10 h after its administration, compared with the control group (p < 0.01; Figure 4B), and at 3 and 8 h after hCG administration, compared with PGE2 levels in the pre-treatment time (p < 0.01; Figure 4B). No differences in the total amount of PGE2 were observed between mares with intra-U administration and intra-CL injection (p > 0.05; Table 4).
Discussion
Until now, many studies have been focusing on the different application route or sites of luteolytic/luteotropic factors that may be used in veterinary practices to regulate the estrous cycle in mares. In the literature, different ways of PGE2 or hCG administrations have been demonstrated, for example, i.m., i.v., s.c., intrafollicular, or intracervical (10, 14, 17, 18, 58, 59). The ultrasound-guided intra-CL injection as a method for studying the direct effect of PGF2α on reproductive function in mares was evaluated by Weber et al. (61). However, this technique is not widely known by practitioners. While intra-U administration of luteotropic PGE2 on the CL function was described by Vanderwall et al. (32), in our study we demonstrated the effect of luteotropic factor PGE2 on P4 secretion, depending on the application site: intra-CL vs. intra-U in mares at day 10 of the estrous cycle. To the best of our knowledge, for the first time, we have showed that application of PGE2 supports equine CL secretory function, regardless of the application site, consequently leading to differences in both P4 and PGE2 concentrations in blood plasma.
The role of PGE2 on equine CL function is not fully understood. A previous in vitro study in cows confirmed that PGE2 participates in luteoprotective mechanisms required for CL formation and maintenance (62), and stimulates the P4 production by luteal steroidogenic cells (63). Moreover, in cows and ewes, there have been evidences that PGE1 or PGE2 prevented P4-induced premature luteolysis by suppressing the loss of luteal LH receptors (64, 65).
Interestingly, our study shows that the action of PGE2 on CL secretory function is determined by the application site and dose. An intra-CL injection of PGE2 increased P4 concentrations in blood plasma of mares at day 10 of the estrous cycle compared with the control group, suggesting its direct action. The aforementioned data are in agreement with a preliminary study conducted by our group (33, 34), showing that in mares PGE2 plays a luteotropic role as an auto-paracrine factor stimulating P4 production by luteal steroidogenic cells and CL tissues in vitro. Some decades ago, Vanderwall et al. (32) reported that a single intra-U administration of PGE2 was capable to maintain prolonged luteal function in the mare in vivo. In the experiment of Vanderwall et al. (32), non-pregnant mares were continuously infused with 0.24 mg of PGE2, from day 10 to 16 postestrus, using an osmotic minipump surgically placed into the uterine lumen. In our study, intra-U administration of PGE2 increased P4 concentrations in blood plasma on day 10 of the estrous cycle in mares, compared with the control group. Simple comparison between data obtained in our study and in the study of Vanderwall et al. (32) cannot be made because of differences in methodology of PGE2 application. We should take into account that in our study, whereas P4 concentrations increased at 3–4 h after direct intra-CL injection of PGE2, the positive effect of intra-U administration of PGE2 on P4 concentrations was observed between 8 and 24 h after treatment. We suppose that the aforementioned effect is a result of indirect action of PGE2 on PGE2 receptors in the uterus, involving the regulation of vasculature events and induction of other luteotropic factors engaged in luteal support, in the equine endometrium (e.g., growth factors, nitric oxide, and cytokines). Galvão et al. (42, 66) showed that cytokines interact with nitic oxide synthases and influence luteal angiogenesis in mares as angiogenic factors themselves can also modulate luteal secretory function. Previously, Otzen et al. (67) found that PGE2 stimulates vascular endothelial growth factor (VEGF), which participates in the regeneration and expansion of the equine uterine blood vessel network. Moreover, VEGF has been reported to effectively modulate luteal secretory function of equine CL (P4 and PGE2 production) (66).
In the first experiment, the dose of PGE2 5 mg/5 ml was chosen as an effective dose based on an increase in P4 concentrations in blood samples after intra-U treatments in mares. We demonstrated that the highest dose of PGE2 administered into the uterus does not affect P4 concentrations in blood plasma. Therefore, we can suspect the possibility of the conversion of PGE2 by the PGE2-9-K into PGF2α. It is known that PGE2-9-K enzyme has also a 20 α-HSD activity, and in fact converts P4 into 20α-OH-P4, which may contribute to the decrease of P4 induced by PGF2α (4, 48). To check and confirm this fact, we examined the effect of an intra-U administration of different doses of PGE2 on total PGF2α (sum of PGF2α and its main metabolite—PGFM) concentrations in blood plasma on day 10 of the estrous cycle in mares. Interestingly, we observed higher total PGF2α concentrations in blood plasma between 1 and 3 h after intra-U administration of PGE2 at the highest dose (20 mg/5 ml), compared with its concentrations in the pre-treatment time. Hence, our in vivo results should be interpreted carefully and our hypothesis that the lack of the effect of PGE2 in the highest dose on P4 concentrations may be related to its conversion into PGF2α by PGE2-9-K needs further studies in mares.
In our study, we assume that intra-CL injection and intra-U administration of PGE2 increased its own concentration in blood plasma. There is evidence that in the endometrium of mare, PGF2α has an auto-amplification system, stimulating its own production (40). Therefore, future study should be planned to assume whether there is a positive PGE2 feedback loop and whether PGE2 has a positive effect on its own production.
There are a large number of in vivo studies concerning the effect of hCG on CL function in mare (16–19, 68). Kelly et al. (18) and Watson et al. (19) demonstrated the positive luteotropic effect of hCG on P4 secretion. Therefore, in our study, we decided to assign mares treated with intra-CL injection or intra-U administration of hCG as positive control group. In the present study, we observed an increase in P4 concentration in blood plasma after intra-U administration of 1,500 IU of hCG. No effect on CL function was reported by Brito et al. (68), using one i.v. injection of this same dose-−1,500 IU of hCG at day 10 after ovulation. In agreement with our results, a positive effect on P4 secretion was observed in diestrus mares, using repeated i.m. injections of 1,000 IU of hCG (days 3, 4, 5) (18) or a single i.v. injection of 1,500 IU of hCG (day 8) (19). Interestingly, in our study one intra-U administration of hCG at the doses 3,000 IU or 4,500 IU did not affect P4 secretion from equine CL. Likewise, Köhne et al. (16) did not observe any increase in P4 concentration and luteal size after i.v. administration of 5,000 IU of hCG at day 5 after ovulation. Therefore, it might be suggested that higher doses of hCG are not related to their effectiveness. We have noted that both a single intra-CL injection of hCG and a single intra-U administration of hCG increased blood P4 concentrations, supporting P4 secretion from mare CL. The intra-CL injection of hCG seems to directly influence the luteal steroidogenic cells. An additional in vitro study should be conducted to explore molecular mechanisms involved in the CL secretory function in response to intra-CL injection of hCG. Unexpectedly, the intra-U administration of hCG was more effective in increasing P4 secretion by CL (Table 3), throughout its indirect effect on equine PGE2 receptors in the uterus, affecting regulation of vasculature events and induction of luteotropic factors involved in luteal support.
Human chorionic gonadotropin has structural and functional similarities with LH, sharing the same receptor with this luteotropic hormone (1). The evidence for the presence of the LH/CGR receptor in the reproductive tract of humans and other domestic animals is well described (11, 12). In mares, LH receptor expression occurs in the CL (69) and in the endometrium and myometrium during the estrous cycle and anestrus (13). Therefore, the presence of LH/CGR receptors in equine reproductive tract could mediate the indirect effect of intra-U administration of hCG and the direct effect of hCG injection into the CL. Interestingly, in the present study, we show that only one intra-U administration of hCG increases PGE2 concentration in blood plasma. We have previously demonstrated that LH stimulated PGE2 secretion by equine endometrium and myometrium (45). We postulate that hCG through LH/CGR receptors in the mare uterus affects the luteotropic PGE2 production. Moreover, PGE2 has a positive effect on P4 secretion. However, further studies are needed to clarify the mechanism of action of hCG on PGE2 production within the equine reproductive tract.
In conclusion, the aforementioned results indicate the importance of proper application site of drugs and may influence drug delivery strategies in veterinary medicine. Application of PGE2 supports equine CL function via augmentation of P4 and PGE2 secretions. Progesterone secretion in response to PGE2 depends on their application site. In the present study, we found more effective increase in P4 secretion after intra-U administration of luteotropic factors (especially hCG) than their intra-CL injections. Therefore, the efficacy of intra-CL site of application warrants further in vitro and in vivo studies. We confirm that therapeutic use of intra-U administration of luteotropic factors is an easily applicable, valuable method in veterinary practice that may be used to support early pregnancy in mares. However, this knowledge is still insufficient and needs better understanding of the endocrine, cellular, receptor, and molecular mechanism action of luteotropic factors on equine CL function.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by Local Ethics Committee for Experiments on Animals, University of Warmia and Mazury in Olsztyn, Poland (Approval No. 51/2011). Written informed consent was obtained from the owners for the participation of their animals in this study.
Author Contributions
KKP-T: conceptualization, investigation, methodology, formal analysis, visualization, writing—original draft, and writing—review and editing. AWJ: investigation, methodology, formal analysis, visualization, writing—original draft, and writing—review and editing. AZS-M: conceptualization, investigation, methodology, formal analysis, writing—original draft, and writing—review and editing. EŻ: formal analysis. GF-D: supervision and writing—review and editing. DJS: conceptualization, investigation, formal analysis, supervision, funding acquisition, and writing—review and editing. All authors have read, critically revised, and approved the final version of the article.
Funding
This work was supported by the National Science Center in Poland (2011/02/A/NZ5/00338).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank M. Szwichtenberg (Private horse stable Stajnia pod Klonem, Wiezyca, Poland) for his cooperation in providing animals for the study from the farm in Wiezyca. We are grateful to P. Warmowski (Private Veterinary Clinic Taurus, Kartuzy, Poland) for his veterinary assistance during the experiments. The authors wish to thank K. Jankowska, A. Bacławska, W. Krzywiec, and the Institute of Animal Reproduction and Food Research, Polish Academy of Science, Olsztyn, Poland, for technical support in the experiments.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2021.753796/full#supplementary-material
References
1. Niswender GD, Juengel JL, Silva PJ, Rollyson MK, McIntush EW. Mechanisms controlling the function and life span of the corpus luteum. Physiol Rev. (2000) 80:1–29. doi: 10.1152/physrev.2000.80.1.1
2. Ginther OJ. Endocrinology of the ovulatory season. In: Ginther OJ, editor. Reproductive Biology of the Mare, 2nd edn. Wisconsin: Equiservices. (1992). p. 233–90.
3. Pinto CRF. Impact of the corpus luteum on survival of the developing embryo and early pregnancy in mares. Theriogenology. (2020) 1:374–81. doi: 10.1016/j.theriogenology.2020.02.011
4. Zerani M, Polisca A, Boiti C, Maranesi M. Current knowledge on the multifactorial regulation of corpora lutea lifespan: the rabbit model. Animals. (2021) 11:296. doi: 10.3390/ani11020296
5. Weems CW, Weems YS, Randel RD. Prostaglandins and reproduction in female farm animals. Vet J. (2006) 171:206–28. doi: 10.1016/j.tvjl.2004.11.014
6. Skarzynski DJ, Piotrowska-Tomala KK, Lukasik K, Galvão A, Farberov S, Zalman Y, et al. Growth and regression in bovine corpora lutea: regulation by local survival and death pathways. Reprod Domest Anim. (2013) 48:25–37. doi: 10.1111/rda.12203
7. McCracken JA, Custer EE, Lamsa JC. Luteolysis: a neuroendocrine- mediated event. Physiol Rev. (1999) 79:263–323. doi: 10.1152/physrev.1999.79.2.263
8. Masello M, Scarbolo M, Schneck MV, Perez MM, Schillkowsky EM, Sitko EM, et al. Intravaginal instillation of prostaglandin F2α was as effective as intramuscular injection for induction of luteal regression in lactating dairy cows. J Dairy Sci. (2020) 103:2743–55. doi: 10.3168/jds.2019-17589
9. Dordas-Perpinyà M, Normandin L, Dhier T, Terris H, Cochard A, Frilley C, et al. Single injection of triptorelin or buserelin acetate in saline solution induces ovulation in mares the same as a single injection of hCG. Reprod Domest Anim. (2020) 55:374–83. doi: 10.1111/rda.13632
10. Morel MCGD, Newcombe JR. The efficacy of different hCG dose rates and the effect of hCG treatment on ovarian activity: ovulation, multiple ovulation, pregnancy, multiple pregnancy, synchrony of multiple ovulation in the mare. Anim Reprod Sci. (2008) 109:189–99. doi: 10.1016/j.anireprosci.2007.10.005
11. Fields MJ, Shemesh M. Extragonadal luteinizing hormone receptors in the reproductive tract of domestic animals. Biol Reprod. (2004) 71:1412–8. doi: 10.1095/biolreprod.104.027201
12. Cole LA. Biological functions of hCG and hCG-related molecules. Reprod Biol Endocrinol. (2010) 8:102–15. doi: 10.1186/1477-7827-8-102
13. Esmeraldino AT, Malschitzky E, Fiala SM, Santarém L, Wolf CA, Jobim MIM, et al. Immunohistochemical identification of luteinizing hormone receptors in the extra-gonadal reproductive tract of the mare. Anim Reprod Sci. (2010) 121:38–9. doi: 10.1016/j.anireprosci.2010.04.132
14. Kilicarslan MR, Horoz H, Senunver A, Konuk SC, Tek C, Carioglu B. Effect of GnRH and hCG on ovulation and pregnancy in mares. Vet Rec. (1996) 3:119–20. doi: 10.1136/vr.139.5.119
15. Wilson CG, Downie CR, Hughes JP, Roser J. Effects of repeated hCG injections on reproductive efficiency in mares. J Equine Vet Sci. (1990) 10:301–8. doi: 10.1016/S0737-0806(06)80015-8
16. Köhne M, Kuhl J, Ille N, Erber R, Aurich C. Treatment with human chorionic gonadotrophin before ovulation increases progestin concentration in early equine pregnancies. Anim Reprod Sci. (2014) 149:187–93. doi: 10.1016/j.anireprosci.2014.07.002
17. Alonso MA, Silva LA, Affonso FJ, Lemes KM, Celeghini ECC, Lançoni R, et al. Effect of hCG application at different moments of the estrous cycle on corpus luteum and uterine vascularization and serum progesterone concentration in mares. Anim Reprod. (2019) 16:317–27. doi: 10.21451/1984-3143-AR2018-0103
18. Kelly CM, Hoyer PB, Wise ME. In- vitro and in-vivo responsiveness of the corpus luteum of the mare and effect of hCG. J Reprod Fertil. (1988) 84:593–600. doi: 10.1530/jrf.0.0840593
19. Watson ED, Colston M, Broadley C. LH and progesterone concentrations during diestrus in the mare and the effect of hCG. Theriogenology. (1995) 43:1325–37. doi: 10.1016/0093-691X(95)00117-Q
20. Barbacini S, Zavaglia G, Gulden P, Marchi V, Necchi D. Retrospective study on the efficacy of hCG in an equine artificial insemination program using frozen semen. Equine Vet Educ. (2000) 12:312–7. doi: 10.1111/j.2042-3292.2000.tb00067.x
21. Fricke PM, Reynolds LP, Redmer DA. Effect human chorionic gonadotropin administered early in the estrous cycle on ovulation and subsequent luteal function in cows. J Anim Sci. (1993) 71:1242–6. doi: 10.2527/1993.7151242x
22. Breuel KF, Spitzer JC, Thompson CE, Breuel JF. First-service pregnancy rate in beef heifers as influenced by human chorionic gonadotropin administration before and/or after breeding. Theriogenology. (1990) 34:139–45. doi: 10.1016/0093-691X(90)90585-H
23. Santos JE, Thatcher WW, Pool L, Overton MW. Effect of human chorionic gonadotropin on luteal function and reproductive performance of high-producing lactating Holstein dairy cows. J Anim Sci. (2001) 79:2881–94. doi: 10.2527/2001.79112881x
24. Nishigai M, Kamomae H, Tanaka T, Kaneda Y. Improvement of pregnancy rate in Japanese Black cows by administration of hCG to recipients of transferred frozen- thawed embryos. Theriogenology. (2002) 58:1597–606. doi: 10.1016/S0093-691X(02)01062-2
25. Diaz FJ, Anderson LE, Wu YL, Rabot A, Tsai SJ, Wiltbank MC. Regulation of progesterone and prostaglandin F2α production in the CL. Mol. Cell Endocrinol. (2002) 191:65–80. doi: 10.1016/S0303-7207(02)00056-4
26. Aroshi JA, Banu SK, Chapdelaine P, Madore E, Sirois J, Fortier MA. Prostaglandin biosynthesis, transport, and signaling in corpus luteum: a basis for autoregulation of luteal function. Endocrinology. (2004) 145:2551–60. doi: 10.1210/en.2003-1607
27. Zerani M, Dall'Aglio C, Maranesi M, Gobbetti A, Brecchia G, Mercati, et al. Intraluteal regulation of prostaglandin F2α-induced prostaglandin biosynthesis in pseudopregnant rabbits. Reproduction. (2007) 133:1005–116. doi: 10.1530/REP-06-0107
28. Skarzynski DJ, Siemieniuch MJ, Pilawski W, Woclawek-Potocka I, Bah MM, Majewska M, et al. In vitro assessment of progesterone and prostaglandin e(2) production by the corpus luteum in cattle following pharmacological synchronization of estrus. J Reprod Dev. (2009) 55:170–6. doi: 10.1262/jrd.20121
29. Parillo F, Catone G, Maranesi M, Gobbetti A, Gasparrini B, Russo, et al Immunolocalization, gene expression, and enzymatic activity of cyclooxygenases, prostaglandin E2-9-ketoreductase, and nitric oxide synthases in Mediterranean buffalo (Bubalus bubalis) corpora lutea during diestrus. Microsc Res Tech. (2012) 75:1682–90. doi: 10.1002/jemt.22116
30. Kozai K, Hojo T, Tokuyama S, Szóstek AZ, Takahashi M, Sakatani M. Expression of aldo-keto reductase 1C23 in the equine corpus luteum in different luteal phases. J Reprod Dev. (2014) 60:150–4. doi: 10.1262/jrd.2013-120
31. Zavy MT, Bazer FW, Sharp DC, Frank M, Thatcher WW. Uterine luminal prostaglandin F in cycling mares. Prostaglandins. (1978) 16:643–50. doi: 10.1016/0090-6980(78)90194-6
32. Vanderwall DK, Woods GL, Weber JA, Lichtenwalner AB. Corpus luteal function in nonpregnant mares following intrauterine administration of prostaglandin E(2) or estradiol-17b. Theriogenology. (1994) 42:1069–83. doi: 10.1016/0093-691X(94)90855-9
33. Lukasik K, Gola B, Galvao AM, Ferreira-Dias GM, Skarzynski DJ. Effect of prostaglandin E2 and F2α on progesterone production of equine corpus luteum cells. In: Reproduction in Domestic Animal 45. 14th Annual Conference of the European Society for Domestic Animal Reproduction. Eger (2010).
34. Lukasik K, Szóstek A, Galvao A, Hojo T, Okuda K, Skarzynski DJ. Auto-paracrine action of prostaglandins E2 and F2α in equine corpus luteum. J Equine Vet Sci. (2014) 34:120. doi: 10.1016/j.jevs.2013.10.081
35. Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and function. Physiol Rev. (1999) 79:1193–226. doi: 10.1152/physrev.1999.79.4.1193
36. Silva ESM, Scoggin KE, Canisso IF, Troedsson MHT, Squires EL, Ball BA. Expression of receptors for ovarian steroids and prostaglandin E2 in the endometrium and myometrium of mares during estrus, diestrus and early pregnancy. Anim Reprod Sci. (2014) 30:169–81. doi: 10.1016/j.anireprosci.2014.11.001
37. Lytton FD, Poyser NL. Prostaglandin production by the rabbit uterus and placenta in vitro. J Reprod Fertil. (1982) 66:591–9. doi: 10.1530/jrf.0.0660591
38. Ginther OJ, Beg MA. The hour of transition into luteolysis in horses and cattle: a species comparison. Theriogenology. (2012) 77:1731–40. doi: 10.1016/j.theriogenology.2012.01.001
39. Ginther OJ, Beg MA. Dynamics of circulating progesterone concentrations before and during luteolysis: a comparison between cattle and horses. Biol Reprod. (2012) 86:170, 1–12. doi: 10.1095/biolreprod.112.099820
40. Kozai K, Tokuyama S, Szóstek AZ, Toishi Y, Tsunoda N, Taya K, et al. Evidence for a PGF2α auto-amplification system in the endometrium in mares. Reproduction. (2016) 151:517–26. doi: 10.1530/REP-15-0617
41. Ginther OJ, Rodrigues BL, Ferreira JC, Araujo RR, Beg MA. Characterisation of pulses of 13,14- dihydro-15-keto-PGF2 alpha (PGFM) and relationships between PGFM pulses and luteal blood flow before, during, and after luteolysis in mares. Reprod Fertil Dev. (2008) 20:684–93. doi: 10.1071/RD08077
42. Galvão AM, Szóstek AZ, Skarzynski DJ, Ferreira-Dias GM. Role of tumor necrosis factor-α, interferon-γ and Fas-ligand on in vitro nitric oxide activity in the corpus luteum. Cytokine. (2013) 64:18–21. doi: 10.1016/j.cyto.2013.07.015
43. Galvão AM, Ferreira-Dias GM, Chełmonska-Soyta A, Wocławek-Potocka I, Skarzynski DJ. Immune-endocrine cross-talk in reproductive biology and pathology. Mediators Inflamm. (2014) 2014:856465. doi: 10.1155/2014/856465
44. Szóstek AZ, Galvão AM, Ferreira-Dias GM, Skarzynski DJ. Ovarian steroids affect prostaglandin production in equine endometrial cells in vitro. J Endocrinol. (2014) 30:263–76. doi: 10.1530/JOE-13-0185
45. Piotrowska-Tomala KK, Jonczyk AW, Skarzynski DJ, Szóstek-Mioduchowska AZ. Luteinizing hormone and ovarian steroids affect in vitro prostaglandin production in the equine myometrium and endometrium. Theriogenology. (2020) 153:1–8. doi: 10.1016/j.theriogenology.2020.04.039
46. Szóstek-Mioduchowska AZ, Shiotani H, Yamamoto Y, Sadowska A, Wójtowicz A, Kozai K, et al. Effects of cortisol on prostaglandin F2α secretion and expression of genes involved in the arachidonic acid metabolic pathway in equine endometrium- in vitro study. Theriogenology. (2021) 173:221–9. doi: 10.1016/j.theriogenology.2021.08.009
47. Simmons DL, Botting RM, Hla T. Cyclooxygenase isozymes: the biology of prostaglandin synthesis and inhibition. Pharmacol Rev. (2004) 56:387–437. doi: 10.1124/pr.56.3.3
48. Brown KA, Boerboom D, Bouchard N, Doré M, Lussier JG, Sirois J. Human chorionic gonadotropin-dependent induction of an equine aldo-keto reductase (AKR1C23) with 20α-hydroxysteroid dehydrogenase activity during follicular luteinization in vivo. J Mol Endocrinol. (2006) 36:449–61. doi: 10.1677/jme.1.01987
49. El-Sheikh Ali H, Legacki EL, Scoggin KE, Loux SC, Dini P, Esteller-Vico A. Steroid synthesis and metabolism in the equine placenta during placentitis. Reproduction. (2020) 159:289–302. doi: 10.1530/REP-19-0420
50. Sayasith K, Bouchard N, Doré M, Sirois J. Cloning of equine prostaglandin dehydrogenase and its gonadotropin-dependent regulation in theca and mural granulosa cells of equine preovulatory follicles during the ovulatory process. Reproduction. (2007) 133:455–66. doi: 10.1530/REP-06-0210
51. Ousey JC, Fowden AL. Prostaglandins and the regulation of parturition in mares. Equine Vet J. (2012) 44:140–8. doi: 10.1111/j.2042-3306.2011.00506.x
52. Han X, Rossdale PD, Ousey JC, Holdstock NB, Allen WR, Silver M. Localisation of 15-hydroxy prostaglandin dehydrogenase (PGDH) and steroidogenic enzymes in the equine placenta. Equine Vet J. (1995) 27:334–9. doi: 10.1111/j.2042-3306.1995.tb04067.x
53. Schlegel W, Daniels D, Krüger S. Partial purification of prostaglandin E2-9-ketoreductase and prostaglandin-15-hydroxydehydrogenase from ovarian tissues of rabbits. Clin Physiol Biochem. (1987) 5:336–42.
54. Wintergalen N, Thole HH, Galla HJ, Schlegel W Prostaglandin-E2 9-reductase from corpus luteum of pseudopregnant rabbit is a member of the aldo-keto reductase superfamily featuring 20 alpha-hydroxysteroid dehydrogenase activity. Eur J Biochem. (1995) 15:264–70. doi: 10.1111/j.1432-1033.1995.264_c.x
55. Kankofer M, Wierciński J, Zerbe H. Prostaglandin E(2) 9-keto reductase activity in bovine retained and not retained placenta. Prostaglandins Leukot Essent Fatty Acids. (2002) 66:413–7. doi: 10.1054/plef.2002.0367
56. Kankofer M, Wierciński J. Prostaglandin E2 9-keto reductase from bovine term placenta. Prostaglandins Leukot Essent Fatty Acids. (1999) 61:29–32. doi: 10.1054/plef.1999.0069
57. Gastal MO, Gastal EL, Torres CA, Ginther OJ. Effect of PGE2 on uterine contractility and tone in mares. Theriogenology. (1998) 50:989–99. doi: 10.1016/S0093-691X(98)00202-7
58. Martínez-Boví R, Cuervo-Arango J. Intrafollicular treatment with prostaglandins PGE2 and PGF2alpha inhibits the formation of luteinised unruptured follicles and restores normal ovulation in mares treated with flunixin-meglumine. Equine Vet J. (2016) 48:211–7. doi: 10.1111/evj.12396
59. Witkowski M, Pawłowski K. Clinical observations on the course of oxytocin- or prostaglandin E2/oxytocin-induced parturition in mares. Pol J Vet Sci. (2014) 17:347–51. doi: 10.2478/pjvs-2014-0047
60. Shrestha HK, Beg MA, Burnette RR, Ginther OJ. Plasma clearance and half-life of prostaglandin F2alpha: a comparison between mares and heifers. Biol Reprod. (2012) 87:1–6. doi: 10.1095/biolreprod.112.100776
61. Weber JA, Causey RC, Emmans EE. Induction of luteolysis in mares by ultrasound-guided intraluteal treatment with PGF2alpha. Theriogenology. (2001) 55:1769–76. doi: 10.1016/S0093-691X(01)00519-2
62. Korzekwa A, Jaroszewski JJ, Bogacki M, Deptula KM, Maslanka TS, Acosta TJ, et al. Effects of prostaglandin F(2alpha) and nitric oxide on the secretory function of bovine luteal cells. J Reprod Dev. (2004) 50:411–7. doi: 10.1262/jrd.50.411
63. Kotwica J, Skarzynski D, Mlynarczuk J, Rekawiecki R. Role of prostaglandin E2 in basal and noradrenaline-induced progesterone secretion by the bovine corpus luteum. Prostaglandins Other Lipid Mediat. (2003) 70:351–9. doi: 10.1016/S0090-6980(02)00149-1
64. Weems YS, Bridges PJ, Jeoung M, Arreguin-Arevalo JA, Nett TM, Vann RC, et al. In vivo intra-luteal implants of prostaglandin (PG) E1 or E2 (PGE1, PGE2) prevent luteolysis in cows. II: mRNA for PGF2α, EP1, EP2, EP3 (A-D), EP3A, EP3B, EP3C, EP3D, and EP4 prostanoid receptors in luteal tissue. Prostaglandins Other Lipid Mediat. (2012) 97:60–5. doi: 10.1016/j.prostaglandins.2011.11.006
65. Weems YS, Raney A, Pang J, Uchima T, Lennon E, Johnson D, et al. Prostaglandin E1 or E2 (PGE1, PGE2) prevents premature luteolysis induced by progesterone given early in the estrous cycle in ewes. Theriogenology. (2013) 80:507–12. doi: 10.1016/j.theriogenology.2013.05.014
66. Galvão A, Henriques S, Pestka D, Lukasik K, Skarzynski D, Mateus LM, et al. Equine luteal function regulation may depend on the interaction between cytokines and vascular endothelial growth factor: an in vitro study. Biol Reprod. (2012) 22:187. doi: 10.1095/biolreprod.111.097147
67. Otzen H, Sieme H, Oldenhof H, Kassens A, Ertmer F, Rode K, et al. Equine endometrial vascular pattern changes during the estrous cycle examined by Narrow Band Imaging hysteroscopy. Anim Reprod Sci. (2016) 166:80–9. doi: 10.1016/j.anireprosci.2016.01.006
68. Brito LFC, Baldrighi JM, Wolf CA, Ginther OJ. Effect of GnRH and hCG on progesterone concentrations and ovarian and luteal blood flow in diestrous mares. Anim Reprod Sci. (2017) 176:64–9. doi: 10.1016/j.anireprosci.2016.11.010
Keywords: prostaglandin E2, human chorion gonadotropin, corpus luteum, progesterone, mare
Citation: Piotrowska-Tomala KK, Jonczyk AW, Szóstek-Mioduchowska AZ, Żebrowska E, Ferreira-Dias G and Skarzynski DJ (2022) The Effects of Prostaglandin E2 Treatment on the Secretory Function of Mare Corpus Luteum Depends on the Site of Application: An in vivo Study. Front. Vet. Sci. 8:753796. doi: 10.3389/fvets.2021.753796
Received: 05 August 2021; Accepted: 28 December 2021;
Published: 15 February 2022.
Edited by:
Margherita Maranesi, University of Perugia, ItalyReviewed by:
Cecilia Dall'Aglio, University of Perugia, ItalyMassimo Zerani, University of Perugia, Italy
Copyright © 2022 Piotrowska-Tomala, Jonczyk, Szóstek-Mioduchowska, Żebrowska, Ferreira-Dias and Skarzynski. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dariusz J. Skarzynski, d.skarzynski@pan.olsztyn.pl
†ORCID: Katarzyna K. Piotrowska-Tomala orcid.org/0000-0001-6989-9243
Agnieszka W. Jonczyk orcid.org/0000-0002-6867-0823
Anna Z. Szóstek-Mioduchowska orcid.org/0000-0003-4204-1698
Graca Ferreira-Dias orcid.org/0000-0003-0622-6513
Dariusz J. Skarzynski orcid.org/0000-0001-9537-3560
 Katarzyna K. Piotrowska-Tomala
Katarzyna K. Piotrowska-Tomala Agnieszka W. Jonczyk
Agnieszka W. Jonczyk Anna Z. Szóstek-Mioduchowska
Anna Z. Szóstek-Mioduchowska Ewelina Żebrowska1
Ewelina Żebrowska1  Graca Ferreira-Dias
Graca Ferreira-Dias Dariusz J. Skarzynski
Dariusz J. Skarzynski