Differential Effects of Dietary Oregano Essential Oil on the Inflammation Related Gene Expression in Peripheral Blood Mononuclear Cells From Outdoor and Indoor Reared Pigs
- 1Dipartimento di Medicina Veterinaria, University of Perugia, Perugia, Italy
- 2Dipartimento di Farmacia, University of Chieti, Chieti, Italy
Intensive farming systems represent a stressful environment for pigs and negatively influence neuroendocrine functions, behavior, and performance. Outdoor farming is an alternative option, which is thought to imply several beneficial effects for the animal. Dietary essential oils are known to be an innovative strategy to improve pig health and performance, and oregano essential oil (ORE) possesses beneficial effects due to its antimicrobial, anti-fungal, and antioxidant properties. We tested the effect of dietary ORE on peripheral blood mononuclear cells (PBMCs) in 36 growing pigs, either reared under indoor or outdoor conditions. Quantitative real-time PCR (RT-qPCR) assay was used to evaluate the effect of diet (control vs. ORE) and the time of sampling (T1−120 days vs. T2−190 days) on the expression of inflammatory and immune-related genes (TNF, IL1β, IL8, IL18, IL10, IL1RN, STAT3, HSP90, ICAM-1, and NFKB1). Under outdoor condition, the majority of transcripts were upregulated (p < 0.05), assuming a general inflammatory status (TNF, HSP90, NFKB1, IL1β, and STAT3). However, an interaction between diet and the farming system was observed: HSP90, NFKB1, and STAT3 were downregulated (p < 0.05) in the outdoor reared pigs when fed the ORE diet. Our study showed that bioactive compounds of ORE exert their activity, especially when the animals are exposed to stressful stimuli. Dietary ORE can be an acceptable strategy to help pigs tolerate the stress related to the harsh, outdoor, rearing conditions.
Introduction
The increase in demand of meat production has led to a worldwide increase in intensive farming systems, which usually represent a stressful environment for the animals (1). In intensively reared pigs farms, high temperature, small space, and noise are some of the stress factors which negatively affect animal growth and meat quality (2). Prolonged stressful stimuli influence the immune system, altering leukocyte activation and the release of immunoglobulin, cytokines, and inflammatory factors (3–5).
The outdoor farming system is generally considered a better strategy to improve animal welfare and health compared to the intensive indoor farming, even though beneficial and stressful stimuli in both rearing systems can influence performance (2, 6–11). With increased space being available and the more natural environment in outdoor farming representing advantages to improve pig welfare (12, 13), the harsh environmental conditions can negatively influence the performance of the animals (6, 7, 14, 15). Pigs in the outdoor rearing system, indeed, are exposed to environmental stressors (i.e., extreme climatic events, physical exercise, and predators), which can negatively affect their immune system and increase their susceptibility to diseases (16–18). Lymphocyte proliferation and natural killer cell activity can be impaired by acute exposure to thermal stress (19–21) and, in addition to the environmental stressor, exercise exacerbates the reaction of the immune system and activates pro-inflammatory and anti-inflammatory pathways, depending on the intensity and duration of stress (22, 23).
Moreover, the genetic background of current pig breeds, which have been selected to optimize the performance in indoor breeding systems, probably compounds the negative effects of the outdoor rearing systems. Thus, many management practices need to be reassessed when certain genotypes are recruited (24).
Among the possible management interventions, the incorporation of plant-derived compounds into the diet appears to be a strategy to improve swine health, performance, and food-derived quality (25, 26). Essential oils, from plant derivatives, are used for their antimicrobial, antioxidant, and anti-inflammatory properties (27–30). Among essential oils, oregano essential oil (ORE) is used in the pig industry for its beneficial effects, ranging from improved performance to increased pork quality (31, 32). It has been shown that oregano has anti-inflammatory and antimicrobial effects (33–35), by modulating the cytokine levels and immunity-related transcription factors (36, 37). In addition, oregano stimulates digestion with effects on enterocytes, by accelerating their renewal rate, improving their capacity for nutrient absorption, and reducing pathogen contamination (38).
In a previous study, we have observed that outdoor rearing conditions have a major, negative impact on the growth rate of pigs and that dietary ORE is effective in reducing these performance losses due to the rearing system by increasing the oxidative status of the animals (11).
In this study, we hypothesized that these environmental factors may influence the expression of inflammatory and immune response-related genes in the peripheral blood mononuclear cells (PBMCs) of growing pigs, either under indoor or outdoor conditions, and that the dietary supplementation with ORE can affect this response. The PBMC population has already been chosen to correctly monitor the immune response in pigs (39, 40) and the effect of the rearing system on this response (3–5).
Materials and Methods
Housing, Animals, and Dietary Treatments
The Department of Pathology, Diagnostics and Veterinary Clinics of the University of Perugia approved the experimental project, and all procedures were in accordance with the European recommendations (European Parliament and the Council of the European Union, 2010) for the protection of animals used for scientific purposes.
The experiment was conducted from June 2012 to January 2013. A total of 36 male Suffolk® hybrid pigs, with an average live weight (LW) of 41.87 ± 1.23 kg, were allowed 35 days to adapt to the experimental conditions prior to the commencement of the study.
Animals were then balanced for LW and litter and blocked into four groups according to a 2 × 2 factorial design: two rearing systems, (a) Outdoor (OUT): ~280 m2/pig in outdoor pens provided with huts for shelter and (b) Indoor (IN): 2 m2/pig in a building with natural ventilation and wheat straw for bedding), and two dietary treatments. The two diets, formulated to be isonitrogenous and isoenergetic and to meet the nutrient requirements of National Research Council (NRC) for growing pigs (41), were as follows: (a) a commercial pelleted feed (CTRL) and (b) a CTRL with 0.2% (as fed) ORE (Origanum vulgare L.) adsorbed on inert adsorbents (ORE).
The experimental diets were administered for 190 days until slaughter; each of them consisted of two feeds administered in two consecutive phases: early finisher (up to 100 kg LW) and late finisher (100 kg LW to slaughter). During the first 35 days of the experiment (adaptation period—T0), all groups of pigs were fed the CTRL diet. More details concerning the composition of the diets, the housing conditions, and the climatic details recorded during the experiment are described in Forte et al. (11).
Both outdoor and indoor pigs were reared in a farm located in Umbria region, Central Italy. The climatic classification of the region according to the Köppen-Geiger system (42) is Cfa (warm temperate climate, fully humid, with hot summer) (11). The natural photoperiod (43°110 northern latitude and 12°610 eastern longitude) was maintained for the whole experimental period, and the main registered meteorological variables were as follows: maximum and minimum temperatures in July and January were 40.6–13.2 °C and 16.1–3.5 °C, respectively; the relative humidity ranged from 55.5 to 90.3%; the mean wind speed at the animal level in July and January ranged from 9 to 39 km/h and from 6 to 41 km/h, respectively; the average rainfall was 48 mm in July and 120 mm in December (average rainfall per year: 901 mm).
Phytochemical Characterization of the ORE
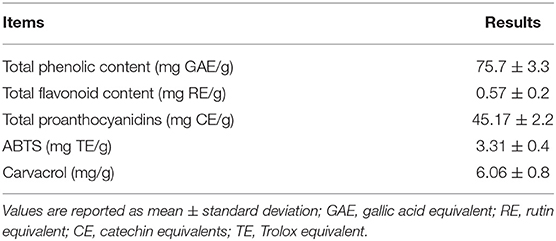
A commercial sample of ORE in the form of white powder was used as a raw material. A commercial sample of ORE, adsorbed on calcium carbonate, calcium aluminum silicate, and potassium aluminum silicate (OR200 Greenvet, Apa-CT, Forlì, Italy), was formulated to be used in animal feeding as a feed supplement. An aliquot of powder (2 g) was transferred in a conic vial and subjected to ultrasound-assisted extraction for 30 min with 25 ml of methanol 70% in water. Afterward, the sample was centrifuged (5,000 g, 10 min), and the residue was diluted in fresh solvent and was extracted for a second time. After centrifugation, the two liquids were combined, taken to final volume, and directly used for phytochemical investigation in terms of total phenols (by Folin-Cicalteau method), total flavonoids (AlCl3 method), total proanthocyanidins (p-dimethylaminocinnamaldehyde assays), and total antioxidant capacity [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) test]. The detailed procedures for each method are described in previously published paper (43).
For quantitative determination of the carvacrol content, the extract was filtered (0.45 μm pore filter) and injected in an analytical HPLC-PDA system according to Skendi et al. (44) with a slight modification. Briefly, the chromatographic system consisted of a binary system pump (Jasco PU-2080, Tokyo, Japan) and a diode array detector (Jasco MD-2010, Tokyo, Japan) equipped with a reversed-phase Kinetex C18 column (250 × 4.5 mm, particle size; Phenomenex, Torrance, USA). The mobile phase consisted of 0.1% acetic acid (A) and methanol (B) using a gradient elution from 70% A to 35% A in 50 min. The flow rate was 1 ml/min. Peak identification was performed by a direct comparison of retention times and UV–vis spectra with carvacrol pure standard (Sigma Aldrich, St. Louis, MO, USA). Quantification was performed by the comparison with a calibration curve (R2 = 0.998). Analyses were performed in triplicate. Results of the phytochemical profile are reported in Table 1.
Sample Collection, RNA Extraction, and Reverse Transcription
Blood samples were drawn from the jugular vein of all animals at the three time-points after the adaptation period of 35 days (T0), at 120 days (T1), and at the end of the experiment (T2, 190 d), and peripheral blood mononuclear cells (PBMCs) were isolated with gradient centrifugation of Ficoll-Paque™ PLUS Media (Cytiva, Marlborough, MA, USA) according to the manufacturer's instructions. In brief, we diluted total blood with a phosphate-buffered saline (PBS) medium 1:2 and then stratified on Ficoll-Paque gradient 1:4 centrifugation. After centrifugation, we withdrew the PBMC rings that were washed twice in the PBS solution.
Total RNA was extracted from PBMCs using the Aurum Total RNA Fatty and Fibrous Tissue Kit (Bio-Rad, Herculers CA, USA) according to the manufacturer's instructions, and treatment with the Ambion TURBO DNA-free kit (ThermoFisher Scientific, Waltham, MA, USA) was carried out, to avoid DNA contamination.
RNA quantity and integrity were evaluated with the Quant-iT RNA Assay Kit (Invitrogen, Doret, UK) in a VersaFluor fluorometer (Bio-Rad, Herculers CA, USA) and denaturing agarose gel electrophoresis, respectively. Purified RNA (500 ng) was reverse transcribed using random hexamers and the Superscript III Reverse Transcriptase (Invitrogen, Dorset, UK), according to the manufacturer's instructions. Successful reverse transcription was confirmed by PCR amplification of the Sus scrofa β-actin gene (XM_003357928).
Primers Design, Reference Genes (RGs) Selection, and Quantitative Real-Time PCR (RT-qPCR)
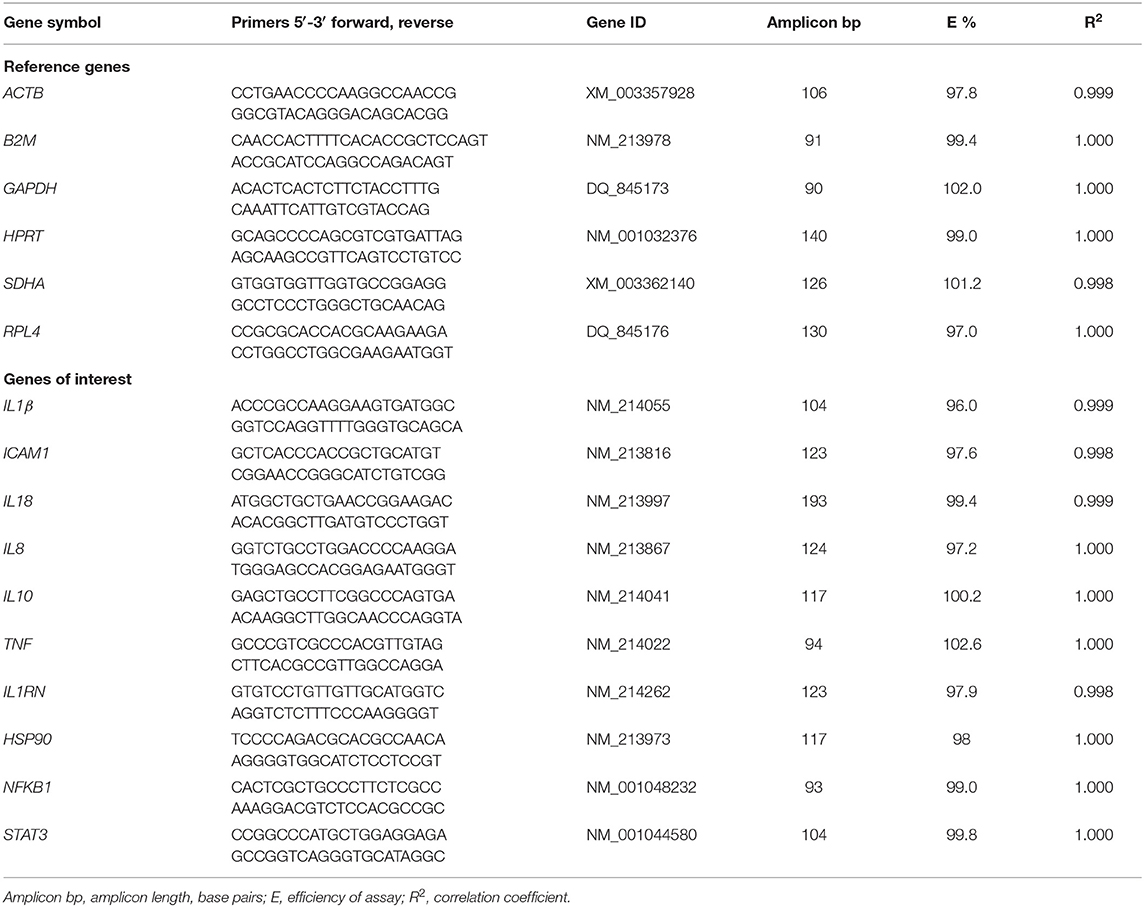
Primers were designed using the Primer-BLAST software (45) for six RGs: β-actin (ACTB), glyceraldehyde-3P-dehydrogenase (GAPDH), hypoxanthine ribosyltransferase (HPRT), β-2-microglobin (B2M), succinate dehydrogenase complex subunit A (SDHA), and ribosomal protein L4 (RPL4). There were ten genes of interest: intercellular Adhesion Molecule 1 (ICAM1), tumor necrosis factor-α (TNF-α), nuclear factor kappa-light-chain-enhancer of activated B cells (NFKB1), interleukin 1 β (IL1β), interleukin 8 (IL8), interleukin 18 (IL18), interleukin 10 (IL10), signal transducer and activator of transcription 3 (STAT3), heat shock protein 90 (HSP90), interleukin-1 receptor antagonist protein (IL1RN). Primer sequences are reported in Table 2.
Whenever possible, primers were designed on different exons or at an exon–exon junction to minimize inaccuracies due to possible residual genomic DNA contamination. Amplicon lengths were optimized to 90/193 bp to ensure optimal amplification efficiency. The specificity of amplification was confirmed by sequencing. For each pair of primers, a preliminary qRT-PCR was performed and the efficiency (E) was assessed by assigning slope values and correlation coefficients (R2) reported in Table 2.
Reactions were carried out in a final volume of 20 μl using 5 μl of a 10-fold diluted cDNA template, FastStart SYBR Green Master mix (Roche Applied Science, Penzberg, Germany), ROX fluorochrome as an internal check on an MX3000P instrument (Stratagene, Santa Clara, CA, USA) following the same conditions for all primer pairs: 95 °C for 10 min, 40 cycles of 95 °C for 30 s, 58 °C for 30 s. Fluorescence data were collected at the end of the extension step. Each reaction was run in triplicate and, at the end of each run, the melting curve was determined in the range of 58–95 °C with a temperature increment of 0.01 °C/s. For analysis, the raw Cq values were imported to the GenEx Pro (version 6) software package (MultiD, Göteborg, Sweden). The stability of the six RGs was tested using geNorm (46) and the NormFinder algorithms (47) included in the GenEx Pro software (MultiD, Göteborg, Sweden).
Analysis of RT-qPCR Data
The raw Cq values were analyzed at three time-points: T0, T1, and T2. During the pre-processing step, data were corrected for the E values of primers (Table 2), normalized for the two most stable RGs (HPRT, GAPDH), quantified relative to the maximum Cq value, and converted in log2.
Statistical Analysis
The gene expression data from the GenEx Pro software (MultiD, Göteborg, Sweden) were analyzed using the GLM procedure of SAS (JMP 9; SAS Institute Inc., Cary, NC, 2010). Before the analysis, data distributions for each gene were checked for normality using the Shapiro–Wilk test. A mixed design ANOVA, where the rearing system (two levels: IN and OUT) and diet (two levels: CTRL and ORE) were included as between-subjects factors, time as a repeated factor, and T0 as covariate, was used. Except for diet*rearing system, all other tested interactions between the main factors were not significant (p > 0.05) and were removed from the model. The Tukey–Kramer adjusted t-test was applied to assess the difference between means. We considered statistically significant differences when p-value was lower than 0.05. Data were expressed as least squares (LS) means ± standard error (SE).
Results
During the whole experimental period, the animals underwent regular veterinary checks and no pathologies were observed.
Selection of Optimal RGs
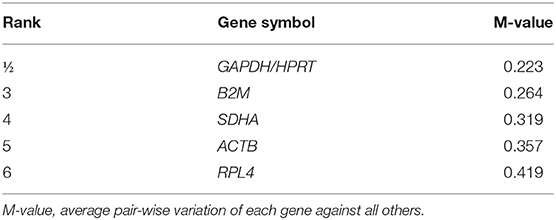
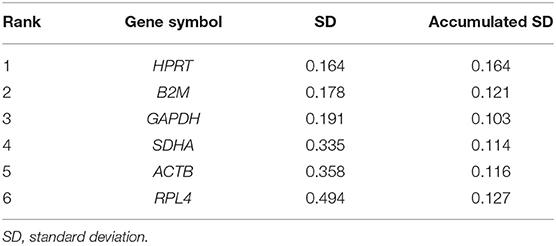
To evaluate the most stable genes to be used for normalization purposes, six potential RGs (ACTB, B2M, GAPDH, HPRT, RPL4, and SDHA) were tested in 48 samples, randomly chosen at each time point (T0, T1, T2), at the farming system (IN, OUT), and at diet treatment (CTRL, ORE). The six genes were analyzed by qRT-PCR to estimate their efficiency according to Cappelli et al. (48) (Table 3). The Cq value for the six RGs tested ranged between 18.0 (ACTB) and 25.5 (HPRT). Tables 3, 4 show the ranking of the six candidate RGs, based on their stability values calculated using geNorm and NormFinder, respectively.
The results obtained using geNorm and NormFinder showed that three RGs (HPRT, GAPDH, and B2M) were always classified among the top positions in the stability rankings produced by both algorithms.
Overall, on the basis of the expression stability values collected in this study, an optimal normalization factor for qRT-PCR data could be calculated using the two most stable genes, HPRT and GAPDH.
Gene Expression Analysis
Effect of Dietary Treatment (CTRL vs. ORE)
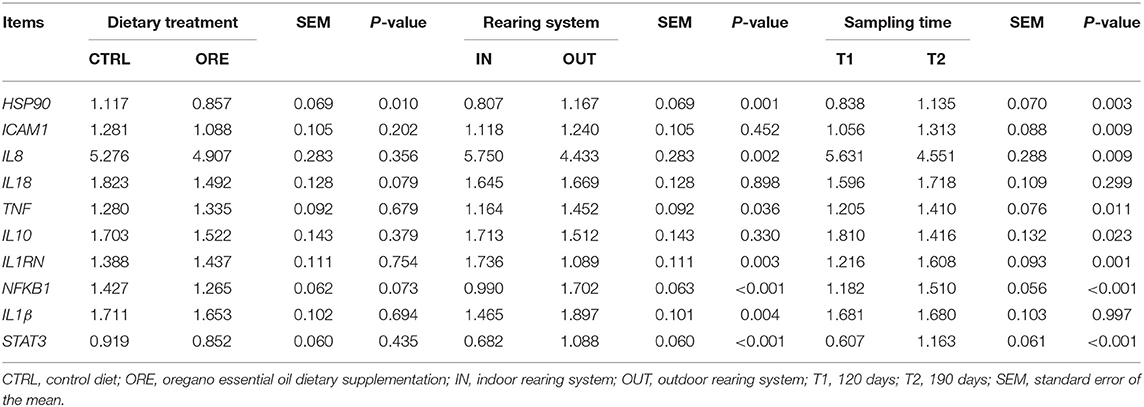
When comparing the effect of the dietary treatment on gene expression, only HSP90 was downregulated in pigs fed with the ORE diet (P < 0.01). Results are shown in Table 5.
Effect of Rearing System (IN vs. OUT)
The expression of seven genes changed between pigs reared in the IN vs. OUT systems (Table 5). The expression of IL8 and IL1RN was upregulated in pigs reared in the IN system (p < 0.01), whereas the expression of HSP90, TNF, NFKB1, IL1β, and STAT3 was upregulated in pigs reared in the OUT system (p < 0.05).
Effect of Sampling Time
Comparison at T0, before the administration of the experimental diets, showed no significant differences (p > 0.05) between groups for all the tested genes before the administration of the experimental diets.
With the exception of IL18 and IL1β, all genes had a differential expression in T1 vs. T2 (Table 5). The expression of IL8 and IL10 was higher in T1, whereas the expression of HSP90, ICAM-1, TNF, IL1RN, NFKB1, and STAT3 was higher in T2 (p < 0.05).
Effect of the Interaction Between Rearing System (IN vs. OUT) and Diet (CTRL vs. ORE)
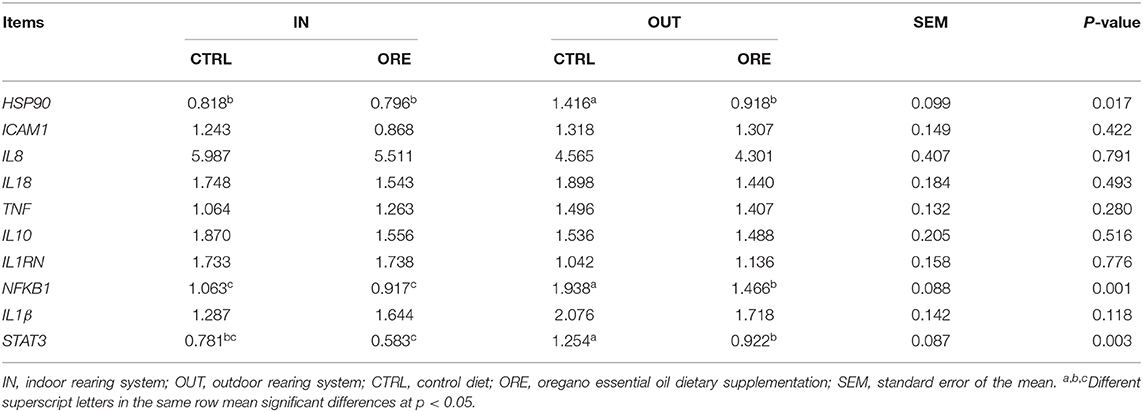
As shown in Table 6, the expression of HSP90, NFKB1, and STAT3 was significantly downregulated in the OUT reared pigs fed with the ORE diet while, in the IN pigs, these genes were basically less activated and not modulated by the dietary treatment (rearing system × diet: p < 0.05, p < 0.001, and p < 0.01, respectively).
Discussion
Our molecular data collectively support the hypothesis that the interaction between the rearing system (IN vs. OUT) and the dietary supplementation with the ORE influences a group of inflammatory response genes in pigs. Hereafter, we will begin by discussing this interaction; then, we will evaluate the main effects exerted by the rearing system and time of sampling, which is in turn associated with the climatic changes of the year.
Effect of Interaction Between Rearing System and Diet
Our data showed a significant interaction between dietary treatment and the rearing system for a number of genes (Table 6). This suggests that the oregano bioactive compounds act in modulating the inflammatory signaling involved in response to stressful stimuli, only when these are in action.
NFKB1 is a transcription factor and also a signaling hub in leukocytes (49, 50); indeed, the transcript of this protein was upregulated in the outdoor conditions when compared to the indoor conditions (Table 5). The lower expression of NFKB1 in the OUT pigs fed with the ORE diet indicates that oregano could be effective in mitigating pro-inflammatory pathways by means of a leukocyte response driven by the NFKB1 transcription. Our data confirm previous findings on the role of oregano as an inhibitor of NFKB1 (37, 51). Indeed, oregano exhibited a significant ability to inhibit the NFKB1 activity induced by lipopolysaccharide in a monocyte cell line in vitro (51). Moreover, the expression of NFKB1 in the small intestine of broilers challenged with lipopolysaccharide was reduced after oral administration of carvacrol (52), one of the major components of ORE (Table 1) (53, 54). Carvacrol is an immunomodulant and an anti-inflammatory molecule known to reduce protein concentration as well as the gene expression modulation of interleukins (55, 56).
In addition to this well-established pathway, oregano was shown to modulate microbial species in the intestine (37) and reduce pathogen contamination (38). It is known that the modulation of gastrointestinal microbiota can influence systemic immune responses (57). It has also been demonstrated that oregano has positive effects on enterocytes by accelerating their renewal rate, improves their capacity for nutrient absorption, and promotes integrity of the intestinal barrier in pigs (37). Enterocytes, in turn, are in close relationship with the intestinal immune system and constitute a functional barrier to dietary and microbial antigens. For these reasons, we cannot exclude that, in our experimental conditions, oregano has indirectly affected gut mucosa functionality and digestion in a beneficial manner.
Finally, it should be stressed that NFKB1 and STAT3 are transcription factors known to modulate the heat shock protein (HSPs) expression during the immune response (58–60). These proteins are chaperones in protein folding, activation, and assembly and prevent tissue damage due to stress stimuli. It is known that HSPs are upregulated and modulate inflammatory pathways in pigs under inflammatory conditions (61), especially heat stress (62–64). In our experiment, samples at T1 and T2 were collected at the end of summer and in full winter, respectively, in an undoubtedly stressful situation for the animals. The modulating influence exerted by the ORE diet was observed under the OUT conditions, where the genes of the response to thermal stress were more actively upregulated (Table 5).
STAT3 encodes a protein which activates in response to cytokines, especially IL10 (65). STAT3 protein translocate into the cell nucleus and acts in blocking the release of pro-inflammatory cytokines (66). The upregulation of this gene under OUT conditions (Table 5) may depend on the need to moderate the inflammatory process due to chronic stressful stimuli, such as exercise or climate adaptation, a mechanism already known to protect the organism from activated macrophage “overshooting,” with potential tissue damage via the IL10/STAT3 pathway (67, 68). In our data, IL10 was not significantly modulated by the rearing system, whereas IL8 was downregulated; we can hypothesize that, at T1, STAT3 activator transcripts IL10 had already returned to their baseline transcription after inducing the transcription of STAT3 (Table 5).
Our data show that, when administered under OUT conditions, the ORE diet led to a significant decrease in the STAT3 expression, and its inductor transcripts (pro-inflammatory cytokines NFKB1, IL8, IL18, and anti-inflammatory IL10) were not modulated in these groups of animals (Table 6).
Moreover, in the same group of animals, the ORE diet prevented IL1β from increasing. IL1β is a prototypic proinflammatory cytokine, which exerts effects on a variety of cells and plays a key role in acute and chronic inflammation. IL1β has important homeostatic functions in the normal organism. However, the overproduction of IL1β is implicated in the pathophysiological changes, which occur during different disease states (69).
Effect of Rearing System (IN vs. OUT)
It is known that various stressors involved in the OUT rearing condition can alter the physiology of an intestinal barrier and can affect the production of pro-inflammatory cytokines and chemokines in pigs (70, 71). These factors, coupled with changes in the diet associated to the OUT environment, have the potential to modulate the microbiome at the mucosal level and, consequently, at the intestinal immune response (70). Early life, especially the time of weaning, is crucial in establishing microbiota and the immune system in pigs (57). Indoor or outdoor environments can have a differential impact on these phenomena (72). For instance, an increased physical complexity of rearing environment seems to generate a less diverse microbial community when compared to indoor-raised pigs (73).
Differences in intestinal microbial population can be related to transcriptional modulations in a number of genes. When comparing two groups of pigs, either reared under outdoor or indoor conditions, large differences in the ileal mucosa-adherent microorganisms were found (72). These differences were associated to an increased expression of major histocompatibility complex class I (MCHI) and chemokines that indicate the presence of an immune-activated gut microenvironment (72).
Under our experimental conditions, the majority of transcripts, which refer to a general inflammatory status (TNF, HSP90, NFKB1, IL1β, and STAT3), showed an upregulation in the OUT environment, whereas transcripts encoding IL8 [inhibited by STAT3 (74)] and IL1RN were downregulated as shown in Table 5. This pattern is possibly related to a stimulation of the immune system by the stressful stimuli present in outdoor rearing, such as exercise and harsh climatic conditions, which can activate the inflammatory response pathways. As previously detailed, the environmental conditions of the OUT pigs have most likely affected the level of stress in both hot and cold seasons of the year (cold winds and rain in winter, solar irradiation in summer, the absence of straw bedding, physical exercise, and the presence of a thick layer of mud in rainy periods).
Both IL1β and TNF transcripts, upregulated in the OUT conditions, transcribe well-known pro-inflammatory cytokines released from various immune cells (mainly monocytes and macrophages), and their production suggests the onset of an inflammatory response (75–78). The circulating levels of TNF have also been indicated as a marker of inflammation in pigs challenged with common pathogens, such as Escherichia coli (79–82). However, in our experimental conditions, no pathologies were found. The upregulation of these two cytokines in our study might reflect the exposure to thermal stress and to the environmental stressors, like physical exercise, that exacerbate this response.
In addition, pro-inflammatory cytokine stimuli, such as TNF-α and IL-1 (49, 50, 83–85), can activate NFKB1; its roles as a transcription factor and a signaling hub in leukocytes were previously described. Together with NFKB1, we have discussed how the ORE diet was able to modulate the expression of two other major genes (STAT3 and HSP90) involved in the inflammatory process and markedly (p < 0.001) upregulated under the OUT conditions (Table 5).
In contrast, IL1RN transcripts were downregulated under the OUT conditions. The IL1RN gene encodes IL1Ra, a protein that binds non-productively to the outer cell surface domain of the IL-1R. IL1Ra is secreted by various types of cells, including immune cells, and is a natural inhibitor of the pro-inflammatory effect of IL1β. The balance between IL1β and IL1RN determines whether the initial pro-inflammatory response will persist or regress (86). The downregulation of this gene, together with the upregulation of IL1β, contributes to explain the inflammatory response recorded under the OUT conditions.
Effect of Sampling Time
The expression of most tested genes was affected by time: six genes out of the eight were upregulated in T2 as compared to T1 (Table 5).
Since the pigs were acclimated to the environment prior to the study, this suggests that, during the experiment, the environment changed over time. Indeed, blood samples were taken in September (T1), after hot summer weather conditions, and in January (T2), in the middle of winter with opposite weather conditions.
The inflammatory response is likely associated with the upregulation of TNF, HSP90, IL8, NFKB1, and ICAM1. The ICAM1 gene encodes a cell surface glycoprotein, typically expressed on cells of the immune system and binding integrins to activate the immune response. It is usually upregulated during inflammatory conditions (87) in response to the pro-inflammatory stimuli of cytokine, especially IL8 and TNF (88, 89).
In contrast, it is likely that the observed increase of IL1RN transcription at T2 was intended to antagonize the pro-inflammatory cytokine stimuli (Table 5).
Taken all together, these results lead to the same direction as other encouraging number of studies regarding the molecular mechanism underlying the beneficial effects of dietary oregano were administered under stressful conditions. They report a decrease in the inflammatory cytokine levels in the pig jejunum, following transport stress (32), an inhibition of the NFKB1 transcription factor on PBMCs in vitro (36, 51), and a decrease in the mortality rate. They also show an acceleration in the recovery of a gain in body weight, a reduction in tissue damage, and a decrease in the mRNA levels of pro-inflammatory cytokines (90).
Conclusion
Our overall observations seem to suggest that the ORE diet can have a mitigation effect on the inflammation state induced by non-infective stress. Indeed, the observed interaction between diet and the farming systems indicates that the beneficial effects of bioactive compounds contained in the OREs are likely to exert their activity when the animals are exposed to stressful stimuli. It is known that stressors involved in outdoor rearing conditions can have an impact on the gut ecosystem and favor inflammatory processes. These factors, often associated with changes in the diet, can influence the intestinal microbiota and alter immune systems at both systemic and local levels.
We can speculate that the ORE dietary supplement can be an acceptable strategy to help pigs tolerate the stress related to harsh, outdoor, rearing conditions. Indeed, these findings based on the molecular data are confirmed by growth performance results reported by our research group in another study, where the same animal cohort was used (11).
In view of the consumer positive attitudes toward extensive rearing systems and animal welfare, further studies should be encouraged to better understand the effects of outdoor environmental constraints and the possible beneficial role of phytoderivatives in livestock diets.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The animal study was reviewed and approved by Council of the Pathology, Diagnostics and Veterinary Clinics Department, University of Perugia (Department Council board minutes no. 8 of 28 June 2010). All blood samples were collected during 2012–2013, before the implementation of Italian Legislation Decree n. 26/2014 which requires a specific authorization of Ministry of Health.
Author Contributions
MT-M, KC, and GA conceived the study and participated in its design. KC choose the research methodology. SC, AV-S, and MT-M carried out the formal analysis. KC, GA, MS, and LM performed the investigation. AV-S, MT-M, and LM provided the funding acquisition. SC, GA, and MT-M performed the data curation. KC, MT-M, and MS participated in writing-original draft preparation. AV-S, SC, and GA participated in writing-review and editing. AV-S and MT-M supervised the research. All authors have read and contributed to the final manuscript and approved the submitted version.
Funding
This research was funded by Regione Umbria PSR 07-13, Mis. 1.2.4 (FITOPIG Project—SIAN no. 84750328829).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors thank Mr. Gianluca Alunni, Dr. Gabriella Cobellis, and Dr. Michela Felicetti for their valuable technical support. Mr. Francesco Rustici's cooperation in animal management and care was greatly acknowledged. Nuovo Molino di Assisi, Apa-CT S.r.l., and Dr. Clarita Cavallucci were gratefully acknowledged for providing technical support and advice in formulating experimental feeds.
References
1. Barnett JL, Hemsworth PH, Cronin GM, Jongman EC, Hutson GD. A review of the welfare issues for sows and piglets in relation to housing. Aust J Agric Res. (2001) 52:1–28. doi: 10.1071/AR00057
2. Martínez-Miró S, Tecles F, Ramón M, Escribano D, Hernández F, Madrid J, et al. Causes, consequences and biomarkers of stress in swine: an update. BMC Vet Res. (2016) 12:171. doi: 10.1186/s12917-016-0791-8
3. Wrona D, Trojniar W, Borman A, Ciepielewski Z, Tokarski J. Stress-induced changes in peripheral natural killer cell cytotoxicity in pigs may not depend on plasma cortisol. Brain Behav Immun. (2001) 15:54–64. doi: 10.1006/brbi.2000.0583
4. Dhabhar FS. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation. (2009) 16:300–17. doi: 10.1159/000216188
5. Tuchscherer M, Kanitz E, Puppe B, Tuchscherer A. Altered immunomodulation by glucocorticoids in neonatal pigs exposed to a psychosocial stressor. Pediatr Res. (2010) 68:473–8. doi: 10.1203/PDR.0b013e3181f70f08
6. Beattie VE, O'Connell NE, Moss BW. Influence of environmental enrichment on the behaviour, performance and meat quality of domestic pigs. Livestock Prod Sci. (2000) 65:71–9. doi: 10.1016/S0301-6226(99)00179-7
7. Edwards SA. Product quality attributes associated with outdoor pig production. Livestock Prod Sci. (2005) 94:5–14. doi: 10.1016/j.livprodsci.2004.11.028
8. Chaloupková H, Illmann G, Neuhauserová K, Tománek M, Valis L. Preweaning housing effects on behavior and physiological measures in pigs during the suckling and fattening periods. J Anim Sci. (2007) 85:1741–9. doi: 10.2527/jas.2006-504
9. Victoria Sanz Fernandez M, Johnson JS, Abuajamieh M, Stoakes SK, Seibert JT, Cox L, et al. Effects of heat stress on carbohydrate and lipid metabolism in growing pigs. Physiol Rep. (2015) 3:e12315. doi: 10.14814/phy2.12315
10. Grimberg-Henrici CGE, Vermaak P, Elizabeth Bolhuis J, Nordquist RE, van der Staay FJ. Effects of environmental enrichment on cognitive performance of pigs in a spatial holeboard discrimination task. Anim Cogn. (2016) 19:271–83. doi: 10.1007/s10071-015-0932-7
11. Forte C, Ranucci D, Beghelli D, Branciari R, Acuti G, Todini L, et al. Dietary integration with oregano (Origanum vulgare L.) essential oil improves growth rate and oxidative status in outdoor-reared, but not indoor-reared, pigs. J Anim Physiol Anim Nutr (Berl). (2017) 101:e352–61. doi: 10.1111/jpn.12612
12. Razzuoli E, Villa R, Sossi E, Amadori M. Characterization of the interferon-α response of pigs to the weaning stress. J Interferon Cytokine Res. (2011) 31:237–47. doi: 10.1089/jir.2010.0041
13. Razzuoli E, Dotti S, Archetti IL, Amadori M. Clinical chemistry parameters of piglets at weaning are modulated by an oral, low-dose interferon-alpha treatment. Vet Res Commun. (2010) 34:S189–92. doi: 10.1007/s11259-010-9402-5
14. Klont RE, Hulsegge B, Hoving-Bolink AH, Gerritzen MA, Kurt E, Winkelman-Goedhart HA, et al. Relationships between behavioral and meat quality characteristics of pigs raised under barren and enriched housing conditions. J Anim Sci. (2001) 79:2835–43. doi: 10.2527/2001.79112835x
15. Yonezawa T, Takahashi A, Imai S, Okitsu A, Komiyama S, Irimajiri M, et al. Effects of outdoor housing of piglets on behavior, stress reaction and meat characteristics. Asian Australas J Anim Sci. (2012) 25:886–94. doi: 10.5713/ajas.2011.11380
16. Blecha F, Kelley KW. Effects of cold and weaning stressors on the antibody-mediated immune response of pigs. J Anim Sci. (1981) 53:439–47. doi: 10.2527/jas1981.532439x
17. Che TM, Johnson RW, Kelley KW, Van Alstine WG, Dawson KA, Moran CA, et al. Mannan oligosaccharide modulates gene expression profile in pigs experimentally infected with porcine reproductive and respiratory syndrome virus. J Anim Sci. (2011) 89:3016–29. doi: 10.2527/jas.2010-3366
18. Yong Y, Zhao Y, Gooneratne R, Liao M, Ju X. T Regulatory and T helper 17 populations with transcription factors in pig tissues in response to chronic heat stress. Int J Agric Biol. (2019) 21:3–10. doi: 10.17957/IJAB/15.0854
19. Hicks TA, McGlone JJ, Whisnant CS, Kattesh HG, Norman RL. Behavioral, endocrine, immune, and performance measures for pigs exposed to acute stress. J Anim Sci. (1998) 76:474–83. doi: 10.2527/1998.762474x
20. Webster Marketon JI, Glaser R. Stress hormones and immune function. Cell Immunol. (2008) 252:16–26. doi: 10.1016/j.cellimm.2007.09.006
21. He Y, Maltecca C, Tiezzi F, Soto EL, Flowers WL. Transcriptome analysis identifies genes and co-expression networks underlying heat tolerance in pigs. BMC Genetics. (2020) 21:44. doi: 10.1186/s12863-020-00852-4
22. de Nadal E, Ammerer G, Posas F. Controlling gene expression in response to stress. Nat Rev Genet. (2011) 12:833–45. doi: 10.1038/nrg3055
23. Abdelnour SA, Abd El-Hack ME, Khafaga AF, Arif M, Taha AE, Noreldin AE. Stress biomarkers and proteomics alteration to thermal stress in ruminants: a review. J Therm Biol. (2019) 79:120–34. doi: 10.1016/j.jtherbio.2018.12.013
24. Kleinbeck SN, McGlone JJ. Intensive indoor versus outdoor swine production systems: genotype and supplemental iron effects on blood hemoglobin and selected immune measures in young pigs. J Anim Sci. (1999) 77:2384–90. doi: 10.2527/1999.7792384x
25. Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. (2008) 86:E140–8. doi: 10.2527/jas.2007-0459
26. Cappai MG, Wolf P, Pinna W, Kamphues J. Digestibility coefficients of crude nutrients in raw hulled acorns (Quercus pubescens Willd.) fed to growing pigs. Anim Feed Sci Tech. (2014) 197:148–54. doi: 10.1016/j.anifeedsci.2014.08.001
27. Peana AT, D'Aquila PS, Panin F, Serra G, Pippia P, Moretti MDL. Anti-inflammatory activity of linalool and linalyl acetate constituents of essential oils. Phytomedicine. (2002) 9:721–6. doi: 10.1078/094471102321621322
28. Riella KR, Marinho RR, Santos JS, Pereira-Filho RN, Cardoso JC, Albuquerque-Junior RLC, et al. Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J Ethnopharmacol. (2012) 143:656–63. doi: 10.1016/j.jep.2012.07.028
29. Florou-Paneri P, Christaki E, Giannenas I. Feed Additives: Aromatic Plants and Herbs in Animal Nutrition and Health. London; San Diego, CA; Cambridge, MA; Oxford: Academic Press (2019). doi: 10.1016/B978-0-12-814700-9.00002-9
30. Chen ZG, Xu GR, Yuan QL, Chen HY, Lei HY, Su JM. Quercetin inhibits porcine intestinal inflammation in vitro. Trop J Pharma Res. (2019) 17:1947. doi: 10.4314/tjpr.v17i10.8
31. Cheng C, Liu Z, Zhou Y, Wei H, Zhang X, Xia M, et al. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. (2017) 133:103–9. doi: 10.1016/j.meatsci.2017.06.011
32. Zou Y, Hu X, Zhang T, Wei H, Zhou Y, Zhou Z, et al. Effects of dietary oregano essential oil and vitamin E supplementation on meat quality, stress response and intestinal morphology in pigs following transport stress. J Vet Med Sci. (2017) 79:328–35. doi: 10.1292/jvms.16-0576
33. Silva FV, Guimarães AG, Silva ERS, Sousa-Neto BP, Machado FDF, Quintans-Júnior LJ, et al. Anti-inflammatory and anti-ulcer activities of carvacrol, a monoterpene present in the essential oil of Oregano. J Med Food. (2012) 15:984–91. doi: 10.1089/jmf.2012.0102
34. Sadeghi A, Bastin AR, Ghahremani H, Doustimotlagh AH. The effects of rosmarinic acid on oxidative stress parameters and inflammatory cytokines in lipopolysaccharide-induced peripheral blood mononuclear cells. Mol Biol Rep. (2020) 47:3557–66. doi: 10.1007/s11033-020-05447-x
35. Listorti V, Battistini R, Ercolini C, Tramuta C, Razzuoli E, Vencia W, et al. In vitro susceptibility of multidrug resistant strains of salmonella to essential oils. Nat Prod Commun. (2020) 15:1934578X19878904. doi: 10.1177/1934578X19878904
36. Paur I, Balstad TR, Kolberg M, Pedersen MK, Austenaa LM, Jacobs DR, et al. Extract of oregano, coffee, thyme, clove, and walnuts inhibits NF-kappaB in monocytes and in transgenic reporter mice. Cancer Prev Res (Phila). (2010) 3:653–63. doi: 10.1158/1940-6207.CAPR-09-0089
37. Zou Y, Xiang Q, Wang J, Peng J, Wei H. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. Biomed Res Int. (2016) 2016:5436738. doi: 10.1155/2016/5436738
38. Amrik B, Bilkei G. Influence of farm application of oregano on performances of sows. Can Vet J. (2004) 45:674–7.
39. Mach N, Gao Y, Lemonnier G, Lecardonnel J, Oswald IP, Estellé J, et al. The peripheral blood transcriptome reflects variations in immunity traits in swine: towards the identification of biomarkers. BMC Genomics. (2013) 14:894. doi: 10.1186/1471-2164-14-894
40. Jégou M, Gondret F, Vincent A, Tréfeu C, Gilbert H, Louveau I. Whole blood transcriptomics is relevant to identify molecular changes in response to genetic selection for feed efficiency and nutritional status in the pig. PLoS ONE. (2016) 11:e0146550. doi: 10.1371/journal.pone.0146550
41. National Research Council. Nutrient Requirements of Swine: Eleventh Revised Edition. Washington, DC: National Academies Press (2012).
42. Kottek M, Grieser J, Beck C, Rudolf B, Rubel F. World map of the Köppen-Geiger climate classification updated. Meteorol Z. (2006) 15:259–63. doi: 10.1127/0941-2948/2006/0130
43. Cobellis G, Acuti G, Forte C, Menghini L, De Vincenzi S, Orrù M, et al. Use of Rosmarinus officinalis in sheep diet formulations: effects on ruminal fermentation, microbial numbers and in situ degradability. Small Rumin Res. (2015) 126:10–8. doi: 10.1016/j.smallrumres.2015.01.018
44. Skendi A, Irakli M, Chatzopoulou P, Papageorgiou M. Aromatic plants of Lamiaceae family in a traditional bread recipe: effects on quality and phytochemical content. J Food Biochem. (2019) 43:e13020. doi: 10.1111/jfbc.13020
45. Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden TL. Primer-BLAST: a tool to design target-specific primers for polymerase chain reaction. BMC Bioinformatics. (2012) 13:134. doi: 10.1186/1471-2105-13-134
46. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. (2002) 3:RESEARCH0034. doi: 10.1186/gb-2002-3-7-research0034
47. Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. (2004) 64:5245–50. doi: 10.1158/0008-5472.CAN-04-0496
48. Cappelli K, Felicetti M, Capomaccio S, Spinsanti G, Silvestrelli M, Supplizi AV. Exercise induced stress in horses: selection of the most stable reference genes for quantitative RT-PCR normalization. BMC Mol Biol. (2008) 9:49. doi: 10.1186/1471-2199-9-49
49. Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb Perspect Biol. (2009) 1:a000034. doi: 10.1101/cshperspect.a000034
50. Pahl HL. Activators and target genes of Rel/NF-kappaB transcription factors. Oncogene. (1999) 18:6853–66. doi: 10.1038/sj.onc.1203239
51. Paur I, Austenaa LM, Blomhoff R. Extracts of dietary plants are efficient modulators of nuclear factor kappa B. Food Chem Toxicol. (2008) 46:1288–97. doi: 10.1016/j.fct.2007.09.103
52. Liu SD, Song MH, Yun W, Lee JH, Kim HB, Cho JH. Effect of carvacrol essential oils on immune response and inflammation-related genes expression in broilers challenged by lipopolysaccharide. Poultry Sci. (2019) 98:2026–33. doi: 10.3382/ps/pey575
53. Wogiatzi E, Gougoulias N, Papachatzis A, Vagelas I, Chouliaras N. Chemical composition and antimicrobial effects of Greek Origanum species essential oil. Biotechnol Biotechnol Equip. (2009) 23:1322–4. doi: 10.1080/13102818.2009.10817662
54. Napoli EM, Curcuruto G, Ruberto G. Screening the essential oil composition of wild Sicilian oregano. Biochem Syst Ecol. (2009) 37:484–93. doi: 10.1016/j.bse.2009.07.008
55. Kara M, Uslu S, Demirci F, Temel HE, Baydemir C. Supplemental carvacrol can reduce the severity of inflammation by influencing the production of mediators of inflammation. Inflammation. (2015) 38:1020–7. doi: 10.1007/s10753-014-0066-0
56. Zuntres ZE, Coccimiglio J, Alipour M. The bioactivity and toxicological actions of carvacrol. Crit Rev Food Sci Nutr. (2015) 55:304–18. doi: 10.1080/10408398.2011.653458
57. Schachtschneider KM, Yeoman CJ, Isaacson RE, White BA, Schook LB, Pieters M. Modulation of systemic immune responses through commensal gastrointestinal microbiota. PLoS ONE. (2013) 8:e53969. doi: 10.1371/journal.pone.0053969
58. Stephanou A, Amin V, Isenberg DA, Akira S, Kishimoto T, Latchman DS. Interleukin 6 activates heat-shock protein 90β gene expression. Biochem J. (1997) 321:103–6. doi: 10.1042/bj3210103
59. Ripley BJM, Stephanou A, Isenberg DA, Latchman DS. Interleukin-10 activates heat-shock protein 90β gene expression. Immunology. (1999) 97:226–31. doi: 10.1046/j.1365-2567.1999.00773.x
60. Ammirante M, Rosati A, Gentilella A, Festa M, Petrella A, Marzullo L, et al. The activity of hsp90α promoter is regulated by NF-κB transcription factors. Oncogene. (2007) 27:1175–8. doi: 10.1038/sj.onc.1210716
61. Hartl FU, Martin J, Neupert W. Protein folding in the cell: the role of molecular chaperones Hsp70 and Hsp60. Annu Rev Biophys Biomol Struct. (1992) 21:293–322. doi: 10.1146/annurev.bb.21.060192.001453
62. Sevin M, Girodon F, Garrido C, de Thonel A. HSP90 and HSP70: implication in inflammation processes and therapeutic approaches for myeloproliferative neoplasms. Mediat Inflamm. (2015) 2015:e970242. doi: 10.1155/2015/970242
63. Bambou JC, Gourdine JL, Grondin R, Vachiery N, Renaudeau D. Effect of heat challenge on peripheral blood mononuclear cell viability: comparison of a tropical and temperate pig breed. Trop Anim Health Prod. (2011) 43:1535–41. doi: 10.1007/s11250-011-9838-9
64. Hassan F, Nawaz A, Rehman MS, Ali MA, Dilshad SMR, Yang C. Prospects of HSP70 as a genetic marker for thermo-tolerance and immuno-modulation in animals under climate change scenario. Anim Nutr. (2019) 5:340–50. doi: 10.1016/j.aninu.2019.06.005
65. Lang R, Patel D, Morris JJ, Rutschman RL, Murray PJ. Shaping gene expression in activated and resting primary macrophages by IL-10. J Immunol. (2002) 169:2253–63. doi: 10.4049/jimmunol.169.5.2253
66. Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. (2006) 6:379–86. doi: 10.1016/j.coph.2006.01.010
67. Elenkov IJ, Chrousos GP. Stress hormones, Th1/Th2 patterns, pro/anti-inflammatory cytokines and susceptibility to disease. Trends Endocrinol Metab. (1999) 10:359–68. doi: 10.1016/S1043-2760(99)00188-5
68. Hu D, Wan L, Chen M, Caudle Y, LeSage G, Li Q, et al. Essential role of IL-10/STAT3 in chronic stress-induced immune suppression. Brain Behav Immun. (2014) 36:118–27. doi: 10.1016/j.bbi.2013.10.016
69. Garlanda C, Dinarello CA, Mantovani A. The interleukin-1 family: back to the future. Immunity. (2013) 39:1003–18. doi: 10.1016/j.immuni.2013.11.010
70. Christoforidou Z, Burt R, Mulder I, Gill BP, Pluske J, Kelly D, et al. Development of immune cells in the intestinal mucosa can be affected by intensive and extensive farm environments, and antibiotic use. Front Immunol. (2018) 9:1061. doi: 10.3389/fimmu.2018.01061
71. Raqib R, Cravioto A. Nutrition, immunology, and genetics: future perspectives. Nutr Rev. (2009) 67:S227–36. doi: 10.1111/j.1753-4887.2009.00244.x
72. Mulder IE, Schmidt B, Stokes CR, Lewis M, Bailey M, Aminov RI, et al. Environmentally-acquired bacteria influence microbial diversity and natural innate immune responses at gut surfaces. BMC Biol. (2009) 7:79. doi: 10.1186/1741-7007-7-79
73. Megahed A, Zeineldin M, Evans K, Maradiaga N, Blair B, Aldridge B, et al. Impacts of environmental complexity on respiratory and gut microbiome community structure and diversity in growing pigs. Sci Rep. (2019) 9:13773. doi: 10.1038/s41598-019-50187-z
74. de la Iglesia N, Konopka G, Lim K-L, Nutt CL, Bromberg JF, Frank DA, et al. Deregulation of a STAT3-interleukin 8 signaling pathway promotes human glioblastoma cell proliferation and invasiveness. J Neurosci. (2008) 28:5870–8. doi: 10.1523/JNEUROSCI.5385-07.2008
75. Harada A, Sekido N, Akahoshi T, Wada T, Mukaida N, Matsushima K. Essential involvement of interleukin-8 (IL-8) in acute inflammation. J Leukoc Biol. (1994) 56:559–64. doi: 10.1002/jlb.56.5.559
76. Sikora JP, Chlebna-Sokół D, Krzyzańska-Oberbek A. Proinflammatory cytokines (IL-6, IL-8), cytokine inhibitors (IL-6sR, sTNFRII) and anti-inflammatory cytokines (IL-10, IL-13) in the pathogenesis of sepsis in newborns and infants. Arch Immunol Ther Exp (Warsz). (2001) 49:399–404.
77. Zhang JM, An J. Cytokines, inflammation and pain. Int Anesthesiol Clin. (2007) 45:27–37. doi: 10.1097/AIA.0b013e318034194e
78. Ocaña-Fuentes A, Arranz-Gutiérrez E, Señorans FJ, Reglero G. Supercritical fluid extraction of oregano (Origanum vulgare) essentials oils: anti-inflammatory properties based on cytokine response on THP-1 macrophages. Food Chem Toxicol. (2010) 48:1568–75. doi: 10.1016/j.fct.2010.03.026
79. Jesmok G, Lindsey C, Duerr M, Fournel M, Emerson T. Efficacy of monoclonal antibody against human recombinant tumor necrosis factor in E. coli-challenged swine. Am J Pathol. (1992) 141:1197–207.
80. Maeda K, Schoeniger LO, Shimada M, Winchurch RA, Buchman TG, Robotham JL. Regulation of acute phase gene expression following surgery and endotoxin administration in the anesthetized pig. Anesthesiology. (1993) 79:1324–37. doi: 10.1097/00000542-199312000-00024
81. Frank JW, Carroll JA, Allee GL, Zannelli ME. Effects of thermal environment and spray-dried plasma on the acute-phase response of pigs challenged with lipopolysaccharide. J Anim Sci. (2003) 81:1166–76. doi: 10.2527/2003.8151166x
82. Hohnstein FS, Meurer M, de Buhr N, von Köckritz-Blickwede M, Baums CG, Alber G, et al. Analysis of porcine pro- and anti-inflammatory cytokine induction by S. suis in vivo and in vitro. Pathogens. (2020) 9:40. doi: 10.3390/pathogens9010040
83. Tak PP, Firestein GS. NF-κB: a key role in inflammatory diseases. J Clin Invest. (2001) 107:7–11. doi: 10.1172/JCI11830
84. Asadullah K, Sterry W, Volk HD. Interleukin-10 therapy–review of a new approach. Pharmacol Rev. (2003) 55:241–69. doi: 10.1124/pr.55.2.4
85. Ross JW, Ashworth MD, Mathew D, Reagan P, Ritchey JW, Hayashi K, et al. Activation of the transcription factor, nuclear factor kappa-B, during the estrous cycle and early pregnancy in the pig. Reprod Biol Endocrinol. (2010) 8:39. doi: 10.1186/1477-7827-8-39
86. Goldbach-Mansky R. Immunology in clinic review series; focus on autoinflammatory diseases: update on monogenic autoinflammatory diseases: the role of interleukin (IL)-1 and an emerging role for cytokines beyond IL-1. Clin Exp Immunol. (2012) 167:391–404. doi: 10.1111/j.1365-2249.2011.04533.x
87. Papa A, Danese S, Urgesi R, Grillo A, Guglielmo S, Roberto I, et al. Intercellular adhesion molecule 1 gene polymorphisms in inflammatory bowel disease. Eur Rev Med Pharmacol Sci. (2004) 8:187–91.
88. Chiang CH. Effects of anti-tumor necrosis factor-alpha and anti-intercellular adhesion molecule-1 antibodies on ischemia/reperfusion lung injury. Chin J Physiol. (2006) 49:266–74.
89. Ying B, Yang T, Song X, Hu X, Fan H, Lu X, et al. Quercetin inhibits IL-1 beta-induced ICAM-1 expression in pulmonary epithelial cell line A549 through the MAPK pathways. Mol Biol Rep. (2009) 36:1825–32. doi: 10.1007/s11033-008-9386-1
Keywords: immune response to environment, gene expression, oregano essential oil, pig, rearing system, PBMC, inflammation
Citation: Cappelli K, Sabino M, Trabalza-Marinucci M, Acuti G, Capomaccio S, Menghini L and Verini-Supplizi A (2021) Differential Effects of Dietary Oregano Essential Oil on the Inflammation Related Gene Expression in Peripheral Blood Mononuclear Cells From Outdoor and Indoor Reared Pigs. Front. Vet. Sci. 8:602811. doi: 10.3389/fvets.2021.602811
Received: 04 September 2020; Accepted: 01 February 2021;
Published: 25 February 2021.
Edited by:
Dennis Jewell, Kansas State University, United StatesReviewed by:
Maria Grazia Cappai, University of Sassari, ItalyYosra Ahmed Soltan, Alexandria University, Egypt
Copyright © 2021 Cappelli, Sabino, Trabalza-Marinucci, Acuti, Capomaccio, Menghini and Verini-Supplizi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Trabalza-Marinucci, massimo.trabalzamarinucci@unipg.it; Gabriele Acuti, gabriele.acuti@unipg.it
†These authors have contributed equally to this work
 Katia Cappelli
Katia Cappelli Marcella Sabino
Marcella Sabino Massimo Trabalza-Marinucci
Massimo Trabalza-Marinucci Gabriele Acuti
Gabriele Acuti Stefano Capomaccio
Stefano Capomaccio Luigi Menghini
Luigi Menghini Andrea Verini-Supplizi
Andrea Verini-Supplizi