Pro-Inflammatory Response of Bovine Polymorphonuclear Cells Induced by Mycoplasma mycoides subsp. mycoides
- 1Unit of Basic and Applied Biosciences, Faculty of Biosciences and Technology for Food, Agriculture and Environmental, University of Teramo, Teramo, Italy
- 2Molecular Biology and Genomic Unit, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise “G. Caporale”, Teramo, Italy
- 3Immunology and Serology Department, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise “G. Caporale”, Teramo, Italy
- 4Bacterial Vaccines and Diagnostics Department, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise “G. Caporale”, Teramo, Italy
- 5Cooperation Office, Istituto Zooprofilattico Sperimentale dell'Abruzzo e del Molise “G. Caporale”, Teramo, Italy
- 6Faculty of Veterinary Medicine, University of Teramo, Teramo, Italy
Mycoplasma mycoides subsp. mycoides (Mmm) is the etiological agent of contagious bovine pleuropneumonia (CBPP), one of the major diseases affecting cattle in sub-Saharan Africa. Some evidences suggest that the immune system of the host (cattle) plays an important role in the pathogenic mechanism of CBPP, but the factors involved in the process remain largely unknown. The present study aimed to investigate the cell response of bovine polymorphonuclear neutrophils (PMNs) after Mmm in vitro exposure using one step RT-qPCR and Western blotting. Data obtained indicate that gene and protein expression levels of some pro-inflammatory factors already change upon 30 min of PMNs exposure to Mmm. Of note, mRNA expression level in Mmm exposed PMNs increased in a time-dependent manner and for all time points investigated; targets expression was also detected by Western blotting in Mmm exposed PMNs only. These data demonstrate that when bovine PMN cells are triggered by Mmm, they undergo molecular changes, upregulating mRNA and protein expression of specific pro-inflammatory factors. These results provide additional information on host-pathogen interaction during CBPP infection.
Introduction
Mycoplasma mycoides subsp. mycoides (Mmm) is the etiological agent of contagious bovine pleuropneumonia (CBPP), a severe respiratory disease of cattle notifiable to the World Organization for Animal Health (Office International des Epizooties [OIE]) (1). CBPP has been eradicated in most countries worldwide (http://www.oie.int/en/animal-health-in-the-world/official-disease-status/cbpp/en-cbpp) but remains widespread in sub-Saharan Africa, where it strongly impacts livestock productivity, draining financial resources to Governments for high cost of the control measures (2, 3).
Mmm belongs to mycoplasmas, the smallest wall-less and self-replicating microorganisms. So far, no typical virulence factors have been detected for Mmm and its virulence is probably multifactorial (4, 5). Some evidences suggest that the production of reactive oxygen species (ROS) (6, 7) and other immune-driven mechanisms (8) could contribute to CBPP lung injury but the factors promoting and sustaining those processes remain largely unknown.
Polymorphonuclear cells (PMNs) are the first line of cellular defense against invading pathogens, playing a critical role in innate immunity and influencing adaptive immune responses (9–11). In adult cattle PMNs represent the second most abundant leukocyte population with a neutrophil-to-lymphocyte ratio of ~1:2, which is lower compared to other domestic animals where PMNs represent up to 75% of the population of circulating leukocytes (12, 13). These cells are rapidly recruited to inflammatory and infection sites to provide early defense against invading microorganisms. At respiratory level, PMNs are among the major innate immune effector cells recruited during acute inflammation (14). PMNs are professional phagocytes and take part in pathogen clearance through several mechanisms like degranulation, phagocytosis, antibody derived cell cytotoxicity, and release of neutrophil extracellular traps (NETs) (15, 16). Beside their involvement in primary host defense against infections, PMNs also contribute to regulate inflammatory and immune responses (17). However, dysregulation of inflammatory stimuli leading to excessive neutrophils recruitment and activation may contribute to tissue injury (18). In CBPP, lungs showing acute-to-subacute stages of infection are characterized by an abundant cell inflammatory infiltrate containing PMNs and alveolar macrophages, suggesting the possible involvement of these cells in defensive and pathological mechanisms (19, 20).
Previous in vitro studies investigated the role of PMNs in the mechanism of interaction between cattle PMNs and M. bovis (21, 22) but few data are available on the interaction between Mmm and PMNs (7). Thus, this study aimed to investigate in vitro the expression of pro-inflammatory cytokines and inflammatory mediators induced in PMNs after Mmm exposure.
Materials and Methods
Mycoplasma mycoides subsp. mycoides Strain
Experiments were conducted using Mmm “Caprivi,” a highly virulent African strain available at the OIE Reference Laboratory for CBPP in Teramo (Italy), isolated in Namibia in 2003 (23). Cultures were grown in modified PPLO broth at 37°C in a 5% CO2 atmosphere for 2 days, then sub cultured and expanded to log phase for additional 44–48 h. Bacterial cells were obtained by centrifugation at 9,000 × g at 4°C for 40 min followed by two washes with isotonic phosphate-buffered saline (PBS, pH 7.2). Bacteria were re-suspended in PBS at a cell density of 108 per ml.
Isolation of Bovine PMNs and Exposure to Mmm
Blood samples were collected in EDTA containing tubes from clinically healthy and regularly slaughtered cattle (n = 3), selected in a CBPP free area (Italy). PMNs were isolated by density gradient using Ficoll Paque Plus (Merck KGaA, Darmstadt, Germany), according to manufacturer's instructions. Cell precipitate was treated with a hypotonic lysis buffer (155 mM NH4Cl, 10 mM KHCO3, 0.12 mM EDTA) to remove red blood cells. Then, PMNs were re-suspended in RPMI media (Merck KGaA, Darmstadt) to a concentration of 106 per ml with a viability ≥ 90%, which was determined using an automated cell analyser (Vi-Cell, Beckman Coulter).
Re-suspended PMNs were seeded at a density of 5 × 105 cells per well (500 μl) in 24-well flat-bottom plates (Falcon, Corning incorporated) with or without Mmm (5 × 107cells per well) (500 μl) to obtain a multiplicity of infection (MOI) of 100. The plates were incubated at 37°C in 5% CO2 in mild shaking and cell suspensions were sampled at 30 min and 1, 2, 3, 6, and 18 h after Mmm exposure. Each sample (exposed PMNs and not exposed PMNs) was assessed in duplicate for every time point considered.
RNA Extraction and RT-qPCR Analysis
After incubation in the absence or presence of Mmm, PMNs were pelleted, and RNA from the pellet was extracted using Direct Zoll RNA Kit (Zymo Research), which included a DNA digestion step (DNase I). Total RNA was quantified by Qubit RNA HS (High Sensitivity) Assay Kit (Thermo Fischer Scientific).
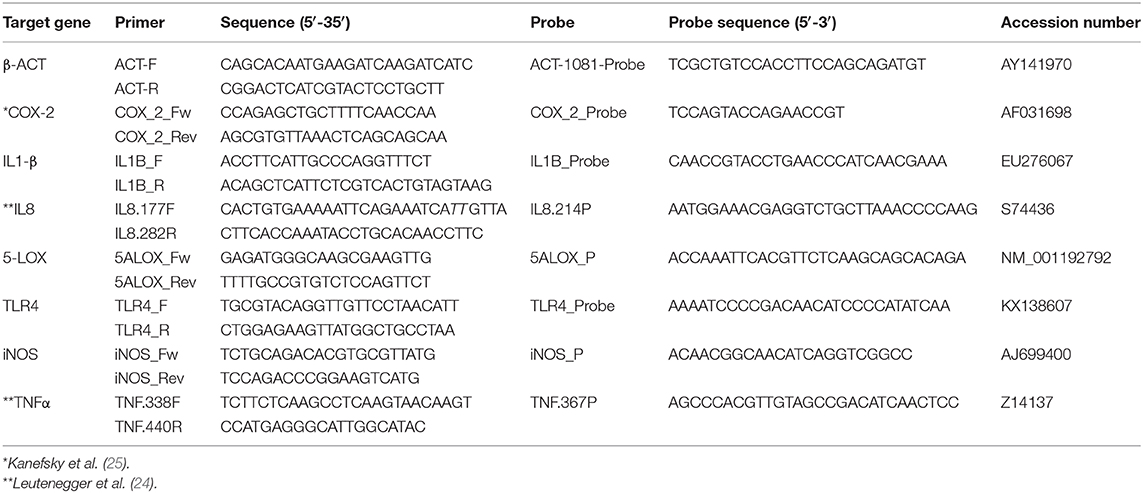
One step RT-qPCR assays were developed to quantify the relative expression of a panel of 7 target genes (interleukin-1β, IL-1β;interleukin 8, IL8; 5-lipoxygenase, 5-LOX; cyclooxygenase-2, COX-2; inducible nitric oxide synthase, iNOS; toll-like receptor 4, TLR4; tumor necrosis factor α, TNFα) involved in the inflammatory process. Primers and TaqMan probes (Eurofins Genomics) targeting IL8, TNFα (24), and COX-2 (25) were used as previously described in literature, while primers and probes for the other target genes were designed using Primer Express software (Applied Biosystem) (Table 1). RT-qPCR assay for each considered target was optimized and validated using lung sampled from CBPP infected cattle.
RT-qPCR analysis were performed using SuperScript™ III Platinum™ One-Step qRT-PCR Kit (Invitrogen) according to manufacturer's instructions. The retro transcription and amplification were carried out in 96 well-plates employing 5 μl of RNA suspension in a 20 μl of reaction volume using QuantStudio 7 Flex Real-Time PCR System instrument (Applied Biosystem) using the following thermal cycling conditions: 15 min at 50°C (retro transcription), 2 min at 95°C (Taq polymerase activation), and 40 cycle of 15 s at 95°C and 30 s at 60°C (amplification reaction).
Target gene expression was evaluated against β-actin (β-ACT) housekeeping gene target using 2(−ΔΔCT) method (26).
Western Blotting and ELISA
Western blotting analyses were carried on culture supernatans collected after incubation of PMNs in the absence or presence of Mmm. PMNs supernatants were separated by NuPAGE 4–12% Bis-Tris gel (Novex, Life Technologies) at 200 V and then transferred onto iBlot2 NC stacks nitrocellulose membranes (Life Technologies) by iBlot2® Dry Blotting System (Life Technologies). Membranes were blocked with PBS containing 0.05% Tween 20 (PBST) and 5% skimmed milk for 2 h at room temperature. Membranes were incubated overnight at 4°C with specific antibodies-Rabbit anti-bovine: IL-1β (AHP851Z, Bio-Rad), 5-LOX (NB110-58749, Novus Biologicals), COX-2 (AB5118, Merck Millipore), TLR4 (A00017, Boster Biological Technology), iNOS (ADI-KAS-NO001-D, Enzo Life Sciences), and Mouse anti-bovine: IL8 polyclonal (Anti-IL8 antibody ab193818, Abcam) and monoclonal (Anti-bovine IL8 (CXCL8) mAb MT8H6, Mabtech AB) and TNFα (MCA2334, Bio-Rad). All the antibodies were diluted 1:1000 in PBST containing 2.5% skimmed milk.
After washing with PBST, membranes were incubated for 1 h at room temperature with goat anti-rabbit IgG-HRP (Bio-Rad) diluted 1:3000 or anti-mouse IgG-HRP (GE Healthcare) diluted 1:8000 in PBST containing 2.5% skimmed milk. Antigen-antibody reactions were visualized by adding chemiluminescent substrates (GE Healthcare). Images were acquired using the ChemiDoc MP (Bio-Rad) and the Image Lab Software, version 4.0.1 (Bio-Rad).
Additional tests for IL8 and TNFα were carried out on PMNs culture supernatants using commercial quantitative ELISA tests (Bovine interleukin 8 ELISA kit, MBS008105; Bovine tumor necrosis alpha ELISA kit, MBS4502967 MyBiosource) following manufacturer instructions.
Statistical Analyses
Statistical analyses were performed applying a t-test for a sample (unilateral test compared to a theoretical mean of 1). The test was considered significant when observed mean was >1 with a P < 0.05.
Results
Data obtained by RT-qPCR and Western blotting indicate that gene and protein expression levels of pro-inflammatory factors are modified following PMNs exposure to Mmm.
Figure 1 shows the fold change in mRNA levels quantified by RT-qPCR (normalized to β-ACT) after PMNs exposure to Mmm for all considered time points, except for 18 h where signals for all targeting genes and for all samples (exposed PMNs and not exposed PMNs) were not appreciated.
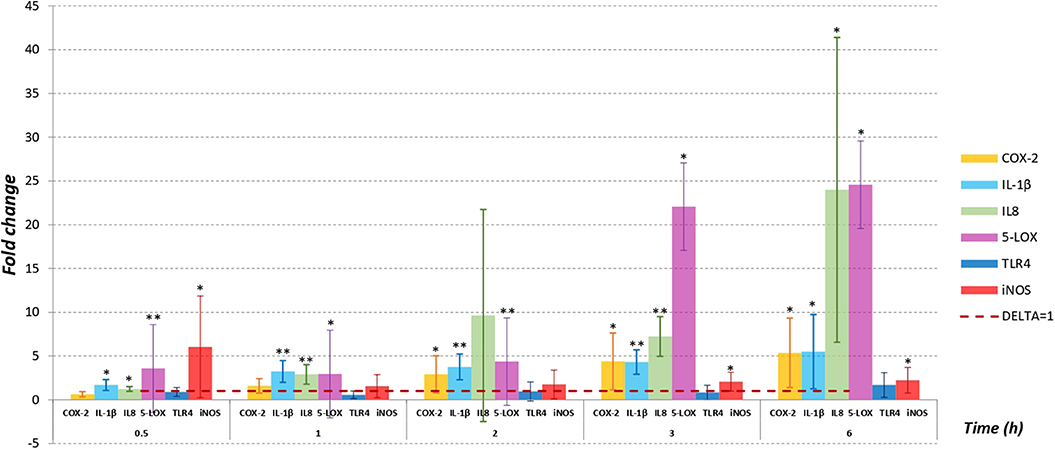
Figure 1. PMNs were infected with Mmm Caprivi at MOI = 100. RT-qPCR was applied to examine the mRNA levels and fold changes were calculated by 2−(ΔΔCT) method as compared to unexposed control cells. Endogenous β-actin mRNA level was used for normalization. Fold changes were expressed as mean ± SD from three sets of independent experiments. No mRNA expression for TNFα was recorded in exposed PMNs and in control cells. No signals were appreciated at 18 h time sampling for all targeting genes. *P < 0.05; **P < 0.01.
IL-1β, IL8, and 5-LOX gene expression levels in PMNs exposed to Mmm were significantly higher to control values (P < 0.05) in all time point tested. Moreover, mRNA expression levels in treated PMNs increased in a time-dependent manner in all tested time points, with highest values observed for IL8 and 5-LOX at 3 (IL8 at 7-fold, 5-LOX at 22-fold, P < 0.5) and 6 h (IL8 at 24-fold, 5-LOX at 24.5-fold, P < 0.05) post PMNs treatment. Also, COX-2 mRNA expression level increased over time, but the fold changes values are significantly different (P < 0.05) starting from 2 h after PMNs exposure to Mmm.
Instead, iNOS showed a different expression profile. In fact, iNOS displayed the highest mRNA level (6-fold) at the first time point considered (30 min) while no differences to control values (P > 0.05) were observed at 1 h and 2 h after Mmm exposure.
For all time points tested by RT-qPCR, the differences observed in TLR4 expression levels were not significantly different (P > 0.05) compared to control values.
Western blotting detected the presence of IL-1β, TLR4, iNOS, COX-2, and 5-LOX proteins, for all considered time points, starting from 30 min, only in PMNs culture supernatants of samples exposed to Mmm (Figure 2).
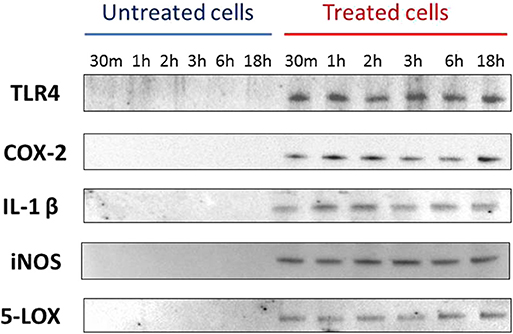
Figure 2. Western blotting data for TLR4, COX-2, IL-1β, iNOS, 5-LOX. Data are representative of three independent experiment.
Conversely, no evidence of IL8 was detected in Western blotting while a concentration of ~100 pg/ml was detected by quantitative ELISA in both Mmm exposed and control samples (data not shown).
Finally, no expression for TNFα was recorded by Western blotting, ELISA and RT-qPCR assays in any of the time point tested.
Discussion
Although investigated for long time, the pathogenesis of CBPP remains mostly unknown. This is due to several reasons including difficulties and costs to reproduce the disease experimentally in the natural host and the lack of appropriate laboratory animal models (27). Recently, ex vivo models based on bovine respiratory explants were developed, providing additional knowledge about host-specificity of Mmm (28) and its selective tropism for lower respiratory airways (bronchioles and alveoli) which represent the primary infection site (27). Acute-subacute CBPP pulmonary lesions from infected animals are characterized by a massive infiltration of inflammatory cells, with a relevant component represented by neutrophil granulocytes, together with the presence of high levels of some pro-inflammatory factors (TNFα, IL-1β, and IL-17A) (29). However, despite the clear inflammatory picture associated to CBPP lesions, transcriptomic analysis of blood samples collected from CBPP affected cattle showed that genes involved in inflammation mechanisms (as TNFα) were not upregulated during the infection (30). These findings highlight how the peripheral condition observed in blood does not necessarily reflect local inflammation, confirming the complexity of CBPP pathogenesis in which different cell types contribute to the disease development and progression. Taking this into consideration, the use of simplified in vitro models may contribute to dissect and clarify CBPP pathogenic mechanisms. In this research, the effects of the early interaction between Mmm and bovine PMNs were investigated in vitro, for the first time in terms of gene and protein expression, providing additional information on the involvement of these cells in the lung inflammatory response, typically observed during CBPP infection.
The obtained data indicate that Mmm is able to promote PMNs response in vitro, modulating the expression of some pro-inflammatory cytokines and inflammatory mediators released by those cells.
IL-1β and TNFα are expressed rapidly during the first stage of inflammatory response and are crucial to orchestrate a systemic and local signaling network able to promote the recognition of tissue damage (31, 32). IL-1β is expressed by different cell types through stimulation of TLRs and CD14, PMNs included, and it contributes to promote proliferation of T and B lymphocytes, activate Natural Killer cells, and stimulate the production of other inflammatory mediators including COX-2 and 5-LOX (33, 34). Data obtained in the current study indicate that Mmm induces a significant and progressive increase of IL-1β expression in PMNs upon to 30 min post exposure, supporting previous studies where high levels of IL-1β were observed in CBPP lung lesions (8, 29).
TNFα is also produced by different cells in response to TLRs stimulation, including PMNs. This cytokine is an important factor involved in the inflammatory process, regulating the expression of adhesion molecules and inducing dendritic cell maturation and chemokines production (32). The presence of TNFα in bovine affected by CBPP was demonstrated both in plasma and pulmonary lesions of CBPP infected animals (8, 29) but in this study PMNs exposed to Mmm did not express TNFα in terms of gene and protein expression. In our opinion the lack of TNFα expression may be due to the absence within the in vitro model tested of other important immune cells involved in TNFα signaling such as alveolar macrophages that represent the main source of this cytokine (32).
TLR4 is generally associated to the recognition of lipopolysaccharide antigen (LPS), but it has also been reported to be involved in mycoplasma infections (35), triggering the production of pro-inflammatory mediators involved in the immune response to bacterial infection (36). Data obtained by RT-qPCR showed no significant differences for TLR4 mRNA level between the two considered conditions (PMNs exposed to Mmm and PMNs not exposed) in all time point tested despite Western blotting revealed the protein expression of TLR4 only in PMNs exposed to Mmm. This may be due to the lysis of PMNs induced by Mmm exposure, causing the release of TLR4 in the treated cells supernatant.
Mmm induced a relevant, progressive and significant increase of IL8 expression—a chemotactic factor involved in the recruitment step—that reached its maximum pick at 6 h post-infection. IL8 can be produced by different cell types, comprising neutrophils, confirming that PMNs products trigger different immune cell types, comprising neutrophils themselves (37). This means that under Mmm stimulation, PMNs are induced to recruit other PMNs, causing an exacerbation of the inflammatory reaction. In addition to chemotaxis, IL8 also induces the production of reactive oxygen species (ROS) (38), which are suggested to play a role in the pathogenesis of CBPP (4, 7). However, at least in the in vitro condition tested, the amount of IL8 secreted by PMNs in culture medium did not correlate with the increased IL8 mRNA level observed. The poor correlation of IL8 protein abundance in the supernatant with high IL8 mRNA expression level may be due to post-transcriptional mechanisms that could be related to the experiment set up (39).
Although less effective than ROS, nitric oxide (NO) is a highly reactive product of nitric oxide oxidation (40) and represents an effector molecule and key mediator of non-specific immunity (41). NO is released by stimulated neutrophils in order to protect the host from harmful microorganisms and in this study, its production was very rapid. In fact, iNOS, the inducible form of the enzyme nitric oxide synthase, was the gene target more expressed by neutrophils after 30 min post Mmm exposure while its mRNA level decreased just after 1 h post-treatment.
Similarity to IL8, 5-LOX mRNA expression levels increased in a time dependent manner, reaching the highest fold change values at 3 and 6 h post PMNs exposure to Mmm. Likewise, the expression of COX-2 increased over the time, even if less pronounced then 5-LOX. COX-2 and 5-LOX are key enzymes in arachidonic acid (AA) metabolism, mediating the production of eicosanoids (42, 43). Data obtained in this study suggest that Mmm is able to modulate COX-2 and 5-LOX pathways, inducing the release of eicosanoid which are effective autocrine and paracrine bioactive mediators promoting the inflammatory cascade (44). Both COX-2 and 5-LOX have just been demonstrated to be involved in other mycoplasma lung infection (45) but never reported for CBPP. In fact, data showed in this study represent the first report describing the involvement of COX-2 and 5-LOX in CBPP pathogenic mechanism.
In conclusion, the achieved data indicate that Mmm is able to induce an early PMNs in vitro response in terms of gene and protein expression of some inflammatory mediators, supporting the hypothesis that Mmm exerts its pathogenic activity by modulating host immune response. In this case, Mmm directly induces PMNs activation, upregulating some pro-inflammatory mediators, such as IL-1β, IL8, 5-LOX, COX-2, and iNOS, that directly and indirectly contribute to amplify the immune and inflammatory responses taking place during CBPP infection and that may result in host tissue damage. Similar mechanisms of direct cell activation by Mmm could be investigated for other cells such as alveolar macrophages, endothelial cells or pneumocytes and bronchial epithelial cells, which support PMNs recruitment and probably are involved in the early stages of CBPP infection.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
MD and MA planned and conducted the experiments, performed RT-qPCR analysis, and wrote the manuscript. VD gave technical support for RT-qPCR analysis. IK, TD, and ML conducted the immunoblotting analysis, discussed the result, and revised the manuscript. GO contributed to antigen preparation. CC, FS, GM, MS, and MM co-supervised the work, discussed the results, and revised the manuscript.
Funding
Research activities have been supported with internal funding of IZSAM, OIE Reference Laboratory for Contagious Bovine Pleuropneumonia (CBPP), and National Reference Centre for Foreign Animal Diseases (CESME).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors acknowledge the technical support of Emanuela Rossi and Romolo Salini.
References
1. World Organisation for Animal Health. Contagious bovine pleuropneumonia. In: Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, 6th ed. Paris: Office International des Epizooties (2014). p. 1–16.
2. Onono JO, Wieland B, Rushton J. Estimation of impact of contagious bovine pleuropneumonia on pastoralists in Kenya. Prev Vet Med. (2014) 115:122–9. doi: 10.1016/j.prevetmed.2014.03.022
3. Tambi NE, Maina WO, Ndi C. An estimation of the economic impact of contagious bovine pleuropneumonia in Africa. Rev Sci Tec. (2006) 25:999–1011. doi: 10.20506/rst.25.3.1710
4. Pilo P, Vilei EM, Peterhans E, Bonvin-Klotz L, Stoffel MH, Dobbelaere D, et al. A metabolic enzyme as a primary virulence factor of Mycoplasma mycoides subsp. mycoides small colony. J Bacteriol. (2005) 187:6824–31. doi: 10.1128/JB.187.19.6824-6831.2005
5. Pilo P, Frey J, Vilei EM. Molecular mechanisms of pathogenicity of Mycoplasma mycoides subsp. Mycoides SC. Vet J. (2007) 174:513–21. doi: 10.1016/j.tvjl.2006.10.016
6. El-Benna J, Hurtado-Nedelec M, Marzaioli V, Marie JC, Gougerot-Pocidalo MA, Dang PM. Priming of the neutrophil respiratory burst: role in host defence and inflammation. Immunol Rev. (2016) 273:180–93. doi: 10.1111/imr.12447
7. Di Teodoro G, Marruchella G, Mosca F, Di Provvido A, Sacchini F, Tiscar PG, et al. Polymorphonuclear cells and reactive oxygen species in contagious bovine pleuropneumonia: new insight from in vitro investigations. Vet Immunol Immunopathol. (2018) 201:16–9. doi: 10.1016/j.vetimm.2018.04.011
8. Sacchini F, Luciani M, Salini R, Scacchia M, Pini A, Lelli R, et al. Plasma levels of TNF-α, IFN-γ, IL-4 and IL-10 during a course of experimental contagious bovine pleuropneumonia. BMC Vet Res. (2012) 8:44. doi: 10.1186/1746-6148-8-44
9. Mantovani A, Cassatella MA, Costantini C, Jaillon S. Neutrophils in the activation and regulation of innate and adaptive immunity. Nat Rev Immunol. (2011) 11:519–31. doi: 10.1038/nri3024
10. Mócsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. (2013) 210:1283–99. doi: 10.1084/jem.20122220
11. Ear T, Tatsiy O, Allard FL, McDonald PP. Regulation of discrete functional responses by Syk and Src family tyrosine kinases in human neutrophils. J Immunol Res. (2017) 2017:4347121. doi: 10.1155/2017/4347121
12. Roland L, Drillich M, Iwersen M. Hematology as a diagnostic tool in bovine medicine. R J Vet Diagn Invest. (2014) 26:592–8. doi: 10.1177/1040638714546490
13. Paape MJ, Bannerman DD, Zhao X, Lee JW. The bovine neutrophil: structure and function in blood and milk. J Vet Res. (2003) 34:597–627. doi: 10.1051/vetres:2003024
14. Ackermann MR. Acute inflammation. In: McGavin MD, Zachary JF, editors. Pathologic Basis of Veterinary Disease. St. Louis, MO: Mosby Elsevier Inc. (2007). p. 101–43.
15. Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, et al. Neutrophil extracellular traps kill bacteria. Science. (2004) 303:1532–5. doi: 10.1126/science.1092385
16. Nauseef WM. How human neutrophils kill and degrade microbes: an integrated view. Immunol Rev. (2007) 219:88–102. doi: 10.1111/j.1600-065X.2007.00550.x
17. Tecchio C, Micheletti A, Cassatella MA. Neutrophil derived cytokines: facts beyond expression. Front Immunol. (2014) 5:508. doi: 10.3389/fimmu.2014.00508
18. Kruger P, Saffarzadeh M, Weber AN, Rieber N, Radsak M, von Bernuth H, et al. Neutrophils: between host defence, immune modulation, and tissue injury. PLoS Pathog. (2015) 11:e1004651. doi: 10.1371/journal.ppat.1004651
19. Thiaucourt F, Var Der Lugt JJ, Provost A. Contagious bovine pleuropneumonia. In: Coetzer JAW, Tustin RC, editors. Infectious Diseases of Livestock. Cape Town: Oxford University Press (2004). pp. 2045–59.
20. Nicholas R, Ayling R, McAuliffe L Contagious bovine pleuropneumonia. In: Nicholas R, Ayling R, McAuliffe L, editors. Mycoplasma Disease of Ruminants. Weybridge: CABI, Oxfordshire University (2007). pp. 69–97. doi: 10.1079/9780851990125.0069
21. Jimbo S, Suleman M, Maina T, Prysliak T, Mulongo M, Perez-Casal J. Effect of Mycoplasma bovis on bovine neutrophils. Vet Immunol Immunopathol. (2017) 188:27–33. doi: 10.1016/j.vetimm.2017.04.011
22. Gondaira S, Higuchi H, Nishi K, Iwano H, Nagahata H. Mycoplasma bovis escapes bovine neutrophil extracellular traps. Vet Microbiol. (2017) 199:68–73. doi: 10.1016/j.vetmic.2016.12.022
23. Scacchia M, Tjipura-Zaire G, Lelli R, Sacchini F, Pini A. Contagious bovine pleuropneumonia: humoral and pathological events in cattle infected by endotracheal intubation or by exposure to infected animals. Vet Ital. (2011) 47:407–13
24. Leutenegger CM, Alluwaimi AM, Smith WL, Perani L, Cullor JS. Quantitation of bovine cytokine mRNA in milk cells of healthy cattle by real-time TaqMan polymerase chain reaction. Vet Immunol Immunopathol. (2000) 77:275–87 doi: 10.1016/S0165-2427(00)00243-9
25. Kanefsky J, Lenburg M, Hai CM. Cholinergic receptor and cyclic stretch-mediated inflammatory gene expression in intact ASM. Am J Respir Cell Mol Biol. (2006) 34:417–25. doi: 10.1165/rcmb.2005-0326OC
26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. (2001) 25:402–8. doi: 10.1006/meth.2001.1262
27. Di Teodoro G, Marruchella G, Di Provvido A, Orsini G, Ronchi GF, D'Angelo AR, et al. Respiratory explants as a model to investigate early events of contagious bovine pleuropneumonia infection. Vet Res. (2018) 49:5 doi: 10.1186/s13567-017-0500-z
28. Weldearegay YB, Müller S, Hänske J, Schulze A, Kostka A, Rüger N, et al. Host-pathogen interactions of Mycoplasma mycoides in caprine and bovine Precision-Cut Lung Slices (PCLS) models. Pathogens. (2019) 8:E82. doi: 10.3390/pathogens8020082
29. Sterner-Kock A, Haider W, Sacchini F, Liljander A, Means J, Poole J, et al. Morphological characterization and immunohistochemical detection of the proinflammatory cytokines IL-1β, IL-17A, and TNF-α in lung lesions associated with contagious bovine pleuropneumonia. Trop Anim Health Prod. (2016) 48:569–76. doi: 10.1007/s11250-016-0994-9
30. Rodrigues V, Holzmuller P, Puech C, Wesonga H, Thiaucourt F, Manso-Silván L. Whole blood transcriptome analysis of Mycoplasma mycoides Subsp. mycoides infected cattle confirms immunosuppression but does not reflect local inflammation. PLoS ONE. (2015) 10:e0139678. doi: 10.1371/journal.pone.0139678
31. Mohammadi N, Midiri A, Mancuso G, Patanè F, Venza M, Venza I, et al. Neutrophils directly recognize Group B streptococci and contribute to interleukin 1β production during infection. PLos ONE. (2016) 11:e0160249. doi: 10.1371/journal.pone.0160249
32. Allie N, Grivennikov SI, Keeton R, Hsu NJ, Bourigault ML, Court N, et al. Prominent role for T cell-derived tumour necrosis factor for sustained control of Mycobacterium tuberculosis infection. Sci Rep. (2013) 3:1809. doi: 10.1038/srep01809
33. Machado-Carvalho L, Martín M, Torres R, Gabasa M, Alobid I, Mullol J, et al. Low E-prostanoid 2 receptor levels and deficient induction of the IL-1β/IL-1 type I receptor/COX-2 pathway: vicious circle in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. (2016) 137:99–107.e7. doi: 10.1016/j.jaci.2015.09.028
34. Luheshi NM, Rothwell NJ, Brough D. Dual functionality of interleukin-1 family cytokines: implications for anti-interleukin-1 therapy. Br J Pharmacol. (2009) 157:1318–29. doi: 10.1111/j.1476-5381.2009.00331.x
35. Shimizu T, Kimura Y, Kida Y, Kuwano K, Tachibana M, Hashino M, et al. Cytoadherence of Mycoplasma pneumoniae induces inflammatory responses through autophagy and toll-like receptor 4. Infect Immun. (2014) 82:3076–86. doi: 10.1128/IAI.01961-14
36. Płóciennikowska A, Hromada-Judycka A, Borzecka K, Kwiatkowska K. Co-operation of TLR4 and raft proteins in LPS-induced pro-inflammatory signaling. Cell Mol Life Sci. (2015) 72:557–81. doi: 10.1007/s00018-014-1762-5
37. Lyadova IV. Neutrophils in tuberculosis: heterogeneity shapes the way?. Mediators Inflamm. (2017) 2017:8619307. doi: 10.1155/2017/8619307
38. Galligan CL, Coomber BL. Effects of human IL-8 isoforms on bovine neutrophil function in vitro. Vet Immunol Immunopathol. (2000) 74:71–85. doi: 10.1016/S0165-2427(00)00162-8
39. Greenbaum D, Colangelo C, Williams K, Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. (2003) 4:117. doi: 10.1186/gb-2003-4-9-117
40. Mayadas TN, Cullere X, Lowell CA. The multifaceted functions of neutrophils. Ann Rev Pathol. (2014) 9:181–218. doi: 10.1146/annurev-pathol-020712-164023
41. Billack B. Macrophage activation: role of toll-like receptors, nitric oxide, and nuclear factor kappa B. Am J Pharm Educ. (2006) 70:102. doi: 10.5688/aj7005102
42. Hanna VS, Hafez EAA. Synopsis of arachidonic acid metabolism: a review. J Adv Res. (2018) 11:23–32. doi: 10.1016/j.jare.2018.03.005
43. Hedi H, Norbert G. 5-Lipoxygenase pathway, dendritic cells, and adaptive immunity. J Biomed Biotechnol. (2004) 2004:99–105. doi: 10.1155/S1110724304310041
44. Tianqi W, Xianjun F, Qingfa C, Patra JK, Dongdong W, Wang Z, et al. Arachidonic acid metabolism and kidney inflammation. Int J Mol Sci. (2019) 20. doi: 10.3390/ijms20153683
Keywords: Mycoplasma mycoides subsp. mycoides, contagious bovine pleuropneumonia, polymorphonuclear cells, inflammatory mediators, gene expression
Citation: Di Federico M, Ancora M, Luciani M, Krasteva I, Sacchini F, Orsini G, Di Febo T, Di Lollo V, Mattioli M, Scacchia M, Marruchella G and Cammà C (2020) Pro-Inflammatory Response of Bovine Polymorphonuclear Cells Induced by Mycoplasma mycoides subsp. mycoides. Front. Vet. Sci. 7:142. doi: 10.3389/fvets.2020.00142
Received: 03 December 2019; Accepted: 25 February 2020;
Published: 27 March 2020.
Edited by:
Jasim Muhammad Uddin, Bangladesh Agricultural University, BangladeshReviewed by:
Jan Naessens, International Livestock Research Institute, KenyaPascal Rainard, Institut National de la Recherche Agronomique, France
Copyright © 2020 Di Federico, Ancora, Luciani, Krasteva, Sacchini, Orsini, Di Febo, Di Lollo, Mattioli, Scacchia, Marruchella and Cammà. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Massimo Ancora, m.ancora@izs.it
 Marta Di Federico
Marta Di Federico Massimo Ancora
Massimo Ancora Mirella Luciani
Mirella Luciani Ivanka Krasteva3
Ivanka Krasteva3  Flavio Sacchini
Flavio Sacchini Gianluca Orsini
Gianluca Orsini Tiziana Di Febo
Tiziana Di Febo Giuseppe Marruchella
Giuseppe Marruchella Cesare Cammà
Cesare Cammà