Hip-preserving reconstruction using a customized cemented femoral endoprosthesis with a curved stem in patients with short proximal femur segments: Mid-term follow-up outcomes
- 1Department of Orthopedics, Orthopedic Research Institute, West China Hospital, Sichuan University, Chengdu, China
- 2Sichuan Model Worker and Craftsman Talent Innovation Resaerch Studio, China
Background: Short metaphyseal segments that remain following extensive distal femoral tumor resection can be challenging to manage, as the residual short segments may not be sufficient to accept an intramedullary cemented stem of standard length. The present study was developed to detail preliminary findings and experiences associated with an intra-neck curved stem (INCS) reconstructive approach, with a particular focus on mechanical stability.
Method: From March 2013 to August 2016, 11 total patients underwent reconstructive procedures using a customized cemented femoral endoprosthesis (CCFE) with an INCS. Measurements of femoral neck-shaft angle values were made before and after this procedure. Radiological outcomes associated with this treatment strategy over an average 63-month follow-up period were additionally assessed. Functionality was assessed based upon Musculoskeletal Tumor Society (MSTS) scores, while a visual analog scale (VAS) was used to rate pre- and postoperative pain, and any complications were noted.
Results: Relative to the preoperative design, no significant differences in femoral neck–shaft angle were observed after this reconstructive procedure (p = 0.410). Postoperatively, the tip of the stem was primarily positioned within the middle third of the femoral head in both lateral and posterior-anterior radiographic, supporting the accuracy of INCS positioning. The average MSTS score for these patients was 25 (range: 21–28), and VAS scores were significantly reduced after surgery (p < 0.0001). One patient exhibited local disease recurrence and ultimately succumbed to lung metastases, while two patients exhibited aseptic loosening. None of the treated patients exhibited complications such as infections, periprosthetic fractures, or prosthetic fractures as of most recent follow-up.
Conclusion: CCFE with an INCS represents a viable approach to massive femoral diaphyseal defect with short proximal femur repair, as patients can achieve good functional outcomes and early weight-bearing with proper individualized rehabilitative interventions, all while exhibiting low rates of procedure-related complications.
Introduction
The surgical removal of large femoral malignancies can yield short femoral metaphyseal juxta-articular segments that can be difficult to accurately reconstruct (1). Reconstructive approaches in these patients include total femur replacement (TFR) (2), the use of inactivated autologous bone grafts (3), osteoarticular allografts (4), or a combination of both autografts and allofrafts (5, 6). While TFR can obviate the need to amputate the affected limb and is associated with positive functional outcomes during the early stages of patient follow-up, this procedure is often associated with undesirable outcomes including infection, local recurrence, aseptic loosening, hip disarticulation, and limb-length discrepancies (2, 7, 8). The use of inactivated autologous bone grafts offers several advantages including lower operative costs, appropriate anatomical matching, physiological reconstruction, and the lack of any need for a bone bank, but this procedure is also subject to limitations including the potential for infection, internal fixation failure, nonunion, and fracture of the inactivated bone (3). While the use of osteoarticular allografts can support the physiological reconstruction of target defect sites while preserving host bone integrity (9), larger allografts are generally associated with an elevated risk of infection, delayed union, nonunion, or graft fracture (4). Combined autografts and allografts combine the biological activity of free vascularized fibular grafts (FVFGs) with the initial mechanical strength of allografts. The Capanna technique has been reported to lessen the impact of complication (graft fracture, and delayed union or nonunion) (10–13). However, the risk of anastomosis failure by thrombosis is a concern (14). In addition, previous study showed that there was little difference in the percentage of graft fractures when comparing allografts with and without this vascularized graft (15).
The surgical removal of distal femoral tumors with proximal metaphyseal extension can often lead to the incidence of massive femoral diaphyseal defects (MFDD) with a short proximal femur (SPF), and optimal approaches to treating these defects remain to be established. In contrast to other forms of reconstructive surgery, the literature pertaining to the use of large femoral endoprosthesis is somewhat limited. However, the use of customized femoral endoprostheses can obviate the requirement for prolonged immobilization following allograft- or autograft-based reconstructive procedures, offering a means of immediately improving stability while promoting rapid recovery, early weight-bearing, a shorter duration of hospitalization, and a more rapid return to daily life and postoperative neoadjuvant chemotherapy or radiotherapy treatment, as appropriate (16, 17). However, these endoprostheses are often associated with both mechanical and non-mechanical complications including infection, periprosthetic fracture, breakage of the implant, and aseptic loosening (18). Insufficient contact area is available between the endoprosthetic stem and the cancellous bone in cases of MFDD with an SPF, and the inadequacy of cancellous bone in the trochanteric region can impact cement interdigitation for the straight cemented stem.
In our institution, SPF is defined as a residual proximal femur of ≤110 mm in length when measured from the pyriform fossa to the level of the osteotomy. In these cases, the residual segment is likely to be insufficient to accept a standard 150 mm intramedullary cemented stem (19). In an effort to better match the residual proximal femur while increasing the surface area of contact between the cancellous bone and endoprosthetic stem, a customized cemented femoral endoprosthesis (CCFE) with an intra-neck curved stem (INCS) was thus utilized for reconstructive procedures in cases of MFDD with an SPF. This study is the first to our knowledge to report clinical outcomes associated with such a reconstructive approach. As such, this analysis was primarily developed with the aim of detailing preliminary clinical outcomes and other experiences associated with INCS-based reconstruction, with a particular focus on associated mechanical stability.
Materials and methods
Ethical considerations
This retrospective study received ethical approval from the institutional ethics committee, and all patients provided written informed consent.
Patients
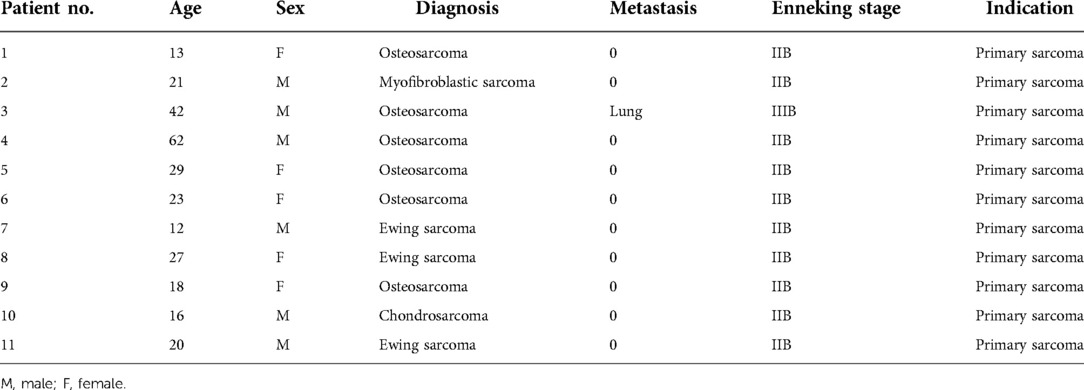
From 2013 to 2016, 11 total patients (6 male, 5 female; mean age: 26 years, range: 12–62 years) underwent distal femoral reconstruction procedures performed using a CCFE with an INCS. The average follow-up duration for these patients was 63 months (range: 17–102 months), during which three patients succumbed to lung metastases at 17, 22 and 29 months post-reconstruction, respectively (Figure 1A). Two patients developed aseptic loosening at 48 and 59 months post-reconstruction (Figure 1B). One patient developed local disease recurrence and ultimately succumbed to lung metastases. Surgical staging was performed as per the Enneking bone and soft tissue sarcoma staging system (20) (Table 1). Prior to definitive surgery, all patients underwent biopsy procedures. Preoperative x-ray, magnetic resonance imaging (MRI), computed tomography (CT), and single-photon emission CT approaches were used to establish the required length of bone to be resected (Figure 2). Patient demographic and clinical characteristics such as age, sex, tumor size, defect length, and residual proximal femur length were recorded.
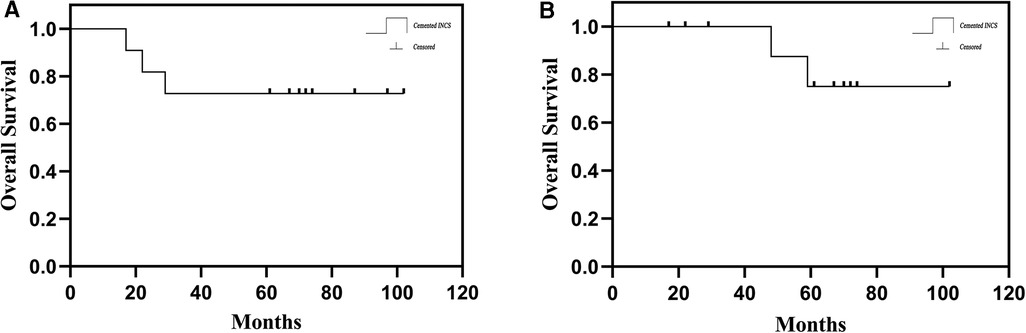
Figure 1. (A) the overall patient survival of hip-preserving reconstruction using a customized cemented femoral endoprosthesis with an INCS. (B) Survival to aseptic loosening of cemented INCS. (INCS: intra-neck curved stem).
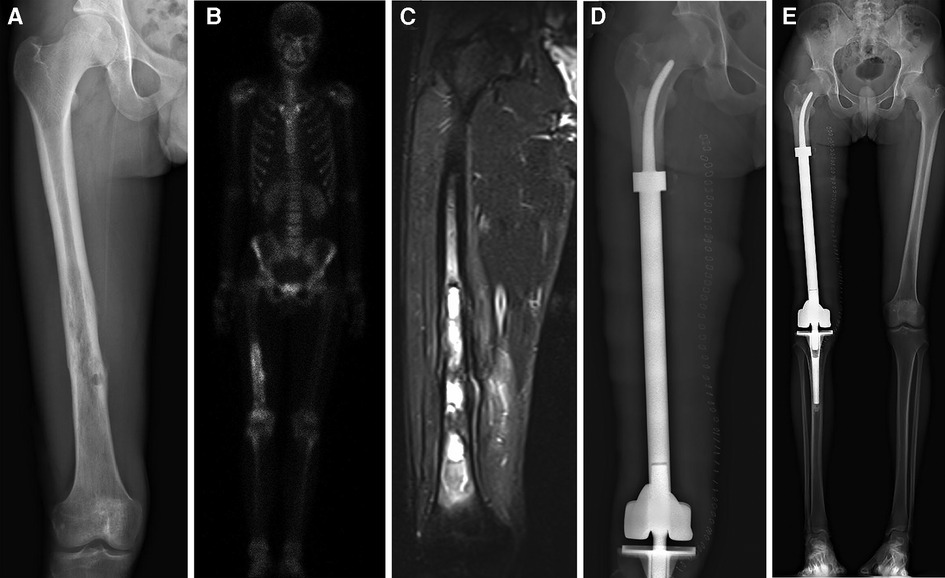
Figure 2. All patients underwent en bloc tumor resection followed by reconstruction with the customized cemented femoral endoprosthesis with an intra-neck curved stem. (A) Anteroposterior radiograph of the right femur of a patient with a distal femoral myofibroblastic sarcoma. (B) Single-photon emission whole-body Computed tomography image. (C) Magnetic resonance image of the patient's right upper leg. (D) Postoperative radiograph of the femur. (E) Full-length x-ray films of lower extremities in anteroposterior view at 7 days after surgery.
Stem design and fabrication
After preoperative imaging-based determination of the tumor margins in each patient, the osteotomy plane was established. The stem was designed as an arc-shaped solid structure with a base that had a diameter that was 2–3 mm smaller than that of the inner surface of the inner femoral cortices. Stem curvature was designed in accordance with the medial cortex of the femoral neck. Strength was maintained by gradually reducing the stem diameter such that the diameter at the end of the curved stem region remained >10 mm. Our clinical team designed all stems, which were subsequently fabricated by Chunlizhengda Medical Instruments (Tong Zhou, Beijing, China).
Surgical approach
One senior surgeon (Chongqi Tu) performed all procedures described in this study. All operations were conducted via a lateral approach with patients in the lateral recumbent position. Initially, the distal and medial segments of the femur were exposed. Tumors were then exposed, subjected to en bloc resection, and soft tissue was removed as appropriate based on preoperative simulations, with medullary tissue from the proximal femur being sent for frozen biopsy to confirm en bloc resection. Previous sites and needle biopsy tracks were additionally subjected to en block removal. Precise control of the osteotomy plane was maintained to minimize any risk of misfit between the residual proximal femur and the customized stem. Following tumor resection, the tip of a customized guide needle was inserted into the center of the femoral head using a mobile C-arm, after which a flexible reamer with different diameters and the customized guide needle were used to facilitate the gradual enlargement of the medullary cavity with the residual femur, ultimately producing a cone-shaped cavity. The prosthesis was then prepared to match the curvature of the residual femur and to correct for lower extremity alignment. A vacuum-mixing cement gun was used to inject bone cement into the medullary cavity, after which the curved stem was inserted into the residual proximal femur, with care being taken to ensure that no cement remained between the prosthesis and the soft tissue.
Postoperative management
Following surgery, patients were routinely administered intravenous prophylactic antibiotics for 48 h. Rehabilitative programs were developed in an individualized manner based on the intraoperative assessment of each patient. In general, patients were subject to bed rest for 3–5 days, with their lower extremities being maintained in a neutral position with knee and ankle flexion and extension exercises being conducted in bed after 8 h. After 3 days, patients initiated hip flexion and abduction exercises, while after 7 days, patients began partial weight-bearing with the assistance of two crutches. After 21 days, patients began to progress to full weight-bearing.
During the initial 3 months after surgery, patients underwent monthly follow-up, followed by follow-up visits every 3 months for 2 years, with yearly visits thereafter. At each follow-up visit, a physical examination of the affected limb was conducted. A visual analog scale (VAS) was used to rate pain. Radiographic imaging of the reconstructed limb was conducted monthly during the first 3 months, every 3 months for the first year, every 6 months during the second year, and once per year thereafter. Lower limb function was assessed as per the Musculoskeletal Tumor Society (MSTS) scoring system (21). Postoperative stem positioning was assessed via x-ray. Femoral neck-shaft angles were measured before and after surgery. Procedure-related complications such as infection, periprosthetic fractures, aseptic loosening, and implant breakage were assessed.
Statistical analysis
Differences between pre- and postoperative measurements were made via paired t-tests, with p < 0.05 as the threshold of significance. All data were analyzed using SPSS v 19.0 (IBM Corp, NY, USA).
Results
Radiographic analysis
x-ray and T-smart approaches were used to assess the bone-cement and cement-prosthesis interfaces in treated patients. In two cases, x-ray images revealed radiolucent lines at 48 and 59 months post-surgery, and these patients ultimately underwent revision surgical procedures and total femur replacement (Figure 3). No other patients exhibited such abnormalities. In one case, a patient underwent reconstructive surgery using a CCFE with an INCS following the resection of 82% of the femoral length in the context of massive tumor resection, and the most recent follow-up imaging of this patient revealed stable bone-cement and cement-prosthesis interfaces and neocortex formation (Figure 4). With the exception of two of the treated patients, lateral and posterior-anterior radiographic imaging revealed the stem tip to be located primarily in the middle third of the femoral head. No significant differences in pre- and postoperative femoral neck-shaft angle were evident in these patients (p = 0.410) (Table 2), consistent with acceptable INCS positioning.
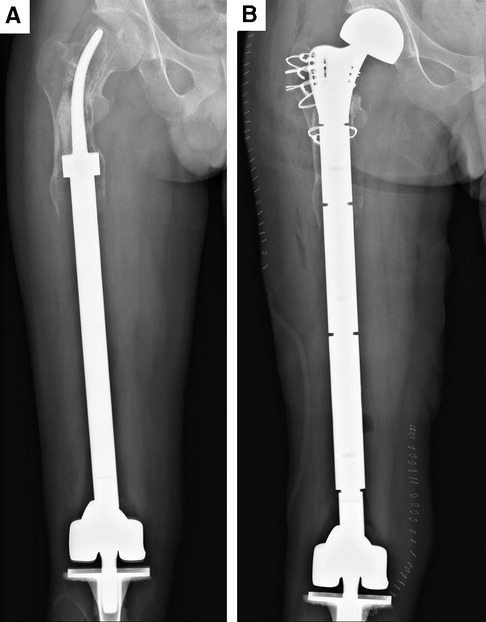
Figure 3. Aseptic loosening of the curved stem and revision surgery of case. (A) Aseptic loosening of the curved stem at the postoperative 97th month. (B) Aseptic loosening of the curved stem in case of revision surgery.
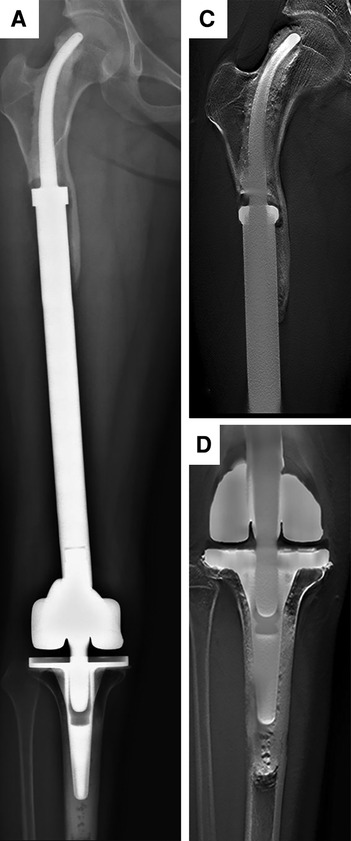
Figure 4. Radiographs showing the 102-month postoperative views of the customized cemented femoral endoprosthesis with an intra-neck curved stem placed during treatment for an osteosarcoma. (A) Posteroanterior radiograph of the entire femur. (B) Posteroanterior with Shimadzu Metal Artefact Reduction Technology (T-smart) views of the stem insertion region in the proximal femur. (C) Posteroanterior T-smart views of the stem insertion region in the proximal tibial.
Functional analyses
The average MSTS score for this patient population as of most recent follow-up was 25 (range: 21–28). No surviving patients required crutches or other devices to aid walking at most recent follow-up. Two patients reported lower extremity pain when walking supported for over 3,000 m, with VAS scores of 3 and 2. Relative to preoperative VAS scores, patients exhibited significant overall reductions in pain (p < 0.0001) (Table 3). No other patients reported pain or Trendelenburg gait as of most recent follow-up (Figure 5).
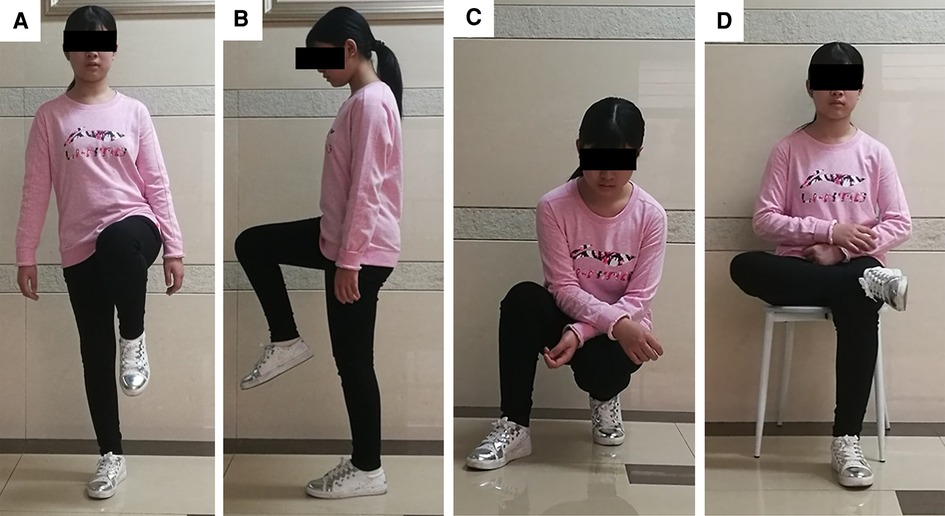
Figure 5. Limb function after INCS reconstruction at the postoperative 6th month of case. (A) This patient could stand up just with affected limb without any pain; (B) The knee and hip flexion of this patient was normal; (C) This patient could squat and stand up without any difficulty; (D) This patient could cross legs without any pain. (INCS: intra-neck curved stem).
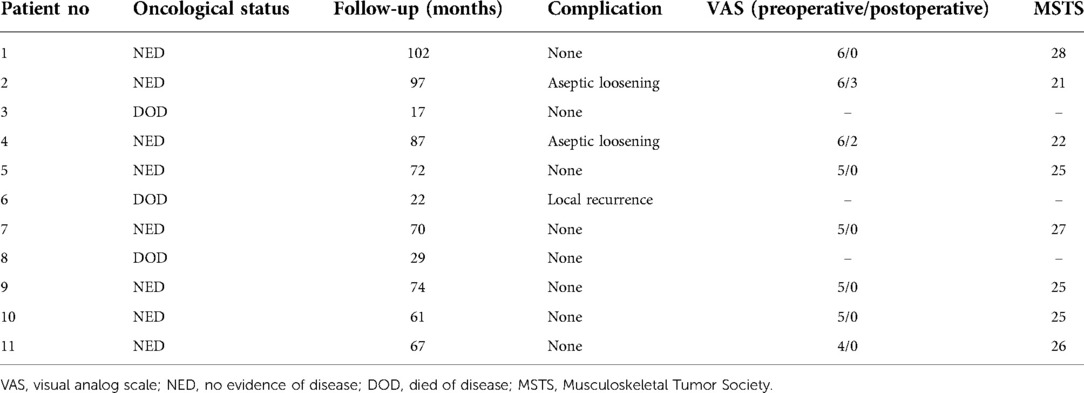
Table 3. Results for patients undergoing distal femoral reconstruction with an intra-neck curved stem endoprosthesis.
Complications
One patient exhibited locally recurrent disease and ultimately succumbed to lung metastases at 22 months post-surgery. With the exception of the two patients that underwent revision surgery described above, there were no instances of aseptic loosening. None of the treated patients developed periprosthetic infections, periprosthetic fractures, neuropathy, implant breakage, or vascular incidents.
Discussion
Optimal approaches to treating distal femoral tumors with proximal metaphyseal extension remain the subject of controversy. TFR is associated with both mechanical and non-mechanical complications (2). In addition, proximal femoral resection can also result in the opening of an additional compartment, and the hip joint may be affected in cases of local infection or recurrence, necessitating hemipelvectomy in certain cases to achieve the margins necessary to avoid further local recurrence (22) Hip joint preservation is critical to the improvement of lower limb function, and bone stock preservation is critical to permit future revision.
Reported hip-preserving reconstructive surgical approaches to date have included customized short medullary stems (23), stems with extra-cortical plates (19), allograft prosthetic composite (APC) (1, 24), stems with cross-fixation pins (16, 25) or the Compress® implant (26, 27). Short-stemmed endoprostheses have the potential to exhibit higher aseptic loosening and implant failure rates (28), with extracortical plates thus being employed in an effort to reduce these risks via supplemental fixation. APC preconstruction has been proposed as an alternative reconstructive approach for tibial and femoral sites (24, 29), offering advantages including endoprosthetic durability, intraoperative flexibility, and local bone stock availability. This approach, however, is subject to limitations such as infection, nonunion, and implant fracture incidence, and postoperative weight-bearing is generally delayed to permit the formation of an allograft-host junction (1). Stems with cross-fixation pins offer advantages including relatively low complication rates (30), but can be costly and necessitate time to facilitate the design and manufacturing process, making their use impractical for patients subject to time limitations associated with neoadjuvant chemotherapy treatment (24). Compressive osseointegration fixation can generate a stable, high-pressure bone-implant interface with the potential to avoid stress shielding (31, 32), but this approach is contraindicated when the cortical thickness at the bone-implant interface is <2.5 mm (33). This compression approach may thus be infeasible in children or patients that have undergone prior reconstructive procedures (24). Chemotherapy has also been shown to lower rates of bone-implant interface cortical hypertrophy, contributing to a trend towards decreased prosthetic survivorship in one report (34).
Promising short-stem endoprostheses available at present include the Compress® implant (26) and the Buxtehude stem (16). Rates of early aseptic loosening associated with the Compress® implant in prior studies range from 3.8%–14% (26, 35). The Buxtehude stem has been used in studies of patients with an SPF, exhibiting instances of fixation screw breakage and 12.5% early aseptic loosening incidence over the course of follow-up (16). In the present study, 2/11 patients developed aseptic loosening following femoral reconstruction, with this incidence rate being in line with rates reported previously for the Compress® implant and Buxtehude stem prostheses. Reasons for these rates of aseptic loosening may include the following: (1) The endoprostheses used for MFDD with an SPF reconstruction in the present study were designed to fit well with the proximal femur anatomy, with accurate INCS positioning being confirmed postoperatively; (2) Relative to a straight stem, the tip of the INCS yields a smaller offset distance such that the bending moment is smaller, potentially contributing to low rates of endoprosthesis loosening (36) (Figure 6). In addition, INCS make the force distribution of the residual proximal femur more even (Supplementary Figure S1); and (3) Achieving lasting fixation between bone and endoprostheses when using cemented straight femoral endoprostheses can be challenging owing to a lack of sufficient residual proximal femoral length. In cases of SPF, the proximal endpoint of the straight intramedullary stem is often present within the trochanteric region, which not only exhibits a large offset, but also does not contain sufficient cancellous bone (28). This lack of sufficient cancellous bone can adversely impact bone cement interdigitation, with the resultant distribution and thickness of this bone cement influencing the stability of the intramedullary endoprosthesis (37).
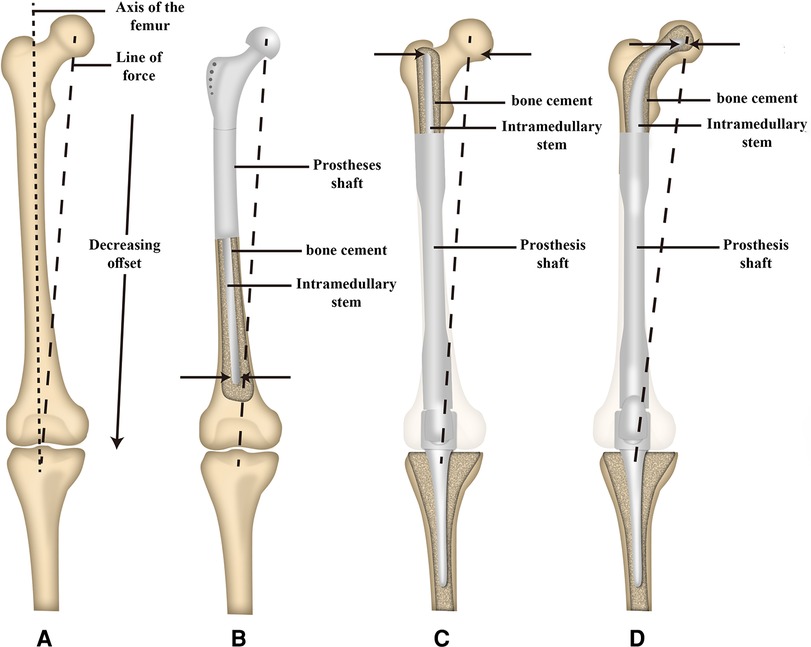
Figure 6. Schematic illustration of the offset distance between the line of force and the long axis of the femur and the offset distance of the tip of intramedullary stem between a proximal and distal femoral replacement. (A) The offset distance between the line of force and the long axis of the femur. (B) The offset distance of the tip of the intramedullary stem of proximal femoral replacement. (C) The offset distance of the tip of the intramedullary straight stem of distal femoral replacement. (D) The offset distance of the tip of the intramedullary curved stem of distal femoral replacement. [Adapted from ref. (26) with permission].
To enable greater intraoperative proximal femur retention, the tip of the stem was pressed to the femoral head-neck junction in two patients. While these patients exhibited good lower extremity function at early postoperative time points, they ultimately developed aseptic loosening of the prosthesis. We believe the reasons as follow: (1) Relative to the center of the femoral head, the stem tip will exhibit a increased distance of offset when located at the femoral head-neck junction; and (2) Relative to the center of the femoral head, the stem tip was subject to greater stress in the medial femoral head-neck junction region. In later procedures, the tip of the curved stem was pressed into the center of the femoral head when feasible during this reconstructive procedure. The positioning of the stem tip was deemed acceptable when located in the middle third of the femoral head in posterior-anterior and lateral radiographic views. Intramedullary stem stability can also be impacted by bone cement distribution and thickness (38). Lee et al. (39) reported a 2–5 mm mantle to be sufficient for bone cement penetration, with increasing thickness representing an effective means of reducing associated stress. For the present study in an effort to improve INCS stability, bone cement thickness at the stem base was increased slightly to 3–4 mm.
The average MSTS score among surviving patients in the present study (25 points) is in line with values reported in other prior studies (16, 26, 40). While complete lower extremity functional rehabilitation was not achieved, these patients did experience substantial pain relief and the ability to retain sufficient limb function to permit self-care. In addition, this operative approach was associated with a relatively quick postoperative recovery and allows for early weight-bearing, both of which are beneficial to patients. Patients in this study did not report any postoperative limitations in lower limb function in daily life. This approach yielded these positive outcomes for several reasons: (1) native hip joint preservation can decrease the potential for surgical disruption, minimizing muscular damage and preventing the degeneration of the articular surface that has the potential to occur when using prosthetic joints or osteoarticular allografts (41), thus allowing for maximal lower extremity functional restoration; (2) both the stability of the utilized endoprostheses and natural bodyweight transmission were beneficial to restoring limb function; and (3) this rehabilitative program was conducive to early functional training, leading to better function of the lower extremities. Periprosthetic infections and fractures are complications that are often observed following distal femur or diaphysis reconstruction (25, 42, 43). As of most recent follow-up, however, none of the patients included in this study had developed either of these complications.
There are several limitations to this study. For one, this is a single-center description of the experiences associated with procedures performed by one surgeon. The sample size in this study was limited, and the follow-up period was relatively short, potentially leading to a failure to note any uncommon complications associated with this operative approach. Moreover, this was a retrospective study with a noncomparative design owing to the rarity of prosthetic reconstruction procedures for MFDD with an SPF following malignant tumor resection, limiting the power of these results. However, the authors believe that, despite these limitations, this study can be instructive to other surgeons and researchers.
Conclusion
In summary, the present study described preliminary outcomes associated with the use of a CCFE with an INCS as an alternative surgical procedure for cases of MFDD with an SPF, providing support for the safety and feasibility of this operative approach. This strategy has the potential to avoid risks associated with proximal femur resection, including dislocation, Trendelenburg limp, and the opening of an additional oncological compartment, while also allowing patients to achieve early weight-bearing and good lower limb function, all while maintaining low rates of procedure-related complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by institutional ethics committee of West China Hospital, Sichuan University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
QY: Data curation, Formal analysis, Investigation, Writing – original draft. ML: Data curation, Formal analysis, Investigation, Writing – original draft. LM: Data curation, Formal analysis, Methodology. YL: Data curation, Formal analysis, Validation. YZ: Conceptualization, Investigation, Visualization, riting – review / editing. CT: Conceptualization, Investigation, Visualization, riting – review / editing. All authors contributed to the article and approved the submitted version.
Funding
The study was approved by the Science and Technology Research Program of Sichuan Province (2020YFS0036), 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (No. ZYJC18036) and Qing Dao research institutes of Si Chuan University, Research of Biomedical Materials and 3D printing related products (20GZ30301) and Project funded by China Postdoctoral Science Foundation (2021M702342).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fsurg.2022.991168/full#supplementary-material.
References
1. Moon BS, Gilbert NF, Cannon CP, Lin PP, Lewis VO. Distal femur allograft prosthetic composite reconstruction for short proximal femur segments following tumor resection. Adv Orthop. (2013) 2013:397456. doi: 10.1155/2013/397456
2. Sevelda F, Schuh R, Hofstaetter JG, Schinhan M, Windhager R, Funovics PT. Total femur replacement after tumor resection: limb salvage usually achieved but complications and failures are common. Clin Orthop Relat Res. (2015) 473(6):2079–87. doi: 10.1007/s11999-015-4282-1
3. Li Y, Yang Y, Huang Z, Shan H, Xu H, Niu X. Bone defect reconstruction with autologous bone inactivated with liquid nitrogen after resection of primary limb malignant tumors. Medicine (Baltimore). (2020) 99(24):e20442. doi: 10.1097/MD.0000000000020442
4. Bus MPA., van de Sande MAJ., Taminiau AHM., Dijkstra PDS.. Is there still a role for osteoarticular allograft reconstruction in musculoskeletal tumour surgery? A long-term follow-up study of 38 patients and systematic review of the literature. Bone / Joint Journal. (2017) 99-B(4):522–30. doi: 10.1302/0301-620X.99B4.BJJ-2016-0443.R2
5. Errani C, Ceruso M, Donati DM, Manfrini M. Microsurgical reconstruction with vascularized fibula and massive bone allograft for bone tumors. Eur J Orthop Surg Traumatol. (2019) 29(2):307–11. doi: 10.1007/s00590-018-2360-2
6. Ayvaz M, Goker B, Leblebicioglu G. Hip-preserving reconstruction of the proximal femur with a vascularized fibula autograft and liquid-nitrogen-treated tumor bearing bone. Jt Dis Relat Surg. (2021) 32(3):792–7. doi: 10.52312/jdrs.2021.12
7. Liu T, Zhang X, Zhang Q, Zhang X, Guo X. Total femoral reconstruction with custom prosthesis for osteosarcoma. World J Surg Oncol. (2016) 14:93. doi: 10.1186/s12957-016-0852-2
8. Sevelda F, Waldstein W, Panotopoulos J, Kaider A, Funovics PT. Reinhard windhager is total femur replacement a reliable treatment option for patients with metastatic carcinoma of the femur? Clin Orthop Relat Res. (2018) 476(5):977–83. doi: 10.1007/s11999.0000000000000125
9. Bus MP, Dijkstra PD, van de Sande MA, Taminiau AH, Schreuder HW, Jutte PC, et al. Intercalary allograft reconstructions following resection of primary bone tumors: a nationwide multicenter study. J Bone Joint Surg Am. (2014) 96(4):e26. doi: 10.2106/JBJS.M.00655
10. Li J, Wang Z, Guo Z, Chen GJ, Fu J, Pei GX. The use of allograft shell with intramedullary vascularized fibula graft for intercalary reconstruction after diaphyseal resection for lower extremity bony malignancy. J Surg Oncol. (2010) 102(5):368–74. doi: 10.1002/jso.21620
11. Houdek MT, Wagner ER, Stans AA, Shin AY, Bishop AT, Sim FH, et al. What is the outcome of allograft and intramedullary free fibula (capanna technique) in pediatric and adolescent patients with bone tumors? Clin Orthop Relat Res. (2016) 474(3):660–8. doi: 10.1007/s11999-015-4204-2
12. Errani C, Ceruso M, Donati DM, Manfrini M. Microsurgical reconstruction with vascularized fibula and massive bone allograft for bone tumors. Eur J Orthop Surg Traumatol: Orthopedie Traumatologie. (2019) 29(2):307–11. doi: 10.1007/s00590-018-2360-2
13. Campanacci DA, Totti F, Puccini S, Beltrami G, Scoccianti G, Delcroix L, et al. Intercalary reconstruction of femur after tumour resection: is a vascularized fibular autograft plus allograft a long-lasting solution? Bone Joint J. (2018) 100-b(3):378–86. doi: 10.1302/0301-620X.100B3.BJJ-2017-0283.R2
14. Rabitsch K, Maurer-Ertl W, Pirker-Fruhauf U, Wibmer C, Leithner A. Intercalary reconstructions with vascularised fibula and allograft after tumour resection in the lower limb. Sarcoma. (2013) 2013:160295. doi: 10.1155/2013/160295
15. Frisoni T, Cevolani L, Giorgini A, Dozza B, Donati DM. Factors affecting outcome of massive intercalary bone allografts in the treatment of tumours of the femur. J Bone Joint Surg Br. (2012) 94(6):836–41. doi: 10.1302/0301-620X.94B6.28680
16. Dieckmann R, Henrichs MP, Gosheger G, Holl S, Hardes J, Streitburger A. Short-stem reconstruction for megaendoprostheses in case of an ultrashort proximal femur. BMC Musculoskelet Disord. (2014) 15:190. doi: 10.1186/1471-2474-15-190
17. Qu H, Guo W, Yang R, Tang X, Yan T, Li D, et al. Cortical strut bone grafting and long-stem endoprosthetic reconstruction following massive bone tumour resection in the lower limb. Bone Joint J. (2015) 97(4):544–9. doi: 10.1302/0301-620X.97B4.34695
18. You Q, Lu M, Min L, Zhang Y, Wang J, Wang Y, et al. Hip-Preserved reconstruction using a customized cementless intercalary endoprosthesis with an intra-neck curved stem in patients with an ultrashort proximal femur: midterm follow-up outcomes. Front Bioeng Biotechnol. (2022) 10:795485. doi: 10.3389/fbioe.2022.795485
19. Stevenson JD, Wigley C, Burton H, Ghezelayagh S, Morris G, Evans S, et al. Minimising aseptic loosening in extreme bone resections custom-made tumour endoprostheses with short medullary stems and extra-cortical plates. Bone Joint J. (2017) 99-B:1689–95. doi: 10.1302/0301-620X.99B12.BJJ-2017-0213.R1
20. Enneking WF. A system of staging musculoskeletal neoplasms. Clin Orthop Relat Res. (1986) 204:9–24. doi: 10.1097/00003086-198603000-00003
21. Enneking WF, Dunham W, Gebhardt MC, Malawar M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. (1993) (286):241–6. PMID: 8425352
22. Kalra S, Abudu A, Murata H, Grimer RJ, Tillman RM, Carter SR. Total femur replacement: primary procedure for treatment of malignant tumours of the femur. Eur J Surg Oncol. (2010) 36(4):378–83. doi: 10.1016/j.ejso.2009.11.002
23. Krauspe PRR. Transposition of a vascularised distal femoral bone graft after wide resection of a diaphyseal ewing's sarcoma–a salvage procedure. Arch Orthop Trauma Surg. (1998) 117:402–4. doi: 10.1007/s004020050279
24. Hindiskere S, Staals E, Donati DM, Manfrini M. What is the survival of the telescope allograft technique to augment a short proximal femur segment in children after resection and distal femur endoprosthesis reconstruction for a bone sarcoma? Clin Orthop Relat Res. (2021) 479(8):1780–90. doi: 10.1097/CORR.0000000000001686
25. Bernthal NM, Upfill-Brown A, Burke ZDC, Ishmael CR, Hsiue P, Hori K, et al. Long-term follow-up of custom cross-pin fixation of 56 tumour endoprosthesis stems a single-institution experience. Bone Joint J. (2019) 101-B:724–31. doi: 10.1302/0301-620X.101B6.BJJ-2018-0993.R1
26. Calvert GT, Cummings JE, Bowles AJ, Jones KB, Wurtz LD, Randall RL. A dual-center review of compressive osseointegration for fixation of massive endoprosthetics: 2- to 9-year followup. Clin Orthop Relat Res. (2014) 472(3):822–9. doi: 10.1007/s11999-013-2885-y
27. Monument MJ, Bernthal NM, Bowles AJ, Jones KB, Randall RL. What are the 5-year survivorship outcomes of compressive endoprosthetic osseointegration fixation of the femur? Clin Orthop Relat Res. (2015) 473(3):883–90. doi: 10.1007/s11999-014-3724-5
28. Unwin PS, Cannon SR, Grimer RJ, Kemp HB, Sneath RS, Walker PS. Aseptic loosening in cemented custom-made prosthetic replacements for bone tumours of the lower limb. J Bone Joint Surg Br. (1996) 78(1):5–13. doi: 10.1302/0301-620X.78B1.0780005
29. Biau DJ, Dumaine V, Babinet A, Tomeno B, Anract P. Allograft-prosthesis composites after bone tumor resection at the proximal tibia. Clin Orthop Relat Res. (2007) 456:211–7. doi: 10.1097/BLO.0b013e31802ba478
30. Cannon CP, Eckardt JJ, Kabo JM, Ward WG Sr, Kelly CM, Wirganowicz PZ, et al. Custom cross-pin fixation of 32 tumor endoprostheses stems. Clin Orthop Relat Res. (2003) 417:285–92. doi: 10.1097/01.blo.0000096801.78689.9e
31. Kramer MJ, Tanner BJ, Horvai AE, O'Donnell RJ. Compressive osseointegration promotes viable bone at the endoprosthetic interface: retrieval study of compress implants. Int Orthop. (2008) 32(5):567–71. doi: 10.1007/s00264-007-0392-z
32. Kagan R, Adams J, Schulman C, Laursen R, Espana K, Yoo J, et al. What factors are associated with failure of compressive osseointegration fixation? Clin Orthop Relat Res. (2017) 475(3):698–704. doi: 10.1007/s11999-016-4764-9
33. Healey JH, Morris CD, Athanasian EA, Boland PJ. Compress knee arthroplasty has 80% 10-year survivorship and novel forms of bone failure. Clin Orthop Relat Res. (2013) 471(3):774–83. doi: 10.1007/s11999-012-2635-6
34. Avedian RS, Goldsby RE, Kramer MJ, O'Donnell RJ. Effect of chemotherapy on initial compressive osseointegration of tumor endoprostheses. Clin Orthop Relat Res. (2007) 459:48–53. doi: 10.1097/BLO.0b013e3180514c66
35. Pedtke AC, Wustrack RL, Fang AS, Grimer RJ, O'Donnell RJ. Aseptic failure: how does the compress((R)) implant compare to cemented stems? Clin Orthop Relat Res. (2012) 470(3):735–42. doi: 10.1007/s11999-011-2159-5
36. Wyatt MC, Kieser DC, Kemp MA, McHugh G, Frampton CMA, Hooper GJ. Does the femoral offset affect replacements? The results from a national joint registry. Hip Int. (2019) 29(3):289–98. doi: 10.1177/1120700018780318
37. Ebramzadeh E, Sarmiento A, McKellop HA, Llinas A, Gogan W. The cement mantle in total hip arthroplasty. Analysis of long-term radiographic results. J Bone Joint Surg Am. (1994) 76(1):77–87. doi: 10.2106/00004623-199401000-00010
38. Cawley DT, Kelly N, McGarry JP, Shannon FJ. Cementing techniques for the tibial component in primary total knee replacement. Bone Joint J. (2013) 95-B:295–300. doi: 10.1302/0301-620X.95B3.29586
39. Lee IY, Skinner HB, Keyak JH. Effects of variation of cement thickness on bone and cement stress at the tip of a femoral implant. Iowa Orthop J. (1993) 13:155–9. PMID: 7820736
40. Zheng K, Yu XC, Hu YC, Shao ZW, Xu M, Wang BC, et al. Outcome of segmental prosthesis reconstruction for diaphyseal bone tumors: a multi-center retrospective study. BMC Cancer. (2019) 19(1):638. doi: 10.1186/s12885-019-5865-0
41. Ramkumar DB, Kelly SP, Ramkumar N, Ercolano LB, Lozano-Calderon S, Gebhardt MC, et al. Oncological and functional outcomes in joint-sparing resections of the proximal femur for malignant primary bone tumors. J Pediatr Orthop. (2021) 41(8):e680–e5. doi: 10.1097/BPO.0000000000001878
42. Choi HS, Nho JH, Kim CH, Kwon SW, Park JS, Suh YS. Revision arthroplasty using a MUTARS(R) prosthesis in comminuted periprosthetic fracture of the distal femur. Yonsei Med J. (2016) 57(6):1517–22. doi: 10.3349/ymj.2016.57.6.1517
Keywords: customized cemented femoral endoprosthesis, intra-neck curved stem, massive femoral diaphyseal defects, short proximal femur segment, reconstructive surgery
Citation: You Q, Lu M, Min L, Zhang Y, Luo Y, Zhou Y and Tu C (2022) Hip-preserving reconstruction using a customized cemented femoral endoprosthesis with a curved stem in patients with short proximal femur segments: Mid-term follow-up outcomes. Front. Surg. 9:991168. doi: 10.3389/fsurg.2022.991168
Received: 11 July 2022; Accepted: 5 September 2022;
Published: 22 September 2022.
Edited by:
Jason Pui Yin Cheung, The University of Hong Kong, Hong Kong, SAR ChinaReviewed by:
Jiazhen Li, First Affiliated Hospital of Zhengzhou University, ChinaLianghao Zhang, Peking University, China
© 2022 You, Lu, Min, Zhang, Luo, Zhou and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chongqi Tu Tucq@scu.edu.cn Yong Zhou Zhouyonggk@163.com
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Orthopedic Surgery, a section of the journal Frontiers in Surgery
 Qi You
Qi You Minxun Lu
Minxun Lu Li Min1,2
Li Min1,2  Yong Zhou
Yong Zhou Chongqi Tu
Chongqi Tu