Clinical outcome comparison of laparoscopic radical antegrade modular pancreatosplenectomy vs. laparoscopic distal pancreatosplenectomy for left-sided pancreatic ductal adenocarcinoma surgical resection
- 1Department of Surgery, The Second Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, China
- 2Department of General Surgery, Cancer Center, Division of Gastrointestinal and Pancreatic Surgery, Zhejiang Provincial People’s Hospital, Affiliated People’s Hospital, Hangzhou Medical College, Hangzhou, China
- 3Department of Surgery, Bengbu Medical College, Bengbu, China
- 4Department of Surgery, Qingdao University, Qingdao, China
Background: Laparoscopic radical antegrade modular pancreatosplenectomy (LRAMPS) is a validated surgical treatment for patients with left-sided pancreatic ductal adenocarcinoma (PDAC). In addition, laparoscopic distal pancreatectomy (LDPS) has purported benefits. However, there is a limited analysis comparing the results between LRAMPS and LDPS. Thus, this study aims to compare the short-term and long-term outcomes of patients who underwent LRAMPS and LDPS for PDAC treatment.
Methods: Patients with left-sided PDAC that underwent LRAMPS or LDPS from 2015 to 2021 were retrospectively identified. Demographic and clinic pathologic data were collected. Disease-free survival (DFS) and overall survival (OS) probabilities were obtained.
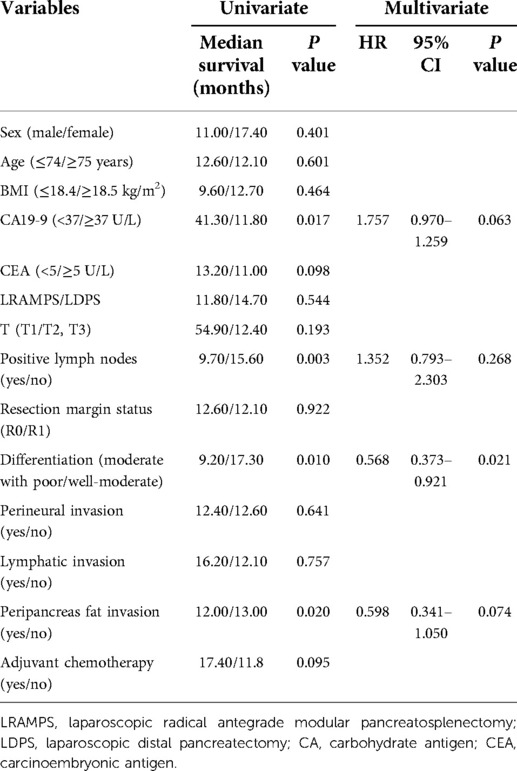
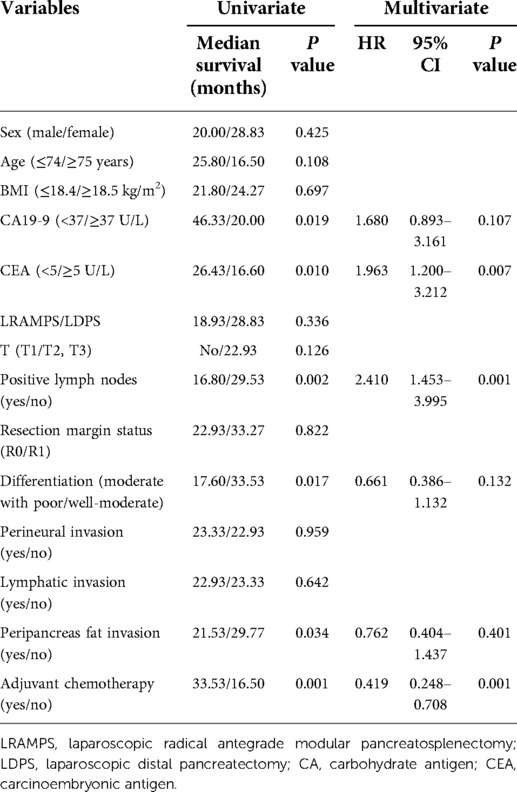
Results: The number of lymph nodes retrieved was significantly greater in the LRAMPS group than in the LDPS group. Several clinicopathological factors, including CA19-9 levels greater than 37 U/ml, positive lymph nodes, moderate to poor tumor differentiation, and peripancreas fat invasion, were associated with DFS. Moderate with poor tumor differentiation was associated with poor DFS (HR 0.568; 95% CI 0.373–0.921; P = 0.021). Levels of CA19-9 greater than 37 U/ml, CEA levels greater than 5 μg/ml, larger tumor size, positive lymph nodes, moderate with poor tumor differentiation, peripancreas fat invasion, and adjuvant chemotherapy were all associated with OS. LRAMPS nearly improved OS but did not reach statistical significance. Serum carcinoembryonic antigen (CEA) levels greater than 5 ug/ml (HR 1.693; 95% CI 1.200–1.132; P = 0.001), and positive lymph nodes (HR 2.410; 95% CI 1.453–3.995; P = 0.001) were independently associated with poor OS. Treatment with adjuvant chemotherapy was associated with improved OS (HR 0.491; 95% CI 0.248–0.708; P = 0.001).
Conclusions: The LRAMPS procedure achieved comparable results to standard LDPS in terms of postoperative outcomes. Treatment with chemotherapy is important for the prognosis of patients with left-sided pancreatic cancer.
Introduction
Surgical resection offers the only curative treatment for pancreatic ductal adenocarcinoma (PDAC). Specifically, distal pancreatectomy (DPS) is the standard procedure for left-sided PDAC resection, but it has a high rate of retroperitoneal margin positivity (62%), which indicates that cancerous tissue persists post-surgery. In 2003, Dr. Steven Strasberg introduced the radical antegrade modular pancreatosplenectomy (RAMPS) procedure (1). Compared to DPS, RAMPS attempts to achieve negative retroperitoneal margins and higher lymph node retrieval in order to improve survival outcomes (2, 3). Several studies have demonstrated that RAMPS increases the negative tangential margin rate and lymph node harvest (4). Furthermore, laparoscopic distal pancreatectomy (LDPS) has purported benefits for short-term and long-term outcomes compared to open DPS (5, 6). Similarly, laparoscopic RAMPS (LRAMPS) has been demonstrated to be more feasible (7–9). In a recent meta-analysis to date comparing minimally invasive RAMPS (MI-RAMPS) against open RAMPS, intra-operative blood loss was observed to be significantly reduced in MI-RAMPS, while lymph node yield was higher in O-RAMPS and there was no difference in overall survival (OS) (10). Modern chemotherapy has proven to be effective and improves the survival of PDAC. A previous study revealed chemotherapy to be an independent factor for OS after RAMPS and DPS (11). However, there is limited clinical outcome analysis comparing the LRAMPS and LDPS procedures with chemotherapy. Thus, we conducted this study to compare the short-term and long-term outcomes of patients who underwent LRAMPS and LDPS for left-sided PDAC treatment.
Methods
Patients
From May 2015 to May 2021, patients with left-sided PDAC that underwent LRAMPS or LDPS procedures performed by one surgeon were retrospectively identified from the database at Zhejiang Provincial People's Hospital. Patients with left-sided PDAC without any evidence of distant metastasis or vascular invasion beyond the celiac axis were included. Patients who underwent neoadjuvant therapy and vascular reconstruction were excluded. All acquisition methods were approved by the Institutional Review Board.
Demographic and clinic pathologic data were collected. Postoperative pancreatic fistula (POPF) was defined according to the International Study Group on Pancreatic Fistula Definition (ISGPF) (12). All patients with grades B and C were defined as having clinically significant POPF. The severity of complications was defined according to Clavien–Dindo classification system (13). Pathological data were classified using the eighth AJCC/UICC TNM. R1 margin status was defined as <1 mm from the edge of the specimen. Overall survival (OS) was defined as the duration of time between the date of surgery and the date of death. Disease-free survival (DFS) was similarly calculated as the interval between the date of surgery and the date of tumor recurrence. Adjuvant chemotherapy was recommended as routine therapy for patients who could tolerate the adverse effects. Chemotherapy regimens and duration were left to the discretion of the oncologist.
Operative procedures
The LRAMPS procedure was performed in our institution since 2017 as described by Strasberg et al. (1). Briefly, patients were placed in the supine position with their heads slightly elevated. Five trocars, including a camera port (10 mm) below the umbilicus and four additional working ports (one 12 mm and three 5 mm), were placed in the right flank, right upper flank, left upper flank, and left flank. The peritoneal cavity and liver surface were inspected to rule out metastasis. The gastrocolic ligament was divided to expose the anterior surface of the pancreas. The inferior border of the pancreas was mobilized to visualize the superior mesenteric vein and the portal vein. Furthermore, the pancreas was mobilized along the superior border to explore the common hepatic artery and to dissect the lymph nodes along the common hepatic and gastroduodenal arteries. The pancreas neck was then transected with an endoscopic linear stapler (Ethicon Endo-Surgery, PSE45A, Cincinnati, OH, USA). The splenic artery and vein were ligated and divided, respectively. Lymph nodes were dissected from the celiac axis down to the left side of the superior mesenteric artery. The distal pancreas was dissected along with the soft tissue of the retroperitoneum in a medial-to-lateral manner. The short gastric vessels were ligated to mobilize the spleen and the lymph nodes along the splenic artery and hilum were removed. After complete resection of the distal pancreas and spleen, the tissue was removed through an enlarged umbilical incision with a specimen bag. The LDPS procedure was performed as previously described (14). The laparoscopic procedure was performed as the LRAMPS procedure. The lymph nodes along the celiac axis and superior mesenteric artery were dissected but the soft tissue of the retroperitoneum was reserved.
Statistical analysis
All statistical analyses were performed using SPSS v.22.0 and GraphPad Prism 8 software. All continuous variables were presented as median summative values with interquartile ranges (IQRs). Continuous variables were compared using the student's t-test or Wilcoxon rank test for parametric or nonparametric distributions, respectively. The chi-square or Fisher's exact tests were used to analyze categorical variables, as appropriate. OS and DFS were assessed using the Kaplan–Meier estimate method, and comparisons were conducted using the log-rank test. Only variables with P-value less than 0.05 in univariate analysis were included in a Cox proportional hazards model. P-values less than 0.05 were considered statistically significant.
Results
In total, 109 patients with left-sided PDAC underwent surgical resection. Clinic pathological characteristics of patients are summarized in Table 1.
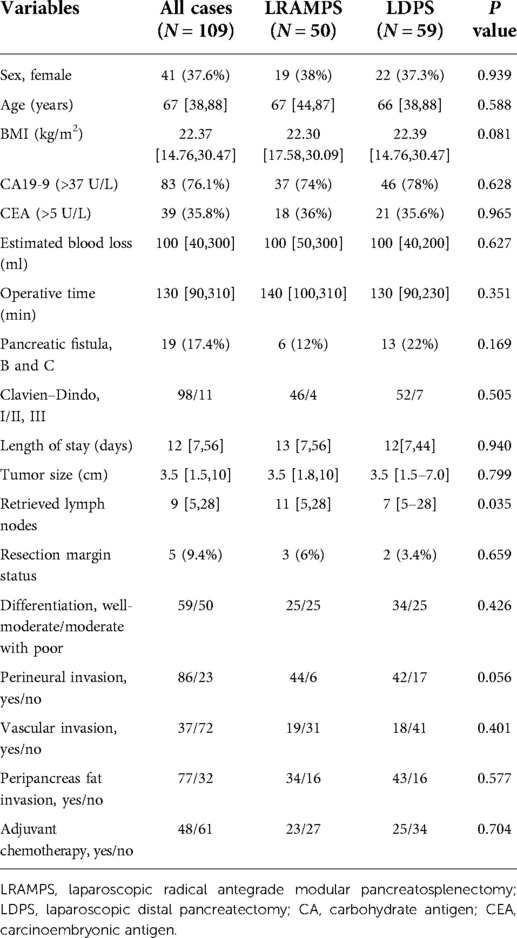
Table 1. Clinicopathological characteristics of patients with left-side pancreatic ductal adenocarcinoma.
Fifty (45.9%) patients underwent LRAMPS, and 59 (54.1%) patients underwent LDPS. Factors including sex, age, BMI, serum carbohydrate antigen (CA) 19-9 values, and serum carcinoembryonic antigen (CEA) values were confirmed to have no significant difference between the LRAMPS and LDPS groups. A total of 19 patients developed clinically significant POPF. Thirteen cases of grade B POPF occurred in the LDPS group. Moreover, one case of grade C and five cases of grade B POPF were confirmed in the LRAMPS group. No meaningful differences were found in operative time, estimated blood loss (EBL), clinically significant POPF, complications, or length of stay (Table 1).
There were no significant differences in the tumor size, level of differentiation, R0 resection margin, perineural invasion, vascular invasion, or peripancreas fat invasion. The number of lymph nodes retrieved was significantly greater in the LRAMPS group than in the LDPS group (P = 0.035). Furthermore, 49 (42.2%) patients received adjuvant chemotherapy and others rejected chemotherapy. Gemcitabine-based chemotherapy was administered as first-line chemotherapy for most patients; the remaining patients received FOLFIRINOX. The median follow-up period was 46.7 months.
By the end of the follow-up period, disease recurrence occurred in 77 (70.64%) patients and 69 (63.3%) patients had died. The median DFS was 12.6 months and the median OS was 22.9 months. Finally, the 1-year, 3-year, and 5-year OS rates were 84.4%, 25.7%, and 12.8%, respectively.
Several clinicopathological factors were associated with DFS upon univariate analysis, including CA19-9 levels greater than 37 U/ml, positive lymph nodes, moderate with poor tumor differentiation, and peripancreas fat invasion. Neither surgical procedure nor adjuvant chemotherapy was found to affect DFS. Additionally, moderate with poor tumor differentiation was associated with poor DFS upon multivariate analysis (HR 0.568; 95% CI 0.373–0.921; P = 0.021) (Table 2).
Univariate analysis revealed CA19-9 levels greater than 37 U/ml, CEA levels greater than 5 ug/ml, larger tumor size, positive lymph nodes, moderate with poor tumor differentiation, peripancreas fat invasion, and adjuvant chemotherapy were associated with OS. LRAMPS trended to improve OS but did not reach statistical significance (28.83 months vs. 18.93 months, P = 0.336). Serum CEA levels greater than 5 μg/ml (HR 1.693; 95% CI 1.200–1.132; P = 0.001) and positive lymph nodes (HR 2.410; 95% CI 1.453–3.995; P = 0.001) were independently associated with poor OS. Treatment with adjuvant chemotherapy was associated with improved OS (HR 0.491; 95% CI 0.248–0.708; P = 0.001) (Table 3).
Cancer-positive lymph nodes were found to affect both DFS and OS. The LRAMPS procedure retrieved more lymph nodes than LDPS. However, the median OS of patients with positive lymph nodes that underwent LRAMPS and LDPS was 22.1 and 13.6 months, respectively, and did not reach statistical significance (P = 0.156). OS in patients with positive lymph nodes and treated with adjuvant chemotherapy was significantly different from untreated patients (33.5 months vs. 13.4 months, P = 0.001) (Figure 1). In the cancer-negative lymph node cohort, neither surgical procedure (P = 0.502) nor treatment with adjuvant chemotherapy (P = 0.065) was found to affect OS (Figure 2).
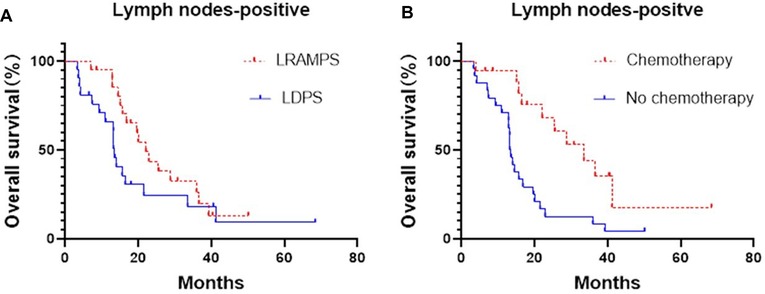
Figure 1. Median OS of positive lymph nodes patients, (A) LRAMPS: 22.1 months; LDPS: 13.57 months. (B) Chemotherapy: 33.5 months; no chemotherapy: 13.4 months.
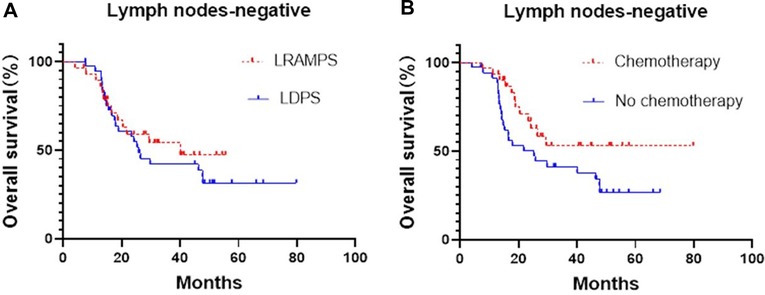
Figure 2. Median OS of negative lymph nodes patients, (A) LRAMPS: 40.1 months; LDPS: 25.8 months. (B) Chemotherapy: not reach; no chemotherapy: 25.3 months.
Discussion
Curative surgery plays an essential role in the treatment of PDAC. R0 resection margin and radical N1 lymph node resection are major prognostic factors for patients with left-sided PDAC (15, 16). Many reports (11, 17) showed that RAMPS achieved improved R0 resection and regional lymph node retrieval compared to DPS as evident by reported outcomes. Due to these results, RAMPS became a mainstream surgical procedure for patients with left-sided PDAC. Over the past decade, the safety and feasibility of LDPS improved and demonstrated fewer complications with shortened hospital stays worldwide (5). Therefore, the LRAMPS procedure was mainly performed in high-volume pancreatic centers, where several reports indicated the improved safety and feasibility of LRAMPS (10).
The LRAMPS procedure is more complex than LDPS. Therefore, surgeons worried about longer operative times and perioperative complications when deciding between the two procedures. However, in the present study, there were no significant differences found in operative time, EBL, clinically significant POPF, complications, and length of stay between LRAMPS and LDPS groups. A large, multi-institutional study also revealed that there were no differences in rates of postoperative pancreatic fistula (16.5% vs. 17.8%, P = 1.000) and postoperative hemorrhage (5.9% vs. 3.6%, P = 0.385) between RAMPS and DPS groups (18). Furthermore, a meta-analysis indicated no significant differences were observed between RAMPS and DPS in terms of postoperative complications (P = 0.87), POPF (P = 0.15), mortality (P = 0.80), and length of stay (P = 0.53) (4).
This study demonstrated no significant difference in DFS or OS between LRAMPS and LDPS procedures and supported the findings of previous studies and meta-analyses of RAMPS and DPS (11, 18). Moreover, the results showed that adjuvant chemotherapy was associated with improved OS. It supported the consensus that adjuvant chemotherapy is indispensable for patients with PDAC. Survival is slowly increasing with a reported median OS of 54.4 months with adjuvant chemotherapy with FOLFIRINOX after surgery (19, 20).
Many RAMPS proponents argued that R0 resection and more lymph node retrieval can improve prognosis. In this study, the number of lymph nodes retrieved was significantly greater in the LRAMPS group than in the LDPS group. However, there was no significant difference in the survival of patients with positive lymph nodes that underwent LRAMPS or LDPS (22.1 months vs. 13.6 months, P = 0.156). In addition, we found no significant difference in the R0 resection margin. Moreover, OS in patients with positive lymph nodes and treated with adjuvant chemotherapy was significantly longer than in patients that did not receive adjuvant chemotherapy (33.5 vs. 13.4, P = 0.001). It was consistent with several reports between open RAMPS and DPS (11, 18). We hypothesize the survival of patients with positive lymph nodes was more likely determined by adjuvant chemotherapy because of biological properties rather than surgical procedures (21). Modern chemotherapy has proven to be effective and improve survival, even in the setting of an R1 resection (22, 23).
We acknowledge that this study has limitations. First, this was a retrospective, cohort study, which has inherent weaknesses. Second, some data, such as pancreatic parenchyma texture and the thickness of the pancreatic stump, were not elaborately collected and may affect the quality of the reported short-term outcomes. Finally, due to the limited number of patients who received adjuvant chemotherapy, the efficacy of different chemotherapy regimens could not be thoroughly evaluated.
Conclusions
The LRAMPS procedure achieves comparable results to standard LDPS regarding postoperative outcomes. Comparatively, LRAMPS retrieves more lymph nodes. In addition, positive lymph nodes correlated with both DFS and OS. However, LRAMPS is not associated with an improvement in either DFS or OS over LDPS. OS in patients with positive lymph nodes and treated with adjuvant chemotherapy was significantly better than in untreated patients. Treatment with chemotherapy is an important predictive factor procedure for the prognosis of patients with left-sided pancreatic cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by Zhejiang Provincial People's Hospital Institutional Review Board. The patients/participants provided their written informed consent to participate in this study.
Author contributions
NN, YM, and TX contributed to the conception and design of the study. YHH, YCZ, WWJ, CL, QCZ, and YX organized the database. SM, PX, TX performed the statistical analysis. TX wrote the first draft of the manuscript. NN and YH wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the Key Medical Science and Technology Project of Zhejiang Province (no. WKJ-ZJ-2201 to YPM), and Key Projects of Zhejiang Provincial Science and Technology (no. 2022C03099 to YPM).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Strasberg SM, Drebin JA, Linehan D. Radical antegrade modular pancreatosplenectomy. Surgery. (2003) 133(5):521–7. doi: 10.1067/msy.2003.146
2. Dai M, Zhang H, Li Y, Xing C, Ding C, Liao Q, et al. Radical antegrade modular pancreatosplenectomy (RAMPS) versus conventional distal pancreatosplenectomy (CDPS) for left-sided pancreatic ductal adenocarcinoma. Surg Today. (2021) 51(7):1126–34. doi: 10.1007/s00595-020-02203-3
3. Chun YS. Role of radical antegrade modular pancreatosplenectomy (RAMPS) and pancreatic cancer. Ann Surg Oncol. (2018) 25(1):46–50. doi: 10.1245/s10434-016-5675-4
4. Zhou Q, Fengwei G, Gong J, Xie Q, Liu Y, Wang Q, et al. Assessment of postoperative long-term survival quality and complications associated with radical antegrade modular pancreatosplenectomy and distal pancreatectomy: a meta-analysis and systematic review. BMC Surg. (2019) 19(1):12. doi: 10.1186/s12893-019-0476-x
5. Lee JM, Kim H, Kang JS, Byun Y, Choi YJ, Sohn HJ, et al. Comparison of perioperative short-term outcomes and oncologic long-term outcomes between open and laparoscopic distal pancreatectomy in patients with pancreatic ductal adenocarcinoma. Ann Surg Treat Res. (2021) 100(6):320–8. doi: 10.4174/astr.2021.100.6.320
6. de Rooij T, van Hilst J, van Santvoort H, Boerma D, van den Boezem P, Daams F, et al. Minimally invasive versus open distal pancreatectomy (LEOPARD): a multicenter patient-blinded randomized controlled trial. Ann Surg. (2019) 269(1):2–9. doi: 10.1097/SLA.0000000000002979
7. Larkins K, Rowcroft A, Pandanaboyana S, Loveday BPT. A systematic scoping review of the initial experience with laparoscopic radical antegrade modular pancreatosplenectomy for pancreatic malignancy. Surg Endosc. (2021) 35(9):4930–44. doi: 10.1007/s00464-021-08528-5
8. Hirashita T, Iwashita Y, Fujinaga A, Nakanuma H, Tada K, Masuda T, et al. Surgical and oncological outcomes of laparoscopic versus open radical antegrade modular pancreatosplenectomy for pancreatic ductal adenocarcinoma. Surg Today. (2021) 52(2):224–30. doi: 10.1007/s00595-021-02326-1
9. Zhang H, Li Y, Liao Q, Xing C, Ding C, Zhang T, et al. Comparison of minimal invasive versus open radical antegrade modular pancreatosplenectomy (RAMPS) for pancreatic ductal adenocarcinoma: a single center retrospective study. Surg Endosc. (2021) 35(7):3763–73. doi: 10.1007/s00464-020-07938-1
10. Wu EJ, Kabir T, Zhao JJ, Sarr M, Abu Hilal M, Adham M, et al. Minimally invasive versus open radical antegrade modular pancreatosplenectomy: a meta-analysis. World J Surg. (2022) 46(1):235–45. doi: 10.1007/s00268-021-06328-5
11. Kim HS, Hong TH, You YK, Park JS, Yoon DS. Radical antegrade modular pancreatosplenectomy (RAMPS) versus conventional distal pancreatectomy for left-sided pancreatic cancer: findings of a multicenter, retrospective, propensity score matching study. Surg Today. (2021) 51(11):1775–86. doi: 10.1007/s00595-021-02280-y
12. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. (2017) 161(3):584–91. doi: 10.1016/j.surg.2016.11.014
13. Clavien PA, Barkun J, DE Oliveira ML, Vauthey JN, Dindo D, Schulick RD, et al. The Clavien–Dindo classification of surgical complications: five-year experience. Ann Surg. (2009) 250(2):187–96. doi: 10.1097/SLA.0b013e3181b13ca2
14. Xia T, Zhou JY, Mou YP, Xu XW, Zhang RC, Zhou YC, et al. Risk factors for postoperative pancreatic fistula after laparoscopic distal pancreatectomy using stapler closure technique from one single surgeon. PLoS One. (2017) 12(2):e0172857. doi: 10.1371/journal.pone.0172857
15. Rho SY, Hwang HK, Chong JU, Yoon DS, Lee WJ, Kang CM. Association of preoperative total lymphocyte count with prognosis in resected left-sided pancreatic cancer. ANZ J Surg. (2019) 89(5):503–8. doi: 10.1111/ans.15030
16. Sugawara T, Ban D, Nishino J, Watanabe S, Maekawa A, Ishikawa Y, et al. Prediction of early recurrence of pancreatic ductal adenocarcinoma after resection. PLoS One. (2021) 16(4):e0249885. doi: 10.1371/journal.pone.0249885
17. Trottman P, Swett K, Shen P, Sirintrapun J. Comparison of standard distal pancreatectomy and splenectomy with radical antegrade modular pancreatosplenectomy. Am Surg. (2014) 80(3):295–300. doi: 10.1177/000313481408000326
18. Sham JG, Guo S, Ding D, Shao Z, Wright M, Jing W, et al. Radical antegrade modular pancreatosplenectomy versus standard distal pancreatosplenectomy for pancreatic cancer, a dual-institutional analysis. Chin Clin Oncol. (2020) 9(4):54. doi: 10.21037/cco-20-6
19. Oneda E, Zaniboni A. Are we sure that adjuvant chemotherapy is the best approach for resectable pancreatic cancer? Are we in the era of neoadjuvant treatment? A review of current literature. J Clin Med. (2019) 8(11):1922. doi: 10.3390/jcm8111922
20. Motoi F, Unno M. Adjuvant and neoadjuvant treatment for pancreatic adenocarcinoma. Jpn J Clin Oncol. (2020) 50(5):483–9. doi: 10.1093/jjco/hyaa018
21. Wijetunga AR, Chua TC, Nahm CB, et al. Survival in borderline resectable and locally advanced pancreatic cancer is determined by the duration and response of neoadjuvant therapy. Eur J Surg Oncol. (2021) 47(10):2543–50. doi: 10.1016/j.ejso.2021.04.005
22. Zhang E, Wang L, Shaikh T, Pavlakis N, Clarke S, Chan DL, et al. Neoadjuvant chemoradiation impacts the prognostic effect of surgical margin status in pancreatic adenocarcinoma. Ann Surg Oncol. (2022) 29(1):354–63. doi: 10.1245/s10434-021-10219-3
Keywords: pancreatic cancer, laparoscopic distal pancreatosplenectomy, laparoscopic RAMPS, chemotherapy, survivability
Citation: Niu N, He Y, Mou Y, Meng S, Xu P, Zhou Y, Jin W, Lu C, Xu Y, Zhu Q and Xia T (2022) Clinical outcome comparison of laparoscopic radical antegrade modular pancreatosplenectomy vs. laparoscopic distal pancreatosplenectomy for left-sided pancreatic ductal adenocarcinoma surgical resection. Front. Surg. 9:981591. doi: 10.3389/fsurg.2022.981591
Received: 29 June 2022; Accepted: 10 August 2022;
Published: 1 September 2022.
Edited by:
Chang-In Choi, Pusan National University Hospital, South KoreaReviewed by:
Ludovico Carbone, University of Siena, ItalyFrancesca Marcon, IRCCS Ca 'Granda Foundation Maggiore Policlinico Hospital, Italy
© 2022 Niu, He, Mou, Meng, Xu, Zhou, Jin, Lu, Xu, Zhu and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yiping Mou yipingmou@126.com; Tao Xia xiatao13@163.com
†These authors have contributed equally to this work
Specialty Section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Nan Niu
Nan Niu Yuhui He1,2,†
Yuhui He1,2,†  Yiping Mou
Yiping Mou Tao Xia
Tao Xia