Risk Factors and Clinical Impacts of Post-Pancreatectomy Acute Pancreatitis After Pancreaticoduodenectomy: A Single-Center Retrospective Analysis of 298 Patients Based on the ISGPS Definition and Grading System
- 1Department of Hepatobiliary Surgery, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 2Department of Physiology and Pathophysiology, School of Basic Medicine, Xi’an Jiaotong University, Xi’an, China
- 3Department of Radiology, the First Affiliated Hospital of Xi’an Jiaotong University, Xi’an, China
- 4Department of Epidemiology and Biostatistics, School of Public Health, Xi’an Jiaotong University Health Science Center, Xi’an, China
Background: The definition and grading system of post-pancreatectomy acute pancreatitis (PPAP) has recently been proposed by ISGPS. This study aimed to put this definition and classification into practice and investigate the potential risk factors and clinical impacts of PPAP.
Methods: Demographic and perioperative data of consecutive patients who underwent pancreaticoduodenectomy (PD) from January 2019 to July 2021 were collected and analyzed retrospectively. The diagnostic criteria of PPAP published by ISGPS, consisting of biochemical, radiologic, and clinical parameters, were adopted. The risk factors were analyzed by univariate and multivariate analyses.
Results: A total of 298 patients were enrolled in this study, and the total incidence of PPAP was 52.4% (150 patients). Stratified by clinical impacts of PPAP, the incidences of grades B and C PPAP were 48.9% and 3.5%, respectively. PPAP after PD was significantly associated with pancreatic fistula and other unfavorable complications. Soft pancreatic texture (OR 3.0) and CRP ≥ 180 mg/L (OR 3.6) were the independent predictors of PPAP, AUC 0.613. Stratified by the grade of PPAP, soft pancreatic texture (OR 2.7) and CRP ≥ 180 mg/L (OR 3.4) were the independent predictors of grade B PPAP, and soft pancreatic texture (OR 19.3), operation duration >360 min (OR 13.8), and the pancreatic anastomosis by using conventional duct to mucosa methods (OR 10.4) were the independent predictors of grade C PPAP. PPAP complicated with pancreatic fistula significantly increased the severe complications and mortality compared to only PPAP occurrence.
Conclusion: PPAP was not an uncommon complication after PD and was associated with unfavorable clinical outcomes, especially since it was complicated with pancreatic fistula. Soft pancreatic texture and CRP ≥ 180 mg/L were the independent predictors of PPAP. Higher-volume multicenter and prospective studies are strongly needed.
Introduction
Post-ERCP pancreatitis (PEP) has been widely recognized, and its clinical practice guidelines have been published (1). Under the same postoperative background, postpancreatectomy acute pancreatitis (PPAP) was not comprehensively recognized. Previous studies regarded PPAP as an indirect manifestation of pancreatic fistula (PF) (2, 3). PPAP has attracted attention since Connor proposed the first definition based on the systematic review (4). Several medical centers carried out their clinical studies relevant to PPAP (5–9), and the incidence reported in previous studies varied widely from 1.5% to 67.9% due to the lack of authoritative definitions and terminology.
Recently, the international study group of pancreatic surgeons (ISGPS) developed a consensus definition, diagnostic, and grading criterion of PPAP. PPAP is defined as acute inflammation of the remnant pancreas within the first 3 days after partial pancreatectomy. The ISGPS group come up with the term “PPAP” instead of postoperative pancreatitis (POAP) (4) to refer specifically to pancreatitis after partial pancreatectomy. This group also clarified the definition of postoperative serum hyperamylasemia (POH), which had previously been confused with PPAP (10). The diagnostic criteria of PPAP (11) require three dimensions: sustained POH, clinical impacts relevant to PPAP, and radiologic features of acute pancreatitis (12, 13). The grading system of PPAP is based on clinical impacts, including POH (biochemical change only), grade B (mild or moderate clinical impacts), and grade C (severe clinical impacts).
Here, in this study, we used the definition and grading system of PPAP that had just been published by ISGPS to review our clinical data and aimed to assess PPAP in our clinical practice and recognize potential risk factors of PPAP.
Methods
Patients and Data Collection
This retrospective study was performed on all patients who consecutively underwent pancreaticoduodenectomy (PD) from January 2019 to July 2021 at the First affiliated Hospital of Xi’an Jiaotong University. Patients who got a PD procedure in the Department of Hepatobiliary Surgery were enrolled in this study. Patients without a detailed record of postoperative complications, serum amylase, and abdominal CT scan in the early postoperative period were excluded. This study was approved by the local ethics committee (ethical approval number: XJTU1AF2015LSL-057), and informed consent was obtained from the patients.
To control bias, demographics, preoperative clinical parameters, and postoperative clinical parameters were collected by different individuals to reduce the behavior of artificial adjustment and the influence of personal tendency in the data collection phase. Demographic characteristics included age, gender, body mass index (BMI), smoking, and drinking conditions. Past medical history and comorbidities included cardiovascular and pulmonary diseases, hepatitis, kidney diseases, cholelithiasis, type 2 diabetes mellitus, history of acute pancreatitis attack and previous abdominal surgery, preoperative jaundice, and the American Society of Anaesthesiologists (ASA) score.
Operative details included blood loss, transfusion, and operative duration. The surgical procedure details also contained whether pylorus-preserving pancreaticoduodenectomy (PPPD) (14) or Whipple procedure (15), standard or extended resection (vascular resection and/or extended organ resection), the usage of pancreatic duct stent, and the diameter of the pancreatic duct. The pancreatic texture was assessed by the primary surgeon and documented in the surgical records. The fistula risk scores (16) were calculated. The management of pancreatic stump in operations was all treated with pancreatojejunostomy (PJ), none of the pancreatogastric anastomosis (PG), including modified Blumgart pancreatic duct–mucous anastomosis, double-layer duct-to-mucosa (conventional duct to the mucosa), and end-to-side or end-to-end invagination. Pathology types were divided into three groups: the first was pancreatic ductal adenocarcinoma (PDAC) and chronic pancreatitis (CP), the second was another malignant group (periampullary carcinoma, duodenal carcinoma), and the third was the benign and low malignant group (pancreatic cyst tumor, pancreatic neuroendocrine tumor, duodenal stromal tumor, and other benign or precancerous lesions).
The removal time and volume of drainage tubes, including gastric, urine, and abdominal drainage tubes, were collected by the medical orders and medical records. The number of patients who accepted the neoadjuvant therapy was collected. The usage of the pancreatic exocrine inhibitory drug (octreotide or somatostatin) and ulinastatin was recorded. The length of hospital stay, postoperative hospital stay, intensive care unit (ICU) stay, postoperative mortality, and gross cost were collected. Perioperative serum biochemical markers including CRP (on POD 0–3), total bilirubin (TB), direct bilirubin, albumin (ALB), calcium, and amylase were also recorded.
Definitions
The preoperative jaundice state means serum TB ≥ 34.2 μmol/L (more than 2 times the upper normal serum level) before the surgery. The liquid intake/output volume was defined as the difference between all intake volume and output (urine volume plus blood loss) on the day of operation (POD 0) including intraoperative. About 2000 ml (roughly the physiological requirements) was the cutoff to assess the liquid intake/output volume. The measures of preoperative biliary drainage include percutaneous transhepatic biliary drainage (PTBD), endoscopic nasobiliary drainage (ENBD), and T-shaped tube placed in the previous operation. The volume of abdominal drainage, namely, extraintestinal drainage on the postoperative day, did not include the intestinal drainage such as the pancreatic duct stents, biliary stents, PTBD, and gastric tubes. Hypoalbuminemia was defined as the serum concentration on POD 1 of less than 3.5 g/dL (35 g/L), and serum calcium on POD 1 below the lower limit was defined as hypocalcemia. The ΔTB was equal to the postoperative minus preoperative TB value on POD 1. The unchanged ΔTB ranged from −5 μmol/ L to 5μmol/ L, higher than that defined as elevation and lower than that defined as decrease.
The definition and severity of PPAP (11), PF (17), delayed gastric empty (DGE) (18), and postpancreatectomy hemorrhage (PPH) (19) were according to ISGPS. Bile leakage (BL) was defined as the concentration of bilirubin in the drainage fluid >3 times of the serum bilirubin on or POD 3 or requiring radiological or surgical intervention due to biliary collection or biliary peritonitis (20). Intra-abdominal infection was supported by evidence of bacterial culture etiology in the abdominal drainage fluid. Wound infection was proved by purulent discharge or the need to remove the suture and drainage. Acute kidney injury (AKI) was according to the Kidney Disease Improving Global Outcomes (KDIGO) classification (21). The abdominal fluid collection was confirmed by imaging (ultrasound or CT scans). Percutaneous drainage was guided by ultrasound or CT scans under local infiltration anesthesia. Unplanned reoperation meant the unplanned need for laparotomy or interventional surgery during the hospital stay. The severity of complications was according to the Clavien–Dindo classification (22); the ≥IIIb complications were defined as serious complications. Postoperative mortality was stipulated as mortality within 30 days after surgery.
Evaluation of Postoperative CT
Postoperative CT scans were evaluated by the team of the Department of Radiology at the First Affiliated Hospital of Xi’an Jiaotong University, which consisted of one professor and two associate professors majoring in the abdominal area. This team reached a consensus on the manifestations of PPAP on postoperative CT images (Figure 1). Based on the radiological features in the early postoperative period (11, 12), PPAP can be stratified into acute edematous pancreatitis and acute necrotizing pancreatitis. Interstitial edematous pancreatitis shows relatively homogeneous enhancement or attenuation, inflammatory change, and peripancreatic fluid collection (Figure 1A), while acute necrotizing pancreatitis shows inhomogeneous enhancement or attenuation, necrosis of the pancreatic parenchyma and/or the peripancreatic tissue (Figure 1B).
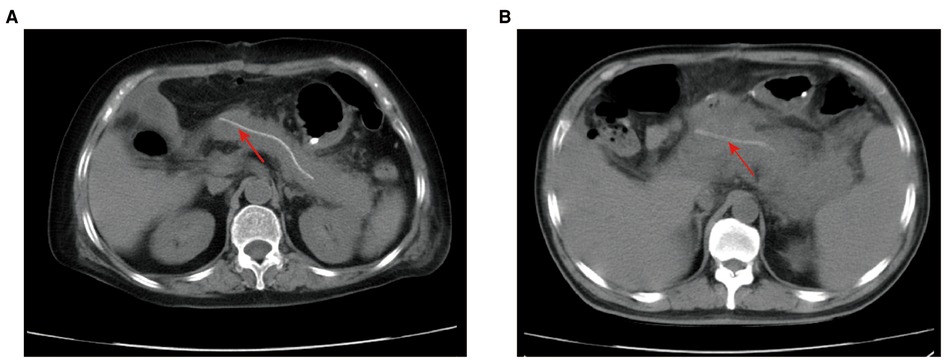
Figure 1. Postoperative CT scans of PPAP. (A) Acute edematous pancreatitis after PD: the boundary of the remnant pancreas is coarse, extensive exudation and inflammatory change around the remnant pancreas, not the surgical field. (B). Acute necrotizing pancreatitis after PD: the borderline of remnant pancreas is not distinct, inflammatory changes and exudate surrounding the remnant pancreas, necrosis change in the pancreatic parenchyma and the peripancreatic tissue. Red arrow: internal pancreatic duct stent. PPAP, postoperative acute pancreatitis; PD, pancreaticoduodenectomy.
Statistics
SPSS 21.0 software package was used for data processing. The normal distribution data are described by , the non-normal distribution data are described by median (IQR), and the counting data are described by proportion, relative ratio, and composition ratio. The Mann–Whitney U test for two independent samples was used for non-normal distribution. Pearson’s chi-square test was used for counting data, and the t-test was used for two independent samples in accordance with normal distribution. Univariate analysis was used to judge the association between perioperative parameters and PPAP. Multivariate analyses, including binary and Firth logistic regression, were used to recognize the risk factors of PPAP and PF. The efficiency of the predicting model was measured by ROC curve analysis. P values <0.05 were defined as statistically significant.
Results
Patients’ Characteristics
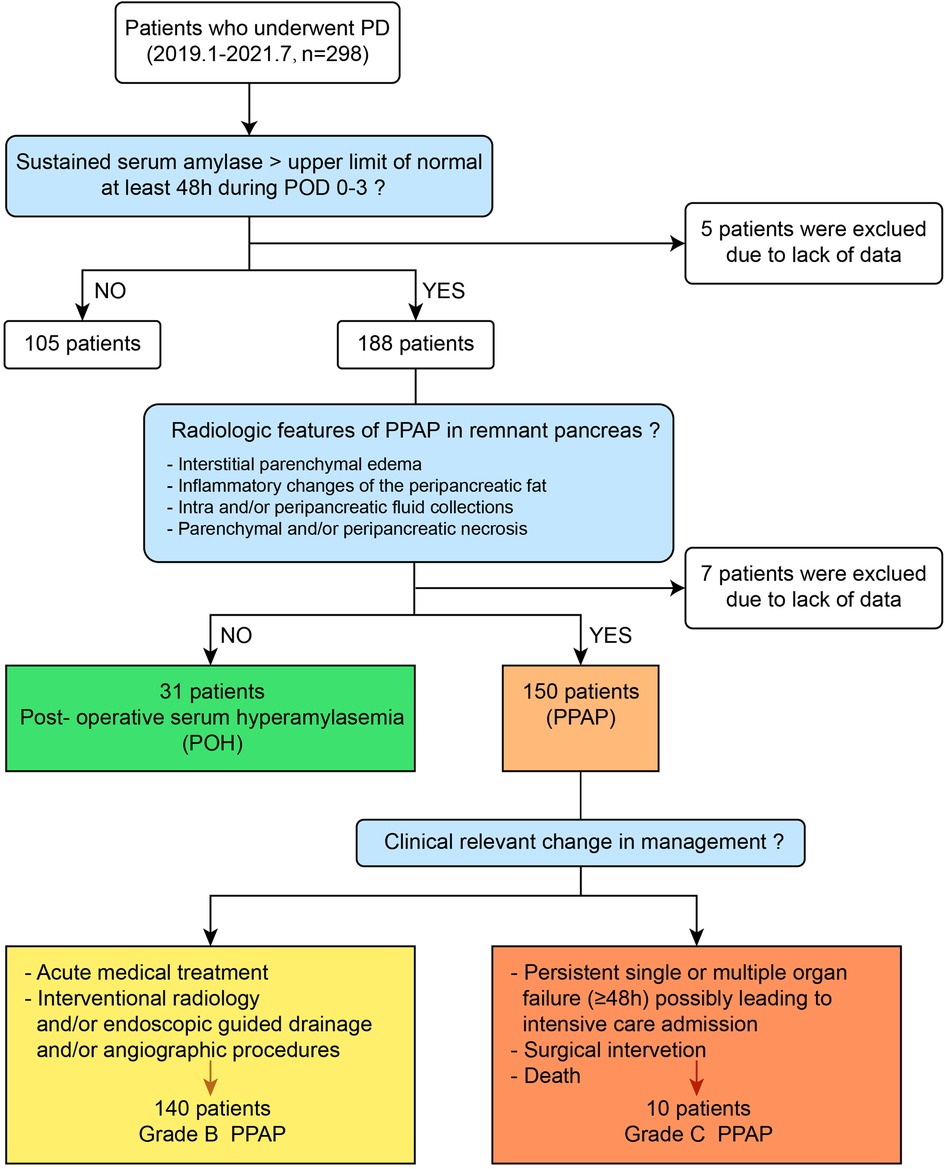
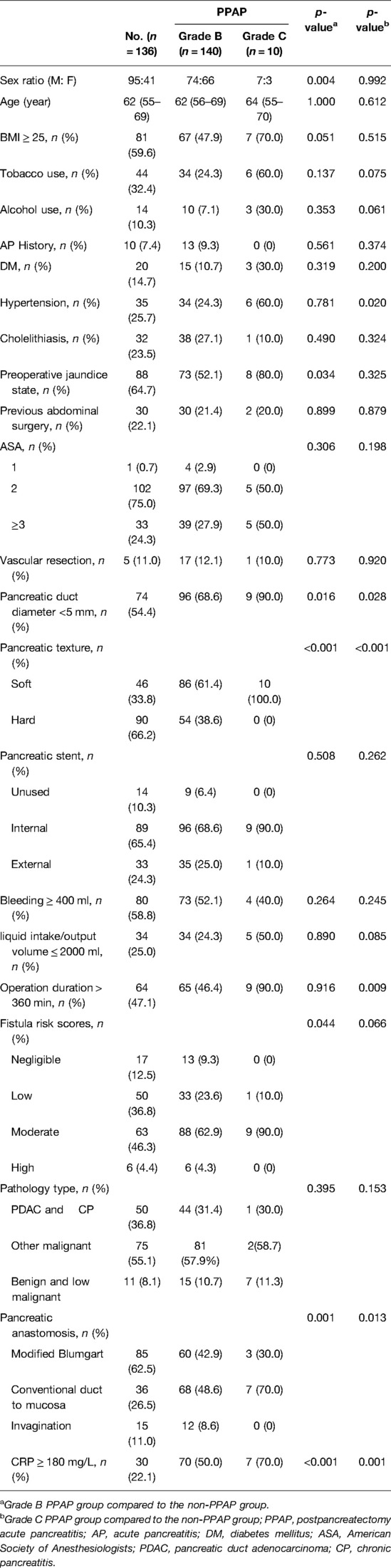
A total of 298 consecutive patients who underwent PD from January 2019 to July 2021 at the First affiliated Hospital of Xi’an Jiaotong University were enrolled in this study. Twelve (4.0%) patients were excluded due to the lack of detailed records of postoperative complications, serum amylase, and abdominal CT scan data. The sex ratio of men to women was 1.6:1. The mean age of the patients was 62 (55–69) years. Twenty-three (8%) patients had a history of acute pancreatitis attack, and 71 (24.8%) had cholelithiasis. Sixty-two (21.7%) patients had abdominal surgery previously. The most frequent indications for PD were malignant tumors, including 92 (32.2%) pancreatic ductal adenocarcinoma, 157 (54.9%) periampullary carcinoma, and 6 (2.1%) duodenal carcinoma. The residual contains 16 (5.6%) pancreatic cyst tumor, 4 (1.4%) pancreatic neuroendocrine tumor, 3 (1.0%) chronic pancreatitis, 2 (0.7%) duodenal stromal tumor, and 6 (2.1%) other benign or precancerous lesions. According to diagnostic criteria of PPAP formulated by ISGPS, the patients were divided into the PPAP group and non-PPAP group (the diagnostic flow chart is shown in Figure 2); the total incidence of PPAP was 52.4% (150 patients). Stratified by clinical impacts of PPAP, grade B PPAP was 48.9% (140 patients) and grade C PPAP was 3.5% (10 patients). The serum amylase level on POD 1–3 is shown in Supplementary Table 1 (the normal upper limit of serum amylase in our institution is 135 U/L). The incidence of clinically relevant pancreatic fistula (CR-PF) was 23.4% (67 patients). Of the patients with CR-PF, 56 (19.6%) patients had grade B PF and 11 (3.8%) patients had grade C PF.
PPAP After PD Was Associated with Unfavorable Complications
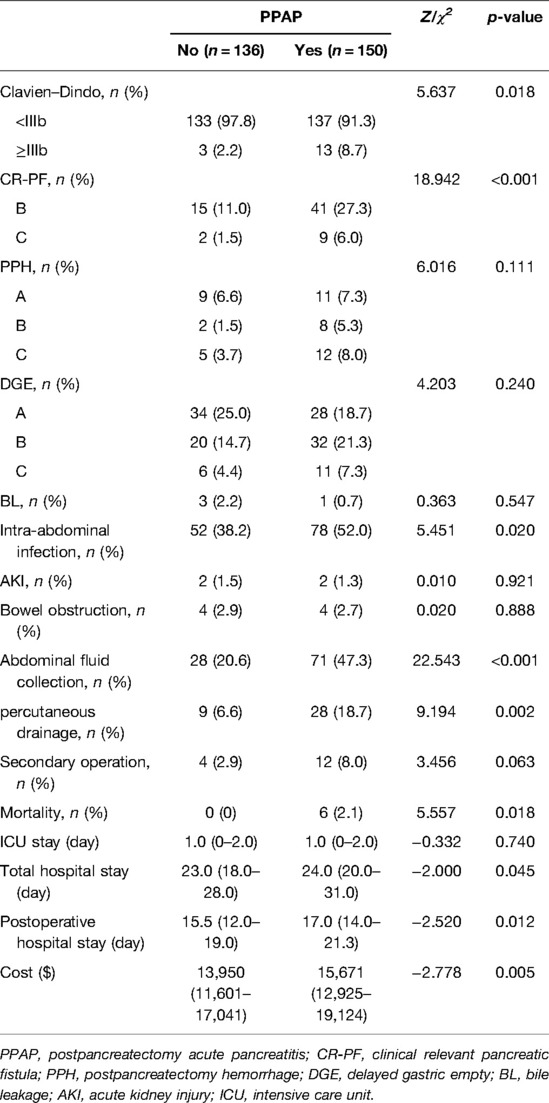
The postoperative outcomes of patients grouped by the occurrence of PPAP are shown in Table 1. The complications of Clavien–Dindo ≥ IIIb were significantly increased in patients with PPAP, and CR-PF, intra-abdominal infection, abdominal fluid collection, puncture, and drainage treatment, and mortality were also significantly increased. The total lengths of hospital stay and postoperative hospital stay were longer in the PPAP group. The hospitalization cost in the PPAP group was also significantly increased by $1,721 ($15,671 and $13,950, respectively).
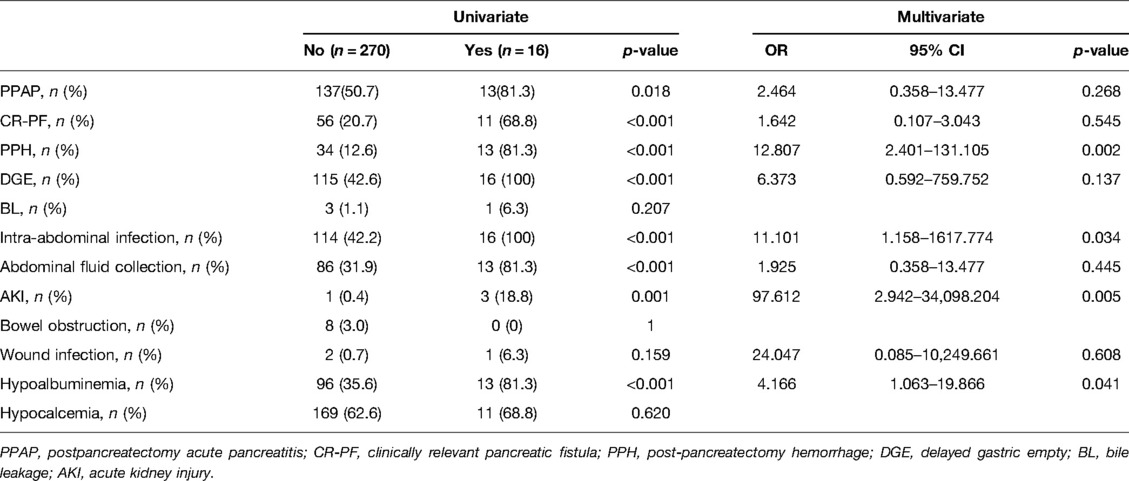
To further evaluate the effects of PPAP on serious postoperative complications, univariate and multivariate analyses were conducted on patients with Clavien–Dindo ≥ IIIb (Table 2). This indicated that PPH (OR 12.807, 95% CI 2.401–131.105), intra-abdominal infection (OR 11.101, 95% CI 1.158–1617.774), AKI (OR 97.612, 95% CI 2.942–34098.204), and postoperative hypoalbuminemia (OR 4.166, 95% CI 1.063–19.866) were independent predictors of Clavien–Dindo ≥ IIIb. It' is worth noting that in univariate analyses, there was statistical difference in PPAP, suggesting that PPAP did not increase the incidence of Clavien–Dindo ≥ IIIb directly.
Risk Factors for PPAP After PD
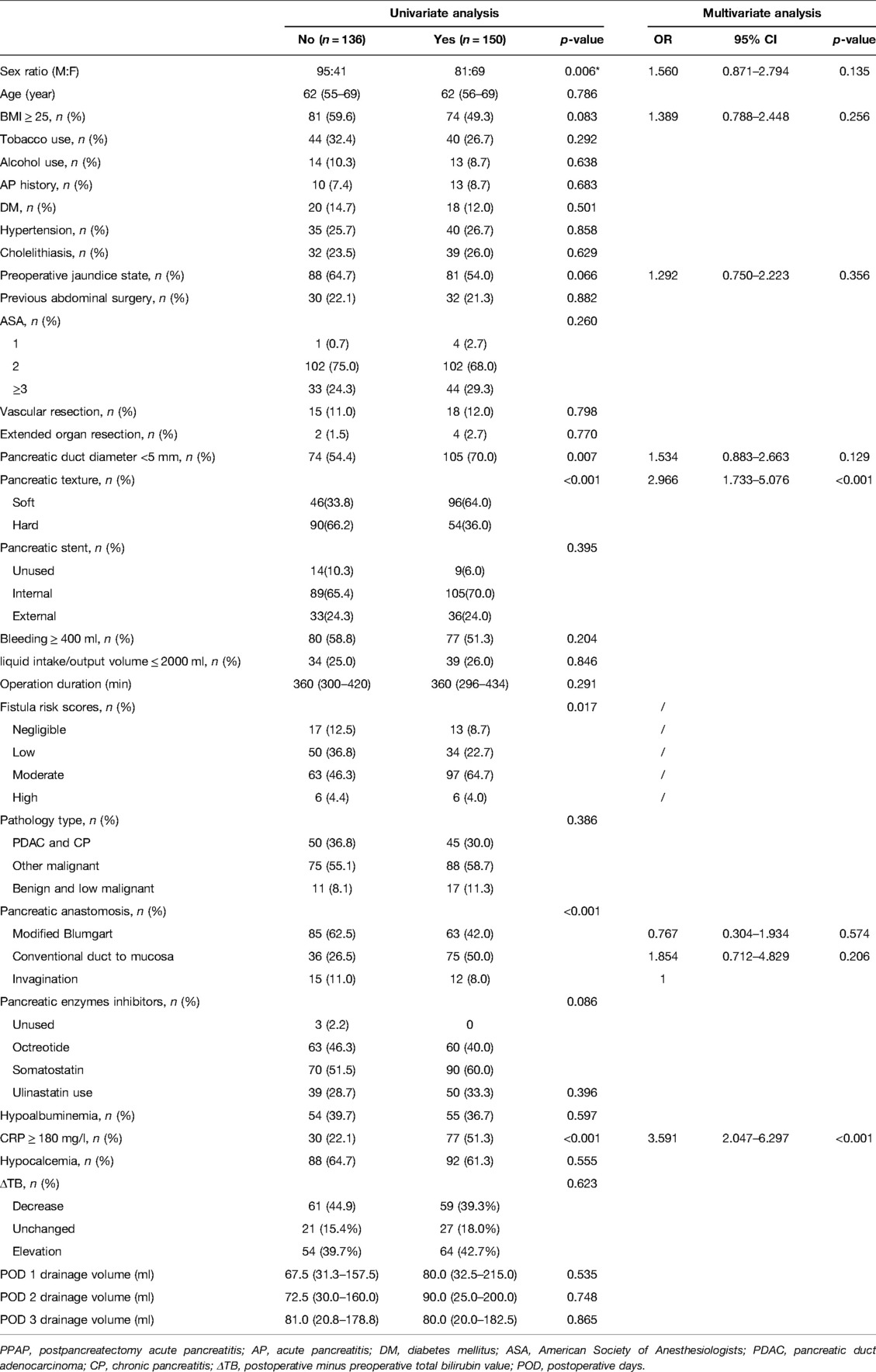
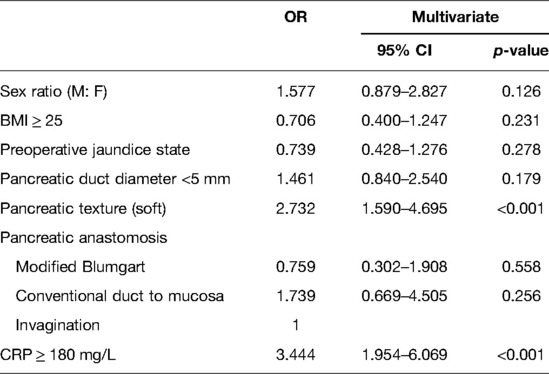
Stratified by the PPAP occurrence, the perioperative characteristics as well as univariate and multivariate analyses are shown in Table 3. Female, BMI ≥ 25, preoperative jaundice state, pancreatic texture, diameter of pancreatic duct, the techniques of pancreatic anastomosis, and CRP ≥ 180 mg/L were enrolled into the multivariate analysis model. Due to multicollinearity of the diameter of pancreatic duct and pancreatic texture, fistula risk scores were excluded from multivariate analysis. The soft texture of pancreatic stump and CRP ≥ 180 mg/L were defined as independent predictors of PPAP through multivariate analysis (OR 2.953, 95% CI 1.764–4.943 and OR 3.591, 95% CI 2.047–6.297, respectively). The area under the ROC curve was 0.613 (Figure 3).
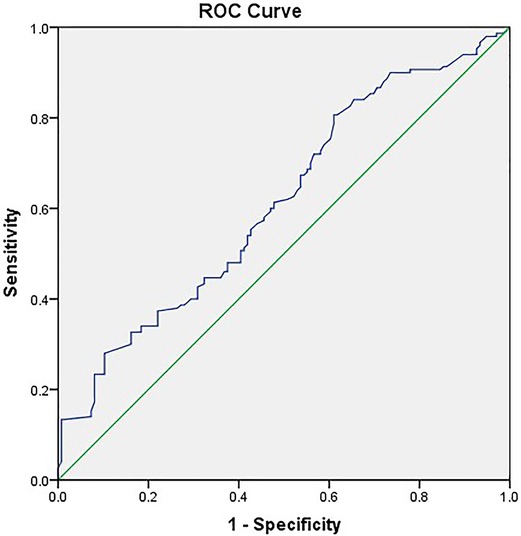
Figure 3. Receiver operating characteristic (ROC) curve for threshold analysis of predicting PPAP (AUC 0.613).
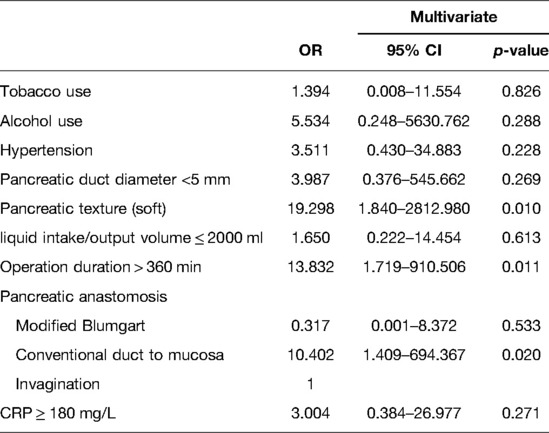
In this study, 10 patients occurred gade C PPAP in the patients’ cohort, and the incidence of grade C PPAP was 3.5%. Grade C PPAP was a rare but life-threatening complication after PD. Stratified by the grade of PPAP, the perioperative characteristics as well as univariate analysis are shown in Table 4. Compared to the non-PPAP group, male, BMI ≥ 25, preoperative jaundice state, pancreatic duct diameter <5 mm, soft pancreatic texture, the methods of pancreatic anastomosis, and CRP ≥ 180 mg/L were enrolled into the multivariate analysis model for predicting grade B PPAP. For the reasons mentioned above, fistula risk scores were excluded from multivariate analysis. As shown in Table 5, soft pancreatic texture (OR 2.732, 95% CI 1.590–4.695) and CRP ≥ 180 mg/L (OR 3.444, 95% CI 1.954–6.069) were the independent predictors of grade B PPAP. For predicting the model of grade C PPAP, tobacco and alcohol use, hypertension, pancreatic duct diameter <5 mm, soft pancreatic texture, liquid intake/output volume ≤ 2000 ml, operation duration >360 min, the methods of pancreatic anastomosis, and CRP ≥ 180 mg/L were enrolled compared to the non-PPAP group. As shown in Table 6, soft pancreatic texture (OR 19.298, 95% CI 1.840–2812.980), operation duration >360 min (OR 13.832, 95% CI 1.719–910.506), and the pancreatic anastomosis by using conventional duct-to-mucosa methods (OR 10.402, 95%CI 1.409-694.367) were the independent predictors of grade C PPAP.
Influence of PPAP and PF on Postoperative Complications
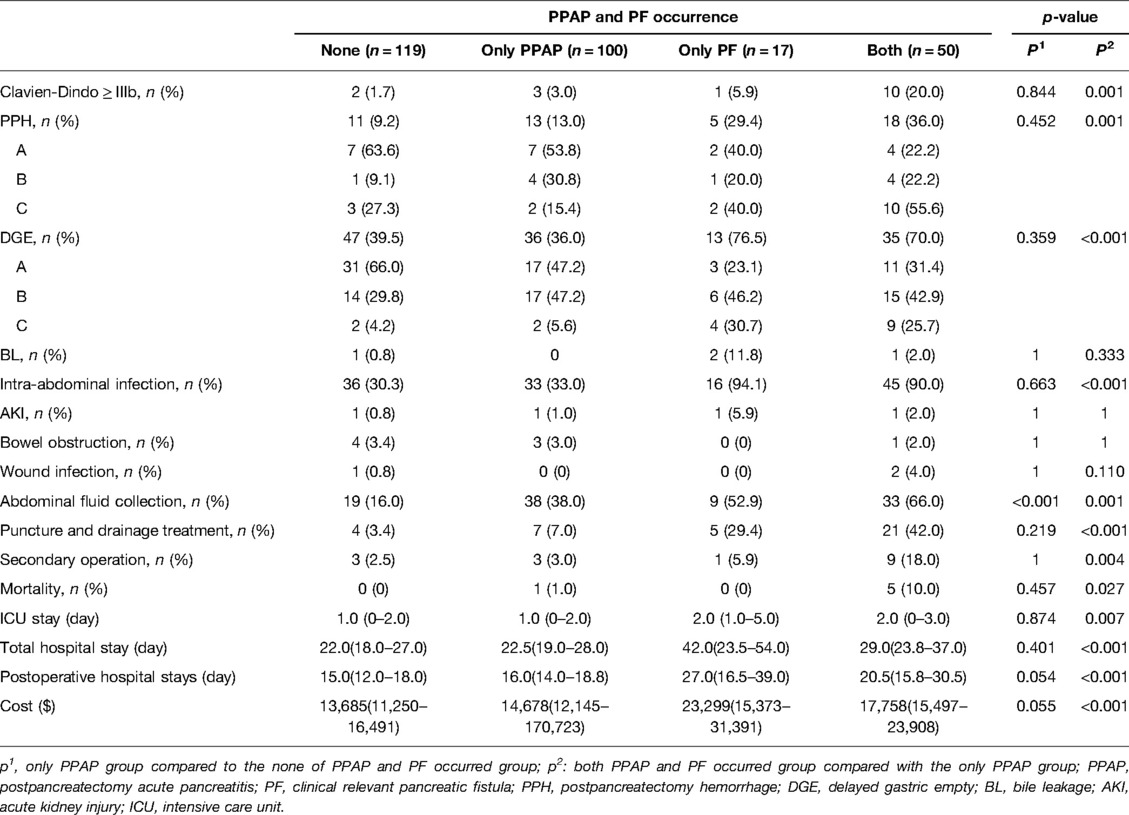
To explore the influence of PPAP and PF on postoperative complications, the patients was divided into none of PPAP and PF occurred group, only PPAP occurred group, only PF group, and both PPAP and PF occurred group. The postoperative complications among different groups are given in Table 4. It shows that the occurrence of PPAP was independent of PF by observing 100 patients with PPAP but was not complicated with PF. Meanwhile, 50 patients suffered from both PPAP and PF.
Two questions of concern to us were statistically analyzed. First, what were the clinical consequences for the patients with PPAP compared to the patients with neither PPAP nor PF? Shown in Table 7, PPAP occurrence just significantly increased the incidence of abdominal fluid collection (p < 0.001); however, other severe complications such as PPH were not significantly increased. What is more, the only PPAP occurred group had longer postoperative hospital stay and spent more money on hospitalization but did not show significant difference at the level of p value <0.05. Second, what were clinical impacts of PPAP complicated with PF compared to PPAP alone? PPAP complicated with PF significantly increased the mortality and incidence of Clavien–Dindo ≥ IIIb complications, PPH, DGE, intraabdominal infection, abdominal fluid collection, percutaneous drainage, and unplanned secondary operation compared to the only PPAP occurred group. In addition, PPAP complicated with PF significantly increased the length of ICU and hospital stay and hospital expenses.
Discussion
Incidence of PPAP
Postoperative pancreatitis after PD was brought into our attention after a patient died inevitably from severe pancreatitis in remnant pancreas in January 2016; then, we started to do targeted inspections (serum enzymology and CT examination) on suspected patients to provide an actual aid for patients' management. Therefore, the patients' cohort in this retrospective study had relatively complete data of serum enzymology and abdominal CT images. We retrieved the PubMed database; acute pancreatitis after partial pancreatectomy was first reported in 1952 (23). Recent literature started to focus on PPAP, and the occurrence of PPAP was an independent predictor of PF (5). However, there was considerable heterogeneity in previous studies due to the lack of uniform diagnostic criteria and grading system. Recently, ISGPS published the definition of PPAP (11), which was a milestone in the research of PPAP.
The incidence of PPAP varied greatly between current and past studies. According to Kriger et al. (24), the incidence of PPAP was 58.9% (178/302) by the Atlanta classification and definitions (12). Based on Connor's definition, Nahm et al. (7) found the incidence of PPAP was 62% (38/61). Most recently, Bassi et al. (5) reviewed 292 PD patients, and the incidence was 55.8% (163/292) in 2018. In the same year, the German team (6) reported that the incidence was 53% (100/190). Here, we practiced this definition published by ISGPS, and the incidence of PPAP was 52.4% in this study, which was at the same level as in current studies. However, past literature works reported the incidence of PPAP at a very low level of about 2%–3% (25, 26). Possibly due to a lack of standardized definitions in past, PPAP was diagnosed only in the condition of typical or severe clinical symptoms or life-threatening complications caused by pancreatitis. In other words, postpancreatectomy pancreatitis mentioned in the past literature probably meant the grade C PPAP (11). In this study, the incidence of grade C PPAP was 3.5%, which was comparable to the past literature (25, 26). This result provided a possible explanation for the polarization of PPAP incidence in the literature. Recent study (27) showed that necrotizing pancreatitis of the remnant pancreas confirmed by histological section was found in 33 out of 79 (41%) patients who underwent completion pancreatectomy after initial PD due to unfavorable complications. The postoperative pancreatitis not only occurred in the PD or other partial pancreatectomy but also was reported in scoliosis surgery (28), aortic dissection (29), renal transplantation (30), and gynecologic and obstetric surgery (31), and the incidence ranged from 0.29% to 5.9%.
Diagnostic Parameters of PPAP
The diagnostic criteria of PPAP consisted of three parameters: biochemical, radiological, and clinical evidence. The elevated serum amylase greater than the upper limit showed the same diagnostic efficacy as the elevation of three times (32). Early and sustained elevation of serum amylase was thought to be more associated with postoperative complications than its peak value detected (33). The available literature (4) agreed that PPAP occurred in the early phase of postoperative period (POD 0–3). Thus, it is distinguished from the time of PF occurrence, which was defined at the later phase (POD ≥ 3) (17). Abdominal pain is an essential criterion in usual acute pancreatitis. However, abdominal pain is not a reliable diagnostic criterion after partial pancreatectomy because it could be concealed by postoperative analgesia to varying degrees (11). Early postoperative CT scans were helpful to evaluate the recovery in the surgical field. The remnant pancreas (pancreatic body and tail) was not conventionally dissected during PD procedure; however, the signs of pancreatic exudation or parenchymal changes in postoperative CT scans suggest the formation of PPAP (34). Palumbo et al. (35) suggested that the routinely postoperative CT scan after laparoscopic sleeve gastrectomy was helpful to early stratification of leakage risk. Contrast-enhanced CT in our retrospective study was less adopted except when necessary, mainly because it usually has a long waiting time for examination and might put extra burden on the kidneys, which was not suitable for patients in early postoperative phase.
Clinical Significance of PPAP
PPAP was significantly associated with unfavorable outcomes in our study. PPAP and PF are reciprocal causation, and they can also occur independently. In this study, PF complicated with PPAP was found in 50 (74.6%) out of 67 PF patients. On the one hand, PPAP could cause cellular injury by releasing active zymogens and stimulate inflammatory response in the pancreatic parenchyma (36). In the setting of pancreatojejunostomy, zymogens can be activated by digestive juice in reconstructed digestive tract, which causes autodigestive injury in anastomotic tissue. PPAP could probably prolong the healing time of anastomosis and provide a pre-condition for the occurrence of pancreatic leakage, which then leads to PF (11). On the other hand, activated pancreatic juice leaking from dehiscence of the anastomosis pervades the remnant pancreas, which causes inflammatory damage (10). Besides, we found that 66.7% (100/150) PPAP, which was not complicated with PF, did not lead to serious complications; however, PF complicated with PPAP could cause serious complications and increase the mortality rate (10.0%). Rudis et al. found that grade C PF complicated with PPAP was observed in 4 out of 160 patients, and none of these patients survived (37). We also noticed that a large proportion of PPAP with unfavorable outcomes was complicated with PF, and PF appeared to be the major factor on outcome. Only the occurrence of PPAP may not lead to serious clinical impacts, unless grade C PPAP, which could be the cause of persistent organ failure and other severe complications.
Risk Factors for PPAP
In this study, we found that soft pancreatic texture and CRP ≥ 180 mg/L were the independent predictors of grade B PPAP. In addition, soft pancreatic texture, operation duration >360 min, and pancreatic anastomosis by using conventional duct-to-mucosa methods were the independent risk factors for grade C PPAP. Due to the small sample size in our study, some variables occurred complete separation and quasi-complete separation. To improve the stability of the prediction model, we used Firth logistic regression to perform multivariate analysis. A retrospective study from the University of Heidelberg (6) reported a comparable incidence of PPAP and the association with CRP to our study. Notably, the pancreatic texture was observed in all patients with grade C PPAP. However, the soft texture of pancreas is a subjective index. Nahm et al. (7) reported that the acinar cell density at the pancreatic resection margin can better describe the residual pancreas than “texture,” and the density of acinar was significantly associated with PPAP. Univariate analysis showed that PPAP was more likely to take place in females, and female is also one of the independent risk factors for PEP (38). Women have a higher percentage of body fat than men, which makes the pancreas softer; hpwever, gender did not show statistical difference in multivariate analysis in this study. Bassi et al. (5) found that independent risk factors for PPAP included preoperative exocrine insufficiency, neoadjuvant therapy, additional resection of the pancreatic stump margin, soft pancreatic texture, and main pancreatic duct diameter ≤3 mm. From our retrospective data, details of extended pancreatic stump resection were not routinely recorded, and in 92 patients with pancreatic ductal adenocarcinoma, only 5 (5.4%) patients with borderline resectable pancreatic cancer received neoadjuvant chemotherapy and (or) radiotherapy, so these variables were not analyzed. Intraoperative pancreatic ischemia was thought to be a mechanism for pancreatitis (39, 40); nevertheless, this study cannot produce effective comparisons between the two groups. For the average liquid intake/output volume on the surgery day, our patients’ cohort was far beyond the “near-zero fluid” (5) (PPAP group: 2729 ml, non-PPAP group: 2721 ml).
Managements of PPAP
Currently, the methods to prevent PPAP were very limited due to the lack of RCTs and prospective studies. The usage of pancreatic enzyme inhibitors, including octreotide and somatostatin, was not a protective factor for PPAP occurrence in this study. This was in accordance with the results of PF study (41–43). Somatostatin drugs can reduce splanchnic blood flow (44), which may increase the occurrence of pancreatitis and PF. One RCT study (45) demonstrated that prophylactic administration of ulinastatin can reduce the incidence of PPAP and also reduce the levels of amylase in serum and drain. Hydrocortisone (46) and rectal indomethacin (47) had proved that they can reduce the incidence of postoperative complications; however, these factors were not enrolled in this study due to very small sample size (n = 2).
The deficiency of this study was its retrospective design. Moreover, the patients were not stratified by preoperative features and surgical techniques to avoid small sample sizes, which may reduce the statistical efficiency and make clinical significance unstable. The data of preoperative amylase were lacking in this study, and the delta could better describe the inflammatory changes in the remnant pancreas than the specified threshold (48). The study is focused on the early postoperative period; however, and the impacts of PPAP on the long-term, such as recurrent pancreatitis, chronic pancreatitis, diabetes, fatty liver, and survival, were not calculated.
In conclusion, based on the structured definition and grading system of PPAP published by ISGPS, we found the incidence of PPAP after PD was at a high level (52.4%), which was in accordance with current research. Stratified by the grade of PPAP, soft pancreatic texture and CRP ≥ 180 mg/L were the independent predictors of grade B PPAP, and soft pancreatic texture, operation duration >360 min, and the pancreatic anastomosis by using conventional duct-to-mucosa methods were the independent predictors of grade C PPAP. PPAP had certain clinical practical significance on the clinical outcomes, especially when it was complicated with PF. Higher-volume multicenter and prospective studies are needed to promote a better understanding of PPAP.
Data Availability Statements
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the First Affiliated Hospital of Xi’an Jiao tong University. The patients/participants provided their written informed consent to participate in this study.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SW and HW collected the data and wrote the original draft. SW and FX analyzed data. GX performed the radiological evaluation. YZ, SD and LH analyzed and interpreted the data. ZW and ZW revised the manuscript. ZW conceived and designed the study. All authors contributed to the article and approved the submitted version script.
Funding
This work was supported by the Open and Sharing platform of Science and Technology Resources of Shaanxi Province [grant numbers 2022PT-35], the Key Research and Development Programs of Shaanxi Province [grant numbers 2022SF-437], and the Natural Science Basic Research Program of Shaanxi Province [grant number 2020JQ-510].
Acknowledgments
The authors thank the clinicians of the Department of Radiology at the First Affiliated Hospital of Xi’an Jiaotong University for providing conditions about radiological assessment.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/article/10.3389/fsurg.2022.916486/full#supplementary-material.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mine T, Morizane T, Kawaguchi Y, Akashi R, Hanada K, Ito T, et al. Clinical practice guideline for post-ERCP pancreatitis. J Gastroenterol. (2017) 52(9):1013–22. doi: 10.1007/s00535-017-1359-5
2. Jin S, Shi XJ, Wang SY, Zhang P, Lv GY, Du XH, et al. Drainage fluid and serum amylase levels accurately predict development of postoperative pancreatic fistula. World J Gastroenterol. (2017) 23(34):6357–64. doi: 10.3748/wjg.v23.i34.6357
3. Palani Velu LK, Chandrabalan VV, Jabbar S, McMillan DC, McKay CJ, Carter CR, et al. Serum amylase on the night of surgery predicts clinically significant pancreatic fistula after pancreaticoduodenectomy. HPB (Oxford). (2014) 16(7):610–9. doi: 10.1111/hpb.12184
4. Connor S. Defining postoperative pancreatitis as a new pancreatic specific complication following pancreatic resection. HPB (Oxford. (2016) 18(8):642–51. doi: 10.1016/j.hpb.2016.05.006
5. Bannone E, Andrianello S, Marchegiani G, Masini G, Malleo G, Bassi C, et al. Postoperative acute pancreatitis following pancreaticoduodenectomy: a determinant of Fistula potentially driven by the intraoperative fluid management. Ann Surg. (2018) 268(5):815–22. doi: 10.1097/SLA.0000000000002900
6. Birgin E, Reeg A, Téoule P, Rahbari NN, Post S, Reissfelder C, et al. Early postoperative pancreatitis following pancreaticoduodenectomy: what is clinically relevant postoperative pancreatitis. HPB (Oxford). (2019) 21(8):972–80. doi: 10.1016/j.hpb.2018.11.006
7. Nahm CB, Brown KM, Townend PJ, Colvin E, Howell VM, Gill AJ, et al. Acinar cell density at the pancreatic resection margin is associated with post-pancreatectomy pancreatitis and the development of postoperative pancreatic fistula. HPB (Oxford). (2018) 20(5):432–40. doi: 10.1016/j.hpb.2017.11.003
8. Andrianello S, Bannone E, Marchegiani G, Malleo G, Paiella S, Esposito A, et al. Characterization of postoperative acute pancreatitis (POAP) after distal pancreatectomy. Surgery. (2021) 169:724–31. doi: 10.1016/j.surg.2020.09.008
9. Nakeeb A E, Salah T, Sultan A, El Hemaly M, Askr W, Ezzat H, et al. Pancreatic anastomotic leakage after pancreaticoduodenectomy. Risk factors, clinical predictors, and management (single center experience). World J Surg. (2013) 37:1405–18. doi: 10.1007/s00268-013-1998-5
10. Bannone E, Andrianello S, Marchegiani G, Malleo G, Paiella S, Salvia R, et al. Postoperative hyperamylasemia (POH) and acute pancreatitis after pancreatoduodenectomy (POAP): state of the art and systematic review. Surgery. (2021) 169:377–87. doi: 10.1016/j.surg.2020.04.062
11. Marchegiani G, Barreto SG, Bannone E, Sarr M, Vollmer CM, Connor S, et al. Postpancreatectomy acute pancreatitis (PPAP): definition and grading from the international study group for pancreatic surgery (ISGPS). Ann Surg. (2022) 275:663–72. doi: 10.1097/SLA.0000000000005226
12. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62(1):102–11. doi: 10.1136/gutjnl-2012-302779
13. Tonolini M, Ierardi AM, Carrafiello G. Elucidating early CT after pancreatico-duodenectomy: a primer for radiologists. Insights Imaging. (2018) 9(4):425–36. doi: 10.1007/s13244-018-0616-3
14. Traverso LW, Longmire WP. Preservation of the pylorus in pancreaticoduodenectomy. Surg Gynecol Obstet. (1978) 146(6):959–62. doi: 10.1097/00000658-198009000-00005
15. Winter JM, Cameron JL, Campbell KA, Arnold MA, Chang DC, Coleman J, et al. 1423 Pancreaticoduodenectomies for pancreatic cancer: a single-institution experience. J Gastrointest Surg. (2006) 10(9):1199–210. discussion 1210-1. doi: 10.1016/j.gassur.2006.08.018
16. McMillan MT, Soi S, Asbun HJ, Ball CG, Bassi C, Beane JD, et al. Risk-adjusted outcomes of clinically relevant pancreatic Fistula following pancreatoduodenectomy: a model for performance evaluation. Ann Surg. (2016) 264(2):344–52. doi: 10.1097/SLA.0000000000001537
17. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu HM, Adham M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. (2017) 161:584–91. doi: 10.1016/j.surg.2016.11.014
18. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. (2007) 142(5):761–8. doi: 10.1016/j.surg.2007.05.005
19. Wente MN, Veit JA, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, et al. Postpancreatectomy hemorrhage (PPH): an International Study Group of Pancreatic Surgery (ISGPS) definition. Surgery. (2007) 142(1):20–5. doi: 10.1016/j.surg.2007.02.001
20. Koch M, Garden OJ, Padbury R, Rahbari NN, Adam R, Capussotti L, et al. Bile leakage after hepatobiliary and pancreatic surgery: a definition and grading of severity by the International Study Group of Liver Surgery. Surgery. (2011) 149(5):680–8. doi: 10.1016/j.surg.2010.12.002
21. Thomas ME, Blaine C, Dawnay A, Devonald MA, Ftouh S, Laing C, et al. The definition of acute kidney injury and its use in practice. Kidney Int. (2015) 87(1):62–73. doi: 10.1038/ki.2014.328
22. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
23. Dunphy JE, Brooks JR, Archroyd F. Acute postoperative pancreatitis. N Engl J Med. (1952) 248(11):445–51. doi: 10.1056/NEJM195303122481102
24. Kriger AG, Kubishkin VA, Karmazanovskiĭ GG, Svitina KA, Kochatkov AV, Berelavichus SV, et al. The postoperative pancreatitis after the pancreatic surgery. Khirurgiia (Mosk). (2012) (4):14–9. https://pubmed.ncbi.nlm.nih.gov/25263473/22810339
25. Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. (2006) 244(1):10–5. doi: 10.1097/01.sla.0000217673.04165.ea
26. Adam U, Makowiec F, Riediger H, Schareck WD, Benz S, Hopt UT. Risk factors for complications after pancreatic head resection. Am J Surg. (2004) 187(2):201–8. doi: 10.1016/j.amjsurg.2003.11.004
27. Globke B, Timmermann L, Klein F, Fehrenbach U, Pratschke J, Bahra M, et al. Postoperative acute necrotizing pancreatitis of the pancreatic remnant (POANP): a new definition of severe pancreatitis following pancreaticoduodenectomy. HPB (Oxford). (2019) (2020). 22(43):445–51. doi: 10.1016/j.hpb.2019.07.016.31431414
28. El BM, Leveque C, Miladi L, Irtan S, Hamza J, Oualha M. Acute pancreatitis following scoliosis surgery: description and clinical course in 14 adolescents. Eur Spine J. (2016) 25(10):3316–23. doi: 10.1007/s00586-016-4595-0
29. Wang R, Zhu JM, Qi RD, Liu YM, Zheng J, Zhang N, et al. Acute ischemic pancreatitis secondary to aortic dissection. Ann Vasc Surg. (2018) 52:85–9. doi: 10.1016/j.avsg.2018.03.007
30. Fernández-Cruz L, Targarona EM, Cugat E, Alcaraz A, Oppenheimer F. Acute pancreatitis after renal transplantation. Br J Surg. (1989) 76(11):1132–5. doi: 10.1002/bjs.1800761108
31. Ramsey PS, Podratz KC. Acute pancreatitis after gynecologic and obstetric surgery. Am J Obstet Gynecol. (1999) 181(3):542–6. doi: 10.1016/S0002-9378(99)70490-4
32. Partelli S, Tamburrino D, Andreasi V, Mazzocato S, Crippa S, Perretti E, et al. Implications of increased serum amylase after pancreaticoduodenectomy: toward a better definition of clinically relevant postoperative acute pancreatitis. HPB (Oxford). (2020) 22(11):1645–53. doi: 10.1016/j.hpb.2020.03.010.32291175
33. Bannone E, Marchegiani G, Balduzzi A, Procida G, Vacca PG, Salvia R, et al. Early and sustained elevation in serum pancreatic amylase activity: a novel predictor of morbidity after pancreatic surgery. Ann Surg. (2021). doi: 10.1097/SLA.0000000000004921. [Epub ahead of print]33938491
34. Loos M, Strobel O, Mehrabi A, Mihaljevic AL, Ramouz A, Dietrich M, et al. Postoperative acute pancreatitis is a serious but rare complication after distal pancreatectomy. HPB (Oxford). (2021) 23:1339–48. doi: 10.1016/j.hpb.2021.01.004.33546896
35. Palumbo D, Socci C, Martinenghi C, Guazzarotti G, Leone R, Nicoletti R, et al. Leakage risk stratification after laparoscopic sleeve gastrectomy (LSG): is there a role for routine postoperative CT scan. Obes Surg. (2020) 30(9):3370–7. doi: 10.1007/s11695-020-04586-1.32291703
36. Lankisch PG, Apte M, Banks PA. Acute pancreatitis. Lancet. (2015) 386(9988):85–96. doi: 10.1016/S0140-6736(14)60649-8
37. Rudis J, Ryska M. Pancreatic leakage and acute postoperative pancreatitis after proximal pancreatoduodenectomy. Rozhl Chir. (2014) 93:380–85.25263473
38. Jamry A. Risk factors of pancreatitis after endoscopic sphincterotomy. Review of literature and practical remarks based on approximately 10,000 ERCPs. Pol Przegl Chir. (2017) 89(5):29–33. doi: 10.5604/01.3001.0010.5409
39. Ansorge C, Regner S, Segersvärd R, Strömmer L. Early intraperitoneal metabolic changes and protease activation as indicators of pancreatic fistula after pancreaticoduodenectomy. Br J Surg. (2012) 99(1):104–11. doi: 10.1002/bjs.7730
40. Strasberg SM, Drebin JA, Mokadam NA, Green DW, Jones KL, Ehlers JP, et al. Prospective trial of a blood supply-based technique of pancreaticojejunostomy: effect on anastomotic failure in the Whipple procedure. J Am Coll Surg. (2002) 194(6):746–58. discussion 759-60. doi: 10.1016/S1072-7515(02)01202-4
41. Gurusamy KS, Koti R, Fusai G, Davidson BR. Somatostatin analogues for pancreatic surgery. Cochrane Database Syst Rev. (2013) 2013:CD008370. 1002/14651858.CD008370.pub3
42. Yeo CJ, Cameron JL, Lillemoe KD, Sauter PK, Coleman J, Sohn TA, et al. Does prophylactic octreotide decrease the rates of pancreatic fistula and other complications after pancreaticoduodenectomy? Results of a prospective randomized placebo-controlled trial. Ann Surg. (2000) 232(3):419–29. doi: 10.1097/00000658-200009000-00014
43. McMillan MT, Christein JD, Callery MP, Behrman SW, Drebin JA, Kent TS, et al. Prophylactic octreotide for pancreatoduodenectomy: more harm than good. HPB (Oxford). (2014) 16(10):954–62. doi: 10.1111/hpb.12314
44. Barthelmes D, Parviainen I, Vainio P, Vanninen R, Takala J, Ikonen A, et al. Assessment of splanchnic blood flow using magnetic resonance imaging. Eur J Gastroenterol Hepatol. (2009) 21(6):693–700. doi: 10.1097/MEG.0b013e32831a86e0
45. Uemura K, Murakami Y, Hayashidani Y, Sudo T, Hashimoto Y, Ohge H, et al. Randomized clinical trial to assess the efficacy of ulinastatin for postoperative pancreatitis following pancreaticoduodenectomy. J Surg Oncol. (2008) 98(5):309–13. doi: 10.1002/jso.21098
46. Laaninen M, Sand J, Nordback I, Vasama K, Laukkarinen J. Perioperative hydrocortisone reduces major complications after pancreaticoduodenectomy: a randomized controlled trial. Ann Surg. (2016) 264(5):696–702. doi: 10.1097/SLA.0000000000001883
47. Elmunzer BJ, Scheiman JM, Lehman GA, Chak A, Mosler P, Higgins PD, et al. A randomized trial of rectal indomethacin to prevent post-ERCP pancreatitis. N Engl J Med. (2012) 366(15):1414–22. doi: 10.1056/NEJMoa1111103
Keywords: pancreaticoduodenectomy, acute pancreatitis, postoperative complications, risk factors, retrospective analysis
Citation: Wu S, Wu H, Xu G, Zhao Y, Xue F, Dong S, Han L, Wang Z and Wu Z (2022) Risk Factors and Clinical Impacts of Post-Pancreatectomy Acute Pancreatitis After Pancreaticoduodenectomy: A Single-Center Retrospective Analysis of 298 Patients Based on the ISGPS Definition and Grading System. Front. Surg. 9:916486. doi: 10.3389/fsurg.2022.916486
Received: 9 April 2022; Accepted: 13 June 2022;
Published: 4 July 2022.
Edited by:
Domenico Tamburrino, San Raffaele Hospital (IRCCS), ItalyReviewed by:
Donal Brendan O’Connor, Trinity College Dublin, IrelandYu-Liang Hung, Chang Gung Memorial Hospital, Taiwan
Matteo De Pastena, University of Verona, Italy
Copyright © 2022 Wu, Wu, Xu, Zhao, Xue, Dong, Han, Wang and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zheng Wu woozheng@xjtu.edu.cn
Specialty section: This article was submitted to Surgical Oncology, a section of the journal Frontiers in Surgery
 Shuai Wu
Shuai Wu Hanxue Wu2
Hanxue Wu2  Liang Han
Liang Han Zheng Wu
Zheng Wu