Effect of Marital Status on Upper Digestive Tract Tumor Survival: Married Male Patients Exhibited a Better Prognosis
- State Key Laboratory of Oral Diseases, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, China
Purpose: Marital status has been associated with the outcomes in several types of cancer, but less is known about upper digestive tract tumors (UDTTs). The study aims to explore the effect of marital status on the survival outcomes of UDTT.
Methods: We collected patient cases of UDTT using the Surveillance, Epidemiology, and End Results (SEER) database between 1975 and 2016. The univariate analyses of overall survival (OS) and cancer-specific survival (CSS) were performed using the Kaplan–Meier method. The multivariate survival analyses were performed using Cox proportional hazard model.
Results: A total of 282,189 patients were included, with 56.42, 16.30, 13.33, and 13.95% of patients married, never married, divorced or separated, and widowed, respectively. The significant differences were observed among married, never-married, divorced or separated, and widowed patients with regard to the year of diagnosis, sex, age, race, pathological type, anatomical site, the number of primary tumor, grade, rate of surgery performed, radiotherapy, chemotherapy (p < 0.001). The proportions of patients with 3-year and 5-year OS were 54.22 and 48.02% in the married group, 46.96 and 41.12% in the never-married group, 44.24 and 38.06% in the divorced or separated group, 34.59 and 27.57% in the widowed group, respectively (p < 0.001); the proportions of patients with 3-year and 5-year CSS were 70.76 and 68.13% in the married group, 62.44 and 59,93% in the never-married group, 63.13 and 60.53% in the divorced or separated group, 62.11 and 58.89% in the widowed group, respectively (p < 0.001); all these data indicated married patients exhibited favorable OS and CSS than never-married, divorced or separated, and widowed patients. Men in the married group showed better OS (HR, 1.16; 95%CI: 1.11–1.22) and CSS (HR, 0.96; 95%CI: 0.92–1.23) than those in the never-married group.
Conclusion: This study reveals that marital status is an independent prognostic factor for OS and CSS of patients with UDTT. Married male patients with UDTT trend to have a better prognosis.
Introduction
Social supports are emerging and closely related to the cancer prognosis as the society develops attracting more attention (1, 2). Marital status as one of the most important social relationships has significant implications for human health and well-being. The numerous studies have identified significant differences in morbidity and prognosis of different diseases in different marital statuses (3–6). Aizer et al. used the Surveillance, Epidemiology, and End Results (SEER) database for analysis and found that unmarried patients have higher risks of cancer metastasis, under-treatment, and death compared to married patients in a sample size of nearly 1 million patients (7). Marital status has been increasingly considered as an independent factor in the prognostic assessment of many cancers (7–9).
The upper digestive tract tumors (UDTTs), accounted for 6.8% of new on-set cancers and 8.9% of cancer deaths worldwide in 2018, are the seventh most frequent cancer type and the seventh most common cause of death from cancer worldwide (10). The upper digestive tract (UDT), which includes oral cavity, larynx, and esophagus, is the passage through which food enters the body and is covered by squamous epithelium, and the most frequent pathological type of UDTT is squamous cell carcinoma (SCC) (11–13). Sociological behaviors and psychosocial factors such as smoking, drinking, HPV infection, and upset emotion contribute enormously to UDTT (14–18). Psychosocial factors are involved in the pathogenesis of mental disorders by acting via mechanisms involving epigenetics (19), and the impact of different marital statuses on sociological behavior and psychosocial factor is critical (20, 21). Marriage has been a protective factor in many previous studies of cancer associations (7, 8). However, as far as we are concerned, first, UDTT is closely related to many sociological factors, and whether marriage, as an important sociological factor, is related to UDTT has not been studied before. Second, on the research methods, many other tumor-related studies only discuss the relationship between marital status and overall survival (OS) rate, but the lack of research on relationship about marital status and cancer-specific survival (CSS) rate which is better at showing the relationship between the tumor and survival. Therefore, the aim of our study was to explore the effects of different marital statuses, which include married, never married, divorced or separated, widowed on the prognosis of patients with UDTTs according to the multiple stratified studies based on the SEER population-based database.
Materials and Methods
Patient Selection and Data Collection
Patient cases of UDTT included between 1975 and 2016 were collected from the SEER database, which is the largest population-based cancer registry in the world and included 18 cancer registries as released on 2019 (22). UDT is comprised of three anatomical sites: (1) oral department consisting of lip (C000–C009), tongue (C019–C029), and floor of mouth (C040–C049); (2) pharyngeal department consisting of nasopharynx (C110–C119), tonsil (C090–C099), oropharynx (C100–C109), hypopharynx (C129–C130–139), and other oral cavity and pharynx (C140, C142–C148); (3) esophagus (C150–C159). Accordingly, three groups were classified based on the anatomical sites, oral, pharyngeal, and esophagus. Cases with different histological types were identified using the codes of International Classification of Oncology (ICD-O-3/WHO 2008) for tumor morphology (SCC = 8050–8084). Tumors of the salivary gland were excluded due to the differences in the type of epithelium, pathological type, and etiology. The flow chart is shown in Figure 1. Collected UDTT patients must have age more than 18 years, with demographic and clinical information that includes years of diagnosis, age, sex, race, marital status, anatomical site, pathological type, number of primary tumors, grade, stage, surgery, radiotherapy, chemotherapy, 3-year OS rate, 5-year OS, 3-year CSS rate, 5-year CSS, total OS rate, and total CSS rate. Grade was rated from I to IV based on the cancer cell differentiation. Stage was also rated from I to IV based on tumor metastasis. OS or CSS was defined as the date when the patient diagnosed with cancer to the date of the patient's death or cancer-specific death. When CSS calculated, deaths from other causes were treated as censored observations. It should be pointed out that, in many studies of UDTT and cancers, 45 and 75 are respectively regarded as the recognized age of a young patient and old patient (10, 23). Patients were age-stratified into <45 years, 45–60 years, 61–75 years, and >75 years. Marital status was classified as married, never married including those reported to cohabitate with an unmarried, domestic partner (same gender, opposite gender, or unregistered), divorced or separated, and widowed. Patients should be excluded if they had unknown parameters of stage, partnership status, treatment, or performance of sentinel lymph node biopsy.
Statistical Analysis
Categorical data are shown as percentage and analyzed by chi-square test. The OS and CSS of patients were plotted using the Kaplan–Meier method according to the different marital statuses and examined by log rank test. The survival is calculated as the number of months after the date of cancer diagnosis until the date of death in the SEER. Multivariate analysis was conducted to calculate OS rate and CSS rate with Cox proportional hazard ratios (HRs), respectively, and the nomograms were plotted to show the results of Cox regression. All statistical analyses were performed with R version 3.6.8 (R Core Team, Vienna, Austria), and the significance level was set as 0.05.
Results
Patient Characteristics
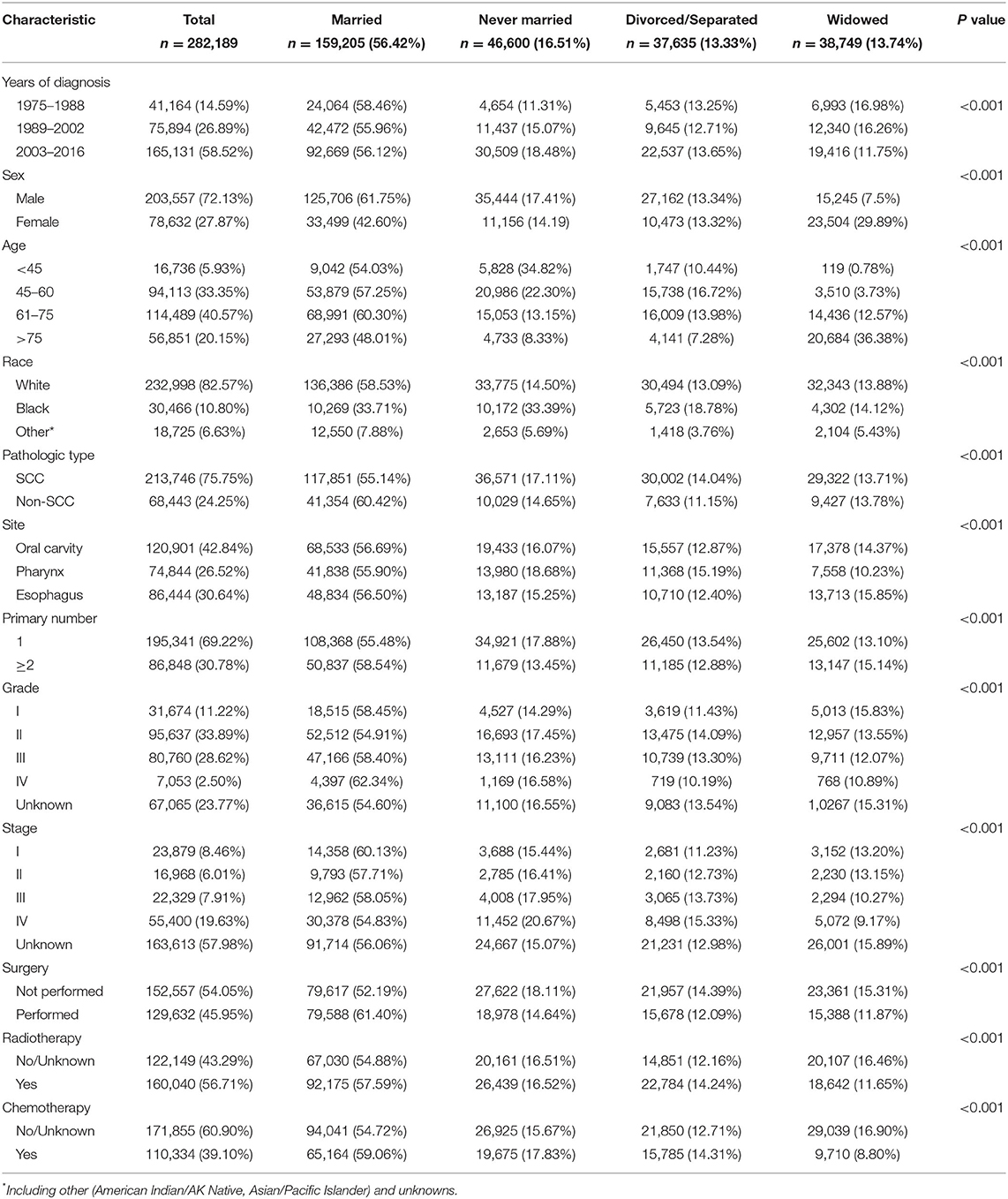
A total of 282,189 eligible patients with UDTTs in the SEER database during a 42-year study period (1975–2016) were analyzed in this study. Their demographic and clinical information for all study population is shown in Table 1. The proportions were 56.42% (159,205/282,189) for married patients, 16.51% (46,600/282,189) for never-married patients, 13.33% (37,635/282,189) for divorced or separated patients, and 13.74% (38,749/282,189) for widowed patients. The highest proportion of new diagnoses of UDTTs was found in the period of 2002–2016 (58.52%, 165,131/282,189), possibly due to the advances in diagnose of UDTTs. In each diagnosis period with a 14-year interval, married patients accounted for the majority (p <0.001). The proportion of never-married patients continued to increase, from 11.31% at the beginning to 18.48% at the end. The male patients accounted for 72.13% (203,557/282,189). With regard to age stratification, new diagnoses of UDTTs were found in age ranging from 45 to 75 years (73.92% in total). There were 82.57% of patients (232,998/282189) being white, 75.75% (213,746/282189) with SCC, 42.84% (120,901/282189) occurring in oral cavity, 69.22% (195,341/282189) with a primary lesion, 33.89% (95,637/282189) with grade II, and 19.63% (55,400/282189) with stage IV (p < 0.001).
Association Between Patient's Characteristics and Marital Status
Significant differences were observed among married, never-married, divorced or separated, and widowed patients with regard to the year of diagnosis, sex, age, race, pathological type, anatomical site, number of primary tumor, grade, rate of surgery performed, radiotherapy, and chemotherapy (Table 1, p < 0.001). In detail, a higher proportion of married patients and a lower proportion of never-married patients were found in the diagnosis period of 1975–1988 compared with other two periods (p < 0.001). A higher proportion of never-married patients and a lower proportion of widowed patients were found in the diagnosis period of 2003–2016 compared with other two periods (p < 0.001). Men had higher proportions in married (61.75 vs. 42.60%, p < 0.001) and never-married (17.41 vs. 14.19%, p < 0.001) patients but a lower proportion in widowed patients (7.5 vs. 29.89%, p < 0.001) when comparable to women. In each marital status, four age stratifications showed significant differences, so did race (p < 0.001). Higher proportions of SCC cases were noted in never-married (17.11 vs. 14.65%, p < 0.001) and divorced or separated (14.04 vs. 11.15%, p < 0.001) patients but a lower proportion of SCC cases were noted in married patients (55.14 vs. 60.42%, p < 0.001) than non-SCC cases. Most patients were diagnosed with grade IV in married group (62.34%), fewest patients with grade II in never-married group (14.29%), and fewest patients with grade IV in divorced or separated group (10.19%) and widowed group (10.89%). Concerning tumor stage, most stage I cases were found in married group (60.13%), most stage IV cases in never-married group (20.67%), and fewest stage IV cases in widowed group (9.17%). The proportion of patients undergoing surgery was higher than those not in married group (61.40 vs. 52.19%, p < 0.001), while the proportion of patients undergoing surgery was lower than those not in never-married (18.11 vs. 14.64%, p < 0.001), divorced or separated (14.39 vs. 12.09%, p < 0.001), and widowed (15.31 vs. 11.87%, p < 0.001) patients. More patients underwent radiotherapy and chemotherapy in married group (59.06 vs. 54.72%, p < 0.001), but fewer in widowed group (8.80 vs. 16.90%, p < 0.001).
Association Between Patient's Characteristics, Marital Status, and Survival Outcomes of Patients With UDTTs
The median survival time of all patients with UDTTs was 21 months, the median survival time was 26 months in the married group, 17 months in the never-married group, 17 months in the divorced or separated group, and 12 months in widowed group. As shown in Figure 2, the proportions of patients with 3-year and 5-year OS were 54.22 and 48.02% in the married group, 46.96 and 41.12% in the never-married group, 44.24 and 38.06% in the divorced or separated group, 34.59 and 27.57% in the widowed group, respectively (p < 0.001), which indicated that married patients exhibited favorable OS than never-married, divorced or separated, and widowed patients. Likewise, the proportions of patients with 3-year and 5-year CSS were 70.76 and 68.13% in the married group, 62.44 and 59.93% in the never-married group, 63.13 and 60.53% in the divorced or separated group, 62.11 and 58.89% in the widowed group, respectively (p < 0.001), which indicated that married patients exhibited favorable CSS than never-married, divorced or separated, and widowed patients. An early year of diagnosis (1975–1988), male, age older than 75 years, Black race, widowed, non-SCC, tumor in esophagus, number of primary tumors=1, tumor grade=III, stage IV, surgery not performed, and radiotherapy were considered as the significant risk factors (p < 0.001) of OS and CSS rate. Furthermore, chemotherapy predicted better 3-year OS rate, but worse rates of 5-year OS, 3-year CSS, and 5-year CSS (Table 2).
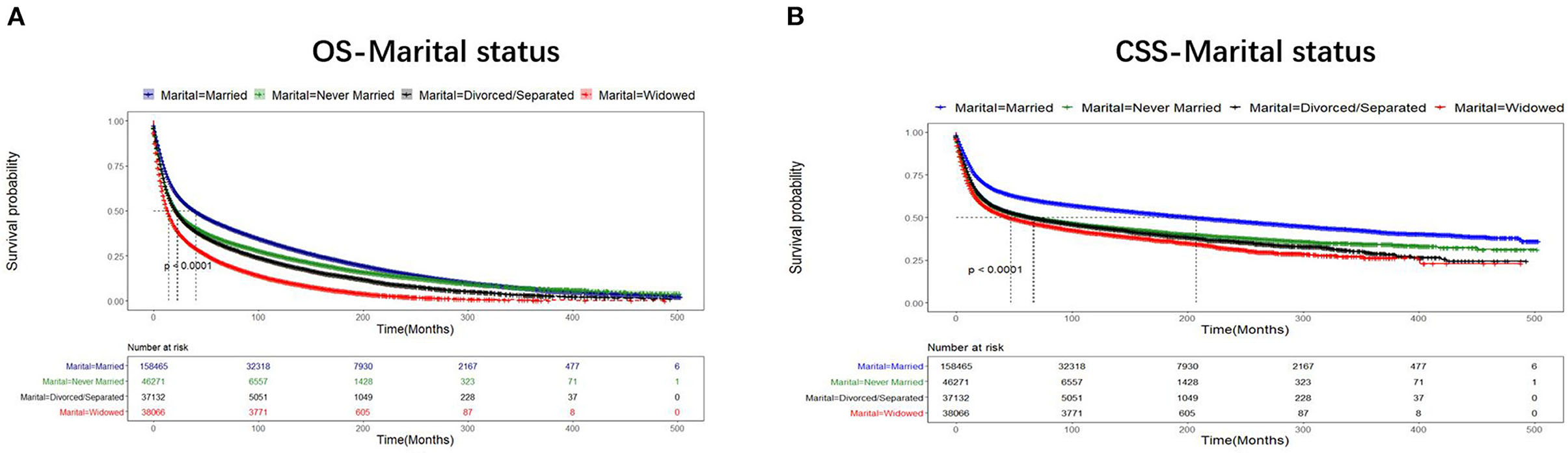
Figure 2. Comparison of OS and CSS of the patients with UDTT in different marital statuses, 1975–2016 (p < 0.001). Censoring marks are indicated with small vertical lines. (A) OS- Marital status; (B) CSS- Marital status.
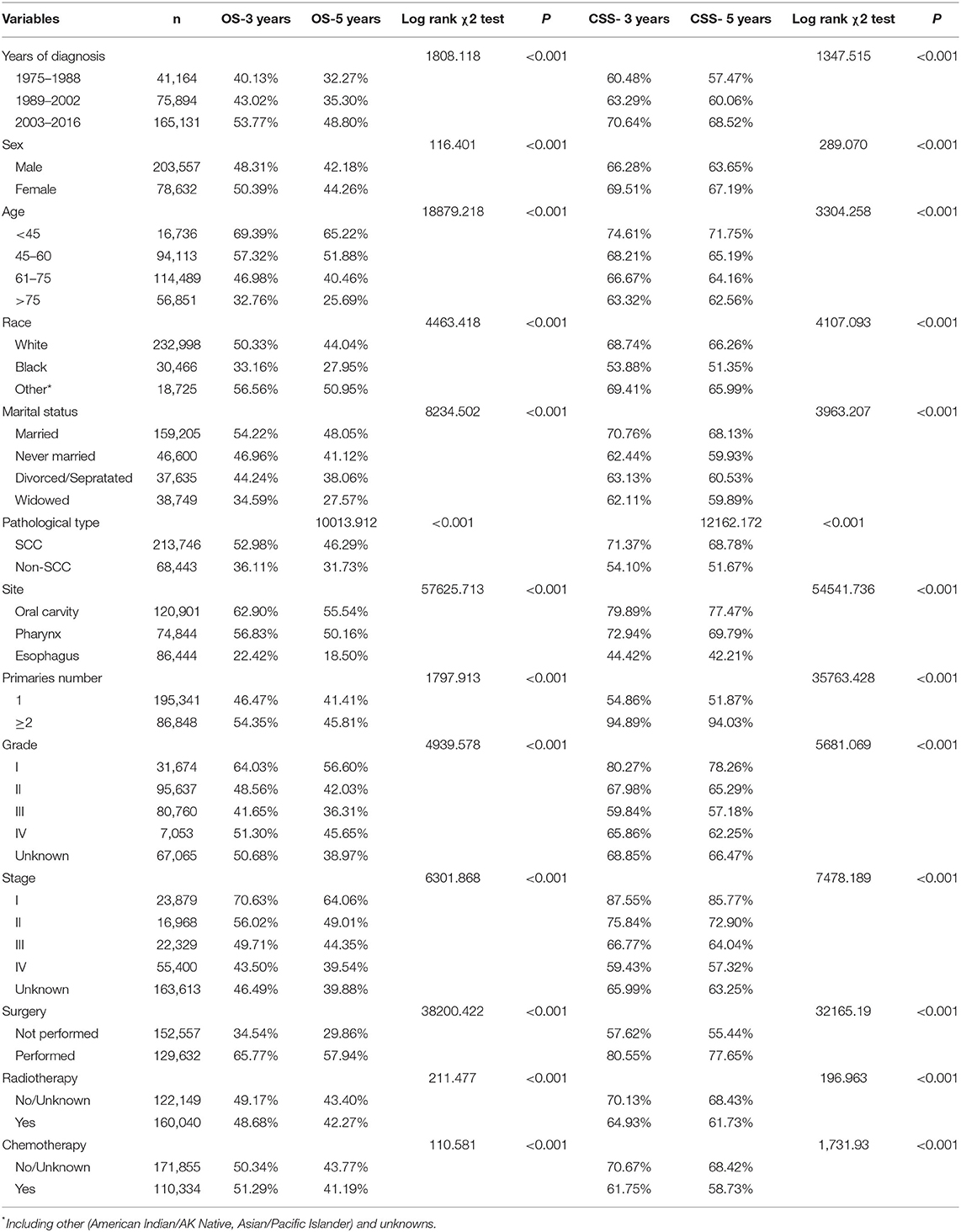
Table 2. Univariate survival analyses of patients with UDTT according to various clinicopathological variables.
Marital Status as an Independent Factor Influencing Survival Outcomes of Patients With UDTTs
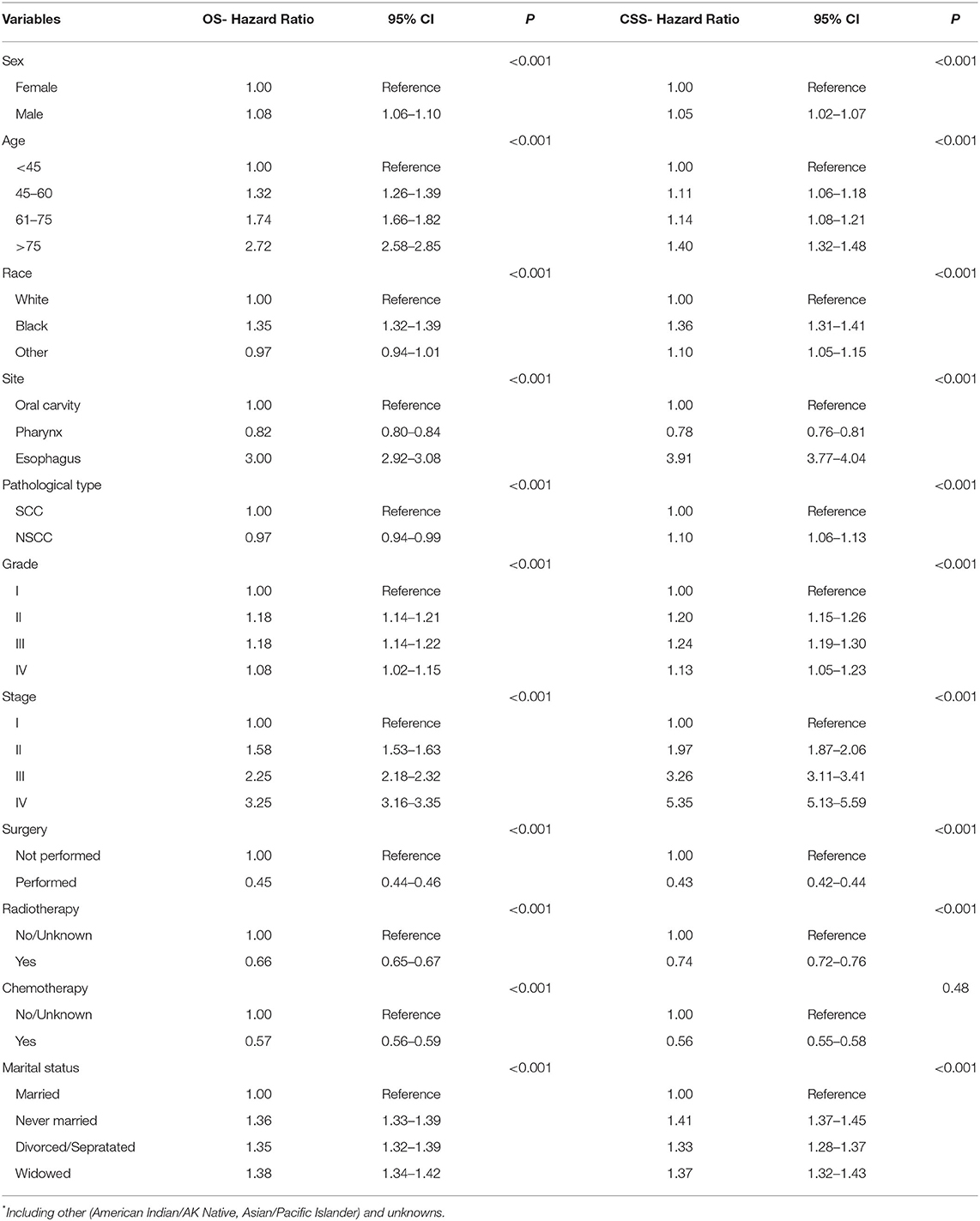
The variables that include sex, age, race, anatomical site, pathological type, grade, stage, surgery, radiotherapy, chemotherapy, and marital status were analyzed with Cox regression model for their correlations with the prognosis of patients with UDTTs. All variables were found to be independent prognostic factors of OS of patients with UDTTs (Figure 3, Table 3): sex (male: HR, 1.08; 95%CI: 1.06–1.10), age (45–60 years: HR, 1.32; 95%CI: 1.26–1.39; 61–75 years: HR, 1.74; 95%CI: 1.66–1.82; > 75 years: HR, 2.72; 95%CI: 2.58–2.85), race (Black: HR, 1.35; 95%CI: 1.32–1.39; other: HR, 0.97; 95%CI: 0.94–1.01), anatomical site (pharynx: HR, 0.82; 95%CI: 0.80–0.84; esophagus: HR, 3.00; 95%CI: 2.92–3.08), pathological type (NSCC: HR, 0.97; 95%CI: 0.94–0.99), grade (II: HR, 1.18; 95%CI: 1.14–1.21; III: HR, 1.18; 95%CI: 1.14–1.22; IV: HR, 1.08; 95%CI: 1.02–1.15;), stage (II: HR, 1.58; 95%CI: 1.53–1.63; III: HR, 2.25; 95%CI: 2.18–2.32; IV: HR, 3.25; 95%CI: 3.16–3.35;), surgery (performed: HR, 0.45; 95%CI: 0.44–0.46), radiotherapy (Yes: HR, 0.66; 95%CI: 0.65–0.67), chemotherapy (yes: HR, 0.57; 95%CI: 0.56–0.59), and marital status (never married: HR,1.36; 95%CI: 1.33–1.39; divorced/separated: HR, 1.35; 95%CI: 1.32–1.39; widowed: HR, 1.38; 95%CI: 1.34–1.42). Only in race, other race showed no significant difference (p=0.134), and other variables are significantly different. Likewise, all variables were found to be the independent prognostic factors of CSS of patients with UDTTs: sex (male: HR, 1.05; 95%CI: 1.02–1.07), age (45–60 years: HR, 1.11; 95%CI: 1.06–1.18; 61–75 years: HR, 1.74; 95%CI: 1.08–1.21; > 75 years: HR, 1.40; 95%CI: 1.32–1.48), race (Black: HR, 1.36; 95%CI: 1.31–1.41; other: HR, 1.10; 95%CI: 1.05–1.15), anatomical site (pharynx: HR, 0.78; 95%CI: 0.76–0.81; esophagus: HR, 3.91; 95%CI: 3.77–4.04), pathological type (NSCC: HR, 1.10; 95%CI: 1.06–1.13), grade (II: HR, 1.20; 95%CI: 1.15–1.26; III: HR, 1.24; 95%CI: 1.19–1.30; IV: HR, 1.13; 95%CI: 1.05–1.23), stage (II: HR, 1.97; 95%CI: 1.87–2.06; III: HR, 3.26; 95%CI: 3.11–3.41; IV: HR, 5.35; 95%CI: 5.13–5.59;), surgery (performed: HR, 0.43; 95%CI: 0.42–0.44), radiotherapy (Yes: HR, 0.74; 95%CI: 0.72–0.76), chemotherapy (yes: HR, 0.56; 95%CI: 0.55–0.58), and marital status (never married: HR,1.41; 95%CI: 1.37–1.45; divorced or separated: HR, 1.33; 95%CI: 1.28–1.37; widowed: HR, 1.32; 95%CI: 1.32–1.43;).
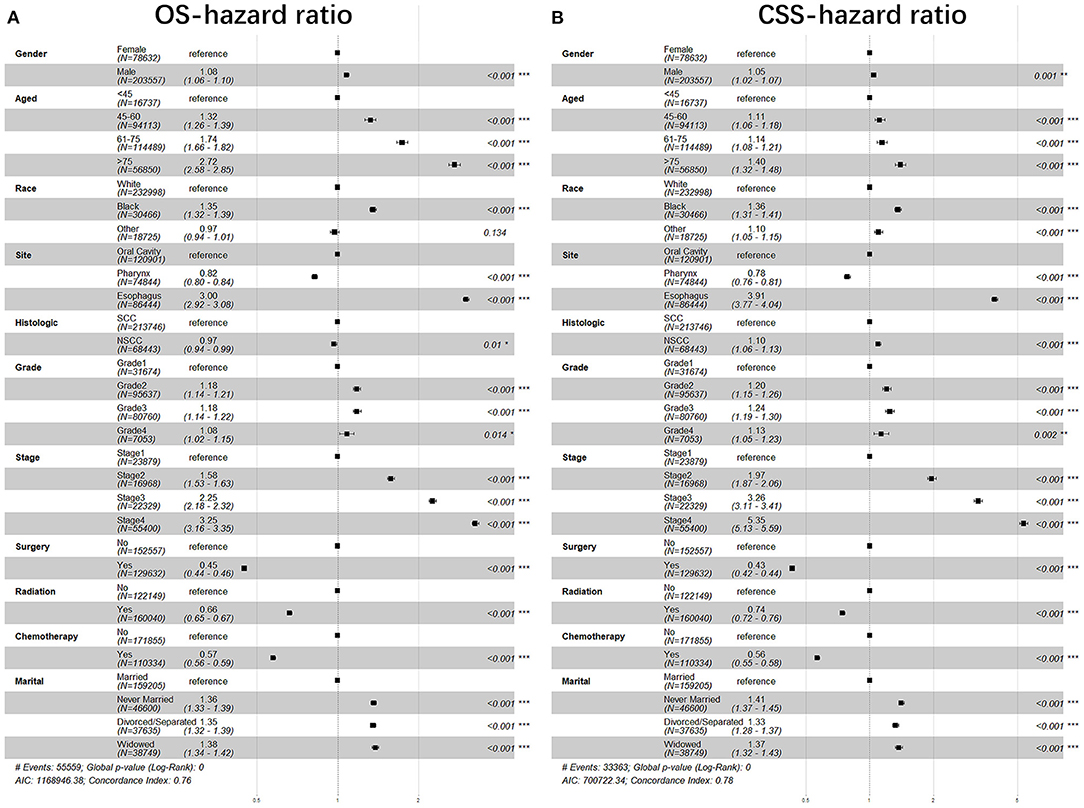
Figure 3. The forest plot of HR (95% CI) for OS and CSS of the patients with UDTT. HR, hazard ratio; CI, confidence interval. (A) OS- hazard ratio; (B) CSS- hazard ratio.
Stratified Analysis of Marital Status
Furthermore, we studied the effect of sex, age, race, anatomic site, pathological type, grade, stage, surgery, radiotherapy, chemotherapy, and marital status on OS and CSS of patients with UDTTs by COX proportional hazard regression model (Figure 4). Men had poor OS and CSS than women in never married and divorced or separated by comparing the HR in OS (never married: HR, 1.40; 95%CI: 1.37–1.44; divorced or separated: HR, 1.39; 95%CI: 1.35–1.43) and CSS (never married: HR, 1.45; 95%CI: 1.41–1.50; divorced/separated: HR, 1.39; 95%CI: 1.34–1.44). Compared with marital status, older women were more risk in never married (Figure 4). So, we made subgroup analysis according to married and never married (Figure 5). Regarding the OS patients with UDTTs, men in the married group showed better OS than those in the never-married group (HR, 1.16; 95%CI: 1.11–1.22). Regarding the CSS patients with UDTTs, men in the married group showed better CSS than those in the never-married group (HR, 0.96; 95%CI: 0.92–1.23). Notably, compared with the unmarried group, whether for OS or CSS, age is a greater risk factor for the married group. These data showed married male patients owed better survival outcome.
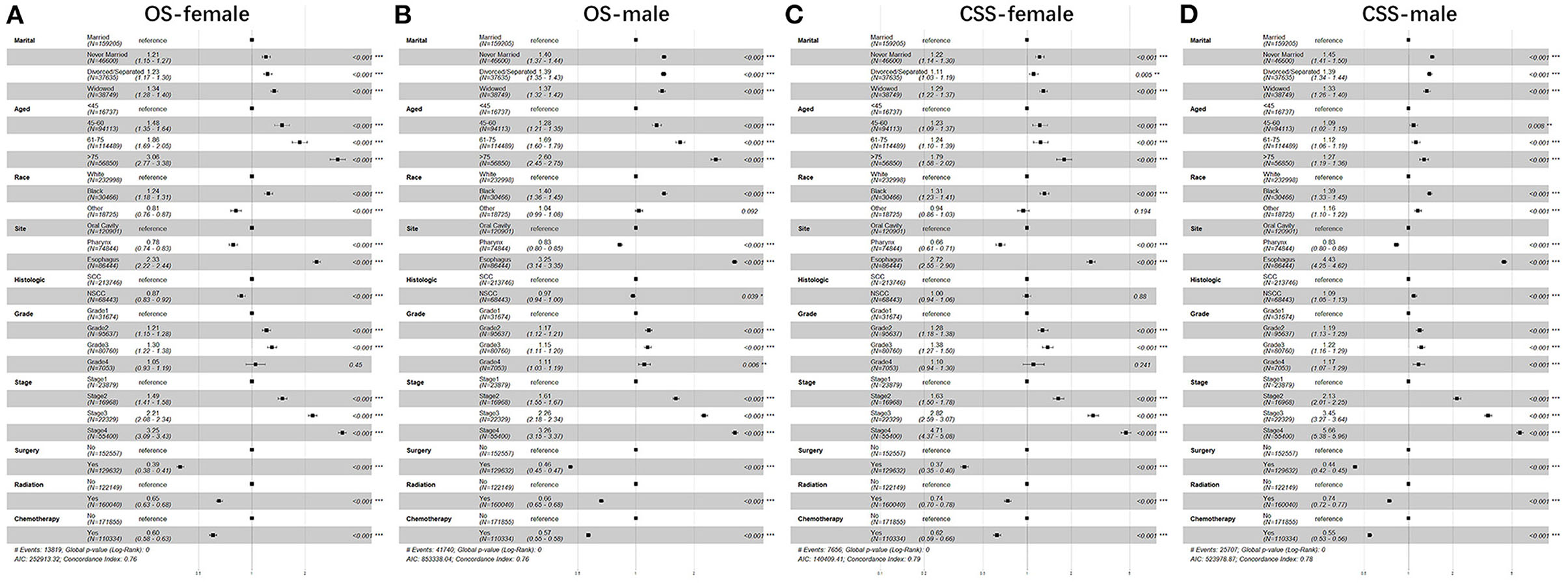
Figure 4. The forest plot of HR (95% CI) for OS and CSS of the patients with UDTT in each gender. HR, hazard ratio; CI confidence interval; (A) OS of female; (B) OS of male; (C) CSS of female; (D) CSS of male.
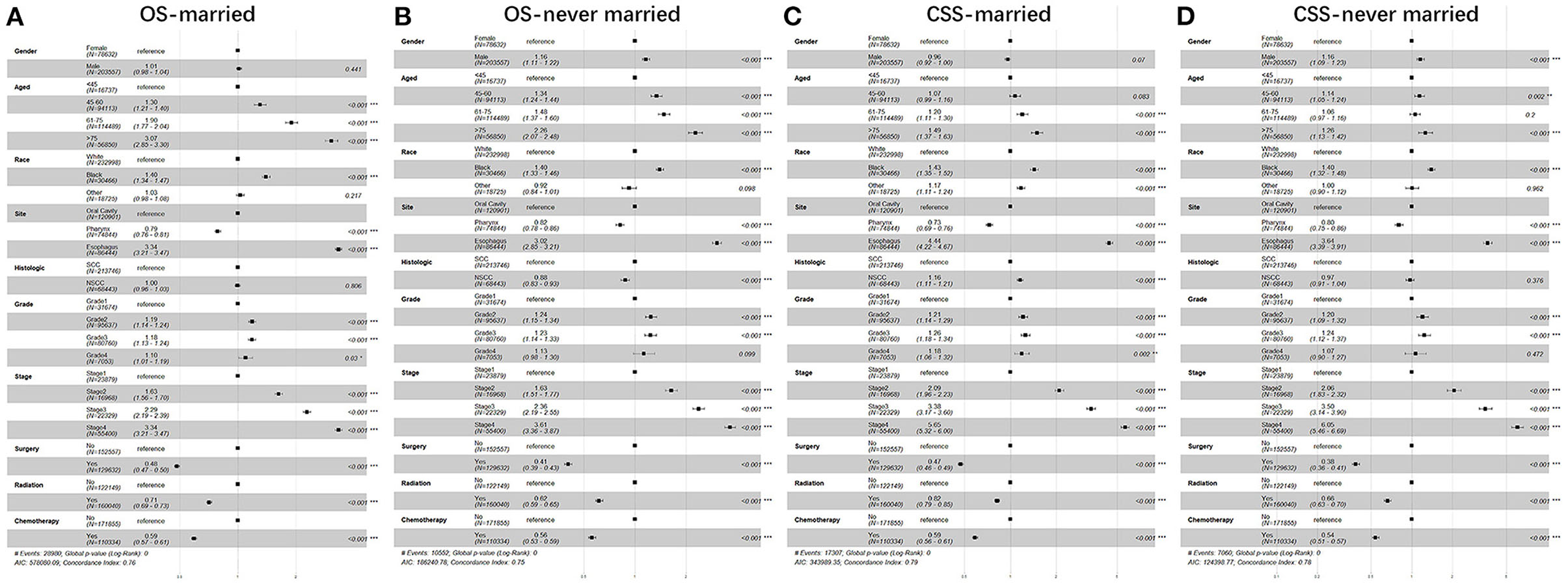
Figure 5. The forest plot of HR (95% CI) for OS and CSS of the patients with UDTT in subgroups of married and never married. HR, hazard ratio; CI confidence interval; (A) OS of married; (B) OS of never married; (C) CSS of married; (D) CSS of never married.
Discussion
As far as we are concerned, few researches have focused on the heterogeneity of patients with UDTT in different marital statuses with stratified comparisons. UDTT, as a type of tumor with a higher incidence and a poor prognosis (24), is closely related to psychological and behavioral factors (14, 15), and the marital relationship has a significant impact on it through psychological and behavioral differences (20, 21). Our study indicated that married patients had better OS/CSS rate of UDTT, while widowed patients were had worse OS/CSS rate. This similar survival difference was also observed in each gender, stage, and treatments.
In our study, the never-married group had gradually increased from 1975 to 2016, which is consistent with the declining marriage rate in the United States (25). At the same time, the proportion of male patients was significantly higher than that of female patients, which may be due to the fact that UDTT is related to smoking, drinking, and other living habits. The 3-year and 5-year survival rates gradually improved as the time of diagnosis approached, which indicates that with the advancement of medicine, both diagnosis and treatment of UDTT have made significant progress. Both 3-year and 5-year OS and CSS were significantly lower for men than for women. The 3-year and 5-year survival rates for Blacks were also significantly lower than Whites and other races. For the 3-year and 5-year survival rates, being married is an obvious protective factor.
Most studies suggested that unmarried patients have worse survival rates due to delayed diagnosis and under-treatment (3, 7, 26, 27). Hinyard et al. found that the probability of late-stage diagnosis among unmarried female patients was 1.18-folds higher than married female patients (28). Hershman et al. stated that unmarried subjects tended to postpone the start of adjuvant chemotherapeutic treatment after receiving the surgeries of breast carcinoma, which led to higher mortality (29). Our study found something similarly in Cox multivariate regression analysis, and we found that never-married and divorced patients in OS and CSS had greater risks for men than women, and increased age was riskier for women. From the site of UDTT, the prognosis of esophageal cancer in men was worse than that in women. Women can benefit more from the surgery. In the further stratified comparison of married and never-married status, it was found that men can benefit more from marriage than women, thus avoiding premature death caused by UDTT. The never-married group received more benefits in treatment than the married group.
There are several studies that have emphasized the importance of marriage to the patients with cancer. On the one hand, it is analyzed by psychological differences. Unmarried patients more likely display greater stress and depression when diagnosed with cancer, which can change immune function and cause tumor progression (30, 31). Meanwhile, unmarried patients lack the support and care from their spouses, so they often suffer from distressed psychological state and indulge in bad habits, just like smoking and excessive drinking, which lead to an exacerbation of tumor and poor treatment outcomes (32–34). Widowhood is a serious emotion stress, which means that social support and material support are reduced that could lead the patients to pay less attention to health and causing more non-definitive treatment even when symptoms are present (27). On the other hand, the lack of marriage has a corresponding effect on the hormones and mediators of the patient's body. The lack of social support and chronic stress may promote the secretion of cortisol (35, 36). Other studies have shown that increased psychological stress by pass through the hypothalamic-pituitary-adrenal axis to decrease the immune response and promote development of tumor. Furthermore, the release of glucocorticoids and catecholamines is regulated, which further directly influences the tumor microenvironment (37, 38), and has been implicated in cancer survival (39, 40). In contrast, it has been suggested that mates tend to encourage screening and compliance to treatment and therefore could improve treatment outcomes (4, 7).
Our study was based on a big database and involved a huge population and could give light on the impact of marital status on the prognosis in patients with UDTT. However, there are still some limitations. First, the SEER database only records marital status of patient at the time of diagnosis, not dynamically, so changes in marital status as the tumor progresses may affect the outcome of a different marital status on survival outcomes. Second, SEER database lacks corresponding records of marital quality, because disharmony and depression in marriage may negatively affect the prognosis (41). Third, a considerable proportion of patients may not have a legal marriage, but live in de facto same or opposite sex or partnerships (4). It was not until June 2015, the United States officially legalized same-sex marriage (42), and the SEER does not record it, so it is impossible to know whether there is a difference in the prognosis of tumors between same-sex and heterosexual marriages. Finally, SEER also has limited the information on adverse habits such as smoking and drinking.
In summary, our study has revealed that marital status is an independent prognostic factor of OS and CSS rates in patients with UDTTs. Compared with other marital status groups, married patients gained significantly better outcome, irrespective of different variables we studied. The never-married group performed significantly worse in OS and CSS, while men with UDTTs benefited more from marriage than women. Men with UDTTs who were never married had at higher risk than women. Thus, married status plays a significant role as a protective factor in patients with UDTTs, especially for men. Therefore, the marital status of the patients is recommended for predicting the prognosis as a clinical routine during the treatment of UDTTs, and never-married men with UDTTs also need more attention.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethical Statement
The SEER belongs to public databases. The patients involved in the database have obtained ethical approval. Users can download relevant data for free for research and publish relevant articles. Our study is based on open-source data, so there are no ethical issues and other conflict of interests.
Author Contributions
MFQ as the first author and corresponding author conceived the study, wrote the initial draft, and edited the final version. JKP prepared the experimental resources and the software. QHS and HX performed data analysis and interpretation, and contributed to charts. QMC as a corresponding author assisted in manuscript revision. All authors contributed significantly to the initiation and design of the study and manuscript approval.
Funding
This study was supported by the National Natural Science Foundation of China (No. 81771086).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mankarious A, Dave F, Pados G, Tsolakidis D, Gidron Y, Pang Y, et al. The pro-social neurohormone oxytocin reverses the actions of the stress hormone cortisol in human ovarian carcinoma cells in vitro. Int J Oncol. (2016) 48:1805–14. doi: 10.3892/ijo.2016.3410
2. Zhai Z, Zhang F, Zheng Y, Zhou L, Tian T, Lin S, et al. Effects of marital status on breast cancer survival by age, race, and hormone receptor status: a population-based Study. Cancer Med. (2019) 8:4906–17. doi: 10.1002/cam4.2352
3. Liu YL, Wang DW, Yang ZC, Ma R, Li Z, Suo W, et al. Marital status is an independent prognostic factor in inflammatory breast cancer patients: an analysis of the surveillance, epidemiology, and end results database. Breast Cancer Res Treat. (2019) 178:379–88. doi: 10.1007/s10549-019-05385-8
4. Wu SG, Zhang QH, Zhang WW, Sun JY, Lin Q, He YZ. The effect of marital status on nasopharyngeal carcinoma survival: a surveillance, epidemiology and end results study. J Cancer. (2018) 9:1870–6. doi: 10.7150/jca.23965
5. Wang N, Bu Q, Liu Q, Yang J, He H, Liu J, et al. Effect of marital status on duodenal adenocarcinoma survival: a surveillance epidemiology and end results population analysis. Oncol Lett. (2019) 18:1904–14. doi: 10.3892/ol.2019.10475
6. Zhang W, Wang X, Huang R, Jin K, Zhangyuan G, Yu W, et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci Rep. (2017) 7:41695. doi: 10.1038/srep41695
7. Aizer AA, Chen MH, McCarthy EP, Mendu ML, Koo S, Wilhite TJ, et al. Marital status and survival in patients with cancer. J Clin Oncol. (2013) 31:3869–76. doi: 10.1200/JCO.2013.49.6489
8. Denberg TD, Beaty BL, Kim FJ, Steiner FJ. Marriage and ethnicity predict treatment in localized prostate carcinoma. Cancer. (2005) 103:1819–25. doi: 10.1002/cncr.20982
9. Wang L, Wilson SE, Stewart DB, Hollenbeak SC. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. (2011) 35:417–22. doi: 10.1016/j.canep.2011.02.004
10. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2018) 68:394–424. doi: 10.3322/caac.21492
11. Kostareli E, Hielscher T, Zucknick M, Baboci L, Wichmann G, Holzinger D, et al. Gene promoter methylation signature predicts survival of head and neck squamous cell carcinoma patients. Epigenetics. (2016) 11:61–73. doi: 10.1080/15592294.2015.1137414
12. Ma G, Zhang F, Dong X, Wang X, Ren Y. Low expression of microRNA-202 is associated with the metastasis of esophageal squamous cell carcinoma. Exp Ther Med. (2016) 11:951–6. doi: 10.3892/etm.2016.3014
13. Madsen SJ, Shih EC, Peng Q, Christie C, Krasieva T, Hirschberg H. Photothermal enhancement of chemotherapy mediated by gold-silica nanoshell-loaded macrophages: in vitro squamous cell carcinoma study. J Biomed Opt. (2016) 21:18004. doi: 10.1117/1.JBO.21.1.018004
14. Scoccianti C, Straif K, Romieu I. Recent evidence on alcohol and cancer epidemiology. Future Oncol. (2013) 9:1315–22. doi: 10.2217/fon.13.94
15. Cui R, Kamatani Y, Takahashi A, Usami M, Hosono N, Kawaguchi T, et al. Functional variants in ADH1B and ALDH2 coupled with alcohol and smoking synergistically enhance esophageal cancer risk. Gastroenterology. (2009) 137:1768–75. doi: 10.1053/j.gastro.2009.07.070
16. Leemans CR, Snijders PJF, Brakenhoff HR. The molecular landscape of head and neck cancer. Nat Rev Cancer. (2018) 18:269–82. doi: 10.1038/nrc.2018.11
17. Adams AK, Wise-Draper TM, Wells IS. Human papillomavirus induced transformation in cervical and head and neck cancers. Cancers (Basel). (2014) 6:1793–820. doi: 10.3390/cancers6031793
18. Petrick JL, Wyss AB, Butler AM, Cummings C, Sun X, Poole C, et al. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. (2014) 110:2369–77. doi: 10.1038/bjc.2014.96
19. Peedicayil J. Psychosocial factors may act via epigenetic mechanisms in the pathogenesis of mental disorders. Med Hypothese. (2008) 70:700–1 doi: 10.1016/j.mehy.2007.07.015
20. Odigie VI, Tanaka R, Yusufu LM, Gomna A, Odigie EC, Dawotola DA, et al. Psychosocial effects of mastectomy on married African women in Northwestern Nigeria. Psychooncology. (2010) 19:893–7. doi: 10.1002/pon.1675
21. Tuinman MA, Lehmann V, Hagedoorn M. Do single people want to date a cancer survivor? A vignette study. PLoS One. (2018) 13:e0194277. doi: 10.1371/journal.pone.0194277
22. Parikh-Patel A, Bates JH, Campleman S. Colorectal cancer stage at diagnosis by socioeconomic and urban/rural status in California, 1988-2000. Cancer. (2006) 107(5 Suppl):1189–95. doi: 10.1002/cncr.22016
23. Rex DK, Johnson DA, Anderson JC, Schoenfeld PS, Burke CA, Inadomi JM, et al. American College of Gastroenterology guidelines for colorectal cancer screening (2009) [corrected]. Am J Gastroenterol. (2009) 104:739–50. doi: 10.1038/ajg.2009.104
24. Ribeiro IP, Caramelo F, Ribeiro M, Machado A, Migueis J, Marques F, et al. Upper aerodigestive tract carcinoma: Development of a (epi)genomic predictive model for recurrence and metastasis. Oncol Lett. (2020) 19:3459–68. doi: 10.3892/ol.2020.11459
25. Curtin SC. Marriage Rates in the United States, 1900–2018. National Center for Health Statistics. Available online at: https://www.cdc.gov/nchs/products/hestats.htm
26. Osborne C, Ostir GV, Du X, Peek MK, Goodwin SJ. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. (2005) 93:41–7. doi: 10.1007/s10549-005-3702-4
27. Reyes Ortiz CA, Freeman JL, Kuo YF, Goodwin SJ. The influence of marital status on stage at diagnosis and survival of older persons with melanoma. J Gerontol A Biol Sci Med Sci. (2007) 62:892–8. doi: 10.1093/gerona/62.8.892
28. Hinyard L, Wirth LS, Clancy JM, Schwartz T. The effect of marital status on breast cancer-related outcomes in women under 65: a SEER database analysis. Breast. (2017) 32:13–7. doi: 10.1016/j.breast.2016.12.008
29. Hershman DL, Wang X, McBride R, Jacobson JS, Grann VR, Neugut IA. Delay of adjuvant chemotherapy initiation following breast cancer surgery among elderly women. Breast Cancer Res Treat. (2006) 99:313–21. doi: 10.1007/s10549-006-9206-z
30. Moreno-Smith M, Lutgendorf SK, Sood KA. Impact of stress on cancer metastasis. Future Oncol. (2010) 6:1863–81. doi: 10.2217/fon.10.142
31. Sklar LS, Anisman H. Stress and coping factors influence tumor growth. Science. (1979) 205:513–5. doi: 10.1126/science.109924
32. Surman M, Janik EM. Stress and its molecular consequences in cancer progression. Postepy Hig Med Dosw. (2017) 71:485–99. doi: 10.5604/01.3001.0010.3830
33. Goldzweig G, Andritsch E, Hubert A, Brenner B, Walach N, Perry S, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann Oncol. (2010) 21:877–83. doi: 10.1093/annonc/mdp398
34. Goldzweig G, Andritsch E, Hubert A, Walach N, Perry S, Brenner B, et al. How relevant is marital status and gender variables in coping with colorectal cancer? A sample of middle-aged and older cancer survivors. Psychooncology. (2009) 18:866–74. doi: 10.1002/pon.1499
35. McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol Rev. (2007) 87:873–904. doi: 10.1152/physrev.00041.2006
36. Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. (2000) 92:994–1000. doi: 10.1093/jnci/92.12.994
37. Antoni MH, Lutgendorf SK, Cole SW, Dhabhar FS, Sephton SE, McDonald PG, et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat Rev Cancer. (2006) 6:240–8. doi: 10.1038/nrc1820
38. Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res. (2006) 12:369–75. doi: 10.1158/1078-0432.CCR-05-1698
39. Garssen B, Goodkin K. On the role of immunological factors as mediators between psychosocial factors and cancer progression. Psychiatry Res. (1999) 85:51–61. doi: 10.1016/S0165-1781(99)00008-6
40. Reiche EM, Nunes SO, Morimoto KH. Stress, depression, the immune system, and cancer. Lancet Oncol. (2004) 5:617–25. doi: 10.1016/S1470-2045(04)01597-9
41. Manzoli L, Villari P, Pirone GM, Boccia A. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med. (2007) 64:77–94. doi: 10.1016/j.socscimed.2006.08.031
Keywords: upper digestive tract tumors, marital status, SEER, prognostic factors, cancer-specific survival
Citation: Qing MF, Peng JK, Shang QH, Xu H and Chen QM (2022) Effect of Marital Status on Upper Digestive Tract Tumor Survival: Married Male Patients Exhibited a Better Prognosis. Front. Surg. 9:880893. doi: 10.3389/fsurg.2022.880893
Received: 22 February 2022; Accepted: 10 March 2022;
Published: 11 April 2022.
Edited by:
Weiguo Li, Harbin Institute of Technology, ChinaReviewed by:
Zhi Zhao, Second Hospital of Hebei Medical University, ChinaPengcheng Sun, Forth Affiliated Hospital of Harbin Medical University, China
Copyright © 2022 Qing, Peng, Shang, Xu and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maofeng Qing, doudouwq@163.com; Qianming Chen, qmchen@scu.edu.cn
 Maofeng Qing
Maofeng Qing Jiakuan Peng
Jiakuan Peng  Hao Xu
Hao Xu