Clinical analysis of transurethral holmium laser enucleation in the treatment of benign prostatic hyperplasia with prostatic inflammation: A prospective research study
- 1Department of Clinical Medicine, Weifang Medical University, Weifang, China
- 2Department of Urology, Affiliated Hospital of Weifang Medical University, Weifang, China
Objective: To investigate the clinical efficacy of holmium laser enucleation of the prostate (HoLEP) in the treatment of benign prostatic hyperplasia (BPH) with prostatic inflammation (PI).
Methods: We prospectively collected and followed up data on patients with BPH who underwent HoLEP at the Affiliated Hospital of Weifang Medical University between July 2021 and July 2022. According to the postoperative pathological results, the patients were divided into two groups: BPH without PI group (BPH group) and BPH with PI group. Statistical analysis was performed on clinical data, including age and body mass index (BMI), prostate volume (PV), postoperative residual urine volume (PVR), preoperative serum total prostate-specific antigen (tPSA), serum-free prostate-specific antigen (fPSA), preoperative and postoperative maximum urinary flow rate (Qmax), International Prostate Symptom Score (IPSS) before and 3 months after surgery, quality of life index (QoL) before and 3 months after surgery, and postoperative complications.
Results: A total of 41 patients were included in this study, including 16 in the BPH group and 25 in the BPH with PI group. There were no significant differences in preoperative age, BMI, PV, PVR, tPSA, fPSA, and f/tPSA between the BPH and BPH with PI groups (P > 0.05). The preoperative mean Qmax of the BPH and BPH with PI groups were 9.44 ± 2.449 and 7.52 ± 2.946 [mean ± standard deviation (SD)] ml/s, mean IPSS were 17.75 ± 5.335 and 24.24 ± 5.861 (mean ± SD), and mean QoL were 4.13 ± 0.806 and 4.48 ± 0.8 (mean ± SD), respectively. The postoperative mean Qmax of the BPH and BPH with PI groups were 20.38 ± 4.787 and 14.32 ± 3.827 (mean ± SD) ml/s, mean IPSS were 2.69 ± 1.25 and 5.84 ± 3.579 (mean ± SD), and mean QoL were 0.13 ± 0.342 and 0.92 ± 0.759 (mean ± SD), respectively. In both groups, Qmax significantly increased (P < 0.05) and IPSS and QoL significantly decreased after HoLEP (P < 0.05). Before and after surgery, the Qmax in the BPH with PI group was lower than that in the BPH group, and the IPSS and QoL levels in the BPH with PI group were higher than those in the BPH group (P < 0.05). Compared with the BPH group, the increase in Qmax in the BPH with PI group was smaller and the decrease in IPSS was larger (P < 0.05), but the variation in QoL was not statistically significant (P > 0.05).
Conclusion: Improvements in Qmax, IPSS, and QoL in BPH patients with PI after HoLEP surgery were lower than those in BPH patients alone. PI may be a predictor of a worse response to surgical treatment. However, more multicenter randomized controlled trials with larger samples and long-term follow-up are needed to verify this.
Introduction
Benign prostatic hyperplasia (BPH) is one of the most common diseases in older men, with a prevalence of 30% in those over the age of 50 years (1). Prostatic inflammation (PI) is also a common genitourinary disease in men, with a prevalence of 12.4% for prostatitis-like symptoms in the total population (2). The prevalence rate among young men aged 18–30 years is as high as 16% (3). However, a combination of these two diseases is present in approximately 5%–20% of cases (4). One study reported that inflammatory cell infiltration was found in 81% of postoperative pathological tissues in patients with BPH (5). Moreover, prostate inflammation plays a major role in BPH development and pathogenesis (6, 7). In recent years, with advancements in minimally invasive techniques, holmium laser enucleation of the prostate (HoLEP) has been applied effectively in clinical practice. Compared with traditional minimally invasive methods, such as transurethral plasma enucleation and transurethral resection of the prostate, HoLEP shows superior clinical efficacy and safety in the treatment of patients with BPH (8). In this study, we collected data from patients who underwent HoLEP at our hospital. We aimed to explore the clinical characteristics of BPH complicated with PI before and after surgery and provide new ideas to improve the clinical outcomes of these patients.
Materials and methods
Subjects
The study was approved by the Medical Research Ethics Review of the Affiliated Hospital of Weifang Medical University (approval number: wyfy-2022-ky-172). Using a prospective design, we included patients who met the following criteria: (i) patients who underwent HoLEP at the Affiliated Hospital of Weifang Medical University from July 2021 to July 2022 and (ii) patients with or without PI, as long as the postoperative pathological findings confirmed BPH. PI was defined as pathological inflammation of the prostate specimen after resection, including postoperative pathology suggestive of chronic or acute prostatitis. We excluded patients with a previous history of prostatic surgery and/or biopsy, acute urinary retention, urinary tumors, severe metabolic diseases, multiple organ dysfunction and other diseases, or incomplete clinical data.
Grouping
According to the postoperative pathological results, the patients were divided into two groups: BPH without PI group (BPH group) and BPH with PI group.
Methods
The following parameters were obtained and recorded: (i) patient age and body mass index (BMI); (ii) prostate volume (PV) and postvoid residual urine (PVR): PV and PVR determined by urological ultrasound = anterior-posterior diameter × superior-inferior diameter × left-right diameter × 0.523; (iii) preoperative prostate-specific antigen (PSA): fasting serum PSA in the morning was determined by enzyme-linked immunosorbent assay (ELISA), including total PSA (tPSA) and free PSA (fPSA); (iv) maximum flow rate (Qmax) before surgery and 1 day after catheter removal post-surgery: measured using Flowmaster wireless Uroflowmeter (Laborie Medical Technologies, USA); (v) International prostate symptom score (IPSS) before and 3 months after surgery, where the higher score, the more severe the lower urinary tract symptoms (LUTS); (vi) quality of life (QoL) before and 3 months after surgery, where the higher the score, the more distressed the patients are.
Statistical analysis
THE IBM SPSS Statistics for Windows, version 26.0 (IBM Corp., Armonk, N.Y., USA) was used for statistical analysis. We used the Shapiro-Wilk test to verify the normality of the data. The F-test was used to determine the homogeneity of variances. Normally distributed continuous variables are expressed as mean ± standard deviation and compared using t-tests. Two independent sample t-tests were used for comparisons between the groups, and a paired t-test was used for comparisons before and after surgery. Statistical significance was set at P < 0.05.
Results
Comparison of clinical indicators between the two groups before surgery
A total of 41 patients were included in this study: 16 in the BPH group and 25 in the BPH with PI group. In this study, there were no significant differences in preoperative age, BMI, PV, PVR, tPSA, fPSA, and f/tPSA between the two groups (Table 1).
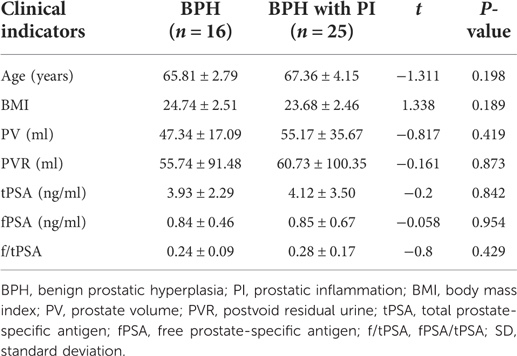
Table 1. Comparison of clinical indicators between two groups of patients before surgery (mean ± SD).
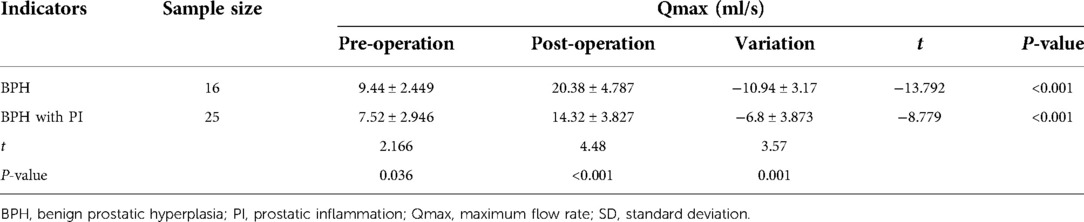
Comparison of Qmax data between the two groups before and after surgery
Preoperative mean Qmax of the BPH and BPH with PI groups were 9.44 ± 2.449 and 7.52 ± 2.946 [mean ± standard deviation (SD)] ml/s, and postoperative mean Qmax were 20.38 ± 4.787 and 14.32 ± 3.827 (mean ± SD) ml/s, respectively. The results of the intra-group comparison showed that the postoperative Qmax in the BPH with PI and BPH groups was significantly higher than the preoperative Qmax in the BPH with PI and BPH groups (P < 0.001). Moreover, compared with the BPH group, the variation in Qmax values in the BPH with PI group was lower in the preoperative, postoperative, and pre- and postoperative Qmax, and all differences were statistically significant (P < 0.05) (Table 2).
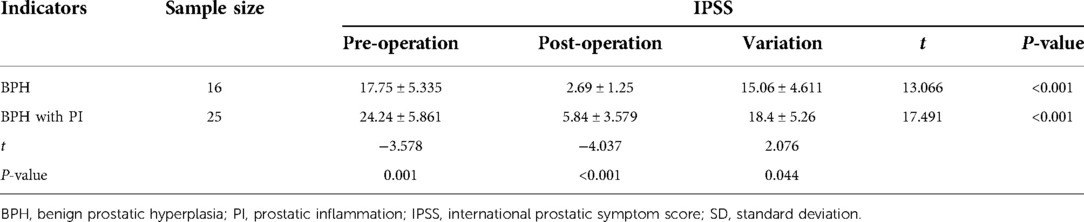
Comparison of IPSS data between the two groups before and after surgery
Preoperative mean IPSS of the BPH and BPH with PI groups were 17.75 ± 5.335 and 24.24 ± 5.861 (mean ± SD), and postoperative mean IPSS were 2.69 ± 1.25 and 5.84 ± 3.579 (mean ± SD), respectively. The results of the intra-group comparison showed that the postoperative IPSS of the BPH with PI and BPH groups were significantly lower than those before surgery, and the differences before and after surgery were statistically significant (P < 0.001). Moreover, the IPSS of the BPH with PI group was higher than that of the BPH group before and after surgery, and the difference between the two groups was statistically significant (P < 0.05). The variation in the IPSS before and after surgery in the BPH with PI group was larger than that in the BPH group, and the difference between the two groups was statistically significant (P < 0.05) (Table 3).
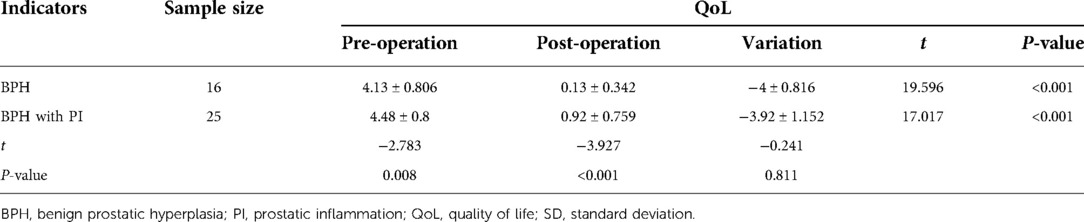
Comparison of QoL data between the two groups before and after surgery
The preoperative mean QoL of the BPH and BPH with PI groups were 4.13 ± 0.806 and 4.48 ± 0.8 (mean ± SD), and the postoperative mean QoL was 0.13 ± 0.342 and 0.92 ± 0.759 (mean ± SD), respectively. The results of the intra-group comparison showed that the postoperative QoL in the BPH with PI and BPH groups were significantly lower than those before surgery, and the differences were statistically significant (P < 0.001). Furthermore, QoL in the BPH with PI group was higher than that in the BPH group before and after surgery, and the difference between the two groups was statistically significant (P < 0.05). There was no significant difference in the variation in QoL between the two groups before and after surgery (Table 4).
Discussion
BPH and PI are common urinary diseases in middle-aged and older men and mainly affect their QoL (9, 10). Tang et al. found that the incidence of BPH complicated with PI was as high as 78.3% (11). A multicenter study by Nickel et al. showed that 77.6% of 8,224 patients with BPH had PI (12). In a study by Cao et al., 91.7% of the patients with BPH had PI (13). The BPH rate of combined PI was as high as 78.6% in a study by Li et al. (14). However, in a study by Chang et al., only 37.5% of pathological specimens from patients with BPH had PI (15). Similarly, only 34.2% of the patients with BPH in the study by Du et al. had PI (16). In contrast, the percentage of patients with BPH and PI in the present study was 61.0%. Such large differences may be related to the different pathological specimen collections and pathological diagnostic criteria.
The present study showed no statistically significant difference in PV between the BPH with PI and BPH groups (P > 0.05). However, Long et al. showed that the PV of BPH patients with PI was significantly higher than that of BPH patients without PI (17). Gerstenbluth et al. also showed that patients with BPH who had PI had significantly larger PV than those with BPH only (18). Inflammation of the prostate leads to repeated destruction, healing, and regeneration of prostate tissue, causing enlargement and remodeling of prostate nodules (19). The difference in the results of the current study may be related to the insufficient sample size or the lower degree of inflammation found in the included patients. There were no statistical differences between the two groups in the present study in the indicators of PVR, tPSA, fPSA, and f/tPSA., which is the same as the results of Du et al. and Li et al. (14, 16). However, Feng et al. found that tPSA was significantly higher in the BPH with PI group than in the BPH group (20). Most of the current studies suggest that inflammatory cells in PI infiltrate the prostate epithelium, causing its destruction and release of excessive PSA, and that the prostatic ducts and the original physiological barrier in BPH patients with PI are severely disrupted by inflammation and PSA leaks into the circulation, leading to elevated serum PSA levels (21–23). PI may be an important factor influencing the elevation of PSA levels; however, its significance needs to be supported by more data.
The present study focused on evaluating the clinical outcomes of two groups of patients undergoing HoLEP using two subjective indicators (IPSS and QoL) and one objective indicator (Qmax). The American Urological Association recommends the former to evaluate the severity of LUTS and is the best method for evaluating the severity of symptoms in patients with BPH (24). The QoL score is mainly used to evaluate the degree of LUTS distress and tolerance in patients with BPH (25). Qmax can be used as an indicator to assess disease progression and the preoperative and postoperative surgical outcomes in patients with BPH. Our study found that the Qmax in the BPH with PI group was lower than that in the BPH group, whereas the IPSS and QoL in the inflammation group were higher than those in the BPH group before and after surgery (P < 0.05). It has been suggested that inflammation may aggravate the clinical symptoms of patients with BPH, especially LUTS. Robert et al. and the REDUCE study similarly showed a higher preoperative IPSS in BPH patients with inflammation than in patients with BPH alone (5, 12). A study by Du et al. also showed that, both preoperatively and postoperatively, the group with inflammatory hyperplasia had lower Qmax and higher IPSS and QoL than the group with simple hyperplasia (16). Although that study was based on patients with BPH after plasma resection, similar results confirm that PI promotes disease progression and affects the QoL of patients. Related studies have shown that PI can induce T-cell activation under certain initial stimulations, thus producing and releasing related inflammatory factors (such as IL-6 and IL-8) that lead to cell injury (26). Related inflammatory factors can also react with prostatic stromal and other cells to stimulate prostate tissue hyperplasia (27). The processes of lymphocyte activation, cytokine release, and tissue hyperplasia can act as a self-perpetuating cycle, leading to chronic inflammation and a gradual increase in the volume of the prostate, thus forming a “vicious circle” (28).
Furthermore, the present study showed that compared with the BPH group, the increase in Qmax was smaller and the decrease in IPSS was larger in the group with inflammation, but there was no significant difference in the variation in QoL. In contrast, a study by Huang et al. showed no statistically significant difference in IPSS variation after transurethral resection of the prostate (TURP) in patients with BPH with or without PI (29). Can we speculate that HoLEP surgery can reduce IPSS to a greater extent than TURP in patients with BPH with PI, that is, are HoLEP surgeries more effective in relieving LUTS? The mechanism of HoLEP treatment, that is, vaporization, rapid coagulation, and thin-layer engagement, has this basis (30). Further research is required to confirm this hypothesis.
The present study had some limitations. First, the sample size of the present study was relatively small, and the sample was limited to a single medical center. Second, our research did not investigate or restrict patients’ pre-surgical medications, such as whether they were taking antibiotic-like or antispasmodic drugs. This may have influenced the results. Furthermore, the same surgeon did not complete each patient's operation, which may have affected the outcome to a certain extent. The follow-up period of this study was short, and the results obtained were limited and one-sided. We lacked long-term relevant data to confirm the reliability of the present study. However, the sample in this study was strictly screened and the results were relatively robust, which can still serve as a guide for clinical work.
In conclusion, improvements in Qmax, IPSS, and QoL in BPH patients with PI undergoing HoLEP were lower than those in patients with BPH alone. PI may be a predictor of a worse response to surgical treatment. However, the next step is to expand the sample size to a multicenter study and conduct a comparative study on the safety and clinical efficacy of different clinical interventions in BPH patients with PI undergoing HoLEP.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The study was approved by the Medical Research Ethics Review of the Affiliated Hospital of Weifang Medical University (approval number: wyfy-2022-ky-172). The patients/participants provided their written informed consent to participate in this study.
Author contributions
WZ: project development, data collection, study design, data analysis, manuscript writing, and revision. DM: data collection and manuscript writing. LL: data collection and analysis. GL: project development and data collection. GG: project development and data collection. HL: data collection. DG: project development, study design, and revision. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Blankstein U, Van Asseldonk B, Elterman DS. BPH update: medical versus interventional management. Can J Urol. (2016) 23(Suppl 1):10–5. PMID: 2692459026924590
2. Zhang Z, Li Z, Yu Q, Wu C, Lu Z, Zhu F, et al. The prevalence of and risk factors for prostatitis-like symptoms and its relation to erectile dysfunction in Chinese men. Andrology. (2015) 3(6):1119–24. doi: 10.1111/andr.12104
3. Gao DJ, Guo YS, Yu HY, Wang YJ, Cui WG. Prevalence and related factors of prostatitis-like symptoms in young men. Natl J Androl. (2007) 12:1087–90. doi: 10.13263/j.cnki.nja.2007.12.015
4. Yu YJ, Xia J, Qian SB, Zhang L, Li Q, Bai Q. Study on the relationship between chronic prostatitis and lower urinary tract symptoms and bladder neck contracture after transurethral resection of the prostate. Natl J Androl. (2017) 31(05):12–4. doi: 10.26914/c.cnkihy.2019.011549
5. Robert G, Descazeaud A, Nicolaïew N, Terry S, Sirab N, Vacherot F, et al. Inflammation in benign prostatic hyperplasia: a 282 patients’ immunohistochemical analysis. Prostate. (2009) 69(16):1774–80. doi: 10.1002/pros.21027
6. Gandaglia G, Briganti A, Gontero P, Mondaini N, Novara G, Salonia A, et al. The role of chronic prostatic inflammation in the pathogenesis and progression of benign prostatic hyperplasia (BPH). BJU Int. (2013) 112(4):432–41. doi: 10.1111/bju.12118
7. Lloyd GL, Marks JM, Ricke WA. Benign prostatic hyperplasia and lower urinary tract symptoms: what is the role and significance of inflammation? Curr Urol Rep. (2019) 20(9):54. doi: 10.1007/s11934-019-0917-1
8. Dai H, Yu ZH, Guo YC, Yu K. Efficacy and safety of HoLEP, TUERP and TURP in the treatment of elderly patients with BPH. Chin J Gerontol. (2022) 42(08):1902–6. doi: 10.3969/j.issn.1005-9202.2022.08.035
9. Nickel JC. Alpha-blockers for treatment of the prostatitis syndromes. Rev Urol. (2005) 7(Suppl 8):S18–25. PMID: 16985886; PMCID: PMC147763616985886
10. Zhang YM, Fan WL, Lin GT, Lin YP, Chen R. The role of chronic prostatitis in the pathogenesis and progression of prostatic hyperplasia. Int J Urol Nephrol. (2016) 36(04):542–4. doi: 10.3760/cma.j.issn.1673-4416.2016.04.020
11. Tang LK, Qu WL, Tian F, Wang ZL, Song L, Yu ZW, et al. Correlation between benign prostatic hyperplasia and chronic prostatitis. Chin J Urol. (2009) 02:124–6.v
12. Nickel JC, Roehrborn CG, O'Leary MP, Bostwick DG, Somerville MC, Rittmaster RS. The relationship between prostate inflammation and lower urinary tract symptoms: examination of baseline data from the REDUCE trial. Eur Urol. (2008) 54(6):1379–84. doi: 10.1016/j.eururo.2007.11.026
13. Cao DH, Liu LR, Li L, Fang Y, Tang P, Li T, et al. Pathological characteristics of CD40/CD40l and NF-κB proteins related to inflammation in benign prostatic hyperplasia tissue: a retrospective study of 120 cases. Int J Clin Exp Pathol. (2017) 10(11):10863–72. PMID: 31966429; PMCID: PMC696582831966429
14. Li J, Li Y, Cao D, Huang Y, Peng L, Meng C, et al. The association between histological prostatitis and benign prostatic hyperplasia: a single-center retrospective study. Aging Male. (2022) 25(1):88–93. doi: 10.1080/13685538.2022.2050360
15. Chang DG, Li GS, Zhang PH, Wu TL, Mei XF, Cao J, et al. Clinical features of benign prostatic hyperplasia complicated by chronic prostatitis. Natl J Androl. (2010) 16(09):830–3. doi: 10.13263/j.cnki.nja.2010.09.013
16. Du GW, Xiong J, Chen Z, Chen HB, Que H, Zhen J, et al. Clinical features and postoperative complications of patients with benign prostatic hyperplasia complicated with histological prostatitis. J Mod Urol. (2020) 25(07):596–600. doi: 10.3969/j.issn.1009-8291.2020.07.006
17. Long Z, He LY, Zhong KB, Zhang YC, Yue D. Clinical analysis of benign prostate hyperplasia with prostatitis. J Cent South Univ. (2010) 35(04):381–5. doi: 10.3969/j.issn.1672-7347.2010.04.018
18. Gerstenbluth RE, Seftel AD, MacLennan GT, Rao RN, Corty EW, Ferguson K, et al. Distribution of chronic prostatitis in radical prostatectomy specimens with up-regulation of bcl-2 in areas of inflammation. J Urol. (2002) 167(5):2267–70. doi: 10.1016/S0022-5347(05)65140-3
19. De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schröder F, et al. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. (2011) 60(1):106–17. doi: 10.1016/j.eururo.2011.03.055
20. Zhang F, Si-mu-jiang-abula A, Zhang LD. Influence of histological prostatitis on the clinical features of benign prostatic hyperplasia and prostate cancer. Natl J Androl. (2014) 20(04):354–8. doi: 10.13263/j.cnki.nja.2014.04.013
21. Battikhi MN, Hussein I. Age-specific reference ranges for prostate specific antigen-total and free in patients with prostatitis symptoms and patients at risk. Int Urol Nephrol. (2006) 38(3-4):559–64. doi: 10.1007/s11255-006-0073-7
22. Irani J, Levillain P, Goujon JM, Bon D, Doré B, Aubert J. Inflammation in benign prostatic hyperplasia: correlation with prostate specific antigen value. J Urol. (1997) 157(4):1301–3. doi: 10.1016/S0022-5347(01)64957-7
23. Anim JT, Udo C, John B. Characterisation of inflammatory cells in benign prostatic hyperplasia. Acta Histochem. (1998) 100(4):439–49. doi: 10.1016/S0065-1281(98)80040-8
24. Barry MJ, Fowler FJ Jr, O'Leary MP, Bruskewitz RC, Holtgrewe HL, Mebust WK, et al. The American urological association symptom index for benign prostatic hyperplasia. The measurement committee of the American urological association. J Urol. (1992) 148(5):1549–57; discussion 64. doi: 10.1016/S0022-5347(17)36966-5
25. Mazzariol O J, Reis LO, Palma PR. Correlation of tools for objective evaluation of infravesical obstruction of men with lower urinary tract symptoms. Int Braz J Urol. (2019) 45(4):775–81. doi: 10.1590/s1677-5538.ibju.2018.0706
26. Penna G, Fibbi B, Amuchastegui S, Cossetti C, Aquilano F, Laverny G, et al. Human benign prostatic hyperplasia stromal cells as inducers and targets of chronic immuno-mediated inflammation. J Immunol. (2009) 182(7):4056–64. doi: 10.4049/jimmunol.0801875
27. Meng Y, Yu W, Liu Z, Zhang M, Chen Y, Li S, et al. The inflammation patterns of different inflammatory cells in histological structures of hyperplasic prostatic tissues. Transl Androl Urol. (2020) 9(4):1639–49. doi: 10.21037/tau-20-448
28. Devlin CM, Simms MS, Maitland NJ. Benign prostatic hyperplasia - what do we know? BJU Int. (2021) 127(4):389–99. doi: 10.1111/bju.15229
29. Huang XH, Tan B, Liang YW, Wu QG, Li CZ, Wei GS, et al. LUTS in BPH patients with histological prostatitis before and after transurethral resection of the prostate. Natl J Androl. (2013) 19(01):35–9. doi: 10.13263/j.cnki.nja.2013.01.010
Keywords: benign prostatic hyperplasia, prostatic inflammation, HoLEP, clinical efficacy, prospective research
Citation: Zhou W, Mao D, Li L, Liu G, Gao G, Li H and Gao D (2023) Clinical analysis of transurethral holmium laser enucleation in the treatment of benign prostatic hyperplasia with prostatic inflammation: A prospective research study. Front. Surg. 9:1026657. doi: 10.3389/fsurg.2022.1026657
Received: 24 August 2022; Accepted: 7 November 2022;
Published: 6 January 2023.
Edited by:
Afonso Morgado, Centro Hospitalar Universitário de São João (CHUSJ), PortugalReviewed by:
Nicolo' Schifano, San Raffaele Hospital (IRCCS), ItalyMargarida Manso, São João University Hospital Center, Portugal
© 2023 Zhou, Mao, Li, Liu, Gao, Li and Gao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dianjun Gao sdwfgdj@163.com
Specialty Section: This article was submitted to Genitourinary Surgery, a section of the journal Frontiers in Surgery
 Weijian Zhou
Weijian Zhou Dongdong Mao2
Dongdong Mao2