Changes in membrane fatty acids of a halo-psychrophile exposed to magnesium perchlorate and low temperatures: Implications for Mars habitability
- 1Centro de Astrobiología (CAB, CSIC-INTA), Torrejón de Ardoz, Madrid, Spain
- 2IMDEA Water Institute, Alcalá de Henares, Madrid, Spain
- 3Department of Astronomy, Cornell University, Ithaca, NY, United States
The presence of perchlorate salts in aqueous solutions bears two opposite effects on habitability. On the one hand, perchlorate salts trigger a decrease in the freezing point of the aqueous solutions, resulting in stable aqueous solutions at subzero temperatures, thereby widening the habitable conditions for potential microbial life. On the other hand, the presence of perchlorates in solution imposes a significant osmotic stress that compromises the integrity of microbial cell membranes, thereby restricting the habitable conditions in the same aqueous environment. Here we investigated the survivability and the changes in the composition of membrane fatty acids (FAs) of the bacterium Rhodococcus sp. JG-3 cells under warm (20°C), cold (4°C), and subzero temperatures (−10°C and −16°C), and in the presence (8 wt% and 16 wt%) and absence of magnesium perchlorate (Mg(ClO4)2). Bacterial cell survivability decreased with decreasing temperature and presence of magnesium perchlorate. However, Rhodococcus sp. JG-3 was able to tolerate up to 8 wt% Mg(ClO4)2 at −16°C. The presence of magnesium perchlorate in the medium decreased the concentration of total FAs, likely due to a destabilization of the molecules by the chaotropic effect of the perchlorate anion. At the maximum stress (both subzero temperatures and 16 wt% magnesium perchlorate), the composition of FAs changed, i.e., Rhodococcus sp. JG-3 cells increased the relative abundance of saturated FAs (SFAs) over the unsaturated (UFAs) or branched (BFAs). These changes in the proportion of FAs types may be a physiological response during cooling, aimed to improve lipid membrane stability. Interestingly, the composition and relative abundance of fatty acid types (i.e., SFAs, UFAs and BFAs) of Rhodococcus sp. JG-3 when simultaneously exposed to subzero temperatures and 16 wt% magnesium perchlorate was similar to that following freezing stress alone, suggesting that either both conditions triggered a similar response or that one response dominated over the other. Our findings contribute to understand the survivability and adaptation of extremophilic microorganisms under polyextreme conditions, such as those existing in the Martian subsurface today and/or in the past, which include the documented presence of magnesium perchlorate salts in ancient sediments and global cold temperatures.
Introduction
Studying the adaptability of microorganisms (as the simplest life forms on Earth) to extreme environmental conditions is key for a comprehensive understanding of the emergence of life, its limits and evolution in our planet and beyond. A better knowledge of the molecules involved in the processes of microbial adaptation and survival to different environmental conditions would provide valuable information for the search for traces of life on other planetary bodies, with special implications for Mars.
The identification of highly saline intervals (deposits of different salts from episodes of overflow and drying) in the Gale crater lakes (Rapin et al., 2019; Thomas et al., 2019) supports the hypothesis of a ‘‘cold and wet’’ early Mars (Fairén, 2010; Fairén, 2020), in which the stability of liquid water could be linked to a variable enrichment of dissolved salts in cold brines. In these brines, the presence of perchlorate salts is of particular relevance and has significant astrobiological implications (Soudi et al., 2017; Garcia-Descalzo et al., 2020). The main perchlorate parent salt on Mars is still to be determined (Kounaves et al., 2014), but the perchlorate anion (ClO4−) has been found or inferred at >0.5 wt% in multiple locations, including the Mars Phoenix lander site near the north pole (Hecht et al., 2009), in soil samples at Gale Crater (Glavin et al., 2013) and Jezero Crater (Scheller et al., 2022), and arguably at recurring slope lineae locations (Ojha et al., 2015).
The eutectic point of the magnesium perchlorate in solution occurs at −57°C (Stillman and Grimm, 2011), and its hexahydrate solid-phase [Mg(ClO4)2:6H2O] deliquesces when exposed to conditions above 40% relative humidity (aw = 0.4) (Besley and Bottomley, 1969; Robertson and Bish, 2011). These properties enable the formation of films of transient brines that can remain stable at very low temperatures, even at current Mars surface conditions (Chevrier et al., 2009; Fairén et al., 2009; Robertson and Bish, 2011), which therefore could represent an opportunity for microbial life. In this scenario, potential microbial life forms would have needed to cope with both cold conditions and salinity at the same time, in addition to the perchlorate chaotropicity. For instance, previous laboratory experiments carried out with the bacterium Rhodococcus sp. JG-3 and the yeast Debaryomyces hansenii showed tolerance under low temperatures and perchlorate concentrations similar or higher to those detected on Mars (Garcia-Descalzo et al., 2020; Heinz et al., 2021).
The stressful conditions of combined cold temperatures and salinity challenge the fluidity of the cell membrane by affecting the conformation and arrangement of membrane phospholipids (Turk et al., 2004; Cray et al., 2015; Bajerski et al., 2017). The microbial membrane is a permeable natural barrier of the cell that controls and regulates the transport of nutrients and other substances and solutes between the cell and the environment. Its main structure consists of a bilayer of phospholipids with polar head-groups facing the outer sides, and fatty acid (FA) acyl-chains in the interior. Keeping its integrity and functionality is crucial to maintain microbial survival. At cold temperatures, the conformation of the lipid membrane changes from the crystalline liquid phase into the rigid gel phase (crossing the transition midpoint—Tm), affecting its fluidity (Siliakus et al., 2017). When this phase transition occurs, membrane lipids turn into a more rigid conformation in which hydrocarbon chains form an ordered package perpendicular to the plane of the bilayer (Eze, 1991).
Apart from cold temperatures, the fluidity of the membrane also changes with high concentration of salts (Imhoff and Thiemann, 1991; Turk et al., 2004) and chaotropic agents (Cray et al., 2015). Perchlorate salts are especially detrimental for cell survival since they challenge the osmotic balance of cells and causes destabilization of biomacromolecules due to the chaotropic effect of the perchlorate anion (Heinz et al., 2021). As a result, perchlorate salts affect metabolic pathways crucial for the survival of microorganisms. Often, microorganisms employ the same adaptive mechanisms to adjust to conditions of cold and salt stress, like an increase in the synthesis of osmotically compatible solutes, such as trehalose (De Maayer et al., 2014; Cray et al., 2015), or changes in membrane lipids (Taha et al., 2013) by variations in the conformation of the fatty acyl chains (Russell, 1984).
Bacteria that grow below their optimal temperature perform several cellular modifications to decrease the Tm of the lipid membrane and therefore keep its fluidity by arranging a more disordered gel phase or preventing the gel phase formation (Siliakus et al., 2017). The rearrangement and composition of the bacterial membrane due to changes in environmental conditions is known as homeoviscous or homeophasic adaptation (Sinensky, 1974). Overall, this rearrangement entails variations in the composition and level of saturation/unsaturation of membrane FAs, the length of acyl-chains, their branching, or the iso and ante-iso ratio (Taha et al., 2013; Yoon et al., 2015; Siliakus et al., 2017; Willdigg and Helmann, 2021). These variations could differ from one bacterium to another depending, for instance, on the Gram type (Kaneda, 1991; Weber and Marahiel, 2002; Yoon et al., 2015). Studying the responses of microorganisms facing simultaneous stress conditions (e.g., low temperatures and salinity), is key to better understand the potential habitability of cold and salty environments on Earth and other planetary bodies, like Mars or the icy moons of the outer Solar System.
Here, we analyzed the physiological responses of the halotolerant and eurypsychrophile Rhodococcus sp. JG-3 (Goordial et al., 2015a; Goordial et al., 2015b) under polyextreme environmental conditions: cold and subzero temperatures, and high salinity and chaotropicity using different magnesium perchlorate concentrations. Specifically, we studied the limits of survival of the bacterium Rhodococcus sp. JG-3, and characterized the modifications in its FA composition and relative abundance under different low temperatures and magnesium perchlorate concentrations.
Materials and methods
Strain and culture conditions
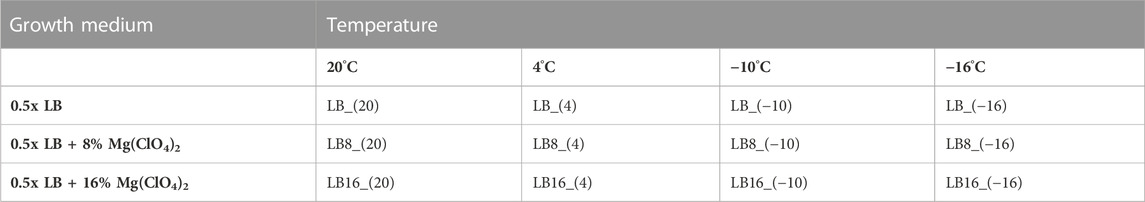
The bacterium Rhodococcus sp. JG-3, originally isolated from permafrost in the Antarctic Dry Valleys, is a Gram-positive eurypsycrophile and halotolerant bacterium (Goordial et al., 2015a; Goordial et al., 2015b). In order to investigate the survivability and variations in FAs composition of this bacterium, triplicate cultures (12 mL) were incubated in 25 mL flasks during 10 days under different temperature and medium conditions (Table 1): i) 0.5x LB (Lysogeny Broth) medium incubated at 20°C (optimum), 4°C, −10°C and −16°C; ii) 0.5x LB with 8 wt% Mg(ClO4)2 incubated at the same four temperatures; and iii) 0.5x LB with 16 wt% Mg(ClO4)2 incubated at the same four temperatures. We used 0.5x LB medium to reduce the concentration of medium salts to better understand the effect of the magnesium perchlorate on the bacterium cells, while providing sufficient nutrients for growth. The magnesium perchlorate concentrations used here were 1.5 and 2.5 times lower than those used in García-Descalzo et al., 2020, since in that study we reported a low relative cell survival rate (%) in LB medium with 20 wt% magnesium perchlorate. Antifreezes were not used here because we were precisely testing the antifreeze capacity of the magnesium perchlorate. All bacterial cultures started from a pellet corresponding to 4 mL of a preinoculum at the late exponential phase (optical density (OD) of ∼0.5 measured at 600 nm) in LB at 20°C (optimal temperature of Rhodococcus sp. JG-3).
Survivability tests
To test the survivability of bacterial cells, OD measured at 600 nm was recorded at the beginning (day 0), middle (day 5) and at the end (day 10) of the incubation period. In addition, colony counts, expressed as colony-forming units per millilitre (CFU/mL), were performed adding an aliquot of previous experiments on LB plates incubated at 20°C to test the viability of the cells at the beginning and at the end of the treatments. A negative control with LB medium and without bacteria was included in both liquid and solid media experiments at 20°C.
Extraction, fractionation and analysis of fatty acids
To compare the FA composition among the samples exposed to the different treatments, we selected the bacterial cultures treated with the highest concentration of perchlorates (16 wt%), as well as those with the lowest temperatures (−10 and −16°C), as these were the most extreme conditions to compare with the control (LB without perchlorates incubated at 20°C). We wanted to test whether the bacterium could have modified its FA composition and concentration during cooling and the presence of 16 wt% magnesium perchlorate during incubation.
FAs extractions were performed in triplicate (i.e., each bacterial replicate of each treatment). The sample incubated in LB without perchlorate at 20°C (LB_(20)) was used as a control. At the end of the incubation time (10 days), the whole volume of each culture was collected, centrifuged at 13,200 rpm for 10 min, and supernatants were discarded. Then, the pellets were washed once in PBS and centrifuged again in the same conditions to remove any trace of LB medium. Finally, the pellets were lyophilized overnight prior to FA extraction.
FAs from the lyophilized bacterial samples (ca. 0.1–0.2 g, which may also include traces of the PBS used in the washing step) were extracted with a mixture of dichloromethane and methanol (DCM:MeOH, 3:1, v/v) by ultrasound sonication (3 × 10 min cycles, 37 kHz) (Elma, Elmasonic P, Germany). The concentrated total lipid extracts were digested overnight at room temperature in a mixture of methanolic potassium (6% KOH w/w), and then separated into neutral and acidic fractions with n-hexane following a protocol previously described (Grimalt et al., 1992; Carrizo et al., 2019). Afterward, the acidic fraction was analyzed by gas chromatography-mass spectrometry (GC-MS) after derivatization with BF3 in MeOH to form fatty acid methyl esters (FAMEs). The GC-MS analysis was performed using a 6850 GC system coupled to a 5975 VL MSD with a triple-axis detector (Agilent Technologies, United States), operating with electron ionization at 70 eV and scanning from m/z 50 to 650. Analytical details are described in Carrizo et al. (2019). Compounds were identified based on the comparison of mass spectra with reference materials, while their quantifications were obtained using an external calibration curve of FAMEs (from C8 to C24). All chemicals and standards were supplied by Sigma Aldrich (San Luis, Missouri, United States). Quantification of FAMEs was normalized by dry weight (mg · g−1 dw), and classified in saturated (SFA), unsaturated (UFA) and branched (BFA) fatty acids. In addition, the relative abundance (%) of each fatty acid type per sample was calculated.
Statistical analysis
The one-way non-parametric Kruskal-Wallis test was used to determine whether there were statistically significant differences between samples based on the concentration (mg · g−1 dw) and relative abundance (%) of FAs types (SFA, UFA, and BFA) using temperature and culture media as independent variables. In our experiments, temperature is a factor with three levels (20°C, −10°C and −16°C) and culture media is a factor with two levels (with and without 16 wt% magnesium perchlorate). These analyses were performed with the Kruskal test function in R 4.1.2 (R Core Team, 2022). ANOVA tests were not performed due to the lack of normality (Shapiro Wilks tests) in the distribution of the data. Moreover, a Principal Coordinate Analysis (PCoA) based on the Bray-Curtis dissimilarity matrix was performed using the software CANOCO5 v.5.12 (Microcomputer Power, Ithaca, NY) to evaluate the similarities between the samples (i.e., LB_(20), LB_(−10), LB_(−16), LB16_(20), LB16_(−10) and LB16_(−16)) based on the relative abundance (%) of the three FA types (SFA, UFA, and BFA).
Results
Survivability of Rhodococcus sp. JG-3 under different temperature and magnesium perchlorate concentrations
The experiments performed with the different treatments varied from liquid, to semi-solid (like a slush, with more or less liquid) or to a solid state (at −16°C) after the 10 days of incubation. As expected, bacterial growth (measured as an increase in O.D.600 nm) was only observed at 20°C and 4°C in LB medium without the perchlorate salt (Figure 1). For the rest of the experiments (i.e., LB medium without magnesium perchlorate at −10°C and −16°C, or in the presence of 8 wt% or 16 wt% magnesium perchlorate at all temperatures), Rhodococcus sp. JG-3 did not show growth.
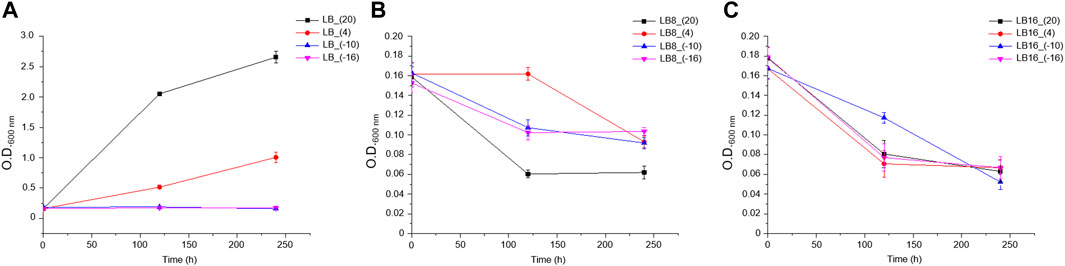
FIGURE 1. Rhodococcus sp. JG-3 growth curves measured by optical density (600 nm) in (A) 0.5x LB medium, (B) 0.5x LB + 8% Mg(ClO4)2, and (C) 0.5x LB + 16% Mg(ClO4)2, at 20°C, 4°C, −10°C and −16°C. Points are means and error bars indicate standard error of triplicates. Please, note the different scales in the Y-axis.
To evaluate the viability of the bacterium after the incubation under each condition, CFU/mL were measured at the beginning (day 0) and the end of the incubation time (day 10) (Figure 2). The ability of the bacterial cells to recover (i.e., divide and form colonies on solid medium) after the incubation period, was observed for all bacterial cultures either above or below 0°C in LB medium without the magnesium perchlorate or with the perchlorate salt up to 8 wt%. Therefore, our most interesting result is that Rhodococcus sp. JG-3 was able to tolerate incubation in LB medium with 8 wt% magnesium perchlorate down to −16°C for 10 days (CFU/mL at the end of the experiments were similar to those at the beginning). In contrast, bacterial cultures incubated with 16 wt% Mg(ClO4)2 showed a lack of colony formation after the incubation period at any temperature, indicating that the presence of 16 wt% magnesium perchlorate in the medium caused an irreparable cell death.
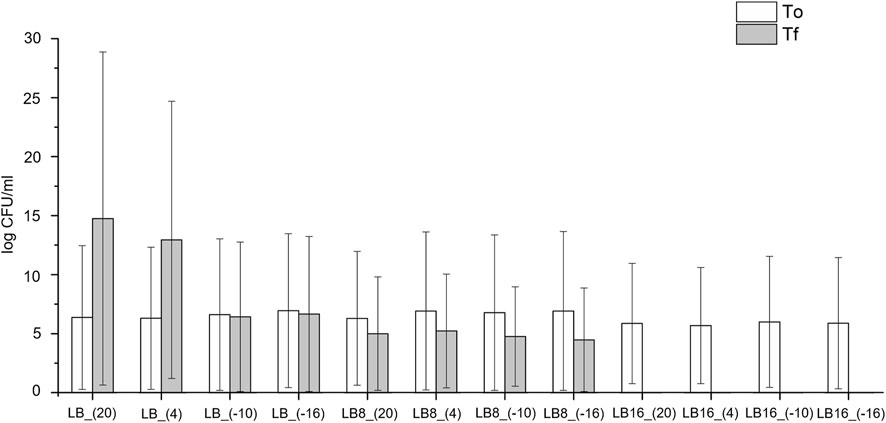
FIGURE 2. Viable Rhodococcus sp. JG-3 cells expressed as colony forming units (CFU)/mL that grew on LB medium plates at the beginning (day 0) and at the end (day 10) of the treatments: 0.5x LB (sample labels preceded by LB), 0.5x LB + 8% Mg(ClO4)2 (sample labels preceded by LB8), and 0.5x LB + 16% Mg(ClO4)2 (sample labels preceded by LB16), incubated at 20°C, 4°C, −10°C and −16°C. Bars indicate means and error bars indicate standard errors of triplicates.
Fatty acids profile of Rhodococcus sp. JG-3 at different temperatures and in the presence/absence of magnesium perchlorate
The composition of fatty acids analysed as methyl esters (FAMEs) of Rhodococcus sp. JG-3 cells incubated at 20°C, −10°C and −16°C, with (16 wt%) and without magnesium perchlorate, were measured by GC-MS and are listed in Table 2. We used the 16 wt% Mg(ClO4)2 to induce the maximum possible stress to the bacterium and therefore obtain clearer results when comparing with the control, despite the previously described lack of colony formation after the incubation period at 16 wt% magnesium perchlorate.
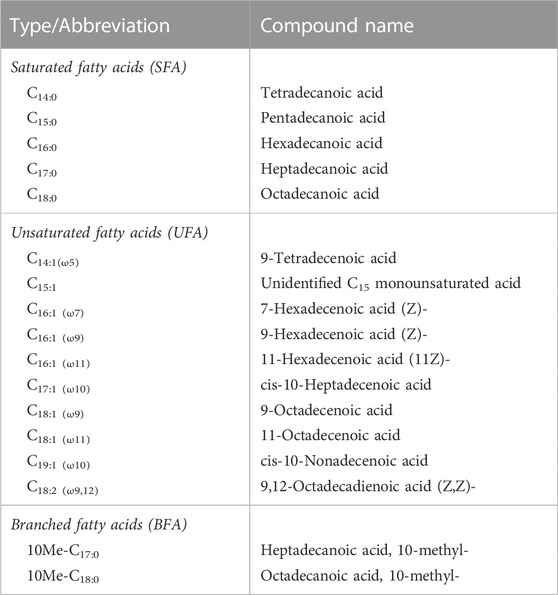
TABLE 2. Fatty acids (FAs) identified in Rhodococcus sp. JG-3 cultures incubated in 0.5x LB, and in 0.5x LB + 16 wt% Mg(ClO4)2, at 20°C, −10°C and −16°C.
The concentration of total FAs (mg · g−1 dw) in Rhodococcus sp. JG-3 cells were higher when the strain was incubated in LB medium without magnesium perchlorate than in the presence of 16 wt% of the perchlorate salt, regardless of the temperature (Figure 3A). The FA type present in the highest concentration in all treatments was the SFA (up to 28 mg · g−1 dw), except in the LB medium at 20°C, whose highest concentration was the UFA (up to 8 mg · g−1 dw). The next highest concentration of FAs in all treatments was the UFA (up to 11 mg · g−1 dw) and, finally, the BFA (up to 2 mg · g−1 dw) (Figure 3B). The concentration of SFAs, UFAs and BFAs in Rhodococcus sp. JG-3 cells incubated in the presence of 16 wt% of magnesium perchlorate were lower compared to those incubated in the absence of the perchlorate salt at any temperature, except for the SFAs at 20°C (LB16_(20)). The influence of magnesium perchlorate on the concentration of the three FA types was statistically supported by Kruskal-Wallis tests (SFAs p-value = 0.00016, UFAs p-value = 0.00698 and BFAs p-value = 0.03853). In contrast, the influence of temperature on the concentration of the three FAs types, either in the presence or absence of the perchlorate salt, was not statistically supported (p-values > 0.05).
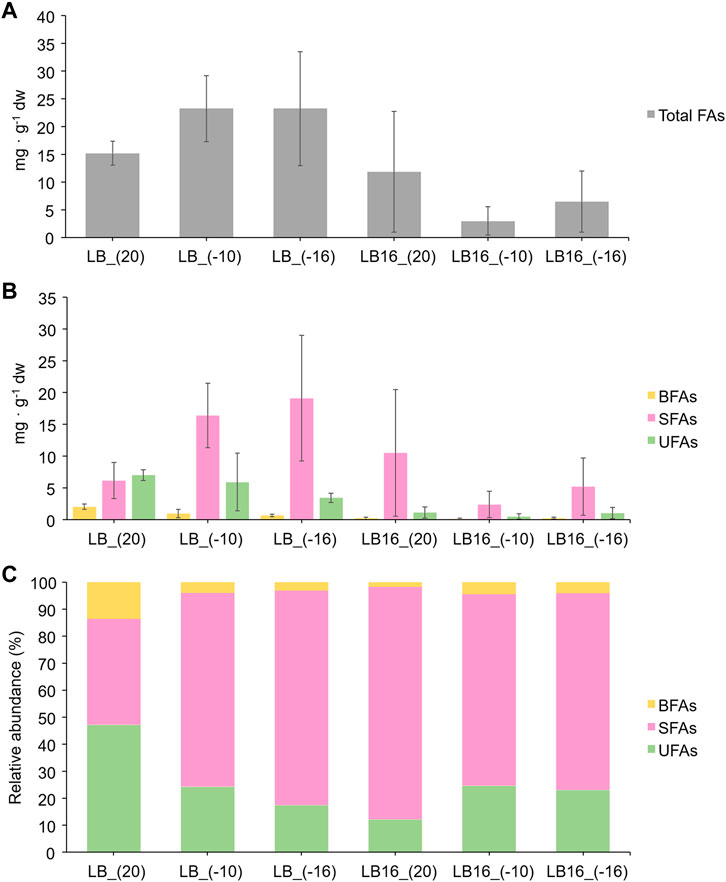
FIGURE 3. Fatty acids (FAs) concentration of Rhodococcus sp. JG-3 cells incubated in 0.5x LB (sample labels preceded by LB), and in 0.5x LB + 16 wt% Mg(ClO4)2 (sample labels preceded by LB16) at 20°C, −10°C and −16°C. In (A) total FAs concentration (mg · g−1 dw), in (B) FAs concentration (mg · g−1 dw) per type (saturated or SFA, unsaturated or UFA, and branched or BFA), and in (C) relative abundance (%) of FAs types (SFA, UFA and BFA). Bars indicate means and error bars indicate standard deviation of triplicates.
To analyse the variation in the proportion of FA types among samples, the concentration of SFAs, UFAs and BFAs was relativized per sample (%) (Figure 3C). Rhodococcus sp. JG-3 cells incubated at 20°C in LB medium without magnesium perchlorate (LB_(20), used as a control sample) showed higher proportion of UFAs (47% ± 11%) than SFAs (39% ± 15%) or BFAs (14% ± 4%). In contrast, the rest of the samples (i.e., treated at subzero temperatures and/or in the presence of 16 wt% magnesium perchlorate) showed higher proportion of SFAs (71%–86%), followed by UFAs (12%–25%) and BFAs (2%–4%). The Kruskal-Wallis test did not show a statistically significant difference between % FA types as a function of temperature or medium (SFA, UFA and BFA p-values < 0.05). Therefore, neither temperature nor presence/absence of 16% wt Mg(ClO4)2 in the medium had a significative impact on the proportion of FA types in Rhodococcus sp. JG-3 cells. Still, a trend in increasing the relative abundance of SFAs with decreasing temperature or in the presence of magnesium perchlorate in the medium was observed. In addition, an increase in the relative abundance of BFAs and UFAs in Rhodococcus sp. JG-3 cells incubated at subzero temperatures with 16 wt% magnesium perchlorate as compared to that at 20°C was also observed. To confirm these trends, however, a larger number of replicates would be necessary to reduce the uncertainties in FAs concentrations.
The lipid compounds of SFAs, UFAs and BFAs in Rhodococcus sp. JG-3 cells at each treatment are shown in Figure 4. The main compounds of SFAs in Rhodococcus sp. JG-3 cells in all sample treatments were C16:0 (24%–43%) and C18:0 (10%–45%), those of UFAs were C18:1(ω9) (8%–29%) and C16:1(ω11) (2%–13%), and that of BFAs was 10Me-C18:0 (2%–13%) (Figure 4). However, this composition of FA types varied between samples. For instance, the compound present in the highest proportion at subzero temperatures and/or in the presence of magnesium perchlorate was C18:0, especially when compared with the control (LB_(20)). In contrast, C16:1(ω11), C18:1(ω9) and 10Me-C18:0 decreased in relative abundance at subzero temperatures or in the presence of magnesium perchlorate compared to the LB_(20) control. As a minor change, these three compounds increased in relative abundance in the presence of magnesium perchlorate at subzero temperatures compared to the sample at 20°C (LB16_(20)). Overall, the samples at subzero temperatures, with or without magnesium perchlorate, were more similar to each other than with the sample in LB medium at 20°C (LB_(20)). These similarities in the composition of FAs of Rhodococcus sp. JG-3 cells at subzero temperatures were also reflected in the PCoA analysis (Figure 5). In the PCoA, samples at subzero temperatures were clustered together in the ordination plot, whereas samples at 20°C (i.e., LB_(20) and LB16_(20)) were located far away in the graph. In addition, LB_(20) and LB16_(20) were separated from each other, due to their differences in the relative abundances of FA types.
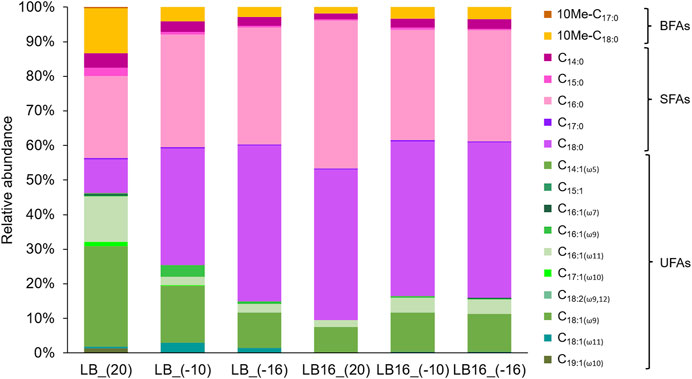
FIGURE 4. Relative abundance (%) of saturated (SFAs), unsaturated (UFAs), and branched (BFAs) compounds of Rhodococcus sp. JG-3 cells incubated in 0.5x LB (sample labels preceded by LB), and in 0.5x LB + 16 wt% Mg(ClO4)2 (sample labels preceded by LB16), at 20°C, −10°C and −16°C.
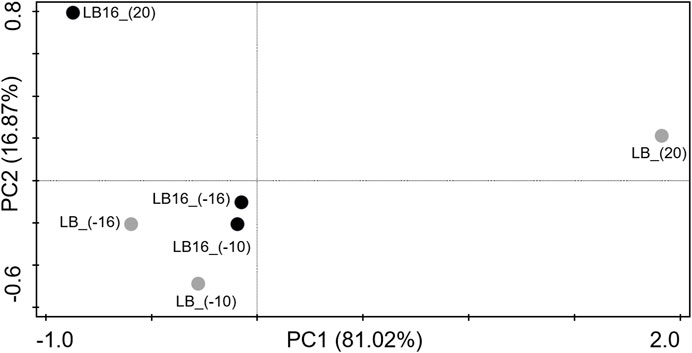
FIGURE 5. Principal Coordinate Analysis (PCoA) based on Bray-Curtis dissimilarity matrix using the relative abundances of the saturated (SFAs), unsaturated (UFAs) and branched (BFAs) fatty acids from Rhodococcus sp. JG-3 cells incubated in 0.5x LB (black dots, sample labels preceded by LB), and in 0.5x LB + 16 wt% Mg(ClO4)2 (grey dots, sampled preceded by LB16) at 20°C, −10°C and −16°C.
Discussion
The lowest limit of temperature for life remains unsettled, as new records of new species are described as research advances. It is known that most microorganisms could remain alive for a long time at −80°C (cryopreservation), although inactive in preservative conditions. Then, they are able to recover afterwards when optimal conditions are restored (Feller, 2017). Rhodococcus sp. JG-3 is able to grow in a temperature range from 30°C to −5°C (optimal at ∼20°C), keeping a minimum metabolism used for maintenance of cells down to −15°C (Goordial et al., 2015a, Goordial et al., 2015b). According to our results, and in agreement with its original description, the bacterium Rhodococcus sp. JG-3 grew well in a general culture medium like LB at cold temperatures (4°C). However, the bacterium showed a lack of growth when incubated at subzero temperatures, i.e., at −10°C and −16°C for 10 days (Figure 1), although it was still viable at such temperatures (Figure 2), possibly because freezing left the cells to a dormant state.
Rhodococcus sp. JG-3 is also described as a halotolerant microorganism able to tolerate 7 wt% NaCl (Goordial et al., 2015a, Goordial et al., 2015b). Our results showed that this bacterium could also tolerate temporarily a medium containing 8 wt% of the chaotropic magnesium perchlorate salt at warm (20°C), cold (4°C) and subzero (−10 and −16°C) temperatures (Figure 2). Incubation times longer than 10 days would be necessary to test if bacterial survival can be maintained for a longer period of time. Other halotolerant microorganisms exhibited tolerance to perchlorate and non-perchlorate salty solutions at different concentrations. Several bacteria isolated from Big Soda Lake showed resistance to solutions up to 20 wt% NaCl, and up to 2 wt% Mg(ClO4)2 at 37°C (Matsubara et al., 2017). The bacterium Planococcus halocryophilus showed tolerance up to 12 wt% NaClO4 at 25°C, and up to 7 wt% at 4°C (Heinz et al., 2019). The bacterium Halomonas venusta (HL12) has been reported to resist 10 wt% Mg(ClO4)2 at room temperature (Soudi et al., 2017) and the archaea Halorubrum lacusprofundi showed growth up to 18.5 wt% Mg(ClO4)2 at 37°C (Laye and DasSarma, 2018). Moreover, the yeast Debaryomyces hansenii exhibited resistance up to 23 wt% NaClO4 at 25°C (Heinz et al., 2020). Therefore, the viability of Rhodococcus sp. JG-3 in medium with 8 wt% Mg(ClO4)2 at subzero temperatures (−10 and −16°C) in this study represents a milestone in the tolerance of a bacterium in the presence of a relatively high concentration of magnesium perchlorate below zero. In contrast, an increase of magnesium perchlorate to 16 wt% resulted in an absence of bacterium growth on LB plates, likely due to the osmotic, and mainly chaotropic stress caused by perchlorates.
In this study, we found that Rhodococcus sp. JG-3 cells did not survive when 16 wt% magnesium perchlorate was present. However, this bacterium showed, at least temporarily, high tolerance after exposure to a highly stressful environment, i.e., subzero temperatures and 8 wt% Mg(ClO4)2. The capability of Rhodococcus sp. JG-3 cells to recover an active metabolism after its exposure to cold-brine conditions down to −16°C supports the temperature tolerance range of this bacterium described so far by −15°C (Goordial et al., 2015b). Similarly, Pseudomonas putida GR12 was also able to recover after incubations at −20°C and −50°C for 24 h when optimal conditions were restored (Sun et al., 1995). Therefore, the resistance of the bacterium Rhodococcus sp. JG-3 against a combination of freezing and salinity conditions supports the possibility of a microbial life in cold-brine environments on Mars (either extinct or extant), especially if temperature oscillations could provide time intervals for microorganisms to recover their metabolism (Nelson and Parkinson, 1978).
As a consequence of the stress to temperature and salinity conditions in Rhodococcus sp. JG-3, we observed a variation in the composition of different types of fatty acids (i.e., saturated, unsaturated and branched fatty acids). Rhodococcus sp. JG-3 cells showed significant differences in the concentration (mg · g−1 dw) of total FAs between the set of cultures incubated with and without magnesium perchlorate in the medium (Figure 3A). The low concentration of FAs in samples with the perchlorate salt may be related to the strong chaotropic effect of the perchlorate anion, and even the magnesium cation (Hallsworth et al., 2007; dC Rubin et al., 2017), which could have destabilized FAs during the incubation time. This chaotropicity drives to a structural disorder of macromolecules, further affecting the membranes (Cray et al., 2015) by abnormally increasing their permeability. Modifications of FAs are important for regulating membrane fluidity in a process known as homeoviscous adaptation (Willdigg and Helmann, 2021). For this reason, an increase in saturated fatty acids (i.e., SFAs) and decrease in unsaturated and branched chains (i.e., BFAs), would allow to restore certain rigidity and minimize osmotic stress, as we observed with Rhodococcus sp. JG-3 cells at subzero temperatures and in the presence of magnesium perchlorate (Figures 3B, C).
To the best of our knowledge, no consensus exists on the impact of both low temperature and presence of magnesium perchlorate on the modifications of the FA content/composition in bacteria, and it has been shown to depend on the specific microorganism studied and the precise culture conditions used, for instance, the Gram type (Kaneda, 1991; Weber and Marahiel, 2002; Yoon et al., 2015), species (Siliakus et al., 2017) or temperature (Hassan et al., 2020). Despite this, studies have shown that FA adaptations to low temperatures and osmotic stress generally increase the content of BFAs and UFAs to avoid membrane damage and rigidity (Taha et al., 2013; Yoon et al., 2015; Hassan et al., 2020). Microorganisms living in cold environments usually keep high levels of UFAs to maintain membrane fluidity by avoiding an excessive packing, so the SFA/UFA ratio is low. For instance, psychrotrophic or psychrophilic bacteria, such as those inhabiting most Antarctic environments, possess an elevated content of UFAs (Gounot, 1986; Garba et al., 2016; Hassan et al., 2020). These modifications allow for a less compact and rigid conformation of the membrane, and thus preserve the transmembrane transport function (Yoon et al., 2015). In contrast to this trend, Mykytczuk et al. (2013) found that cytoplasmatic membranes from Planococcus halocryophilus Or1 were remodelled favouring higher ratio of saturated over branched FAs when incubated at −15°C. In our study, we observed that the SFA/UFA ratio of Rhodococcus sp. JG-3 cells was higher when the bacterium was incubated at subzero temperatures than at 20°C in media without magnesium perchlorate (Figure 3C). Therefore, our results supported those found by Mykytczuk et al. (2013). Moreover, in the presence of magnesium perchlorate, both BFAs and UFAs from Rhodococcus sp. JG-3 cells slightly increased in proportion, although SFA/UFA or SFA/BFA ratios were still higher than that of the control sample (LB (20)). We propose that the chaotropicity of the perchlorate anion (Heinz et al., 2021), and even the magnesium cation (Hallsworth et al., 2007; dC Rubin et al., 2017), may have contributed to partially explain the differences between SFA, UFA and BFA in the samples with magnesium perchlorate relative to the control (LB_(20)).
The similarities in the relative abundance of FAs types in the presence or absence of magnesium perchlorate at subzero temperatures could have different explanations. On the one hand, it may be explained by a buffering effect of osmotically compatible solutes that could be influencing the composition of FAs. This could be the case of trehalose, which has a kosmotropic role (Cray et al., 2015), but also confers adaptive responses to microorganisms against temperature stress (hot and cold), and other stressing conditions like high osmolarity or oxidation (Kandror et al., 2002; Reina-Bueno et al., 2012). On the other hand, the simultaneous low temperature and high salinity conditions studied here may have triggered several concerted stress responses (Willdigg and Helmann, 2021), whereby microorganisms in these concomitant conditions combine strategies (Mudge et al., 2021), sometimes with one effect/response dominating over the other (de Lima Alves et al., 2015). In particular, the relative abundance of UFAs decreased with temperature in the absence of magnesium perchlorate, but slightly increased when magnesium perchlorate was present in the medium. The response of Rhodococcus sp. JG-3 cells against the two simultaneous stressful conditions (cold and high salt content), may result into a counteracting effect in the response of Rhodococcus sp. JG-3 cells against low temperature and magnesium perchlorate that led to a similar proportion of SFAs, UFAs and BFAs among samples at subzero temperatures with or without the perchlorate salt. It should be also note that, although halophiles and psychrophiles often share molecular strategies to cope with cold and salinity, they sometimes do not. For instance, while some psychrophiles increase BFAs, halophiles tend to either increase or decrease UFAs (Turk et al., 2004; Mudge et al., 2021). In addition, FA composition may be also affected by media, growth phase, temperature and oxygen concentration/availability (Beranová et al., 2008; Cray et al., 2015). The potential presence of osmotically compatible solutes, like trehalose, could also overlap with the stress response of cells against cold and/or salinity. Overall, these factors could have also affected Rhodococcus sp. JG-3 cells in our experiments and contribute to explain FAs changes in composition and concentration among samples.
The SFAs C16:0 and C18:0, the UFAs C16:1(ω11) and C18:1(ω9), and the BFA 10Me-C18:0 were the major contributors for the overall variations in the concentration of FAs in Rhodococcus sp. JG-3 cells under different temperatures and concentrations of magnesium perchlorate (Figure 4). This is in accordance with previous studies indicating the SFAs C18:0 and C16:0, and the UFAs C16:1 and C18:1, as the most abundant FAs in bacteria (Taha et al., 2013; Yoshida et al., 2016), especially in psychrophiles (Moyer and Morita, 2007). The detection of these FAs (i.e., carboxylic chains of FAs with 16 or 18 carbons, either with a saturated, unsaturated or branched conformation) under the harsh cold and salinity conditions tested in this study suggests them as good molecular biomarkers for the search for potential Earth-like microbial life for planetary exploration (Eigenbrode, 2008; Carrizo et al., 2020).
Our results support the need of studying polyextreme environments (i.e., those that harbor two or more harsh conditions for life, e.g., Horne et al., 2022) and polyextremophilic microorganisms to understand life’s adaptation to extreme environments. This is key in the research of habitability, understand limits of life and search for biosignatures on Mars and icy moons of the outer Solar System, where high content of salts and cold temperatures are present simultaneously. The presence of salts in aqueous solutions, allowing liquid water to exist at lower temperatures (inferred both for Mars and icy moons, e.g., Fairén et al., 2009; Vance et al., 2018) and therefore expanding the potential habitability conditions in different bodies of the Solar System and beyond, bears the negative effect of an increased osmotic stress, compromising the integrity of microbial cell membranes and therefore limiting the habitability conditions in those same places. Understanding the limits, balances and countereffects of both processes acting simultaneously on living beings is necessary to adequately define the limits of habitability in our cosmic neighborhood.
Conclusion
The adaptation of microbes to low temperatures allows them to succeed in diverse cold environments owing to molecular strategies. Many of these natural cold environments also include additional stressing conditions, like high concentrations of salts, which force cells to develop strategies to cope with cold and salinity at the same time. This makes polyextremophiles, like the bacterium Rhodococcus sp. JG-3, prime candidates to study the limits of microbial life and the molecular traces they could have left behind when they respond simultaneously to cold and salinity, conditions that are of high astrobiological interest, particularly for early Mars and icy moons.
Among the strategies used by microorganisms to adapt to polyextreme environments, it is crucial the modulation of the cell membrane permeability, for instance, by changing the composition of fatty acids (FAs). In this study we have reported two main results. First, we have documented the survival of Rhodococcus sp. JG-3 in LB medium with up to 8 wt% magnesium perchlorate and down to −16°C. And second, we have characterized the variations in FAs types composition (saturated, unsaturated and branched) under warm (20°C) and subzero (−10°C and −16°C) temperatures in the presence (16 wt%) and absence of magnesium perchlorate in the medium. The 16 wt% magnesium perchlorate in the medium caused a decrease in the concentration of total FAs. Moreover, saturated FAs (SFAs) tend to increase over unsaturated FAs (UFAs) and branched FAs (BFAs) with decreasing temperature, either in the absence or presence of 16 wt% perchlorates. In addition, the proportion of SFAs, UFAs and BFAs were similar at subzero temperatures both with and without magnesium perchlorate in the medium, suggesting a counteracting effect of low temperature and magnesium perchlorate on the bacterium response. Future work will require exploring differences in FAs composition and concentration with bacterium cells at different growth stages and larger incubation times.
Data availability statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Author contributions
LG-D: Conceptualization, work design, laboratory experiments performance, data analysis and manuscript writing. ML: Statistical analysis, manuscript writing, editing and review. DC: FAs experiments, data acquisition, and manuscript editing and review. AF: Conceptualization, work design, funding acquisition, and manuscript editing and review. All authors approved the submitted version.
Funding
This research has been funded by the European Research Council through the project “MarsFirstWater”, Consolidator Grant No. 818602, and from the Spanish Ministry of Science and Innovation/State Agency of Research MCIN/AEI/10.13039/501100011033 and ERDF “A way of making Europe” through the project No. MDM-2017-0737 (Unidad de Excelencia María de Maeztu - Centro de Astrobiología (CSIC-INTA)) and a Juan de la Cierva postdoctoral grant (FJC 2018-037246-I). The strain of Rhodococcus sp. JG-3 was courteously donated by Dr. Lyle Whyte (McGill University).
Acknowledgments
This paper is published in loving memory of Laura, the first author, and is dedicated to her effort and dedication to this work, as well as to her endless joy while we worked with her. We miss you and love you.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Bajerski, F., Wagner, D., and Mangelsdorf, K. (2017). Cell membrane fatty acid composition of chryseobacterium frigidisoli PB4T, isolated from antarctic glacier forefield soils, in response to changing temperature and pH conditions. Front. Microbiol. 8, 677–711. doi:10.3389/fmicb.2017.00677
Beranová, J., Jemioła-Rzemińska, M., Elhottová, D., Strzałka, K., and Konopásek, I. (2008). Metabolic control of the membrane fluidity in Bacillus subtilis during cold adaptation. Biochim. Biophys. Acta - Biomembr. 1778, 445–453. doi:10.1016/j.bbamem.2007.11.012
Besley, L. M., and Bottomley, G. A. (1969). The water vapour equilibria over magnesium perchlorate hydrates. J. Chem. Thermodyn. 1, 13–19. doi:10.1016/0021-9614(69)90032-9
Carrizo, D., Muñoz-Iglesias, V., Fernández-Sampedro, M. T., Gil-Lozano, C., Sánchez-García, L., Prieto-Ballesteros, O., et al. (2020). Detection of potential lipid biomarkers in oxidative environments by Raman spectroscopy and implications for the ExoMars 2020-Raman laser spectrometer instrument performance. Astrobiology 20, 405–414. doi:10.1089/ast.2019.2100
Carrizo, D., Sánchez-García, L., Menes, R. J., and García-Rodríguez, F. (2019). Discriminating sources and preservation of organic matter in surface sediments from five Antarctic lakes in the Fildes Peninsula (King George Island) by lipid biomarkers and compound-specific isotopic analysis. Sci. Total Environ. 672, 657–668. doi:10.1016/j.scitotenv.2019.03.459
Chevrier, V. F., Hanley, J., and Altheide, T. S. (2009). Stability of perchlorate hydrates and their liquid solutions at the Phoenix landing site, Mars. Geophys. Res. Lett. 36, L10202. doi:10.1029/2009gl037497
Cray, J. A., Stevenson, A., Ball, P., Bankar, S. B., Eleutherio, E. C. A., Ezeji, T. C., et al. (2015). Chaotropicity: A key factor in product tolerance of biofuel-producing microorganisms. Curr. Opin. Biotechnol. 33, 228–259. doi:10.1016/j.copbio.2015.02.010
dC Rubin, S. S., Marín, I., Gómez, M. J., Morales, E. A., Zekker, I., San Martín-Uriz, P., et al. (2017). Prokaryotic diversity and community composition in the Salar de Uyuni, a large scale, chaotropic salt flat. Environ. Microbiol. 19, 3745–3754. doi:10.1111/1462-2920.13876
de Lima Alves, F., Stevenson, A., Baxter, E., Gillion, J. L. M., Hejazi, F., Hayes, S., et al. (2015). Concomitant osmotic and chaotropicity-induced stresses in Aspergillus wentii: Compatible solutes determine the biotic window. Curr. Genet. 61, 457–477. doi:10.1007/s00294-015-0496-8
De Maayer, P., Anderson, D., Cary, C., and Cowan, D. A. (2014). Some like it cold: Understanding the survival strategies of psychrophiles. EMBO Rep. 15, 508–517. doi:10.1002/embr.201338170
Eigenbrode, J. L. (2008). Fossil lipids for life-detection: A case study from the early Earth record. Space Sci. Rev. 135, 161–185. doi:10.1007/s11214-007-9252-9
Eze, M. O. (1991). Phase transitions in phospholipid bilayers: Lateral phase separations play vital roles in biomembranes. Biochem. Educ. 19, 204–208. doi:10.1016/0307-4412(91)90103-f
Fairén, A. G. (2020). Organic chemistry on a cool and wet young Mars. Nat. Astron. 4, 446–447. doi:10.1038/s41550-020-1098-z
Fairén, A. G., Davila, A. F., Gago-Duport, L., Amils, R., and McKay, C. P. (2009). Stability against freezing of aqueous solutions on early Mars. Nature 459, 401–404. doi:10.1038/nature07978
Feller, G. (2017). Cryosphere and psychrophiles: Insights into a cold origin of life? Life 7, 25. doi:10.3390/life7020025
Garba, L., Latip, W., Al, M., Oslan, S. N., and Binti Raja, R. N. Z. (2016). Unsaturated fatty acids in antarctic bacteria. Res. J. Microbiol. 11, 146–152. doi:10.3923/jm.2016.146.152
Garcia-Descalzo, L., Gil-Lozano, C., Muñoz-Iglesias, V., Prieto-Ballesteros, O., Azua-Bustos, A., and Fairén, A. G. (2020). Can halophilic and psychrophilic microorganisms modify the freezing/melting curve of cold salty solutions? Implications for Mars habitability. Astrobiology 20, 1067–1075. doi:10.1089/ast.2019.2094
Glavin, D. P., Freissinet, C., Miller, K. E., Eigenbrode, J. L., Brunner, A. E., Buch, A., et al. (2013). Evidence for perchlorates and the origin of chlorinated hydrocarbons detected by SAM at the Rocknest aeolian deposit in Gale Crater. J. Geophys. Res. Planets 118, 1955–1973. doi:10.1002/jgre.20144
Goordial, J., Raymond-Bouchard, I., Ronholm, J., Shapiro, N., Woyke, T., Whyte, L., et al. (2015a). Improved-high-quality draft genome sequence of Rhodococcus sp. JG-3, a eurypsychrophilic actinobacteria from antarctic dry valley permafrost. Stand. Genomic Sci. 10, 61. doi:10.1186/s40793-015-0043-8
Goordial, J., Raymond-Bouchard, I., Zolotarov, Y., de Bethencourt, L., Ronholm, J., Shapiro, N., et al. (2015b). Cold adaptive traits revealed by comparative genomic analysis of the eurypsychrophile Rhodococcus sp. FEMS Microbiol. Ecol. 92, fiv154. doi:10.1093/femsec/fiv154
Gounot, A. M. (1986). Psychrophilic and psychrotrophic microorganisms. Experientia 42, 1192–1197. doi:10.1007/bf01946390
Grimalt, J. O., de Wit, R., Teixidor, P., and Albaigés, J. (1992). Lipid biogeochemistry of phormidium and microcoleus mats. Org. Geochem. 19, 509–530. doi:10.1016/0146-6380(92)90015-p
Hallsworth, J. E., Yakimov, M. M., Golyshin, P. N., Gillion, J. L. M., D’Auria, G., de Lima Alves, F., et al. (2007). Limits of life in MgCl 2 -containing environments: Chaotropicity defines the window. Environ. Microbiol. 9, 801–813. doi:10.1111/j.1462-2920.2006.01212.x
Hassan, N., Anesio, A. M., Rafiq, M., Holtvoeth, J., Bull, I., Haleem, A., et al. (2020). Temperature driven membrane lipid adaptation in glacial psychrophilic bacteria. Front. Microbiol. 11, 824–910. doi:10.3389/fmicb.2020.00824
Hecht, M. H., Kounaves, S. P., Quinn, R. C., West, S. J., Young, S. M. M., Ming, D. W., et al. (2009). Detection of perchlorate and the soluble chemistry of martian soil at the phoenix lander site. Sci. 325, 64–67. doi:10.1126/science.1172466
Heinz, J., Krahn, T., and Schulze-Makuch, D. (2020). A new record for microbial perchlorate tolerance: Fungal growth in NaClO4 brines and its implications for putative life on Mars. Life 10, 53. doi:10.3390/life10050053
Heinz, J., Rambags, V., and Schulze-Makuch, D. (2021). Physicochemical parameters limiting growth of Debaryomyces hansenii in solutions of hygroscopic compounds and their effects on the habitability of martian brines. Life 11, 1194. doi:10.3390/life11111194
Heinz, J., Waajen, A. C., Airo, A., Alibrandi, A., Schirmack, J., and Schulze-Makuch, D. (2019). Bacterial growth in chloride and perchlorate brines: Halotolerances and salt stress responses of Planococcus halocryophilus. Astrobiology 19, 1377–1387. doi:10.1089/ast.2019.2069
Horne, W. H., Volpe, R. P., Korza, G., Depratti, S., Conze, I. H., Shuryak, I., et al. (2022). Effects of desiccation and freezing on microbial ionizing radiation survivability: Considerations for Mars sample return. Considerations Mars Sample Return 22, 1337–1350. doi:10.1089/ast.2022.0065
Imhoff, J. F., and Thiemann, B. (1991). Influence of salt concentration and temperature on the fatty acid compositions of Ectothiorhodospira and other halophilic phototrophic purple bacteria. Arch. Microbiol. 156, 370–375. doi:10.1007/bf00248713
Kandror, O., DeLeon, A., and Goldberg, A. L. (2002). Trehalose synthesis is induced upon exposure of Escherichia coli to cold and is essential for viability at low temperatures. Proc. Natl. Acad. Sci. 99, 9727–9732. doi:10.1073/pnas.142314099
Kaneda, T. (1991). Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 55, 288–302. doi:10.1128/mr.55.2.288-302.1991
Kounaves, S. P., Chaniotakis, N. A., Chevrier, V. F., Carrier, B. L., Folds, K. E., Hansen, V. M., et al. (2014). Identification of the perchlorate parent salts at the Phoenix Mars landing site and possible implications. Icarus 232, 226–231. doi:10.1016/j.icarus.2014.01.016
Laye, V. J., and DasSarma, S. (2018). An antarctic extreme halophile and its polyextremophilic enzyme: Effects of perchlorate salts. Astrobiology 18, 412–418. doi:10.1089/ast.2017.1766
Matsubara, T., Fujishima, K., Saltikov, C. W., Nakamura, S., and Rothschild, L. J. (2017). Earth analogues for past and future life on Mars: Isolation of perchlorate resistant halophiles from Big Soda Lake. Insternational J. Astrobiol. 16, 218–228. doi:10.1017/s1473550416000458
Moyer, C. L., and Morita, R. Y. (2007). “Psychrophiles and psychrotrophs,” in Encyclopedia of Life Sciences (New York, NY: John Wiley & Sons). doi:10.1002/9780470015902.a0000402.pub2
Mudge, M. C., Nunn, B. L., Firth, E., Ewert, M., Hales, K., Fondrie, W. E., et al. (2021). Subzero, saline incubations of Colwellia psychrerythraea reveal strategies and biomarkers for sustained life in extreme icy environments. Environ. Microbiol. 23, 3840–3866. doi:10.1111/1462-2920.15485
Mykytczuk, N. C. S., Foote, S. J., Omelon, C. R., Southam, G., Greer, C. W., and Whyte, L. G. (2013). Bacterial growth at −15 °C; molecular insights from the permafrost bacterium Planococcus halocryophilus Or1. ISME J. 7, 1211–1226. doi:10.1038/ismej.2013.8
Nelson, L. M., and Parkinson, D. (1978). Effect of freezing and thawing on survival of three bacterial isolates from an arctic soil. Can. J. Microbiol. 24, 1468–1474. doi:10.1139/m78-236
Ojha, L., Wilhelm, M. B., Murchie, S. L., McEwen, A. S., Wray, J. J., Hanley, J., et al. (2015). Spectral evidence for hydrated salts in recurring slope lineae on Mars. Nat. Geosci. 8, 829–832. doi:10.1038/ngeo2546
R Core Team (2022). R: A language and environment for statistical computing. Available at: http://www.r-project.org/.
Rapin, W., Ehlmann, B. L., Dromart, G., Schieber, J., Thomas, N. H., Fischer, W. W., et al. (2019). An interval of high salinity in ancient Gale crater lake on Mars. Nat. Geosci. 12, 889–895. doi:10.1038/s41561-019-0458-8
Reina-Bueno, M., Argandoña, M., Nieto, J. J., Hidalgo-García, A., Iglesias-Guerra, F., Delgado, M. J., et al. (2012). Role of trehalose in heat and desiccation tolerance in the soil bacterium Rhizobium etli. BMC Microbiol. 12, 207. doi:10.1186/1471-2180-12-207
Robertson, K., and Bish, D. (2011). Stability of phases in the Mg(ClO4)2·nH2O system and implications for perchlorate occurrences on Mars. J. Geophys. Res. 116, E07006. doi:10.1029/2010je003754
Russell, N. J. (1984). Mechanisms of thermal adaptation in bacteria: Blueprints for survival. Trends biochem. Sci. 9, 108–112. doi:10.1016/0968-0004(84)90106-3
Scheller, E. L., Razzell Hollis, J., Cardarelli, E. L., Steele, A., Beegle, L. W., Bhartia, R., et al. (2022). Aqueous alteration processes in Jezero Crater, Mars—Implications for organic geochemistry. Science 378, 1105–1110. doi:10.1126/science.abo5204
Siliakus, M. F., van der Oost, J., and Kengen, S. W. M. (2017). Adaptations of archaeal and bacterial membranes to variations in temperature, pH and pressure. Extremophiles 21, 651–670. doi:10.1007/s00792-017-0939-x
Sinensky, M. (1974). Homeoviscous adaptation—a homeostatic process that regulates the viscosity of membrane lipids in Escherichia coli. Proc. Natl. Acad. Sci. 71, 522–525. doi:10.1073/pnas.71.2.522
Soudi, A., Farhat, O., Chen, F., Clark, B. C., and Schneegurt, M. A. (2017). Bacterial growth tolerance to concentrations of chlorate and perchlorate salts relevant to Mars. Int. J. Astrobiol. 16, 229–235. doi:10.1017/s1473550416000434
Stillman, D. E., and Grimm, R. E. (2011). Dielectric signatures of adsorbed and salty liquid water at the Phoenix landing site, Mars. J. Geophys. Res. 116, E09005. doi:10.1029/2011JE003838
Sun, X., Griffith, M., Pasternak, J. J., and Glick, B. R. (1995). Low temperature growth, freezing survival, and production of antifreeze protein by the plant growth promoting rhizobacterium Pseudomonas putida GR12-2. Can. J. Microbiol. 41, 776–784. doi:10.1139/m95-107
Taha, A. I. B. H. M., Ahmed, R. Z., Motoigi, T., Watanabe, K., Kurosawa, N., and Okuyama, M. (2013). “Lipids in cold-adapted microorganisms,” in Cold-adapted microorganisms (Caister Academic Press).
Thomas, N. H., Ehlmann, B. L., Meslin, P. Y., Rapin, W., Anderson, D. E., Rivera-Hernández, F., et al. (2019). Mars science laboratory observations of chloride salts in Gale Crater, Mars. Geophys. Res. Lett. 46, 10754–10763. doi:10.1029/2019gl082764
Turk, M., Méjanelle, L., Šentjurc, M., Grimalt, J. O., Gunde-Cimerman, N., and Plemenitaš, A. (2004). Salt-induced changes in lipid composition and membrane fluidity of halophilic yeast-like melanized fungi. Extremophiles 8, 53–61. doi:10.1007/s00792-003-0360-5
Vance, S. D., Panning, M. P., Stähler, S., Cammarano, F., Bills, B. G., Tobie, G., et al. (2018). Geophysical investigations of habitability in ice-covered ocean worlds. J. Geophys. Res. Planets 123, 180–205. doi:10.1002/2017je005341
Weber, M. H. W., and Marahiel, M. A. (2002). Coping with the cold: The cold shock response in the gram-positive soil bacterium Bacillus subtilis. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 357, 895–907. doi:10.1098/rstb.2002.1078
Willdigg, J. R., and Helmann, J. D. (2021). Mini review: Bacterial membrane composition and its modulation in response to stress. Front. Mol. Biosci. 8, 634438–634511. doi:10.3389/fmolb.2021.634438
Yoon, Y., Lee, H., Lee, S., Kim, S., and Choi, K. H. (2015). Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 72, 25–36. doi:10.1016/j.foodres.2015.03.016
Keywords: fatty-acids, freezing, perchlorate, halo-psychrophiles, Mars habitability
Citation: García-Descalzo L, Lezcano MÁ, Carrizo D and Fairén AG (2023) Changes in membrane fatty acids of a halo-psychrophile exposed to magnesium perchlorate and low temperatures: Implications for Mars habitability. Front. Astron. Space Sci. 10:1034651. doi: 10.3389/fspas.2023.1034651
Received: 01 September 2022; Accepted: 22 February 2023;
Published: 07 March 2023.
Edited by:
Daniela Billi, University of Rome Tor Vergata, ItalyReviewed by:
Mihaela Glamoclija, Rutgers University, United StatesRosa Santomartino, University of Edinburgh, United Kingdom
Jacob Heinz, Technical University of Berlin, Germany
Copyright © 2023 García-Descalzo, Lezcano, Carrizo and Fairén. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: María Ángeles Lezcano, mangeles.lezcano@gmail.com; Daniel Carrizo, dcarrizo@cab.inta-csic.es; Alberto G. Fairén, agfairen@cab.inta-csic.es
†Deceased
 Laura García-Descalzo
Laura García-Descalzo María Ángeles Lezcano
María Ángeles Lezcano Daniel Carrizo
Daniel Carrizo Alberto G. Fairén
Alberto G. Fairén