Patient decision support resources inform decisions about cancer susceptibility genetic testing and risk management: a systematic review of patient impact and experience
- 1Centre for Psychosocial Research in Cancer: CentRIC, School of Health Sciences, University of Southampton, Southampton, United Kingdom
- 2Clinical Genetics, St George's University Hospitals NHS Foundation Trust, London, United Kingdom
- 3Southampton Health Technology Assessments Centre, University of Southampton, Southampton, United Kingdom
- 4Engagement Services, University of Southampton Library, University of Southampton, Southampton, United Kingdom
- 5Faculty of Medicine, University of Southampton, Southampton, United Kingdom
Background: Patients with genetic cancer susceptibility are presented with complex management options involving difficult decisions, for example about genetic testing, treatment, screening and risk-reducing surgery/medications. This review sought to explore the experience of patients using decision support resources in this context, and the impact on decision-making outcomes.
Methods: Systematic review of quantitative, qualitative and mixed-methods studies involving adults with or without cancer who used a decision support resource pre- or post-genetic test for any cancer susceptibility. To gather a broad view of existing resources and gaps for development, digital or paper-based patient resources were included and not limited to decision aids. Narrative synthesis was used to summarise patient impact and experience.
Results: Thirty-six publications describing 27 resources were included. Heterogeneity of resources and outcome measurements highlighted the multiple modes of resource delivery and personal tailoring acceptable to and valued by patients. Impact on cognitive, emotional, and behavioural outcomes was mixed, but mainly positive. Findings suggested clear potential for quality patient-facing resources to be acceptable and useful.
Conclusions: Decision support resources about genetic cancer susceptibility are likely useful to support decision-making, but should be co-designed with patients according to evidence-based frameworks. More research is needed to study impact and outcomes, particularly in terms of longer term follow-up to identify whether patients follow through on decisions and whether any increased distress is transient. Innovative, streamlined resources are needed to scale up delivery of genetic cancer susceptibility testing for patients with cancer in mainstream oncology clinics. Tailored patient-facing decision aids should also be made available to patients identified as carriers of a pathogenic gene variant that increases future cancer risks, to complement traditional genetic counselling.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020220460, identifier: CRD42020220460.
1. Introduction
Patients who have cancer susceptibility gene testing and are found to carry a pathogenic variant that causes increased future cancer risks (“carriers”) are presented with choices about primary prevention (e.g., risk-reducing surgery, chemoprevention, changes in diet), screening (e.g., earlier, more frequent mammogram/colonoscopy) and treatment for cancer or premalignant conditions (1, 2). They are also encouraged to communicate with at-risk relatives so they can be offered cascade predictive testing (3). Genetic testing has traditionally been supported through genetic counselling: the “process of helping people understand and adapt to the medical, psychological and familial implications of genetic contributions to disease” (4). Genetic counselling promotes personalised, values-based decision-making made possible through formation of a therapeutic alliance during the consultation (5). Increased importance of genomic test results to guide cancer treatment (6–10) and calls for population screening to identity carriers and offer targeted treatment, prevention and surveillance options to high risk groups (11–13) have created increased pressure on already stretched genetics and oncology services. Knowledge can be increased through genetic counselling (14), but people may not accept that risks apply to them (15, 16) or events will happen to them personally, for example due to framing (17) and anchoring-and-adjustment biases (18). Therefore, communicating risks in a personally meaningful way is crucial to quality decision-making.
Shared decision-making between healthcare providers and patients is recommended (19, 20), particularly where choices are personal and complex, as is typical regarding cancer susceptibility genes. Patient decision support resources have been employed in many areas of medicine, including genetics, to promote shared decision-making, streamline clinical consultations and improve decision quality. Patient decision aids (ptDA) are a type of decision support resource that encourage patients to become engaged in difficult decisions by considering not only information about the options, pros and cons but also how personal values influence their decision (21, 22). This is often achieved through the inclusion of a values-based exercise, for example a sliding scale or worksheet for patients to record the importance of personal values relevant to the particular decision. PtDA are useful when there is no clearly preferred option, or when feelings and choices may differ according to individual values. A Cochrane review of 105 studies including 31,043 patients using ptDA before/during clinic compared to usual care revealed increased knowledge and confidence about decisions aligned with personal values, without harmful effects (23). However, clinicians must be mindful that the design and effectiveness of ptDAs is variable, with many not meeting the International Patient Decision Aids Standards (IPDAS) (24) or lacking a theoretical framework (25) to inform content delivery. Also, whilst web-based education may be highly acceptable (26), patients will often seek online sources of information themselves (27), value individual choice, and may not view information if asked to do so at home rather than in clinic (28).
There are two contexts in which patient resources could be relevant to support decisions regarding genetic cancer susceptibility:
i) pre-genetic testing (29): to support patients making a decision about whether to have a genetic test, either with or without a diagnosis of cancer (30).
ii) post-genetic testing: to complement shared decision-making with a healthcare professional for patients identified to have a genetic cancer susceptibility, and their relatives (31, 32).At a time of increasing demand for genetic testing and limited in-person resources to support patients, there is a need to better understand the impact and experience of PtDA for genetic cancer susceptibility in both contexts.
This systematic review aimed to identify patient resources to support decision-making pre- testing for any genetic cancer susceptibility, or regarding cancer management options for carriers. The net was cast widely to find any existing resources, including brief educational materials or interventions as well as ptDA, to explore their potential for supporting patients. A secondary goal was to highlight gaps to guide future work co-designing a PtDA with patients.
The extent to which existing decision support resources meet patients' needs and preferences was explored in terms of:
i) impact on outcomes, e.g. cognitive, emotional, or behavioural.
ii) patient experience.
Recommendations for clinical practice and future research were proposed.
2. Methods
The Centre for Reviews and Dissemination's guidance for reviews in health care (33) and the Preferred Reporting Items for Systematic Reviews (PRISMA) statement (34) guided methods and reporting for this systematic review. The protocol was published on PROSPERO: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID = 220460.
2.1. Co- design
Stakeholders were consulted from the earliest planning phases and throughout this review, includingpatient engagement in design and data synthesis. Patients have been included from the Cancer Research UK-funded CanGene-CanVar programme. An International Lynch Decision Aid Stakeholder Panel has been established to provide advice and guidance. Individuals have been invited based on expertise in clinical care, academic work, public policy, patient charities, peer support groups and public bodies.
2.2. Literature searching
The following databases (and host platforms) were searched and re-run prior to final analysis (from database inception to 02/07/2021, English language only): MEDLINE (EBSCOhost), PsycINFO (EBSCOhost), Embase (OVID), CINAHL (EBSCOhost), Web of Science Core Collection, and the Cochrane Library (Cochrane Database of Systematic Reviews (CDSR); Cochrane Central Register of Controlled Trials (CENTRAL)). The search strategy combined key word and subject terms targeting three concepts: cancer genetics, decision-making, and written resources (Supplementary Material Table S1).
In addition, other relevant studies were identified by examining bibliographies of included publications and using forward citation searching in Web of Science Core Collection. Grey literature was searched to identify unpublished resources (Supplementary Material Table S2).
2.3. Study selection
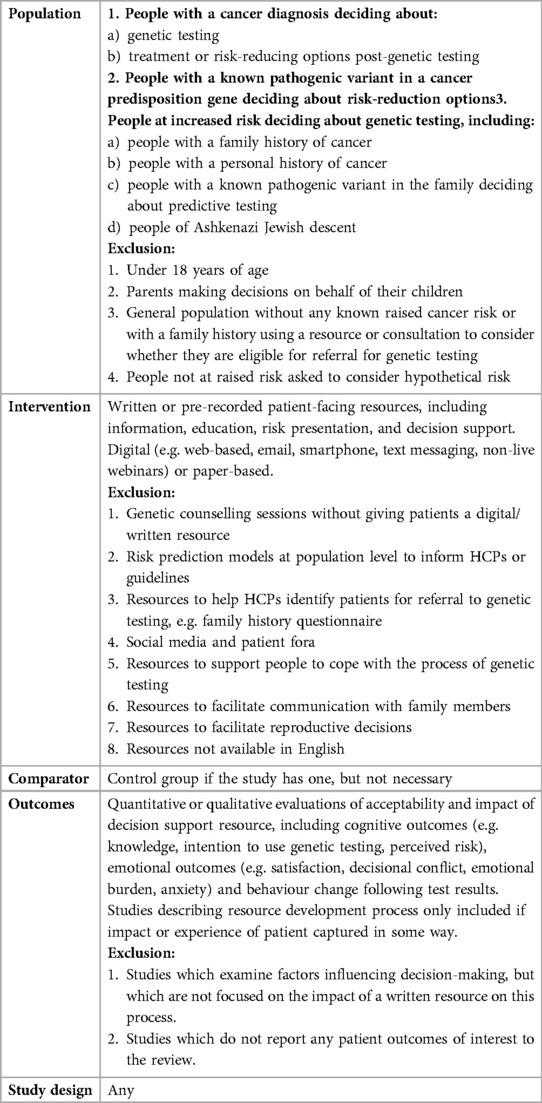
Inclusion and exclusion criteria are detailed in Table 1. In brief, studies were included if they involved adults (with or without cancer) who used a decision support resource pre- or post- testing for any cancer susceptibility genes. These could be delivered digitally or paper-based, and included one or more of the following: information, education, visual presentation of cancer risk and personalised resources, including ptDA. Quantitative, qualitative and mixed methods studies were included.
All search results were exported to EndNote X9 software for de-duplication. Two reviewers (KK, KM) independently screened 20% of titles and abstracts (sampled in alphabetical order) as a pilot to test whether the inclusion/exclusion criteria were appropriate and to assess whether their application was accurately applied by both reviewers. Rayyan, a web application for collaboration on systematic reviews (35) was used. After each batch of 100 references in the 20% pilot, reviewers' decisions were unblinded and compared. There were 29 disagreements out of 500 and these were all resolved through discussion. Informed by the pilot, the eligibility criteria were adjusted following discussion with the wider research team (DE, CF, CG, LT). The remaining 80% of titles and abstracts were screened by the lead author (KK). Both reviewers completed full text screening. Where discrepancies arose, discussion took place (involving the wider research team where necessary) until agreement was reached.
2.4. Data extraction and critical appraisal
Data from eligible publications were extracted into an Excel database with fields guided by the TIDieR (template for intervention description and replication) checklist (36). Both reviewers performed independent data extraction for 10% of the included studies. Disagreements were resolved through discussion. Subsequently, KK extracted data from the remaining studies, checked by the second reviewer.
Critical appraisal was performed by KK and KM (Supplementary Material Table S3). Depending upon the type of study, this was guided by National Institute for Health and Care Excellence (NICE) checklists for quantitative intervention studies and qualitative studies (37) or the mixed-methods appraisal tool (38). Aspects of study design and reporting were appraised. Each study was awarded an overall study quality grading for internal validity and external validity and subject to an overall assessment grading of how well the study was conducted, as far as could be ascertained from the paper. Appraisal of study criteria in Supplementary Material Table S3 is presented as yes, no, or N/A (not applicable or not able to ascertain from the paper).
2.5. Data synthesis
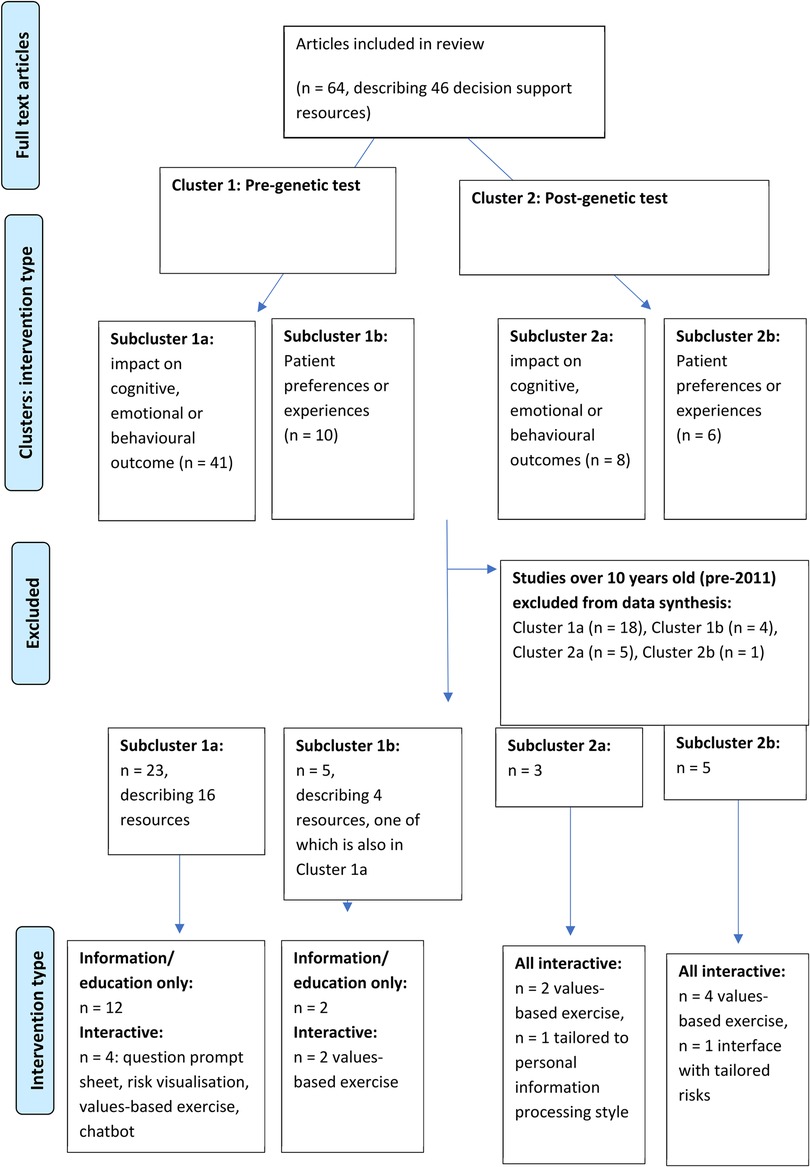
The review aims necessitated inclusion of a wide range of studies, mostly of quantitative but also mixed methods and qualitative design. Meta-analysis was not possible due to the heterogeneity of methodologies, populations and outcome measures. Narrative synthesis, often used for systematic reviews of healthcare interventions (39), was selected as the most appropriate method to synthesise findings without diluting the individual value contributed by different study designs (40, 41). Tabulated data were examined to describe patterns and summarise estimates of effect direction and size (39, 42). Studies were grouped into clusters and subclusters based on pre- or post-genetic test setting and patient outcomes (Figure 1) to facilitate post-hoc subgroup analysis (40). Qualitative data were subjected to thematic analysis to interpret primary themes and concepts, and representative patient narratives were chosen to be presented in their original form to highlight these themes (41).
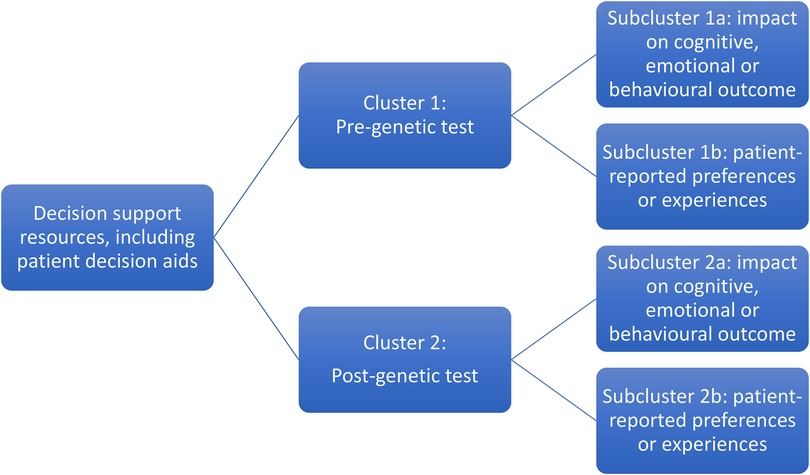
Figure 1. Studies included in the systematic review were grouped into clusters based on the context of the patient decision support resource (pre- and post-genetic testing) and subclusters relating to the impact on outcomes and patient-reported experiences.
3. Results
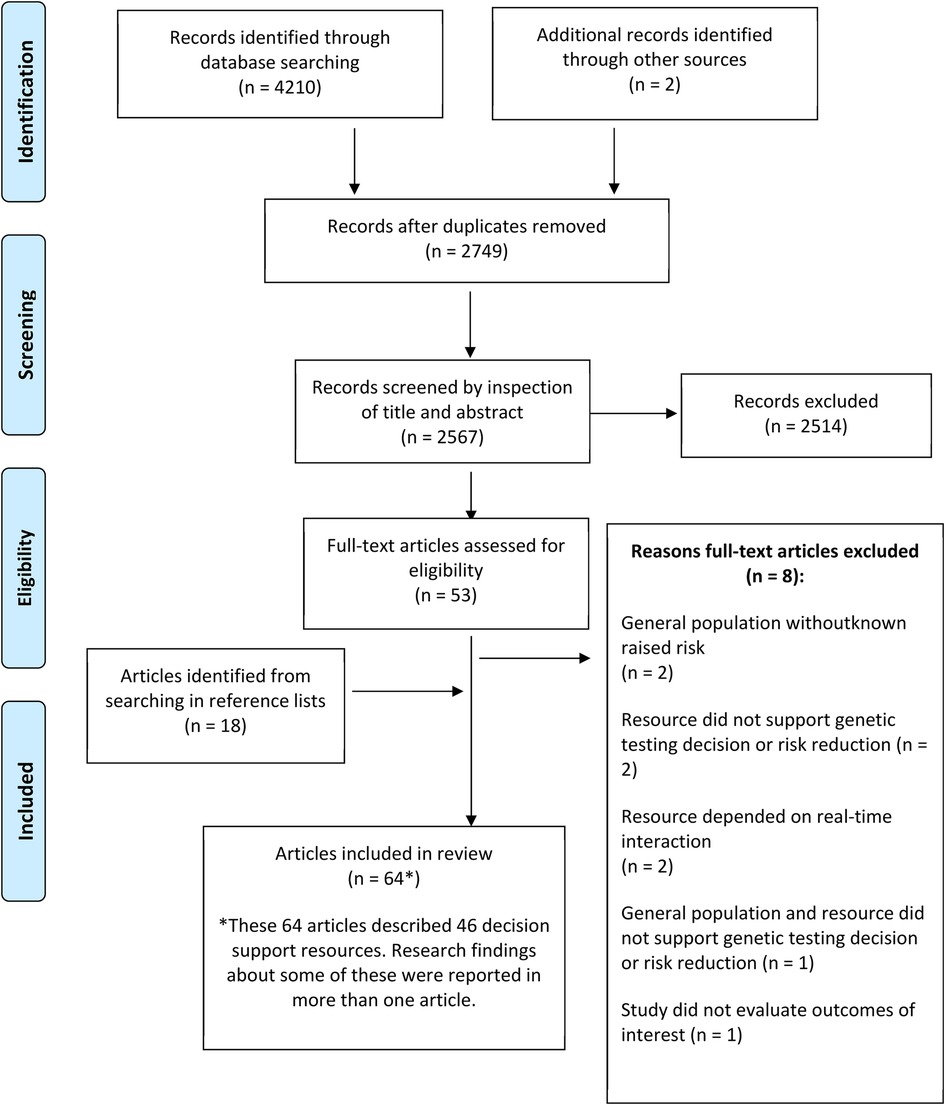
Sixty-four publications regarding 46 patient decision support resources were found to be eligible from the searches (more than one publication was included regarding some of these). Figure 2 shows the flow of study selection using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram (34). Most studies were from United States (n = 26), followed by Australia (n = 12) and Netherlands (n = 12). A pragmatic decision was taken to focus data synthesis on studies published from 2011 onwards, and the protocol was amended accordingly. Older studies were based on outdated guidelines and less likely to be relevant to our study aim of identifying resources that could be used or easily adapted for current practice. As shown in Figure 3, 36 publications were retained, describing 27 resources divided into two clusters (pre-and post-genetic test) and four subclusters (impact on cognitive, emotional or behavioural outcomes and patient-reported preferences/experience for each cluster) as illustrated in Figure 1.
3.1. Critical appraisal of studies
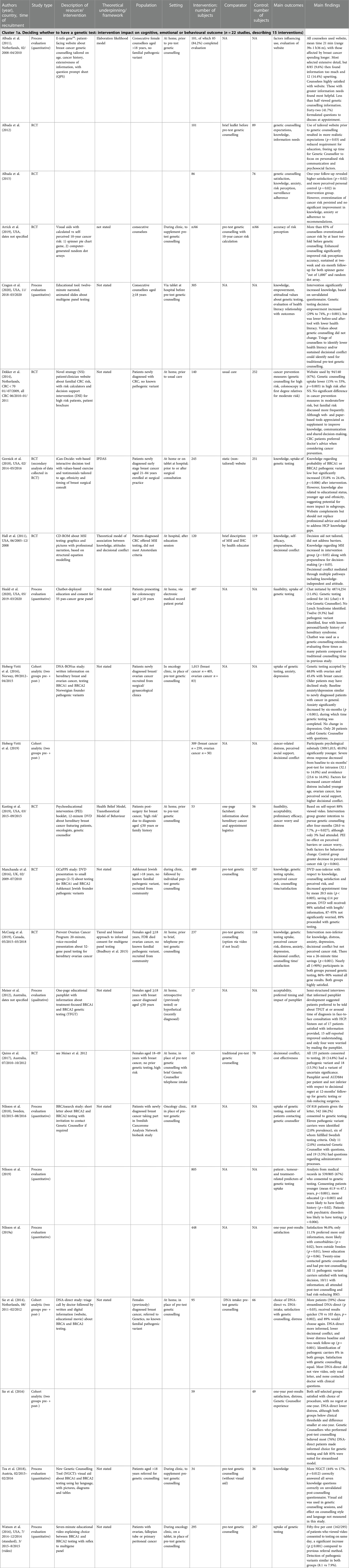
Most (n = 17) studies contained strong certainty of evidence/low risk of bias, 13 contained medium certainty of evidence/moderate risk of bias and three contained weak certainty of evidence/high risk of bias (Supplementary Appendix S3). Study design varied, from publications describing development of resources with some preliminary patient evaluation in a hypothetical decision-making setting (32, 43–50), to larger randomised controlled trials in target populations (51–56). Follow-up was often short (less than two months), with only a few studies recording outcomes as long as 12 months after resource use (53, 55, 57–59). Qualitative study designs resulted in some rich findings (60–64) but some lacked rigorous analysis methods leading to more shallow data (45, 60). Overall, external validity was limited by lack of patient diversity.
3.2. Target population
Cluster 1 resources supported pre-genetic test decisions for patients diagnosed with breast (28, 57, 60, 61, 64, 65, 66, 67), ovarian (68), breast/ovarian (69) and colorectal cancer (70, 71) as well as patients (mostly) unaffected by cancer with a family history (48, 49, 53, 54, 72, 73) or of Ashkenazi Jewish heritage (51, 62) (see Table 1). All Cluster 2 resources targeted BRCA1 or BRCA2 carriers to support decisions about risk management post-genetic test. These were designed for carriers unaffected by cancer (45, 55), with personal history of breast cancer (63), separate versions tailored to breast cancer history (32) or not specified (47, 46, 52, 59).
3.2.1. Setting for delivery
Cluster 1 resources were designed to replace face-to-face genetic counselling pre-test (28, 48, 57, 62, 67, 68, 69) or to supplement genetic counselling (44, 49, 51, 53, 54, 60, 64, 66, 70, 72, 73), which may have been delivered in the mainstream setting by oncology professionals (52, 65, 71). Cluster 2 resources were exclusively to supplement standard of care genetic counselling post-test for carriers.
3.3. Characteristics of decision support resources
3.3.1. Conceptual/theoretical framework
In most publications, a conceptual/theoretical framework to inform design was not specified. However, six resources were based on underlying theory (43, 49, 53, 54, 59, 64, 66, 71, 74) (see Table 2). These included theories related to information tailoring (43, 53, 74), such as the elaboration likelihood model of communication persuasion, which examines how presenting personalised messages can encourage more thoughtful decision-making (75, 76). The health belief model (77) suggests behaviour change is maximised if resources address threat severity and personal benefits and the transtheoretical model of health behaviour change (78) describes stages of change that patients move through before taking action. These two behaviour change theories informed content in psychoeducational resources and measured intention to pursue genetic counselling and testing (66). Another theory informed information presentation (64, 66): the fuzzy-trace model postulates decision-making is influenced by a quick, intuitive “getting the gist” which is personal and values-based, and can be more important than memory of information learned verbatim (76).
Whilst not all ptDA were informed by theory, some (32, 45, 49, 55, 59, 63, 64) followed recommended guidelines such as the Ottawa Decision-Support Framework (ODSF) (79) which includes a values-based exercise to improve decision quality and process. Similarly, the multiattribute value and utility models (80, 81) guided inclusion of pros and cons of cancer risk management choices that patients rated according to personal importance (59), and the informed choice model (82, 83) was used to theorise that increased knowledge would improve decision-making quality and genetic testing uptake (49).
3.3.2. Format and content delivery mode varied widely
For studies aimed at replacing the need for in-person counselling pre-test, a brief information letter was used (57, 69, 67), or letter and digital options including a website (49), video (28) or chatbot (48). Educational presentations to supplement genetic counselling ranged in length from seven-minute video (68), 12 minute DVD (66) or animated slides (73), 15 minute DVD delivered to small groups (51), 20 minute voice-recorded presentation (54) and CD-ROM about microsatellite instability testing in colorectal cancers that took on average 24 min to view (71).
Paper-based resources were either brief (one-page) and focused on treatment-related implications of genetic testing for people with breast cancer (60) or longer (15-page) including a worksheet to record personal weighing up of options (32, 47, 55). Others made resources available online with printable content (45, 64). Visual aids were created to improve genetic cancer risk communication using pictures, diagrams and tables (44) or by comparing an interactive spinner-game format to random dot icon arrays (72). Digital decision support resources were mostly interactive (62, 63, 64, 52, 59, 70) and some also included computer-tailoring for personal characteristics to present more relevant information (46, 49, 53, 65).
3.4. Impact of decision support resources
A summary of the main findings is presented in Table 2, with narrative synthesis below.
3.4.1. Knowledge, understanding and expectations
Improvement in genetic cancer susceptibility knowledge or realistic expectations was statistically significant for many (44, 51, 53, 54, 65, 71) but not all resources (70). However, measurement was often by self-report, using unvalidated questionnaires and/or with lack of pre- and post-counselling measures or control group. In one study, patients stated viewing an educational video increased knowledge, however qualitative data gathered via semi-structured interviews suggested gaps remained, for example many thought genetic counselling was the same as genetic testing (61).
Accuracy of cancer risk perception significantly improved by adding personalised visual aids to genetic counselling (72), with effects sustained up to six-months. However, recorded presentations before a shortened session had mixed results when compared to traditional counselling in randomised trials. An educational video followed by abbreviated genetic counselling was non-inferior to traditional counselling for increased knowledge, satisfaction and risk perception for community-recruited participants offered Ashkenazi Jewish founder BRCA1 and BRCA2 gene testing (51). Patients with a relative who died of ovarian cancer were offered 52-gene panel testing using a digital presentation viewed at home followed by short telephone genetic counselling, which was non-inferior to traditional counselling for knowledge, satisfaction and psychological factors but not for ovarian cancer risk perception (54).
3.4.2. Distress, anxiety and cancer worry
Mental well-being outcomes such as distress, anxiety, cancer worry and depression tended to be below clinically relevant thresholds at baseline and follow-up, indicating no significant evidence of psychological harm from pre-test resources (51, 53, 54, 66, 67, 69, 84). For people newly diagnosed with cancer, levels were transiently increased as expected at this challenging stage of life;genetic testing using decision support resources did not increase symptoms (28, 58, 66, 67, 69). However, several studies excluded patients with psychological conditions (28, 57, 58, 60, 85, 86), so it is not known how resources might have influenced mental well-being in these groups.
Some post-test resources showed time-dependent results, with cancer-related distress higher in the PtDA group compared to usual care at one month, but lower from one- to six-months, possibly indicative of a deliberative decision-making process (59) or declining over time but similar in ptDA and usual care groups (55). Distress was also shown to vary by topic, with lower levels regarding chemoprevention compared to risk-reducing breast and ovarian surgery decisions (55).
3.4.3. Genetic testing uptake
Written information instead of pre-test counselling led to genetic testing uptake in 405/1015 (45.4%) (84) and 542/818 (66.2%) (85) patients with breast and 83/1,015 (68.0%) with ovarian cancer (84), although there was no comparator group and older people were less likely to take part. An educational video followed by brief counselling in community-recruited patients led to high testing uptake, with 92% of video vs. 96% of the traditional counselling group electing testing, and video delivered a significant time saving (19.4 vs. 45.8 min, p < 0.001) (54). In a similar study, 89% across DVD and traditional counselling groups had testing, with the DVD saving 20.5 min of counselling time (51). However, there was lower uptake of testing amongst patients with ovarian cancer shown a video in their oncology clinic instead of referral for genetic counselling (162/295, 55%) (68). Despite interventions increasing knowledge and interest in testing, patients particularly valued their doctor's advice and did not always follow through on scheduling a genetic counselling appointment (65, 66). Some resources were used to manage expectations about being offered testing (48, 56), a successful approach for people at lower risk who do not meet current eligibility guideline criteria.
3.4.4. Decisional conflict
Decisional conflict describes level of uncertainty about making a choice. The decisional conflict scale measures contributing factors such as support, information and personal values (87). Results from this review suggest patients with higher decisional conflict may need additional decision support and genetic counselling (57, 73, 65).
An educational resource to shorten pre-test genetic counselling was non-inferior to standard care with respect to decisional conflict in patients with a family history of ovarian cancer (video (54),) and patients diagnosed with breast cancer before age 50 years (pamphlet (67),). Baseline levels of decisional conflict were moderate in patients referred for genetic risk assessment, and showed significant decrease at two-months after using an interactive, web-based ptDA about breast reconstruction after risk-reducing mastectomies, compared to standard of care counselling (52). One study in 239 high risk patients with colorectal cancer (71) showed that a ptDA impacted decisional conflict by increasing knowledge and preparedness to make a decision. Decisional conflict was also influenced by knowledge-independent factors such as attitudes about testing and learning about hereditary cancer risk which, along with other barriers, are often not addressed in ptDA.
BRCA1 and BRCA2 carriers with no history of cancer had low baseline decisional conflict about breast risk management options in intervention and control groups, which declined with time up to 12-months and was not significantly influenced by a paper-based ptDA used at home after post-test genetic counselling (55).
3.5. Evaluation of decision support resources
3.5.1. Satisfaction and acceptability by patient report
Satisfaction and acceptance was high across clinical settings and patient groups; however, resource usage was often untracked, and many studies did not compare to standard care in a randomised controlled trial. Where optional usage was monitored or self-reported, this revealed 64/100 (64%) used an interactive CD-ROM (59), 94/140 (67%) used a website (70), and 53/60 (88%) viewed a video (66). A much lower percentage (487/4,254, 11.4%) of patients presenting for colonoscopy screening engaged with a chatbot to answer questions about their family history to determine eligibility for genetic testing (48). Most (95/161, 59%) patients with breast cancer chose to use streamlined pre-test information instead of genetic counselling; when presented with letter and video options, most only read the letter and none contacted the doctor with questions (28). There was no regret at 12-months about choosing streamlined testing, which identified a pathogenic BRCA variant in 8/95 (8%) of patients (58). Similarly, 96% of patients with breast cancer were satisfied at 12-months with a short letter instead of pre-test counselling and only 11/818 (2%) contacted the genetic counsellor for support (57). Only 20/1,015 (1.9%) of patients with breast or ovarian cancer who received brief written pre-test information in oncology contacted the genetic counsellor (84).
3.5.2. Experience and emotional outcomes by patient report
Two studies explored the experience of patients with breast cancer using resources to decide about genetic counselling/testing. In a telephone structured interview study to evaluate acceptability and emotional impact of a one-page pamphlet about treatment-focused genetic testing, 7/17 people thought the pamphlet sufficient for decision making, whilst 10/17 believed more information was needed, e.g., discussion with healthcare professional (HCP) or searching online (60). Four out of 17 were worried by reading the pamphlet: three were reminded of their breast cancer diagnosis and one was worried about their relatives. Think-aloud interviews reviewing a web-based ptDA revealed patients with breast cancer preferred less text, to get the “gist”, with optional, more detailed information, and wanted a “friendlier” feel to patient pictures (64). This is in contrast to study findings about another web-based ptDA tailored for personal characteristics (43), in which patients with breast cancer spent more time looking at information and selected to receive extensive detail, however when looking at the information 12/85 (14.4%) then found it upsetting.
The JeneScreen web-based programme for Ashkenazi Jewish BRCA testing was evaluated in a pre- and post-test interview study of 11 patients without cancer (62). Similar to findings from another study involving patients with breast cancer (64), some wanted less pre-test information up front and suggested a staged approach: “…But you may not get that result, so you wouldn”t need to go into as much detail about that topic…only if you need the information”. Ten out of 11 were satisfied with online consent to testing and suggested it was more convenient: “Online, everybody prefers online”; “If it required me going somewhere to meet someone, then it probably would have taken me longer to get around to doing it”. However, one patient referred to her age as the reason she would prefer in-person support: “Well, I am over 70, I prefer to do things where I am speaking to someone”.
A psychoeducational intervention (PEI) containing information about breast cancer genetic testing was explored by focus groups (paper version (50),) and semi-structured interviews (video format (61),). The paper PEI was visually attractive and culturally acceptable: “I like the cover; you have a variety of ethnic groups and ages” and appreciated as a take-home resource: “you”re only going to remember a little piece of what [your health care professional says]…but hand me books…I can flip through it and then…write down notes to ask the next time I see somebody”; “I would see it; I can hold it; I can turn the pages…it prompts me to start thinking” (50). Patients with breast cancer had emotional reactions to patient narratives in the video PEI: “It just makes you realize [sic] that other people feel or felt like that….touching to watch the stories because I can relate, I kind of teared up” (61).
Focus groups with 15 BRCA carriers with breast cancer guided development and evaluation of a web-based ptDA (63). Key decision-making motivating factors were identified, such as feeling obligated e.g., “do the right thing” to save life by having risk-reducing ovarian surgery, or HCP being strong influencers, e.g., “my surgeon was gung-ho”, describing consultations about risk-reducing mastectomies. Inclusion of a values-based exercise was appreciated: “When it is in black and white in front of me and I am able to block out everybody else, what they want, what they think I should do and I can look at it and say what is the best thing step by step for me and then get a print out—that is huge”. PtDA timing was debated, suggesting need for personalisation: “The patient will probably let you know if they are ready for it or not”. Patients preferred to use ptDAs in clinical settings: “more geared up….more serious about it if it was in an office than at home”, while clinicians (also included in focus groups) were keen for at-home use but cautioned that support was needed: “…are they really going to know how to self-interpret with what they”ve just done?”
4. Discussion
4.1. Discussion
This systematic literature review identified a range of decision support resources about genetic cancer susceptibility testing or cancer risk management for carriers. The heterogeneity of resources and study designs precluded meta-analysis. However, to meet study aims it was important that the systematic review search strategy was inclusive and broad to capture any types of resources, including digital, paper-based or educational that might benefit patients with different preferences and backgrounds. Any existing resources that could be delivered in current clinical practice or easily adapated might improve decision-making experience and outcomes, and needed to be considered.
Regarding the aims of this review:
i) decision support resources used to streamline cancer susceptibility genetic testing were non-inferior in terms of knowledge and decision satisfaction, however there was limited rigorous evaluation in randomised control trials compared to usual care. Decision support resources for carriers did not have a sustained effect on cancer-related distress, with transiently increased levels in one study possibly indicative of more deliberative decision-making. However, few studies included longer term follow-up beyond one to two months after resource use. Measures such as distress could change over time due to impact of the cancer diagnosis trajectory with changes in prognosis due to worsening disease. It is therefore important to make comparisons at multiple timepoints between patients with cancer who have used decision support resources to those who have not, to investigate the effect. Decisional conflict was low or moderate at baseline and with use of the intervention.
ii) all studies evaluating patient experience reported positive feedback on satisfaction and usefulness, however this was often by self-report, and many studies lacked a control group receiving usual care. There was a lack of patient diversity.
On balance, there was clear potential for decision support resources to be useful to help patients make more deliberative decisions in line with their personal values and situation, and may save time in clinic and healthcare resources. Taking into account the importance of varied patient preferences, there was no one best method of delivery, suggesting a flexible, multi-modal approach should be considered for future co-design of patient resources. This review highlighted several gaps in the availability of patient decision support resources to fulfil what patients in focus groups have told our research team that they want: trusted, up-to-date sources of information from experts that can also help them to educate their healthcare professionals and relatives (88).
Our findings align with results from a systematic review of Ottawa Decision Support Framework (ODSF)-based resources in 24 randomised controlled trials which showed PtDA used across a variety of medical specialities resulted in higher quality decisions and less HCP resource, compared to usual care (89). Some of the resources included in this review used a framework such as ODSF/IPDAS, but many did not which could have impacted effectiveness. The ODSF was recently updated following a review of use across 18 countries and >50,000 patients (90) to include decisional outcomes such as proportion of patients undecided, feeling uninformed, unsupported or unsure of values. There has been limited evaluation of these outcomes in decision support for genetic cancer susceptibility, and their inclusion should be given consideration in future research.
Streamlined, cost-effective pathways and patient resources are needed for HCP to deliver genomic testing “routinely to all people with cancer”, a commitment of the NHS Genomic Medicine Service (91) to inform surgical/treatment options, future cancer risks and risk to relatives. This is particularly relevant as genetic testing is moved into “mainstream” care, with patient-facing resources presenting an opportunity to more safely scale up delivery of testing without compromising informed decisions. Written or digital educational resources can be non-inferior to genetic counselling in the pre-test setting to increase knowledge (51, 54, 92).
Setting, mode of delivery and accessibility should be given due consideration; there was limited evaluation of this for the resources identified in this review. The results presented suggest people may not use a resource if asked to view a video/website/chatbot, or look at something at home, and they will rarely contact HCP with questions. It is not known whether these people did not need support or did not realise what support and benefit genetic counselling could provide. Those with more decision support needs should be referred to genetic counselling along with being offered tailored paper- and/or web-based patient resources because there is a suggestion that those with the greatest information needs may benefit most from additional decision support (43).
Quantifying genetic cancer susceptibility (known as “penetrance”) depends on the gene variant as well as age, medical and family history (93–96). Understanding of risk conferred by variants in cancer susceptibility genes has progressed at pace due to advances in genomic sequencing technology and large consortium studies (97–99). However, uncertainty remains about the likelihood that a carrier will develop cancer, which type of cancer and at what age. This is often experienced as trading one type of uncertainty for another, first finding out the genetic test result and then diverting thoughts and energy to thinking (or worrying) about what happens next. In patient-centred healthcare, uncertainty is multidimensional, including ethical (100), as well as scientific, system-based and personal factors linked to values, understanding and context (101, 102). Communication about uncertainty in a transparent, accessible way that engenders trust is a challenge for creators of ptDA, but if done successfully could improve understanding and emotional response (103–105) and make patients feel part of a team with their HCP (106). The findings of this review suggest the importance of including visual presentations of cancer risk to improve understanding of genetic cancer susceptibility and inform personalised decision-making.
Emerging research suggests that using digital technology such as smartphone applications could be acceptable and accessible for patients with lower literacy to consent for genetic testing (107), however continued bioethics exploration is needed to optimise inclusivitys. One size does not fit all, but harnessing digital technology to personalise decision support resources could empower more patients to take an active role in their care plan and improve health outcomes, consistent with the goals of the NHS Long Term Plan (108) and Universal Personalised Care Action Plan (109). This review has highlighted the usefulness, acceptability and time-saving nature of patient decision support resources, but has not identified one best method of delivery or any resources suitable for implementation in current clinical practice.
4.2. Future work
This review provides the groundwork to inform co-design with patients and other expert stakeholders from clinical genetics, oncology, charities, ethics, academic and health care bodies of a ptDA for Lynch syndrome, a genetic predisposition to certain cancers, mainly colorectal (bowel), endometrial (womb/uterine), ovarian and gastro-intestinal. The learning will be applied to create an adaptable template ptDA for genetic predispositions to other cancers. Most ptDA have focussed on pre-test decisions rather than genetic cancer susceptibility risk management. This highlighted the need for more resources for carriers, particularly for genes other than BRCA1 and BRCA2. The ptDA we are co-designing is tailored based on personal characteristics to present risk estimates and relevant options spanning from targeted treatment of cancer to primary prevention of future cancers. This will be multi-modal to allow wider dissemination, using an interactive website with the option to print personalised paper-based versions, question checklists and summaries of values-based exercises to take to an appointment with a healthcare professional. The Person-Based Approach (110) is being used to develop and iteratively optimise content and delivery of the ptDA, with attention to accessibility such as for patients with lower health literacy.
Future research is needed in the following areas:
• to determine the best modes of implementation in clinical practice
• co-design of decision support resources with patients, including from diverse communities
4.3. Strengths and limitations
Non-peer reviewed, published resources may not have been identified. The decision to exclude pre-2011 publications resulted in loss of some relevant (but dated) evidence. Since genetics and technology are evolving so rapidly, it was decided that the review would be more relevant if it was focussed on more recent publications. However, even recent studies lacked exploration of the impact of more complex genetic testing, for example large gene panel tests or whole genome sequencing, which lead to more additional, unexpected findings and variants of uncertain significance requiring more interpretation and uncertainty about recommended management options.
The findings of this systematic review may be subject to uncertainty due to methodological limitations of the included studies, including: lack of comparison to usual care using randomised controlled trials; use of unvalidated and patient-reported outcome measures; potential bias due to missing data (included untracked usage of resources) and short-term follow-up. Samples often lacked carriers (where included, these were exclusively BRCA1 and BRCA2). Studies were commonly conducted in a single centre or one country and therefore may not be generalisable.
4.4. Conclusions
More longitudinal research is needed regarding whether people complete actions in line with the decisions they make about cancer susceptibility genetic testing, cancer treatment and prevention. Longer term studies are important to understand whether people retain knowledge and accurate risk perception and maintain low levels of distress and decisional conflict over time after using decision support resources, compared to usual care. However, there has been little exploration of how measures such as distress might change over the longer term, and how this could be related to the psychological effects of a cancer diagnosis with changing prognosis, vs. the effect from using a PtDA which may be more transient. Evaluation should drive an iterative process of development and refinement of ptDA and determine the most effective mode of delivery in oncology and genetics services to improve health outcomes. Sustainable funding to update and securely host ptDA is essential to provide personalised cancer risk estimates and options based on current evidence and clinical guidelines.
Overall, the findings of this review suggest there is clear potential for ptDA and other decision support resources to complement the usual standard of care involving shared decision-making with healthcare professionals about genetic cancer susceptibility, but further development is needed to meet needs in current clincial practice. Psychological behavioural theory and a proven framework e.g., the ODSF (79) should underpin co-design, and quality standards should be met, e.g., IPDAS (24).
4.5. Practice implications
There are two main settings in which genetic cancer susceptibility patient decision support resources should be considered. First, healthcare professionals in oncology offering genetic cancer susceptibility testing for patients with cancer, where decisions to inform personalised treatment are often needed urgently. Patient-facing resources in this context could be brief and paper-based, with the option to delve into interactive, web-based resources and/or genetic counselling referral for those requiring or desiring a higher level of decision support. Secondly, post-genetic testing, carriers will need ongoing management of their lifelong condition, and ptDA can complement genetic counselling, encouraging decisions in accordance with personal values. Relatives of carriers will similarly need genetic counselling with a healthcare professional, but could additionally benefit from ptDA to increase knowledge pre-test.
A heterogeneous group of decision support resources has been identified in this review, each designed for a specific local care pathway and patient poulation. Clinical implementation shuld involves evaluating resources in complex care pathways, dealing with the realities of funding and staffing shortages present in the healthcare setting. Further research is needed to understand what decision support resources for genetic cancer susceptibility work for whom, how, why and in what setting (111, 112), with particular attention to patient values and preferences and ensuring inclusive accessibility.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
KK: conceptualisation, methodology, formal analysis, writing- original draft preparation. KM: conceptualisation, methodology, investigation, writing- reviewing and editing. LT: conceptualisation, formal analysis, writing- reviewing and editing. JS: methodology, writing- reviewing and editing. VF: methodology, writing- reviewing and editing. LW: methodology, writing- reviewing and editing. DE: conceptualisation, writing- reviewing and editing, supervision. CG: conceptualisation, writing- reviewing and editing, supervision. CF: conceptualisation, writing- reviewing and editing, supervision. All authors contributed to the article and approved the submitted version
Funding
This work was supported by Cancer Research UK [CG1296/A27223].
Acknowledgments
We would like to thank our CanGene-CanVar Patient Reference Panel members: Caroline Dale, Sue Duncombe, Rochelle Gold, Sonia Patton, Warren Rook, Richard Stephens, Lesley Turner, Frankie Vale, Helen White, Ivan Woodward, Steve Worrall, and Julie Young, other Patient and Public Involvement contributors from the community and our International Lynch Syndrome Decision Aid Stakeholder panel: Munaza Ahmed, Lyndsy Ambler, Antonis Antoniou, Stephanie Archer, Ruth Armstrong, Elizabeth Bancroft, Kristine Barlow-Stewart, Elizabeth Barnett, Marion Bartlett, Julian Barwell, Dany Bell, Cheryl Berlin, Matilda Bradford, John Burn, Sarah Cable, Dharmisha Chauhan, Ruth Cleaver, Elizabeth Coad, Gaya Connolly, Gillian Crawford, Emma Crosbie, Victoria Cuthill, Tabib Dahir, Karina Dahl Steffensen, Eleanor Davies, Glyn Elwyn, Mary Jane Esplen, D Gareth Evans, Pia Fabricius, Andrea Forman, Kaisa Fritzell, Claire Giffney, Joana Gomes, Rebecca Hall, Helen Hanson, Menna Hawkins, Deborah Holliday, Roberta Horgan, Karen Hurley, Margaret James, Ros Jewell, Sarah John, Siobhan John, Victoria Kiesel, Anna Koziel, Anjana Kulkarni, Fiona Lalloo, Helen Liggett, Aela Limbu, Kate Lippiett, Anne Lowry, Manami Matsukawa, Tracie Miles, Shakira Milton, Pål Møller, Kevin Monahan, Laura Monje-Garcia, Alex Murray, Jennie Murray, Kai-Ren Ong, Anbu Paramasivam, Alison Pope, Sarah Pugh, Gabriel Recchia, Nicola Reents, Peter Risby, Neil Ryan, Sibel Saya, Raza Sayyed, Salma Schickh, Lucy Side, Sian Smith, Tracy Smith, Dawn Stacey, Eriko Takamine, Katrina Tatton-Brown, Helle Vendel Petersen, Robert Volk, Jennifer Wiggins, Lisa Wilde, Jennet Williams, Catherine Willis, Elizabeth Winchester, Kristi Withington, Emma Woodward.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/frhs.2023.1092816/full#supplementary-material.
References
1. Excellence, N. I. f. H., & Care. Clinical Guideline [CG164] Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer | Guidance | NICE. 2013, NICE.
2. Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut. (2010) 59(5):666–89. doi: 10.1136/gut.2009.179804
3. Royal College of Physicians, Royal College of Pathologists and British Society for Genetic Medicin. Consent and confidentiality in genomic medicine: Guidance on the use of genetic and genomic information in the clinic. 2019, Report of the Joint Committee on Genomics in Medicine.: London.
4. Resta R, Biesecker BB, Bennett RL, Blum S, Hahn SE, Strecker MN, et al. A new definition of genetic counseling: national society of genetic Counselors’ task force report. J Genet Couns. (2006) 15(2):77–83. doi: 10.1007/s10897-005-9014-3
5. Biesecker B, Austin J, Caleshu C. Theories for psychotherapeutic genetic counseling: fuzzy trace theory and cognitive behavior theory. J Genet Couns. (2017) 26(2):322–30. doi: 10.1007/s10897-016-0023-1
6. Pilié PG, Gay CM, Byers LA, O’Connor MJ, Yap TA. PARP Inhibitors: extending benefit beyond BRCA-mutant cancers. Clin Cancer Res. (2019) 25(13):3759–71. doi: 10.1158/1078-0432.CCR-18-0968
7. Luchini C, Bibeau F, Ligtenberg MJL, Singh N, Nottegar A, Bosse T. ESMO Recommendations on microsatellite instability testing for immunotherapy in cancer, and its relationship with PD-1/PD-L1 expression and tumour mutational burden: a systematic review-based approach. Ann Oncol. (2019) 30(8):1232–43. doi: 10.1093/annonc/mdz116
8. Schon K, Tischkowitz M. Clinical implications of germline mutations in breast cancer: tP53. Breast Cancer Res Treat. (2018) 167(2):417–23. doi: 10.1007/s10549-017-4531-y
9. Mosele F, Remon J, Mateo J, Westphalen CB, Barlesi F, Lolkema MP, et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: a report from the ESMO precision medicine working group. Ann Oncol. (2020) 31(11):1491–505. doi: 10.1016/j.annonc.2020.07.014
10. Mandelker D, Donoghue M, Talukdar S, Bandlamudi C, Srinivasan P, Vivek M, et al. Germline-focussed analysis of tumour-only sequencing: recommendations from the ESMO precision medicine working group. Ann Oncol. (2019) 30(8):1221–31. doi: 10.1093/annonc/mdz136
11. Turnbull C, Sud A, Houlston RS. Cancer genetics, precision prevention and a call to action. Nat Genet. (2018) 50(9):1212–8. doi: 10.1038/s41588-018-0202-0
12. Grzymski JJ, Elhanan G, Morales Rosado JA, Smith E, Schlauch KA, Read R, et al. Population genetic screening efficiently identifies carriers of autosomal dominant diseases. Nat Med. (2020) 26(8):1235–9. doi: 10.1038/s41591-020-0982-5
13. Manchanda R, Burnell M, Gaba F, Desai R, Wardle J, Gessler S, et al. Randomised trial of population-based BRCA testing in ashkenazi Jews: long-term outcomes. Bjog. (2020) 127(3):364–75. doi: 10.1111/1471-0528.15905
14. Madlensky L, Trepanier AM, Cragun D, Lerner B, Shannon KM, Zierhut H. A rapid systematic review of outcomes studies in genetic counseling. J Genet Couns. (2017) 26(3):361–78. doi: 10.1007/s10897-017-0067-x
15. Bayne M, Fairey M, Silarova B, Griffin SJ, Sharp SJ, Klein WMP, et al. Effect of interventions including provision of personalised cancer risk information on accuracy of risk perception and psychological responses: a systematic review and meta-analysis. Patient Educ Couns. (2020) 103(1):83–95. doi: 10.1016/j.pec.2019.08.010
16. Turbitt E, Roberts MC, Taber JM, Waters EA, McNeel TS, Biesecker BB, et al. Genetic counseling, genetic testing, and risk perceptions for breast and colorectal cancer: results from the 2015 national health interview survey. Prev Med. (2019) 123:12–9. doi: 10.1016/j.ypmed.2019.02.027
17. Tversky A, Kahneman D. The framing of decisions and the psychology of choice. Science. (1981) 211(4481):453. doi: 10.1126/science.7455683
18. Senay I, Kaphingst KA. Anchoring-and-Adjustment bias in communication of disease risk. Med Decis Making. (2008) 29(2):193–201. doi: 10.1177/0272989X08327395
19. Montgomery v Lanarkshire Health Board (2015). Available at: https://www.supremecourt.uk/cases/docs/uksc-2013-0136-judgment.pdf
20. Excellence, N. I. f. H. a. C. Clinical Guideline [CG138] Patient experience in adult NHS services: improving the experience of care for people using adult NHS services | Guidance | NICE. (2021). NICE Available at:https://www.nice.org.uk/guidance/cg138.
21. O’Connor AM, Bennett CL, Stacey D, Barry M, Col NF, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2009) 3. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001431.pub2/full
22. Elwyn G, O’Connor AM, Bennett C, Newcombe RG, Politi M, Durand MA, et al. Assessing the quality of decision support technologies using the international patient decision aid standards instrument (IPDASi). PLoS One. (2009) 4(3):e4705. doi: 10.1371/journal.pone.0004705
23. Stacey D, Legare F, Lewis K, Barry MJ, Bennett CL, Eden KB, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. (2017) 4. https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD001431.pub5/full28402085
24. O’Connor AM, Bennett C, Stacey D, Barry MJ, Col NE, Eden KB, et al. Do patient decision aids meet effectiveness criteria of the international patient decision aid standards collaboration? A systematic review and meta-analysis. Med Decis Making. (2007) 27(5):554–74. doi: 10.1177/0272989X07307319
25. Durand M-A, Stiel M, Boivin J, Elwyn G. Where is the theory? Evaluating the theoretical frameworks described in decision support technologies. Patient Educ Couns. (2008) 71(1):125–35. doi: 10.1016/j.pec.2007.12.004
26. Hilgart JS, Hayward JA, Coles B, Iredale R. Telegenetics: a systematic review of telemedicine in genetics services. Genet Med. (2012) 14(9):765–76. doi: 10.1038/gim.2012.40
27. Morgan D, Sylvester H, Lucas FL, Miesfeldt S. Hereditary breast and ovarian cancer: referral source for genetic assessment and communication regarding assessment with nongenetic clinicians in the community setting. Genet Med. (2010) 12(1):25–31. doi: 10.1097/GIM.0b013e3181c60955
28. Sie AS, van Zelst-Stams WA, Spruijt L, Mensenkamp AR, Ligtenberg MJ, Brunner HG, et al. More breast cancer patients prefer BRCA-mutation testing without prior face-to-face genetic counseling. Fam Cancer. (2014) 13(2):143–51. doi: 10.1007/s10689-013-9686-z
29. Sturm AC, Schmidlen T, Scheinfeldt L, Hovick S, McElroy JP, Toland AE, et al. Early outcome data assessing utility of a post-test genomic counseling framework for the scalable delivery of precision health. J Pers Med. (2018) 8(3). doi: 10.3390/jpm8030025
30. Grimmett C, Pickett K, Shepherd J, Welch K, Recio-Saucedo A, Streit E, et al. Systematic review of the empirical investigation of resources to support decision-making regarding BRCA1 and BRCA2 genetic testing in women with breast cancer. Patient Educ Couns. (2018) 101(5):779–88. doi: 10.1016/j.pec.2017.11.016
31. Krassuski L, Vennedey V, Stock S, Kautz-Freimuth S. Effectiveness of decision aids for female BRCA1 and BRCA2 mutation carriers: a systematic review. BMC Med Inform Decis Mak. (2019) 19. doi: 10.1186/s12911-019-0872-2. https://bmcmedinformdecismak.biomedcentral.com/articles/10.1186/s12911-019-0872-231370837
32. Kautz-Freimuth S, Redaèlli M, Rhiem K, Vodermaier A, Krassuski L, Nicolai K, et al. Development of decision aids for female BRCA1 and BRCA2 mutation carriers in Germany to support preference-sensitive decision-making. BMC Med Inform Decis Mak. (2021) 21(1):180–180. doi: 10.1186/s12911-021-01528-4
33. Systematic Reviews: Guidance from the Centre for Reviews and Dissemination. (2009) [05/03/2021]; Available at: https://www.york.ac.uk/crd/guidance/
34. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
35. Rayyan . (cited 2021 01/05/2021); Available at: https://rayyan.ai
36. Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ: British Medical Journal. (2014) 348:g1687. doi: 10.1136/bmj.g1687
37. Excellence, N. I. f. H., & Care. Process and methods [PMG4] Methods for the development of NICE public health guidance (third edition).(2012). Available at: https://www.nice.org.uk/process/pmg4/chapter/appendix-f-quality-appraisal-checklist-quantitative-intervention-studies.
38. Hong Q, Pluye P, Fàbregues S, Bartlett G, Boardman F, Cargo M, et al. Mixed Methods Appraisal Tool (MMAT). 2018, Canadian Intellectual Property Office, Industry Canada.
39. Campbell M, McKenzie JE, Sowden A, Katikireddi SV, Brennan SE, Ellis S, et al. Synthesis without meta-analysis (SWiM) in systematic reviews: reporting guideline. Br Med J. (2020) 368:l6890. doi: 10.1136/bmj.l6890
40. Popay J. Guidance on the Conduct of Narrative Synthesis in Systematic Reviews. (2006) [12/03/2021]; Available at: https://www.lancaster.ac.uk/media/lancaster-university/content-assets/documents/fhm/dhr/chir/NSsynthesisguidanceVersion1-April2006.pdf
41. Mays N, Pope C, Popay J. Systematically reviewing qualitative and quantitative evidence to inform management and policy-making in the health field. J Health Serv Res Policy. (2005) 10(Suppl 1):6–20. doi: 10.1258/1355819054308576
42. Mulrow C, Langhorne P, Grimshaw J. Integrating heterogeneous pieces of evidence in systematic reviews. Ann Intern Med. (1997) 127(11):989–95. doi: 10.7326/0003-4819-127-11-199712010-00008
43. Albada A, Ausems M, Otten R, Bensing JM, van Dulmen S. Use and evaluation of an individually tailored website for counselees prior to breast cancer genetic counseling. J Cancer Educ. (2011) 26(4):670–81. doi: 10.1007/s13187-011-0227-x
44. Tea MKM, Tan YY, Staudigl C, Eibl B, Renz R, Asseryanis E, et al. Improving comprehension of genetic counseling for hereditary breast and ovarian cancer clients with a visual tool. Plos One. (2018) 13(7). doi: 10.1371/journal.pone.0200559 30001421
45. Jabaley T, Underhill-Blazey ML, Berry DL. Development and testing of a decision aid for unaffected women with a BRCA1 or BRCA2 mutation. J Cancer Educ. (2020) 35(2):339–44. doi: 10.1007/s13187-019-1470-9
46. Schackmann EA, Munoz DF, Mills MA, Plevritis SK, Kurian AW. Feasibility evaluation of an online tool to guide decisions for BRCA1/2 mutation carriers. Fam Cancer. (2013) 12(1):65–73. doi: 10.1007/s10689-012-9577-8
47. Harmsen MG, Steenbeek MP, Hoogerbrugge N, van Doorn HC, Gaarenstroom KN, Vos MC, et al. A patient decision aid for risk-reducing surgery in premenopausal BRCA1/2 mutation carriers: development process and pilot testing. Health Expect. (2018) 21(3):659–67. doi: 10.1111/hex.12661
48. Heald B, Keel E, Marquard J, Burke CA, Kalady MF, Church JM, et al. Using chatbots to screen for heritable cancer syndromes in patients undergoing routine colonoscopy. J Med Genet. (2020) 58(12):807–14. doi: 10.1136/jmedgenet-2020-107294
49. Peshkin BN, Ladd MK, Isaacs C, Segal H, Jacobs A, Taylor KL, et al. The genetic education for men (GEM) trial: development of web-based education for untested men in BRCA1/2-positive families. J Cancer Educ. (2021) 36(1):72–84. doi: 10.1007/s13187-019-01599-y
50. Vadaparampil ST, Malo TL, Nam KM, Nelson A, de la Cruz CZ, Quinn GP. From observation to intervention: development of a psychoeducational intervention to increase uptake of BRCA genetic counseling among high-risk breast cancer survivors. J Cancer Educ. (2014) 29(4):709–19. doi: 10.1007/s13187-014-0643-9
51. Manchanda R, Burnell M, Loggenberg K, Desai R, Wardle J, Sanderson SC, et al. Cluster-randomised non-inferiority trial comparing DVD-assisted and traditional genetic counselling in systematic population testing for BRCA1/2 mutations. J Med Genet. (2016) 53(7):472–80. doi: 10.1136/jmedgenet-2015-103740
52. Sherman KA, Kilby CJ, Shaw LK, Winch C, Kirk J, Tucker K, et al. Facilitating decision-making in women undergoing genetic testing for hereditary breast cancer: bRECONDA randomized controlled trial results. Breast. (2017) 36:79–85. doi: 10.1016/j.breast.2017.10.001
53. Albada A, van Dulmen S, Spreeuwenberg P, Ausems MG. Follow-up effects of a tailored pre-counseling website with question prompt in breast cancer genetic counseling. Patient Educ Couns. (2015) 98(1):69–76. doi: 10.1016/j.pec.2014.10.005
54. McCuaig JM, Tone AA, Maganti M, Romagnuolo T, Ricker N, Shuldiner J, et al. Modified panel-based genetic counseling for ovarian cancer susceptibility: a randomized non-inferiority study. Gynecol Oncol. (2019) 153(1):108–15. doi: 10.1016/j.ygyno.2018.12.027
55. Metcalfe KA, Dennis CL, Poll A, Armel S, Demsky R, Carlsson L, et al. Effect of decision aid for breast cancer prevention on decisional conflict in women with a BRCA1 or BRCA2 mutation: a multisite, randomized, controlled trial. Genet Med. (2017) 19(3):330–6. doi: 10.1038/gim.2016.108
56. Albada A, van Dulmen S, Ausems M, Bensing JM. A pre-visit website with question prompt sheet for counselees facilitates communication in the first consultation for breast cancer genetic counseling: findings from a randomized controlled trial. Genet Med. (2012) 14(5):535–42. doi: 10.1038/gim.2011.42
57. Nilsson MP, Nilsson ED, Borg Å, Brandberg Y, Silfverberg B, Loman N. High patient satisfaction with a simplified BRCA1/2 testing procedure: long-term results of a prospective study. Breast Cancer Res Treat. (2019) 173(2):313–8. doi: 10.1007/s10549-018-5000-y
58. Sie AS, Spruijt L, van Zelst-Stams WA, Mensenkamp AR, Ligtenberg MJ, Brunner HG, et al. High satisfaction and low distress in breast cancer patients one year after BRCA-mutation testing without prior face-to-face genetic counseling. J Genet Couns. (2016) 25(3):504–14. doi: 10.1007/s10897-015-9899-4
59. Hooker GW, Leventhal KG, DeMarco T, Peshkin BN, Finch C, Wahl E, et al. Longitudinal changes in patient distress following interactive decision aid use among BRCA1/2 carriers: a randomized trial. Med Decis Making. (2011) 31(3):412–21. doi: 10.1177/0272989X10381283
60. Meiser B, Gleeson M, Watts K, Peate M, Zilliacus E, Barlow-Stewart K, et al. Getting to the point: what women newly diagnosed with breast cancer want to know about treatment-focused genetic testing. Oncol Nurs Forum. (2012) 39(2):E101–11. doi: 10.1188/12.ONF.E101-E111
61. Scherr CL, Nam K, Augusto B, Kasting ML, Caldwell M, Lee MC, et al. A framework for pilot testing health risk video narratives. Health Commun. (2020) 35(7):832–41. doi: 10.1080/10410236.2019.1598612
62. Yuen J, Cousens N, Barlow-Stewart K, O’Shea R, Andrews L. Online BRCA1/2 screening in the Australian Jewish community: a qualitative study. J Community Genet. (2020) 11(3):291–302. doi: 10.1007/s12687-019-00450-7
63. Culver JO, MacDonald DJ, Thornton AA, Sand SR, Grant M, Bowen DJ, et al. Development and evaluation of a decision aid for BRCA carriers with breast cancer. J Genet Couns. (2011) 20(3):294–307. doi: 10.1007/s10897-011-9350-4
64. Grimmett C, Brooks C, Recio-Saucedo A, Armstrong A, Cutress RI, Evans DG, et al. Development of breast cancer choices: a decision support tool for young women with breast cancer deciding whether to have genetic testing for BRCA1/2 mutations. Support Care Cancer. (2019) 27(1):297–309. doi: 10.1007/s00520-018-4307-x
65. Gornick MC, Kurian AW, An LC, Fagerlin A, Jagsi R, Katz SJ, et al. Knowledge regarding and patterns of genetic testing in patients newly diagnosed with breast cancer participating in the iCanDecide trial. Cancer. (2018) 124(20):4000–9. doi: 10.1002/cncr.31731
66. Kasting ML, Conley CC, Hoogland AI, Scherr CL, Kim J, Thapa R, et al. A randomized controlled intervention to promote readiness to genetic counseling for breast cancer survivors. Psychooncology. (2019) 28(5):980–8.30883986
67. Quinn VF, Meiser B, Kirk J, Tucker KM, Watts KJ, Rahman B, et al. Streamlined genetic education is effective in preparing women newly diagnosed with breast cancer for decision making about treatment-focused genetic testing: a randomized controlled noninferiority trial. Genet Med. (2017) 19(4):448–56. doi: 10.1038/gim.2016.130
68. Watson CH, Ulm M, Blackburn P, Smiley L, Reed M, Covington R, et al. Video-assisted genetic counseling in patients with ovarian, fallopian and peritoneal carcinoma. Gynecol Oncol. (2016) 143(1):109–12. doi: 10.1016/j.ygyno.2016.07.094
69. Høberg-Vetti H, Eide GE, Siglen E, Listøl W, Haavind MT, Hoogerbrugge N, et al. Cancer-related distress in unselected women with newly diagnosed breast or ovarian cancer undergoing BRCA1/2 testing without pretest genetic counseling. Acta Oncol. (2019) 58(2):175–81. doi: 10.1080/0284186X.2018.1502466
70. Dekker N, Hermens RP, de Wilt JH, van Zelst-Stams WA, Hoogerbrugge N. RISCO Study Group. Improving recognition and referral of patients with an increased familial risk of colorectal cancer: results from a randomized controlled trial. Colorectal Dis. (2015) 17(6):499–510. doi: 10.1111/codi.12880
71. Hall MJ, Manne SL, Winkel G, Chung DS, Weinberg DS, Meropol NJ. Effects of a decision support intervention on decisional conflict associated with microsatellite instability testing. Cancer Epidemiol Biomark Prev. (2011) 20(2):249–54. doi: 10.1158/1055-9965.EPI-10-0685
72. Arrick BA, Bloch KJ, Colello LS, Woloshin S, Schwartz LM. Visual representations of risk enhance long-term retention of risk information: a randomized trial. Med Decis Making. (2019) 39(2):100–7. doi: 10.1177/0272989X18819493
73. Cragun D, Weidner A, Tezak A, Zuniga B, Wiesner GL, Pal T. A web-based tool to automate portions of pretest genetic counseling for inherited cancer. J Natl Compr Canc Netw. (2020) 18(7):841–7. doi: 10.6004/jnccn.2020.7546
74. Albada A, van Dulmen S, Lindhout D, Bensing JM, Ausems M. A pre-visit tailored website enhances counselees’ realistic expectations and knowledge and fulfils information needs for breast cancer genetic counselling. Fam Cancer. (2012) 11(1):85–95. doi: 10.1007/s10689-011-9479-1
75. Petty RE, Cacioppo JT. The elaboration likelihood model of persuasion. In: Berkowitz L, editors. Advances in experimental social psychology. New York: Academic Press (1986). p. 123–205.
76. Reyna VF. A theory of medical decision making and health: fuzzy trace theory. Med Decis Making. (2008) 28(6):850–65. doi: 10.1177/0272989X08327066
77. Janz NK, Becker MH. The health belief model: a decade later. Health Educ Q. (1984) 11(1):1–47. doi: 10.1177/109019818401100101
78. Prochaska JO, Velicer WF. The transtheoretical model of health behavior change. Am J Health Promot. (1997) 12(1):38–48. doi: 10.4278/0890-1171-12.1.38
79. Ottawa Decision Support Framework. Available at: https://decisionaid.ohri.ca/odsf.html
80. Keeney RL, Raiffa H. Decisions with multiple objectives: Preferences and value trade-offs. Cambridge: Cambridge University Press (1993).
81. Edwards W, Newman WEJR, Newman JR, Snapper K. Multiattribute evaluation. New York: SAGE Publications (1982).
82. Michie S, Dormandy E, Marteau TM. The multi-dimensional measure of informed choice: a validation study. Patient Educ Couns. (2002) 48(1):87–91. doi: 10.1016/S0738-3991(02)00089-7
83. Michie S, Dormandy E, Marteau TM. Informed choice: understanding knowledge in the context of screening uptake. Patient Educ Couns. (2003) 50(3):247–53. doi: 10.1016/S0738-3991(03)00044-2
84. Høberg-Vetti H, Bjorvatn C, Fiane BE, Aas T, Woie K, Espelid H, et al. BRCA1/2 Testing in newly diagnosed breast and ovarian cancer patients without prior genetic counselling: the DNA-BONus study. Eur J Hum Genet. (2016) 24(6):881–8. doi: 10.1038/ejhg.2015.196
85. Nilsson MP, Törngren T, Henriksson K, Kristoffersson U, Kvist A, Silfverberg B, et al. BRCAsearch: written pre-test information and BRCA1/2 germline mutation testing in unselected patients with newly diagnosed breast cancer. Breast Cancer Res Treat. (2018) 168(1):117–26. doi: 10.1007/s10549-017-4584-y
86. Nilsson MP, Nilsson ED, Silfverberg B, Borg Å, Loman N. Written pretest information and germline BRCA1/2 pathogenic variant testing in unselected breast cancer patients: predictors of testing uptake. Genet Med. (2019) 21(1):89–96. doi: 10.1038/s41436-018-0021-9
87. O’Connor AM. Validation of a decisional conflict scale. Med Decis Making. (1995) 15(1):25–30. doi: 10.1177/0272989X9501500105
88. Morton K, Kohut K, Turner L, Smith S, Crosbie E. J, Ryan N, et al. Person-based co-design of a decision aid template for people with a genetic predisposition to cancer. Front in Dig Health. (2022) 4. doi: 10.3389/fdgth.2022.1039701
89. Hoefel L, Lewis KB, O’Connor A, Stacey D. 20th Anniversary update of the Ottawa decision support framework: part 2 subanalysis of a systematic review of patient decision aids. Med Decis Making. (2020) 40(4):522–39. doi: 10.1177/0272989X20924645
90. Stacey D, Légaré F, Boland L, Lewis KB, Loiselle M-C, Hoefel L, et al. 20th Anniversary Ottawa decision support framework: part 3 overview of systematic reviews and updated framework. Med Decis Making. (2020) 40(3):379–98. doi: 10.1177/0272989X20911870
91. England NHS. NHS Genomic Medicine Service. 2021 27/07/2021]; Available at: https://www.england.nhs.uk/genomics/nhs-genomic-med-service/
92. Biesecker BB, Lewis KL, Umstead KL, Johnston JJ, Turbitt E, Fishier KP, et al. Web platform vs in-person genetic counselor for return of carrier results from exome sequencing A randomized clinical trial. JAMA Intern Med. (2018) 178(3):338–46. doi: 10.1001/jamainternmed.2017.8049
93. Shin SJ, Dodd-Eaton EB, Peng G, Bojadzieva J, Chen J, Amos CI, et al. Penetrance of different cancer types in families with li-fraumeni syndrome: a validation study using multicenter cohorts. Cancer Res. (2020) 80(2):354–60. doi: 10.1158/0008-5472.CAN-19-0728
94. Tung N, Domchek SM, Stadler Z, Nathanson KL, Couch F, Garber JE, et al. Counselling framework for moderate-penetrance cancer-susceptibility mutations. Nat Rev Clin Oncol. (2016) 13(9):581–8. doi: 10.1038/nrclinonc.2016.90
95. Valle L, Vilar E, Tavtigian SV, Stoffel EM. Genetic predisposition to colorectal cancer: syndromes, genes, classification of genetic variants and implications for precision medicine. J Pathol. (2019) 247(5):574–88. doi: 10.1002/path.5229
96. Batalini F, Peacock EG, Stobie L, Robertson A, Garber J, Weitzel JN, et al. Li-Fraumeni syndrome: not a straightforward diagnosis anymore-the interpretation of pathogenic variants of low allele frequency and the differences between germline PVs, mosaicism, and clonal hematopoiesis. Breast Cancer Res. (2019) 21(1):107. doi: 10.1186/s13058-019-1193-1
97. Dominguez-Valentin M, Sampson JR, Seppälä TT, Ten Broeke SW, Plazzer JP, Nakken S, et al. Cancer risks by gene, age, and gender in 6350 carriers of pathogenic mismatch repair variants: findings from the prospective lynch syndrome database. Genet Med. (2020) 22(1):15–25. doi: 10.1038/s41436-019-0596-9
98. Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. Jama. (2017) 317(23):2402–16. doi: 10.1001/jama.2017.7112
99. Mai PL, Best AF, Peters JA, DeCastro RM, Khincha PP, Loud JT, et al. Risks of first and subsequent cancers among TP53 mutation carriers in the national cancer institute li-fraumeni syndrome cohort. Cancer. (2016) 122(23):3673–81. doi: 10.1002/cncr.30248
100. Cribb A. Managing ethical uncertainty: implicit normativity and the sociology of ethics. Sociol Health Illn. (2020) 42(S1):21–34. doi: 10.1111/1467-9566.13010
101. Han PKJ, Klein WMP, Arora NK. Varieties of uncertainty in health care: a conceptual taxonomy. Med Decis Making. (2011) 31(6):828–38. doi: 10.1177/0272989X10393976
102. Han PKJ, Umstead KL, Bernhardt BA, Green RC, Joffe S, Koenig B, et al. A taxonomy of medical uncertainties in clinical genome sequencing. Genet Med. (2017) 19(8):918–25. doi: 10.1038/gim.2016.212
103. Newson AJ, Leonard SJ, Hall A, Gaff CL. Known unknowns: building an ethics of uncertainty into genomic medicine. BMC Med Genomics. (2016) 9(1):57–57. doi: 10.1186/s12920-016-0219-0
104. Stivers T, Timmermans S. Negotiating the diagnostic uncertainty of genomic test results. Soc Psychol Q. (2016) 79(3):199–221. doi: 10.1177/0190272516658770
105. Brashers DE. Communication and uncertainty management. Journal of Communication. (2001) 51(3):477–97. doi: 10.1111/j.1460-2466.2001.tb02892.x
106. Clarke AJ, Wallgren-Pettersson C. Ethics in genetic counselling. J Community Genet. (2019) 10(1):3–33. doi: 10.1007/s12687-018-0371-7
107. Kraft SA, Porter KM, Duenas DM, Guerra C, Joseph G, Lee SS-J, et al. Participant reactions to a literacy-focused, web-based informed consent approach for a genomic implementation study. AJOB Empir Bioeth. (2021) 12(1):1–11. doi: 10.1080/23294515.2020.1823907
108. England N. NHS Long term plan. (2019) :24–6. https://www.longtermplan.nhs.uk/
109. England NHS. Universal Personalised Care: Implementing the Comprehensive Model.(2019). Available at: https://www.england.nhs.uk/publication/universal-personalised-care-implementing-the-comprehensive-model/
110. Yardley L, Morrison L, Bradbury K, Muller I. The person-based approach to intervention development: application to digital health-related behavior change interventions. J Med Internet Res. (2015) 17(1):e30. doi: 10.2196/jmir.4055
111. Pawson R. Evidence-based policy: the promise of `realist synthesis’. Evaluation. (2002) 8(3):340–58. doi: 10.1177/135638902401462448
Keywords: cancer genetics, genetic counselling, patient decision aid, shared decision-making, decision support
Citation: Kohut K, Morton K, Turner L, Shepherd J, Fenerty V, Woods L, Grimmett C, Eccles DM and Foster C (2023) Patient decision support resources inform decisions about cancer susceptibility genetic testing and risk management: a systematic review of patient impact and experience. Front. Health Serv. 3:1092816. doi: 10.3389/frhs.2023.1092816
Received: 18 November 2022; Accepted: 26 April 2023;
Published: 31 May 2023.
Edited by:
Nisha Shah, University of Oxford, United KingdomReviewed by:
Leonieke Kranenburg, Erasmus Medical Center, NetherlandsErin Turbitt, University of Technology Sydney, Australia
© 2023 Kohut, Morton, Turner, Shepherd, Fenerty, Woods, Grimmett, Eccles and Foster. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kelly Kohut k.e.kohut@soton.ac.uk
 Kelly Kohut
Kelly Kohut Kate Morton
Kate Morton Lesley Turner1
Lesley Turner1  Diana M. Eccles
Diana M. Eccles Claire Foster
Claire Foster