Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration
- 1Department of Cell and Molecular Biology, Tulane University, New Orleans, LA, United States
- 2Department of Ophthalmology, Tulane University, New Orleans, LA, United States
- 3Tulane Personalized Health Institute, Tulane University, New Orleans, LA, United States
Retinal pigment epithelial (RPE) cells form a monolayer between the neuroretina and choroid. It has multiple important functions, including acting as outer blood-retina barrier, maintaining the function of neuroretina and photoreceptors, participating in the visual cycle and regulating retinal immune response. Due to high oxidative stress environment, RPE cells are vulnerable to dysfunction, cellular senescence, and cell death, which underlies RPE aging and age-related diseases, including age-related macular degeneration (AMD). Mitochondria are the powerhouse of cells and a major source of cellular reactive oxygen species (ROS) that contribute to mitochondrial DNA damage, cell death, senescence, and age-related diseases. Mitochondria also undergo dynamic changes including fission/fusion, biogenesis and mitophagy for quality control in response to stresses. The role of mitochondria, especially mitochondrial dynamics, in RPE aging and age-related diseases, is still unclear. In this review, we summarize the current understanding of mitochondrial function, biogenesis and especially dynamics such as morphological changes and mitophagy in RPE aging and age-related RPE diseases, as well as in the biological processes of RPE cellular senescence and cell death. We also discuss the current preclinical and clinical research efforts to prevent or treat RPE degeneration by restoring mitochondrial function and dynamics.
1 Introduction
1.1 Functions of Retinal Pigmented Epithelial Cells
The RPE comprises a monolayer of polarized epithelial cells located in between the neuroretina and choroid. It functions as the outer blood-retina barrier and as a conduit for oxygen, nutrients, and waste between the neuroretina and choriocapillaris. RPE cells are highly polarized with regard to proteins and organelles distribution or secretion (Burke, 2008). On the apical side, microvilli of the RPE cells envelop and interact with photoreceptor outer segments (POS) of rods and cones; while on the basal side, the RPE cells display highly convoluted microfolds with close interaction with Bruch’s membrane (BrM) and the underlying choroid capillaries. RPE cells have many important functions including: 1) Maintaining function of the neuroretina. RPE cells synthesize and store melanin which absorbs reflected light that may otherwise degrade the visual image (Hu et al., 2008). 2) Providing a stable environment for RPE and nearby cells by maintaining the volume, ion concentrations and chemical composition of the subretinal space through transporters such as sodium/potassium adenosine triphosphatase (Na+/K+-ATPase) on RPE membrane (Wimmers et al., 2007). 3) Maintaining the function of photoreceptors. The POS regenerates every 7–12 days, RPE cells function to clear the old POS through phagocytosis, which protects photoreceptors from chronic oxidative stress exposure (Bok, 1985; Mazzoni et al., 2014). 4) Participating in the visual cycle. Through the process of visual cycle, retinoids cycle between the rod outer segments and the RPE. Light isomerizes 11-cis retinal into all-trans retinal causing visual pigment activation. RPE cells then take the photoproducts and regenerate 11-cis retinal before its returning to photoreceptors (McBee et al., 2001). 5) Regulating retinal immune response. Cytokines secreted by RPE, such as interleukin (IL)-1α, IL-1β, IL-7, tumor necrosis factor (TNF)-α, interferon (IFN)-γ, transforming growth factor (TGF)-β, play an important role in the homeostasis and inflammatory responses of the retina by activating resident immune cells and attracting circulating inflammatory cells (Holtkamp et al., 2001). Overall, RPE cells are fundamentally important for the metabolism and homeostasis of neuroretina. For more information of RPE function, refer to a review by Sparrrow et al. (Sparrow et al., 2010).
1.2 Overview of Cellular Senescence and Cell Death
Cellular senescence and cell death play important roles in aging and age-related diseases. Here we briefly summarize the two processes. Cellular senescence is a stable cell cycle arrest, which involves metabolic reprogramming, chromatin rearrangement and autophagy modulation (Kuilman et al., 2010). Senescent cells present enlarged cell size, increased reactive oxygen species (ROS) levels, persistent DNA damage response, arrested growth, apoptosis resistance, disorganized chromatin and changed gene expression (Chen et al., 2000; Hampel et al., 2004; Herbig et al., 2004). They also release chemokines, cytokines, proteases, and growth factors, which is collectively called senescence-associated secretory phenotype that would affect neighboring cells (Nelson et al., 2012). The accumulation of senescent cells could drive aging and age-related diseases (Childs et al., 2015). Multiple types of cell death could exist in RPE cells. Apoptosis is a classic type of programmed cell death which is regulated by the caspase family of proteins. Regulated necrosis also happens in RPE cells which includes but not limited to necroptosis, pyroptosis and ferroptosis. Necroptosis is morphologically characterized by cell swelling and bursting, associated with the release of intracellular contents (Hanus et al., 2013; Hanus et al., 2016). Activation of necrosomes is a marker of necroptosis. Pyroptosis is mediated by inflammasome activation and release of proinflammatory intracellular contents, including IL-1β and IL-18. Ferroptosis is characterized by lipid peroxidation and iron involvement. Ferroptotic cells usually do not have typical morphological characteristics of necrosis, but display mitochondrial shrinkage, increased mitochondrial membrane density and reduced mitochondrial cristae (Yagoda et al., 2007; Yang and Stockwell, 2008). For more in-depth review of cell death and senescence in RPE cells, please refer to our recent reviews (Hanus et al., 2015; Tong and Wang, 2020).
1.3 Mitochondrial Functions and Dynamics
Mitochondria are double membrane organelles in the cell responsible for energy production. Electron transport chain (ETC) and adenosine triphosphate (ATP) synthase are located within mitochondrial inner membrane, while enzymes of the tricarboxylic acid (TCA) cycle and fatty acid oxidation are in the matrix. Thus, mitochondria are very important for cellular energy metabolism, generating the majority of cellular ATP in eukaryotes (Wallace, 2009). Mitochondrial ATP production and membrane potential require the universal cofactor nicotinamide adenine dinucleotide (NAD). As an essential coenzyme, NAD gains two electrons and a proton from substrates at multiple TCA cycle steps, being reduced to NADH. An optimal NAD/NADH ratio is essential for efficient mitochondrial metabolism as TCA cycle ETC require NAD and NADH respectively (Stein and Imai, 2012). Mitochondria can provide energy to transport calcium (Ca2+) against its concentration gradient. They also modulate Ca2+ concentrations in the cytosol, sequestering the ion in the mitochondrial matrix which helps to maintain the appropriate concentrations of Ca2+ inside the endoplasmic reticulum (ER) and near the sites of exocytosis (Rizzuto et al., 2012; Finkel et al., 2015). Mitochondria also provide activation signals, such as mitochondrial ROS and oxidized mitochondrial DNA (mtDNA), and structural platform for inflammasome assembly and activation (Shimada et al., 2012; Elliott et al., 2018). What’s more, mitochondria can regulate ketone body formation, heme biosynthesis and the urea cycle (Boyman et al., 2020). In addition, mitochondria are also responsible for producing cell signaling molecules (Boyman et al., 2020), and many cellular processes including cellular senescence and cell death (Davalli et al., 2016). Mitochondria are highly dynamic and undergo fusion/fission, biogenesis, and mitophagy in response to energy needs and stresses. Together, these constitute mitochondrial quality control, which is essential for maintaining mitochondrial homeostasis and function (Meyer et al., 2017; Ren et al., 2020). The processes that are involved in mitochondrial quality control include:
1) Mitochondrial fusion is the process by which the outer membrane guanosine triphosphatases (GTPase) proteins mitofusins (MFN) fuse two outer mitochondrial membranes and the inner membrane GTPase optic atrophy 1 (OPA1) fuses two mitochondrial inner membranes to form one mitochondrion. It is a potential mitochondrial repair mechanism through the diffusion of mtDNA and proteins (van der Bliek et al., 2013; Tilokani et al., 2018).
2) Mitochondrial fission increases the number of mitochondria. In this process, dynamin-related protein 1 (DRP1) is recruited by mitochondrial outer membrane proteins including mitochondrial fission factor, and forms a ring around the mitochondrion, clinching it to eventually form two separate mitochondria. Mitochondrial fission is a regular event during cell division and can be induced by oxidative stress and DNA damage (van der Bliek et al., 2013; Tilokani et al., 2018). Defective or imbalanced mitochondrial fission/fusion may affect mitochondrial motility and energy production, promote oxidative stress and mtDNA deletion, and impair Ca2+ buffering, all of which could lead to cell death (Chan, 2012).
3) Mitochondrial biogenesis is the process of mitochondrial self-replication, involving replication and expression of mtDNA-encoded genes, as well as the synthesis and import of nuclear-encoded mitochondrial genes (Ploumi et al., 2017). It occurs in response to the energy demand triggered by developmental signals and environmental stressors. Peroxisome proliferator-activated receptor gamma coactivator (PGC)-1α and nuclear respiratory factors (NRFs) are key regulators of mitochondrial biogenesis. The interplay between NRF2 and PGC-1α through their interaction and regulation loop collectively controls mitochondrial biogenesis (Gureev et al., 2019).
4) Mitophagy is a selective engulfment process of dysfunctional mitochondria in lysosome by autophagy under adverse conditions, such as oxidative stress, hypoxia, mitochondrial membrane potential loss, accumulation of unfolded protein and iron starvation (Palikaras et al., 2018). Mitophagy can occur through different pathways based on the targeting signals on damaged mitochondria that initiate mitophagy: 1) Ubiquitin-dependent mitophagy: a) Parkin dependent: PTEN-induced putative kinase 1 (Pink1)-Parkin pathway; b) Parkin independent but ubiquitin dependent mitophagy; 2) Ubiquitin-independent or receptor based mitophagy: a) Apoptosis related proteins as mitophagy receptors or inhibitors; b) Other mitophagy receptors; 3) Lipid based mitophagy: a) Cardiolipin based; b) Sphingolipid based; 4) Micromitophagy. Pink1-Parkin pathway is the best-studied mitophagy pathway among all others. Pink1 becomes lodged in the translocase of the outer membrane (TOM) and leads to the recruitment of Parkin, an E3 ubiquitin ligase, which polyubiquitinates proteins on the outer mitochondrial membrane and triggers the recruitment of autophagy receptors and autophagy machinery to degrade the mitochondrion (Narendra et al., 2008; Youle and Narendra, 2011). The adenosine5′-monophosphate-activated protein kinase (AMPK) is recently described as a master sensor of cell stress and is emerging as a crucial regulatory factor of mitochondrial metabolism and mitophagy. It has been reported that AMPK plays a role in mitochondrial fission and autophagosomal engulfment, and interplays with Pink1-Parkin signaling (Herzig and Shaw, 2018).
As a part of the quality control mechanism, mitophagy enables the degradation of damaged and superfluous mitochondria, which prevent detrimental effects and reinstates cellular homeostasis in response to stress. Mitochondrial fission facilitates mitophagy by dividing mitochondria into fragments or segregating damaged mitochondrial subdomains for autophagosome engulfment, therefore promoting mitophagy. Impaired mitophagy indicates less mitochondrial turnover, which leads to the accumulation of dysfunctional senescent mitochondria and age-related disorders (Doblado et al., 2021). The ubiquitin proteasome system (UPS) can ubiquitinate mitochondrial proteins via a cascade of E1, E2, and E3 enzymes, and redirect them for proteasome degradation (Livnat-Levanon and Glickman, 2011). The importance of UPS in mitophagy largely attributes to the E3 ligase Parkin. Ubiquitin-dependent degradation of key mitochondrial proteins also participates in regulating mitochondrial energy metabolism, including regulating the turnover of several oxidative phosphorylation (OXPHOS) proteins.
In summary, mitochondria are critical for energy production and the integrity and quality control of mitochondria are important for cellular processes in response to different stresses.
1.4 Current Mitochondrial Assays Used in Research
Many methods have been used to assess the parameters of mitochondrial morphology, function, mtDNA and mitochondrial protein damage, mitochondrial metabolism and autophagy regulation to evaluate mitochondrial quality: 1) Assessing autophagy and mitophagy: a) Autophagy: Given the importance of autophagy in maintaining healthy mitochondrial populations, microtubule-associated protein 1 light chain 3 (LC3)-II western blot and LC3 puncta imaging can be used to determine if aberrations in autophagy alter mitochondrial quality (Tanida et al., 2008). b) Mitochondrial morphology: Confocal microscopy can be used to measure both the changes of morphology of the mitochondrial network under stresses, and mitophagy. The mitochondrial network can change primarily through fission or fusion. The level of fission/fragmentation in response to stress can be measured by quantifying the length of a cell’s mitochondrial population (Mitra and Lippincott-Schwartz, 2010). Transmission electron microscopy (TEM) can be used to obtain high-resolution micrographs of mitochondria, but mitochondrial ultrastructure (Tobias et al., 2018). c) Mitophagy: Mitophagy events can be determined from the co-localization between MitoTracker (MT) red (a red-fluorescent dye that stains mitochondria in live cells) and LysoTracker (LT) green (a cell-permeable green dye that stains lysosome in live cells). Imaging MT/LT co-localization can be performed to assess the increase or decrease of flux (Dolman et al., 2013). MitoTimer is another fluorescent reporter protein that can detect mitochondrial turnover within cells. It encodes a mitochondria-targeted green fluorescent protein when newly synthesized, which shifts irreversibly to red fluorescence when oxidized (Laker et al., 2014; Gottlieb and Stotland, 2015). Mito-Keima is a pH-sensitive, dual-excitation ratiometric fluorescent protein that can detect the delivery of mitochondria to the lysosome. In the alkaline environment, the shorter-wavelength (green) excitation predominates, while within the acidic lysosome, the Keima protein gradually shifts to the longer-wavelength (red) excitation, with partial overlap in the emission spectra. These properties of mito-Keima can be used to determine whether Keima-tagged mitochondria are at the physiological pH of the mitochondria (pH 8.0) or the lysosome (pH 4.5) (Sun et al., 2017). d) Mitochondrial mass protein levels and mtDNA copy: The protein complexes in the mitochondria can be turned over at different rates and a decrease in mitochondrial quality can be detected as decreased specific activity of the mitochondrial enzymes and damaged mtDNA. The levels of all five mitochondrial complexes can be assessed by measuring representative subunits from each complex which can be done using separate antibodies and probing each complex individually. This approach can be extended to evaluate levels of mitochondrial proteins involved in other metabolic pathways such as the TCA cycle. Measurement of mtDNA copy number can be used as an additional indicator of mitochondrial mass which can be done by real-time PCR using mtDNA directed primers; 2) Assessing metabolism: Metabolomics mass spectrometry can be used to analyze TCA cycle; glycolysis and glutaminolysis (Gowda and Djukovic, 2014); 3) Assessing mitochondrial bioenergetic function: The development of seahorse extracellular flux technology has allowed for the high throughput measurement of cellular bioenergetics and the activity of individual mitochondrial complexes within the cell and in isolated mitochondria (Dranka et al., 2011; Salabei et al., 2014).
2 Retinal Pigmented Epithelial Structural and Functional Changes During Aging
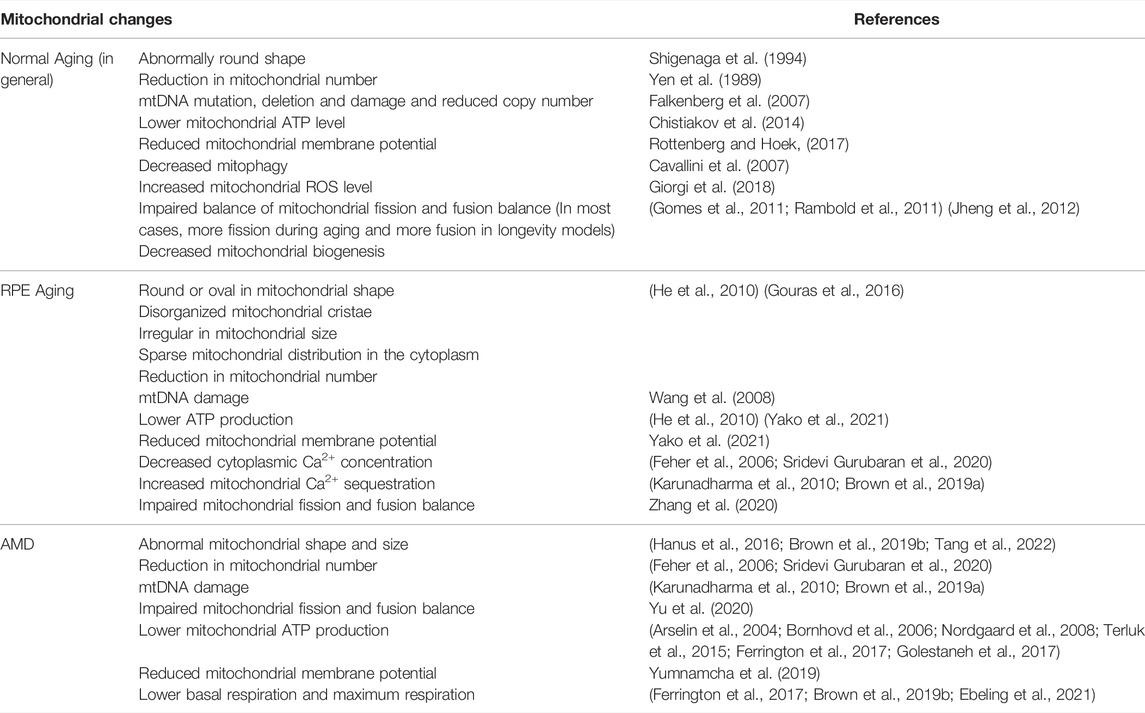
The distinct functions of RPE cells make them susceptible to oxidative stress, due to their high metabolism and exposure to high oxygen, oxidized POS and polyunsaturated fatty acids (PUFAs). In addition, environmental factors, such as visible or ultraviolet (UV) light exposure and cigarette smoking, also pose oxidative stress to the RPE cells. These could lead to elevated levels of ROS and reactive nitrogen species in the cells, which can modify and damage carbohydrates, membrane lipids, proteins and nucleic acids, and eventually lead to pathological consequences. RPE cells are equipped with enzymatic and non-enzymatic antioxidative systems to protect against oxidative stress. However, the antioxidative capabilities diminish with aging, which leads to diminished RPE functions. RPE structural and functional changes associated with aging have been reviewed by Bonilha (Bonilha, 2008) and are summarized below (Table 1; Figure 1): 1) Aged RPE from human donors showed loss of melanin granules on their apical surface and accumulation of age pigment lipofuscin. Accumulation of secondary lysosomes and residual bodies containing lipofuscin, has been observed in post-mitotic and intermitotic cells during aging, and has been used as a universal index for cellular senescence (Schmucker and Sachs, 2002; Kubasik-Juraniec et al., 2004; Morales et al., 2004). 2) Shortening of RPE microvilli, which could affect some RPE key functions, such as phagocytosis of shed POS (Boulton and Dayhaw-Barker, 2001). 3) BrM is an acellular and pentalaminar structure formed by the RPE and choroid. Increased thickness, lipid content and advanced glycation products have been reported in the aging BrM, likely due to the entrapment of proteins and lipids in the extracellular matrix (Zarbin, 2004; Harris et al., 2017). Functionally, the elasticity of human BrM-choroid complex decreases linearly with aging. 4) Drusen are extracellular deposits of biomaterials below the RPE along BrM. Drusen can be originated from blood and/or RPE and is a clinical hallmark for age-related macular degeneration (AMD) and other age-related diseases including Alzheimer’s disease (Bergen et al., 2019). Drusen are ubiquitous in people over 50 years old and are considered as part of normal aging. 5) RPE cell density/size changes during aging. An earlier study using normal human donors showed that RPE density in the fovea decreased significantly by 0.3% per year with increasing age. An increase in RPE cell size and multinucleation has been observed in mice (Chen et al., 2016). 6) Age-related accumulation of iron in RPE/choroid has been observed in rats, while its increase in the retina was modest (Chen et al., 2009a; Chen et al., 2009b). Exposure of ARPE-19 cells (a spontaneously arising RPE cell line derived from the normal eyes of a 19-year-old male) to increased iron markedly decreased their phagocytosis activity, suggesting that iron accumulation with age may impair the phagocytosis and lysosomal functions of the RPE. In human, phagocytosis level in RPE from donors showed a moderate decrease with aging (Inana et al., 2018). 7) RPE secretome changes during aging. Secretome includes soluble proteins and proteins secreted as part of extracellular vesicles which function as mediators of cell-to-cell signaling and modulate cell activities. The secreted proteins could be used as biomarkers of some diseases. It has been reported that RPE secreted over 1,000 proteins, many of which change significantly due to ROS accumulated during aging (Meyer et al., 2019).
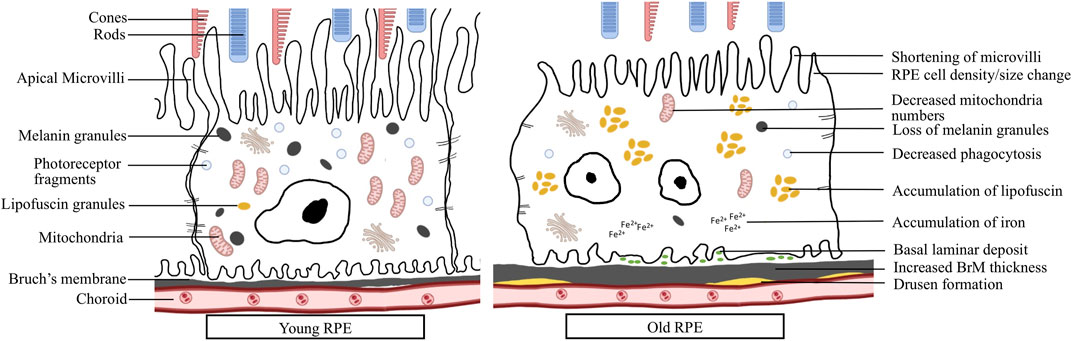
FIGURE 1. RPE changes during aging: Young RPE cell shows elongated microvilli, tight contact with nearby cells, containing plenty of mitochondria, melanin granules and photoreceptor fragments. Aged RPE cell shows larger size, multinucleation, shortened microvilli, decreased mitochondria numbers, loss of melanin granules, decreased phagocytosis, accumulation of lipofuscin and iron, basal laminar deposits, increased BrM thickness and accumulation of drusen.
3 Retinal Pigmented Epithelial Changes in Age-Related Macular Degeneration
Aging, together with genetic and environmental factors, cause RPE dysfunction and degeneration, which significantly contributes to age-related retinal diseases including AMD. The RPE changes involve RPE functional decline, morphological changes, epithelial-mesenchymal transition (EMT), senescence and cell death. AMD is a leading cause of blindness among the elderly population. Late AMD has two forms, “dry” and “wet” AMD. Geographic atrophy (GA) is the advanced form of dry AMD. It is featured by the irreversible loss of the RPE, photoreceptors and choriocapillaris, which eventually lead to vision loss. The features and phenotypes of RPE in AMD have been under extensive studies by numerous groups. Here we summarize the RPE changes in AMD (Table 1; Figure 1): 1) Structural changes of RPE can be visualized by spectral domain optical coherence tomography (SD-OCT), autofluorescence imaging, combined with physiopathological analysis under electromicroscopy. Large soft drusen and hyper- or hypopigmentation in the RPE were identified as key features preceding RPE cell dysfunction and late form of AMD (Wang et al., 2003). Lipofuscin aggregation has been observed almost exclusively in AMD eyes, implying more cellular senescence in AMD (Ach et al., 2015). In an independent longitudinal study, increased fundus autofluorescence was observed preceding GA development, suggesting the involvement of cellular senescence and subsequent cell death in atrophic AMD (Holz et al., 2001; Hwang et al., 2006). 2) RPE layer becomes highly variable and overall thicker in both GA and wet AMD eyes, which could be explained by hypertrophy or cellular senescence (Zanzottera et al., 2015). 3) Shedding, dissociating and sloughing RPE cells were observed in GA, suggesting death, transdifferentiation or emigration of RPE cells (Zanzottera et al., 2015). Apoptosis of RPE cells in AMD was also detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay, the most widely used method to detect fragmented DNA in apoptotic cells or tissue samples (Dunaief et al., 2002). However, cells may become TUNEL positive during other types of cell death. Therefore, more studies are needed to confirm the RPE cell death mechanisms in AMD, especially during the process of GA. 4) RPE phagocytosis, while showing a moderate decline with age, was significantly reduced in AMD RPE, more than expected during normal aging (Inana et al., 2018) 5) Induced pluripotent stem cell (iPSC)-RPE cells from homozygous ARMS2/HTRA1 risk genotype showed significantly higher complement and inflammatory factors expression, and iPSC-RPEs from complement factor H (CFH) (Y402H) risk genotype showed reduced mitochondrial function and increased inflammation markers (Saini et al., 2017; Ebeling et al., 2021). 6) By analyzing the secretome from RPE cells of AMD and age-matched control donors, RPE cells were found to secrete a variety of extracellular matrix proteins, complement factors, and protease inhibitors that have been reported to be major constituents of drusen (An et al., 2007). AMD RPE cells secrete more galectin 3 binding protein, fibronectin, clusterin, matrix metalloproteinase -2 and pigment epithelium derived factor, but less secreted protein acidic and rich in cysteine than RPE cells from age-matched healthy donors. Overall, significant progress has been made regarding the morphological changes and pathogenic mechanisms of RPE in AMD. More mechanistic studies of RPE in AMD, including molecular mechanism, the involvement of EMT, cell senescence and different types of cell death, are still needed.
4 Mitochondrial Changes During Retinal Pigmented Epithelial Aging
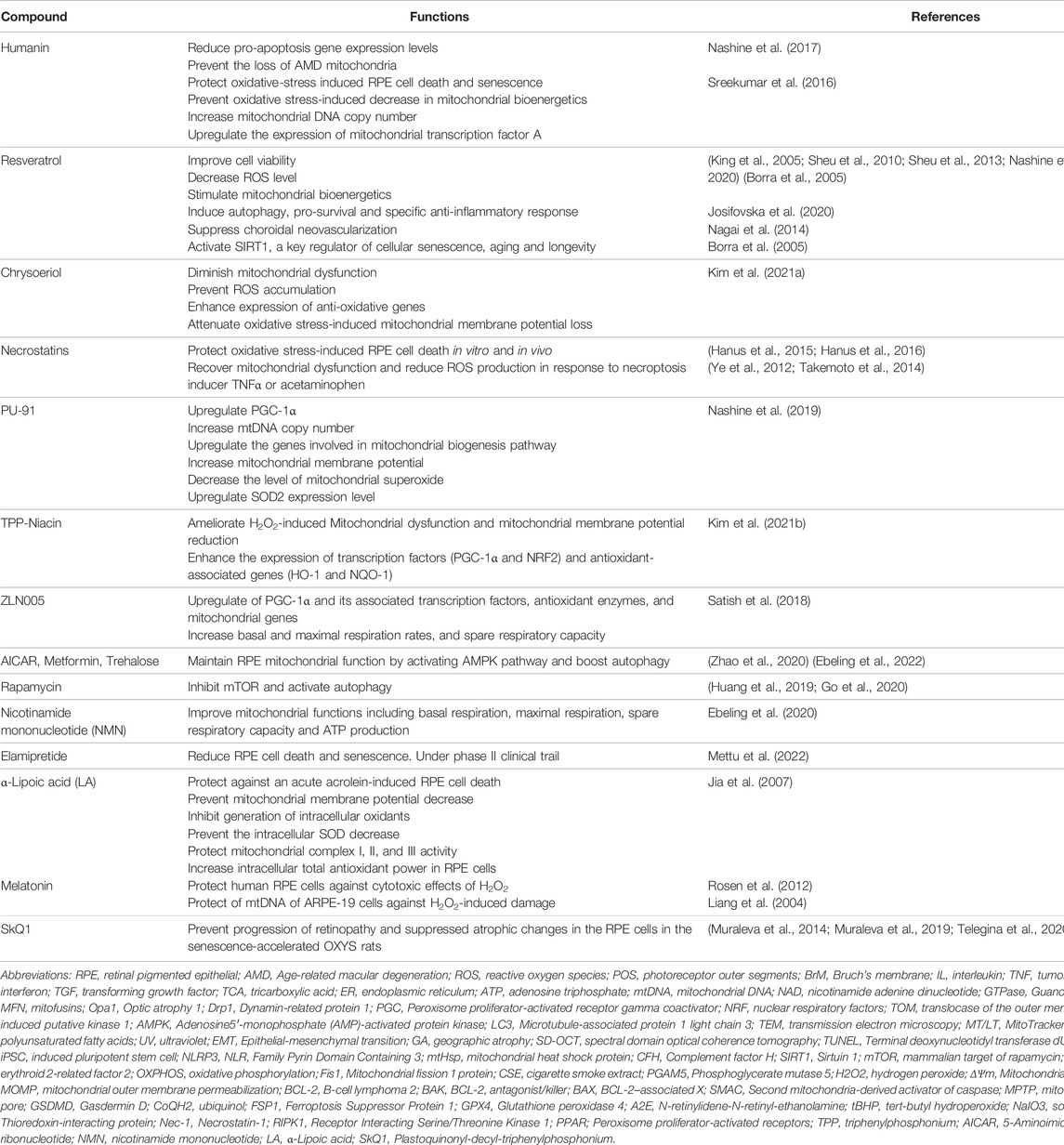
Numerous studies support the involvement of mitochondria in aging (Table 2). In the 1950s, Denham Harman proposed the free radical theory of aging, which he later expanded it to the mitochondrial free radical theory of aging. In this theory, free radicals produced by mitochondrial activities damage cellular components and lead to aging. Mitochondrial changes during aging include: 1) Morphological changes, such as abnormally rounded mitochondria (Shigenaga et al., 1994), decreased number of mitochondria (Yen et al., 1989), concurrent with decreases in mtDNA copy number and mitochondrial protein levels (Stocco et al., 1977). 2) Many studies have reported mitochondrial functional decline during aging, including lower oxidative capacity, reduced OXPHOS, decreased ATP production (Chistiakov et al., 2014). There is an average decline of 8% per decade in ATP producing capacity (Short et al., 2005). 3) Mitochondrial ROS significantly increases with aging and in aging-related diseases. Moderate ROS helps to maintain cellular energy production and to regulate mitochondrial protective signaling pathways, which increase lifespan in lower organisms (Giorgi et al., 2018). However, excess ROS are pathogenic and may induce cell degeneration or even cell death. For example, ROS and mitochondrial Ca2+ overload can cause the opening of the mitochondrial permeability transition pore, which leads to apoptosis (Rottenberg and Hoek, 2017). During normal aging, increased ROS level promotes oxidative damage to mitochondrial DNA, lipids, and proteins (Jarrett et al., 2008). 4) Impaired balance between mitochondrial fission and fusion is related to age-dependent decline in mitochondrial biogenesis. Mitochondrial fusion has beneficial effect to prolong lifespan by increasing bioenergetics efficiency, maintaining ATP production (Gomes et al., 2011; Rambold et al., 2011). Meanwhile, mitochondrial fission is associated with aging due to increased oxidative stress, mitochondrial depolarization, and reduced ATP production (Jheng et al., 2012). Fission maintains mitochondrial quality and integrity by involving in the selection of dysfunctional mitochondria. Mitophagy selectively removes defective mitochondria which have impaired oxidative capacity and declined integrity (Ding and Yin, 2012). With age, mitophagy was observed to decrease (Cavallini et al., 2007), which leads to an accumulation of damaged mitochondria, advanced oxidative stress, and increased apoptosis (Masiero and Sandri, 2010). 5) Mitochondrial density appears to decline gradually during aging, suggesting a decrease in mitochondrial biogenesis during aging, which could result from age-dependent reduction in levels of PGC-1α (Crane et al., 2010). 6) mtDNA damage, mutation and deletion during aging. mtDNA encodes essential components of OXPHOS and protein synthesis machinery (Falkenberg et al., 2007). Thus, oxidative stress-induced mtDNA damage and mutations impair either the assembly or the function of the respiratory chain which then trigger further accumulation of ROS which could be lethal (Hiona and Leeuwenburgh, 2008). Besides point mutations, deletions of mtDNA are detected at higher frequency in aged human and animal tissues (Nekhaeva et al., 2002; Kujoth et al., 2007). In addition, mtDNA abundance also declines with age in various tissues of human and rodent (Wallace, 2001; Miller et al., 2003; Welle et al., 2003). MtDNA containing unmethylated CpG dinucleotides and can trigger inflammation that aggravates tissue injury by activating toll-like receptor 9, inflammasomes, and the stimulator of interferon genes pathway (Nie et al., 2020). It has been reported to be pro-inflammatory in various diseases such as Alzheimer’s disease and heart failure (Wilkins et al., 2014; Nakayama and Otsu, 2018). Intracellular mtDNA has been shown to induce the secretion of inflammatory cytokines IL-6 and IL-8, and the priming of the NLR Family Pyrin Domain Containing 3 (NLRP3) inflammasome in RPE cells (Dib et al., 2015).
Only a few papers have reported the mitochondrial structural and functional changes during RPE aging in humans. Using electron microscopy, mitochondria in young RPE were found to be numerous, most bacillus-like shaped, rich in well preserved cristae, and orientated parallel with the apical-basal axis (Feher et al., 2006). In aged eyes, mitochondria of the RPE were decreased in number, variable in size, usually oval shaped, sometimes with disorganization of cristae (Figure 2). In another study, using isolated primary RPE cells from young (9–20)-, mid-age (48–60)-, and >60 (62–76)-year-old donors, some different morphological changes in mitochondria were observed. Mitochondria from the two younger groups were found to be numerous, regular in size, and with round or oval shapes (He et al., 2010). Cristae were distinctly visible and the outer membranes appeared to be intact. However, mitochondria from the >60 age group RPE cells were sparsely distributed in the cytoplasm, irregular in size, tubular in shape, larger, and with electron-dense matrices, less distinct cristae, and disrupted outer membranes. Length of the mitochondria in this group (Length/width ratio) was almost seven folds greater compared to the other ages. These mitochondrial abnormalities correlated with lower ATP levels, reduced mitochondrial membrane potential, decreased cytoplasmic Ca2+ concentration, and increased Ca2+sequestration in the mitochondria in cells with advanced aging (He et al., 2010).
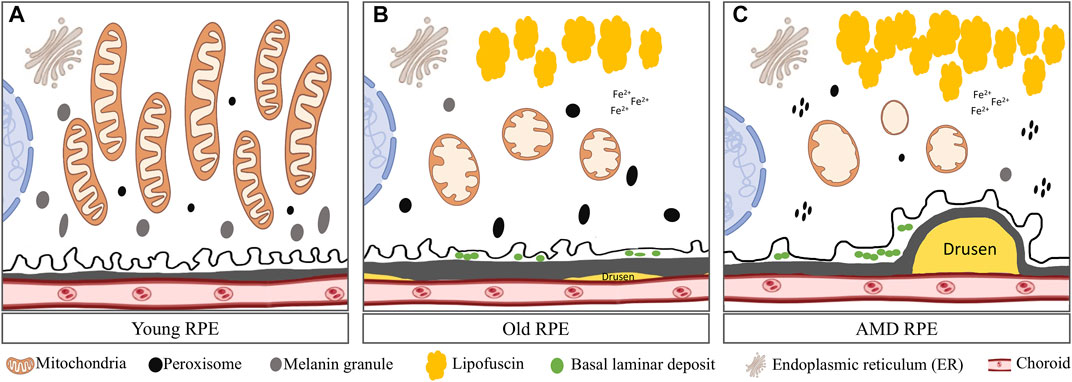
FIGURE 2. Comparison of mitochondria in young, aged and AMD RPE: (A) Young RPE cell contains numerous mitochondria with long axes, usually oriented from the apical to the basal surfaces of the RPE and are parallel to one another. The mitochondrial cristae are well preserved. Several peroxisomes appeared as small, round, electron-dense organelles. Plenty of melanin granules exist in the cells. (B) In aged RPE cell, mitochondria show membrane disorganization and loss of cristae. Accumulated lipofuscin presents in the cell. Several peroxisomes of various density, shape and size were distributed randomly in the cytoplasm. Less melanin granules appear in the cell. Also, small drusen forms underneath BrM and basal lamina deposits forms in between the cell and BrM. (C) In AMD RPE, advanced mitochondrial alterations occur. Most mitochondria had severe disorganization of membranes that varied from focal to complete loss of cristae. Peroxisomes are clustered and aggregated in the cell. Large and soft drusen forms underneath the BrM.
There are several reports regarding age-related mitochondrial changes in animal models of RPE aging. Using monkeys up to 35 years old, Gouras et al. found decreased mitochondrial number and density, increased mitochondrial length and increased clustering of very elongated mitochondria during aging (Gouras et al., 2016). Using C57BL/6 mice (up to 22 months old) and rats (up to 26 months old), Wang et al. found an increased mtDNA damage in aged RPE and choroid (Wang et al., 2008). Yako et al. reported that in the RPE of 12-month-old C57BL/6J mice, mitochondrial number was decreased, but cristae width was increased compared to young mice (Yako et al., 2021). They also found that pharmacological suppression of mitochondrial fission improved POS phagocytosis, suggesting that mitochondrial dysfunction and fission in RPE could impede phagocytosis and cause retardation of the visual cycle. Metabolomics analysis in the RPE/choroid of young (6 weeks) and old (73 weeks) C57BL6/J mice identified 45 significantly changed metabolites, with most of which are involved in the mitochondrial metabolism, glucose metabolism and amino acid metabolism pathways (Wang Y et al., 2018). NAD and riboflavin (a precursor for flavin adenine dinucleotide) levels were reduced to less than half, but nicotinamide (substrate for NAD synthesis) and the substrates for mitochondrial metabolism such as pyruvate and dihydroxyacetone phosphate were accumulated to higher levels in the old mice, suggesting impaired mitochondrial energy metabolism during RPE/choroid aging. Taken together, the research on the mitochondrial changes in RPE aging is still limited, more systematic studies with even older groups, with focus on mitochondrial functions, mitochondrial dynamics and quality control, are still needed.
5 Retinal Pigmented Epithelial Mitochondrial Changes in Age-Related Macular Degeneration
Several studies have reported RPE mitochondrial changes in AMD (Table 2). A reduction in mitochondrial number has been observed in AMD donor RPE compared to age-matched controls (Feher et al., 2006). A proteomic study revealed decreased expression of ATP synthase subunits in AMD RPE (Nordgaard et al., 2008). ATP synthase complexes participate in OXPHOS and maintain mitochondrial morphology and mitochondrial membrane potential. Decreased expression of ATP synthase subunits with AMD could lead to defects in these critical mitochondrial functions (Arselin et al., 2004; Bornhovd et al., 2006). Another change in the mitochondrial proteome is the decreased expression of the mitochondrial heat shock protein (mtHsp70) in AMD RPE (Nordgaard et al., 2008). mtHsp70 functions as a molecular chaperone that regulates the ATP-dependent import of nuclear-encoded proteins into the mitochondrial matrix (Nordgaard et al., 2006; Nordgaard et al., 2008; Kaarniranta et al., 2009). Therefore, decreased mtHsp70 levels could be detrimental to overall mitochondrial function and limit energy production in AMD RPE. mtDNA damages increase with age, more mtDNA damage in the macula of human AMD RPE has been detected compared to age-matched controls (Karunadharma et al., 2010). The extent of RPE mtDNA damage correlates with AMD severity. Damage to the mtDNA could be mitochondrial genome-wide, including the region encoding the subunit of ETC and the D-loop, the site of initiation for mtDNA transcription and replication of one mtDNA strand (Karunadharma et al., 2010; Terluk et al., 2015). Damage to either the D-loop or region encoding the ETC proteins could lead to negative functional outcomes for the mitochondria (Terluk et al., 2015). The role of mitochondria in AMD was also studied using transmitochondrial cybrids (Miceli and Jazwinski, 2005; Nashine et al., 2017; Nashine et al., 2020). Transmitochondrial cybrids were created by fusing mitochondrial DNA-deficient APRE-19 cell line with platelets isolated from either AMD patients or age-matched normal subjects. Therefore, the cybrids had identical nuclei but different mitochondria. AMD cybrids showed: 1) reduced cell viability, lower mtDNA copy numbers, and downregulated mitochondrial replication/transcription genes and antioxidant enzyme genes; and 2) elevated levels of genes related to apoptosis, autophagy and ER stress along with increased mtDNA fragmentation and higher susceptibility to amyloid-β-induced toxicity compared to control cybrids. These studies support an important role for mitochondria in AMD.
Mitochondrial functions have also been studied using in the cultured RPE or iPSC-RPE cells derived from AMD donors. Ferrington et al. found that mitochondria of RPE from AMD donors have significantly declined functions such as lower basal respiration, ATP production, and maximum respiration compared to age-matched controls (Ferrington et al., 2017). There is no difference in mtDNA content when comparing healthy and AMD donors. In another study by Golestaneh et al., accumulation of lipid droplets and glycogen granules, damaged mitochondria, and increased autophagosomes were observed in cultured AMD RPE cells (Golestaneh et al., 2017). Decreased NAD+ and Sirtuin 1 (SIRT1), increased PGC-1α acetylation (inactive form), lower AMPK activity, and overactive mammalian target of rapamycin (mTOR) pathway were also observed in AMD RPE cells, suggesting RPE metabolic dysregulation in AMD (Zhang et al., 2020). Compared with normal RPE, AMD RPE exhibit increased susceptibility to oxidative stress, produce higher ROS levels under stress conditions, and showed reduced mitochondrial activity with decreased ATP production. Of note, Ferrington et al.‘s study showed that AMD RPE are more resistant to acute oxidative stress (Ferrington et al., 2017). iPSC-RPE cells have been derived from AMD patients, and reduced mitochondrial function has been observed in iPSC-RPE cells from the CFH Y402H risk genotype RPE (Ebeling et al., 2021).
Although no AMD mouse models can recapitulate human AMD phenotypes due to their lack of macula, AMD mouse models can provide some insight into the mechanism of AMD. Here we present several mouse models with connection to mitochondria. RPE-specific knockout of mitochondrial antioxidant enzyme MnSOD (or SOD2) resulted in reduced RPE function with age (Brown et al., 2019a). Less electron dense and swollen mitochondria, disorganized mitochondrial cristae, reduced ATP production, and a compensatory increase in glycolytic metabolism have been observed in the SOD2−/− mice, supporting that mitochondrial oxidative stress can lead to mitochondrial malfunction, RPE metabolic reprogramming and RPE dysfunction. In another model, global knockout of NRF2 and PGC-1α, the master regulators of antioxidant production and mitochondrial biogenesis, led to disturbed autophagy, an accumulation of drusen-like deposits, and the infiltration of Iba-1 positive immune cells mimicking clinical features of the dry AMD phenotype (Felszeghy et al., 2019). In the NRF2/PGC-1α−/− mice, a decline in viable mitochondria and higher mitochondrial damage were observed in the RPE cells. Damaged mitochondria were marked by Pink1/Parkin and autophagosomes with mitochondrial cargo. These data support that defective mitochondrial antioxidative system and biogenesis in the RPE could lead to AMD-like phenotypes (Wang K et al., 2018; Wang et al., 2019). For more information about RPE mitochondria in AMD, refer to reviews by Kaarniranta (Kaarniranta et al., 2020) and Blasiak (Blasiak et al., 2020).
6 Role of Mitochondria in Retinal Pigmented Epithelial Senescence
Cellular senescence and cell death are important processes involved in aging and age-related diseases. Mitochondria have been established as important regulators of senescence and cell death. In this and next section, we summarize the role of mitochondria in RPE cellular senescence (Figure 3) and cell death (Figure 4).
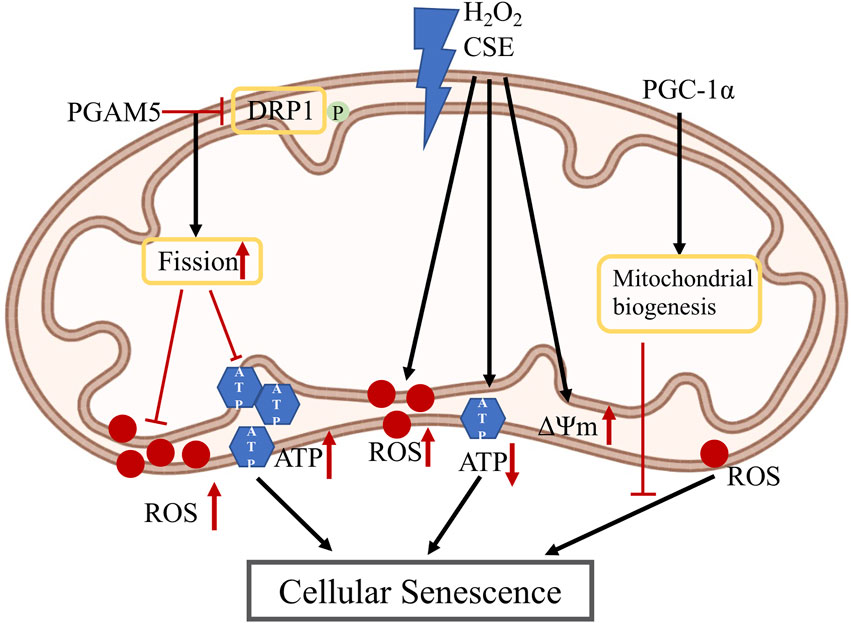
FIGURE 3. Mitochondria changes in RPE senescence: PGAM5 dephosphorylates DRP-1 which promotes mitochondrial fission, which then inhibits the increase of ROS and ATP in RPE cellular senescence; H2O2 and CSE induce increased mitochondrial ROS and membrane potential, also induce decreased ATP level which cause RPE cellular senescence; PGC-1α is a master regulator of mitochondria biogenesis and could reduce ROS level which may inhibit RPE cellular senescence. PGAM5: phosphoglycerate mutase 5; DRP1: dynamin-related protein 1; H2O2: hydrogen peroxide; CSE: cigarette smoke extract; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator-1α; ATP: adenosine triphosphate; ROS: reactive oxygen species; ΔΨm: mitochondrial membrane potential.
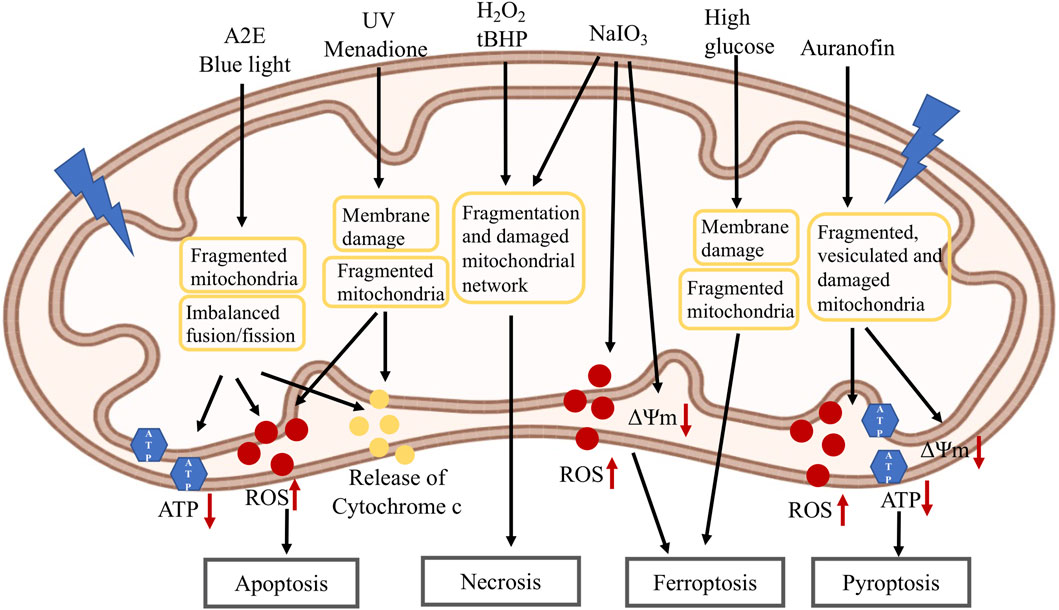
FIGURE 4. Mitochondria changes in RPE cell death induced by different stressors: A2E and blue light lead to fragmented mitochondria, imbalanced mitochondrial fusion/fission, decreased ATP level, increased ROS level and release of cytochrome C which then induce apoptosis; UV and menadione cause mitochondrial membrane damage, fragmented mitochondria, increased ROS level and release of cytochrome C which then induce apoptosis; H2O2, tBHP and NaIO3 cause fragmented mitochondria and damaged mitochondrial network, and lead to necrosis. NaIO3 also induces decreased mitochondrial membrane potential and increased ROS level, and leads to ferroptosis. High glucose induces mitochondrial membrane damage, fragmented mitochondria and cause ferroptosis; Auranofin causes decreased mitochondrial membrane potential, fragmented, vesiculated and damaged mitochondria, increased ROS level and decreased ATP level which lead to pyroptosis. A2E: N-retinylidene-N-retinyl-ethanolamine; UV: ultraviolet; H2O2: hydrogen peroxide; tBHP: tert-butyl hydroperoxide; NaIO3: sodium iodate; ATP: adenosine triphosphate; ROS: reactive oxygen species; ΔΨm: mitochondrial membrane potential.
Deregulation of mitochondrial homeostasis, shown by impaired mitochondrial biogenesis, metabolism and dynamics, has emerged as a hallmark of cellular senescence, which also drives the senescent phenotypes (Vasileiou et al., 2019). Defects in mitochondrial OXPHOS, reduced ATP production, and increased mitochondrial ROS production have been reported in cells undergoing senescence. In senescent cells, mitochondrial ROS can further aggravate cellular senescence by increasing telomere shortening, DNA damage and sustaining DNA damage response signaling pathway (Passos et al., 2010). In addition, mtDNA damage induced by ROS can impair OXPHOS function and further increase ROS release, forming a vicious cycle in aggravating senescence. Mitochondrial dynamics (including biogenesis, fusion, fission and mitophagy) are also linked to senescence. In aging endothelial cells, mitochondrial membrane potential, mitochondrial fusion and fission are reduced (Jendrach et al., 2005). In stress-induced senescent cells, highly elongated mitochondria with enhanced cristae structure have been reported (Yoon et al., 2006). Depletion of mitochondrial fission 1 protein (Fis1) caused sustained elongation of mitochondria and senescent phenotypes, whereas reintroduction of Fis1 protein restored mitochondrial fission and partially reversed the senescent phenotypes (Lee et al., 2007). Depletion of both Fis1 and mitochondrial fusion protein OPA1 resulted in mitochondrial fragmentation phenotypes and rescued senescence-associated changes. Intriguingly, sustained mitochondrial elongation is associated with reduced mitochondrial membrane potential, increased ROS level and DNA damage. These indicate that sustained mitochondrial elongation can trigger senescence-associated changes. However, in a cellular senescent model induced by cigarette smoke extract (CSE), more mitochondrial fragmentation was observed. In addition, mitochondrial fragmentation induction by knockdown of fusion proteins, OPA1 or MFN, increased mitochondrial ROS production and cellular senescence. For in vivo studies, inhibition of mitochondrial fission by deleting a mitochondrial fission protein DRP1 or maintenance of the fused mitochondrial network is necessary for longevity in yeast or C. elegans (Scheckhuber et al., 2007; Chaudhari and Kipreos, 2017). However, disruption of either mitochondrial fission or fusion significantly reduces medium lifespan in C. elegans, while in another study, promoting mitochondrial fission in midlife prolongs healthy lifespan of D. melanogaster (Rana et al., 2017; Byrne et al., 2019). Together, these conflicting data warrant more research to study the role of mitochondrial fusion or fission, or the imbalance of mitochondrial fusion/fission, in cellular senescence. The other mitochondrial quality control processes include mitochondrial biogenesis that propagates pre-existing pool of mitochondria, and mitophagy that eliminates malfunctional mitochondria. Mitochondrial biogenesis occurs constantly at basal levels and increases during cell renewal, development and stress conditions. Although in general mitochondrial biogenesis decreases during aging, expression of mitochondrial biogenesis regulators is upregulated in models of cellular senescence, which may reflect a compensatory response (Lee et al., 2002; Moiseeva et al., 2009). Mitophagy generally positively impacts lifespan and healthspan. Decreased mitophagy was observed during aging, while overexpression of Parkin and Pink1 extended lifespan in flies (Todd and Staveley, 2012; Rana et al., 2013; Bakula and Scheibye-Knudsen, 2020). Regarding mitophagy in senescence, mitophagy activities have been shown to be reduced in senescent cells in vitro and in vivo (Dalle Pezze et al., 2014; Garcia-Prat et al., 2016). This may be the consequence of reduced mitochondrial fission, autophagy and lysosomal activities, since mitochondrial fission is required for the separation of dysfunctional mitochondrial from the mitochondrial network, while lysosome is required for the terminal events of both autophagy and mitophagy.
Regarding the role of mitochondrial in RPE senescence, PGC-1α, a key protein for mitochondrial biogenesis and redox control, has been hypothesized to repress RPE senescence (Kaarniranta et al., 2018). We have recently reported that mitochondrial hyperfusion drives RPE senescence (Yu et al., 2020). We found that deletion of mitochondrial phosphatase phosphoglycerate mutase 5 (PGAM5) leads to accelerated RPE senescence in vitro and in vivo. Mechanistically, PGAM5 is required for mitochondrial fission through dephosphorylating DRP1. PGAM5 deletion leads to increased mitochondrial fusion and decreased mitochondrial turnover. As results, cellular ATP and ROS levels are elevated, mTOR and IRF/IFN-β signaling pathways are enhanced, leading to cellular senescence. Overexpression of DRP1-K38A mutant overexpression attenuated mitochondrial fission and elongated mitochondrial branches, which phenocopies PGAM5−/− senescent phenotypes, while overexpression of DRP1-S37A mutant dramatically inhibited mitochondria fusion and rescued mTOR activation and senescence in PGAM5−/− cells. These data support that mitochondrial dynamics can regulate signaling pathways linking to RPE senescence. In hydrogen peroxide (H2O2)-mediated premature RPE senescence model and natural passage-mediated replicative RPE senescence model, levels of ROS and mitochondrial membrane potential were increased, while coculture of RPE cells with embryonic stem cells reversed RPE senescence phenotypes in vitro (Wang et al., 2020). Cigarette smoke is one of the major risk factors for AMD. CSE can induce RPE senescence (Marazita et al., 2016). CSE increased superoxide while decreasing ATP production in RPE cells, suggesting an uncoupling of OXPHOS and mitochondrial dysfunction. Mice with intravitreal injection of CSE showed decreased TOM20 protein and ATP levels in the injected eyes (Cano et al., 2014). The exact function of mitochondria in CSE-induced RPE senescence is yet to be established.
7 Role of Mitochondria in Retinal Pigmented Epithelial Cell Death
Mitochondria also have significant involvement in different types of cell death. The role of mitochondria in apoptosis is well-established. Mitochondrial outer membrane permeabilization (MOMP) induced by B-cell lymphoma 2 (BCL-2) antagonist/killer (BAK) and BCL-2–associated X (BAX), causes the release of intermembrane space proteins including cytochrome c, second mitochondria-derived activator of caspase (SMAC) and Omi to the cytoplasm. Omi and SMAC promote caspase-9 activation by cleaving or binding to inhibitor of apoptosis proteins, while cytochrome c binds to apoptotic peptidase activating factor-1. These lead to apoptosome formation and caspase recruitment, caspase cascade activation and dismantling of the cellular contents (Vringer and Tait, 2019). Although apoptosis is generally believed to be immunotolerant, MOMP was recently shown to induce potent pro-inflammatory signaling. The role of mitochondria in necroptosis, a regulated form of necrosis, has been controversial (Marshall and Baines, 2014). Mitochondrial fragmentation has been observed in necroptosis (Wang et al., 2012). BAX and BAK function as the outer membrane component of the mitochondrial permeability pore (MPTP) and regulate MPTP opening through oligomerization during necrotic cell death (Karch et al., 2013; Karch et al., 2015). Numerous studies support the role of mitochondrial dysfunction, including mitochondrial ROS, activation of mitochondrial phosphatase PGAM5, and induction of mitochondrial permeability transition in necroptosis. However, recent studies suggest none of these are involved in necroptosis. Necroptosis was still functional in cells with mitochondria depleted by mitophagy (Tait et al., 2013). Further work is needed to confirm whether mitochondria are fully deleted in those cells and whether necroptosis is mitochondrial independent. During pyroptosis, rounded and fragmented mitochondria and mitochondrial outer membrane permeabilization were observed after the opening of gasdermin D (GSDMD)-dependent pores before the sudden rupture of the plasma membrane, and there is little evidence that mitochondria are involved in pyroptosis (de Vasconcelos et al., 2019). However, mitochondrial ROS was recently shown to mediate pyroptosis (Evavold et al., 2021). In that study, Ragulator-Rag complex controls mTORC1-dependent events to promote mitochondrial ROS, which operates downstream of GSDMD cleavage to promote GSDMD oligomerization and pore formation. The role of mitochondria in ferroptosis is still controversial (Gan, 2021). Diverse metabolic activities in mitochondria can facilitate ferroptosis, but mitochondria are also equipped with strong anti-ferroptosis defense systems. For example, ubiquinol (CoQH2) generated in mitochondria can defend against ferroptosis, but mitochondrial ROS and ATP can promote ferroptosis. Ferroptotic cells do not have typical necrotic morphological features, but mainly display mitochondrial shrinkage, increased mitochondrial membrane density and reduced mitochondrial cristae (Yagoda et al., 2007; Yang and Stockwell, 2008). However, cells lacking mitochondria are as sensitive to ferroptosis as parental cells with intact mitochondria, supporting the existence of mitochondrial independent ferroptosis (Dixon et al., 2012). Ferroptosis Suppressor Protein 1 (FSP1) was recently shown to exert strong anti-ferroptosis function on plasma membrane in the absence of glutathione peroxidase 4 (GPX4), supporting the existence of mitochondrial-independent ferroptosis (Bersuker et al., 2019; Doll et al., 2019).
Different kinds of RPE cell death have been reported depending on the types of stressors (Hanus et al., 2015; Tong and Wang, 2020). Mitochondrial changes have been associated with RPE cell death. N-retinylidene-N-retinyl-ethanolamine (A2E) is a by-product of the visual cycle formed by the reaction of two trans-retinal molecules with phosphatidyl-ethanolamine (Sparrow et al., 2003). It is a major fluorophore in lipofuscin and is accumulated in aging RPE (Sparrow and Boulton, 2005). A2E has been shown to induce RPE apoptosis by inhibiting cytochrome C oxidase, associated with declined mitochondrial activity and release of cytochrome c (Shaban et al., 2001). Blue light could potentially produce retinal toxicity leading to the development of degenerative eye diseases, such as AMD (Contin et al., 2013; Kuse et al., 2014). Blue light could lead to RPE cell necrosis or apoptosis, possibly depending on the intensity and duration of the light (Pang et al., 1999; Seko et al., 2001). In A2E-loaded RPE cells, blue light reduced cell viability, associated with decreased ATP and increased ROS levels (Marie et al., 2018; Alaimo et al., 2019). Blue light caused the imbalance of mitochondrial fusion/fission towards mitochondrial fragmentation in both non-loaded and A2E-loaded cells, which is correlated with decreased OPA1 and increased DRP1 expression levels. It was also showed that A2E treated cells led to an increase in both mitochondrial fusion and fission. Blue light induced mitochondrial fragmentation in A2E-loaded cells, consistent with their increased propensity to die. Exposure to UV radiation can induce oxidative stress and is associated with ocular pathologies (Marchitti et al., 2011). UV has been shown to induce RPE apoptosis (Yao et al., 2013). In UV-treated RPE cells, upregulation of cytochrome c protein and increased ROS production were observed, suggesting that mitochondrial membrane integrity was compromised (Balaiya et al., 2010). Bidirectional movement of short and elongated mitochondria was noted in control RPE cells. Immediately after UV irradiation, mitochondria became shorter and no longer moving, with very few branched mitochondria remaining within the cells (Bantseev and Youn, 2006; Youn et al., 2010). Loss of mitochondrial membrane potential and the number of mitochondria were also reported in UV-treated RPE cells (Youn et al., 2007; Hsieh et al., 2018). Menadione (or Vitamin K3) induces RPE apoptosis, associated with mitochondrial depolarization and cytochrome C release (Zhang et al., 2003). Exposure of RPE cells to a lethal dose of H2O2 (1 mM) has been shown to induce BAX translocation to the mitochondria and the release of apoptosis-inducing factor from the mitochondria (Ho et al., 2006).
Our lab has shown that H2O2 or tert-butyl hydroperoxide (tBHP) treatment induces RPE necrosis, which can be prevented by necrosis inhibitors necrostatins but not caspase inhibitor z-VAD (Hanus et al., 2013). Fragmentation and degeneration of mitochondrial network were observed in the treated cells. Sodium iodate (NaIO3) injection has been extensively used as a pre-clinical model of RPE dystrophy and GA (Wang J., 2013). Similar necrotic phenotypes were observed in NaIO3-treated RPE cells, which was associated with fragmented and clustered mitochondrial network in the perinuclear region (Hanus et al., 2016). Auranofin, an inhibitor of redox proteins TrxR1 and TrxR2, induced pyroptosis in RPE cells, which was repressed by NLRP3 and Caspase-1 inhibitors (Yumnamcha et al., 2019). In these cells, reduced ATP production and mitochondrial membrane potential, increased ROS, accumulation of damaged, fragmented and vesiculated mitochondria, and mitophagic flux to lysosomes, were observed. NaIO3 and high glucose have also been shown to induce RPE ferroptosis, which can be repressed by ferroptosis inhibitors (Liu et al., 2021; Tang et al., 2022). In an independent study, high glucose promoted RPE apoptosis and inhibited cell proliferation and mitophagy by inactivating ROS/Pink1/Parkin signal pathway (Zhang et al., 2019). Reduced mitochondrial membrane potential, increased ROS production were observed in NaIO3-treated cells, and reduced mitochondrial size and ridge, disrupted mitochondrial membrane were observed in high glucose-treated cells. Further study indicated that high glucose increased the expression of thioredoxin-interacting protein (TXNIP), which is associated with mitochondrial membrane depolarization, fragmentation and mitophagic flux to lysosomes. Elongated mitochondrial network was observed under low glucose, while fragmented mitochondria were observed in high glucose. TXNIP knockdown by shRNA prevented mitochondrial fragmentation and mitophagic flux under high glucose (Devi et al., 2019). Taken together, different stressors could induce different types of RPE cell death, mitochondrial dysfunction including reduced mitochondrial membrane potential, decreased ATP and increased ROS levels, and in many cases mitochondrial fragmentation and/or mitochondrial fission/fusion imbalance, were generally observed, supporting a role for mitochondria in RPE cell death.
8 Restoring Mitochondrial Function as Treatment Option for Retinal Pigmented Epithelial Aging and Age-Related Macular Degeneration
Given the critical role of mitochondrial function and dynamics in senescence and cell death, extensive efforts are being made to target mitochondria for RPE aging and age-related diseases, especially AMD. Here we summarize the current preclinical experiments and clinical studies on this topic (Table 3).
1) Humanin is a mitochondrial-derived peptide with cytoprotective function in different disease models. Using cybrid containing AMD mitochondria, Nashine et al. found that humanin has a pivotal role in protecting cells with AMD mitochondria, reducing pro-apoptosis gene expression and increasing protection against amyloid-β-induced damage (Nashine et al., 2017). Mechanistically, humanin acts via both intracellular (BAX) and extracellular (gp130) pathways and prevents the loss of AMD mitochondria. In an independent study, humanin was shown to protect oxidative-stress induced RPE cell death and senescence (Sreekumar et al., 2016). Humanin treatment prevented oxidative stress-induced decrease in mitochondrial bioenergetics, increased mtDNA copy number and upregulated the expression of mitochondrial transcription factor A, a key biogenesis regulator protein. These studies suggest the potential for humanin therapy for prevention of retinal degeneration, including AMD.
2) Resveratrol is a phytoalexin synthesized by numerous plants including vines with strong antioxidative properties. Resveratrol improved cell viability and decreased ROS levels in AMD cybrid model and stimulated mitochondrial bioenergetics in RPE cells (King et al., 2005; Sheu et al., 2010; Sheu et al., 2013; Nashine et al., 2020; Neal et al., 2020). It also induced autophagy, pro-survival and specific anti-inflammatory response in RPE cells (Josifovska et al., 2020). In addition, resveratrol suppressed choroidal neovascularization in an animal model of wet AMD (Nagai et al., 2014). Other studies showed that resveratrol activates SIRT1, a key regulator of cellular senescence, aging and longevity (Borra et al., 2005). Based on these, resveratrol has great potential as therapeutic targets for RPE aging and AMD.
3) Chrysoeriol is a flavonoid compound that is commonly found in plants of the genus Perilla frutescens. This compound possesses several health-beneficial properties, including antioxidant (Mishra et al., 2003; Nickavar et al., 2016), anti-inflammatory (Choi et al., 2005) and anti-tumor activities (Min et al., 2020). Chrysoeriol protected H2O2-induced RPE cell death by diminishing mitochondrial dysfunction, preventing ROS accumulation and enhancing the expression of anti-oxidative genes including NRF2 (Kim et al., 2021a). It attenuated oxidative stress-induced MMP loss and upregulated mitochondrial related gene expression, including OXPHOS genes, mitochondrial process genes, and mtDNA replication and transcription genes. It also significantly increased TOM20, MFN2, and OPA1 expression and decreased H2O2-induced DRP1 expression and phosphorylation (at Ser 616 position). Further work is needed to directly test the role of chrysoeriol in mitochondrial biogenesis and quality control, as well as RPE aging and degeneration in vivo.
4) Necrostatins are inhibitors of necroptosis (Degterev et al., 2005). Necrostatin-1 (Nec-1) is the most common inhibitor of necrosis that targets receptor Interacting Serine/Threonine Kinase 1 (RIPK1); Nec-5 is the necrosis inhibitor that inhibits RIPK1 indirectly (Wang et al., 2007); while Nec-7 targets RIPK1-independent necrosis pathways (Zheng et al., 2008). Nec-1 has been shown to block necroptosis and ameliorate inflammatory response in multiple disease models (Cao and Mu, 2021). Our lab has shown that necrostatins protect oxidative stress-induced RPE cell death in vitro and in vivo (Hanus et al., 2015; Hanus et al., 2016). Other studies showed that Nec-1 can recover mitochondrial dysfunction and reduce ROS production in response to necroptosis inducer TNF-α or acetaminophen (Ye et al., 2012; Takemoto et al., 2014). Based on these, necrotstatins could have potential in regulating mitochondrial function and RPE degeneration, which awaits future work to confirm.
5) PGC-1α enhancers. PGC1-α is a critical regulator of mitochondrial biogenesis and redox control. PU-91, an FDA-approved mitochondrion-stabilizing drug, was shown to improve cell survival, mitochondrial health and anti-oxidative potential by upregulating PGC-1α in AMD cybrid model (Nashine et al., 2019). It increases mitochondrial DNA copy number, upregulates the genes involved in mitochondrial biogenesis pathway including PGC-1α, NRF-1, NRF-2, peroxisome proliferator-activated receptors (PPAR)-α, and PPAR-γ, increases mitochondrial membrane potential, decreases mitochondrial superoxides levels, upregulates SOD2 expression level and increases the production of mitochondrial derived peptides. Triphenylphosphonium (TPP) is a well-known mitochondrial targeting moiety. Vitamin B3 (niacin) is a powerful antioxidant with lipid lowering functions (Ganji et al., 2009; Boden et al., 2014). TPP-conjugated Niacin (TPP-Niacin) has been shown to improve cell viability, reduce ROS generation, and increase the antioxidant enzymes in H2O2-treated ARPE-19 cells (Kim et al., 2021b). It ameliorated H2O2-induced mitochondrial dysfunction and mitochondrial membrane potential reduction. It also markedly enhanced the expression of transcription factors (PGC-1α and NRF2) and antioxidant-associated genes (especially heme oxygenase-1 and NAD(P)H Quinone Dehydrogenase 1). ZLN005, a selective PGC-1α transcriptional regulator, protected RPE from cytotoxic oxidative damage (Satish et al., 2018). ZLN005-treated ARPE-19 cells showed robust upregulation of PGC-1α and its associated transcription factors, antioxidant enzymes, and mitochondrial genes, and enhanced mitochondrial function shown by increasing basal and maximal respiration rates, and spare respiratory capacity. In addition, ZLN005 protected ARPE-19 cells from cell death caused by H2O2, oxidized low-density lipoprotein, and NaIO3 without any cytotoxicity under basal conditions. ZLN005 protection effect is PGC-1α-dependent as it was lost in PGC-1α-silenced cells. Taken together, PGC-1α regulators, including PU-91, TPP-Niacin and ZLN005, could serve as novel therapeutic agents for RPE degeneration.
6) Autophagy boosters (AMPK activators). As autophagy (including mitophagy) has significant implications in aging and age-related diseases, autophagy regulators have been tested in RPE cells for protective response to oxidative stress. Metformin is the first-line anti-type 2 diabetes drug and has been known to stimulate autophagy in many tissues. In RPE cells, metformin conferred protection against H2O2-induced oxidative damage by activating AMPK pathway (Zhao et al., 2020). It also protected photoreceptors from light damage, delayed inherited retinal degeneration, and protected RPE from NaIO3-induced injury in vivo (Xu L et al., 2018). The protection was associated with decreased oxidative stress, decreased DNA damage, and increased mitochondrial energy production. A retrospective study indicates that metformin use is associated with decreased odds of developing AMD (Brown et al., 2019b). iPSC-RPE cells derived from AMD patients have been established to test the efficacy of drugs in AMD. Three AMPK activators AICAR (5-Aminoimidazole-4-carboxamide ribonucleotide), metformin, trehalose, have been tested for maintaining mitochondrial function in AMD iPSC-RPE cells (Ebeling et al., 2022). Rapamycin is a drug used to prevent organ transplant rejection. It inhibits mTOR and activates autophagy. Increased mTORC activity by RPE-specific deletion of mTOR suppressor tuberous sclerosis 1 led to RPE degeneration (Huang et al., 2019; Go et al., 2020). Inhibition of mTORC1 by rapamycin partially rescued RPE degeneration, supporting the potential therapeutic role for rapamycin in RPE degeneration.
7) Mitochondrial metabolism regulators. Reductive carboxylation is a major metabolic pathway in RPE cells (Du et al., 2016). It regulates cell viability, redox balance, mitochondrial function, and response to oxidative stress. Nicotinamide mononucleotide (NMN) is a key intermediate of NAD+, which is required to support reductive carboxylation and ATP production. NAD+ decreases with age. Oxidative stress depletes NAD+, and supplementation with NMN completely prevented RPE cell death induced by H2O2. Using RPE cells from AMD and control donors, only RPE cells from AMD donors show improvements in mitochondrial functions, including basal respiration, maximal respiration, spare respiratory capacity, and ATP production, after NMN treatment (Ebeling et al., 2020). Of note, NMN has been shown to mitigate age-related physiology decline in mice and is being pursued as anti-aging molecule in humans (Mills et al., 2016; Shade, 2020). These support a potential role of NMN in RPE aging and AMD.
8) Elamipretide is a mitochondria-targeted tetrapeptide that has been evaluated in mitochondrial diseases including primary mitochondrial myopathy and Barth syndrome (Sabbah, 2021). It acts by stabilizing cardiolipin and therefore increasing cellular ATP production and reducing mitochondrial ROS (Birk et al., 2014; Nickel et al., 2014; Szeto, 2014). In RPE-specific SOD2 knockout mice, daily topical Elamipretide treatment led to the prevention of RPE cell size increase, suggesting reduced RPE cell death and RPE senescence. Based on its mechanisms of action, phase 1 clinical trial has been conducted to evaluate its potential in dry AMD and noncentral GA after daily subcutaneous injection (Mettu et al., 2022). It appeared to be well tolerated without serious adverse effects. Phase 2A clinical trial is underway.
9) α-Lipoic acid (LA) is a mitochondria-targeted antioxidant and mitochondrial nutrient (Packer et al., 1997; Liu and Ames, 2005). Jia et al. reported that LA protected against acute acrolein (a toxicant present in cigarette smoke)-induced RPE cell death and mitochondrial membrane potential decrease. It also inhibited acrolein-induced generation of intracellular oxidants, prevented the intracellular SOD decrease, protected mitochondrial complex I, II, and III activity and increased intracellular total antioxidant power in RPE cells (Jia et al., 2007).
10) Melatonin protects mitochondria by scavenging ROS, inhibiting the MPTP, and activating uncoupling proteins. Thus, melatonin maintains the optimal mitochondrial membrane potential, preserves mitochondrial functions, and regulates mitochondrial biogenesis and dynamics. In most cases, melatonin reduces mitochondrial fission and elevates their fusion. It also has been found to promote mitophagy and improve homeostasis of mitochondria (Tan et al., 2016). In the retina, melatonin is released mainly by photoreceptor cells but can be also produced by other cell types in pathological conditions (Sakamoto et al., 2004). It has been reported that melatonin protects human RPE cells against the cytotoxic effects of H2O2 (Rosen et al., 2012). It was also shown to be effective in the protection of mtDNA of ARPE-19 cells against H2O2-induced damage (Liang et al., 2004). However, high exogenous concentrations of melatonin increase light-induced damage to photoreceptor (Tan et al., 2003). The antioxidant effect of melatonin may indicate its protective role in AMD.
11) Plastoquinonyl-decyl-triphenylphosphonium (SkQ1) is another mitochondrial targeted antioxidant. It has been reported that the treatment with SkQ1 (250 nmol/kg body weight) during the period of active disease progression (from 12 to 18 months of age) significantly prevented the progression of retinopathy and suppressed atrophic changes in the RPE cells in the senescence-accelerated OXYS rats (Muraleva et al., 2014; Muraleva et al., 2019; Telegina et al., 2020).
9 Conclusion Marks and Future Directions
RPE cells are critical for the metabolism and homeostasis of retina. Due to high metabolism, high exposure to light, oxidized POS and PUFAs, RPE cells are vulnerable to oxidative stress and other relevant stresses which make them more susceptible to aging and age-related disease. Mitochondria are the powerhouse of cells and can be a major source of cellular ROS that contribute to mtDNA damage, cell death, senescence, and age-related diseases. Mitochondria directly participate in cell death and senescence processes, and can undergo dynamic changes including fission/fusion, biogenesis and mitophagy for quality control in response to stresses. In this minireview, we described the RPE changes during aging and in AMD. The role of mitochondria in RPE aging and AMD was also discussed. Particularly, the involvement of mitochondria in RPE cellular senescence and death, two processes critical for RPE degeneration, and the current translational approaches to prevent RPE aging and degeneration, were summarized. Changes in the mitochondria of RPE, including mtDNA deletion and mutation, decreased ATP production, mitochondrial fission/fusion imbalance, decreased mitochondrial biogenesis and mitophagy and et al., were commonly observed during RPE aging and degeneration. More longitudinal studies, especially in vivo studies, are required to confirm some of the findings, since mitochondria could undergo dynamic changes during aging and in response to stresses, with adaptive response in the short term and pathological response in the long term. Based on the current research, although RPE senescence and cell death are involved in RPE aging and degeneration, the extent of their contribution and whether we can target RPE senescence and/or cell death for RPE aging and/or degeneration are still a matter of debate. Elimination of senescence using a senolytic approach increases mouse lifespan and has shown promise in human trials (Xu M et al., 2018; Justice et al., 2019). If RPE senescence is proved to be the major mechanism for RPE aging and degeneration, senolytic approach could be used to treat these conditions. Restoring mitochondrial function for preventing RPE aging and degeneration is an exciting idea, and many compounds listed above have shown promise in preclinical and clinical models. However, the understanding of mitochondria, especially mitochondrial dynamics and quality control, in RPE cell death and senescence, as well as RPE aging and degeneration, is still incomplete and awaits future studies. Stringent and new technologies, including genetic functional study, genetic lineage tracing, and single cell multi-omics studies, would be powered to answer these questions. New drug targets and drug candidates, more preclinical and clinical studies are also needed to test their efficacy and safety for RPE degeneration and AMD. Work on AMD-derived iPSC-RPE cells has shown considerable variability in drug response across patient cell lines (Ebeling et al., 2022). Therefore, a personalized medicine approach, including stratifying patients based on genotyping and more clinically relevant features, is needed in the future.
Author Contributions
YT, ZZ and SW contributed to writing the review.
Funding
YT was supported by American Federation for Aging Research (AFAR) Scholarship. SW was supported by a Startup fund from Tulane University, BrightFocus Foundation Grant in AMD, and NIH Grants EY021862 and EY026069. The fundings are not responsible for the content of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ach, T., Tolstik, E., Messinger, J. D., Zarubina, A. V., Heintzmann, R., and Curcio, C. A. (2015). Lipofuscin Redistribution and Loss Accompanied by Cytoskeletal Stress in Retinal Pigment Epithelium of Eyes with Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 56, 3242–3252. doi:10.1167/iovs.14-16274
Alaimo, A., Liñares, G. G., Bujjamer, J. M., Gorojod, R. M., Alcon, S. P., Martínez, J. H., et al. (2019). Toxicity of Blue Led Light and A2E Is Associated to Mitochondrial Dynamics Impairment in ARPE-19 Cells: Implications for Age-Related Macular Degeneration. Arch. Toxicol. 93, 1401–1415. doi:10.1007/s00204-019-02409-6
Ambati, J., Ambati, B. K., Yoo, S. H., Ianchulev, S., and Adamis, A. P. (2003). Age-related Macular Degeneration: Etiology, Pathogenesis, and Therapeutic Strategies. Surv. Ophthalmol. 48, 257–293. doi:10.1016/s0039-6257(03)00030-4
An, E., Lu, X., Flippin, J., Devaney, J. M., Halligan, B., Hoffman, E., et al. (2006). Secreted Proteome Profiling in Human RPE Cell Cultures Derived from Donors with Age Related Macular Degeneration and Age Matched Healthy Donors. J. Proteome Res. 5, 2599–2610. doi:10.1021/pr060121j
An, E., Lu, X., Flippin, J., Devaney, J. M., Halligan, B., Hoffman, E., et al. (2007). Secreted Proteome Profiling in Human RPE Cell Cultures Derived from Donors with Age Related Macular Degeneration and Age Matched Healthy Donors J. Proteome Res. 2006, 5, 2599−2610. J. Proteome Res. 6, 1615. doi:10.1021/pr078003z
Arselin, G., Vaillier, J., Salin, B., Schaeffer, J., Giraud, M.-F., Dautant, A., et al. (2004). The Modulation In Subunits E and G Amounts Of Yeast ATP Synthase Modifies Mitochondrial Cristae Morphology. J. Biol. Chem. 279, 40392–40399. doi:10.1074/jbc.M404316200
Bakula, D., and Scheibye-Knudsen, M. (2020). MitophAging: Mitophagy in Aging and Disease. Front. Cell Dev. Biol. 8, 239. doi:10.3389/fcell.2020.00239
Bantseev, V., and Youn, H.-Y. (2006). Mitochondrial "Movement" and Lens Optics Following Oxidative Stress from UV-B Irradiation: Cultured Bovine Lenses and Human Retinal Pigment Epithelial Cells (ARPE-19) as Examples. Ann. N. Y. Acad. Sci. 1091, 17–33. doi:10.1196/annals.1378.051
Bergen, A. A., Arya, S., Koster, C., Pilgrim, M. G., Wiatrek-Moumoulidis, D., van der Spek, P. J., et al. (2019). On the Origin of Proteins in Human Drusen: The Meet, Greet and Stick Hypothesis. Prog. Retin. Eye Res. 70, 55–84. doi:10.1016/j.preteyeres.2018.12.003
Bersuker, K., Hendricks, J. M., Li, Z., Magtanong, L., Ford, B., Tang, P. H., et al. (2019). The CoQ Oxidoreductase FSP1 Acts Parallel to GPX4 to Inhibit Ferroptosis. Nature. 575, 688. doi:10.1038/s41586-019-1705-2
Birk, A. V., Chao, W. M., Bracken, C., Warren, J. D., and Szeto, H. H. (2014). Targeting Mitochondrial Cardiolipin and the Cytochromec/cardiolipin Complex to Promote Electron Transport and Optimize Mitochondrial ATP Synthesis. Br. J. Pharmacol. 171, 2017–2028. doi:10.1111/bph.12468
Blasiak, J., Pawlowska, E., Sobczuk, A., Szczepanska, J., and Kaarniranta, K. (2020). The Aging Stress Response and its Implication for AMD Pathogenesis. Ijms 21, 8840. doi:10.3390/ijms21228840
Boden, W. E., Sidhu, M. S., and Toth, P. P. (2014). The Therapeutic Role of Niacin in Dyslipidemia Management. J. Cardiovasc Pharmacol. Ther. 19, 141–158. doi:10.1177/1074248413514481
Bok, D. (1985). Retinal Photoreceptor-Pigment Epithelium Interactions. Friedenwald Lecture. Investig. Ophthalmol. Vis. Sci. 26, 1659.
Bonilha, V. (2008). Age and Disease-Related Structural Changes in the Retinal Pigment Epithelium. Opth 2, 413–424. doi:10.2147/opth.s2151
Bornhövd, C., Vogel, F., Neupert, W., and Reichert, A. S. (2006). Mitochondrial Membrane Potential Is Dependent on the Oligomeric State of F1F0-ATP Synthase Supracomplexes. J. Biol. Chem. 281, 13990–13998. doi:10.1074/jbc.M512334200
Borra, M. T., Smith, B. C., and Denu, J. M. (2005). Mechanism of Human SIRT1 Activation by Resveratrol. J. Biol. Chem. 280, 17187–17195. doi:10.1074/jbc.M501250200
Boulton, M., and Dayhaw-Barker, P. (2001). The Role of the Retinal Pigment Epithelium: Topographical Variation and Ageing Changes. Eye 15, 384–389. doi:10.1038/eye.2001.141
Boyman, L., Karbowski, M., and Lederer, W. J. (2020). Regulation of Mitochondrial ATP Production: Ca2+ Signaling and Quality Control. Trends Mol. Med. 26, 21–39. doi:10.1016/j.molmed.2019.10.007
Brown, E. E., DeWeerd, A. J., Ildefonso, C. J., Lewin, A. S., and Ash, J. D. (2019b). Mitochondrial Oxidative Stress in the Retinal Pigment Epithelium (RPE) Led to Metabolic Dysfunction in Both the RPE and Retinal Photoreceptors. Redox Biol. 24, 101201. doi:10.1016/j.redox.2019.101201
Brown, E. E., Ball, J. D., Chen, Z., Khurshid, G. S., Prosperi, M., and Ash, J. D. (2019a). The Common Antidiabetic Drug Metformin Reduces Odds of Developing Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 60, 1470–1477. doi:10.1167/iovs.18-26422
Burke, J. (2008). Epithelial Phenotype and the RPE: Is the Answer Blowing in the Wnt? Prog. Retin. Eye Res. 27, 579–595. doi:10.1016/j.preteyeres.2008.08.002
Byrne, J. J., Soh, M. S., Chandhok, G., Vijayaraghavan, T., Teoh, J.-S., Crawford, S., et al. (2019). Disruption of Mitochondrial Dynamics Affects Behaviour and Lifespan in Caenorhabditis elegans. Cell. Mol. Life Sci. 76, 1967–1985. doi:10.1007/s00018-019-03024-5
Cano, M., Wang, L., Wan, J., Barnett, B. P., Ebrahimi, K., Qian, J., et al. (2014). Oxidative Stress Induces Mitochondrial Dysfunction and a Protective Unfolded Protein Response in RPE Cells. Free Radic. Biol. Med. 69, 1–14. doi:10.1016/j.freeradbiomed.2014.01.004
Cao, L. Y., and Mu, W. (2021). Necrostatin-1 and Necroptosis Inhibition: Pathophysiology and Therapeutic Implications. Pharmacol. Res. 163, 105297. doi:10.1016/j.phrs.2020.105297
Cavallini, G., Donati, A., Taddei, M., and Bergamini, E. (2007). Evidence for Selective Mitochondrial Autophagy and Failure in Aging. Autophagy 3, 26–27. doi:10.4161/auto.3268
Balaiya, K., Murthy, R. K., Brar, V. S., and Chalam, K. V. (2010). Evaluation of Ultraviolet Light Toxicity on Cultured Retinal Pigment Epithelial and Retinal Ganglion Cells. Opth 4, 33–39. doi:10.2147/opth.s7979
Chan, D. C. (2012). Fusion and Fission: Interlinked Processes Critical for Mitochondrial Health. Annu. Rev. Genet. 46, 265–287. doi:10.1146/annurev-genet-110410-132529
Chaudhari, S. N., and Kipreos, E. T. (2017). Increased Mitochondrial Fusion Allows the Survival of Older Animals in Diverse C. elegans Longevity Pathways. Nat. Commun. 8, 182. doi:10.1038/s41467-017-00274-4
Chen, H., Attieh, Z. K., Dang, T., Huang, G., van der Hee, R. M., and Vulpe, C. (2009a). Decreased Hephaestin Expression and Activity Leads to Decreased Iron Efflux from Differentiated CaCO2 Cells. J. Cell. Biochem. 107, 803–808. doi:10.1002/jcb.22178
Chen, H., Liu, B., Lukas, T. J., Suyeoka, G., Wu, G., and Neufeld, A. H. (2009b). Changes in Iron-Regulatory Proteins in the Aged Rodent Neural Retina. Neurobiol. Aging 30, 1865–1876. doi:10.1016/j.neurobiolaging.2008.01.002
Chen, H., Lukas, T. J., Du, N., Suyeoka, G., and Neufeld, A. H. (2009c). Dysfunction of the Retinal Pigment Epithelium with Age: Increased Iron Decreases Phagocytosis and Lysosomal Activity. Investig. Ophthalmol. Vis. Sci. 50, 1895–1902. doi:10.1167/iovs.08-2850
Chen, M., Rajapakse, D., Fraczek, M., Luo, C., Forrester, J. V., and Xu, H. (2016). Retinal Pigment Epithelial Cell Multinucleation in the Aging Eye - a Mechanism to Repair Damage and Maintain Homoeostasis. Aging Cell 15, 436–445. doi:10.1111/acel.12447
Chen, Q. M., Liu, J., and Merrett, J. B. (2000). Apoptosis or Senescence-like Growth Arrest: Influence of Cell-Cycle Position, P53, P21 and Bax in H2O2 Response of Normal Human Fibroblasts. Biochem. J. 347, 543–551. doi:10.1042/0264-6021:347054310.1042/bj3470543
Childs, B. G., Durik, M., Baker, D. J., and van Deursen, J. M. (2015). Cellular Senescence in Aging and Age-Related Disease: from Mechanisms to Therapy. Nat. Med. 21, 1424–1435. doi:10.1038/nm.4000
Chistiakov, D. A., Sobenin, I. A., Revin, V. V., Orekhov, A. N., and Bobryshev, Y. V. (2014). Mitochondrial Aging and Age-Related Dysfunction of Mitochondria. BioMed Res. Int. 2014, 1. doi:10.1155/2014/238463
Choi, D.-Y., Lee, J. Y., Kim, M.-R., Woo, E.-R., Kim, Y. G., and Kang, K. W. (2005). Chrysoeriol Potently Inhibits the Induction of Nitric Oxide Synthase by Blocking AP-1 Activation. J. Biomed. Sci. 12, 949–959. doi:10.1007/s11373-005-9028-8
Contín, M. A., Arietti, M. M., Benedetto, M. M., Bussi, C., and Guido, M. E. (2013). Photoreceptor Damage Induced by Low-Intensity Light: Model of Retinal Degeneration in Mammals. Mol. Vis. 19, 1614–1625.
Crane, J. D., Devries, M. C., Safdar, A., Hamadeh, M. J., and Tarnopolsky, M. A. (2010). The Effect of Aging on Human Skeletal Muscle Mitochondrial and Intramyocellular Lipid Ultrastructure. Journals Gerontology Ser. A Biol. Sci. Med. Sci. 65A, 119–128. doi:10.1093/gerona/glp179
Dalle Pezze, P., Nelson, G., Otten, E. G., Korolchuk, V. I., Kirkwood, T. B. L., von Zglinicki, T., et al. (2014). Dynamic Modelling of Pathways to Cellular Senescence Reveals Strategies for Targeted Interventions. PLoS Comput. Biol. 10, e1003728. doi:10.1371/journal.pcbi.1003728
Davalli, P., Mitic, T., Caporali, A., Lauriola, A., and D’Arca, D. (2016). ROS, Cell Senescence, and Novel Molecular Mechanisms in Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2016, 1. doi:10.1155/2016/3565127
de Vasconcelos, N. M., Van Opdenbosch, N., Van Gorp, H., Parthoens, E., and Lamkanfi, M. (2019). Single-cell Analysis of Pyroptosis Dynamics Reveals Conserved GSDMD-Mediated Subcellular Events that Precede Plasma Membrane Rupture. Cell Death Differ. 26, 146–161. doi:10.1038/s41418-018-0106-7
Degterev, A., Huang, Z., Boyce, M., Li, Y., Jagtap, P., Mizushima, N., et al. (2005). Erratum: Corrigendum: Chemical Inhibitor of Nonapoptotic Cell Death with Therapeutic Potential for Ischemic Brain Injury. Nat. Chem. Biol. 1, 234. doi:10.1038/nchembio0905-234a
Devi, T. S., Yumnamcha, T., Yao, F., Somayajulu, M., Kowluru, R. A., and Singh, L. P. (2019). TXNIP Mediates High Glucose-Induced Mitophagic Flux and Lysosome Enlargement in Human Retinal Pigment Epithelial Cells. Biol. Open 8, 8521. doi:10.1242/bio.038521
Dib, B., Lin, H., Maidana, D. E., Tian, B., Miller, J. B., Bouzika, P., et al. (2015). Mitochondrial DNA Has a Pro-inflammatory Role in AMD. Biochimica Biophysica Acta (BBA) - Mol. Cell Res. 1853, 2897–2906. doi:10.1016/j.bbamcr.2015.08.012
Ding, W.-X., and Yin, X.-M. (2012). Mitophagy: Mechanisms, Pathophysiological Roles, and Analysis. Biol. Chem. 393, 547–564. doi:10.1515/hsz-2012-0119
Dixon, S. J., Lemberg, K. M., Lamprecht, M. R., Skouta, R., Zaitsev, E. M., Gleason, C. E., et al. (2012). Ferroptosis: An Iron-dependent Form of Nonapoptotic Cell Death. Cell 149, 1060–1072. doi:10.1016/j.cell.2012.03.042
Doblado, L., Lueck, C., Rey, C., Samhan-Arias, A. K., Prieto, I., Stacchiotti, A., et al. (2021). Mitophagy in Human Diseases. Ijms 22, 3903. doi:10.3390/ijms22083903
Doll, S., Freitas, F. P., Shah, R., Aldrovandi, M., da Silva, M. C., Ingold, I., et al. (2019). FSP1 Is a Glutathione-independent Ferroptosis Suppressor. Nature. 575, 693, 698-+. doi:10.1038/s41586-019-1707-0
Dolman, N. J., Chambers, K. M., Mandavilli, B., Batchelor, R. H., and Janes, M. S. (2013). Tools and Techniques to Measure Mitophagy Using Fluorescence Microscopy. Autophagy 9, 1653–1662. doi:10.4161/auto.24001
Dranka, B. P., Benavides, G. A., Diers, A. R., Giordano, S., Zelickson, B. R., Reily, C., et al. (2011). Assessing Bioenergetic Function in Response to Oxidative Stress by Metabolic Profiling. Free Radic. Biol. Med. 51, 1621–1635. doi:10.1016/j.freeradbiomed.2011.08.005
Du, J., Yanagida, A., Knight, K., Engel, A. L., Vo, A. H., Jankowski, C., et al. (2016). Reductive Carboxylation Is a Major Metabolic Pathway in the Retinal Pigment Epithelium. Proc. Natl. Acad. Sci. U.S.A. 113, 14710–14715. doi:10.1073/pnas.1604572113
Dunaief, J. L., Dentchev, T., Ying, G. S., and Milam, A. H. (2002). The Role of Apoptosis in Age-Related Macular Degeneration. Arch. Ophthalmol. 120, 1435–1442. doi:10.1001/archopht.120.11.1435
Ebeling, M. C., Polanco, J. R., Qu, J., Tu, C., and Montezuma, S. R. (2020). .Improving Retinal Mitochondrial Function as a Treatment for Age-Related Macular Degeneration. Redox Biol 34, 101552. doi:10.1016/j.redox.2020.101552
Ebeling, M. C., Geng, Z., Kapphahn, R. J., Roehrich, H., Montezuma, S. R., Dutton, J. R., et al. (2021). Impaired Mitochondrial Function in iPSC-Retinal Pigment Epithelium with the Complement Factor H Polymorphism for Age-Related Macular Degeneration. Cells 10, 789. doi:10.3390/cells10040789
Ebeling, M. C., Geng, Z., Stahl, M. R., Kapphahn, R. J., Roehrich, H., Montezuma, S. R., et al. (2022). Testing Mitochondrial-Targeted Drugs in iPSC-RPE from Patients with Age-Related Macular Degeneration. Pharmaceuticals 15, 62. doi:10.3390/ph15010062
Elliott, E. I., Miller, A. N., Banoth, B., Iyer, S. S., Stotland, A., Weiss, J. P., et al. (2018). Cutting Edge: Mitochondrial Assembly of the NLRP3 Inflammasome Complex Is Initiated at Priming. J. Immunol. 200, 3047–3052. doi:10.4049/jimmunol.1701723
Evavold, C. L., Hafner-Bratkovič, I., Devant, P., D’Andrea, J. M., Ngwa, E. M., Boršić, E., et al. (2021). Control of Gasdermin D Oligomerization and Pyroptosis by the Ragulator-Rag-mTORC1 Pathway, Cell, 184, 4495, 4511. doi:10.1016/j.cell.2021.06.028
Falkenberg, M., Larsson, N.-G., and Gustafsson, C. M. (2007). DNA Replication and Transcription in Mammalian Mitochondria. Annu. Rev. Biochem. 76, 679–699. doi:10.1146/annurev.biochem.76.060305.152028
Feher, J., Kovacs, I., Artico, M., Cavallotti, C., Papale, A., and Balacco Gabrieli, C. (2006). Mitochondrial Alterations of Retinal Pigment Epithelium in Age-Related Macular Degeneration. Neurobiol. Aging 27, 983–993. doi:10.1016/j.neurobiolaging.2005.05.012
Felszeghy, S., Viiri, J., Paterno, J. J., Hyttinen, J. M. T., Koskela, A., Chen, M., et al. (2019). Loss of NRF-2 and PGC-1α Genes Leads to Retinal Pigment Epithelium Damage Resembling Dry Age-Related Macular Degeneration. Redox Biol. 20, 1–12. doi:10.1016/j.redox.2018.09.011
Ferrington, D. A., Ebeling, M. C., Kapphahn, R. J., Terluk, M. R., Fisher, C. R., Polanco, J. R., et al. (2017). Altered Bioenergetics and Enhanced Resistance to Oxidative Stress in Human Retinal Pigment Epithelial Cells from Donors with Age-Related Macular Degeneration. Redox Biol. 13, 255–265. doi:10.1016/j.redox.2017.05.015
Finkel, T., Menazza, S., Holmström, K. M., Parks, R. J., Liu, J., Sun, J., et al. (2015). The Ins and Outs of Mitochondrial Calcium. Circ. Res. 116, 1810–1819. doi:10.1161/CIRCRESAHA.116.305484
Gan, B. Y. (2021). Mitochondrial Regulation of Ferroptosis. J. Cell Biol. 220, e202105043. doi:10.1083/jcb.202105043
Ganji, S. H., Qin, S., Zhang, L., Kamanna, V. S., and Kashyap, M. L. (2009). Niacin Inhibits Vascular Oxidative Stress, Redox-Sensitive Genes, and Monocyte Adhesion to Human Aortic Endothelial Cells. Atherosclerosis 202, 68–75. doi:10.1016/j.atherosclerosis.2008.04.044
García-Prat, L., Martínez-Vicente, M., Perdiguero, E., Ortet, L., Rodríguez-Ubreva, J., Rebollo, E., et al. (2016). Autophagy Maintains Stemness by Preventing Senescence. Nature 529, 37–42. doi:10.1038/nature16187
Giorgi, C., Marchi, S., Simoes, I. C. M., Ren, Z., Morciano, G., Perrone, M., et al. (2018). Mitochondria and Reactive Oxygen Species in Aging and Age-Related Diseases. Int. Rev. Cell Mol. Biol. 340, 209–344. doi:10.1016/bs.ircmb.2018.05.006
Go, Y. M., Zhang, J., Fernandes, J., Litwin, C., Chen, R., Wensel, T. G., et al. (2020). MTOR‐initiated Metabolic Switch and Degeneration in the Retinal Pigment Epithelium. FASEB J. 34, 12502–12520. doi:10.1096/fj.202000612R
Golestaneh, N., Chu, Y., Xiao, Y.-Y., Stoleru, G. L., and Theos, A. C. (2017). Dysfunctional Autophagy in RPE, a Contributing Factor in Age-Related Macular Degeneration. Cell Death Dis. 8, e2537. doi:10.1038/cddis.2016.453
Gomes, L. C., Benedetto, G. D., and Scorrano, L. (2011). During Autophagy Mitochondria Elongate, Are Spared from Degradation and Sustain Cell Viability. Nat. Cell Biol. 13, 589–598. doi:10.1038/ncb2220
Gottlieb, R. A., and Stotland, A. (2015). MitoTimer: a Novel Protein for Monitoring Mitochondrial Turnover in the Heart. J. Mol. Med. 93, 271–278. doi:10.1007/s00109-014-1230-6
Gouras, P., Ivert, L., Neuringer, M., and Nagasaki, T. (2016). Mitochondrial Elongation in the Macular RPE of Aging Monkeys, Evidence of Metabolic Stress. Graefes Arch. Clin. Exp. Ophthalmol. 254, 1221–1227. doi:10.1007/s00417-016-3342-x
Gowda, G. A. N., and Djukovic, D. (2014). Overview of Mass Spectrometry-Based Metabolomics: Opportunities and Challenges. Methods Mol. Biol. 1198, 3–12. doi:10.1007/978-1-4939-1258-2_1
Gureev, A. P., Shaforostova, E. A., and Popov, V. N. (2019). Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 10, 435. doi:10.3389/fgene.2019.00435
Hampel, B., Malisan, F., Niederegger, H., Testi, R., and Jansen-Dürr, P. (2004). Differential Regulation of Apoptotic Cell Death in Senescent Human Cells. Exp. Gerontol. 39, 1713–1721. doi:10.1016/j.exger.2004.05.010
Hanus, J., Anderson, C., Sarraf, D., Ma, J., and Wang, S. (2016). Retinal Pigment Epithelial Cell Necroptosis in Response to Sodium Iodate. Cell Death Discov. 2, 16054. doi:10.1038/cddiscovery.2016.54
Hanus, J., Anderson, C., and Wang, S. (2015). RPE Necroptosis in Response to Oxidative Stress and in AMD. Ageing Res. Rev. 24, 286–298. doi:10.1016/j.arr.2015.09.002
Hanus, J., Zhang, H., Wang, Z., Liu, Q., Zhou, Q., and Wang, S. (2013). Induction of Necrotic Cell Death by Oxidative Stress in Retinal Pigment Epithelial Cells. Cell Death Dis. 4, e965. doi:10.1038/cddis.2013.478
Harris, J., Subhi, Y., and Sørensen, T. L. (2017). Effect of Aging and Lifestyle on Photoreceptors and Retinal Pigment Epithelium: Cross-Sectional Study in a Healthy Danish Population. Pathobiology Aging. Age-related Dis. 7, 1398016. doi:10.1080/20010001.2017.1398016
He, Y., Ge, J., Burke, J. M., Myers, R. L., Dong, Z. Z., and Tombran-Tink, J. (2010). Mitochondria Impairment Correlates with Increased Sensitivity of Aging RPE Cells to Oxidative Stress. J. Ocul. Biol. Dis. Inf. 3, 92–108. doi:10.1007/s12177-011-9061-y
Herbig, U., Jobling, W. A., Chen, B. P. C., Chen, D. J., and Sedivy, J. M. (2004). Telomere Shortening Triggers Senescence of Human Cells through a Pathway Involving ATM, P53, and p21CIP1, but Not p16INK4a. Mol. Cell 14, 501–513. doi:10.1016/s1097-2765(04)00256-4
Herzig, S., and Shaw, R. J. (2018). AMPK: Guardian of Metabolism and Mitochondrial Homeostasis. Nat. Rev. Mol. Cell Biol. 19, 121–135. doi:10.1038/nrm.2017.95
Hiona, A., and Leeuwenburgh, C. (2008). The Role of Mitochondrial DNA Mutations in Aging and Sarcopenia: Implications for the Mitochondrial Vicious Cycle Theory of Aging. Exp. Gerontol. 43, 24–33. doi:10.1016/j.exger.2007.10.001
Ho, T.-C., Yang, Y.-C., Cheng, H.-C., Wu, A.-C., Chen, S.-L., Chen, H.-K., et al. (2006). Activation of Mitogen-Activated Protein Kinases Is Essential for Hydrogen Peroxide -induced Apoptosis in Retinal Pigment Epithelial Cells. Apoptosis 11, 1899–1908. doi:10.1007/s10495-006-9403-6
Holtkamp, G. M., Kijlstra, A., Peek, R., and de Vos, A. F. (2001). Retinal Pigment Epithelium-Immune System Interactions: Cytokine Production and Cytokine-Induced Changes. Prog. Retin. Eye Res. 20, 29–48. doi:10.1016/s1350-9462(00)00017-3
Holz, F. G., Bellman, C., Staudt, S., Schütt, F., and Völcker, H. E. (2001). Fundus Autofluorescence and Development of Geographic Atrophy in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 42 (5), 1051–1056.
Hsieh, F.-C., Hung, C.-T., Cheng, K.-C., Wu, C.-Y., Chen, Y.-C., Wu, Y.-J., et al. (2018). Protective Effects of Lycium Barbarum Extracts on UVB-Induced Damage in Human Retinal Pigment Epithelial Cells Accompanied by Attenuating ROS and DNA Damage. Oxidative Med. Cell. Longev. 2018, 12. doi:10.1155/2018/4814928
Hu, D.-N., Simon, J. D., and Sarna, T. (2008). Role of Ocular Melanin in Ophthalmic Physiology and Pathology. Photochem Photobiol. 84, 639–644. doi:10.1111/j.1751-1097.2008.00316.x
Huang, J., Gu, S., Chen, M., Zhang, S.-j., Jiang, Z., Chen, X., et al. (2019). Abnormal mTORC1 Signaling Leads to Retinal Pigment Epithelium Degeneration. Theranostics 9, 1170–1180. doi:10.7150/thno.26281
Hwang, J. C., Chan, J. W. K., Chang, S., and Smith, R. T. (2006). Predictive Value of Fundus Autofluorescence for Development of Geographic Atrophy in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 47, 2655–2661. doi:10.1167/iovs.05-1027
Inana, G., Murat, C., An, W., Yao, X., Harris, I. R., and Cao, J. (2018). RPE Phagocytic Function Declines in Age-Related Macular Degeneration and Is Rescued by Human Umbilical Tissue Derived Cells. J. Transl. Med. 16, 63. doi:10.1186/s12967-018-1434-6
Jarrett, S., Lin, H., Godley, B., and Boulton, M. (2008). Mitochondrial DNA Damage and its Potential Role in Retinal Degeneration. Prog. Retin. Eye Res. 27, 596–607. doi:10.1016/j.preteyeres.2008.09.001
Jendrach, M., Pohl, S., Vöth, M., Kowald, A., Hammerstein, P., and Bereiter-Hahn, J. (2005). Morpho-dynamic Changes of Mitochondria during Ageing of Human Endothelial Cells. Mech. Ageing Dev. 126, 813–821. doi:10.1016/j.mad.2005.03.002
Jheng, H.-F., Tsai, P.-J., Guo, S.-M., Kuo, L.-H., Chang, C.-S., Su, I.-J., et al. (2012). Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Mol. Cell Biol. 32, 309–319. doi:10.1128/MCB.05603-11
Jia, L., Liu, Z., Sun, L., Miller, S. S., Ames, B. N., Cotman, C. W., et al. (2007). Acrolein, a Toxicant in Cigarette Smoke, Causes Oxidative Damage and Mitochondrial Dysfunction in RPE Cells: Protection by (R)-α-Lipoic Acid. Investig. Ophthalmol. Vis. Sci. 48, 339–348. doi:10.1167/iovs.06-0248
Josifovska, N., Albert, R., Nagymihály, R., Lytvynchuk, L., Moe, M. C., Kaarniranta, K., et al. (2020). Resveratrol as Inducer of Autophagy, Pro-survival, and Anti-inflammatory Stimuli in Cultured Human RPE Cells. Int. J. Mol. Sci. 21. 813. doi:10.3390/ijms21030813
Justice, J. N., Nambiar, A. M., Tchkonia, T., LeBrasseur, N. K., Pascual, R., Hashmi, S. K., et al. (2019). Senolytics in Idiopathic Pulmonary Fibrosis: Results from a First-In-Human, Open-Label, Pilot Study. EBioMedicine 40, 554–563. doi:10.1016/j.ebiom.2018.12.052
Kaarniranta, K., Kajdanek, J., Morawiec, J., Pawlowska, E., and Blasiak, J. (2018). PGC-1α Protects RPE Cells of the Aging Retina against Oxidative Stress-Induced Degeneration through the Regulation of Senescence and Mitochondrial Quality Control. The Significance for AMD PathogenesisThe Significance for AMD Pathogenesis. Int. J. Mol. Sci. 19. 2317. doi:10.3390/ijms19082317
Kaarniranta, K., Salminen, A., Eskelinen, E.-L., and Kopitz, J. (2009). Heat Shock Proteins as Gatekeepers of Proteolytic Pathways-Implications for Age-Related Macular Degeneration (AMD). Ageing Res. Rev. 8, 128–139. doi:10.1016/j.arr.2009.01.001
Kaarniranta, K., Uusitalo, H., Blasiak, J., Felszeghy, S., Kannan, R., Kauppinen, A., et al. (2020). Mechanisms of mitochondrial dysfunction and their impact on age-related macular degeneration. Prog. Retin. Eye Res. 79, 100858. doi:10.1016/j.preteyeres.2020.100858
Karch, J., Kwong, J. Q., Burr, A. R., Sargent, M. A., Elrod, J. W., Peixoto, P. M., et al. (2013). Bax and Bak Function As the Outer Membrane Component of the Mitochondrial Permeability Pore in Regulating Necrotic Cell Death in Mice. Elife 2, e00772. doi:10.7554/eLife.00772
Karch, J., Kanisicak, O., Brody, M. J., Sargent, M. A., Michael, D. M., and Molkentin, J. D. (2015). Necroptosis Interfaces With MOMP and the MPTP in Mediating Cell Death. Plos One 10, e013052. doi:10.1371/journal.pone.0130520
Karunadharma, P. P., Nordgaard, C. L., Olsen, T. W., and Ferrington, D. A. (2010). Mitochondrial DNA Damage As A Potential Mechanism for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 51, 5470–5479. doi:10.1167/iovs.10-5429
Kim, M. H., Kim, D.-H., Yang, S. G., and Kim, D. Y. (2021a). Improved Effect of a Mitochondria-Targeted Antioxidant on Hydrogen Peroxide-Induced Oxidative Stress in Human Retinal Pigment Epithelium Cells. BMC Pharmacol. Toxicol. 22, 7. doi:10.1186/s40360-020-00471-w
Kim, M. H., Kwon, S. Y., Woo, S.-Y., Seo, W. D., and Kim, D. Y. (2021b). Antioxidative Effects of Chrysoeriol Via Activation of the Nrf2 Signaling Pathway And Modulation of Mitochondrial Function. Molecules 26, 313. doi:10.3390/molecules26020313
King, R. E., Kent, K. D., and Bomser, J. A. (2005). Resveratrol Reduces Oxidation and Proliferation of Human Retinal Pigment Epithelial Cells Via Extracellular Signal-Regulated Kinase Inhibition. Chemico-Biological Interact. 151, 143–149. doi:10.1016/j.cbi.2004.11.003
Kubasik-Juraniec, J., Kmieć, Z., Tukaj, C., Rudzińska-Kisiel, T., Kotlarz, G., Pokrywka, L., et al. (2004). The Effect Of Fasting and Refeeding on the Ultrastructure of the Hypothalamic Paraventricular Nucleus in Young and Old Rats. Folia Morphol. (Warsz) 63 (1), 25–35.
Kuilman, T., Michaloglou, C., Mooi, W. J., and Peeper, D. S. (2010). The Essence of Senescence: Figure 1. Genes. Dev. 24, 2463–2479. doi:10.1101/gad.1971610
Kujoth, G. C., Bradshaw, P. C., Haroon, S., and Prolla, T. A. (2007). The Role of Mitochondrial DNA Mutations in Mammalian Aging. PLoS Genet. 3, e24. doi:10.1371/journal.pgen.0030024
Kuse, Y., Ogawa, K., Tsuruma, K., Shimazawa, M., and Hara, H. (2014). Damage of Photoreceptor-Derived Cells in Culture Induced by Light Emitting Diode-Derived Blue Light. Sci. Rep. 4, 5223. doi:10.1038/srep05223
Laker, R. C., Xu, P., Ryall, K. A., Sujkowski, A., Kenwood, B. M., Chain, K. H., et al. (2014). A Novel Mitotimer Reporter Gene for Mitochondrial Content, Structure, Stress, and Damage In Vivo. J. Biol. Chem. 289, 12005–12015. doi:10.1074/jbc.M113.530527
Lee, H.-C., Yin, P.-H., Chi, C.-W., and Wei, Y.-H. (2002). Increase In Mitochondrial Mass in Human Fibroblasts Under Oxidative Stress and During Replicative Cell Senescence. J. Biomed. Sci. 9, 517–526. doi:10.1159/00006472410.1007/bf02254978
Lee, S., Jeong, S.-Y., Lim, W.-C., Kim, S., Park, Y.-Y., Sun, X., et al. (2007). Mitochondrial Fission and Fusion Mediators, Hfis1 and OPA1, Modulate Cellular Senescence. J. Biol. Chem. 282, 22977–22983. doi:10.1074/jbc.M700679200
Liang, F.-Q., Green, L., Wang, C., Alssadi, R., and Godley, B. F. (2004). Melatonin Protects Human Retinal Pigment Epithelial (RPE) Cells Against Oxidative Stress. Exp. Eye Res. 78, 1069–1075. doi:10.1016/j.exer.2004.02.003
Liu, B., Wang, W., Shah, A., Yu, M., Liu, Y., He, L., et al. (2021). Sodium Iodate Induces Ferroptosis in Human Retinal Pigment Epithelium ARPE-19 Cells. Cell Death Dis. 12, 230. doi:10.1038/s41419-021-03520-2
Liu, J., and Ames, B. N. (2005). Reducing Mitochondrial Decay With Mitochondrial Nutrients to Delay and Treat Cognitive Dysfunction, Alzheimer's Disease, and Parkinson's Disease. Nutr. Neurosci. 8, 67–89. doi:10.1080/10284150500047161
Livnat-Levanon, N., and Glickman, M. H. (2011). Ubiquitin-Proteasome System and Mitochondria - Reciprocity. Biochimica Biophysica Acta (BBA) - Gene Regul. Mech. 1809, 80–87. doi:10.1016/j.bbagrm.2010.07.005
Marazita, M. C., Dugour, A., Marquioni-Ramella, M. D., Figueroa, J. M., and Suburo, A. M. (2016). Oxidative Stress-Induced Premature Senescence Dysregulates VEGF And CFH Expression in Retinal Pigment Epithelial Cells: Implications for Age-Related Macular Degeneration. Redox Biol. 7, 78–87. doi:10.1016/j.redox.2015.11.011
Marchitti, S. A., Chen, Y., Thompson, D. C., and Vasiliou, V. (2011). Ultraviolet Radiation: Cellular Antioxidant Response and the Role of Ocular Aldehyde Dehydrogenase Enzymes. Eye Contact Lens 37, 206–213. doi:10.1097/ICL.0b013e3182212642
Marie, M., Bigot, K., Angebault, C., Barrau, C., Gondouin, P., Pagan, D., et al. (2018). Light Action Spectrum on Oxidative Stress and Mitochondrial Damage in A2E-Loaded Retinal Pigment Epithelium Cells. Cell Death Dis. 9, 287. doi:10.1038/s41419-018-0331-5
Marshall, K. D., and Baines, C. P. (2014). Necroptosis: is There a Role for Mitochondria? Front. Physiol. 5, 323. doi:10.3389/fphys.2014.00323
Masiero, E., and Sandri, M. (2010). Autophagy Inhibition Induces Atrophy and Myopathy in Adult Skeletal Muscles. Autophagy 6, 307–309. doi:10.4161/auto.6.2.11137
Mazzoni, F., Safa, H., and Finnemann, S. C. (2014). Understanding Photoreceptor Outer Segment Phagocytosis: Use and Utility of RPE Cells in Culture. Exp. Eye Res. 126, 51–60. doi:10.1016/j.exer.2014.01.010
McBee, J. K., Van Hooser, J. P., Jang, G.-F., and Palczewski, K. (2001). Isomerization of 11-cis- Retinoids to All-trans-retinoids In Vitro and In Vivo. J. Biol. Chem. 276, 48483–48493. doi:10.1074/jbc.M105840200
Mettu, P. S., Mettu, M. J. A., Allingham, M. J., and Cousins, S. W. (2022). Phase 1 Clinical Trial of Elamipretide in Dry Age-Related Macular Degeneration and Noncentral Geographic Atrophy. Ophthalmol. Sci. 2, 100086. doi:10.1016/j.xops.2021.100086
Meyer, J. G., Garcia, T. Y., Schilling, B., Gibson, B. W., and Lamba, D. A. (2019). Proteome and Secretome Dynamics of Human Retinal Pigment Epithelium in Response to Reactive Oxygen Species. Sci. Rep. 9, 15440. doi:10.1038/s41598-019-51777-7
Meyer, J. N., Leuthner, T. C., and Luz, A. L. (2017). Mitochondrial Fusion, Fission, and Mitochondrial Toxicity. Toxicology 391, 42–53. doi:10.1016/j.tox.2017.07.019
Miceli, M. V., and Jazwinski, S. M. (2005). Nuclear Gene Expression Changes due to Mitochondrial Dysfunction in ARPE-19 Cells: Implications for Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 46, 1765–1773. doi:10.1167/iovs.04-1327
Miller, F. J., Rosenfeldt, F. L., Zhang, C., Linnane, A. W., and Nagley, P. (2003). Precise Determination of Mitochondrial DNA Copy Number in Human Skeletal and Cardiac Muscle by a PCR-Based Assay: Lack of Change of Copy Number With Age. Nucleic Acids Res. 31, 61e–61. doi:10.1093/nar/gng060
Mills, K. F., Yoshida, S., Stein, L. R., Grozio, A., Kubota, S., Sasaki, Y., et al. (2016). Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 24, 795–806. doi:10.1016/j.cmet.2016.09.013
Min, D. Y., Jung, E., Ahn, S. S., Lee, Y. H., Lim, Y., and Shin, S. Y. (2020). Chrysoeriol Prevents TNFα-Induced CYP19 Gene Expression via EGR-1 Downregulation in MCF7 Breast Cancer Cells. Ijms 21, 7523. doi:10.3390/ijms21207523
Mishra, B., Priyadarsini, K. I., Kumar, M. S., Unnikrishnan, M. K., and Mohan, H. (2003). Effect of O -Glycosilation on the Antioxidant Activity and Free Radical Reactions of a Plant Flavonoid, Chrysoeriol. Bioorg. Med. Chem. 11, 2677–2685. doi:10.1016/s0968-0896(03)00232-3
Mitra, K., and Lippincott-Schwartz, J. (2010). Analysis of Mitochondrial Dynamics and Functions Using Imaging Approaches. Curr. Protoc. Cell Biol. Chapter 4, Unit1–214. doi:10.1002/0471143030.cb0425s46
Moiseeva, O., Bourdeau, V., Roux, A., Deschênes-Simard, X., and Ferbeyre, G. (2009). Mitochondrial Dysfunction Contributes to Oncogene-Induced Senescence. Mol. Cell Biol. 29, 4495–4507. doi:10.1128/Mcb.01868-08
Morales, E., Horn, R., Pastor, L. M., Santamaría, L., Pallarés, J., Zuasti, A., et al. (2004). Involution of Seminiferous Tubules in Aged Hamsters: an Ultrastructural, Immunohistochemical and Quantitative Morphological Study. Histol. Histopathol. 19, 445–455. doi:10.14670/HH-19.445
Muraleva, N. A., Kozhevnikova, O. S., Fursova, A. Z., and Kolosova, N. G. (2019). Suppression of AMD-like Pathology by Mitochondria-Targeted Antioxidant SkQ1 is Associated with a Decrease in the Accumulation of Amyloid β and in mTOR Activity. Antioxidants 8, 177. doi:10.3390/antiox8060177
Muraleva, N. A., Kozhevnikova, O. S., Zhdankina, A. A., Stefanova, N. A., Karamysheva, T. V., Fursova, A. Z., et al. (2014). The Mitochondria-Targeted Antioxidant Skq1 Restoresαb-Crystallin Expression and Protects Against AMD-Like Retinopathy in OXYS Rats. Cell Cycle 13, 3499–3505. doi:10.4161/15384101.2014.958393
Nagai, N., Kubota, S., Tsubota, K., and Ozawa, Y. (2014). Resveratrol Prevents the Development of Choroidal Neovascularization by Modulating AMP-Activated Protein Kinase in Macrophages and Other Cell Types. J. Nutr. Biochem. 25, 1218–1225. doi:10.1016/j.jnutbio.2014.05.015
Nakayama, H., and Otsu, K. (2018). Mitochondrial DNA as an Inflammatory Mediator in Cardiovascular Diseases. Biochem. J. 475, 839–852. doi:10.1042/BCJ20170714
Narendra, D., Tanaka, A., Suen, D.-F., and Youle, R. J. (2008). Parkin is Recruited Selectively to Impaired Mitochondria and Promotes Their Autophagy. J. Cell Biol. 183, 795–803. doi:10.1083/jcb.200809125
Nashine, S., Cohen, P., Chwa, M., Lu, S., Nesburn, A. B., Kuppermann, B. D., et al. (2017). Humanin G (HNG) Protects Age-Related Macular Degeneration (AMD) Transmitochondrial ARPE-19 Cybrids From Mitochondrial and Cellular Damage. Cell Death Dis. 8, e2951. doi:10.1038/cddis.2017.348
Nashine, S., Nesburn, A. B., Kuppermann, B. D., and Kenney, M. C. (2020). Role of Resveratrol in Transmitochondrial AMD RPE Cells. Nutrients 12, 159. doi:10.3390/nu12010159
Nashine, S., Subramaniam, S. R., Chwa, M., Nesburn, A., Kuppermann, B. D., Federoff, H., et al. (2019). PU-91 Drug Rescues Human Age-Related Macular Degeneration RPE Cells; Implications for AMD Therapeutics. Aging 11, 6691–6713. doi:10.18632/aging.102179
Neal, S. E., Buehne, K. L., Besley, N. A., Yang, P., Silinski, P., Hong, J., et al. (2020). Resveratrol Protects Against Hydroquinone-Induced Oxidative Threat in Retinal Pigment Epithelial Cells. Investig. Ophthalmol. Vis. Sci. 61, 32. doi:10.1167/iovs.61.4.32
Nekhaeva, E., Bodyak, N. D., Kraytsberg, Y., McGrath, S. B., Van Orsouw, N. J., Pluzhnikov, A., et al. (2002). Clonally Expanded Mtdna Point Mutations are Abundant in Individual Cells of Human Tissues. Proc. Natl. Acad. Sci. U.S.A. 99, 5521–5526. doi:10.1073/pnas.072670199
Nelson, G., Wordsworth, J., Wang, C., Jurk, D., Lawless, C., Martin‐Ruiz, C., et al. (2012). A Senescent Cell Bystander Effect: Senescence‐Induced Senescence. Aging Cell 11, 345–349. doi:10.1111/j.1474-9726.2012.00795.x
Nickavar, B., Rezaee, J., and Nickavar, A. (2016). Effect-Directed Analysis for the Antioxidant Compound in Salvia verticillata. Iran. J. Pharm. Res. 15, 241
Nickel, A., Kohlhaas, M., and Maack, C. (2014). Mitochondrial Reactive Oxygen Species Production and Elimination. J. Mol. Cell. Cardiol. 73, 26–33. doi:10.1016/j.yjmcc.2014.03.011
Nie, S., Lu, J., Wang, L., and Gao, M. (2020). Pro‐Inflammatory Role of Cell‐Free Mitochondrial Dna in Cardiovascular Diseases. IUBMB Life 72, 1879–1890. doi:10.1002/iub.2339
Nordgaard, C. L., Berg, K. M., Kapphahn, R. J., Reilly, C., Feng, X., Olsen, T. W., et al. (2006). Proteomics of the Retinal Pigment Epithelium Reveals Altered Protein Expression at Progressive Stages of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 47, 815–822. doi:10.1167/iovs.05-0976
Nordgaard, C. L., Karunadharma, P. P., Feng, X., Olsen, T. W., and Ferrington, D. A. (2008). Mitochondrial Proteomics Of The Retinal Pigment Epithelium At Progressive Stages of Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 49, 2848–2855. doi:10.1167/iovs.07-1352
Packer, L., Roy, S., and Sen, C. K. (1997). Alpha-Lipoic Acid: a Metabolic Antioxidant and Potential Redox Modulator of Transcription. Adv. Pharmacol. 38, 79–101. doi:10.1016/s1054-3589(08)60980-1
Palikaras, K., Lionaki, E., and Tavernarakis, N. (2018). Mechanisms of Mitophagy in Cellular Homeostasis, Physiology and Pathology. Nat. Cell Biol. 20, 1013–1022. doi:10.1038/s41556-018-0176-2
Pang, J., Seko, Y., and Tokoro, T. (1999). Processes of Blue Light-Induced Damage to Retinal Pigment Epithelial Cells Lacking Phagosomes. Jpn. J. Ophthalmol. 43, 103–108. doi:10.1016/s0021-5155(98)00073-2
Passos, J. F., Nelson, G., Wang, C., Richter, T., Simillion, C., Proctor, C. J., et al. (2010). Feedback Between P21 and Reactive Oxygen Production is Necessary for Cell Senescence. Mol. Syst. Biol. 6, 347.doi:10.1038/msb.2010.5
Ploumi, C., Daskalaki, I., and Tavernarakis, N. (2017). Mitochondrial Biogenesis and Clearance: A Balancing Act. FEBS J. 284, 183–195. doi:10.1111/febs.13820
Rambold, A. S., Kostelecky, B., Elia, N., and Lippincott-Schwartz, J. (2011). Tubular Network Formation Protects Mitochondria from Autophagosomal Degradation During Nutrient Starvation. Proc. Natl. Acad. Sci. U.S.A. 108, 10190–10195. doi:10.1073/pnas.1107402108
Rana, A., Oliveira, M. P., Khamoui, A. V., Aparicio, R., Rera, M., Rossiter, H. B., et al. (2017). Promoting Drp1-Mediated Mitochondrial Fission in Midlife Prolongs Healthy Lifespan of Drosophila Melanogaster. Nat. Commun. 8, 448. doi:10.1038/s41467-017-00525-4
Rana, A., Rera, M., and Walker, D. W. (2013). Parkin Overexpression During Aging Reduces Proteotoxicity, Alters Mitochondrial Dynamics, and Extends Lifespan. Proc. Natl. Acad. Sci. U.S.A. 110, 8638–8643. doi:10.1073/pnas.1216197110
Ren, L., Chen, X., Chen, X., Li, J., Cheng, B., and Xia, J. (2020). Mitochondrial Dynamics: Fission and Fusion in Fate Determination of Mesenchymal Stem Cells. Front. Cell Dev. Biol. 8, 580070. doi:10.3389/fcell.2020.580070
Rizzuto, R., De Stefani, D., Raffaello, A., and Mammucari, C. (2012). Mitochondria as Sensors and Regulators of Calcium Signalling. Nat. Rev. Mol. Cell Biol. 13, 566–578. doi:10.1038/nrm3412
Rosen, R. B., Hu, D. N., Chen, M., McCormick, S. A., Walsh, J., and Roberts, J. E. (2012). Effects of Melatonin and Its Receptor Antagonist on Retinal Pigment Epithelial Cells Against Hydrogen Peroxide Damage. Mol. Vis. 18, 1640–1648.
Rottenberg, H., and Hoek, J. B. (2017). The Path From Mitochondrial ROS to Aging Runs Through the Mitochondrial Permeability Transition Pore. Aging Cell 16, 943–955. doi:10.1111/acel.12650
R. Sparrrow, J., Hicks, D., and P. Hamel, C. (2010). The Retinal Pigment Epithelium in Health and Disease. Cmm 10, 802–823. doi:10.2174/156652410793937813
Sabbah, H. N. (2021). Barth Syndrome Cardiomyopathy: Targeting the Mitochondria With Elamipretide. Heart Fail Rev. 26, 237–253. doi:10.1007/s10741-020-10031-3
Saini, J. S., Corneo, B., Miller, J. D., Kiehl, T. R., Wang, Q., Boles, N. C., et al. (2017). Nicotinamide Ameliorates Disease Phenotypes in a Human iPSC Model of Age-Related Macular Degeneration. Cell Stem Cell 20, 635–647. doi:10.1016/j.stem.2016.12.015
Sakamoto, K., Liu, C., and Tosini, G. (2004). Circadian Rhythms in the Retina of Rats With Photoreceptor Degeneration. J. Neurochem. 90, 1019–1024. doi:10.1111/j.1471-4159.2004.02571.x
Salabei, J. K., Gibb, A. A., and Hill, B. G. (2014). Comprehensive Measurement of Respiratory Activity in Permeabilized Cells Using Extracellular Flux Analysis. Nat. Protoc. 9, 421–438. doi:10.1038/nprot.2014.018
Sarna, T., Burke, J. M., Korytowski, W., Różanowska, M., Skumatz, C. M. B., Zaręba, A., et al. (2003). Loss of Melanin From Human RPE With Aging: Possible Role of Melanin Photooxidation. Exp. Eye Res. 76, 89–98. doi:10.1016/s0014-4835(02)00247-6
Satish, S., Philipose, H., Rosales, M. A. B., and Saint-Geniez, M. (2018). Pharmaceutical Induction of PGC-1α Promotes Retinal Pigment Epithelial Cell Metabolism and Protects against Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 9, doi:10.1155/2018/9248640
Scheckhuber, C. Q., Erjavec, N., Tinazli, A., Hamann, A., Nyström, T., and Osiewacz, H. D. (2007). Reducing Mitochondrial Fission Results in Increased Life Span and Fitness of Two Fungal Ageing Models. Nat. Cell Biol. 9, 99–105. doi:10.1038/ncb1524
Schmucker, D. L., and Sachs, H. (2002). Quantifying Dense Bodies and Lipofuscin During Aging: a Morphologist's Perspective. Archives Gerontology Geriatrics 34, 249–261. doi:10.1016/s0167-4943(01)00218-7
Seko, Y., Pang, J., Tokoro, T., Ichinose, S., and Mochizuki, M. (2001). Blue Light-Induced Apoptosis in Cultured Retinal Pigment Epithelium Cells of the Rat. Graefe's Arch. Clin. Exp. Ophthalmol. 239, 47–52. doi:10.1007/s004170000220
Shaban, H., Gazzotti, P., and Richter, C. (2001). Cytochrome C Oxidase Inhibition By N-Retinyl-N-Retinylidene Ethanolamine, a Compound Suspected to Cause Age-Related Macula Degeneration. Archives Biochem. Biophysics 394, 111–116. doi:10.1006/abbi.2001.2535
Shade, C. (2020). The Science Behind NMN-A Stable, Reliable NAD+Activator and Anti-Aging Molecule. Integr. Med. (Encinitas) 19 (1), 12–14.
Sheu, S.-J., Liu, N.-C., and Chen, J.-L. (2010). Resveratrol protects human retinal pigment epithelial cells from acrolein-induced damage. J. Ocular Pharmacol. Ther. 26, 231–236. doi:10.1089/jop.2009.0137
Sheu, S.-J., Liu, N.-C., Ou, C.-C., Bee, Y.-S., Chen, S.-C., Lin, H.-C., et al. (2013). Resveratrol Stimulates Mitochondrial Bioenergetics to Protect Retinal Pigment Epithelial Cells From Oxidative Damage. Investig. Ophthalmol. Vis. Sci. 54, 6426–6438. doi:10.1167/iovs.13-12024
Shigenaga, M. K., Hagen, T. M., and Ames, B. N. (1994). Oxidative Damage and Mitochondrial Decay in Aging. Proc. Natl. Acad. Sci. U.S.A. 91, 10771–10778. doi:10.1073/pnas.91.23.10771
Shimada, K., Crother, T. R., Karlin, J., Dagvadorj, J., Chiba, N., Chen, S., et al. (2012). Oxidized Mitochondrial DNA Activates the NLRP3 Inflammasome During Apoptosis. Immunity 36, 401–414. doi:10.1016/j.immuni.2012.01.009
Short, K. R., Bigelow, M. L., Kahl, J., Singh, R., Coenen-Schimke, J., Raghavakaimal, S., et al. (2005). Decline in Skeletal Muscle Mitochondrial Function With Aging in Humans. Proc. Natl. Acad. Sci. U.S.A. 102, 5618–5623. doi:10.1073/pnas.0501559102
Sparrow, J. R., and Boulton, M. (2005). RPE Lipofuscin and its Role in Retinal Pathobiology. Exp. Eye Res. 80, 595–606. doi:10.1016/j.exer.2005.01.007
Sparrow, J. R., Fishkin, N., Zhou, J., Cai, B., Jang, Y. P., Krane, S., et al. (2003). A2E, a Byproduct of the Visual Cycle. Vis. Res. 43, 2983–2990. doi:10.1016/s0042-6989(03)00475-9
Sreekumar, P. G., Ishikawa, K., Spee, C., Mehta, H. H., Wan, J., Yen, K., et al. (2016). The Mitochondrial-Derived Peptide Humanin Protects RPE Cells From Oxidative Stress, Senescence, and Mitochondrial Dysfunction. Investig. Ophthalmol. Vis. Sci. 57, 1238–1253. doi:10.1167/iovs.15-17053
Sridevi Gurubaran, I., Viiri, J., Koskela, A., Hyttinen, J. M. T., Paterno, J. J., Kis, G., et al. (2020). Mitophagy in the Retinal Pigment Epithelium of Dry Age-Related Macular Degeneration Investigated in the NFE2L2/PGC-1α-/- Mouse Model. Ijms 21, 1976. doi:10.3390/ijms21061976
Stein, L. R., and Imai, S.-i. (2012). The Dynamic Regulation of NAD Metabolism in Mitochondria. Trends Endocrinol. Metabolism 23, 420–428. doi:10.1016/j.tem.2012.06.005
Stocco, D. M., Cascarano, J., and Wilson, M. A. (1977). Quantitation of Mitochondrial DNA, RNA, and Protein in Starved and Starved-Refed Rat Liver. J. Cell. Physiol. 90, 295–306. doi:10.1002/jcp.1040900215
Sun, N., Malide, D., Liu, J., Rovira, , Combs, C. A., and Finkel, T. (2017). A Fluorescence-Based Imaging Method to Measure in Vitro and in Vivo Mitophagy Using Mt-Keima. Nat. Protoc. 12, 1576–1587. doi:10.1038/nprot.2017.060
Szeto, H. H. (2014). First-in-Class Cardiolipin-Protective Compound as a Therapeutic Agent to Restore Mitochondrial Bioenergetics. Br. J. Pharmacol. 171, 2029–2050. doi:10.1111/bph.12461
Tait, S. W. G., Oberst, A., Quarato, G., Milasta, S., Haller, M., Wang, R., et al. (2013). Widespread Mitochondrial Depletion Via Mitophagy Does not Compromise Necroptosis. Cell Rep. 5, 878–885. doi:10.1016/j.celrep.2013.10.034
Takemoto, K., Hatano, E., Iwaisako, K., Takeiri, M., Noma, N., Ohmae, S., et al. (2014). Necrostatin-1 Protects Against Reactive Oxygen Species (ROS)-Induced Hepatotoxicity in Acetaminophen-Induced Acute Liver Failure. FEBS Open Bio 4, 777–787. doi:10.1016/j.fob.2014.08.007
Tan, D.-X., Manchester, L. C., Hardeland, R., Lopez-Burillo, S., Mayo, J. C., Sainz, R. M., et al. (2003). Melatonin: a Hormone, a Tissue Factor, an Autocoid, a Paracoid, and an Antioxidant Vitamin. J. Pineal Res. 34, 75–78. doi:10.1034/j.1600-079x.2003.02111.x
Tan, D.-X., Manchester, L., Qin, L., and Reiter, R. (2016). Melatonin: A Mitochondrial Targeting Molecule Involving Mitochondrial Protection and Dynamics. Ijms 17, 2124. doi:10.3390/ijms17122124
Tang, X., Li, X., Zhang, D., and Han, W. (2022). Astragaloside-IV Alleviates High Glucose-Induced Ferroptosis In Retinal Pigment Epithelial Cells by Disrupting the Expression of Mir-138-5p/Sirt1/Nrf2. Bioengineered 13, 8240–8254. doi:10.1080/21655979.2022.2049471
Tanida, I., Ueno, T., and Kominami, E. (2008). LC3 and Autophagy. Methods Mol. Biol. 445, 77–88. doi:10.1007/978-1-59745-157-4_4
Telegina, D. V., Kozhevnikova, O. S., Fursova, A. Z., and Kolosova, N. G. (2020). Autophagy as a Target for the Retinoprotective Effects of the Mitochondria-Targeted Antioxidant SkQ1. Biochem. Mosc. 85, 1640–1649. doi:10.1134/S0006297920120159
Terluk, M. R., Kapphahn, R. J., Soukup, L. M., Gong, H., Gallardo, C., Montezuma, S. R., et al. (2015). Investigating Mitochondria as a Target for Treating Age-Related Macular Degeneration. J. Neurosci. 35, 7304–7311. doi:10.1523/JNEUROSCI.0190-15.2015
Tilokani, L., Nagashima, S., Paupe, V., and Prudent, J. (2018). Mitochondrial Dynamics: Overview of Molecular Mechanisms. Essays Biochem. 62, 341–360. doi:10.1042/EBC20170104
Tobias, I. C., Khazaee, R., and Betts, D. H. (2018). Analysis of Mitochondrial Dimensions and Cristae Structure in Pluripotent Stem Cells Using Transmission Electron Microscopy. Curr. Protoc. Stem Cell Biol. 47, e67. doi:10.1002/cpsc.67
Todd, A. M., and Staveley, B. E. (2012). Expression of Pink1 With Α-Synuclein in the Dopaminergic Neurons of Drosophila Leads to Increases in Both Lifespan and Healthspan. Genet. Mol. Res. 11, 1497–1502. doi:10.4238/2012.May.21.6
Tong, Y., and Wang, S. (2020). Not All Stressors are Equal: Mechanism of Stressors on RPE Cell Degeneration. Front. Cell Dev. Biol. 8, 591067. doi:10.3389/fcell.2020.591067
van der Bliek, A. M., Shen, Q., and Kawajiri, S. (2013). Mechanisms of Mitochondrial Fission and Fusion. Cold Spring Harb. Perspect. Biol. 5, a011072. doi:10.1101/cshperspect.a011072
van der Schaft, T. L., de Bruijnz, W. C., Mooy, C. M., and de Jong, P. T. V. M. (1993). Basal Laminar Deposit in the Aging Peripheral Human Retina. Graefe's Arch. Clin. Exp. Ophthalmol. 231, 470–475. doi:10.1007/BF02044234
Vasileiou, P., Evangelou, K., Vlasis, K., Fildisis, G., Panayiotidis, M., Chronopoulos, E., et al. (2019). Mitochondrial Homeostasis and Cellular Senescence. Cells 8, 686. doi:10.3390/cells8070686
Vringer, E., and Tait, S. W. G. (2019). Mitochondria and Inflammation: Cell Death Heats Up. Front. Cell Dev. Biol. 7, 100. doi:10.3389/fcell.2019.00100
Wallace, D. C. (2009). Mitochondria, Bioenergetics, and the Epigenome in Eukaryotic and Human Evolution. Cold Spring Harb. Symposia Quantitative Biol. 74, 383–393. doi:10.1101/sqb.2009.74.031
Wallace, D. C. (2001). Mouse Models for Mitochondrial Disease. Am. J. Med. Genet. 106, 71–93. doi:10.1002/ajmg.1393
Wang, A. L., Lukas, T. J., Yuan, M., and Neufeld, A. H. (2008). Increased Mitochondrial DNA Damage and Down-Regulation of DNA Repair Enzymes in Aged Rodent Retinal Pigment Epithelium and Choroid. Mol. Vis. 14, 644–651.
Wang, J. J., Foran, S., Smith, W., and Mitchell, P. (2003). Risk of Age-Related Macular Degeneration in Eyes With Macular Drusen or Hyperpigmentation: the Blue Mountains Eye Study cohort. Arch. Ophthalmol. 121, 658–663. doi:10.1001/archopht.121.5.658
Wang, K., Zheng, M., Lester, K. L., and Han, Z. (2019). Light-Induced Nrf2-/- Mice as Atrophic Age-Related Macular Degeneration Model and Treatment With Nanoceria Laden Injectable Hydrogel. Sci. Rep. 9, 14573. doi:10.1038/s41598-019-51151-7
Wang, K., Li, J., Degterev, A., Hsu, E., Yuan, J., and Yuan, C. (2007). Structure-Activity Relationship Analysis of a Novel Necroptosis Inhibitor, Necrostatin-5. Bioorg. Med. Chem. Lett. 17, 1455–1465. doi:10.1016/j.bmcl.2006.11.056
Wang, S., Liu, Y., Liu, Y., Li, C., Wan, Q., Yang, L., et al. (2020). Reversed Senescence of Retinal Pigment Epithelial Cell by Coculture With Embryonic Stem Cell via the TGFβ and PI3K Pathways. Front. Cell Dev. Biol. 8, 588050. doi:10.3389/fcell.2020.588050
Wang, Z., Jiang, H., Chen, S., Du, F., and Wang, X. (2012). The Mitochondrial Phosphatase PGAM5 Functions at the Convergence Point of Multiple Necrotic Death Pathways. Cell 148, 228–243. doi:10.1016/j.cell.2011.11.030
Wang, K, K., Zheng, M., and Han, Z. C. (2018). Light-Induced Nrf2 Knockout Mice as Atrophic Age-Related Macular Degeneration Model & the Treatment with Nanoceria Laden Injectable Hydrogel. Mol. Ther. 26, 267. doi:10.1038/s41598-019-51151-7
Wang, Y, Y., Grenell, A., Zhong, F., Yam, M., Hauer, A., Gregor, E., et al. (2018). Metabolic Signature of the Aging Eye in Mice. Neurobiol. Aging 71, 223–233. doi:10.1016/j.neurobiolaging.2018.07.024
Welle, S., Bhatt, K., Shah, B., Needler, N., Delehanty, J. M., and Thornton, C. A. (2003). Reduced Amount of Mitochondrial DNA in Aged Human Muscle. J. Appl. Physiology 94, 1479–1484. doi:10.1152/japplphysiol.01061.2002
Wilkins, H. M., Carl, S. M., Greenlief, A. C. S., Festoff, B. W., and Swerdlow, R. H. (2014). Bioenergetic Dysfunction and Inflammation in Alzheimerâ€s Disease: A Possible Connection. Front. Aging Neurosci. 6, 311. doi:10.3389/fnagi.2014.00311
Wimmers, S., Karl, M., and Strauss, O. (2007). Ion channels in the RPE. Prog. Retin. Eye Res. 26, 263–301. doi:10.1016/j.preteyeres.2006.12.002
Xu, L, L., Kong, L., Wang, J., and Ash, J. D. (2018). Stimulation of AMPK Prevents Degeneration of Photoreceptors and the Retinal Pigment Epithelium. Proc. Natl. Acad. Sci. U.S.A. 115, 10475–10480. doi:10.1073/pnas.1802724115
Xu, M, M., Pirtskhalava, T., Farr, J. N., Weigand, B. M., Palmer, A. K., Weivoda, M. M., et al. (2018). Senolytics Improve Physical Function and Increase Lifespan in Old Age. Nat. Med. 24, 1246–1256. doi:10.1038/s41591-018-0092-9
Yagoda, N., von Rechenberg, M., Zaganjor, E., Bauer, A. J., Yang, W. S., Fridman, D. J., et al. (2007). RAS-RAF-MEK-Dependent Oxidative Cell Death Involving Voltage-Dependent Anion Channels. Nature 447, 865–869. doi:10.1038/nature05859
Yako, T., Nakamura, M., Otsu, W., Nakamura, S., Shimazawa, M., and Hara, H. (2021). Mitochondria Dynamics in the Aged Mice Eye and the Role in the RPE Phagocytosis. Exp. Eye Res. 213, 108800. doi:10.1016/j.exer.2021.108800
Yang, W. S., and Stockwell, B. R. (2008). Synthetic Lethal Screening Identifies Compounds Activating Iron-Dependent, Nonapoptotic Cell Death in Oncogenic-RAS-Harboring Cancer Cells. Chem. Biol. 15, 234–245. doi:10.1016/j.chembiol.2008.02.010
Yao, J., Bi, H.-E., Sheng, Y., Cheng, L.-B., Wendu, R.-L., Wang, C.-H., et al. (2013). Ultraviolet (UV) and Hydrogen Peroxide Activate Ceramide-ER Stress-AMPK Signaling Axis to Promote Retinal Pigment Epithelium (RPE) Cell Apoptosis. Ijms 14, 10355–10368. doi:10.3390/ijms140510355
Ye, Y.-C., Wang, H.-J., Yu, L., Tashiro, S.-I., Onodera, S., and Ikejima, T. (2012). RIP1-Mediated Mitochondrial Dysfunction and ROS Production Contributed to Tumor Necrosis Factor Alpha-Induced L929 Cell Necroptosis and Autophagy. Int. Immunopharmacol. 14, 674–682. doi:10.1016/j.intimp.2012.08.003
Yen, T. C., Chen, Y. S., King, K. L., Yeh, S. H., and Wei, Y. H. (1989). Liver Mitochondrial Respiratory Functions Decline With Age. Biochem. Biophys. Res. Commun. 165, 944–1003. doi:10.1016/0006-291x(89)92701-0
Yoon, Y.-S., Yoon, D.-S., Lim, I. K., Yoon, S.-H., Chung, H.-Y., Rojo, M., et al. (2006). Formation of Elongated Giant Mitochondria in DFO-Induced Cellular Senescence: Involvement of Enhanced Fusion Process Through Modulation of Fis1. J. Cell. Physiol. 209, 468–480. doi:10.1002/jcp.20753
Youle, R. J., and Narendra, D. P. (2011). Mechanisms of Mitophagy. Nat. Rev. Mol. Cell Biol. 12, 9–14. doi:10.1038/nrm3028
Youn, H.-Y., Bantseev, V., Bols, N. C., Cullen, A. P., and Sivak, J. G. (2007). In Vitro Assays for Evaluating the Ultraviolet B-Induced Damage in Cultured Human Retinal Pigment Epithelial Cells. J. Photochem. Photobiol. B Biol. 88, 21–28. doi:10.1016/j.jphotobiol.2007.04.012
Youn, H. Y., Cullen, B. R., and Sivak, J. G. (2010). Phototoxicity of Ultraviolet (UV) Radiation: Evaluation of UV-Blocking Efficiency of Intraocular Lens (IOL) Materials Using Retinal Cell Culture and In Vitro Bioassays. Totoxij 4, 13–20. doi:10.2174/1874340401004010013
Yu, B., Ma, J., Li, J., Wang, D., Wang, Z., and Wang, S. (2020). Mitochondrial Phosphatase PGAM5 Modulates Cellular Senescence by Regulating Mitochondrial Dynamics. Nat. Commun. 11, 2549. doi:10.1038/s41467-020-16312-7
Yumnamcha, T., Devi, T. S., and Singh, L. P. (2019). Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front. Neurosci. 13, 1065. doi:10.3389/fnins.2019.01065
Zanzottera, E. C., Messinger, J. D., Ach, T., Smith, R. T., Freund, K. B., and Curcio, C. A. (2015). The Project MACULA Retinal Pigment Epithelium Grading System for Histology and Optical Coherence Tomography in Age-Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 56, 3253–3268. doi:10.1167/iovs.15-16431
Zarbin, M. A. (2004). Current Concepts in the Pathogenesis of Age-Related Macular Degeneration. Arch. Ophthalmol. 122, 598–614. doi:10.1001/archopht.122.4.598
Zhang, C., Baffi, J., Cousins, S. W., and Csaky, K. G. (2003). Oxidant-Induced Cell Death in Retinal Pigment Epithelium Cells Mediated Through the Release of Apoptosis-Inducing Factor. J. Cell Sci. 116, 1915–1923. doi:10.1242/jcs.00390
Zhang, M., Jiang, N., Chu, Y., Postnikova, O., Varghese, R., Horvath, A., et al. (2020). Dysregulated Metabolic Pathways in Age-Related Macular Degeneration. Sci. Rep. 10, 2464. doi:10.1038/s41598-020-59244-4
Zhang, Y., Xi, X., Mei, Y., Zhao, X., Zhou, L., Ma, M., et al. (2019). High-Glucose Induces Retinal Pigment Epithelium Mitochondrial Pathways of Apoptosis and Inhibits Mitophagy by Regulating ROS/PINK1/Parkin Signal Pathway. Biomed. Pharmacother. 111, 1315–1325. doi:10.1016/j.biopha.2019.01.034
Zhao, X., Liu, L. L., Jiang, Y. Z., Silva, M., Zhen, X. C., and Zheng, W. H. (2020). Protective Effect of Metformin against Hydrogen Peroxide-Induced Oxidative Damage in Human Retinal Pigment Epithelial (RPE) Cells by Enhancing Autophagy through Activation of AMPK Pathway. Oxidative Med. Cell. Longev. 2020, 2524174. doi:10.1155/2020/2524174
Keywords: RPE, aging, degeneration, mitochondria, senescense, cell death, age-related macula degeneration
Citation: Tong Y, Zhang Z and Wang S (2022) Role of Mitochondria in Retinal Pigment Epithelial Aging and Degeneration. Front. Aging 3:926627. doi: 10.3389/fragi.2022.926627
Received: 22 April 2022; Accepted: 21 June 2022;
Published: 15 July 2022.
Edited by:
Terytty Yang Li, Swiss Federal Institute of Technology Lausanne, SwitzerlandReviewed by:
Oyuna S. Kozhevnikova, Institute of Cytology and Genetics (RAS), RussiaJon Ambæk Durhuus, Hvidovre Hospital, Denmark
Copyright © 2022 Tong, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shusheng Wang, swang1@tulane.edu
 Yao Tong
Yao Tong Zunyi Zhang1
Zunyi Zhang1  Shusheng Wang
Shusheng Wang