Erratum: The impact of COVID-19 on pulmonary, neurological, and cardiac outcomes: evidence from a Mendelian randomization study
- 1Division of Data Analytics, Bioinformatics and Structural Biology, Yenepoya Research Centre, Yenepoya (Deemed to be University), Mangalore, India
- 2Department of Community Medicine, Yenepoya Medical College and Hospital, Yenepoya (Deemed to be University), Mangalore, India
- 3Department of Statistics, Yenepoya (Deemed to be University), Mangalore, India
- 4Department of Medical Genetics, Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, India
Background: Long COVID is a clinical entity characterized by persistent health problems or development of new diseases, without an alternative diagnosis, following SARS-CoV-2 infection that affects a significant proportion of individuals globally. It can manifest with a wide range of symptoms due to dysfunction of multiple organ systems including but not limited to cardiovascular, hematologic, neurological, gastrointestinal, and renal organs, revealed by observational studies. However, a causal association between the genetic predisposition to COVID-19 and many post-infective abnormalities in long COVID remain unclear.
Methods: Here we employed Mendelian randomization (MR), a robust genetic epidemiological approach, to investigate the potential causal associations between genetic predisposition to COVID-19 and long COVID symptoms, namely pulmonary (pneumonia and airway infections including bronchitis, emphysema, asthma, and rhinitis), neurological (headache, depression, and Parkinson’s disease), cardiac (heart failure and chest pain) diseases, and chronic fatigue. Using two-sample MR, we leveraged genetic data from a large COVID-19 genome-wide association study and various disorder-specific datasets.
Results: This analysis revealed that a genetic predisposition to COVID-19 was significantly causally linked to an increased risk of developing pneumonia, airway infections, headache, and heart failure. It also showed a strong positive correlation with chronic fatigue, a frequently observed symptom in long COVID patients. However, our findings on Parkinson’s disease, depression, and chest pain were inconclusive.
Conclusion: Overall, these findings provide valuable insights into the genetic underpinnings of long COVID and its diverse range of symptoms. Understanding these causal associations may aid in better management and treatment of long COVID patients, thereby alleviating the substantial burden it poses on global health and socioeconomic systems.
1 Introduction
Long-term residual health problems or the onset of new diseases following Coronavirus disease 2019 (COVID-19) that is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) are termed as post-acute sequalae of SARS-CoV-2 (PASC) or Long COVID that is a recognized and multi-systemic clinical entity with onset ≥3 months after a probable or confirmed diagnosis of COVID-19 and lasting for ≥2 months, with symptoms not explained by any other alternative diagnosis (1). It can manifest with a broad range of clinical phenotypes owing to respiratory, cardiovascular, neurological, gastrointestinal, renal, immunological, reproductive and other organ system dysfunction (2). Its global prevalence varies widely, ranging from 7.5–45% individuals including children and adolescents (3–7) and even conservatively it is estimated to affect ~65 million individuals globally (2); though it is likely this number is much higher due to the large number of unreported cases. The etiology of long COVID is complex and influenced by several factors, such as severity of initial infection, age, sex, presence of comorbidities, e.g., obesity and host genetic variations, such as at the FOXP4 locus (8–13). The burden of long COVID was also found to be significant in individuals with SARS-CoV-2 breakthrough infections (BTI) that occur post receiving vaccine booster dose (14). However, vaccination partially ameliorated the risk of mortality and extreme adverse outcomes associated with BTI in long COVID subjects (14, 15).
Frequently observed long COVID symptoms include chronic fatigue and respiratory abnormalities, e.g., dyspnea or shortness of breath (5, 11, 16). Dyspnea and cough were found to persist in 40 and 20% long COVID subjects, respectively (17). Follow ups in individuals hospitalized with COVID-19 induced pneumonia, using radiological and pulmonary function investigations, uncovered attenuated lung function, fibrotic-like changes in the lungs and parenchymal lung disease in long COVID patients (18–20). Cognitive and neurological derangements are another major cluster of long COVID symptoms, including anosmia, ageusia, headache, cognitive impairments, e.g., memory and attention deficits, brain fog, neuropsychiatric symptoms, such as depression, anxiety, psychosis, and insomnia (16, 21–25). Few cases of parkinsonism have also been reported in the older long COVID patients (26, 27). In addition, observational studies note that the cardiovascular sequalae of long COVID include chest pain, heart failure, dysrhythmias, cerebrovascular disorders, inflammatory heart disease, ischemic heart disease and thromboembolic disease (28, 29). Further the adverse health outcomes in long COVID include the new onset of diseases, such as type 2 diabetes (30) and multi-organ damage (31). Given that a plethora of long COVID manifestations is not only observed in acute cases of COVID-19 but also significantly impact individuals with asymptomatic, mild or moderate initial SARS-CoV-2 infections that did not warrant hospitalization (7, 32, 33), severely exacerbates the health and socio-economic burden of long COVID worldwide.
The causal association between the genetic predisposition to COVID-19 and many post-infective abnormalities in long COVID remain unclear. Mendelian randomization (MR) is a genetic epidemiological strategy that utilizes genetic variants, such as single nucleotide polymorphisms (SNPs) associated with an exposure as instrumental variables to determine the causal relationship between the exposure and health outcome (34). It is less susceptible to confounding, reverse causality and regression dilution biases that limit observational studies. So far MR studies have been used to dissect a causal association of COVID-19 with the increased risk of development of Alzheimer’s disease (35), hypothyroidism (36), psychosis and schizophrenia (37), and specific cancers (38).
In the present study we used two sample MR to investigate the potential causal association of genetic predisposition to COVID-19 (exposure) with the onset of long COVID symptoms (outcomes), namely pulmonary disease (airway infections and pneumonia), neurological deficits (headache, depression, and parkinsonism), cardiac anomalies (chest pain and heart failure) and chronic fatigue.
2 Methods
2.1 Study design
Mendelian randomization (MR) relies on genetic variations as tools to investigate the lifelong and causal impacts of an exposure on an outcome (39). It closely resembles randomized controlled trials, as alleles are randomly assigned at conception, reducing susceptibility to reverse causation and unaccounted-for variables compared to traditional cohort studies. Consequently, it offers more robust evidence for establishing causal relationships. In the current study, we employed a two-sample MR approach by extracting exposure and outcome summary data from separate publicly available datasets. Notably, the effectiveness and reliability of these findings hinge on three key assumptions: (a) genetic instruments are linked to the exposure; (b) genetic instruments are not associated with any confounding factors that influence the exposure-outcome connection; (c) genetic instruments solely impact the outcome through the exposure, without involving other pathways (39). To statistically evaluate these assumptions, we performed pleiotropy and heterogeneity tests. The study design and underlying assumptions for MR is shown in Figure 1.
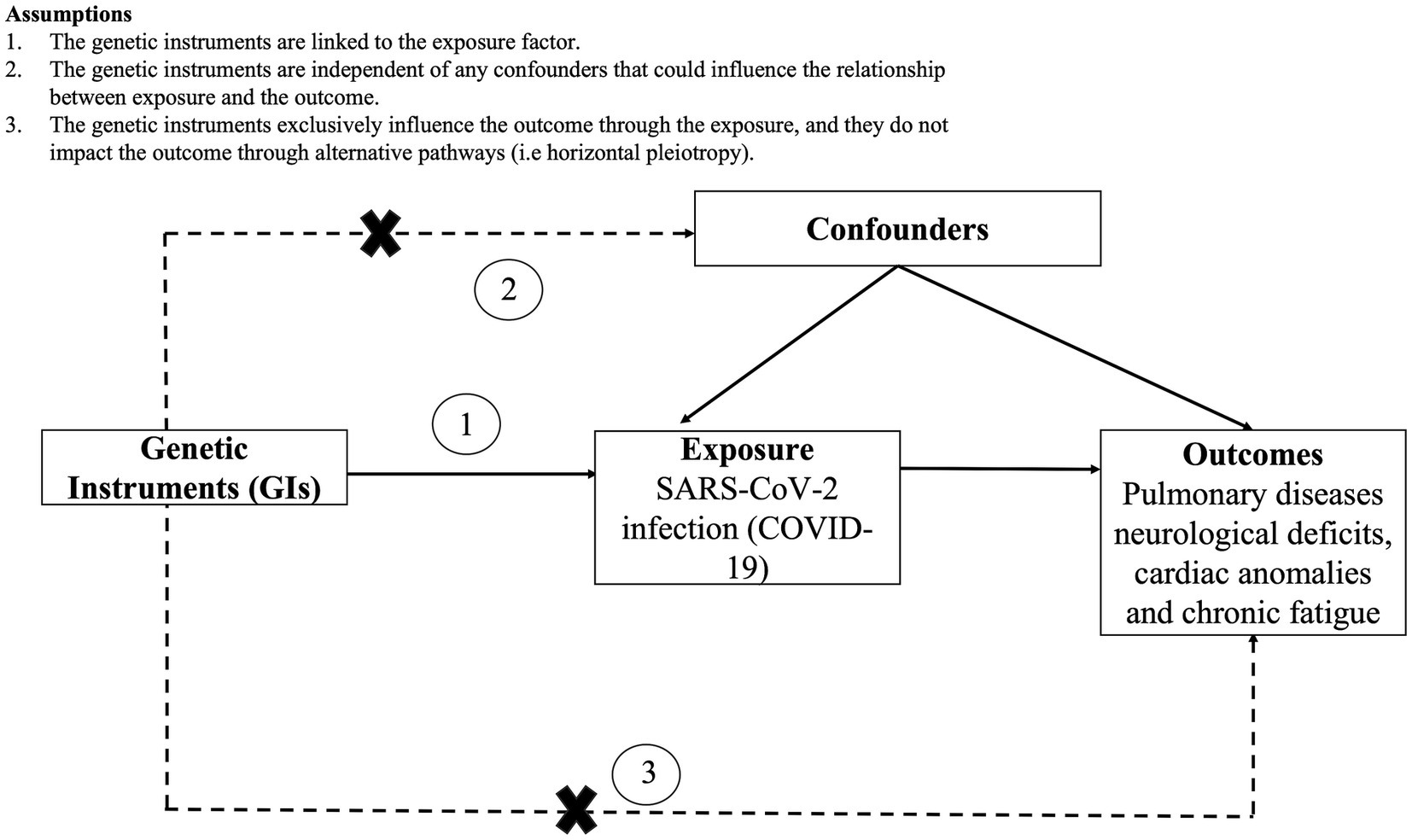
Figure 1. Study design and assumptions of Mendelian randomization (MR) analysis along with the assumptions.
2.2 Data source: COVID-19
Genome Wide Association Study (GWAS) summary statistics were retrieved from one of the largest GWAS data set for COVID-19 (hospitalized vs. non-hospitalized) described by the COVID-19 Host Genetics Initiative Release 6: B1_ALL_leave_23andme, released on June 15, 2021. B1_ALL_leave_23andme consists of trans-ancestry GWAS summary statistics of individuals who were hospitalized (n = 14,480; cases) vs. those who were not hospitalized (n = 73,191; controls) due to COVID-19 (Table 1). 1,35,110 highly significant SNPs were identified (p ≤ 0.01). To ensure the independence of these SNPs, they were first extracted from the Genome Asia dataset (40) and subsequently pruned for linkage disequilibrium (LD) using PLINK v1.9 (41). The LD proxies were limited to a minimum r2 ≧ 0.6. 1,02,354 SNPs were pruned out and the remaining 32,756 SNPs were used for downstream analyses.
2.3 Data source: disorders
Information about genetic association of pulmonary diseases (airway infections and pneumonia), neurological deficits (headache, depression and Parkinson’s Disease), cardiac anomalies (chest pain and heart failure) and chronic fatigue were obtained from the ieu open gwas project1 (Table 1). The pneumonia dataset (ieu-b-4976) consisted of individuals with pneumonia (n = 22,567; cases) and individuals without pneumonia (n = 4,63,917; controls) of European ancestry. The dataset for a group of diseases that cause airflow blockage and breathing-related problems including bronchitis, emphysema, asthma, and rhinitis (ukb-e-6152_p1_CSA) consisted of 5,986 cases and 2,576 controls of South Asian ancestry. The GWAS summary statistics of anxiety, tension, depression (ukb-e-2100_CSA) consisted of individuals with depression (n = 828; cases) and individuals without depression (n = 7,610; controls) of South Asian ancestry. The Parkinson’s disease dataset (ieu-b-7) consisted of individuals with Parkinson’s disease (n = 33,674; cases) and individuals without Parkinson’s disease (n = 4,49,056; controls) of European ancestry. The headache dataset (ukb-e-339_CSA) consisted of individuals with headache (n = 348; cases) and individuals without headache (n = 8,472; controls) of South Asian ancestry. The summary statistics of heart failure (ebi-a-GCST009541) consisted of individuals with heart failure (n = 47,309; cases) and individuals without heart failure (n = 930,014; controls) of European ancestry. The chest pain dataset (ukb-e-418_CSA) consisted of individuals with chest pain (n = 1,267; cases) and individuals without chest pain (n = 7,521; controls) of South Asian ancestry. Notably, this dataset consists of patients with “nonspecific” chest pain, which indicates that the cause of the pain was unclear and may be caused by heart or lung abnormalities. Finally, the fatigue (tiredness/lethargy) dataset (ukb-e-2080_CSA) consisted of individuals with tiredness (n = 7,878; cases) of South Asian ancestry (Table 1).
2.4 Pleiotropy test
The intercept from MR-Egger regression (variants uncorrelated, random-effect model) implemented in the R package MendelianRandomization v0.9 (42), was utilized to test the pleiotropy of the SNPs associated with COVID-19 in pulmonary diseases (airway infections and pneumonia), neurological deficits (headache, depression and Parkinsonism), cardiac anomalies (chest pain and heart failure) and chronic fatigue (Table 2). In particular, MR assumes no pleiotropy, so a p > 0.05 implies no significant pleiotropy of the COVID-19 associated SNPs in above mentioned outcomes. Sensitivity analysis utilizing the “leave-one-out” method was performed using the mr_loo function in MendelianRandomization to evaluate whether the analysis could be influenced by a solitary SNP with significant and broad horizontal pleiotropic impact. In addition, funnel plots were generated using the mr_funnel function, were used to evaluate diversity among different SNPs.
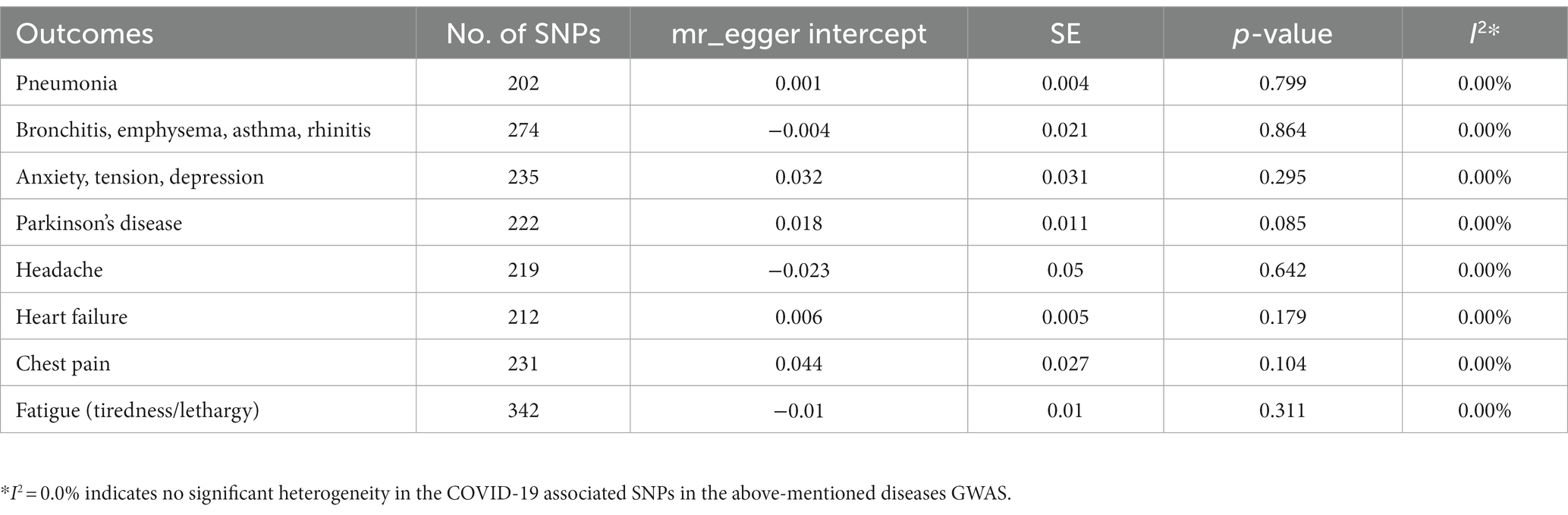
Table 2. Pleiotropy test of COVID-19 associated SNPs in the diseases’ Genome Wide Association Study (GWAS).
2.5 Mendelian randomization analysis
To evaluate the causal association between COVID-19 and various pulmonary, neurological, and cardiac disorders and chronic fatigue, we performed MR analysis using inverse variance weighted (IVW), Penalized IVW, Robust-IVW, Penalized robust IVW, Penalized MR-Egger, Robust MR-Egger, Mr-Egger, Penalized robust MR-Egger, weighted median, simple median and Penalized weighted median estimators implemented in the R package MendelianRandomization v0.9. p value < 0.05 represents a causal association of COVID-19 with various disorders.
2.6 Single SNP effect analysis
The mr_plot function in MendelianRandomization v0.9 was used to visualize the individual potential causal effects of COVID-19 associated SNPs on various pulmonary, neurological, and cardiac disorders and chronic fatigue. Furthermore, the mr_forest function was used to determine single SNP effect size for COVID-19 on various disorders.
3 Results
3.1 Pleiotropy test
In terms of pleiotropy and sensitivity, the MR-Egger regression analysis demonstrated no indications of biased pleiotropy for any of the disorders under study (Table 2). The leave-one-out analysis and funnel plots also indicated no breaches of the instrumental variable assumptions (Supplementary Figure S1, respectively). The leave-one-out sensitivity analysis depicted that removing a specific SNP among COVID-19 SNPs did not change the results for any of the disease under study (Supplementary Figure S1).
The overall Mendelian Randomization analysis results are summarized in Figure 2. Only penalized methods (penalized weighted median, penalized IVW and penalized MR-Egger) are shown. The plot was generated using the R package “forestplot.”
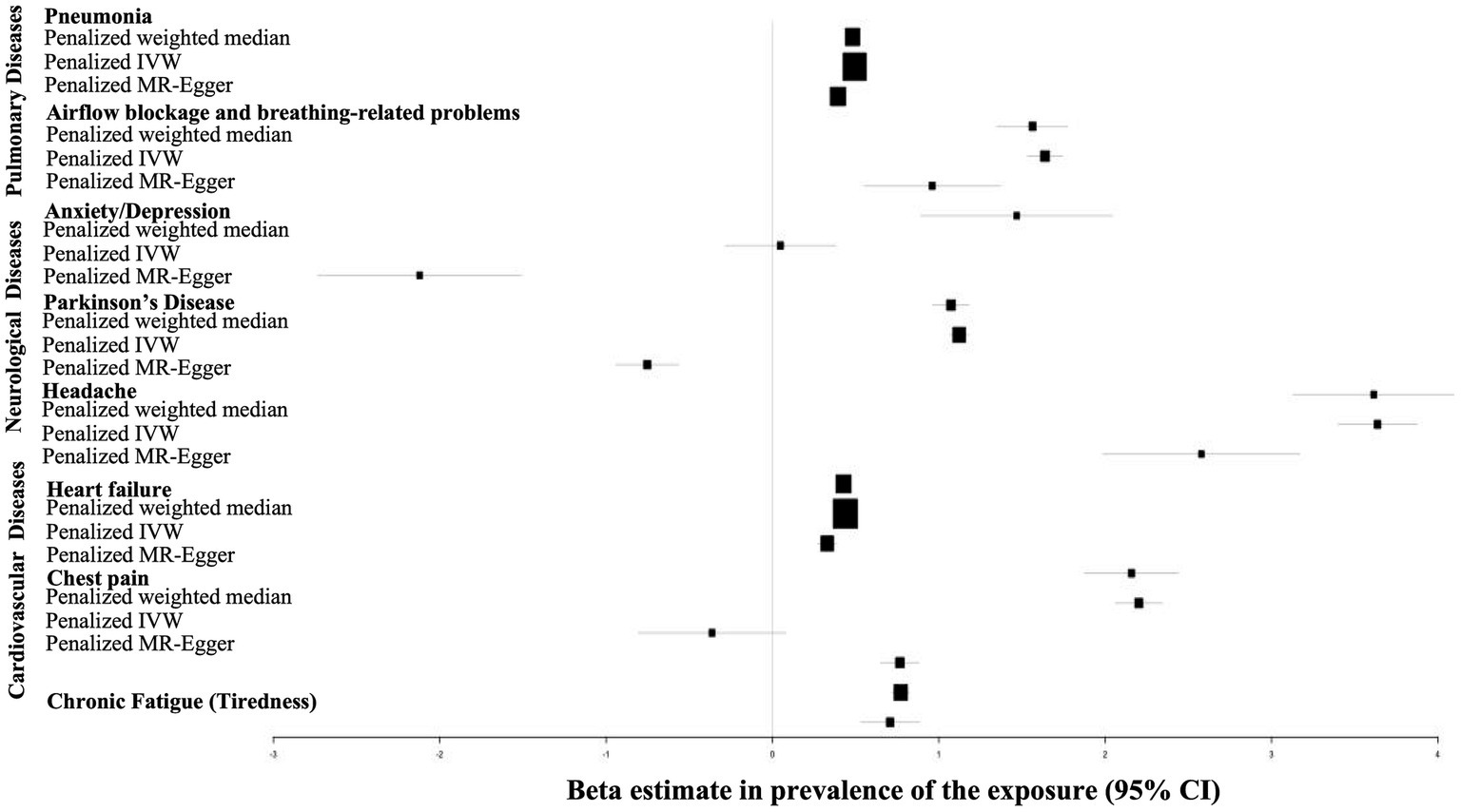
Figure 2. Forrest plot of MR analysis evaluating causal effects of genetic liability to COVID-19 on various pulmonary, cardiovascular and nueropsychiatric disorders. The plot was generated using the R package forestplot . The x-axis shows MR effect size for COVID-19 on various disorders. The y-axis shows the analysis for all SNPs together in a single instrument with the penalized IVW, penalized MR-Egger and penalized weighted median methods.
3.2 Effect of COVID-19 on lung function
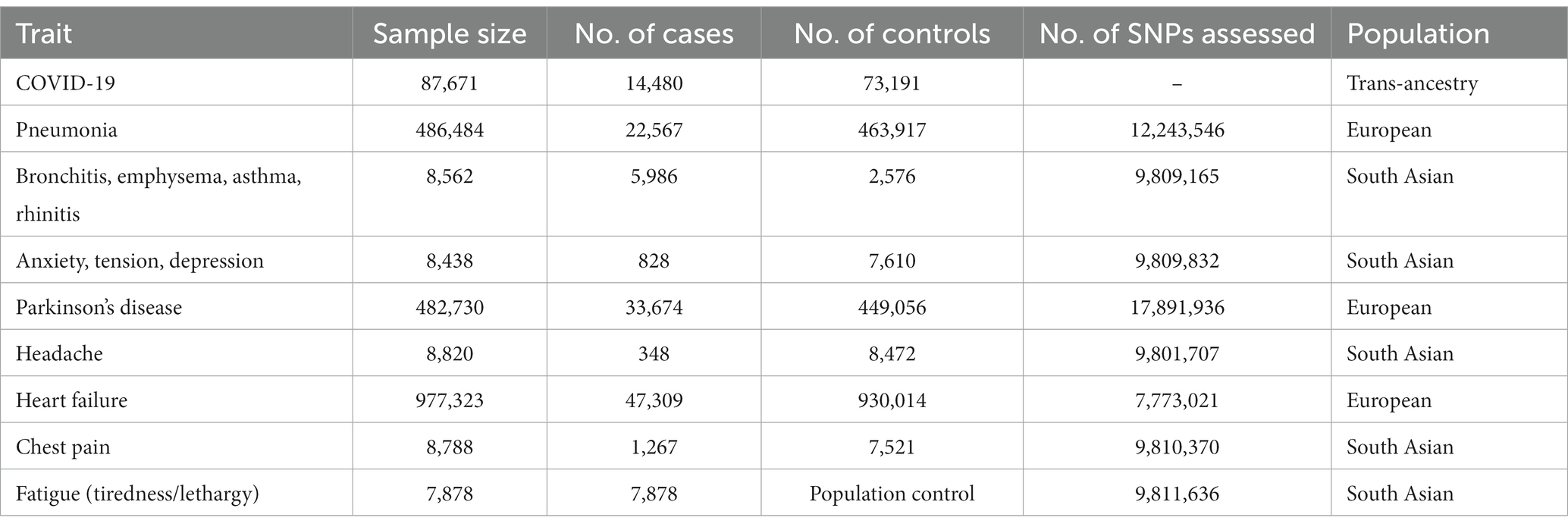
A strong positive association between history of COVID-19 and development of pneumonia was discerned by simple median analysis (β: 0.427, 95% CI 0.391 to 0.463, p < 0.0001), weighted median (β: −0.400, 95% CI 0.362 to 0.438, p < 0.0001), penalized weighted median (β: 0.481, 95% CI 0.437 to 0.526, p < 0.0001), IVW (β: 0.111, 95% CI 0.059 to 0.63, p < 0.0001), penalized IVW (β: 0.494, 95% CI 0.471 to 0.518, p < 0.0001), robust IVW (β: 0.356, 95% CI −0.018 to 0.730, p = 0.062), penalized robust IVW (β: 0.505, 95% CI 0.404 to 0.607, p < 0.0001), MR-Egger (β: 0.102, 95% CI 0.014 to 0.190, p = 0.023), penalized MR-Egger (β: 0.394, 95% CI 0.353 to 0.434, p < 0.0001), robust MR-Egger (β: 0.406, 95% CI 0.315 to 0.497, p < 0.0001) and penalized robust MR-Egger (β: 0.398, 95% CI 0.374 to 0.422, p < 0.0001) methods (Table 3A, Figure 3A).
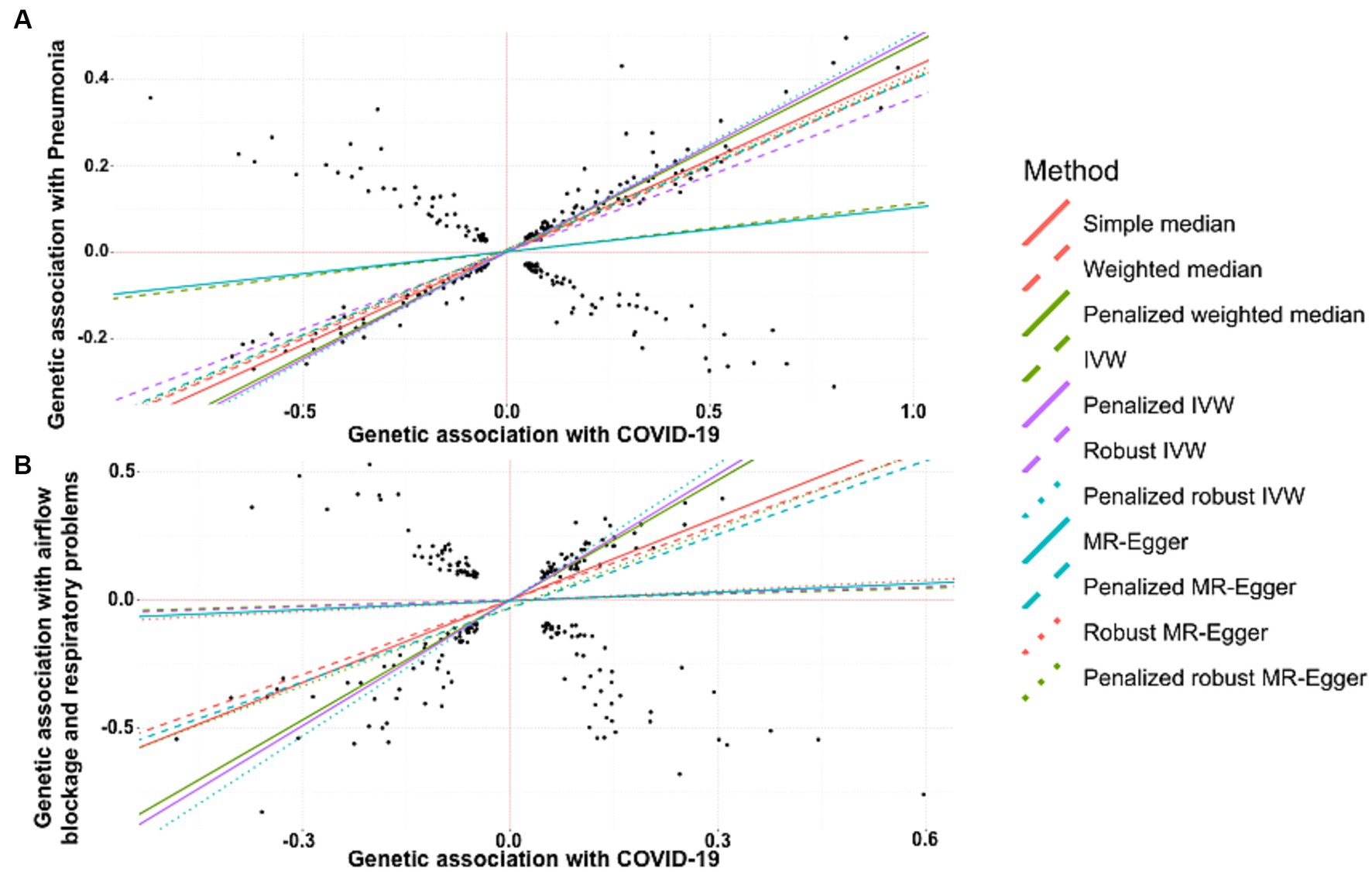
Figure 3. Scatter plot of SNPs associated with COVID-19 and pulmonary diseases. (A) pneumonia and (B) a group of diseases associated with airflow blockage and breathing-related problems including bronchitis, emphysema, asthma and rhinitis. The slopes of each line represent the causal association for each method. The plot was generated using the R package MendelianRandomization v0.9.
MR revealed a positive and causal association between COVID-19 and onset of the group of diseases associated with airflow blockage and respiratory problems including bronchitis, emphysema, asthma, and rhinitis. This was evidenced by the simple median estimate (β: 1.077, 95% CI 0.862 to 1.292, p < 0.0001), weighted median (β: 0.971, 95% CI 0.751 to 1.191, p < 0.0001), penalized weighted median (β: 1.563, 95% CI 1.350 to 1.776, p < 0.0001), penalized IVW (β: 1.638, 95% CI 1.533 to 1.743, p < 0.0001), penalized robust IVW (β: 1.771, 95% CI 1.488 to 2.055, p < 0.0001), penalized MR-Egger (β: 0.960, 95% CI 0.550 to 1.371, p < 0.0001), and the penalized robust MR-Egger (β: 1.025, 95% CI 0.667 to 1.382, p < 0.0001) methods. While the estimates from IVW (β: 0.077, 95% CI −0.145 to 0.298, p = 0.497), robust IVW (β: 0.088, 95% CI −0.171 to 0.347, p = 0.505), MR-Egger (β: 0.115, 95% CI −0.371 to 0.600, p = 0.644) and robust MR-Egger (β: 0.136, 95% CI −0.420 to 0.692, p = 0.632) method also revealed positive correlation between COVID-19 occurrence and risk of bronchitis, they were statistically non-significant (Table 3B, Figure 3B).
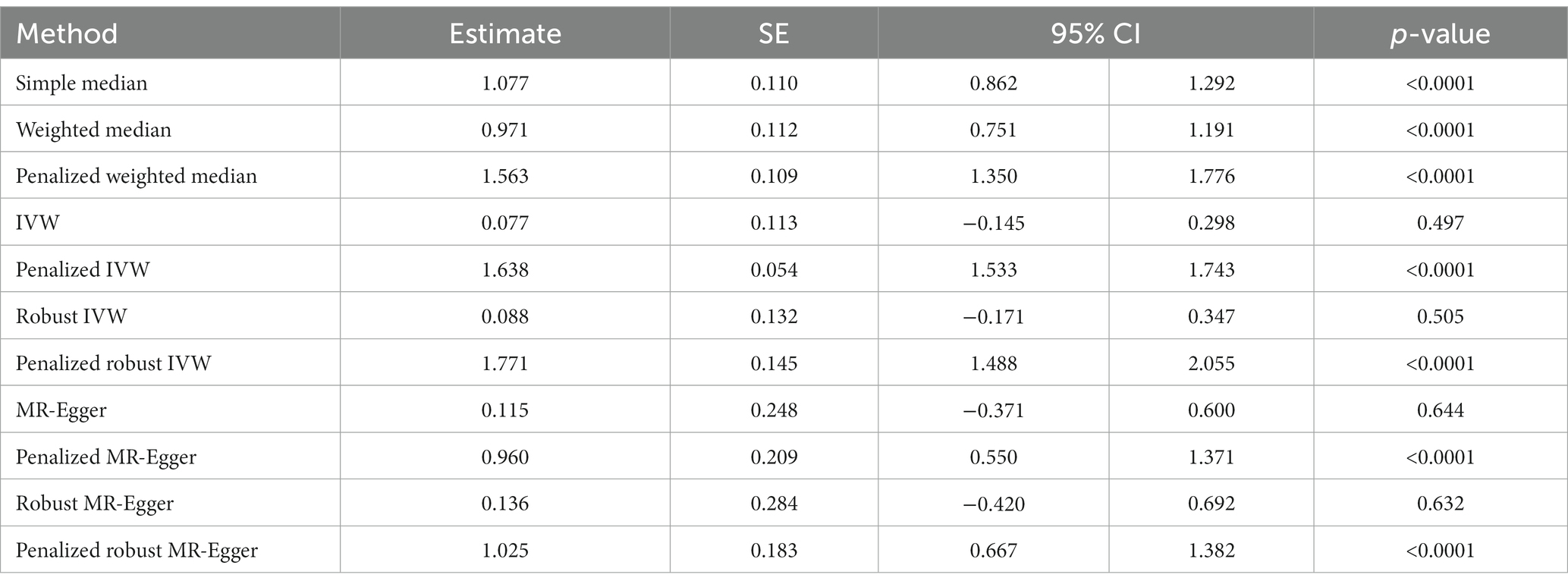
Table 3B. The MR estimates of the causal effect of COVID-19 on group of diseases associated with airflow blockage and breathing-related problems.
3.3 Effect of COVID-19 on neurological abnormalities
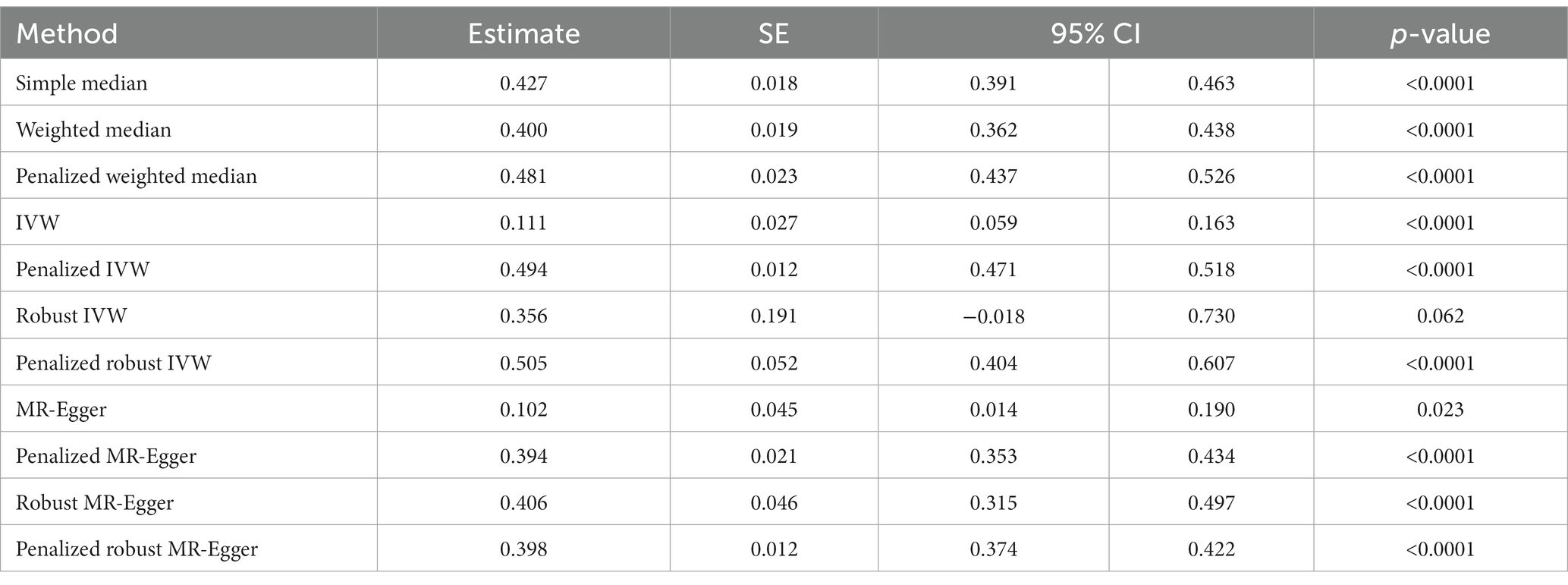
We did not find any significant association between COVID-19 and the risk of developing anxiety or depression through simple median method (β: 0.007, 95% CI −0.723 to 0.737, p = 0.986), weighted median (β: 0.121, 95% CI −0.572 to 0.813, p = 0.733), IVW (β: 0.006, 95% CI −0.352 to 0.364, p = 0.974), penalized IVW (β: 0.048, 95% CI −0.282 to 0.378, p = 0.776), robust IVW method (β: 0.006, 95% CI −0.384 to 0.397, p = 0.976), penalized robust IVW (β: 0.055, 95% CI -0.418 to 0.527, p = 0.820), MR-Egger (β: −0.328, 95% CI −1.048 to 0.392, p = 0.372), and robust MR-Egger (β: −0.370, 95% CI −1.184 to 0.444, p = 0.373) (Table 4A). On the contrary, penalized weighted median (β: 1.468, 95% CI 0.892 to 2.044, p < 0.0001) indicated a positive association between the two, and penalized MR-Egger (β: −2.121, 95% CI −2.731to −1.510, p < 0.0001) and penalized robust MR-Egger (β: −2.220, 95% CI −2.871 to −1.570, p < 0.0001) depicted a negative association. Therefore, the association between a history of COVID-19 and risk of anxiety or depression is inconclusive (Table 4A, Figure 4A).
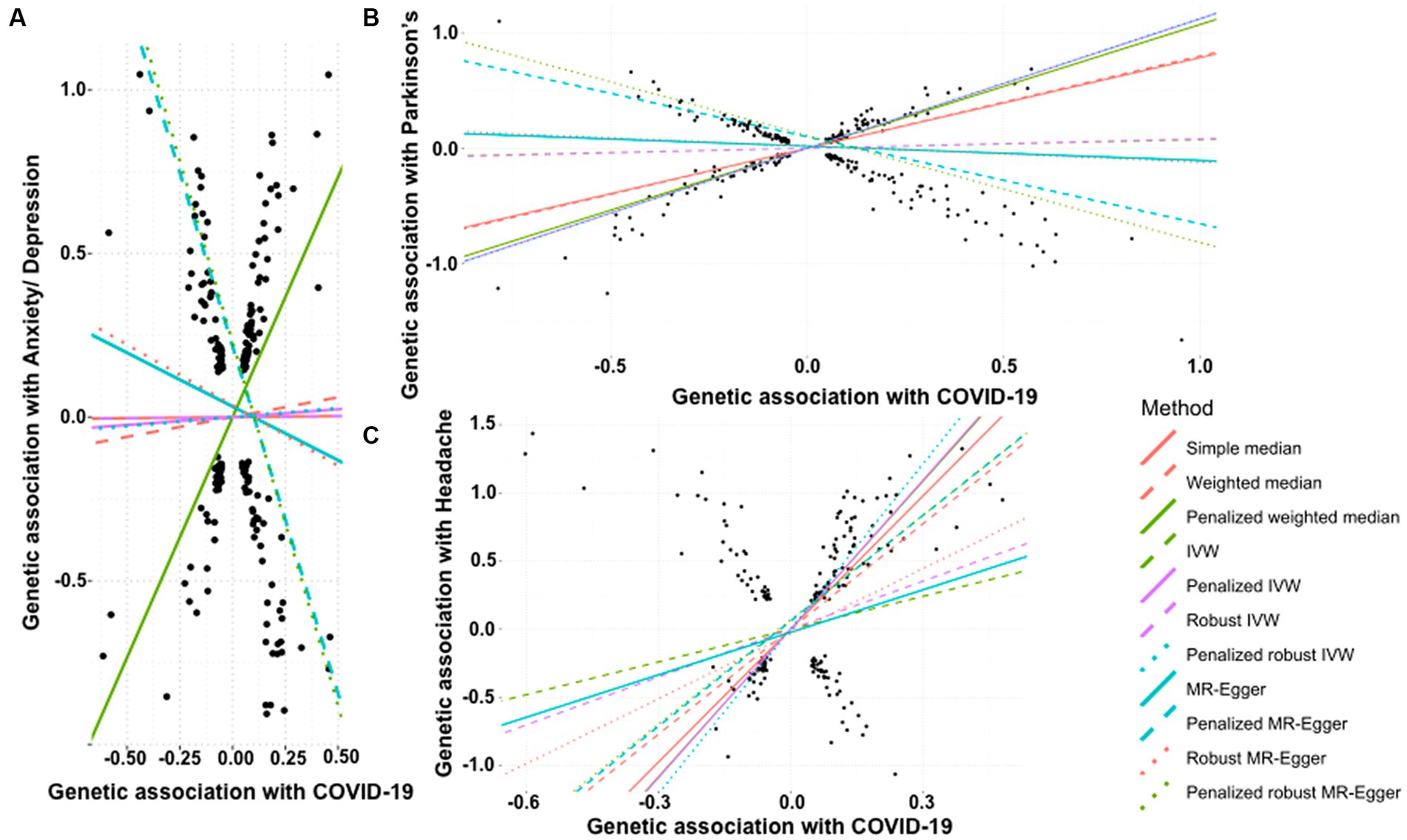
Figure 4. Scatter plot of SNPs associated with COVID-19 and neurological abnormalities. (A) anxiety/depression, (B) Parkinson’s Disease, and (C) headache. The slopes of each line represent the causal association for each method. The plot was generated using the R package MendelianRandomization v0.9.
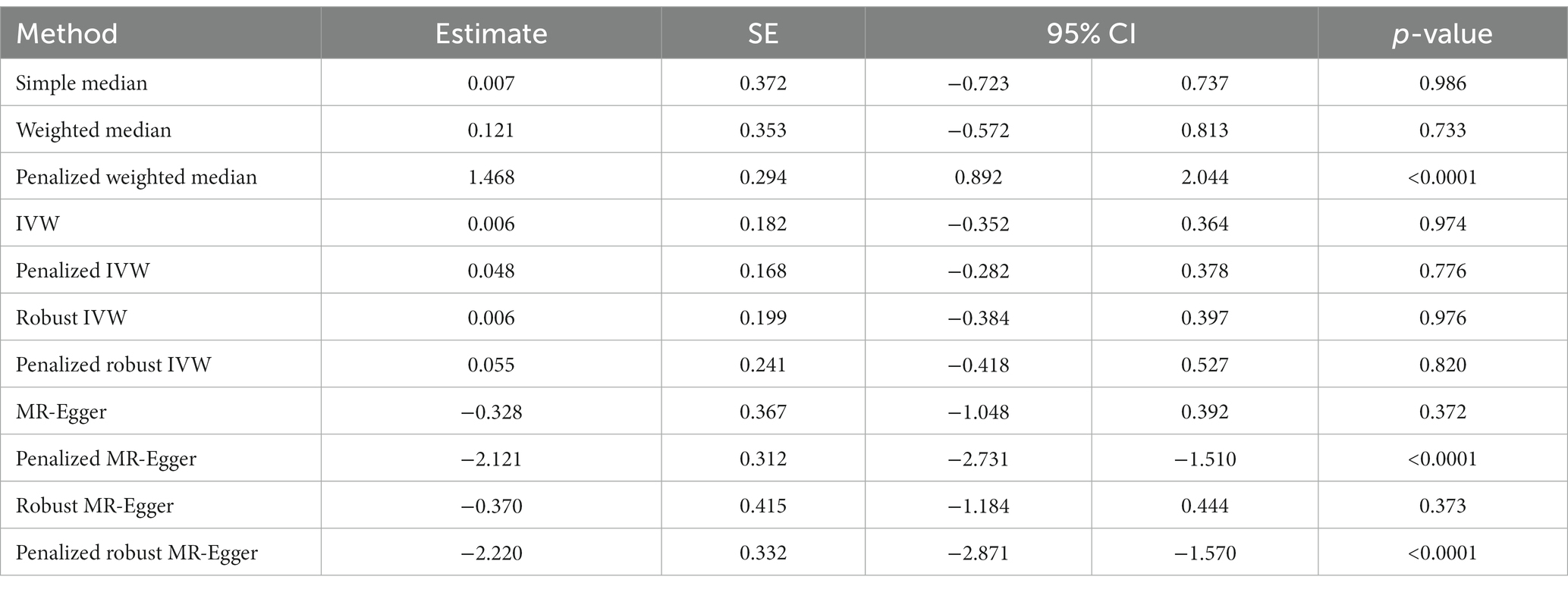
We tested the effect of COVID-19 on risk of development of Parkinson’s disease. While the simple median (β: 0.785, 95% CI 0.633 to 0.938, p < 0.0001) and IVW based methods, i.e., weighted median (β: 0.801, 95% CI 0.697 to 0.905, p < 0.0001), penalized weighted median (β: 1.073, 95% CI 0.965 to 1.181, p < 0.0001) suggested that the occurrence of COVID-19 is positively associated with an increased risk of Parkinson’s disease. This was also supported by the penalized IVW (β: 1.122, 95% CI 1.066 to 1.177, p < 0.0001) and penalized robust IVW (β: 1.124, 95% CI 0.940 to 1.309, p < 0.0001) methods. The positive association was also revealed by the IVW (β: 0.075, 95% CI −0.048 to 0.198, p = 0.232), robust IVW (β: 0.081, 95% CI −0.063 to 0.225, p = 0.270), though non-significant. However, the Egger based methods, MR-Egger (β: −0.124, 95% CI −0.381 to 0.134, p = 0.347), penalized MR-Egger (β: −0.753, 95% CI −0.942 to −0.565, p < 0.0001), robust MR-Egger method (β: −0.141, 95% CI −0.431 to 0.148, p = 0.338) and penalized robust MR-Egger (β: −0.928, 95% CI −1.160 to −0.696, p < 0.0001) suggested a negative association between the two. Therefore, the association between a history of COVID-19 and onset of Parkinson’s disease remains undetermined (Table 4B, Figure 4B).
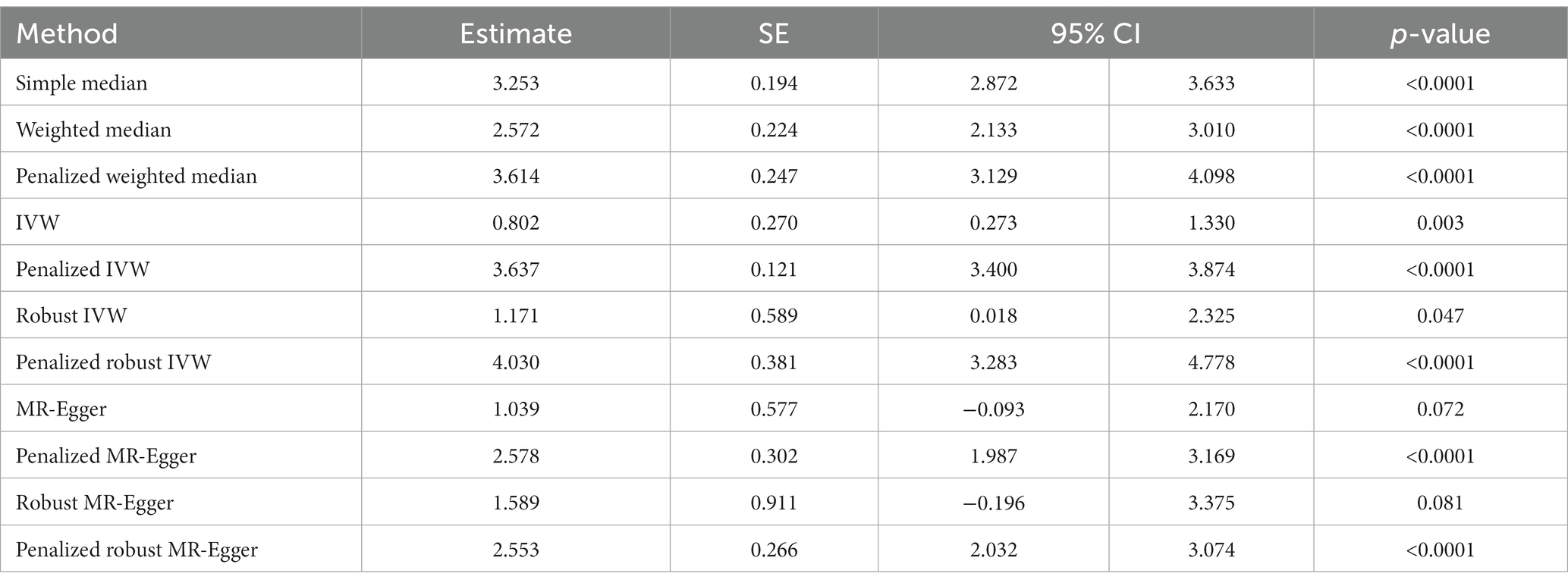
In contrast to the above, we note a strong positive causal association of a history of COVID-19 with the development of headache by simple median analysis (β: 3.253, 95% CI 2.872 to 3.633, p < 0.0001), weighted median (β: 2.572, 95% CI 2.133 to 3.010, p < 0.0001), penalized weighted median (β: 3.614, 95% CI 3.129 to 4.098, p < 0.0001), IVW (β: 0.802, 95% CI 0.273 to 1.330, p = 0.003), penalized IVW (β: 3.637, 95% CI 3.400 to 3.874, p < 0.0001), robust IVW (β: 1.171, 95% CI 0.018 to 2.325, p = 0.047), penalized robust IVW (β: 4.030, 95% CI 3.283 to 4.778, p < 0.0001), penalized MR-Egger (β: 2.578, 95% CI 1.987 to 3.169, p < 0.0001) and penalized robust MR-Egger (β: 2.553 95% CI 2.032 to 3.074, p < 0.0001) methods. The positive association was also revealed by MR-Egger (β: 1.039, 95% CI −0.093 to 2.170, p = 0.072) and robust MR-Egger (β: 1.589, 95% CI −0.196 to 3.375, p = 0.081) methods, however they were marginally significant (Table 4C, Figure 4C).
3.4 Effect of COVID-19 on cardiovascular diseases
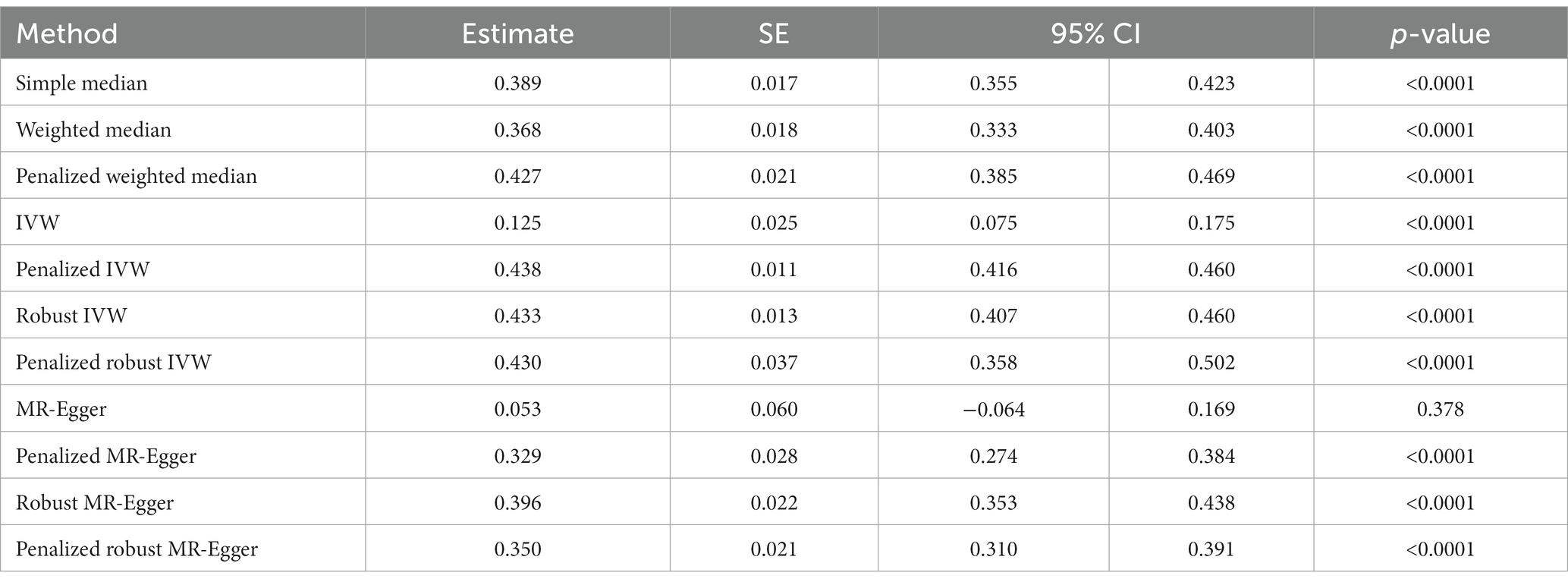
The history of COVID-19 showed a strong positive causal association with the risk of heart failure. This was estimated by simple median analysis (β: 0.389, 95% CI 0.355 to 0.423, p < 0.0001), weighted median (β: 0.368, 95% CI 0.333 to 0.403, p < 0.0001), penalized weighted median (β: 0.427, 95% CI 0.385 to 0.469, p < 0.0001), IVW (β: 0.125, 95% CI 0.075 to 0.175, p < 0.0001), penalized IVW (β: 0.438, 95% CI 0.416 to 0.460, p < 0.0001), robust IVW (β: 0.433, 95% CI 0.407 to 0.460, p < 0.0001), penalized robust IVW (β: 0.430, 95% CI 0.358 to 0.502, p < 0.0001), penalized MR-Egger (β: 0.329, 95% CI 0.274 to 0.384, p < 0.0001), robust MR-Egger (β: 0.396, 95% CI 0.353 to 0.438, p < 0.0001) and penalized robust MR-Egger (β: 0.350 95% CI 0.310 to 0.391, p < 0.0001) methods. The MR-Egger method also showed a positive association though statistically non-significant (β: 0.053, 95% CI −0.064 to 0.169, p = 0.378) (Table 5A, Figure 5A).
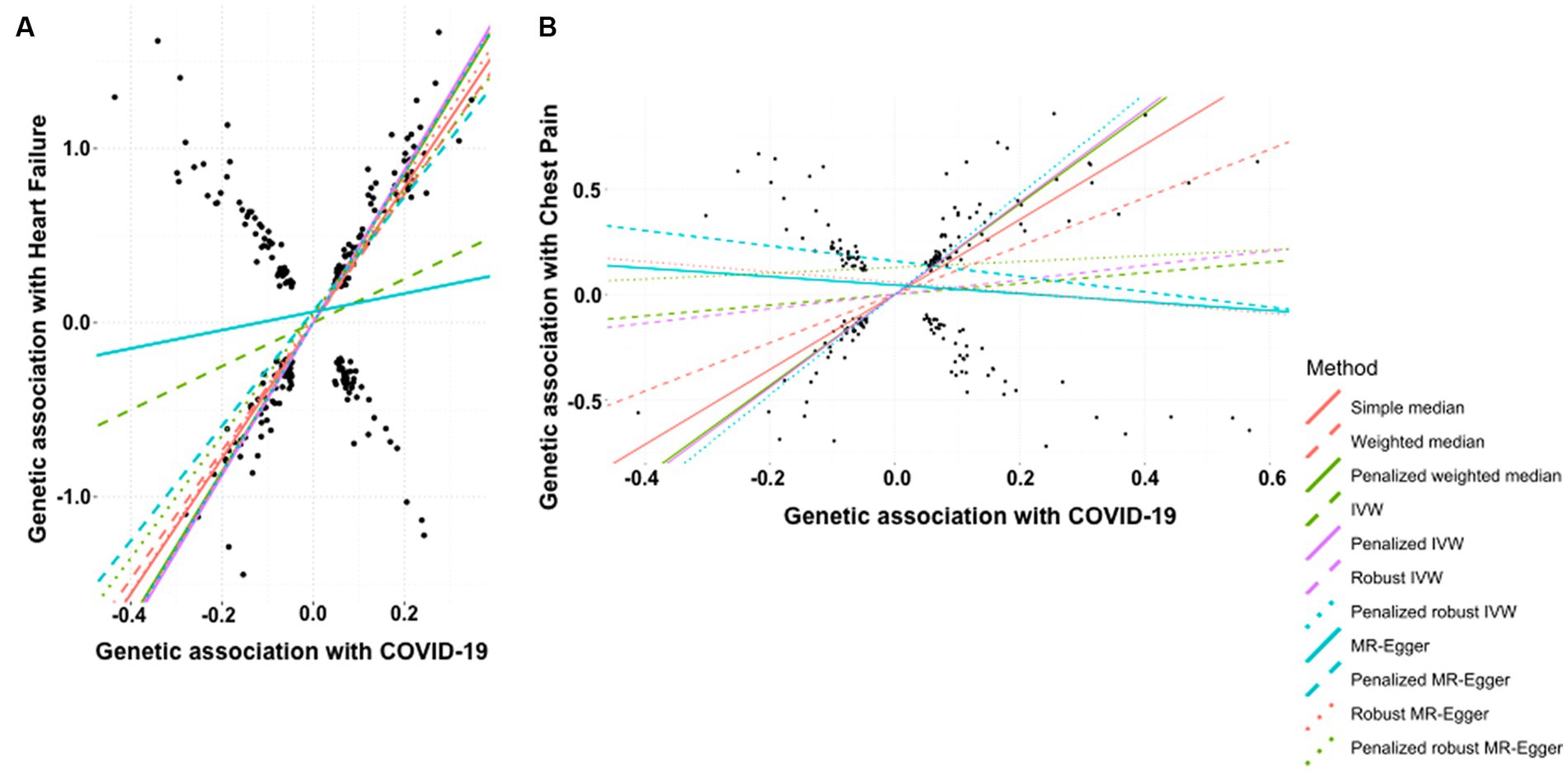
Figure 5. Scatter plot of SNPs associated with COVID-19 and cardiovascular diseases. (A) heart failure and (B) chest pain. The slopes of each line represent the causal association for each method. The plot was generated using the R package MendelianRandomization v0.9.
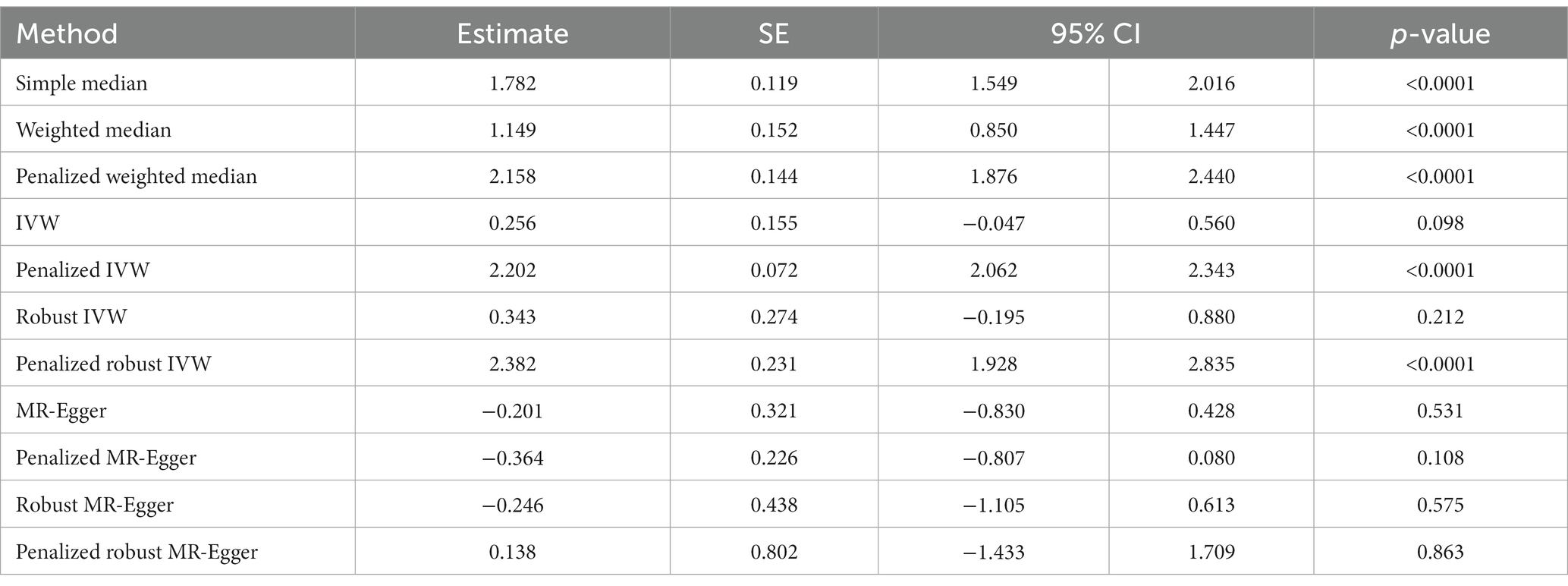
According to the simple median analysis, COVID-19 is positively and causatively correlated with the risk of developing chest pain (β: 1.782, 95% CI 1.549 to 2.016, p < 0.0001). Similar positive association emerged using weighted median (β: 1.149, 95% CI 0.850 to 1.447, p < 0.0001), penalized weighted median (β: 2.158, 95% CI 1.876 to 2.440, p < 0.0001), penalized IVW method (β: 2.202, 95% CI 2.062 to 2.343, p < 0.0001) and penalized robust IVW (β: 2.382, 95% CI 1.928 to 2.835, p < 0.0001) methods. The positive association was further replicated by the IVW (β: 0.256, 95% CI −0.047 to 0.560, p = 0.098) and robust IVW (β: 0.343, 95% CI −0.195 to 0.880, p = 0.212) methods, however they were statistically non-significant. In contrast all MR-Egger based methods, namely MR-Egger (β: −0.201, 95% CI −0.830 to 0.428, p = 0.531), penalized MR-Egger (β: −0.364, 95% CI −0.807 to 0.080, p = 0.108) and robust MR-Egger (β: −0.246, 95% CI −1.105 to 0.613, p = 0.575) showed negative association between occurrence of COVID-19 and risk of chest pain. Accordingly, the association of COVID-19 with chest pain remains inconclusive (Table 5B, Figure 5B).
3.5 Effect of COVID-19 on chronic fatigue
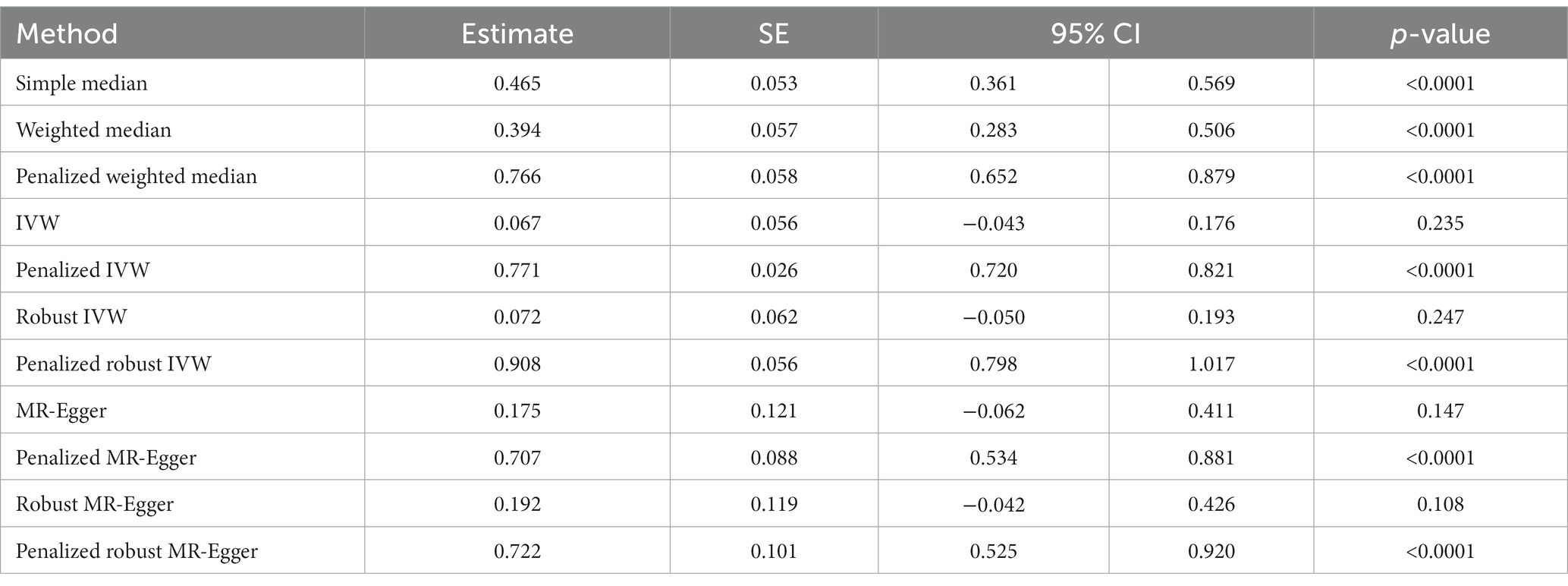
Finally, the history of COVID-19 showed a strong and positive association with the risk of developing chronic fatigue (tiredness/lethargy). This was supported by simple median (β: 0.465, 95% CI 0.361 to 0.569, p < 0.0001), weighted median (β: 0.394, 95% CI 0.283 to 0.506, p < 0.0001), penalized weighted median (β: 0.766, 95% CI 0.652 to 0.879, p < 0.0001), penalized IVW (β: 0.771, 95% CI 0.720 to 0.821, p < 0.0001), penalized robust IVW (β: 0.908, 95% CI 0.798 to 1.017, p < 0.0001), penalized MR-Egger (β: 0.707, 95% CI 0.534 to 0.881, p < 0.0001) and penalized robust MR-Egger (β: 0.722, 95% CI 0.525 to 0.920, p < 0.0001) methods. IVW (β: 0.067, 95% CI −0.043 to 0.176, p = 0.235), robust IVW (β: 0.072 95% CI −0.050 to 0.193, p = 0.247), MR-Egger (β: 0.175, 95% CI −0.062 to 0.411, p = 0.147) and robust MR-Egger (β: 0.192, 95% CI −0.042 to 0.426, p = 0.108) methods also indicted positive association between the two, though statistically non-significant (Table 6, Figure 6).
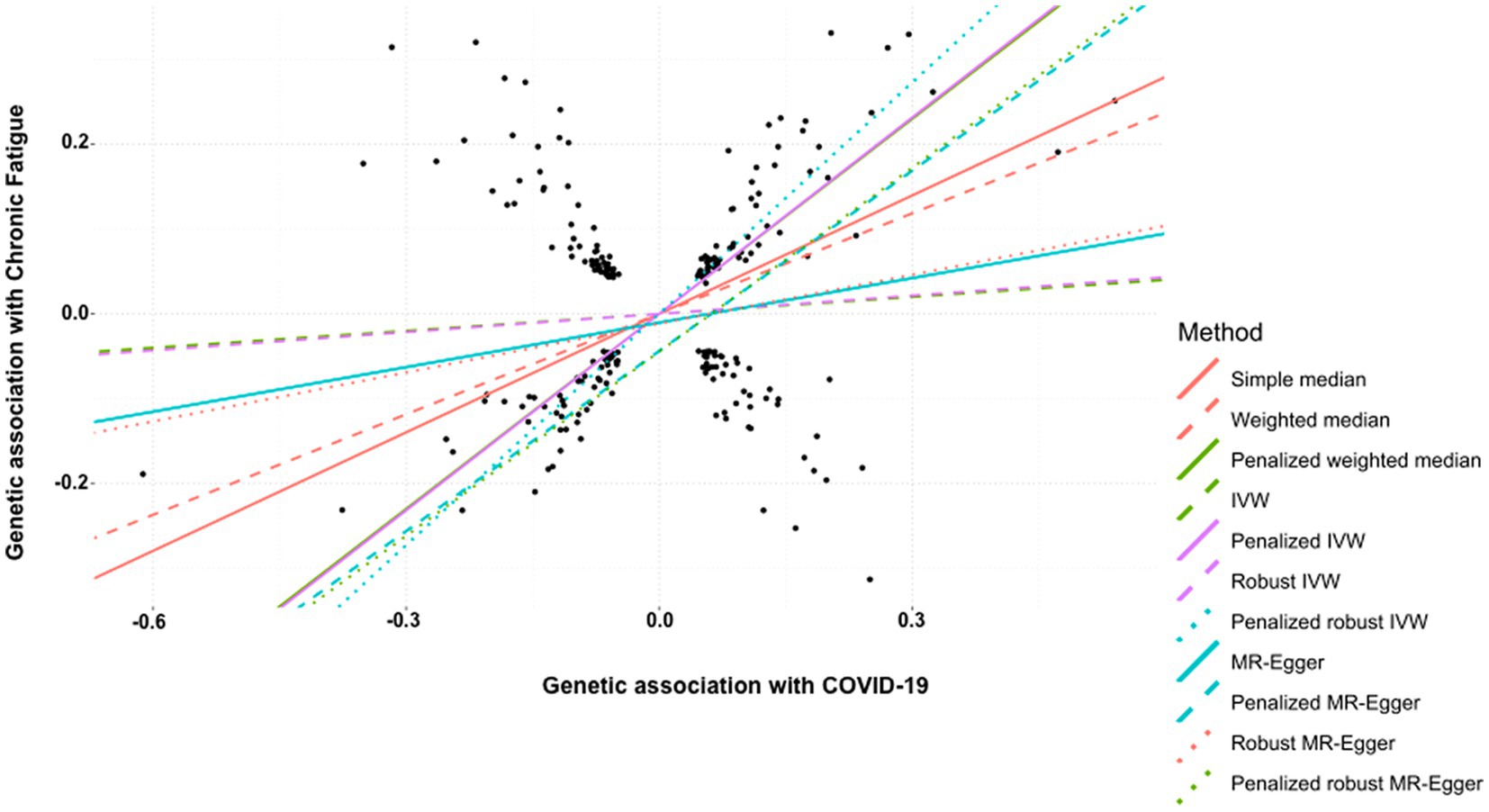
Figure 6. Scatter plot of SNPs associated with COVID-19 and chronic fatigue (tiredness/lethargy). The slopes of each line represent the causal association for each method. The plot was generated using the R package MendelianRandomization v0.9.
4 Discussion
The COVID-19 pandemic remains an ongoing challenge globally with ~77,04,37,327 confirmed cases worldwide that have not only led to ~69,56,900 deaths (43) (last accessed 11th September, 2023), but has been followed by the emergence of long COVID, which is a complex, multi-systemic entity affecting at least 10% of SARS-CoV-2 infections (2). The heterogeneity of symptoms (≥200) in long COVID patients makes it challenging to dissect which symptoms arise as a cause of SARS-CoV-2 infection vs. those resulting from exacerbation of pre-existing or coincidental conditions. These factors present significant challenges for understanding the pathomechanisms at play and for developing treatment strategies for long COVID. Here we tested the causal association of a genetic predisposition to COVID-19 with several health problems observed among subjects with long COVID, including respiratory, neurological, and cardiovascular dysfunction. Two-sample MR studies in this study suggest that a genetic predisposition for COVID-19 is causally associated with increased risk of fatigue, development of airflow blockage and respiratory problems including bronchitis, emphysema, asthma and rhinitis, pneumonia, headache, and heart failure. However, the association of COVID-19 with Parkinson’s disease, depression and chest pain was inconclusive.
Persistent fatigue is one of the most reported long COVID symptoms worldwide (1, 16). Studies showed that a subset of subjects, ~20% who recover from SARS-COV-2 infections, particularly mild infections, manifest with dyspnea and persistent fatigue despite normal cardiac and pulmonary function (44, 45). Especially in cohorts of long COVID patients with a history of SARS-CoV-2 induced hospitalization chronic fatigue was observed in a higher proportion ~40–60% of affected individuals (46, 47). Myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) that is triggered by many pathogens, e.g., Epstein–Barr (EBV) and Giardia lamblia in a subset of infections (48, 49) was also noted in a subgroup, ~50% of long COVID subjects (44) including in young individuals with mild or moderate SARS-CoV-2 infections (50). Congruently, MR studies here showed a strong positive association of a genetic predisposition for COVID-19 and persistent fatigue (tiredness/lethargy). While fatigue is likely multisystemic, the pathomechanisms underlying chronic fatigue in long COVID involve neural dysregulation, such as underactivity in particular cortical circuits, abnormal autonomic function, and myopathic changes in skeletal muscle (51). This has also been correlated with structural changes in the thalamus and basal ganglia in long COVID patients with sustained fatigue (52).
A spectrum of neurological symptoms has been reported at varying frequencies in the post-infection phase among long COVID patients. This is consistent with the neurotropic, neuroinvasive and neurovirulent nature of SARS-CoV-2 (53). In some subjects long COVID neuropathogenesis is marked by structural brain anomalies, such as pronounced decrease in its overall size, reduction in gray matter thickness and tissue damage in primary olfactory cortex (54), encephalopathy (55), hemorrhagic posterior reversible encephalopathy (56), and demyelinating lesions in the central nervous system (CNS) (57) in addition to cognitive decline (25). Other factors influencing the neuropathology of long COVID may include neuroinflammation, anti-neural auto-immune dysfunction, hypometabolism of the brain and brain stem, and abnormal cerebrospinal fluid (58–61). Congruently neuroinflammation injury and apoptosis, brain hypoxia and microhaemorrhages have been observed in non-human models of SARS-CoV-2 infection (62). Further neurological and cognitive impairment marked by structural brain abnormalities have also been noted among long COVID subjects following mild or moderate initial SARS-CoV-2 infections (54, 61).
Frequency of chronic headache range widely from ~8–40% among long COVID cases (46, 63–65). Individuals mildly affected by SARS-CoV-2 infections seemed more prone to post-COVID headaches (64, 66), which were exacerbated with a prior history of migraines (63, 64). Our MR analysis showed a strong positive causal association between COVID-19 and chronic headache in long COVID patients. Recent evidence suggests that long COVID headaches appear to be triggered by hyperinflammation and are sustained by chronic inflammatory activation, and dysregulation of neurotransmitters and metabolic inflammation (66).
Based on observational studies, among individuals affected with long COVID, ~20% may develop mood disorders, e.g., anxiety and depression (47). In one study the risk of mood disorders returned to baseline within 2 months following initial COVID-19, but some conditions such as cognitive impairment, seizures, psychosis, and dementia lingered up to 2 years (25). Another study noted that limbic atrophy and significantly abnormal cerebral functional connectivity underlie anxiety and depression among long COVID patients with mild SARS-CoV-2 infections (67). In contrast several studies inferred that anxiety and depression were not strongly linked to COVID-19 (68) with neuropsychiatric symptoms elevated disproportionately in long COVID subjects with acute initial SARS-CoV-2 infection (68, 69) or other post-infection health complications and psychiatric history (70). The onset and progression of mood disorders may also be modulated by dysfunctional regulatory cells of the innate and adaptive immune system that may contribute to chronic systemic and neuroinflammation (71). Therefore, the observations of increased psychiatric manifestations in long COVID subjects could be a result of compromised immunoregulatory mechanisms. Using MR in the present study the causal association of genetic predisposition to COVID-19 with anxiety and depression remained obscure, warranting further research to clarify this.
Parkinson’s disease is a progressive motor disorder that is highly prevalent in older adults (72). It may be triggered following infections by viruses, e.g., Influenza A, EBV and Herpes simplex virus 1 (73). Some cases of post-COVID parkinsonism have also been recorded (26, 27). Nevertheless, we detected no causal association of COVID-19 with Parkinson’s disease using MR studies.
A range of cardiovascular manifestations have been noted in long COVID patients, including those without any evidence of pre-existing cardiovascular disease or risk factors or COVID-19 related hospitalization (29). While the pathophysiology of long COVID linked cardiovascular disease is far from certain, it may involve viral invasion of cardiomyocytes and cell death, downregulation of Angiotensin-converting enzyme 2 (ACE2), endothelial cell infection, complement activation, deregulation of renin-angiotensin-aldosterone system, autonomic abnormalities, myocarditis and cardiac tissue fibrosis (74–79). The proportion of long COVID subjects with chest pain varied from ~3–20% up to 6 months post initial SARS-CoV-2 infection in different cohorts (80, 81). While the overall association of genetic predisposition to COVID-19 with chest pain in this study was inconclusive, five out of twelve MR strategies showed a strong positive causal link that was statistically significant, two techniques showed a positive association that was non-significant, and the rest showed negative association. This strongly warrants further studies to explore a putative causal link of COVID-19 with chest pain.
Heart failures as part of cardiovascular sequalae in long COVID have been observed in patients with pre-existing cardiac complications (82, 83), as well as those without (84–86). This is also supported by anecdotal accounts of increased cardiovascular abnormalities particularly among mildly symptomatic or asymptomatic subjects in the post SARS-CoV-2 infection period. Studies note that autonomic imbalance and increased sympathetic activity in the post-acute phase in ~30 days after mild SARS-CoV-2 infection may explain the cardiovascular complications during this time (87, 88). Although, the involvement of autonomic dysfunction in heart complications in long COVID is uncertain (87). All 11 methods employed for MR analysis in this study suggested a positive causal association between genetic predisposition to COVID-19 and heart failures. Ten out of eleven methods concur on this with high significance (p < 0.0001). Moreover, the effect size (β) range is highly consistent across all methods with narrow confidence intervals. Taken together MR analysis suggested a strong and positive causal association between genetic predisposition to COVID-19 and heart failure.
Finally, respiratory complications, e.g., cough are one of the commonest features of long COVID subjects (4) and are exacerbated in patients with preexisting respiratory comorbidities (89). Subjects with moderate-to-severe SARS-CoV-2 related pneumonia sustain lung abnormalities, such as parenchymal lung disease, fibrosis, and bronchiectasis even a year following initial infection (90). New onset of airway abnormalities, e.g., asthma is not common but has been strongly associated with COVID-19 (91). Increased lung emphysema was noted at 30 days following initial SARS-CoV-2 infection (92). In this study MR results support a strong positive causal association of a history of COVID-19 with increased risk of disorders with chronic lung and airway inflammation, as well as pneumonia.
This study has some limitations. First since it utilizes publicly available genetic datasets, the vaccination and BTI status of the included individuals is unknown. Accordingly, it cannot account for how these factors influence long COVID outcomes. It is noteworthy that BTI of SARS-CoV-2 following vaccination may be influenced by several factors, e.g., age and previous infection (93). Moreover, the serological response to vaccines was also reported to be insufficient in individuals with two or more pre-existing chronic conditions (94). These factors may influence long COVID phenotypes and cannot be evaluated in the present study. Second, the dataset for chest pain used here is non-specific, and may include subjects where it is caused due to cardiac, lung or abdominal organ abnormalities. This may also explain why the association of a history of COVID-19 with chest pain in this study was inconclusive despite it being a commonly noted long COVID complexity (2).
In conclusion, this study has employed MR to shed light on the genetic underpinnings of long COVID, uncovering significant causal associations between genetic predisposition to COVID-19 and several prevailing health complications. MR findings in this study corroborate the multiple adverse outcomes of long COVID, linking it to an increased risk of developing pneumonia, airway infections, headache, chronic fatigue, and heart failure. Our findings on Parkinson’s disease, depression, and chest pain were inconclusive. These insights are critical in enhancing our understanding of the lasting health implications of COVID-19. As we continue to grapple with the long-term challenges posed by the COVID-19 pandemic, a deeper comprehension of the genetic factors contributing to long COVID will be a precursor to developing targeted strategies to support those symptomatic with these long COVID symptoms. Further research is warranted to explore the intricate web of genetic determinants underlying the diverse manifestations of long COVID, which will spearhead global efforts in combating this healthcare crisis effectively.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://gwas.mrcieu.ac.uk/ and https://www.covid19hg.org/results/r6/.
Ethics statement
Ethical approval was not required for the study involving humans in accordance with the local legislation and institutional requirements. Written informed consent to participate in this study was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and the institutional requirements.
Author contributions
PS: Methodology, Visualization, Writing – review & editing, Data curation, Formal Analysis, Software. HU: Writing – review & editing, Funding acquisition, Investigation, Project administration, Resources. JKS: Writing – review & editing, Data curation, Formal Analysis, Software. SB: Data curation, Formal Analysis, Software, Writing – review & editing. NJ: Data curation, Formal Analysis, Software, Writing – review & editing. NK: Data curation, Formal Analysis, Software, Writing – review & editing. RD: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing – review & editing. PU: Writing – review & editing, Conceptualization, Investigation, Methodology, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was funded by Yenepoya (Deemed to be University) Seed Grant to HU (YU/Seed Grant/110-2022).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1303183/full#supplementary-material
Footnotes
References
1. (WHO) WHO. A clinical case definition of post COVID-19 condition by a Delphi consensus. (2021) Available at: https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-21.1 (Accessed October 6, 2021).
2. Davis, HE, McCorkell, L, Vogel, JM, and Topol, EJ. Long COVID: major findings, mechanisms and recommendations. Nat Rev Microbiol. (2023) 21:133–46. doi: 10.1038/s41579-022-00846-2
3. Lopez-Leon, S, Wegman-Ostrosky, T, Ayuzo Del Valle, NC, Perelman, C, Sepulveda, R, Rebolledo, PA, et al. Long-COVID in children and adolescents: a systematic review and meta-analyses. Sci Rep. (2022) 12:9950. doi: 10.1038/s41598-022-13495-5
4. Prevention CfDCa. Post-COVID conditions. (2023) Available at: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html.
5. Chen, C, Haupert, SR, Zimmermann, L, Shi, X, Fritsche, LG, and Mukherjee, B. Global prevalence of post-coronavirus disease 2019 (COVID-19) condition or long COVID: a Meta-analysis and systematic review. J Infect Dis. (2022) 226:1593–607. doi: 10.1093/infdis/jiac136
6. Perlis, RH, Santillana, M, Ognyanova, K, Safarpour, A, Lunz Trujillo, K, Simonson, MD, et al. Prevalence and correlates of long COVID symptoms among US adults. JAMA Netw Open. (2022) 5:e2238804. doi: 10.1001/jamanetworkopen.2022.38804
7. Caze, AB, Cerqueira-Silva, T, Bomfim, AP, de Souza, GL, Azevedo, AC, Brasil, MQ, et al. Prevalence and risk factors for long COVID after mild disease: a cohort study with a symptomatic control group. J Glob Health. (2023) 13:06015. doi: 10.7189/jogh.13.06015
8. Bai, F, Tomasoni, D, Falcinella, C, Barbanotti, D, Castoldi, R, Mule, G, et al. Female gender is associated with long COVID syndrome: a prospective cohort study. Clin Microbiol Infect. (2022) 28:611.e9–e16. doi: 10.1016/j.cmi.2021.11.002
9. Malkova, A, Kudryavtsev, I, Starshinova, A, Kudlay, D, Zinchenko, Y, Glushkova, A, et al. Post COVID-19 syndrome in patients with asymptomatic/mild form. Pathogens. (2021) 10:1408. doi: 10.3390/pathogens10111408
10. Fernandez-de-Las-Penas, C, Martin-Guerrero, JD, Florencio, LL, Navarro-Pardo, E, Rodriguez-Jimenez, J, Torres-Macho, J, et al. Clustering analysis reveals different profiles associating long-term post-COVID symptoms, COVID-19 symptoms at hospital admission and previous medical co-morbidities in previously hospitalized COVID-19 survivors. Infection. (2023) 51:61–9. doi: 10.1007/s15010-022-01822-x
11. Subramanian, A, Nirantharakumar, K, Hughes, S, Myles, P, Williams, T, Gokhale, KM, et al. Symptoms and risk factors for long COVID in non-hospitalized adults. Nat Med. (2022) 28:1706–14. doi: 10.1038/s41591-022-01909-w
12. Cerqueira-Silva, T, Shah, SA, Robertson, C, Sanchez, M, Katikireddi, SV, de Araujo, OV, et al. Effectiveness of mRNA boosters after homologous primary series with BNT162b2 or ChAdOx1 against symptomatic infection and severe COVID-19 in Brazil and Scotland: a test-negative design case-control study. PLoS Med. (2023) 20:e1004156. doi: 10.1371/journal.pmed.1004156
13. Lammi, V, Nakanishi, T, Jones, SE, Andrews, SJ, Karjalainen, J, Cortés, B, et al. Genome-wide association study of long COVID. medRxiv 2023.06.29.23292056. (2023). doi: 10.1101/2023.06.29.23292056
14. Al-Aly, Z, Bowe, B, and Xie, Y. Long COVID after breakthrough SARS-CoV-2 infection. Nat Med. (2022) 28:1461–7. doi: 10.1038/s41591-022-01840-0
15. Taquet, M, Dercon, Q, and Harrison, PJ. Six-month sequelae of post-vaccination SARS-CoV-2 infection: a retrospective cohort study of 10,024 breakthrough infections. Brain Behav Immun. (2022) 103:154–62. doi: 10.1016/j.bbi.2022.04.013
16. Global Burden of Disease Long COVID CollaboratorsWulf Hanson, S, Abbafati, C, Aerts, JG, al-Aly, Z, Ashbaugh, C, et al. Estimated global proportions of individuals with persistent fatigue, cognitive, and respiratory symptom clusters following symptomatic COVID-19 in 2020 and 2021. JAMA. (2022) 328:1604–15. doi: 10.1001/jama.2022.18931
17. Davis, HE, Assaf, GS, McCorkell, L, Wei, H, Low, RJ, Re'em, Y, et al. Characterizing long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine. (2021) 38:101019. doi: 10.1016/j.eclinm.2021.101019
18. Arnold, DT, Hamilton, FW, Milne, A, Morley, AJ, Viner, J, Attwood, M, et al. Patient outcomes after hospitalisation with COVID-19 and implications for follow-up: results from a prospective UK cohort. Thorax. (2021) 76:399–401. doi: 10.1136/thoraxjnl-2020-216086
19. Han, X, Fan, Y, Alwalid, O, Li, N, Jia, X, Yuan, M, et al. Six-month follow-up chest CT findings after severe COVID-19 pneumonia. Radiology. (2021) 299:E177–86. doi: 10.1148/radiol.2021203153
20. Fabbri, L, Moss, S, Khan, FA, Chi, W, Xia, J, Robinson, K, et al. Parenchymal lung abnormalities following hospitalisation for COVID-19 and viral pneumonitis: a systematic review and meta-analysis. Thorax. (2023) 78:191–201. doi: 10.1136/thoraxjnl-2021-218275
21. Nasserie, T, Hittle, M, and Goodman, SN. Assessment of the frequency and variety of persistent symptoms among patients with COVID-19: a systematic review. JAMA Netw Open. (2021) 4:e2111417. doi: 10.1001/jamanetworkopen.2021.11417
22. Crivelli, L, Palmer, K, Calandri, I, Guekht, A, Beghi, E, Carroll, W, et al. Changes in cognitive functioning after COVID-19: a systematic review and meta-analysis. Alzheimers Dement. (2022) 18:1047–66. doi: 10.1002/alz.12644
23. Holdsworth, DA, Chamley, R, Barker-Davies, R, O'Sullivan, O, Ladlow, P, Mitchell, JL, et al. Comprehensive clinical assessment identifies specific neurocognitive deficits in working-age patients with long-COVID. PLoS One. (2022) 17:e0267392. doi: 10.1371/journal.pone.0267392
24. Tana, C, Bentivegna, E, Cho, SJ, Harriott, AM, Garcia-Azorin, D, Labastida-Ramirez, A, et al. Long COVID headache. J Headache Pain. (2022) 23:93. doi: 10.1186/s10194-022-01450-8
25. Taquet, M, Sillett, R, Zhu, L, Mendel, J, Camplisson, I, Dercon, Q, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry. (2022) 9:815–27. doi: 10.1016/S2215-0366(22)00260-7
26. Cohen, ME, Eichel, R, Steiner-Birmanns, B, Janah, A, Ioshpa, M, Bar-Shalom, R, et al. A case of probable Parkinson's disease after SARS-CoV-2 infection. Lancet Neurol. (2020) 19:804–5. doi: 10.1016/S1474-4422(20)30305-7
27. Rao, AR, Hidayathullah, SM, Hegde, K, and Adhikari, P. Parkinsonism: an emerging post COVID sequelae. IDCases. (2022) 27:e01388. doi: 10.1016/j.idcr.2022.e01388
28. Roca-Fernandez, A, Wamil, M, Telford, A, Carapella, V, Borlotti, A, Monteiro, D, et al. Cardiac impairment in long Covid 1-year post SARS-CoV-2 infection. Eur Heart J. (2022) 43:43(Supplement_2). doi: 10.1093/eurheartj/ehac544.219
29. Xie, Y, Xu, E, Bowe, B, and Al-Aly, Z. Long-term cardiovascular outcomes of COVID-19. Nat Med. (2022) 28:583–90. doi: 10.1038/s41591-022-01689-3
30. Xie, Y, and Al-Aly, Z. Risks and burdens of incident diabetes in long COVID: a cohort study. Lancet Diabetes Endocrinol. (2022) 10:311–21. doi: 10.1016/S2213-8587(22)00044-4
31. Dennis, A, Cuthbertson, DJ, Wootton, D, Crooks, M, Gabbay, M, Eichert, N, et al. Multi-organ impairment and long COVID: a 1-year prospective, longitudinal cohort study. J R Soc Med. (2023) 116:97–112. doi: 10.1177/01410768231154703
32. Adler, L, Gazit, S, Pinto, Y, Perez, G, Mizrahi Reuveni, M, Yehoshua, I, et al. Long-COVID in patients with a history of mild or asymptomatic SARS-CoV-2 infection: a Nationwide cohort study. Scand J Prim Health Care. (2022) 40:342–9. doi: 10.1080/02813432.2022.2139480
33. Bygdell, M, Leach, S, Lundberg, L, Gyll, D, Martikainen, J, Santosa, A, et al. A comprehensive characterization of patients diagnosed with post-COVID-19 condition in Sweden 16 months after the introduction of the international classification of diseases tenth revision diagnosis code (U09.9): a population-based cohort study. Int J Infect Dis. (2023) 126:104–13. doi: 10.1016/j.ijid.2022.11.021
34. Smith, GD, and Ebrahim, S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol. (2003) 32:1–22. doi: 10.1093/ije/dyg070
35. Baranova, A, Cao, H, and Zhang, F. Causal effect of COVID-19 on Alzheimer's disease: a Mendelian randomization study. J Med Virol. (2023) 95:e28107. doi: 10.1002/jmv.28107
36. Li, GH, Tang, CM, and Cheung, CL. COVID-19 and thyroid function: a bi-directional two-sample Mendelian randomization study. Thyroid. (2022) 32:1037–50. doi: 10.1089/thy.2022.0243
37. Liu, N, Tan, JS, Liu, L, Wang, Y, Hua, L, and Qian, Q. Genetic predisposition between COVID-19 and four mental illnesses: a bidirectional, two-sample Mendelian randomization study. Front Psych. (2021) 12:746276. doi: 10.3389/fpsyt.2021.746276
38. Li, J, Bai, H, Qiao, H, Du, C, Yao, P, Zhang, Y, et al. Causal effects of COVID-19 on cancer risk: a Mendelian randomization study. J Med Virol. (2023) 95:e28722. doi: 10.1002/jmv.28722
39. Davies, NM, Holmes, MV, and Davey, SG. Reading Mendelian randomisation studies: a guide, glossary, and checklist for clinicians. BMJ. (2018) 362:k601. doi: 10.1136/bmj.k601
40. GenomeAsia, KC. The GenomeAsia 100K project enables genetic discoveries across Asia. Nature. (2019) 576:106–11. doi: 10.1038/s41586-019-1793-z
41. Chang, CC, Chow, CC, Tellier, LC, Vattikuti, S, Purcell, SM, and Lee, JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. (2015) 4:7. doi: 10.1186/s13742-015-0047-8
42. Teumer, A. Common methods for performing Mendelian randomization. Front. Cardiovasc. Med. (2018) 5:51. doi: 10.3389/fcvm.2018.00051
43. Dashboard WCC. (2023) Available at: https://covid19.who.int.
44. Mancini, DM, Brunjes, DL, Lala, A, Trivieri, MG, Contreras, JP, and Natelson, BH. Use of cardiopulmonary stress testing for patients with unexplained dyspnea post-coronavirus disease. JACC Heart Fail. (2021) 9:927–37. doi: 10.1016/j.jchf.2021.10.002
45. van den Borst, B, Peters, JB, Brink, M, Schoon, Y, Bleeker-Rovers, CP, Schers, H, et al. Comprehensive health assessment 3 months after recovery from acute coronavirus disease 2019 (COVID-19). Clin Infect Dis. (2021) 73:e1089–98. doi: 10.1093/cid/ciaa1750
46. Gentilotti, E, Gorska, A, Tami, A, Gusinow, R, Mirandola, M, Rodriguez Bano, J, et al. Clinical phenotypes and quality of life to define post-COVID-19 syndrome: a cluster analysis of the multinational, prospective ORCHESTRA cohort. EClinicalMedicine. (2023) 62:102107. doi: 10.1016/j.eclinm.2023.102107
47. Laurent, R, Correia, P, Lachand, R, Diconne, E, Ezingeard, E, Bruna, F, et al. Long-term outcomes of COVID-19 intensive care unit survivors and their family members: a one year follow-up prospective study. Front Public Health. (2023) 11:1236990. doi: 10.3389/fpubh.2023.1236990
48. Naess, H, Nyland, M, Hausken, T, Follestad, I, and Nyland, HI. Chronic fatigue syndrome after Giardia enteritis: clinical characteristics, disability and long-term sickness absence. BMC Gastroenterol. (2012) 12:13. doi: 10.1186/1471-230X-12-13
49. Hickie, I, Davenport, T, Wakefield, D, Vollmer-Conna, U, Cameron, B, Vernon, SD, et al. Post-infective and chronic fatigue syndromes precipitated by viral and non-viral pathogens: prospective cohort study. BMJ. (2006) 333:575. doi: 10.1136/bmj.38933.585764.AE
50. Kedor, C, Freitag, H, Meyer-Arndt, L, Wittke, K, Hanitsch, LG, Zoller, T, et al. A prospective observational study of post-COVID-19 chronic fatigue syndrome following the first pandemic wave in Germany and biomarkers associated with symptom severity. Nat Commun. (2022) 13:5104. doi: 10.1038/s41467-022-32507-6
51. Baker, AME, Maffitt, NJ, Del Vecchio, A, McKeating, KM, Baker, MR, Baker, SN, et al. Neural dysregulation in post-COVID fatigue. Brain Commun. (2023) 5:fcad122. doi: 10.1093/braincomms/fcad122
52. Heine, J, Schwichtenberg, K, Hartung, TJ, Rekers, S, Chien, C, Boesl, F, et al. Structural brain changes in patients with post-COVID fatigue: a prospective observational study. EClinicalMedicine. (2023) 58:101874. doi: 10.1016/j.eclinm.2023.101874
53. Bauer, L, Laksono, BM, de Vrij, FMS, Kushner, SA, Harschnitz, O, and van Riel, D. The neuroinvasiveness, neurotropism, and neurovirulence of SARS-CoV-2. Trends Neurosci. (2022) 45:358–68. doi: 10.1016/j.tins.2022.02.006
54. Douaud, G, Lee, S, Alfaro-Almagro, F, Arthofer, C, Wang, C, McCarthy, P, et al. SARS-CoV-2 is associated with changes in brain structure in UK biobank. Nature. (2022) 604:697–707. doi: 10.1038/s41586-022-04569-5
55. Helms, J, Kremer, S, Merdji, H, Clere-Jehl, R, Schenck, M, Kummerlen, C, et al. Neurologic features in severe SARS-CoV-2 infection. N Engl J Med. (2020) 382:2268–70. doi: 10.1056/NEJMc2008597
56. Franceschi, AM, Ahmed, O, Giliberto, L, and Castillo, M. Hemorrhagic posterior reversible encephalopathy syndrome as a manifestation of COVID-19 infection. AJNR Am J Neuroradiol. (2020) 41:1173–6. doi: 10.3174/ajnr.A6595
57. Zanin, L, Saraceno, G, Panciani, PP, Renisi, G, Signorini, L, Migliorati, K, et al. SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir. (2020) 162:1491–4. doi: 10.1007/s00701-020-04374-x
58. Spudich, S, and Nath, A. Nervous system consequences of COVID-19. Science. (2022) 375:267–9. doi: 10.1126/science.abm2052
59. Guedj, E, Campion, JY, Dudouet, P, Kaphan, E, Bregeon, F, Tissot-Dupont, H, et al. (18)F-FDG brain PET hypometabolism in patients with long COVID. Eur J Nucl Med Mol Imaging. (2021) 48:2823–33. doi: 10.1007/s00259-021-05215-4
60. Hugon, J, Queneau, M, Sanchez Ortiz, M, Msika, EF, Farid, K, and Paquet, C. Cognitive decline and brainstem hypometabolism in long COVID: a case series. Brain Behav. (2022) 12:e2513. doi: 10.1002/brb3.2513
61. Apple, AC, Oddi, A, Peluso, MJ, Asken, BM, Henrich, TJ, Kelly, JD, et al. Risk factors and abnormal cerebrospinal fluid associate with cognitive symptoms after mild COVID-19. Ann Clin Transl Neurol. (2022) 9:221–6. doi: 10.1002/acn3.51498
62. Rutkai, I, Mayer, MG, Hellmers, LM, Ning, B, Huang, Z, Monjure, CJ, et al. Neuropathology and virus in brain of SARS-CoV-2 infected non-human primates. Nat Commun. (2022) 13:1745. doi: 10.1038/s41467-022-29440-z
63. Garcia-Azorin, D, Layos-Romero, A, Porta-Etessam, J, Membrilla, JA, Caronna, E, Gonzalez-Martinez, A, et al. Post-COVID-19 persistent headache: a multicentric 9-months follow-up study of 905 patients. Cephalalgia. (2022) 42:804–9. doi: 10.1177/03331024211068074
64. Fernandez-de-Las-Penas, C, Navarro-Santana, M, Gomez-Mayordomo, V, Cuadrado, ML, Garcia-Azorin, D, Arendt-Nielsen, L, et al. Headache as an acute and post-COVID-19 symptom in COVID-19 survivors: a meta-analysis of the current literature. Eur J Neurol. (2021) 28:3820–5. doi: 10.1111/ene.15040
65. Rodrigues, AN, Dias, ARN, Paranhos, ACM, Silva, CC, Bastos, TDR, de Brito, BB, et al. Headache in long COVID as disabling condition: a clinical approach. Front Neurol. (2023) 14:1149294. doi: 10.3389/fneur.2023.1149294
66. Foo, S-S, Chen, W, Jung, KL, Azamor, T, Choi, UY, Zhang, P, et al. Immunometabolic rewiring in long COVID patients with chronic headache. bioRxiv 2023.03.06.531302. (2023). doi: 10.1101/2023.03.06.531302
67. Costa, BAD, Silva, L, Mendes, M, Karmann, Í, Campos, B, Nogueira, M, et al. Anxiety and depression are associated with limbic atrophy and severe disruption of brain functional connectivity after mild COVID-19 infection (S21.007). Neurology. (2023) 100:1998. doi: 10.1212/WNL.0000000000202252
68. Caspersen, IH, Magnus, P, and Trogstad, L. Excess risk and clusters of symptoms after COVID-19 in a large Norwegian cohort. Eur J Epidemiol. (2022) 37:539–48. doi: 10.1007/s10654-022-00847-8
69. Rogers, JP, Chesney, E, Oliver, D, Pollak, TA, McGuire, P, Fusar-Poli, P, et al. Psychiatric and neuropsychiatric presentations associated with severe coronavirus infections: a systematic review and meta-analysis with comparison to the COVID-19 pandemic. Lancet Psychiatry. (2020) 7:611–27. doi: 10.1016/S2215-0366(20)30203-0
70. Re’em, Y, Stelson, EA, Davis, HE, McCorkell, L, Wei, H, Assaf, G, et al. Factors associated with psychiatric outcomes and coping in long COVID. Nat. Mental Health. (2023) 1:361–72. doi: 10.1038/s44220-023-00064-6
71. Bauer, ME, and Teixeira, AL. Neuroinflammation in mood disorders: role of regulatory immune cells. Neuroimmunomodulation. (2021) 28:99–107. doi: 10.1159/000515594
72. Lee, A, and Gilbert, RM. Epidemiology of Parkinson disease. Neurol Clin. (2016) 34:955–65. doi: 10.1016/j.ncl.2016.06.012
73. Limphaibool, N, Iwanowski, P, Holstad, MJV, Kobylarek, D, and Kozubski, W. Infectious etiologies of parkinsonism: Pathomechanisms and clinical implications. Front Neurol. (2019) 10:652. doi: 10.3389/fneur.2019.00652
74. Delorey, TM, Ziegler, CGK, Heimberg, G, Normand, R, Yang, Y, Segerstolpe, A, et al. COVID-19 tissue atlases reveal SARS-CoV-2 pathology and cellular targets. Nature. (2021) 595:107–13. doi: 10.1038/s41586-021-03570-8
75. Song, WC, and FitzGerald, GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. (2020) 130:3950–3. doi: 10.1172/JCI140183
76. Varga, Z, Flammer, AJ, Steiger, P, Haberecker, M, Andermatt, R, Zinkernagel, AS, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet. (2020) 395:1417–8. doi: 10.1016/S0140-6736(20)30937-5
77. Chung, MK, Zidar, DA, Bristow, MR, Cameron, SJ, Chan, T, Harding, CV 3rd, et al. COVID-19 and cardiovascular disease: from bench to bedside. Circ Res. (2021) 128:1214–36. doi: 10.1161/CIRCRESAHA.121.317997
78. Liu, PP, Blet, A, Smyth, D, and Li, H. The science underlying COVID-19: implications for the cardiovascular system. Circulation. (2020) 142:68–78. doi: 10.1161/CIRCULATIONAHA.120.047549
79. Nishiga, M, Wang, DW, Han, Y, Lewis, DB, and Wu, JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. (2020) 17:543–58. doi: 10.1038/s41569-020-0413-9
80. Carvalho-Schneider, C, Laurent, E, Lemaignen, A, Beaufils, E, Bourbao-Tournois, C, Laribi, S, et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin Microbiol Infect. (2021) 27:258–63. doi: 10.1016/j.cmi.2020.09.052
81. Ballering, AV, van Zon, SKR, Olde Hartman, TC, and Rosmalen, JGM, Lifelines Corona Research Initiative. Persistence of somatic symptoms after COVID-19 in the Netherlands: an observational cohort study. Lancet. (2022) 400:452–61. doi: 10.1016/S0140-6736(22)01214-4
82. Shi, S, Qin, M, Shen, B, Cai, Y, Liu, T, Yang, F, et al. Association of Cardiac Injury with Mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. (2020) 5:802–10. doi: 10.1001/jamacardio.2020.0950
83. Guo, T, Fan, Y, Chen, M, Wu, X, Zhang, L, He, T, et al. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol. (2020) 5:811–8. doi: 10.1001/jamacardio.2020.1017
84. Bajaj, R, Sinclair, HC, Patel, K, Low, B, Pericao, A, Manisty, C, et al. Delayed-onset myocarditis following COVID-19. Lancet Respir Med. (2021) 9:e32–4. doi: 10.1016/S2213-2600(21)00085-0
85. Dennis, A, Wamil, M, Alberts, J, Oben, J, Cuthbertson, DJ, Wootton, D, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open. (2021) 11:e048391. doi: 10.1136/bmjopen-2020-048391
86. Rohun, J, Dorniak, K, Faran, A, Kochańska, A, Zacharek, D, and Daniłowicz-Szymanowicz, L. Long COVID-19 myocarditis and various heart failure presentations: a case series. J. Cardiovasc. Dev. Dis. (2022) 9:427. doi: 10.3390/jcdd9120427
87. Liviero, F, Scapellato, ML, Folino, F, Moretto, A, Mason, P, and Pavanello, S. Persistent increase of sympathetic activity in post-acute COVID-19 of Paucisymptomatic healthcare workers. Int J Environ Res Public Health. (2023) 20:1256512. doi: 10.3390/ijerph20010830
88. Baldi, E, Sechi, GM, Mare, C, Canevari, F, Brancaglione, A, Primi, R, et al. Out-of-hospital cardiac arrest during the Covid-19 outbreak in Italy. N Engl J Med. (2020) 383:496–8. doi: 10.1056/NEJMc2010418
89. Laorden, D, Dominguez-Ortega, J, Carpio, C, Barranco, P, Villamanan, E, Romero, D, et al. Long COVID outcomes in an asthmatic cohort and its implications for asthma control. Respir Med. (2023) 207:107092. doi: 10.1016/j.rmed.2022.107092
90. Kanne, JP, Little, BP, Schulte, JJ, Haramati, A, and Haramati, LB. Long-term lung abnormalities associated with COVID-19 pneumonia. Radiology. (2023) 306:e221806. doi: 10.1148/radiol.221806
91. Lee, H, Kim, BG, Chung, SJ, Park, DW, Park, TS, Moon, JY, et al. New-onset asthma following COVID-19 in adults. J Allergy Clin Immunol Pract. (2023) 11:2228–31. doi: 10.1016/j.jaip.2023.03.050
92. Celik, E, Nelles, C, Kottlors, J, Fervers, P, Goertz, L, Pinto Dos Santos, D, et al. Quantitative determination of pulmonary emphysema in follow-up LD-CTs of patients with COVID-19 infection. PLoS One. (2022) 17:e0263261. doi: 10.1371/journal.pone.0263261
93. Porru, S, Monaco, MGL, Spiteri, G, Carta, A, Pezzani, MD, Lippi, G, et al. SARS-CoV-2 breakthrough infections: incidence and risk factors in a large European multicentric cohort of health workers. Vaccines (Basel). (2022) 10:1193. doi: 10.3390/vaccines10020272
94. Violan, C, Carrasco-Ribelles, LA, Collatuzzo, G, Ditano, G, Abedini, M, Janke, C, et al. Multimorbidity and serological response to SARS-CoV-2 nine months after 1st vaccine dose: European cohort of healthcare workers-Orchestra project. Vaccines (Basel). (2023) 11:1340. doi: 10.3390/vaccines11081340
Keywords: long COVID, Mendelian randomization, COVID-induced neurological disorders, COVID-induced fatigue, COVID-induced cardiac disease
Citation: Shenoy PU, Udupa H, KS J, Babu S, K N, Jain N, Das R and Upadhyai P (2023) The impact of COVID-19 on pulmonary, neurological, and cardiac outcomes: evidence from a Mendelian randomization study. Front. Public Health. 11:1303183. doi: 10.3389/fpubh.2023.1303183
Edited by:
Elisa Frullanti, University of Siena, ItalyReviewed by:
Filippo Liviero, University of Padua, ItalyJosé Pedro Elizalde Díaz, National Institute of Genomic Medicine (INMEGEN), Mexico
Copyright © 2023 Shenoy, Udupa, KS, Babu, K, Jain, Das and Upadhyai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranajit Das, das.ranajit@gmail.com; Priyanka Upadhyai, priyanka.u@manipal.edu
†These authors have contributed equally to this work and share last authorship
 Pooja U. Shenoy
Pooja U. Shenoy Hrushikesh Udupa
Hrushikesh Udupa Jyothika KS
Jyothika KS Sangeetha Babu
Sangeetha Babu Nikshita K3
Nikshita K3 Ranajit Das
Ranajit Das Priyanka Upadhyai
Priyanka Upadhyai