- 1The Department of Obstetrics and Gynecology, West China Second University Hospital of Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
Objective: To investigate the application value of different dose of HPV vaccine in young females.
Data sources: The following databases were searched: Cochrane Library, PubMed, Embase, Web of Science, SINOMED, and Wanfang Data, from the establishment of the database to August 1st, 2022.
Study eligibility criteria: The inclusion criterias were: healthy young women younger than 25 years old as the research object, randomized controlled study as the research type, and the efficacy and safety of single-dose, two-dose or three-dose HPV vaccines as the intervention measures and research endpoints.
Study appraisal and synthesis methods: Meta-analysis was performed to analyze the protective effects of single-dose, 2-dose and 3-dose HPV vaccine series on young females.
Results: A total of eight eligible studies involving 16 publications were included. There is no difference in the immunogenicity between the 2-dose and 3-dose series within 12 months after the last dose of HPV vaccine. However, 3-dose series was better than the 2-dose series, which performed better than the single-dose vaccine, after 12 months. With respect to the prevention of HPV16/18 infection or HPV31/33/45 infection, the single-dose vaccine worked better than 2-dose or 3-dose series.
Conclusions: The present study showed that the immunogenicity of low-dose HPV vaccine was significantly less, but it reduced the risk of high-risk HPV infection. The low-dose HPV vaccine series may not offer a preventive effect on cervical lesions, though it needs to be further confirmed by additional studies.
Highlights
- Different HPV vaccine dose for young females.
- Low-dose HPV vaccine may be perfect.
- The results is beneficial to promote the application of HPV vaccine.
1. Introduction
The Human Papillomavirus and Related Diseases Report, China, 2021 released by the Catalan Institute of Oncology (ICO) showed that there were nearly 110,000 new cases of cervical cancers each year, with nearly 60,000 deaths in China. The infection rate of the top 10 high-risk human papillomaviruses (HR-HPV) was as high as 97.2%, and a persistent infection of HR-HPV was the culprit behind the cervical cancer (1, 2). HPV vaccines have been widely used clinically. One hundred and ten countries and regions around the world have incorporated HPV vaccines into their national immunization programs until Oct 2020. Global HPV vaccine coverage is increasing year by year. In some countries, HPV vaccine coverage has reached 90% (2).
In 2018, the World Health Organization (WHO) called for the elimination of cervical cancer worldwide, requiring no <90% HPV vaccination rate of young girls under the age of 15. The widespread adoption of HPV vaccine has dramatically reduced the incidence of cervical lesions. A systematic review published by the Lancet in 2019 showed that after 5–9 years of HPV vaccine (bivalent and quadrivalent) in a total of 60 million people in 14 countries around the world, the incidence of cervical intraepithelial neoplasia (CIN) 2+ was significantly reduced by 51 and 31% in women aged from 15 to 19 and 20 to 24 years, respectively (3). However, at present, the insufficient supply of HPV vaccine and the economic burden of people in low-income countries that do not include HPV vaccine in the national immunization plan make the dose and effectiveness of HPV vaccine a new research hotspot. Studies by Bruni et al. (2) have shown that the single-dose HPV vaccine was found to be more feasible in the low- and middle-income countries and may achieve high coverage faster. A Cochrane systematic review has studied the efficacy and safety of different HPV vaccine formulations in various gender and age groups, and the results suggested a no significant difference in the vaccine efficacy between the 2-dose and 3-dose series in young girls (4). A systematic review of non-randomized controlled studies revealed no significant immunological difference between the 2-dose and 3-dose HPV vaccine series in high-income countries (5). Another systematic review of randomized controlled studies investigated the safety and efficacy of the single-dose HPV vaccine and found that it was as effective as that of the multiple doses in preventing HPV infection in healthy young females (6).
At present, there is a lack of high-quality systematic reviews on the effectiveness of the single-dose, 2-dose, and 3-dose HPV vaccines. In this pursuit, this study retrieved the data related to the published randomized controlled studies of HPV vaccines using various doses, and for the first time, systematically studied the preventive effect of the three HPV vaccine dose on cervical lesions in young females in terms clinical efficacy. Our study could serve as a data reference for promoting the broader adoption of HPV vaccine, and further improve its coverage.
2. Materials and methods
A pair-wise meta-analysis and network meta-analysis were performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (7, 8).
2.1. Literature search
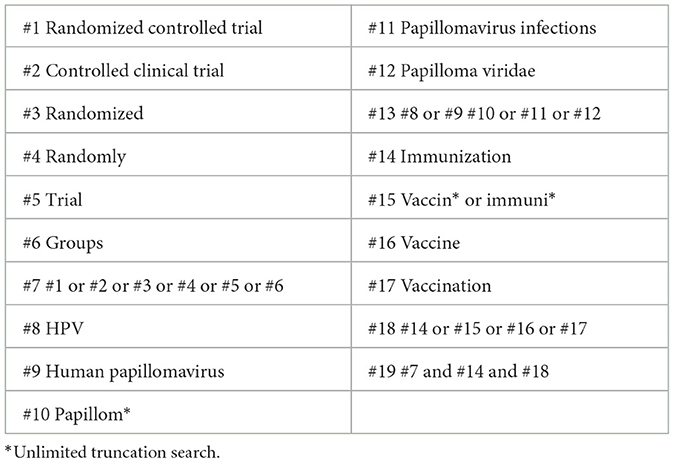
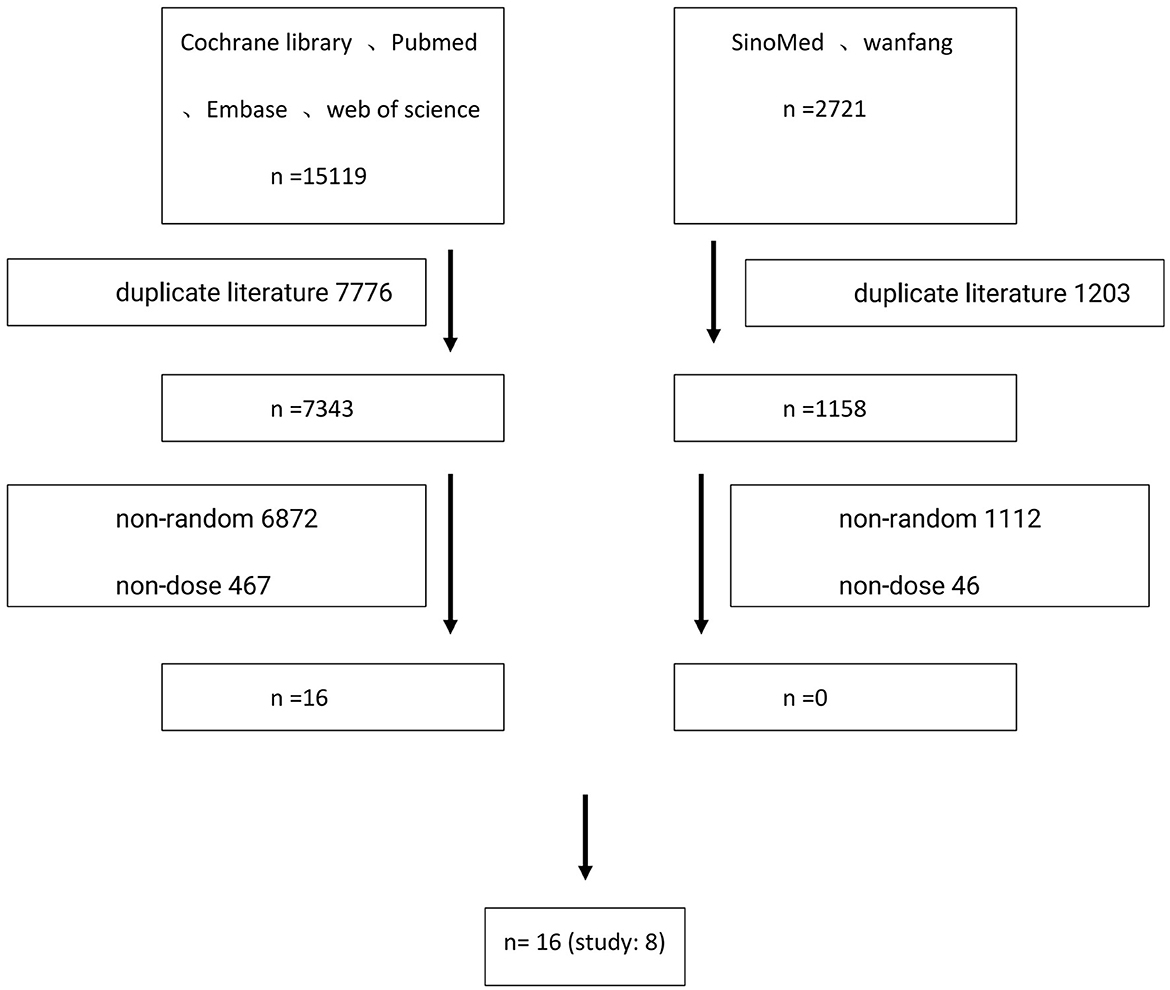
According to the search strategy using Medical Subject Headings (MeSH) terms, the following databases were searched: Cochrane Library, PubMed, Embase, Web of Science, SINOMED, and Wanfang Data, from the establishment of the database to August 1st, 2022, by two researchers independently. Any disagreement would be discussed and resolved between them. If not, a third methodology researcher was consulted to reach a final agreement. Search keywords included: HPV, human papillomavirus, vaccine, immunogenicity, randomized controlled studies, and cervical lesions. The detailed inspection strategy is listed in Table 1, and the literature screening process is shown in Figure 1.
2.2. Inclusion and exclusion criteria
The inclusion and exclusion criteria were set according to PICOS (patients, intervention, comparison, outcomes, and study design) principle. The inclusion criterias were: healthy young women younger than 25 years old as the research object, randomized controlled study as the research type, and the efficacy and safety of single-dose, two-dose, or three-dose HPV vaccines as the intervention measures and research endpoints. Efficacy included the immunogenicity and clinical efficacy in preventing HPV infection. Immunogenicity was defined as the geometric mean titers (GMTs) of HPV neutralizing antibodies. HPV vaccines were bivalent, quadrivalent, or non-avalent. The included studies were published studies in English or Chinese.
The study objects older than 25 years old or those in males were not included in this study. The subgroup analysis of randomized controlled trials comparing the efficacy and safety of HPV vaccines using HPV vaccines vs. placebo were not included in this study.
2.3. Data extraction
The two scholars extracted the data according to the designed data extraction table and then cross-checked the results. Disagreements were resolved through discussion or with the help of peer experts, if necessary. The data information included the basic characteristics such as study authors, time, study center, study type, intervention measures, study subjects, sample size, and age. It included efficacy and safety indicators such as the short-term immunogenicity within 12 months after the last dose of HPV vaccination, the long-term immunogenicity of over 12 months, clinical indicators of HPV infection prevention (infection rate of various HPV subtypes), toxicity and side effects. It also included the methodological indicators such as randomization plan, blinding plan, and research reports.
2.4. Risk of bias and quality assessment of included studies
The risk of bias and quality of included studies were assessed using the randomized controlled study quality assessment tool recommended by the Cochrane Collaboration (9). The evaluation indicators included the following seven items: (1) Random sequence generation (selection bias); (2) Allocation concealment (selection bias); (3) Blinding of participants and personnel (performance bias); (4) Blinding of outcome assessment (detection bias); (5) Incomplete outcome data (attrition bias); (6) Selective reporting (reporting bias); and (7) Other bias. The risk of bias and quality assessment was conducted item-by-item for each study that met the inclusion criteria.
2.5. Research endpoints
The research endpoints were immunogenicity and clinical efficacy in preventing the HPV infection. In terms of the immunogenicity of the HPV vaccine, the studies were divided into a short-term group within 12 months and a long-term group of over 12 months after the last dose of HPV vaccination. According to HPV genotypes, the studies were divided into HPV 16 and HPV 18. In terms of the clinical effect of preventing HPV infection, the patients were followed up for more than 1 year after HPV vaccination, and they were divided into HPV16/18 and HPV31/33/45 according to HPV subtypes.
2.6. Data analysis
The mean and standard deviation (M + SD) values of the geometric mean titers of HPV neutralizing antibodies were extracted in this study. If the reported results were mean and 95% confidence interval (M + 95% CI), the RevMan 5.4 tool was used to convert the results to M + SD format.
The ADDIS 1.16.8 software (Aggregate Data Drug Information System) was employed for meta-analysis. The odds ratio was used for enumeration data, while the mean difference was adopted for measurement data. First, the chi-square test was used to test the heterogeneity, and I2 was used to evaluate the heterogeneity. An I2 ≤ 50% was considered as small heterogeneity and the meta-analysis was considered to be feasible. I2 > 50% was considered as substantial heterogeneity. The cause of heterogeneity was analyzed, and meta typing was performed after excluding the clinical heterogeneity. When only two intervention measures were combined, a pair-wise meta-analysis (DerSimonian-Laird random effect meta-analysis) was used, and a Markov Chain Monte Carlo Network meta-analysis was conducted when three intervention measures were combined.
3. Research results
3.1. Basic characteristics of included studies
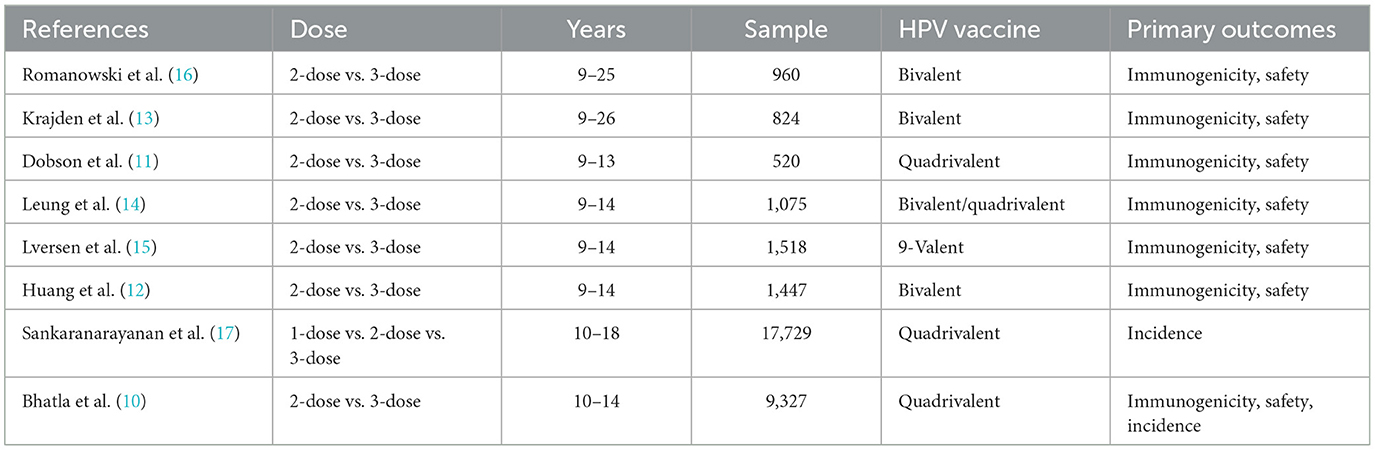
A total of 17,840 primary screening documents (15,119 in English and 2,721 in Chinese) were retrieved using the search strategy with MeSH terms. After removing the duplicate documents, non-randomized controlled studies and other documents that failed the inclusion criteria, a total of eight studies were included, involving 16 publications (10–25). A total of 32,423 young females aged in the range of 9–25 were included. Six studies reported the short-term immunogenicity of HPV16/18 subtypes (11–16), eight studies reported the long-term immunogenicity of HPV16/18 subtypes (10–17), while two studies reported the clinical efficacy of the HPV vaccine in preventing the HPV infection (10, 17). The basic characteristics of the included studies are shown in Table 2.
3.2. Risk of bias and quality assessment of included studies
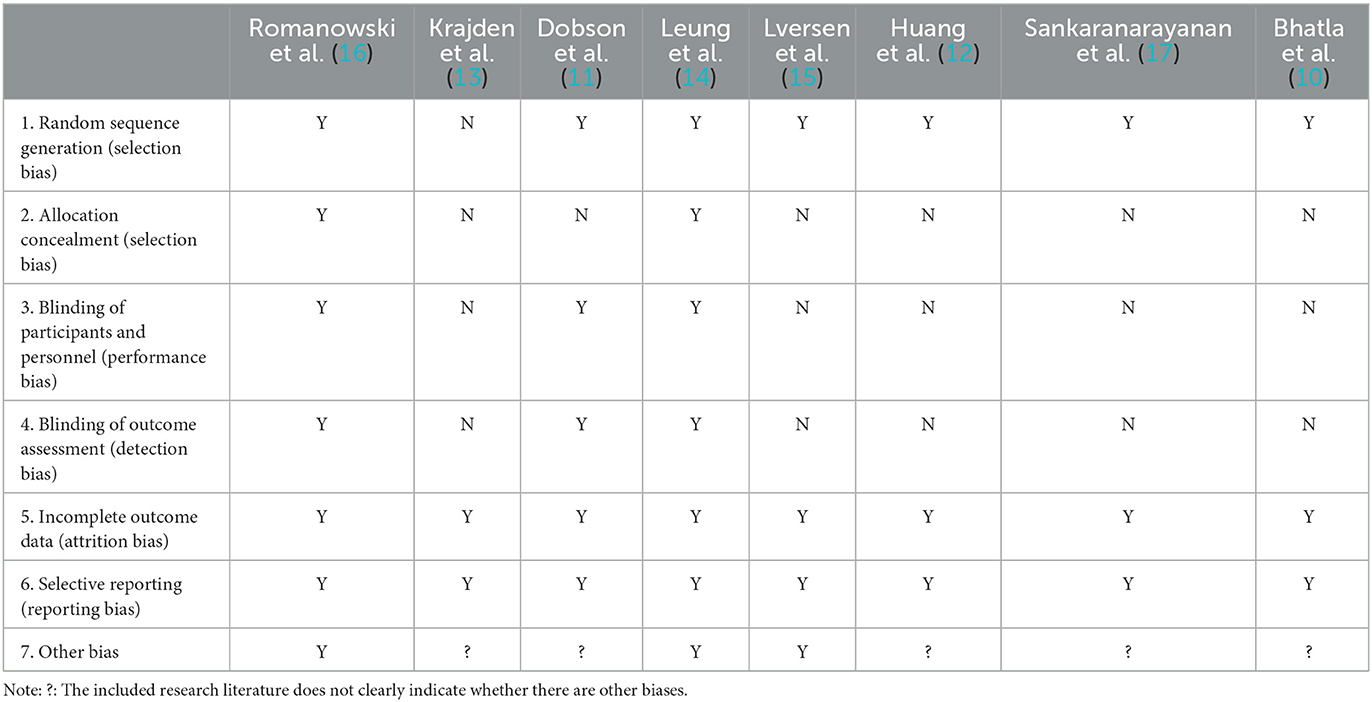
All the eight studies were randomized controlled studies, seven studies clearly stated the randomization design (10–12, 14–17), and three studies were blinded (11, 14, 16). All the eight studies were high-quality randomized controlled studies. The results of risk of bias and quality assessment of the included studies are shown in Table 3.
3.3. Short-term immunogenicity of different HPV vaccines
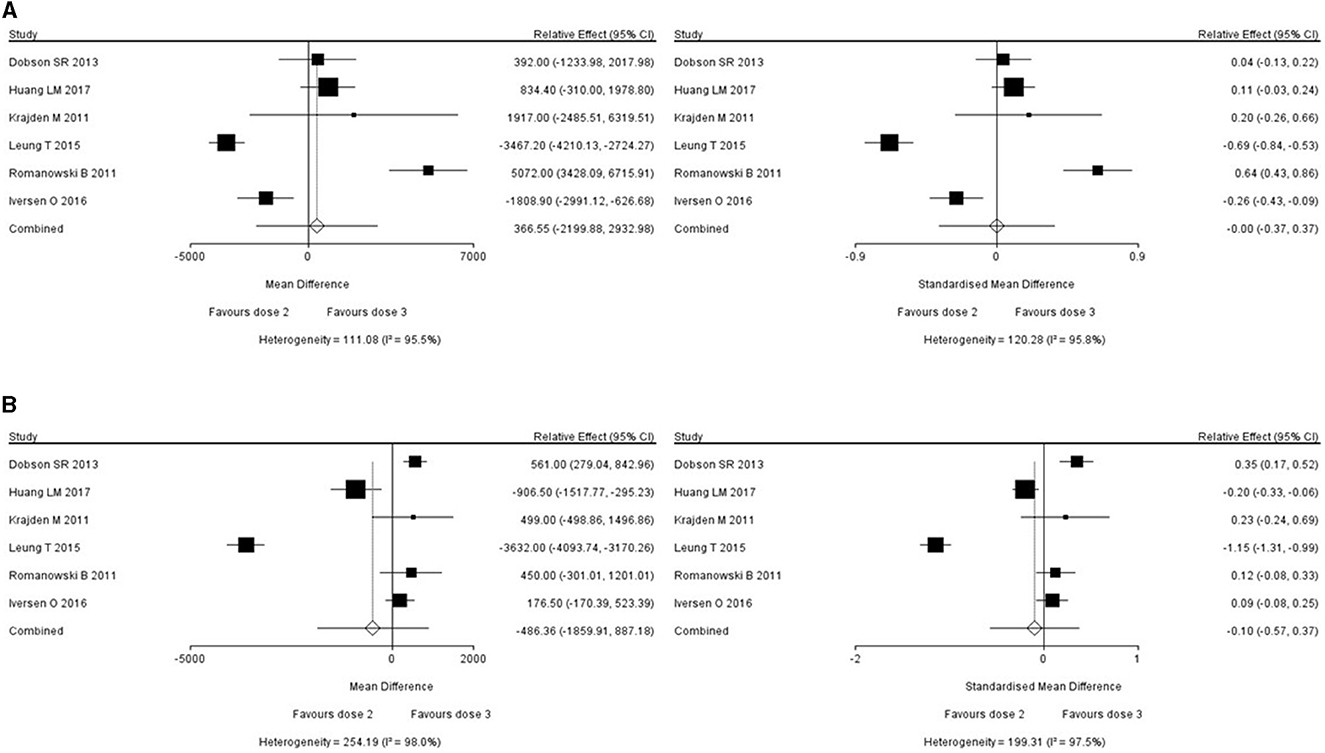
Six studies reported the short-term immunogenicity of HPV16/18 subtypes (11–16), all of which were studies comparing the efficacy and safety of 2- and 3-dose series. For studies with an I2 value of above 50% (which meant large heterogeneity), the reasons for the heterogeneity were investigated to exclude the clinical heterogeneity, and then the meta typing was performed. When combining two interventions, a pair-wise meta-analysis and DerSimonian-Laird Random effect meta-analysis was adopted. The relative effect and 95% CI of short-term immunogenicity of HPV16 and HPV18 were found to be 366.55 (−2199.88, 293.98) and −486.36 (−1859.91, 887.18). No statistically significant difference between the 2- and 3-dose series was observed in terms of short-term immunogenicity. The short-term immunogenicity of the two HPV vaccine formulations against HPV16 and HPV18 is shown in Figure 2.
3.4. Long-term immunogenicity of different HPV vaccines
Eight studies reported the long-term immunogenicity of HPV16/18 subtypes (10–17), seven of which compared the efficacy and safety of 2-dose with 3-dose series (10–16) and one study compared the efficacy and safety of single-, 2- and 3-dose HPV vaccines (17). When combining the three intervention measures, a Markov Chain Monte Carlo Network meta-analysis was adopted.
Consistency is good. Consistency model for HPV 16 shows the OR value and 95%CI of dose 1 vs. dose 2 is −4173.7(−10983.06, 19185.03), the OR value and 95%CI of dose 1 vs. dose 3 is −5333.4(−12425.87, 420.02), the OR value and 95%CI of dose 2 vs. dose 3 is −1159.37(−4060.16, 1344.65). Consistency model for HPV 18 shows the OR value and 95%CI of dose 1 vs. dose 2 is −778.46(−2631.63, 925.83), the OR value and 95%CI of dose 1 vs. dose 3 is −1040.01(−2986.96, 648.22), the OR value and 95%CI of dose 2 vs. dose 3 is −263.18(−1069.69, 398.60). Inconsistency model results show that the stability of the research results is good. Inconsistency model for HPV 16 shows the OR value and 95%CI of dose 1 vs. dose 2 is −422.68(−11051.02, 17542.04), the OR value and 95%CI of dose 1 vs. dose 3 is −5391.51(−12651.41, 501.02), the OR value and 95%CI of dose 2 vs. dose 3 is −1177.56(−4278.95, 1463.41). Inconsistency model for HPV 18 shows the OR value and 95%CI of dose 1 vs. dose 2 is −793.15(−12602.02, 928.05), the OR value and 95%CI of dose 1 vs. dose 3 is −1054.96(−2976.77, 600.55), the OR value and 95%CI of dose 2 vs. dose 3 is −259.44(−1042.52, 394.56).
Beyond 12 months of the last dose of vaccination, it was observed that the 3-dose series was better than that of 2-dose series, and 2-dose series was better than single-dose vaccine considering the immunogenicity against HPV16 and HPV18. The evidence map and ranking map of the network meta-analysis is shown in Figures 3, 4.
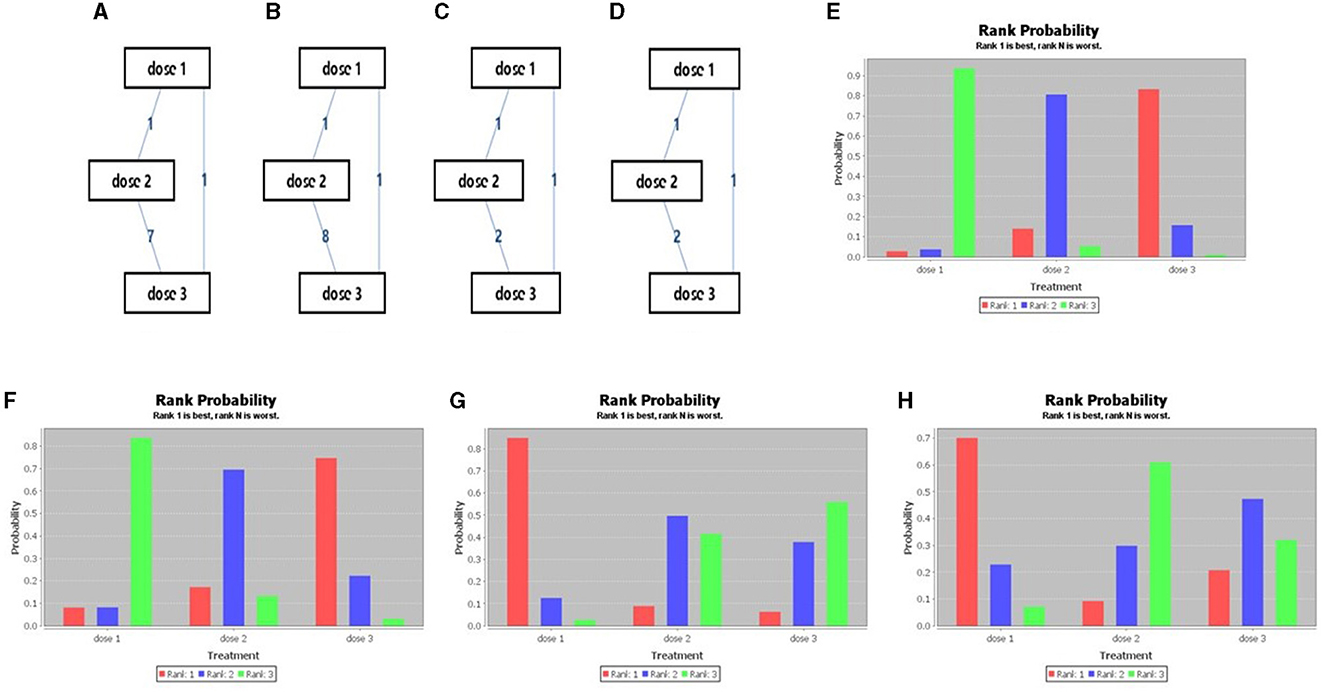
Figure 3. Network meta-analysis evidence network diagram (A: HPV 16, B: HPV 18, C: incidence of HPV 16/18, D: incidence of HPV 31/33/45) and Sequence of effects of HPV dosage forms (E: HPV 16, F: HPV 18, G: incidence of HPV 16/18, H: incidence of HPV 31/33/45).
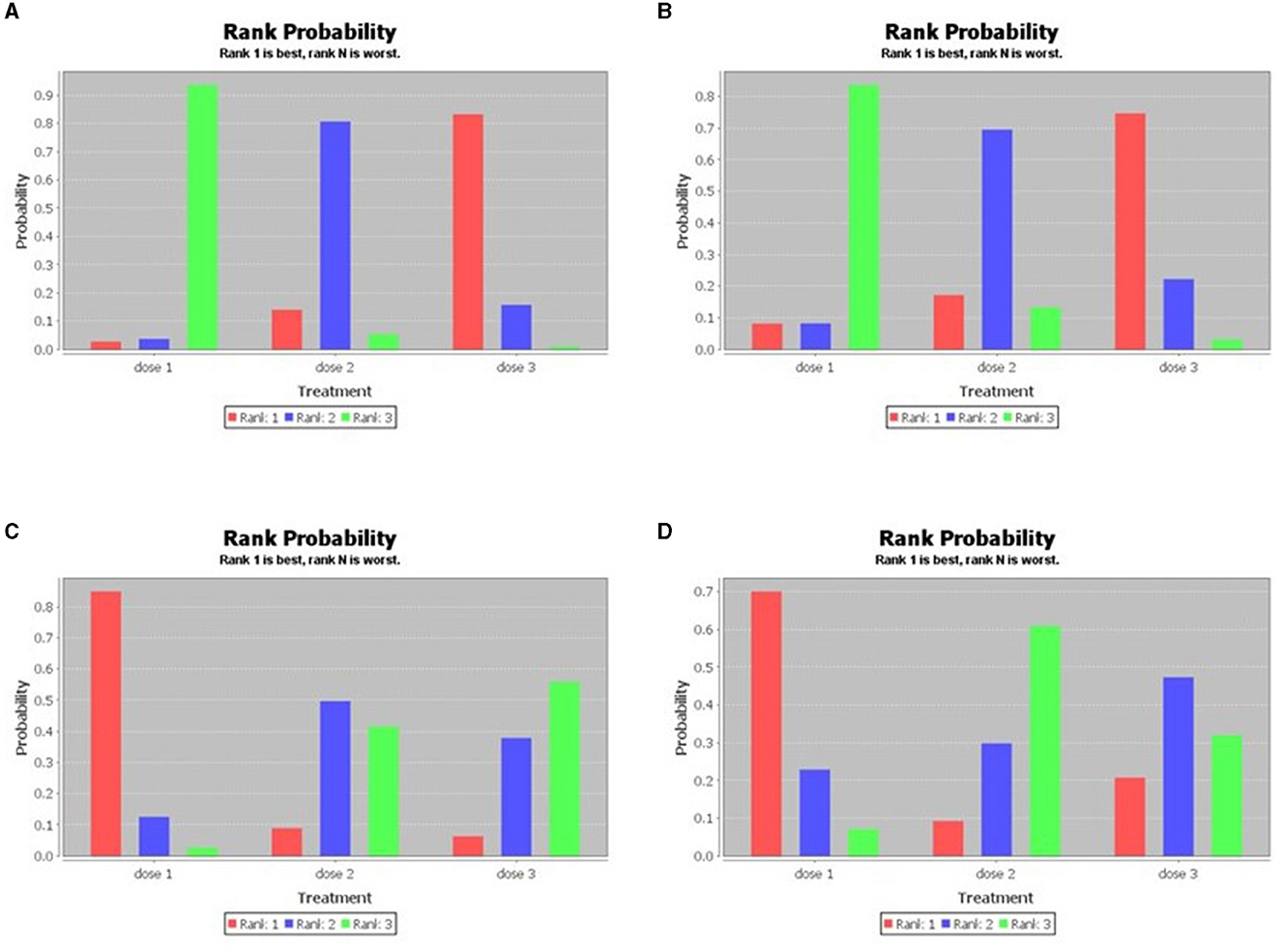
Figure 4. Sequence of effects of HPV dosage forms (the consistency test of the network meta-analysis; A: HPV 16, B: HPV 18, C: incidence of HPV 16/18, D: incidence of HPV 31/33/45).
3.5. Clinical effect of HPV vaccine in preventing HPV infection
Two studies reported the HPV infection rates after a minimum follow-up of 3 years after HPV vaccination. One study compared the effects of 2- and 3-dose series, and another study compared the effects of single-, 2-, and 3-dose series.
Consistency is good. Consistency model for HPV 16/18 shows the OR value and 95%CI of dose 1 vs. dose 2 is 1.75(0.72, 4.34), the OR value and 95%CI of dose 1 vs. dose 3 is 1.90(0.76, 4.88), the OR value and 95%CI of dose 2 vs. dose 3 is 1.08(0.41, 2.79). Consistency model for HPV31/33/45 shows the OR value and 95%CI of dose 1 vs. dose 2 is 1.25(0.82, 1.88), the OR value and 95%CI of dose 1 vs. dose 3 is 1.14(0.77, 1.74), the OR value and 95%CI of dose 2 vs. dose 3 is 0.92(0.62, 1.39). Inconsistency model results show that the stability of the research results is good. Inconsistency model for HPV 16/18 shows the OR value and 95%CI of dose 1 vs. dose 2 is 1.75(0.67, 4.92), the OR value and 95%CI of dose 1 vs. dose 3 is 2.03(0.83, 5.27), the OR value and 95%CI of dose 2 vs. dose 3 is 1.14(0.42, 3.14). Inconsistency model for HPV31/33/45 shows the OR value and 95%CI of dose 1 vs. dose 2 is 1.24(0.83, 1.87), the OR value and 95%CI of dose 1 vs. dose 3 is 1.15(0.78, 1.77), the OR value and 95%CI of dose 2 vs. dose 3 is 0.93(0.62, 1.40).
In the prevention of HPV16/18 infection, single-dose vaccine was superior to 2- and 3- dose series, and the 2-dose series was superior to 3-dose series. While in the prevention of HPV31/33/45 infection, the single-dose vaccine was superior to the 2- and 3-dose series, and the 3-dose series was superior to the 2-dose series. The evidence and ranking maps of the network meta-analysis is shown in Figures 3, 4.
4. Discussion
4.1. Main findings
The immunogenicity of low-dose HPV vaccine was significantly less, but it reduced the risk of high-risk HPV infection. The low-dose HPV vaccine series may not offer a preventive effect on cervical lesions, though it needs to be further confirmed by additional studies.
4.2. Strengths and limitations
The present study included randomized controlled studies involving young healthy females, large sized samples, and mostly international multi-center studies, in order to reduce the correlation bias due to confounding factors such as ethnicity, sample size, and age. The results of our study support the fact that the reduced doses of HPV vaccine may not affect the ability of the HPV in preventing the cervical lesions, and it is beneficial to promote the application of HPV vaccine, expanding the coverage of HPV vaccination, and achieving the goal of eliminating cervical cancer called by the WHO. However, the present study still presents some limitations. First, the heterogeneity between the studies was large. The follow-up time nodes of immunogenicity indicators and clinical indicators of HPV infection were different, and the sample sizes were different, thereby increasing the heterogeneity of the studies. Only one study compared three HPV vaccine formulations. The time points of HPV vaccination were different in different studies, increasing the unreliability of the study results. HPV Persistent Infection and CIN Incidence are Closely Related to Cervical Lesions. However, only two studies reported the HPV infection rates after a minimum follow-up of 3 years after HPV vaccination, there were no studies with persistent HPV infection or incidence of CIN as the end point. The present study did not investigate the effect of different doses of HPV vaccine on the incidence of cervical lesions, and to date, no related randomized controlled trials (RCT) studies have been reported. These factors may significantly affect the accuracy of the research conclusions. 2#, 4#, or 9# vaccines have different immune targets and use immune adjuvants, it may be not suitable for analyzed these three different vaccines combinedly. We attempted to perform a subgroup analysis by HPV vaccine type, because of data limitations, we finally had no choice but to give up. We hope that more research appears later, we will update this part of the problem.
4.3. Comparison with existing literature
Human papilloma virus (HPV) vaccination is an effective primary preventive measure against HPV infection and infection-related diseases (26). HPV vaccine induces a humoral immune response, and the produced neutralizing antibodies can bind to the virus antigen when HPV enters the body, thereby preventing the HPV infection. The occurrence and progression of cervical pre-cancer may be blocked by preventing the primary HPV infection and reducing the persistent HPV infection. The antibodies induced by the vaccine can penetrate the blood vessel wall and accumulate in the local epithelial tissue in a high concentration. When the HPV particles reaches the basal layer cells via a micro-wound in the mucosal epithelium, antibodies located in the epithelial tissue can bind to the viral particles and neutralize them. Studies at home and abroad have shown that after the completion of the whole course of immunization with bivalent, quadrivalent or nonavalent HPV vaccines, high rates of vaccine-related antibody seroconversion and serological antibody titers (96–100%) were observed (27–29). The results of the clinical studies in China showed that females aged 9–17 exhibited stronger immune responses after being vaccinated with bivalent and quadrivalent HPV vaccines, and their serological antibody titers were 1.42–3.00 times higher than those of females aged 18–26, whose antibody titers were similar to women aged 26–45 (30–32). A phase III clinical trial exhibited that the antibody titers of HPV (6/11/16/18) in females aged 16–17 after receiving the non-avalent HPV vaccine were higher than those aged 18–26, while their immune responses were comparable to those receiving the quadrivalent HPV vaccine (33).
Some studies have shown that by reducing the number of HPV vaccine doses may be beneficial for expanding the HPV vaccination coverage (2). Meta-analysis demonstrated that the immunogenicity of HPV vaccines of various dosage forms could be similar (5, 6). However, these studies were meta-analyses of non-randomized controlled studies or systematic reviews comparing only two dosage forms. There is currently a lack of quality and evidence-based investigation for the superiority of the three HPV vaccine formulations. The present study is the first to discuss the effectiveness of the single-dose, 2-dose, and 3-dose HPV vaccines in young females in a network meta-analysis format. We found no statistically significant difference between the 2- and 3-dose series was observed in terms of short-term immunogenicity, that is, the short-term immunogenicity of 2-dose is similar with 3-dose's short-term immunogenicity. Consistency is good, and inconsistency model results show that the stability of the research results is good in long-term immunogenicity of different HPV vaccines. Beyond 12 months after the last dose, the 3-dose series displayed the strongest immunogenicity, followed by the 2-dose series and the single-dose one, respectively.
HPV vaccines have exhibited 87.3–100.0% protective efficacy in the prevention of HPV type-related diseases in clinical trials. The quadrivalent HPV vaccine has a good protective effect in females aged 18–25. One study with 78-month follow-up for Chinese females aged 20–45 showed that the quadrivalent HPV vaccine provided 100% protection against HPV16/18-related CIN 2/3, adenocarcinoma in situ (AIS), and cervical cancer (29). The non-avalent HPV vaccine showed a comparable protective efficacy as that of the quadrivalent HPV vaccine against the HPV6/11/16/18-related persistent infection and cervical cancer in females aged 16–26 (32). The non-avalent HPV vaccine exhibited 100% protective efficacy against HPV 31/33/45/52/58-related CIN1+ in the subgroup of East Asian females aged 16–26, and showed 95.8% protective efficacy against the 6-month and above persistent HPV 31/33/45/52/58-related infections in cervix, vagina, vulva, and anus (34). However, the differences in the preventive effect of various HPV vaccine dose on HPV infection remains controversial. Few studies compared the prevention of HPV infection between the different dose of HPV vaccines. Consistency is good, and inconsistency model results show that the stability of the research results is good in clinical effect of HPV vaccine in preventing HPV infection. The present meta-analysis showed that the single-dose vaccine was the most effective in preventing HPV16/18 infection, followed by the 2-dose and the 3-dose series, respectively. In terms of preventing the HPV31/33/45 infection, the single-dose performed the best, followed by the 3-dose series, and the 2-dose series, respectively. The results of preventing the HPV infection were inconsistent to the immunogenicity results of HPV vaccine, and presently it is difficult to explain the vaccine mechanism. The reason may be that Antibody responses to a single dose of the HPV vaccine more closely resemble those to a live virus infection, which can persist indefinitely at a relatively steady-state level (35). Other antivirion antibody responses induced by viral infection or live viral vaccines, which present the same type of high density repetitive display of surface epitopes as HPV VLPs, have been shown to persist at essentially constant levels for many decades. Yet the sustained immunological responses observed among single-dose HPV-vaccinated participants were unexpected because it has not been observed with other subunit vaccines. For example, anti-Hepatitis B (HB) positivity in young adults (median age of 25 years) after single-dose HB vaccination was 4% and dropped to 0% after 2 years, with antibody levels below the threshold considered necessary for protection against HBV infection. However, the subviral particles that comprise the HBV vaccine may not be sufficiently “virus-like” to efficiently induce durable antibody responses. It may be also related to factors such as sample size, heterogeneity of studies, age, and ethnicity of the research subjects, and needs further clarification.
5. Conclusions and implications
In conclusion, although the low-dose series of the HPV vaccine showed no advantages in terms of immunogenicity, nevertheless they demonstrated clear advantages in preventing the high-risk HPV infection. It is proposed that the low-dose HPV vaccine may not show less protection from HPV. This vaccination strategy can further expand the HPV vaccination rate of young girls under the age of 15. It may have significant value for cervical cancer prevention in developing countries. However, this should be verified by systematically prospective large sample randomized controlled studies on the three formulations, and special attention should be paid to the effect of preventing the cervical lesions.
Author contributions
Conception: LK and YR. Design and final manuscript approval: All authors. Data collection and manuscript writing: LK and ZM. Data analysis: LK. All authors contributed to the article and approved the submitted version.
Funding
The Key Project of Sichuan Provincial Department of Science and Technology: Study on the key factors affecting the diagnosis and treatment of major diseases in obstetrics and gynecology (19ZDYF).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. ICO/IARC Information Centre. Human Papillomavirus and Related Diseases Report, China. ICO/IARC Information Centre (2021). Available online at: https://hpvcentre.net/ (accessed January 25, 2023).
2. Bruni L, Saura-Lázaro A, Montoliu A, Brotons M, Alemany L, Diallo MS, et al. HPV vaccination introduction worldwide and WHO and UNICEF estimates of national HPV immunization coverage 2010-2019. Prev Med. (2021) 144:106399. doi: 10.1016/j.ypmed.2020.106399
3. Drolet M, Bénard E, Pérez N, Brisson M, HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: updated systematic review and meta-analysis. Lancet. (2019) 394:497–509. doi: 10.1016/S0140-6736(19)30298-3
4. Bergman H, Buckley BS, Villanueva G, Petkovic J, Garritty C, Lutje V, et al. Comparison of different human papillomavirus (HPV) vaccine types and dose schedules for prevention of HPV-related disease in females and males. Cochrane Database Syst Rev. (2019) 2019:CD013479. doi: 10.1002/14651858.CD013479
5. D'Addario M, Redmond S, Scott P, Egli-Gany D, Riveros-Balta AX, Henao Restrepo AM, et al. Two-dose schedules for human papillomavirus vaccine: systematic review and meta-analysis. Vaccine. (2017) 35:2892–901. doi: 10.1016/j.vaccine.2017.03.096
6. Whitworth HS, Gallagher KE, Howard N, Mounier-Jack S, Mbwanji G, Kreimer AR, et al. Efficacy and immunogenicity of a single dose of human papillomavirus vaccine compared to no vaccination or standard three and two-dose vaccination regimens: a systematic review of evidence from clinical trials. Vaccine. (2020) 38:1302–14. doi: 10.1016/j.vaccine.2019.12.017
7. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
8. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
9. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
10. Bhatla N, Nene BM, Joshi S, Esmy PO, Poli URR, Joshi G, et al. Are two doses of human papillomavirus vaccine sufficient for girls aged 15-18 years? Results from a cohort study in India. Papillomavirus Res. (2018) 5:163–71. doi: 10.1016/j.pvr.2018.03.008
11. Dobson SRM, McNeil S, Dionne M, Dawar M, Ogilvie G, Krajden M, et al. Immunogenicity of 2 doses of HPV vaccine in younger adolescents vs. 3 doses in young women: a randomized clinical trial. JAMA. (2013) 309:1793–802. doi: 10.1001/jama.2013.1625
12. Huang L-M, Puthanakit T, Cheng-Hsun C, Ren-Bin T, Schwarz T, Pellegrino A, et al. Sustained immunogenicity of 2-dose human papillomavirus 16/18 AS04-adjuvanted vaccine schedules in girls aged 9-14 years: a randomized trial. J Infect Dis. (2017) 215:1711–9. doi: 10.1093/infdis/jix154
13. Krajden M, Cook D, Yu A, Chow R, Mei W, McNeil S, et al. Human papillomavirus 16 (HPV 16) and HPV 18 antibody responses measured by pseudovirus neutralization and competitive Luminex assays in a two- versus three-dose HPV vaccine trial. Clin Vaccine Immunol. (2011) 18:418–23. doi: 10.1128/CVI.00489-10
14. Leung TF, Liu AP-Y, Lim FS, Thollot F, Oh HML, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and HPV-6/11/16/18 vaccine administered according to 2- and 3-dose schedules in girls aged 9-14 years: results to month 12 from a randomized trial. Hum Vaccin Immunother. (2015) 11:1689–702. doi: 10.1080/21645515.2015.1050570
15. Iversen O-E, Miranda MJ, Ulied A, Soerdal T, Lazarus E, Chokephaibulkit K, et al. Immunogenicity of the 9-valent HPV vaccine using 2-dose regimens in girls and boys vs a 3-dose regimen in women. JAMA. (2016) 316:2411–21. doi: 10.1001/jama.2016.17615
16. Romanowski B, Schwarz TF, Ferguson LM, Peters K, Dionne M, Schulze K, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose schedule compared with the licensed 3-dose schedule: results from a randomized study. Hum Vaccin. (2011) 7:1374–86. doi: 10.4161/hv.7.12.18322
17. Sankaranarayanan R, Smita Joshi S, Muwonge R, Esmy PO, Basu P, Prabhu P, et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. (2018) 36(32 Pt A):4783–91. doi: 10.1016/j.vaccine.2018.02.087
18. Puthanakit T, Huang L-M, Chiu C-H, Tang R-B, Schwarz TF, Esposito S, et al. Randomized open trial comparing 2-dose regimens of the human papillomavirus 16/18 AS04-adjuvanted vaccine in girls aged 9-14 years versus a 3-dose regimen in women aged 15-25 years. J Infect Dis. (2016) 214:525–36. doi: 10.1093/infdis/jiw036
19. Ogilvie G, Sauvageau C, Dionne M, McNeil S, Krajden M, Money D, et al. Immunogenicity of 2 vs 3 doses of the quadrivalent human papillomavirus vaccine in girls aged 9 to 13 years after 60 months. JAMA. (2017) 317:1687–8. doi: 10.1001/jama.2017.1840
20. Donken R, Dobson SRM, Marty KD, Cook D, Sauvageau C, Gilca V, et al. Immunogenicity of 2 and 3 doses of the quadrivalent human papillomavirus vaccine up to 120 months postvaccination: follow-up of a randomized clinical trial. Clin Infect Dis. (2020) 71:1022–9. doi: 10.1093/cid/ciz887
21. Krajden M, Cook D, Yu A, Chow R, Su Q, Mei W, et al. Assessment of HPV 16 and HPV 18 antibody responses by pseudovirus neutralization, Merck cLIA and Merck total IgG LIA immunoassays in a reduced dosage quadrivalent HPV vaccine trial. Vaccine. (2014) 32:624–30. doi: 10.1016/j.vaccine.2013.09.007
22. Leung TF, Liu AP-Y, Lim FS, Thollot F, Oh HML, Lee BW, et al. Comparative immunogenicity and safety of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine and 4vHPV vaccine administered according to two- or three-dose schedules in girls aged 9-14 years: results to month 36 from a randomized trial. Vaccine. (2018) 36:98–106. doi: 10.1016/j.vaccine.2017.11.034
23. Romanowski B, Schwarz TF, Ferguson LM, Ferguson M, Peters K, Dionne M, et al. Immune response to the HPV-16/18 AS04-adjuvanted vaccine administered as a 2-dose or 3-dose schedule up to 4 years after vaccination: results from a randomized study. Hum Vaccin Immunother. (2014) 10:1155–65. doi: 10.4161/hv.28022
24. Romanowski B, Schwarz TF, Ferguson L, Peters K, Dionne M, Behre U, et al. Sustained immunogenicity of the HPV-16/18 AS04-adjuvanted vaccine administered as a two-dose schedule in adolescent girls: Five-year clinical data and modeling predictions from a randomized study. Hum Vaccin Immunother. (2016) 12:20–9. doi: 10.1080/21645515.2015.1065363
25. Sankaranarayanan R, Prabhu PR, Pawlita M, Gheit T, Bhatla N, Muwonge R, et al. Immunogenicity and HPV infection after one, two, and three doses of quadrivalent HPV vaccine in girls in India: a multicentre prospective cohort study. Lancet Oncol. (2016) 17:67–77. doi: 10.1016/S1470-2045(15)00414-3
26. Zou M, Liu H, Liu H, Wang M, Zou Z, Zhang L. Chinese expert consensus on clinical application of human papilloma virus vaccine. Prog Obstet Gynecol. (2021) 30:81–91.
27. Colombara DV, Wang SM. The impact of HPV vaccination delays in China: lessons from HBV control programs. Vaccine. (2013) 31:4057–9. doi: 10.1016/j.vaccine.2013.06.031
28. Zeng Z, Yang H, Li Z, He X, Griffith CC, Chen X, et al. Prevalence and genotype distribution of HPV infection in China: analysis of 51,345 HPV genotyping results from China's largest CAP certified laboratory. J Cancer. (2016) 7:1037–43. doi: 10.7150/jca.14971
29. Wei L, Xie X, Liu J, Zhao Y, Chen W, Zhao C, et al. Efficacy of quadrivalent human papill omavirus vaccine against persistent infection and genital disease in Chinese women: a randomized, placebo-controlled trial with 78-month follow-up. Vaccine. (2019) 37:3617–24. doi: 10.1016/j.vaccine.2018.08.009
30. Zhu F, Li J, Hu Y, Zhang X, Yang X, Zhao H, et al. Immunogenicity and safety of the HPV-16/18 AS04-adjuvanted vaccine in healthy Chinese girls and women aged 9 to 45 years. Hum Vaccin Immunother. (2014) 10:1795–806. doi: 10.4161/hv.28702
31. Li R, Li Y, Radley D, Liu Y, Huang T, Sings HL, et al. Safety and immunogenicity of a vaccine targeting human papillomavirus types 6, 11, 16 and 18: a randomized, double-blind, placebo-controlled trial in Chinese males and females. Vaccine. (2012) 30:4284–91. doi: 10.1016/j.vaccine.2012.02.079
32. Hu Y-M, Guo M, Li C-G, Chu K, He W-G, Zhang J, et al. Immunogenicity noninferiority study of 2 doses and 3 doses of an Escherichia coli-produced HPV bivalent vaccine in girls vs. 3 doses in young women. Sci China Life Sci. (2020) 63:582–91. doi: 10.1007/s11427-019-9547-7
33. Van Damme P, Meijer CJLM, Kieninger D, Schuyleman A, Thomas S, Luxembourg A, et al. A phase III clinical study to compare the immunogenicity and safety of the 9-valent and quadrivalent HPV vaccines in men. Vaccine. (2016) 34:4205–12. doi: 10.1016/j.vaccine.2016.06.056
34. Garland SM, Pitisuttithum P, Ngan HYS, Cho CH, Lee CY, Chen CA, et al. Efficacy, immunogenicity, and safety of a 9-valent human papillomavirus vaccine: subgroup analysis of participants from Asian countries. J Infect Dis. (2018) 218:95–108. doi: 10.1093/infdis/jiy133
Keywords: HPV vaccine, different dosage series, randomized controlled study, meta-analysis, network
Citation: Kemin L, Mengpei Z, Jing Z and Rutie Y (2023) Different dose series of human papillomavirus vaccine in young females: a pair-wise meta-analysis and network meta-analysis from randomized controlled trials. Front. Public Health 11:1152057. doi: 10.3389/fpubh.2023.1152057
Received: 27 January 2023; Accepted: 29 August 2023;
Published: 21 September 2023.
Edited by:
Rogelio González-González, Juárez University of the State of Durango, MexicoReviewed by:
Manuela Tamburro, University of Molise, ItalyRupsa Basu, Humane Genomics, United States
Copyright © 2023 Kemin, Mengpei, Jing and Rutie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Rutie, yrtt2013@163.com
 Li Kemin
Li Kemin Zhang Mengpei1,2
Zhang Mengpei1,2 Yin Rutie
Yin Rutie