- 1Department of Clinical Tropical Medicine, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- 2Division of Ambulatory Medicine, Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 3Department of Tropical Hygiene, Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand
- 4Department of Medicine, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 5Department of Microbiology, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 6Department of Pediatrics, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
- 7Siriraj Institute of Clinical Research, Faculty of Medicine Siriraj Hospital, Mahidol University, Bangkok, Thailand
Introduction: This study aims to assess the economic impact of introducing the 13-valent pneumococcal conjugate vaccine (PCV13) and 23-valent pneumococcal polysaccharide vaccine (PPSV23) to Thai older adult aged ≥ 65 years who are healthy or with chronic health conditions and immunocompromised conditions from a societal perspective in order to introduce the vaccine to Thailand’s National Immunization Program for the older adult.
Methods: A Markov model was adopted to simulate the natural history and economic outcomes of invasive pneumococcal diseases using updated published sources and Thai databases. We reported analyses as incremental cost-effectiveness ratios (ICER) in USD per quality-adjusted life year (QALY) gained. In addition, sensitivity analyses and budget impact analyses were conducted.
Results: The base-case analysis of all interventions (no vaccinations [current standard of care in Thailand], PPSV23, and PCV13) showed that PPSV23 was extendedly dominated by PCV13. Among healthy individuals or those with chronic health conditions, ICER for PCV13 was 233.63 USD/QALY; meanwhile, among individuals with immunocompromised conditions, ICER for PCV13 was 627.24 USD/QALY. PCV13 are economical vaccine for all older adult Thai individuals when compared to all interventions.
Conclusions: In the context of Thailand, PCV13 is recommended as the best buy and should be primarily prioritized when both costs and benefits are considered. Also, this model will be beneficial to the two-next generation pneumococcal vaccines implementation in Thailand.
1. Introduction
Two next-generation higher valency pneumococcal conjugate vaccines (15-valent or 20-valent pneumococcal conjugate vaccines, PCV15 or PCV20) are expected to be implemented for adult use in high-income countries in the near future before pediatric licensing (1). However, Thailand has not yet to incorporate any pneumococcal vaccines into the National Immunization Program (NIP) as of 2022 (2). There is strong evidence that pneumococcal vaccines reduce the incidence of invasive pneumococcal diseases (IPDs) or pneumococcal diseases (3). IPDs afflicted not only young children but also the older adult. In Thailand, the incidence of IPDs among older adult was 26 per 100,000 individuals (4), and the hospitalization rate was 30.5 per 100,000 person-years (5). Total costs by age groups for all types of pneumococcal diseases ranged from 680.7 million USD to 1,346.7 million USD in 2009 with the highest cost among patients aged 75–84 years (6). While, Thai children would lose 453,401 years of life and 457,598 productivity-adjusted life years (PALYs) which were equivalent to 5,586 USD million to pneumococcal diseases (7). Introducing a pneumococcal vaccine is an effective strategy for reducing disease-burden nasopharyngeal carriage and generating an indirect herd effect in the community.
In recent years, there has been much discussion about including pneumococcal vaccines in Thai NIP. This is because policymakers must weigh the costs and benefits of vaccination. The major considerations before vaccine implementation are that current economic evaluations are limited to pediatric populations and yield conflicting results. Although studies among children from the Philippines and Singapore, demonstrated that implementing pneumococcal vaccine in the health system was cost-effective compared with no vaccination program (8, 9), several studies from Thailand focused on children and yielded conflicting results. Kulpeng et al. (10) demonstrated that in 2013, the 10-valent pneumococcal polysaccharide nontypeable Haemophilus influenzae protein D-conjugated vaccine (PHiD-CV) and PCV13 were not cost-effective when compared with no vaccine due to their high costs. In 2019, Dilokthornsakul et al. (11) incorporated herd immunity effects and showed that both vaccines were considered cost-effective among Thai children. However, economic studies focusing on Thai older adult are scarce.
According to some economic studies, either PCV13 or the 23-valent pneumococcal polysaccharide vaccine (PPSV23) is cost-effective among the older adult. Igarashi et al. (12) performed a cost-effectiveness analysis of PCV13 and PPSV23 in older adult aged ≥ 60 years in a Japanese health care setting. The study showed that PCV13 was more cost-effective than PPSV23 (12). Meanwhile, the study conducted in Denmark suggested that implementing a PPSV23 vaccination program for all older adult aged ≥ 65 years is cost-effective (13). Even though there are many economic studies on the older adult from various countries, the generalizability of the results should be considered due to each country’s diverse backgrounds such as pneumococcal serotype distributions and vaccine support from non-profit organization.
Adult pneumococcal vaccine policies vary across countries as a result of epidemiological changes in the disease burden caused by the indirect effect of child vaccination programs. Currently, the Thai older adult have two options for pneumococcal vaccine: (1) PCV13 and (2) PPSV23. The (14) suggests older adult immunization schedules, either PCV13 or PPSV23, among older adult aged ≥ 65 years with or without chronic health conditions or immunocompromised conditions. Meanwhile, the Pediatric Infectious Disease Society of Thailand (15) recommend either PCV10 or PCV13 as an optional vaccine for healthy children aged 2, 4, and 12–15 months. Even though two pneumococcal vaccines are available in Thailand, disease burden remains high while vaccine uptake rate is low (16). All Thai individuals need to afford the out-of-pocket costs of pneumococcal vaccines regardless of their immune status as the vaccines are not listed in NIP. The cost of vaccines is the significant barrier to national implementation in Thailand; therefore, negotiations with manufacturers is encouraged. Using available local data, this study aimed to perform an economic evaluation of both PCV13 and PPSV23 compared with no vaccine and each other among Thai older adult aged ≥ 65 years in order to introduce the vaccine to Thailand’s NIP for the older adult.
2. Methods
We conducted a cost-effectiveness analysis to determine whether the benefits derived from the different vaccine interventions [no vaccinations (current standard of care in Thailand), PPSV23, and PCV13], measured as quality-adjusted life year (QALY) gained, offered value for money. We applied a decision rule for cost-effectiveness analysis as mentioned in Karlsson et al. (17) to compare the cost-effectiveness of interventions and excluded dominated vaccines from the analyses. If a vaccine was less effective and more costly than other interventions or the incremental cost-effectiveness ratio (ICER) was higher when compared to the next more effective vaccine will be dominated (known as extended dominance). This study defined the older adult population as those aged 65 years and older. Currently, Thailand’s NIP does not include pneumococcal vaccination for any age group. We classified targeted populations into two groups based on pneumococcal vaccine recommendations (18): (1) healthy individuals and/or those with chronic health conditions (i.e., chronic heart, lung, or liver diseases) and (2) individuals with immunocompromised conditions (i.e., HIV infection, lymphoma, or generalized malignancy). The information regarding chronic and immunocompromised conditions was defined by Matanock et al. (18). The lifetime horizon was selected for the analysis because some cases of IPDs have long-term effects, such as hearing loss or neurofunctional impairment after meningitis. The annual discount rate of 3% was applied to both costs and outcomes. We presented an ICER in United State dollars (USD) per QALY gained from the perspective of Thai society.
2.1. Model overview
Based on disease characteristics, vaccine effectiveness and previous literature (19, 20), this study developed a Markov model. The model’s cycle length was set to 1 year. The virtual cohort was followed on an annual basis until death. We assumed that all individuals who were not infected would have the same life expectancy as the general Thai population and all transition states were mutually exclusive (19). Once he or she had pneumococcal infection, the mortality rate would change depending on the condition. Our model took into account four conditions/consequences following a pneumococcal infection: (1) meningitis, (2) bacteremia without pneumonia, (3) bacteremia with pneumonia, and (4) pneumococcal pneumonia. Disseminated infection and adverse events associated with PCV13 and PPSV23 vaccination were not considered because they were of rare condition and mild or moderate severity, respectively (21, 22).
Our Markov model has seven health states that are mutually exclusive (Figure 1). All virtual patients would enter the model in the non-infection state (State A: older adult aged ≥ 65 years without infection). They could then either (1) become infected with pneumococcal bacteria and progress to one of the four health states (States B–E) that represent the conditions/results of pneumococcal bacterial infection, (2) remain in the same non-infection health state (State A), or (3) die (State G). The model allowed for some patients to experience complications following the infection (State F), whereas others would fully recover and return to the non-infection state. The complications were permanent conditions such as hearing loss, neurofunctional impairment following meningitis, and chronic lung diseases after pneumonia. The arrows in Figure 1 depict transitions between health states and staying in the same states. Transitions from a non-infection health state to a pneumococcal disease state were determined by the subjects’ health status, the incidence of each consequence and the effect of vaccination.
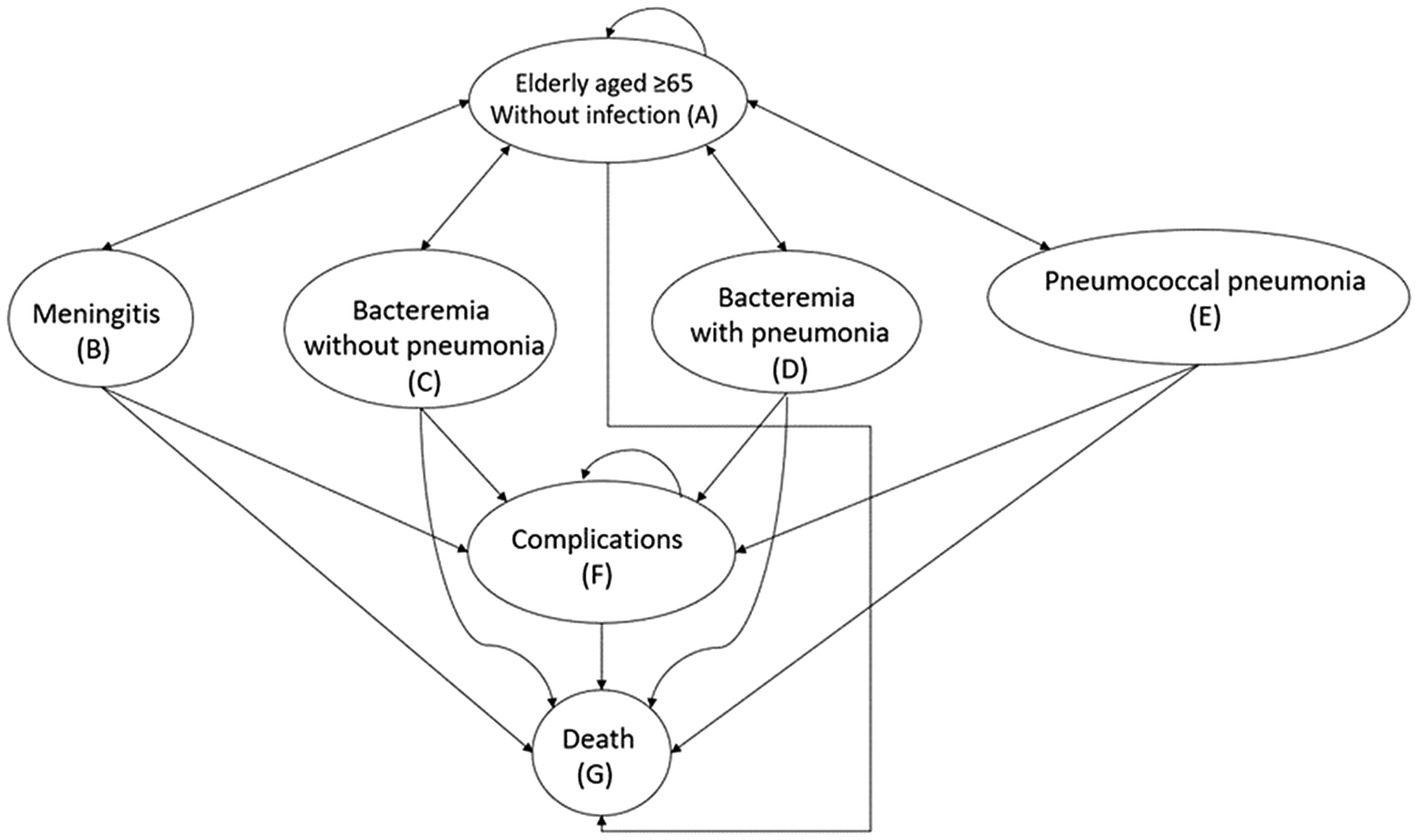
Figure 1. Markov model for pneumococcal vaccination and infection. Health states are in oval shapes. Transitions between states or remaining in the same state are represented by arrows.
2.2. Model input parameters
2.2.1. Epidemiology data
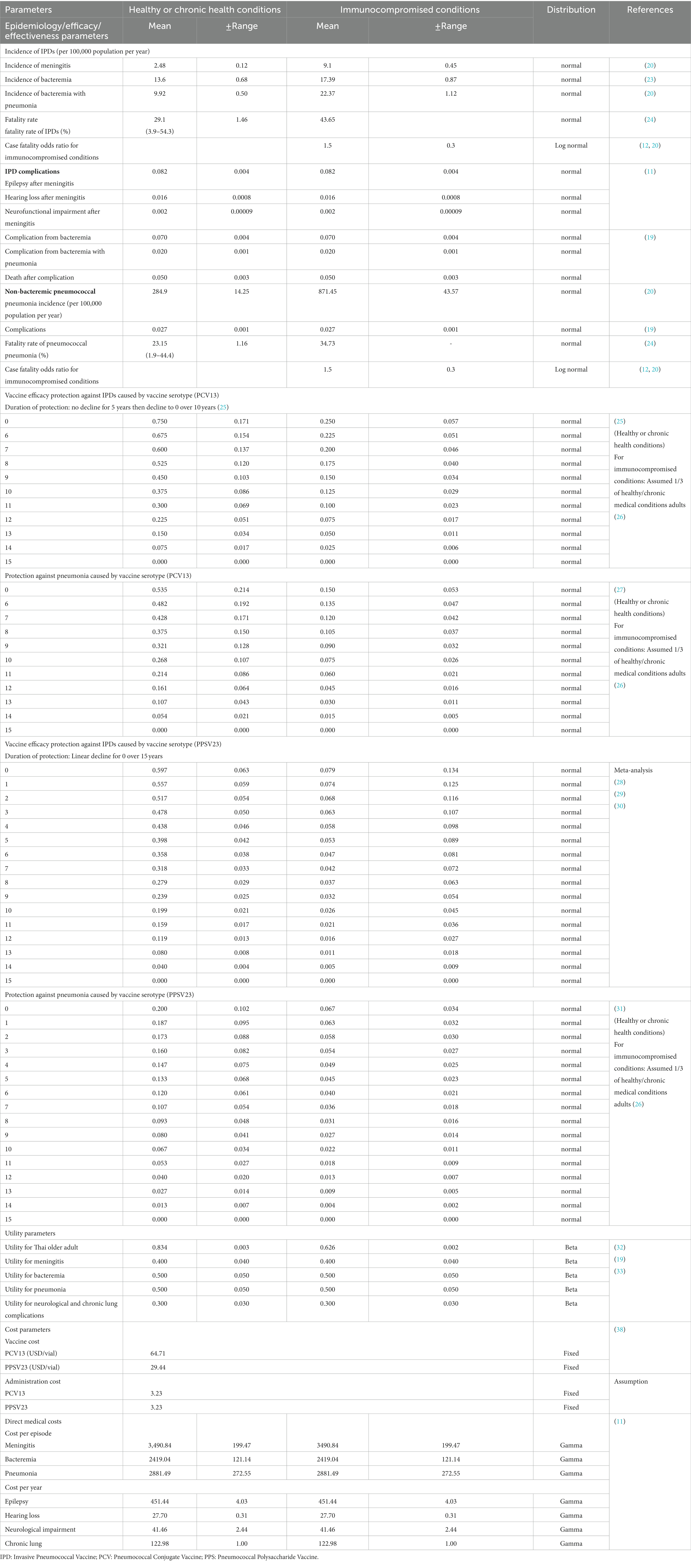
The input variables were obtained from published literature (Table 1). Meningitis, bacteremia without pneumonia, and bacteremia with pneumonia incidence rates were calculated separately based on the incidence of IPDs among Thai individuals with and without chronic health conditions. The rates were derived from a population-based survey in Sa Kaeo and Nakhon Phanom provinces (located in Northeast of Thailand) (4). Incidence of IPDs among individuals with immunocompromised conditions was derived from Smith et al. (20) using data collected in the United States prior to the 2010 introduction of the pneumococcal vaccine. In our model, we assumed that three IPD consequences were mutually exclusive and that the probabilities of transitioning from a non-infected state to meningitis, bacteremia without pneumonia, and pneumococcal bacteremia were proportional. The ratio was derived from previously published articles (20) that report the age-specific value of individuals stratified by their medical conditions. Case fatality rate were derived using data from Asia-Pacific countries (24) and assumed with an odds ratio for immunocompromised conditions between 1.3 and 1.8. This similar approach was used in the previous published economic analysis (12, 20). The proportion of IPD outcomes and incidence of pneumococcal disease complications were assumed to be equal for infected healthy individuals, those with chronic health conditions and those with immunocompromised conditions. We extracted the data from Smith et al. (20) and Hoshi et al. (19) as there was evidence that incidence rates of pneumococcal pneumonia without bacteremia and its complications (chronic lung diseases following infection) were higher in immunocompromised conditions than in healthy older adult. The incidence of adverse events was excluded for both PCV13 and PPSV23 because the majority was minimal, non-serious and indifferent from placebo (25, 34, 35).
2.2.2. Vaccine efficacy/effectiveness
We adopted the vaccine effectiveness model based on the Centers for Disease Control and Prevention (CDC) model, which was updated in the Advisory Committee on Immunization Practices meeting 2021 (36). According to various resources, the CDC model included both clinical trial phases 3 and 4. Therefore, either vaccine efficacy or effectiveness was present in the input parameters. The duration of PCV13 protection is 15 years, with no decline for the first 5 years, but declines linearly to 0 after 10 years. However, the protection duration of PPSV23 has been decreasing linearly since the first year, and its protective effect will be zero in 15 years. Because pneumococcal vaccine has not yet been implemented in Thailand’s NIP, the indirect effect of vaccine was not considered in our model.
2.2.3. Utilities
The utility of Thai healthy individuals and those with chronic health conditions in the absence of infection was based on the average utility of Thai adults aged 45–70 years. We estimated the utility for immunocompromised older adult without infection proportionally based on the utility ratio of populations at high and average risk in the same age range (20). In the absence of local data regarding the utility of adults with infection, we based our model on the National Health Interview Survey data from 1990 to 2000 from the resident civilian non-institutionalized population of the United States. The utility for episode of infection was assumed to be equivalent in both scenarios.
2.2.4. Costs
Because the model was developed from a societal standpoint, direct medical and non-medical costs were included. We exclude indirect costs because we assumed that lost or impaired ability to work or engage in leisure activities due to morbidity would be accounted for in the disutility of QALY (37). PCV13 and PPSV23 prices were obtained from the Drug and Medical Supply Information Center (38). The cost of vaccination acquisition and wastage was obtained from a Thailand’s survey study (39) (in Thai). The cost of vaccine administration was 3.23 USD which was referred to the standard cost list for health technology assessment and adjusted with consumer price index (40). We refer to a published cost-effectiveness study using the NHSO database (2011–2016) (11) to estimate age-specific treatment costs for bacteremia, meningitis, pneumonia and complications. We anticipated that all four infection conditions would necessitate hospitalization. Direct non-medical costs, such as transportation, meals, accommodation, and facilities, were derived from a previous published cost-effectiveness study (11). The cost per episode for healthy or chronic health conditions was assumed to be the same as the cost for immunocompromised conditions. The average of meningitis and pneumonia complications was used to estimate bacteremia complications. We applied consumer price index to convert the value of all past costs to the value of 2021. All costs were converted to USD using the official exchange rate of the world bank of Thai Baht (THB) 31.98 = 1 USD.
2.3. Base-case analysis
The incremental costs, life year gained, QALYs gained, and ICER were the study outcomes. For the base-case analysis, the expected lifetime costs and health-related outcomes of all interventions (no vaccinations, PPSV23, and PCV13) were presented and selected as comparators with the least lifetime costs and outcomes.
In our study, two scenarios were categorized by the target population of the vaccination program. The first scenario was based on healthy older adult with or without chronic health conditions. The latter study examined the cost-effectiveness of vaccines in immunocompromised individuals. Each scenario featured two pairs of comparisons. The willingness-to-pay (WTP) threshold of 5,003 USD/QALY gained according to the Thai Health Economic Working Group for drug listing in the National List of Essential Medicines in 2012 (41) determined the results of the cost-effectiveness analysis.
2.4. Sensitivity analyses
We ran sensitivity analyses to determine how ICER would change as a result of parameter uncertainty. In each scenario, one-way and probabilistic sensitivity analyses were performed. The lower and upper limits of the parameter values used in one-way sensitivity analyses were determined using each parameter’s standard error. Because the standard errors of incidence proportions and utilities were not available, we varied them within the range of ± 5 and ± 10% from the base-case values, respectively. The results of one-way sensitivity analyses were represented as tornado diagrams. The effect of all variable’s uncertainty was examined using probabilistic sensitivity analyses (PSA). We ran a Monte Carlo simulation in Microsoft Excel 2018 for 1,000 iterations to demonstrate a range of total cost, health-related outcomes, and ICER values. The PSA results were illustrated as cost-effectiveness planes and cost-effectiveness acceptability curves. Threshold analyses were also performed to determine the vaccines’ acceptable costs in Thailand. The analyses were carried out by lowering vaccine prices (USD per vial) until the ICER of each scenario became cost-effective (i.e., ≤ 5,003 USD/QALY gained) or cost-saving (i.e., 0 USD/QALY gained).
2.5. Budget impact analysis
We analyzed the 5-year budget impact to estimate the total budget for the national PCV13 and PPSV23 vaccination program for Thai older adult in 2022–2026. The total number of Thai older adult population aged ≥ 65 years were retrieved from the database of the Official Statistic Registration System, Department of Provincial Administration, Thailand (42). Prior to the implementation of NIP, approximately 15% of the older adult population in Thailand had been vaccinated, as alternative vaccines had been available in Thailand since 2011 (43). In Southeast Asia, however, the pneumococcal vaccine uptake rate was approximately 29%, which was lower than other regions (16). The acceptance rate of pneumococcal vaccines in the Thai population was estimated using the acceptance rate of influenza vaccine. According to published data, between 40 and 50% of the Thai population had received a flu vaccine (44). We predicted that the vaccines access rate would increase by 10% annually. We calculated the budgetary impact of two policy strategies: (1) ‘active policy strategy’ which permitted vaccination of the population aged ≥ 65 years (those who aged more than 65 years were supported by Thai government) and (2) ‘passive policy strategies,’ which allowed population to receive the vaccine only at the age of 65 years (only those at 65 years were subsidized by Thai government).
3. Results
3.1. Base-case analysis
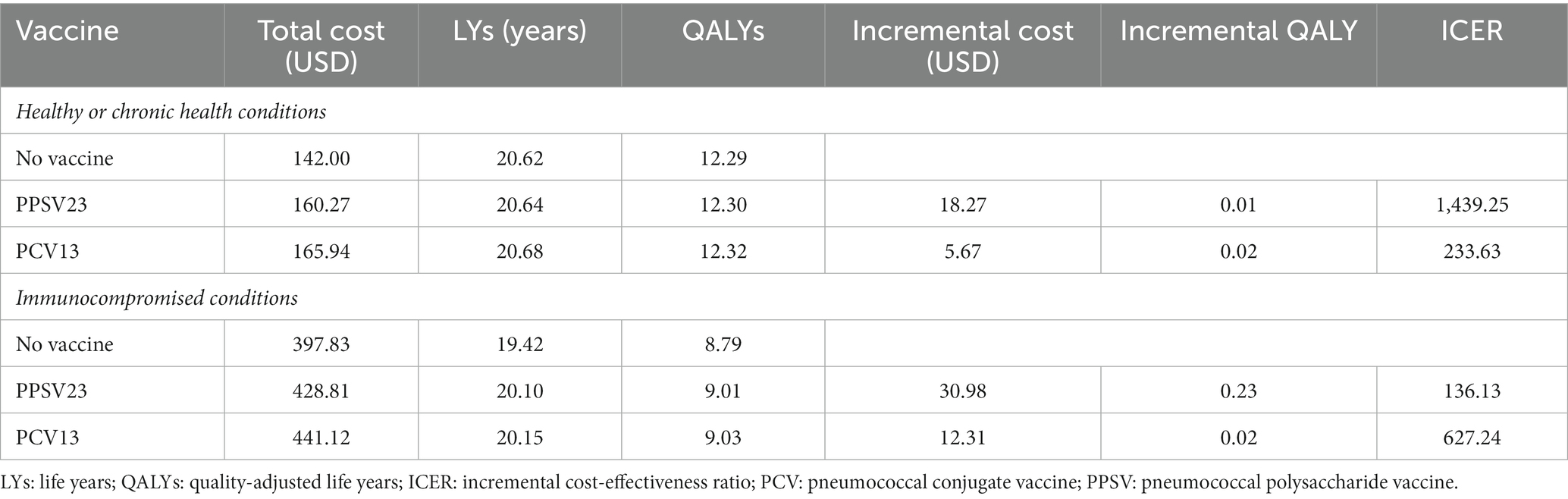
In healthy or chronic health conditions, total lifetime QALYs of no vaccination, PPSV23, and PCV13 were 12.29, 12.30, and 12.32 years, respectively. Total lifetime costs of no vaccination, PPSV23, and PCV13 were 142.0, 160.3, and 166.0 USD, respectively. Meanwhile, total lifetime cost and QALY of PCV13 in immunocompromised conditions were greater than PPSV23 and then no vaccination (Figures 2A,B). Therefore, no vaccination was described as the comparator with the least total lifetime cost and QALYs, followed by PPSV23 and PCV13.
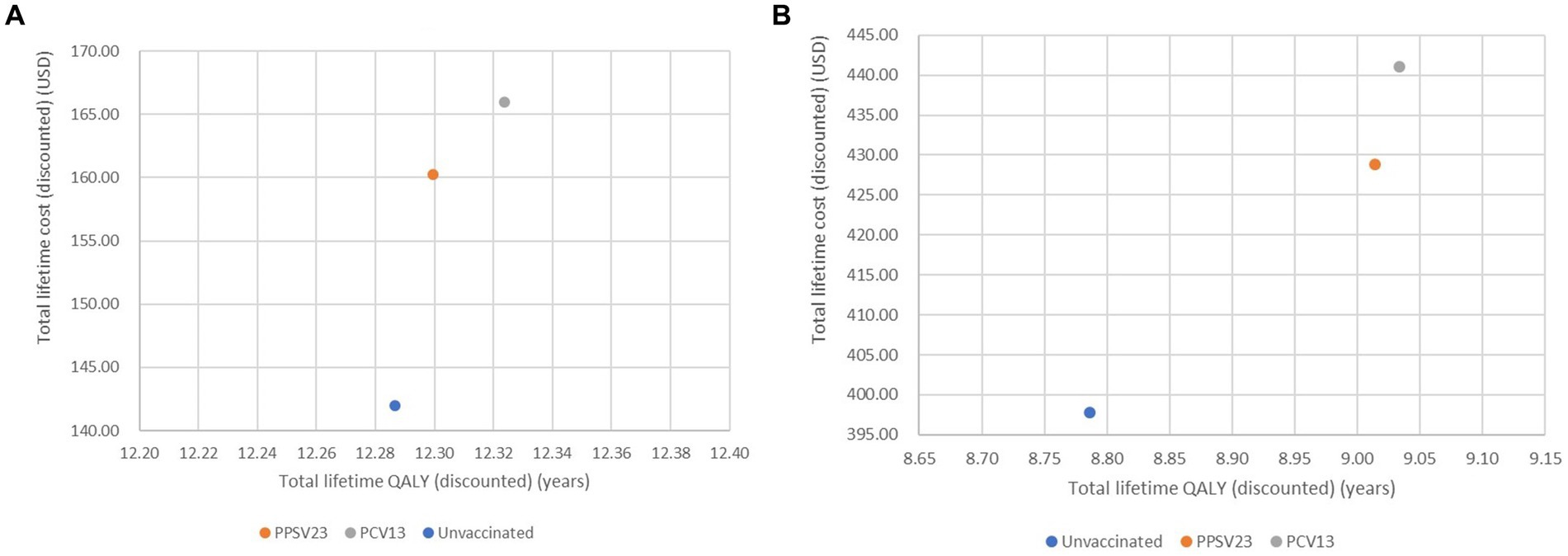
Figure 2. Cost-effectiveness plane of all interventions analysis of healthy or with chronic health conditions (A) older adult with immunocompromised conditions (B).
For one-time PPSV23 vaccination programs increased both cost and QALYs compared to no vaccination policy among healthy or chronic health conditions (Table 2). The incremental cost, life year, QALYs gained, and ICER of PPSV23 were 18.27 USD, 20.64 years, 0.01 QALYs, and 1439.25 USD/QALY gained, respectively. PPSV23 was extendedly dominated by PCV13 as PCV13 yielded more 0.02 QALYs/lifetime per individual with an incremental total lifetime cost of 5.67 USD/individual compared to PPSV23; hence the ICER of PCV13 vs. PPSV23 was 233.63 USD/QALY gained.
In the scenario focusing on immunocompromised persons, the incremental cost, life year, QALYs gained, and ICER of PPSV23, compared with no vaccination, was 30.98 USD, 20.10 years, 0.23 QALYs and 136.13 USD/QALY gained, respectively. In addition, PCV13 would gain 0.02 QALYs/individual while costing 12.31 USD more per individual than PPSV23, resulting in an ICER of 627.24 USD/QALY gained. Comparing PCV13 with PPSV23 among healthy older adult, older adult with chronic health conditions and immunocompromised people, we found that PCV13 was a cost-effective vaccine, with an ICER of 233.63USD/QALY gained and 627.24 USD/QALY gained, respectively.
3.2. Sensitivity analyses
3.2.1. One-way sensitivity analyses
Case fatality of non bacteremic pneumococcal pneumonia and PCV13 efficacy protection against pneumonia were found to be the most sensitive input parameter in one-way sensitivity analyses of PPSV23 compared to no vaccination and PCV13, respectively (Figures 3A,B) that focused on older adult with healthy or chronic health conditions. The utility of the older adult in a non-infected health state was the input parameter that had the greatest impact on the results of immunocompromised older adult in PPSV23 vs. no vaccination. PCV13 efficacy protection against pneumonia demonstrated higher sensitivity parameters than PPSV23 when compared in all types of older adult (Figures 3C,D).
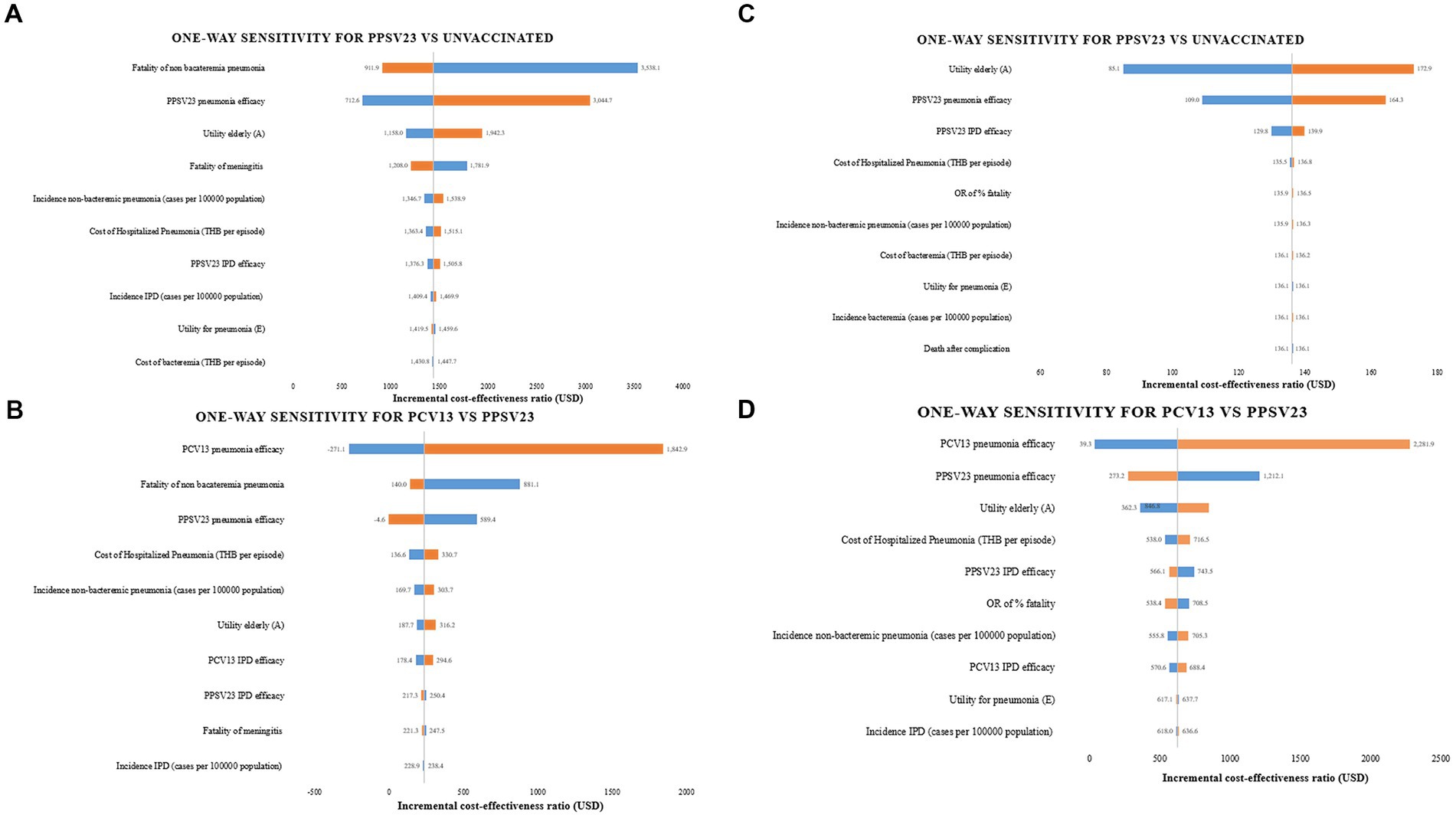
Figure 3. One-way sensitivity analyses. (A) PPSV23 vs. unvaccinated in healthy or chronic health conditions (B) PPSV23 vs. PCV13 in healthy or chronic health conditions (C) PPSV23 vs. unvaccinated in immunocompromised conditions (D) PPSV23 vs. PCV13 in immunocompromised conditions.
3.2.2. Multivariate probabilistic sensitivity analyses
The cost-effectiveness planes displayed PSA results based on 1,000 Monte Carlo simulations (Figure 4). In all scenarios, the majority of simulated ICERs were in the upper-right quadrant. These findings showed that PCV13 policy implementation provided higher QALYs at a higher cost than PPSV23 policy among all groups of Thai older adult. When compared to PPSV23, the probability of PCV13 being cost-saving among immunocompromised people was more than 90%, which was higher than the probability of healthy or chronic health conditions (80%; Figure 5).
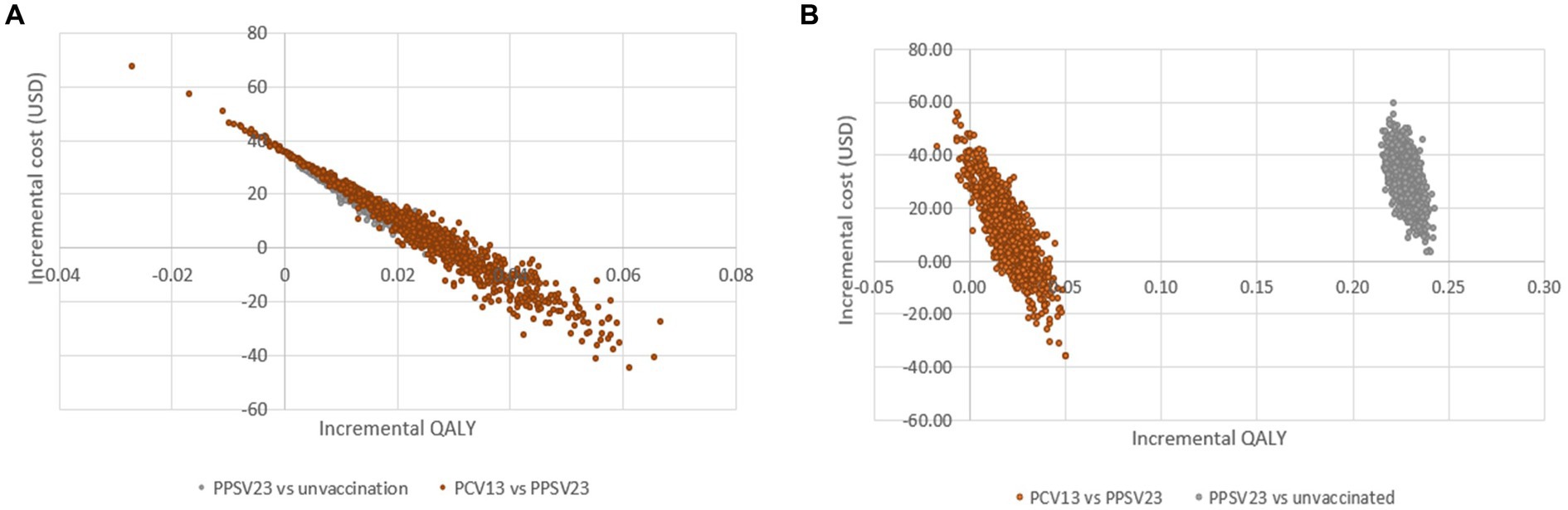
Figure 4. Cost-effectiveness scatter plot demonstrating probabilistic sensitivity analysis of healthy or with chronic health conditions (A) older adult with immunocompromised conditions (B).
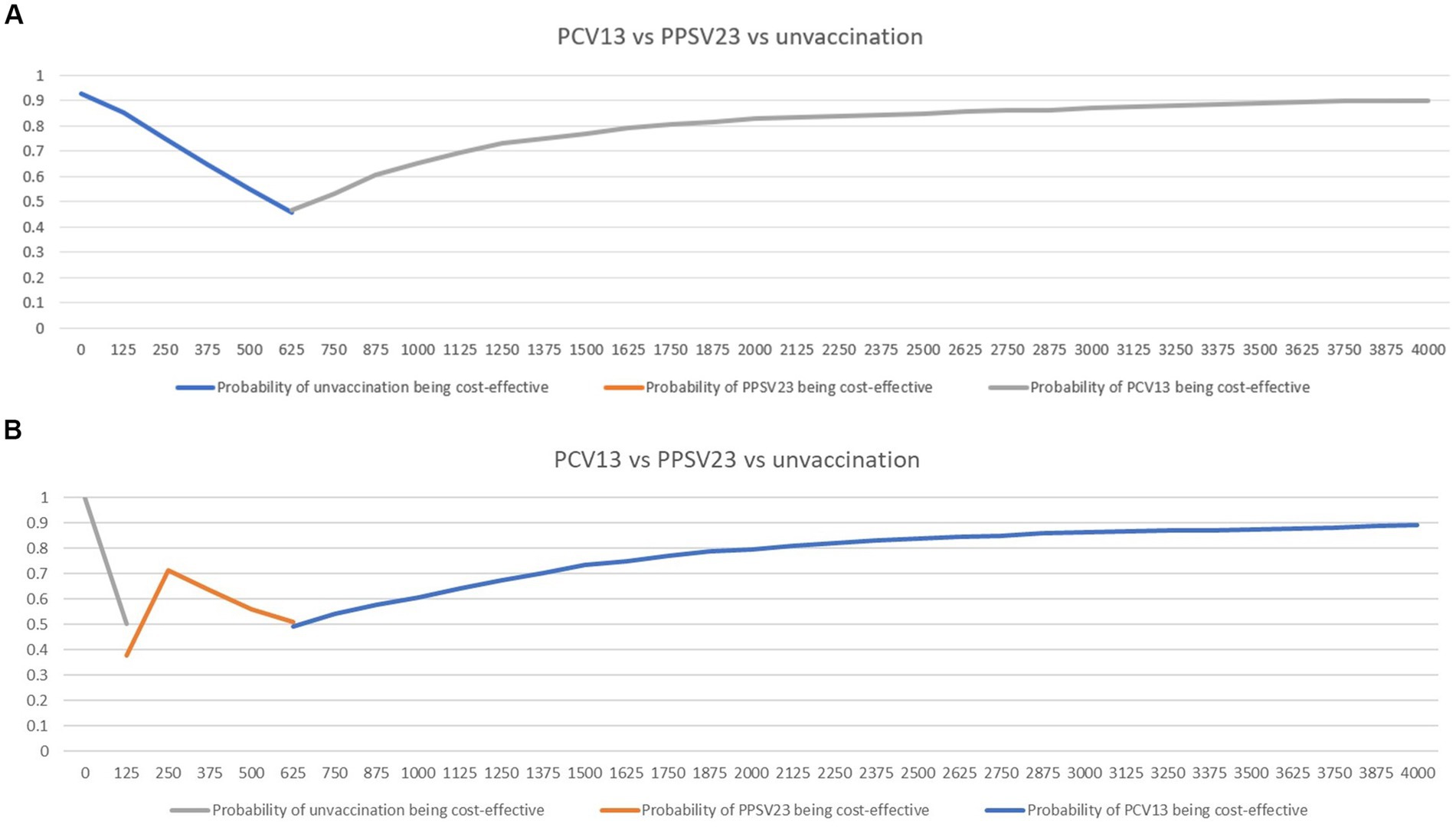
Figure 5. Cost-effectiveness acceptability curves showing the probability of being cost-effective and cost-saving when comparing PCV13 and PPSV23 among healthy or chronic health conditions (A) or immunocompromised conditions (B).
3.2.3. Threshold analysis
Thai Health Technology Assessment guidelines recommended that the result of disease prevention policies, such as vaccination program, should be cost-saving compared with no policy to consider adopting the policy for implementation. In terms of cost-saving, the cost of PCV13 vaccination needed to be reduced by 8.8 and 19.0% of the current price, when compared to PPSV23 among healthy older adult or older adult with chronic health conditions and people with immunocompromised conditions, respectively (Table 3).
3.3. Budget impact analysis
Budget impact analysis (BIA) revealed that the Thai government may have to invest approximately 12–19 million USD per year for PCV13 implementation in passive policy strategies; however, the budget for PPSV23 policy implementation was lower than twice that of PCV13 policy implementation (Table 4). Active policy strategies clearly required higher budget investment (50–79 million USD per year for PCV13) than passive policy strategies. However, vaccine coverage was much higher in active policy strategies than in passive policy strategies [active policy (77%) vs. passive policy (25%)].
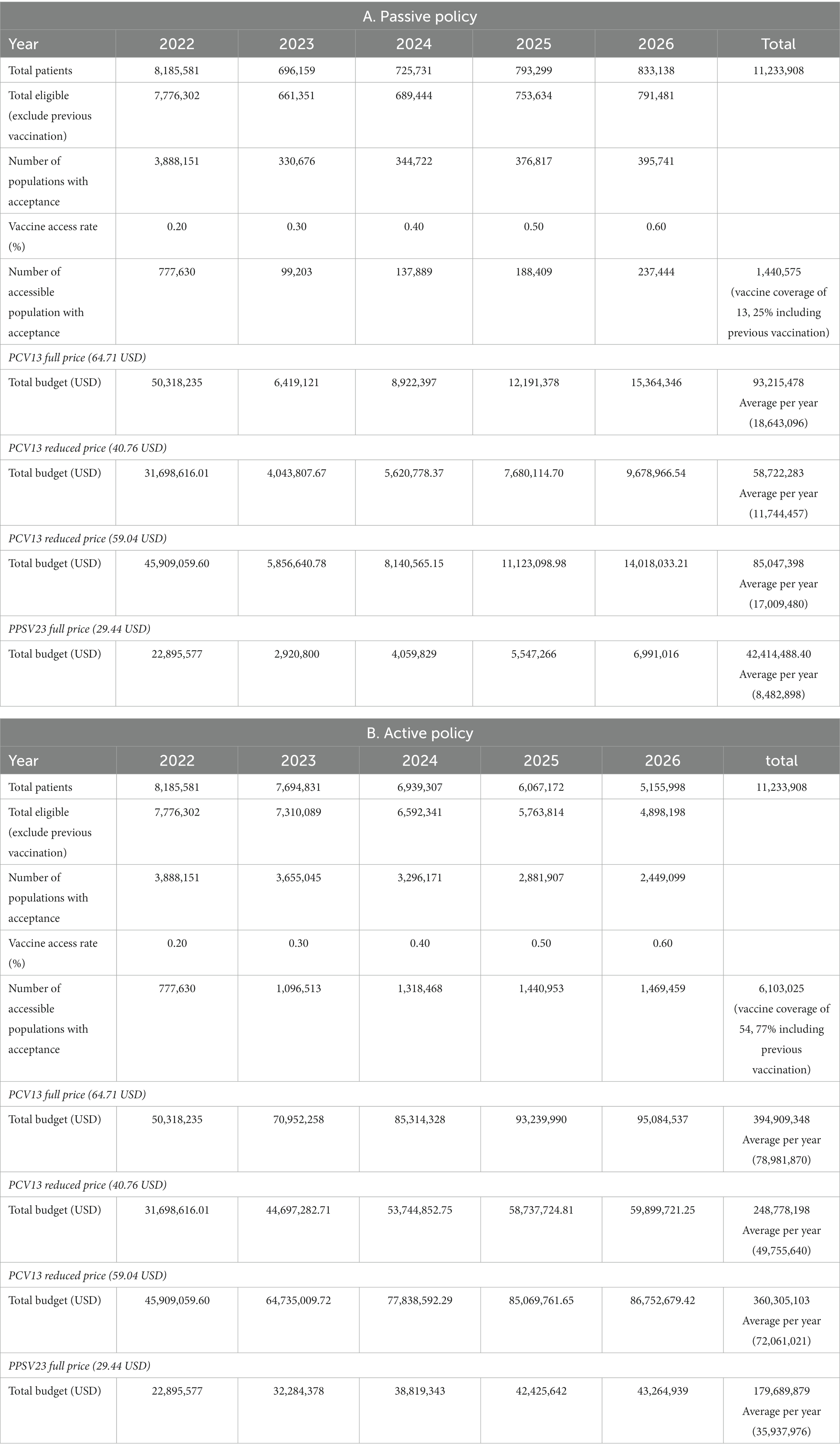
Table 4. Budget impact analysis of PCV13 stratified by policy types: (A) Passive policy (B) Active policy.
4. Discussion
Cost-effectiveness analysis is a crucial information to support decision making for policy makers (45). This analysis has a critical role in low- and middle-income countries (LMICs) for prioritizing vaccines. Our analyses revealed that comparison one vaccine (PPSV23 or PCV13) over another showed that PCV13 are economical vaccine for all older adult Thai individuals when compared to PPSV23 and no vaccinations. These findings are consistent with previous analyses of the cost-effectiveness of these vaccines in adults aged ≥ 50 years residing in low- and middle-income countries (46). We anticipated that ICER between immunocompromised and non-immunocompromised subgroups were different. This is because input parameters between those are different, particularly vaccine efficacy and epidemiological data. Still, both are vaccine target group in clinical practice these day in Thailand. The main determinant factor was PCV13 efficacy on pneumococcal pneumonia across all populations according to the sensitivity analyses and CDC data which protection against pneumonia was lower than IPD. Consequently, when policy makers intend to conduct a similar analysis, they should carefully pay attention to input data of vaccine efficacy or effectiveness against pneumonia since they are the most sensitive parameter. Although the incremental cost among immunocompromised group was higher than healthy and chronic health conditions, the incremental QALY when PPSV23 compared to no vaccination were more than PCV13 compared to one another. This is because utility played a major sensitive factor in immunocompromised conditions according to our sensitivity analyses. Therefore, the direction of ICERs of scenario based on individual with immunocompromised conditions when comparing PPSV23 and no vaccination was lower; meanwhile, the direction of ICER PCV13 comparing one another was higher than that of healthy individuals or those with chronic health conditions.
The majority of previous studies indicated that PCV13 or PPSV23 were cost-saving in specific countries; however, our findings indicated that, using the current price of vaccines, PCV13 and PPSV23 were both cost-effective but not cost-saving. The prices of PCV13 must be reduced by 8.8–19.0% of actual price, depending on the age and conditions. The older adult has lower vaccination implementation costs than children (11). Moreover, the price of the vaccine is the primary barrier to pneumococcal vaccine implementation in middle-income countries (47, 48). Even though our study demonstrated that both vaccines are cost-effective, price reductions are still required to achieve cost-saving results. We anticipated that if we included the cost of complications from deteriorated medical condition resulting from pneumococcal infection, the ICER of vaccine would be lower.
The outcomes of studies comparing one vaccine (PCV13 or PPSV23) to another displayed PCV13 was cost-saving compared to PPSV23 (46). Our findings indicate that PCV13 is superior to PPSV23 because it extends life expectancy and is more effective. In addition, the probability of PCV13 being cost-saving was significantly greater than that of PPSV23. We proposed that PCV13 should be prioritized before PPSV23 in NIP. However, before vaccine implementation, stakeholders must engage in discussion. This is because BIA is typically preferred by the Thai government when the annual budget is less than 4.7 million USD (49–51). Based on our research, the Thai government must invest at least 8 million USD annually for PPSV23 and more than 12 million USD annually for PCV13. In addition, we designed active and passive policy alternatives to determine whether or not Thailand would reach its vaccine coverage goal. The Thai government must spend a substantial amount of money on active policy, but the high vaccine coverage rate and herd immunity threshold may be attained in a relatively short period. While the passive policy may hardly represent the real-world situations, its advantage is budget capability. We recommend that the costs of pneumococcal disease-burden reduction after vaccine and policy deployment be incorporated in our analysis when the Thai government considers this vaccination program. In addition, the duration of pneumococcal vaccination campaigns may be extended to reduce the annual fiscal burden.
Moreover, our results were sensitive to the vaccine efficacy against pneumonia among the Thai older adult. The efficacy of PCV13 against pneumonia was determined to be superior to that of PPSV23. Pneumococcal pneumonia is more prevalent than IPDs despite its restricted serotype coverage. In addition, PCV13 is significantly more effective against pneumonia than PPSV23. Our findings were consistent with those of Smith et al. (20).
Our research has several strengths. First, most of our input data were selected based on Thailand-specific information to produce data and make the outcomes more country-specific. The probabilistic sensitivity analytic model indicated that the constructed model was robust and valid. We also adopted the recent updated pneumococcal vaccine efficacy/effectiveness from the CDC model, which included several significant changes that were closed to real world data: (1) the duration of PCV protection was shorter and (2) vaccine efficacy/effectiveness was lower for PCV13 and PPSV23 in immunocompromised conditions. In addition, this model will be beneficial to the two-next generation pneumococcal vaccines implementation. Lastly, our BIAs incorporated vaccine uptake rate and were stratified by policy types, i.e., active or passive to create different scenarios for decision making. However, this study has some limitations. First, our model was developed using static model, whose results should be interpreted with caution. Second, herd immunity was not included in the model due to the low vaccine uptake rate. The model should be updated when the nationwide pneumococcal vaccine deployment among children reaches herd immunity threshold, or the valent vaccine are implemented. Lastly, as the absence of local data regarding the utility of adults with infection, the utility ratio of populations at high and average risk in the same age range were derived from population in Western countries.
In conclusion, PCV13 is cost-effective among Thai older adult. PCV13 should be prioritized first when both costs and benefits are considered. The cost of vaccines is the most significant barrier to national implementation in Thailand; therefore, negotiations with manufacturers is encouraged. A proactive policy can contribute rapidly to herd immunity in a community, but substantial resources are required. New generation of pneumococcal vaccines with a higher valency will soon be available in high-income countries. Further economic analysis of these new generation vaccines in the context of Thai society is needed.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
TN, CK, and PP contributed to the original idea and conceived the paper. TN wrote the initial draft of the paper under CK and PP guidance. SL, PR, VL, PC, JK, and KC contributed to the revision of the manuscript, and the final version was reviewed by TN, CK, and PP. The authors acknowledge CK’s essential intellectual contribution. All authors contributed to the article and approved the submitted version.
Funding
This research was supported by Mahidol University (MU-miniRC grant: MU-MiniRC04/2564, fiscal year 2021; SL). The APC was funded by the Faculty of Tropical Medicine, Faculty of Medicine Siriraj Hospital Mahidol University, and Mahidol University.
Acknowledgments
This publication fulflls part of the degree programme for a Doctor of Philosophy (Clinical Tropical Medicine), Faculty of Tropical Medicine, Mahidol University (TN). The authors also would like to thank Prof. Terapong Tantawichien for idea sharing and Enago™ (http://www.enago.com/) for the English language review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Vyse, A, Campling, J, Czudek, C, Ellsbury, G, Mendes, D, Reinert, RR, et al. A review of current data to support decision making for introduction of next generation higher valency pneumococcal conjugate vaccination of immunocompetent older adults in the UK. Expert Rev Vaccines. (2021) 20:1311–25. doi: 10.1080/14760584.2021.1984888
2. Gamil, A, Chokephaibulkit, K, Phongsamart, W, Techasaensiri, C, Piralam, B, and Thamaree, R. Pneumococcal disease in Thailand. Int J Infect Dis. (2020) 102:429–36. doi: 10.1016/j.ijid.2020.10.048
3. Ladhani, SN, Collins, S, Djennad, A, Sheppard, CL, Borrow, R, Fry, NK, et al. Rapid increase in non-vaccine serotypes causing invasive pneumococcal disease in England and Wales, 2000-17: a prospective national observational cohort study. Lancet Infect Dis. (2018) 18:441–51. doi: 10.1016/s1473-3099(18)30052-5
4. Bravo, LC. Overview of the disease burden of invasive pneumococcal disease in Asia. Vaccine. (2009) 27:7282–91. doi: 10.1016/j.vaccine.2009.04.046
5. Piralam, B, Tomczyk, SM, Rhodes, JC, Thamthitiwat, S, Gregory, CJ, Olsen, SJ, et al. Incidence of pneumococcal pneumonia among adults in rural Thailand, 2006-2011: implications for pneumococcal vaccine considerations. Am J Trop Med Hyg. (2015) 93:1140–7. doi: 10.4269/ajtmh.15-0429
6. Wu, DB, Roberts, CS, Huang, YC, Chien, L, Fang, CH, and Chang, CJ. A retrospective study to assess the epidemiological and economic burden of pneumococcal diseases in adults aged 50 years and older in Taiwan. J Med Econ. (2014) 17:312–9. doi: 10.3111/13696998.2014.898644
7. Ounsirithupsakul, T, Dilokthornsakul, P, Kongpakwattana, K, Ademi, Z, Liew, D, and Chaiyakunapruk, N. Estimating the productivity burden of pediatric pneumococcal disease in Thailand. Appl Health Econ Health Policy. (2020) 18:579–87. doi: 10.1007/s40258-020-00553-0
8. Haasis, MA, Ceria, JA, Kulpeng, W, Teerawattananon, Y, and Alejandria, M. Do pneumococcal conjugate vaccines represent good value for money in a lower-middle income country? A cost-utility analysis in the Philippines. PLoS One. (2015) 10:e0131156. doi: 10.1371/journal.pone.0131156
9. Tyo, KR, Rosen, MM, Zeng, W, Yap, M, Pwee, KH, Ang, LW, et al. Cost-effectiveness of conjugate pneumococcal vaccination in Singapore: comparing estimates for 7-valent, 10-valent, and 13-valent vaccines. Vaccine. (2011) 29:6686–94. doi: 10.1016/j.vaccine.2011.06.091
10. Kulpeng, W, Leelahavarong, P, Rattanavipapong, W, Sornsrivichai, V, Baggett, HC, Meeyai, A, et al. Cost-utility analysis of 10- and 13-valent pneumococcal conjugate vaccines: protection at what price in the Thai context? Vaccine. (2013) 31:2839–47. doi: 10.1016/j.vaccine.2013.03.047
11. Dilokthornsakul, P, Kengkla, K, Saokaew, S, Permsuwan, U, Techasaensiri, C, Chotpitayasunondh, T, et al. An updated cost-effectiveness analysis of pneumococcal conjugate vaccine among children in Thailand. Vaccine. (2019) 37:4551–60. doi: 10.1016/j.vaccine.2019.06.015
12. Igarashi, A, Hirose, E, Kobayashi, Y, Yonemoto, N, and Lee, B. Cost-effectiveness analysis for PCV13 in adults 60 years and over with underlying medical conditions which put them at an elevated risk of pneumococcal disease in Japan. Expert Rev Vaccines. (2021) 20:1153–65. doi: 10.1080/14760584.2021.1952869
13. Birck, AM, Nordin Christensen, L, Pedersen, MH, Olsen, J, Johnson, KD, Bencina, G, et al. Health economic evaluation of introducing a PPSV23-based vaccination programme to adults aged 65 and above, and an extension to the 60-64 age group in Denmark. Expert Rev Vaccines. (2021) 20:1327–37. doi: 10.1080/14760584.2021.1977627
14. Infectious Disease Association of Thailand (2018). Recommended adult and elderly immunization schedule [Online]. Available at: https://www.idthai.org/Contents/Views/?d=Jk3P!17!4!!390!JatsDZz5 [Accessed September 11, 2022].
15. Pediatric Infectious Disease Society of Thailand. Recommended Thai Children Immunization Schedule [Internet]. (2023). Available at: http://www.pidst.or.th/A1291.html
16. World Health Organization (2022). Pneumococcal vaccination coverage [Online]. Available at: https://immunizationdata.who.int/pages/coverage/pcv.html?CODE=SEAR&ANTIGEN=&YEAR= [Accessed Aug 28, 2022].
17. Karlsson, G, and Johannesson, M. The decision rules of cost-effectiveness analysis. PharmacoEconomics. (1996) 9:113–20. doi: 10.2165/00019053-199609020-00003
18. Matanock, A, L.G., Gierke, R, Kobayashi, M, Leidner, A, and Pilishvili, T (2019). Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥ 65 years: updated recommendations of the advisory committee on immunization practices. MMWR Morb Mortal Wkly Rep 68, 1069–1075. doi: 10.15585/mmwr.mm6846a5
19. Hoshi, SL, Kondo, M, and Okubo, I. Economic evaluation of immunisation Programme of 23-valent pneumococcal polysaccharide vaccine and the inclusion of 13-valent pneumococcal conjugate vaccine in the list for single-dose subsidy to the elderly in Japan. PLoS One. (2015) 10:e0139140. doi: 10.1371/journal.pone.0139140
20. Smith, KJ, Wateska, AR, Nowalk, MP, Raymund, M, Nuorti, JP, and Zimmerman, RK. Cost-effectiveness of adult vaccination strategies using pneumococcal conjugate vaccine compared with pneumococcal polysaccharide vaccine. JAMA. (2012) 307:804–12. doi: 10.1001/jama.2012.169
21. Jackson, LA, Gurtman, A, Rice, K, Pauksens, K, Greenberg, RN, Jones, TR, et al. Immunogenicity and safety of a 13-valent pneumococcal conjugate vaccine in adults 70 years of age and older previously vaccinated with 23-valent pneumococcal polysaccharide vaccine. Vaccine. (2013a) 31:3585–93. doi: 10.1016/j.vaccine.2013.05.010
22. Jackson, LA, Gurtman, A, van Cleeff, M, Frenck, RW, Treanor, J, Jansen, KU, et al. Influence of initial vaccination with 13-valent pneumococcal conjugate vaccine or 23-valent pneumococcal polysaccharide vaccine on anti-pneumococcal responses following subsequent pneumococcal vaccination in adults 50 years and older. Vaccine. (2013b) 31:3594–602. doi: 10.1016/j.vaccine.2013.04.084
23. Rhodes, J, Dejsirilert, S, Maloney, SA, Jorakate, P, Kaewpan, A, Salika, P, et al. Pneumococcal bacteremia requiring hospitalization in rural Thailand: an update on incidence, clinical characteristics, serotype distribution, and antimicrobial susceptibility, 2005-2010. PLoS One. (2013) 8:e66038. doi: 10.1371/journal.pone.0066038
24. Tantawichien, T, Hsu, LY, Zaidi, O, Bernauer, M, du, F, Yamada, E, et al. Systematic literature review of the disease burden and vaccination of pneumococcal disease among adults in select Asia-Pacific areas. Expert Rev Vaccines. (2022) 21:215–26. doi: 10.1080/14760584.2022.2016399
25. Bonten, MJ, Huijts, SM, Bolkenbaas, M, Webber, C, Patterson, S, Gault, S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. (2015) 372:1114–25. doi: 10.1056/NEJMoa1408544
26. Cho, BH, Stoecker, C, Link-Gelles, R, and Moore, MR. Cost-effectiveness of administering 13-valent pneumococcal conjugate vaccine in addition to 23-valent pneumococcal polysaccharide vaccine to adults with immunocompromising conditions. Vaccine. (2013) 31:6011–21. doi: 10.1016/j.vaccine.2013.10.024
27. Suaya, JA, Jiang, Q, Scott, DA, Gruber, WC, Webber, C, Schmoele-Thoma, B, et al. Post hoc analysis of the efficacy of the 13-valent pneumococcal conjugate vaccine against vaccine-type community-acquired pneumonia in at-risk older adults. Vaccine. (2018) 36:1477–83. doi: 10.1016/j.vaccine.2018.01.049
28. Andrews, NJ, Waight, PA, George, RC, Slack, MP, and Miller, E. Impact and effectiveness of 23-valent pneumococcal polysaccharide vaccine against invasive pneumococcal disease in the elderly in England and Wales. Vaccine. (2012) 30:6802–8. doi: 10.1016/j.vaccine.2012.09.019
29. Rudnick, W, Liu, Z, Shigayeva, A, Low, DE, Green, K, Plevneshi, A, et al. Pneumococcal vaccination programs and the burden of invasive pneumococcal disease in Ontario, Canada, 1995-2011. Vaccine. (2013) 31:5863–71. doi: 10.1016/j.vaccine.2013.09.049
30. Djennad, A, Ramsay, ME, Pebody, R, Fry, NK, Sheppard, C, Ladhani, SN, et al. Effectiveness of 23-valent polysaccharide pneumococcal vaccine and changes in invasive pneumococcal disease incidence from 2000 to 2017 in those aged 65 and over in England and Wales. EClinicalMedicine. (2018) 6:42–50. doi: 10.1016/j.eclinm.2018.12.007
31. Lawrence, H, Pick, H, Baskaran, V, Daniel, P, Rodrigo, C, Ashton, D, et al. Effectiveness of the 23-valent pneumococcal polysaccharide vaccine against vaccine serotype pneumococcal pneumonia in adults: a case-control test-negative design study. PLoS Med. (2020) 17:e1003326. doi: 10.1371/journal.pmed.1003326
32. Kimman, M, Vathesatogkit, P, Woodward, M, Tai, ES, Thumboo, J, Yamwong, S, et al. Validity of the Thai EQ-5D in an occupational population in Thailand. Qual Life Res. (2013) 22:1499–506. doi: 10.1007/s11136-012-0251-2
33. Erickson, P, Wilson, R, and Shannon, I. Years of healthy life. Healthy People 2000 Stat Notes. (1995):1–15. doi: 10.1037/e583992012-001
34. Ohshima, N, Akeda, Y, Nagai, H, and Oishi, K. Immunogenicity and safety after the third vaccination with the 23-valent pneumococcal polysaccharide vaccine in elderly patients with chronic lung disease. Hum Vaccin Immunother. (2020) 16:2285–91. doi: 10.1080/21645515.2020.1718975
35. Zou, X, He, J, Zheng, J, Liang, M, Gao, J, Huang, J, et al. Evaluation of effectiveness, safety and cost-benefit of the 23-valent pneumococcal capsular polysaccharide vaccine for HIV-infected patients. Vaccine. (2022) 40:37–42. doi: 10.1016/j.vaccine.2021.11.058
36. Leidner, AJ. Summary of three economic models assessing pneumococcal vaccines in US adults In: ACIP Meeting. (2021).
38. Ministry of Public Health (2022). drug and medical supply information center [Online]. Available at: dmsic.moph.go.th/. Accessed August 28, 2022.
39. Techathawat, S, Yoochareon, P, Varinsathien, P, and Rasdjarmrearnsook, A. Wastage of multi-dose measles vaccine survey in public health facilities (in Thai). Dis Control J. (2008) 34:311–26.
40. Ministry of Public Health (2010). Standard Cost List for Health Technology Assessment [Online]. Available from: http://www.hitap.net/costingmenu/. Accessed April 27, 2023 (in Thai).
41. Thavorncharoensap, M, Teerawattananon, Y, Natanant, S, Kulpeng, W, Yothasamut, J, and Werayingyong, P. Estimating the willingness to pay for a quality-adjusted life year in Thailand: does the context of health gain matter? Clinicoecon Outcomes Res. (2013) 5:29–36. doi: 10.2147/ceor.S38062
42. Bureau of Registration Administration, Department of Provincial Administration [Internet]. Official statistics registration systems (in Thai). (2022) [cited 2022] Available at: https://stat.bora.dopa.go.th/stat/statnew/statMONTH/statmonth/#/view.
43. Thewjitcharoen, Y, Butadej, S, Malidaeng, A, Yenseung, N, Nakasatien, S, Lekpittaya, N, et al. Trends in influenza and pneumococcal vaccine coverage in Thai patients with type 2 diabetes mellitus 2010-2018: experience from a tertiary diabetes center in Bangkok. J Clin Transl Endocrinol. (2020) 20:100227. doi: 10.1016/j.jcte.2020.100227
44. Leewongtrakul, T. Acceptance of influenza vaccination among pregnant women attending the antenatal care clinic, King Chulalongkorn Memorial Hospital. Thai Journal of Obstetrics and Gynaecology. (2017) 25:75. doi: 10.14456/tjog.2017.12
45. Neumann, PJ, Thorat, T, Zhong, Y, Anderson, J, Farquhar, M, Salem, M, et al. A systematic review of cost-effectiveness studies reporting cost-per-DALY averted. PLoS One. (2016) 11:e0168512. doi: 10.1371/journal.pone.0168512
46. Shao, Y, and Stoecker, C. Cost-effectiveness of pneumococcal vaccines among adults over 50 years old in low- and middle-income countries: a systematic review. Expert Rev Vaccines. (2020) 19:1141–51. doi: 10.1080/14760584.2020.1874929
47. Lieu, TA, Finkelstein, JA, Adams, MM, Miroshnik, IL, Lett, SM, Palfrey, S, et al. Pediatricians' views on financial barriers and values for pneumococcal vaccine for children. Ambul Pediatr. (2002) 2:358–66. doi: 10.1367/1539-4409(2002)002<0358:pvofba>2.0.co;2
48. Tricarico, S, McNeil, HC, Cleary, DW, Head, MG, Lim, V, Yap, IKS, et al. Pneumococcal conjugate vaccine implementation in middle-income countries. Pneumonia (Nathan). (2017) 9:6. doi: 10.1186/s41479-017-0030-5
49. Kotirum, S, Muangchana, C, Techathawat, S, Dilokthornsakul, P, Wu, DB, and Chaiyakunapruk, N. Economic evaluation and budget impact analysis of vaccination against Haemophilus influenzae type b infection in Thailand. Front Public Health. (2017) 5:289. doi: 10.3389/fpubh.2017.00289
50. Leelahavarong, P, Chaikledkaew, U, Hongeng, S, Kasemsup, V, Lubell, Y, and Teerawattananon, Y. A cost-utility and budget impact analysis of allogeneic hematopoietic stem cell transplantation for severe thalassemic patients in Thailand. BMC Health Serv Res. (2010) 10:209. doi: 10.1186/1472-6963-10-209
51. Thongsri, W, Bussabawalai, T, Leelahavarong, P, Wanitkun, S, Durongpisitkul, K, Chaikledkaew, U, et al. Cost-utility and budget impact analysis of drug treatments in pulmonary arterial hypertension associated with congenital heart diseases in Thailand. Expert Rev Pharmacoecon Outcomes Res. (2016) 16:525–36. doi: 10.1586/14737167.2016.1120672
Keywords: 13-valent pneumococcal conjugated vaccine (PCV13), 23-valent pneumococcal polysaccharide vaccine (PPSV23), cost-effectiveness, economic evaluation, Thailand, older adult
Citation: Ngamprasertchai T, Kositamongkol C, Lawpoolsri S, Rattanaumpawan P, Luvira V, Chongtrakool P, Kaewkungwal J, Chokephaibulkit K and Phisalprapa P (2023) A cost-effectiveness analysis of the 13-valent pneumococcal conjugated vaccine and the 23-valent pneumococcal polysaccharide vaccine among Thai older adult. Front. Public Health 11:1071117. doi: 10.3389/fpubh.2023.1071117
Edited by:
Piyameth Dilokthornsakul, Chiang Mai University, ThailandReviewed by:
Poukwan Arunmanakul, Chiang Mai University, ThailandRatree Sawangjit, Mahasarakham University, Thailand
Copyright © 2023 Ngamprasertchai, Kositamongkol, Lawpoolsri, Rattanaumpawan, Luvira, Chongtrakool, Kaewkungwal, Chokephaibulkit and Phisalprapa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pochamana Phisalprapa, pochamana.phi@mahidol.ac.th
 Thundon Ngamprasertchai
Thundon Ngamprasertchai Chayanis Kositamongkol
Chayanis Kositamongkol Saranath Lawpoolsri3
Saranath Lawpoolsri3 Viravarn Luvira
Viravarn Luvira Piriyaporn Chongtrakool
Piriyaporn Chongtrakool Jaranit Kaewkungwal
Jaranit Kaewkungwal Kulkanya Chokephaibulkit
Kulkanya Chokephaibulkit Pochamana Phisalprapa
Pochamana Phisalprapa