- 1Department of Psychiatry, BronxCare Health System, New York, NY, United States
- 2BronxCare Health System, New York, NY, United States
- 3Department of Psychiatry, Icahn School of Medicine at Mount Sinai, New York, NY, United States
Myocardial infarction (MI) can have significant physical and mental consequences. Depression is a prevalent psychiatric condition after MI which can reduce the quality of life and increase the mortality rates of patients. However, the connection between MI and depression has remained under-appreciated. This review examines the potential connection between depression and MI by overviewing the possible pathophysiologic mechanisms including dysregulation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system, coagulation system dysfunction, inflammation, environmental factors, as well as, genetic factors. Furthermore, depression can be an adverse event of medications used for MI treatment including beta-blockers, statins, or anti-platelet agents. The need for early detection and management of depression in patients with MI is, therefore, crucial for improving their overall prognosis. Adherence to treatments and regular follow-up visits can ensure the best response to treatment.
1. Introduction
Depression is a highly prevalent mental disorder that imposes significant economic and social burdens (1). Over 300 million (4.4%) individuals are estimated to be affected by depression worldwide, with higher rates among females (5.1%) than males (3.6%). Additionally, the prevalence of depressive disorders increases with age, affecting over 7.5% of females and 5.5% of males aged over 55 years (2). According to projections, depressive disorders are expected to become the first leading cause of the burden of disease in high-income countries by 2030 and the second leading cause worldwide (3).
Cardiovascular disorders are the leading causes of mortality worldwide (4). Ischemic heart disease (IHD) is a subtype of cardiovascular disorder and was found to cause about 8.4 to 9.7 million deaths in 2019 and was more common in developed countries than in developing nations (4). Myocardial infarction (MI) as the most severe form of IHD can cause several physical and mental issues. Depression is one of the most prevalent psychological reactions after MI (5–10). A recent meta-analysis of over 12,000 subjects with MI reported that about 29% of individuals experienced depression (11). Depression after MI can lead to lower quality of life and increase the mortality of patients (8–10). Post-MI depression was found to be associated with a 2 to 2.5-fold increased risk of cardiovascular complications (12).
Despite its prevalence, post-MI depression is often overlooked as a natural emotional reaction to physical illness. Furthermore, emerging evidence has shown that depression is a risk factor for MI (13). In this study, we aim to shed light on the pathways connecting MI and depression by outlining the possible pathophysiologic mechanisms. Our study provides an updated comprehensive overview of the association between post-MI depression and different factors, including dysregulation of the hypothalamic-pituitary-adrenal axis and autonomic nervous system, coagulation system dysfunction, inflammation, as well as, genetic factors (Table 1).
2. Hypothalamic-pituitary-adrenal axis and autonomic nervous system
MI elicits various consequential responses, such as the activation of the hypothalamic-pituitary-adrenal (HPA) axis and dysregulation of the autonomic nervous system (ANS) (14). These effects can further cause selective dysfunction in the prefrontal cortex and anterior cingulate gyrus which develop depression (14–16) (Figure 1).
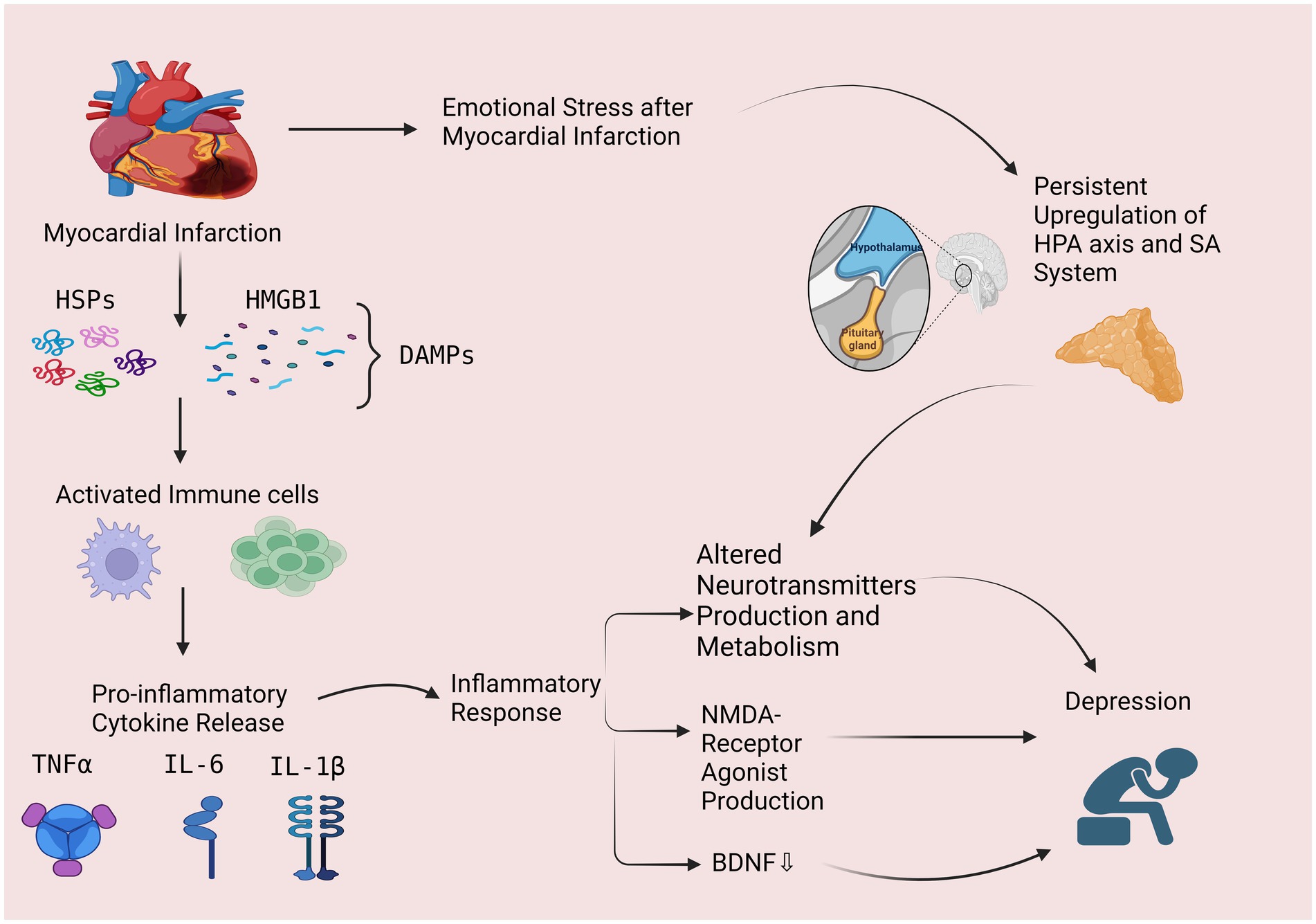
Figure 1. The possible role of inflammatory response and hypothalamic pituitary axis as possible mechanisms in post-MI depression. Myocardial infarction leads to a lack of oxygen and the release of damage-associated molecular patterns (DAMPs), such as high-mobility group box 1 (HMGB1) and heat shock proteins (HSPs). These DAMPs activate immune cells, such as macrophages, which phagocytose the damaged tissue and release pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α), leading to an inflammatory response. Inflammation can lead to depression through 3 mechanisms including: (1) altering the production and metabolism of neurotransmitters, (2) increased production of N-methyl-D-aspartate (NMDA) receptor agonist and, (3) decreased levels of brain-derived neurotrophic factor (BDNF). Myocardial Infarction also cause emotional stress which activates the hypothalamic-pituitary-adrenal (HPA) axis and sympathetic-adrenomedullary (SA) system and can cause altered metabolism and production of neurotransmitters, leading to depression. HPA, hypothalamic-pituitary-adrenal; SA, sympathetic-adrenomedullary; DAMPs, damage-associated molecular patterns; HMGB1, high-mobility group box-1; HSPs, heat shock proteins, TNF-α, tumor necrosis factor-alpha, interleukin 6 (IL-6), Interleukin 1β (IL-1β), NMDA, N-methyl-D-aspartate; BDNF, brain-derived neurotrophic factor. (This figure is created by Bioreneder.com).
According to a recent study on post-MI patients (15), an immediate increase in cortisol concentration due to the activation of the HPA axis was observed after MI, which returned to baseline within 72 h. There was no difference between morning and afternoon cortisol levels of individuals with post-MI depression. Patients with depression lasting for more than 3 months exhibit a more pronounced flattened daily rhythm of cortisol secretion. Among post-MI patients without depression, however, the afternoon cortisol level was significantly lower than the morning level. An abnormal cortisol rhythm has been linked to cognitive impairment and reduced stress-coping abilities, which may increase the risk of developing depressive symptoms (15, 17).
Emotional stress associated with post-MI depression can activate the sympathetic-adrenomedullary (SA) system, which in conjunction with the HPA axis activation, can cause dysregulation of serotonin and may contribute to the maintenance of depressive symptoms (18–20).
3. Environmental factors
Several lifestyle and environmental factors have been identified as potential contributors to the development of post-MI depression, including lack of social support, lifestyle changes, financial stress, and health-related fears and anxiety (21–29).
3.1. Lack of social support
Lack of social support or a poor support system has been linked to an increased risk of post-MI depression (21). Limited emotional or practical support from family, friends, or healthcare providers can exacerbate feelings of isolation, sadness, and distress. On the other hand, strong social support can help individuals cope better with the emotional challenges following MI (21, 22).
3.2. Lifestyle changes
Following MI, individuals are often advised to make significant lifestyle modifications, such as adopting a healthier diet, engaging in regular physical activity, quitting smoking, and reducing alcohol consumption (23). Difficulties in implementing and maintaining these lifestyle changes and unhealthy lifestyle habits, including physical inactivity, poor dietary choices, smoking, and excessive alcohol consumption, have been associated with a higher risk of post-MI depression. These habits can worsen physical health outcomes, impact mood regulation, and contribute to a negative emotional state (23, 24).
3.3. Financial stress
Financial strain resulting from medical expenses, loss of income, or inability to work due to a heart attack can contribute to post-MI depression (25). Financial difficulties can heighten anxiety, worry, and uncertainty about the future, which may negatively impact mental well-being (25).
3.4. Health-related anxiety
After experiencing MI, individuals may develop health-related anxiety, including fear of another cardiac event, fear of physical exertion, or hypochondriasis. These fears can lead to increased distress, avoidance of physical activity, and impaired quality of life, potentially contributing to the development of depression (26).
Additionally, several other factors can exacerbate these environmental factors and influence the development of post-MI depression. Pre-existing mental health conditions, particularly a prior history of anxiety or depression (27, 28), as well as complications during hospitalization (29), are notable examples.
4. Coagulation system
Tissue-type plasminogen activator, or tPA, is a thrombolytic enzyme that converts plasminogen to plasmin and plays an important role in promoting neuronal synaptic plasticity (30). The plasminogen activator inhibitor 1 (PAI-1) is a major endogenous inhibitor of tPA within the extracellular space (31) and is encoded by the SERPINE1 gene. SERPINE1 has been linked to increased susceptibility to depression and may influence the therapeutic response to SSRIs (30, 31). While the relationship between tPA levels and depression remains unclear, evidence suggests that PAI-1 levels increase during psychological stress and depression (30). Lower PAI-1 levels in patients with anxiety and depression who were treated with serotonergic antidepressants have been reported (32). One study found that depressed patients had lower tPA levels prior to antidepressant treatment. After 8 weeks of treatment, however, levels of tPA significantly increased (33). This may show the possible correlation between depression and MI, as high fibrinogen levels and high PAI-1 levels present an increased risk for ischemic cardiovascular events such as MI. Because PAI-1 inhibits tPA, there is an important link between the fibrinolytic processes of this inhibition and the increased risk for cardiovascular disease.
The possible correlation between the coagulation system and the development of depression can be further discovered via the production of brain-derived neurotrophic factor (BDNF), as the tPA-plasmin pathway cleaves the precursor to BDNF, pro-BDNF, to BDNF (34, 35). Neurotrophins are key regulators of synaptic plasticity and neuronal connectivity (34), and BDNF is a small dimeric neurotrophin that is strongly implicated in the pathophysiology of depression due to its high expression in brain regions responsible for mood regulation, including the hippocampus, prefrontal cortex, and amygdala (35, 36). Preclinical and clinical studies have consistently shown that levels of BDNF decrease within the brain during periods of emotional and psychological stress and depression (34–36).
5. Inflammation
Inflammation plays a crucial role in the pathophysiology of MI and subsequent depression (37). During MI, the lack of oxygen and nutrients causes damage to the heart muscle, leading to the release of damage-associated molecular patterns (DAMPs), such as high-mobility group box 1 (HMGB1) and heat shock proteins (HSPs) (37). These DAMPs activate immune cells, such as macrophages, which phagocytose the damaged tissue and release pro-inflammatory cytokines, such as interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) (37, 38) (Figure 1).
Inflammatory cytokines have been shown to contribute to the development of depression in both animal and human studies. Studies in rodents have demonstrated that administration of IL-1β or TNF-α induces depressive-like behavior, while blockade of these cytokines attenuates depressive-like behavior in response to stress (39, 40). In humans, elevated levels of inflammatory cytokines have been found in patients with depression, including those with post-MI depression (41). The mechanisms by which inflammatory cytokines contribute to depression are complex and not fully understood. It is thought that these cytokines may activate the kynurenine pathway, leading to increased production of quinolinic acid, an N-methyl-D-aspartate (NMDA) receptor agonist that has been implicated in the pathophysiology of depression (42). In addition, inflammatory cytokines can also affect the production and metabolism of neurotransmitters, such as serotonin, dopamine, and norepinephrine, which are involved in the regulation of mood (43, 44). For example, IL-6 can induce the expression of indoleamine 2,3-dioxygenase (IDO), an enzyme that metabolizes tryptophan, the precursor of serotonin. This can lead to a decrease in serotonin production and an increase in the production of kynurenine, which has been linked to the development of depression (45). Furthermore, inflammatory cytokines have been linked to decreased levels of brain-derived neurotrophic factor (BDNF), a protein that is important for the survival and function of neurons. Studies have shown that decreased levels of BDNF are associated with depression, and inflammatory cytokines can decrease the expression of BDNF in the brain (46–48).
In summary, inflammation and inflammatory cytokines play a crucial role in the development of post-MI depression. Future research on the relationship between inflammation and post-MI depression may lead to new treatments and preventative strategies for this debilitating condition.
6. Genetic factors
Recent studies utilizing Mendelian randomization (MR) have discovered a noteworthy correlation between genetic susceptibility to depression and an increased risk of cardiovascular disorders (CVD) and MI. Moreover, genetic-related depression is linked to a higher risk of heart failure and small-vessel stroke. These findings demonstrated that depression has enduring and stable effects on the risk of MI (49, 50).
Genetic and environmental factors contribute to the pathophysiology of depression after MI. One study found that a variant of the serotonin transporter gene or 5-HTTLPR was associated with an increased risk of depression after MI and that patients with this variant had a poorer response to antidepressant treatment (51). Another study found that genetic variations in the interleukin-1 (IL-1) gene were associated with an increased risk of depression after MI, possibly due to the role of IL-1 in the inflammatory response (52). A study found that patients with a family history of depression were more likely to develop depression after MI and this risk was further increased in patients who experienced a high level of stress during the MI (53). Based on a review by Schins et al. (54), the increased risk of thromboembolic events in patients with depression and cardiovascular disease may be linked to the upregulation and/or heightened sensitivity of serotonin receptors 5-HT2A/1B, as well as the downregulation of serotonin transporter (5-HTT) receptors. Additionally, the S allele of the serotonin transporter (5-HTT) gene-linked polymorphic region was found to be associated with both depressive symptoms and cardiac events (55). Although these studies suggest a role for genetics in the pathophysiology of depression after MI, it is important to note that depression is a complex disorder and further research is needed to fully understand the genetic basis of post-MI depression and to identify potential targets for treatment and prevention.
7. Post-MI medications and their potential association with post-MI depression
Certain medications prescribed after MI may contribute to the development of depression in patients. The use of beta-blockers after MI is a standard therapeutic approach aimed at reducing the risk of future cardiovascular events and improving overall cardiac function (56). Research examining the relationship between beta-blocker use and depression after MI has produced mixed results. Some studies have reported a higher incidence of depressive symptoms in patients treated with beta-blockers (57–59) while others have found no significant association (60–64). It is important to note that the evidence is not conclusive and further research is needed to establish a clearer understanding of this relationship. The mechanism behind the potential association between beta blockers and depression is not fully understood. It has been proposed that beta-blockers may have an impact on the central nervous system, influencing neurotransmitters and hormonal pathways that are involved in mood regulation. However, the exact biological mechanisms linking beta-blocker use and depression after MI remain speculative and require more investigation.
Statins are other commonly prescribed medications that are used to decrease cholesterol levels and prevent cardiovascular events, including MI (65). Several studies have investigated the potential link between statin use and the risk of depression (66–74). The findings have been controversial, with some studies suggesting a possible protective effect of statins against depression (66–70) while others have found no significant association (70) or even an increased risk (71–74). One proposed mechanism by which statins might influence depression risk is through their anti-inflammatory properties. It is believed that inflammation plays a role in the development of depression, and statins have been shown to reduce the expression of hippocampal pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6. By modulating the inflammatory response, statins could potentially have a positive impact on mood and depressive symptoms (67). Given the conflicting findings in the existing literature, more research is needed to better understand the relationship between statin use after MI and the risk of depression.
Antiplatelet agents, such as aspirin, are often used after MI to prevent blood clots and lower the risk of recurrent MI (75). Aspirin was found to protect against depression based on several studies (67, 74), while others found no effect (76, 77) or even an increased risk of depression (78). One of the possible mechanisms for the depressogenic effects of aspirin can be its potential impact on the arachidonic acid pathway. Arachidonic acid, which is associated with mood disorders, has been linked to depression when its levels are higher compared to other fatty acids (79). Moreover, arachidonic acid can directly affect brain serotonin transporters (80). Therefore, by inhibiting the metabolism of arachidonic acid, aspirin could potentially interfere with serotonin systems that regulate mood (80).
8. Implications for clinical practice
An overview of clinical points can be seen in Table 2. Post-MI depression is common and associated with poor outcomes and increased healthcare costs. Ongoing research aims to improve clinical practice and patient outcomes, with some implications including:
1. Screening: Detecting depression early is vital for prompt treatment. Tools like PHQ-9 and HADS can screen and identify patients with depression (81, 82).
2. Treatment: Post-MI depression treatment involves pharmacological and non-pharmacological interventions, such as selective serotonin reuptake inhibitors (SSRIs) and serotonin and norepinephrine reuptake inhibitors (SNRIs). Effective psychosocial interventions include cognitive behavioral therapy (CBT) and cardiac rehabilitation programs (83).
3. Adherence: Adherence to treatment is crucial for the successful management of depression. Patients should be educated about the importance of adherence to their medication and therapy sessions (84).
4. Follow-up: Regular follow-up visits with healthcare providers is essential for the monitoring of depression symptoms and the adjustment of treatment as needed (84).
Tailored treatment approaches based on individual needs could enhance post-MI depression treatment outcomes. Identifying biomarkers to match specific treatments is actively investigated. Digital interventions like mHealth apps and telehealth can boost treatment access and adherence by providing real-time support and feedback (85–87). Combining pharmacological and non-pharmacological approaches can improve outcomes. For instance, combining CBT with antidepressants has proved effective in post-MI depression treatment (83). Therefore, prompt identification and treatment of post-MI depression are crucial, and personalized, digital, and combined interventions can improve outcomes (83). Further research, including RCTs, will provide a better understanding of the best post-MI depression treatment approaches.
9. Discussion
The precise cause of post-MI depression remains unclear. Other possible underlying mechanisms have been proposed in some studies such as the possible role of abnormal lipid profiles in developing depression (88–90). Some studies have reported a positive correlation between unipolar depression and elevated levels of low-density lipoprotein (LDL) cholesterol (88–90) and total cholesterol (91), along with lower levels of high-density lipoprotein cholesterol (HDL) (86, 87). However, some evidence reported a negative correlation between LDL (92, 93) and total cholesterol (94–98) levels with depression and suicidal behaviors. There is also an increasing amount of literature suggesting no correlation between serum lipids and depressive episodes (99–104). These discrepancies in the findings could be attributed to various factors, including uncontrolled confounding variables and differences in study settings. To gain a better understanding of the relationship between lipid profiles and depression, it is imperative to conduct further studies with larger sample sizes.
It should be noted that depression is also a risk factor for MI development (13). Several mechanisms including platelets activation and thrombosis, behavioral and lifestyle factors, inflammatory processes, as well as HPA axis and ANS have been proposed (105).
9.1. Platelets activation and thrombosis
There is a strong link between depression and platelet reactivity that can cause cardiovascular morbidities (106–109). Depressed patients have been found to demonstrate enhanced platelet reactivity and increased expression of activated glycoprotein (GP) IIb/IIIa, GP Ib/IX receptors, P selectin, β thromboglobulin and platelet factor four, and monoamine oxidase in comparison to healthy individuals (110, 111). GP Ib/IX receptors lead to a conformational change and activation of GP IIb/IIIa receptors. GP IIb/IIIa complex is a receptor for fibrinogen, fibronectin, vitronectin, Von Willebrand factor, and thrombospondin which enhance platelet activation (106, 108, 109). This heightened platelet activation may contribute to ischemic heart disease and post-MI mortality (112).
9.2. Behavioral and lifestyle factors
Chronic depression often leads to unhealthy lifestyle practices (113) such as physical inactivity (114), poor dietary habits, smoking, and non-adherence to medication regimens (115). These behaviors can increase the risk of developing cardiovascular disease, including MI (113). Additionally, depressed patients who have experienced an acute MI are less inclined to follow the suggested behavioral and lifestyle modifications aimed at decreasing the likelihood of future cardiac events (116).
9.3. Inflammatory processes
Chronic depression has been associated with an increased risk of systemic inflammation (117). Persistent elevation of pro-inflammatory markers, such as C-reactive protein (CRP), IL-6, and IL-1 in depression (118) can promote the development and progression of atherosclerosis, a condition characterized by the buildup of fatty plaques in the arterial walls. These plaques can eventually rupture, leading to the formation of blood clots that can block coronary arteries, resulting in myocardial infarction (118–120).
9.4. HPA axis and ANS
Previous studies have shown that patients with depression have an excessive rate of norepinephrine entry into plasma from the sympathetic nerves and a rapid elimination phase from the bloodstream which corresponds with an increased neuronal uptake (120). This excessive sympathetic outflow results in coronary vasoconstriction and reduced cardiac blood flow (121), major ventricular arrhythmias (121, 122), left ventricular hypertrophy (123), endothelial dysfunction (124), and MI (125). Current evidence also reported that changes in ANS can increase the risk of developing recurrent MI and higher mortality rate in post-MI patients (126–129). Individuals with depression often experience a reduction in cardiac parasympathetic tone and vagal activity leading to a decrease in heart rate variability (126–128). Decreased heart rate variability is strongly correlated with mortality in post-MI patients (126, 129). Furthermore, overstimulation of the HPA axis and increased levels of cortisol in depression (130, 131) can lead to metabolic syndrome (132). Metabolic syndrome in turn is associated with coronary heart disease (CHD), CVD, increased mortality rate (133) and increased sympathetic nervous system activity (134).
The relationship between chronic depression and MI is complex, and the mechanisms described above are not mutually exclusive. They likely interact and influence each other, contributing to an increased risk of MI in individuals with chronic depression (105). Additionally, other factors such as genetic predisposition (135), cardiovascular side effects of depression treatment (136), and comorbid conditions may also play a role in this association.
Conclusion
Several factors including the dysregulation of the autonomic nervous system and HPA axis, inflammatory cytokines, coagulation system, platelet aggregation, various environmental factors, medications, and genetics can be contributed to the correlation between MI and depression. The co-occurrence of these two conditions can significantly impact the quality of life of the affected individuals.
Author contributions
EG, TK, BS, GY, and GG: conceived and designed the study, collected and analyzed the data, and drafted the manuscript. MS and SG: contributed to the study design, editing, and critically revised the manuscript for important intellectual content and provided critical feedback on the manuscript and approved the final version for submission. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA: American Psychiatric Publishing (2013).
2. World Health Organization. Depression and other common mental disorders: global health estimates. Geneva: World Health Organization (2017).
3. Mathers, CD, and Loncar, D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med (2006) 3:e442. doi: 10.1371/journal.pmed.0030442
4. Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) results. Seattle, United States: Institute for Health Metrics and Evaluation (IHME) (2020).
5. Feng, HP, Chien, WC, Cheng, WT, Chung, CH, Cheng, SM, and Tzeng, WC. Risk of anxiety and depressive disorders in patients with myocardial infarction. Medicine (2016) 95:e4464. doi: 10.1097/MD.0000000000004464
6. JHC, F, NASE, S, Pereira, BB, and GMM, O. Major depression and acute coronary syndrome-related factors. Arq Bras Cardiol (2017) 108:217–27. doi: 10.5935/abc.20170028
7. Lane, D, Carroll, D, Ring, C, Beevers, DG, and Lip, GY. The prevalence and persistence of depression and anxiety following myocardial infarction. Br J Health Psychol (2002);7:11–21, doi: 10.1348/135910702169321
8. Hosseini, SH, Ghaemian, A, Mehdizadeh, E, and Ashraf, H. Contribution of depression and anxiety to impaired quality of life in survivors of myocardial infarction. Int J Psychiatry Clin Pract (2014) 18:175–81. doi: 10.3109/13651501.2014.940049
9. Bush, DE, Ziegelstein, RC, Tayback, M, Richter, D, Stevens, S, Zahalsky, H, et al. Even minimal symptoms of depression increase mortality risk after acute myocardial infarction. Am J Cardiol (2001) 88:337–41. doi: 10.1016/S0002-9149(01)01675-7
10. Meijer, A, Conradi, HJ, Bos, EH, Thombs, BD, van Melle, JP, and de Jonge, P. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis of 25 years of research. Gen Hosp Psychiatry (2011) 33:203–16. doi: 10.1016/j.genhosppsych.2011.02.007
11. Feng, L, Li, L, Liu, W, Yang, J, Wang, Q, Shi, L, et al. Prevalence of depression in myocardial infarction: a PRISMA-compliant meta-analysis. Medicine (2019) 98:e14596. doi: 10.1097/MD.0000000000014596
12. van Melle, JP, de Jonge, P, Spijkerman, TA, Tijssen, JG, Ormel, J, van Veldhuisen, DJ, et al. Prognostic association of depression following myocardial infarction with mortality and cardiovascular events: a meta-analysis. Psychosom Med (2004) 66:814. doi: 10.1097/01.psy.0000146294.82810.9c
13. Lett, HS, Blumenthal, JA, Babyak, MA, Sherwood, A, Strauman, T, Robins, C, et al. Depression as a risk factor for coronary artery disease: evidence, mechanisms, and treatment. Psychosom Med (2004) 66:305–15. doi: 10.1097/01.psy.0000126207.43307.c0
14. Ter Horst, GJ. Central autonomic control of the heart, angina, and pathogenic mechanisms of post-myocardial infarction depression. Eur J Morphol (1999) 37:257. doi: 10.1076/ejom.37.4.257.4722
15. Wilkowska, A, Rynkiewicz, A, Wdowczyk, J, and Landowski, J. Morning and afternoon serum cortisol level in patients with post-myocardial infarction depression. Cardiol J (2019) 26:550. doi: 10.5603/CJ.a2017.0123
16. Granville, SI, Parker, G, Cvejic, E, and Vollmer-Conna, U. Acute coronary syndrome-associated depression: the salience of a sickness response analogy? Brain Behav Immun (2015) 49:18. doi: 10.1016/j.bbi.2015.02.025
17. Sjögren, E, Leanderson, P, and Kristenson, M. Diurnal saliva cortisol levels and relations to psychosocial factors in a population sample of middle-aged Swedish men and women. Int J Behav Med (2006) 13:193–200. doi: 10.1207/s15327558ijbm1303_2
18. Jacobs, BL, van Praag, H, and Gage, FH. Adult brain neurogenesis and psychiatry: a novel theory of depression. Mol Psychiatry (2000) 5:262. doi: 10.1038/sj.mp.4000712
19. Elhwuegi, AS. Central monoamines and their role in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry (2004) 28:435. doi: 10.1016/j.pnpbp.2003.11.018
20. Pariante, CM. Depression, stress and the adrenal axis. J Neuroendocrinol (2003) 15:811–2. doi: 10.1046/j.1365-2826.2003.01058.x
21. Barefoot, JC, Burg, MM, Carney, RM, Cornell, CE, Czajkowski, SM, Freedland, KE, et al. Aspects of social support associated with depression at hospitalization and follow-up assessment among cardiac patients. J Cardiopulm Rehabil Prev (2003) 23:404–12. doi: 10.1097/00008483-200311000-00002
22. Frasure-Smith, N, Lespérance, F, Gravel, G, Masson, A, Juneau, M, Talajic, M, et al. Social support, depression, and mortality during the first year after myocardial infarction. Circulation (2000) 101:1919–24. doi: 10.1161/01.CIR.101.16.1919
23. Myers, V, Gerber, Y, Benyamini, Y, Goldbourt, U, and Drory, Y. Post-myocardial infarction depression: increased hospital admissions and reduced adoption of secondary prevention measures—a longitudinal study. J Psychosom Res (2012) 72:5–10. doi: 10.1016/j.jpsychores.2011.09.009
24. Johnston, DW. Lifestyle changes after a myocardial infarction. Heart (1999) 82:543–4. doi: 10.1136/hrt.82.5.543
25. Murphy, B, Le Grande, M, Alvarenga, M, Worcester, M, and Jackson, A. Anxiety and depression after a cardiac event: prevalence and predictors. Front Psychol (2020) 10:3010. doi: 10.3389/fpsyg.2019.03010
26. Tully, PJ, Cosh, SM, and Baune, BT. A review of the affects of worry and generalized anxiety disorder upon cardiovascular health and coronary heart disease. Psychol Health Med (2013) 18:627–44. doi: 10.1080/13548506.2012.749355
27. Strik, JJ, Honig, A, and Maes, M. Depression and myocardial infarction: relationship between heart and mind. Prog Neuro-Psychopharmacol Biol Psychiatry (2001) 25:879–92. doi: 10.1016/S0278-5846(01)00150-6
28. Larsen, KK, Vestergaard, M, Søndergaard, J, and Christensen, B. Screening for depression in patients with myocardial infarction by general practitioners. Eur J Prev Cardiol (2013) 20:800–6. doi: 10.1177/2047487312444994
29. Strik, JJ, Honig, A, Lousberg, R, Van Os, J, Van Den Berg, EJ, and Van Praag, HM. Clinical correlates of depression following myocardial infarction. Int J Psychiatry Med (2001) 31:255–64. doi: 10.2190/EJBR-DWLH-EV3P-TWHX
30. Tsai, SJ. Role of tissue-type plasminogen activator and plasminogen activator inhibitor-1 in psychological stress and depression. Oncotarget (2017) 8:113258. doi: 10.18632/oncotarget.19935
31. Tsai, SJ, Hong, CJ, Liou, YJ, Yu, YW, and Chen, TJ. Plasminogen activator inhibitor-1 gene is associated with major depression and antidepressant treatment response. Pharmacogenet Genomics (2008) 18:869. doi: 10.1097/FPC.0b013e328308bbc0
32. Geiser, F, Conrad, R, Imbierowicz, K, Meier, C, Liedtke, R, Klingmüller, D, et al. Coagulation activation and fibrinolysis impairment are reduced in patients with anxiety and depression when medicated with serotonergic antidepressants. Psychiatry Clin Neurosci (2011) 65:518. doi: 10.1111/j.1440-1819.2011.02241.x
33. Jiang, H, Chen, S, Li, C, Lu, N, Yue, Y, Yin, Y, et al. The serum protein levels of the tPA-BDNF pathway are implicated in depression and antidepressant treatment. Transl Psychiatry (2017) 7:e1079. doi: 10.1038/tp.2017.43
34. Karege, F, Perret, G, Bondolfi, G, Schwald, M, Bertschy, G, and Aubry, JM. Decreased serum brain-derived neurotrophic factor levels in major depressed patients. Psychiatry Res (2002) 109:143. doi: 10.1016/S0165-1781(02)00005-7
35. Hofer, M, Pagliusi, SR, Hohn, A, Leibrock, J, and Barde, YA. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J (1990) 9:2459.
36. Sen, S, Duman, R, and Sanacora, G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry (2008) 64:527. doi: 10.1016/j.biopsych.2008.05.005
37. Frangogiannis, NG. The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol (2014) 11:255–65. doi: 10.1038/nrcardio.2014.28
38. Libby, P, Ridker, PM, and Hansson, GK. Progress and challenges in translating the biology of atherosclerosis. Nature (2011) 473:317–25. doi: 10.1038/nature10146
39. Konsman, JP, Luheshi, GN, Bluthe, RM, and Dantzer, R. The vagus nerve mediates behavioural depression, but not fever, in response to peripheral immune signals; a functional anatomical analysis. Eur J Neurosci (2000) 12:4434–46. doi: 10.1046/j.0953-816X.2000.01319.x
40. Hodes, GE, Pfau, ML, Leboeuf, M, Golden, SA, Christoffel, DJ, Bregman, D, et al. Individual differences in the peripheral immune system promote resilience versus susceptibility to social stress. Proc Natl Acad Sci U S A (2014) 111:16136–41. doi: 10.1073/pnas.1415191111
41. Penninx, BW, Kritchevsky, SB, Yaffe, K, Newman, AB, Simonsick, EM, Rubin, S, et al. Inflammatory markers and depressed mood in older persons: results from the health, aging and body composition study. Biol Psychiatry (2003) 54:566–72. doi: 10.1016/S0006-3223(02)01811-5
42. Dantzer, R, O’Connor, JC, Lawson, MA, and Kelley, KW. Inflammation-associated depression: from serotonin to kynurenine. Psychoneuroendocrinology (2011) 36:426–36. doi: 10.1016/j.psyneuen.2010.09.012
43. Maes, M, Bosmans, E, De Jongh, R, Vandoolaeghe, E, and Neels, H. Increased serum IL-6 and IL-1 receptor antagonist concentrations in major depression and treatment resistant depression. Cytokine (1997) 9:853–8. doi: 10.1006/cyto.1997.0238
44. Raison, CL, Capuron, L, and Miller, AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol (2006) 27:24–31. doi: 10.1016/j.it.2005.11.006
45. O‘Connor, JC, Lawson, MA, André, C, Moreau, M, Lestage, J, Castanon, N, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry (2009) 14:511–22. doi: 10.1038/sj.mp.4002148
46. Krishnan, V, and Nestler, EJ. The molecular neurobiology of depression. Nature (2008) 455:894–902. doi: 10.1038/nature07455
47. Hashimoto, K. Brain-derived neurotrophic factor as a biomarker for mood disorders: an historical overview and future directions. Psychiatry Clin Neurosci (2010) 64:341–57. doi: 10.1111/j.1440-1819.2010.02113.x
48. Miller, AH, Maletic, V, and Raison, CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry (2009) 65:732–41. doi: 10.1016/j.biopsych.2008.11.029
49. Khandaker, GM, Zuber, V, Rees, JMB, Carvalho, L, Mason, AM, Foley, CN, et al. Shared mechanisms between coronary heart disease and depression: findings from a large UK general population-based cohort. Mol Psychiatry (2019) 25:1477–86. doi: 10.1038/s41380-019-0395-3
50. Lu, Y, Wang, Z, Georgakis, MK, Lin, H, and Zheng, L. Genetic liability to depression and risk of coronary artery disease, myocardial infarction, and other cardiovascular outcomes. J Am Heart Assoc (2021) 10:e017986. doi: 10.1161/JAHA.120.017986
51. Otte, C, McCaffery, J, Ali, S, and Whooley, MA. Association of a serotonin transporter polymorphism (5-HTTLPR) with depression, perceived stress, and norepinephrine in patients with coronary disease: the heart and soul study. Am J Psychiatry (2007) 164:1379. doi: 10.1176/appi.ajp.2007.06101617
52. Bujak, M, and Frangogiannis, NG. The role of Interleukin-1 in the pathogenesis of heart disease. Arch Immunol Ther Exp (2009) 57:165. doi: 10.1007/s00005-009-0024-y
53. Lesperance, F, Frasure-Smith, N, and Talajic, M. Major depression before and after myocardial infarction: its nature and consequences. Psychosom Med (1996) 58:99–110. doi: 10.1097/00006842-199603000-00001
54. Schins, A, Honig, A, Crijns, H, Baur, L, and Hamulyák, K. Increased coronary events in depressed cardiovascular patients: 5-HT2A receptor as missing link? Psychosom Med (2003) 65:729–37. doi: 10.1097/01.PSY.0000088596.42029.10
55. Nakatani, D, Sato, H, Sakata, Y, Shiotani, I, Kinjo, K, Mizuno, H, et al. Influence of serotonin transporter gene polymorphism on depressive symptoms and new cardiac events after acute myocardial infarction. Am Heart J (2005) 150:652–8. doi: 10.1016/j.ahj.2005.03.062
56. Riemer, TG, Villagomez Fuentes, LE, Algharably, EA, Schäfer, MS, Mangelsen, E, Fürtig, MA, et al. Do β-blockers cause depression? Systematic review and meta-analysis of psychiatric adverse events during β-blocker therapy. Hypertension (2021) 77:1539–48. doi: 10.1161/HYPERTENSIONAHA.120.16590
57. Ringoir, L, Pedersen, SS, Widdershoven, JW, Pouwer, F, Keyzer, JM, Romeijnders, AC, et al. Beta-blockers and depression in elderly hypertension patients in primary care. Fam Med (2014) 46:447.
58. Avorn, J, Everitt, DE, and Weiss, S. Increased antidepressant use in patients prescribed β-blockers. JAMA (1986) 255:357–60. doi: 10.1001/jama.1986.03370030077031
59. Thiessen, BQ, Wallace, SM, Blackburn, JL, Wilson, TW, and Bergman, U. Increased prescribing of antidepressants subsequent to ß-blocker therapy. Arch Intern Med (1990) 150:2286–90. doi: 10.1001/archinte.1990.00390220044009
60. Crane, PB, Oles, KS, and Kennedy-Malone, L. Beta-blocker medication usage in older women after myocardial infarction. J Am Acad Nurse Pract (2006) 18:463–70. doi: 10.1111/j.1745-7599.2006.00164.x
61. van Melle, JP, Verbeek, DE, van den Berg, MP, Ormel, J, van der Linde, MR, and de Jonge, P. Beta-blockers and depression after myocardial infarction: a multicenter prospective study. J Am Coll Cardiol (2006) 48:2209. doi: 10.1016/j.jacc.2006.07.056
62. Schleifer, SJ, Slater, WR, Macari-Hinson, MM, Coyle, DA, Kahn, M, Zucker, HD, et al. Digitalis and β-blocking agents: effects on depression following myocardial infarction. Am Heart J (1991) 121:1397–402. doi: 10.1016/0002-8703(91)90144-7
63. Ko, DT, Hebert, PR, Coffey, CS, Sedrakyan, A, Curtis, JP, and Krumholz, HM. β-blocker therapy and symptoms of depression, fatigue, and sexual dysfunction. JAMA (2002) 288:351–7. doi: 10.1001/jama.288.3.351
64. Ranchord, AM, Spertus, JA, Buchanan, DM, Gosch, KL, and Chan, PS. Initiation of β-blocker therapy and depression after acute myocardial infarction. Am Heart J (2016) 174:37–42. doi: 10.1016/j.ahj.2015.11.018
65. Koella, WP. CNS-related (side-) effects of β-blockers with special reference to mechanisms of action. Eur J Clin Pharmacol (1985) 28:55–63. doi: 10.1007/BF00543711
66. Kim, SW, Kang, HJ, Bae, KY, Shin, IS, Hong, YJ, Ahn, YK, et al. Interactions between pro-inflammatory cytokines and statins on depression in patients with acute coronary syndrome. Prog Neuro-Psychopharmacol Biol Psychiatry (2018) 80:250–4. doi: 10.1016/j.pnpbp.2017.07.003
67. Yu, XB, Zhang, HN, Dai, Y, Zhou, ZY, Xu, RA, Hu, LF, et al. Simvastatin prevents and ameliorates depressive behaviors via neuroinflammatory regulation in mice. J Affect Disord (2019) 245:939–49. doi: 10.1016/j.jad.2018.11.086
68. Chuang, CS, Yang, TY, Muo, CH, Su, HL, Sung, FC, and Kao, CH. Hyperlipidemia, statin use and the risk of developing depression: a nationwide retrospective cohort study. Gen Hosp Psychiatry (2014) 36:497–501. doi: 10.1016/j.genhosppsych.2014.05.008
69. Redlich, C, Berk, M, Williams, LJ, Sundquist, J, Sundquist, K, and Li, X. Statin use and risk of depression: a Swedish national cohort study. BMC Psychiatry (2014) 14:1–9. doi: 10.1186/s12888-014-0348-y
70. Parsaik, AK, Singh, B, Hassan, MM, Singh, K, Mascarenhas, SS, Williams, MD, et al. Statins use and risk of depression: a systematic review and meta-analysis. J Affect Disord (2014) 160:62–7. doi: 10.1016/j.jad.2013.11.026
71. Köhler-Forsberg, O, Gasse, C, Petersen, L, Nierenberg, AA, Mors, O, and Østergaard, SD. Statin treatment and the risk of depression. J Affect Disord (2019) 246:706–15. doi: 10.1016/j.jad.2018.12.110
72. Mandas, A, Congiu, MG, Abete, C, DessI, S, Manconi, PE, Musio, M, et al. Cognitive decline and depressive symptoms in late-life are associated with statin use: evidence from a population-based study of Sardinian old people living in their own home. Neurol Res (2014) 36:247–54. doi: 10.1179/1743132813Y.0000000287
73. Cham, S, Koslik, HJ, and Golomb, BA. Mood, personality, and behavior changes during treatment with statins: a case series. Drug Saf Case Rep (2016) 3:1–3. doi: 10.1007/s40800-015-0024-2
74. Hyyppä, MT, Kronholm, E, Virtanen, A, Leino, A, and Jula, A. Does simvastatin affect mood and steroid hormone levels in hypercholesterolemic men? A randomized double-blind trial. Psychoneuroendocrinology (2003) 28:181–94. doi: 10.1016/s0306-4530(02)00014-8
75. Zhang, L, Bao, Y, Tao, S, Zhao, Y, and Liu, M. The association between cardiovascular drugs and depression/anxiety in patients with cardiovascular disease: a meta-analysis. Pharmacol Res (2022) 175:106024. doi: 10.1016/j.phrs.2021.106024
76. Berk, M, Woods, RL, Nelson, MR, Shah, RC, Reid, CM, Storey, E, et al. Effect of aspirin vs placebo on the prevention of depression in older people: a randomized clinical trial. JAMA Psychiatry (2020) 77:1012–20. doi: 10.1001/jamapsychiatry.2020.1214
77. Berk, M, Mohebbi, M, Dean, OM, Cotton, SM, Chanen, AM, Dodd, S, et al. Youth depression alleviation with anti-inflammatory agents (YoDA-A): a randomised clinical trial of rosuvastatin and aspirin. BMC Med (2020) 18:1–2. doi: 10.1186/s12916-019-1475-6
78. Berk, M, Agustini, B, Woods, RL, Nelson, MR, Shah, RC, Reid, CM, et al. Effects of aspirin on the long-term management of depression in older people: a double-blind randomised placebo-controlled trial. Mol Psychiatry (2021) 26:5161–70. doi: 10.1038/s41380-021-01020-5
79. Lin, PY, Huang, SY, and Su, KP. A meta-analytic review of polyunsaturated fatty acid compositions in patients with depression. Biol Psychiatry (2010) 68:140–7. doi: 10.1016/j.biopsych.2010.03.018
80. Gopaldas, M, Zanderigo, F, Zhan, S, Ogden, RT, Miller, JM, Rubin-Falcone, H, et al. Brain serotonin transporter binding, plasma arachidonic acid and depression severity: a positron emission tomography study of major depression. J Affect Disord (2019) 257:495–503. doi: 10.1016/j.jad.2019.07.035
81. Kroenke, K, Spitzer, RL, and Williams, JBW. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med (2001) 16:606. doi: 10.1046/j.1525-1497.2001.016009606.x
82. Zigmond, AS, and Snaith, RP. The hospital anxiety and depression scale. Acta Psychiatr Scand (1983) 67:361. doi: 10.1111/j.1600-0447.1983.tb09716.x
83. Post-Myocardial Infarction Depression Clinical Practice Guideline Panel. AAFP guideline for the detection and management of post–myocardial infarction depression. Ann Fam Med (2009) 7:71–9. doi: 10.1370/afm.918
84. Thota, AB, Sipe, TA, Byard, GJ, Zometa, CS, Hahn, RA, McKnight-Eily, LR, et al. Collaborative care to improve the management of depressive disorders: a community guide systematic review and meta-analysis. Am J Prev Med (2012) 42:525–38. doi: 10.1016/j.amepre.2012.01.019
85. Krzowski, B, Peller, M, Boszko, M, Hoffman, P, Żurawska, N, Jaruga, K, et al. Mobile app and digital system for patients after myocardial infarction (after AMI): study protocol for a randomized controlled trial. Trials (2022) 23:522. doi: 10.1186/s13063-022-06463-x
86. Kang, SH, Baek, H, Cho, J, Kim, S, Hwang, H, Lee, W, et al. Management of cardiovascular disease using a mHealth tool: a randomized clinical trial. NPJ Digit Med (2021) 4:1–7. doi: 10.1038/s41746-021-00535-z
87. Marvel, FA, Spaulding, EM, Lee, MA, Yang, WE, Demo, R, Ding, J, et al. Digital health intervention in acute myocardial infarction. Circ Cardiovasc Qual Outcomes (2021) 14:e007741. doi: 10.1161/CIRCOUTCOMES.121.007741
88. Wagner, CJ, Musenbichler, C, Böhm, L, Färber, K, Fischer, AI, von Nippold, F, et al. LDL cholesterol relates to depression, its severity, and the prospective course. Prog Neuro-Psychopharmacol Biol Psychiatry (2019) 92:405–11. doi: 10.1016/j.pnpbp.2019.01.010
89. Gupta, A, Jadhav, AA, Petkar, SB, and Dubey, V. Study of lipid derangement in pyschiatric disorder. (2013).
90. Wysokiński, A, Strzelecki, D, and Kłoszewska, I. Levels of triglycerides, cholesterol, LDL, HDL and glucose in patients with schizophrenia, unipolar depression and bipolar disorder. Diabetes Metab Syndr Clin Res Rev (2015) 9:168–76. doi: 10.1016/j.dsx.2015.04.004
91. Swartz, CM. Albumin decrement in depression and cholesterol decrement in mania. J Affect Disord (1990) 19:173–6. doi: 10.1016/0165-0327(90)90088-P
92. Persons, JE, and Fiedorowicz, JG. Depression and serum low-density lipoprotein: a systematic review and meta-analysis. J Affect Disord (2016) 206:55–67. doi: 10.1016/j.jad.2016.07.033
93. Rabe-Jabłońska, J, and Poprawska, I. Levels of serum total cholesterol and LDL-cholesterol in patients with major depression in acute period and remission. Med Sci Monit (2000) 6:CR539-47.
94. Ernst, E, Saradeth, T, Seidl, S, Resch, KL, and Frischenschlager, O. Cholesterol and depression. Arch Intern Med (1994) 154:1166. doi: 10.1001/archinte.1994.00420100153025
95. Terao, T, Iwata, N, Kanazawa, K, Takano, T, Takahashi, N, Hayashi, T, et al. Low serum cholesterol levels and depressive state in human dock visitors. Acta Psychiatr Scand (2000) 101:231. doi: 10.1034/j.1600-0447.2000.101003231.x
96. Horsten, M, Wamala, SP, Vingerhoets, AD, and Orth-Gomer, K. Depressive symptoms, social support, and lipid profile in healthy middle-aged women. Psychosom Med (1997) 59:521–8. doi: 10.1097/00006842-199709000-00009
97. Su, KP, Tsai, SY, and Huang, SY. Cholesterol, depression and suicide. Br J Psychiatry (2000) 176:3.
98. Olusi, SO, and Fido, AA. Serum lipid concentrations in patients with major depressive disorder. Biol Psychiatry (1996) 40:1128–31. doi: 10.1016/S0006-3223(95)00599-4
99. Cepeda, MS, Kern, DM, Blacketer, C, and Drevets, WC. Low levels of cholesterol and the cholesterol type are not associated with depression: results of a cross-sectional NHANES study. J Clin Lipidol (2020) 14:515–21. doi: 10.1016/j.jacl.2020.06.001
100. Han, AL. Association between lipid ratio and depression: a cross-sectional study. Sci Rep (2022) 12:6190. doi: 10.1038/s41598-022-10350-5
101. Zhang, Q, Liu, Z, Wang, Q, and Li, X. Low cholesterol is not associated with depression: data from the 2005-2018 National Health and Nutrition Examination Survey. Lipids Health Dis (2022) 21:35. doi: 10.1186/s12944-022-01645-7
102. So, HC, Chau, CK, Cheng, YY, and Sham, PC. Causal relationships between blood lipids and depression phenotypes: a mendelian randomisation analysis. Psychol Med (2021) 51:2357–69. doi: 10.1017/S0033291720000951
103. Brown, SL, Salive, ME, Harris, TB, Simonsick, EM, Guarlnik, JM, and Kohout, FJ. Low cholesterol concentrations and severe depressive symptoms in elderly people. BMJ (1994) 308:1328–32. doi: 10.1136/bmj.308.6940.1328
104. Uguz, S, Bozdemir, N, Güzel, R, Burgut, R, Saatçi, E, and Akpinar, E. The relationship between cholesterol levels and depression in the elderly. Minerva Med (1995) 86:251–6.
105. Carney, RM, Freedland, KE, Miller, GE, and Jaffe, AS. Depression as a risk factor for cardiac mortality and morbidity: a review of potential mechanisms. J Psychosom Res (2002) 53:897–902. doi: 10.1016/S0022-3999(02)00311-2
106. Nemeroff, CB, and Musselman, DL. Are platelets the link between depression and ischemic heart disease? Am Heart J (2000) 140:57. doi: 10.1067/mhj.2000.109978
107. Markovitz, JH, and Matthews, KA. Platelets and coronary heart disease: potential psychophysiological mechanisms. Psychosom Med (1991) 53:643. doi: 10.1097/00006842-199111000-00006
108. Musselman, DL, Marzec, U, Davidoff, M, Manatunga, AK, Gao, F, Reemsnyder, A, et al. Platelet activation and secretion in patients with major depression, thoracic aortic atherosclerosis, or renal dialysis treatment. Depress Anxiety (2002) 15:91–101. doi: 10.1002/da.10020
109. Musselman, DL, Tomer, A, Manatunga, AK, Knight, BT, Porter, MR, Kasey, S, et al. Exaggerated platelet reactivity in major depression. Am J Psychiatry (1996) 153:1313. doi: 10.1176/ajp.153.10.1313
110. Williams, MS. Platelets and depression in cardiovascular disease: a brief review of the current literature. World J Psychiatry (2012) 2:114. doi: 10.5498/wjp.v2.i6.114
111. Pollock, BG, Laghrissi-Thode, F, and Wagner, WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. J Clin Psychopharmacol (2000) 20:137. doi: 10.1097/00004714-200004000-00004
112. Serebruany, VL, Glassman, AH, Malinin, AI, Sane, DC, Finkel, MS, Krishnan, RR, et al. Enhanced platelet/endothelial activation in depressed patients with acute coronary syndromes: evidence from recent clinical trials. Blood Coagul Fibrinolysis (2003) 14:563–7. doi: 10.1097/00001721-200309000-00008
113. Bonnet, F, Irving, K, Terra, JL, Nony, P, Berthezène, F, and Moulin, P. Anxiety and depression are associated with unhealthy lifestyle in patients at risk of cardiovascular disease. Atherosclerosis (2005) 178:339–44. doi: 10.1016/j.atherosclerosis.2004.08.035
114. Ruo, B, Rumsfeld, JS, Pipkin, S, and Whooley, MA. Relation between depressive symptoms and treadmill exercise capacity in the heart and soul study. Am J Cardiol (2004) 94:96–9. doi: 10.1016/j.amjcard.2004.03.035
115. Gehi, A, Haas, D, Pipkin, S, and Whooley, MA. Depression and medication adherence in outpatients with coronary heart disease: findings from the heart and soul study. Arch Intern Med (2005) 165:2508–13. doi: 10.1001/archinte.165.21.2508
116. Ziegelstein, RC, Fauerbach, JA, Stevens, SS, Romanelli, J, Richter, DP, and Bush, DE. Patients with depression are less likely to follow recommendations to reduce cardiac risk during recovery from a myocardial infarction. Arch Intern Med (2000) 160:1818–23. doi: 10.1001/archinte.160.12.1818
117. Miller, AH, and Raison, CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol (2016) 16:22–34. doi: 10.1038/nri.2015.5
118. Howren, MB, Lamkin, DM, and Suls, J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med (2009) 71:171–86. doi: 10.1097/PSY.0b013e3181907c1b
119. Empana, JP, Sykes, DH, Luc, G, Juhan-Vague, I, Arveiler, D, Ferrieres, J, et al. Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the prospective epidemiological study of myocardial infarction (PRIME). Circulation (2005) 111:2299–305. doi: 10.1161/01.CIR.0000164203.54111.AE
120. Yudkin, JS, Kumari, M, Humphries, SE, and Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: is interleukin-6 the link? Atherosclerosis (2000) 148:209–14. doi: 10.1016/S0021-9150(99)00463-3
121. L’abbate, A, Simonetti, I, Carpeggiani, C, and Michelassi, C. Coronary dynamics and mental arithmetic stress in humans. Circulation (1991) 83:II94.
122. Meredith, IT, Broughton, A, Jennings, GL, and Esler, MD. Evidence of a selective increase in cardiac sympathetic activity in patients with sustained ventricular arrhythmias. N Engl J Med (1991) 325:618–24. doi: 10.1056/NEJM199108293250905
123. Schlaich, MP, Kaye, DM, Lambert, E, Sommerville, M, Socratous, F, and Esler, MD. Relation between cardiac sympathetic activity and hypertensive left ventricular hypertrophy. Circulation (2003) 108:560–5. doi: 10.1161/01.CIR.0000081775.72651.B6
124. Spieker, LE, Hürlimann, D, Ruschitzka, F, Corti, R, Enseleit, F, Shaw, S, et al. Mental stress induces prolonged endothelial dysfunction via endothelin-a receptors. Circulation (2002) 105:2817–20. doi: 10.1161/01.CIR.0000021598.15895.34
125. Dhar, AK, and Barton, DA. Depression and the link with cardiovascular disease. Front Psychiatry (2016) 7:33. doi: 10.3389/fpsyt.2016.00033
126. Carney, RM, Blumenthal, JA, Stein, PK, Watkins, L, Catellier, D, Berkman, LF, et al. Depression, heart rate variability, and acute myocardial infarction. Circulation (2001) 104:2024. doi: 10.1161/hc4201.097834
127. Carney, RM, Freedland, KE, and Veith, RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med (2005) 67:29–33. doi: 10.1097/01.psy.0000162254.61556.d5
128. Stein, PK, Carney, RM, Freedland, KE, Skala, JA, Jaffe, AS, Kleiger, RE, et al. Severe depression is associated with markedly reduced heart rate variability in patients with stable coronary heart disease. J Psychosom Res (2000) 48:493. doi: 10.1016/s0022-3999(99)00085-9
129. Kleiger, RE, Miller, JP, Bigger, JT Jr, and Moss, AJ. Decreased heart rate variability and its association with increased mortality after acute myocardial infarction. Am J Cardiol (1987) 59:256–62. doi: 10.1016/0002-9149(87)90795-8
130. Otte, C, Marmar, CR, Pipkin, SS, Moos, R, Browner, WS, and Whooley, MA. Depression and 24-hour urinary cortisol in medical outpatients with coronary heart disease: the heart and soul study. Biol Psychiatry (2004) 56:241–7. doi: 10.1016/j.biopsych.2004.06.003
131. Wong, ML, Kling, MA, Munson, PJ, Listwak, S, Licinio, J, Prolo, P, et al. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A (2000) 97:325–30. doi: 10.1073/pnas.97.1.325
132. Björntorp, P, and Rosmond, R. The metabolic syndrome—a neuroendocrine disorder? Br J Nutr (2000) 83:S49–57. doi: 10.1017/S0007114500000957
133. Malik, S, Wong, ND, Franklin, SS, Kamath, TV, L’Italien, GJ, Pio, JR, et al. Impact of the metabolic syndrome on mortality from coronary heart disease, cardiovascular disease, and all causes in United States adults. Circulation (2004) 110:1245–50. doi: 10.1161/01.CIR.0000140677.20606.0E
134. Straznicky, NE, Lambert, EA, Lambert, GW, Masuo, K, Esler, MD, and Nestel, PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metabol (2005) 90:5998–6005. doi: 10.1210/jc.2005-0961
135. McCaffery, JM, Frasure-Smith, N, Dubé, MP, Théroux, P, Rouleau, GA, Duan, Q, et al. Common genetic vulnerability to depressive symptoms and coronary artery disease: a review and development of candidate genes related to inflammation and serotonin. Psychosom Med (2006) 68:187–200. doi: 10.1097/01.psy.0000208630.79271.a0
Keywords: major depressive disorder, post myocardial infarction, HPA axis, coagulation, inflammation, pathophysiological
Citation: Garrels E, Kainth T, Silva B, Yadav G, Gill G, Salehi M and Gunturu S (2023) Pathophysiological mechanisms of post-myocardial infarction depression: a narrative review. Front. Psychiatry 14:1225794. doi: 10.3389/fpsyt.2023.1225794
Edited by:
Sen Li, Beijing University of Chinese Medicine, ChinaReviewed by:
Jane Elizabeth Persons, The University of Iowa, United StatesJennifer Glaus, Centre Hospitalier Universitaire Vaudois (CHUV), Switzerland
Copyright © 2023 Garrels, Kainth, Silva, Yadav, Gill, Salehi and Gunturu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sasidhar Gunturu, sashi.dr@gmail.com
 Eric Garrels
Eric Garrels Tejasvi Kainth
Tejasvi Kainth Briana Silva
Briana Silva Garima Yadav
Garima Yadav Gurtej Gill1
Gurtej Gill1 Mona Salehi
Mona Salehi Sasidhar Gunturu
Sasidhar Gunturu