- 1Department of Rehabilitation, The Hong Kong Polytechnic University, Kowloon, Hong Kong, SAR China
- 2Mental Health Research Centre, The Hong Kong Polytechnic University, Kowloon, Hong Kong, SAR China
Objectives: Transcranial electric stimulation (TES) and repetitive transcranial magnetic stimulation (rTMS) have experienced significant development in treating insomnia. This review aims to examine the effectiveness of randomized sham-controlled trials of TES and rTMS in improving insomnia and examine potential moderators associated with the effect of the treatment.
Methods: Nine electronic databases were searched for studies comparing the effects of TES/rTMS with sham group on insomnia from the inception of these databases to June 25, 2021, namely, Medline, Embase, PsycINFO, CINAHL, Cochrane Library, Web of Science, PubMed, ProQuest Dissertation and Thesis, and CNKI. Meta-analyses were conducted to examine the effect of TES and rTMS in treating insomnia. Univariate meta-regression was performed to explore potential treatment moderators that may influence the pooled results. Risk of bias was assessed by using the Cochrane Risk of Bias Tool.
Results: A total of 16 TES studies and 27 rTMS studies were included in this review. The pooled results indicated that there was no significant difference between the TES group and the sham group in improving objective measures of sleep. rTMS was superior to its sham group in improving sleep efficiency, total sleep time, sleep onset latency, wake up after sleep onset, and number of awakenings (all p < 0.05). Both TES and rTMS were superior to their sham counterparts in improving sleep quality as measured by the Pittsburgh Sleep Quality Index at post-intervention. The weighted mean difference for TES and rTMS were −1.17 (95% CI: −1.98, −0.36) and −4.08 (95% CI: −4.86, −3.30), respectively. Gender, total treatment sessions, number of pulses per session, and length of treatment per session were associated with rTMS efficacy. No significant relationship was observed between TES efficacy and the stimulation parameters.
Conclusions: It seems that TES and rTMS have a chance to play a decisive role in the therapy of insomnia. Possible dose-dependent and gender difference effects of rTMS are suggested.
Introduction
As one of the most commonly reported sleep complaints, insomnia affects approximately 10–35% of the general population (1). According to the diagnosis criteria described in the fifth edition of the Diagnostic and Statistical Manual of Mental Disorders and the third edition of the International Classification of Sleep Disorders, insomnia disorder is a predominant complaint of dissatisfaction with sleep quantity or quality, associated with one (or more) of the following symptoms: difficulty initiating sleep, difficulty maintaining sleep, and early-morning awakening. The symptom is presented for at least three nights per week for at least 3 months. It is associated with distress or impairments in daytime function (2, 3). Insomnia could exist alone or be comorbid with other physical or psychiatric disorders, such as chronic pain, Parkinson's disease, cancer, anxiety, or depression (4–7). It decreases the psychological wellbeing and quality of life in people suffering from it and is frequently associated with mood disorders, driving accidents, and a greater prevalence of physical impairment (7, 8).
The cortical hyperarousal plays a central role in the etiology of insomnia. Many studies reported that people with insomnia have a higher level of physiological arousal (9, 10). Therefore, reducing the arousal level may facilitate sleep. Various therapeutic approaches have been used and investigated to improve the sleep of people with insomnia. Pharmacological treatments are proven to be effective and available but related to abuse, dependence, and adverse effects (11). Psychological and behavioral therapies, such as cognitive behavior therapy, targeting somatic and cognitive arousal, have demonstrated promising efficacy for relieving insomnia (12, 13) but remained underutilized due to highly demanding resources (14).
In recent years, non-invasive brain stimulation (NIBS) techniques have experienced significant development and gained increasing attention from researchers. Transcranial electric stimulation (TES) and repetitive transcranial magnetic stimulation (rTMS) are the two most popular types of NIBS. They share common characteristics of being relatively painless, safe, and well-tolerated with different mechanisms (15). TES is a neuromodulation approach that applies a low-intensity electrical current to the cerebral cortex of the brain. It includes cranial electrotherapy stimulation (CES), transcranial direct current stimulation (tDCS), transcranial alternative current stimulation (tACS), and transcranial random noise stimulation (tRNS). CES is a portable device that usually applies pulsed and low-level micro-current (<1 mA) stimulation to the brain via electrodes clipped onto the earlobes. It was approved by the US Food and Drug Administration for the treatment of insomnia, anxiety, and depression (16). tDCS modulates cortical activity by employing a constant, low-intensity current (0.5–2 mA) to the scalp over a pair of saline-sponge electrodes (17). The two electrodes are placed according to the international 10–20 electrode placement system (18). Generally speaking, anodal stimulation increases cortical excitability, while cathodal stimulation induces an opposite effect, i.e., reducing the cortical excitability (19). tACS and tRNS are relatively new TES techniques, which aim to increase the cortical excitability in a way similar to tDCS. However, instead of giving a steady and constant current between the two sites, tACS delivers a non-constant current to the brain so as to modulate the neural oscillations (20), and tRNS gives random frequencies between 0.1 and 640 Hz with a random noise distribution (21, 22). Currently, the mechanism of the effect of TES on insomnia is not well-established. It is hypothesized that TES could interfere with slow oscillation in the brain, which could increase the slow wave activity and enhance the low-frequency electroencephalogram (EEG) activity (i.e., a marker of arousal) (23–25). Due to its portable features and convenience to use, TES is suitable for self-administration at home (26, 27). However, TES is not free of limitations, and it has been criticized for poor spatial accuracy (28).
rTMS, on the other hand, is another appealing approach that combines both neurostimulation and neuromodulation techniques (29). It was developed in the 1980s and had shown therapeutic potential in improving insomnia (30, 31). Unlike TES, which stimulates the brain by delivering a weak current, rTMS utilizes electromagnetic induction. During rTMS stimulation, an electromagnetic coil is placed over the scalp. The coil could generate rapidly changing focal magnetic pulses that induce an electrical current to stimulate the neurons (29, 32, 33). rTMS has been classified into high (fast) frequency (>1 Hz) and low (slow) frequency (≤1 Hz) (34). rTMS at high frequency tends to have an excitatory effect, while rTMS at low frequency appears to have an inhibitory effect on the cortex (35). rTMS is regarded as a parameter-dependent technique. Its therapeutic effect could be influenced by the characteristics of the participants and a range of stimulation parameters, such as frequency, number of sessions, number of pulses/session, length of treatment/session, total number of pulses, and stimulation site (36, 37). Regarding the mechanism of rTMS on insomnia, it has been suggested that rTMS may reduce the state of hyperarousal and regulate brain plasticity by increasing the release of sleep-related hormones, such as brain-derived neurotrophic factor and gamma-aminobutyric acid (38, 39). rTMS is usually delivered in clinical settings. Compared to TES, rTMS has a better focality of stimulation and time resolution. Meanwhile, rTMS has significant limitations in terms of cost and poor portability. It also requires constant attention from the therapist during the treatment (40).
Although several reviews have been conducted to summarize the effectiveness of NIBS techniques for insomnia (15, 41–45), they were either narrative summaries on available evidence without meta-analysis (15, 41) or merely focused on one form of NIBS (42–45). Moreover, the moderators of the therapeutic effect of TES and rTMS on insomnia have not been extensively studied. Therefore, we aimed to review the therapeutic effects of TES and rTMS for the treatment of insomnia and investigate differences between them and the potential moderators associated with the treatment while restricting our review to randomized sham-controlled trials.
Methods
Search Strategy
Nine electronic databases were searched from the inception of these databases to June 25, 2021, including Medline, Embase, PsycINFO, CINAHL, Cochrane Library, Web of Science, PubMed, ProQuest Dissertation and Thesis, and CNKI. The retrieved abstracts and full-text articles were screened according to the PICOS framework.
The included studies should meet the following criteria: (1) Population: people with insomnia according to clinical diagnosis or had insomnia secondary to or comorbid with other physical or mental diseases or had a subjective complaint of insomnia without a clinical diagnosis; (2) Intervention: TES/rTMS techniques being employed as monotherapy or augmentation therapy for insomnia, such as TES/rTMS plus usual care or other types of intervention, were both eligible if the main aim of the study was to examine the effect of TES/rTMS and the sole difference between intervention and control was TES/rTMS. The search terms included transcranial electric stimulation or TES or cranial electrical stimulation OR CES OR cranial electric stimulat* OR electrotherap* OR fisher wallace stimulat* OR alpha-stim OR Neuroelectric therapy OR Transcerebral electrotherapy OR Transcranial stimulation OR tDCS OR Brain Polarization OR Electric Stimulation OR Electric Polarization OR transcranial alternative current stimulation OR tACS OR transcranial random noise stimulation OR tRNS OR transcranial magnetic stimulation OR TMS OR non-invasive brain stimulation OR NIBS; (3) Comparison: studies compare TES/rTMS with a sham group; (4) Outcome: each study must have reported at least one of the following objective or subjective measurements of insomnia: sleep onset latency (SOL), total sleep time (TST), wake after sleep onset (WASO), sleep efficiency (SE), number of awakenings (NA), or subjective sleep quality—for example, polysomnography (PSG) is considered a “gold standard” for the diagnosis of sleep disorders. The Pittsburgh Sleep Quality Index (PSQI) and Insomnia Severity Index (ISI) are popular subjective instruments of sleep quality and severity of sleep disturbance; and (5) Study design: only randomized controlled trial (RCT) was included. The search was limited to articles in English and Chinese languages. Studies which failed to meet the abovementioned inclusion criteria were excluded.
Two authors (JL and DL) independently screened the title, abstract, and full text of the studies and determined the study eligibility. Any disagreement was resolved by consensus through a discussion or further consultation with a third author (HX) if needed.
Data Extraction
Two authors independently extracted data from the included articles. The characteristics of the study were extracted and tabulated according to authors, year of publication, country, types of insomnia, diagnosis, age, percentage of males, sample size, attrition rate, treatment parameters, sham procedure, main instruments used for outcome measurements, and assessment time point. The treatment parameters included electrode/coil position, current intensity, stimulation frequency, magnetic field strength, (resting) motor threshold, number of pulses per session, duration of the treatment per session, and total number of sessions.
Assessment of the Risk of Bias
The risk of bias of the included study was assessed using the Cochrane Risk of Bias Tool. The assessment was done across seven domains of bias: (1) random sequence generation, (2) allocation concealment, (3) blinding of participants and personnel, (4) blinding of care providers, (5) blinding of outcome assessment, (6) incomplete outcome data, and (7) selective reporting. Each study was ranked as having low, high, or unclear risk of bias for each of the potential sources of bias. Discrepancies were discussed until a consensus was reached.
Statistical Analysis
The data was analyzed using the Review Manager (version 5.4). Changes in the continuous outcome were expressed as weighted mean difference (WMD) when the outcome was measured with the same scale. Otherwise, standard mean difference was used. Changes in dichotomous outcomes were expressed as relative risks (RR). The corresponding 95% confidence interval (CI) was calculated. To estimate the statistical heterogeneity of the intervention effects among studies, the I2 statistic was used, in which I2 < 25%, 25–50%, and >50% were considered low, moderate, and high heterogeneity, respectively. Fixed-effects model was performed to calculate the pooled mean difference if I2 < 50%. Otherwise, random-effects model was performed. When data was available, immediate, short-term, and long-term effects were also analyzed and compared. In this review, the immediate follow-up was defined as 0 to <1 week post-intervention, the short-term follow-up was defined as 1 to 4 weeks post-intervention, and the long-term follow-up was defined as >4 weeks post-intervention. To further explore the heterogeneity of the results, sensitivity analyses were limited to studies among participants with primary insomnia and having a lower or unclear risk of bias.
To explore potential treatment moderators that may influence the pooled results, univariate meta-regression of continuous moderators was performed using Comprehensive Meta-Analysis software (version 3.0). The analyses were restricted to studies with at least six effect sizes for a continuous variable and four effect sizes per group for a categorical variable (46). The following possible moderators were considered: mean age of the participants, percentage of males, stimulation intensity (milliampere), frequency (Hz), number of pulses per session, total number of sessions, number of weekly sessions, length of each session, and stimulation site. Multivariable meta-regression analyses were not conducted to avoid exceeding the power of the pooled studies (47). All p-values were set at 0.05 level (two-tailed).
In the presence of potential publication bias, funnel plots and Egger's regression test were applied using Comprehensive Meta-Analysis software (version 3.0). The funnel plots were analyzed when at least 10 studies were included in the meta-analysis.
Results
A total of 843 citations were identified from the databases, and 129 duplicates were removed. After screening the title and abstract, 44 full-text articles were retrieved for further assessment. Of these, eight studies were excluded for the following reasons: abstract without full text (n = 2), ongoing trial without outcome data (n = 2), completed RCT without reporting data (n = 2), duplicated publication with data from the same source of study (n = 1), and research proposal (n =1). Besides these, seven additional studies were identified from the hand search of the reference lists. In total, 43 studies were included in this review, including 16 TES studies and 27 rTMS studies (see Figure 1).
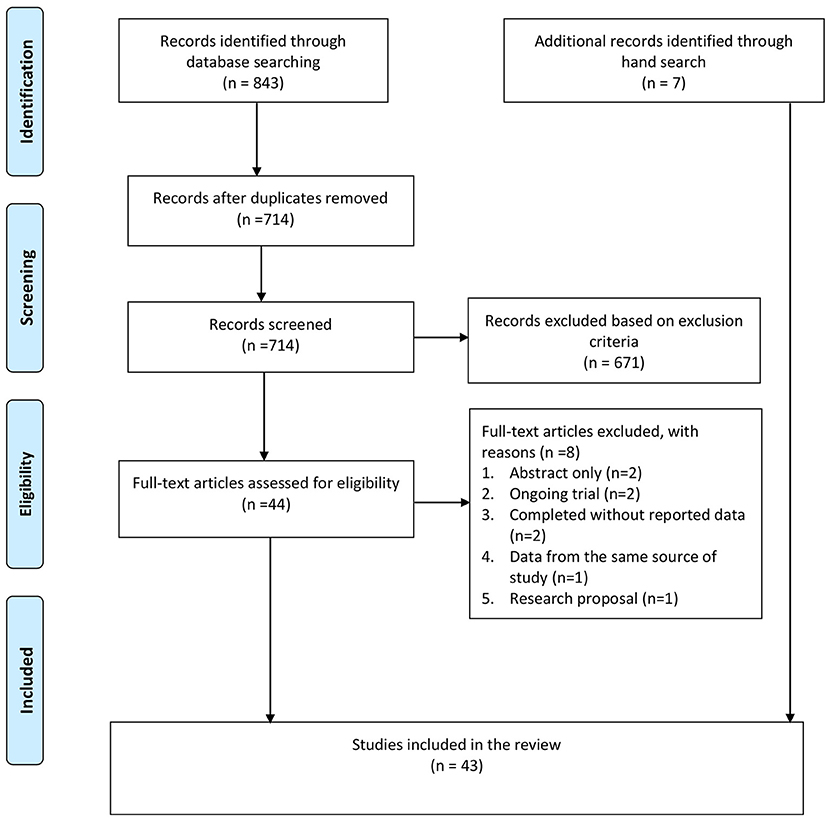
Figure 1. PRISMA flow chart. Adapted from Moher et al. (48).
Among the TES studies, eight of them used CES (49–56), seven applied tDCS (57–63), and one examined tACS (64). No study was identified to have examined the effectiveness of tRNS in insomnia. A summary of the characteristics of TES and rTMS is described in the following section.
Characteristics of TES Studies
Participants
The groups of individuals treated by TES were heterogeneous, including people with cancer, fibromyalgia, post-polio, HIV, substance abuse, or women after menopause. The diagnosis of insomnia varied across studies. Of nine studies that reported a diagnosis of insomnia, three used DSM, ICSD, or International Statistical Classification of Diseases and Related Health Problems (ICD-10) criteria, and three studies used the cutoff scores of PSQI, ISI, and General Sleep Disturbance Scale (GSDS). One study employed the diagnosis of neurotic and personality disorders combined with insomnia, and one study adopted sleep parameters to determine insomnia. The sample size in each study ranged from 10 to 167, with a median number of 32. The mean age of all participants was 52.5 (SD = 7.36) years. There were considerably more female subjects than male subjects, with the male-to-female ratio being 1:3.2. The attrition rate ranged from 0.0 to 19.3%.
Electrode Position
Usually, most CES studies used ear-clip electrodes attached to the earlobes (n = 5). All tDCS studies applied one anode and one cathode, except that one study (57) used two anodes and one cathode. The anode was located according to the International 10–20 EEG system. Four studies applied the anode over the C3/C4 area. Three tDCS studies located the anode at the right or left dorsolateral prefrontal cortex (DLPFC) area, and one study placed the anode at the right inferior frontal cortex near F10. The cathode locations also varied. Three studies located the cathode at the contralateral supraorbital region, two studies chose the right/left DLPFC area, one study put it on the left shoulder, and another study placed it on the contralateral upper arm. The tACS study was composed of three electrodes; one was placed over the forehead, and two others were placed over the mastoid area (see Supplementary Table 1).
Stimulation Parameters
A low current of 0.1 mA was used in the majority of CES studies. The current intensity ranged from 1.5 to 2 mA in the tDCS studies and was 15 mA in the tACS study. The dosage and follow-up frame of the intervention varied widely. The duration of each session lasted from 5 to 90 min, with the majority of CES studies lasting for 60 min and of tDCS studies lasting for 20 min. The majority of TES was administered once daily for a duration of 5 days to 4 weeks. The majority of the studies only measured the outcome immediately after the end of the intervention. Five studies collected follow-up assessment at 1–4 weeks post-intervention, and one study investigated the effect of CES at 2 years of follow-up (see Supplementary Table 1).
Sham TES Procedure
All TES studies used a similar type of sham procedure, which involved no electrical current or gave a few seconds of electrical stimulation at the beginning/end of the intervention.
Characteristics of rTMS Studies
Participants
As summarized in Supplementary Table 2, a total of 27 studies applied rTMS technique (65–91). The diagnosis of insomnia also differed. Of 25 studies that reported the diagnosis of insomnia, 14 studies used the DSM or ICD criteria, six studies used the Chinese Classification of Mental Disorders, three studies adopted the diagnosis and treatment of adult insomnia in China, and two studies employed the cutoff score of PSQI. Most rTMS studies include patients with primary insomnia (n = 18). The sample size in each study varied from 19 to 160, with a median number of 78. The mean age of all participants was 47.5 (SD = 10.26), and the male-to-female ratio was 1:1.3. The attrition rate ranged from 0.0 to 13.3%.
Stimulation Site
Regarding rTMS trials, the majority of them targeted the right DLPFC (n = 19). Other sites included the left prefrontal cortex (PFC) (n = 2), the right lateral and middle PFC (n = 1), the vertex (n = 1), the right posterior parietal cortex (P4 electrode site) (n = 1), the raphe nuclei (n = 1), the middle of the bilateral frontal/occipital/temple cortex (n = 1), and certain acupoints (n = 1). One rTMS study did not describe the stimulation site (see Supplementary Table 2).
rTMS Stimulation Parameters
The intensity of the rTMS studies also varied. The stimulation frequency in most studies ranged from 0.5 to 1 Hz, the stimulation intensity ranged from 80 to 130% motor threshold, and the number of pulses per session ranged from 1,100 to 2,400. The duration of each session lasted from 10 to 90 min, with the majority of interventions lasting for 20 min (n = 15). The majority of rTMS was administered on consecutive days or 5 days per week for a duration of 2 to 4 weeks. Most studies measured the outcome immediately after the intervention. Seven studies also collected data at 1–22 weeks of follow-up (see Supplementary Table 2).
Sham rTMS Procedure
The common sham methods applied in rTMS studies were using a 90°/180° tilted coil (n = 13) or an inactive coil with/without a sound effect (n = 11).
Outcome Measurements of TES and rTMS Studies
Various measures have been applied in the included studies. Laboratory-based PSG, sleep diary, EEG, and actigraphy provided an objective evaluation of the sleep parameters. The most popular objective measurement was PSG-measured SOL (n =13), followed by PSG-measured WASO (n = 10), PSG-measured SE (n = 9), PSG-measured TST (n = 9), and PSG-measured NA (n = 6). Among the subjective measurements of sleep, the PSQI was the most frequently used measurement (n = 33), followed by the GSDS (n = 3), ISI (n = 2), and Krakow Sleep Score (n = 1) (see Supplementary Tables 1, 2).
Quality Assessment
Overall, the risk of bias of the included studies was considered mediocre. The majority of the studies failed to report a detailed methodology. Moreover, 23 of them did not report an adequate method of random sequence generation. Only three studies described the allocation concealment. All studies were rated as having a low risk of bias in blinding the participants because of the sham procedure. However, it was difficult to blind the practitioner of the assigned intervention in most of the RCTs. Only one study blinded the practitioner via adopting pre-set sham devices provided by a device manufacturer. A total of 14 studies reported blinding of outcome assessment, while the rest of the 29 studies did not. Regarding the outcome data, 39 studies were considered as having a low risk of attrition bias (dropout rate <10%, used intention-to-treat analysis). In comparison, a high risk of attrition bias was reported in the remaining four studies (dropout rate >10%). All studies, except one, reported complete outcome data (see Supplementary Figures 1, 2).
Synthesis of Results
TES Studies
1) Objective Measures of Sleep Parameters
Supplementary Table 3 presents the results of a meta-analysis on PSG and EEG measures of sleep parameters in TES studies. Two RCTs evaluated the effectiveness of TES on SE and total TST, and three RCTs reported the results of TES on SOL. The findings from the random-effects model indicated that there was no significant difference between the TES group and the sham group in improving SE (WMD: −4.86, 95% CI: 17.29, 7.57, p = 0.0003), TST (WMD: −7.75, 95% CI: −42.25, 26.74, p = 0.66), or SOL (WMD: 1.24, 95% CI: −10.05, 12.52, p = 0.83). Between studies, substantial heterogeneity among these sleep parameters existed, which ranged from 79 to 95%.
2) Subjective Measurement of Sleep Quality—PSQI
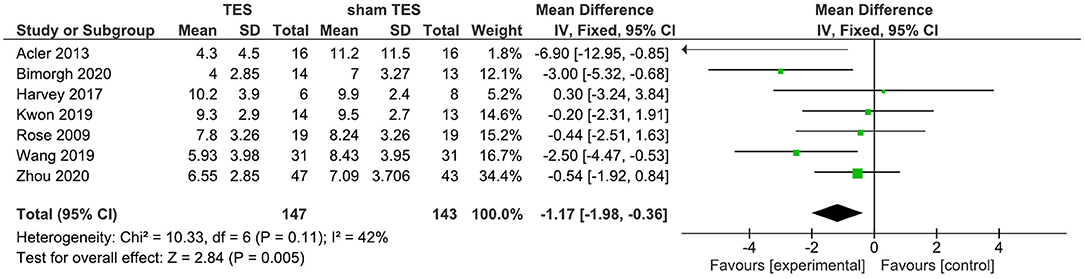
A total of seven studies reported that the results of TES contrast with those of sham TES in terms of changes of the PSQI total score immediately after the intervention. Findings from the fixed-effects model showed that active TES were superior to their sham counterparts in improving the PSQI total score. The WMD for TES was −1.17 (95% CI: −1.98, −0.36) (Figure 2). Nevertheless, moderate heterogeneity existed among TES studies (I2 = 42%).
Supplementary Table 4 displays the results of univariate analyses of moderators for effects of TES on sleep quality as measured by PSQI. The results of the meta-regression of TES studies revealed that age, percentage of males, current intensity, total number of treatment sessions, number of weekly sessions, and duration of each session were not significant moderators for the effects of TES on sleep quality as measured by the PSQI.
rTMS Studies
1) Objective Measures of Sleep Parameters
The results of the meta-analyses on PSG and the actigraphy measures of SE, TST, SOL, WASO, and NA in rTMS studies are shown in Supplementary Table 5. Eight studies reported on the effectiveness of rTMS on SE and TST. The pooled results indicated that rTMS was superior to the sham group in improving SE (random-effects model: WMD −7.91; 95% CI −3.70, 12.12; p < 0.00001) and TST (random-effects model: WMD −37.25, 95% CI −21.51, 52.98). A total of 12 studies reported the effect of rTMS on SOL, and the WMD for rTMS was −9.78 (95% CI: −13.25, −6.31). Eleven studies examined the effect of rTMS on WASO. The findings from the random-effects model indicated that rTMS was superior to the sham counterpart in improving WASO (random-effects model: WMD: −27.86; 95% CI: −38.70, −17.02; p < 0.00001). However, the pooled data on SE, TST, SOL, and WASO had substantial heterogeneity, and the I2 ranged from 80 to 96%. In addition, seven studies evaluated the effect of rTMS on NA. According to the fixed-effects model, rTMS significantly reduced the NA (WMD: −1.06; 95% CI:−1.53, 0.59; p < 0.00001). Mild heterogeneity between studies was found (I2 = 22%).
2) Subjective Measurements of Sleep Quality—PSQI
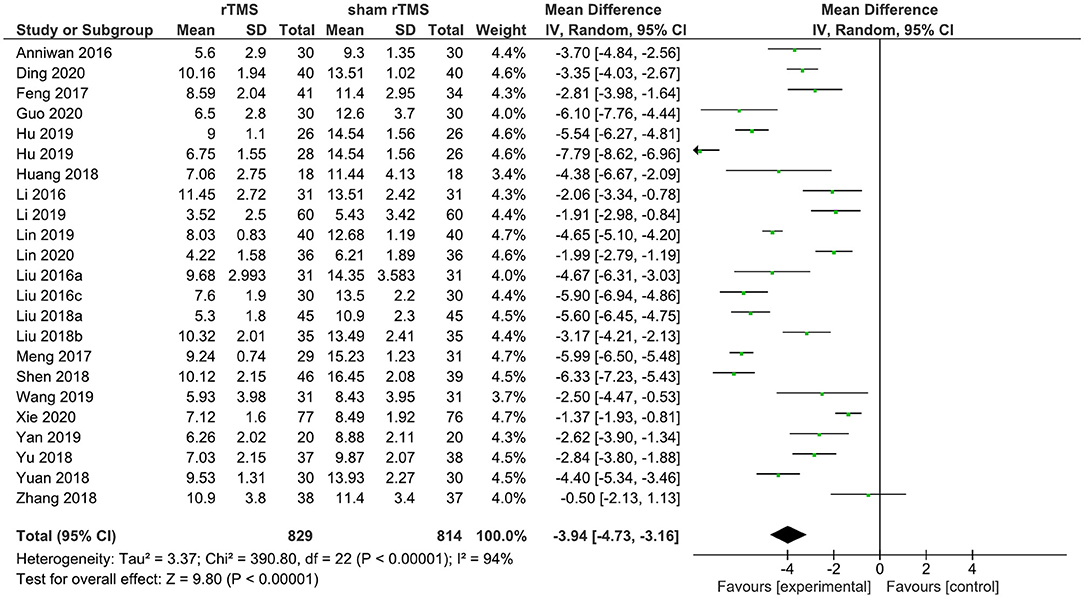
A total of 22 rTMS studies provided data on the PSQI total score after the completion of the intervention. The meta-analysis showed the evidence of a positive effect of rTMS on sleep quality compared to the sham group (WMD: −4.08; 95% CI: −4.86, −3.30, p < 0.00001). However, pronounced heterogeneity was also recorded between studies (I2 = 94%; Figure 3).
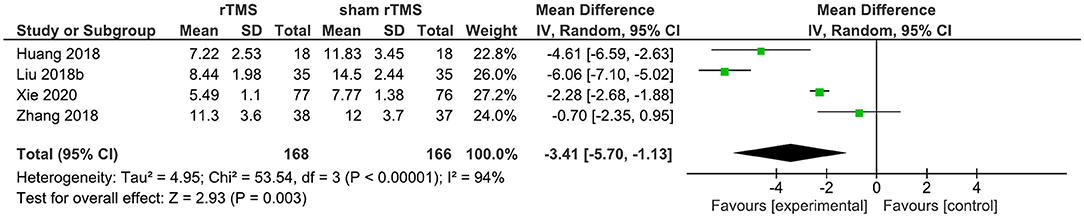
Four studies provided data on the PSQI total score in the short term (1–4 weeks post-intervention). rTMS, as compared to sham rTMS, resulted in a statistically significant improvement in the PSQI total score (WMD: −3.41; 95% CI: −5.70, −1.13; p = 0.003). Significant heterogeneity existed (I2 = 94%; Figure 4).
Supplementary Table 6 displays the results of the univariate analyses of moderators for the effects of rTMS on insomnia. The results of the meta-regression of rTMS studies showed that a greater number of total treatment sessions was associated with a greater improvement in SE and PSQI score (both p < 0.05). The results also indicated a significant inverse relationship between the length of rTMS treatment per session and the improvement in the TST and PSQI scores (both p < 0.05). Male gender and a greater number of pulses per session were associated with a significant improvement in the PSQI total score at post-intervention (all p < 0.05), whereas age and stimulation site were insignificant.
Sensitivity Analysis
Regarding the high level of heterogeneity in the studies, especially in TES studies, we restricted the meta-analysis to participants with primary insomnia and the results of the sensitivity analysis by removing five TES studies that confirmed the findings of the entire dataset of seven TES studies (see Supplementary Figure 3). The results of the sensitivity analysis by removing four rTMS studies also confirmed the findings of the entire dataset of 22 rTMS studies (see Supplementary Figure 4). Furthermore, the sensitivity analysis, by excluding one TES study and two rTMS studies with a high risk of bias, did not change the overall estimate of the effects of TES and rTMS (see Supplementary Figures 5, 6).
Publication Bias
Egger's test was used to assess the publication bias for the effect of rTMS on the objective and subjective measures of insomnia. Egger's test indicated that there was no evidence of significant publication bias in rTMS studies that reported WASO (intercept = −12.90; two-tailed 95% CI, −32.89, 7.09; p = 0.18) (Supplementary Figure 7), while potential publication bias was found in rTMS studies that reported SOL (intercept = −10.02; two-tailed 95% CI, −19.69, −0.34; p = 0.04) (Supplementary Figure 8) and PSQI score (intercept = −11.77; two-tailed 95% CI, −14.99, −8.56; p = 0.000) (Supplementary Figure 9).
Adverse Events
Mild and temporary adverse events were reported in tDCS and rTMS studies. The most frequently observed adverse events in tDCS studies were dizziness, discomfort, or itching at the stimulation site. The frequently reported adverse events in rTMS studies were headache, dizziness, pain at the stimulation site, discomfort, itchiness, muscle spasm, and constipation (see Supplementary Table 7).
The meta-analyses of the safety outcomes are summarized in Supplementary Table 8. The occurrence of any adverse events, dizziness, or headache did not differ significantly between the NIBS group and the sham NIBS group and in their subgroup analysis. However, a marginally significant association was found between NIBS and the complaints of discomfort by the participants (RR = 5.00; 95% CI: 0.89, 27.97; p = 0.07, I2 = 0%). Furthermore, pain was a common side effect of rTMS and was reported in six studies. The participants in the rTMS group were significantly more likely to experience more pain at the stimulation site than those in the sham rTMS group (RR = 2.58; 95% CI: 1.14, 5.84; p = 0.02, I2 = 0%).
Discussion
This review extends and improves previous reviews on the effectiveness of NIBS on insomnia. It examined the effects of TES and rTMS using a meta-analytical approach in treating insomnia and examining the potential moderators associated with the treatment. Overall, both techniques could be, respectively, considered as an effective and safe approach for insomnia, while the data suggested a greater effect size with rTMS than TES in improving SE, SOL, TST, and PSQI total score. In the following section, the possible explanations of differences in the treatment effectiveness between TES and rTMS, the dose-dependent effect of rTMS, and the gender difference in the effect of rTMS are discussed.
Findings from our review support the use of TES for insomnia. TES was superior compared to their sham counterparts in improving PSQI total score. However, it failed to demonstrate superiority in objective measures of sleep parameters. Compared to rTMS, TES also showed less strong evidence in improving sleep-related outcomes. The differences in effectiveness may be explained by a number of factors. Firstly, given the differences in the characteristics of the participants and the intervention, clinical heterogeneity should be considered. Secondly, the analysis was based on a relatively small number of TES studies. More TES studies should be conducted to confirm the superiority of either approach. Thirdly, the underlying mechanism of the two techniques could be another possible explanation for the difference in the therapeutic outcomes. For TES, it was assumed that only some fractions of the current could pass through the scalp. For rTMS, the magnetic field generated by stimulating the coil could pass through the scalp directly and reach the deep cortex cortical without energy loss (37, 92). The mechanism underlying the therapeutic effects of TES and rTMS need further exploration, while the findings from this review are still encouraging since TES devices have many advantages and have the potential to be easily promoted in the community—for example, most TES devices are portable and wearable, and they could even be self-administered at home by people with sleep problems. With the portable character, their effect on facilitating sleep could be enlarged.
The results of this review are in line with the prior meta-analysis that rTMS is effective in improving PSQI (43). Furthermore, the results from this review extend our knowledge of rTMS in improving objective sleep parameters, including SE, SOL, TST, WASO, and NA. Regarding the stimulation parameters, the included studies ranged from 10 to 30 sessions, with each session consisting of 1,100 to 2,000 pulses for 10 to 30 min. The finding from this review suggests a potential dose-dependent effect of rTMS in treating insomnia. A greater number of treatment sessions is associated with better SE and sleep quality as measured by PSQI, and a greater number of pulses per session is associated with an improved PSQI. These findings are consistent with many previous studies among people with other mental disorders (93–97). Meanwhile, the inverse associations between the length of treatment per session and TST and PSQI are also noteworthy. There is evidence that the rTMS technique could induce a cumulative effect on cortical excitability that outlasts the stimulation period (98). However, prolonged rTMS stimulation could have a reversed after-effect (99, 100). The abovementioned findings raise several interesting questions about designing an optimal rTMS treatment protocol for people with insomnia: What is the optimal number of pulses per session/duration of stimulation per session/total number of sessions? Does the cumulative number of pulses show the same relationship with the therapeutic outcome as the cumulative number of sessions? How long will the cumulative effect last?
Previous studies on gender differences have reported that gender, age, and menopausal status could predict rTMS response (101, 102). This review also showed that male subjects had a higher response to rTMS treatment in insomnia as measured by the PSQI. We speculate that such differences may be attributed to age and the associated level of sex hormones. As significant confounding factors for the association between gender and rTMS response, older age and the menopausal status of females could predict worse rTMS response (101, 102). In general, the average age of menopause is approximately 52.5 years (103), and the average age of the participants in our pooled analysis was 50.59 (SD = 11.05) years. It is thus plausible that a significant number of women were in the stage of perimenopause or menopause, resulting in a decreased rTMS response in females. However, due to the lack of a detailed description of the clinical characteristics of the participants, especially the age of male and female subjects, the gender ratio in the active rTMS group compared to the sham group, and menopausal status, future studies are recommended to explore the influence of confounding factors, such as age, gender, and sex hormones level, on rTMS response among people with insomnia.
Strengths and Limitations
One of the strengths of this review is that it examined two forms of NIBS technique with more RCTs and more participants. Furthermore, it described the trends in outcomes across the immediate post-intervention and short-term follow-up and considered the moderators of effects of TES and rTMS.
There are also some limitations in this review. Firstly, due to the poor reporting of random sequence generation and allocation concealment in most of the included studies, it was difficult to evaluate the methodology quality. Secondly, substantial heterogeneity existed, which may partially be explained by the differences in the characteristics of the participants, diagnosis of insomnia, and stimulation parameters. This review also included participants with insomnia comorbid with other chronic conditions without control by the use of medication. The results should be interpreted with caution. Thirdly, due to the lack of studies evaluating the long-term effect of TES and rTMS in treating insomnia, only the short-term effect could be examined. The small number of studies may also limit the generalization of the findings. To further elucidate whether the effects could be sustained over time, future studies are suggested to adopt a longer follow-up period. The ideal follow-up period may be 3 months as insomnia is characterized by the sleep difficulty symptom that lasts for at least 3 months.
Conclusion
Overall, TES and rTMS are promising approaches in improving the symptoms of insomnia. rTMS was better studied and showed a larger effect size than TES in both the objective and subjective measures of sleep, with therapeutic effect maintained at 1–4 weeks of follow-up. Individual characteristics and stimulation parameters, such as gender, number of pulses per session, total number of treatment sessions, and length of treatment per session, were associated with the effect of rTMS and should be considered when developing optimal treatment protocols. This review highlighted the paucity of research on TES study. Future research with a longer follow-up period is also recommended.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author Contributions
HT initiated the idea and conceptualized the framework for the article. JH and DL performed the literature search and data extraction. HM conducted the data synthesis and wrote the first draft of the manuscript. JL and HT commented on previous versions of the manuscript. All authors have read and approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2021.744475/full#supplementary-material
References
1. Grewal RG, Doghramji K. Epidemiology of insomnia. In Attarian HP, editor. Clinical Handbook of Insomnia. Cham: Springer International Publishing (2016). p. 13–25. doi: 10.1007/978-3-319-41400-3_2
2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-5. Arlington, VA: American Psychiatric Association (2013). doi: 10.1176/appi.books.9780890425596
3. Sateia MJMD. International classification of sleep disorders-third edition. Chest. (2014) 146:1387–94. doi: 10.1378/chest.14-0970
4. O'Donnell JF. Insomnia in cancer patients. Clin Cornerstone. (2004) 6:S6–14. doi: 10.1016/S1098-3597(05)80002-X
5. Tang NKY, Wright KJ, Salkovskis PM. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J Sleep Res. (2007) 16:85–95. doi: 10.1111/j.1365-2869.2007.00571.x
6. Gjerstad MD, Wentzel-Larsen T, Aarsland D, Larsen JP. Insomnia in Parkinson's disease: frequency and progression over time. J Neurol Neurosurg Psychiatry. (2007) 78:476–9. doi: 10.1136/jnnp.2006.100370
7. Jansson M, Linton SJ. The role of anxiety and depression in the development of insomnia: cross-sectional and prospective analyses. Psychol Health. (2006) 21:383–97. doi: 10.1080/14768320500129015
8. Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. (2007) 3:S10. doi: 10.5664/jcsm.26929
9. Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. (2010) 14:9–15. doi: 10.1016/j.smrv.2009.05.002
10. Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. (2010) 14:19–31. doi: 10.1016/j.smrv.2009.04.002
11. Kripke DF. Do hypnotics cause death and cancer? The burden of proof. Sleep Med. (2009) 10:275–6. doi: 10.1016/j.sleep.2009.01.002
12. Kwon C-Y, Lee B, Cheong MJ, Kim T-H, Jang B-H, Chung SY, et al. Non-pharmacological treatment for elderly individuals with insomnia: a systematic review and network meta-analysis. Front Psychiatry. (2021) 11:608896. doi: 10.3389/fpsyt.2020.608896
13. Harvey AG, Tang NKY, Browning L. Cognitive approaches to insomnia. Clin Psychol Rev. (2005) 25:593–611. doi: 10.1016/j.cpr.2005.04.005
14. Morin CM, Bootzin RR, Buysse DJ, Edinger JD, Espie CA, Lichstein KL. Psychological and behavioral treatment of insomnia: update of the recent evidence (1998-2004). Sleep. (2006) 29:1398–414. doi: 10.1093/sleep/29.11.1398
15. Provencher T, Charest J, Bastien CH. Non-invasive brain stimulation for insomnia - a review of current data and future implications. OBM Integr Complement Med. (2019) 5:1. doi: 10.21926/obm.icm.2001001
16. Food and Drug Administration. Neurological Devices; Reclassification of Cranial Electrotherapy Stimulator Devices Intended To Treat Anxiety and/ or Insomnia; Effective Date of Requirement for Premarket Approval for Cranial Electrotherapy Stimulator Devices Intended To Treat Depression. (2019).
17. Moreno-Duarte I, Gebodh N, Schestatsky P, Guleyupoglu B, Reato D, Bikson M, et al. Transcranial electrical stimulation: transcranial direct current stimulation (tDCS), transcranial alternating current stimulation (tACS), transcranial pulsed current stimulation (tPCS), and transcranial random noise stimulation (tRNS). Stimul Brain. (2014) 2014:35–59. doi: 10.1016/B978-0-12-404704-4.00002-8
18. Woods AJ, Antal A, Bikson M, Boggio PS, Brunoni AR, Celnik P, et al. A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol. (2016) 127:1031–48. doi: 10.1016/j.clinph.2015.11.012
19. Nitsche MA, Seeber A, Frommann K, Klein CC, Rochford C, Nitsche MS, et al. Modulating parameters of excitability during and after transcranial direct current stimulation of the human motor cortex. J Physiol. (2005) 568:291–303. doi: 10.1113/jphysiol.2005.092429
20. Antal A, Paulus W. Transcranial alternating current stimulation (tACS). Front Hum Neurosci. (2013) 7:317. doi: 10.3389/fnhum.2013.00317
21. Terney D, Chaieb L, Moliadze V, Antal A, Paulus W. Increasing human brain excitability by transcranial high-frequency random noise stimulation. J Neurosci. (2008) 28:14147–55. doi: 10.1523/JNEUROSCI.4248-08.2008
22. Reed T, Cohen Kadosh R. Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J Inherit Metab Dis. (2018) 41:1123–30. doi: 10.1007/s10545-018-0181-4
23. Frase L, Piosczyk H, Zittel S, Jahn F, Selhausen P, Krone L, et al. Modulation of total sleep time by transcranial direct current stimulation (tDCS). Neuropsychopharmacology. (2016) 41:2577–86. doi: 10.1038/npp.2016.65
24. Voss U, Holzmann R, Hobson A, Paulus W, Koppehele-Gossel J, Klimke A, et al. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat Neurosci. (2014) 17:810–2. doi: 10.1038/nn.3719
25. Antal AP, Boros KMD, Poreisz CMD, Chaieb LMS, Terney DMD, Paulus WMD. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. (2008) 1:97–105. doi: 10.1016/j.brs.2007.10.001
26. Borges H, Dufau A, Paneri B, Woods AJ, Knotkova H, Bikson M. Updated technique for reliable, easy, and tolerated transcranial electrical stimulation including transcranial direct current stimulation. J Visual Exp. (2020) 155:e59204. doi: 10.3791/59204
27. KirschDaniel L, Francine N. Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin. (2013) 36:169–76. doi: 10.1016/j.psc.2013.01.006
28. Prehn K, Flöel A. Potentials and limits to enhance cognitive functions in healthy and pathological aging by tDCS. Front Cell Neurosci. (2015) 9:355. doi: 10.3389/fncel.2015.00355
29. Pascual-Leone FFaA. Technology Insight: noninvasive brain stimulation in neurology-perspectives on the therapeutic potential of rTMS and tDCS. Nat Clin Pract Neurol. (2007) 3:383–93. doi: 10.1038/ncpneuro0530
30. Barker AT, Jalinous R. Freeston LL. Non-invasive magnetic stimulation of human motor cortex. Lancet. (1985) 325:1106–7. doi: 10.1016/S0140-6736(85)92413-4
31. Aleman A. Use of repetitive transcranial magnetic stimulation for treatment in psychiatry. Clin Psychopharmacol Neurosci. (2012) 11:53–9. doi: 10.9758/cpn.2013.11.2.53
32. Ridding MC, Rothwell JC. Is there a future for therapeutic use of transcranial magnetic stimulation? Nat Rev Neurosci. (2007) 8:559–67. doi: 10.1038/nrn2169
33. Arias-Carrión O. Basic mechanisms of rTMS: implications in Parkinson's disease. Int Arch Med. (2008) 1:2. doi: 10.1186/1755-7682-1-2
34. Hoffman RE, Cavus I. Slow transcranial magnetic stimulation, long-term depotentiation, and brain hyperexcitability disorders. Am J Psychiatry. (2002) 159:1093–102. doi: 10.1176/appi.ajp.159.7.1093
35. Speer AM, Kimbrell TA, Wassermann EMD, Repella J, Willis MW, Herscovitch P, et al. Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry. (2000) 48:1133–41. doi: 10.1016/S0006-3223(00)01065-9
36. Fidalgo TM, Morales-Quezada L, Muzy GSC, Chiavetta NM, Mendonça ME, Santana MVB, et al. Biological markers in non-invasive brain stimulation trials in major depressive disorder: a systematic review. J ECT. (2014) 30:47–61. doi: 10.1097/YCT.0b013e31828b34d8
37. Klomjai W, Katz R, Lackmy-Vallée A. Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Ann Phys Rehabil Med. (2015) 58:208–13. doi: 10.1016/j.rehab.2015.05.005
38. Wang H-Y, Crupi D, Liu J, Stucky A, Cruciata G, di Rocco A, et al. Repetitive transcranial magnetic stimulation enhances BDNF-TrkB signaling in both brain and lymphocyte. J Neurosci. (2011) 31:11044–54. doi: 10.1523/JNEUROSCI.2125-11.2011
39. Feng J, Zhang Q, Zhang C, Wen Z, Zhou X. The effect of sequential bilateral low-frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. Brain Behav. (2019) 9:e01206. doi: 10.1002/brb3.1206
40. Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): A tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. (2006) 117:845–50. doi: 10.1016/j.clinph.2005.12.003
41. Herrero Babiloni A, Bellemare A, Beetz G, Vinet S-A, Martel MO, Lavigne GJ, et al. The effects of non-invasive brain stimulation on sleep disturbances among different neurological and neuropsychiatric conditions: a systematic review. Sleep Med Rev. (2021) 55:101381. doi: 10.1016/j.smrv.2020.101381
42. Shekelle PG, Cook IA, Miake-Lye IM, Booth MS, Beroes JM, Mak S. Benefits and harms of cranial electrical stimulation for chronic painful conditions, depression, anxiety, and insomnia a systematic review. Ann Intern Med. (2018) 168:414–21. doi: 10.7326/M17-1970
43. Sun N, He Y, Wang Z, Zou W, Liu X. The effect of repetitive transcranial magnetic stimulation for insomnia: a systematic review and meta-analysis. Sleep Med. (2020) 77:226–3. doi: 10.1016/j.sleep.2020.05.020
44. Klawansky S, Yeung A, Berkey C, Shah N, Phan HAI, Chalmers TC. Meta-analysis of randomized controlled trials of cranial electrostimulation: efficacy in treating selected psychological and physiological conditions. J Nerv Ment Dis. (1995) 183:478–84. doi: 10.1097/00005053-199507000-00010
45. Jiang B, He D, Guo Z, Mu Q, Zhang L. Efficacy and placebo response of repetitive transcranial magnetic stimulation for primary insomnia. Sleep Med. (2019) 63:9–13. doi: 10.1016/j.sleep.2019.05.008
46. Fu R, Gartlehner G, Grant M, Shamliyan T, Sedrakyan A, Wilt TJ, et al. Conducting quantitative synthesis when comparing medical interventions: AHRQ and the Effective Health Care Program. J Clin Epidemiol. (2011) 64:1187–97. doi: 10.1016/j.jclinepi.2010.08.010
47. Baker WL, Michael White C, Cappelleri JC, Kluger J, Coleman CI. Understanding heterogeneity in meta-analysis: the role of meta-regression. Int J Clin Pract. (2009) 63:1426–34. doi: 10.1111/j.1742-1241.2009.02168.x
48. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. (2009) 339:e78–336. doi: 10.1136/bmj.b2535
49. Cartwright RD, Cartwright RD, Weiss MF, Weiss MF. The effects of electrosleep on insomnia revisited. J Nerv Ment Dis. (1975) 161:134–7. doi: 10.1097/00005053-197508000-00008
50. Lande RG, Gragnani C. Efficacy of cranial electric stimulation for the treatment of insomnia: a randomized pilot study. Complement Ther Med. (2013) 21:8–13. doi: 10.1016/j.ctim.2012.11.007
51. Lyon D, Kelly D, Walter J, Bear H, Thacker L, Elswick RK. Randomized sham controlled trial of cranial microcurrent stimulation for symptoms of depression, anxiety, pain, fatigue and sleep disturbances in women receiving chemotherapy for early-stage breast cancer. Springerplus. (2015) 4:369. doi: 10.1186/s40064-015-1151-z
52. Rose KM, Taylor AG, Bourguignon C. Effects of cranial electrical stimulation on sleep disturbances, depressive symptoms, and caregiving appraisal in spousal caregivers of persons with Alzheimer's disease. Appl Nurs Res. (2009) 22:119–25. doi: 10.1016/j.apnr.2007.06.001
54. Weiss MF. The treatment of insomnia through the use of electrosleep: an eeg study. J Nerv Ment Dis. (1973) 157:108–20. doi: 10.1097/00005053-197308000-00003
55. Kwon DR, Park JA, Lee YS, Kwak JH, Do JK, Kim JE. Synergic effects of cranial electrotherapy stimulation with sleep hygiene in patients with chronic insomnia. J Sleep Med. (2019) 16:36–40. doi: 10.13078/jsm.19029
56. Taylor AG, Anderson JG, Riedel SL, Lewis JE, Kinser PA, Bourguignon C. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Manage Nurs. (2013) 14:327–35. doi: 10.1016/j.pmn.2011.07.002
57. Acler M, Bocci T, Valenti D, Turri M, Priori A, Bertolasi L. Transcranial direct current stimulation (tDCS) for sleep disturbances and fatigue in patients with post-polio syndrome. Restor Neurol Neurosci. (2013) 31:661–8. doi: 10.3233/RNN-130321
58. Bianchi MS, Ferreira CF, Fregni F, Schestatsky P, Caumo W, Wender MCO. Transcranial direct current stimulation effects on menopausal vasomotor symptoms. Menopause. (2017) 24:1122–8. doi: 10.1097/GME.0000000000000905
59. Sadeghi Bimorgh M, Omidi A, Ghoreishi FS, Rezaei Ardani A, Ghaderi A, Banafshe HR. Exploring the therapeutic effects of transcranial direct current stimulation on sleep quality among patients under methadone maintenance treatment. Int J Med Toxicol Forensic Med. (2020) 10:29088. doi: 10.32598/ijmtfm.v10i2.29088
60. Cody SL, Fazeli PL, Crowe M, Kempf M-C, Moneyham L, Stavrinos D, et al. Effects of speed of processing training and transcranial direct current stimulation on global sleep quality and speed of processing in older adults with and without HIV: a pilot study. Appl Neuropsychol Adult. (2020) 27:267–78. doi: 10.1080/23279095.2018.1534736
61. Harvey MP, Lorrain D, Martel M, Bergeron-Vezina K, Houde F, Séguin M, et al. Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? - Results from a randomized controlled pilot study. Clin Intervent Aging. (2017) 12:937–47. doi: 10.2147/CIA.S133423
62. Roizenblatt S, Fregni F, Gimenez R, Wetzel T, Rigonatti SP, Tufik S, et al. Site-specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham-controlled study. Pain Pract. (2007) 7:297–306. doi: 10.1111/j.1533-2500.2007.00152.x
63. Zhou Q, Yu C, Yu HH, Zhang YY, Liu ZW, Hu ZY, et al. The effects of repeated transcranial direct current stimulation on sleep quality and depression symptoms in patients with major depression and insomnia. Sleep Med. (2020) 70:17–26. doi: 10.1016/j.sleep.2020.02.003
64. Wang H-X, Wang L, Zhang W-R, Xue Q, Peng M, Sun Z-C, et al. Effect of transcranial alternating current stimulation for the treatment of chronic insomnia: a randomized, double-blind, parallel-group, placebo-controlled clinical trial. Psychother Psychosom. (2020) 89:38–47. doi: 10.1159/000504609
65. Hu S, Shen Y, Mo F, Gu X, Guan Z. The influence of low- frequency repetitive transcranial magnetic stimulation sites on the elderly patients with chronic insomnia. Chin J Rehabil Med. (2019) 34:433–9. doi: 10.3969/j.issn.1001-1242.2019.04.012
66. Huang Z, Li Y, Bianchi MT, Zhan S, Jiang F, Li N, et al. Repetitive transcranial magnetic stimulation of the right parietal cortex for comorbid generalized anxiety disorder and insomnia: a randomized, double-blind, sham-controlled pilot study. Brain Stimul. (2018) 11:1103–9. doi: 10.1016/j.brs.2018.05.016
67. Li Z, He W, Zhen L, Gan X, Huang S, Aowen W, et al. Clinical efficacy of lorazepam combined with low-frequency repetitive transcranial magnetic stimulation on chronic insomnia disorder. Sichuan Mental Health. (2019) 32:337–41. doi: 10.11886/j.issn.1007-3256.2019.04.010
68. Li M, Lu J, Li W, Hao Z, Mu H. Efficacy of ultra-low frequency transcranial magnetic stimulation combined with pharmacological treatment of sleep disorders associated in patients with Alzheimer's disease. Guizhou Med J. (2016) 40:274–6.
69. Liu Q, Wei L, Chen W, Lu Y. The effect of low-frequency rTMS in people with sleep disorders of Chronic fatigue syndrome: a randomized controlled trial. Foreign Med Sci Sect Med Geogr. (2018) 39:315–9. doi: 10.3969/j.issn.1001-8883.2018.04.010
70. Shen X, Wang Z. Curative effect of low-frequency repetitive transcranial magnetic stimulation on primary insomnia. J Military Surg Southwest China. (2018) 20:28–32. doi: 10.3969/j.issn.1672-7193.2018.01.010
71. Yuan J, Li X, Xu Y, Su F, Wang N, Guo T. Randomized controlled trial of treatment with low -frequency repetitive transcranial magnetic stimulation for patients with primary insomnia. Chin Modern Med. (2018) 25:57–60.
72. Yu Z, Yang Y, Wang H, Mao H, Tang G, Song M, et al. Evaluation of the efficacy of low-frequency repetitive transcranial magnetic stimulation in combination with zolpidem for the treatment of primary insomnia. Chin J Geriatr. (2017) 38:3949–51. doi: 10.3969/j.issn.1005-9202.2018.16.044
73. Feng X, Gai H, Wang X. Repetitive transcranial magnetic stimulation in the treatment of primary insomnia. J Clin Psychiatry. (2017) 27:415–7.
74. Liang X, Gan J, Liu L, Zhang W, Gao C, Zhao L, et al. controlled study of low-frequency repetitive transcranial magnetic stimulation for the treatment of insomnia in military personnel. China Brain Med Brain Sci. (2012) 21:622–3. doi: 10.3760/cma.j.issn.1674-6554.2012.07.016
75. Liu H, Wu X, Yi L, Xu L, Ma H, Shen Z, et al. Therapeutical effects of repetitive transcranial magnetic stimulation combined with cognitive-behavioral therapy for insomnia. Med J Chin Peoples Health. (2016) 28:10–2. doi: 10.3969/j.issn.1672-0369.2016.12.004
76. Liu C, Wang T, Sun Z. Clinical observation of a repetitive transcranial magnetic stimulation in the treatment of insomnia with alcohol dependence. China J Drug Depend. (2016) 25:206–8. doi: 10.13936/j.cnki.cjdd1992.2016.02.014
77. Liu C, Duan N, Yongdong Z, Wang L. Clinical observation of repetitive transcranial magnetic stimulation in the treatment of intractable insomnia. J Int Psychiatry. (2016) 43:263–5.
78. Zhang Y-P, Liao W-J, Xia W-G. Effect of acupuncture cooperated with low-frequency repetitive transcranial magnetic stimulation on chronic insomnia: a randomized clinical trial. Curr Med Sci. (2018) 38:491–8. doi: 10.1007/s11596-018-1905-2
79. Arias P, Vivas J, Grieve KL, Cudeiro J. Double-blind, randomized, placebo controlled trial on the effect of 10 days low-frequency rTMS over the vertex on sleep in Parkinson's disease. Sleep Med. (2010) 11:759–65. doi: 10.1016/j.sleep.2010.05.003
80. Lin J, Liu X, Li H, Yu L, Shen M, Lou Y, et al. Chronic repetitive transcranial magnetic stimulation (rTMS) on sleeping quality and mood status in drug dependent male inpatients during abstinence. Sleep Med. (2019) 58:7–12. doi: 10.1016/j.sleep.2019.01.052
81. Hu X, Zhou H, Tang B, Lei G, Su C. Clinical observation of repetitive transcranial magnetic stimulation in the treatment of anxiety insomnia in 38 patients. J Chin Pract Diagn Therapy. (2014) 28:158–62.
82. Anniwan M, Maimaitiming N, Wang Q, Xiao C, Chen J. Thirty cases curative effect observation of repetitive transcranial magnetic stimulation in the treatment of non organic insomnia. World J Sleep Med. (2016) 3:275–9.
83. Gao MH, Bu S, Chunlei, Meng X, Yuan S, Yang H. Clinical study of low frequency repetitive transcranial magnetic stimulation in treatment of insomnia. J Qiqihar Univers Med. (2017) 38:895–7.
84. Li G. Clinical observation of repeated transcranial magnetic stimulation for the treatment of anxiety disorder with insomnia. J Clin Med. (2017) 4:15707–9. doi: 10.16281/j.cnki.jocml.2017.80.050
85. Xie Y, Li Y, Chen Y, Li X. Influence of combined treatment scheme on sleep quality, depression and sleep structure index of patients with primary insomnia. Anhui Med Pharmaceut J. (2020) 24:771–4. doi: 10.3969/j.issn.1009-6469.2020.04.034
86. Lin W, Bai J, Peng Z, Yu B, Wang C, Chen X. Observation of 36 cases of primary insomnia treated with repetitive transcranial magnetic stimulation of head and acupuncture points. J Traditional Chin Med. (2020) 61:800–3. doi: 10.13288/j.11-2166/r.2020.09.016
87. Guo F. Effects of low frequency repeatitive transcranial magnetic stimulation in treatment of primary insomnia. Med J Chin Peoples Health. (2020) 32:14–5. doi: 10.3969/j.issn.1672-0369.2020.11.006
88. Ding X, Tang C, Liang W. Observation of the effectiveness of repeated transcranial magnetic stimulation in the treatment of insomnia. China Pract Med. (2020) 15:94–6. doi: 10.14163/j.cnki.11-5547/r.2020.25.042
89. He M, Wang X, Xu B, Li Z, Jiang H. Transcranial magnetic stimulation in the treatment of primary insomnia. China J Phys Med Rehabil. (2009) 31:763–6. doi: 10.3760/cma.j.issn.0254-1424.2009.11.014
90. Liu C, Lin H, Huang G. Huang Q, Huang Y. Clinical curative effect of repeated transcranial magnetic stimulation on treating insomnia of patients with ketamine dependence. J Int Psychiatry. (2018) 45:285–7.
91. Yan W, Zhan J, Zhao J, Dong Y, Zhao S. Observation on the effect of ultra -low frequency transcranial magnetic stimulation in the treatment of mental sub-health patients with insomnia. China Modern Doctor. (2019) 57:80–4.
92. Habib S, Hamid U, Jamil A, Zainab AZ, Yousuf T, Habib S, et al. Transcranial magnetic stimulation as a therapeutic option for neurologic and psychiatric illnesses. Cureus. (2018) 10:e3456. doi: 10.7759/cureus.3456
93. Dell'Osso B, Camuri G, Castellano F, Vecchi V, Benedetti M, Bortolussi S, et al. Meta-review of metanalytic studies with repetitive transcranial magnetic stimulation (rTMS) for the treatment of major depression. Clin Pract Epidemiol Ment Health. (2011) 7:167–77. doi: 10.2174/1745017901107010167
94. Jiang Y, Guo Z, McClure MA, He L, Mu Q. Effect of rTMS on Parkinson's cognitive function: a systematic review and meta-analysis. BMC Neurol. (2020) 20:377. doi: 10.1186/s12883-020-01953-4
95. Zhang JJQ, Fong KNK. Ouyang Rg, Siu AMH, Kranz GS. Effects of repetitive transcranial magnetic stimulation (rTMS) on craving and substance consumption in patients with substance dependence: a systematic review and meta-analysis. Addiction. (2019) 114:2137–49. doi: 10.1111/add.14753
96. de Jesus DR. Favalli GPdS, Hoppenbrouwers SS, Barr MS, Chen R, Fitzgerald PB, et al. Determining optimal rTMS parameters through changes in cortical inhibition. Clin Neurophysiol. (2013) 125:755–62. doi: 10.1016/j.clinph.2013.09.011
97. Teng S, Guo Z, Peng H, Xing G, Chen H, He B, et al. High-frequency repetitive transcranial magnetic stimulation over the left DLPFC for major depression: session-dependent efficacy: a meta-analysis. Eur Psychiatry. (2016) 41:75–84. doi: 10.1016/j.eurpsy.2016.11.002
98. Bäumer T, Lange R, Liepert J, Weiller C, Siebner HR, Rothwell JC, et al. Repeated premotor rTMS leads to cumulative plastic changes of motor cortex excitability in humans. Neuroimage. (2003) 20:550–60. doi: 10.1016/S1053-8119(03)00310-0
99. Müller-Dahlhaus F, Ziemann U. Metaplasticity in human cortex. Neuroscientist. (2015) 21:185–202. doi: 10.1177/1073858414526645
100. Gamboa OL, Antal A, Moliadze V, Paulus W. Simply longer is not better: reversal of theta burst after-effect with prolonged stimulation. Exp Brain Res. (2010) 204:181–7. doi: 10.1007/s00221-010-2293-4
101. Huang CC, Wei IH, Chou YH, Su TP. Effect of age, gender, menopausal status, and ovarian hormonal level on rTMS in treatment-resistant depression. Psychoneuroendocrinology. (2008) 33:821–31. doi: 10.1016/j.psyneuen.2008.03.006
102. Chung SW, Thomson CJ, Lee S, Worsley RN, Rogasch NC, Kulkarni J, et al. The influence of endogenous estrogen on high-frequency prefrontal transcranial magnetic stimulation. Brain Stimul. (2019) 12:1271–9. doi: 10.1016/j.brs.2019.05.007
Keywords: insomnia, transcranial electric stimulation, repetitive transcranial magnetic stimulation, meta-analysis, meta-regression
Citation: Ma H, Lin J, He J, Lo DHT and Tsang HWH (2021) Effectiveness of TES and rTMS for the Treatment of Insomnia: Meta-Analysis and Meta-Regression of Randomized Sham-Controlled Trials. Front. Psychiatry 12:744475. doi: 10.3389/fpsyt.2021.744475
Received: 20 July 2021; Accepted: 03 September 2021;
Published: 22 October 2021.
Edited by:
Bin Zhang, Southern Medical University, ChinaReviewed by:
Barbara Galland, University of Otago, New ZealandAxel Steiger, Ludwig Maximilian University of Munich, Germany
Copyright © 2021 Ma, Lin, He, Lo and Tsang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hector W. H. Tsang, hector.tsang@polyu.edu.hk
 Haixia Ma
Haixia Ma Jingxia Lin
Jingxia Lin Jiali He
Jiali He Dilys Hoi Ting Lo
Dilys Hoi Ting Lo Hector W. H. Tsang
Hector W. H. Tsang