- 1German Center for Neurodegenerative Diseases (DZNE), RG Psychosocial Epidemiology and Public Health, Greifswald, Germany
- 2Center for Cognitive Science, University of Kaiserslautern, Kaiserslautern, Germany
- 3Institute of Social Medicine, Occupational Medicine and Public Health, University of Leipzig, Leipzig, Germany
Introduction
With life expectancy increasing, many of us can hope to live a long life. However, older age comes with a higher risk for a variety of chronic health conditions such as for cardiovascular, respiratory, musculoskeletal, and neurological diseases that can make it difficult to enjoy getting older (1). One of the most prevalent neurological diseases in old age is dementia. Its prevalence increases with older age. While in the 70–74 years' age group, the prevalence of dementia is only around 3%, already about 12% in the 80–84 years' age group and more than 20% in the 90+ years' age group have dementia (2). Accordingly, the number of people with dementia will be increasing, as populations grow older. Estimations suggest that the prevalence worldwide is around 50 million people with nearly 10 million new cases every year (3). Therefore, dementia does not only affect the individual but society as a whole.
For more than a century, research on dementia has focused on biological aspects of cognitive disorders such as brain imaging and biomarkers (4). However, the neurobiological changes in the aging brain (5) are influenced by behavior and by the environment (6). More and more ongoing research reveals the relevance of social determinants and lifestyle factors for the risk of developing dementia (7).
Life-Course Approach
Taking a life-course approach to cognitive health, which explicitly includes social and lifestyle factors, can contribute to a better prevention. Research taking this approach aims at understanding how biological, behavioral, social, and psychological exposures are important for health in later life (8). The Life Course Health Development (LCHD) framework considers health to be the result of multiple interactions between the environment and the bio-behavioral regulatory systems of the human body (9). From that perspective, health trajectories are the cumulative product of risks and protective factors (9). Disparities in health between people of varying socioeconomic status are the result of psychological and social influences e.g., stress, social exclusion, working conditions, unemployment, healthy food etc. (10). In that sense, healthy aging is a multifaceted phenomenon that can only be understood by holistically studying the complexity of social and lifestyle factors in the context of aging (11). The “biological capital” that an individual has acquired in their lifetime determines how long they remain above a critical threshold for adverse health outcomes (12). This is a perspective adopted by the World Health Organization (WHO), which states that “Healthy aging is about creating the environments and opportunities that enable people to be and do what they value throughout their lives” (13).
Cognitive health also depends on multiple determinants and their dynamics throughout the life-course. The Cognitive Health Environment Life Course Model (CHELM) summarizes lifestyle factors that influence cognitive health in old age (14). Lifestyle can positively or negatively moderate disease risk via neuropathological changes (14). Indeed, lifestyle can affect cognitive health to a great extent. Estimates suggest that up to 3 million dementia cases worldwide could be prevented if it was possible to reduce known risk factors by 25% (15). This type of evidence calls for modification of public health policies to prevent cognitive decline and dementia (16).
The Role of Intellectual Stimulation in the Form of Education and Occupation
Ever since researchers started investigating dementia, they observed that people with higher education develop dementia less frequently and express less severe dementia symptoms in comparison to low-educated people with the same level of Alzheimer's disease (AD) pathology in the brain (17). In research, this effect is called “cognitive reserve” (18). The interesting aspect is that a simple socio-environmental factor can possibly provide such resilience against a biologically devastating disease. Health status or income do not explain this resilience (19, 20). Rather, intellectual stimulation across the lifespan seems to be the central factor in this cognitive reserve effect. Moreover, intellectual stimulation by the environment can help the brain to remain healthy for longer by a process called “brain maintenance” (21). Indications on how intellectual stimulation leads to better brain maintenance come from rodent studies. Relevant studies have shown that exposure to enriched housing (i.e., an enriched environment) compared to standard housing conditions can protect against neurodegenerative diseases (22). Exposure to enriched environments seems to up-regulate synaptogenesis and synaptic plasticity and to activate epigenetic mechanisms (22). In this way, the intellectual stimulation provided by enriched environments can improve brain maintenance.
Evidence from longitudinal studies largely confirms that intellectually stimulating activities over the life-course protect cognitive health in old age. As summarized in the Life Course Model of Cognitive Reserve (LCMCR), an intellectually engaged lifestyle builds up a “cognitive reserve” that protects cognitive health in old age (23). The model highlights that all stages across the life-course—starting from cognitive development in childhood to hobbies in later life—can modify cognitive health (23). Results from the majority of longitudinal studies confirm that higher education is associated with benefits for cognitive functioning in later life (24). The socioeconomic attainment related to education has little impact on dementia risk, even in minority populations (20). Instead, it appears that the difference in the number of years of education can explain ethnic and racial disparities in dementia prevalence (20). However, high education does not prevent cognitive decline. Instead, it seems that highly educated individuals have a seven to 8 year period of only limited cognitive decline before their cognitive abilities deteriorate to the same extent as they do in low-educated individuals (25). Biological evidence reinforces the idea that education does not prevent dementia, as higher education does not seem to be associated with a reduction in AD biomarkers of Alzheimer's disease (26). Rather, studies have pointed out that higher education is associated with a higher brain network efficiency, even in the presence of such biomarkers (tau pathology) in the brain (26). This may reflect compensatory mechanisms in the brains of highly educated people.
In addition, evidence from longitudinal studies also accentuates the relevance of intellectual stimulation at work. Higher mental demands in the occupational context are shown to be associated with a lower dementia risk (27). Specifically, people who were working in jobs that challenge executive cognitive abilities have a lower dementia risk (27) and slower cognitive decline (28). Similar observations are made in people who were working in jobs with complex information processing (29). Importantly, these and other studies have shown that not all types of mental demands at work provide this beneficial effect. The complexity of information processing required appears to be critical (29). Based on Wickens's model of human information processing, tasks that are more complex require more attentional resources, a higher workload, and more integration of long-term memories (30). It is possible that this simultaneous activation of several cognitive resources will help to protect the brain longer.
Yet, the exact mechanisms remain unclear. On a cognitive level, people with high education seem to have superior semantic memory and executive cognitive abilities (31). These abilities provide them with a larger knowledge base and skills that constitute an advantage in terms of employing memory compensation strategies (32). Observations from computerized cognitive training show structural and functional brain changes related to the domain that was trained (33). Accordingly, a training in semantic memory and executive cognitive abilities might delay cognitive decline. Moreover, we would assume that maximizing the complexity of information processing in cognitive training tools might maximize training effects. However, differentiated evidence on this is not yet available.
Benefits for High-Risk Groups
People with a greater risk for dementia can benefit from intellectual stimulation throughout the life-course. Genetically, people who carry an Apolipoprotein E (APOE) e4 allele are considered to be a high-risk group for dementia (34). Research has shown that associations between lifestyle factors and cognitive functioning are generally similar in APOE e4 allele carriers and non-carriers (35, 36). Accordingly, a preventive lifestyle approach is also beneficial for this group. Increasing education, in particular, may be relevant to this high-risk group as low education comes with a higher dementia risk in APOE e4 allele carriers than in non-carriers (37). Looking specifically at low-educated people, findings demonstrate that low-educated APOE e4 allele carriers had a larger decrease in the metabolism in medial-temporal and prefrontal areas in old age than low-educated non-carriers (38). Low education might thus be inflating dementia risk among people with the high-risk variant of the APOE e4 gene.
Another important risk factor for dementia is social isolation (39, 40). Evidence suggests that intellectual stimulation may also be beneficial for the socially isolated high-risk group. Regarding education, research findings indicate that the association between social isolation and memory performance is stronger among people with low education than high education (41). These findings suggest that low-educated individuals experience greater adverse effects from isolation. Education might thus shield against the negative impact of social isolation. Similar findings are made regarding people with high mental demands at work, who experienced less adverse effects from social isolation on their cognitive functioning than people with low mental demands at work (41, 42). The cause of this effect is yet unknown. Yet, the evidence suggests that intellectual stimulation is clearly advisable for people who live socially isolated.
Prevention Strategies
Intellectual stimulation in the form of education, occupation, or other cognitive activities is associated with important protective effects for cognitive health. Accordingly, it should be integrated in effective prevention strategy programs. First initiatives to offer cognitive training programs have been created, e.g., the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) (43) and the Improvement in Memory with Plasticity-based Adaptive Cognitive Training (IMPACT) Study (44). In addition, the first multi-dimensional training programs (including cognitive training, diet, exercise, and vascular risk monitoring), such as the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER, 45) are being implemented. As far as publicly known, FINGER was the first study of this kind. It started in 2009 and offered a 2-year intervention for people at a high risk for developing dementia. After the 2 years, performance in all cognitive sub-domains was improved (45). By today, FINGER has inspired further large multi-domain intervention studies worldwide, which are connected in the World-Wide FINGERS, the first global network of multi-domain lifestyle intervention trials (https://www.alz.org/wwfingers/overview.asp).
WHO guidelines likewise recommend a proactive multi-dimensional approach to risk reduction of cognitive decline and dementia (46). Recommendations comprise physical activity, nutritional interventions, social activity, tobacco and alcohol abuse cessation, and better management of chronic diseases (46). Further, the WHO report includes a conditional recommendation for cognitive training for people with mild cognitive impairments. Even though many studies show significant effects of cognitive training, high quality evidence is still lacking—a conclusion also reached by the Lancet commission on dementia prevention (47). Irrespective of any benefit from short-term trainings, it is important to acknowledge the greater benefit of cumulative exposure to intellectual stimulation in all of its form throughout the life-course (48), which so far has been underestimated.
While the above-mentioned studies constitute important progress, major problems persist: First, the programs typically end when the respective study ends. Second, only a limited number of people in the population had the chance to benefit from the program. Third, the infrastructure necessary to implement such programs on a population scale is, as of now, rarely available. Table 1 summarizes a number of challenges that need to be addressed. Given the potential of intellectual stimulation to shape the expression of symptoms at each disease level, it should be a persistent part of secondary and tertiary as well as primary prevention for the entire population. Yet, preventative efforts on the population level are rare. Current efforts in prevention focus mainly on physical activity and obesity (49). Prevention, however, can and must encompass multiple factors, including access to higher education. Education is not only important to promote a longer lifetime period with better cognitive functioning, but education also strengthens the cognitive competencies necessary for social and economic participation of the elderly population. Hence, implementing programs that encourage and support higher education must be a major focus of public policy.
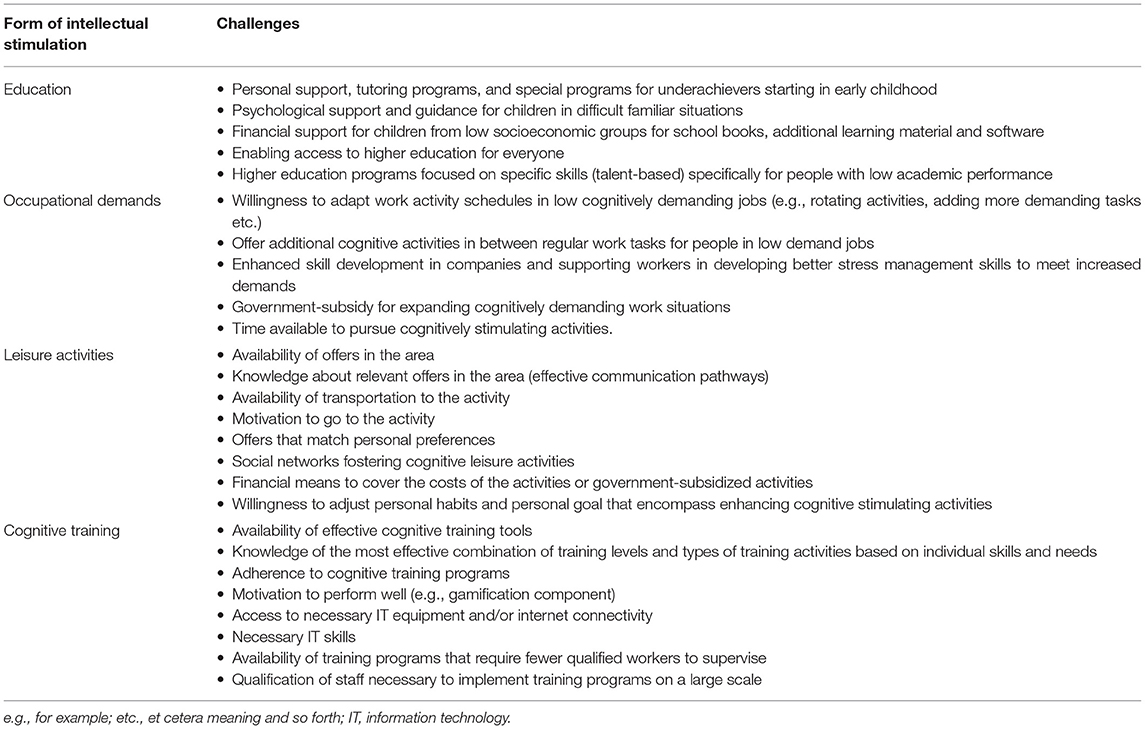
Table 1. Some important challenges for implementing intellectual stimulation within prevention programs.
The process of translating research into policy is generally slow because the implementation of such programs requires a collaborative approach in raising awareness, reducing risk, informing local communities, targeting social inequalities, developing programs for high-risk groups, and establishing effective healthcare structures (49)—some challenges are mentioned in Table 1. Overall, it is useful to offer programs tailored to each person's individual risk profile. Personalized interventions can include social engagement strategies for the socially isolated people, nutrition plans for those with high blood sugar and high blood lipids, cognitive trainings for those with low education and those who worked in jobs with low mental demands, and others. Personalizing interventions is a more efficient use of resources than offering standardized multimodal programs. This is because the latter requires each participant to undergo the same program irrespective of individual need and characteristics, which is more expensive and stressful to the individual. Interventions for cognitive health that are tailored to the person are more likely to bring the best benefits with minimum burden. There are first tailored interventions implemented on a local level for managing behavioral disturbances of patients with frontotemporal dementia (50) and for preventing falls (51). Yet, to our knowledge, there seem to be no publicly known tailored intervention program for dementia prevention. This should be addressed urgently.
Conclusion
Aging is our future and every one of us wants to enjoy a long and happy life. To protect our cognitive abilities in old age, we need to be active now. Prevention starts today and evidence has shown that intellectual stimulation, such as our education and what we do every day at work, can strengthen our resilience against dementia. Despite the evidence, public policy is slow in implementing relevant prevention strategies. Encouraging higher education for everyone and supporting continuous education in mid- and old-age, including cognitive stimulation with complex information processing in medical treatment plans, and developing person-tailored intervention programs are just some steps in the right direction. Given that maintaining cognitive health in old age is one of the major challenges of aging societies like ours, we urgently need to recognize the potential of non-biological risk factors across the life-course in medical treatment plans and public policy.
Author Contributions
FR wrote the manuscript and approved of the final version to be submitted for publication.
Funding
This work was supported by the German Research Foundation (DFG, grant no. TH2137/3-1) and the Hans und Ilse Breuer Foundation.
Conflict of Interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Prince, M. J., Wu, F., Guo, Y., Gutierrez Robledo, L. M., O'donnell, M., Sullivan, R., et al. (2015). The burden of disease in older people and implications for health policy and practice. Lancet 385:549–62. doi: 10.1016/S0140-6736(14)61347-7
2. Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, et al. Prevalence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts. Neurol Dis Elderly Res Group Neurol. (2000) 54:S4–9.
3. World Health Organization. Dementia: Key Facts. (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed June 16, 2021).
4. Weiner MW, Veitch DP, Aisen PS, Beckett LA, Cairns NJ, Green RC, et al. The Alzheimer's Disease Neuroimaging Initiative: a review of papers published since its inception. Alzheimers Dement. (2013) 9:e111–194. doi: 10.1016/j.jalz.2013.05.1769
5. Park DC, Reuter-Lorenz P. The adaptive brain: aging and neurocognitive scaffolding. Annu Rev Psychol. (2009) 60:173–96. doi: 10.1146/annurev.psych.59.103006.093656
6. Whalley LJ, Dick FD, Mcneill G. A life-course approach to the aetiology of late-onset dementias. Lancet Neurol. (2006) 5:87–96. doi: 10.1016/S1474-4422(05)70286-6
7. Jiang T, Yu JT, Tian Y, Tan L. Epidemiology and etiology of Alzheimer's disease: from genetic to non-genetic factors. Curr Alzheimer Res. (2013) 10:852–67. doi: 10.2174/15672050113109990155
8. Lynch J, Smith GD. A life course approach to chronic disease epidemiology. Annu Rev Public Health. (2005) 26:1–35. doi: 10.1146/annurev.publhealth.26.021304.144505
9. Halfon N, Hochstein M. Life course health development: an integrated framework for developing health, policy, and research. Milbank Q. (2002) 80:433–79. doi: 10.1111/1468-0009.00019
10. Wilkinson RG, Marmot M. Social Determinants of Health: The Solid Facts. Geneva: World Health Organization (2003).
11. Crosnoe R, Elder GH. Successful adaptation in the later years: a life course approach to aging. Soc Psychol Q. (2002) 65:309–28. doi: 10.2307/3090105
12. Kuh D, New Dynamics of Ageing Preparatory N. A life course approach to healthy aging, frailty, and capability. J Gerontol A Biol Sci Med Sci. (2007) 62:717–21. doi: 10.1093/gerona/62.7.717
13. World Health Organization. Ageing: Healthy Ageing and Functional Ability. (2020). https://www.who.int/westernpacific/news/q-a-detail/ageing-healthy-ageing-and-functional-ability (accessed June 16, 2021).
14. Anstey KJ. Optimizing cognitive development over the life course and preventing cognitive decline: Introducing the Cognitive Health Environment Life Course Model (CHELM). Int J Behav Dev. (2014) 38:1–10. doi: 10.1177/0165025413512255
15. Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. (2011) 10:819–28. doi: 10.1016/S1474-4422(11)70072-2
16. Mora F. Successful brain aging: plasticity, environmental enrichment, and lifestyle. Dialogues Clin Neurosci. (2013) 15:45–52. doi: 10.31887/DCNS.2013.15.1/fmora
17. Stern Y. Cognitive reserve in ageing and Alzheimer's disease. Lancet Neurol. (2012) 11:1006–12. doi: 10.1016/S1474-4422(12)70191-6
18. Stern Y. What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. (2002) 8:448–60. doi: 10.1017/S1355617702813248
19. Habeck C, Razlighi Q, Gazes Y, Barulli D, Steffener J, Stern Y. Cognitive reserve and brain maintenance: orthogonal concepts in theory and practice. Cereb Cortex. (2017) 27:3962–9. doi: 10.1093/cercor/bhw208
20. Rodriguez FS, Aranda MP, Lloyd DA, Vega WA. Racial and ethnic disparities in dementia risk among individuals with low education. Am J Geriatr Psychiatry. (2018) 26:966–76. doi: 10.1016/j.jagp.2018.05.011
21. Barulli D, Stern Y. Efficiency, capacity, compensation, maintenance, plasticity: emerging concepts in cognitive reserve. Trends Cogn Sci. (2013) 17:502–9. doi: 10.1016/j.tics.2013.08.012
22. Nithianantharajah J, Hannan AJ. Enriched environments, experience-dependent plasticity and disorders of the nervous system. Nat Rev Neurosci. (2006) 7:697–709. doi: 10.1038/nrn1970
23. Richards M, Deary IJ. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. (2005) 58:617–22. doi: 10.1002/ana.20637
24. Xu W, Tan L, Wang HF, Tan MS, Tan L, Li JQ, et al. Education and risk of dementia: dose-response meta-analysis of prospective cohort studies. Mol Neurobiol. (2016) 53:3113–23. doi: 10.1007/s12035-015-9211-5
25. Amieva H, Mokri H, Le Goff M, Meillon C, Jacqmin-Gadda H, Foubert-Samier A, et al. Compensatory mechanisms in higher-educated subjects with Alzheimer's disease: a study of 20 years of cognitive decline. Brain. (2014) 137(Pt 4):1167–75. doi: 10.1093/brain/awu035
26. Weiler M, Casseb RF, De Campos BM, De Ligo Teixeira CV, Carletti-Cassani A, Vicentini JE, et al. Cognitive reserve relates to functional network efficiency in Alzheimer's disease. Front Aging Neurosci. (2018) 10:255. doi: 10.3389/fnagi.2018.00255
27. Then FS, Luppa M, Schroeter ML, Konig HH, Angermeyer MC, Riedel-Heller SG. Enriched environment at work and the incidence of dementia: results of the Leipzig longitudinal study of the aged (LEILA 75+). PLoS ONE. (2013) 8:e70906. doi: 10.1371/journal.pone.0070906
28. Then FS, Luck T, Luppa M, Konig HH, Angermeyer MC, Riedel-Heller SG. Differential effects of enriched environment at work on cognitive decline in old age. Neurology. (2015) 84:2169–76. doi: 10.1212/WNL.0000000000001605
29. Then FS, Luck T, Heser K, Ernst A, Posselt T, Wiese B, et al. Which types of mental work demands may be associated with reduced risk of dementia? Alzheimers Dement. (2017) 13:431–40. doi: 10.1016/j.jalz.2016.08.008
30. Wickens CD. Processing resources and attention. In: Damos DL, editors. Multiple Task Performance. 1st ed. London: CRC Press (1991). p. 3–34. doi: 10.1201/9781003069447-2
31. Rodriguez FS, Zheng L, Chui HC. Psychometric characteristics of cognitive reserve: how high education might improve certain cognitive abilities in aging. Dement Geriatr Cogn Disord. (2019) 47:335–44. doi: 10.1159/000501150
32. Rodriguez FS. Cognitive reserve: cognitive abilities that shield against dementia symptomatology. OBM Geriatr. (2018) 2. doi: 10.21926/obm.geriatr.1804017
33. Brinke LFT, Davis JC, Barha CK, Liu-Ambrose T. Effects of computerized cognitive training on neuroimaging outcomes in older adults: a systematic review. BMC Geriatr. (2017) 17:139. doi: 10.1186/s12877-017-0529-x
34. Liu C-C, Liu C-C, Kanekiyo T, Xu H, Bu G. Apolipoprotein E and Alzheimer disease: risk, mechanisms and therapy. Nat Rev Neurol. (2013) 9:106–18. doi: 10.1038/nrneurol.2012.263
35. Garibotto V, Borroni B, Sorbi S, Cappa S, Padovani A, Perani D. Education and occupation provide reserve in both ApoE ε4 carrier and noncarrier patients with probable Alzheimer's disease. Neurolo Sci. (2012) 33:1037–42. doi: 10.1007/s10072-011-0889-5
36. Rodriguez FS, Schroeter ML, Arelin K, Witte AV, Baber R, Burkhardt R, et al. APOE e4-genotype and lifestyle interaction on cognitive performance: results of the LIFE-Adult-study. Health Psychol. (2018) 37:194–205. doi: 10.1037/hea0000515
37. Wang H-X, Gustafson DR, Kivipelto M, Pedersen NL, Skoog I, Windblad B, et al. Education halves the risk of dementia due to apolipoprotein ε4 allele: a collaborative study from the Swedish Brain Power initiative. Neurobiol Aging. (2012) 33:1007–e1001. doi: 10.1016/j.neurobiolaging.2011.10.003
38. Arenaza-Urquijo EM, Gonneaud J, Fouquet M, Perrotin A, Mezenge F, Landeau B, et al. Interaction between years of education and APOE epsilon4 status on frontal and temporal metabolism. Neurology. (2015) 85:1392–9. doi: 10.1212/WNL.0000000000002034
39. Penninkilampi R, Casey AN, Singh MF, Brodaty H. The association between social engagement, loneliness, and risk of dementia: a systematic review and meta-analysis. J Alzheimers Dis. (2018) 66:1619–33. doi: 10.3233/JAD-180439
40. Rodriguez FS, Pabst A, Luck T, Konig HH, Angermeyer MC, Witte AV, et al. Social Network Types in Old Age and Incident Dementia. J Geriatr Psychiatry Neurol. (2018) 31:163–70. doi: 10.1177/0891988718781041
41. Shankar A, Hamer M, Mcmunn A, Steptoe A. Social isolation and loneliness: relationships with cognitive function during 4 years of follow-up in the english longitudinal study of ageing. Psychosom Med. (2013) 75:161–70. doi: 10.1097/PSY.0b013e31827f09cd
42. Rodriguez FS, Schroeter ML, Witte AV, Engel C, Loffler M, Thiery J, et al. Could high mental demands at work offset the adverse association between social isolation and cognitive functioning? Results of the population-based LIFE-adult-study. Am J Geriatr Psychiatry. (2017) 25:1258–69. doi: 10.1016/j.jagp.2017.05.014
43. Tennstedt SL, Unverzagt FW. The ACTIVE study: study overview and major findings. J Aging Health. (2013) 25:3S−20S. doi: 10.1177/0898264313518133
44. Smith GE, Housen P, Yaffe K, Ruff R, Kennison RF, Mahncke HW, et al. A cognitive training program based on principles of brain plasticity: results from the improvement in memory with plasticity-based adaptive cognitive training (IMPACT) study. J Am Geriatr Soc. (2009) 57:594–603. doi: 10.1111/j.1532-5415.2008.02167.x
45. Ngandu T, Lehtisalo J, Solomon A, Levalahti E, Ahtiluoto S, Antikainen R, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet. (2015) 385:2255–63. doi: 10.1016/S0140-6736(15)60461-5
46. World Health Organization. Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization. (2019).
47. Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet. (2020) 396:413–46. doi: 10.1016/S0140-6736(20)30367-6
48. Wang HX, MacDonald SW, Dekhtyar S, Fratiglioni L. Association of lifelong exposure to cognitive reserve-enhancing factors with dementia risk: a community-based cohort study. PLoS Med. (2017) 14:e1002251. doi: 10.1371/journal.pmed.1002251
49. Collins R, Silarova B, Clare L. Dementia primary prevention policies and strategies and their local implementation: a scoping review using england as a case study. J Alzheimers Dis. (2019) 70:S303–18. doi: 10.3233/JAD-180608
50. O'connor CM, Clemson L, Brodaty H, Low LF, Jeon YH, Gitlin LN, et al. The tailored activity program (TAP) to address behavioral disturbances in frontotemporal dementia: a feasibility and pilot study. Disabil Rehabil. (2019) 41:299–310. doi: 10.1080/09638288.2017.1387614
Keywords: old age, dementia, cognitive decline, prevention, life-course
Citation: Rodriguez FS (2021) Life-Course Pathways to Cognitive Aging: The Significance of Intellectual Stimulation in the Form of Education and Occupation for Public Policy and Prevention Plans. Front. Psychiatry 12:719609. doi: 10.3389/fpsyt.2021.719609
Received: 02 June 2021; Accepted: 29 June 2021;
Published: 22 July 2021.
Edited by:
Debanjan Banerjee, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaReviewed by:
Sanchari Mukhopadhyay, National Institute of Mental Health and Neurosciences (NIMHANS), IndiaMigita Michael D'Cruz, National Institute of Mental Health and Neurosciences (NIMHANS), India
Copyright © 2021 Rodriguez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francisca S. Rodriguez, francisca-saveria.rodriguez@dzne.de
 Francisca S. Rodriguez
Francisca S. Rodriguez