- 1Department of Psychology, University of Tübingen, Tübingen, Germany
- 2LEAD Graduate School & Research Network, University of Tübingen, Tübingen, Germany
- 3Belgorod National Research University, Belgorod, Russia
- 4Leibniz-Institut für Wissensmedien, Tübingen, Germany
- 5Department of Psychiatry and Psychotherapy, University Hospital of Tübingen, Tübingen, Germany
In this review, we aim to highlight the application of functional near-infrared spectroscopy (fNIRS) as a useful neuroimaging technique for the investigation of cognitive development. We focus on brain activation changes during the development of mathematics and language skills in schoolchildren. We discuss how technical limitations of common neuroimaging techniques such as functional magnetic resonance imaging (fMRI) have resulted in our limited understanding of neural changes during development, while fNIRS would be a suitable and child-friendly method to examine cognitive development. Moreover, this technique enables us to go to schools to collect large samples of data from children in ecologically valid settings. Furthermore, we report findings of fNIRS studies in the fields of mathematics and language, followed by a discussion of the outlook of fNIRS in these fields. We suggest fNIRS as an additional technique to track brain activation changes in the field of educational neuroscience.
Introduction: Mathematics and Language Development
Understanding the processes underlying acquisition and learning of academic skills, such as mathematics and language, are of interest for both educational science and neuroscience. Mastering mathematics and language are central both to career and life perspectives of an individual and also to a society at large (Butterworth et al., 2011). For instance, mathematics has become an inseparable part of everyday life and plays an important role in modern society on every level: from finding a page in a book and selecting a TV channel, to calculating the profits on investments in business and estimating the long-term impacts of political decisions, economic proceedings and social events. Individuals who experience severe difficulties in learning to count and calculate are at a great disadvantage in both academic and professional life (Kadosh and Dowker, 2015). Therefore, the development of numerical abilities is crucial at every stage of the life span from infancy to adulthood (Geary, 2000). Regarding language acquisition, some universal features that are common in all human languages need to be understood by the learning child. This requires a wide-ranging skill set, from domain-specific language-related abilities (e.g., the ability to identify and understand phonemes) to domain-general cognitive abilities (e.g., mental flexibility as bilingual learners switch between languages). Furthermore, languages are built into a social-cultural context; their use is influenced greatly by metalinguistic peculiarities of any society (Obrig et al., 2010), which needs to be taken into account as well.
While multiple valuable behavioral studies of mathematics and language acquisition in childhood have dramatically improved our understanding, in recent years, the educational neuroscience approach has suggested that going beyond behavioral data by means of neurocognitive methods will promote our understanding of cognitive development (Howard-Jones et al., 2016). One neurocognitive method to study cognitive and academic learning and development in children is functional near-infrared spectroscopy (fNIRS). The goal of this review is to outline the contribution of fNIRS to our understanding of the neurocognitive development of mathematics and language skills, particularly in schoolchildren. We briefly explain the concept of educational neuroscience and application of fNIRS. Thereafter, findings of fNIRS studies in these two domains are reported and further discussed.
Educational Neuroscience Perspective
In 1997, John T. Bruer wrote a seminal article entitled “Education and the brain: A bridge too far” and concluded back then that we did “not know enough about brain development and neural function to link that understanding directly, in any meaningful, defensible way, to instruction and educational practice” (Bruer, 1997, p. 4). Despite this rather negative conclusion, one needs to be aware that this was about five years after the first functional magnetic resonance imaging (fMRI) and fNIRS studies had been published. Since then neuroscience has progressed rapidly. Over the last decade, neuroscientific methods have been applied to investigate the structural and functional changes in the developing brain across the life span (e.g., Munakata et al., 2004). This has augmented our basic knowledge but is still not directly useful for instruction and educational practice (see below)–despite the initial hope to directly apply neuroscientific findings to learning and teaching strategies. However, the scientific efforts have boosted interest in a new interdisciplinary research field, known as educational neuroscience or neuroeducation. The growth of educational neuroscience has been regarded a two-way street (Geake, 2004), where learning and educational scientists and neuroscientists influence each other (Spelke, 2002; De Smedt et al., 2010).
The field's focus lies on the elucidation of general and specific mechanisms relevant for learning and development. This knowledge, in turn, may help to improve diagnosis and treatment of developmental disorders. It still may have potential for improvements in the current education systems and teaching methods and to make learning most effective according to “sensitive periods” in development (Ansari and Coch, 2006; Goswami, 2006). However, one has to be very realistic and cautious not to predict something unattainable; not everyone agrees that educational neuroscience might contribute to direct innovative educational applications or perspective teaching methods (Bowers, 2016). Recently, there has been a controversial debate over whether neurocognitive data actually aid understanding and facilitation of cognitive development and learning (Bowers, 2016; Gabrieli, 2016; Howard-Jones et al., 2016). Furthermore, few of educators' expectations regarding neuroscience research and how they might find educational neuroscience professionally useful have been met (Hook and Farah, 2013). Thus, the biggest challenges facing educational neuroscience include applying neuroscience findings directly to developmental patterns and educational settings, and improving interdisciplinary communication between teachers and neuroscientists (Ansari et al., 2012).
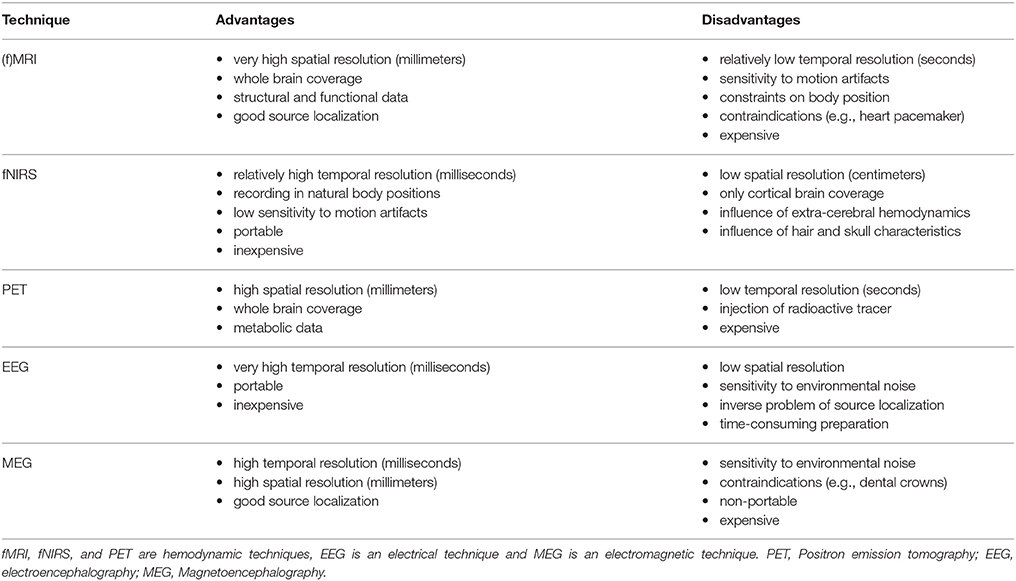
Different neuroimaging tools have been used to measure underlying neural mechanisms during such cognitive processes as mathematics and language tasks in children. While each tool has specific benefits, its limitations might make it less applicable in developmental populations such as children. As shown in Table 1, fNIRS might be considered one of the most suitable tools to investigate brain activation changes in the frame of educational neuroscience.
We have scarce knowledge about the neurocognitive foundations of mathematics and language (particularly reading) development in children, in large part due to the limiting factors of commonly used neuroimaging tools. Given that one of the main aims of educational neuroscience is to advance diagnostic and interventional approaches in learning disabilities, a suitable tool should allow us to measure these cognitive processes in natural settings, such as in schools (Mücke et al., 2018).
FNIRS as an Imaging Technique
FNIRS is an optical imaging technique that uses near-infrared light (wavelengths 650–950 nm) to measure concentration changes of oxygenated and deoxygenated hemoglobin in cortical brain structures (for more in-depth reviews see Ferrari and Quaresima, 2012; Scholkmann et al., 2014). Light from the near-infrared range has the ability to penetrate biological tissue (e.g., skin, skull, brain) and is mainly absorbed by oxygenated and deoxygenated hemoglobin. With the most commonly used continuous-wave systems, near-infrared light is continuously sent via light emitters (emitting optodes) through brain tissue and then collected by light detectors (detecting optodes). A pair of emitter-detector optodes represents a measuring channel. A simplified illustration can be found in Figure 1A. The average trajectory of photons from the emitter to the detector can be represented by a “banana-shaped” form that in part pervades cortical tissue (red-shaded area in the figure). Owing to the above-mentioned absorption and scattering of near-infrared light, there is a loss of light intensity at the detector. Using the modified Beer-Lambert Law for light attenuation in scattering biological tissue and specific assumptions, concentration changes of oxygenated and deoxygenated hemoglobin can be calculated from the intensity loss. Technically, fNIRS does not assess brain activity directly, but measures concentration changes of oxygenated and deoxygenated hemoglobin in the blood vessels. As neuronal activity leads to an increase in regional blood flow, combined with an increase in oxygenated and decrease in deoxygenated hemoglobin (neurovascular coupling), neural activity can be inferred from the concentration changes of the chromophores. Hence, similar to fMRI, fNIRS measures an optical blood oxygen level dependent (BOLD) signal, which can be analyzed using methods similar to those applied to fMRI data (i.e., general linear model, channel-wise analysis, region-of-interest analysis, and functional connectivity). These days, with the availability of multi-channel systems (cf. Figure 1B), fNIRS can be considered cost-effective, in comparison with fMRI, as a non-invasive brain imaging technique that offers several additional advantages (cf. Table 1). These advantages make it suitable for investigating neurocognitive measures in ecologically valid settings such as schools or kindergartens with a natural response type—a verbal or written production paradigm—which is the core concept of the current review. Similar to the other neuroimaging techniques, it also facilitates the study of questions of innateness, as neural responses to language or numerical stimuli can be measured in the absence of conscious language understanding or verbal or manual responses.
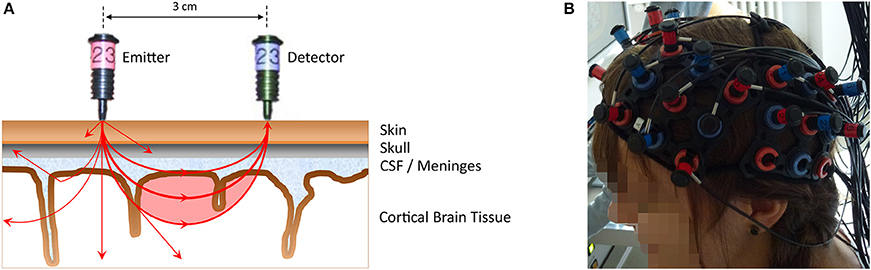
Figure 1. (A) A simplified illustration of an emitter-detector optode pair (i.e., one measurement channel) representing the principles of NIRS measurement (distance 3 cm, Hitachi ETG-4000). Near-infrared light from the emitter (red optode) penetrates the scalp to pass through different biological tissues (e.g., skin, skull, CSF [cerebro-spinal fluid]/meninges, cortical brain tissue). The near-infrared light that is subsequently detected (blue optode) on average travels through a “banana-shaped” form (red-shaded area), allowing hemodynamic changes within this area to be assessed. Note that due to the properties of the penetrated medium (e.g., resulting in scattering, reflection, absorption by oxygenated, and deoxygenated hemoglobin), only a fraction of the emitted light reaches the detector. This is illustrated by exemplary photon paths on the left side. From the intensity loss at the detector site, concentration changes in oxygenated and deoxygenated hemoglobin can be calculated. The near-infrared light originating from one emitter can be detected by several detectors surrounding that emitter, thus resulting in neighboring channels (e.g., photons propagating to the left). (B) The placement of a multi-channel fNIRS probe sets.
Several commercial NIRS machines are available, which differ regarding various parameters such as time resolution, number of emitters/detectors, adjustable, or fixed emitter-detector-distances, employed wavelengths, etc. (see Scholkmann et al., 2014).
FNIRS and fMRI (the most common neuroimaging technique) should be considered complementary techniques, since both have their advantages and disadvantages. The use of a specific method should always be dependent on the research question and the respective samples. In a sample without anxiety and specific MRI contraindications, or in simple research paradigms without movement artifacts, fMRI may be the better choice. In young children and especially in tasks where movements are present, fNIRS may be the better choice. In this article, we concentrate on the contribution of fNIRS to two major fields of cognitive and educational development: mathematics and language.
Application of fNIRS to the Investigation of Mathematics
FMRI studies showed that in infants and preschoolers the right parietal cortex is sensitive to changes in the cardinality of a set of objects (Cantlon et al., 2006; Izard et al., 2009; Park et al., 2014). Activation shifts progressively from the right intraparietal sulcus to bilateral intraparietal sulcus in numerical-magnitude processing due to the acquisition of the exact number system (Ansari et al., 2006; Cantlon et al., 2006; Piazza et al., 2007; Emerson and Cantlon, 2015). Moreover, there is a developmental fronto-parietal shift representing the change from more effortful procedural strategies to more automatic and retrieval strategies between 8 and 19 years of age (e.g., Rivera et al., 2005). This shift accompanies a decreased activation of the hippocampus in adults and adolescents compared to 8- to 9-year-old children (Qin et al., 2014) and an increased activation of the left supramarginal gyrus and angular gyrus by involving language-related areas in retrieving facts from long-term memory between 8 and 14 years of age (Ansari, 2008; Prado et al., 2014). However, because of scarce neuroimaging studies in children, it is not easy to distinguish which changes are due to specific mathematical learning and general cognitive development, and which are due to the maturation of the brain (Arsalidou et al., 2017; Peters and De Smedt, 2017). Moreover, the generalization of such findings in experimental settings to ecological settings is not trivial since mathematics in real life is not conducted lying down in a noisy environment without movement, which can affect numerical learning.
FNIRS research in this field has only started to emerge in recent years as it allows overcoming some of the aforementioned challenges. For example, fNIRS studies revealed activity in the right intraparietal sulcus—a key region for number processing—in response to a numerical change in a visual pattern in awake 5.5–6.5-month-old infants (Hyde et al., 2010; Edwards et al., 2016). In other words, this technique makes it possible to see the brain regions responding in awake infants. In the study by Hyde et al. (2010), infants were adapted to a set of 16 dots and in an oddball paradigm they were shown images changing in numerosity (8 or 16 dots) and shape (16 squares or triangles). In the study by Edwards et al. (2016), they were shown either the same number of dots (8 or 16) or sets varying in numerosity (8 and 16) in different blocks. In both studies, an increased activation was found in the right intraparietal sulcus in response to the change in the number of dots, in comparison with other conditions. These findings provided evidence that infants start mentally operating with non-symbolic numerosity very early, and rely on their approximate number system before acquiring language and symbolic number system experience. As for neural correlates of numerical cognition in preschoolers, there are no fNIRS studies yet.
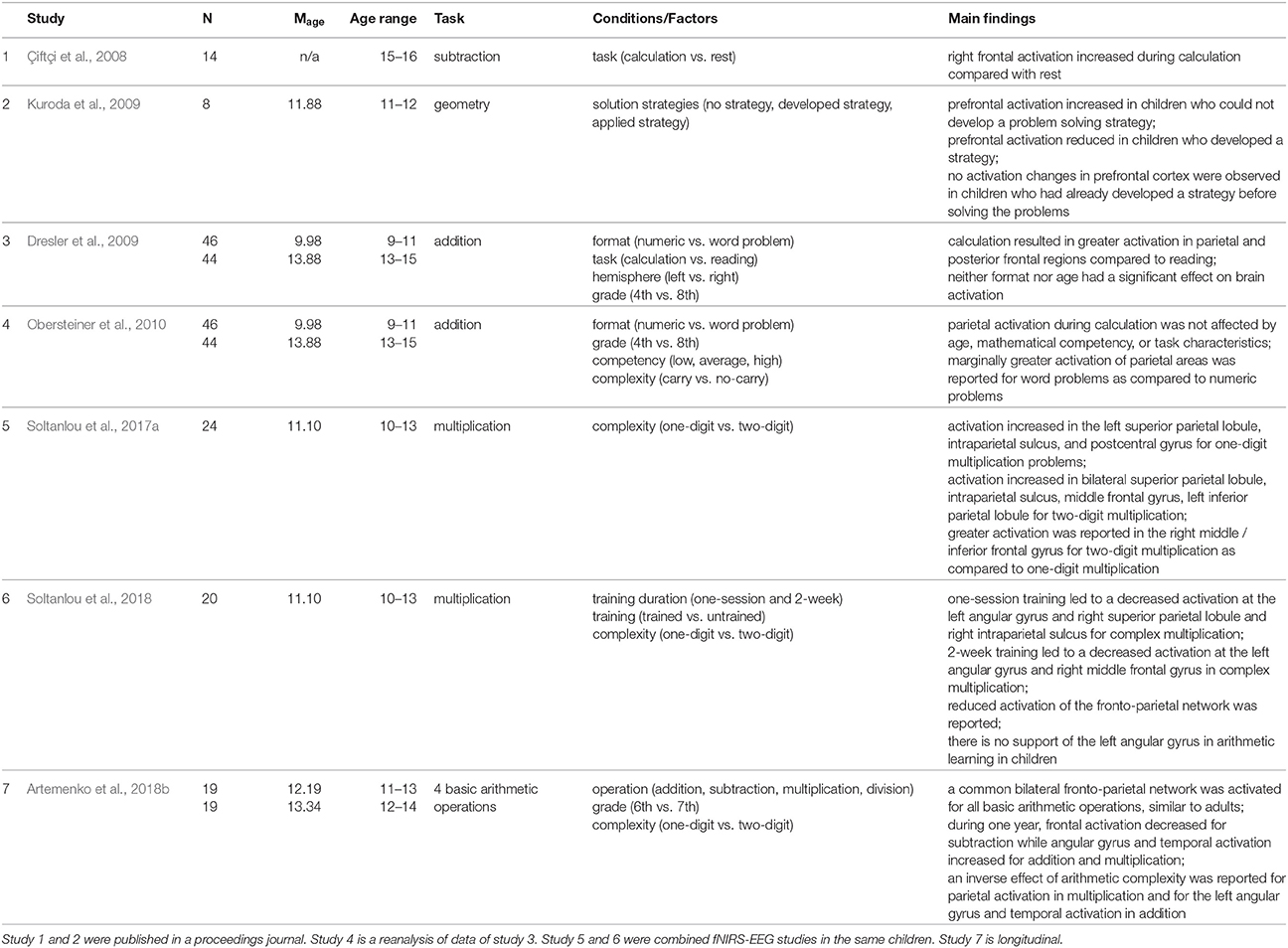
FNIRS studies of arithmetic performance in primary- and secondary-school children (e.g., Dresler et al., 2009; Soltanlou et al., 2017a) revealed bilateral activation of a frontal-parietal network, the network that has been observed in fMRI neuroimaging studies in adults and children (Arsalidou and Taylor, 2011; Arsalidou et al., 2017). Dresler et al. (2009) presented an arithmetic problem either in numeric format or embedded in text to primary- and secondary-school children (cf. Table 2). In this study, a sample of 90 children was measured, which is very difficult to achieve with other techniques such as fMRI. They observed greater activation in parietal and posterior frontal regions for the calculation than for reading in both primary- and secondary-school children, which is in line with fMRI results in children (Rivera et al., 2005; Ansari et al., 2006; Kucian et al., 2006) and fNIRS results in adults (Richter et al., 2009; but see Artemenko et al., 2018a for basic tasks of copying numbers and letters). Moreover, greater task-related activation in bilateral frontal areas (precentral premotor and motor areas) was observed in younger children than in older children. This activation is due to less automatized calculation processing and more speech-related activity. In line with previous studies (Rivera et al., 2005; Kaufmann et al., 2006; Kucian et al., 2008), this fNIRS finding in schoolchildren pointed out a developmental frontal-to-parietal shift in brain activation.
This developmental shift was also reported in another longitudinal fNIRS study (Artemenko et al., 2018b). The applicability of fNIRS in a natural setting let them measure brain activation during all four basic operations in a written production paradigm. Because of uncomfortable positioning in the fMRI scanner and sensitivity of this technique to movement artifacts, most fMRI studies have not used written production, which is the most ecologically valid way to solve such tasks, as they would be solved in schools. Artemenko et al. (2018b) reported decreased activation of frontal regions for subtraction—less effortful calculation—from 6th to 7th grade and at the same time increased activation of the angular gyrus and temporal regions for addition and multiplication—more automatic calculation and fact retrieval (cf. Table 2). Importantly, in such a natural setting, they not only found a shift between activation regions of interest, but also more efficient (or less effortful) processing within those regions.
However, in children, not only the operation and the age play a role in neurocognitive processing in arithmetic, but also the mathematical competency. Obersteiner et al. (2010) investigated the fNIRS data of Dresler et al. (2009) in more detail and besides the format and grade further explored mathematical competency (low, average, high) and task complexity (addition with and without a carry operation) in the calculation condition (cf. Table 2). For these factors, no significant activation differences were found in the targeted parietal regions, with a trend for higher activation for word than for numeric problems. However, they observed higher parietal activation in no-carry addition than carry addition in the group with average mathematical competency. Interestingly, this study was conducted at school, which is impossible in the case of several non-portable neuroimaging techniques such as fMRI. This situation probably leads to less anxiety in children then when they come to experimental labs, which might influence the activation patterns.
In another fNIRS study in secondary-school children, Kuroda et al. (2009) utilized fNIRS to measure brain activation changes during spatial manipulation with objects, which is important for geometry (cf. Table 2). Prefrontal activation was measured in 6th graders while they were solving tangram puzzle tasks. Different activation patterns were detected in accordance with strategies the children used to solve problems, which led to three groups of children based on their solution methods. In children who were not able to solve the tangram puzzle, prefrontal activation continually increased. In children who were able to develop a strategy in the process of manipulating tangram pieces, prefrontal activation steadily declined. No changes in prefrontal activation were found in a group where children had already developed a strategy before solving the problems. In general, the findings are in line with neuroimaging studies of prefrontal activation: when the level of task complexity increases, the activation may increase correspondingly (Kuroda et al., 2009; see also Mücke et al., 2018). Again, the possibility of testing in a natural setting let them measure brain activities during tangram puzzle solving (see also Soltanlou et al., 2017b), which cannot be done in an fMRI scanner.
In a recent combined fNIRS-EEG study, Soltanlou et al. (2017a) investigated arithmetic complexity in multiplication problems in 5th graders. This study shows the feasibility of fNIRS in combination with other techniques such as EEG, which does not lead to additional artifacts in EEG signals (cf. Table 2). Soltanlou et al. (2017a) observed significant activation in the left superior parietal lobule, intraparietal sulcus, and postcentral gyrus for one-digit multiplication problems, while bilateral superior parietal lobule, intraparietal sulcus, middle frontal gyrus, and left inferior parietal lobule were activated when children were solving complex multiplication problems. The contrast of complex vs. simple revealed greater activation in the right middle frontal gyrus but not in parietal regions. This finding shows that in children, increasing mathematics complexity promotes domain-general cognitive processes, i.e., working memory, sustained attention and planning (see also Mücke et al., 2018). Furthermore, the authors suggested that at this stage in development, children rely on domain-specific magnitude processing for both simple and complex calculations (Soltanlou et al., 2017a). This finding was in line with the findings of Artemenko et al. (2018b) showing a decrease in activation of middle and inferior frontal gyri from 6th to 7th grade. Therefore, we might conclude that dependency on domain-general cognitive processes in mental calculation declines during development. However, another fNIRS study of mental calculation in high-school children between the ages of 15–16 years (Çiftçi et al., 2008) revealed greater activation of the right prefrontal cortex during subtraction compared to rest (cf. Table 2). This finding points out that improvement in solving subtraction problems relies on fast procedural processes rather than fact retrieval processes (Prado et al., 2014) because adolescents still rely on this activation to solve subtraction problems but are fast in problem solving.
In another fNIRS-EEG learning study on multiplication problems with 5th graders, Soltanlou et al. (2018) reported decreased activation of the right middle frontal gyrus during trained vs. untrained sets after 2 weeks of training (cf. Table 2), which was in line with previous arithmetic learning studies in adults (Zamarian et al., 2009) and children (Arsalidou et al., 2017; Peters and De Smedt, 2017). Additionally, the authors found decreased activation of the left angular gyrus in trained vs. untrained sets via multiplication learning in children, which was contradictory to adults' studies (Soltanlou et al., 2018). This finding is line with a recent meta-analysis showing that a brain activation network underlying arithmetic processing and development in children differs from that in adults (Arsalidou et al., 2017). Moreover, they argue that one of the aims of this study was to investigate brain activation changes due to training in an ecologically valid setting.
To conclude, the consistency of the findings in the few above-mentioned fNIRS studies with the findings of other approaches, particularly fMRI, in schoolchildren (Peters and De Smedt, 2017), reveals the feasibility of this approach in educational neuroscience. Usually, a frontal-parietal network is observed, which varies with task complexity, age, and the expertise of the children. Greater task complexity, younger age, and lesser expertise usually require more effortful processing and more involvement of frontal regions, corresponding with domain-general contributions to arithmetic problem solving for such groups and problems.
Application of fNIRS to the Investigation of Language
FMRI studies showed that the major brain regions responsible for many aspects of language development and processing make up the left perisylvian area in human cortex, including Broca's and Wernicke's areas (Gazzaniga, 2004). The brain specialization for language acquisition in neonates and infants is subserved by a temporo-frontal loop. Thereafter, when reading skills are acquired in children aged from 7 to 17 years, visual forms of words are represented in the occipital-temporal areas (Shaywitz et al., 2002). Interestingly, the processing of written and spoken words similarly rely on posterior multi-modal areas including Wernicke's area (Booth et al., 2001). However, understanding and appropriately using language in different contexts, evaluating humor and emotional expressiveness, together with visuospatial processing, which is needed for reading skill, also involve the right hemisphere or other brain areas in the left hemisphere (Kensinger and Choi, 2009). In sum, while a left lateralization for language can be already observed in infancy, the right hemisphere also plays an essential role in some aspects of language processing and reading in particular. However, because of the advantages of fNIRS, it is worthwhile to measure these processes in more natural settings to test the feasibility of this generalization.
During the last decade, fNIRS has been used in several language studies conducted with neonates, infants, children and adults (for a review see Minagawa-Kawai et al., 2008; Ferrari and Quaresima, 2012; Quaresima et al., 2012; Vanderwert and Nelson, 2014; Aslin et al., 2015). FNIRS has been successfully applied to study neural correlates of linguistic and non-linguistic processing in native and non-native languages in newborns (Pena et al., 2003; Telkemeyer et al., 2009; Arimitsu et al., 2011; May et al., 2011; Vannasing et al., 2016) and 3- to 11-month-old infants (Homae et al., 2006, 2007). The ability to perceive acoustic and speech stimuli helps infants process segmental and suprasegmental information from birth. It was shown that the auditory cortex of neonates is sensitive both to phonemic and prosodic information, but with different patterns of brain activation. Arimitsu et al. (2011) revealed a right-dominant superior temporal sulcus and mid temporal gyrus activation in neonates (3–8 days old) in response to intonation changes in speech (eg., itta vs. itta?), and a left-dominant activation in temporal and inferior parietal regions (supramarginal gyrus) in response to the phonemic changes (e.g., itta vs. itte) due to the verbal-auditory short-term memory. FNIRS has been also successfully combined with other neuroimaging and neurophysiological techniques to study language. For instance, in a combined fNIRS-EEG study in 2- to 6-day-old neonates, Telkemeyer et al. (2009) found an increased activation in right inferior and posterior temporal areas in response to prosodic information, and a left hemisphere (temporoparietal) dominance for fast acoustic modulations that are most relevant to phonetic processing. In sum, the results converge to show that newborns process language properties bilaterally, and activation is mostly observed in temporal and inferior parietal regions known as language areas from adult studies. Studies using fNIRS with infants and preschoolers revealed similar results (Homae et al., 2006, 2007, 2011; Wartenburger et al., 2007; Telkemeyer et al., 2011).
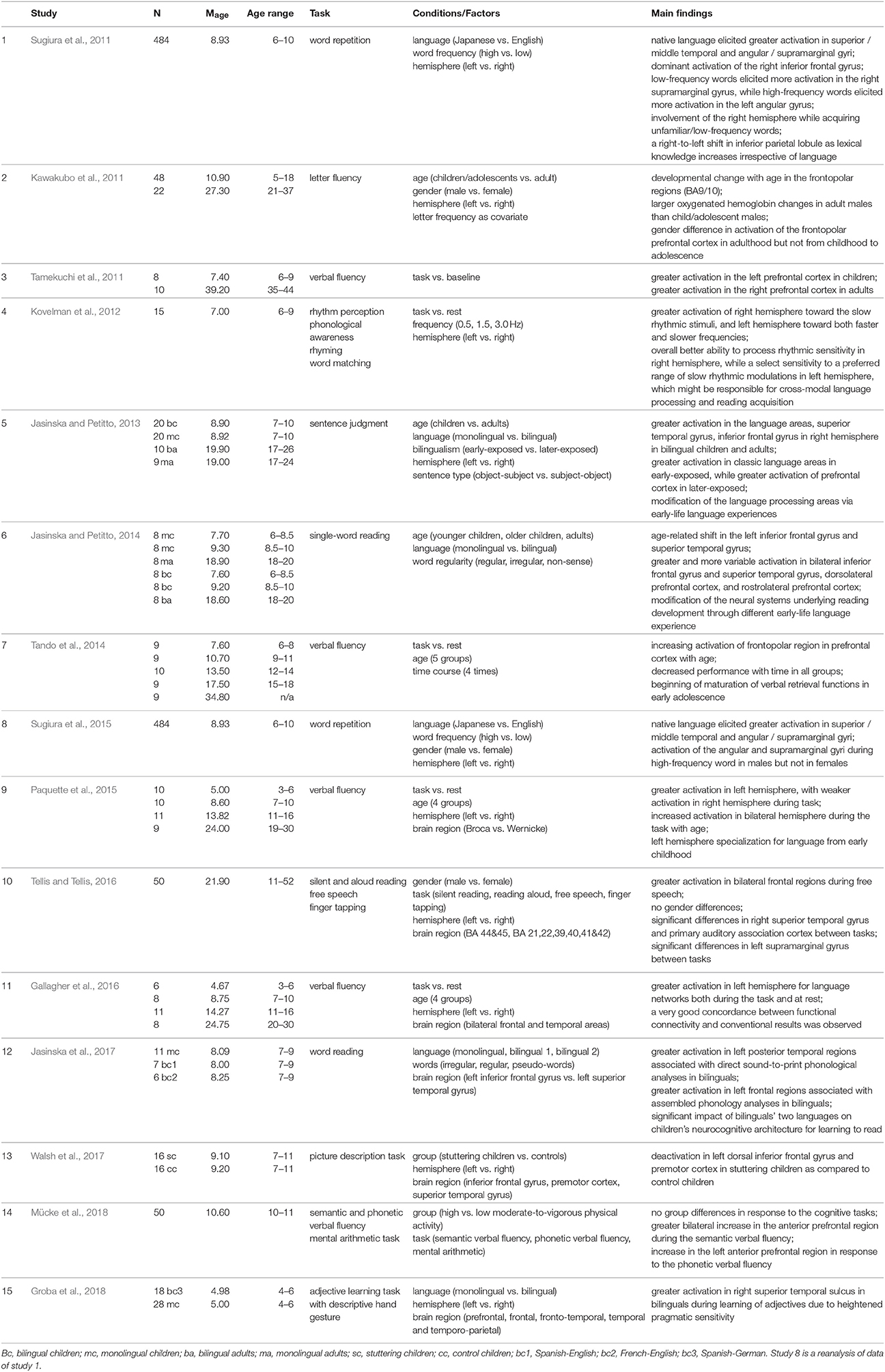
In a combined fNIRS-EEG study of 3- and 6-month-old infants, Telkemeyer et al. (2011) observed right temporal activation in response to different changes in prosodic information (slow modulations), while left tempo-parietal regions responded to speech processing (fast modulations). Later, at the age of 4 years, this right lateralized pattern of processing the prosodic components of speech remained significant (Wartenburger et al., 2007). Wartenburger et al. (2007) showed that linguistic information is processed in left fronto-temporal regions, whereas prosodic information engages right fronto-temporal activation. Similar findings (cf. Table 3) were observed in older children at the ages of 6–9 years (Kovelman et al., 2012). These fNIRS findings were interpreted as showing a tendency of the right hemisphere to process slow rhythmic stimuli, and a selective sensitivity of the left hemisphere to a specific range of slow rhythmic modulations that are important for reading acquisition (Kovelman et al., 2012). These slow modulations correlate with reading ability in 10-year-old children (Goswami, 2011). These findings suggest that the prosodic processing is innate or at least very quickly developed, and the ability to use it to identify utterance enhances during the first year. However, this processing changes with age: the more linguistic information is processed, the more the left hemisphere is involved. Moreover, these findings demonstrate that suprasegmental information plays a crucial role even at an early age and corresponds to adult-like activation in response to intonation or loudness of the speaker (Meyer et al., 2004; Obrig et al., 2010). Therefore, for language as for numerosity development, fNIRS is especially suited to investigate innate or quickly emerging neural responses to language in the absence of any language production in infants.
Some characteristics of fNIRS, such as robustness to muscle movements in the case of reading and speaking (speech production) can be considered a significant advantage in neuroimaging studies of language development in preschoolers and schoolchildren (Gallagher et al., 2016; Walsh et al., 2017). For instance, Tellis and Tellis (2016) measured brain activation changes during three different reading tasks in children and adults: silent reading, reading out loud, and free speech on a given topic. They observed the highest activation during free speech in bilateral frontal regions. In another example, Kawakubo et al. (2011) observed developmental changes from 5 to 37 years in the frontopolar region during a letter fluency task (see also Tamekuchi et al., 2011). Furthermore, they found a gender difference in the frontopolar region in adulthood, which was not observed in childhood or adolescence (Kawakubo et al., 2011). Similar results were found in an fNIRS study by Tando et al. (2014), who observed increased activation of the frontopolar region during a verbal fluency task from 6 to 18 years (cf. Table 3). They concluded that maturation of the verbal retrieval functions starts in early adolescence and continues into adulthood (Tando et al., 2014). Moreover, Tamekuchi et al. (2011) reported higher activation in the left prefrontal cortex in children (6–9 years) than in adults (35–44 years) during a verbal fluency task, while the opposite pattern was observed in the right prefrontal cortex. In line with this finding, Paquette et al. (2015) investigated age-related changes in lateralization of brain activation during expressive language from 3 to 30 years. However, they observed a developmental increase in both hemispheres during a verbal fluency task (Paquette et al., 2015). They found greater activation in left temporal and frontal areas compared with right hemispheric areas in all ages, and concluded that left hemispheric specialization for expressive language is established in very young children and develops until adulthood (Paquette et al., 2015). Interestingly, Walsh et al. (2017) observed deactivation in the left hemisphere, i.e., dorsal inferior frontal gyrus and premotor cortex during speech production in 7- to 11-year-old children with stuttering compared to typically developing peers. The above-mentioned tasks with verbal production are rarely used in fMRI studies, while they can be readily utilized during fNIRS measurement (cf. Table 3).
The application of fNIRS to the detection of brain mechanisms of bilingual children has been another interesting area for language studies. Jasinska and Petitto (2014) investigated the neural basis for reading by applying tasks with three word-type conditions (regular, irregular, and nonsense words). They tested two groups of monolingual and bilingual primary-school children: younger (ages 6–8) and older (ages 8–10) in comparison to adults (cf. Table 3). Younger children showed bilateral superior temporal gyrus activation for both regular and irregular words due to a high level of control of matching phonological processing and orthography. Older children revealed left inferior frontal gyrus activation for irregular in comparison to regular words, and in contrast, inferior parietal lobule activation for regular in comparison to irregular words, due to paying attention to lexical word complexity and whole-word processing in general (Jasinska and Petitto, 2014). Compared to monolingual readers, bilinguals in all age groups showed greater bilateral activation in classic language areas (left inferior frontal gyrus, superior temporal gyrus, and inferior parietal lobule), and homologous areas in the right hemisphere. They also revealed greater activation in the prefrontal cortex including the rostrolateral prefrontal cortex and dorsolateral prefrontal cortex due to employing such cognitive processes as reasoning, working memory, and attention, which are necessary for switching between languages. This finding is further supported by work showing increased activation of bilateral prefrontal cortex during a verbal fluency test in 10- to 11-year-olds (Mücke et al., 2018). Bilingual children are supposed to have greater cognitive plasticity, better sensitivity to language functional and structural peculiarities, and more flexibility in their way of thinking than many monolingual children between the ages of 5 and 10 years (Bialystok et al., 2012; see also Groba et al., 2018). For instance, Jasinska et al. (2017) observed greater activation in left posterior temporal regions—associated with direct sound-to-print (transparent) phonology—and decreased activation in left frontal regions—associated with assembled phonology—in Spanish-English and French-English bilingual children from ages 6 to 10, but not in English monolingual children during an overt word reading task (cf. Table 3). The fNIRS findings suggest that these neural circuitries are highly relevant for reading skills in bilinguals (Jasinska et al., 2017). In line with these findings, Jasinska and Petitto (2013) observed greater activation in left-hemispheric language areas, and additionally, right-hemispheric homologs, i.e., right superior temporal gyrus, and inferior frontal gyrus, in bilingual children (ages 7-10) and adults as compared with monolinguals (cf. Table 3).
In a large-sample study, 484 elementary-school children (6- to 10-year-olds) performed word repetition tasks in their native language and second language, while their brain activation was recorded by means of fNIRS in a neuroimaging vehicle (Sugiura et al., 2011), showing the portability of fNIRS devices. FNIRS findings revealed that the cortical activation pattern associated with language processing involved a bilateral frontal, temporal, and parietal network (Sugiura et al., 2011). Native language words evoked significantly greater left brain activation than foreign words in the superior and middle temporal gyri, and in inferior parietal regions (angular and supramarginal gyri). Foreign language words elicited activation in the right hemisphere, as primary-school children had only started learning foreign languages and did not know many non-native words (see also Groba et al., 2018). Moreover, low-frequency words in both languages led to significant activation in the right supramarginal gyrus, while left-sided activation was detected in the angular gyrus for high-frequency words in the native language, as the lexical meanings of most these words were familiar to children. Sugiura et al. (2015) showed that high frequency word processing leads to increased activation in the angular and supramarginal gyri in boys but not in girls. While the main effect of sex in these two regions was significant, its interaction with hemisphere did not reach significance level. They suggested that this activation differs between boys and girls (Sugiura et al., 2015). This tendency shows the right-to-left shift in the inferior parietal areas when lexical proficiency increases in acquiring knowledge in any language. Sugiura et al. (2011) also found a statistically significant tendency in the age-related dynamics: the older the children were, the less activation was detected in auditory and temporal areas for the native language. In line with fMRI studies, the right temporo-parieto-frontal network revealed activation in response to low-frequency words, and non-linguistic information played an important role in the development of language competencies in native and non-native languages. This kind of large-scale brain imaging study is feasible (because of lower cost and portability) with the help of fNIRS rather than several other imaging techniques. This finding is further corroborated by greater activation in the right superior temporal sulcus in bilingual as compared to monolingual children at the age of 5 years. Groba et al. (2018) observed that in the absence of a behavioral difference, fNIRS results show that Spanish-German bilingual children rely more on the right superior temporal sulcus than German monolingual children during adjective learning.
There are similarities between the mathematics and language studies. More difficult stimuli (here low-frequency words), different age groups and expertise groups (here bilingual children) lead to more frontal activation that supposedly underlies more domain-general processes, as in mathematics studies. FNIRS seems to be a suitable technique not only to investigate language acquisition and comprehension, but also reading and speaking both in native and foreign languages and in bilingual settings in children of different age groups.
Future Research
In this review, we present research using fNIRS in two developmentally and educationally important domains: mathematics and language. We acknowledge the importance of cognitive variables like working memory, attention, executive functions, and sensorimotoric development as well as emotional-motivational variables like anxiety, depression, motivation, and so on.
While some studies in schoolchildren have investigated basic arithmetic skills and differences between calculating and reading arithmetic problems, others have used the advantages of fNIRS, by letting children solve geometric tasks like tangram puzzles. The studies converge on their observation of a frontal-parietal network, with more frontal and/or more pronounced activation for younger children, children with less expertise or in solving more difficult arithmetic problems. With respect to language, many aspects of segmental, and suprasegmental information processing in developing children have been investigated. However, some domains are not sufficiently studied in preschoolers and schoolchildren, such as reading and speech production under different circumstances, probably because some of these paradigms are hard to realize in fMRI in children. This is one reason why, in our opinion, fNIRS should be more frequently used for studying mathematics and language.
To date, fNIRS has already contributed to investigating brain activation changes underlying several cognitive processes, particularly in infants and adults, but it has not been used as often in schoolchildren. We believe that fNIRS has the potential to become a viable and widely-used technique in educational neuroscience as well, where the target sample is children under direct academic training. It measures the hemodynamic responses and offers a procedure of high ecological validity, which makes it possible to bring fNIRS to schools so that students can be tested in a familiar environment. Therefore, its application can be extended to:
• study different cognitive functions effectively due to a silent, noiseless procedure that does not interfere with task solving and does not lead to such problems as anxiety about the unfamiliar situation or the restrictions needed in certain techniques like fMRI (Soltanlou et al., 2017a);
• investigate embodied cognition, as it is less sensitive to movements than fMRI, and enables sitting or standing positions (Bahnmueller et al., 2014). Indeed, fNIRS allows the study of cognitive development during movement. Movements can be of different types, such as finger counting and grasping, moving a dominant hand in a written production task (Artemenko et al., 2018a), or even whole body movement as in the investigation of embodied numerosity (Dackermann et al., 2017; for whole-body embodied learning);
• measure larger samples of participants for short periods of time because of lower cost (e.g., it is a one-time purchase, whereas fMRI requires additional funds per use) and portability (Dresler et al., 2009; Obersteiner et al., 2010; Sugiura et al., 2011);
• take repeated or continuous measurements for monitoring purposes (Soltanlou et al., 2018);
• combine effortlessly with other neuroimaging techniques such as EEG without any measurement interference, in order to provide a better understanding of brain mechanisms (Telkemeyer et al., 2009; Soltanlou et al., 2017a);
• investigate brain activation changes in populations with atypical development, such as in children with dyslexia or attention deficit and hyperactivity disorder (ADHD) (Moser et al., 2009; Cutini et al., 2016);
• use the method as a neurofeedback and interventional tool in cognitive development studies (Hosseini et al., 2016).
Conclusion
Rapid constant technical improvement of fNIRS might bring us closer to bridging the gap between education and neuroscience (Ansari and Coch, 2006). FNIRS can provide novel insight into the neural basis of numerical cognition and of language acquisition or production by studying these processes in natural academic settings (Soltanlou et al., 2017a,b), where most other neuroimaging techniques, such as fMRI, are unsuitable. Furthermore, fNIRS can trace changes on the neural level during development (Artemenko et al., 2018b) and eventually the life span (Wilcox and Biondi, 2015; Gallagher et al., 2016) to understand how particular brain structures stay constant or change with maturation, experience, and learning (Gervain et al., 2011). FNIRS may also be applicable to studying the learning of mathematics and language in real life (Soltanlou et al., 2018). Usually, fNIRS is used to observe brain activation in response to cognitive and motor tasks; however, few fNIRS studies have attempted to find out how these skills are learned (Gervain et al., 2008). Furthermore, while most of the neuroimaging techniques are prone to motion artifacts, fNIRS is more flexible to movement (Bahnmueller et al., 2014; Herold et al., 2017). Notably, movement and embodied cognition can be an important intervention technique in supporting mathematics learning (e.g., physically moving the body along a number line; Dackermann et al., 2017).
Application of fNIRS has been also extended to study atypical development. Recently, Cutini et al. (2016) successfully used fNIRS for the first time to investigate hemispheric asymmetry effects of prosodic processing in children with developmental dyslexia. Furthermore, fNIRS is fortunately resilient to subtle movements, which makes it suitable for measuring children with ADHD (Weber et al., 2005; Moser et al., 2009). Moreover, fNIRS was shown to be an effective tool for neurofeedback in ADHD children (Marx et al., 2014; Blume et al., 2017) and in improving cognitive and motor performance (Hosseini et al., 2016; Lapborisuth et al., 2017). These studies suggest a good outlook for the application of fNIRS as a neurofeedback tool in cognitive development training, including mathematics and language in children with developmental dyscalculia and dyslexia. In general, because of the very fast development of fNIRS devices, analysis toolboxes, and its reliable findings in different fields, the fNIRS technique can be regarded as a versatile and promising instrument in educational neuroscience.
Author Contributions
MS, MAS, and TD: wrote the first draft of the manuscript; all authors edited the manuscript in various versions and commented on it.
Funding
MS was supported by the DFG grant [NU 265/3-1] to H-CN. MS, TD, and H-CN are further supported by the LEAD Graduate School & Research Network [GSC1028], which is funded within the framework of the Excellence Initiative of the German federal and state governments.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We would like to thank Julianne Skinner for proofreading the manuscript. We further thank our reviewers for very constructive comments.
References
Ansari, D. (2008). Effects of development and enculturation on number representation in the brain. Nat. Revi. Neurosci. 9:278. doi: 10.1038/nrn2334
Ansari, D., and Coch, D. (2006). Bridges over troubled waters: education and cognitive neuroscience. Trends Cogn. Sci. 10, 146–151. doi: 10.1016/j.tics.2006.02.007
Ansari, D., De Smedt, B., and Grabner, R. H. (2012). Neuroeducation–a critical overview of an emerging field. Neuroethics 5, 105–117. doi: 10.1007/s12152-011-9119-3
Ansari, D., Dhital, B., and Siong, S. C. (2006). Parametric effects of numerical distance on the intraparietal sulcus during passive viewing of rapid numerosity changes. Brain Res. 1067, 181–188. doi: 10.1016/j.brainres.2005.10.083
Arimitsu, T., Uchida-Ota, M., Yagihashi, T., Kojima, S., Watanabe, S., Hokuto, I., et al. (2011). Functional hemispheric specialization in processing phonemic and prosodic auditory changes in neonates. Front. Psychol. 2:202. doi: 10.3389/fpsyg.2011.00202
Arsalidou, M., Pawliw-Levac, M., Sadeghi, M., and Pascual-Leone, J. (2017). Brain areas needed for numbers and calculations in children: meta-analyses of fMRI studies. Dev. Cogn. Neurosci. doi: 10.1016/j.dcn.2017.08.002. [Epub ahead of print].
Arsalidou, M., and Taylor, M. J. (2011). Is 2 + 2 = 4? Meta-analyses of brain areas needed for numbers and calculations. Neuroimage 54, 2382–2393. doi: 10.1016/j.neuroimage.2010.10.009
Artemenko, C., Coldea, A., Soltanlou, M., Dresler, T., Nuerk, H.-C., and Ehlis, A.-C. (2018a). The neural circuits of number and letter copying: an fNIRS study. Exp. Brain Res. 1–10. doi: 10.1007/s00221-018-5204-8
Artemenko, C., Soltanlou, M., Ehlis, A.-C., Nuerk, H.-C., and Dresler, T. (2018b). The neural correlates of mental arithmetic in adolescents: a longitudinal fNIRS study. Behav. Brain Funct. 14:5. doi: 10.1186/s12993-018-0137-8
Aslin, R. N., Shukla, M., and Emberson, L. L. (2015). Hemodynamic correlates of cognition in human infants. Annu. Rev. Psychol. 66, 349–379. doi: 10.1146/annurev-psych-010213-115108
Bahnmueller, J., Dresler, T., Ehlis, A.-C., Cress, U., and Nuerk, H.-C. (2014). NIRS in motion—unraveling the neurocognitive underpinnings of embodied numerical cognition. Front. Psychol. 5:743. doi: 10.3389/fpsyg.2014.00743
Bialystok, E., Craik, F. I., and Luk, G. (2012). Bilingualism: consequences for mind and brain. Trends Cogn. Sci. 16, 240–250. doi: 10.1016/j.tics.2012.03.001
Blume, F., Hudak, J., Dresler, T., Ehlis, A.-C., Kühnhausen, J., Renner, T. J., et al. (2017). NIRS-based neurofeedback training in a virtual reality classroom for children with attention-deficit/hyperactivity disorder: study protocol for a randomized controlled trial. Trials 18:41. doi: 10.1186/s13063-016-1769-3
Booth, J. R., Burman, D. D., Santen, F. W. V., Harasaki, Y., Gitelman, D. R., Parrish, T. B., et al. (2001). The development of specialized brain systems in reading and oral-language. Child Neuropsychol. 7, 119–141. doi: 10.1076/chin.7.3.119.8740
Bowers, J. S. (2016). Psychology, not educational neuroscience, is the way forward for improving educational outcomes for all children: reply to Gabrieli (2016) and Howard-Jones et al. (2016). Psychol. Rev. 123, 628–635. doi: 10.1037/rev0000043
Bruer, J. T. (1997). Education and the brain: a bridge too far. Educ. Res. 26, 4–16. doi: 10.3102/0013189X026008004
Butterworth, B., Varma, S., and Laurillard, D. (2011). Dyscalculia: from brain to education. Science 332, 1049–1053. doi: 10.1126/science.1201536
Cantlon, J. F., Brannon, E. M., Carter, E. J., and Pelphrey, K. A. (2006). Functional imaging of numerical processing in adults and 4-y-old children. PLoS Biol. 4:e125. doi: 10.1371/journal.pbio.0040125
Çiftçi, K., Sankur, B., Kahya, Y. P., and Akin, A. (2008). “Functional clusters in the prefrontal cortex during mental arithmetic,” in 16th European Signal Processing Conference (EUSIPCO 2008) (Lausanne), 1–4.
Cutini, S., Szucs, D., Mead, N., Huss, M., and Goswami, U. (2016). Atypical right hemisphere response to slow temporal modulations in children with developmental dyslexia. Neuroimage 143, 40–49. doi: 10.1016/j.neuroimage.2016.08.012
Dackermann, T., Fischer, U., Nuerk, H.-C., Cress, U., and Moeller, K. (2017). Applying embodied cognition: from useful interventions and their theoretical underpinnings to practical applications. ZDM 49, 545–557. doi: 10.1007/s11858-017-0850-z
De Smedt, B., Ansari, D., Grabner, R. H., Hannula, M. M., Schneider, M., and Verschaffel, L. (2010). Cognitive neuroscience meets mathematics education. Educ. Res. Rev. 5, 97–105. doi: 10.1016/j.edurev.2009.11.001
Dresler, T., Obersteiner, A., Schecklmann, M., Vogel, A. C. M., Ehlis, A.-C., Richter, M. M., et al. (2009). Arithmetic tasks in different formats and their influence on behavior and brain oxygenation as assessed with near-infrared spectroscopy (NIRS): a study involving primary and secondary school children. J. Neural Transm. 116:1689. doi: 10.1007/s00702-009-0307-9
Edwards, L. A., Wagner, J. B., Simon, C. E., and Hyde, D. C. (2016). Functional brain organization for number processing in pre-verbal infants. Dev. Sci. 19, 757–769. doi: 10.1111/desc.12333
Emerson, R. W., and Cantlon, J. F. (2015). Continuity and change in children's longitudinal neural responses to numbers. Dev. Sci. 18, 314–326. doi: 10.1111/desc.12215
Ferrari, M., and Quaresima, V. (2012). A brief review on the history of human functional near-infrared spectroscopy (fNIRS) development and fields of application. Neuroimage 63, 921–935. doi: 10.1016/j.neuroimage.2012.03.049
Gabrieli, J. D. (2016). The promise of educational neuroscience: comment on Bowers (2016). Psychol. Rev. 123, 613–619. doi: 10.1037/rev0000034
Gallagher, A., Tremblay, J., and Vannasing, P. (2016). Language mapping in children using resting-state functional connectivity: comparison with a task-based approach. J. Biomed. Opt. 21:125006. doi: 10.1117/1.JBO.21.12.125006
Geake, J. (2004). Cognitive neuroscience and education: two-way traffic or one-way street? Westminster Stud. Educ. 27, 87–98. doi: 10.1080/0140672040270107
Geary, D. C. (2000). From infancy to adulthood: the development of numerical abilities. Eur. Child Adolesc. Psychiatry 9, S11–S16. doi: 10.1007/s007870070004
Gervain, J., Macagno, F., Cogoi, S., Peña, M., and Mehler, J. (2008). The neonate brain detects speech structure. Proc. Natl. Acad. Sci. U.S.A. 105, 14222–14227. doi: 10.1073/pnas.0806530105
Gervain, J., Mehler, J., Werker, J. F., Nelson, C. A., Csibra, G., Lloyd-Fox, S., et al. (2011). Near-infrared spectroscopy: a report from the McDonnell infant methodology consortium. Dev. Cogn. Neurosci. 1, 22–46. doi: 10.1016/j.dcn.2010.07.004
Goswami, U. (2006). Neuroscience and education: from research to practice? Nat. Rev. Neurosci. 7, 406–413. doi: 10.1038/nrn1907
Goswami, U. (2011). A temporal sampling framework for developmental dyslexia. Trends Cogn. Sci. 15, 3–10. doi: 10.1016/j.tics.2010.10.001
Groba, A., De Houwer, A., Mehnert, J., Rossi, S., and Obrig, H. (2018). Bilingual and monolingual children process pragmatic cues differently when learning novel adjectives. Bilingualism Lang. Cogn. 21, 384–402. doi: 10.1017/S1366728917000232
Herold, F., Wiegel, P., Scholkmann, F., Thiers, A., Hamacher, D., and Schega, L. (2017). Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks. Neurophotonics 4:041403. doi: 10.1117/1.NPh.4.4.041403
Homae, F., Watanabe, H., Nakano, T., Asakawa, K., and Taga, G. (2006). The right hemisphere of sleeping infant perceives sentential prosody. Neurosci. Res. 54, 276–280. doi: 10.1016/j.neures.2005.12.006
Homae, F., Watanabe, H., Nakano, T., and Taga, G. (2007). Prosodic processing in the developing brain. Neurosci. Res. 59, 29–39. doi: 10.1016/j.neures.2007.05.005
Homae, F., Watanabe, H., Nakano, T., and Taga, G. (2011). Large-scale brain networks underlying language acquisition in early infancy. Front. Psychol. 2:93. doi: 10.3389/fpsyg.2011.00093
Hook, C. J., and Farah, M. J. (2013). Neuroscience for educators: what are they seeking, and what are they finding? Neuroethics 6, 331–341. doi: 10.1007/s12152-012-9159-3
Hosseini, S. H., Pritchard-Berman, M., Sosa, N., Ceja, A., and Kesler, S. R. (2016). Task-based neurofeedback training: a novel approach toward training executive functions. Neuroimage 134, 153–159. doi: 10.1016/j.neuroimage.2016.03.035
Howard-Jones, P., Varma, S., Ansari, D., Butterworth, B., De Smedt, B., Goswami, U., et al. (2016). The principles and practices of educational neuroscience: commentary on bowers (2016). Psychol. Rev. 123, 620–627. doi: 10.1037/rev0000036
Hyde, D. C., Boas, D. A., Blair, C., and Carey, S. (2010). Near-infrared spectroscopy shows right parietal specialization for number in pre-verbal infants. Neuroimage 53, 647–652. doi: 10.1016/j.neuroimage.2010.06.030
Izard, V., Sann, C., Spelke, E. S., and Streri, A. (2009). Newborn infants perceive abstract numbers. Proc. Natl. Acad. Sci. U.S.A. 106, 10382–10385. doi: 10.1073/pnas.0812142106
Jasinska, K., Berens, M., Kovelman, I., and Petitto, L. (2017). Bilingualism yields language-specific plasticity in left hemisphere's circuitry for learning to read in young children. Neuropsychologia 98, 34–45. doi: 10.1016/j.neuropsychologia.2016.11.018
Jasinska, K. K., and Petitto, L. A. (2013). How age of bilingual exposure can change the neural systems for language in the developing brain: a functional near infrared spectroscopy investigation of syntactic processing in monolingual and bilingual children. Dev. Cogn. Neurosci. 6, 87–101. doi: 10.1016/j.dcn.2013.06.005
Jasinska, K., and Petitto, L. (2014). Development of neural systems for reading in the monolingual and bilingual brain: new insights from functional near infrared spectroscopy neuroimaging. Dev. Neuropsychol. 39, 421–439. doi: 10.1080/87565641.2014.939180
Kadosh, R. C., and Dowker, A. (2015). The Oxford Handbook of Numerical Cognition. Oxford: Oxford University Press.
Kaufmann, L., Koppelstaetter, F., Siedentopf, C., Haala, I., Haberlandt, E., Zimmerhackl, L.-B., et al. (2006). Neural correlates of the number–size interference task in children. Neuroreport 17:587. doi: 10.1097/00001756-200604240-00007
Kawakubo, Y., Kono, T., Takizawa, R., Kuwabara, H., Ishii-Takahashi, A., and Kasai, K. (2011). Developmental changes of prefrontal activation in humans: a near-infrared spectroscopy study of preschool children and adults. PLoS ONE 6:e25944. doi: 10.1371/journal.pone.0025944
Kensinger, E. A., and Choi, E. S. (2009). When side matters: hemispheric processing and the visual specificity of emotional memories. J. Exp. Psychol. 35:247. doi: 10.1037/a0013414
Kovelman, I., Mascho, K., Millott, L., Mastic, A., Moiseff, B., and Shalinsky, M. H. (2012). At the rhythm of language: brain bases of language-related frequency perception in children. Neuroimage 60, 673–682. doi: 10.1016/j.neuroimage.2011.12.066
Kucian, K., Loenneker, T., Dietrich, T., Dosch, M., Martin, E., and Von Aster, M. (2006). Impaired neural networks for approximate calculation in dyscalculic children: a functional MRI study. Behav. Brain Funct. 2:31. doi: 10.1186/1744-9081-2-31
Kucian, K., von Aster, M., Loenneker, T., Dietrich, T., and Martin, E. (2008). Development of neural networks for exact and approximate calculation: a FMRI study. Dev. Neuropsychol. 33, 447–473. doi: 10.1080/87565640802101474
Kuroda, Y., Okamoto, N., Chance, B., Nioka, S., Eda, H., and Maesako, T. (2009). “Visualization of children's mathematics solving process using near infrared spectroscopic approach,” in Proceedings Volume 7174, Optical Tomography and Spectroscopy of Tissue VIII (San Jose, CA).
Lapborisuth, P., Zhang, X., Noah, A., and Hirsch, J. (2017). Neurofeedback-based functional near-infrared spectroscopy upregulates motor cortex activity in imagined motor tasks. Neurophotonics 4:021107. doi: 10.1117/1.NPh.4.2.021107
Marx, A.-M., Ehlis, A.-C., Furdea, A., Holtmann, M., Banaschewski, T., Brandeis, D., et al. (2014). Near-infrared spectroscopy (NIRS) neurofeedback as a treatment for children with attention deficit hyperactivity disorder (ADHD)—a pilot study. Front. Hum. Neurosci. 8:1038. doi: 10.3389/fnhum.2014.01038
May, L., Byers-Heinlein, K., Gervain, J., and Werker, J. F. (2011). Language and the newborn brain: does prenatal language experience shape the neonate neural response to speech? Front. Psychol. 2:22. doi: 10.3389/fpsyg.2011.00222
Meyer, M., Steinhauer, K., Alter, K., Friederici, A. D., and von Cramon, D. Y. (2004). Brain activity varies with modulation of dynamic pitch variance in sentence melody. Brain Lang. 89, 277–289. doi: 10.1016/S0093-934X(03)00350-X
Minagawa-Kawai, Y., Mori, K., Hebden, J. C., and Dupoux, E. (2008). Optical imaging of infants' neurocognitive development: recent advances and perspectives. Dev. Neurobiol. 68, 712–728. doi: 10.1002/dneu.20618
Moser, S. J., Cutini, S., Weber, P., and Schroeter, M. L. (2009). Right prefrontal brain activation due to Stroop interference is altered in attention-deficit hyperactivity disorder—a functional near-infrared spectroscopy study. Psychiatry Res. 173, 190–195. doi: 10.1016/j.pscychresns.2008.10.003
Mücke, M., Andrä, C., Gerber, M., Pühse, U., and Ludyga, S. (2018). Moderate-to-vigorous physical activity, executive functions and prefrontal brain oxygenation in children: a functional near-infrared spectroscopy study. J. Sports Sci. 36, 630–636. doi: 10.1080/02640414.2017.1326619
Munakata, Y., Casey, B., and Diamond, A. (2004). Developmental cognitive neuroscience: progress and potential. Trends Cogn. Sci. 8, 122–128. doi: 10.1016/j.tics.2004.01.005
Obersteiner, A., Dresler, T., Reiss, K., Vogel, A. C. M., Pekrun, R., and Fallgatter, A. J. (2010). Bringing brain imaging to the school to assess arithmetic problem solving: chances and limitations in combining educational and neuroscientific research. ZDM 42, 541–554. doi: 10.1007/s11858-010-0256-7
Obrig, H., Rossi, S., Telkemeyer, S., and Wartenburger, I. (2010). From acoustic segmentation to language processing: evidence from optical imaging. Front. Neuroenerget. 2:13. doi: 10.3389/fnene.2010.00013
Paquette, N., Lassonde, M., Vannasing, P., Tremblay, J., González-Frankenberger, B., Florea, O., et al. (2015). Developmental patterns of expressive language hemispheric lateralization in children, adolescents and adults using functional near-infrared spectroscopy. Neuropsychologia 68, 117–125. doi: 10.1016/j.neuropsychologia.2015.01.007
Park, J., Li, R., and Brannon, E. M. (2014). Neural connectivity patterns underlying symbolic number processing indicate mathematical achievement in children. Dev. Sci. 17, 187–202. doi: 10.1111/desc.12114
Pena, M., Maki, A., Kovacić, D., Dehaene-Lambertz, G., Koizumi, H., Bouquet, F., et al. (2003). Sounds and silence: an optical topography study of language recognition at birth. Proc. Natl. Acad. Sci. U.S.A. 100, 11702–11705. doi: 10.1073/pnas.1934290100
Peters, L., and De Smedt, B. (2017). Arithmetic in the developing brain: a review of brain imaging studies. Dev. Cogn. Neurosci. doi: 10.1016/j.dcn.2017.05.002. [Epub ahead of print].
Piazza, M., Pinel, P., Le Bihan, D., and Dehaene, S. (2007). A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron 53, 293–305. doi: 10.1016/j.neuron.2006.11.022
Prado, J., Mutreja, R., and Booth, J. R. (2014). Developmental dissociation in the neural responses to simple multiplication and subtraction problems. Dev. Sci. 17, 537–552. doi: 10.1111/desc.12140
Qin, S., Cho, S., Chen, T., Rosenberg-Lee, M., Geary, D. C., and Menon, V. (2014). Hippocampal-neocortical functional reorganization underlies children's cognitive development. Nat. Neurosci. 17, 1263–1269. doi: 10.1038/nn.3788
Quaresima, V., Bisconti, S., and Ferrari, M. (2012). A brief review on the use of functional near-infrared spectroscopy (fNIRS) for language imaging studies in human newborns and adults. Brain Lang. 121, 79–89. doi: 10.1016/j.bandl.2011.03.009
Richter, M. M., Zierhut, K. C., Dresler, T., Plichta, M. M., Ehlis, A.-C., Reiss, K., et al. (2009). Changes in cortical blood oxygenation during arithmetical tasks measured by near-infrared spectroscopy. J. Neural Transm. 116, 267–273. doi: 10.1007/s00702-008-0168-7
Rivera, S. M., Reiss, A., Eckert, M. A., and Menon, V. (2005). Developmental changes in mental arithmetic: evidence for increased functional specialization in the left inferior parietal cortex. Cerebral cortex 15, 1779–1790. doi: 10.1093/cercor/bhi055
Scholkmann, F., Kleiser, S., Metz, A. J., Zimmermann, R., Pavia, J. M., Wolf, U., et al. (2014). A review on continuous wave functional near-infrared spectroscopy and imaging instrumentation and methodology. Neuroimage 85, 6–27. doi: 10.1016/j.neuroimage.2013.05.004
Shaywitz, B. A., Shaywitz, S. E., Pugh, K. R., Mencl, W. E., Fulbright, R. K., Skudlarski, P., et al. (2002). Disruption of posterior brain systems for reading in children with developmental dyslexia. Biol. Psychiatry 52, 101–110. doi: 10.1016/S0006-3223(02)01365-3
Soltanlou, M., Artemenko, C., Dresler, T., Haeussinger, F. B., Fallgatter, A. J., Ehlis, A.-C., et al. (2017a). Increased arithmetic complexity is associated with domain-general but not domain-specific magnitude processing in children: a simultaneous fNIRS-EEG study. Cogn. Affect. Behav. Neurosci. 17, 724–736. doi: 10.3758/s13415-017-0508-x
Soltanlou, M., Artemenko, C., Ehlis, A.-C., Huber, S., Fallgatter, A. J., Dresler, T., et al. (2018). Reduction but no shift in brain activation after arithmetic learning in children: a simultaneous fNIRS-EEG study. Sci. Rep. 8:1707. doi: 10.1038/s41598-018-20007-x
Soltanlou, M., Jung, S., Roesch, S., Ninaus, M., Brandelik, K., Heller, J., et al. (2017b). “Behavioral and neurocognitive evaluation of a web-platform for game-based learning of orthography and numeracy,” in Informational Environments, eds J. Buder and F. W. Hesse (New York, NY: Springer), 149–176.
Spelke, E. S. (2002). Developmental neuroimaging: a developmental psychologist looks ahead. Dev. Sci. 5, 392–396. doi: 10.1111/1467-7687.00378
Sugiura, L., Ojima, S., Matsuba-Kurita, H., Dan, I., Tsuzuki, D., Katura, T., et al. (2011). Sound to language: different cortical processing for first and second languages in elementary school children as revealed by a large-scale study using fNIRS. Cereb. Cortex 21, 2374–2393. doi: 10.1093/cercor/bhr023
Sugiura, L., Ojima, S., Matsuba-Kurita, H., Dan, I., Tsuzuki, D., Katura, T., et al. (2015). Effects of sex and proficiency in second language processing as revealed by a large-scale fNIRS study of school-aged children. Hum. Brain Mapp. 36, 3890–3911. doi: 10.1002/hbm.22885
Tamekuchi, Y., Hashimoto, K., Honda, M., Miyamura, K., and Abo, M. (2011). Activation of the prefrontal cortex during verbal fluency tasks as measured with 2-channel near-infrared spectroscopy in children (originals). Jikeikai Med. J. 58, 77–82.
Tando, T., Kaga, Y., Ishii, S., Aoyagi, K., Sano, F., Kanemura, H., et al. (2014). Developmental changes in frontal lobe function during a verbal fluency task: a multi-channel near-infrared spectroscopy study. Brain Dev. 36, 844–852. doi: 10.1016/j.braindev.2014.01.002
Telkemeyer, S., Rossi, S., Koch, S. P., Nierhaus, T., Steinbrink, J., Poeppel, D., et al. (2009). Sensitivity of newborn auditory cortex to the temporal structure of sounds. J. Neurosci. 29, 14726–14733. doi: 10.1523/JNEUROSCI.1246-09.2009
Telkemeyer, S., Rossi, S., Nierhaus, T., Steinbrink, J., Obrig, H., and Wartenburger, I. (2011). Acoustic processing of temporally modulated sounds in infants: evidence from a combined near-infrared spectroscopy and EEG study. Front. Psychol. 2:62. doi: 10.3389/fpsyg.2011.00062
Tellis, G., and Tellis, C. (2016). Using functional near infrared spectroscopy with fluent speakers to determine haemoglobin changes in the brain during speech and non-speech tasks. NIR News 27, 4–7. doi: 10.1255/nirn.1599
Vanderwert, R. E., and Nelson, C. A. (2014). The use of near-infrared spectroscopy in the study of typical and atypical development. Neuroimage 85, 264–271. doi: 10.1016/j.neuroimage.2013.10.009
Vannasing, P., Florea, O., González-Frankenberger, B., Tremblay, J., Paquette, N., Safi, D., et al. (2016). Distinct hemispheric specializations for native and non-native languages in one-day-old newborns identified by fNIRS. Neuropsychologia 84, 63–69. doi: 10.1016/j.neuropsychologia.2016.01.038
Walsh, B., Tian, F., Tourville, J., Yücel, M., Kuczek, T., and Bostian, A. (2017). Hemodynamics of speech production: an fNIRS investigation of children who stutter. Sci. Rep. 7:4034. doi: 10.1038/s41598-017-04357-6
Wartenburger, I., Steinbrink, J., Telkemeyer, S., Friedrich, M., Friederici, A. D., and Obrig, H. (2007). The processing of prosody: evidence of interhemispheric specialization at the age of four. Neuroimage 34, 416–425. doi: 10.1016/j.neuroimage.2006.09.009
Weber, P., Lütschg, J., and Fahnenstich, H. (2005). Cerebral hemodynamic changes in response to an executive function task in children with attention-deficit hyperactivity disorder measured by near-infrared spectroscopy. J. Dev. Behav. Pediatr. 26, 105–111. doi: 10.1097/00004703-200504000-00005
Wilcox, T., and Biondi, M. (2015). fNIRS in the developmental sciences. Wiley Interdiscip. Rev. Cogn. Sci. 6, 263–283. doi: 10.1002/wcs.1343
Keywords: cognitive development, numerical development, mathematical development, language development, reading acquisition, fNIRS, educational neuroscience
Citation: Soltanlou M, Sitnikova MA, Nuerk H-C and Dresler T (2018) Applications of Functional Near-Infrared Spectroscopy (fNIRS) in Studying Cognitive Development: The Case of Mathematics and Language. Front. Psychol. 9:277. doi: 10.3389/fpsyg.2018.00277
Received: 15 September 2017; Accepted: 19 February 2018;
Published: 03 April 2018.
Edited by:
Elena Nava, Università degli Studi di Milano Bicocca, ItalyReviewed by:
Ruth Ford, Anglia Ruskin University, United KingdomJennifer Wagner, College of Staten Island, United States
Copyright © 2018 Soltanlou, Sitnikova, Nuerk and Dresler. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hans-Christoph Nuerk, hc.nuerk@uni-tuebingen.de
†These authors have contributed equally to this work and should be regarded as joint first authors.
 Mojtaba Soltanlou
Mojtaba Soltanlou Maria A. Sitnikova
Maria A. Sitnikova Hans-Christoph Nuerk
Hans-Christoph Nuerk Thomas Dresler
Thomas Dresler