- 1College of Pharmacy, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, China
- 2Centre of Biomedical Research & Diversity of Development, Fujian University Traditional Chinese Medicine, Fuzhou, Fujian, China
- 3Innovation and Transformation Center of Science and Technology, Fujian University of Traditional Chinese Medicine, Fuzhou, Fujian, China
- 4Department of Pharmacology, School of Pharmaceutical Sciences, University Sains Malaysia, Minden, Penang, Malaysia
- 5Research and Development Department, Fujian Xianzhilou Biological Science & Technology Co., Ltd., Fuzhou, China
Rationale: Ganoderma lucidum (G. lucidum) is a popular medicinal fungus that has been used in traditional medicine for decades, with its provenance influencing its medicinal and commercial worth. The amount of active ingredients and the price of G. lucidum from different origins vary significantly; hence, fraudulent labeling is common. Reliable techniques for G. lucidum geographic verification are urgently required to safeguard the interests of consumers, producers, and honest dealers. A stable isotope is widely acknowledged as a useful traceability technique and could be developed to confirm the geographical origin of G. lucidum.
Methods: G. lucidum samples from various sources and in varying stages were identified by using δ13C, δD, δ18O, δ15N, C, and N contents combined with chemometric tools. Chemometric approaches, including PCA, OPLS-DA, PLS, and FLDA models, were applied to the obtained data. The established models were used to trace the origin of G. lucidum from various sources or track various stages of G. lucidum.
Results: In the stage model, the δ13C, δD, δ18O, δ15N, C, and N contents were considered meaningful variables to identify various stages of G. lucidum (bud development, growth, and maturing) using PCA and OPLS-DA and the findings were validated by the PLS model rather than by only four variables (δ13C, δD, δ18O, and δ15N). In the origin model, only four variables, namely δ13C, δD, δ18O, and δ15N, were used. PCA divided G. lucidum samples into four clusters: A (Zhejiang), B (Anhui), C (Jilin), and D (Fujian). The OPLS-DA model could be used to classify the origin of G. lucidum. The model was validated by other test samples (Pseudostellaria heterophylla), and the external test (G. lucidum) by PLS and FLDA models demonstrated external verification accuracy of up to 100%.
Conclusion: C, H, O, and N stable isotopes and C and N contents combined with chemometric techniques demonstrated considerable potential in the geographic authentication of G. lucidum, providing a promising method to identify stages of G. lucidum.
1 Introduction
G. lucidum, a traditional medicinal mushroom that has been used for several millennia to treat various common ailments, is one of the best-known medicinal macrofungi in the world. The pharmacological activities of G. lucidum are widely recognized and have been used for centuries to promote health and longevity. Moreover, G. lucidum is listed in the Herbal Medicines of the USP, the Chinese Pharmacopoeia, and the Japanese Pharmacopoeia. China is the main source of the G. lucidum industry worldwide; for example, Fujian G. lucidum has been certified as an organic food in the United States, Europe, Japan, and China. G. lucidum has recently been used to produce various food and health products due to its potentially pharmacologically active components and healthful features, thereby attaining immense economic value. The perceived nutritional and healthy values of G. lucidum have been accepted globally, and its annual economic output is more than US$2.5 billion (Ren et al., 2020).
Certain production regions suitable for G. lucidum growth, called “daodi” in China, have specific strains and cultivation practices. Triterpenoids and polysaccharides are the prime bioactive components responsible for the therapeutic activities of G. lucidum, which have been reported to be anti-inflammatory and anti-cancer, to decrease obesity, and ameliorate metabolic dysfunctions (Chang et al., 2015; Ahmad, 2020; Sang et al., 2021; Wang et al., 2019). The composition of its chemical components, including triterpenoids, polysaccharides, proteins, alkaloids, and vitamins, which are key sources of its pharmacological active constituents, is affected by its growing conditions. Studies have revealed discrepancies in the protein and trace element content of G. lucidum grown in China and Iberia and also differences in the ganoderic acid and triterpenoid content of G. lucidum from various sources (Ha et al., 2015; Zhang et al., 2019; Fraile et al., 2021), resulting in an unstable medicinal value and curative effect of extended products. Therefore, the geographical origin is considered to be a key factor affecting the safety and quality of G. lucidum. This factor raises concerns regarding the authenticity and traceability of mushroom species and the impact of commercial medicinal plant extracts on the G. lucidum extraction and fractionation sector. The capacity to trace the geographical origin of G. lucidum will increase its value in traditional Chinese medicine (TCM) and cuisine.
Different stages of G. lucidum show different pharmacological activities. Basswood cultivation (one way of log planting) is a certified organic food of G. lucidum cultivation in the United States, European Union, Republic of Korea, Japan, and China (Surya et al., 2018). Temperature, humidity, CO2 content, and other environmental factors affect the growth of G. lucidum during the planting process (Bijalwan et al., 2021), which may lead to changes in stable isotopes or bioactive compounds in different stages of G. lucidum. The changes in bioactive compounds may also result in variations in stable isotopes at different stages. Stripes during the spore maturity stage had the highest total phenols and flavonoids, resulting in a high level of antioxidant activity, but bud-developing stage stripes had substantially high levels of ganoderic acid B, C2, and G based on previous research (Ren et al., 2020). Several G. lucidum-based products are available in the form of nutriceuticals. Some of these products are marketed as dietary supplements and are widely consumed in some countries. Changes in the nutritional content of G. lucidum during its growth will undoubtedly result in alterations to its efficacy (Chen et al., 2012). Therefore, developing a technology for distinguishing different stages of G. lucidum is necessary to provide a scientific basis for the advancement and utilization of G. lucidum.
The authors are aware that numerous chemical strategies based on chromatographic and spectral analysis methodologies have been developed for determining the geographical origin and applied to a range of TCM, such as techniques based on FT-NIR, UHPLC-QqQ-MS/MS, GC-MS, and LC-MS (Chen et al., 2008; Fu et al., 2017; Zhao et al., 2021; Singh et al., 2022). However, they cannot effectively distinguish the geographic origin of TCM due to insufficient compound information. A chemometrics strategy that combines stable isotope ratios has emerged as a promising approach for distinguishing regional origins, particularly in rice, honey, milk, and wolfberry (Magdas et al., 2008; Wang et al., 2020; Zhao et al., 2020; Gong et al., 2022). A stoichiometric strategy incorporating stable isotope ratios has also emerged as a highly promising approach for identifying the geographic origin of various products. This strategy has been successfully applied to high-value food or medical products, such as honey’s authenticity and global geographic origin (Zhou et al., 2018). The application of stable isotopes to the tracing of G. lucidum, a valuable traditional Chinese medicinal ingredient, has not been documented in any study.
The current study aims to further investigate the potential of stable isotopes for the geographic authentication of G. lucidum. The isotope ratios of δ13C, δD, δ18O, δ15N, C%, and N% in G. lucidum samples produced in four provinces of China were measured by isotope ratio mass spectrometry. Chemometric methods, including principal component analysis (PCA), orthogonal partial least squares-discriminant analysis (OPLS-DA), PLS, and Fisher linear discriminant analysis (FLDA), were applied to evaluate the discriminant power of these data.
2 Materials and methods
2.1 G. Lucidum samples
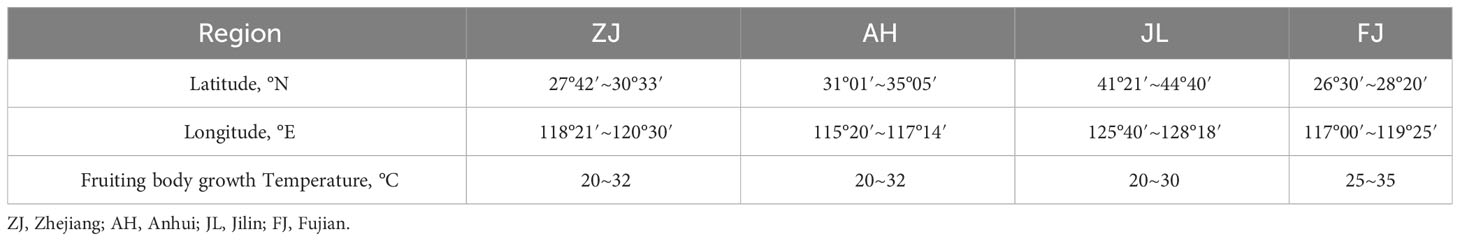
The G. lucidum from Fujian was provided by Fujian Xian-Zhi-Lou Technology Co., Ltd. The strain of G. lucidum was JB-A119-20191010. It was incubated for 7 days at 28°C on potato dextrose agar (PDA) (medium: potato, 200 g; dextrose, 20 g; agar, 20 g; and deionized water, 1 L). The inoculated substrate was incubated at 28°C for mycelial colonization. The air temperature in the cultivation room was maintained at 28°C, and the relative humidity was set at 70%–80%. The grown mycelium was homogenized after 15–20 days of cultivation. The culture medium was autoclaved at 121°C for 2 h. The fruiting body samples were cultivated by the solid method using wood (the wood of Castanopsis sclerophylla used for cultivating G. lucidum was taken from local forests). Before planting, the cultivation site had been aired, cleaned, and slightly lime-disinfected and had proper ventilation (CO2 0.1%–0.3%). The experiment was conducted at the three growth stages of bud development, growth, and maturing. Bud development stage: After inoculation, the mycelia of G. lucidum began to germinate. Primordium culture conditions were maintained at 28°C–30°C, 90%–95% relative humidity, 0.03%–0.1% CO2, and a light intensity of 100 lux. The initial sampling was conducted approximately 35–40 days after inoculation, which is when the bud-developing stage and differentiation had begun. Growth stage: The second sampling was conducted at the growth stage, approximately 55–60 days after inoculation, which is when the primordial grows long and stripes form. The growth stage began after elongation. The color of the caps deepens as they grow, demonstrating a deep center and white edges. The culture conditions of fruiting bodies were maintained at 25°C–28°C, relative humidity at 80%–95%, CO2 of 0.03%–0.1%, and a light intensity of 150–200 lux. Maturing stage: The last sampling was performed at the maturing stage approximately 70–80 days after the inoculation. The culture conditions of fruiting bodies were maintained at 25°C–28°C, relative humidity at 80%–95%, CO2 at 0.03%–0.05%, and a light intensity of 150–200 lux. G. lucidum at different stages, when the white edge of the cap disappears and hardens, is shown in Figure 1.
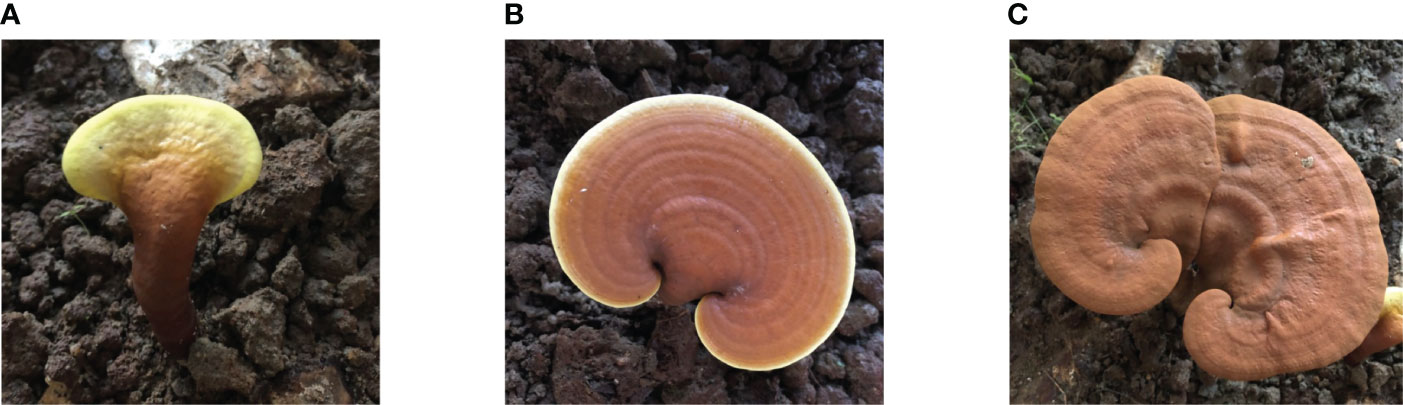
Figure 1 G. lucidum at different stages. (A) Bud-developing stage, (B) Growth stage, and (C) Maturing stage.
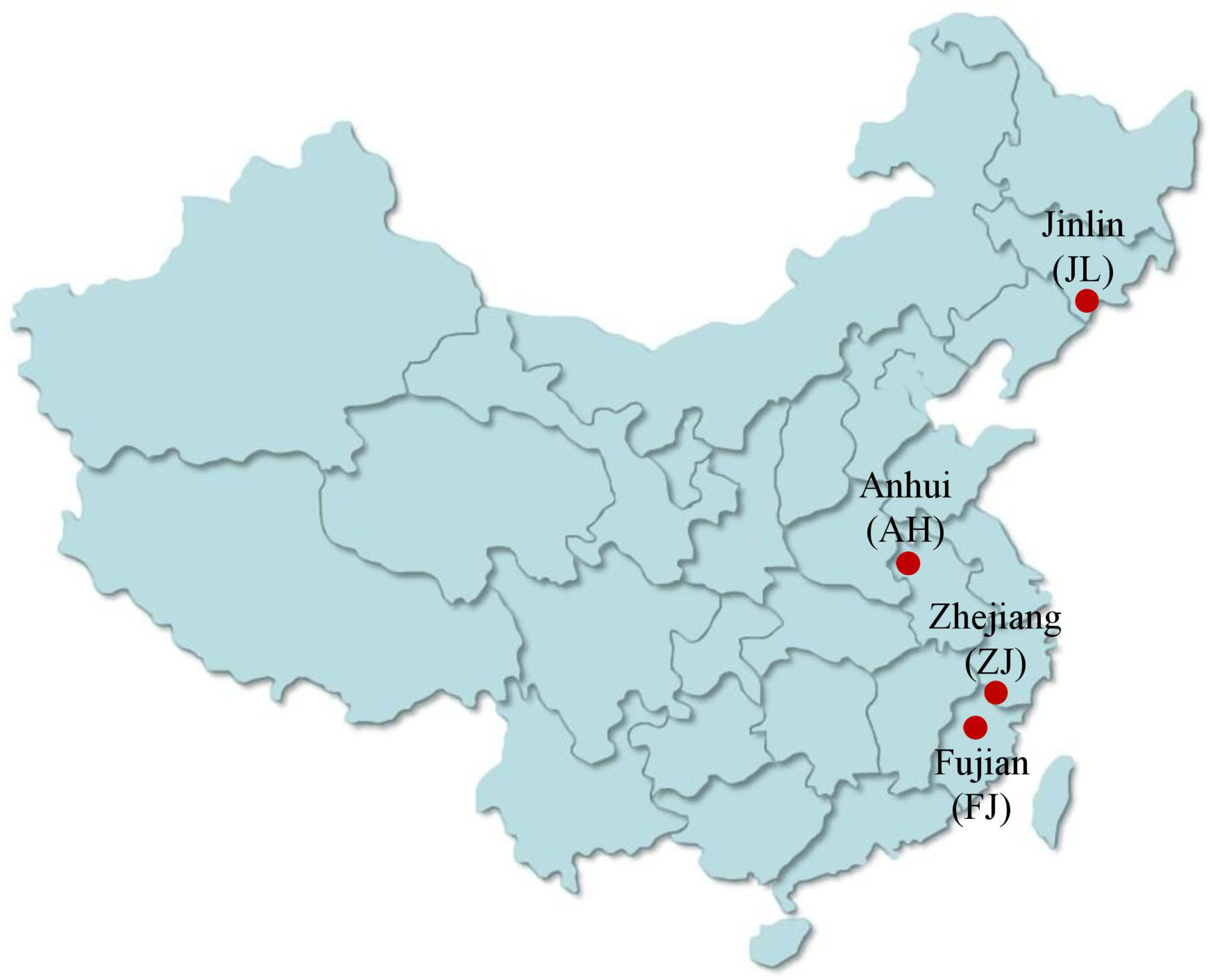
G. Lucidum collected from Zhejiang, Anhui, and Jilin was grown on wood logs and purchased from the local “Daodi” market (maturity stage). The origin of G. lucidum includes latitude, longitude, and specific parameters of the planting process as shown in Table 1. The pictures of G. lucidum from different origins and the specific sampling locations are presented in Figures 2 and 3, respectively. The Pseudostellaria heterophylla samples used for heterogeneous validation were collected from Anhui (n = 3) and Fujian (n = 3). All the samples were dried at 45°C in a blast drying oven (9240A, Shanghai Jing Hong Laboratory Instrument Co., Ltd.). The dried samples were then ground into a fine powder with a ball mill, sieved through a 100-mesh sieve, and then subjected to stable isotope ratio analysis.

Figure 2 G. lucidum from different areas. (A) Zhejiang (ZJ), (B) Anhui (AH), (C) Jilin (JL), and (D) Fujian (FJ).
2.2 Stable isotope ratios analysis
The stable isotope ratios (C and N) and the carbon and nitrogen contents were measured with stable isotope mass spectrometry (Element analyzer-isotope ratio mass spectrometers (EA-IRMS), Thermo). The EA-IRMS conditions were set as follows: the reactor temperature was 960°C, and gases produced by the oxidation reactor were changed into He in the reducing furnace. He was then used as a carrier gas at a flow rate of 90 mL·min−1. The gases were subsequently separated and measured by EA-IRMS. Reference materials, including IAEA-600 and USGS40, were used for calibration. The sample powders were accurately weighed at 1 mg and then packed in a tin cup for the analysis of EA-IRMS.
The stable isotope ratios (O and H) were measured with elemental analysis isotope ratio mass spectrometry (high-temperature pyrolysis element analyzer and gas isotope ratio mass spectrometer (HT-IRMS), Thermo). The HT-IRMS conditions were set as follows: the reactor temperature was 1,380°C, He was used as carrier gas at a flow rate of 100 mL·min−1, and the column temperature was 600°C. Reference materials, including EMA-P1, EMA-P2, and IAEA-601, were used for calibration. The sample powders were accurately weighed at 3 mg and then packed in a tin cup for the analysis of HT-IRMS.
Stability checks of the used reference gases were continuously performed by determining International Atomic Energy Agency standards with defined 13C/12C, 15N/14N, 18O/16O, and D/1H ratios. Isotope ratios were expressed in per mil (‰) deviation relative to the V-PDB, V-SMOW, and N2 international standards. Values were calculated using the following formula:
where Rsample is the ratio of the heaviest to the lightest stable isotope in the sample, and Rstandard is the ratio of the heaviest to the lightest stable isotope in the international reference standards, namely, V-PDB, air, and V-SMOW for δ13C, δ15N, and δ18O, and δD values, respectively.
2.3 Statistical analysis
The results were presented as mean values ± SD (standard deviation). One-way analysis of variance was utilized for the statistical analysis (ANOVA). The FLDA was examined with SPSS 26.0 (SPSS Inc., Chicago, IL, USA). Chemometric analyses, including principal component analysis (PCA) and orthogonal partial least squares-discriminant analysis (OPLS-DA), were applied using the SIMCA-P Version 14.1 (Umetrics AB, Umea, Sweden).
3 Results
3.1 C, H, O, and N stable isotopes from different origins
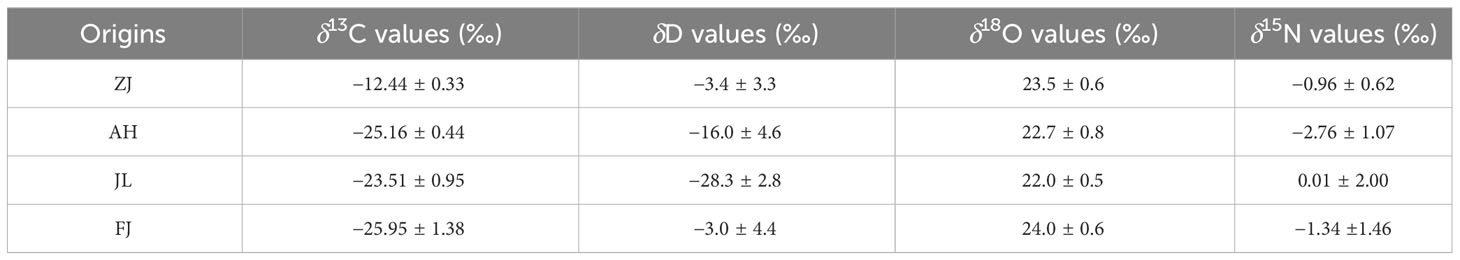
Six variables, including C, H, O, and N stable isotopes and the C and N contents in G. lucidum samples from different provinces (ZJ, AH, JL, and FJ), are compiled in Table 2. G. lucidum isotope ratios showed δ13C values ranging from -29.61 to -12.01, δD values ranging from -31.9 to 4.1, δ18O values ranging from 21.3 to 24.7, and δ15N values ranging from -4.36 to 3.59. The distribution of four stable isotopes is shown in Figure 4 to demonstrate the differences among origins.
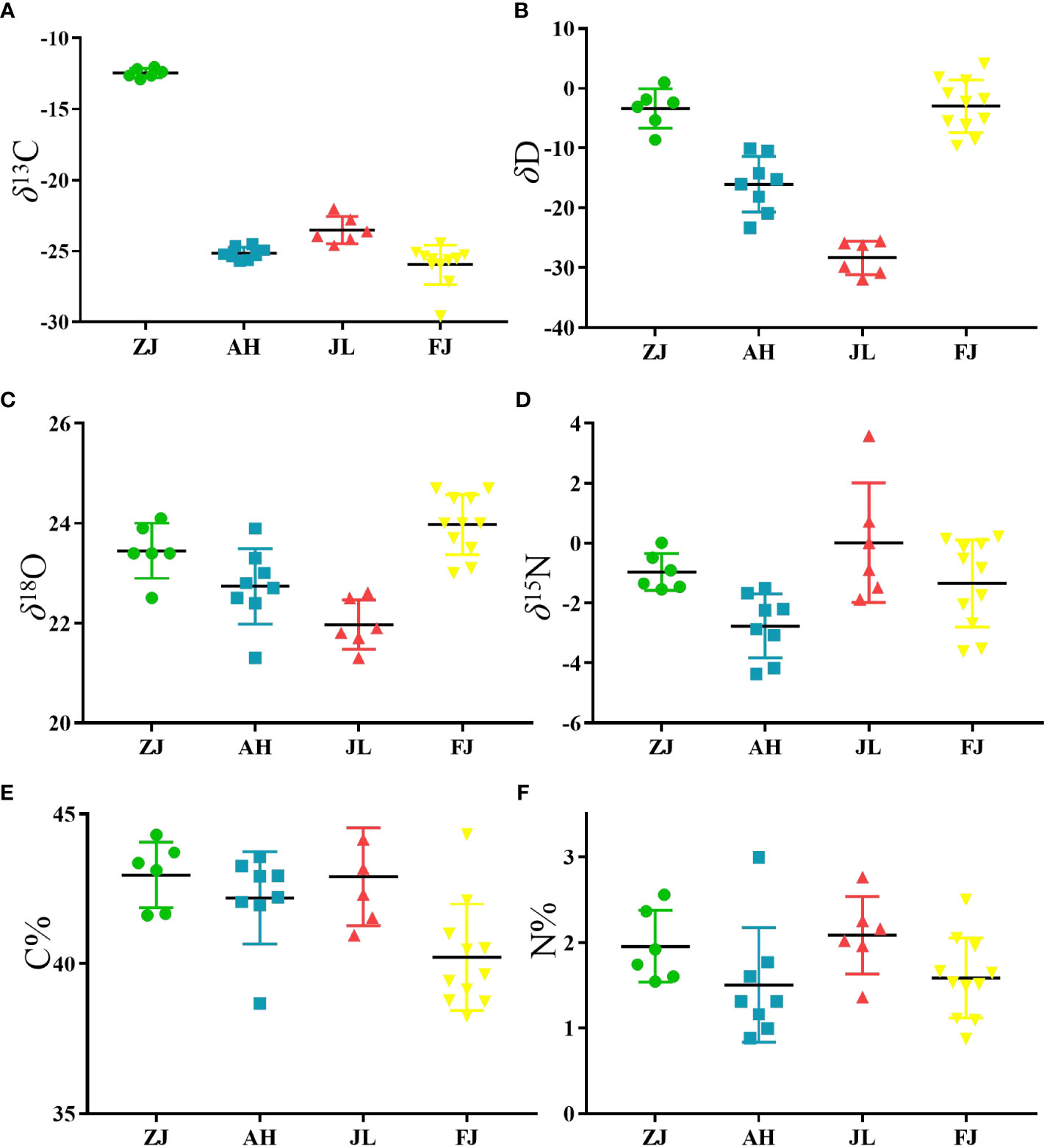
Figure 4 Scatter plot of the variation in (A) δ13C, (B) δD, (C) δ18O, (D) δ15N, (E) C contents and (F) N contents of G.lucidum samples from different origins in China. (ZJ, n=6, AH, n=8, JL, n=6, FJ, n=11).
3.2 Evaluation of chemometric models for G. lucidum authentication from different origins
Using the data of isotope ratios (δ13C, δD, δ18O, and δ15N), an unsupervised multivariate data analysis method, that is, PCA, was performed to minimize dimension and visualize the separation effects of geographical origins. The 2D score plot of the PCA divided the samples into four independent clusters (Figure 5A). The results of the classification were consistent with those of the original data. The results are based on the 95% confidence interval (Figure 5B).
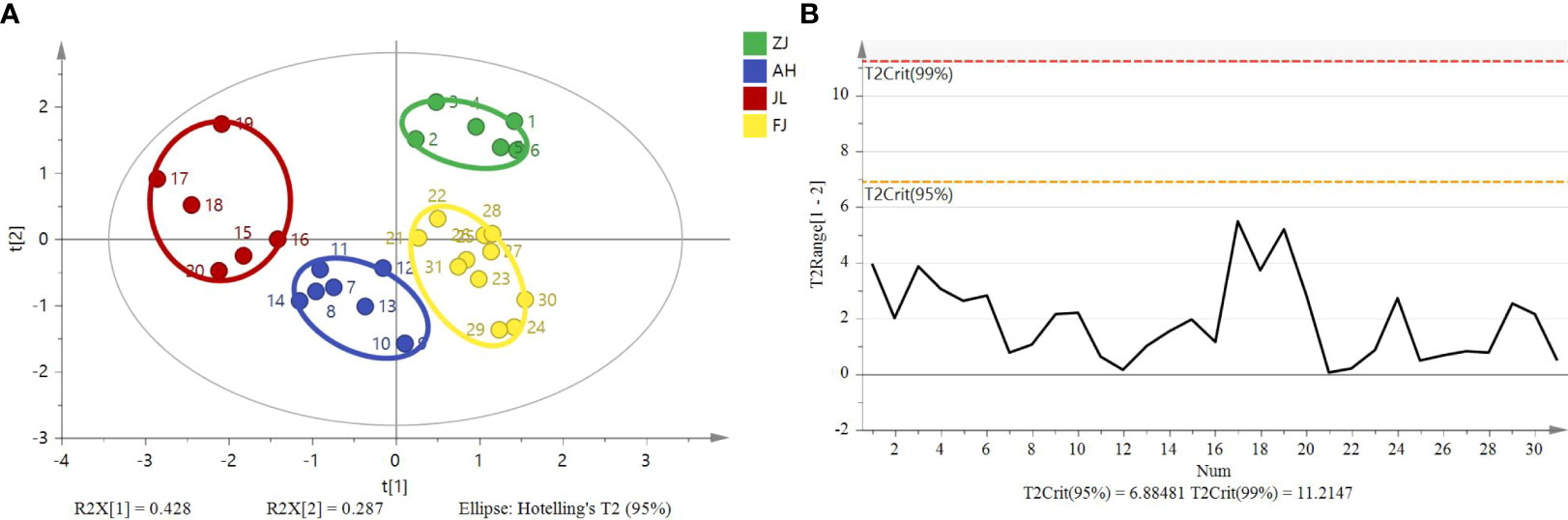
Figure 5 Principal component analysis score plot and Hotelling’s T2 ellipse from the isotope ratios (δ13C, δD, δ18O, and δ15N) of G. lucidum samples from ZJ, AH, JL, and FJ. (A) Principal component analysis score plot. (B) Hotelling’s T2 ellipse was constructed from the PCA score plot. The results are based on the 95% confidence interval.
OPLS-DA is a modification of the PLS-DA method, where the variation in the data (X matrix) is separated into two parts: one that is linearly related to the response variable (Y matrix) and one that is orthogonal or unrelated. In SIMCA version 14.1 by Umetrics, the OPLS-DA method is implemented to allow multigroup data analysis by performing several binary (one-against-all) comparisons. In the context of a four-group study, four separate OPLS-DA models would be constructed, each focusing on the discrimination of one group against the remaining three. Therefore, these models can then be collectively interpreted to understand the differences among the four groups. OPLS-DA models in SIMCA 14.1 also allow users to visualize and interpret the predictive and orthogonal components separately, which aids in effectively understanding the underlying structure of the dataset. Therefore, OPLS-DA is a supervised discriminant analysis model that can assist in understanding inter-class separation and identifying potential categorical variables, increasing its suitability for multivariate discriminant analysis. The OPLS-DA model was developed utilizing stable isotope ratios as variables to visualize and investigate their intrinsic correlation and explore their contribution to the origin discrimination of G. lucidum. The OPLS-DA 2D score plot showed that samples were divided into four categories (Figure 6A). The results are based on the 95% confidence interval (Figure 6B). The model can explain 91.6% of the variation (R2X = 0.916, R2Y = 0.726), demonstrating a high predictive capability (Q2 = 0.67) and showing a good fit and predictive capability of the training set. Theoretically, in OPLS-DA, VIP values larger than 1 indicate that variables are essential. The VIP (Figure 6C) suggested that the δ13C value was the most important contributor to the discrimination (VIP > 1). Cross-validation with 200 permutation tests showed that the OPLS-DA model has good robustness (R2 = 0.0295, Q2 = −0.276, Figure 6D).
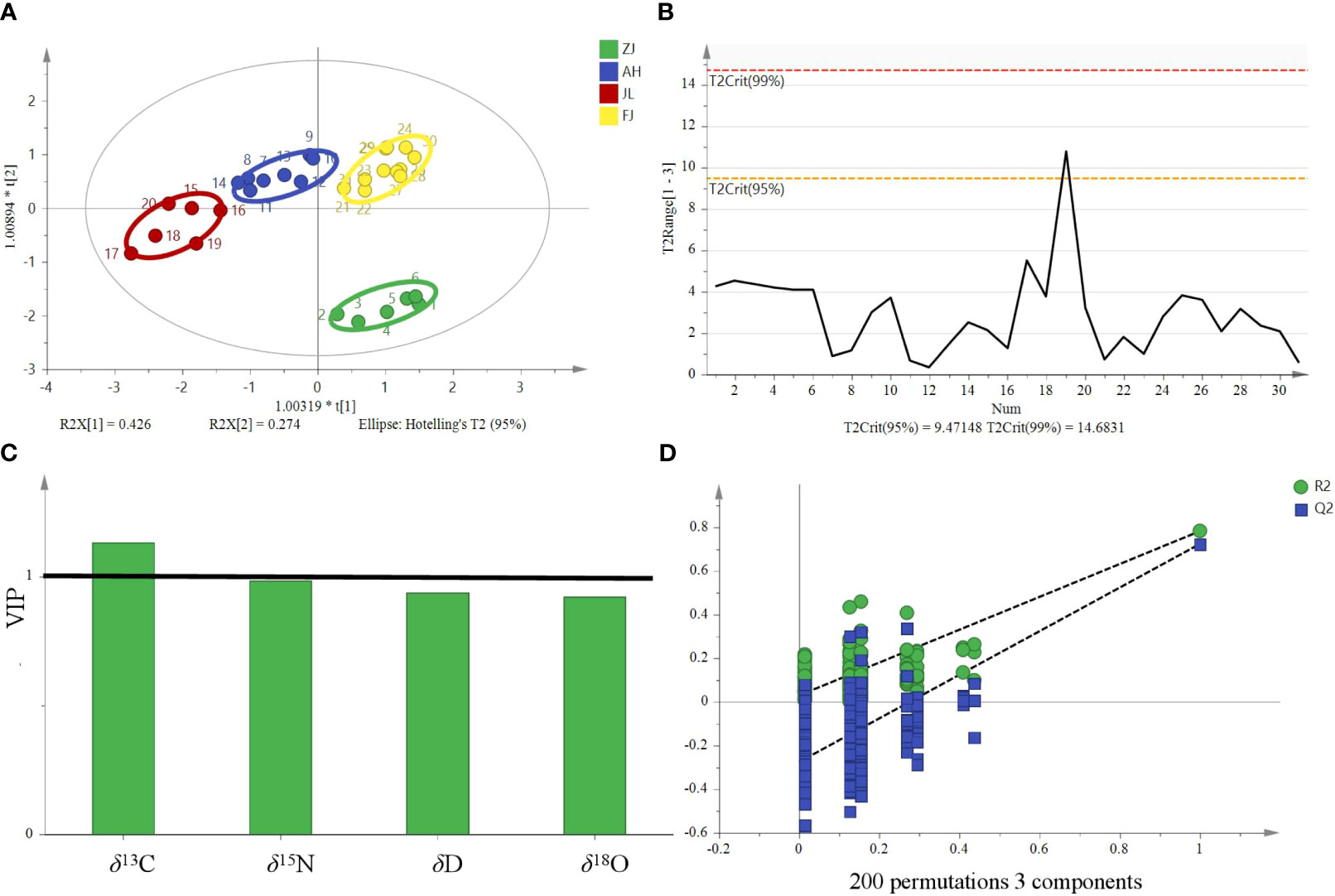
Figure 6 OPLS-DA model based on four stable isotopes (δ13C, δD, δ18O, and δ15N) for tracing the origins of G. lucidum. (A) OPLS-DA score, (B) Hotelling’s T2 ellipse was constructed from the OPLS-DA score plot. The results are based on the 95% confidence interval. (C) VIP plots and (D) The permutation test plots of the model for the samples.
G. lucidum samples (ZJ = 2, AH = 2, JL = 2, and FJ = 3) were used for external source validation. Figure 7 shows the predicted and actual values of the PLS validation according to the isotope ratios (RMSEE = 0.62, RMSECV = 0.66). The results revealed that the model could identify nine external samples in their classified group with 100% accuracy.
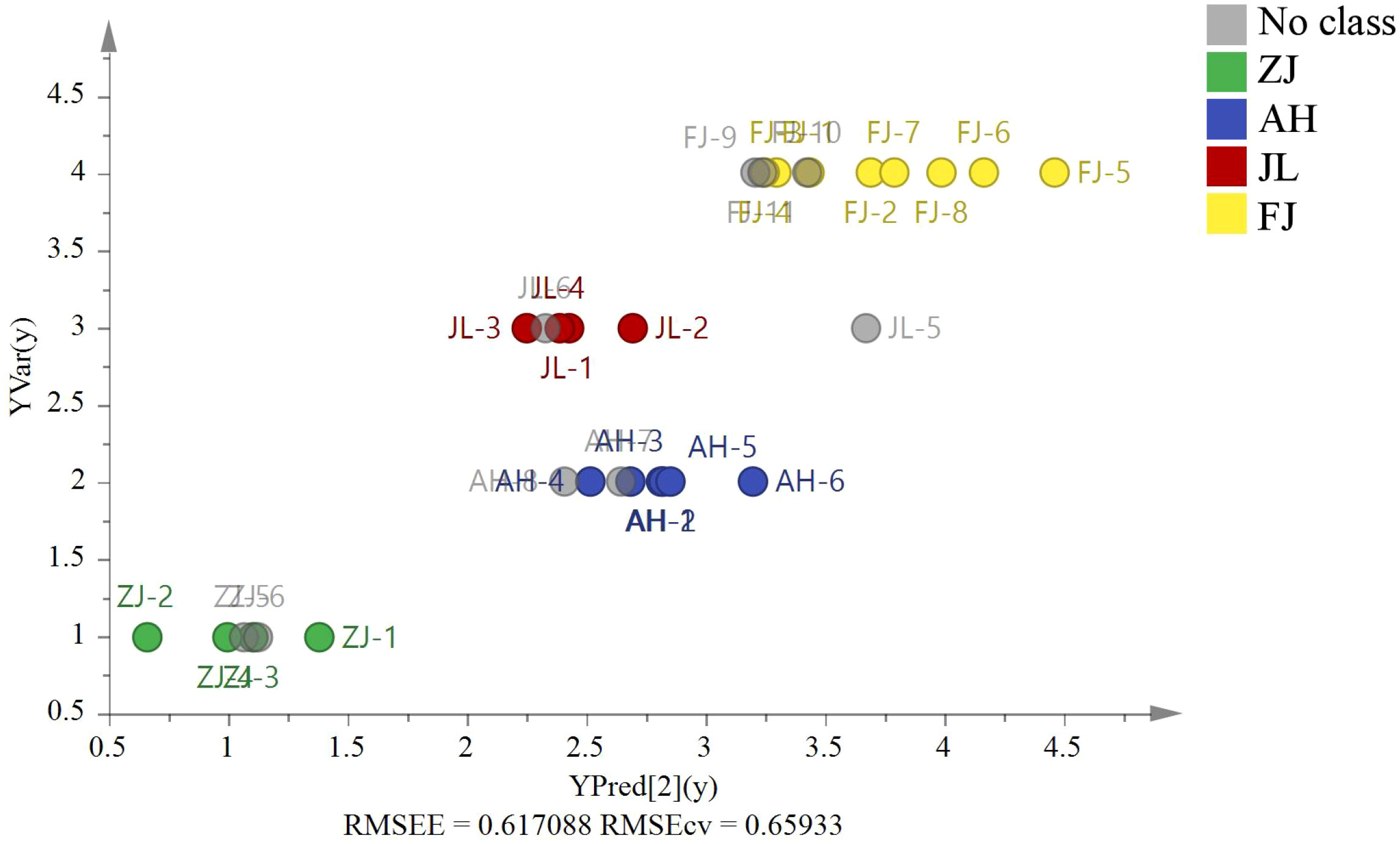
Figure 7 The PLS model based on four stable isotopes (δ13C, δD, δ18O, and δ15N) of origin validation by nine external G. lucidum samples (ZJ=2, AH=2, JL=2, and FJ=3).
Six Pseudostellaria heterophylla heterogeneous samples were incorporated at random, including three samples from Fujian and others from Anhui, to further confirm the reliability of the model. δ13C, δD, δ18O, and δ15N were acquired by using the same isotope ratio mass spectrometry for verification of other heterogeneous samples (Figure 8, RMSEE = 0.61, RMSECV = 0.60). Figure 8 shows the classification of G. lucidum and Pseudostellaria heterophylla samples with a cutoff value of 4.5. The result indicated that G. lucidum has its own unique stable isotopes. Moreover, the results revealed that the model could identify other heterogeneous samples (Pseudostellariae Radix) in other groups with 100% accuracy. By contrast, these samples (G. lucidum and Pseudostellaria heterophylla) were again analyzed by PCA (Figure S1). The analysis results showed that G. lucidum was clearly isolated from samples of Pseudostellaria heterophylla. Therefore, G. lucidum is efficiently and precisely identified using the established model.
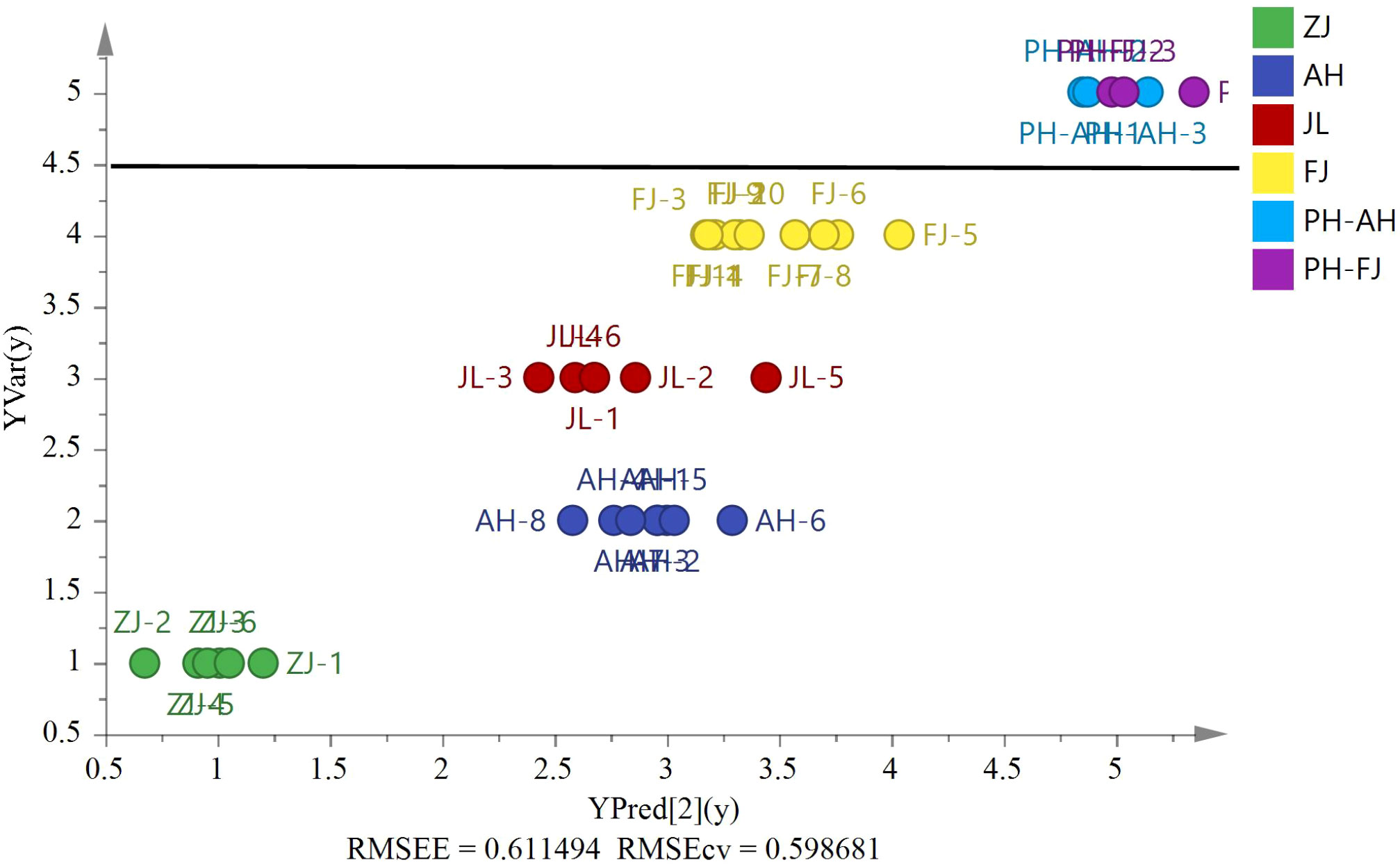
Figure 8 The PLS model based on four stable isotopes (δ13C, δD, δ18O, and δ15N) of origin validation by the heterogeneous six Pseudostellariae Radix samples (AH, n=3 and FJ, n=3).
FLDA was employed to establish a predictive model to facilitate the clustering of group members based on observed characteristics. The discriminant function based on linear combinations of predictor variables was utilized to discriminate further and classify the unknown members. The FLDA model of the geographical origin of G. lucidum was established, and the classification results are shown in Table 3. The FLDA function is as follows: Y(ZJ) = −18.131 δ13C + 3.131 δ15N + 70.205 δ18O − 0.316 δD − 932.103, Y(AH) = −47.559 δ13C − 3.887 δ15N + 62.028 δ18O − 6.324 δD − 1371.246, Y(JL) = −46.931 δ13C − 2.976 δ15N + 57.780 δ18O − 7.040 δD − 1275.817, and Y(FJ) = −43.875 δ13C − 1.462 δ15N + 67.994 δ18O − 4.505 δD − 1394.239. The FLDA linear discriminant with stable isotopes as discriminant variables has been evaluated with back-replacement accuracy of 100% and cross-validation reaching 95.7%.
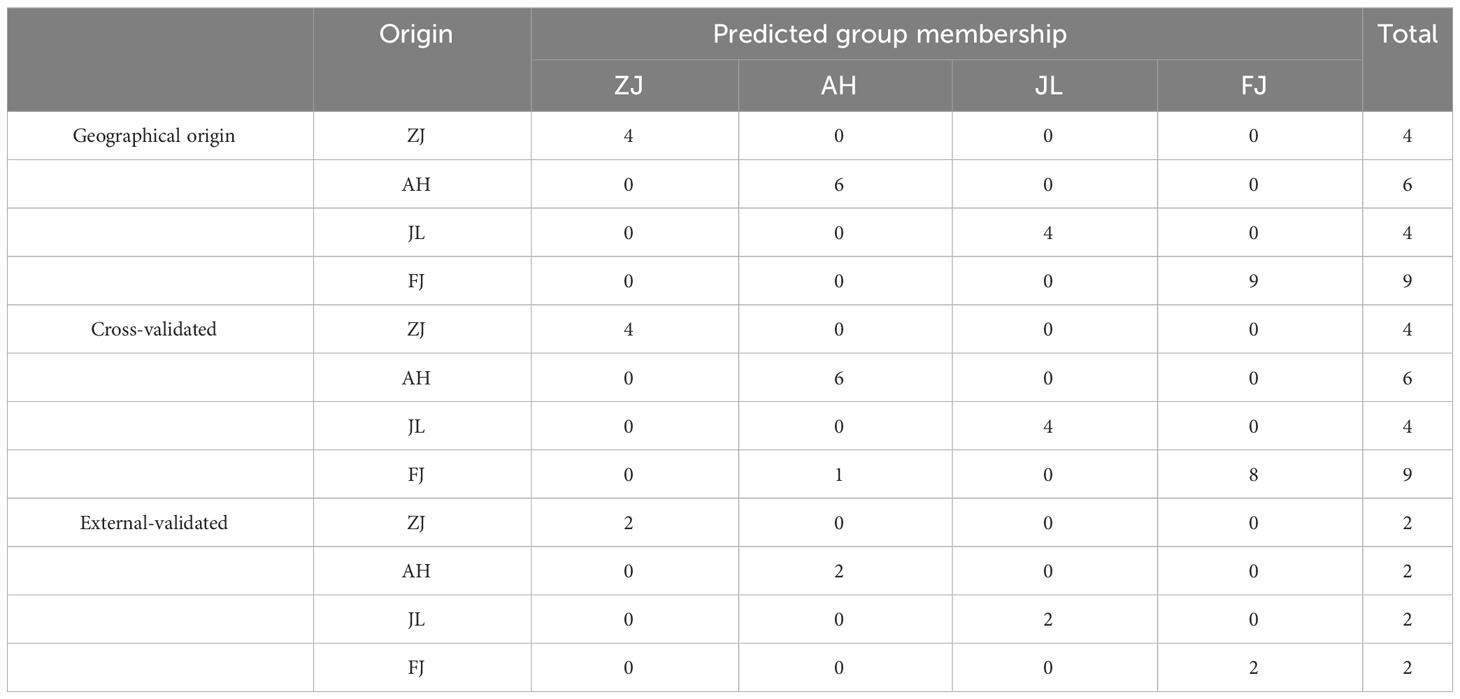
Table 3 The discrimination model based on four stable isotopes (δ13C, δD, δ18O, and δ15N) of the geographical origin of G. lucidum.
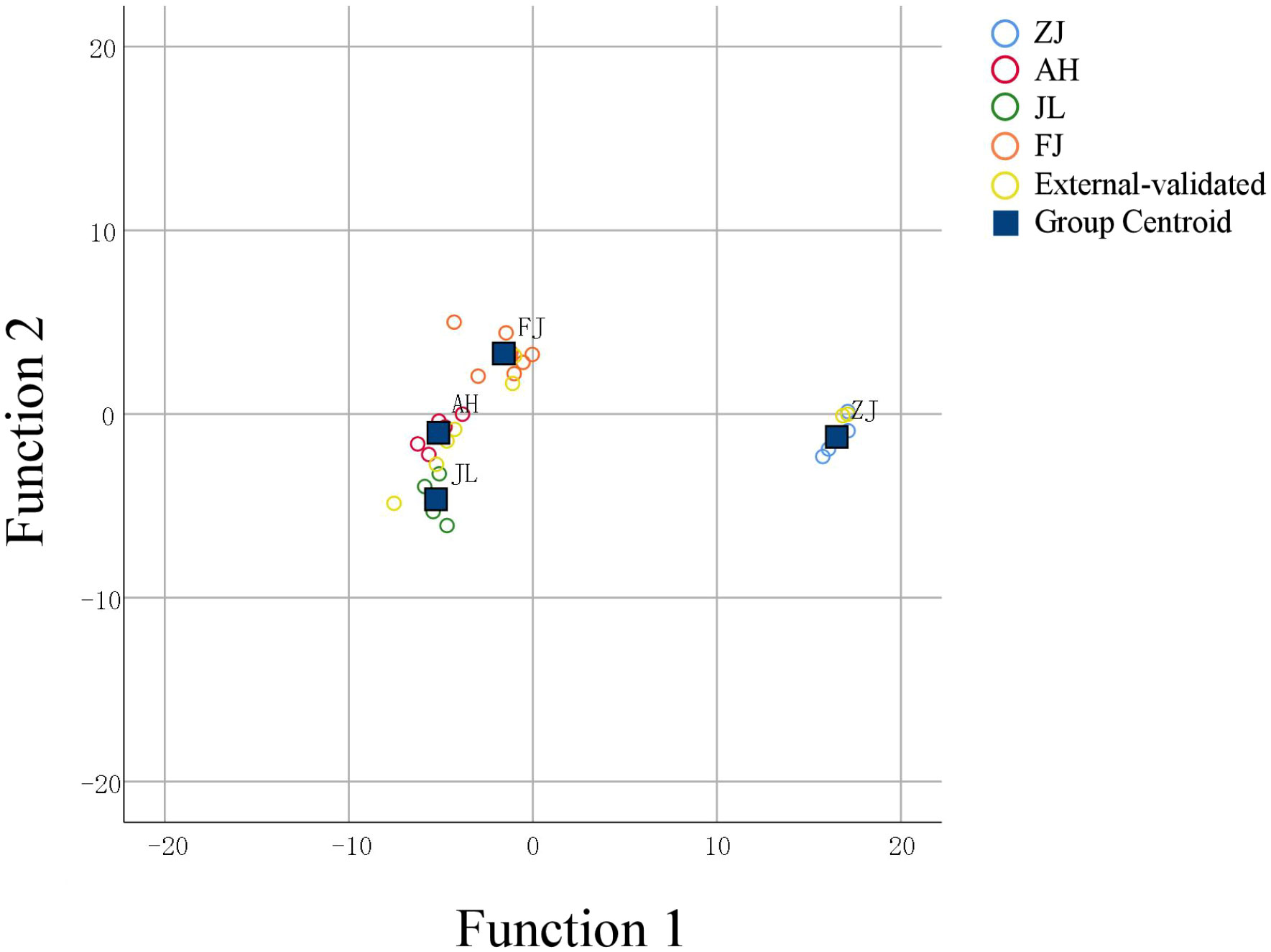
Scatter plots were used to evaluate the distribution of the geographical origins to distinguish the discrepancies explicitly. As shown in Figure 9, geographical origins could be successfully divided into four groups. The discriminant function is based on linear combinations of predictor variables to discriminate further and classify the unknown members. The model for geographical origin discrimination was used to verify the origins of nine samples outside the model. The nine G. lucidum samples were accurately recognized, demonstrating the precision and consistency of the model.
3.3 C, H, O, and N stable isotopes from different stages
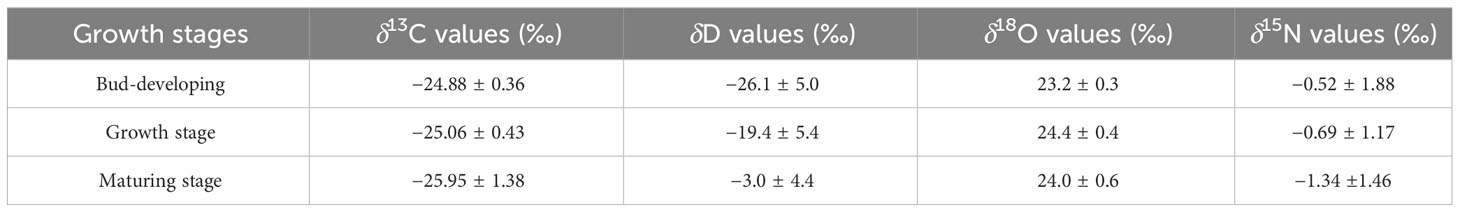
Thus far, studies that have exhaustively examined the relationship between stable isotopes and geographical origins at various phases are currently unavailable. Four stable isotopes and C and N contents are shown in Table 4 and Figure 10, respectively. G. lucidum stable isotope showed δ13C values ranging from −29.61 to −24.30, δD values ranging from −31.8 to 4.1, δ18O values ranging from 22.8 to 25.0, and δ15N values ranging from −4.22 to 1.53. The average δD values of samples at the maturing stage were highest (−3.0‰), followed by the growth and bud development stages.
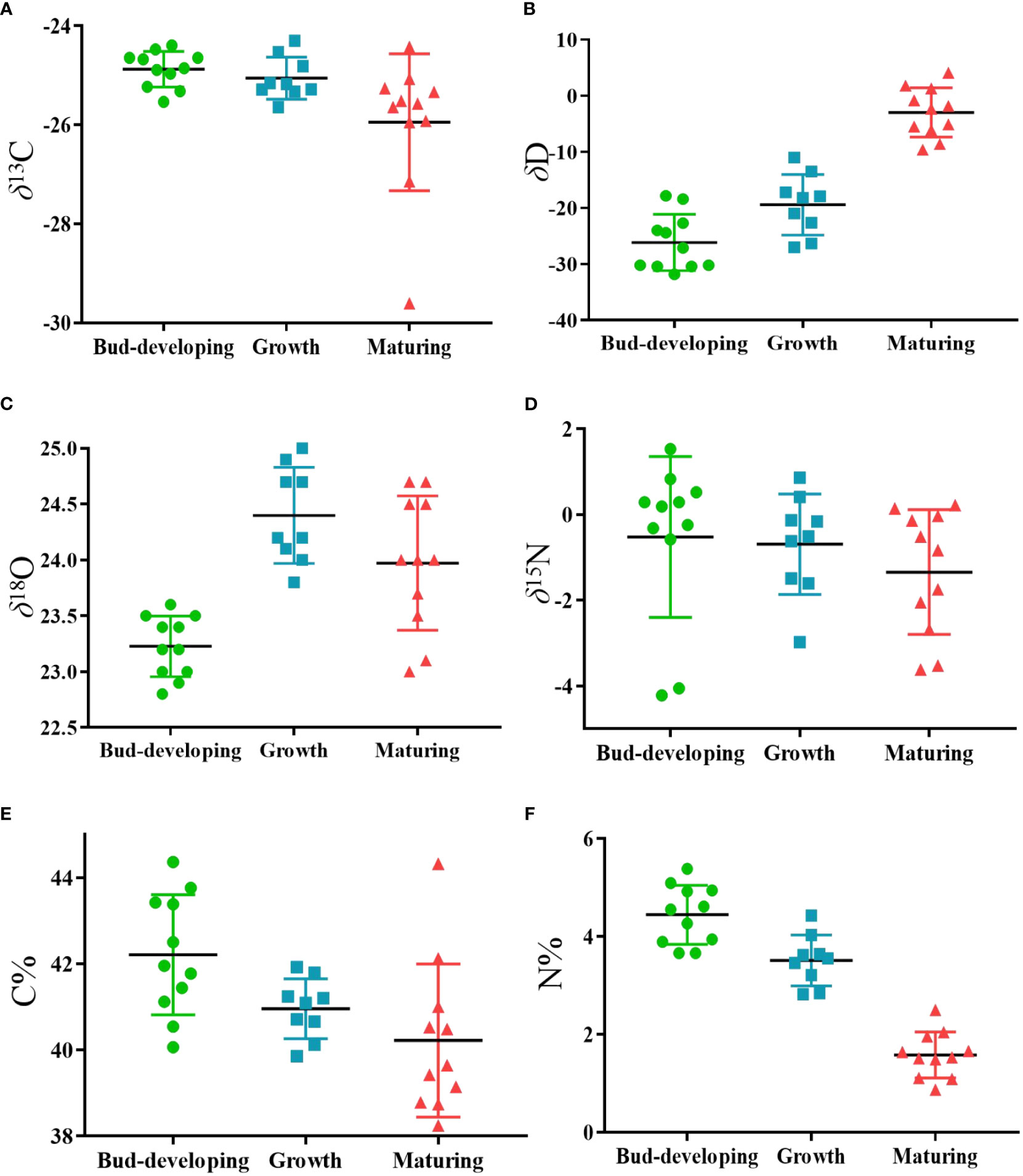
Figure 10 Scatter plot of the variation in (A) δ13C, (B) δD, (C) δ18O, (D) δ15N, (E) C contents and (F) N contents of G.lucidum samples at different stages. (Bud-developing stage, n=11, Growth stage, n=9, Growth stage, n=11).
3.4 Correlation analysis of δ13C and δ15N values with C and N contents from different stages
The correlation between carbon and nitrogen can reflect the nutrient acquisition of G. lucidum during the growth process, which plays an important role in its growth (Hsieh and Yang, 2004). Correlations of δ13C and δ15N values with C and N contents in G. lucidum are shown in Table 5. A significantly negative relationship is observed between the C/N ratio and the N content (r = −0.894, p < 0.001). The relationship between the carbon-to-nitrogen ratio and nitrogen content is one of mutual inhibition.
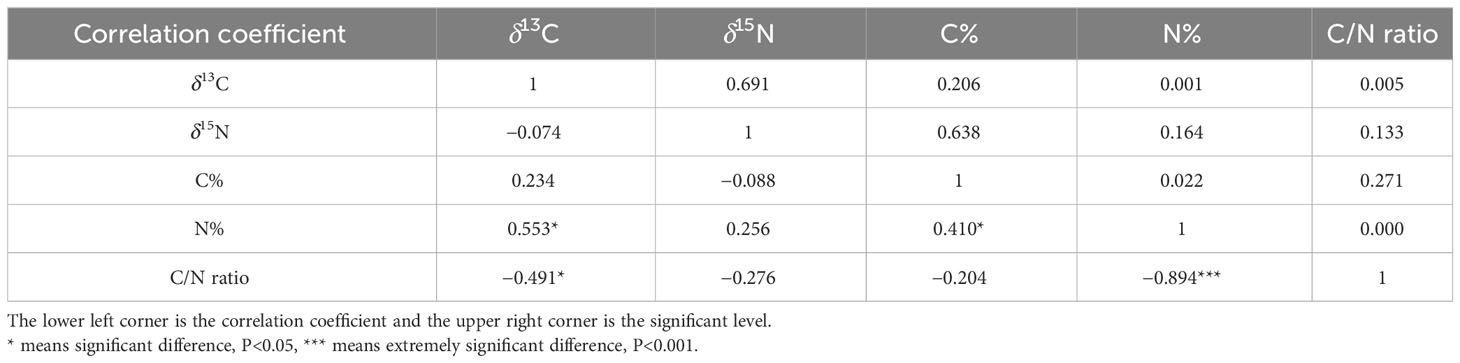
Table 5 Correlation analysis table of δ13C, δ15N, and C, N contents stoichiometry in G. lucidum at different stages.
3.5 Evaluation of chemometric models for G. lucidum authentication from different stages
The model based on stable isotope ratios fails to distinguish samples at different stages and overlaps in some areas (Figure 11A). Compared with the model of stable isotopes (four variables, including δ13C, δ15N, δD, and δ18O), the PCA model, after increasing C% and N%, showed that G. lucidum samples at each stage were highly centralized (Figure 11C). Different stages were clustered on the left of PC1. The results are based on the 95% confidence interval (Figures 11B, D). The OPLS-DA score plot showed that the samples were divided into three categories, with significant differences (Figure 12A). The model could explain 99.0% of the variation (R2X = 0.991, R2Y = 0.74) and had high prediction capability (Q2 = 0.638). The results are based on the 95% confidence interval (Figure 12B). The VIP of six variables showed that δ18O, N%, and δ15N are the most important contributors to the discrimination (VIP > 1, Figure 12C). Cross-validation with 200 permutation tests showed that the OPLS-DA model has good robustness (R2 = 0.0915 and Q2 = −0.508, Figure 12D).
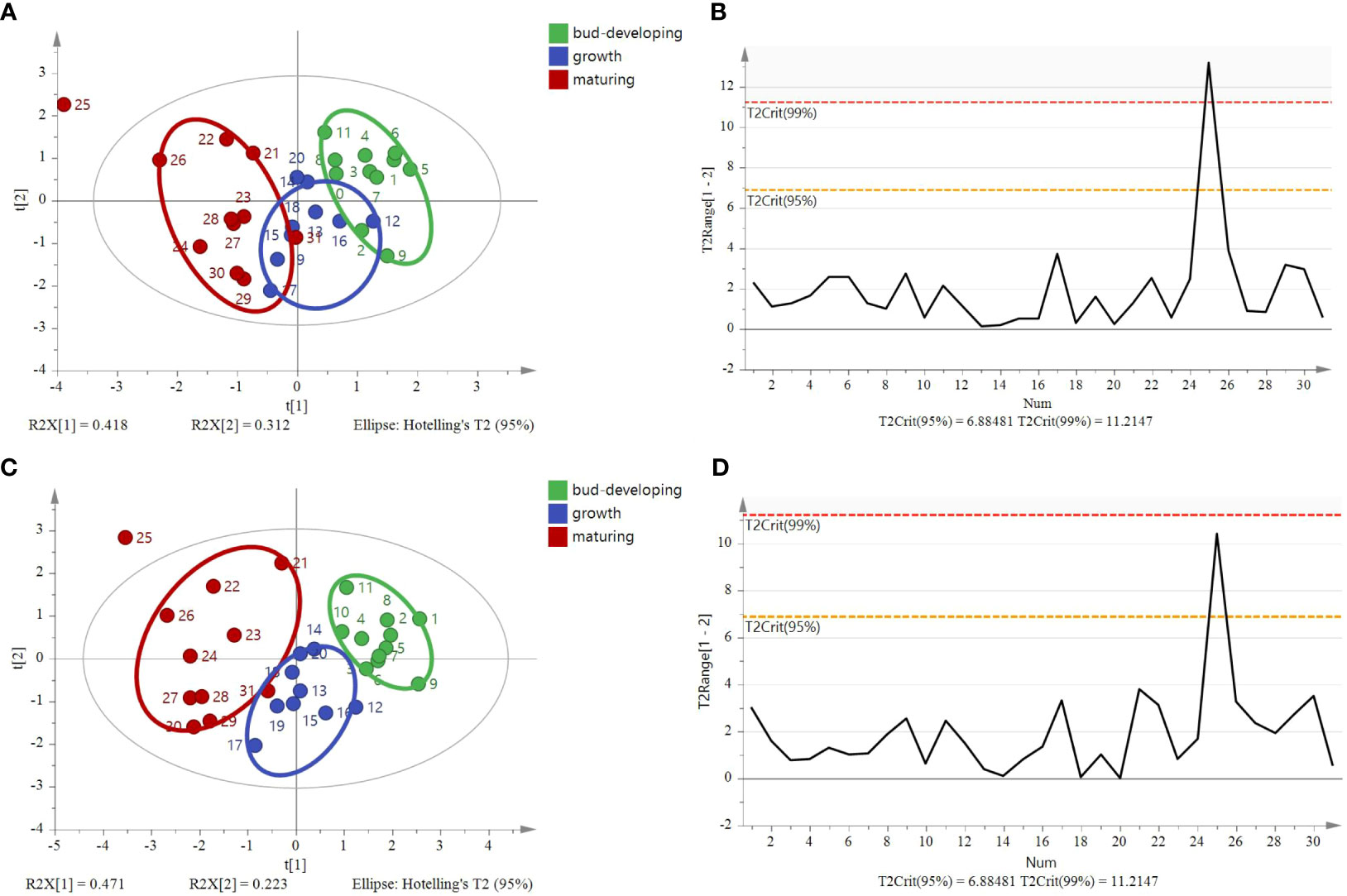
Figure 11 Principal component analysis (PCA) and Hotelling’s T2 ellipse of G. lucidum samples at stages of the bud-developing stage, growth stage, and maturing stage. (A) Principal component analysis score plot of four variables (δ13C, δD, δ18O, δ15N), (B) Hotelling’s T2 ellipse of four variables was constructed from the PCA score plot. The results are based on the 95% confidence interval. (C) Principal component analysis score plot of six variables (δ13C, δD, δ18O, δ15N C%, and N%) and (D) Hotelling’s T2 ellipse of six variables constructed from the PCA score plot. The results are based on the 95% confidence interval.
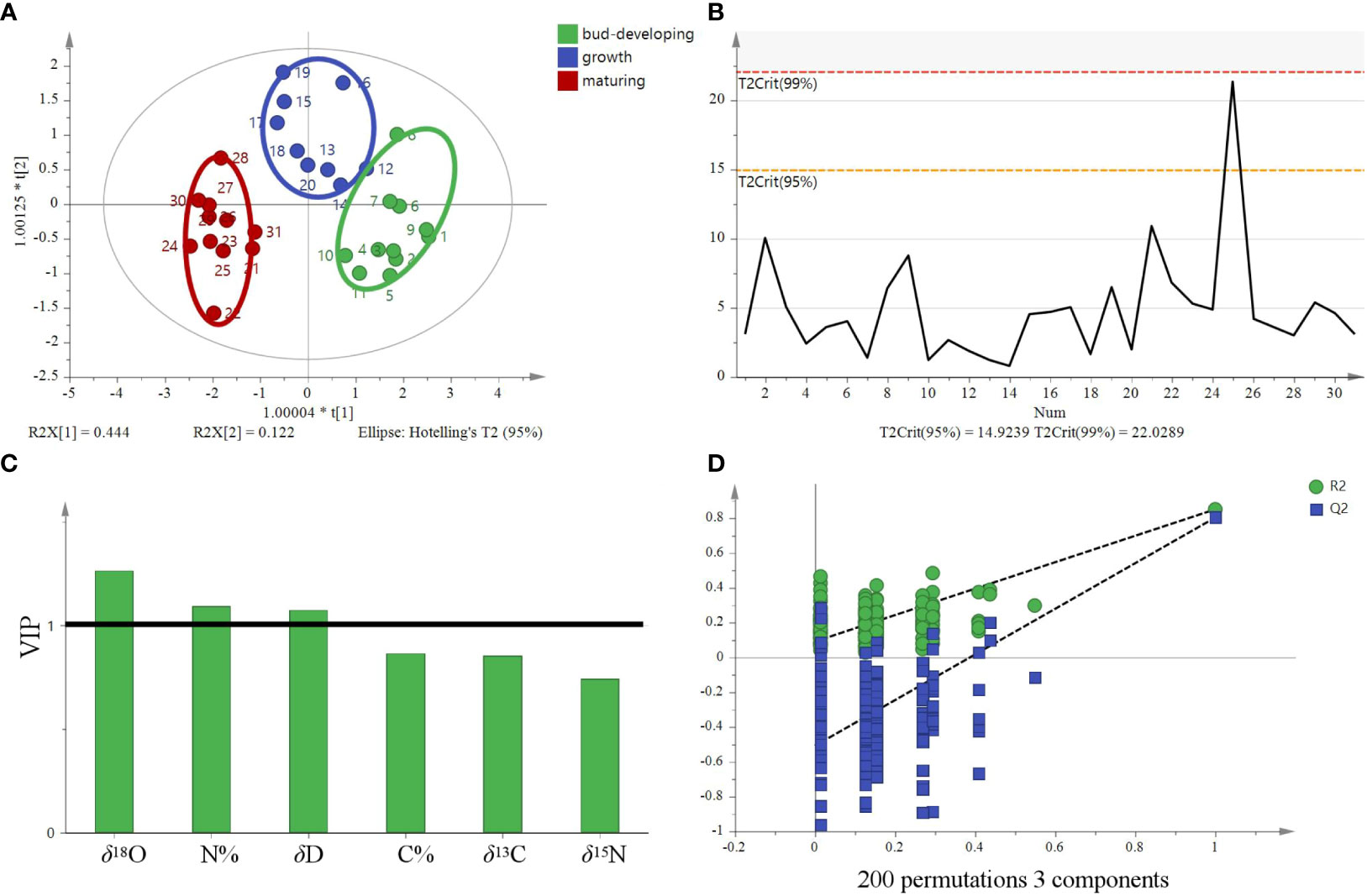
Figure 12 OPLS-DA model based on six variables (δ13C, δD, δ18O, δ15N, C%, and N%) for tracing the different stages of G. lucidum. (A) OPLS-DA score, (B) Hotelling’s T2 ellipse constructed from the OPLS-DA score plot. The results are based on the 95% confidence interval. (C) VIP plots and (D) The permutation test plots of the model for the samples.
Growth stage samples were used for the model verification. Figure 13 shows the predicted and actual values of the PLS model according to the four isotope ratios (δ13C, δD, δ18O, and δ15N) and C% and N% (RMSEE = 0.27, RMSECV = 0.34). All external sample validations of G. lucidum were classified correctly.
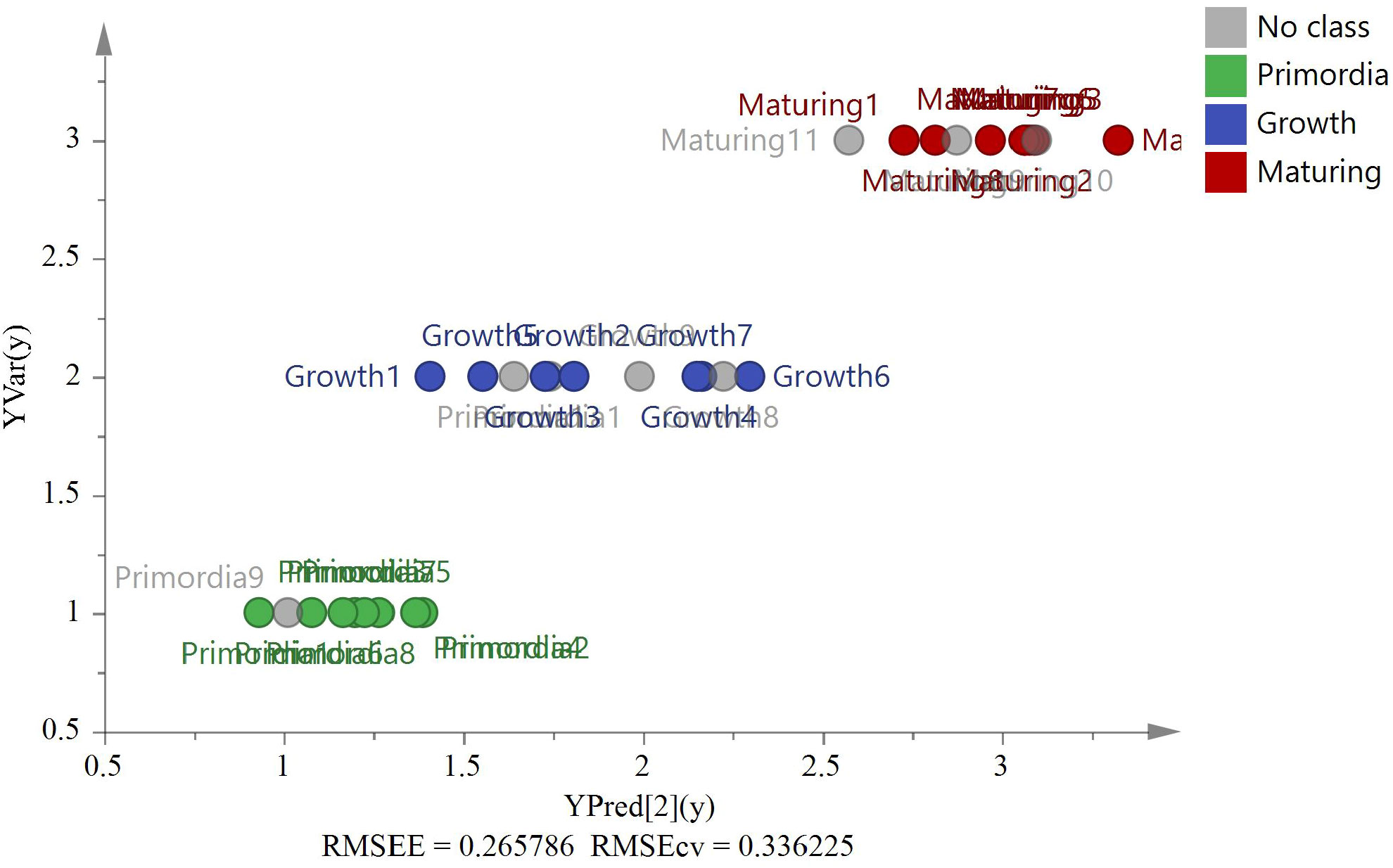
Figure 13 The PLS model based on six variables (δ13C, δD, δ18O, δ15N, C%, and N%) of stages validation by eight external G. lucidum samples (Bud-developing stage n=3, Growth stage n=2, and Maturing stage n=3).
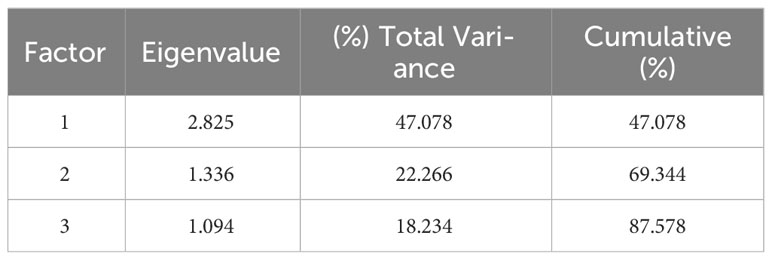
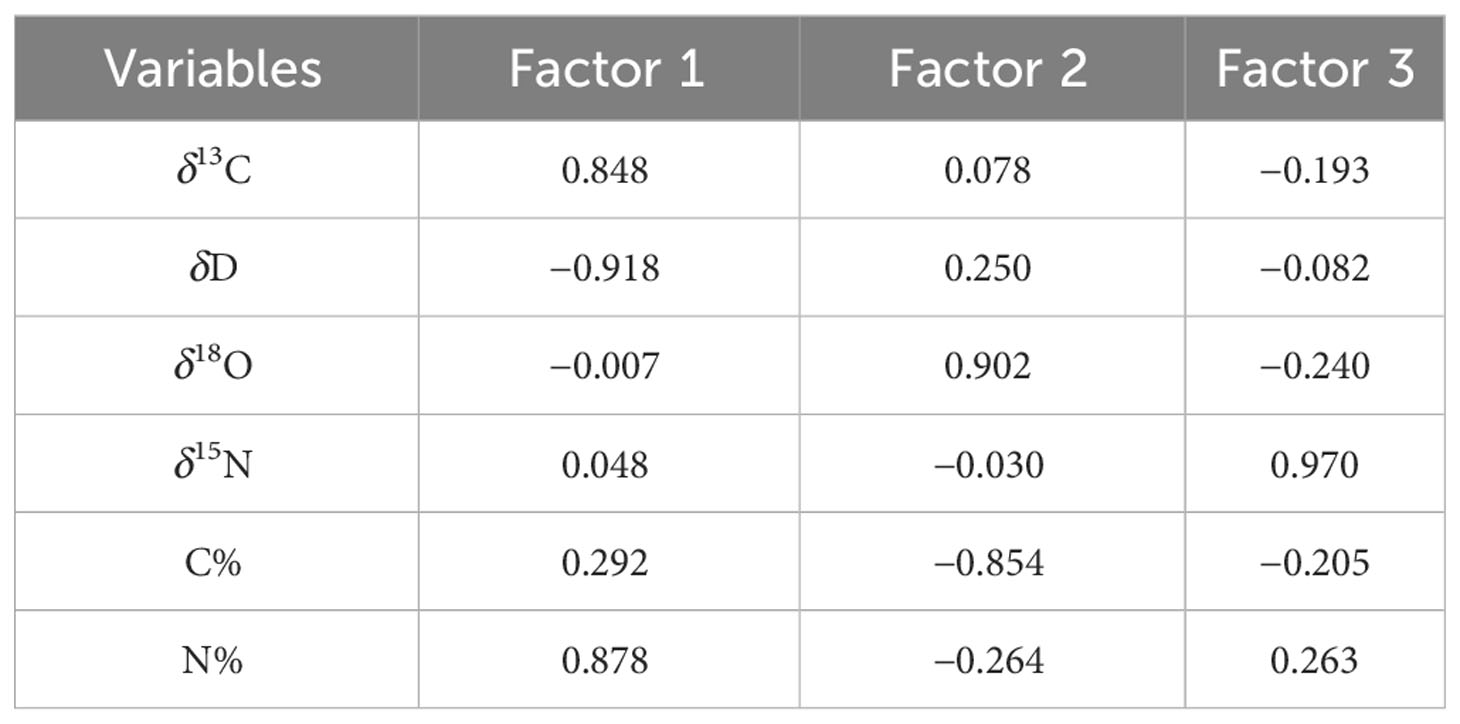
The eigenvalues of the sample correlation matrix were calculated, and the eigenvalue demonstrates the importance of each new factor in explaining data variability. The contribution of six variables, namely, δ13C, δD, δ18O, δ15N, C%, and N%, was analyzed by PCA at the PCA model, and the results are presented in Table 6. Three major factors generally accounted for 87.578% (factors 1, 2, and 3 explained 47.078%, 22.266%, and 18.234% of the data variability, respectively) of the six variations inherent in the input model data. Among them, N% was strongly associated with factor 1 (0.878), and C% was highly correlated with factor 2 (−0.854), indicating that the contribution rates of C% and N% were highly correlated with the first two factors, thereby boosting the model’s accuracy, especially N% (see Table 7). The contribution of six variables was also analyzed using the VIP factor in the OPLS-DA model, and the results are presented in Figure 12C. δD, N%, and δ15N VIP values >1 indicate that these markers contribute to differentiation at different stages. This finding also showed the importance of N% in the model.
4 Discussion
G. lucidum is a popular medicinal fungus that has been used in traditional medicine for decades, with its origin influencing its medicinal and commercial worth. The amount of active ingredients and the price of G. lucidum from different origins varies significantly; hence, fraudulent labeling is common. Reliable techniques for G. lucidum geographic verification are urgently required to safeguard the interests of consumers, producers, and honest dealers. A stable isotope is widely acknowledged as a useful traceability technique and could be developed to confirm the geographical origin of G. lucidum. The chemical composition of G. lucidum was affected by different regions and planting stages caused by temperature, humidity, CO2 content, and other environmental factors (Sudheer et al., 2018a; Sudheer et al., 2018b). Particularly during the planting process of G. lucidum, temperature, humidity, and CO2 content directly affect its growth states (bud development, growth, and maturing stages), followed by changes in G. lucidum stable isotopes along with bioactive compounds in these stages. The changes in bioactive compounds may contribute to the variations of stable isotopes at different stages. Therefore, the variation in stable isotopes in different regions and stages should be comprehensively considered.
The latitude is one of the main factors affecting the δ13C values; theoretically, high δ13C values will have high latitudes (Guo et al., 2017; Li et al., 2011). The latitudes of the four provinces are ranked as follows: Jilin > Anhui > Zhejiang > Fujian. Thus, the theoretical arrangement of δ13C values should follow the order: Jilin > Anhui > Zhejiang > Fujian. However, in the actual data, the Zhejiang samples with δ13C values were the highest, and those of the other producing regions were within acceptable bounds. Moreover, δ13C values had minimal effect on the separation of G. lucidum at different growth stages (VIP < 1). In the origin model, δ13C values showed the strongest influence on the separation of different regions (Figure 6A). The significance of this effect was also observed in Figure 6C (VIP > 1). Thus, δ13C values were an important variable in G. lucidum from different origins. Six other heterogeneous samples (Pseudostellaria heterophylla) of the same origin, including three from Fujian (FJ) and three from Anhui (AH), were randomly introduced to further verify the reliability of the model. δ13C, δD, δ18O, and δ15N were acquired by using the same isotope ratio mass spectrometry. The result of G. lucidum PCA, OPLS-DA, and PLS analysis to further verify the reliability of the model showed that other samples (Pseudostellaria heterophylla) could be identified by the model in another group with 100% accuracy. These samples (G. lucidum and Pseudostellaria heterophylla) were then again analyzed by PCA, which revealed that G. lucidum samples were separated from Pseudostellaria heterophylla samples. These results demonstrated that G. lucidum had its own unique stable isotopes, while other TCM from the same location and the known model could be used to distinguish G. lucidum accurately and effectively. For the model validation, nine of the G. lucidum as external samples for the OPLS-DA model showed that external samples could be identified by the model in their group with 100% accuracy. In the FLDA method, the eight G. lucidum samples consistent with PLS for discrimination and classification results were the same at 100% accuracy, indicating the accuracy and stability of the established model.
Studies have shown that the contents of different substances vary significantly in different stages. The highest content of ganoderic acids A, B, D, and F was detected during the bud development stage. The highest contents of ganoderiol and ganoderic acids S and T were detected during the maturing stage (Zhou et al., 2018). These components are not only related to growing conditions (i.e., latitude, longitude, temperature, and altitude) but also have different growth stages. However, methods for distinguishing between the various growth stages of G. lucidum are currently unavailable. Samples of G. lucidum at various stages were collected in Fujian for this experiment to further examine the formation mechanism of stable isotopes. Focusing only on the maturing stage, the stable isotope ratio of G. lucidum was stable (RSD < 4.4%). The ANOVA results showed differences in δ18O (p < 0.05), N% (p < 0.001), and δD (p < 0.001), indicating that δD, N%, and δ18O values can be used to distinguish G. lucidum at different stages. C% and N% showed the maximum concentrations at the bud development stage. These concentrations declined with the growth of G. lucidum, which may be associated with the maturation stage. The discriminative stage model of G. lucidum had six variables (δ13C, δD, δ18O, δ15N, C%, and N%) whereas the origin model had four variables (δ13C, δD, δ18O, and δ15N). Evidently, PCA results demonstrated an overlapping phenomenon when only four variables were employed to separate samples at different stages, and miscalculation was observed when PLS was used to validate the model at the overlapped sample (e.g., S12, S15, S17, and S19). The patterns of G. lucidum samples at each stage were distinguishable following the addition of the C% and N% variables, and the model validation of the PLS demonstrated that the accuracy had reached 100%. Further study of the contribution of six variables using principal component factor analysis in the PCA model revealed that the contribution rates of C% and N% in the phase model could not be disregarded, especially N%. Similar to PCA and the contribution of six variables by the VIP factor in the OPLS-DA model, N% was the major VIP marker at different stages. Therefore, the stage model with six variables was superior to the model with only four variables. This finding may be due to the same geographical location of samples collected at successive phases, which was difficult to identify with only four variables (δ13C, δD, δ18O, and δ15N). After increasing the C% and N% with stage characteristics, the model could be successfully applied to the differentiation of stages and accurate validation. By contrast, the six variables (δ13C, δD, δ18O, δ15N, C%, and N%) in different stages of the current study may also provide a reference for the cultivation of G. lucidum. These variables could not only be used to trace geographical origin but also for tracking the migration of the flux of nutritional elements such as N during cultivation (Tan et al., 2019).
5 Conclusions
This study determined the C, H, O, and N stable isotopes in G. lucidum samples from different regions. The data were evaluated using chemometric analyses, including PCA, OPLS-DA, PLS, and FLDA. These results suggested that the geographic origin significantly impacted these G. lucidum indicators. The findings of this study could be used to boost the transparency of G. lucidum supply chains. In the future, it will be necessary to collect additional samples from different years and integrate mean temperature, precipitation, and geographic information systems to develop a comprehensive chemometric prediction model that comprehends the effect of the geographical environment or climate and serves as a standard for quality control.
Data availability statement
The original contributions presented in the study are included in the Supplementary Material, and further inquiries can be directed to the corresponding authors.
Author contributions
YZ conducted experiments and data analysis. CW and KJ provided and analyzed sample sources. WenX and MY contributed to the conception and design of the study. SC, LW, XZ, WeiX, and YL designed, supervised the work, and prepared the manuscript. All authors contributed to the manuscript revision, read, and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (2019YFC1710505) and Fujian Province Science and Technology Department project (No. 2022Y4017). The previous version omitted information on funding from the Department of Science and Technology Department project (No. 2022Y4017).
Acknowledgments
We want to thank the Third Institute of Oceanography, Ministry of Natural Resources for the experimental support. We would like to thank all authors for their joint efforts.
Conflict of interest
CW was employed by the company Fujian Xianzhilou Biological Science & Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1234729/full#supplementary-material
References
Ahmad, M. F. (2020). Ganoderma lucidum: A rational pharmacological approach to surmount cancer. J. Ethnopharmacol. 260, 113047. doi: 10.1016/j.jep.2020.113047
Bijalwan, A., Bahuguna, K., Vasishth, A., Singh, A., Chaudhary, S., Dongariyal, A., et al. (2021). Growth performance of Ganoderma lucidum using billet method in Garhwal Himalaya, India. Saudi J. Biol. Sci. 28 (5), 2709–2717. doi: 10.1016/j.sjbs.2021.03.030
Chang, C. J., Lin, C. S., Lu, C. C., Martel, J., Ko, Y. F., Ojcius, D. M., et al. (2015). Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 6, 7489. doi: 10.1038/ncomms8489
Chen, S., Xu, J., Liu, C., Zhu, Y., Nelson, D. R., Zhou, S., et al. (2012). Genome sequence of the model medicinal mushroom Ganoderma lucidum. Nat. Commun. 3, 913. doi: 10.1038/ncomms1923
Chen, Y., Yan, Y., Xie, M. Y., Nie, S. P., Liu, W., Gong, X. F., et al. (2008). Development of a chromatographic fingerprint for the chloroform extracts of Ganoderma lucidum by HPLC and LC-MS. J. Pharm. BioMed. Anal. 47 (3), 469–477. doi: 10.1016/j.jpba.2008.01.039
Fraile-Fabero, R., Ozcariz-Fermoselle, M. V., Oria-de-Rueda-Salgueiro, J. A., Garcia-Recio, V., Cordoba-Diaz, D., Del P Jiménez-López, M., et al. (2021). Differences in Antioxidants, Polyphenols, Protein Digestibility and Nutritional Profile between Ganoderma lingzhi from Industrial Crops in Asia and Ganoderma lucidum from Cultivation and Iberian Origin. Foods 10 (8), 1750. doi: 10.3390/foods10081750
Fu, H., Yin, Q., Xu, L., Wang, W., Chen, F., Yang, T. (2017). A comprehensive quality evaluation method by FT-NIR spectroscopy and chemometric: Fine classification and untargeted authentication against multiple frauds for Chinese Ganoderma lucidum. Spectrochim Acta A Mol. Biomol Spectrosc 182, 17–25. doi: 10.1016/j.saa.2017.03.074
Gong, H., Rehman, F., Li, Z., Liu, J., Yang, T., Liu, J., et al. (2022). Discrimination of geographical origins of wolfberry (Lycium barbarum L.) fruits using stable isotopes, earth elements, free amino acids, and saccharides. J. Agric. Food Chem. 70 (9), 2984–2997. doi: 10.1021/acs.jafc.1c06207
Guo, L. X., Hong, Y. H., Zhou, Q. Z., Zhu, Q., Xu, X. M., Wang, J. H. (2017). Fungus-larva relation in the formation of Cordyceps sinensis as revealed by stable carbon isotope analysis. Sci. Rep. 7 (1), 7789. doi: 10.1038/s41598-017-08198-1
Ha, T., Loan le, T., Hung, T. M., Han le, V. N., Khoi, N. M., Dung le, V., et al. (2015). An improved HPLC-DAD method for quantitative comparisons of triterpenes in Ganoderma lucidum and its five related species originating from Vietnam. Molecules 20 (1), 1059–1077. doi: 10.3390/molecules20011059
Hsieh, C., Yang, F. C. (2004). Reusing soy residue for the solid-state fermentation of Ganoderma lucidum. Bioresour Technol. 91 (1), 105–109. doi: 10.1016/s0960-8524(03)00157-3
Li, G. C., Wu, Z. J., Wang, Y. H., Dong, X. C., Li, B., He, W. D., et al. (2011). Identification of geographical origins of Schisandra fruits in China based on stable carbon isotope ratio analysis. Eur. Food Res. Technol. 232, 797–802. doi: 10.1007/s00217-011-1445-5
Magdas, D. A., Guyon, F., Puscas, R., Vigouroux, A., Gaillard, L., Dehelean, A., et al. (2008). Applications of emerging stable isotopes and elemental markers for geographical and varietal recognition of Romanian and French honeys. Food Chem. 334, 127599. doi: 10.1016/j.foodchem.2020.127599
Ren, X., Wang, J., Huang, L., Cheng, K., Zhang, M., Yang, H. (2020). Comparative Studies on Bioactive Compounds, Ganoderic Acid Biosynthesis, and Antioxidant Activity of Pileus and Stipes of Lingzhi or Reishi Medicinal Mushroom, Ganoderma lucidum (Agaricomycetes) Fruiting Body at Different Growth Stages. Int. J. Med. Mushrooms 22 (2), 133–144. doi: 10.1615/IntJMedMushrooms.2020033683
Sang, T., Guo, C., Guo, D., Wu, J., Wang, Y., Wang, Y., et al. (2021). Suppression of obesity and inflammation by polysaccharide from sporoderm-broken spore of Ganoderma lucidum via gut microbiota regulation. Carbohydr Polym. 256, 117594. doi: 10.1016/j.carbpol.2020.117594
Singh, S., Sharma, P. C. (2022). Gas chromatography-mass spectrometry (GC-MS) profiling reveals substantial metabolome diversity in seabuckthorn (Hippophae rhamnoides L.) berries originating from different geographical regions in the Indian Himalayas. Phytochem. Anal. 33 (2), 214–225. doi: 10.1002/pca.3081
Sudheer, S., Alzorqi, I., Ali, A., Cheng, P. G., Siddiqui, Y., Manickam, S. (2018a). Determination of the biological efficiency and antioxidant potential of lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), cultivated using different agro-wastes in Malaysia. Int. J. Med. Mushrooms 20 (1), 89–100. doi: 10.1615/IntJMedMushrooms.2017024588
Sudheer, S., Taha, Z., Manickam, S., Ali, A., Cheng, P. G. (2018b). Development of antler-type fruiting bodies of Ganoderma lucidum and determination of its biochemical properties. Fungal Biol. 122 (5), 293–301. doi: 10.1016/j.funbio.2018.01.007
Surya, S., Ibrahim, A., Sivakumar, M., Asgar, A. (20182018). Bioactive compounds of the wonder. Medicinal mushroom “Ganoderma lucidum”. Bioact Mol. Food 45, 1. doi: 10.1007/978-3-319-54528-8_45-1
Tan, H., Kohler, A., Miao, R., Liu, T., Zhang, Q., Zhang, B., et al. (2019). Multi-omic analyses of exogenous nutrient bag decomposition by the black morel Morchella importuna reveal sustained carbon acquisition and transferring. Environ. Microbiol. 21 (10), 3909–3926. doi: 10.1111/1462-2920.14741
Wang, J., Chen, T., Zhang, W., Zhao, Y., Yang, S., Chen, A. (2020). Tracing the geographical origin of rice by stable isotopic analyses combined with chemometrics. Food Chem. 313, 126093. doi: 10.1016/j.foodchem.2019.126093
Wang, K., Liao, M., Zhou, N., Bao, L., Ma, K., Zheng, Z., et al. (2019). Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 26 (1), 222–235.e5. doi: 10.1016/j.celrep.2018.12.028
Zhang, C., Fu, D., Chen, G., Guo, M. (2019). Comparative and chemometric analysis of correlations between the chemical fingerprints and anti-proliferative activities of ganoderic acids from three Ganoderma species. Phytochem. Anal. 30 (4), 474–480. doi: 10.1002/pca.2830
Zhao, S. L., Fu, F., Jia, Y. R., Yu, X. A., Yu, B. Y., Li, R. S. (2021). Quality assessment and traceability study of Angelicae Sinensis Radix via binary chromatography, triple quadrupole tandem mass spectrometry, and multivariate statistical analysis. J. Sep Sci. 44 (5), 1062–1071. doi: 10.1002/jssc.202001087
Zhao, S., Zhao, Y., Rogers, K. M., Chen, G., Chen, A., Yang, S. (2020). Application of multi-element (C, N, H, O) stable isotope ratio analysis for the traceability of milk samples from China. Food Chem. 310, 125826. doi: 10.1016/j.foodchem.2019.125826
Zhou, S., Tang, Q., Tang, C., Liu, Y., Ma, F., Zhang, X., et al. (2018). Triterpenes and soluble polysaccharide changes in lingzhi or reishi medicinal mushroom, Ganoderma lucidum (Agaricomycetes), during fruiting growth. Int. J. Med. Mushrooms 20 (9), 859–871. doi: 10.1615/IntJMedMushrooms.2018027357
Keywords: Ganoderma lucidum, stable isotope ratio, chemometrics, growth stages, geographical origin
Citation: Zhang Y, Jiang K, Chen S, Wang L, Zhang X, Xu W, Yam MF, Wu C, Xu W and Lin Y (2023) Quality control of Ganoderma lucidum by using C, H, O, and N stable isotopes and C and N contents for geographical traceability. Front. Plant Sci. 14:1234729. doi: 10.3389/fpls.2023.1234729
Received: 05 June 2023; Accepted: 11 September 2023;
Published: 11 October 2023.
Edited by:
Zhentian Lei, University of Missouri, United StatesReviewed by:
José Juan Ordaz-Ortiz, National Polytechnic Institute of Mexico (CINVESTAV), MexicoHao Tan, Sichuan Academy of Agricultural Sciences, Chengdu, China
Copyright © 2023 Zhang, Jiang, Chen, Wang, Zhang, Xu, Yam, Wu, Xu and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wen Xu, 2012029@fjtcm.edu.cn; Mun Fei Yam, yammunfei@usm.my; Yu Lin, yulam@163.com
 Ying Zhang
Ying Zhang Kunxia Jiang
Kunxia Jiang Sisi Chen1
Sisi Chen1 Lina Wang
Lina Wang Xun Zhang
Xun Zhang Wen Xu
Wen Xu Mun Fei Yam
Mun Fei Yam Wei Xu
Wei Xu Yu Lin
Yu Lin