- 1Department of Agricultural Biotechnology, Akdeniz University, Antalya, Türkiye
- 2Institute of Molecular Biology and Biotechnologies, Ministry of Science and Education, Republic of Azerbaijan, Baku, Azerbaijan
- 3Department of Microbiology, Medical Faculty, Erciyes University, Kayseri, Türkiye
- 4Vaccine Research, Development and Application Center, Erciyes University, Kayseri, Türkiye
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel and highly pathogenic coronavirus that caused an outbreak in Wuhan City, China, in 2019 and then spread rapidly throughout the world. Although several coronavirus disease 2019 (COVID-19) vaccines are currently available for mass immunization, they are less effective against emerging SARS-CoV-2 variants, especially the Omicron (B.1.1.529). Recently, we successfully produced receptor-binding domain (RBD) variants of the spike (S) protein of SARS-CoV-2 and an antigen cocktail in Nicotiana benthamiana, which are highly produced in plants and elicited high-titer antibodies with potent neutralizing activity against SARS-CoV-2. In this study, based on neutralization ability, we demonstrate that plant-produced RBD and cocktail-based vaccine candidates are highly effective against SARS-CoV-2, independently of its emerging variants. These data demonstrate that plant-produced RBD and cocktail-based proteins are the most promising vaccine candidates and may protect against Delta and Omicron-mediated COVID-19. This is the first report describing vaccines against SARS-CoV-2, which demonstrate significant activities against Delta and Omicron variants.
1 Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that causes the coronavirus disease 2019 (COVID-19) infection, a highly infectious RNA virus, has undergone numerous mutations with the formation of genetically diverse linkages since first appearing in the city of Wuhan in 2019. Mutations could potentially have an impact on viral transmission as they affect the interaction between the S protein and its receptor, ACE-2. Among variants of concern (VOCs), the Alpha (B.1.1.7) and Delta (B.1.617.2) variants are associated with a high viral transmission rate and virulence compared to the parental Wuhan strain. Nine mutations were found in the S protein of the Delta variant, including in the amino-terminal domain (NTD) (five mutations) and receptor-binding domain (RBD) (two mutations, L452R, T478K). One mutation (P681R) was found near the furin cleavage site, and the other (D950N) in the S2 domain (Planas et al., 2021). Notably, mutations of G476, F486, T500, and N50, observed within the RBD are close to the receptor (ACE-2) binding site.
On 26 November 2021, a new variant named Omicron (B.1.1.529) was designated the fifth VOC (He et al., 2021), which carries more than 60 mutations compared to the original Wuhan strain. In the new Omicron variant, 36 mutations were found in the S protein sequence, including 30 amino acid substitutions, three deletions, and one insertion. It should be noted that 15 of the 30 amino acid substitutions are in the RBD (Tiecco et al., 2022).
Vaccination is currently the most effective way to prevent pathogenic diseases, including COVID-19. However, currently, available vaccines are less effective or not effective against emerging SARS-CoV-2 variants, such as the Delta strain (B.1.617) and the Omicron (B.1.1.529). Therefore, developing COVID-19 vaccines that are effective against emerging new variants of SARS-CoV-2, such as the Omicron, will be a very challenging task. Recently, we reported the successful production of RBD variants of the S protein of SARS-CoV-2 (Mamedov et al., 2021a) and of an antigen cocktail (Mamedov et al., 2021b) in Nicotiana benthamiana, which are highly produced in plants and elicited high-titer antibodies with potent neutralizing activity against SARS-CoV-2. Since the SARS-CoV-2 virus has mutated over time, in this study we tested how RBD or cocktail antigens could elicit specific immune responses against existing SARS-CoV-2 variants, Delta and Omicron. We demonstrate that plant-produced RBD or cocktail antigen-elicited antibodies are capable of neutralizing the Delta or Omicron variants.
2 Materials and method
2.1 Cloning, expression, and purification of gRBD, dRBD, and N+RBD proteins
Cloning, expression, and purification of glycosylated RBD (gRBD), deglycosylated RBD (dRBD), and N+RBD (nucleocapsid protein + receptor binding domain) proteins from N. benthamiana plant were performed as described recently (Mamedov et al., 2021a; Mamedov et al., 2021b). The genes encoding RBD of SARS-CoV-2 spike protein (RBD, 319-591 aa, GenBank: QHO60594.1) and nucleocapsid (N, 1–419 aa, GenBank: YP_009724397) were codon optimized using N. benthamiana codons and de novo synthesized (Biomatik Corp., Kitchener, ON, Canada). The signal peptide (MGFVLFSQLPSFLLVSTLLLFLVISHSCRA) of the tobacco PR-1a gene was added to the N-terminus of the RBD and N proteins. The KDEL sequence (ER retention signal) and the FLAG tag sequence (affinity purification tag) were added to the C-terminus. Genes were inserted into the plant expression pEAQ vector and introduced into the AGL1-1 strain of Agrobacterium tumefaciens to express genes in N. benthamiana. To produce the gRBD protein, pEAQ-RBD was infiltrated into N. benthamiana plant. To produce an N+RBD antigen cocktail, RBD and N genes were co-infiltrated into N. benthamiana leaves via co-agroinfiltration with both pEAQ-RBD and pEAQ-N constructs. To produce dRBD, the RBD and Endo H genes were co-infiltrated into N. benthamiana leaves via co-agroinfiltration with both pEAQ-RBD or pGreenII–Endo H constructs. Leaves were collected at 5 days after post-infiltration (dpi).
Expression levels of gRBD, dRBD, and N+RBD were determined by ELISA and Western blot analysis. To quantify the expression levels of gRBD, dRBD, and N+RBD, N. benthamiana plant leaf extract was filtered through Miracloth and then centrifuged at 20,000×g for 25 min at 4°C. The clear extract was analyzed by ELISA and Western blot.
For ELISA, plates were coated with 50 μl of diluted supernatants containing gRBD, dRBD, or N+RBD. The plates were also coated with commercially available RBD protein (Agr319-Phe541 aa, active protein, MBS2563882, MyBiosource, San Diego, CA, USA) as standard protein. Proteins were detected (i) using purified anti-FLAG antibody to detect plant-produced gRBD, dRBD, or N+RBD antigens; (ii) anti-SARS-CoV-2 S protein S1 mAb (cat. no. 945102, BioLegend, USA) to detect plant produced gRBD, dRBD antigens, and commercial RBD protein; (iii) or human novel coronavirus nucleoprotein (N) (1–419 aa) monoclonal antibody (MBS7135930, MyBioSource, San Diego, CA, USA) to detect plant-produced N protein.
For WB analysis, wells were loaded with 20 μl of diluted supernatants containing gRBD, dRBD, or N+RBD. The wells were also loaded with different amounts (100, 50, and 25 ng) of plant-produced, purified FLAG-tagged (Endo H or PNGase F) or commercially available RBD (Arg319–Phe541 aa, active protein, MBS2563882, MyBiosource) proteins as a standard protein. The expression levels of proteins were quantified using highly sensitive Gene Tools software (Syngene Bioimaging, UK). The expression levels were determined based on at least three replicates for each target protein.
Purification of plant-produced gRBD, dRBD, and N+RBD proteins was performed from 20 g of frozen leaves infiltrated with the pEAQ-RBD (with or without pGreenII-Endo H) or pEAQ-RBD+ pEAQ-N constructs, using anti-FLAG affinity chromatography as described recently (Mamedov et al., 2021a; Mamedov et al., 2021b). Plant-produced antigens were purified using anti-DYKDDDDK affinity gel (cat. no. 651503, BioLegend). gRBD-, dRBD-, and N+RBD-expressing leaves weighing 20 g were extracted in 1× PBS buffer and centrifuged at 13,000×g for 20 min at 4°C. The column was prepared and equilibrated with 10 column volumes of 1× PBS buffer. After washing the column with 10 CV of 1× PBS buffer, proteins from the column were eluted with 200 mM glycine buffer, pH 2.2, containing 150 mM NaCl. Eluted fractions were mixed with 2.0 M Tris for neutralization of glycine in elution buffer and concentrated. Purified antigens were analyzed on the SDS-PAGE and Western blotting.
2.2 SDS-PAGE and Western blot
Purified proteins were separated on 10% polyacrylamide gels. For SDS-PAGE analysis, gels were stained with Coomassie (Gel Code Blue, Pierce Rockford, IL, USA). For Western blot, separated samples were transferred to a polyvinylidene fluoride membrane and blocked with 1% I-block. The plant-produced antigens were detected with an anti-DYKDDDDK antibody (cat. no. 637301, BioLegend), followed by horseradish peroxidase (HRP)-conjugated anti-rat polyclonal antibody (cat. no. 405405, BioLegend). Proteins were visualized using the GeneGnome XRQ Chemiluminescence imaging system.
2.3 Immunogenicity studies
Immunogenicity studies of gRBD, dRBD, and N+RBD in mice were performed in groups of 6–7-week-old Balb/c male animals (six mice/group) as described recently (Mamedov et al., 2021a). Mice were immunized intramuscularly (IM) on days 0 and 21 with 5 μg of gRBD, dRBD, and N+RBD adsorbed to 0.3% Alhydrogel. Blood samples were taken from immunized mice on day 42 and used for microneutralization assay (MNT). Mice studies were conducted at Akdeniz University Experimental Animal Care in compliance with the ARRIVE guidelines and with the permission of the Animal Experiments Local Ethics Committee for Animal Experiments at Akdeniz (under protocol number 1155/2020.07.0) with the supervision of a veterinarian.
2.4 MNT assay
We used to live in the SARS-CoV-2 Wuhan (GB-MT327745; GISAID-EPI_ISL_424366), Delta (GB-OM945721; GISAID-EPI_ISL_10844545), and Omicron variants (GB-OM945722; GISAID-EPI_ISL_10844681). SARS-CoV-2 microneutralization (MN) tests were performed as previously described with minor modifications (Mamedov et al., 2021a). Before the day of the experiment, Vero E6 cells (ATCC, CRL-1586) (2×104 cells/100 μl/well) were passaged to 96-well plates. The sera collected from vaccinated mice were heat-inactivated for 30 min at 56°C and subjected to twofold serial dilutions (from 1:4 to 1:1,024) with serum-free Dulbecco’s modified Eagle’s medium (DMEM). Twofold serial dilutions of the mice sera were mixed with an equal volume of DMEM containing 100 tissue culture infectious dose 50 (100 TCID50) of the SARS-CoV-2 variants and incubated for 90 min at 37°C. Following adsorption, inoculums were removed, and cells were incubated for 72 h with DMEM containing 2% FBS and checked for the cytopathic effect (CPE). Microplates were designed as follows: (i) to test two wells for each serum dilution, (ii) six control wells (virus only), and (iii) three wells of growth media for the blank. The CPE evaluation was performed according to the reduction of infection by 50% or more at the serum dilutions. The 50% microneutralization titer (MNT50) was analyzed by the Spearman–Karber method, which was calculated as the reciprocal of the highest serum dilution at which the infectivity was neutralized in 50% of the cell in wells.
2.5 Statistical analysis
We used GraphPad Prism software for statistical analysis. To compare the neutralization activity of gRBD-, dRBD-, and N+RBD-induced serums against live SARS-CoV-2 Wuhan, Delta, and Omicron variants, one way ANOVA test was used. Significant was accepted as p < 0.05, and p-values are shown as *p < 0.05; **p < 0.01; ***p < 0.001. Each point on the graph was derived from three replicas for each dilution.
3 Results
In this study, glycosylated and nonglycosylated versions of RBD and cocktail antigen obtained by co-expression of RBD with N protein were produced in N. benthamiana, as mentioned in our previous studies (Figure 1) (Mamedov et al., 2021a; Mamedov et al., 2021b).
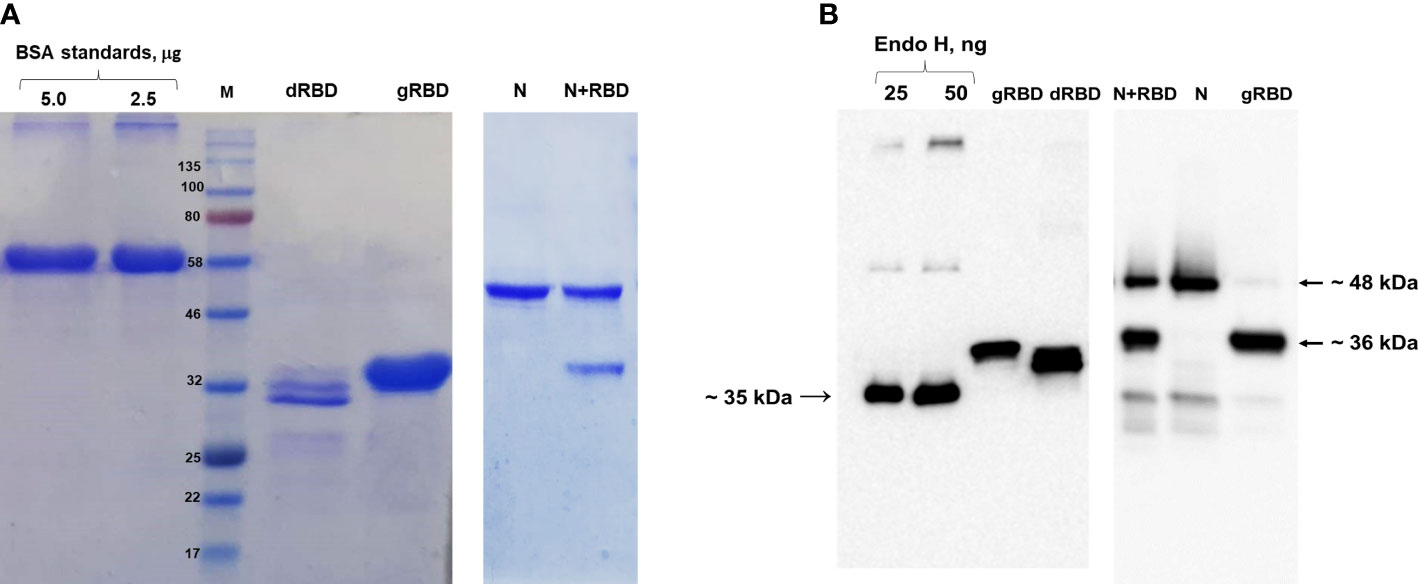
Figure 1 SDS-PAGE and Western blot analysis of gRBD, dRBD, and cocktail antigen (N+RBD) proteins, purified from N. benthamiana plant. Proteins were purified using anti-DYKDDDDK affinity gel. (A) SDS-PAGE for purified antigens. BSA was loaded as a standard, and target proteins were loaded as 5 μg. (B) Western blot analysis for plant-produced antigens. Endo H was used as a standard protein, and target proteins were loaded as 50 ng. Proteins were detected using the anti-FLAG antibody.
The expression levels of gRBD, dRBD, and N+RBD, determined by ELISA and Western blot analysis, were ~42 mg/kg for dRBD and ~45 mg/kg for gRBD and cocktail antigens (N+RBD), similar to what was previously reported (Mamedov et al., 2021a; Mamedov et al., 2021b). RBD and cocktail antigens were purified from N. benthamiana by anti-FLAG affinity chromatography using anti-DYKDDDDK affinity gel, as described in Materials and methods. SDS-PAGE and Western blot analysis of the purified proteins are presented in Figure 1 (and full length gels and blots in the Supplementary Material), which are consistent with the results we recently reported (Mamedov et al., 2021b). Immunogenicity studies of gRBD, dRBD, and N+RBD in mice were performed as described in Materials and methods and as recently reported (Mamedov et al., 2021a; Mamedov et al., 2021b). Antibody levels were determined by ELISA on 42nd-day mouse sera immunized with two doses of plant-produced RBD variants (gRBD or dRBD) or cocktail antigens (N+RBD). As demonstrated in Figure 2, the plant-produced gRBD, dRBD antigens, and cocktail antigens (N+RBD) were able to induce significantly high titers of antibodies with a 5-µg dose. As can be seen from Figure 2, the endpoint titer of N+RBD was 107, higher than that of gRBD or dRBD. It should be noted that the endpoint titer of dRBD is higher than that of gRBD, which is consistent with the results we recently reported (Mamedov et al., 2021a).

Figure 2 Immunogenicity of gRBD, dRBD, and N+RBD antigens in mice on the 42nd day. Mice were immunized with 5 µg of plant-produced proteins in a double injection on days 21 and 42. The endpoint titer was determined as the value corresponding to dilutions of the sera giving an OD value four times greater than the control mouse serum. Data in the graph are shown as the mean standard error of triplicates (SEM) at each sample dilution. The meaning of “**” is that the result is statistically significant. The meaning of “***” is that the result is highly statistically significant. ns, non significant.
To evaluate whether RBD or cocktail antigen-elicited antibodies are capable of neutralizing the Delta or Omicron variant, the latter being dominant at the moment, we tested Wuhan (GB-MT327745; GISAID-EPI_ISL_424366), Delta (GB-OM945721; GISAID-EPI_ISL_10844545), and Omicron variants (GB-OM945722; GISAID-EPI_ISL_10844681) with sera of mice immunized with two doses (5 μg per dose) of the plant-produced RBD variants or cocktail antigen-based COVID-19 vaccines (Figure 2). After two doses, Delta-neutralizing titers were not significantly reduced compared with Wuhan-neutralizing titers. Under the same conditions, Omicron-neutralizing titers were reduced by not more than fourfold compared with neutralizing titers in Wuhan (Figure 3). The plant-produced, deglycosylated RBD vaccine candidate was more effective compared to its glycosylated counterpart, suggesting the negative effect of N-glycosylation on protein functionality.
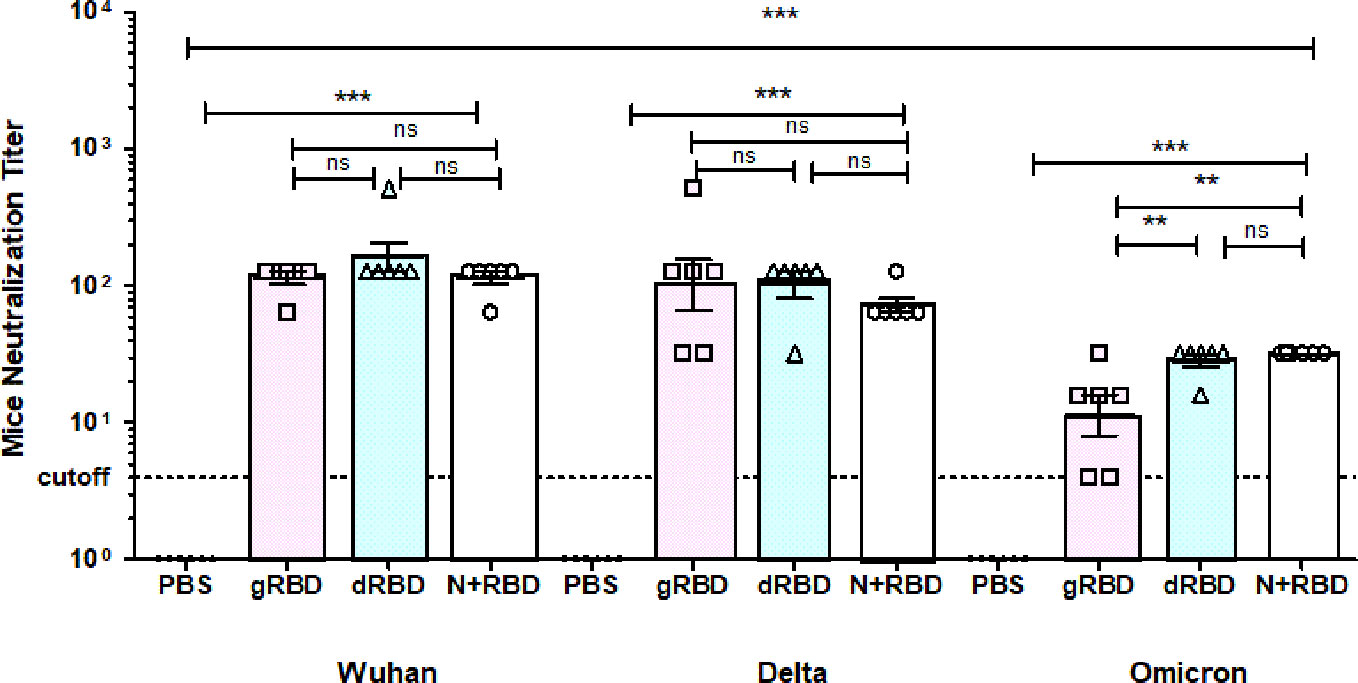
Figure 3 Sera from BALB/c mice immunized with gRBD, dRBD, and N+gRBD (cocktail) proteins demonstrated 50% microneutralization titers (MNT50) against live Wuhan, Delta, and Omicron variants of SARS-CoV-2. Microneutralization assay of 42nd-day mouse sera immunized with plant-produced gRBD, dRBD, and N+RBD (~2.7 μg + 2.3 μg, respectively) against live SARS-CoV-2 Wuhan, Delta, and Omicron variants as indicated. The experiment was performed using 4 to 1,024 dilutions of mouse sera collected on day 42. One-way ANOVA and Tukey’s multiple comparison tests were used to calculate statistical significance (n = 6 mice/group); ***p < 0.001. The meaning of “**” is that the result is statistically significant.
4 Discussion
Recently, we reported the successful production of glycosylated and deglycosylated RBD variants of the S protein of SARS-CoV-2 (Mamedov et al., 2021a) and an antigen cocktail (Mamedov et al., 2021b) in Nicotiana benthamiana, which are highly produced in plants and elicited high-titer antibodies with potent neutralizing activity against SARS-CoV-2. In this study, we demonstrate that plant-produced glycosylated and deglycosylated variants of RBD or cocktail antigen-elicited antibodies are capable of neutralizing the Delta or Omicron variant. It should be noted that after two doses (60 μg total synthetic mRNA), the Omicron-neutralizing titers of the messenger RNA (mRNA)-based COVID-19 vaccine (BNT162b2) were reduced by more than 22 times compared to Wuhan-neutralizing titers (Muik et al., 2022). Cele et al. (2022) demonstrated that in two-dose vaccinated individuals with the BNT162b2, the neutralization protection dropped over 40-fold against the Omicron versus the ancestral D614G virus. These results were expected given that the BNT162b2 vaccine is based on the full-length S protein sequence, while the S protein of the Omicron variant is highly mutated compared to other variants (Cele et al., 2022).
As we have described earlier, plant-produced dRBD demonstrated stronger binding to the SARS-CoV-2 receptor, ACE-2, compared with gRBD (Mamedov et al., 2021a). Moreover, we also demonstrated that both dRBD and dRBD variants induced strong neutralizing antibody responses in mice and sera immunized with the dRBD variant had higher neutralizing activity against live SARS-CoV-2 (Wuhan) compared with gRBD (Mamedov et al, 2021a). The impact of deglycosylation on neutralizing activity can be seen more significantly in the Omicron variant. When comparing Omicron-neutralizing titers, the plant-produced, deglycosylated RBD vaccine candidate was more effective compared to its deglycosylated counterpart, suggesting the negative effect of N-glycosylation on protein functionality. The negative effect of N-glycosylation on the functionality of proteins has been shown for several proteins. Deglycosylation, achieved by mutation of the N-glycosylation sites, increased the expression level and immunogenicity of the RBD protein vaccine (Zhang et al., 2020). The deglycosylation, achieved by the Endo H deglycosylation strategy (Mamedov et al., 2017) in plants significantly enhanced the efficacy of several proteins. For example, deglycosylation improved the SARS-CoV-2 neutralization activity of recombinant ACE2-Fc (Izadi et al., 2023). Deglycosylated Pfs48/45 of Plasmodium falciparum, produced in N. benthamiana plant using Endo H’s in vivo deglycosylation strategy had strong inhibition in SMFA, but under the same conditions, glycosylated Pfs48/45 did not display any significant inhibition (Mamedov et al., 2019b). Plant-produced glycosylated PA83 of Bacillus anthracis was not functionally active and was not able to combine with LF to form a lethal toxin (LeTx) and induce cell death (Mamedov et al., 2016). In contrast, in vivo PNGase F deglycosylated PA83 counterpart was functionally active and showed an EC50 value similar to the Bacillus-produced recombinant PA (Mamedov et al., 2016). When purified plant-produced PNGase (Mamedov et al., 2016) or Endo H (Mamedov et al., 2017) deglycosylated PA83 proteins were assessed for stability, they appeared to be more stable than the glycosylated counterpart. In addition, the plant-produced deglycosylated PA83 elicited significantly higher levels of TNA titers in immunized mice compared with its glycosylated counterpart (Mamedov et al., 2016). The negative effect of N-glycosylation was also demonstrated for plant-produced Pfs25 of Plasmodium falciparum. A nonglycosylated form of Pfs25 antigen (N-linked glycosylation sites mutated) generated higher antibody titers and enhanced TB activity compared to its glycosylated counterpart (Farrance et al., 2011). Likewise, the negative effect of N-glycosylation was also demonstrated for plant-derived human monoclonal antibodies directed against PA of Bacillus anthracis. Deglycosylated (N-linked glycosylation sites mutated), the plant-produced human monoclonal antibody demonstrated superior efficacy compared to a glycosylated form of this mAb in nonhuman primates (Mett et al., 2011).
Plant transient expression systems have proven to be promising alternative expression platforms for the expression of a wide variety of important recombinant proteins, including vaccines (Yusibov & Mamedov, 2010; Mamedov et al., 2016; Mamedov et al., 2019a; Mamedov et al., 2019b; Margolin et al., 2020), therapeutic proteins (Mamedov et al., 2019a), antibodies, and human and industrial enzymes. Importantly, plant expression systems have proven to be capable of high-level production of functionally active SARS-CoV-2 proteins (Mamedov et al., 2020; Mamedov et al., 2021a; Mamedov et al., 2021b) and ACE2 (Mamedov et al., 2021c), receptors of SARS-CoV-2 and SARS-CoV. We recently demonstrated the successful expression of glycosylated and deglycosylated forms of RBD and antigen cocktails, comprising RBD and nucleocapsid (N) proteins, as promising vaccine candidates against COVID-19. We demonstrated that RBD variants of the S protein of SARS-CoV-2 and cocktail antigens are highly produced in the N. benthamiana plant and elicited high-titer antibodies with potent neutralizing activity against SARS-CoV-2. Our hypothesis was that if any SARS-CoV-2 variant infects a human, this means that a mutation in the RBD region did not affect its binding to ACE2, the SARS-CoV-2 S protein receptor. Therefore, the correct selection of RBDs is critical for the successful production of functional RBDs with the ability to induce high neutralizing antibodies against SARS-CoV-2 and its variants. Since SARS-CoV-2 is an mRNA-based virus and mutations were expected, our strategy to eliminate possible emerging mutations was to select not the full sequence of the spike protein (most COVID-19 vaccine developers, including Pfizer-BioNTech and plant-based, Medicago’s VLP, which was approved for use by Health Canada, were targeted on the full-length S sequence) but rather a specific region of the S protein covering the RBD. Moreover, to address mutations, the N-protein + RBD multi-antigen, cocktail-antigen-based vaccine was developed and produced for the first time as a potential COVID-19 vaccine candidate (Mamedov et al., 2021b). In this study, we have shown that the N protein probably significantly contributes to the enhancement of neutralizing activity.
Since RBD of SARS-CoV-2 is the primary target for potent virus-neutralizing antibodies (Barnes et al., 2020; Brouwer et al., 2020; Piccoli et al., 2020; Wang et al., 2020; Yang et al., 2020), it is frequently used for COVID-19 vaccine development (Diego-Martin et al., 2020; Rattanapisit et al., 2020; Shin et al., 2021; Siriwattananon et al., 2021; Phoolcharoen et al., 2023) and as an antigen in serological assays and diagnostic reagents (Amanat and Krammer, 2020; Rattanapisit et al., 2020; Makatsa et al., 2021; Jirarojwattana et al., 2023). Several research groups have utilized N. benthamiana plants to produce different amino acid regions of RBD variants of SARS-CoV-2 (Diego-Martin et al., 2020; Rattanapisit et al., 2020; Shin et al., 2021; Siriwattananon et al., 2021). The amino acid sequence of R319-F541 is the most widely used sequence domain.
The S protein of SARS-CoV-2 contains a relatively high number of cysteine residues, and eight of the nine cysteine residues found in the RBD are involved in disulfide bridge formation (Lan et al., 2020). Notably, for some viruses, such as HIV, the redox state of the fusion protein was shown to be important for the viral fusion to the target cells (Ryser et al., 1994; Manček-Keber et al., 2021). Thus, the proper formation of disulfide bridges is critical for proper folding of the RBD. We produced the RBD variant (R319-S591 aa), where the number of cysteine residues is even, which forms correct disulfide bridges in the molecule that may stabilize the protein conformation, leading to a functional protein. In fact, as we recently reported, the expression levels of plant-produced gRBD and dRBD variants (Mamedov et al., 2020; Mamedov et al., 2021a) were higher than 45 mg/kg of fresh weight. The purification yields of plant-produced gRBD and dRBD were ~22 and ~20 mg pure protein/kg biomass, respectively, which demonstrate the commercialization feasibility of these vaccine candidates. Moreover, in mice, the plant-produced gRBD and dRBD antigens elicited high titers of antibodies with strong SARS-CoV-2 live virus-neutralizing activity (Mamedov et al., 2021a). We concluded that the correct choice of the amino acid sequence within the RBD of the spike protein is critical for achieving high-level production of soluble and functionally active protein (Mamedov et al., 2021a). On this point, in the study of Rattanapisit et al. (2020), RBD of the spike protein of SARS-CoV-2 was produced in N. benthamiana plant, and low yields (2–4 μg/g of fresh weight) were reported (Rattanapisit et al., 2020). In the mentioned study, the amino acid sequence (F318-C617) of RBD was not properly selected, and as a result, cytosine at position 617 remained unpaired (which should form a disulfide bond with cysteine residues at position 649 in the full-length S protein), which may destabilize the protein conformation and lead to a loss of functional activity. In fact, there was no report of neutralization of SARS-CoV-2 in this study. In another study, F318-C617 amino acids of RBD with the Fc region of human immunoglobulin G1 (IgG1) were selected for production in the N. benthamiana plant (Siriwattananon et al., 2021). As in the study of Rattanapisit et al., the amino acid sequence of RBD (F318-C617) was not properly selected, and as a result, cytosine at position 617 remained unpaired (Rattanapisit et al., 2020). The expression level of plant-produced SARS-CoV-2 RBD-Fc was low, at 25 μg/g of fresh weight. Low expression levels (2–4 mg/g of fresh weight) of RBD were also reported for RBD variant (His tagged, aa R319-F541, where the number of cysteine residues was not even) (Diego-Martin et al., 2020; Shin et al., 2021), which is too low to be economical for commercialization of these plant-produced RBD variants (Diego-Martin et al., 2020; Shin et al., 2021).
RBD, or full-length S1, of SARC-CoV-2-based VLPs have been also produced in N. benthamiana for COVID-19 vaccine development by different research groups (Royal et al., 2021; Moon et al., 2022; O'Kennedy et al., 2023). A number of plant-derived SARS-CoV-2 RBD-based antigens and VLPs are in preclinical and clinical trials (Gobeil et al., 2021; Ward et al., 2021; Hager et al., 2022; Pillet et al., 2022; Ruocco and Strasser, 2022). The studies showed that the plant-produced vaccine is safe without any severe allergic reactions and efficacious against SARS-CoV-2, including the Delta variant of concern (Gobeil et al., 2021; Ward et al., 2021; Hager et al., 2022; Pillet et al., 2022). The plant-produced VLP virus-like particle-based (two doses of 3.75 µg of antigen) vaccine has proven to show efficacy of 75.3% against COVID-19 (original Wuhan strain) and has been approved and authorized for use in Canada; however, there are no reports about the efficacy of this vaccine against Omicron. As this plant-based VLP vaccine is targeted on the full-length S sequence, Medicago is preparing to study an Omicron-adapted version of its vaccine, as reported by D’Aoust (https://www.reuters.com/business/healthcare-pharmaceuticals/canada-approves-medicagos-plant-based-covid-19-vaccine-adults-2022-02-24/) even though Canada has already approved a plant-based VLP Medicago vaccine (designed on S protein of SARS-CoV-2 original Wuhan strain) for COVID-19 for adults.
Collectively, all the above findings demonstrate that plant-produced RBD and cocktail antigens are cost-effective, safe, and promising vaccine candidates against SARS-CoV-2, independently of its variants, and may protect against Delta and Omicron-mediated COVID-19.
5 Conclusion
Since the SARS-CoV-2 virus has mutated over time, developing COVID-19 vaccines that are more effective against emerging variants is a very challenging task. Based on our previous and current studies, all our findings demonstrate that plant viral RBDs or cocktail antigens can be used as protein subunit vaccines to elicit specific immune responses against all existing SARS-CoV-2 variants, including Delta and Omicron.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Mice studies were conducted at Akdeniz University Experimental Animal Care in compliance with the ARRIVE guidelines, and with the permission of the Animal Experiments Local Ethics Committee for Animal Experiments at Akdeniz (under protocol number of 1155/2020.07.0) with the supervision of a veterinarian.
Author contributions
TM conceptualized the study. TM designed the experiments. DY, MI, IG, BG, GM, AO, HY, BK, SP, and MU performed the experiments. TM and GH analyzed the data. TM and GH contributed to writing the paper. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by Akdeniz University.
Acknowledgments
The authors are grateful to George P. Lomonossoff (John Innes Center, Biological Chemistry Department) and Plant Bioscience Limited for kindly providing the pEAQ binary expression vector. We thank Philip de Leon at Trade Connections International for his editorial assistance.
Conflict of interest
TM is named as the inventor of the patent applications covering plant-produced COVID-19 vaccine development.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1202570/full#supplementary-material
References
Amanat, F., Krammer, F. (2020). SARS-coV-2 vaccines: status report. Immunity 52, 583–589. doi: 10.1016/j.immuni.2020.03.007
Barnes, C. O., Jette, C. A., Abernathy, M. E., Dam, K. A., Esswein, S. R., Gristick, H. B., et al. (2020). SARS-CoV-2 neutralizing antibody structures inform therapeutic strategies. Nature 588 (7839), 682–687. doi: 10.1038/s41586-020-2852-1
Brouwer, P. J. M., Caniels, T. G., van der Straten, K., Snitselaar, J. L., Aldon, Y., Bangaru, S., et al. (2020). Potent neutralizing antibodies from COVID-19 patients define multiple targets of vulnerability. Science 369 (6504), 643–650. doi: 10.1126/science.abc5902
Cele, S., Jackson, L., Khoury, D. S., Khan, K., Moyo-Gwete, T., Tegally, H., et al. (2022). Omicron extensively but incompletely escapes Pfizer BNT162b2 neutralization. Nature 602, 654–656. doi: 10.1038/s41586-021-04387-1
Diego-Martin, B., González, B., Vazquez-Vilar, M., Selma, S., Mateos-Fernández, R., Gianoglio, S., et al. (2020). Pilot production of SARS-CoV-2 related proteins in plants: a proof of concept for rapid repurposing of indoor farms into biomanufacturing facilities. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.612781
Farrance, C. E., Chichester, J. A., Musiychuk, K., Shamloul, M., Rhee, A., Manceva, S. D., et al. (2011). Antibodies to plant-produced Plasmodium falciparum sexual stage protein Pfs25 exhibit transmission blocking activity. Hum. Vaccin. 7 Suppl, 191–198. doi: 10.4161/hv.7.0.14588
Gobeil, P., Pillet, S., Séguin, A., Boulay, I., Mahmood, A., Vinh, D. C., et al. (2021). Interim report of a phase 2 randomized trial of a plant-produced virus-like particle vaccine for Covid-19 in healthy adults aged 18–64 and older adults Aged 65 and older. medRxiv. doi: 10.1101/2021.05.14.21257248
Hager, K. J., Pérez Marc, G., Gobeil, P., Diaz, R. S., Heizer, G., Llapur, C., et al. (2022). Efficacy and safety of a recombinant plant-based adjuvanted Covid-19 vaccine. N Engl. J. Med. 386 (22), 2084–2096. doi: 10.1056/NEJMoa2201300
He, X., Hong, W., Pan, X., Lu, G., Wei, X. (2021). SARS-CoV-2 Omicron variant: Characteristics and prevention. MedComm 2 (4), 838–845. doi: 10.1002/mco2.110
Izadi, S., Vavra, U., Melnik, S., Grünwald-Gruber, C., Föderl-Höbenreich, E., Sack, M., et al. (2023). In planta deglycosylation improves the SARS-CoV-2 neutralization activity of recombinant ACE2-Fc. Front. Bioeng Biotechnol. 11. doi: 10.3389/fbioe.2023.1180044
Jirarojwattana, P., Shanmugaraj, B., Rattanapisit, K., Phoolcharoen, W. (2023). Development of SARS-CoV-2 neutralizing antibody detection assay by using recombinant plant-produced proteins. Biotechnol. Rep. (Amst) 38, e00796. doi: 10.1016/j.btre.2023.e00796
Lan, J., Ge, J., Yu, J., Shan, S., Zhou, H., Fan, S., et al. (2020). Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 581, 215–220. doi: 10.1038/s41586-020-2180-5
Makatsa, M. S., Tincho, M. B., Wendoh, J. M., Ismail, S. D., Nesamari, R., Pera, F., et al. (2021). SARS-CoV-2 antigens expressed in plants detect antibody responses in COVID-19 patients. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.589940
Mamedov, T., Acsora, R., Gun, N., Gulec, B., Mammadova, G., Cicek, K., et al. (2019a). Engineering, and production of functionally active human Furin in N. benthamiana plant: In vivo post-translational processing of target proteins by Furin in plants. PloS One 14, e0213438. doi: 10.1371/journal.pone.0213438
Mamedov, T., Chichester, J. A., Jones, R. M., Ghosh, A., Coffin, M. V., Herschbach, K., et al. (2016). Production of functionally active and immunogenic non-glycosylated protective antigen from Bacillus anthracis in Nicotiana benthamiana by coexpression with Peptide-N-Glycosidase F (PNGase F) of Flavobacterium meningosepticum. PloS One 11, e0153956. doi: 10.1371/journal.pone.0153956
Mamedov, T., Cicek, K., Gulec, B., Ungor, R., Hasanova, G. (2017). In vivo production of non-glycosylated recombinant proteins in Nicotiana benthamiana plants by co-expression with Endo-β-N-acetylglucosaminidase H (Endo H) of Streptomyces plicatus. PloS One 12 (8), e0183589. doi: 10.1371/journal.pone.0183589
Mamedov, T., Cicek, K., Miura, K., Gulec, B., Akinci, E., Mammadova, G., et al. (2019b). A Plant-Produced in vivo deglycosylated full-length Pfs48/45 as a Transmission-Blocking Vaccine candidate against malaria. Sci. Rep. 9, 9868. doi: 10.1038/s41598-019-46375-6
Mamedov, T., Gurbuzaslan, I., Yuksel, D., Ilgin, M., Mammadova, G., Ozkul, A., et al. (2021c). Soluble human angiotensin- converting enzyme 2 as a potential therapeutic tool for COVID-19 is produced at high levels in Nicotiana benthamiana plant with potent anti-SARS-CoV-2 activity. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.742875
Mamedov, T., Yuksel, D., Ilgin, M., Gurbuzaslan, I., Gulec, B., Mammadova, G., et al. (2020). Engineering, production and characterization of Spike and Nucleocapsid structural proteins of SARS–CoV-2 in Nicotiana benthamiana as vaccine candidates against COVID-19. BioRxiv. doi: 10.1101/2020.12.29.424779
Mamedov, T., Yuksel, D., Ilgin, M., Gurbuzaslan, I., Gulec, B., Yetiskin, H., et al. (2021a). Plant produced glycosylated and in vivo deglycosylated receptor binding domain proteins of SARS-CoV-2 induce potent neutralizing responses in mice. Viruses 13 (8), 1595. doi: 10.3390/v13081595
Mamedov, T., Yuksel, D., Ilgın, M., Gurbuzaslan, I., Gulec, B., Mammadova, G., et al. (2021b). Production and characterization of nucleocapsid and RBD cocktail antigens of SARS-CoV-2 in Nicotiana benthamiana plant as a vaccine candidate against COVID-19. Vaccines 9 (11), 1337. doi: 10.3390/vaccines9111337
Manček-Keber, M., Hafner-Bratkovič, I., Lainšček, D., Benčina, M., Govednik, T., Orehek, S., et al. (2021). Disruption of disulfides within RBD of SARS-CoV-2 spike protein prevents fusion and represents a target for viral entry inhibition by registered drugs. FASEB J. 35 (6), e21651. doi: 10.1096/fj.202100560R
Margolin, E., Crispin, M., Meyers, A., Chapman, R., Rybicki, E. P. (2020). A roadmap for the molecular farming of viral glycoprotein vaccines: engineering glycosylation and glycosylation-directed folding. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.609207
Mett, V., Chichester, J. A., Stewart, M. L., Musiychuk, K., Bi, H., Reifsnyder, C. J., et al. (2011). A non-glycosylated, plant-produced human monoclonal antibody against anthrax protective antigen protects mice and non-human primates from B. anthracis spore challenge. Hum. Vaccin 7 (Suppl), 183–190. doi: 10.4161/hv.7.0.14586
Moon, K. B., Jeon, J. H., Choi, H., Park, J. S., Park, S. J., Lee, H. J., et al. (2022). Construction of SARS-CoV-2 virus-like particles in plant. Sci. Rep. 12 (1), 1005. doi: 10.1038/s41598-022-04883-y
Muik, A., Lui, B. G., Wallisch, A. K., Bacher, M., Mühl, J., Reinholz, J., et al. (2022). Neutralization of SARS-CoV-2 Omicron by BNT162b2 mRNA vaccine-elicited human sera. Science 375 (6581), 678–680. doi: 10.1126/science.abn7591
O'Kennedy, M. M., Abolnik, C., Smith, T., Motlou, T., Goosen, K., Sepotokele, K. M., et al. (2023). Immunogenicity of adjuvanted plant-produced SARS-CoV-2 Beta spike VLP vaccine in New Zealand white rabbits. Vaccine 41 (13), 2261–2269. doi: 10.1016/j.vaccine.2023.02.050
Phoolcharoen, W., Shanmugaraj, B., Khorattanakulchai, N., Sunyakumthorn, P., Pichyangkul, S., Taepavarapruk, P., et al. (2023). Preclinical evaluation of immunogenicity, efficacy and safety of a recombinant plant-based SARS-CoV-2 RBD vaccine formulated with 3M-052-Alum adjuvant. Vaccine 41 (17), 2781–2792. doi: 10.1016/j.vaccine.2023.03.027
Piccoli, L., Park, Y. J., Tortorici, M. A., Czudnochowski, N., Walls, A. C., Beltramello, M., et al. (2020). Mapping neutralizing and immunodominant sites on the SARS-CoV-2 spike receptor-binding domain by structure-guided high-resolution serology. Cell 183 (4), 1024–1042.e21. doi: 10.1016/j.cell.2020.09.037
Pillet, S., Arunachalam, P. S., Andreani, G., Golden, N., Fontenot, J., Aye, P. P., et al. (2022). Safety, immunogenicity, and protection provided by unadjuvanted and adjuvanted formulations of a recombinant plant-derived virus-like particle vaccine candidate for COVID-19 in nonhuman primates. Cell Mol. Immunol. 19, 222–233. doi: 10.1038/s41423-021-00809-2
Planas, D., Veyer, D., Baidaliuk, A., Staropoli, I., Guivel-Benhassine, F., Rajah, M. M., et al. (2021). Reduced sensitivity of SARS-CoV-2 variant Delta to antibody neutralization. Nature 596, 276–280. doi: 10.1038/s41586-021-03777-9
Rattanapisit, K., Shanmugaraj, B., Manopwisedjaroen, S., Purwono, P. B., Siriwattananon, K., Khorattanakulchai, N., et al. (2020). Rapid production of SARS-CoV-2 receptor binding domain (RBD) and spike specific monoclonal antibody CR3022 in Nicotiana benthamiana. Sci. Rep. 10, 17698. doi: 10.1038/s41598-020-74904-1
Royal, J. M., Simpson, C. A., McCormick, A. A., Phillips, A., Hume, S., Morton, J., et al. (2021). Development of a SARS-CoV-2 vaccine candidate using plant-based manufacturing and a tobacco mosaic virus-like nano-particle. Vaccines 9, 1347. doi: 10.3390/vaccines9111347
Ruocco, V., Strasser, R. (2022). Transient expression of glycosylated SARS-coV-2 antigens in Nicotiana benthamiana. Plants 11, 1093. doi: 10.3390/plants11081093
Ryser, H. J., Levy, E. M., Mandel, R., DiSciullo, G. J. (1994). Inhibition of human immunodeficiency virus infection by agents that interfere with thiol-disulfide interchange upon virus-receptor interaction. Proc. Natl. Acad. Sci. 91, 4559–4563. doi: 10.1073/pnas.91.10.4559
Shin, Y. J., König-Beihammer, J., Vavra, U., Schwestka, J., Kienzl, N. F., Klausberger, M., et al. (2021). N-glycosylation of the SARS-CoV-2 receptor binding domain is important for functional expression in plants. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.689104
Siriwattananon, K., Manopwisedjaroen, S., Shanmugaraj, B., Rattanapisit, K., Phumiamorn, S., Sapsutthipas, S., et al. (2021). Plant-produced receptor-binding domain of SARS-CoV-2 elicits potent neutralizing responses in mice and non-human primates. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.682953
Tiecco, G., Storti, S., Degli Antoni, M., Focà, E., Castelli, F., Quiros-Roldan, E. (2022). Omicron genetic and clinical peculiarities that may overturn SARS-CoV-2 pandemic: A literature review. Int. J. Mol. Sci. 23 (4), 1987. doi: 10.3390/ijms23041987
Wang, C., Li, W., Drabek, D., Okba, N. M. A., van Haperen, R., Osterhaus, A. D. M. E., et al. (2020). A human monoclonal antibody blocking SARS-CoV-2 infection. Nat. Commun. 11 (1), 2251. doi: 10.1038/s41467-020-16256-y
Ward, B. J., Gobeil, P., Séguin, A., Atkins, J., Boulay, I., Charbonneau, P. Y., et al. (2021). Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 27, 1071–1078. doi: 10.1038/s41591-021-01370-1
Yang, J., Wang, W., Chen, Z., Lu, S., Yang, F., Bi, Z., et al. (2020). A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 586 (7830), 572–577. doi: 10.1038/s41586-020-2599-8
Yusibov, V. M., Mamedov, T. G. (2010). Plants as an alternative system for expression of vaccine antigens. Proc. ANAS (Biol. Sci.) 65, 195–200.
Keywords: SARS-CoV-2, plant produced RBD, antigen cocktail, virus neutralization, Delta, Omicron
Citation: Mamedov T, Yuksel D, Gurbuzaslan I, Ilgin M, Gulec B, Mammadova G, Ozdarendeli A, Pavel STI, Yetiskin H, Kaplan B, Uygut MA and Hasanova G (2023) Plant-produced RBD and cocktail-based vaccine candidates are highly effective against SARS-CoV-2, independently of its emerging variants. Front. Plant Sci. 14:1202570. doi: 10.3389/fpls.2023.1202570
Received: 08 April 2023; Accepted: 12 July 2023;
Published: 02 August 2023.
Edited by:
Balamurugan Shanmugaraj, Chulalongkorn University, ThailandReviewed by:
Ashwini Malla, Baiya Phytopharm Co., Ltd, ThailandRahul Singh, University of Pennsylvania, United States
Copyright © 2023 Mamedov, Yuksel, Gurbuzaslan, Ilgin, Gulec, Mammadova, Ozdarendeli, Pavel, Yetiskin, Kaplan, Uygut and Hasanova. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tarlan Mamedov, tmammedov@gmail.com
 Tarlan Mamedov
Tarlan Mamedov Damla Yuksel
Damla Yuksel Irem Gurbuzaslan
Irem Gurbuzaslan Merve Ilgin1
Merve Ilgin1 Shaikh Terkis Islam Pavel
Shaikh Terkis Islam Pavel Muhammet Ali Uygut
Muhammet Ali Uygut