- Institute of Plant Genetics, Polish Academy of Sciences, Poznan, Poland
The root system is a key component for plant survival and productivity. In particular, under stress conditions, developing plants with a better root architecture can ensure productivity. The objectives of this study were to investigate the phenotypic variation of selected root- and yield-related traits in a diverse panel of spring barley genotypes. By performing a genome-wide association study (GWAS), we identified several associations underlying the variations occurring in root- and yield-related traits in response to natural variations in soil moisture. Here, we report the results of the GWAS based on both individual single-nucleotide polymorphism markers and linkage disequilibrium (LD) blocks of markers for 11 phenotypic traits related to plant morphology, grain quality, and root system in a group of spring barley accessions grown under field conditions. We also evaluated the root structure of these accessions by using a nondestructive method based on electrical capacitance. The results showed the importance of two LD-based blocks on chromosomes 2H and 7H in the expression of root architecture and yield-related traits. Our results revealed the importance of the region on the short arm of chromosome 2H in the expression of root- and yield-related traits. This study emphasized the pleiotropic effect of this region with respect to heading time and other important agronomic traits, including root architecture. Furthermore, this investigation provides new insights into the roles played by root traits in the yield performance of barley plants grown under natural conditions with daily variations in soil moisture content.
Introduction
Nowadays, barley (Hordeum vulgare L.) is the fourth most important crop in terms of grain production (FAO, 2021). Because of the synteny of grass genomes, it is considered a model crop, being diploid with a genome size of 5.3 Gbp (Hori et al., 2003). Previous studies have shown that barley production is severely affected by abiotic stresses such as drought and heat (Sallam et al., 2019). In order to cope with the effects of climate change, breeders make attempts to improve the adaptability and stability of yield performance of new varieties of barley. As a result, many breeding programs now focus on the adaptation of barley varieties to climate change. For any crop, the root structure is a promising target to increase yield, especially under unfavorable growth conditions. Incorporating genetic information on root traits and root architecture into breeding practices has been reported to possibly improve resource efficiency or stress tolerance.
For the uptake of water and nutrients from the deep zone of the soil, plants require roots with a larger number of root tips (Osmont et al., 2007). From the perspective of breeding, it would be desirable to have genotypes with a larger root volume and a higher dry weight and other related parameters so that they can extract a high amount of water and continue to grow under drought stress (Danakumara et al., 2021).
Nondestructive evaluation of the root growth of plants remains a challenge in research on the root system. Plant roots, in particular, the dynamics of root growth, have not been studied as intensively as shoots, mainly because of physical constraints. Thus far, several methods have been used to study root growth, including core sampling; ingrowth cores; excavation of the root system; X-ray imaging; magnetic resonance imaging; and application of monoliths, ground-penetrating radar, isotopes, rhizotrons, minirhizotrons, and electrical capacitance (Chloupek, 1972; Prokushkin, 1982; van Beem et al., 1998; Hruska et al., 1999). Electrical capacitance is used to study root growth on the basis of the assumption that the capacitance of the root–soil system changes with an increase in the contact area between roots and soil following plant growth (Chloupek, 1977; Dalton, 1995). Dietrich et al. (2013) showed that it is difficult to evaluate root system morphology based on electrical capacitance values. However, the primary advantage of this method is the possibility to measure the electrical capacitance of hundreds of plants per day and to repeat the measurements at different phenological stages. Because dying membranes lose their electrical capacitance, the measured capacitance reflects not only the size of the root system but also the vitality of the membranes. In recent years, the size of the root system, which is measured using the electrical capacitance method, has been used as a criterion to select drought-resistant genotypes in crops such as spring barley (Chloupek et al., 2010; Svačina et al., 2014) and winter wheat (Abdel-Ghani et al., 2013).
economic traits of crops are complex quantitative ones (e.g., yield, quality, earliness, and responses to biotic and abiotic stresses) and have been the focus of interest of plant breeders and researchers for decades. As these traits have been explored only to a limited extent because of their low heritability and environmental sensitivity, they have eventually become the target of traditional breeding approaches; however, expensive and labor-intensive phenotyping procedures are required to successfully explore these quantitative traits (Bhat et al., 2015). Genomic approaches are particularly useful to analyze complex traits as they are usually multigenic in nature and are significantly influenced by the environment. Next-generation sequencing (NGS) has significantly reduced the cost and time needed to sequence and identify single nucleotide polymorphisms (SNPs). Thus, sequence-based genotyping has been developed for the application of NGS in plant breeding and genomic studies. The development of the genotyping-by-sequencing (GBS) approach has led to the introduction of SNPs that are suitable and affordable for genomic selection in both model and nonmodel plant species (Poland et al., 2012). GBS is widely used for genetic mapping of economically important crops (Elshire et al., 2011), including barley (Liu et al., 2014; Yao et al., 2018).
Recently, genome-wide association study (GWAS) has been recognized as a powerful approach to identify genes that regulate complex traits in crops (Famoso et al., 2011; Huang et al., 2012; Morris et al., 2013; Gali et al., 2019; Muhammad et al., 2021). GWAS allows to identify genes that influence natural variations in quantitative traits through the association of markers’ polymorphism with phenotypic variation within diverse panels (Xiao et al., 2017); thus, insights into the genetic architecture of important and yield- and root-related traits can be obtained. This approach has recently become popular for genetic mapping of quantitative traits and for studying natural variations. As reported earlier, linkage disequilibrium (LD) blocks that combine two or more SNPs are more informative than single biallelic SNPs (Rochat et al., 2007). Lorenz et al. (2010) defined barley haplotypes by using different analytical methods and confirmed that the distribution of quantitative trait locus (QTL) alleles in nature does not match that of marker variants; thus, haplotype information could allow to capture associations that would elude individual SNPs. Recent GWASs in wheat (Sehgal et al., 2020) and other crops (Ogawa et al., 2018; Siekmann et al., 2021) have shown that a haplotype-based approach improves prediction accuracy as compared to an individual SNP approach. It has also been suggested that compared to individual markers, haplotype-based GWAS better represents the genetic architecture of complex traits, as this approach can capture epistatic interactions between SNPs at a locus (Bardel et al., 2005) and can more accurately predict whether an allelic series exists at a locus (Hamblin et al., 2011).
The objectives of the present study were as follows: (i) to investigate the phenotypic variations of selected root- and yield-related traits in a diverse panel of spring barley genotypes, (ii) to reveal their genetic architecture by using LD-based GWAS, and (iii) to analyze the potential association between traits linked to root- and yield-related properties of the studied accessions. To assess root architecture in the field, the electrical capacitance characteristics of barley roots and manual root traits were evaluated.
Materials and methods
Plant material and field trials
A total of 149 spring barley genotypes (accession panel) were used in phenotypic and genotypic analyses. The studied accessions were obtained (with few exceptions) from the Polish Breeding Companies. A majority of the barley accessions (85) were registered as cultivars, and 54 accessions were classified as breeding lines. To increase the diversity of the panel, four RILs (seed collection of Institute of Plant Genetics Polish Academy of Sciences – IPG PAS) and eight BC lines (six from IPG PAS collection and two from NordGen collection) were also included. We focused mainly on two-row spring barley accessions from breeding companies’ collections to reduce the confounding effects of population structure in terms of origin, growth, and row types (Comadran et al., 2012; Darrier et al., 2019). The complete list of the accessions used in this study is given in Supplementary Table 1.
The study was conducted over three growing seasons (2017, 2018, 2020) on the experimental fields located at IPG PAS in Poznań, Poland (52°24′30″N 16°56′03″E). All trials were performed in three replications, in randomized blocks, and under standard fertilization and cultivation conditions. The experimentalplots consisted of five rows of 150-cm length that were spaced 20 cm apart. Seeds were planted within each row at 15 cm from each other. In each of the replications, there were 10 individual plants. Before sowing, the seeds were treated with the systemic fungicide Funaben. During the experiment, spraying was carried out as needed to protect the plants against diseases or pests required. Standard agricultural practices of barley production were followed for field management, including irrigation, fertilization, weed control, and pestmanagement. More information in terms of soil fertilization can be found in Supplementary Table 2.
As shownin Table 1, weather data included monthly (March–August) average values from 2017, 2018 and 2020, which were obtained from the Institute of Meteorology and Water Management—National Research Institute database(IMWM–NRI). In addition, soil moisture was evaluated using a handheld moisture meter TDR (Suchorab et al., 2014) attached to an FOM/mts field TDR probe with a 150-mm rod length (E-Test, Poland). The measurements were taken in six randomly selected spots (with three replicates) in the experimental field at three different plant phenological stages (T1, tillering—BBCH 23; T2, stem elongation—BBCH 33; and T3, heading—BBCH 56), as shown in Table 2.
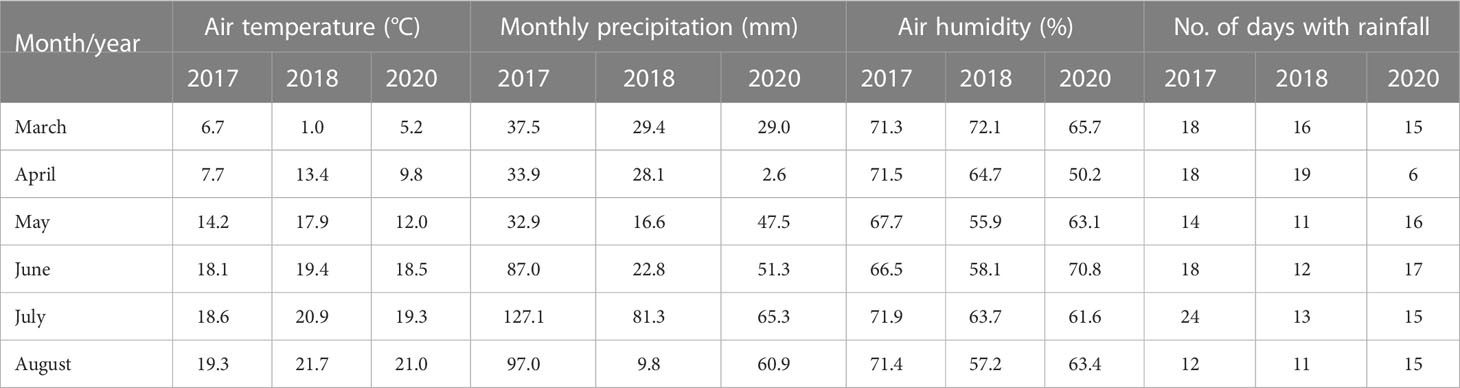
Table 1 Average monthly weather conditions recorded for the growing period (March–August) in 2017, 2018 and 2020.
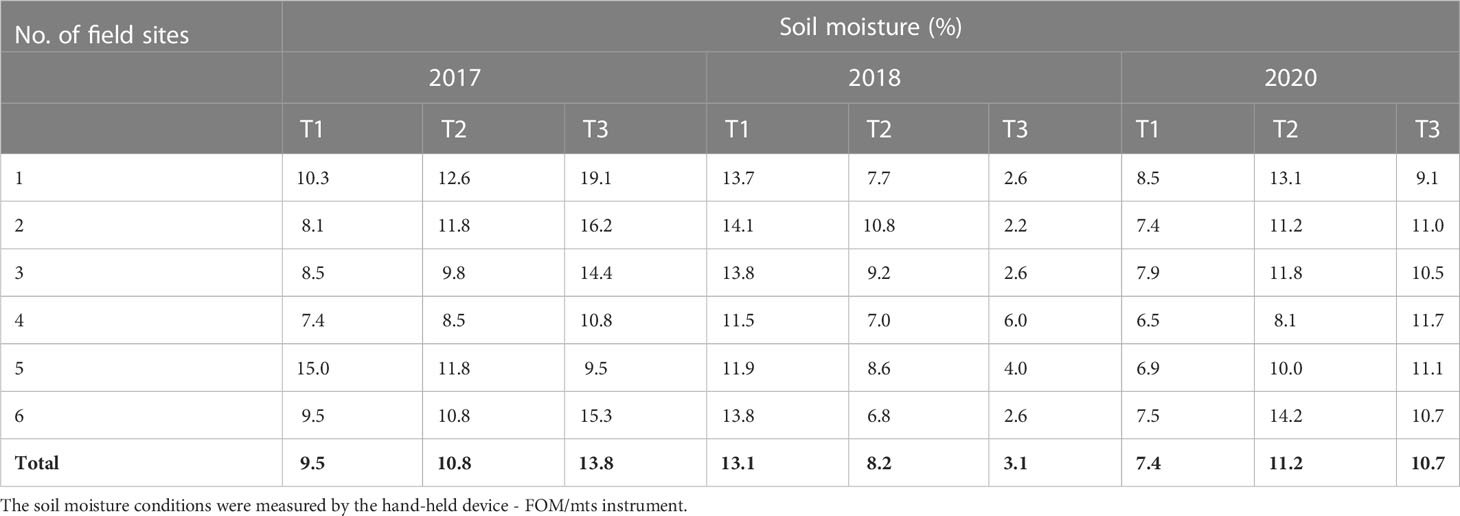
Table 2 Soil moisture (volumetric water content) recorded (mean values) at six randomly chosen spots in the field.
Trait evaluation
Once plants reached full maturity, they were harvested, preserved, and then subjected to biometric analysis in which the following agronomic traits were evaluated: total biomass (g), root biomass (g), root length (cm), number of productive tillers, total number of tillers, plant height (cm), average length of spike (cm), average number of grains per spike (g), average weight of grains per spike (g), weight of grains per plant (g), and weight of a thousand grains (g). A complete list of the analyzed agronomic traits with their abbreviations as well as the methods used for their evaluation is provided in Table 3
Root characterization
At harvest, plants were cut at the soil level and dissected into two main components: roots and shoots. A hose fitted with a fine spray head was used to carefully remove the soil substrate, and the roots (from a 1 m deep piece of soil 1 m deep)were gently teased apart. Because the soil used in this experiment was highly dispersible, excessive manipulation was not required to remove soil particles which can often result in accidental loss of roots.
Electrical capacitance (picofarad—pF) was measured three times at each of the following stages: tillering stage (T1), stem elongation stage (T2), and heading stage (T3). As described by Chloupek et al. (2010) and Středa et al. (2012), capacitances of barley plants were measured using a 380193 Extech LCR meter (Extech Instruments Corporation, USA) at a frequency of 1 kHz. The ground electrode used was a sharpened stainless steel rod (15 cm length and 0.6 cm diameter) inserted up to 10 cm into the substrate, 5 cm away from the stem. The plant electrode was clamped to the stem 1 cm above the substrate level using a 0.5-cm-wide aluminum strip that bent the stem (Wu et al., 2022). A commercial EEG electrode gel was spread under the strip to maintain good electrical contact (Rajkai et al., 2005). Using a standardized methodological protocol, capacitance was measured as described by Středa et al. (2020).
GBS approach
The GWAS panel was genotyped using the GBS method as described by Poland et al. (2012). For DNA extraction, the barley accessions were grown in a growth chamber at the IPG PAS phytotron facility. Young (2-week-old seedlings) first leaf tissue was obtained from each individual from the plant panel and extraction was carried out using the Wizard Genomic DNA Purification Kit (Promega Corporation, Madison, USA) in accordance with the manufacturer’s protocol. In each tube, 3–5 leaves were pooled per genotype for biological replications. Purity of the DNA was checked using the NanoDrop ND-1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA), and its integrity was evaluated by agarose gel electrophoresis. Individual DNA samples were diluted to 20 ng/µl with sterile water and then shipped to LGC Genomics, Germany (http://www.lgcgroup.com).
For the GBS analysis, a pilot project was performed in order to assess the optimal enzyme combination, type of library, and number of reads per sample. PstI/MspI were selected as restriction enzymes (ddRAD approach). Briefly, 200 ng of each DNA sample (10 µl volume) was digested with the restriction enzymes and ligated to unique barcode adapters in the main project. Sequencing was carried out on an Illumina NextSeq 500/550 platform (Illumina Inc., USA), and several runs were performed to obtain ~3 M of 75-bp single-ended reads per sample. Data were quality-trimmed, and variant discovery was done with Freebayes v1.0.2-16 software (https://github.com/ekg/freebayes#readme). The obtained variants were further filtered based on the following criteria: (a) the read count for the locus must exceed eight reads, (b) genotypes must have been observed in at least 66% of samples, and (c) the minimum allele frequency across all samples must exceed 5%. No missing SNP data imputation was performed. The IBSC_v2 (https://plants.ensembl.org) genome assembly was used as the reference (Ensembl Plants rel. 49). The sequencing, variant calling as well as SNP filtering were provided by LGC Genomics.
Statistical analyses
All data were analyzed in Genstat for Windows 21st edition (VSN International, 2019) or R software. Phenotypic data were analyzed in Genstat 21 using analysis of variance (ANOVA) in a mixed linear model (MLM) with fixed effects of year (Y) and random effects of genotype (G) and of G × Y interaction. Heritability was estimated using the method of Cullis et al. (2006). Before analysis outliers were identified by the method of Tukey (1977) in box-and-whisker diagrams and removed from phenotypic data (19 observations).
LD, population structure, and kinship analysis
The kinship coefficient matrix (based on Dice similarity coefficients) was processed via a principal coordinate analysis (PCoA) and used for the hierarchical clustering of accessions to visualize the population structure. LD was estimated for each pair of markers as r2 value in linear regression, with one marker used as the response and another one used as the regressor. Based on the LD matrix, the hierarchical clustering of markers was performed in R software using the group average (UPGMA) agglomerative method.
GWAS analysis
GWAS was carried out by combining the phenotypic data for the 149 barley accessions (genotypic means in individual experiments) and the genotypic data generated using the GBS approach. It was carried out using the method developed by van Eeuwijk et al. (2010) and Malosetti et al. (2013), which allows for the interaction of genetic effects with the environment. The method is based on MLM with the population structure estimated by eigen analysis of the kinship matrix, and with the compound symmetry variance-covariance model used for environmental variation, as implemented in Genstat 21. P values for the effects were corrected for multiple testing using the Benjamini–Hochberg method. An effect was considered significant when the corrected P value was lower than 0.05.
Construction of LD blocks
Based on the significant markers from GWAS, clusters of markers, called LD blocks, were constructed as groups characterized by LD exceeding the mean LD within the chromosome. The second GWAS based on these LD blocks was performed using mean values over all experiments. The analysis of the effects of the LD blocks was carried out using the analysis of covariance in Genstat 21, with the variants within an LD block as the classifying factor and eigen scores of accessions as covariates, with P values corrected as in GWAS. Loci for each chromosome were named following the pattern described by N’Diaye et al. (2017) (with minor modification), as a combination of the prefix “LD-b” (LD block), the chromosome number, and the incrementing number index (1 to N, N being the total number of LD blocks).
Gene and SNP annotations
Positional and functional interpretation of GBS and GWAS results was carried out in relation to two annotation sources (Gene Ontology and InterPro database) provided in Ensembl Plants and the protein descriptions in the nr protein database in OmicsBox 2.1.2 (BioBam Bioinformatics, 2019). Genes corresponding to SNPs (harbouring them or located nearby) and marker translation effects were found using Ensembl Variant Effect Predictor (VEP) (McLaren et al., 2016).
Results
Weather conditions and soil moisture
Monthly precipitation (March–August) ranged from 9.8 to 127.1 mm (Table 1). Relatively high rainfall was recorded in two months of 2017 that are crucial for initial plant development (April and May). The highest mean air temperature for all experiments was recorded in the 2018 growing season (April–June). In addition, a decrease in precipitation was recorded in May in the same year (16.6 mm—the lowest mean value for this month from all studied years). The lowest mean precipitation value (2.6 mm) was recorded in April 2020, with rainfall recorded only 6 days a month. In contrast, this growing season was characterized by a relatively high monthly precipitation value in May (47.5 mm). Soil moisture conditions recorded by the FOM/mts instrument in the experimental field are shown in Table 2. In 2017, soil moisture was low in the T1 stage compared with that in 2018 and 2020, and then, the mean values increased, reaching the highest of all studied years (13.8%) in the T3 stage. In 2018, the mean soil moisture was high in the initial development phase, but then decreased to 3.1% during the heading stage. The variation in soil moisture during the growing season in 2020 was similar to the pattern observed in 2017, and the highest mean value of soil moisture was observed in the T2 stage during stem elongation. Similarly, the lowest mean soil moisture was observed at the T1 stage in 2020 (7.4%).
Phenotypic distribution of yield- and root-associated traits
A total of 149 accessions were evaluated in triplicate under field conditions in 2017, 2018 and 2020 at IPG PAS. Eleven agronomic traits were analyzed by GWAS to identify new candidate loci associated with root traits.
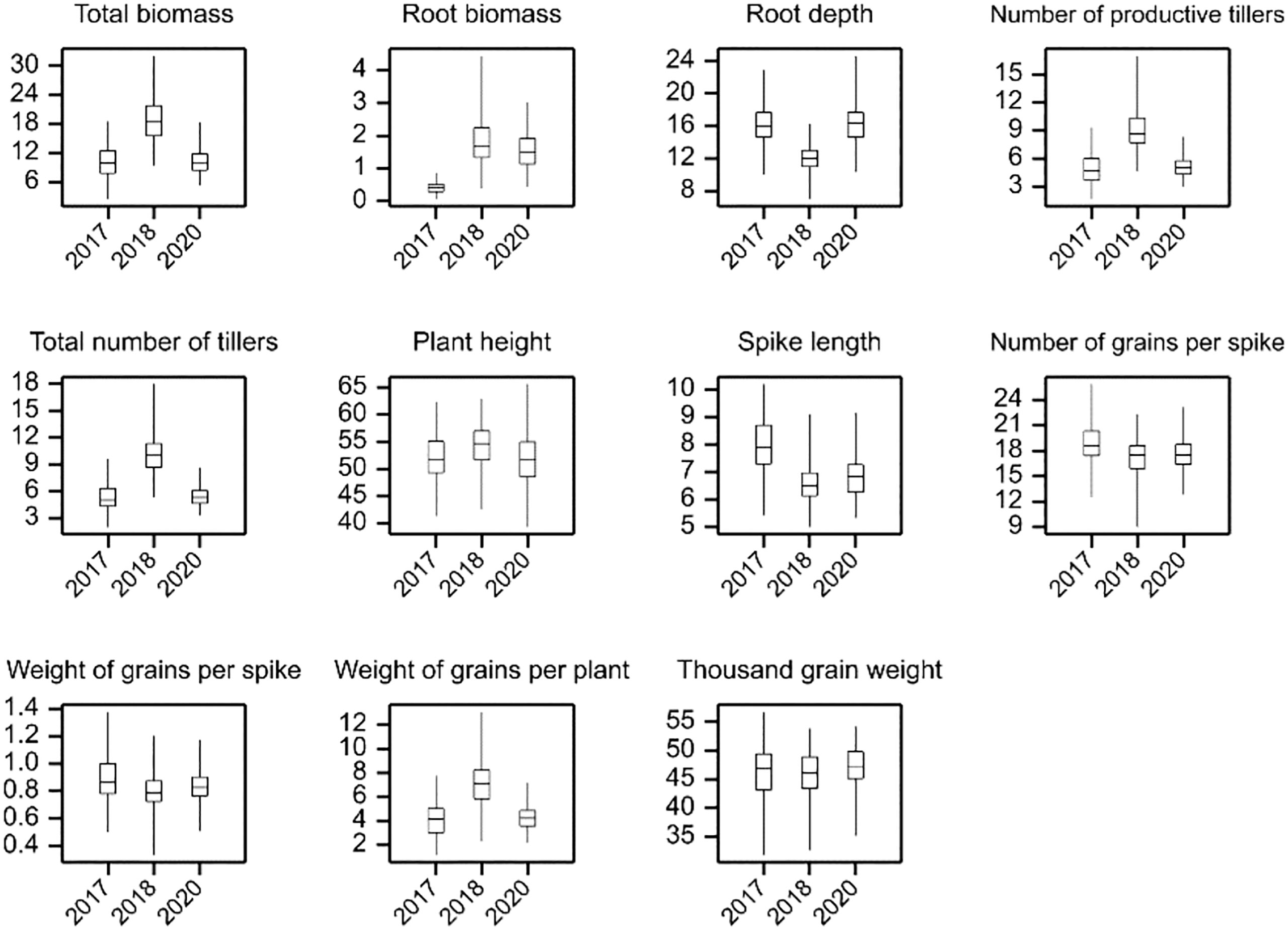
The distribution of the analyzed traits and the results of the comparison of annual means are shown in Figure 1 and Supplementary Table 3, respectively. The yield-related traits mean values recorded for all genotypes are provided in Supplementary Table 4. In the studied years, the highest mean spike length, number of grains per spike, and weight of grains per spike were observed in 2017. On the other hand, in the same year, the mean values of total biomass, root biomass, number of productive tillers, total number of tillers, and weight of grain per plant were lower compared to the next two years of the experiment. A higher mean value of traits related to tillering process (number of productive tillers and total number of tillers) was recorded in 2018. As a result, the total biomass and weight of grains per plant reached the highest mean value in 2018 (18.83 g and 7.16 g, respectively). The mean values of total biomass and root biomass were the highest in 2018, when growing conditions contributed to the better development of the upper part of the plants (shoots). However, the lowest mean root depth was measured in 2018 (11.89 cm), while the mean values for this trait were similar in 2017 and 2020 (16.09 cm and 16.24 cm, respectively). Electrical capacitance measurements on roots
The results of electrical capacitance measurements that were carried out in all years of the experiments are shown in Figure 2. The highest mean values for trait E1-3 were recorded in 2018. Slightly lower E1-3 mean values were recorded in 2017, when root biomass was lowest, compared to the mean values recorded in 2020. Due to large differences between years in terms of both mean values and dispersion of genotypic means, electrical capacitance observations were centered and normalized within the years using observed means and standard deviations.
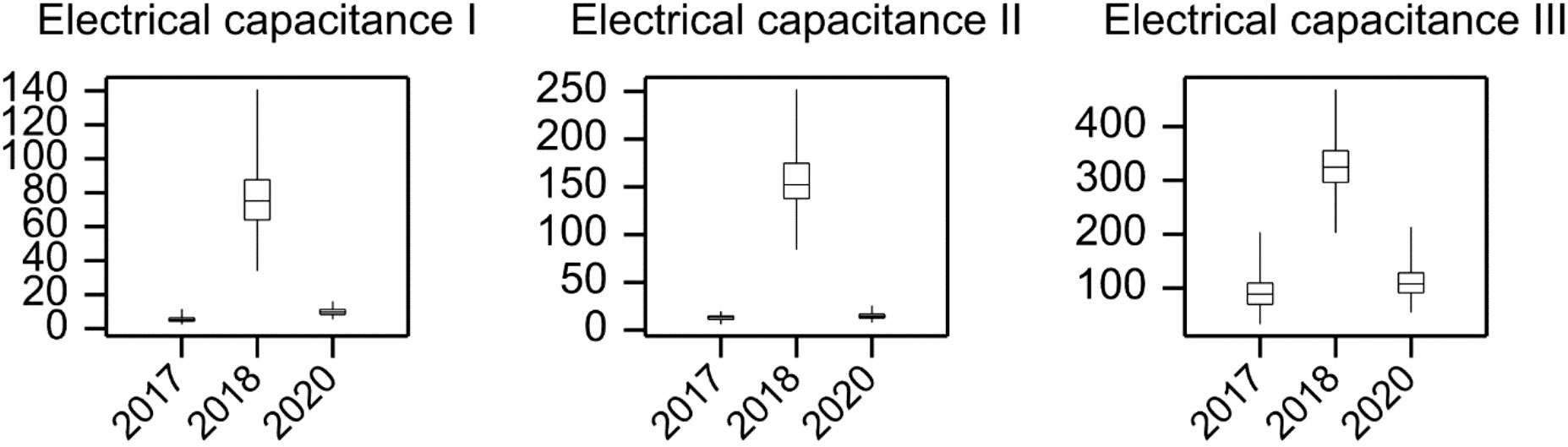
Figure 2 Electrical measurement results for the studied accessions. Electrical capacitance (pico farad – pF) was measured three times - in tillering stage (T1), stem elongation stage (T2) and heading stage (T3).
Analysis of variance and correlations between root- and yield-related traits
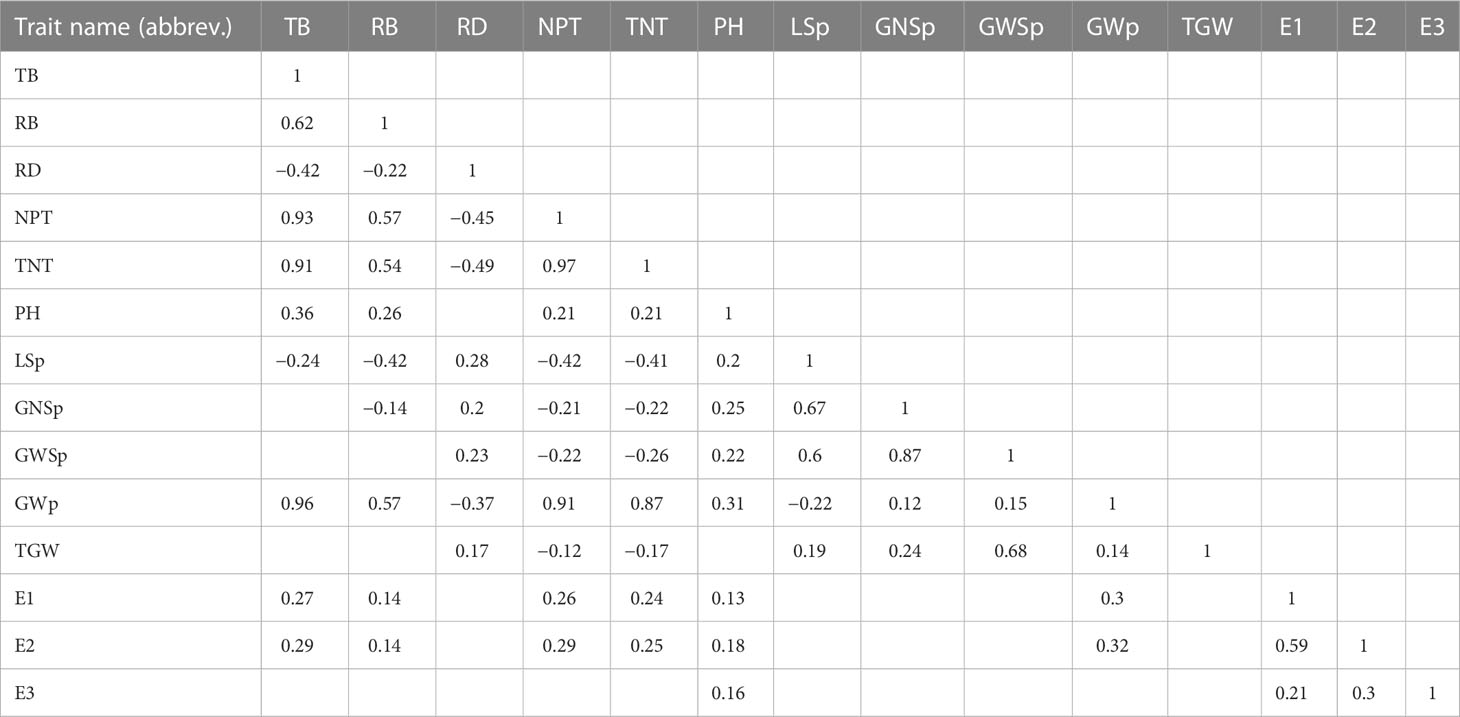
ANOVA based on data from three years of evaluation showed that the variation among genotypes was relatively larger for the traits PH and TGW than for the traits RB and GWSp (Supplementary Table 5). The estimates of the genotypic variance were smaller than the corresponding G × Y interaction variance for almost all traits (except for PH). Root traits (RB and RD) were most affected by G × Y variance. Broad-sense heritability estimates ranged from 7.0% (for RB) to 60.6% (for PH). Strong positive correlations (P value < 0.05) were observed between traits associated with tillering (NPT and TNT) and TB (correlation = 0.93 and 0.91, respectively) (Table 4). TB also showed a significant correlation with GWp (0.96). A negative correlation was found between RD and traits related to plant biomass (TB and RB) and between RD and tillering traits (NPT and TNT). A weak positive correlation was recorded between TB and both E1 and E2 (0.27 and 0.29, respectively).
Genotyping and population structure
Genotyping of the GWAS panel using the GBS method revealed a total of 5739 SNPs (Supplementary Table 6). The physical location (Figure 3) of the SNP markers shown their uneven distribution on and within the barley chromosomes. Wider gaps in marker coverage were observed on chromosomes 1H, 2H, 4H, and 7H, whereas the distribution of SNP markers was more uniform on chromosomes 3H, 5H, and 6H. The number of SNP markers on individual chromosomes ranged from 569 (chromosome 1H) to 977 (chromosome 7H). The population structure (Figure 4) was visualized by PCoA on the kinship (coancestry coefficients) matrix of accessions that were derived from all 5739 SNP markers. PCoA did not divide the accessions into clear clusters; however, some subgroups were observed. Cultivar accessions were mainly grouped on the right side (positive values of PC1), and evenly distributed groups of breeding lines overlapped with the subgroups of cultivars. PCoA separated some breeding lines (offspring with parents) from the rest of the genotypes. As shown in Supplementary Figure 1, the dendrogram obtained by hierarchical clustering based on the kinship matrix confirmed the results of PCoA: the barley breeding lines and cultivars that were used as parents/donors at some stage of the breeding process formed groups (e.g., CamB and the progenies such as MCam53, MCam75, and MPW15.4).
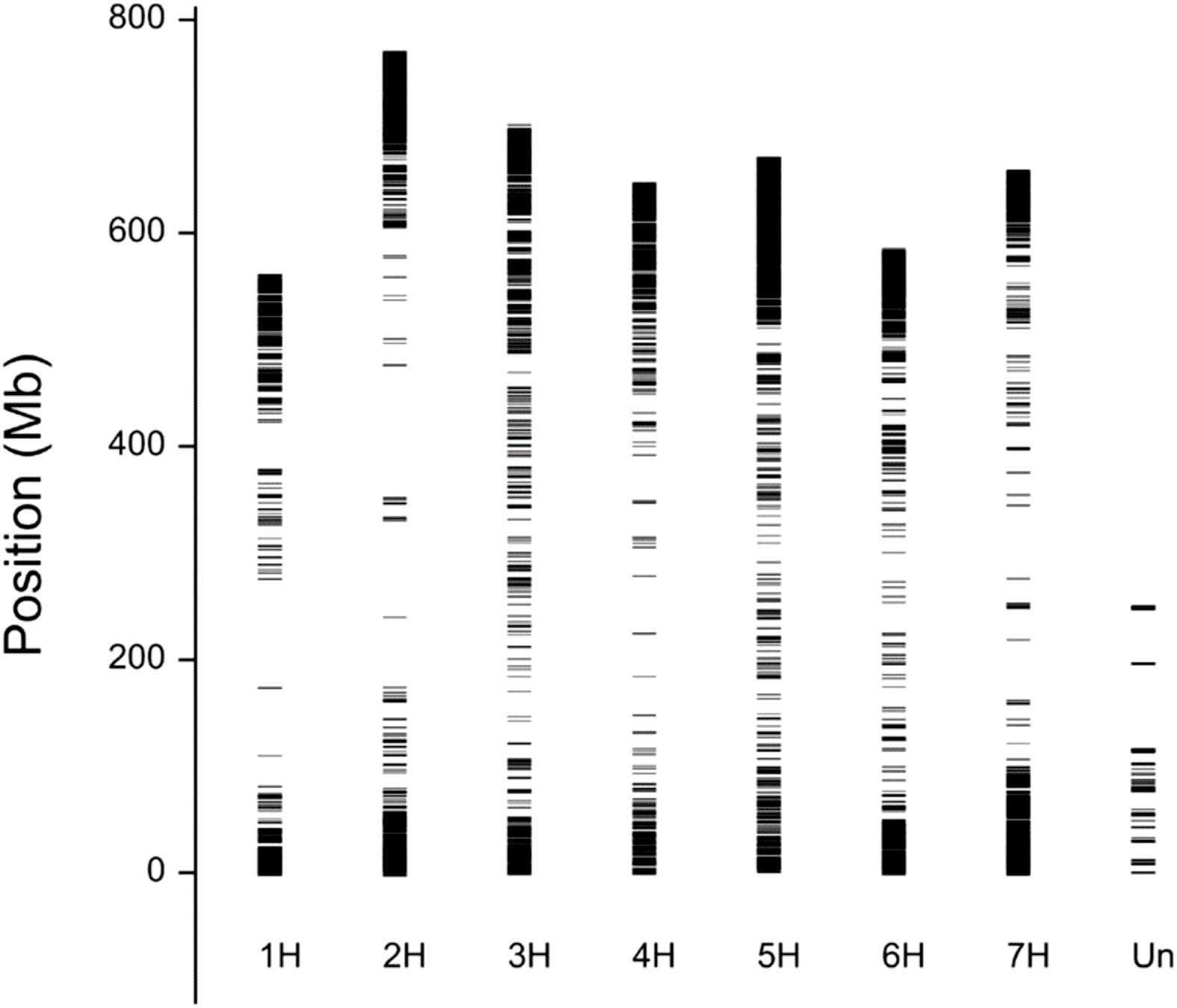
Figure 3 Physical mapping of 5739 SNP markers on barley chromosomes. Marker coverage is lower near the centromere and better further from the centromere, closer to telomere. The reason is not in the method, but in the fact that polymorphisms occur more often closer to the ends of the chromosomes than near to the centromere.
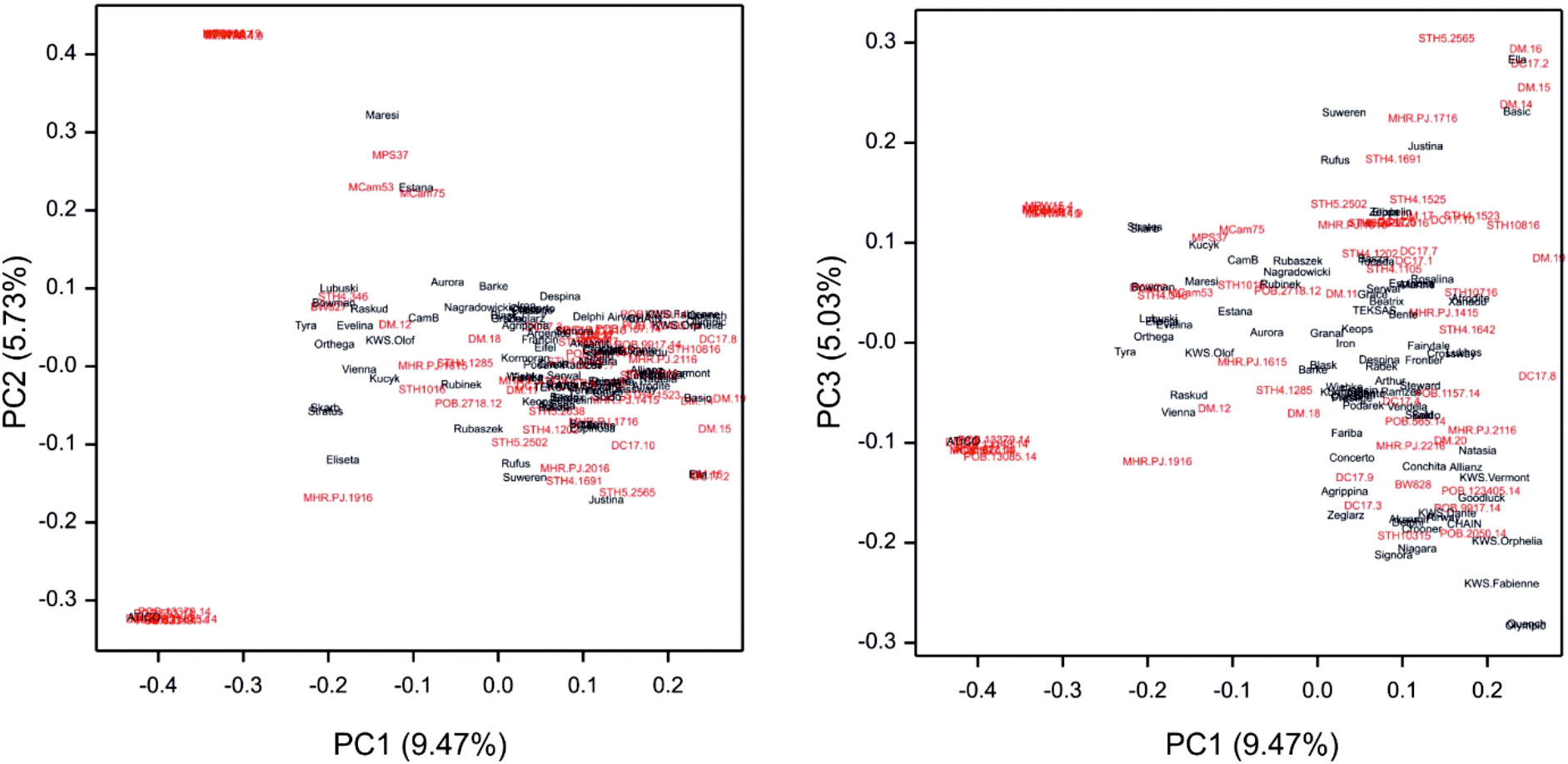
Figure 4 Visualization of the population structure by PCoA of the kinship coefficient matrix for accessions (cultivars – black, breeding lines – red) based on SNP markers. Cultivar accessions were grouped on the right side and evenly distributed groups of breeding lines overlapped with the subgroups of cultivars.
Genome-wide association analysis
A total of 690 marker–trait associations (MTAs) were detected for 8 of 14 analyzed traits at a significance threshold (corrected P value) of 0.05 (Table 5). Manhattan plots depicting the significant SNP markers above the threshold are shown in Figure 5. The highest number of associations was identified for RB a total of 246 MTAs distributed across the whole barley genome, with the majority (61) on chromosome 4H and the lowest on chromosome 6H (Supplementary Table 7). Of the 690 MTAs, 91 were linked to more than one trait (Supplementary Table 7). Five SNP markers on chromosome 7H were associated with four agronomic traits (Table 6): RB, GNSp, GWSp, and TGW (all with negative effects). The marker SNP 2H_759960282 located on chromosome 2H had a positive effect on RB, PH and GNSp, and a negative effect on GWSp. The lowest percentage of significant markers demonstrating interaction with the environment was for TGW (29%), and the highest for TB and LSp (100%), however it was for few associations (3 and 6, respectively). It is worth to mention that 94% of significant marker effects for RB (from 246 associations) were showing interaction with the environment (Table 5), which is in agreement with low heritability and large interaction for this trait revealed by ANOVA; for majority of markers this interaction could be attributed to a larger allelic effect in 2018 (Supplementary Table 7).
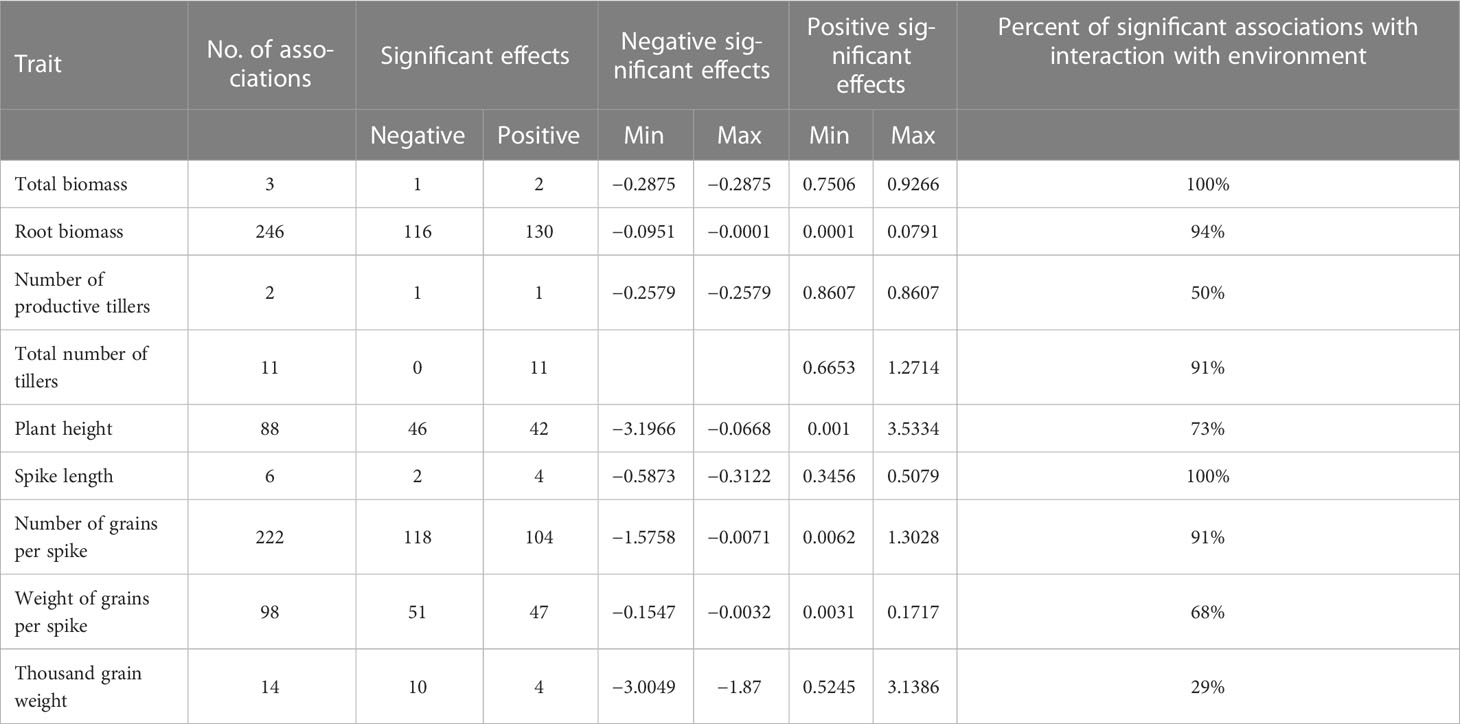
Table 5 Characteristics of allelic effects for SNP markers significantly associated with phenotypic traits.
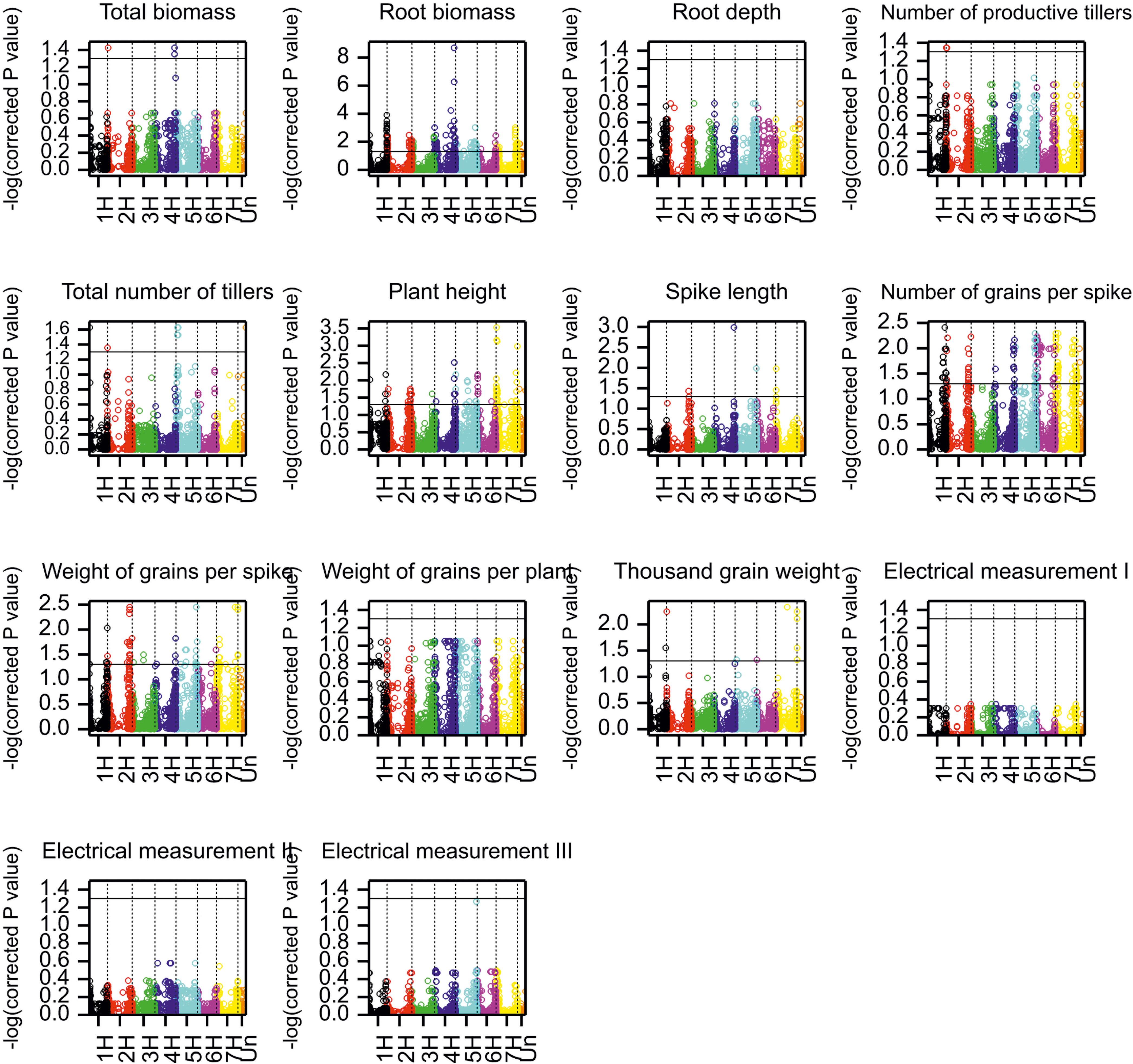
Figure 5 Manhattan plots for the associations of markers with phenotypic traits. Black horizontal line indicates −log(0.05), the significance threshold for log-transformed Benjamini-Hochberg corrected P values.
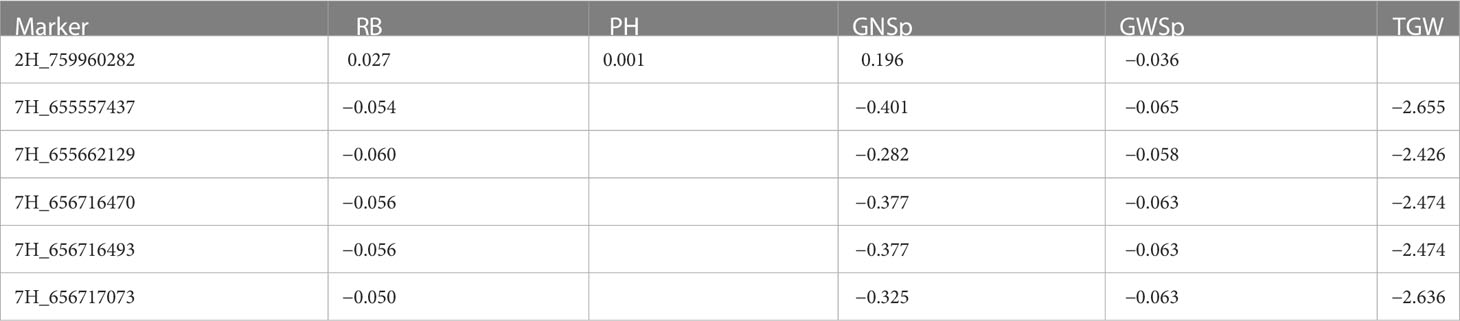
Table 6 Markers significantly associated with four phenotypic traits and their allele substitution effects.
Distribution of LD blocks
Genetic dissection of associations for complex traits was performed using an LD-based haplotype approach. As shown in Supplementary Table 8, a total of 59 LD blocks containing 2–32 SNPs were created as described in the Materials and Methods section. The allele combinations were further filtered based on the following criteria: the minimum number of occurrences of a given combination is eight and LD blocks with at least two combinations remain for further analysis. Therefore, the number of allele combinations varied from two to six for the LD blocks. The highest number of LD blocks was identified on chromosome 2H, whereas the lowest number of blocks on chromosome 3H excluding Un (Supplementary Table 9). The largest number of LD blocks was associated with trait PH (12), whereas the lowest number was linked to TGW (2). Seven LD blocks were associated with each of the traits RB, GNSp, and GWSp. No associated LD blocks were identified for the traits TB, RD, NPT, TNT, LSp and GWp.
Yield-related traits
Plant height
Twelve LD blocks showed significant association with PH. Their distribution on chromosomes was as follows: one on 1H, three on 2H, two on 4H, one on 6H, four on 7H, and one on Un. For the LD-b_7H_55 block, one SNP combination (T/T C/C C/C A/A A/A C/C) resulted in the largest increase in PH (3.09 cm) among three associated marker combinations. This combination was observed in the smallest number of accessions – 9 out of 135. The largest decrease in PH (4.01 cm) was identified for the combination G/G G/G C/C C/C A/A G/G C/C G/G C/C A/A of LD-b_4H_31 block.
Grains number per spike
Seven LD blocks were associated with GNSp, three of which were located on chromosome 2H, three on chromosome 4H, and one on chromosome 7H (LD-b_7H_58). Two LD-b_7H_58 SNP combinations resulted in the largest decrease in GNSp (1.29–1.31 g). LD-b_4H_31 included six SNP combinations, of which the combination C/C G/G C/C C/C A/A G/G C/C G/G C/C A/A resulted in a GNSp increase of 0.70 g.
Grains weight per spike
Six associations (except one on Un) were identified for GWSp on chromosomes 2H, 3H, and 7H. The SNP combination of the block LD-b_7H_58, which included nine accessions, showed the largest negative allelic effect (−0.11 g). The marker combination (T/T G/G) linked to the GWSp block (LD-b_2H_17) resulted in the largest increase of GWSp (0.1 g).
Thousand-grain weight
Two LD blocks were detected for this trait. The largest negative allelic effect (−3.08 g) was found for one of two SNP combinations of LD-b_1H_3. The SNP combination (A/A A/A) of LD-b_2H_6 resulted in the largest increase of TGW (1.56 g).
Root-related traits
Root biomass
Seven LD blocks significantly associated with RB were identified on chromosomes 2H, 4H, 5H, and 7H. In general, eight SNP combinations with positive allelic effects on RB were detected. Combination G/G A/A C/C of LD-b_4H_26 showed the largest allelic effect, resulting in 0.41g increase in RB compared to the general mean RB value of all accessions. This combination was identified in 8 of 146 accessions identified for this block. Twelve combinations with negative effects on the studied trait were identified with the largest decrease in RB (−0.25 g) for the SNP combination T/T G/G A/A of LD-b_7H_58 (Supplementary Table 9).
Marker translation effects on root biomass
VEP analysis revealed three SNP markers with both significant effects on RB and high translation effects (Supplementary Table 7). 1H_551858749 and 2H_21581482 markers showed significant positive effects on RB. The last SNP with a high marker translation (negative) effect was located in chromosome 6H (6H_551431916).
Root-biomass-associated LD blocks collocating with additional traits
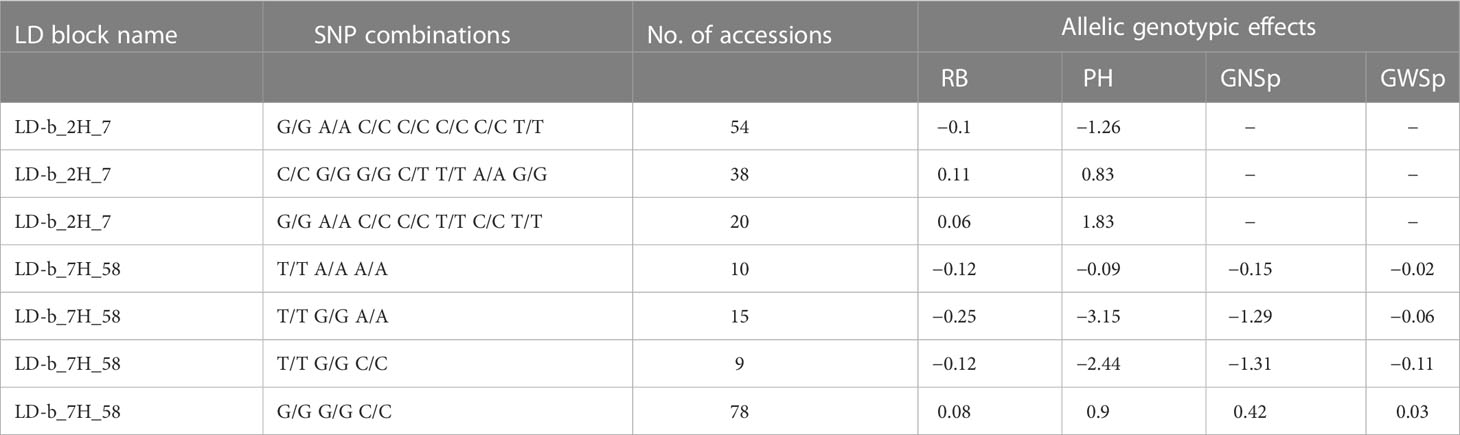
As shown in Table 7, two RB markers that were significantly associated with LD blocks were collocated with additional traits: LD-b_2H_7 was collocated with PH, while LD-b_7H_58 with PH, GNSp and GWSp. LD-b_2H_7 associated with RB also collocated with PH, whereas on chromosome 7H, an SNP combination (G/G G/G C/C) of block LD-b_7H_58 was observed, resulting in an increase in all four traits (RB, PH, GWSp and GNSp). The allelic effects of SNP combinations within LD-b_2H_7 and LD-b_7H_58 are shown in Figure 6. The allelic effects of SNP combinations identified for LD blocks related to both RB- and yield-related traits were larger than those defined for individual SNP markers. Moreover, in some cases (e.g., LD-b_7H_58), the SNP combination had the opposite allelic effect compared with the effects of the individual markers. For the vast majority of these SNP markers, positive interaction with the environment was recorded (e.g., LD-b_2H_7—interactions were found for six of seven markers).
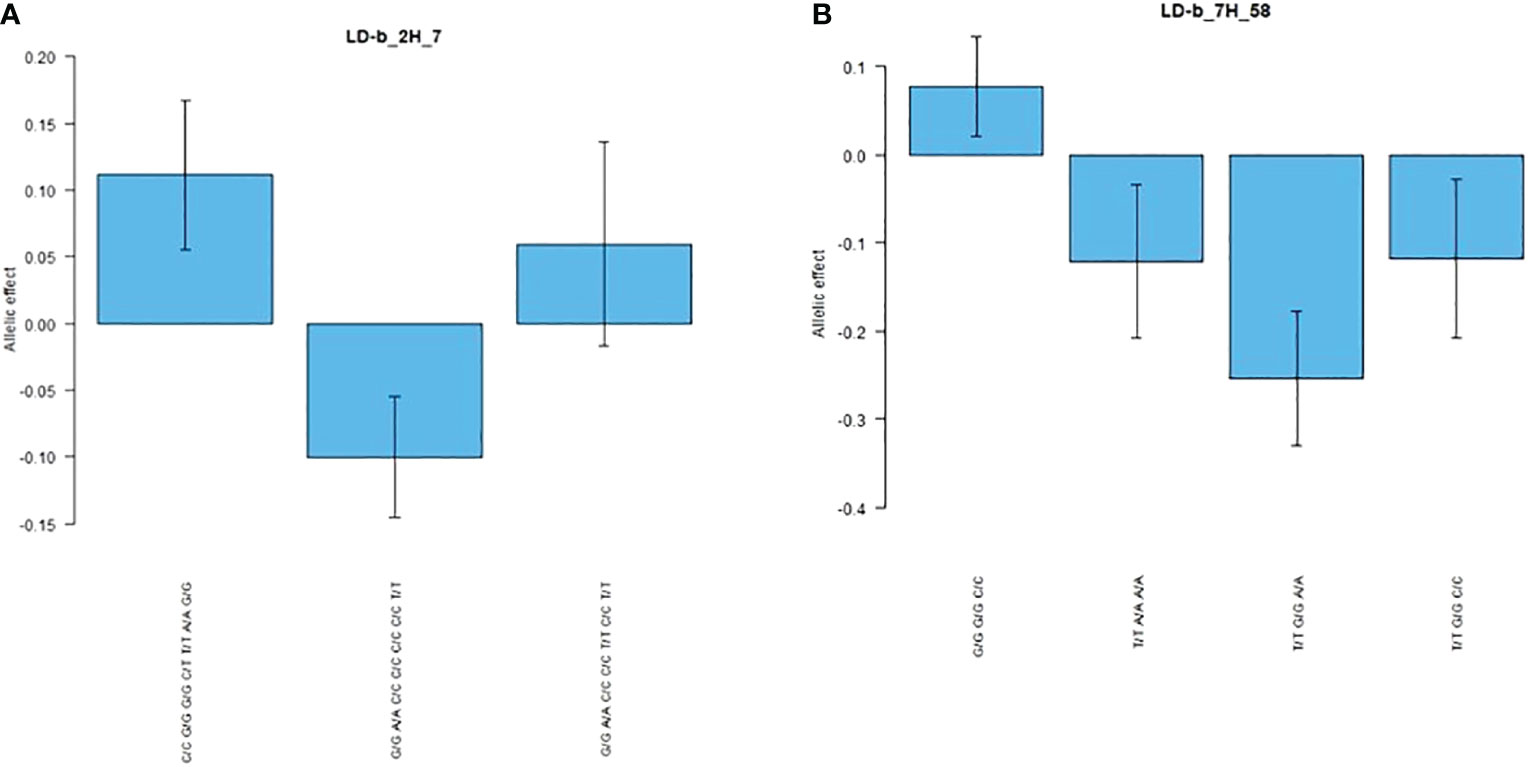
Figure 6 Allelic effects of genotypic combinations within (A) block LD-b_2H_7 and (B) block LD-b_7H_58 for RB trait.
Gene annotations of SNPs associated with RB
The results obtained from functional annotation using different annotation tools for all GWAS results are presented in Supplementary Table 7. Data from three annotation sources, namely Gene Ontology, nr database and InterPro database, provided in Ensembl Plants were collected for SNPs with significant associations for RB with LD blocks (collocated with other yield-related traits; Table 8) and for SNPs with both significant positive effects for RB and high translation effects (Table 9). The annotations belonged to several functional categories, namely protein kinase, transcription factors, or other stress-related candidates, while some of them had unknown functions. For some SNP markers, identical annotations were recorded due to their linkage to the same gene ID.
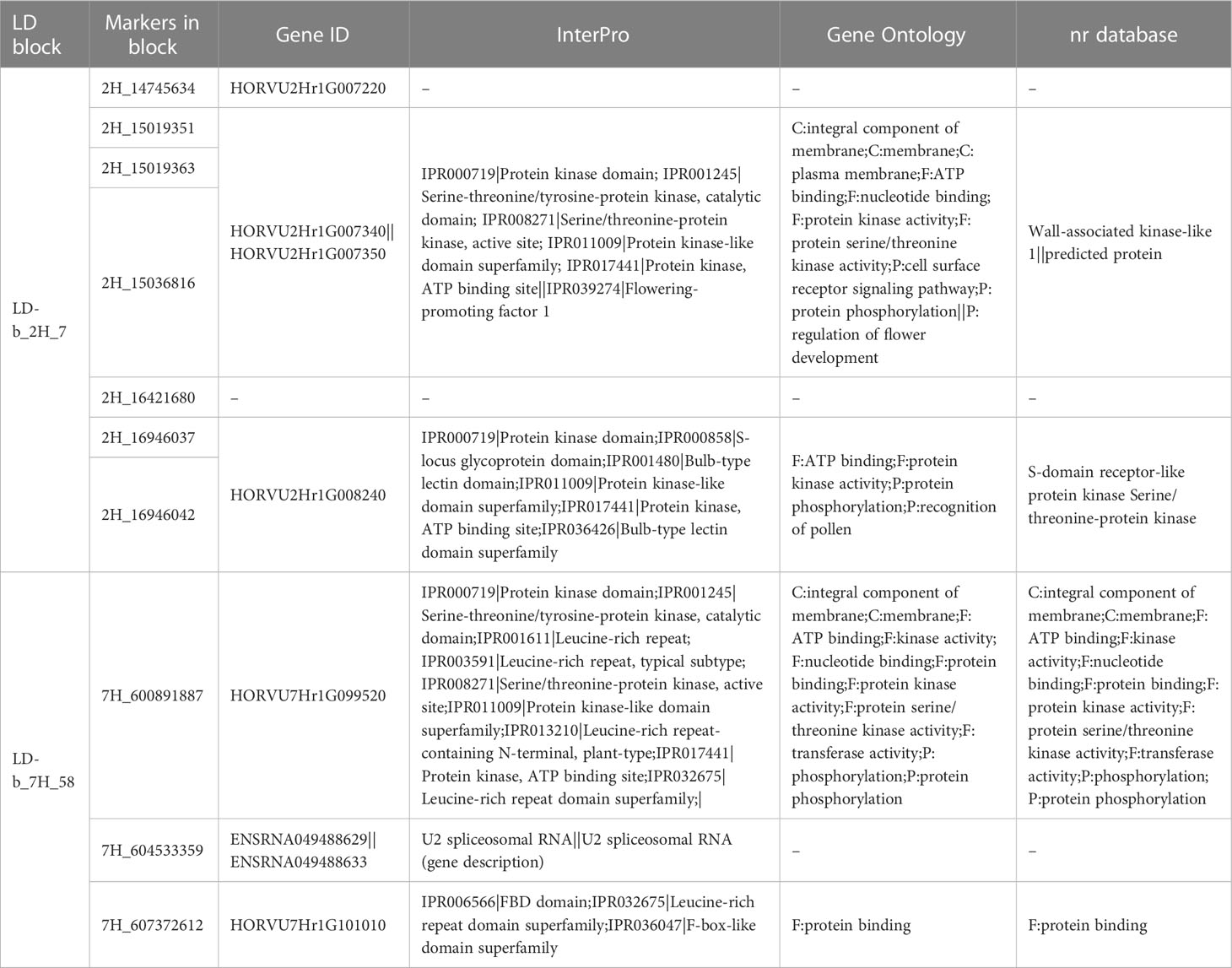
Table 8 Selected gene annotations for LD blocks linked concurrently to root- and yield-related traits.
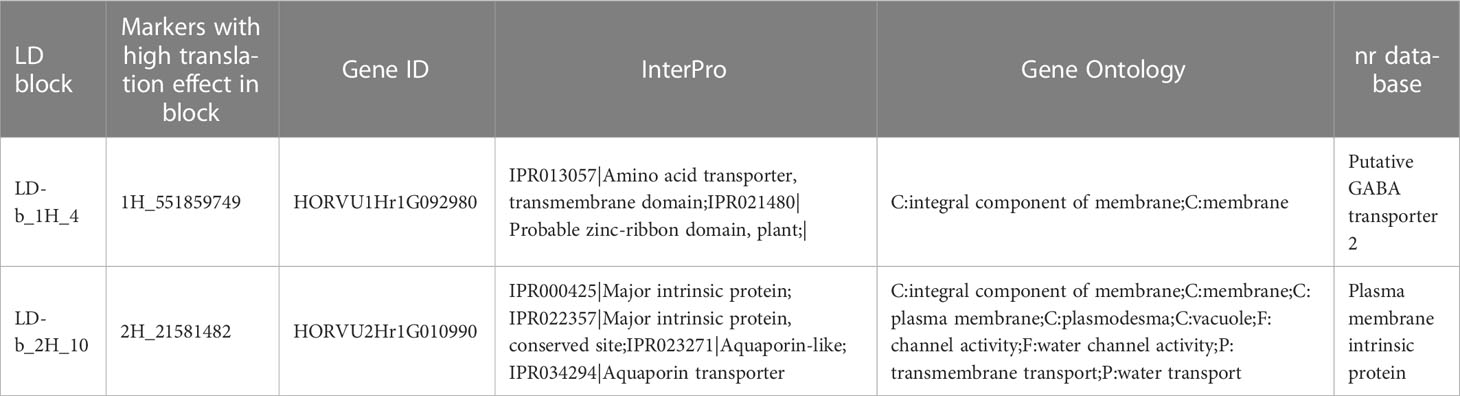
Table 9 Selected gene annotations for SNPs with both significant positive effects for RB and high translation effects.
Discussion
In our previous studies conducted under field conditions, we used linkage mapping to map QTL to determine quantitative traits in spring barley (Ogrodowicz et al., 2020). It is, however, well known that fine-mapping QTL using a linkage analysis needs a population of thousands of individuals (He et al., 2017) and that the limited polymorphic loci between the two parents influence mapping accuracy. Although our accession panel included a sufficient number of plants, we additionally increased the efficiency of single-marker GWAS by using an LD-based haplotype approach to examine root- and yield-related traits in our spring barley panel. In the literature, there are many papers on GWAS for less than 150 plant genotypes, e.g., Ain et al. (2015) used 123 Pakistani historical wheat cultivars, Valluru et al. (2017) used 130 diverse wheat elite lines and landraces, Arora et al. (2017) used 114 wild wheat accessions, Lehnert et al. (2018) used 94 bread wheat genotypes, Ma et al. (2018) used a panel of 66 elite wheat genotypes, and Rohilla et al. (2020) used 94 deep-water rice genotypes. In recent years, association mapping has been widely used to detect quantitative trait loci (Reinert et al., 2016; Abdel-Ghani et al., 2019). In the present study, we used a differentiated accession panel comprising cultivars, recombinant inbred lines, breeding lines, and backcross lines. Nevertheless, the population structure analysis revealed two subgroups, which were clustered mainly in terms of origin; each group contained the donors (cultivars or mutants) used in the breeding processes - e.g., CamB and Maresi – parents in the breeding process of two RILs, namely Mcam53 and Mcam75; Bowman and the backcross line – BW827 (Mikołajczak et al., 2022).
The present study aimed to investigate the role of root architecture in plants grown under natural field conditions. We used the LD-based GWAS to investigate root- and yield-related traits in the spring barley panel. Although several studies have used GWAS to explore the diversity of barley (Nandha and Singh, 2014; Tsai et al., 2020), genetic analysis of root traits has not been commonly performed because of the difficulty in their phenotypic evaluation. Most research on this subject is conducted under greenhouse or growth chamber conditions because of the constraints in conducting field studies (Paez-Garcia et al., 2015). To the best of our knowledge, our study is the first to investigate the major variations in root and related shoot traits by using LD block-based GWAS in spring barley accessions grown under field conditions. Considering the difficulties in root phenotyping in the field, we also used the nondestructive root evaluation method to understand the root structure of the studied accessions.
Because of their ability to retain water and nutrients, roots and their architecture are considered the most important parameters for plant productivity and adaptation to drought stress. The process of development of roots of the same cultivar varies under different environmental conditions such as soil type, water and nutrient availability, and crop management practices (Motzo et al., 1992; Nevo and Chen, 2010; Wasson et al., 2012). Under low water conditions or periods, root plasticity can highly influence crop performance. Some cultivars can better respond to drought through additional root growth than other cultivars (Ehdaie et al., 2012). In some cases, the total root biomass of plants in a water-scarce environment might even increase as compared to that under well-watered conditions (Sharp and Davies, 1985; Thornley, 1998). Although several studies have been conducted to analyze the role of root architecture in different plant species (Ruangsiri et al., 2021; Wu et al., 2022), there remains a gap in our knowledge, particularly regarding how roots are affected by environmental factors (Freschet et al., 2013).
Phenotypic evaluation
In this study, phenotypic evaluation showed significant variations in root- and yield-related traits across the three studied years, thus indicating broad genetic and phenotypic variance within the studied GWAS panel. In 2017, under favorable rainfall conditions, plants did not develop many tillers; however, weather conditions contributed to the development of longer spikes, because of which high mean values of GWSp were recorded. In 2018, the mean values of traits that contribute to yield (TB, RB, NPT, GWp, etc.) and tillering showed a significant increase. In the same year, plants developed much higher root biomass than that in the other two years. Concurrently, the lowest mean root depth values were observed in 2018, which indicated that the studied plants did not develop long roots to search for water resources in the soil. The soil moisture content recorded at the tillering stage (T1) was the highest, which directly contributed to the development of tillers. Under these conditions, plants did not develop deep root systems as the existing root structure allowed sufficient access to water resources. In 2018, a rapid decrease in soil moisture content was recorded only in the heading phase, when the studied plants were mainly in the maturation stage. According to the “balanced growth” hypothesis (Bloom et al., 1985), some plants respond to drought by stimulating or maintaining root growth while reducing shoot growth. In other words, plasticity may be a critical strategy adopted by plants to respond to unstable water conditions. Deep roots that facilitate water acquisition from deeper areas of soil may be particularly important for smaller plants such as wheat, rice, and barley; however, this aspect is generally beneficial for plants that grow under limited soil water conditions in agricultural and natural systems (Henry et al., 2011). Even if the soil becomes completely dry at the surface, water may be available deeper in the profile, which is inaccessible to many agricultural species. Consequently, these species must develop deeper root systems to access this water profile. In contrast, high soil water content or soil density may reduce the root elongation rate and the number of lateral roots, which may also be attributed to a reduction in shoot growth (Bengough et al., 2011), as indicated by the results of the present study in relation to the plant development model observed in 2017.
Content and weather conditions provided information on the dynamic changes in water availability during the course of the field study. The average monthly precipitation values varied across the three years of our investigation; for example, extremely low number of days with rainfall were recorded in 2020. These differences in monthly weather conditions across the year influenced the yield performance of the studied plants to some extent. The studied accessions were not as stable as those grown under greenhouse conditions, but they were rather subject to dynamic, rapid changes in the environment. The monitoring of soil moisture content showed that spring drought occurred under field conditions in both 2017 and 2020, even though rainfall was relatively high in 2017. However, as several constraints limit water use, the use of water resources by plants is not solely dependent on rainfall (e.g., Gavrilescu, 2021).
In this study, the highest values for electrical measurements were observed in 2018, when accessions developed many tillers and both total and root biomass increased. As previously reported, a strong correlation exists between the electrical capacitance of the root and the weight of the root system in crops (Cseresnyés et al., 2018). Therefore, the probability of selecting a larger root system based on electrical capacitance is high. In recent years, root system size (measured by electrical capacitance) has been considered as a criterion for selecting drought-tolerant genotypes in crops, such as spring barley (Chloupek et al., 2010; Svačina et al., 2014) and winter wheat (Abdel-Ghani et al., 2013). We also applied the electrical capacitance method to evaluate the root architecture of barley grown under field conditions. Brown et al. (2012) showed that roots with a diameter of 0.25 mm accounted for almost 95% of the total root length. Thus, a rapid, efficient, and nondestructive method must be used for root system assessments in order to investigate the relationship between root properties and yield. An undeniable advantage of the electrical capacitance method is that it can measure even the finest root structures (root hairs) because it is based on biophysical principles.
Almost all common methods used to observe the below ground parts of the plant have their own advantages and disadvantages (Freschet et al., 2021). Some approaches employed wet-sieving method in a greenhouse (Hudek et al., 2022) and under field conditions (Gan et al., 2009) or monolith excavation method under field condition (Mamedov and Husiyev, 2022). In this study, we excavated the monolith of soil (1 m deep and 0.5 m width) containing a representative root zone of each genotype, since, as reported for barley, wheat or corn, the most of plant roots are concentrated in depth level ∼50-100 cm (ARD, 2013). Roots were then carefully extracted from the soil by washing and drying following the procedure of Gan et al. (2009) (with minor modifications) procedure and then measurements were performed. The results from ANOVA in our study showed that root-associated traits were significantly affected by the G × Y interaction, which was confirmed by the relatively low broad-sense heritability values estimated for RB and RD. Some previous studies have shown that many root-related traits such as root biomass and root length remain relatively stable across different environments (Pantalone et al., 1996; Liu et al., 2005). On the other hand, the root system of plants is influenced by management and environmental conditions and is dependent on the genotype, as plants respond to nutrient limitation and water deficit in soil by increasing their root biomass, and thus the ratio of root to shoot biomass (Abdel-Ghani et al., 2013). Moreover, previous studies have indicated that traits associated with root traits, such as RB and RD, regulate the root architecture, which is essential for successful soil exploration (Falik et al., 2012). In our study, root development was influenced by both dynamic changes in weather and soil moisture conditions and genetic interactions.
The results of the study showed a negative correlation between RD and traits linked to plant biomass (TB and RB), which is consistent with the observations of Sierra Cornejo et al. (2020) who reported that plants develop longer roots to explore the deepest layers of soil where water can still be available. Our study also found a strong positive correlation between RB and traits linked to tillering. Excessive tillering resulted in the formation of a higher number of nodal roots, suggesting that the dependence of the development of shoot and root is in line with the strong positive relationship recorded for RB and NTP with TNT. Among the studied traits, a subgroup of plants can be identified with a similar RB—correlation rate with other traits. This phenomenon confirms the role of genetic background in the development of root architecture.
Marker–trait associations
In this study, the results of a LD-based GWAS for 11 phenotypic traits related to plant morphology, grain quality, and root apparatus in a collection of spring barley were presented. Small association panels were observed to increase both type 1 and type 2 error rates, resulting in the failure to detect true associations while producing a higher rate of false-positive associations (Sesia et al., 2021). The number of genotypes used in this study was similar to the one used for other genomic studies, yet we used a conservative approach of GWAS to reduce false associations. We used an MLM that corrects for population structure using kinship and detected 690 MTAs. The population structure was consistent with the known differences in pedigree and the source of the breeding program. In this study, a large proportion of MTAs (246) were found for the trait RB. These associations were distributed across all chromosomes; this confirms the quantitative architecture of the studied traits. Recently, the importance of root weight has been demonstrated in various studies (e.g., Mandozai et al., 2021; Zhao et al., 2021). Several investigations have shown that a deeper root system is influenced by dry weight and total length of the roots, which is important for improving drought tolerance as well as the final yield under drought stress in crops such as soybean (Hudak and Patterson, 1995) and rice (Suji et al., 2012). Root biomass accumulation is beneficial for drought escape as it could allow efficient uptake of soil water and nutrients (Danakumara et al., 2021). In general, plants growing in nutrient-deficient soils have higher root-to-shoot ratios, suggesting that more resources are allocated to root development, which enables the extraction of nutrients from a deeper and wider zone (Shen et al., 2018). Twenty-nine MTAs observed for RB were simultaneously associated with other yield-related traits. The association between markers and multiple root traits has also been demonstrated in other studies (Long et al., 2013; Chen et al., 2016). These results are well established as a result of the polygenic nature of the investigated traits and confirm the vital role of pleiotropy in synergistic biomass accumulation of roots in barley plants. The finding that MTAs detected in the studied barley panel are simultaneously responsible for root biomass and some yield-related traits suggests that root traits are controlled by multiple loci and highlights the importance of these genomic regions in root growth and architecture.
Analysis of LD blocks
LD blocks were constructed based on the average LD extent in our material for each chromosome. The LD-based approach has been shown to be a reliable tool for analyzing quantitative traits in different crops (N’Diaye et al., 2018). An advantage of this method is that it avoids an arbitrary or suggestive number of markers included in the block. Lorenz et al. (2010) and Contreras-Soto et al. (2017) have shown that the use of haplotype information allows identifying marker–phenotype associations and facilitates the genetic analysis of loci underlying complex traits. In our study, out of the significantly associated LD blocks, 58 contained two or more SNPs that are previously detected in GWAS. This indicates the advantage of haplotypes in the detection of multiple DNA variants. Seven LD blocks associated with the RB trait were found on chromosomes 2H, 4H, 5H and 7H. The largest number of RB blocks was located on chromosome 2H (three LD blocks). This is consistent with the study of Jia et al. (2019) which demonstrated the role of the region on chromosome 2H in shaping root architecture. In addition, these authors identified QTL on chromosomes 2H, 4H, 5H and 7H that were simultaneously associated with root- and yield-related traits. The identification of RB-associated LD blocks on the same chromosome in accordance with previous studies could confirm the associations found in the present study. QTLs that determine root fresh weight were previously found on chromosomes 1H, 2H and 5H (Xu et al., 2012; Broughton et al., 2015). Regions on chromosome 7H have been primarily identified as associated with root length (Ain et al., 2015; Xue et al., 2017), however, associations between the regions on chromosome 7H linked to root biomass have also been reported (Arifuzzaman et al., 2014; Arifuzzaman et al., 2017).
In the present study, two root-related LD blocks were of particular interest because of their colocalization and concomitant allelic effect on plant height and other yield-related traits. LD-b_2H_7 was associated with PH, whereas LD-b_7H_58 was related to three traits (PH, GNSp and GWSp) that directly contributed to yield performance. Colocalization of root architecture loci with other traits is a well-known phenomenon (Khasanova et al., 2019; Danakumara et al., 2021). The allele effect detected in this study is much larger in LD blocks than in single markers, indicating the superiority of LD-based GWAS. In addition, when using single markers, the chances of missing data are higher as only one vector of effects can be observed. When using LD blocks, we can observe both types of effects on the studied traits.
The LD block LD-b_7H_58 could be an interesting target for crop improvement, as it contributes similarly to RB and yield increase. However, this block also results in an increase in plant height, which could contribute to lodging with all the unfavorable consequences for crop yield (Kuczyńska et al., 2013), but the increase in PH is relatively smaller compared with the mean value of MTAs detected in GWAS for this trait. Therefore, LD-b_7H_58 is presumed to be an important and promising target for future validations. Another interesting LD block (LD-b_2H_7) was located on the short arm of chromosome 2H. Near this region, the PPD-H1 locus was identified as the major determinant of response to long photoperiods in barley (Turner et al., 2005). The PPD-H1 locus encodes the pseudo-response regulator (HvPRR37) gene which is orthologous to the Arabidopsis gene PRR7. This gene is part of the plant circadian clock and its activity increases the expression of VRN-H3, the main promoter of flowering, when photoperiods increase above 12 h (Turner et al., 2005; Campoli et al., 2012). Turner et al. (2005) identified an SNP (G/T) at the PPD-H1 locus, resulting in an amino acid change in the CCT domain, which is potentially responsible for the long photoperiod insensitivity. This has also been confirmed in a recent study (Sharma et al., 2020), suggesting that the region on the short arm of chromosome 2H plays an important role in shaping the root architecture. Many association-based studies with extensive germplasm collections have identified PPD-H1 as mainly responsible for variations in flowering duration (Russell et al., 2016; He et al., 2019). In addition to the timing of flowering, pleiotropic effects of PPD-H1 have been observed for many relevant agronomic and morphological traits, such as plant height, leaf size, root growth, and yield components (Ogrodowicz et al., 2017; Alqudah et al., 2018; Wiegmann et al., 2019; Ogrodowicz et al., 2020). PPD-H1 appears to have site-specific effects on yield-related traits, especially (but not only) in association with earliness. The responsive/sensitive allele of PPD-H1 has been associated with increased yield in sites where earliness is advantageous (e.g., early plants can escape drought at the end of the growing season). The yield effect can be explained by the pleiotropic effects of the responsive PPD-H1 allele. This allele shortens the total growing period, prolongs the time of grain formation, and increases grain size. On the other hand, the nonresponsive ppd-H1 allele has been shown to be associated with an increase in yield-related traits at sites where lateness is preferred to achieve higher yields (Wiegmann et al., 2019). However, the current long growing season which is characteristic of Northern Europe may increasingly change toward Mediterranean conditions as a result of climate change so that the ecological benefits of PPD-H1 may disappear in some regions (Herzig et al., 2018). In their study, Jia et al. (2019) found that some QTL associated with root system depth and root spread angle traits were located near the PPD-H1 genes. Root and shoot biomass showed high associations, including the functional SNP in PPD-H1, under osmotic stress and nonstress conditions (Valluru et al., 2017), which demonstrates the effect of HvPPD-H1 on barley root growth as well. Arifuzzaman et al. (2017) also found that the head date gene Vrn-H3 is significantly associated with shoot and root biomass; however, no associations were detected close to it in our GWAS panel. Even though the second promising LD block in the present study was indeed detected on the long arm of chromosome 7H, Vrn-H3 was found on the short arm of this chromosome. Recently, Voss-Fels et al. (2018) identified a context-specific interaction between flowering timing and root development, with early flowering lines with deeper root systems showing higher yield, especially under terminal drought conditions. These findings strongly prove the genetic link between roots and flowering time. Therefore, the regions associated with the abovementioned traits are important and promising targets for future validation in barley breeding.
Marker translation effects on root biomass
In this study, the Ensembl Variant Effect Predictor (VEP) (McLaren et al., 2016) was used to show the effects of translation in selected SNPs on root biomass. The VEP analysis revealed that two SNP markers had a high translation effect, which had simultaneously positive significant effects on RB. SNP 1H_551859749 is located within LD-b_1H_4 and is annotated as a putative GABA transporter 2; it functions as a signaling molecule in plants (Li et al., 2021). Several studies have demonstrated the vital role of GABA (gamma-aminobutyric acid) in drought response of plants. During drought, GABA accumulation is a stress-specific response that helps regulate stomatal opening to prevent water loss (Mekonnen et al., 2016). Various responses to drought have been observed in different plant species, such as increased chlorophyll content, osmoregulation, antioxidant enzyme activity, enhancement of nitrogen recycling, protection of photosystem II, wax biosynthesis, fatty acid desaturase activity, and delay in leaf senescence (Rezaei-Chiyaneh et al., 2018; Hasan et al., 2021). The other SNP with a high marker translation effect was 2H_21581482 within the LD block on chromosome 2H (LD-b_2H_10). This marker was annotated as an aquaporin transporter. Aquaporins are membrane channels that belong to the superfamily of large intrinsic proteins. They play an essential role in maintaining cellular water content and osmotic homeostasis in plants under both control and water-deficient conditions. These proteins are also associated with the tolerance of plants to biotic and abiotic stresses (Li et al., 2015). Overexpression of aquaporins significantly improves the development of the root structure and leads to better tolerance to drought stress in plants such as wheat (Ayadi et al., 2019) and Arabidopsis (Patankar et al., 2019). Although some studies have reported the versatile roles of GABA and aquaporin transporters (Wang et al., 2020; Li et al., 2021), the associations of these genes with root development in spring barley remain elusive.
Putative candidate genes for root-associated LD blocks
Eight genes harbored by the chosen LD blocks (LD-b_2H_7 and LD-b_7H_58) were discovered using selected annotation tools. The SNP markers within the block LD-b_2H_7 were associated with the following genes: HORVU2Hr1G007220, HORVU2Hr1G007340, HORVU2Hr1G007350, and HORVU2Hr1G008240. Wall-associated kinase-like 1 is a HORVU annotation associated with HORVU2Hr1G007340. No association was found for HORVU2Hr1G007220. Both HORVU2Hr1G007340 and HORVU2Hr1G007350 genes were associated with wall-associated kinase-like 1. Wall-associated kinases (WAKs) are receptors of the plasma membrane. They appear to be crosslinked with the cell wall material and can be detected in the cell wall by electron microscopy. WAKs are required for cell expansion and are involved in the response to pathogens. Their expression is activated by several environmental stimuli. According to the available data, WAKs play a role in cell expansion (Li et al., 2009). For example, they are required for cell elongation (Anderson et al., 2001; Wagner and Kohorn, 2001), which in turn is essential for root growth. Furthermore, in the present study, the direct association of HORVU with the flowering process was observed for the genes HORVU2Hr1G007340 and HORVU2Hr1G007350, both of which are associated with flowering promoting factor 1 (FPF1). To the best of our knowledge, this gene annotation has not been reported previously as the gene associated with both root growth and flower development in spring barley. This finding suggests that this region is associated with the previously mentioned spot on chromosome 2H that harbors flowering genes. AtFPF1 in Arabidopsis encodes a small 12.6-kDa protein that regulates flowering and is involved in gibberellin signaling. It is expressed in apical meristems immediately after the photoperiodic induction of flowering. Genetic interactions of this gene with flowering time genes and floral organ identity suggest that it may be involved in modulating the competence to flower. FPF1 is mainly expressed in roots and flowers and, to a lesser extent, in leaves. OsRAA1, a homolog of FPF1 in rice, showed 58% similarity to AtFPF1 at the amino acid level. Overexpression of OsRAA1 induces pleiotropic phenotypes in transgenic rice plants, such as altered leaf shape, heading time, and root development (Kohorn et al., 2009). This observation suggests a possible interaction between the patterns of flowering time and root development. The last gene annotation (S-domain receptor-like protein kinase) for LD blocks discovered on chromosome 2H was registered for the gene HORVU2Hr1G008240. Receptor-like kinase (RLK) is a large protein family that includes more than 600 genes in Arabidopsis and 1100 genes in rice. A possible role in pollen development has been demonstrated for a type of the RLK gene (LecRLK). Disruption of the gene LecRK-IV.2 leads to the formation of smaller pollens that remain stuck together and cannot be detached from the dehiscent anther, eventually resulting in male sterility (Smykal et al., 2004). In the present study, it is worth noting that the annotation and recognition of pollens were recorded for this gene. Although some level of relative expression of OsLecRK was found in both roots and plant tissues associated with anthers in rice (Peng et al., 2020), the functional associations of these genes with both root and pollen development have not been discovered previously in barley. During root growth and development, RLKs control many biological processes (Ou et al., 2021). Moreover, several RLKs have been identified as essential regulators of root hair development in Arabidopsis (Wei et al., 2018). The second LD-based block contained SNP markers associated with the following genes: HORVU7Hr1G099520, ENSRNA049488629, ENSRNA049488633, and HORVU7Hr1G101010. ENSRNA049488629/ENSRNA049488633 was annotated as a U2 spliceosomal RNA. In eukaryotic cells, pre-mRNA splicing is critical for processes such as expression of intron-containing genes, remodeling of protein–protein interaction networks, and regulation of transcript levels (Nilsen and Graveley, 2010). These important cellular functions are mediated by spliceosomes, which comprise five small nuclear ribonucleoproteins (nRNPs) and numerous non-nRNPs (Wahl et al., 2009). Plants are constantly exposed to various biotic and abiotic environmental stresses (Lamers et al., 2020). An interesting finding is that genes involved in stress responses are more likely to undergo an alternative splicing (AS) process (Laloum et al., 2018).
Therefore, AS in living plants may be a result of their adaptation to the new environment during land colonization (Mastrangelo et al., 2012). Thus, these annotations undeniably demonstrate the ability of plants to adapt to rapid changes in the internal and external environment, indicating that plasticity may be a defining feature of plant adaptation (Fritz et al., 2018). The next SNP markers within the LD block of interest were assigned to the following HORVU ID (HORVU7Hr1G099520 and HORVU7Hr1G101010) and were annotated, among others, as a serine–threonine/tyrosine–protein kinase/leucine-rich repeat domain superfamily. The annotation LRR receptor-like serine/threonine–protein kinase EFR was referred to as HORVU2Hr1G007220, indicating the possible links of this resistance gene with root development. Leucine-rich repeat receptor-like protein kinase (LRR-RLK) takes part in plant development and disease defense. In both plants and animals, cell surface receptors are involved in perceiving and processing external and internal signals that arrive at the cell surface. Many LRR-RLKs play an important role in both plant development and defense against pathogens because there is an overlap between these two pathways or because the same receptor recognizes multiple ligands (Oh et al., 2018). A gene that belongs to this gene family in A. thaliana—GSO1 in coordination with GSO2—regulates root growth by controlling cell division and cell fate specification and controls seedling root growth by modulating sucrose response after germination (Racolta et al., 2014).
Conclusions
In summary, the findings of our study offer new insights into the role of root traits in the yield performance of barley plants growing under natural field conditions where soil moisture varies day to day. The barley association panel analyzed in this study showed high variability in most of the agronomic traits. The results demonstrated the importance of the region on the short arm of chromosome 2H in the expression of root- and yield-related traits. Furthermore, the selected SNP markers with high translation effects showed great potential for further investigations of the adaptation of roots to drought. This study also highlighted the pleiotropic effect of the region with respect to heading time and other important agronomic traits, including root architecture. Because barley is widely considered as a model plant, the potential regions of interest identified in our study can be further validated to determine their application in barley breeding programs and to develop drought-tolerant genotypes in different species.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
Conceptualization, PO, KM and AK. Methodology, PO and PK. Software, MM. Validation, PO, MM, PK and AK. Formal Analysis, MM and PK. Investigation, PO and MK. Resources, PO and KM. Data Curation, MM and PK. Writing – Original Draft Preparation, PO. Writing – Review and Editing, KM, MM, PK and AK. Visualization, MM. Supervision, AK. Project Administration, PO, KM and AK. Funding Acquisition, PO, KM and AK. All authors contributed to the article and approved the submitted version.
Funding
Computations were performed in part with the support of Poznań Supercomputing and Networking Centre (http://www.man.poznan.pl). The research was supported by four projects: i) Ministry of Agriculture and Rural Development, Poland, grant no. HOR.hn.802.18.2018 task 106, ii) National Science Centre, Poland, project OPUS 12 no. 2016/23/B/NZ9/03548, iii) National Science Centre, Poland, project SONATA 12 no. 2016/23/D/NZ9/00042 and iv) National Science Centre, Poland, project SONATA 12 no. 2016/23/D/NZ9/00043.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpls.2023.1077631/full#supplementary-material
References
Abdel-Ghani, A. H., Kumar, B., Reyes-Matamoros, J., Gonzales-Portilla, P. J., Jansen, C., San Martin, J. P., et al. (2013). Genotypic variation and relationships between seedling and adult plant traits in maize (Zea mays l.) inbred lines grown under contrasting nitrogen levels. Euphytica 189, 123–133. doi: 10.1007/s10681-012-0759-0
Abdel-Ghani, A. H., Sharma, R., Wabila, C., Dhanagond, S., Owais, S. J., Duwayri, M. A., et al. (2019). Genome-wide association mapping in a diverse spring barley collection reveals the presence of QTL hotspots and candidate genes for root and shoot architecture traits at seedling stage. BMC Plant Biol. 19, 216. doi: 10.1186/s12870-019-1828-5
Ain, Q. U., Rasheed, A., Anwar, A., Mahmood, T., Imtiaz, M., Mahmood, T., et al. (2015). Genome-wide association for grain yield under rainfed conditions in historical wheat c,ultivars from Pakistan. Front. Plant Sci. 22 (6). doi: 10.3389/fpls.2015.00743
Alberta Agriculture and Rural Development (ARD) (2013) Alberta Irrigation management manual. Available at: http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/irr14310.
Alqudah, A. M., Youssef, H. M., Graner, A., Schnurbusch, T. (2018). Natural variation and genetic make-up of leaf blade area in spring barley. Theor. Appl. Genet. 131, 873–886. doi: 10.1007/s00122-018-3053-2
Anderson, C. M., Wagner, T. A., Perret, M., He, Z.-H., Kohorn, B. D. (2001). WAKs: cell wall-associated kinases linking the cytoplasm to the extracellular matrix. Plant Mol. Biol. 47, 197–206. doi: 10.1023/A:1010691701578
Arifuzzaman, M., Günal, S., Bungartz, A., Muzammil, S., Afsharyan, N. P., Léon, J., et al. (2017). Correction: Genetic mapping reveals broader role of vrn-H3 gene in root and shoot development beyond heading in barley. PloS One 12 (5), e0177612. doi: 10.1371/journal.pone.0177612
Arifuzzaman, M., Sayed, M. A., Muzammil, S., Pillen, K., Schumann, H., Naz, A. A., et al. (2014). Detection and validation of novel QTL for shoot and root traits in barley (Hordeum vulgare l.). Mol. Breed. 34, 1373–1387. doi: 10.1007/s11032-014-0122-3
Arora, S., Singh, N., Kaur, S., Bains, N. S., Uauy, C., Poland, J., et al. (2017). Genome-wide association study of grain architecture in wild wheat aegilops tauschii. Front. Plant Sci. 8. doi: 10.3389/fpls.2017.00886
Ayadi, M., Brini, F., Masmoudi, K. (2019). Overexpression of a wheat aquaporin gene, TdPIP2;1, enhances salt and drought tolerance in transgenic durum wheat cv. Maali. Int. J. Mol. Sci. 20 (10), 2389. doi: 10.3390/ijms20102389
Bardel, C., Danjean, V., Hugot, J.-P., Darlu, P., Génin, E. (2005). On the use of haplotype phylogeny to detect disease susceptibility loci. BMC Genet. 6, 24. doi: 10.1186/1471-2156-6-24
Bengough, A. G., McKenzie, B. M., Hallett, P. D., Valentine, T. A. (2011). Root elongation, water stress, and mechanical impedance: a review of limiting stresses and beneficial root tip traits. J. Exp. Bot. 62, 59–68. doi: 10.1093/jxb/erq350
Bhat, J. A., Salgotra, R. K., Dar, M. Y. (2015). Phenomics: a challenge for crop improvement in genomic era. Mol. Plant Breed. 6, 1–11. doi: 10.5376/mpb.2015.06.0020
BioBam Bioinformatics (2019) OmicsBox - bioinformatics made easy (Version 2.1.2). Available at: https://www.biobam.com/omicsbox.
Bloom, A. J., Chapin, F. S., III, Mooney, H. A. (1985). Resource limitation in plants- an economic analog. Ann. Rev. Ecol. Syst. 16, 363–392. doi: 10.1146/annurev.es.16.110185.002051
Broughton, S., Zhou, G., Teakle, N. L., Matsuda, R., Zhou, M., O’Leary, R., et al. (2015). Waterlogging tolerance is associated with root porosity in barley (Hordeum vulgare l.). Mol. Breed. 35, 27. doi: 10.1007/s11032-015-0243-3
Brown, L. K., George, T. S., Thompson, J. A., Wrifht, G., Dupuy, L., Hubbard, S. F., et al. (2012). What are the implications of variation in root hair length on tolerance to phosphorus deficiency in combination with water stress in barley (Hordeum vulgare)? Ann. Bot. 110, 319–328. doi: 10.1093/aob/mcs085
Campoli, C., Shtaya, M., Davis, S. J., von Korff, M. (2012). Expression conservation within the circadian clock of a monocot: natural variation at barley ppd-H1 affects circadian expression of flowering time genes, but not clock orthologs. BMC Plant Biol. 12, 97. doi: 10.1186/1471-2229-12-97
Chen, Y., Shan, F., Nelson, M. N., Siddique, K. H. M., Rengel, Z. (2016). Root trait diversity, molecular marker diversity, and trait-marker associations in a core collection of lupinus angustifolius. J. Exp. Bot. 67 (12), 3683–3697. doi: 10.1093/jxb/erw127
Chloupek, O. (1972). The relationship between electric capacitance and some other parameters of plant roots. Biol. Plant 14, 227–230. doi: 10.1007/BF02921255
Chloupek, O. (1977). Evaluation of the size of a plant's root system using its electrical capacitance. Plant Soil 48, 525–532. doi: 10.1007/BF02187258
Chloupek, O., Dostál, V., Středa, T. (2010). Drought tolerance of barley varieties in relation to their root system size. Plant Breed. 129, 630–636. doi: 10.1111/j.1439-0523.2010.01801.x
Comadran, J., Kilian, B., Russell, J., Ramsay, L., Stein, N., Ganal, M., et al. (2012). Natural variation in a homolog of antirrhinum CENTRORADIALIS contributed to spring growth habit and environmental adaptation in cultivated barley. Nat. Genet. 44 (12), 1388–1392. doi: 10.1038/ng.2447
Contreras-Soto, R. I., Mora, F., de Oliveira, M. A., Higashi, W., Scapim, C. A., Schuster, I. (2017). A genome-wide association study for agronomic traits in soybean using SNP markers and SNP-based haplotype analysis. PloS One 12 (2), e0171105. doi: 10.1371/journal.pone.0171105
Cseresnyés, I., Szitár, K., Rajkai, K., Füzy, A., Mikó, P., Kovács, R., et al. (2018). Application of electrical capacitance method for prediction of plant root mass and activity in field-grown crops. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.00093
Cullis, B. R., Smith, A. B., Coombes, N. E. (2006). On the design of early generation variety trials with correlated data. J. Agric. Biol. Environ. Stat. 11, 381–393. doi: 10.1198/108571106x154443
Dalton, F. N. (1995). In-situ root extent measurements by electrical capacitance methods. Plant Soil 173, 157–165. doi: 10.1007/BF0015552
Danakumara, T., Kumari, J., Singh, A. K., Sinha, S. K., Pradhan, A. K., Sharma, S., et al. (2021). Genetic dissection of seedling root system architectural traits in a diverse panel of hexaploid wheat through multi-locus genome-wide association mapping for improving drought tolerance. Int. J. Mol. Sci. 22 (13), 7188. doi: 10.3390/ijms22137188
Darrier, B., Russell, J., Milner, S. G., Hedley, P. E., Shaw, P. D., Macaulay, M., et al. (2019). A comparison of mainstream genotyping platforms for the evaluation and use of barley genetic resources. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00544
Dietrich, R. C., Bengough, A. G., Jones, H. G., White, P. J. (2013). Can root electrical capacitance be used to predict root mass in soil? Ann. Bot. 112, 457–464. doi: 10.1093/aob/mct044
Ehdaie, B., Layne, A. P., Waines, J. G. (2012). Root system plasticity to drought influences grain yield in bread wheat. Euphytica 186, 219–232. doi: 10.1007/s10681-011-0585-9
Elshire, R. J., Glaubitz, J. C., Sun, Q., Poland, J. A., Kawamoto, K., Buckler, E. S., et al. (2011). A robust, simple genotyping-by-Sequencing (GBS) approach for high diversity species. PloS One 6 (5), e19379. doi: 10.1371/journal.pone.0019379
Falik, O., Mordoch, Y., Ben-Natan, D., Vanunu, M., Goldstein, O., Novoplansky, A. (2012). Plant responsiveness to root–root communication of stress cues. Ann. Bot. 110, 271–280. doi: 10.1093/aob/mcs045
Famoso, A. N., Zhao, K., Clark, R. T., Tung, C. W., Wright, M. H., Bustamante, C., et al. (2011). Genetic architecture of aluminum tolerance in rice (Oryza sativa) determined through genome-wide association analysis and QTL mapping. PloS Genet. 7 (8), e1002221. doi: 10.1371/journal.pgen.1002221
FAO (2021). Available at: https://www.fao.org.
Freschet, G. T., Cornwell, W. K., Wardle, D. A., Elumeeva, T. G., Liu, W., Jackson, B. G., et al. (2013). Linking litter decomposition of above-and below-ground organs to plant–soil feedbacks worldwide. J. Ecol. 101, 943–952. doi: 10.1111/1365-2745.12092
Freschet, G. T., Roumet, C., Comas, L. H., Weemstra, M., Bengough, A. G., Rewald, B., et al. (2021). Root traits as drivers of plant and ecosystem functioning: current understanding, pitfalls and future research needs. New. Phytol. 232, 1123–1158. doi: 10.1111/nph.17072
Fritz, M. A., Rosa, S., Sicard, A. (2018). Mechanisms underlying the environmentally induced plasticity of leaf morphology. Front. Genet. 24 (9). doi: 10.3389/fgene.2018.00478
Gali, K. K., Sackville, A., Tafesse, E. G., Lachagari, V. B. R., McPhee, K., Hybl, M., et al. (2019). Genome-wide association mapping for agronomic and seed quality traits of field pea (Pisum sativum l.). Front. Plant Sci. 10. doi: 10.3389/fpls.2019.01538
Gan, Y. T., Campbell, C. A., Janzen, H. H., Lemke, R., Liu, L. P., Basnyat, P., et al. (2009). Root mass for oilseed and pulse crops: growth and distribution in the soil profile. Can. J. Plant Sci. 89, 883–893. doi: 10.4141/CJPS08154
Gavrilescu, M. (2021). Water, soil, and plants interactions in a threatened environment. Water 13, 2746. doi: 10.3390/w13192746
Hamblin, M. T., Buckler, E. S., Jannink, J. L. (2011). Population genetics of genomics-based crop improvement methods. Trends Genet. 3, 98–106. doi: 10.1016/j.tig.2010.12.003
Hasan, M. M., Skalicky, M., Jahan, M. S., Hossain, M. N., Anwar, Z., Nie, Z.-F., et al. (2021). Spermine: Its emerging role in regulating drought stress responses in plants. Cells 10 (2), 261. doi: 10.3390/cells10020261
He, T., Hill, C. B., Angessa, T. T. (2019). Gene-set association and epistatic analyses reveal complex gene interaction networks affecting flowering time in a worldwide barley collection. J. Exp. Bot. 70, 5603–5616. doi: 10.1093/jxb/erz332
He, Y., Wu, D., Wei, D., Fu, Y., Cui, Y., Dong, H., et al. (2017). GWAS, QTL mapping and gene expression analyses in brassica napus reveal genetic control of branching morphogenesis. Sci. Rep. 7, 15971. doi: 10.1038/s41598-017-15976-4
Henry, A., Gowda, V. R., Torres, R. O. (2011). Variation in root system architecture and drought response in rice (Oryza sativa): phenotyping of the OryzaSNP panel in rainfed lowland fields. Field Crops Res. 120, 205–214. doi: 10.1016/j.fcr.2010.10.003
Herzig, P., Maurer, A., Draba, V., Sharma, R., Draicchio, F., Bull, H., et al. (2018). Contrasting genetic regulation of plant development in wild barley grown in two European environments revealed by nested association mapping. J. Exp. Bot. 69 (7), 1517–1531. doi: 10.1093/jxb/ery002
Hori, K., Kobayashi, T., Shimizu, A., Sato, K., Takeda, K., Kawasaki, S. (2003). Efficient construction of high-density linkage map and its application to QTL analysis in barley. Theor. Appl. Genet. 107, 806–813. doi: 10.1007/s00122-003-1342-9
Hruska, J., Vermak, J., Sustek, S. (1999). Mapping of tree root systems with ground-penetrating radar. Tree Physio 19, 125–130. doi: 10.1093/treephys/19.2.125
Huang, X., Kurata, N., Wei, X., Wang, Z. X., Wang, A., Zhao, Q., et al. (2012). A map of rice genome variation reveals the origin of cultivated rice. Nature 490, 497–501. doi: 10.1038/nature11532
Hudak, C. M., Patterson, R. P. (1995). Vegetative growth analysis of a drought-resistant soybean plant introduction. Crop Sci. 35, 464. doi: 10.2135/cropsci1995.0011183X003500020031x
Hudek, C., Putinica, C., Otten, W., De Baets, S. (2022). Functional root trait-based classification of cover crops to improve soil physical properties. Eur. J. Soil Sci. 73 (1), e13147. doi: 10.1111/ejss.13147
Jia, Z., Liu, Y., Gruber, B. D., Neumann, K., Kilian, B., Graner, A., et al. (2019). Genetic dissection of root system architectural traits in spring barley. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00400
Khasanova, A., Lovell, J. T., Bonnette, J., Weng, X., Jenkins, J., Yoshinaga, Y., et al. (2019). The genetic architecture of shoot and root trait divergence between mesic and xeric ecotypes of a perennial grass. Front. Plant Sci. 10. doi: 10.3389/fpls.2019.00366
Kohorn, B. D., Johansen, S., Shishido, A., Todorova, T., Martinez, R., Defeo, E., et al. (2009). Pectin activation of MAP kinase and gene expression is WAK2 dependent. Plant J. 60, 974–982. doi: 10.1111/j.1365-313X.2009.04016.x
Kuczyńska, A., Surma, M., Adamski, T., Mikołajczak, K., Krystkowiak, K., Ogrodowicz, P. (2013) Effects of the semi-dwarfing sdw1/denso gene in barley. J. Appl. Genet. 54, 381–390. doi: 10.1007/s13353-013-0165-x
Laloum, T., Martín, G., Duque, P. (2018). Alternative splicing control of abiotic stress responses. Trends Plant Sci. 23 (2), 140–150. doi: 10.1016/j.tplants.2017.09.019
Lamers, J., van der Meer, T., Testerink, C. (2020). How plants sense and respond to stressful environments. Plant Physiol. 182 (4), 1624–1635. doi: 10.1104/pp.19.01464
Lehnert, H., Serfling, A., Friedt, W., Ordon, F. (2018). Genome-wide association studies reveal genomic regions associated with the response of wheat (Triticum aestivum l.) to mycorrhizae under drought stress conditions. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01728
Li, H., Zhou, S.-Y., Zhao, W.-S., Peng, Y.-L. (2009). A novel wall-associated receptor-like protein kinase gene, OsWAK1, plays important roles in rice blast disease resistance. Plant Mol. Biol. 69, 337–346. doi: 10.1007/s11103-008-9430-5
Li, L., Dou, N., Zhang, H., Wu, C. (2021). The versatile GABA in plants. Plant Signal Behav. 4 16 (3), 1862565. doi: 10.1080/15592324.2020
Li, L., Wang, H., Gago, J., Cui, H., Qian, Z., Kodama, N., et al. (2015). Harpin Hpa1 interacts with aquaporin PIP1;4 to promote the substrate transport and photosynthesis in arabidopsis. Sci. Rep. 5, 17207. doi: 10.1038/srep17207
Liu, H., Bayer, M., Druka, A., Russell, J. R., Hackett, C. A., Poland, J., et al. (2014). An evaluation of genotyping by sequencing (GBS) to map the breviaristatum-e (ari-e) locus in cultivated barley. BMC Genomics 15, 104. doi: 10.1186/1471-2164-15-104
Liu, Y., Gai, J.-Y., Lü, H.-N., Wang, Y.-J., Chen, S.-Y. (2005). Identification of drought tolerant germplasm and inheritance and QTL mapping of related root traits in soybean (Glycine max (L.) merr.). Acta Genet. Sin. 32, 855–863.
Long, Y., Kong, D., Chen, Z., Zeng, H. (2013). Variation of the linkage of root function with root branch order. PloS One 8 (2), e57153. doi: 10.1371/journal.pone.0057153
Lorenz, A. J., Hamblin, M. T., Jannink, J.-L. (2010). Performance of single nucleotide polymorphisms versus haplotypes for genome-wide association analysis in barley. PloS One 5 (11), e14079. doi: 10.1371/journal.pone.0014079
Ma, F., Xu, Y., Ma, Z., Li, L., An, Z. (2018). Genome-wide association and validation of key loci for yield-related traits in wheat founder parent xiaoyan 6. Mol. Breed. 38, 91. doi: 10.1007/s11032-018-0837-7
Malosetti, M., Ribaut, J.-M., van Eeuwijk, F. A. (2013). The statistical analysis of multi-environment data: modeling genotype-by-environment interaction and its genetic basis. Front. Physiol. 4. doi: 10.3389/fphys.2013.00044
Mamedov, A. I., Husiyev, E. K. (2022). Allometric model for predicting root biomass of field crops in the salt-affected clay soil: Novel approach. environ. Sci. Proc. 16, 11. doi: 10.3390/environsciproc2022016011
Mandozai, A., Moussa, A. A., Zhang, Q., Qu, J., Du, Y., Anwari, G., et al. (2021). Genome-wide association study of root and shoot related traits in spring soybean (Glycine max l.) at seedling stages using SLAF-seq. Front. Plant Sci. 12. doi: 10.3389/fpls.2021.5689
Mastrangelo, A. M., Marone, D., Laidò, G., De Leonardis, A. M., De Vita, P. (2012). Alternative splicing: enhancing ability to cope with stress via transcriptome plasticity. Plant Sci. 185–186, 40–49. doi: 10.1016/j.plantsci.2011.09.006
McLaren, W., Gil, L., Hunt, S. E. (2016). The ensembl variant effect predictor. Genome Biol. 17 (1), 122. doi: 10.1186/s13059-016-0974-4
Mekonnen, D. W., Flügge, U. I., Ludewig, F. (2016). Gamma-aminobutyric acid depletion affects stomata closure and drought tolerance of arabidopsis thaliana. Plant Sci. 245, 25–34. doi: 10.1016/j.plantsci.2016.01.005
Mikołajczak, K., Kuczyńska, A., Ogrodowicz, P., Kiełbowicz-Matuk, A., Ćwiek-Kupczyńska, H., Daszkowska-Golec, A., et al. (2022). High-throughput sequencing data revealed genotype-specific changes evoked by heat stress in crown tissue of barley sdw1 near-isogenic lines. BMC Genomics 23 (177), (2022). doi: 10.1186/s12864-022-08410-1
Morris, G. P., Ramu, P., Deshpande, S. P. C., Hash, T., Shah, T., Upadhyaya, H. U., et al. (2013). Population genomic and genome-wide association studies of agroclimatic traits in sorghum. Proc. Natl. Acad. Sci. U.S.A. 8 110 (2), 453–458. doi: 10.1073/pnas.1215985110
Motzo, R., Attene, G., Deidda, M. (1992). Genotypic variation in durum wheat root systems at different stages of development in a Mediterranean environment. Euphytica 66 (3), 197–206. doi: 10.1007/BF00025303
Muhammad, A., Li, J., Hu, W., Yu, J., Khan, S. U., Khan, M. H. U., et al. (2021). Uncovering genomic regions controlling plant architectural traits in hexaploid wheat using different GWAS models. Sci. Rep. 11, 6767. doi: 10.1038/s41598-021-86127-z
N’Diaye, A., Haile, J. K., Cory, A. T., Clarke, F. R., Clarke, J. M., Knox, R. E., et al. (2017). Single marker and haplotype-based association analysis of semolina and pasta colour in elite durum wheat breeding lines using a high-density consensus map. PloS One 12 (1), e0170941. doi: 10.1371/journal.pone.0170941
N’Diaye, A., Haile, J. K., Nilsen, K. T., Walkowiak, S., Ruan, Y., Singh, A. K., et al. (2018). Haplotype loci under selection in Canadian durum wheat germplasm over 60 years of breeding: Association with grain yield, quality traits, protein loss, and plant height. Front. Plant Sci. 9. doi: 10.3389/fpls.2018.01589
Nandha, P. S., Singh, J. (2014). Comparative assessment of genetic diversity between wild and cultivated barley using gSSR and EST-SSR markers. Plant Breed. 133, 28–35. doi: 10.1111/pbr.12118
Nevo, E., Chen, G. (2010). Drought and salt tolerances in wild relatives for wheat and barley improvement. Plant Cell Environ. 33, 670–685. doi: 10.1111/j.1365-3040.2009.02107.x
Nilsen, T. W., Graveley, B. R. (2010). ). expansion of the eukaryotic proteome by alternative splicing. Nature 463 (7280), 457–463. doi: 10.1038/nature08909
Ogawa, D., Nonoue, Y., Tsunematsu, H., Kanno, N., Yamamoto, T., Yonemaru, J.-I. (2018). Discovery of QTL alleles for grain shape in the Japan-MAGIC rice population using haplotype information. G3 Genes Genomes Genetics 8 (11), 3559–3565. doi: 10.1534/g3.118.200558
Ogrodowicz, P., Adamski, T., Mikołajczak, K., Kuczyńska, A., Surma, M., Krajewski, P., et al. (2017). QTLs for earliness and yield-forming traits in the Lubuski × CamB barley RIL population under various water regimes. J. Appl. Genet. 58, 49–65. doi: 10.1007/s13353-016-0363-4
Ogrodowicz, P., Kuczyńska, A., Mikołajczak, K., Adamski, T., Surma, M., Krajewski, P., et al. (2020). Mapping of quantitative trait loci for traits linked to fusarium head blight in barley. PloS One 15 (2), e0222375. doi: 10.1371/journal.pone.0222375
Oh, E. S., Lee, Y., Chae, W. B. (2018). Biochemical analysis of the role of leucine-rich repeat receptor-like kinases and the carboxy-terminus of receptor kinases in regulating kinase activity in arabidopsis thaliana and brassica oleracea. Molecules 23 (1), 236. doi: 10.3390/molecules23010236
Osmont, K. S., Sibout, R., Hardtke, C. S. (2007). Hidden branches: developments in root system architecture. Annu. Rev. Plant Biol. 58, 93–113. doi: 10.1146/annurev.arplant.58.032806.104006
Ou, Y., Kui, H., Li, J. (2021). Receptor-like kinases in root development: Current progress and future directions. Mol. Plant 14 (1), 166–185. doi: 10.1016/j.molp.2020.12.004
Paez-Garcia, A., Motes, C. M., Scheible, W. R., Chen, R., Blancaflor, E. B., Monteros, M. J. (2015). Root traits and phenotyping strategies for plant improvement. Plants 4, 334–355. doi: 10.3390/plants4020334
Pantalone, V. R., Burton, J. W., Carter, T. E. (1996). Soybean fibrous root heritability and genotypic correlations with agronomic and seed quality traits. Crop Sci. 36, 1120–1125. doi: 10.2135/cropsci1996.0011183X003600050008x
Patankar, H. V., Al-Harrasi, I., Al-Yahyai, R., Yaish, M. W. (2019). Functional characterization of date palm aquaporin gene PdPIP1;2 confers drought and salinity tolerance to yeast and arabidopsis. Genes 10 (5), 390. doi: 10.3390/genes10050390
Peng, X., Wang, M., Li., Y., Yan, W., Chang, Z., Chen, Z., et al. (2020). Lectin receptor kinase OsLecRK-S.7 is required for pollen development and male fertility. J. Integr. Plant Biol. 62 (8), 1227–1245. doi: 10.1111/jipb.12897
Poland, J. A., Brown, P. J., Sorrells, M. E., Jannink, J. L. (2012). Development of high-density genetic maps for barley and wheat using a novel two-enzyme genotyping-by-sequencing approach. PloS One 7 (2), e32253. doi: 10.1371/journal.pone.0032253
Prokushkin, S. G. (1982). Seasonal and daily increment in roots of the scotch pine as a function of soil temperature. Sov. J. Eco. 12, 277–282.
Racolta, A., Bryan, A. C., Tax, F. E. (2014). The receptor-like kinases GSO1 and GSO2 together regulate root growth in arabidopsis through control of cell division and cell fate specification. Dev. Dyn. 243 (2), C1. doi: 10.1002/dvdy.24044
Rajkai, K., Végh, K. R., Nacsa, T. (2005). Electrical capacitance of roots in relation to plant electrodes, measuring frequency and root media. Acta Agron. Hung. 53, 197–210. doi: 10.1556/AAgr.53.2005.2.8
Reinert, S., Kortz, A., Léon, J., Naz, A. A. (2016). Genome-wide association mapping in the global diversity set reveals new QTL controlling root system and related shoot variation in barley. Front. Plant Sci. 7. doi: 10.3389/fpls.2016.01061
Rezaei-Chiyaneh, E., Seyyedi, S. M., Ebrahimian, E., Moghaddam, S. S., Damalas, C. A. (2018). Exogenous application of gamma-aminobutyric acid (GABA) alleviates the effect of water deficit stress in black cumin (Nigella sativa l.). Ind. Crops Prod. 112, 741–748. doi: 10.1016/j.indcrop.2017.12.067
Rochat, R. H., de las Fuentes, L., Stormo, G., Davila-Roman, V. G., Charles Gu, C. (2007). A novel method combining linkage disequilibrium information and imputed functional knowledge for TagSNP selection. Hum. Hered. 64, 4, 243–249. doi: 10.1159/000104227
Rohilla, M., Singh, N., Mazumder, A., Sen, P., Roy, P., Chowdhury, D., et al. (2020). Genome-wide association studies using 50 K rice genic SNP chip unveil genetic architecture for anaerobic germination of deep-water rice population of Assam, India. Mol. Genet. Genom. 295, 1211–1226. doi: 10.1007/s00438-020-01690-w
Ruangsiri, M., Vejchasarn, P., Saengwilai, P., Lynch, J., Bennett, M. J., Brown, K. M., et al. (2021). Genetic control of root architectural traits in KDML105 chromosome segment substitution lines under well-watered and drought stress conditions. Plant Prod. Sci. 24 (4), 512–529. doi: 10.1080/1343943X.2021.1883990
Russell, J., Mascher, M., Dawson, I. K., Kyriakidis, S., Calixto, C., Freund, F., et al. (2016). Exome sequencing of geographically diverse barley landraces and wild relatives gives insights into environmental adaptation. Nat. Genet. 48, 1024–1030. doi: 10.1038/ng.3612
Sallam, A., Alqudah, A. M., Dawood, M. F. A., Baenziger, P. S., Börner, A. (2019). Drought stress tolerance in wheat and barley: Advances in physiology, breeding and genetics research. Int. J. Mol. Sci. 20, 3137. doi: 10.3390/ijms20133137
Sehgal, D., Mondal, S., Crespo-Herrera, L., Velu, G., Juliana, P., Huerta-Espino, J., et al. (2020). Haplotype-based, genome-wide association study reveals stable genomic regions for grain yield in CIMMYT spring bread wheat. Front. Genet. 11. doi: 10.3389/fgene.2020.589490
Sesia, M., Bates, S., Candès, E., Sabatti, C. (2021). False discovery rate control in genome-wide association studies with population structure. Proc. Natl. Acad. Sci. U.S.A. 118 (40), e2105841118. doi: 10.1073/pnas.2105841118
Sharma, R., Shaaf, S., Neumann, K., Go, Y., Mascher, M., David, M., et al. (2020). On the origin of photoperiod non-responsiveness in barley. bioRxiv. doi: 10.1101/2020.07.02.185488
Sharp, R. E., Davies, W. J. (1985). Root growth and water uptake by maize plants in drying soil. J. Exp. Bot. 36, 1441–1456. doi: 10.1093/jxb/36.9.1441
Shen, Q., Wen, Z., Dong, Y., Li, H., Miao, Y., Shen, J. (2018). The responses of root morphology and phosphorus-mobilizing exudations in wheat to increasing shoot phosphorus concentration. AoB Plants 10 (5), ply054. doi: 10.1093/aobpla/ply054
Siekmann, D., Jansen, G., Zaar, A., Kilian, A., Joachim, F., Hackauf, B. (2021). A genome-wide association study pinpoints quantitative trait genes for plant height, heading date, grain quality, and yield in rye (Secale cereale l.). Front. Plant Sci. 12. doi: 10.3389/fpls.2021.718081
Sierra Cornejo, N., Hertel, D., Becker, J. N., Hemp, A., Leuschner, C. (2020). Biomass, morphology, and dynamics of the fine root system across a 3,000-m elevation gradient on mt. Kilimanjaro. Front. Plant Sci. 11. doi: 10.3389/fpls.2020.00013
Smykal, P., Gleissner, R., Corbesier, L., Apel, K., Melzer, S. (2004). Modulation of flowering responses in different nicotiana varieties. Plant Mol. Biol. 55 (2), 253–262. doi: 10.1007/s11103-004-0557-8
Středa, T., Dostál, V., Horáková, V., Chloupek, O. (2012). Effective use of water by wheat varieties with different root system sizes in rain-fed experiments in central Europe. Agric. Water Manage. 104, 203–209. doi: 10.1016/j.agwat.2011.12.018
Středa, T., Haberle, J., Klimešová, J., Klimek-Kopyra, A., Středová, H., Bodner, G., et al. (2020). Field phenotyping of plant roots by electrical capacitance – a standardized methodological protocol for application in plant breeding: a review. Int. Agrophys. 34, 173–184. doi: 10.31545/intagr/117622
Suchorab, Z., Lagod, G., Widomski, M. K., Barnat-Huntek, D. (2014). Methodology of moisture measurement in porous materials using time domain reflectometry. CDEM 19 (1-2), 97–107. doi: 10.1515/cdem-2014-0009
Suji, K. K., Prince, K. S. J., Mankhar, P. S., Kanagaraj, P., Poornima, R., Amutha, K., et al. (2012). Evaluation of rice near isogenic lines with root QTLs for plant production and root traits in rainfed target populations of environment. Field Crop Res. 137, 89–96. doi: 10.1016/j.fcr.2012.08.006
Svačina, P., Středa, T., Chloupek, O. (2014). Uncommon selection by root system size increases barley yield. Agron. Sustain. Dev. 34, 545–551. doi: 10.1007/s13593-013-0160-y
Thornley, J. M. (1998). Modelling shoot [ratio] root relations: The only way forward? Ann. Bot. 81, 165–171. doi: 10.1006/anbo.1997.0529
Tsai, H. Y., Janss, L. L., Andersen, J. R., Orabi, J., Jensen, J. D., Jahoor, A., et al. (2020). Genomic prediction and GWAS of yield, quality and disease-related traits in spring barley and winter wheat. Sci. Rep. 10, 3347. doi: 10.1038/s41598-020-60203-2
Turner, A., Beales, J., Faure, S., Dunford, R. P., Laurie, D. A. (2005). The pseudo-response regulator ppd-H1 provides adaptation to photoperiod in barley. Science 310 (5750), 1031–1034. doi: 10.1126/science.1117619
Valluru, R., Reynolds, M. P., Davies, W. J., Sukumaran, S. (2017). Phenotypic and genome-wide association analysis of spike ethylene in diverse wheat genotypes under heat stress. New Phytol. 214, 271–283. doi: 10.1111/nph.14367
van Beem, J., Smith, M. E., Zobei, R. W. (1998). Estimating root mass in maize using a portable capacitance meter. Agron. J. 90, 566–570. doi: 10.2134/agronj1998.00021962009000040021x
van Eeuwijk, F. A., Bink, M. C. A. M., Chenu, K., Chapman, S. C. (2010). Detection and use of QTL for complex traits in multiple environments. Curr. Opin. Plant Biol. 13, 193–205. doi: 10.1016/j.pbi.2010.01.001
Voss-Fels, K. P., Robinson, H., Mudge, S. R., Richard, C., Newman, S., Wittkop, B., et al. (2018). VERNALIZATION1 modulates root system architecture in wheat and barley. Mol. Plant 11, 226–229. doi: 10.1016/j.molp.2017.10.005
VSN International (2019). Genstat for windows 21nd edition (Hemel Hempstead, UK: VSN International).
Wagner, T. A., Kohorn, B. D. (2001). Wall-associated kinases are expressed throughout plant development and are required for cell expansion. Plant Cell 13 (2), 303–318. doi: 10.1105/tpc.13.2.303
Wahl, M. C., Will, C. L., Lührmann, R. (2009). The spliceosome: design principles of a dynamic RNP machine. Cell 136 (4), 701–718. doi: 10.1016/j.cell.2009.02.009
Wang, Y., Zhao, Z., Liu, F., Sun, L., Hao, F. (2020). Versatile roles of aquaporins in plant growth and development. Int. J. Mol. Sci. 21, 9485. doi: 10.3390/ijms21249485
Wasson, A. P., Richards, R. A., Chatrath, R., Misra, S. C., Sai Prasad, S. V., Rebetzke, G. J., et al. (2012). Traits and selection strategies to improve root systems and water uptake in water-limited wheat crops. J. Exp. Bot. 63 (9), 3485–3498. doi: 10.1093/jxb/ers111
Wei, Z., Li, J. (2018). Receptor-like protein kinases: Key regulators controlling root hair development in arabidopsis thaliana. J. Integr. Plant Biol. 60 (9), 841–850. doi: 10.1111/jipb.12663
Wiegmann, M., Maurer, A., Pham, A., March, T. J., Al-Abdallat, A., Thomas, W. T. B., et al. (2019). Barley yield formation under abiotic stress depends on the interplay between flowering time genes and environmental cues. Sci. Rep. 9, 6397. doi: 10.1038/s41598-019-42673-1
Wu, B., Ren, W., Zhao, L., Li, Q., Sun, J., Chen, F., et al. (2022). Genome-wide association study of root system architecture in maize. Genes 13, 181. doi: 10.3390/genes13020181
Xiao, Y., Liu, H., Wu, L., Warburton, M., Yan, J. (2017). Genome-wide association studies in maize: praise and stargaze. Mol. Plant 10 (3), 359–374. doi: 10.1016/j.molp.2016.12.008
Xu, Y.-F., An, D.-G., Liu, D.-C., Zhang, A.-M., Xu, H.-X., Li, B. (2012). Mapping QTLs with epistatic effects and QTL × treatment interactions for salt tolerance at seedling stage of wheat. Euphytica 186, 233–245. doi: 10.1007/s10681-012-0647-7
Xue, W., Yan, J., Zhao, G., Jiang, Y., Cheng, J., Cattivelli, L., et al. (2017). A major QTL on chromosome 7HS controls the response of barley seedling to salt stress in the Nure × Tremois population. BMC Genet. 18, 79. doi: 10.1186/s12863-017-0545-z
Yao, X., Wu, K., Yao, Y., Bai, Y., Ye, J., Chi, D. (2018). Construction of a high-density genetic map: genotyping by sequencing (GBS) to map purple seed coat color (Psc) in hulless barley. Hereditas 155, 37. doi: 10.1186/s41065-018-0072-6
Keywords: barley, genome-wide association studies, drought, roots architecture, gene annotations
Citation: Ogrodowicz P, Mikołajczak K, Kempa M, Mokrzycka M, Krajewski P and Kuczyńska A (2023) Genome-wide association study of agronomical and root-related traits in spring barley collection grown under field conditions. Front. Plant Sci. 14:1077631. doi: 10.3389/fpls.2023.1077631
Received: 24 October 2022; Accepted: 06 January 2023;
Published: 24 January 2023.
Edited by:
Alessandro Tondelli, Council for Agricultural and Economics Research (CREA), ItalyReviewed by:
Rajiv Sharma, Scotland’s Rural College, United KingdomYin-Gang Hu, Northwest A&F University, China
Copyright © 2023 Ogrodowicz, Mikołajczak, Kempa, Mokrzycka, Krajewski and Kuczyńska. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anetta Kuczyńska, akuc@igr.poznan.pl
 Piotr Ogrodowicz
Piotr Ogrodowicz Krzysztof Mikołajczak
Krzysztof Mikołajczak Michał Kempa
Michał Kempa Monika Mokrzycka
Monika Mokrzycka Paweł Krajewski
Paweł Krajewski Anetta Kuczyńska
Anetta Kuczyńska