- 1Henan Collaborative Innovation Centre of Modern Biological Breeding, Henan Institute of Science and Technology, Xinxiang, China
- 2Foreign Languages College, Henan Institute of Science and Technology, Xinxiang, China
- 3College of Agriculture, Guangxi University, Nanning, China
- 4State Key Laboratory of Cotton Biology, Institute of Cotton Research, Chinese Academy of Agricultural Science, Anyang, China
- 5Hainan Yazhou Bay Seed Laboratory, Sanya, China
- 6National Nanfan Research Institute (Sanya), Chinese Academy of Agriculture Sciences, Sanya, China
- 7Department of Biology, East Carolina University, Greenville, NC, United States
Cotton production is challenged by high costs with multiple management and material inputs including seed, pesticide, and fertilizer application. The production costs can be decreased and profits can be increased by developing efficient crop management strategies, including perennial cotton ratoon cultivation. This review focuses on the role of ratoon cultivation in cotton productivity and breeding. In areas that are frost-free throughout the year, when the soil temperature is suitable for cotton growth in spring, the buds of survived plants begin to sprout, and so their flowering and fruiting periods are approximately 4–6 weeks earlier than those of sown cotton. Due to the absence of frost damage, the ratoon cotton continues to grow, and the renewed plants can offer a higher yield than cotton sown in the following season. Moreover, ratoon cultivation from the last crop without sowing can help conserve seeds, reduce labor inputs, and reduce soil and water loss. In this review, the preservation of perennial cotton germplasm resources, the classification and genome assignment of perennial species in the cotton gene pools, and effective strategies for the collection, preservation, identification, and utilization of perennial cotton germplasms are discussed. Ratoon cultivation is the main driver of cotton production and breeding, especially to maintain male sterility for the utilization and fixation of heterosis. Ratoon cultivation of cotton is worth adopting because it has succeeded in Brazil, China, and India. Therefore, taking advantages of the warm environment to exploit the indeterminant growth habit of perennial cotton for breeding would be an efficiency-increasing, cost-saving, and eco-friendly approach in frost-free regions. In the future, more attention should be given to ratooning perennial cotton for breeding male-sterile lines.
Introduction
Cotton is an industrial textile crop and the most widely grown natural fiber crop (Zhang et al., 2022). Excellent varieties are the basis for high cotton yields, especially those developed in the 1990's, when the breeding of Bt cotton saved cotton production, which was almost destroyed by bollworm. This advancement also reduced the use of chemical pesticides, saving both human and material resources and contributing to environmental protection and the ecological balance. It was therefore rapidly promoted and applied in production. However, Bt-transgenic insect-resistant cotton only has a particular effect on some Lepidoptera pests and is not very effective against aphids, sooty mites, and other pests that are currently causing severe damage in production (Bergman, 1985; Silva et al., 2008; Wan et al., 2017). In addition, as labor inputs are higher for cotton than food crops, while the economic efficiency is low, and cotton is planted in drought-affected and saline areas as well as mudflats, the breeding of cotton varieties with multistress resistance has important and far-reaching significance.
Given the narrow genetic basis of high-yield cotton varieties worldwide and their high degree of homogeneity, as well as the controversy over the ecological safety risks associated with genetically modified cotton, it is extremely important to use semiwild lines of cultivated species and wild species, which make up the majority of Gossypium, as germplasm resources to expand the genetic basis of annual cotton varieties (Teravanesyan and Belova, 1970; Wang, 2007; Migicovsky and Myles, 2017). In the future, it will be necessary to utilize the excellent traits of perennial species and their underlying genes.
The high regeneration potential of cotton has long been recognized, with examples of ratoon cotton being grown in Georgia as early as 1786 (Seabrook, 1844). In 1961, Stroman proposed ratooning F1 plants in Peru to harvest more than one crop of F2 seeds (Stroman, 1961). In 1968, Weaver proposed that ratooning male-sterile plants would provide an excellent way to produce F1 seeds (Weaver, 1968). In the practice of cotton breeding, ratoon cotton could be used to extend the time in which germplasms can be utilized for more than one season (Muhammad et al., 2015). In tropical cotton production areas, the most economical way to take advantage of ratoon cotton is to produce hybrid cotton seeds with high quality and low cost by ratooning male-sterile lines because if ratoon cotton is used for lint production in the tropics, its economic benefit is far lower than that of hybrid seed production and even worse than that of cotton sown annually in temperate areas.
Perennial Conservation of Gossypium Species and Their Classification
Of the Gossypium species, four are cultivated species and the others are perennial wild species (Figure 1). The basis of cotton breeding is the collection and preservation of genetic germplasms, which provide an essential foundation to improve and sustain cotton production (Zhang et al., 2012a). Specifically, the following extraordinary accessions are required for perennial preservation: (1) wild cotton species with short-day flowering behavior, from which it is hard to obtain seeds (Percy et al., 2014); (2) nullisomic, monosomic, telomeric, trisomic, and translocation lines and other cytogenetic stocks of cotton (Kiranga, 2013), the fruiting rate of which is low and the identification of which is difficult and time-consuming; (3) cotton plants infected by some kinds of pathogens, the status of which should be maintained for a long time for research (Mihail et al., 1987; Seo et al., 2006); and (4) cotton hybrids or backcross generations such as F1, F2, and BC1F1 generations, which can be commonly used for only a year but the generations of which can be repeatedly used over many years through perennial growth (D'Eeckenbrugge and Lacape, 2014).
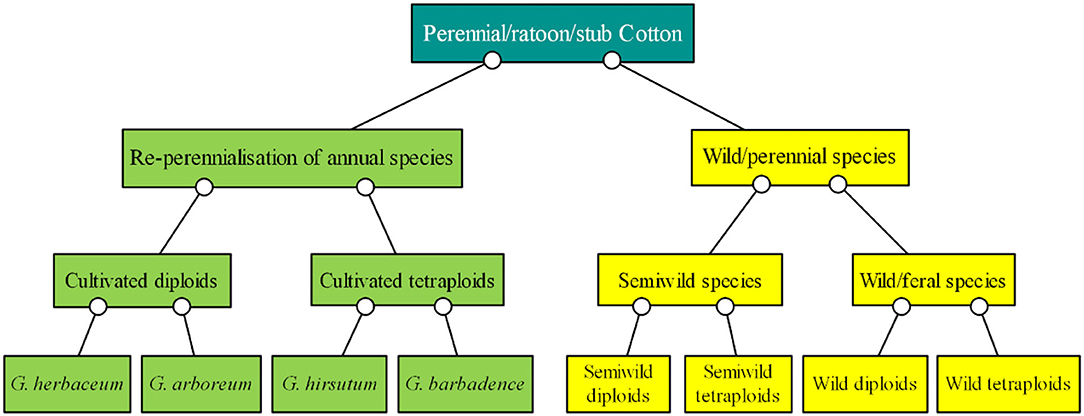
Figure 1. Classification of ratoon cotton based on cultivars, semiwild species, wild/feral species, and chromosome ploidy. The green boxes show the cultivated species, and the yellow boxes show the wild species.
Global Overview of Perennial Germplasms and Perennial Conservation of Cotton
Worldwide, Gossypium germplasms with different ecological niches have much morphological, agronomic, physiological, and genetic variability that is conserved in situ at centers of cotton origin (Castro et al., 2016) and preserved ex situ with a large number of accessions in eight major countries with extensive cotton germplasm collections, namely Australia, Brazil, China, France, India, Russia, the USA, and Uzbekistan (Abdurakhmonov, 2007; Rahmat et al., 2014; Boopathi and Hoffmann, 2016), attaching great importance to the preservation of perennial germplasms. It is worth noting that more than 20,000 cotton germplasm accessions are preserved in Uzbekistan, making that the most extensive collection in the world. Although cotton is now rarely grown in France, it is commendable that more than 3,000 accessions, including approximately 1,000 wild accessions, are preserved in CIRAD (Coopération Internationale en Recherche Agronomique pour le Développement), a French publicly supported agency that specializes in tropical and Mediterranean agriculture (Campbell et al., 2010).
At present, China, India, and the USA are the three largest cotton-producing countries. Of the ~10,000 accessions preserved in the National Cotton Germplasm Collection (NCGC) of the USA, 581 are wild germplasms. Most of the accessions, including photoperiodic germplasms and perennial accessions, are perennially grown at the tropical Cotton Winter Nursery (CWN) in Tecoman, Colima, Mexico (Wallace et al., 2009; Percy et al., 2014). In India, the bank of the Central Institute for Cotton Research has collected a total of 10,227 accessions, including 26 wild species and 32 perennial forms (Boopathi et al., 2014). Although China is not an origin center of cotton, most of the 8,868 accessions, including 32 wild forms, were collected from China, and 2,236 accessions were introduced from 52 foreign countries. The Sanya National Research Station of Wild Cotton Germplasm, which is located within the tropics of China, has 391 wild accessions that are perennially grown for conservation (Jia et al., 2014). In addition, Mexico, Pakistan, and other cotton-planting countries have also collected many germplasms.
Gene Pools and Genome Assignment of Perennial Gossypium Species
At least 48 species of Gossypium, including 7 tetraploid (2n = 4x = 52) species, 41-45 diploid (2n = 2x = 26) species, and other wild species, originate from arid to semiarid regions within the tropics and subtropics (Wendel and Grover, 2015; Shim et al., 2018; Wang et al., 2018). According to the genetic relationship with upland cotton, all the cotton species can be classified into primary, secondary, and tertiary gene pools. Of the 7 tetraploid species (genome AD), G. hirsutum and G. barbadense are mainly cultivated worldwide, so all tetraploid species are in the primary gene pool. Based on the relative genetic approachability and utility of species to improve G. hirsutum and G. barbadense, 20–21 diploid species (genome A/D/F/B) and 21–24 diploid species (genome C/E/G/K) have been classified into secondary and tertiary gene pools, respectively (Figure 2).
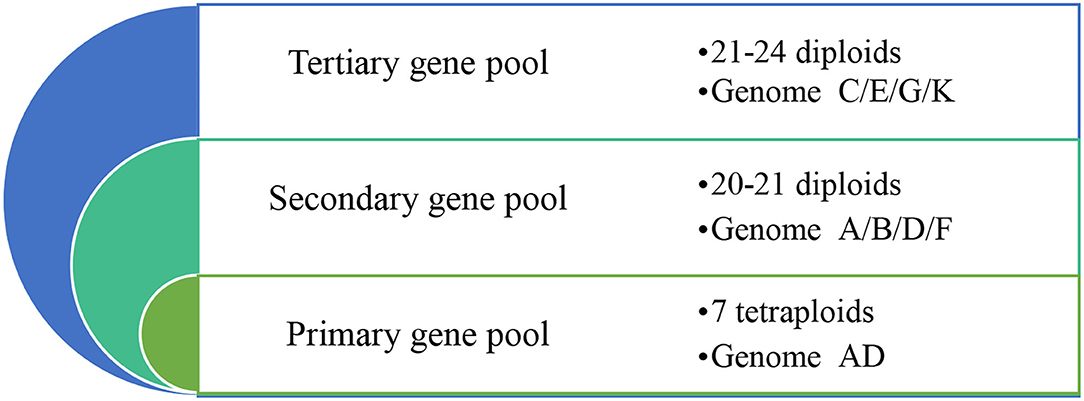
Figure 2. The primary, secondary, and tertiary gene pools based on their genetic relationship with upland cotton. The primary, secondary, and tertiary cotton (Gossypium) gene pools are shown from the inside circle to the outer ring. The farther away the primary gene pool is the further the genetic approachability is from the tetraploids, and the richer the genetic diversity.
Gossypium species with genomic assignments and geographical origins in the primary, secondary, and tertiary cotton gene pools are detailed in Table 1. Responding to the diverse geographic and ecological conditions of frost-free regions, wild cotton species show a broad adaptation range from herbaceous perennial diploid species with a fire-/dry-adapted biseasonal growth pattern in northwest Australia to small cotton trees dropping their leaves to avoid the effects of the dry season in southwest Mexico (Campbell et al., 2010). Therefore, it is widely believed that the extensive genetic diversity within wild cotton increases their opportunities for evolutionary adaptation that reduces their genetic vulnerability to the changing harmful environments (Boopathi and Hoffmann, 2016).

Table 1. Gossypium species with genome assignment and geographic origin in the primary, secondary, and tertiary gene pools.
Efficient Strategies of Collecting, Conserving, and Characterizing Perennial Cotton Germplasm
Perennial cotton germplasms can be widely collected through exploration in tropical origin centers or via exchange with other gene banks. Perennial cotton can be conserved in situ or in vivo in tropical fields. After harvesting enough seeds, the seeds can be preserved in the gene bank. Moreover, perennial cotton germplasm can be preserved as perennial roots and cuttings via grafting and tissue culture in greenhouses or laboratories. Most traits can be evaluated in tropical areas, and some abiotic or biotic stress responses can be characterized in greenhouses. Molecular biological methods, such as genomics, phenomics, and molecular markers, can be used to improve the efficiency of characterizing perennial cotton germplasms.
Ratoon Cultivation of Perennial and Annual Cotton for Breeding
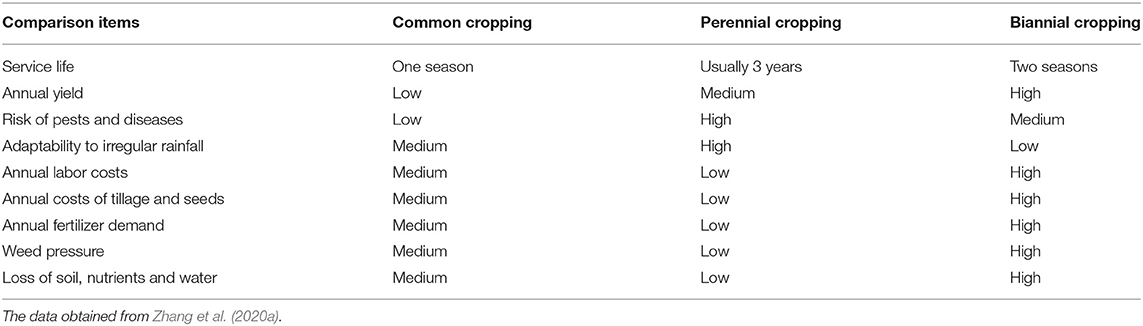
Both perennial and annual cotton species can be cultivated perennially in greenhouses or frost-free regions, and they should be cultivated by ratooning (Figure 1). Otherwise, yields of unratooned perennial cotton forms are meager. Many studies show that the service life, pesticide and fertilizer usage, stress resistance, and yield stability of semiwild races are better than those of annual species in the ratoon cultivation of cotton (Figure 3), and the annual species could be ratoon cultivated for ~3 years (Evenson, 1970; Plucknett et al., 1970; Chen et al., 2010a,b; Zhang et al., 2020a,b). It is worth noting that ratoon cultivation of hybrids between upland cotton and sea island cotton has been performed, which was helpful for fixing the interspecific heterosis of cotton (Komala et al., 2018a,b,c). The benefits of ratoon cultivation of perennial cotton mainly come from three features: (1) the perennial root system, which can save costs for seed and tillage, shorten the vegetative growth period, and provide monetization from an earlier harvest; (2) the low pruned stem, which can reduce plant height and increase the number of fruiting branches; and (3) infinite inflorescences, which can extend the flowering period and increase boll number and yield (Table 2).
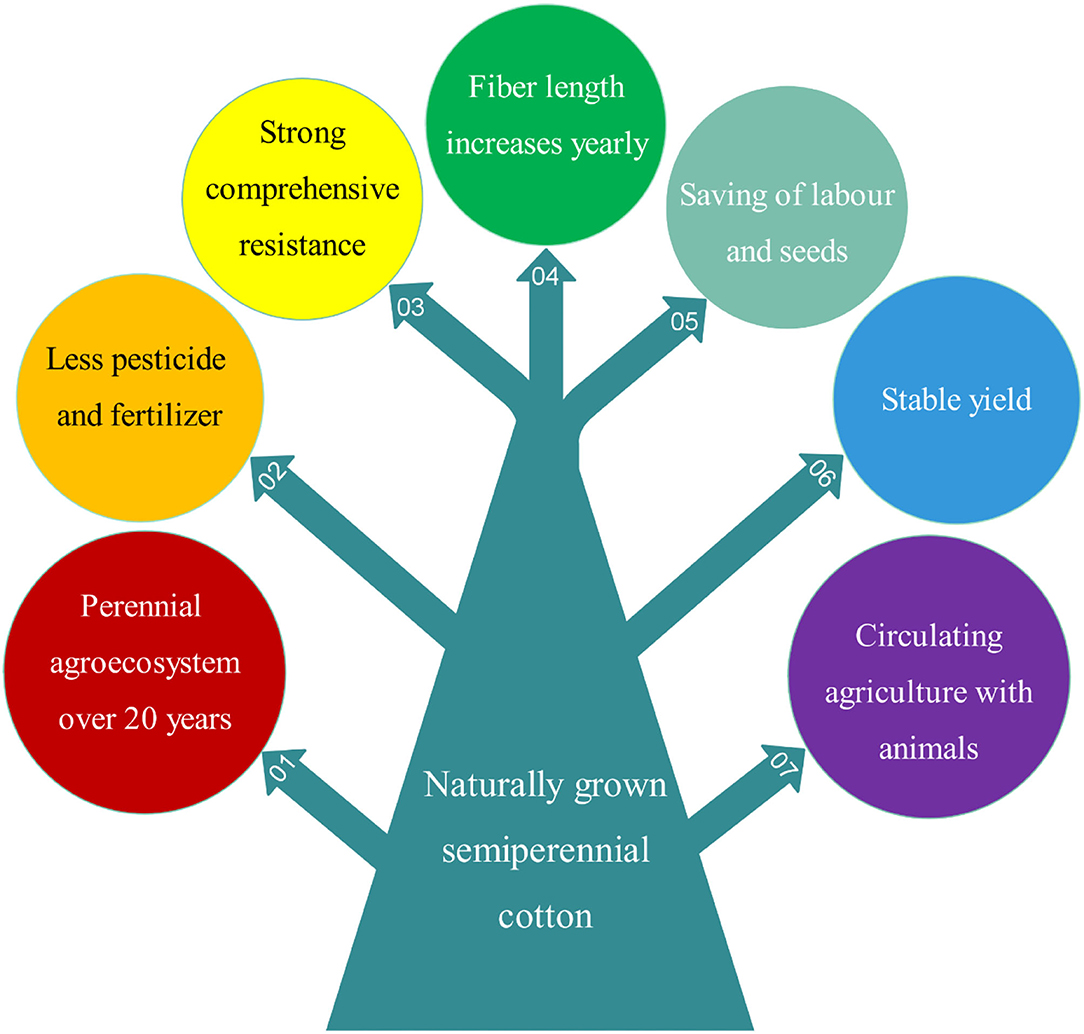
Figure 3. An overview of the seven main advantages of naturally grown semiperennial (semiwild) cotton lines compared to annual cultivars.
Perennial Cropping Methods for Ratoon Cotton
The ratoon cropping system achieves increased cotton yield with less labor input and decreases the cost of producing F1 generation cotton seeds (Bergman et al., 1983). Zhang et al. summarized three methods of ratoon cotton cropping systems, including ratooning semiwild cotton used for perennial cropping, ratooning annual cotton cultivars for perennial cropping, and ratooning annual cotton cultivars for biannual cropping (Zhang et al., 2020a). However, there is abundant evidence that failure to control pests effectively can lead to a severe impact on the yield of ratoon cotton (Flint et al., 1980). Although the perennial cultivation of ratoon cotton has declined and is even banned in some countries (Templeton, 1925; Evenson, 1970; Plucknett et al., 1970; Morris, 1973), ratooning for the second fruiting cycle with an increased yield in the same season can be used in areas with closed season legislation and has great potential and prospects in cotton production (Table 3).
Key Measures to Obtain a High Yield of Ratoon Cotton
Only the annual branches (new branches formed within 1 year) can produce fruits, so pruning, fertilization for rejuvenation, and other proper techniques to increase sprout formation are particularly important for the second fruiting cycle of ratoon cotton in the tropics (Gutstein, 1969; Reddy and Thimmegowda, 1997a; Azevedo et al., 2000; Chen et al., 2010c; Khader and Prakash, 2014; Vukicevich et al., 2016); pruning time and pruning height are the most critical factors for proper fruiting (Reddy and Thimmegowda, 1997b). Moreover, some practical techniques have been proposed for ratoon cultivation of cotton, including winter management, pest control, fertilization, and hormone regulation, based on experimental results or experience (Sachs and Zilkah, 1985).
The main stems of cotton crops were cut at different heights above ground level after harvest, and the remaining stumps regenerated new shoots at the beginning of the next rainy season to provide ratooned cotton crops (Figure 4). Macharia studied the effect of cutting-regenerated cotton sown after harvest at heights of 5, 10, and 15 cm above the ground and showed that the average kapas yields of the three cultivars were 344.0, 381.3, and 408.7 kg/ha, respectively (Macharia, 2013), which showed that the cutting height can be further improved. In addition, the effect of the cutting height on various cultivars was different. When the cutting height of the cultivars “HART 89M” and “F962” was 15 cm, the seed cotton yields were the highest, while “A540” had the highest seed cotton yield when the cutting height was 10 cm. However, because the yield of ratooned crops increased with the proper cutting height, the optimum cutting height may be higher than 15 cm. Moreover, some studies showed that a high yield of ratoon cotton could not be sustained after the third year due to deep pruning at 5–15 cm above the soil level. In particular, it must be noted that the wound left on ratoon cotton by pruning easily becomes a point of water loss and invasion by pests and diseases. Lumps occur at the plant base and necrosis occurs at the top of the stub under the wound, which is the result of no-wax sealing or dressing of the damage.

Figure 4. Ratoon cotton planted in the experimental field on the campus of Guangxi University, Nanning, China. Photos of the population (A) and a single plant (B) after the main stem is pruned.
Grafting Annual Cotton to Achieve Perennial Cultivation in Subtropical Frost-Free Areas
In subtropical frost-free areas, annual cotton cultivars with high yield and good fiber quality grafted onto perennial species with strong resistance to stress, such as drought and low temperature, are conducive to perennial cultivation (Zhang et al., 2013, 2022). Annual cotton cultivated into perennial forms by this method could undergo more growth cycles than normal ratooning crops due to the use of perennial species as rootstocks (Zhang et al., 2022). In addition, the utilization of grafted and perennially maintained male sterility for the production of cotton hybrids has the advantage of eliminating the need for maintainer lines in standard methods and saving agricultural material for sowing, thus reducing the cost of seed production for cotton hybrids. The use of wild cotton as a rootstock can expand the geographical range of seed production using ratooned cotton (Zhou, 2016). Experimental proof for grafting and its role in increasing yield with minimum effort is summarized in Table 4.
Producing Commercial F1 Hybrid Cotton Seeds by Ratoon Cultivation
Producing inexpensive hybrid F1 cotton seeds with high purity and heterosis has significance in commercial breeding. This production approach offers the highest economic potential for cultivating ratoon cotton in frost-free regions (Zhang et al., 2020a, 2022). There was no difference in yield and fiber quality between the hybrid F1 of the male-sterile line “Dong A” with and without ratoon cultivation crossed with the same male parent (Zhang and Zhou, 2009; Zhang et al., 2010). Therefore, breeding out cotton male-sterile lines with strong overwintering survival ability and fine comprehensive traits, and taking advantage of ratoon cultivation to maintain its sterility for producing hybrid seeds could reduce the current production cost of hybrid seeds (Zhang et al., 2015a; Zhou, 2016). This method does not require plowing the land and sowing the seeds each year, reducing the cost of raw materials for production and labor.
Furthermore, rouging and sister crossing would be avoided, which could simplify the breeding procedures of producing hybrid F1 cotton seeds and reduce the cost of breeding GMS lines, and the seed yield and purity of the hybrid cotton seeds could be improved. However, in the case of large-scale production of hybrid F1 cotton seeds, it is worth noting that artificially bred bees can be used, which can further enhance the purity (Zhang et al., 2015a). Additionally, high temperatures may cause some problems in producing hybrid F1 cotton seeds, such as trace fertile pollen occurring on male-sterile cotton in the tropics, which could be addressed by increasing heat tolerance through breeding.
Hybrids That Can Support Ratoon Cropping Patterns in Cotton
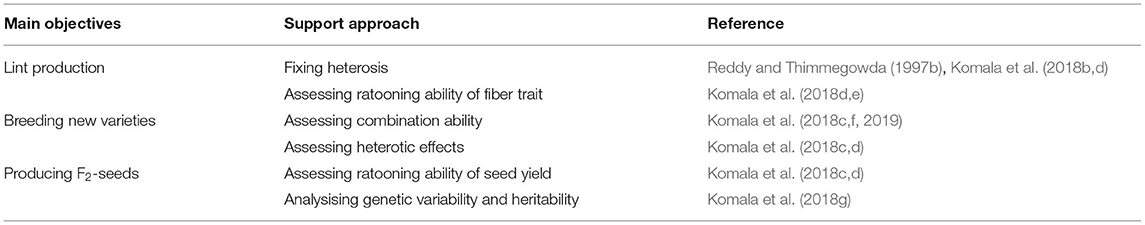
Hybrids can be used in ratoon cotton cropping for three objectives: (1) fixing heterosis for lint production, which requires assessment of the ratoon fiber yield and quality; (2) breeding new varieties for ratoon cropping, which requires the assessment of the combined ability of hybrids and their heterotic effects and (3) producing F2 seeds, which requires the analysis of the ratooning ability, genetic variability, and heritability of seed yield in the F2 generation (Table 5).
Ratooning Perennial Cotton for Genetic Research and Breeding
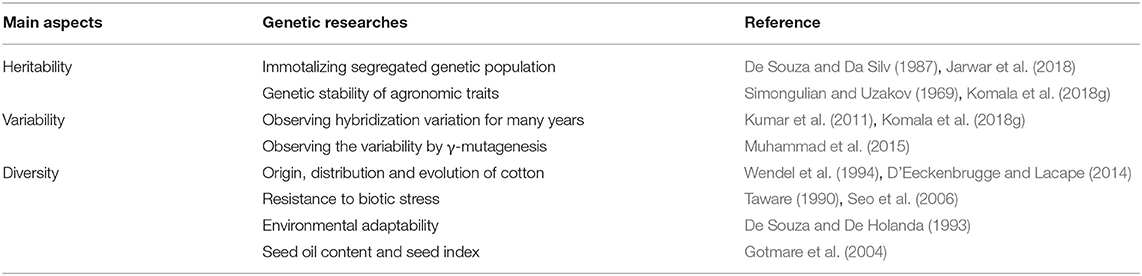
Genetic studies supporting ratoon cropping mainly include the following: (1) investigating heritability such as immortalizing segregated genetic populations and assessing the genetic stability of agronomic traits, (2) studying the variability of traits across generations, such as observing hybridization variation and variability by γ-mutagenesis over many years, and (3) researching the diversity of germplasm resources, such as revealing the origin, distribution and evolution, resistance to biotic stress, environmental adaptability, seed oil content, and seed index of cotton (Table 6).
Identification of Perennial Cotton Germplasm Resources
Some wild cotton (perennial cotton) species contain traits superior to those of annual cultivars in terms of growth and development, physio-biochemical characteristics, and stress resistance (Melo, 1952; Stephens, 1965; Simongulian and Uzakov, 1969; Plnheiro et al., 1970; De Souza and De Holanda, 1993). Among them, the perennial allotetraploids in the primary gene pool of Gossypium species are most easily applied to the breeding of upland cotton and sea island cotton, and their special agronomic traits are shown in Table 7. For example, G. hirsutum ssp. purpurascens, a perennial cotton species with year-round flowering and resistance to several biotic and abiotic stresses, is suitable for ratoon cultivation or breeding as a parent (Zhang et al., 2020a).
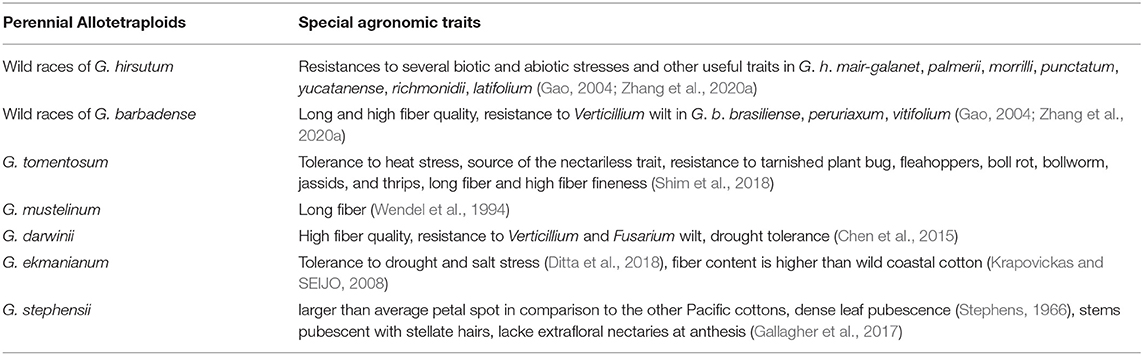
Table 7. Special agronomic traits in perennial allotetraploid cotton species of the primary gene pool.
Perennial cotton, an eco-friendly crop for carbon farming solutions (Zhang et al., 2020a), contains significantly different levels of many metabolites compared to annual cotton. As an eco-friendly carbon farming crop, perennial cotton species contain many metabolites different from those of annual cotton species. From a 3-year study carried out in India, of the 20 wild species and 8 accessions of G. arboretum, the highest seed oil content was recorded in the wild species G. lobatum, followed by G. harknessii, which showed that wild perennial species are helpful in improving the seed oil content of cultivated G. arboreum (Gotmare et al., 2004). In Brazil, a study found that the starch content in the roots of perennial cotton species was much higher than that in the roots of annual cotton (De Souza and Da Silv, 1987). It is thought that the roots of perennial cotton must reserve sufficient carbohydrates to start a subsequent asexual growth cycle when conditions are suitable or to counter a period of drought stress (Sadras, 1996; Wells, 2002).
Genetic Research on Ratoon Cotton
Cryotolerance is an essential trait in ratoon cotton breeding (Zhang et al., 2011). To investigate the interspecific heterosis and cytoplasmic effects of cryotolerance-related traits between annual and 2-year-old cotton, four reciprocal crosses of F1 hybrids and their parents were used. The results showed that the cold tolerance-related traits of the 2-year-old hybrid F1 showed transgressive heterosis, which was better than that of the annual hybrid F1; in addition, there was no significant cytoplasmic effect on the cryotolerance-related traits of annual and 2-year-old cotton; however, there was some effect of nuclear–cytoplasm interaction (Zhang et al., 2011). In another study, the mixed genetic model of the major gene plus polygene was used to research the cryotolerance inheritance of cotton in the overwintering period, and the results were consistent with two major additive genes plus the additive dominance polygene genetic model. These results suggested that inbreeding cryotolerant ratoon cotton, single-cross recombination, or single backcrossing would be helpful for transferring major genes associated with overwintering cryotolerance and selection in the F2 generation would be efficient (Zhang et al., 2012c,d). With the mining, labeling, cloning, and functional identification of perennial-related genes in wild cotton (Bourgou et al., 2017), more and better possibilities for molecular breeding in cotton will become available.
Ratooning Perennial Cotton for Breeding
Ratoon cotton can be used for breeding, such as in conserving germplasms, utilizing heterosis, and analyzing combination ability and heritability (Thomson and Luckett, 1988a,b; Komala et al., 2018d,f,g, 2019). Technological systems with great application value could be established to produce hybrid cotton seeds in frost-free areas for sowing cultivation in temperate zones (Zhang et al., 2020a,b). Researcher-based and company-involved strategies for perennially producing hybrid cotton seeds are in the small-area testing phase in southern China, where almost no cotton is currently planted, so cotton plants for producing hybrids can be easily separated spatially, which is conducive to improving the purity of hybrid seeds. Due to the warm environment, ratoon cotton flowers earlier with a more extended boll-opening period than annuals without the need for breeding bees for pollination or hand-pollination due to rich insect sources.
Future Studies on Ratooning Perennial Cotton for Breeding
Ratooning Perennial Cotton for Breeding Cytoplasmic Male-Sterile Lines
Male sterility plays an impressive role in heterosis utilization by facilitating hybrid breeding and has contributed greatly to the increased yield of many crops globally (Fan and Zhang, 2018). In cotton, due to limited resources and negative cytoplasmic effects, CMS lines have not been widely used (Zheng et al., 2019; Li et al., 2021). Most of the existing male sterility used in production has the genetic background of wild cotton, such as CMS-D2 and CMS-D8 (Zhang et al., 2019). Some wild cotton lines become sterile after transplanting from the origin (Zhang et al., 2022), showing traits such as non-flowering, non-dehiscence of anthers, and self-incompatibility. Through distant hybridization, doubling, and saturation backcrossing, some CMS mutant lines can be screened out (Zheng et al., 2019). Sometimes CMS mutant plants can be found in the field, but the maintainer line cannot be screened out in a timely manner by test crossing. Thus, the perennialization of mutant plants is very important for breeding CMS lines.
Ratooning Perennial Cotton for Breeding Photothermosensitive Genic Male-Sterile Lines
Two-line hybrid rice with high yield potential is becoming increasingly popular, and the PTGMS line is one of the essential components for breeding two-line hybrid rice (Barman et al., 2019). In cotton, increasing attention has been given to photothermosensitive male-sterile lines (Zhou et al., 2007; Sekhar and Khadi, 2012). In China, light and temperature conditions in the south and north are very different (Qian and Zhu, 2001). Based on this, a breeding strategy called “planting in temperate regions and breeding in the tropics” was employed for rice (Liang et al., 2020), maize (Eagles and Lothrop, 1994), and sweet potato (Lu et al., 1989). The ideal photothermosensitive male-sterile line is sterile in the breeding area, convenient for hybrid-seed production, and fertile in the planting area to obtain a high yield. Most PTGMS lines are strongly influenced by the environment, and to explore and take advantage of this feature, multiregional planting is necessary. A high temperature always makes the PTGMS sterile lines (Zhang et al., 2007; Mishra, 2013). For cotton, the temperature at the flowering stage needs to be higher than that at other growth stages (Himanshu et al., 2019). To test the sterile/fertile conversion temperature, transplanting the candidate PTGMS line plants in different areas can be conveniently achieved by a perennial cotton ratooning method.
Conclusion
This study reviews a vital topic within a broader framework for the utilization of perennial germplasm and ratoon cultivation for cotton breeding. Perennial cotton contains a rich diversity of agronomic and stress resistance traits that are important for expanding the economic performance of cotton cultivars. Ratoon cotton can be used to measure the combination ability and heterosis of hybrid combinations, observe the separation of mutagenized populations (Muhammad et al., 2015), and study the performance of a cultivar under different climates and multiple stress conditions for many years (Chamy, 1979). Increased investment is needed for perennial germplasm research, breeding, cultivation, and agroecological research on ratoon cotton. First, breeding efforts should focus on stabilizing the multiyear yield of ratoon cotton and determining the variety adaptability and cropping arrangements suitable for local conditions. Second, proper male-sterile lines for ratoon cultivation in the tropics should be selected or bred. Although ratoon cultivation of cotton has succeeded in some countries such as Brazil, China, and India, due to the lack of evaluation results with commercial production and sales of hybrid cotton seeds from ratooning systems, we can only hypothesize that this method would have good future prospects.
Author Contributions
XZ, QY, and AK wrote the manuscript. XZ, RZ, JZ, YF, BZ, YJ, and XD revised the manuscript. ZZ, XZ, and AK conceived the idea. All authors read and approved the final manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (31571600), the Postgraduate Education Reform and Quality Improvement Project of Henan Province (YJS2022ZX22), the Leading Talent Project in Science and Technology Innovation of Central Plain of China (214200510021), and the Program for Innovative Research Team (in Science and Technology) in University of Henan Province, China (21IRTSTHN023).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdurakhmonov, I. Y. (2007). “Exploiting genetic diversity”. In: Proceedings of World Cotton Research Conference-4 (Lubbock, TX).
Azevedo, D. M. P. D., Dos Santos, J. W., Vieira, D. J., Beltrão, N. E. D. M., Da Nóbrega, L. B., and Pereira, J. R. (2000). Plant population in perennial cotton/maize intercrop, yield components and agronomic efficiency. Revista de Oleaginosas e Fibrosas 4, 75–85.
Barman, H. N., Sheng, Z., Fiaz, S., Zhong, M., Wu, Y., Cai, Y., et al. (2019). Generation of a new thermo-sensitive genic male sterile rice line by targeted mutagenesis of TMS5 gene through CRISPR/Cas9 system. BMC Plant Biol. 19, 109. doi: 10.1186/s12870-019-1715-0
Bergman, D. K. (1985). Boll weevil (Coleoptera: Curculionidae) Overwintering in Arizona. The University of Arizona, Tucson, AZ, United States.
Bergman, D. K., Henneberry, T. J., Bariola, L. A., and Gillespie, J. M. (1983). Studies of Pest and Beneficial Insects in Arizona Stub and Planted Cotton. USDA: Oakland, Calif, 1–2.
Boopathi, N. M., and Hoffmann, L. V. (2016). “Genetic diversity, erosion, and population structure in cotton genetic resources,” in Sustainable Development and Biodiversity, Vol 8, eds M. Ahuja and S. Jain (Cham: Springer), 409–438. doi: 10.1007/978-3-319-25954-3_12
Boopathi, N. M., Sathish, S., Dachinamoorthy, P., Kavitha, P., and Ravikesav, R. (2014). “Usefulness and utilization of Indian cotton germplasm,” in World Cotton Germplasm Resources, eds I.Y. Abdurakhmonov (InTech: Rijeka, Croatia), 315–323.
Bourgou, L., Sawadogo, M., Sanfo, D., and Lacape, J. (2017). SSR-based genetic diversity of traditional and perennial cotton (Gossypium spp.) populations collected in Burkina Faso. Genet. Resour. Crop Ev. 64, 1743–1759. doi: 10.1007/s10722-016-0470-4
Campbell, B. T., Saha, S., Percy, R., Frelichowski, J., Jenkins, J. N., Park, W., et al. (2010). Status of the global cotton germplasm resources. Crop Sci. 50, 1161. doi: 10.2135/cropsci2009.09.0551
Castro, A. A., Hoffmann, L. V., Lima, T. H., Oliveira, A. I. D., Brito, R. R., Mendes, L. D. M. O., et al. (2016). Gossypium barbadense: an approach for in situ conservation in Cerrado, Brazil. J. Agr. Sci. 8, 59–67. doi: 10.5539/jas.v8n8p59
Chamy, A. (1979). Studies on Ratoon Management of Hybrid Cotton Variety CBS 156 (G. hirsutum-barbadense). Coimbatore: Tamil Nadu Agricultural University.
Chen, G. P. (2008). Studies on Biological Basis for Perennial Cultivation of Annual Upland Cotton in Southern Guangxi. Guangxi University, Nanning, China.
Chen, G. P., Zhang, X., Zhou, R. Y., and Zhao, H. T. (2008). Study on economic characteristics of biennial and annual upland cotton. Guihaia 28, 636–639. doi: 10.3969/j.issn.1000-3142.2008.05.017
Chen, G. P., Zhang, X., Zhou, R. Y., and Zhao, H. T. (2010a). Study on law of growth and development for perennial cultivation of annual upland cotton in Southern Guangxi. Southwest China J. Agric. Sci. 23, 650–655. doi: 10.3724/SP.J.1142.2010.40491
Chen, G. P., Zhang, X., Zhou, R. Y., and Zhao, H. T. (2010b). Study on the yield factors and path analyses for perennial cultivation of upland cotton in Southern Guangxi. Guihaia 30, 526–530. doi: 10.3969/j.issn.1000-3142.2010.04.020
Chen, G. P., Zhang, X., Zhou, R. Y., and Zhao, H. T. (2010c). The effect of varieties, sowing date and perennial cultivation on survival rate of upland cotton after winter. Acta Agriculturae Boreali-Occidentalis Sinica 18, 9784–9788. doi: 10.3969/j.issn.1004-1389.2010.12.011
Chen, H., Khan, M. K. R., Zhou, Z., Wang, X., Cai, X., Ilyas, M. K., et al. (2015). A high-density SSR genetic map constructed from a F2 population of Gossypium hirsutum and Gossypium darwinii. Gene 574, 273–286. doi: 10.1016/j.gene.2015.08.022
De Souza, J. G., and Da Silv, J. V. (1987). Partitioning of carbohydrates in annual and perennial cotton (Gossypium hirsutum L.). J. Exp. Bot. 38, 1211–1218. doi: 10.1093/jxb/38.7.1211
De Souza, N. A., and De Holanda, J. S. (1993). Environmental adaptability of perennial cotton in the Seridó. Pesqui. Agropecu. Bras. 28, 797–801.
D'Eeckenbrugge, G. C., and Lacape, J. M. (2014). Distribution and differentiation of wild, feral, and cultivated populations of perennial upland cotton (Gossypium hirsutum L.) in Mesoamerica and the Caribbean. PLoS ONE 9, e107458. doi: 10.1371/journal.pone.0107458
Ditta, A., Zhou, Z., Cai, X., Wang, X., Okubazghi, K. W., Shehzad, M., et al. (2018). Assessment of genetic diversity, population structure, and evolutionary relationship of uncharacterized genes in a novel germplasm collection of diploid and allotetraploid Gossypium accessions using EST and genomic SSR markers. Int. J. Mol. Sci. 19, 2401. doi: 10.3390/ijms19082401
Eagles, H. A., and Lothrop, J. E. (1994). Highland maize from central Mexico—its origin, characteristics, and use in breeding programs. Crop Sci. 34, 11–19. doi: 10.2135/cropsci1994.0011183X003400010002x
Fan, Y., and Zhang, Q. (2018). Genetic and molecular characterization of photoperiod and thermo-sensitive male sterility in rice. Plant Reprod. 31, 3–14. doi: 10.1007/s00497-017-0310-5
Flint, H. M., Salter, S. S., and Walters, S. (1980). Development of Cotton and Associated Beneficial and Pest Insect Populations in a Ratoon Field at Phoenix. Agricultural Reviews and Manuals ARM-W US Dept. of Agriculture, Arizona, United States.
Gallagher, J. P., Grover, C. E., Rex, K., Moran, M., and Wendel, J. F. (2017). A new species of cotton from Wake Atoll, Gossypium stephensii (Malvaceae). Syst. Bot. 42, 115–123. doi: 10.1600/036364417X694593
Gao, Y. H. (2004). Study on the Interspecies Hybrid and Genetics and Systematic Development Between the Four Cultivated Cotton Species. Zhejiang University, Hangzhou, China.
Gotmare, V., Singh, P., Mayee, C. D., Deshpande, V., and Bhagat, C. (2004). Genetic variability for seed oil content and seed index in some wild species and perennial races of cotton. Plant Breeding 123, 207–208. doi: 10.1046/j.1439-0523.2003.00914.x
Gutstein, Y. (1969). Evapotranspiration and Water-Use Efficiency in Seed and Ratoon Growth of Two Species of Cotton Grown Under Dryland Subtropical Conditions. Rehovot Nat Univ Inst Agr Prelim Rep. (Rehovot).
Hao, J. J., Ma, Q. X., Liu, H. M., Jia, X. H., Dong, Z. D., Liu, S. M., et al. (2010). Effects of grafting cotton on Verticillium wilt resistance, yield and fiber quality of cotton. Scientia Agricultura Sinica 43, 3974–3980. doi: 10.1097/MOP.0b013e3283423f35
Himanshu, S. K., Ale, S., Bordovsky, J., and Darapuneni, M. (2019). Evaluation of crop-growth-stage-based deficit irrigation strategies for cotton production in the Southern High Plains. Agr. Water Manage. 225, 105782. doi: 10.1016/j.agwat.2019.105782
Jarwar, A. H., Wang, X. Y., Jarwar, Z. H., Ma, Q. F., and Fan, S. L. (2018). Use of molecular markers in improvement of cotton for agronomic traits. Int. J. Nanotechnol. Allied Sci. 2, 39–60.
Jia, Y. H., Sun, J. L., and Du, X. M. (2014). “Cotton germplasm resources in China,” in World Cotton Germplasm Resources, eds I. Y. Abdurakhmonov (InTech: Rijeka, Croatia), 35–53.
Jin, M. K. (2013). The Research of Germplasm Innovation of Bt Island Cotton and the Grafted Conduction of Insect-Resistant Protein. Guangxi University, Nanning, China.
Khader, S. E. S. A., and Prakash, A. H. (2014). Pruning technique for second fruiting cycle in cotton crop. Cotton Res. J. 6, 46–49.
Kiranga, N. A. (2013). “Morpho-argro-physio-karyotypic Characterization of Wild Cotton (Gossypium spp.), Germplasm From Selected Counties in Kenya”. (Kenyatta University: Kenyatta).
Komala, M., Ganesan, N. M., and Kumar, M. (2018c). Ratooning and combining ability analysis through line × tester mating design in interspecific cotton hybrids (G. hirsutum × G. barbadense). Int. J. Agric. Environ. Biotechnol. 11, 333–343. doi: 10.30954/0974-1712.04.2018.15
Komala, M., Ganesan, N. M., and Kumar, M. (2018d). Assessment of ratooning ability and heterotic effects for yield and yield contributing traits in intraspecific hybrids of upland cotton. Curr. Agric. Res. J. 6, 85–94. doi: 10.12944/CARJ.6.1.11
Komala, M., Ganesan, N. M., and Kumar, M. (2018e). Studies on ratooning ability for yield and fibre quality traits in interspecific cotton hybrids (Gossypium hirsutum x Gossypium barbadense). Res. Crops 19, 752–757. doi: 10.31830/2348-7542.2018.0001.59
Komala, M., Ganesan, N. M., and Kumar, M. (2018f). Combining ability for yield and yield contributing traits in intraspecific hybrids of ratoon upland cotton. Int. J. Basic Appl. Agric. Res. 16, 22–28.
Komala, M., Ganesan, N. M., and Kumar, M. (2018g). Genetic variability, heritability and correlation analysis in F2 populations of ratoon upland cotton hybrids. Int. J. Agric. Environ. Biotechnol. 11, 815–827. doi: 10.30954/0974-1712.12.2018.2
Komala, M., Ganesan, N. M., Kumar, M., Abasianyanga, I., Amalabalu, P., and Premalatha, N. (2018b). Investigations on the ratooning ability of cotton interspecific hybrids (G. hirsutum L× G. barbadense L) and their parents. Int. J. Basic Appl. Agric. Res. 16, 146–153.
Komala, M., Ganesan, N. M., Kumar, M., Manonmani, K., Mahalingam, L., and Premalatha, N. (2018a). Studies on per se performance and ratooning ability for yield and fibre quality traits in intraspecific cotton hybrids. Crop Res. 53, 174–178. doi: 10.31830/2454-1761.2018.0001.14
Komala, M., Kumar, M., and Ganesan, N. M. (2019). Combining ability effects for fibre quality traits in first and ratoon crops of cotton interspecific hybrids (G. hirsutum× G. barbadense). Res. Crops 20, 230–235. doi: 10.31830/2348-7542.2019.033
Kong, X. Q., Luo, Z., Dong, H. Z., Eneji, A. E., and Li, W. J. (2012). Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J. Exp. Bot. 63, 2105–2116. doi: 10.1093/jxb/err420
Krapovickas, A., and SEIJO, G. (2008). Gossypium ekmanianum (Malvaceae), a wild cotton from Dominican Republic. Bonplandia 17, 55–63. doi: 10.30972/bon.1711361
Kumar, S., Kular, J., and Dhaliwal, L. (2011). Seasonal abundance of mealy bug (Phenacoccus solenopsis Tinsley) on Bt cotton in Punjab. Acta Phytopathologica Et Entomologica Hungarica 46, 115–127. doi: 10.1556/APhyt.46.2011.1.9
Li, M., Chen, L., Khan, A., Kong, X., Khan, M. R., Rao, M. J., et al. (2021). Transcriptome and miRNAomics analyses identify genes associated with cytoplasmic male sterility in cotton (Gossypium hirsutum L.). Int. J. Mol. Sci. 22, 4684. doi: 10.3390/ijms22094684
Liang, C. B., Li, J. G., Jiang, H. B., and Yao, J. P. (2020). The strategy of high quality Japonica hybrid rice about planting in the north and breeding in the south. North Rice 50, 53–56. doi: 10.16170/j.cnki.1673-6737.2020.04.016
Lou, X. Y. (2010). The Screened and Grafted Resistant Rootstock's Effect on Improving Resistance of Fusarium and Verticillium wilts of IsIand Cotton. Guangxi University, Nanning, China.
Lu, S. Y., Xue, Q. H., Zhang, D. P., and Song, B. F. (1989). “Sweet potato production and research in China,” in: Improvement of Sweet Potato (Ipomoea batatas) in Asia. (Lima, Peru: International Potato Center).
Luo, Z., Kong, X. Q., Zhang, Y. J., Li, W. J., Zhang, D. M., Dai, J. L., et al. (2019). Leaf-derived jasmonate mediates water uptake from hydrated cotton roots under partial root-zone irrigation. Plant Physiol. 180, 1660–1676. doi: 10.1104/pp.19.00315
Macharia, J. (2013). Effect of Ratooning and Nitrogen Application on Lint Yield and Quality of Cotton Varieties in Central Kenya (Nairobi: University of Nairobi).
Melo, D. N. F. (1952). The Cultivation of Mocó (perennial) Cotton. Ministry of Agriculture, Rio de Janeiro, Brazil.
Migicovsky, Z., and Myles, S. (2017). Exploiting wild relatives for genomics-assisted breeding of perennial crops. Front. Plant Sci. 8, 460. doi: 10.3389/fpls.2017.00460
Mihail, J. D., Brown, J. K., and Nelson, M. R. (1987). The Effects of Cotton Leaf Crumple on Greenhouse-Grown Cotton Incoulated at Five Growth Stages. College of Agriculture, University of Arizona, Tucson, AZ.
Mishra, V. K. (2013). Molecular and genetic basis of male sterility in development of hybrid varieties. A review. Int. J. Curr. Res. 5, 191–197.
Mubvekeri, W., Bare, J., Makaka, C., and Jimu, F. (2014). Assessing the diversity and intensity of pesticide use in communal area cotton production in Zimbabwe. J. Ecol. Nat. Environ. 6, 342–348. doi: 10.5897/JENE2014.0476
Muhammad, A., Rauf, S., and Naz, K. (2015). Induced genetic variability in selected γ-radiated cotton varieties during second year ratooning under rain fed environment. Asian J. Nat. Appl. Sci. 4, 70–81.
Nawaz, B., Naeem, M., Malik, T. A., Muhae-Ud-Din, G., Ahmad, Q., and Sattar, S. (2019). A review about cotton leaf curl viral disease and its control strategies in Pakistan. Int. J. Inn. Appl. Agric. Res 3, 132–147. doi: 10.29329/ijiaar.2019.188.13
Percy, R. G., Frelichowski, J. E., Arnold, M. D., Campbell, T. B., Dever, J. K., Fang, D. D., et al. (2014). “The US national cotton germplasm collection–Its contents, preservation, characterization, and evaluation,” in World Cotton Germplasm Resources, eds I. Y. Abdurakhmonov (InTech: Rijeka, Croatia), 167–201.
Plnheiro, D. M., Fournier, J., and Trellu, A. (1970). Physiology and breeding of the Brazilian perennial cotton “Mocó”. Relationship between annual and total yields. Coton et Fibres Tropicales. 25, 175–179.
Plucknett, D. L., Evenson, J. P., and Sanford, W. G. (1970). Ratoon cropping. Adv. Agron. 22, 285–330. doi: 10.1016/S0065-2113(08)60271-0
Qian, W., and Zhu, Y. (2001). Climate change in China from 1880 to 1998 and its impact on the environmental condition. Clim. Change 50, 419–444. doi: 10.1023/A:1010673212131
Qiu, A. H., Liao, X. F., Wang, C. C., Tang, D. F., Zhou, B. J., Chen, P., et al. (2015). Effect of grafting on improving cotton's resistance to NaCl stress. J. China Agric. Univ. 20, 53–60. doi: 10.11841/j.issn.1007-4333.2015.06.07
Rahmat, Z., Mahmood, A., Abdullah, K., and Zafar, Y. (2014). “Cotton germplasm of Pakistan,” in World Cotton Germplasm Resources, eds I. Y. Abdurakhmonov (InTech: Rijeka, Croatia), 137–166.
Reddy, D. V. S., and Thimmegowda, S. (1997a). Economic analysis of different drip irrigation systems of main and ratoon hybrid cotton. Mysore J Agric Sci. 31, 17–22.
Reddy, D. V. S., and Thimmegowda, S. (1997b). Effect of different systems and levels of irrigation and pruning height on the performance of main and ratoon crop of DCH-32 hybrid cotton. Karnataka J. Agric. Sci. 10, 517–520.
Rui, Y. K., Zhu, B. Z., and Luo, Y. B. (2005). Long-distance transportation of Bt-toxin through xylem sap in Bt-cotton (Gossyposium). Chin. Bulletin Bot. 22, 320–324. doi: 10.1360/aps040074
Sachs, M. H., and Zilkah, S. (1985). Characterization of climatic factors affecting chilling injury in field-grown ratoon cotton. J. Agr. Sci. 105, 475–478. doi: 10.1017/S0021859600056525
Sadras, V. O. (1996). Cotton responses to simulated insect damage: radiation-use efficiency, canopy architecture and leaf nitrogen content as affected by loss of reproductive organs. Field Crop. Res. 48, 199–208. doi: 10.1016/S0378-4290(96)00046-9
Seabrook, W. B. (1844). A Memoir on the Origin, Cultivation and Uses of Cotton. Miller and Browne: Charleston.
Sekhar, L., and Khadi, B. M. (2012). “Genetic, biochemical, histological and molecular analysis of thermosensitive genetic male sterility (TGMS) in cotton (Gossypium arboreum L)” in: Silver Jubilee International Symposium on “Global Cotton Production Technologies vis-a-vis Climate Change” (CCS Haryana Agricultural University, Hisar).
Seo, Y., Zhou, Y., Turini, T. A., Cook, C. G., Gilbertson, R. L., and Natwick, E. T. (2006). Evaluation of cotton germ plasm for resistance to the whitefly and cotton leaf crumple (CLCr) disease and etiology of CLCr in California's Imperial Valley. Plant Dis. 90, 877–884. doi: 10.1094/PD-90-0877
Shim, J., Mangat, P. K., and Angeles-Shim, R. B. (2018). Natural variation in wild Gossypium species as a tool to broaden the genetic base of cultivated cotton. J. Plant Sci. Curr. Res 2, 1–9. doi: 10.24966/PSCR-3743/100005
Silva, F. P. D., Bezerra, A. P. L., and Silva, A. F. D. (2008). Boll weevil (Anthonomus grandis Boheman) oviposition and feed in ratoon cotton of mutants lines of upland cotton. Rev. Cienc. Agron. 39, 85–89. doi: 10.1590/S0100-204X2008000100016
Simongulian, N. G., and Uzakov, I. (1969). Inheritance of fast ripening and photoperiodic response in the hybrids between the annual and the perennial forms of cotton Gossypium mexicanum Tod. Genetika. 6, 24–31.
Stephens, S. G. (1965). The effects of domestication on certain seed and fiber properties of perennial forms of cotton, Gossypium hirsutum L. Am. Naturalist 99, 355–372. doi: 10.1086/282377
Stephens, S. G. (1966). The potentiality for long range oceanic dispersal of cotton seeds. Am. Nat. 100, 199–210. doi: 10.1086/282413
Stroman, G. N. (1961). An approach to hybrid cotton as shown by intra-and interspecific crosses. Crop Sci. 1, 363–366. doi: 10.2135/cropsci1961.0011183X000100050020x
Taware, S. P. (1990). Genetic Studies on Bollworm Resistance and Exploitation of Hybrid Vigour in Perennial Cotton. Savitribai Phule Pune University, Pune, India.
Teravanesyan, D. V., and Belova, Z. F. (1970). Perennial cotton plants as a genetical source for breeding wilt-resistant varieties. Tr Prikladnoi Bot Genet Selek 42, 65–71.
Thomson, N. J., and Luckett, D. J. (1988a). Heterosis and combining ability effects on cotton. I. Combining ability. Aust. J. Agric. Res. 39, 973–990. doi: 10.1071/AR9880973
Thomson, N. J., and Luckett, D. J. (1988b). Heterosis and combining ability effects on cotton. II. Heterosis. Aust. J. Agric. Res. 39, 991–1002. doi: 10.1071/AR9880991
Ullah, R., Akhtar, K. P., Moffett, P., Mansoor, S., Briddon, R. W., and Saeed, M. (2014). An analysis of the resistance of Gossypium arboreum to cotton leaf curl disease by grafting. Eur. J. Plant Pathol. 139, 837–847. doi: 10.1007/s10658-014-0437-2
Vukicevich, E., Lowery, T., Bowen, P., Úrbez-Torres, J. R., and Hart, M. (2016). Cover crops to increase soil microbial diversity and mitigate decline in perennial agriculture. A review. Agron. Sustain. Dev. 36, 1–14. doi: 10.1007/s13593-016-0385-7
Wallace, T. P., Bowman, D., Campbell, B. T., Chee, P., Gutierrez, O. A., Kohel, R. J., et al. (2009). Status of the USA cotton germplasm collection and crop vulnerability. Genet. Resour. Crop Ev. 56, 507–532. doi: 10.1007/s10722-008-9382-2
Wan, P., Xu, D., Cong, S. B., Jiang, Y. Y., Huang, Y. X., Wang, J. T., et al. (2017). Hybridizing transgenic Bt cotton with non-Bt cotton counters resistance in pink bollworm. P. Natl. Acad. Sci. Usa. 114, 5413–5418. doi: 10.1073/pnas.1700396114
Wang, K., Wendel, J. F., and Hua, J. (2018). Designations for individual genomes and chromosomes in Gossypium. J. Cotton Res. 1, 3. doi: 10.1186/s42397-018-0002-1
Wang, K. B. (2007). Introduction and conservation of wild cotton in China. Cotton Sci. 19, 354–361. doi: 10.3969/j.issn.1002-7807.2007.05.005
Weaver, J. B. (1968). Analysis of a genetic double recessive completely male-sterile cotton. Crop Sci. 8, 597–600. doi: 10.2135/cropsci1968.0011183X000800050027x
Wells, R. (2002). Stem and root carbohydrate dynamics of two cotton cultivars bred fifty years apart. Agron. J. 94, 876–882. doi: 10.2134/agronj2002.8760
Wendel, J. F., and Grover, C. E. (2015). “Taxonomy and evolution of the cotton genus, Gossypium,” in Cotton, Agronomy Monograph, 2nd Edn, eds D. D. Fang and R.G. Percy (American Society of Agronomy, Inc., Crop Science Society of America, Inc., and Soil Science Society of America, Inc.: Madison), 25–44.
Wendel, J. F., Rowley, R., and Stewart, J. M. (1994). Genetic diversity in and phylogenetic relationships of the Brazilian endemic cotton, Gossypium mustelinum (Malvaceae). Plant Syst. Evol. 192, 49–59. doi: 10.1007/BF00985907
Zhang, G. L., Chen, L. Y., Zhang, S. T., Liu, G. H., Tang, W. B., Zhi-Zhou, H. E., et al. (2007). Effect of high temperature stress on nitrogen metabolism of flag leaves in rice. Hybrid Rice 22, 57–61. doi: 10.3969/j.issn.1005-3956.2007.04.025
Zhang, J., Abdelraheem, A., and Stewart, J. M. (2019). A comparative analysis of cytoplasmic effects on lint yield and fiber quality between CMS-D2 and CMS-D8 systems in upland cotton. Crop Sci. 59, 624–631. doi: 10.2135/cropsci2018.10.0614
Zhang, J. H., Bie, S., Wang, X. G., Xia, S. B., Qian, A. M., Wu, Z. Y., et al. (2018). The high-efficient grafting and seedling technique of upland cotton and island cotton and its application. Hubei Agric. Sci. 57, 37–39. doi: 10.14088/j.cnki.issn0439-8114.2018.21.009
Zhang, M. J., Xia, Q. Z., and Wu, B. (2012b). Resistance and physiological changes of grafted cotton to Verticillium dahhae Kleb. J. Huazhong Agric. Univ. 31, 414–418.Available online at: http://hnxbl.cnjournals.net/hznydxzren/article/abstract/20120404
Zhang, X., Chen, G. P., Pan, F. Y., Wang, X. Y., and Zhou, R. Y. (2011). Research on interspecific heterosis and cytoplasm effect of cryotolerance and its related traits in perennial cotton. Southwest China J. Agric. Sci. 24, 1669–1675. doi: 10.3969/j.issn.1001-4829.2011.05.011
Zhang, X., Chen, G. P., and Zhou, R. Y. (2010). Effect of perennial cultivation on “Dong A” genic male sterile lines in annual upland cotton. Guihaia 30, 391–394. doi: 10.3969/j.issn.1000-3142.2010.03.021
Zhang, X., Fei, L., Zhou, R., and Chen, G. (2012c). Heterosis utilization of hybrid cotton (G. hirsutum×G. barbadence) in south Guangxi. Chinese J. Trop. Crop. 33, 1164–1169. doi: 10.3969/j.issn.1000-2561.2012.07.003
Zhang, X., Khan, A., Zhou, R., Liu, Y., Zhang, B., Wang, Q., et al. (2022). Grafting in cotton: A mechanistic approach for stress tolerance and sustainable development. Ind. Crop. Prod. 175, 114227. doi: 10.1016/j.indcrop.2021.114227
Zhang, X., Kong, X. J., Zhou, R. Y., Zhang, Z. Y., Zhang, J. B., Wang, L. S., et al. (2020a). Harnessing perennial and indeterminant growth habits for ratoon cotton (Gossypium spp.) cropping. Ecosyst Health Sust. 6, 1715264. doi: 10.1080/20964129.2020.1715264
Zhang, X., Li, C., Wang, X., Chen, G., Zhang, J., and Zhou, R. (2012d). Genetic analysis of cryotolerance in cotton during the overwintering period using mixed model of major gene and polygene. J. Integr. Agr. 11, 537–544. doi: 10.1016/S2095-3119(12)60040-9
Zhang, X., Zhang, Z. Y., Wang, Q. L., Chen, P., Chen, G. P., and Zhou, R. Y. (2013). Effects of rootstocks on cryotolerance and overwintering survivorship of genic male sterile lines in upland cotton (Gossypium hirsutum L.). PLoS ONE 8, e63534. doi: 10.1371/journal.pone.0063534
Zhang, X., Zhang, Z., Zhou, R., Wang, Q., and Wang, L. (2020b). Ratooning annual cotton (Gossypium spp.) for perennial utilization of heterosis. Front. Plant Sci. 11, 1939. doi: 10.3389/fpls.2020.554970
Zhang, X., and Zhou, R. Y. (2009). Cutting propagation and perennial cultivation of genic male sterile upland cotton (Gossypium hirsutum L.) and its heterosis utilization. J. Trop. Subtrop. Botany 17, 489–493. doi: 10.3969/j.issn.1005-3395.2009.05.011
Zhang, X. J., Yue, F. L., Zhang, X. H., Hou, R., Zhang, X. Q., and Li, W. J. (2015a). Technical system of hybrid seed production with perennial plants of cotton sterile lines. Acta Agronomica Sinica 41, 1836–1843. doi: 10.3724/SP.J.1006.2015.01836
Zhang, X. J., Yue, F. L., Zhang, X. H., Li, W. J., and Zhang, X. Q. (2015b). Research on root retention reproduction technique of genetic male-sterile line in cotton for seed production. Seed Sci. Technol. 43, 187–196. doi: 10.15258/sst.2015.43.2.01
Zhang, Y. M., Tian, C., Jiang, L. M., Li, Y. P., Xiao, Z. M., and Li, J. L. (2012a). Advantages of perennial crop on conservation of agroecological environment. Adv. Mat. Res. 518–523, 5213–5216. doi: 10.4028/www.scientific.net/AMR.518-523.5213
Zheng, J., Kong, X., Li, B., Khan, A., Li, Z., Liu, Y., et al. (2019). Comparative transcriptome analysis between a novel allohexaploid cotton progeny CMS line LD6A and its maintainer line LD6B. Int. J. Mol. Sci. 20, 6127. doi: 10.3390/ijms20246127
Zhou, R. Y. (2016). Method of Producing Hybrid Seeds for Annual Cotton by Cultivating Perennially. U.S. Patent No US9265206B2. Washington, DC: U.S. Patent and Trademark Office.
Keywords: Gossypium (cotton), heterosis, indeterminate, male-sterile, stub
Citation: Zhang X, Yang Q, Zhou R, Zheng J, Feng Y, Zhang B, Jia Y, Du X, Khan A and Zhang Z (2022) Perennial Cotton Ratoon Cultivation: A Sustainable Method for Cotton Production and Breeding. Front. Plant Sci. 13:882610. doi: 10.3389/fpls.2022.882610
Received: 24 February 2022; Accepted: 28 April 2022;
Published: 06 June 2022.
Edited by:
Linghe Zeng, United States Department of Agriculture (USDA), United StatesReviewed by:
Narayanan Manikanda Boopathi, Tamil Nadu Agricultural University, IndiaYouxiong Que, Fujian Agriculture and Forestry University, China
Copyright © 2022 Zhang, Yang, Zhou, Zheng, Feng, Zhang, Jia, Du, Khan and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Aziz Khan, aziz.hzau@gmail.com; Zhiyong Zhang, z_zy123@126.com
 Xin Zhang
Xin Zhang Qian Yang2
Qian Yang2 Ruiyang Zhou
Ruiyang Zhou Jie Zheng
Jie Zheng Baohong Zhang
Baohong Zhang Aziz Khan
Aziz Khan