- College of Grassland Science, Nanjing Agricultural University, Nanjing, China
Seashore paspalum (Paspalum vaginatum O. Swartz) is an important warm-season turfgrass species. In this study we generated transgenic seashore paspalum overexpressing CdtNF-YC1, a nuclear factor Y transcription factor from hybrid bermudagrass (Cynodon dactylon × Cynodon transvaalensis). DNA blot hybridization and qRT-PCR analysis showed that CdtNF-YC1 was integrated into the genomes of transgenic seashore paspalum plants and expressed. Reduced relative water content (RWC) and survival rate and increased ion leakage were observed in both wild type (WT) and transgenic plants after drought stress, while transgenic plants had higher levels of RWC and survival rate and lower ion leakage than the WT. Maximal photochemical efficiency of photosystem II (Fv/Fm), chlorophyll concentration and survival rate were decreased after salt stress, while higher levels were maintained in transgenic plants than in WT. In addition, an increased Na+ content and decreased or unaltered K+ in leaves and roots were observed after salt treatment, while lower level of Na+ and higher levels of K+ and K+/ Na+ ratio were maintained in transgenic plants than in WT. The results indicated that overexpressing CdtNF-YC1 resulted in enhanced drought and salt tolerance in transgenic plants. Transcript levels of stress responsive genes including PvLEA3, PvP5CS1, PvABI2, and PvDREB1B were induced in response to drought and salt stress, and higher levels were observed in transgenic seashore paspalum than in WT. The results suggest that the enhanced drought and salt tolerance in transgenic seashore paspalum is associated with induction of a series of stress responsive genes as a result of overexpression of CdtNF-YC1.
Introduction
Plant growth and development are often influenced by abiotic stress such as drought and salinity. Transcription factors are key players in plant adaptation to abiotic stresses by regulating downstream gene expression (Singh et al., 2002). Nuclear factor Y (NF-Y) transcription factor is consisted of three subunits namely NF-YA, NF-YB, and NF-YC. It regulates transcription of downstream genes by binding to CCAAT elements in approximately 25% of eukaryotic gene promoters (Nardini et al., 2013). NF-Y subunits are encoded by multigene families in plants,(Petroni et al., 2012), for example, there are 30 NF-Y members in Arabidopsis (Siefers et al., 2009) and 36 NF-Y members (seven NF-YAs, 17 NF-YBs, and 12 NF-YCs) in the model grass species Brachypodium distachyon (Cao et al., 2011). The multiple NF-Y subunit combinations result in a large variety of NF-Y complexes and diverse functions (Zhao et al., 2016).
Large body of investigations reveal that NF-Y regulate plant growth, development and environmental stress responses (Hou et al., 2014; Zhao et al., 2016; Swain et al., 2017; Zanetti et al., 2017; Lian et al., 2018; Wang et al., 2018). Arabidopsis NF-YC3/4/9 are bound to CCAAT elements of ABI5 promoter to activate its expression and integrated GA and ABA signaling pathways in seed germination (Liu et al., 2016). NF-YA1/2/4, NF-YB1/2/3, and NF-YC1/2/3/4/9 regulate Arabidopsis flowering time (Kumimoto et al., 2008, 2010; Hackenberg et al., 2012; Mu et al., 2013; Hou et al., 2014; Xu et al., 2016). Arabidopsis NF-YC1 confers cold tolerance (Shi et al., 2014), NF-YB3 and NF-YC10 confer heat responses (Sato et al., 2014), NF-YA5, NF-YB1, and NF-YC2 confer drought tolerance (Nelson et al., 2007; Li et al., 2008; Liu and Howell, 2010), and NF-YA1 confers salt tolerance (Li Y.J. et al., 2013). Overexpressing NF-YA5 increased drought tolerance, whereas Arabidopsis nf-ya5 mutants were sensitive to drought compared with the wild type (WT) (Li et al., 2008). OsNF-YA7 expression was up-regulated by drought, and transgenic rice overexpressing OsNF-YA7 showed enhanced drought tolerance (Lee et al., 2015). OsNF-YC13 is identified to be associated with salt tolerance from one activation-tagged salt tolerant indica rice (Manimaran et al., 2017). Our previous study showed that expression of CdtNF-YC1, an ortholog of OsNF-YC4 or AtNF-YC2, is induced by drought and salt stress in hybrid bermudagrass, which depends on ABA; its overexpression results in elevated drought and salt tolerance in transgenic rice with increased ABA sensitivity (Chen et al., 2015). NF-Ys are good candidate genes used for crops improvement of drought and salt tolerance.
Seashore paspalum (Paspalum vaginatum O. Swartz, 2n = 2x = 20) is an important warm-season turfgrass species and commonly used on golf course, athletic fields, and landscape areas. Compared to other turfgrass species, seashore paspalum has superior salt tolerance (Shahba et al., 2012; Uddin et al., 2012), which is associated with maintenance of ion homeostasis by maintaining a high K+ concentration in shoots, reducing the Na+ being transferred from roots to shoots and leading to maintenance of high K+/Na+ ratio under salt conditions (Uddin et al., 2012; Guo et al., 2016). However, it is sensitive to drought (Schiavon et al., 2012; Zhou et al., 2012). There is increasing demand for turfgrass species with improved drought and salt tolerance due to limited water resources and increasing salinity soil worldwide and irrigation using saline water (Pessarakli, 2018). Transgenics has provided an effective tool for turfgrass improvement (Guo et al., 2003; Giri and Praveena, 2015; Luo et al., 2017). For example, broad abiotic stress tolerance is enhanced by overexpressing rice SUMO E3 ligase (OsSIZ1) or a cyanobacterial flavodoxin in transgenic creeping bentgrass (Agrostis palustris L.) (Li Z. et al., 2013; Li et al., 2017). However, transgenic seashore paspalum with improved abiotic stress tolerance has not been reported. It was aimed of this study to generated transgenic seashore paspalum for improved drought and salt tolerance by overexpressing CdtNF-YC1.
Materials and Methods
Generation of Transgenic Seashore Paspalum
The sterilized seeds of the seashore paspalum cultivar ‘Sea Spray’ were placed on callus induction medium (MS2.5) (MS basal medium, 2.5 mg L−1 2, 4-dichlorophenoxy acetic acid (2, 4-D), 0.6 mg L−1 CuSO4, 30 g L−1 sucrose, 8 g L−1 agar, pH 5.8) and incubated at 25°C for induction of callus in the dark. The compact embryogenic callus was propagated for 3 to 5 months with subcultures every 4 weeks, followed by infection with Agrobacterium tumefaciens strain EHA105 (OD600 nm = 0.6) harboring CdtNF-YC1 construct (Chen et al., 2015) for 30 min at room temperature, with occationally shaking. The infected calli were co-cultivated on solid MS2.5 for 2 days in the dark at 25°C. After co-cultivation, the calli were rinsed two to three times in sterilized water containing 200 mg L−1 cefotaxime, drained on filter paper and transferred to callus selection medium (MS2.5 supplemented with 200 mg L−1 hygromycin B and 200 mg L−1 cefotaxime). After 10 to 12 weeks of selection with subcultures every 4 weeks at 28°C, hygromycin B resistant calli were transferred to regeneration medium with selection pressure (MS basal medium containing 0.2 mg L−1 kinetin, 100 mg L−1 hygromycin B, and 100 mg L−1 cefotaxime) for regeneration at 30°C under light. Regenerated shoots were transferred to 1/2 MS containing 100 mg L−1 hygromycin B and 100 mg L−1 cefotaxime for developing roots.
Plant Growth and Treatments
Transgenic plants and WT were planted in soil with a mixture of peat and perlite (3:1) in plastic pots and grown in a greenhouse under nature light. For gene transcription analysis, the third leaves from top were detached and incubated in 0.4 M NaCl solutions for 5 h as salt treatment, or placed in a laminar flow hood for drying gradually as dehydration treatment. The leaf samples were then frozen in liquid nitrogen and used for extraction of total RNA. For physiological measurements, similar size of sods were transplanted into 4 × 8 holes plug tray with the same amount of soil in each hole for growing 2 months. Plants were fully irrigated, followed by withholding irrigation until WT plants showed serious wilting as drought treatment. The leaves were harvested for measurements of relative water content (RWC) and ion leakage, followed by re-irrigating the plants for 7 days of recovery. For salt treatment, plants were irrigated with 0.2 M NaCl solution for 7 days and then 0.4 M NaCl for 7 days, followed by 0.7 M NaCl for 4 days. After the leaves were harvested for measurements of Fv/Fm, chlorophyll, Na+ and K+ concentrations, plants were re-irrigated for 7 days of recovery.
Analysis of PCR and Southern Blot Hybridization
Genomic DNA was extracted using the hexadecyltrimethylammonium bromide (CTAB) method. PCR was conducted for detection of HPT gene, using forward primer HPT-F (5′-ATGTTGGCGACCTCGTATT-3′) and reverse primer HPT-R (5′-CGTTATGTTTATCGGCACTTT-3′). Southern blot was performed as described previously (Luo et al., 2017). Digoxigenin (DIG) labeled hpt was used as DNA probes for hybridization. Probe labeling and hybridization were conducted using DIG High Prime DNA Labeling and Detection Starter Kit II (Roche Diagnostics, Basel, Switzerland) according to the manufacturer’s protocol. Hybridization signals were detected using a chemiluminescence imaging system FUSION SOLO 3S (VILBER, Paris, France).
Quantitative RT-PCR (qRT-PCR)
Total RNA was extracted by using TRIzol reagent (Life Technologies, Carlsbad, CA, United States) according to the manufacturer’s instructions. First-strand cDNA was synthesized from 1 μg of total RNA using the PrimeScript RT reagent Kit with gDNA Eraser (Takara Bio, Inc., Otsu, Shiga, Japan). A diluted cDNA was used as template for qRT-PCR analysis in a total of 10 μl SYBR Green mix (Takara Bio, Inc., Otsu, Shiga, Japan), and ACTIN1 was amplified as an internal control to normalize the amount of template. The PCR primers were listed in Table 1. Melting profiles which showed a single product specific melting temperature was used to validate the primer specificity. Efficiency of all PCR primers was above 95%.
Evaluation of Drought Tolerance
Relative water content and ion leakage and were determined as previously described (Liu et al., 2017). Fresh leaves were weighed (Wf), followed by immersing in distilled water overnight to obtain the weight of water-saturated leaves (WS). Then the leaves were dried in an oven at 80°C for 24 h to obtain the dry weight (Wd). RWC was calculated using the formula RWC = (Wf – Wd)/(WS – Wd) × 100. Survival rate was measured after the drought-treated plants were re-watering for 7 days of recovery. Survival rate (%) = number of survived plants after re-watering/total number of plants (Liu et al., 2017). For measurement of ion leakage, leaf samples were immersed in 10 ml of deionized water overnight and the conductivity of the solution (C1) was measured. The leaf samples were then heated in boiling water bath (100°C) for 20 min. The conductivity of the killed tissues (C2) was measured again after the samples were chilled to room temperature. Ion leakage was calculated as the percentage of C1 to C2.
Assessment of Salt Tolerance
The third leaf from the top was used for measurements of Fv/Fm and chlorophyll concentration after 4 days of treatment with 0.7 M NaCl. Fv/Fm was measured using a pulse-modulated fluorometer (Model FMS-2, Hansatech Instruments, Ltd.) according to the manufacturer’s instructions. For measurement of chlorophyll, the leaves (0.1 g) were ground in a mortar using pestle and extracted for 1 h in 10 ml 80% ethanol (v/v). After filtration, the filtrates were diluted and the absorbance at 663 and 645 nm was determined using a spectrophotometer as described previously (Luo et al., 2017).
Measurements of Na+ and K+ Contents
Na+ and K+ were measured as described by Essah et al. (2003). Leaves and roots were harvested after 4 days of salt treatment with 0.7 M NaCl, followed by drying at 80°C for 24 h. The dry samples were powdered in a mortar with a pestle, and a 100 mg sample was extracted in perchloric acid-nitric acid solution (1:2) overnight, and the solutions were analyzed for Na+ and K+ contents using a flame photometer (Jenway Model PEP7, United States).
Data Analysis
Data from three replicates were analyzed by using one-way ANOVA. Results are showed as mean ± standard error of biological replications. The means were separated using Duncan’s multiple range test (P < 0.05). All statistical analysis was performed by Statistical Package for the Social Sciences (SPSS 17.0).
Results
Analysis of Transgenic Plants
The embryogenic calli of seashore paspalum infected with Agrobacterium tumefaciens containing the CdtNF-YC1 were cultured on the selection medium containing hygromycin B (Figure 1A), followed by transferring the resistant calli to regeneration medium for regeneration (Figure 1B). The putative transgenic plants were planted in plastic pots filled with soil and growing in a greenhouse (Figure 1C). After detection of transgene HPT using PCR (Figure 1D), the PCR positive plants were detected using DNA blot hybridization. One single HPT hybridization signal was detected in two transgenic lines (26 and 37) and two similar HPT hybridization signals were detected in four lines (27, 29, 31, and 33), while no signal was shown in WT plants (Figure 1E), indicating an integration of the transgene into the genomes of transgenic seashore paspalum plants. Relative expression of CdtNF-YC1 was analyzed in the independent transformant lines (26, 27, and 37) using quantitative RT-PCR (qRT-PCR). Compared to the WT, higher transcript levels of CdtNF-YC1 were observed in transgenic lines. Line 27 had the greatest CdtNF-YC1 expression, while the similar transcript level was observed in lines 26 and 37 (Figure 1F).
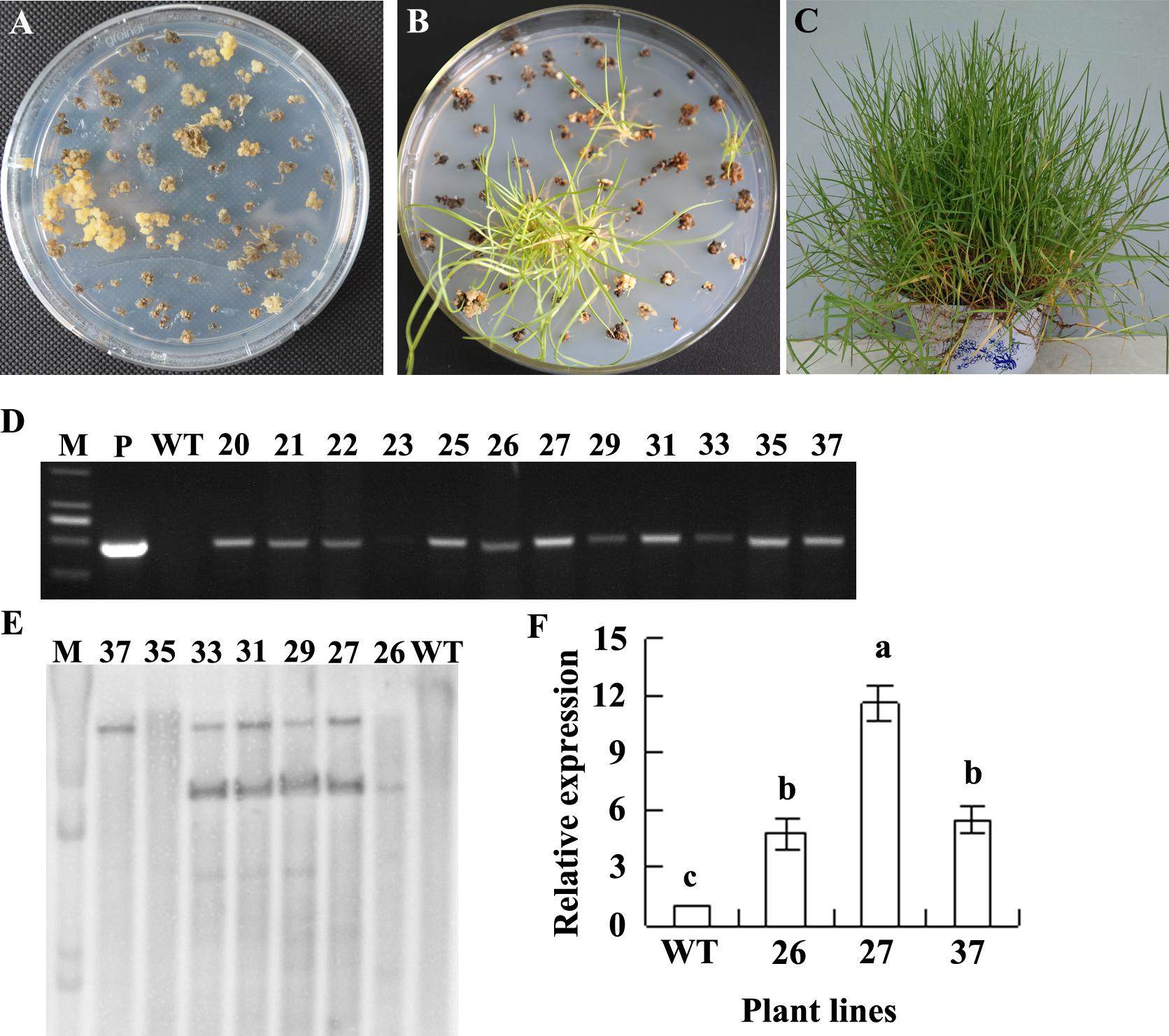
FIGURE 1. Generation and analysis of transgenic plants. Agrobacterium-infected calli were placed on selection medium containing 200 mg L−1 hygromycin (A). The hygromycin-resistant calli were exposed to regeneration (B). The regenerated plants were growing in soil (C). HPT gene was detected in transgenic plants by using PCR (D) and DNA blot hybridization (E). M indicates DNA marker; P indicates plasmid containing CdtNF-YC gene; and WT indicates wild type (WT). Relative expression of CdtNF-YC was detected using real time quantitative RT-PCR, and Actin gene was used as a reference (F). Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at P < 0.05.
Transgenic Plants Had Improved Drought Tolerance
There was no difference in morphologic phenotype between transgenic plants and WT. Previous study showed that CdtNF-YC1 overexpression results in elevated tolerance to drought and salt in transgenic rice (Chen et al., 2015). To investigate the effect of CdtNF-YC1 on drought tolerance in seashore paspalum, we measured RWC and ion leakage. A greatly decreased RWC and increased ion leakage were observed in WT, while RWC and ion leakage were not significantly altered in transgenic plants after drought treatment (Figures 2A,B). Almost all of WT plants became dead after 20 days of withholding irrigation (1.6% survival rate), while transgenic plants had 54 to 56% survival rate (Figures 2C,D). The results indicated that overexpression of CdtNF-YC1 resulted in an enhanced drought tolerance in transgenic seashore paspalum plants.
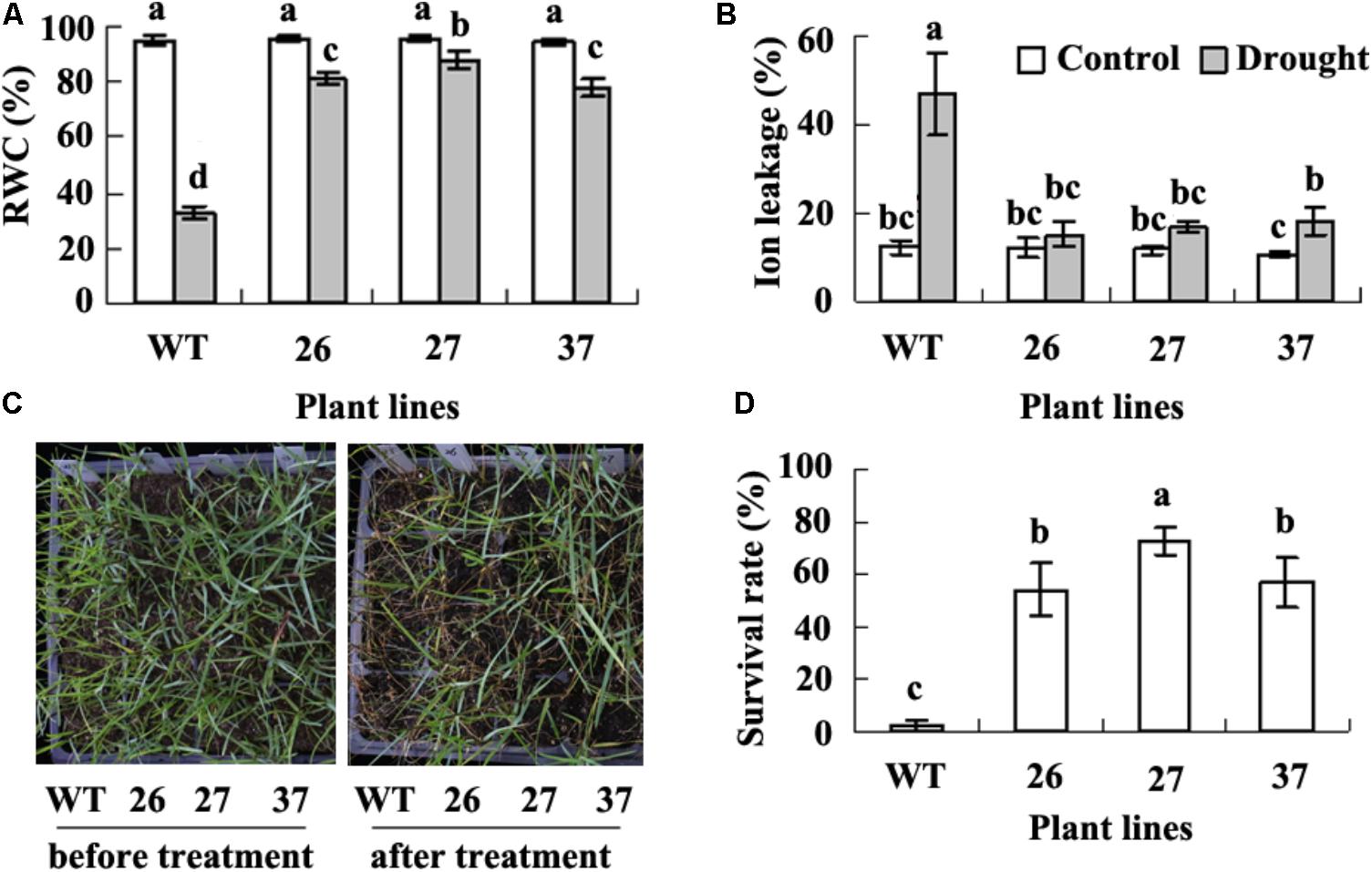
FIGURE 2. Analysis of drought tolerance in transgenic plants in comparison with the WT. Relative water content (RWC, A) and ion leakage (B) was measured when WT plants displayed wilting after withholding irrigation. Photography (C) was taken before withholding irrigation (left) and 7 days after re-watering wilted plants caused by withholding irrigation (right), followed by recording survival plant numbers to calculate survival rate (D). Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at P < 0.05.
Transgenic Plants Showed an Enhanced Salt Tolerance
A serious damage symptom was observed in WT when NaCl concentration was increased to 0.7 M for 4 days, while transgenic plants were little damaged (Figure 3A). Fv/Fm and chlorophyll concentration were decreased greatly in WT plants after salt treatment, while they were unaltered or slightly decreased in transgenic plants (Figures 3B,C). WT plants had a survival rate of 47% after salt treatment, while transgenic had survival rate of 77 to 84% 7 days after watering to remove salt from the soil (Figures 3A,D). The results indicated that transgenic plants had increased salt tolerance compared with WT plants.
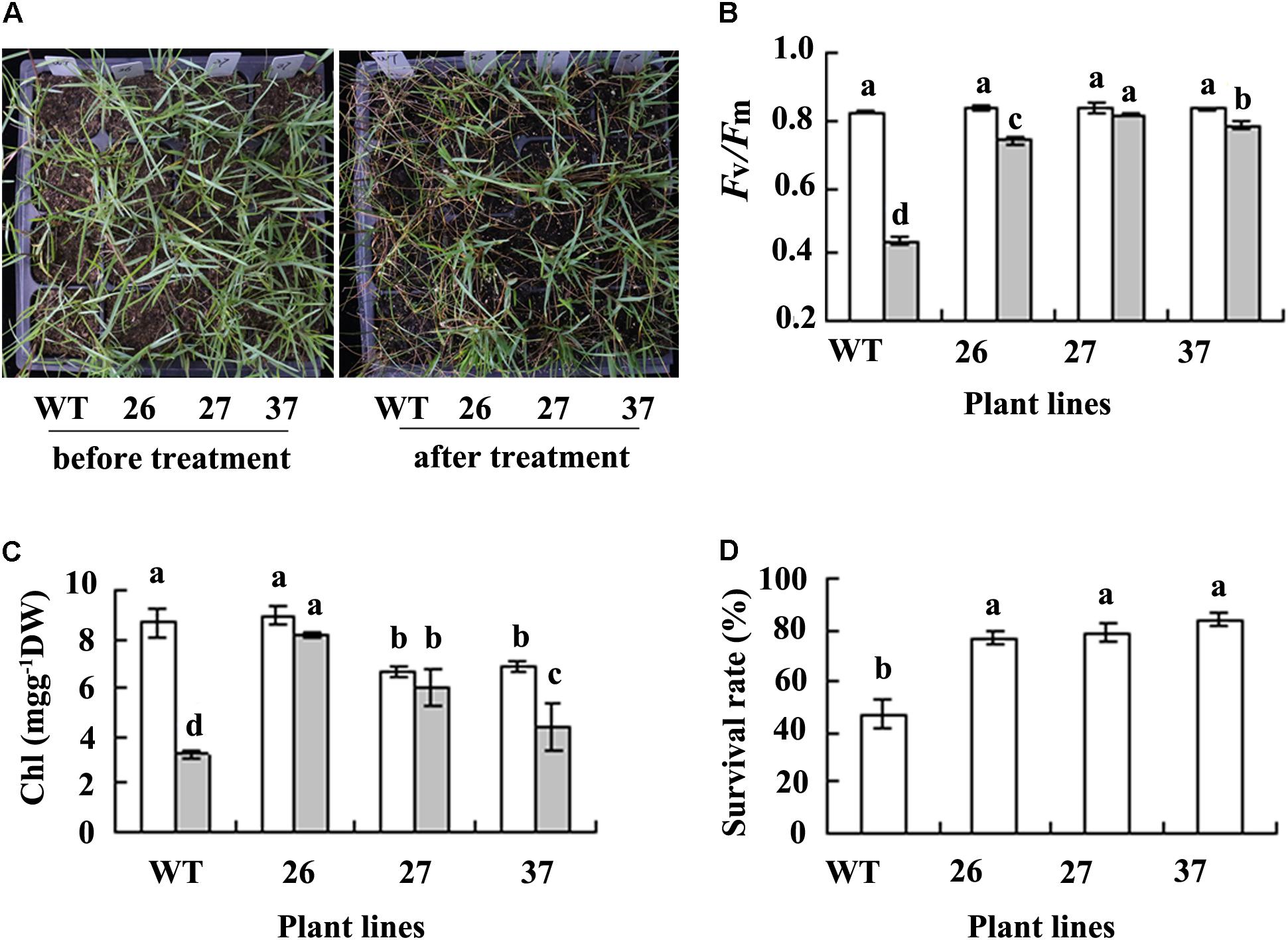
FIGURE 3. Analysis of salt tolerance in transgenic plants in comparison with the WT. Photography (A) was taken before salt treatment (left) and 7 days after watering to dilute slat as a result of treatment with 0.7 M NaCl (right), followed by recording survival plant numbers to calculate survival rate (D). Fv/Fm (B) and chlorophyll (Chl, C) were measured 4 days after NaCl concentration was increased to 0.7 M. Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at P < 0.05.
Maintenance of ion homeostasis is important for plants adaptation to salt environment. Na+ and K+ contents showed no difference in both roots and leaves between transgenic plants and WT under control condition, except for a higher Na+ content in leaves of line 37 (Figures 4A–D). Na+ content was increased in both roots and leaves in all plants after salt treatment, while lower level was maintained in transgenic plants as compared with WT (Figures 4A,B). K+ was decreased in roots of both WT and transgenic lines 26 and 27, but unaltered in line 37 after treatment with salt (Figure 4C). Salt treatment resulted in decrease in K+ level in WT leaves, but did not alter that in transgenic plants (Figure 4D). K+/Na+ ratio was decreased in all plants after salt treatment, and higher K+/Na+ ratio was maintained in transgenic plants than in WT (Figures 4E,F).
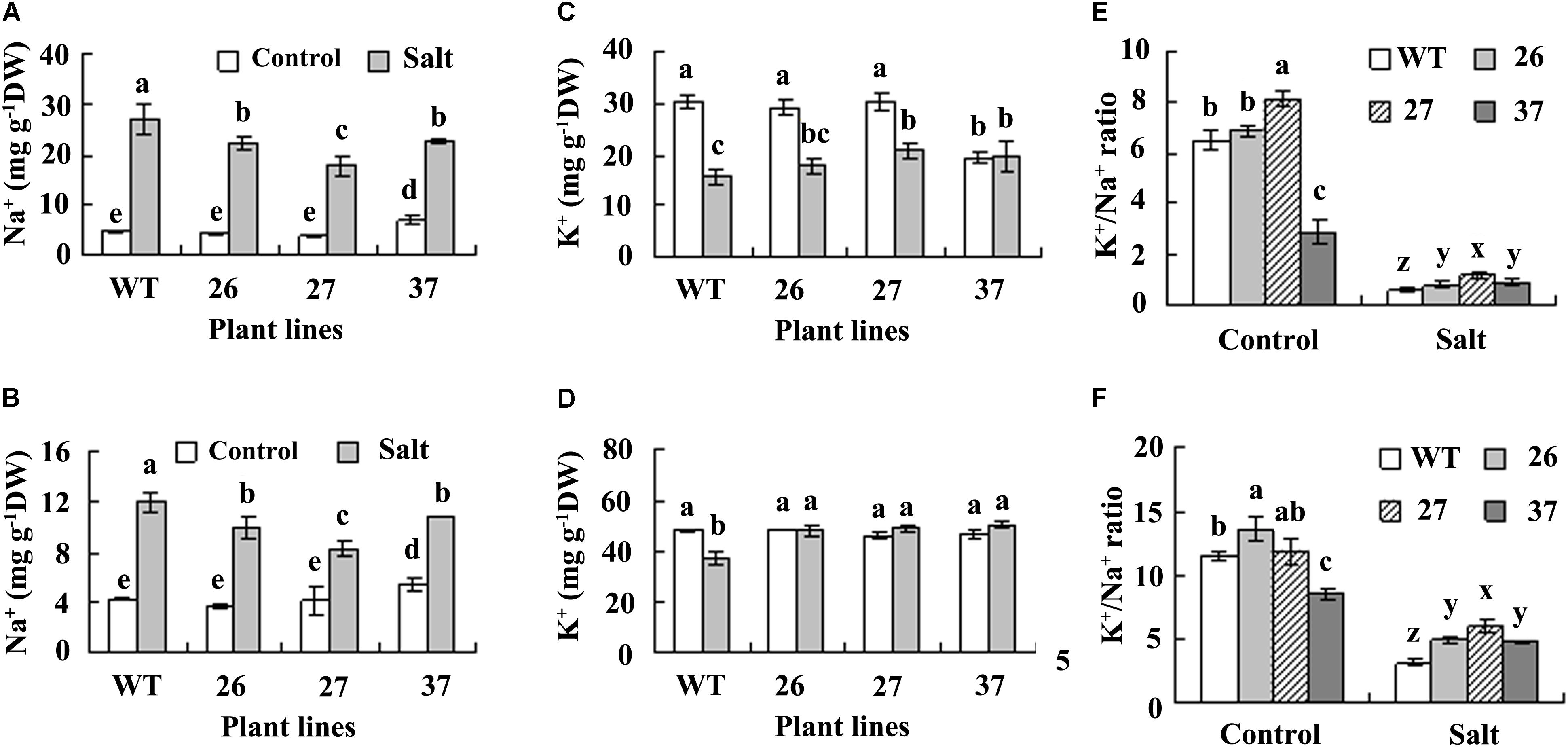
FIGURE 4. Analysis of Na+, K+, and K+/Na+ ratio in roots (A,C,E) and leaves (B,D,F) of transgenic plants and the WT. Roots and leaves were sampled before treatment (control) and 4 days after NaCl concentration was increased to 0.7 M for measurement of Na+ and K+. Means of three independent experiments and standard errors are presented; the same letter above the column indicates no significant difference at P < 0.05.
Analysis of Expression of ABA and Stress Responsive Genes
Three ABA responsive genes, PvLEA3, PvP5CS1, and PvABI2, and one stress responsive gene PvDREB1B, were analyzed under control and stress conditions. Higher transcript levels of PvLEA3, PvABI2, and PvDREB1B were observed in three transgenic lines, and higher PvP5CS1 transcript levels in lines 26 and 37 as compared with WT under control condition (Figure 5A). Dehydration and salt stress resulted in enhanced expression of PvLEA3, PvP5CS1, PvABI2, and PvDREB1B in all plants, while transgenic lines had higher levels than WT (Figures 5B,C).
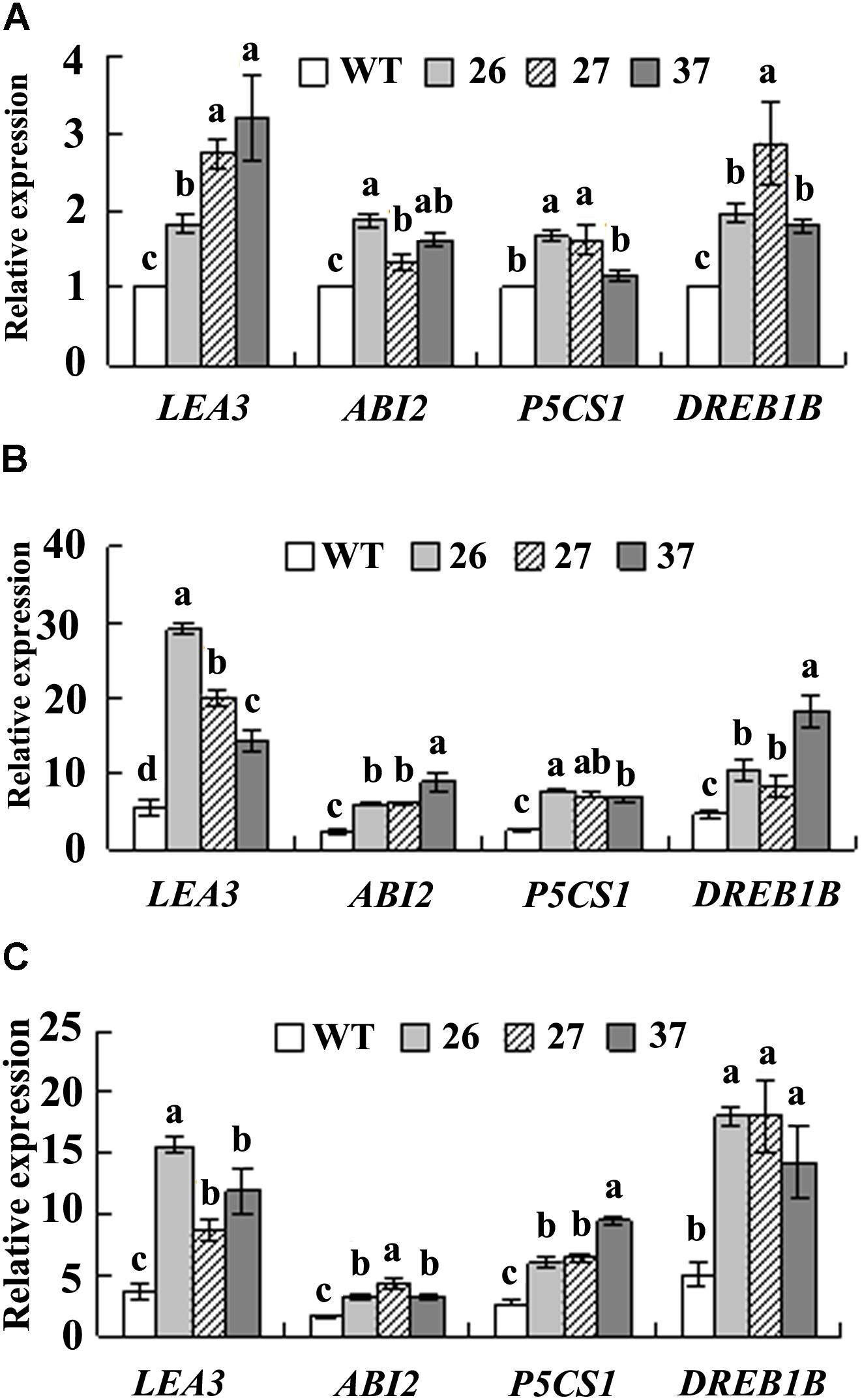
FIGURE 5. Analysis of transcript levels of PvLEA3, PvABI2, PvP5CS1, and PvDREB1B in the transgenic plants in comparison with the WT. Detached leaves were placed in water for 4 h as control (A), or air-dried in a hood for 4 h as dehydration treatment (B), or placed in 0.4 M NaCl solution for 5 h as salt treatment (C). PvACTIN1 was used as a reference gene for qRT-PCR. Relative expression of each gene was calculated by defining the normalized transcript level in WT plant under control condition as one. Means of three replicates and standard errors are presented; the same letter above the column indicates no significant difference at P < 0.05.
Discussion
Transgenic approaches provide an effective tool to improve crops traits. Most of the commercial seashore paspalum cultivars are cultivated using sprigs or sods, while ‘Sea Spray’ is the only seeded cultivar. Agrobacterium-mediated transformation of seashore paspalum has been reported using an unnamed cultivar (Kim et al., 2009). In this study, transgenic seashore paspalum overexpressing CdtNF-YC1 were generated and identified. The transgene was detected in the genomes of transgenic lines based on Southern blot analysis. qRT-PCR assay indicated that CdtNF-YC1 gene was expressed in transgenic lines. NF-Ys are involved in plant adaptation to environmental stresses (Swain et al., 2017). Except for those from model plants, several NF-Y genes have been identified to confer abiotic stress tolerance. For example, overexpression of a drought responsive ZmNF-YB16 improves drought resistance and yield inducing antioxidant protection and enhancing photosynthesis in transgenic maize (Wang et al., 2018). CdtNF-YC1 has been documented to increase drought and salt tolerance in transgenic rice (Chen et al., 2015). Likely, overexpressing CdtNF-YC1 led to increased drought and salt tolerance in transgenic seashore paspalum in this study. This is the first report on transgenic seashore paspalum with modified traits.
Expression of LEA3, P5CS1, ABI2, and DREB1B genes is up-regulated by CdtNF-YC1 in transgenic rice, and CCAAT cis-acting elements exist in the promoter regions of these genes (Chen et al., 2015). Likely, transcript levels of PvLEA3, PvP5CS1, PvABI2, and PvDREB1B were induced by drought and salt stress, and higher levels were observed in transgenic plants compared with WT, although the existence of CCAAT cis-acting elements in the promoter regions of these genes in seashore paspalum was not analyzed due to the absence of genomic information. Up-regulation of these genes leads to increased drought and/or salinity tolerance (Xu et al., 1996; Ohta et al., 2003; Datta et al., 2012; Per et al., 2017). Plant molecular responses to abiotic stress include ABA-dependent and -independent pathways. LEA3, P5CS1, and ABI2 are ABA-dependent stress responsive marker genes (Yamaguchi-Shinozaki and Shinozaki, 2006). NF-Ys have been validated to be involved in ABA signaling in plant responses to drought (Li et al., 2008; Chen et al., 2015; Bi et al., 2017). DREB1B is responsive to stress and functions in ABA-independent pathway (Zhu, 2002), which was induced in CdtNF-YC1 transgenic rice (Chen et al., 2015) and seashore paspalum (this study). It is suggested that the enhanced drought and salt tolerance in transgenic seashore paspalum is associated with up-regulation of a series of stress responsive genes in both ABA-dependent and ABA-independent pathway by CdtNF-YC1.
Maintenance of ion homeostasis is important for plant adaptation to saline environment. Plants may increase salt tolerance either by minimizing the entry of salt into plants or by ion compartmentation mechanism to minimize the salt concentration in the cytoplasm. Plants may restrict uptake of ions like Na+ and Cl− or selectively take up ions to maintain a higher cellular K+/Na+ ratio in saline environments (Munns and Tester, 2008). Among six warm-season turfgrass species, seashore paspalum had the highest salt tolerance with lowest accumulation of Na+, least reduction of K+ and highest K+/Na+ ratio after salt stress (Uddin et al., 2012). In this study, salt treatment resulted in increased Na+ level and decreased K+ level in seashore paspalum plants, while lower levels of Na+ and altered K+ level were maintained in transgenic plants, which led to a higher K+/ Na+ ratio in roots and leaves in transgenic plants than in WT. Many genes such as those encoding Na+/H+ antiporters, NHX antiporters and HKT transporters are involved in homeostasis of K+ and Na+ in plant cells in response to salt stress (Munns and Tester, 2008; Almeida et al., 2017). Although the ion homeostasis related genes regulated by CdtNF-YC1 were not revealed in the present study, our results suggest that overexpression of CdtNF-YC1 influences maintenance of ion homeostasis under salt stress conditions. It will be interesting to investigate the regulation of PvNF-Ys on maintenance of ion homeostasis in response to salt stress for understanding salt tolerance mechanism in seashore paspalum in the future.
In summary, transgenic seashore paspalum plants overexpression CdtNY-YC1 with enhanced drought and salt tolerance were obtained in the present study. The enhanced drought and salt tolerance is associated with induction of a series of stress responsive genes by CdtNF-YC1 in both ABA-dependent and ABA-independent pathway. In addition, the improved salt tolerance was also associated with maintenance of Na+ and K+ homeostasis under salt stress in transgenic plants.
Author Contributions
XW conducted the experiments and wrote the manuscript. HS and ZG designed the experiments and revised the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 31701961), the Natural Science Foundation of Jiangsu Province (Grant No. BK20160728), and the Fundamental Research Funds for the Central Universities (Grant No. Y0201600178).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Almeida, D. M., Oliveira, M. M., and Saibo, N. J. M. (2017). Regulation of Na+and K+ homeostasis in plants: towards improved salt stress tolerance in crop plants. Genet. Mol. Biol. 40, 326–345. doi: 10.1590/1678-4685-GMB-2016-0106
Bi, C., Ma, Y., Wang, X. F., and Zhang, D. P. (2017). Overexpression of the transcription factor NF-YC9 confers abscisic acid hypersensitivity in Arabidopsis. Plant Mol. Biol. 95, 425–439. doi: 10.1007/s11103-017-0661-1
Cao, S., Kumimoto, R. W., Siriwardana, C. L., Risinger, J. R., and Holt, B. F. III (2011). Identification and characterization of NF-Y transcription factor families in the monocot model plant Brachypodium distachyon. PLoS One 6:e21805. doi: 10.1371/journal.pone.0021805
Chen, M., Zhao, Y., Zhuo, C., Lu, S., and Guo, Z. (2015). Overexpression of a NF-YC transcription factor from bermudagrass confers tolerance to drought and salinity in transgenic rice. Plant Biotechnol. J. 13, 482–491. doi: 10.1111/pbi.12270
Datta, K., Baisakh, N., Ganguly, M., Krishnan, S., Shinozaki, K. Y., and Datta, S. K. (2012). Overexpression of Arabidopsis and rice stress genes’ inducible transcription factor confers drought and salinity tolerance to rice. Plant Biotechnol. J. 10, 579–586. doi: 10.1111/j.1467-7652.2012.00688.x
Essah, P. A., Davenport, R., and Tester, M. (2003). Sodium influx and accumulation in Arabidopsis. Plant Physiol. 133, 307–318. doi: 10.1104/pp.103.022178
Giri, C. C., and Praveena, M. (2015). In vitro regeneration, somatic hybridization and genetic transformation studies: an appraisal on biotechnological interventions in grasses. Plant Cell Tissue. Organ Cult. 120, 843–860. doi: 10.1007/s11240-014-0653-7
Guo, H., Wang, Y., Li, D., Chen, J., Zong, J., Wang, Z., et al. (2016). Growth response and ion regulation of seashore paspalum accessions to increasing salinity. Environ. Exp. Bot. 131, 137–145. doi: 10.1016/j.envexpbot.2016.07.003
Guo, Z., Bonos, S., Meyer, W. A., Day, P., and Belanger, F. C. (2003). Transgenic creeping bentgrass with delayed dollar spot symptoms. Mol. Breed. 11, 95–101. doi: 10.1023/A:1022458101221
Hackenberg, D., Wu, Y., Voigt, A., Adams, R., Schramm, P., and Grimm, B. (2012). Studies on differential nuclear translocation mechanism and assembly of the three subunits of the Arabidopsis thaliana transcription factor NF-Y. Mol. Plant 5, 876–888. doi: 10.1093/mp/ssr107
Hou, X., Zhou, J., Liu, C., Liu, L., Shen, L., and Yu, H. (2014). Nuclear factor Y-mediated H3K27me3 demethylation of the SOC1 locus orchestrates flowering responses of Arabidopsis. Nat. Commun. 5:4601. doi: 10.1038/ncomms5601
Kim, K. M., Song, I. J., Lee, H. Y., Raymer, P., Kim, B. S., and Kim, W. (2009). Development of seashore paspalum turfgrass with herbicide resistance. Kor. J. Crop Sci. 54, 427–432.
Kumimoto, R. W., Adam, L., Hymus, G. J., Repetti, P. P., Reuber, T. L., Marion, C. M., et al. (2008). The nuclear factor Y subunits NF-YB2 and NF-YB3 play additive roles in the promotion of flowering by inductive long-day photoperiods in Arabidopsis. Planta 228, 709–723. doi: 10.1007/s00425-008-0773-6
Kumimoto, R. W., Zhang, Y., Siefers, N., and Holt, B. F. (2010). NF-YC3, NF-YC4 and NF-YC9 are required for CONSTANS-mediated, photoperiod-dependent flowering in Arabidopsis thaliana. Plant J. 63, 379–391. doi: 10.1111/j.1365-313X.2010.04247.x
Lee, D.-K., Kim, H. I., Jang, G., Chung, P. J., Jeong, J. S., Kim, Y. S., et al. (2015). The NF-YA transcription factor OsNF-YA7 confers drought stress tolerance of rice in an abscisic acid independent manner. Plant Sci. 241, 199–210. doi: 10.1016/j.plantsci.2015.10.006
Li, W. X., Oono, Y., Zhu, J., He, X. J., Wu, J. M., Iida, K., et al. (2008). The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20, 2238–2251. doi: 10.1105/tpc.108.059444
Li, Y. J., Fang, Y., Fu, Y. R., Huang, J. G., Wu, C. A., and Zheng, C. C. (2013). NFYA1 is involved in regulation of postgermination growth arrest under salt stress in Arabidopsis. PLoS One 8:e61289. doi: 10.1371/journal.pone.0061289
Li, Z., Hu, Q., Zhou, M., Vandenbrink, J., Li, D., Menchyk, N., et al. (2013). Heterologous expression of OsSIZ1, a rice SUMO E3 ligase, enhances broad abiotic stress tolerance in transgenic creeping bentgrass. Plant Biotechnol. J. 11, 432–445. doi: 10.1111/pbi.12030
Li, Z., Yuan, S., Jia, H., Gao, F., Zhou, M., Yuan, N., et al. (2017). Ectopic expression of a cyanobacterial flavodoxin in creeping bentgrass impacts plant development and confers broad abiotic stress tolerance. Plant Biotechnol. J. 15, 433–446. doi: 10.1111/pbi.12638
Lian, C., Li, Q., Yao, K., Zhang, Y., Meng, S., Yin, W., et al. (2018). Populus trichocarpa PtNF-YA9, a multifunctional transcription factor, regulates seed germination, abiotic stress, plant growth and development in Arabidopsis. Front. Plant Sci. 9:954. doi: 10.3389/fpls.2018.00954
Liu, M., Chen, J., Guo, Z., and Lu, S. (2017). Differential responses of polyamines and antioxidants to drought in a centipedegrass mutant in comparison to its wild type plants. Front. Plant Sci. 8:792. doi: 10.3389/fpls.2017.00792
Liu, J. X., and Howell, S. H. (2010). bZIP28 and NF-Y transcription factors are activated by ER stress and assemble into a transcriptional complex to regulate stress response genes in Arabidopsis. Plant Cell 22, 782–796. doi: 10.1105/tpc.109.072173
Liu, X., Hu, P., Huang, M., Tang, Y., Li, Y., Li, L., et al. (2016). The NF-YC-RGL2 module integrates GA and ABA signalling to regulate seed germination in Arabidopsis. Nat. Commun. 7:12768. doi: 10.1038/ncomms12768
Luo, J., Liu, M., Zhang, C., Zhang, P., Chen, J., Guo, Z., et al. (2017). Transgenic centipedegrass (Eremochloa ophiuroides [Munro] Hack.) overexpressing S-adenosylmethionine decarboxylase (SAMDC) gene for improved cold tolerance through involvement of H2O2 and NO signaling. Front. Plant Sci. 8:1655. doi: 10.3389/fpls.2017.01655
Manimaran, P., Venkata Reddy, S., Moin, M., Raghurami Reddy, M., Yugandhar, P., Mohanraj, S. S., et al. (2017). Activation-tagging in indica rice identifies a novel transcription factor subunit, NF-YC13 associated with salt tolerance. Sci. Rep. 7:9341. doi: 10.1038/s41598-017-10022-9
Mu, J., Tan, H., Hong, S., Liang, Y., and Zuo, J. (2013). Arabidopsis transcription factor genes NF-YA1, 5, 6, and 9 play redundant roles in male gametogenesis, embryogenesis, and seed development. Mol. Plant 6, 188–201. doi: 10.1093/mp/sss061
Munns, R., and Tester, M. (2008). Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 59, 651–681. doi: 10.1146/annurev.arplant.59.032607.092911
Nardini, M., Gnesutta, N., Donati, G., Gatta, R., Forni, C., Fossati, A., et al. (2013). Sequence-specific transcription factor NF-Y displays histone-like DNA binding and H2B-like ubiquitination. Cell 152, 132–143. doi: 10.1016/j.cell.2012.11.047
Nelson, D. E., Repetti, P. P., Adams, T. R., Creelman, R. A., Wu, J., Warner, D. C., et al. (2007). Plant nuclear factor Y (NF-Y) B subunits confer drought tolerance and lead to improved corn yields on water-limited acres. Proc. Natl. Acad. Sci. U.S.A. 104, 16450–16455. doi: 10.1073/pnas.0707193104
Ohta, M., Guo, Y., Halfter, U., and Zhu, J.-K. (2003). A novel domain in the protein kinase SOS2 mediates interaction with the protein phosphatase 2C ABI2. Proc. Natl. Acad. Sci. U.S.A. 100, 11771–11776. doi: 10.1073/pnas.2034853100
Per, T. S., Khan, N. A., Reddy, P. S., Masood, A., Hasanuzzaman, M., Khan, M. I. R., et al. (2017). Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 115, 126–140. doi: 10.1016/j.plaphy.2017.03.018
Pessarakli, M. (2018). Screening various cultivars of seashore paspalum (Paspalum vagenitum Swartz) for salt tolerance for potential use as a cover plant in combatting desertification. Int. J. Water Res. Arid Environ. 7, 36–43.
Petroni, K., Kumimoto, R. W., Gnesutta, N., Calvenzani, V., Fornari, M., Tonelli, C., et al. (2012). The promiscuous life of plant NUCLEAR FACTOR Y transcription factors. Plant Cell 24, 4777–4792. doi: 10.1105/tpc.112.105734
Sato, H., Mizoi, J., Tanaka, H., Maruyama, K., Qin, F., Osakabe, Y., et al. (2014). Arabidopsis DPB3-1, a DREB2A interactor, specifically enhances heat stressinduced gene expression by forming a heat stress-specific transcriptional complex with NF-Y subunits. Plant Cell 26, 4954–4973. doi: 10.1105/tpc.114.132928
Schiavon, M., Leinauer, B., Serena, M., Sallenave, R., and Maier, B. (2012). Bermudagrass and seashore paspalum establishment from seed using differing irrigation methods and water qualities. Agron. J. 104, 706–714. doi: 10.2134/agronj2011.0390
Shahba, M. A., Alshammary, S. F., and Abbas, M. S. (2012). Effects of salinity on seashore paspalum cultivars at different mowing heights. Crop Sci. 52, 1358–1370. doi: 10.2135/cropsci2011.06.0337
Shi, H., Ye, T., Zhong, B., Liu, X., Jin, R., and Chan, Z. (2014). AtHAP5A modulates freezing stress resistance in Arabidopsis through binding to CCAAT motif of AtXTH21. New Phytol. 203, 554–567. doi: 10.1111/nph.12812
Siefers, N., Dang, K. K., Kumimoto, R. W., Bynum, W. E., Tayrose, G., and Holt, B. F. (2009). Tissue-specific expression patterns of Arabidopsis NF-Y transcription factors suggest potential for extensive combinatorial complexity. Plant Physiol. 149, 625–641. doi: 10.1104/pp.108.130591
Singh, K. B., Foley, R. C., and Oñate-Sánchez, L. (2002). Transcription factors in plant defense and stress responses. Curr. Opin. Plant Biol. 5, 430–436. doi: 10.1016/S1369-5266(02)00289-3
Swain, S., Myers, Z. A., Siriwardana, C. L., and Holt, B. F. III. (2017). The multifaceted roles of NUCLEAR FACTOR-Y in Arabidopsis thaliana development and stress responses. Biochim. Biophys. Acta 1860, 636–644. doi: 10.1016/j.bbagrm.2016.10.012
Uddin, M. K., Juraimi, A. S., Ismail, M. R., and Alam, M. A. (2012). The effect of salinity on growth and ion accumulation in six turfgrass species. Plant Omics. 5, 244–252.
Wang, B., Li, Z., Ran, Q., Li, P., Peng, Z., and Zhang, J. (2018). ZmNF-YB16 overexpression improves drought resistance and yield by enhancing photosynthesis and the antioxidant capacity of maize plants. Front. Plant Sci. 9:709. doi: 10.3389/fpls.2018.00709
Xu, D., Duan, X., Wang, B., Hong, B., Ho, T. H. D., and Wu, R. (1996). Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol. 110, 249–257. doi: 10.1104/pp.110.1.249
Xu, F., Li, T., Xu, P. B., Li, L., Du, S. S., Lian, H. L., et al. (2016). DELLA proteins physically interact with CONSTANS to regulate flowering under long days in Arabidopsis. FEBS Lett. 590, 541–549. doi: 10.1002/1873-3468.12076
Yamaguchi-Shinozaki, K., and Shinozaki, K. (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 57, 781–803. doi: 10.1146/annurev.arplant.57.032905.105444
Zanetti, M. E., Ripodas, C., and Niebel, A. (2017). Plant NF-Y transcription factors: key players in plant-microbe interactions, root development and adaptation to stress. Biochim. Biophys. Acta 1860, 645–654. doi: 10.1016/j.bbagrm.2016.11.007
Zhao, H., Wu, D., Kong, F., Lin, K., Zhang, H., and Li, G. (2016). The Arabidopsis thaliana nuclear factor Y transcription factors. Front. Plant Sci. 7:2045. doi: 10.3389/fpls.2016.02045
Zhou, Y., Lambrides, C. J., Kearns, R., Ye, C., and Fukai, S. (2012). Water use, water use efficiency and drought resistance among warm-season turfgrasses in shallow soil profiles. Funct. Plant Biol. 39, 116–125. doi: 10.1071/FP11244
Keywords: drought, NF-YC, salinity, seashore paspalum, transformation, turfgrass
Citation: Wu X, Shi H and Guo Z (2018) Overexpression of a NF-YC Gene Results in Enhanced Drought and Salt Tolerance in Transgenic Seashore Paspalum. Front. Plant Sci. 9:1355. doi: 10.3389/fpls.2018.01355
Received: 09 February 2018; Accepted: 28 August 2018;
Published: 21 September 2018.
Edited by:
Zeng-Yu Wang, Noble Research Institute, LLC, United StatesReviewed by:
Hong Luo, Clemson University, United StatesRamesh Katam, Florida A&M University, United States
Man Zhou, Kean University-Wenzhou, China
Copyright © 2018 Wu, Shi and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haifan Shi, shihaifan@njau.edu.cn Zhenfei Guo, zfguo@njau.edu.cn
 Xueli Wu
Xueli Wu Haifan Shi
Haifan Shi Zhenfei Guo
Zhenfei Guo