- 1Department of Entomology and Plant Pathology, Auburn University, Auburn, AL, United States
- 2Department of Biological Sciences, Vanderbilt University, Nashville, TN, United States
- 3Cardiovascular Research Institute, University of California, San Francisco, San Francisco, CA, United States
As one of the most abundant insect orders on earth, most Hemipteran insects are phytophagous, with the few hematophagous exceptions falling into two families: Cimicidae, such as bed bugs, and Reduviidae, such as kissing bugs. Many of these blood-feeding hemipteran insects are known to be realistic or potential disease vectors, presenting both physical and psychological risks for public health. Considerable researches into the interactions between hemipteran insects such as kissing bugs and bed bugs and their human hosts have revealed important information that deepens our understanding of their chemical ecology and olfactory physiology. Sensory mechanisms in the peripheral olfactory system of both insects have now been characterized, with a particular emphasis on their olfactory sensory neurons and odorant receptors. This review summarizes the findings of recent studies of both kissing bugs (including Rhodnius prolixus and Triatoma infestans) and bed bugs (Cimex lectularius), focusing on their chemical ecology and peripheral olfactory systems. Potential chemosensation-based applications for the management of these Hemipteran insect vectors are also discussed.
Introduction
The insect order Hemiptera, one of the most abundant insect orders, encompasses a wide range of different species. Although most hemipteran insects feed on plants or other insects, small invertebrates or even sugars (Díaz-Albiter et al., 2016), a few, such as kissing bugs and bed bugs, utilize blood sources from humans and/or animals [for more details, see the review provided in Reinhardt and Siva-Jothy (2007)]. Bed bugs (Cimicidae) have been reported to be resurgent in many developed countries due to the relaxation of monitoring systems, the development of insecticide resistance, and the increase in international travel in recent years (Doggett et al., 2004, 2012; Ter Poorten and Prose, 2005; Romero et al., 2007; Yoon et al., 2008; Haynes and Potter, 2013; Zhu et al., 2013). Kissing bugs, which are members of the Triatominae subfamily of the family Reduviidae, are typically found in the southern United States, Mexico, Central America, and South America (Justi et al., 2016; Monteiro et al., 2018).
Both kissing bugs and bed bugs are obligate blood-feeding ectoparasites of multiple hosts, including mammals, birds, and reptiles. For human beings, the major concerns related to these two hemipteran insects lie in their biting nuisance and their potential role as disease vectors. Bites from bed bugs result in the victims experiencing clinical symptoms such as a wheal-and-flare response, infiltrated papules, vesicles, and/or blisters (Sansom et al., 1992; Alexander, 1994). In addition to the biting nuisance, bacterial infections such as impetigo, ecthyma, cellulitis, and lymphangitis may occur (Burnett et al., 1986). Another concern is the potential vector capacity of bed bugs. A preliminary study suggested that bed bugs probably share the same role as kissing bugs in transmitting Trypanosoma cruzi, the flagellate protozoan responsible for American trypanosomiasis, which is better known as Chagas disease. Using mice as their animal model, Salazar et al. (2014) found bed bugs to be a competent vector of T. cruzi and that they were able to efficiently and bi-directionally transmit T. cruzi to host mice. Most of the bed bugs fed on experimentally infected mice acquired the parasites, and a majority of the previously uninfected mice became infected after cohabitating with the exposed bed bugs in a laboratory environment. T. cruzi was also transmitted to mice who were directly exposed to the feces of infected bed bugs. Blakely et al. (2018) found live T. cruzi in the gut contents of bed bug adults fed with T. cruzi-contaminated blood and this persisted for at least 97 days post-infection in adult bed bugs. More importantly, they also found that nymphal stage bed bugs that were infected with T. cruzi maintained the parasite after molting, indicating the capacity for transstadial passage of T. cruzi in bed bugs.
As with bed bugs, the reaction to a kissing bug bite depends on the victim’s sensitivity toward the substances introduced during the biting process. A typical light reaction to the kissing bug bite is papular lesions with a central punctum or grouped small vesicles; severe symptoms can include giant urticarial-type lesions with swelling at the site of inoculation; hemorrhagic nodular-to-bullous lesions; conjunctivitis, and a generalized morbilliform eruption (Shields and Walsh, 1956; Hemmige et al., 2012). Kissing bugs are known to be the primary vector of the pathogen T. cruzi (Stevens et al., 2011; Lidani et al., 2019). Surveys conducted in the United States have indicated that about half of the Triatominae species identified were carrying T. cruzi (Davis et al., 1943). Two of the epidemiologically important vectors are Rhodnius prolixus Stal and Triatoma infestans Klug (Coura, 2015). However, unlike the transmission cycle reported for bed bugs, T. cruzi is transmitted by kissing bug through various manners, including vector feces, food contamination, blood transfusion, of which oral transmission by food contamination plays the major role (Pereira et al., 2010; Shikanai-Yasuda and Carvalho, 2012).
As both kissing bugs and bed bugs pose a significant risk to humans and are thus a major concern for public health, remarkable progress has been made in recent decades in elucidating their chemical ecology and olfactory physiology. This review focuses on recent advances in: 1) the factors that regulate the host-seeking behavior of bed bugs and kissing bugs; 2) the mechanisms of peripheral chemosensory system in kissing bug and bed bug, including olfactory sensilla, olfactory receptor neurons (ORNs), odorant binding proteins (OBPs) and chemosensory proteins (CSPs), odorant receptors (ORs), ionotropic receptors (IRs), and gustatory receptors (GRs); and 3) perspectives for chemosensation-based applications in the management of kissing bug and bed bugs. This emerging knowledge is expected to make a positive contribution to the control of these blood-feeding insects and thus reduce the potential disease transmissions.
Host-Seeking Behavior of Kissing Bugs and Bed Bugs
Since both kissing bugs and bed bugs rely on human or animal blood sources for survival and reproduction, host localization is a vital part of their daily activities. In the host-seeking process, heat, host odor, and carbon dioxide (CO2) are important cues for both kissing bugs and bed bugs. Kissing bugs (R. prolixus and/or T. infestans) were found to be attracted to warm temperature (Wigglesworth and Gillett, 1934; Milne et al., 2009), host-related compounds (Bodin et al., 2009; Milne et al., 2009; Ortiz and Molina, 2010; Ortiz et al., 2011), and CO2 (Wiesinger, 1956; Nunez, 1982; Guerenstein and Guerin, 2001; Barrozo and Lazzari, 2004; Guerenstein and Lazarri, 2009; Indacochea et al., 2017). Kissing bug nymphs are attracted by CO2-free traps baited with three host-odor components (ammonia, L-(+)-lactic acid, and hexanoic acid) but not by traps containing either one component alone or two components, suggesting a synergistic effect of host odors in attracting kissing bugs (Guidobaldi and Guerenstein, 2013). Researchers have also found that bed bugs can distinguish temperature differences as low as 1–2°C via the thermosensors on their antennae (Sioli, 1937). Heat baited traps attract significantly more bed bugs than unheated traps (Wang et al., 2009; Anderson et al., 2017). CO2 baited traps are also more attractive for bed bugs than non-CO2 traps and CO2 are more effective than heat in trapping assays (Wang et al., 2009). In addition, bed bugs respond to human skin swabs in the absence of all other host cues (DeVries et al., 2019). However, chemical lures baited with specific human odors displayed more complex results, with the trapping efficiency largely depending on the specific compounds incorporated into the lures. For instance, Wang et al. (2009) found that lures baited with two human odors, 1-octen-3-ol, and L-lactic acid, did not attract significantly more bed bugs than non-baited traps, while Anderson et al. (2017) reported that ammonium bicarbonate and a blend of (E)-2-hexenal and (E)-2-octenal at certain concentrations attracted more bed bugs than the untreated control. In another study, Singh et al. (2012) screened twelve chemicals, evaluated the interactions among chemical lures, CO2, and heat in trapping bed bugs, and revealed a synergistic effect between chemical lures and CO2 but not heat and CO2.
Multiple factors have been determined to regulate the host-search activities of both kissing bugs and bed bugs, including food source availability, mating status, and temporal modulation. Studies have shown that starvation plays a critical role in affecting the olfactory responses of kissing bugs (R. prolixus) to host odors, with starved R. prolixus showing a significant preference for the host-odorant treated arm in a dual-choice olfactometer, while a random distribution was observed in non-starved kissing bugs (Reisenman et al., 2013). Similarly, bed bugs that have been starved for a week were found to be more active in host-searching than those that had received a blood meal 2 days before testing (Romero et al., 2010). Bed bugs that have received a blood meal are also more likely to aggregate in shelters during the scotophase, while those that have not fed tend to spend more time out of the shelters (Reis and Miller, 2011).
Another factor in determining bed bugs’ host-searching activities is mating status. The percentage of females that fed and the amount of blood they ingested were found to be significantly greater in mated females than in unmated females and far more mated than unmated females responded to human odors (DeVries et al., 2019; Saveer et al., 2021). Interestingly, starvation also has a strong impact on the response of mated or unmated female bed bugs to human odors. The response rate of unmated females to skin odor increased with longer starvation periods, while the opposite pattern was observed in mated females (Saveer et al., 2021). Temporal modulation also plays a critical role in determining host-seeking activity. Behavior-related antennal sensitivity is governed by a circadian clock or daily rhythm in multiple insect species, including moths, flies, cockroaches, bed bugs, and kissing bugs (Brady, 1975; Hawkins and Rust, 1977; Van der Goes van Naters et al., 1998; Krishnan et al., 1999; Page and Koelling, 2003; Rosén et al., 2003; Bodin et al., 2008). An endogenous circadian clock has also been found to affect the insect’s orientation toward CO2, but only during the scotophase for both T. infestans and R. prolixus (Barrozo et al., 2004; Barrozo and Lazzari, 2004; Bodin et al., 2008). In addition, Reisenman (2014) reported that the electroantennogram (EAG) response of starved R. prolixus to ammonia (a host odor) was significantly higher than in insects fed only during the night. This modulation of sensory responses at the neural level is believed to trigger host search behavior in starved kissing bugs. In bed bugs, their spontaneous locomotor activity is known to be determined by an inner circadian rhythm, with both adults and nymphs being much more active in the dark than in the light phase (Romero et al., 2010). This is thought to enhance their chance of locating a sleeping human host (Romero et al., 2010).
Mechanism of Peripheral Olfactory System
Kissing bugs and bed bugs, like other insects, sense their chemical environment through their peripheral olfactory system. Their major olfactory appendages are their antennae, where various morphological or functional types of olfactory sensilla are located (Figures 1A,B). Olfactory sensory neurons (OSNs) are housed in each olfactory sensillum and OBPs/CSPs are secreted into the sensillum lymph by the accessory cells. Specific or unique olfactory receptors, including ORs, IRs, and CO2-specific GRs, are expressed on the membrane of these OSNs (Figure 1C). Odorants surrounding the antennae pass through the pores on the sensillum surface and potentially bind with the OBPs/CSPs, after which they are delivered to active sites on the olfactory receptors (Brito et al., 2016). When olfactory receptors are activated by specific ligands, the cation channel formed by the olfactory receptors will be open (Nakagawa et al., 2005; Sato et al., 2008), which leads to the depolarization of OSNs and generation of action potentials. The chemical information is then transformed into electrical signals in the OSNs and transmitted along the axons into the antennal lobe in the central nervous system, where chemical information is further processed before the final behavioral decisions are made (Carey and Carlson, 2011; Leal, 2013). While the peripheral olfactory system of kissing bug is comparable with other blood-feeding insects (e.g. mosquito Anopheles gambiae) in term of the amount of olfactory sensilla and ORs, bed bugs are found to possess a degenerative olfactory system with much fewer olfactory sensilla and ORs (Levinson et al., 1974; Benoit et al., 2016).
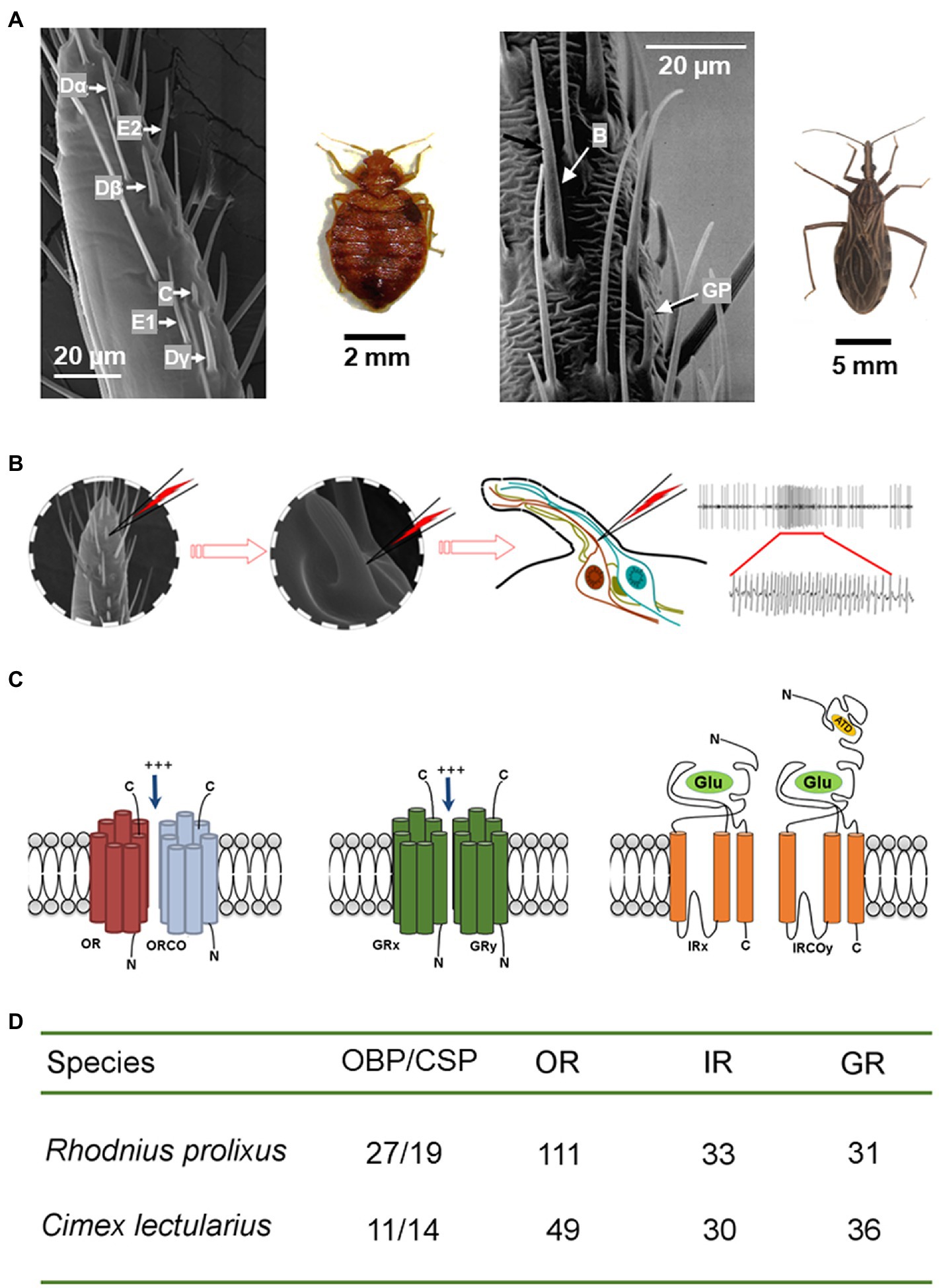
Figure 1. Olfactory mechanism of the peripheral olfactory system in bed bugs and kissing bugs. (A) Scanning electronic microscope images show six functional types of olfactory sensillum (Dα, Dβ, Dγ, E1, E2, and C) for bed bugs (left; Liu and Liu, 2015) and two types (Basiconica and grooved peg) for kissing bugs (right; adapted from Guerenstein and Guerin, 2001, with the permission from Dr. Guerin). (B) The olfactory receptor neurons housed in each olfactory sensillum are responsible for detecting the attractive cues and increasing the firing frequency of the action potentials. Left: one section of a bed bug antennae; middle: a single sensillum is shown at high magnification (x720); right: depiction showing that the recording tungsten electrode is inserted into the shaft of a sensillum to complete the electrical circuit and to extracellularly record the olfactory receptor neuron potentials. (C) Schematic diagrams of the structures of three olfactory receptors (OR/ORCO, GR, and IR/IRCO) expressed in the membranes of the neuron dendrites that are the molecular targets for host cues. (D) The total number of odorant binding proteins and olfactory receptors (OR/ORCO, GR, and IR/IRCO) identified in the genomes of C. lectularius and R. prolixus.
Olfactory Sensilla and Olfactory Receptor Neurons
The olfactory sensilla make up a key structure that plays a critical role in the chemosensation of the insect antennae. Based on their morphological shape, the common bed bug (C. lectularius) has three types of olfactory sensilla: D, C, and E (Table 1). Of these, the majority are distributed along the distal portion of flagellomere II, with just a few located in the pedicel (Harraca et al., 2010; Liu et al., 2013; Olson et al., 2014). Each different type of sensillum houses a varying number of neurons. Three functional types of D sensilla (Dα, Dβ, Dγ), two types of C sensillum (C1, C2), and two E sensilla (E1, E2) have been identified on flagellomere II. A refined distribution map for each type of sensillum was described by Liu et al. (2017c). Dα, Dβ, Dγ, C1, C2, E1, and E2 have all been identified as olfactory sensilla, while the third type of E sensillum (E3) is thought to be a gustatory sensillum (Singh et al., 1996; Olson et al., 2014). The numbers of olfactory sensilla presenting on the antenna gradually increase as C. lectularius progresses from the first nymph instar to the adult stage, but no sexual dimorphism has been observed in either the sensillum number or their distribution along the antenna (Liu et al., 2017c). This is also the case for another bed bug species, the tropical bed bug (C. hemipterus), where the number of chemo-sensilla (olfactory and gustatory sensilla) on the antenna again increase from the nymph to the adult stage, with no sexual dimorphism (Mendki et al., 2013). However, the chemo-sensilla are distributed across all four segments of the antennae in the tropical bed bugs, while no chemo-sensilla have been found in either the base or the flagellomere I of the common bed bug antenna (Mendki et al., 2013; Olson et al., 2014). There are also reports of a few chemo-sensilla being seen in the rostrum of the tropical bed bug but not in the common bed bug (Mendki et al., 2013).
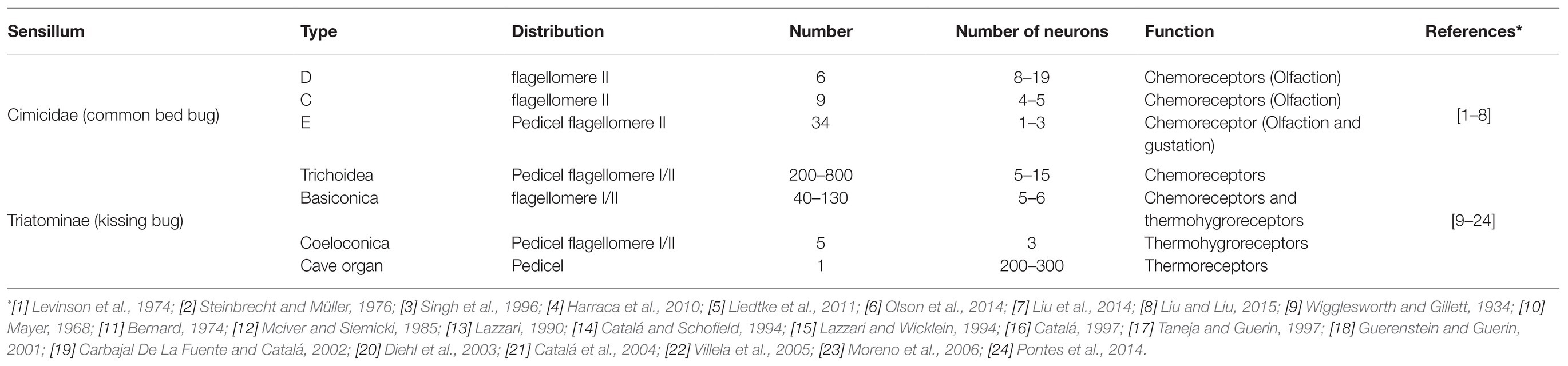
Table 1. Types and functions of the antennal sensilla in the common bed bug (Cimicidae) and kissing bug (Triatominae).
In kissing bugs, four morphological types of sensillum have been characterized in the antenna, namely trichoidea, basiconica, coeloconica, and cave organ (Table 1; Barrozo et al., 2017). Trichoidea and basiconica are the most common types on flagellomeres I and II, both of which function as chemoreceptors. Two subtypes of trichoidea, multi- and uni-porous, have been identified based on the number of pores on individual sensilla (Guidobaldi et al., 2014). Multi-porous trichoidea sensilla sense odors, whereas uni-porous sensilla (with a single pore at the tip) detect tastants (Mayer, 1968; Taneja and Guerin, 1997; Guerenstein and Guerin, 2001; Diehl et al., 2003; Pontes et al., 2014). Sensilla coeloconica are assumed to perform a thermohygrom receptive function in Triatominae; basiconica may also perform the same function (Bernard, 1974; Mciver and Siemicki, 1985; Lazzari, 1990). Only one cave-like sense organ has been found on the pedicel segment and electrophysiological evidence supports a thermoreceptive role for this organ (Catalá and Schofield, 1994; Lazzari and Wicklein, 1994).
The distribution of sensory organs on triatomine antennae displays a genus-, sex-, and habitation-biased pattern. For example, the total number of trichoidea sensilla varies dramatically between Triatoma (400–800) and Rhodnius (200–500; Catalá and Dujardin, 2001; Carbajal De La Fuente and Catalá, 2002; Catalá et al., 2004, 2005; Esteban et al., 2005; Villela et al., 2005; Moreno et al., 2006; Carbajal De La Fuente et al., 2008; Villacís et al., 2010; May-Concha et al., 2016). Triatoma males have trichoidea sensilla that are significantly more thin-walled than those of the females, especially on the pedicel segment (Catalá et al., 2004; Villela et al., 2005; May-Concha et al., 2016), whilst the number of thin-walled trichoidea sensilla in the Rhodnius species exhibit no difference between the sexes (Catalá et al., 2004; Villacís et al., 2010). Interestingly, T. infestans collected from domestic sites have more thin-walled trichoidea sensilla on the pedicel and more thick-walled trichoidea sensillum on both flagellomere I and II than those collected from sylvan sites (Catalá and Dujardin, 2001; Catalá and Torres, 2001) with the specific mechanism yet to be determined.
Potent sensitivities of the kissing bug olfactory sensillum to host odor plumes and a few unitary aldehyde and acid compounds have been described (Guerenstein and Guerin, 2001), while the bed bug olfactory sensilla are particularly sensitive to several chemical classes of odors in human emanation, especially aldehydes, alcohols, aromatics, and ketones (Liu and Liu, 2015), as well as plant-sourced terpenes and terpenoids (Liu et al., 2014). Similar patterns have also been reported for two mosquitoes, Culex quinquefasciatus and Aedes aegypti (Liu et al., 2013; Ye et al., 2016; Chen et al., 2018, 2019). As bed bugs possess far fewer olfactory sensilla/OSNs than either kissing bugs or mosquitoes, their capacity for odor discrimination is likely to be inferior. Indeed, a comparison of the distribution of multiple groups of compounds in the odor space of bed bugs, C. quinquefasciatus and A. aegypti indicates that bed bugs may be less capable of discriminating human-related aldehydes and aromatics and plant-related terpenoids than either Culex or Aedes mosquitoes (Figure 2). These differences in odor-discriminatory capacity probably lie in the much more abundant functional types of olfactory sensilla or OSNs in the antenna of C. quinquefasciatus and A. aegypti compared to bed bugs. Although as yet there is insufficient data to include kissing bugs in this comparison, it is reasonable to speculate that kissing bugs are likely to be endowed with a much stronger ability for odor discrimination than bed bugs as they have a comparable number of olfactory sensilla to mosquitoes and live in a similarly complex chemical environment.

Figure 2. Distribution of odorants in an ORN activity-based odor space. Odor spaces were constructed using the first three principal components of PCA (PAST 3.0, Carey et al., 2010) for the primary sensory responses generated by odorants (Liu et al., 2013, 2014; Liu and Liu, 2015; Ye et al., 2016; Chen et al., 2018, 2019). (A) Aldehydes; (B) aromatics; (C) terpenoids. All odorants from each chemical class are included. The mean inter-odorant distances in three-dimensional space for the set of aldehydes are 0.16 ± 0.01 for C. lectularius, 0.34 ± 0.02 for A. aegypti, and 0.22 ± 0.01 for C. quinquefasciatus (A. aegypti vs. C. lectularius, p < 0.001; C. quinquefasciatus vs. C. lectularius, p < 0.001, t-test). The mean inter-odorant distances for aromatics are 0.05 ± 0.00 for C. lectularius, 0.10 ± 0.01 for A. aegypti, and 0.10 ± 0.01 for C. quinquefasciatus (A. aegypti vs. C. lectularius, p < 0.001; C. quinquefasciatus vs. C. lectularius, p < 0.001, t-test). The mean inter-odorant distances for terpenoids are 0.11 ± 0.01 for C. lectularius, 0.14 ± 0.01 for A. aegypti, and 0.16 ± 0.01 for C. quinquefasciatus (A. aegypti vs. C. lectularius, p < 0.001; C. quinquefasciatus vs. C. lectularius, p < 0.001, t-test).
Odorant-Binding Proteins and Chemosensory Proteins
Odorant-binding proteins (OBPs) and CSPs, low-molecular-weight soluble proteins that are secreted by the accessory cells, are highly concentrated in sensillum lymph. OBPs and CSPs function to transport hydrophobic odorants through the aqueous environment of the sensillum lymph to the ORs’ recognition sites. According to the various models that have been proposed, an OR may be activated either by the odorant molecule itself or the OBP(CSP)/odorant complex (Leal, 2013). For instance, knockdown of OBP1 in the southern house mosquito C. quinquefasciatus results in reduced EAG responses to mosquito oviposition pheromones (Pelletier et al., 2010) and silencing OBP1 leads to a failure to sense indole, a key component of human sweat, in the malaria mosquito Anopheles gambiae (Biessmann et al., 2010). In the tsetse fly, silencing the OBPs that interact with 1-octen-3-ol dramatically abolished flies’ attraction to 1-octen-3-ol (Diallo et al., 2021), while in brown planthopper, silencing one CSP gene (NlugCSP8) induced significant decrease in the behavioral responses to some representative attractants (Waris et al., 2018). With many studies suggesting the essential roles of OBPs and CSPs in the chemosensation of some insect species, there are also opposite discoveries about the odor-transporting role of the OBPs (or CSPs). For example, it is also reported that a fly strain with all obp genes deleted still showed robust responses to odors from diverse chemical groups (Xiao et al., 2019), which suggests other functions of OBPs or CSPs beyond odor transportation in the olfactory sensillum. Actually, only a small number of OBPs or CSPs have been found in the olfactory appendages of various insects and some are expressed in non-sensory tissues such as sex pheromone glands of Lepidoptera, venom glands of wasps, and reproductive organs (Dippel et al., 2014; Brito et al., 2016; Sun et al., 2018), which are thus assumed to function as a carrier of internal chemicals other than external compounds (Pelosi et al., 2014). Other potential roles of OBPs or CSPs, such as contributing to the selectivity of odorant sensation or acting as odorant-degrading enzymes, have also been proposed but remain to be confirmed (Leal, 2013; Larter et al., 2016; Scheuermann and Smith, 2019).
Genome sequencing has contributed greatly to research in this area, which identifies 11 OBPs and 14 CSPs in the common bed bug (C. lectularius) and 27 OBPs and 19 CSPs in kissing bugs (R. prolixus; Figure 1D; Mesquita et al., 2015; Benoit et al., 2016). Transcriptome sequencing of olfactory appendages (antennae or rostrum) in another kissing bug species, Triatoma brasiliensis, also identified 27 OBPs and 17 CSPs, most of which have well-supported orthologs in R. prolixus (Marchant et al., 2016). Proteomic analysis of the antenna of R. prolixus by Oliveira et al. (2017) identified 17 OBPs and 6 CSPs, representing 63 and 31% of all the OBPs and CSPs, respectively, in the genome sequence (Mesquita et al., 2015). Further work by Oliveira et al. (2018) indicated that of the 17 OBP genes identified in the R. prolixus adults, although 11 were expressed in all tissues, six were specific to antennae. Of the six antenna-expressing OBPs, two (RproOBP6 and RproOBP13) were expressed in both sexes; two (RproOBP17 and RproOBP21) were female antenna-enriched, and the rest (RproOBP26 and RproOBP27) were male antenna-specific. RproOBP27 was later confirmed to be involved in the detection of sex pheromones by functional studies (Oliveira et al., 2018). For bed bugs, the functions of OBPs and CSPs have not yet been explored. Given that multiple experimental approaches including RNA interference (Pelletier et al., 2010), CRISPR/Cas9 (Scheuermann and Smith, 2019; Xiao et al., 2019), and competitive binding assays using a fluorescent probe (Brito et al., 2016) have been successfully used to investigate the function of OBPs or CSPs from many other insect species, future studies using similar approaches should yield interesting results about the interactions between bed bug or kissing bug OBPs or CSPs and a wide variety of biologically relevant compounds that have been examined in either electrophysiological or behavioral studies. X-ray crystallography and nuclear magnetic resonance (NMR) are other powerful tools that can provide more details about the unbound or the agonist/antagonist-bound structural complex (Brito et al., 2016), Comparison of the unbound and ligand-bound OBP structures should help identify the amino acid residues involved in ligand binding. All these valuable information will help build our understanding of the mechanisms through which compounds are filtered and transported in the sensillum.
Odorant Receptors
Odorant receptors (ORs) have been extensively studied due to their role in detecting odors from diverse chemical groups (Carey et al., 2010; Joseph and Carlson, 2015; McBride, 2016; Liu et al., 2017a). ORs may evolve from IRs/GRs and are further diversified phylogenetically across different insect taxa (Hansson and Stensmyr, 2011; Missbach et al., 2014; Figure 3). However, the odorant receptor co-receptor (Orco) gene is highly conserved across insects (Jones et al., 2005; Leal, 2013). The ORCO protein is considered to play an important role in 1) the localization and stabilization of ORs in the neuron dendritic membranes; and 2) the transient binding and transduction of odorants via a heteromeric OR/ORCO complex (Larsson et al., 2004; Benton et al., 2006; see also the review in Stengl and Funk, 2013). Studies on the Orco gene of the kissing bug (RproOrco) revealed that when it has been silenced by RNA interference, the kissing bug is unable to locate a vertebrate host in a timely manner, leading to decreased blood ingestion, delayed and decreased molt rate, increased mortality rate, and decreased egg-laying (Franco et al., 2016). The expression level of the RproOrco gene is regulated by both the kissing bug’s feeding status and developmental stage. A significant decrease in RproOrco expression has been observed after blood feeding, while an increase follows an imaginal molt (Latorre-Estivalis et al., 2015). In the common bed bug, the Orco gene has been found in both olfactory appendages (antennae and legs) and other non-olfactory related tissues (Hansen et al., 2014). Interestingly, phylogenetic analysis has indicated that R. prolixus and C. lectularius Orco are closely related, with a relatively close evolutionary distance compared to other insect species in different orders (Liu and Liu, 2015).
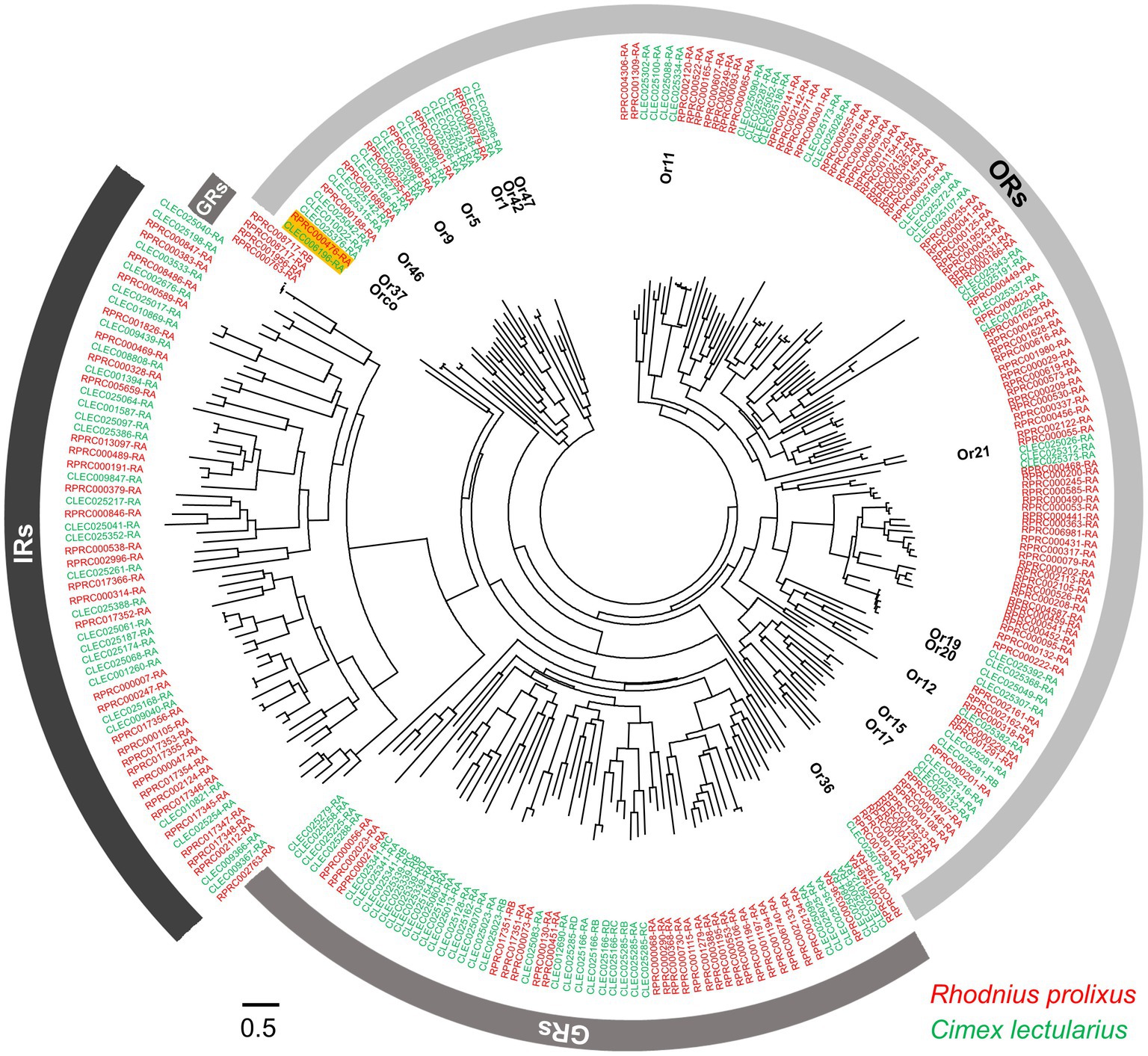
Figure 3. Phylogenetic relationships within the ORs, IRs, and GRs of R. prolixus and C. lectularius. The dendrogram was computed using FastTree based on a MAFFT alignment of 272 amino acid sequences (VectorBase) from R. prolixus (accession number in red color) and C. lectularius (accession number in green color). The accession numbers of two Orco genes are highlighted. Fifteen ORs of C. lectularius that have been tested against around 150 odorants, including human odors and botanical chemical stimuli, are annotated (Liu et al., 2017a).
Whole-genome sequence analyses have revealed 115 and 49 ORs for R. prolixus and C. lectularius, respectively (Figures 1D; Mesquita et al., 2015; Benoit et al., 2016). The striking difference in OR number between these two hemipteran species is thought to be correlated with the complexity of the chemical environment in their respective habitats. The wingless C. lectularius lives in relatively closed and limited spaces, indoors or near the host, while the winged R. prolixus can fly long distances for host/mate searching (Gringorten and Friend, 1979; Zacharias et al., 2010). This natural selection may result in a comparatively stable chemosensory ecology in C. lectularius, which presents rare OR gene expansion in the genome compared to R. prolixus (Liu et al., 2017a). Benefiting from the availability of the genomic information for these species, the expression patterns for some of the ORs in R. prolixus have been characterized for different tissues and developmental stages. Using RT-PCR, Latorre-Estivalis et al. (2016) discovered that the R. prolixus ORs were expressed in every development stage from embryo to nymph and adult antennae. Most of these ORs were found not only in the antennae but also in other tissues such as the rostri, tarsi, tibial pads, and genitalia, suggesting that these appendages may also involve in the chemosensation-mediated behaviors of R. prolixus. Similarly, the ORs in C. lectularius have also been found to be expressed in other structures (e.g. legs) in addition to antennae (Liu and Liu, 2015).
Functional studies aimed at deciphering insect ORs generally use one of the following experimental approaches: 1) Drosophila “Empty Neuron” transgenic system, where exogenous OR genes are expressed in certain fly ORNs without the expression of any native ORs (Hallem and Carlson, 2006); 2) neuron-specific calcium imaging, which monitors calcium activity in GCaMP-expressed tissues or organs, mostly in flies and mosquitoes (Silbering and Galizia, 2007); 3) Xenopus oocyte expression systems, which are coupled with a two-electrode voltage clamp/patch clamp to detect the receptor current through the ion channels on oocyte membrane (Wang et al., 2010); 4) mammalian cell expression system coupled with patch clamp to measure the receptor current and ion conductance of the channels (Jones et al., 2011); 5) chemical informatics, which utilizes in silico modeling to screen large chemical space and identify potential ligands for receptors (Boyle et al., 2013); and 6) gene editing-mediated mutagenesis, which uses gene editing techniques such as clustered regularly interspaced short palindromic repeats/ CRISPR-associated protein 9 (CRISPR/Cas9), Transcription activator-like effector nucleases (TALEN), or zinc-finger nuclease (ZFN) to create mutants and then compares the phenotype changes between the wildtype and mutant insects (McMeniman et al., 2014; Liu et al., 2021). For example, functional studies have been used to investigate the role of four kissing bug ORs in perceiving sex pheromones using a Xenopus oocyte expression system coupled with a two-electrode voltage clamp (Franco et al., 2018). Although none of these ORs were identified as sex pheromone receptors, RproOR80 was found to be extremely sensitive to several compounds that turned out to be repellents for kissing bugs (Franco et al., 2018). In the common bed bug, 15 ORs have been successfully expressed in the Xenopus oocyte and challenged with a large panel of human odors (Liu et al., 2017a). In general, ORs with strong responses were tuned to aldehydes, ketones, alcohols, and aromatic compounds. Functional tests of these ORs in response to the components of aggregation pheromone also revealed that most of these components were encoded by multiple ORs with various tuning properties (Liu et al., 2017b). In addition, three ORs were identified as potent DEET receptors, even though DEET is not very effective in repelling bed bugs. Interestingly, these DEET-sensitive ORs presented even higher sensitivity to certain botanical terpenes/terpenoids that generally displayed much stronger repellency for bed bugs than DEET (Liu et al., 2017c).
Ionotropic Receptors and Gustatory Receptors
Ionotropic glutamate receptors (iGluRs) are chemosensory receptors that mediate neuronal communication between synapses in both vertebrate and invertebrate nervous systems. They comprise one of the three superfamilies used to classify IRs based on their predicted molecular structures, including an extracellular N-terminus, a cytoplasmic C-terminus, a bipartite ligand-binding domain, and an ion channel. However, IRs differ from the well-documented kainate, α-amino-3-hydroxy-5-menthyl-isoxazole-4-propionate (AMPA), or N-menthyl-D-aspartate (NMDA) classes of iGluRs as they (1) lack the characteristic glutamate interacting residues but instead have divergent ligand-binding domains; and (2) accumulate in sensory dendrites rather than at synapses (Benton et al., 2009). Phylogenetic studies have revealed that IRs are conserved across bacteria, plants, and animals, which suggests an evolutionarily ancient function in chemosensation (Benton et al., 2009). IRs in coeloconic OSNs are known to be responsible for detecting organic acids, amines, and polyamines (Benton et al., 2009; Ai et al., 2010; Hussain et al., 2016). Like Orco, IR8a, IR25a, and IR76b are highly conserved across different species and are considered to function as co-receptors with other IRs in mediating the olfactory responses to semiochemicals (Croset et al., 2010). For example, in D. melanogaster, IR64a and IR8a are physically associated in the OSNs and constitute a functional channel when co-expressed in vitro in Xenopus oocytes (Ai et al., 2013). In An. gambiae, both IR25a and IR76b are required for the functional expression of IR41a and IR41c in Xenopus oocytes, while IR8a is needed for the expression of IR75k in oocytes (Pitts et al., 2017). In addition to its role as a co-receptor, Drosophila IR25a has been shown to function as a thermosensor as well as playing a role in establishing the insect’s circadian rhythm (Chen et al., 2015), suggesting other potentially important functions of IRs in insect physiology.
In the kissing bug, R. prolixus, these three IR co-receptors (IRCO) genes (IR8a, 25a, and 76b) have been investigated to determine their expression patterns under different physiological and developmental conditions. IRCOs are known to be transcribed in the antennae of all nymph instar development stages and in both male and female kissing bugs (Latorre-Estivalis et al., 2016) and all three of these IRCOs are down-regulated by blood-feeding and up-regulated after the imaginal molt (Latorre-Estivalis et al., 2015), which underlines the plasticity of triatomine olfactory-mediated behaviors. In addition to the IRCOs, the expression patterns for 15 R. prolixus IRs in different tissues or sexual conditions have been characterized. Although most (11 out of 15) of these RproIRs were expressed in the antennae of all developmental instars, some exceptions have been reported. For example, no RproIR75e expression was observed in embryos and RproIR20a was not detected in first instar nymphs; neither RproIR103 nor RproIR104 were found in the antennae in either the nymph instars or adults of either sex (Latorre-Estivalis et al., 2016).
Based on the genomic data, 33 and 30 IRs have been annotated in R. prolixus and C. lectularius, respectively (Figures 1D). Functional studies of Drosophila IRs have suggested that organic acids and amine compounds are likely to be the primary ligands for IRs (Benton et al., 2009; Ai et al., 2010). Given that the C type sensilla in bed bugs show extreme sensitivity to amine compounds (Liu and Liu, 2015), certain IRs may be expressed in these sensilla. In the kissing bug, R. prolixus, ammonia and amines from vertebrate excretion were found to induce an obvious attraction response, suggesting that some factors in the kissing bug olfactory system (e.g. IRs) are actively sensing these compounds and guiding the host-searching behavior (Otálora-Luna and Guerin, 2014). However, as yet none of the IRs from either kissing bugs or bed bugs have been functionally characterized, further studies on these IRs are therefore necessary to clarify the response profiles of IRs in both insects.
In addition to ORs and IRs, GRs are involved in food searching and feeding stimulation. GRs are known to be responsible for detecting CO2, amines, and polyamines, and compounds in food sources including sugars, bitter tastes, and toxins (Liman et al., 2014; MacWilliam et al., 2018). Based on their genome sequences, there are 36 and 31 GRs in C. lectularius and R. prolixus (Figures 1D, 3), respectively. Among these, no sugar receptors have been identified in either bed bugs (Benoit et al., 2016) or kissing bugs (Mesquita et al., 2015), which explains the lack of phagostimulation by glucose in C. lectularius (Romero and Schal, 2014). This lack of sugar receptors has also been documented in other obligate blood-feeders, including tsetse flies (Obiero et al., 2014) and lice (Kirkness et al., 2010). Interestingly, the CO2 sensory GR subfamily is absent in R. prolixus, while the four putative CO2 sensory GRs that have been identified in bed bugs are phylogenetically conserved with the CO2 receptors in flies, moths, beetles, and one termite species (Terrapon et al., 2014). Future endeavors to investigate the response profiles of GRs from either kissing bugs or bed bugs would thus advance our understanding of chemoreception in both insects considerably.
Chemosensation-Based Applications
Due to the biting nuisance and risk of potential disease transmission, the effective management of both kissing bugs and bed bugs is one of the basic aims of research in this area and a long-term goal for scientists (Boase and Naylor, 2014; Zermoglio et al., 2015). Various strategies have been applied in the battle to control these two pests, with many based on the widespread use of insecticides. However, the intense application of insecticides leads to strong selection pressure, building up resistance in insect populations and dramatically impairing the efficiency of insecticides. Therefore, new approaches are continually being explored as a matter of urgency. Several promising approaches, such as push-pull or stimulo-deterrent diversionary (SDD) strategies (Cook et al., 2007; Figure 4A), are based on the latest research on insect chemosensation. Insects such as bed bugs and kissing bugs can be attracted by host odors (PULL) or repelled by repellents/deterrents (PUSH), while another option is to mask host odors using confusants (MASK). These novel approaches using chemicals, attractants, repellents, and/or confusants are expected to contribute to reducing the vector borne disease transmission.
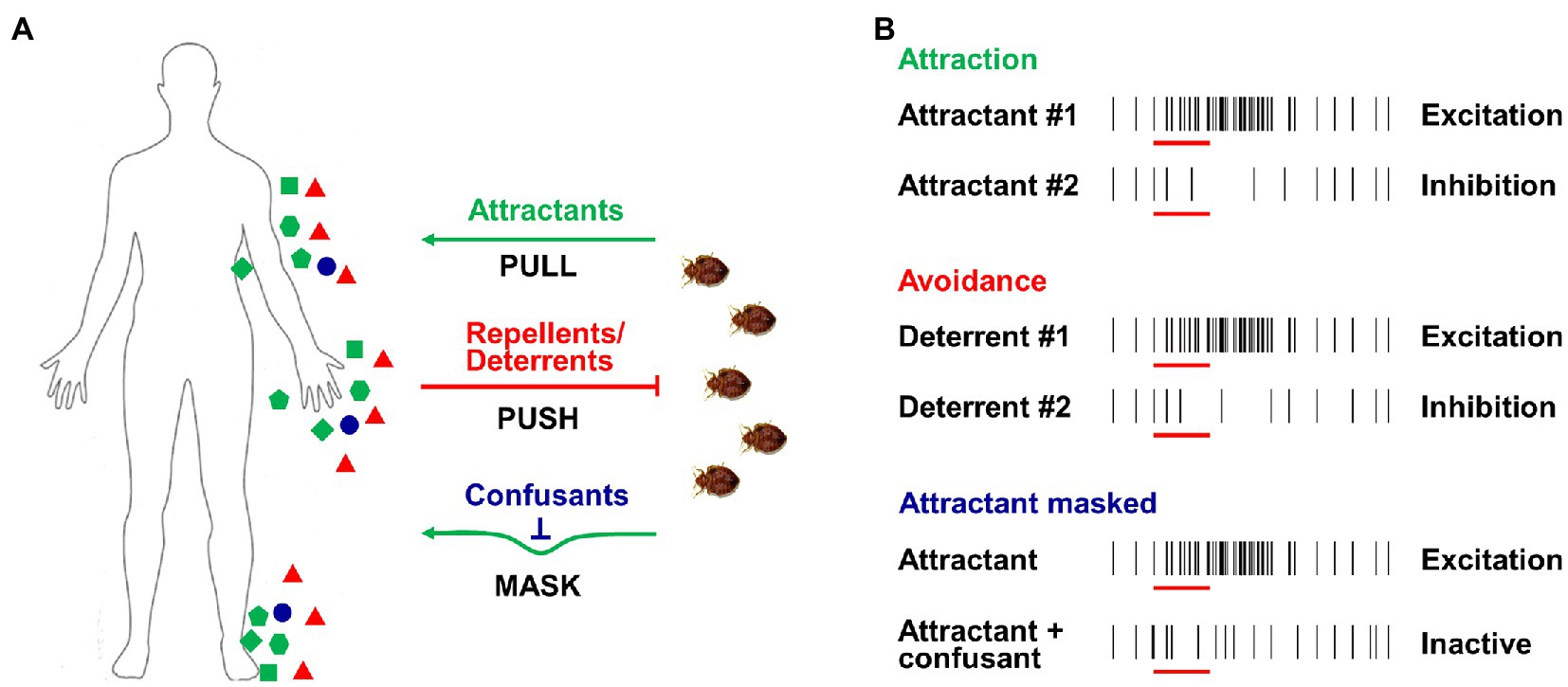
Figure 4. Push-pull strategies and odor-evoked excitation/inhibition activity of ORNs. (A) Push-pull or stimulo-deterrent diversionary (SDD) strategies used for insect control; green dots indicate attractants from hosts (PULL), red dots indicate repellents/deterrents (PUSH), and blue dots indicate confusants (MASK). (B) Excitation or inhibition activities of ORNs caused by attractants, repellents/deterrents and confusants.
Chemical Lures
As one of the most important cues released from human skin and breath, CO2 is highly attractive to most hematophagous insects, including both kissing bugs and bed bugs (Barrozo and Lazzari, 2004; Wang et al., 2009; Singh et al., 2013; Indacochea et al., 2017). It is therefore not surprising that CO2 has been extensively incorporated in many of the bed bug traps that are commercially available as it displays high efficiency in terms of bug catches. Multiple host-related odorants that are generally added to the bait also exert a synergistic effect in attracting kissing bugs or bed bugs. For example, 1-octen-3-ol and nonanal, which are identified in human emanation (Bernier et al., 2000) and bed bug aggregation pheromone (Weeks et al., 2020), display strong activation on OSNs and ORs of bed bugs (Liu and Liu, 2015; Liu et al., 2017a). In laboratory two-choice behavioral assays, 1-octen-3-ol and nonanal have both been shown to attract bed bugs (Singh et al., 2012; Figure 4B). When a chemical mixture containing both 1-octen-3-ol and nonanal is combined with CO2 in a bed bug trap, a synergistic effect has been reported, with increased trap catches (Singh et al., 2012). Since a large number of human odorants have been successively examined in bed bugs using electrophysiological approach and some of them elicit strong neuronal responses in different types of olfactory sensilla (Liu and Liu, 2015), future behavioral studies under laboratory conditions or in the field should identify some promising candidates with potent attraction for bed bugs.
In the kissing bug T. infestans, ammonia is reported to activate two types of grooved peg sensilla, making it a strong attractant for T. infestans (Taneja and Guerin, 1997). Another study found that a carbon dioxide-free attractant containing three human odorants (ammonia, L-(+)-lactic acid, and hexanoic acid) significantly increased bug catches for both R. prolixus and T. infestans (Guidobaldi and Guerenstein, 2013). In addition, male R. prolixus is attracted by a synthetic female-pheromone blend comprised of ten compounds, which elicit neuronal response from basiconic olfactory sensillum (Bohman et al., 2018). Taken together, these behavioral assays indicate the potential utility of using human odors or pheromone components to boost the performance of chemical lures for both bed bugs and kissing bugs, as well as highlighting the need to explore the potency of other OSNs/receptor-sensitive human odors.
Chemical Repellents/Confusants
Several different mechanisms have been proposed for chemical repellency in insect olfaction. First, certain compounds are known to block the attractant binding sites of OBP(s), resulting in insensitivity or reduced sensitivity to attractants. For example, it has been reported that in the mosquito An. gambiae, the synthetic repellent DEET and two natural repellents, 6-menthyl-5-heptene-2-one and eugenyl acetate, occupy the active binding site of OBP1, which is thought to be critical in preventing the transportation of some key attractant odorants (Murphy et al., 2012; Tsitsanou et al., 2012; Affonso et al., 2013). Second, some odorants may cause avoidance behavior by activating or inhibiting the ORN activities in insects (Figure 4B). For instance, in the mosquito C. quinquefasciatus, DEET is known to activate OR136b, triggering an aversive response (Xu et al., 2014); geraniol has also been shown to inhibit the activity of Or10a-ORN and Or42b-ORN and induce avoidance behavior in Drosophila (Cao et al., 2017). Third, some odorants may block/inhibit (or mask) the excitatory responses elicited by attractive compounds or alter the temporal structure of the insect’s ORN response to attractants (Figure 4B). Ditzen et al. (2008) reported that DEET significantly blocks the neuronal response of An. gambiae to one human odorant (1-octen-3-ol), while Pellegrino et al. (2011) found that DEET appears to scramble the olfactory responses of D. melanogaster to some odors, although the precise mechanism is unclear. Bohbot et al. (2011) also suggested that DEET significantly inhibits the function of Aedes mosquitos’ ORs in response to ligands. Odorants that alter the temporal structure of the ORN response may also affect insect olfaction-mediated behavior. It has been reported that in either mosquito or moth, some mimicking compounds evoke continual firing in the primary compound-sensitive ORN, which disrupts the normal attractive behavioral response of those insects (Kramer, 1992; Turner et al., 2011).
In the common bed bug, certain ORNs and ORs were found to be directly activated by DEET while DEET also blocked the excitatory responses of ORNs and ORs to some human odors as well as manipulating the temporal dynamic of the odor-evoked neuronal response, which may result in the significant repellency of DEET agaist the bed bugs (Liu et al., 2017c). The same study also identified some components from essential oils, such as (+)-menthone, linalyl acetate and menthyl acetate, which effectively activated multiple ORNs and ORs and elicited very potent repellency against the bed bugs with a corresponding dose threshold of 10–100 fold lower than that of DEET (Liu et al., 2017c). In R. prolixus, one male-enriched OR (RproOR80) was functionally sensitized to 4-methylcyclohexanol, which turned out to be a strong repellent for kissing bugs by inducing a significant decrease in residence time to the host and a remarkable reduction in blood intake (Franco et al., 2018). This reverse chemical ecology strategy has also been adopted for identifying compounds with biological significance for other blood-feeding insects (Leal et al., 2008; Choo et al., 2018), agricultural insects (Xu et al., 2021), and mammals (Zhu et al., 2017). All these studies highlight the value of conducting further explorations of novel behaviorally active semiochemicals based on the reverse chemical ecology strategy for better controlling insect pests, such as bed bugs and kissing bugs and terminating the potential disease transmission.
Author Contributions
NL, FL, ZC, and YZ: conceived and designed the study, wrote thepaper and reviewed the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by AAES Hatch/Multistate grants ALA08-045 and ALA015-1-10026, and ALA015-1-16009 to NL.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Affonso, R. D. S., Guimarães, A. P., Oliveira, A. A., Slana, G. B., and França, T. C. (2013). Applications of molecular modeling in the design of new insect repellents targeting the odorant binding protein of Anopheles gambiae. J. Braz. Chem. Soc. 24, 473–482. doi: 10.1590/S0103-50532013000300015
Ai, M., Blais, S., Park, J. Y., Min, S., Neubert, T. A., and Suh, G. S. (2013). Ionotropic glutamate receptors IR64a and IR8a form a functional odorant receptor complex in vivo in Drosophila. J. Neurosci. 33, 10741–10749. doi: 10.1523/JNEUROSCI.5419-12.2013
Ai, M., Min, S., Grosjean, Y., Leblanc, C., Bell, R., Benton, R., et al. (2010). Acid sensing by the Drosophila olfactory system. Nature 468, 691–695. doi: 10.1038/nature09537
Alexander, J. O. (ed.) (1994). “Infestation by Hemiptera,” in Arthropods and the human skin. London: Springer Verlag. 57–74.
Anderson, J. F., Ferrandino, F. J., Vasil, M. P., Bedoukian, R. H., Maher, M., and Mckenzie, K. (2017). Relatively small quantities of CO2, Ammonium Bicarbonate, and a blend of (E)-2-Hexenal Plus (E)-2-Octenal attract bed bugs (Hemiptera: Cimicidae). J. Med. Entomol. 54, 362–367. doi: 10.1093/jme/tjw189
Barrozo, R. B., Minoli, S. A., and Lazzari, C. R. (2004). Circadian rhythm of behavioural responsiveness to carbon dioxide in the blood-sucking bug Triatoma infestans (Heteroptera: Reduviidae). J. Insect Physiol. 50, 249–254. doi: 10.1016/j.jinsphys.2004.01.001
Barrozo, R. B., and Lazzari, C. R. (2004). Orientation behaviour of the blood-sucking bug Triatoma infestans to short-chain fatty acids: synergistic effect of L-lactic acid and carbon dioxide. Chem. Senses 29, 833–841. doi: 10.1093/chemse/bjh249
Barrozo, R. B., Reisenman, C. E., Guerenstein, P., Lazzari, C. R., and Lorenzo, M. G. (2017). An inside look at the sensory biology of triatomines. J. Insect Physiol. 97, 3–19. doi: 10.1016/j.jinsphys.2016.11.003
Benoit, J. B., Adelman, Z. N., Reinhardt, K., Dolan, A., Poelchau, M., Jennings, E. C., et al. (2016). Unique features of a global human ectoparasite identified through sequencing of the bed bug genome. Nat. Commn. 7:10165. doi: 10.1038/ncomms10165
Benton, R., Sachse, S., Michnick, S. W., and Vosshall, L. B. (2006). Atypical membrane topology and heteromeric function of Drosophila odorant receptors in vivo. PLoS Biol. 4:e20. doi: 10.1371/journal.pbio.0040020
Benton, R., Vannice, K. S., Gomez-Diaz, C., and Vosshall, L. B. (2009). Variant ionotropic glutamate receptors as chemosensory receptors in Drosophila. Cell 136, 149–162. doi: 10.1016/j.cell.2008.12.001
Bernard, J. (1974). Etude électrophysiologique des récepteurs impliqués dans la orientation vers l’hote et dans l’acte hematophage chez un Hémiptére Triatoma infesfans. Thesis. Rennes, France: Université de Rennes.
Bernier, U. R., Kline, D. L., Barnard, D. R., Schreck, C. E., and Yost, R. A. (2000). Analysis of human skin emanations by gas chromatography/mass spectrometry. 2. Identification of volatile compounds that are candidate attractants for the yellow fever mosquito (Aedes aegypti). Anal. Chem. 72, 747–756. doi: 10.1021/ac990963k
Biessmann, H., Andronopoulou, E., Biessmann, M. R., Douris, V., Dimitratos, S. D., Eliopoulos, E., et al. (2010). The Anopheles gambiae odorant binding protein 1 (AgamOBP1) mediates indole recognition in the antennae of female mosquitoes. PLoS One 5:e9471. doi: 10.1371/journal.pone.0009471
Blakely, B. N., Hanson, S. F., and Romero, A. (2018). Survival and transstadial persistence of Trypanosoma cruzi in the bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 55, 742–746. doi: 10.1093/jme/tjx252
Boase, C., and Naylor, R. (2014). Bed bug management. Urban insect pests: Sustainable management strategies. London, United Kingdom: CABI Press, 8–22.
Bodin, A., Vinauger, C., and Lazzari, C. R. (2009). State-dependency of host-seeking in Rhodnius prolixus: the post-ecdysis time. J. Insect Physiol. 55, 574–579. doi: 10.1016/j.jinsphys.2009.02.004
Bodin, A., Barrozo, R. B., Couton, L., and Lazzari, C. R. (2008). Temporal modulation and adaptive control of the behavioural response to odours in Rhodnius prolixus. J. Insect Physiol. 54, 1343–1348. doi: 10.1016/j.jinsphys.2008.07.004
Bohbot, J. D., Fu, L., Le, T. C., Chauhan, K. R., Cantrell, C. L., and Dickens, J. C. (2011). Multiple activities of insect repellents on odorant receptors in mosquitoes. Med. Vet. Entomol. 25, 436–444. doi: 10.1111/j.1365-2915.2011.00949.x
Bohman, B., Weinstein, A. M., Unelius, C. R., and Lorenzo, M. G. (2018). Attraction of Rhodnius prolixus males to a synthetic female-pheromone blend. Parasit Vectors 11, 1–7. doi: 10.1186/s13071-018-2997-z
Boyle, S. M., McInally, S., and Ray, A. (2013). Expanding the olfactory code by in silico decoding of odor-receptor chemical space. elife 2:e01120. doi: 10.7554/eLife.01120
Brady, J. (1975). Circadian changes in central excitability—the origin of behavioural rhythms in tsetse flies and other animals? Physiol. Entomol. 50, 79–95. doi: 10.1111/j.1365-3032.1975.tb00095.x
Brito, N. F., Moreira, M. F., and Melo, A. C. (2016). A look inside odorant-binding proteins in insect chemoreception. J. Insect Physiol. 95, 51–65. doi: 10.1016/j.jinsphys.2016.09.008
Carbajal De La Fuente, A. L., and Catalá, S. (2002). Relationship between antennal sensilla pattern and habitat in six species of Triatominae. Mem. Inst. Oswaldo Cruz 97, 1121–1125. doi: 10.1590/S0074-02762002000800010
Carbajal De La Fuente, A. L., Noireau, F., and Catalá, S. S. (2008). Inferences about antennal phenotype: the “Triatoma maculata complex,” (Hemiptera: Triatominae) is valid? Acta Trop. 106, 16–21. doi: 10.1016/j.actatropica.2007.12.010
Carey, A. F., Wang, G., Su, C. Y., Zwiebel, L. J., and Carlson, J. R. (2010). Odorant reception in the malaria mosquito Anopheles gambiae. Nature 464, 66–71. doi: 10.1038/nature08834
Carey, A. F., and Carlson, J. R. (2011). Insect olfaction from model systems to disease control. Proc. Natl. Acad. Sci. U. S. A. 108, 12987–12995. doi: 10.1073/pnas.1103472108
Catalá, S. S. (1997). Antennal sensilla of Triatominae (Hemiptera, Reduviidae): a comparative study of five genera. Int. J. Insect Morphol. Embryol. 26, 67–73. doi: 10.1016/S0020-7322(97)00014-7
Catalá, S. S., Maida, D. M., Caro-Riano, H., Jaramillo, N., and Moreno, J. (2004). Changes associated with laboratory rearing in antennal sensilla patterns of Triatoma infestans, Rhodnius prolixus, and Rhodnius pallescens (Hemiptera, Reduviidae, Triatominae). Mem. Inst. Oswaldo Cruz 99, 25–30. doi: 10.1590/S0074-02762004000100005
Catalá, S., and Dujardin, J. P. (2001). Antennal sensilla patterns indicate geographic and ecotopic variability among Triatoma infestans (Hemiptera: Reduviidae) populations. J. Med. Entomol. 38, 423–428. doi: 10.1603/0022-2585-38.3.423
Catalá, S., and Schofield, C. (1994). Antennal sensilla of Rhodnius. J. Morphol. 219, 193–203. doi: 10.1002/jmor.1052190208
Catalá, S., and Torres, M. (2001). Similarity of the patterns of sensilla on the antennae of Triatoma melanosoma and Triatoma infestans. Ann. Trop. Med. Parasitol. 95, 287–295. doi: 10.1080/00034980120051296
Catalá, S., Sachetto, C., Moreno, M., Rosales, R., Salazar-Schettino, P. M., and Gorla, D. (2005). Antennal phenotype of Triatoma dimidiata populations and its relationship with species of phyllosoma and protracta complexes. J. Med. Entomol. 42, 719–725. doi: 10.1093/jmedent/42.5.719
Cao, L. H., Yang, D., Wu, W., Zeng, X., Jing, B. Y., Li, M. T., et al. (2017). Odor-evoked inhibition of olfactory sensory neurons drives olfactory perception in Drosophila. Nat. Commun. 8, 1–13. doi: 10.1038/s41467-017-01185-0
Chen, C., Buhl, E., Xu, M., Croset, V., Rees, J. S., Lilley, K. S., et al. (2015). Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature 527, 516–520. doi: 10.1038/nature16148
Chen, Z., Liu, F., and Liu, N. (2018). Neuronal responses of antennal olfactory sensilla to insect chemical repellents in the yellow fever mosquito, Aedes aegypti. J. Chem. Ecol. 44, 1120–1126. doi: 10.1007/s10886-018-1022-5
Chen, Z., Liu, F., and Liu, N. (2019). Human odour coding in the yellow fever mosquito, Aedes aegypti. Sci. Rep. 9:13336. doi: 10.1038/s41598-019-49753-2
Choo, Y. M., Xu, P., Hwang, J. K., Zeng, F., Tan, K., Bhagavathy, G., et al. (2018). Reverse chemical ecology approach for the identification of an oviposition attractant for Culex quinquefasciatus. Proc. Natl. Acad. Sci. U. S. A. 115, 714–719. doi: 10.1073/pnas.1718284115
Cook, S. M., Khan, Z. R., and Pickett, J. A. (2007). The use of push-pull strategies in integrated pest management. Annu. Rev. Entomol. 52, 375–400. doi: 10.1146/annurev.ento.52.110405.091407
Coura, J. R. (2015). The main sceneries of Chagas disease transmission. The vectors, blood and oral transmissions-A comprehensive review. Mem. Inst. Oswaldo Cruz 110, 277–282. doi: 10.1590/0074-0276140362
Croset, V., Rytz, R., Cummins, S. F., Budd, A., Brawand, D., Kaessmann, H., et al. (2010). Ancient protostome origin of chemosensory ionotropic glutamate receptors and the evolution of insect taste and olfaction. PLoS Genet. 6:e1001064. doi: 10.1371/journal.pgen.1001064
Davis, D. J., McGregor, T., and DeShazo, T. (1943). Triatoma sanguisuga (LeConte) and Triatoma ambigua Neiva as natural carriers of Trypanosoma cruzi in Texas. Public Health Rep. 58, 353–354. doi: 10.2307/4584383
DeVries, Z. C., Saveer, A. M., Mick, R., and Schal, C. (2019). Bed bug (Hemiptera: Cimicidae) attraction to human odors: validation of a two-choice olfactometer. J. Med. Entomol. 56, 362–367. doi: 10.1093/jme/tjy202
Diallo, S., Shahbaaz, M., Makwatta, J. O., Muema, J. M., Masiga, D., Christofells, A., et al. (2021). Antennal enriched odorant binding proteins are required for odor communication in Glossina f. fuscipes. Biomolecules 11:541. doi: 10.3390/biom11040541
Díaz-Albiter, H. M., Ferreira, T. N., Costa, S. G., Rivas, G. B., Gumiel, M., Cavalcante, D. R., et al. (2016). Everybody loves sugar: first report of plant feeding in triatomines. Parasit Vectors 9, 1–8. doi: 10.1186/s13071-016-1401-0
Diehl, P. A., Vlimant, M., Guerenstein, P., and Guerin, P. M. (2003). Ultrastructure and receptor cell responses of the antennal grooved peg sensilla of Triatoma infestans (Hemiptera: Reduviidae). Arthropod Struct. Dev. 31, 271–285. doi: 10.1016/S1467-8039(03)00004-5
Dippel, S., Oberhofer, G., Kahnt, J., Gerischer, L., Opitz, L., and Schachtner, J. (2014). Tissue-specific transcriptomics, chromosomal localization, and phylogeny of chemosensory and odorant binding proteins from the red flour beetle Tribolium castaneum reveal subgroup specificities for olfaction or more general functions. BMC Genomics 15, 1–14. doi: 10.1186/1471-2164-15-1141
Ditzen, M., Pellegrino, M., and Vosshall, L. B. (2008). Insect odorant receptors are molecular targets of the insect repellent DEET. Science 319, 1838–1842. doi: 10.1126/science.1153121
Doggett, S. L., Dwyer, D. E., Penas, P. F., and Russell, R. C. (2012). Bed bugs: clinical relevance and control options. Clin. Microbiol. Rev. 25, 164–192. doi: 10.1128/CMR.05015-11
Doggett, S. L., Geary, M. J., and Russell, R. C. (2004). The resurgence of bed bugs in Australia: with notes on their ecology and control. Environ. Health 4, 30–38. doi: 10.1016/j.actatropica.2009.10.014
Esteban, L., Angulo, V. M., Dora Feliciangeli, M., and Catalá, S. (2005). Analysis of antennal sensilla patterns of Rhodnius prolixus from Colombia and Venezuela. Mem. Inst. Oswaldo Cruz 100, 909–914. doi: 10.1590/S0074-02762005000800014
Franco, T. A., Oliveira, D. S., Moreira, M. F., Leal, W. S., and Melo, A. C. (2016). Silencing the odorant receptor co-receptor RproOrco affects the physiology and behavior of the Chagas disease vector Rhodnius prolixus. Insect Biochem. Mol. Biol. 69, 82–90. doi: 10.1016/j.ibmb.2015.02.012
Franco, T. A., Xu, P., Brito, N. F., Oliveira, D. S., Wen, X., Moreira, M. F., et al. (2018). Reverse chemical ecology-based approach leading to the accidental discovery of repellents for Rhodnius prolixus, a vector of Chagas diseases refractory to DEET. Insect Biochem. Mol. Biol. 103, 46–52. doi: 10.1016/j.ibmb.2018.10.004
Gringorten, J. L., and Friend, W. G. (1979). Wing-beat pattern in Rhodnius prolixus Stål (Heteroptera: Reduviidae) during exhaustive flight. Can. J. Zool. 57, 391–395. doi: 10.1016/j.jinsphys.2016.09.008
Guerenstein, P. G., and Guerin, P. M. (2001). Olfactory and behavioral responses of the blood-sucking bug Triatoma infestans to odours of vertebrate hosts. J. Exp. Biol. 204, 585–597. doi: 10.1242/jeb.204.3.585
Guerenstein, P. G., and Lazzari, C. R. (2009). Host-seeking: how triatomines acquire and make use of information to find blood. Acta Trop. 110, 148–158. doi: 10.1016/j.actatropica.2008.09.019
Guidobaldi, F., and Guerenstein, P. G. (2013). Evaluation of a CO2-free commercial mosquito attractant to capture triatomines in the laboratory. J. Vector. Ecol. 38, 245–250. doi: 10.1111/j.1948-7134.2013.12037.x
Guidobaldi, F., May-Concha, I. J., and Guerenstein, P. G. (2014). Morphology and physiology of the olfactory system of blood-feeding insects. J. Physiol. Paris 108, 96–111. doi: 10.1016/j.jphysparis.2014.04.006
Hallem, E. A., and Carlson, J. R. (2006). Coding of odors by a receptor repertoire. Cell 125, 143–160. doi: 10.1016/j.cell.2006.01.050
Hansen, I. A., Rodriguez, S. D., Drake, L. L., Price, D. P., Blakely, B. N., Hammond, J. I., et al. (2014). The odorant receptor co-receptor from the bed bug, Cimex lectularius L. PloS One 9:e113692. doi: 10.1371/journal.pone.0113692
Hansson, B. S., and Stensmyr, M. C. (2011). Evolution of insect olfaction. Neuron 72, 698–711. doi: 10.1016/j.neuron.2011.11.003
Harraca, V., Ignell, R., Löfstedt, C., and Ryne, C. (2010). Characterization of the antennal olfactory system of the bed bug (Cimex lectularius). Chem. Senses 35, 195–204. doi: 10.1093/chemse/bjp096
Hawkins, W. A., and Rust, M. K. (1977). Factors influencing male sexual response in the American cockroach Periplaneta americana. J. Chem. Ecol. 3, 85–99. doi: 10.1007/BF00988136
Haynes, K. F., and Potter, M. F. (2013). “Recent progress in bed bug management,” in Advanced Technologies for Managing Insect Pests (New York: Springer), 269–278.
Hemmige, V., Tanowitz, H., and Sethi, A. (2012). Trypanosoma cruzi infection: a review with emphasis on cutaneous manifestations. Int. J. Dermatol. 51, 501–508. doi: 10.1111/j.1365-4632.2011.05380.x
Hussain, A., Zhang, M., Üçpunar, H. K., Svensson, T., Quillery, E., Gompel, N., et al. (2016). Ionotropic chemosensory receptors mediate the taste and smell of polyamines. PLoS Biol. 14, 1–30. doi: 10.1371/journal.pbio.1002454
Indacochea, A., Gard, C. C., Hansen, I. A., Pierce, J., and Romero, A. (2017). Short-range responses of the kissing bug Triatoma rubida (Hemiptera: Reduviidae) to carbon dioxide, moisture, and artificial light. Insects 8:E90. doi: 10.3390/insects8030090
Jones, W. D., Nguyen, T. A. T., Kloss, B., Lee, K. J., and Vosshall, L. B. (2005). Functional conservation of an insect odorant receptor gene across 250 million years of evolution. Curr. Biol. 15, 119–121. doi: 10.1016/j.cub.2005.02.007
Jones, P. L., Pask, G. M., Rinker, D. C., and Zwiebel, L. J. (2011). Functional agonism of insect odorant receptor ion channels. Proc. Natl. Acad. Sci. U. S. A. 108, 8821–8825. doi: 10.1073/pnas.1102425108
Joseph, R. M., and Carlson, J. R. (2015). Drosophila chemoreceptors: a molecular interface between the chemical world and the brain. Trends Genet. 31, 683–695. doi: 10.1016/j.tig.2015.09.005
Justi, S. A., Galvao, C., and Schrago, C. G. (2016). Geological changes of the Americas and their influence on the diversification of the Neotropical kissing bugs (Hemiptera: Reduviidae: Triatominae). PLoS Negl. Trop. Dis. 10:e0004527. doi: 10.1371/journal.pntd.0004527
Kirkness, E. F., Haas, B. J., Sun, W., Braig, H. R., Perotti, M. A., Clark, J. M., et al. (2010). Genome sequences of the human body louse and its primary endosymbiont provide insights into the permanent parasitic lifestyle. Proc. Natl. Acad. Sci. U. S. A. 107, 12168–12173. doi: 10.1073/pnas.1003379107
Kramer, E. (1992). Attractivity of pheromone surpassed by time-patterned application of two nonpheromone compounds. J. Insect Behav. 5, 83–97. doi: 10.1007/BF01049160
Krishnan, B., Dryer, S. E., and Hardin, P. E. (1999). Circadian rhythms in olfactory responses of Drosophila melanogaster. Nature 400:375. doi: 10.1038/22566
Larsson, M. C., Domingos, A. I., Jones, W. D., Chiappe, M. E., Amrein, H., and Vosshall, L. B. (2004). Or83b encodes a broadly expressed odorant receptor essential for Drosophila olfaction. Neuron 43, 703–714. doi: 10.1016/j.neuron.2004.08.019
Larter, N. K., Sun, J. S., and Carlson, J. R. (2016). Organization and function of Drosophila odorant binding proteins. elife 5:e20242. doi: 10.7554/eLife.20242
Latorre-Estivalis, J. M., Manuel, J., Omondi, B. A., DeSouza, O., Oliveira, I. H. R., Ignell, R., et al. (2015). Molecular basis of peripheral olfactory plasticity in Rhodnius prolixus, a Chagas disease vector. Front. Ecol. Environ. 3:74. doi: 10.3389/fevo.2015.00074
Latorre-Estivalis, J. M., de Oliveira, E. S., Esteves, B. B., Guimarães, L. S., Ramos, M. N., and Lorenzo, M. G. (2016). Patterns of expression of odorant receptor genes in a Chagas disease vector. Insect Biochem. Mol. Biol. 69, 71–81. doi: 10.1016/j.ibmb.2015.05.002
Lazzari, C. R. (1990). Fisiologia del comportamiento de Triatoma infestarts (Klug, 1834; Heteroptera: Reduviidae), Orientation térmica. Thesis University, Buenos Aires, Buenos Aires, Argentina.
Lazzari, C. R., and Wicklein, M. (1994). The cave-like sense organ in the antennae of Triatominae bugs. Mem. Inst. Oswaldo Cruz 89, 643–648. doi: 10.1590/S0074-02761994000400023
Leal, W. S. (2013). Odorant reception in insects: roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 58, 373–391. doi: 10.1146/annurev-ento-120811-153635
Leal, W. S., Barbosa, R. M., Xu, W., Ishida, Y., Syed, Z., Latte, N., et al. (2008). Reverse and conventional chemical ecology approaches for the development of oviposition attractants for Culex mosquitoes. PLoS One 3:e3045. doi: 10.1371/journal.pone.0003045
Levinson, H. Z., Levinson, A. R., Müller, B., and Steinbrecht, R. A. (1974). Structure of sensilla, olfactory perception, and behaviour of the bed bug, Cimex lectularius, in response to its alarm pheromone. J. Insect Physiol. 20, 1231–1248. doi: 10.1016/0022-1910(74)90229-7
Lidani, K. C. F., Andrade, F. A., Bavia, L., Damasceno, F. S., Beltrame, M. H., Messias-Reason, I. J., et al. (2019). Chagas disease: from discovery to a worldwide health problem. Front. Public Health 7:166. doi: 10.3389/fpubh.2019.00166
Liedtke, H. C., Åbjörnsson, K., Harraca, V., Knudsen, J. T., Wallin, E. A., Hedenström, E., et al. (2011). Alarm pheromones and chemical communication in nymphs of the tropical bed bug Cimex hemipterus (Hemiptera: Cimicidae). PLoS One 6, 1–7. doi: 10.1371/journal.pone.0018156
Liman, E. R., Zhang, Y. V., and Montell, C. (2014). Peripheral coding of taste. Neuron 81, 984–1000. doi: 10.1016/j.neuron.2014.02.022
Liu, F., and Liu, N. (2015). Human odorant reception in the common bed bug, Cimex lectularius. Sci. Rep. 5, 1–14. doi: 10.1038/srep15558
Liu, F., Chen, L., Appel, A. G., and Liu, N. (2013). Olfactory responses of the antennal trichoid sensilla to chemical repellents in the mosquito. Culex quinquefasciatus. J. Insect Physiol. 59, 1169–1177. doi: 10.1016/j.jinsphys.2013.08.016
Liu, F., Chen, Z., and Liu, N. (2017a). Molecular basis of olfactory chemoreception in the common bed bug, Cimex lectularius. Sci. Rep. 7:45531. doi: 10.1038/srep45531
Liu, F., Haynes, K. F., Appel, A. G., and Liu, N. (2014). Antennal olfactory sensilla responses to insect chemical repellents in the common bed bug, Cimex lectularius. J. Chem. Ecol. 40, 522–533. doi: 10.1007/s10886-014-0435-z
Liu, F., Xia, X., and Liu, N. (2017b). Molecular basis of N, N-Diethyl-3-Methylbenzamide (DEET) in repelling the common bed bug, Cimex lectularius. Front. Physiol. 8:418. doi: 10.3389/fphys.2017.00418
Liu, F., Xiong, C., and Liu, N. (2017c). Chemoreception to aggregation pheromones in the common bed bug, Cimex lectularius. Insect Biochem. Mol. Biol. 82, 62–73. doi: 10.1016/j.ibmb.2017.01.012
Liu, F., Wang, Q., Xu, P., Andreazza, F., Valbon, W. R., Bandason, E., et al. (2021). A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat. Commun. 12, 1–9. doi: 10.1038/s41467-021-22847-0
MacWilliam, D., Kowalewski, J., Kumar, A., Pontrello, C., and Ray, A. (2018). Signaling mode of the broad-spectrum conserved CO2 receptor is one of the important determinants of odor valence in Drosophila. Neuron 97, 1153–1167.e4. doi: 10.1016/j.neuron.2018.01.028
Marchant, A., Mougel, F., Jacquin-Joly, E., Costa, J., Almeida, C. E., and Harry, M. (2016). Under-expression of chemosensory genes in domiciliary bugs of the Chagas disease vector Triatoma brasiliensis. PLoS Negl. Trop. Dis. 10, 1–26. doi: 10.1371/journal.pntd.0005067
May-Concha, I., Guerenstein, P. G., Ramsey, J. M., Rojas, J. C., and Catalá, S. (2016). Antennal phenotype of Mexican haplogroups of the Triatoma dimidiata complex, vectors of Chagas disease. Infect. Genet. Evol. 40, 73–79. doi: 10.1016/j.meegid.2016.02.027
Mayer, M. S. (1968). Response of single olfactory cell of Triatoma infestans to human breath. Nature 220, 924–925. doi: 10.1038/220924a0
McBride, C. S. (2016). Genes and odors underlying the recent evolution of mosquito preference for humans. Curr. Biol. 26, 41–46. doi: 10.1016/j.cub.2015.11.032
Mciver, S., and Siemicki, R. (1985). Fine structure of antennal putative thermo-/hygrosensilla of adult Rhodnius prolixus Stal (Hemiptera: Reduviidae). J. Morphol. 183, 15–23. doi: 10.1002/jmor.1051830103
McMeniman, C. J., Corfas, R. A., Matthews, B. J., Ritchie, S. A., and Vosshall, L. B. (2014). Multimodal integration of carbon dioxide and other sensory cues drives mosquito attraction to humans. Cell 156, 1060–1071. doi: 10.1016/j.cell.2013.12.044
Mendki, M. J., Prakash, S., Parashar, B. D., and Agarwal, O. P. (2013). Distribution of sensilla on antenna and rostrum in nymphs and adults of Cimex hemipterus Fabricius (Hemiptera, Cimicidae). Dtsch. Entomol. Z. 60, 213–219. doi: 10.1002/mmnd.201300027
Mesquita, R. D., Vionette-Amaral, R. J., Lowenberger, C., Rivera-Pomar, R., Monteiro, F. A., Minx, P., et al. (2015). Genome of Rhodnius prolixus, an insect vector of Chagas disease, reveals unique adaptations to hematophagy and parasite infection. Proc. Natl. Acad. Sci. U. S. A. 112, 14936–14941. doi: 10.1073/pnas.1506226112
Milne, M. A., Ross, E. J., Sonenshine, D. E., and Kirsch, P. (2009). Attraction of Triatoma dimidiata and Rhodnius prolixus (Hemiptera: Reduviidae) to combinations of host cues tested at two distances. J. Med. Entomol. 46, 1062–1073. doi: 10.1603/033.046.0513
Missbach, C., Dweck, H. K., Vogel, H., Vilcinskas, A., Stensmyr, M. C., Hansson, B. S., et al. (2014). Evolution of insect olfactory receptors. elife 3:e02115. doi: 10.7554/eLife.02115
Moreno, M. L., Gorla, D., and Catalá, S. (2006). Association between antennal phenotype, wing polymorphism and sex in the genus Mepraia (Reduviidae: Triatominae). Infect. Genet. Evol. 6, 228–234. doi: 10.1016/j.meegid.2005.06.001
Monteiro, F. A., Weirauch, C., Felix, M., Lazoski, C., and Abad-Franch, F. (2018). Evolution, systematics, and biogeography of the Triatominae, vectors of Chagas disease. Adv. Parasitol. 99, 265–344. doi: 10.1016/bs.apar.2017.12.002
Murphy, E. J., Booth, J. C., Davrazou, F., Port, A. M., and Jones, D. N. (2012). Interactions of Anopheles gambiae odorant binding proteins with a human-derived repellent: implications for the mode of action of DEET. J. Biol. Chem. 288, 4475–4485. doi: 10.1074/jbc.M112.436386
Nakagawa, T., Sakurai, T., and Nishioka, T. (2005). Insect sex-pheromone signals mediated by specific combinations of olfactory receptors. Science 307, 1638–1642. doi: 10.1126/science.1106267
Nunez, J. A. (1982). Food source orientation and activity in Rhodnius prolixus Stal (Hemiptera: Reduviidae) Bull. Entomol. Res. 72, 253–262. doi: 10.1017/S0007485300010555
Obiero, G. F., et al. (2014). Odorant and gustatory receptors in the tsetse fly Glossina morsitans morsitans. PLoS Negl. Trop. Dis. 8:2663. doi: 10.1371/journal.pntd.0002663
Oliveira, D. S., Brito, N. F., Nogueira, F. C. S., Moreira, M. F., Leal, W. S., Soares, M. R., et al. (2017). Proteomic analysis of the kissing bug Rhodnius prolixus antenna. J. Insect Physiol. 100, 108–118. doi: 10.1016/j.jinsphys.2017.06.004
Oliveira, D. S., Brito, N. F., Franco, T. A., Moreira, M. F., Leal, W. S., and Melo, A. C. (2018). Functional characterization of odorant binding protein 27 (RproOBP27) from Rhodnius prolixus antennae. Front. Physiol. 9:1175. doi: 10.3389/fphys.2018.01175
Olson, J. F., Moon, R. D., Kells, S. A., and Mesce, K. A. (2014). Morphology, ultrastructure and functional role of antennal sensilla in off-host aggregation by the bed bug, Cimex lectularius. Arthropod Struct. Dev. 43, 117–122. doi: 10.1016/j.asd.2013.12.004
Ortiz, M. I., and Molina, J. (2010). Preliminary evidence of Rhodnius prolixus (Hemiptera: Triatominae) attraction to human skin odour extracts. Acta Trop. 113, 174–179. doi: 10.1016/j.actatropica.2009.10.014
Ortiz, M. I., Suárez-Rivillas, A., and Molina, J. (2011). Behavioural responses to human skin extracts and antennal phenotypes of sylvatic first filial generation and long rearing laboratory colony Rhodnius prolixus. Mem. Inst. Oswaldo Cruz 106, 461–466. doi: 10.1590/S0074-02762011000400013
Otálora-Luna, F., and Guerin, P. M. (2014). Amines from vertebrates guide triatomine bugs to resources. J. Insect Physiol. 71, 52–60. doi: 10.1016/j.jinsphys.2014.09.007
Page, T. L., and Koelling, E. (2003). Circadian rhythm in olfactory response in the antennae controlled by the optic lobe in the cockroach. J. Insect Physiol. 49, 697–707. doi: 10.1016/S0022-1910(03)00071-4
Pellegrino, M., Steinbach, N., Stensmyr, M. C., Hansson, B. S., and Vosshall, L. B. (2011). A natural polymorphism alters odour and DEET sensitivity in an insect odorant receptor. Nature 478, 511–514. doi: 10.1038/nature10438
Pelletier, J., Guidolin, A., Syed, Z., Cornel, A. J., and Leal, W. S. (2010). Knockdown of a mosquito odorant-binding protein involved in the sensitive detection of oviposition attractants. J. Chem. Ecol. 36, 245–248. doi: 10.1007/s10886-010-9762-x
Pereira, K. S., Schmidt, F. L., Barbosa, R. L., Guaraldo, A. M., Franco, R. M., Dias, V. L., et al. (2010). Adv. Food Nutr. Res. 59, 63–85. doi: 10.1016/S1043-4526(10)59003-X
Pelosi, P., Mastrogiacomo, R., Iovinella, I., Tuccori, E., and Persaud, K. C. (2014). Structure and biotechnological applications of odorant-binding proteins. Appl. Microbiol. Biotechnol. 98, 61–70. doi: 10.1007/s00253-013-5383-y
Pitts, R. J., Derryberry, S. L., Zhang, Z., and Zwiebel, L. J. (2017). Variant ionotropic receptors in the malaria vector mosquito Anopheles gambiae tuned to amines and carboxylic acids. Sci. Rep. 7:40297. doi: 10.1038/srep40297
Pontes, G., Minoli, S., Insaurralde, I. O., de Brito Sanchez, M. G., and Barrozo, R. B. (2014). Bitter stimuli modulate the feeding decision of a blood-sucking insect via two sensory inputs. J. Exp. Biol. 217, 3708–3717. doi: 10.1242/jeb.107722
Reinhardt, K., and Siva-Jothy, M. T. (2007). Biology of the bed bugs (Cimicidae). Annu. Rev. Entomol. 52, 351–374. doi: 10.1146/annurev.ento.52.040306.133913
Reis, M. D., and Miller, D. M. (2011). Host searching and aggregation activity of recently fed and unfed bed bugs (Cimex lectularius L.). Insects 2, 186–194. doi: 10.3390/insects2020186
Reisenman, C. E. (2014). Hunger is the best spice: effects of starvation in the antennal responses of the blood-sucking bug Rhodnius prolixus. J. Insect Physiol. 71, 8–13. doi: 10.1016/j.jinsphys.2014.09.009
Reisenman, C. E., Lee, Y., Gregory, T., and Guerenstein, P. G. (2013). Effects of starvation on the olfactory responses of the blood-sucking bug Rhodnius prolixus. J. Insect Physiol. 59, 717–721. doi: 10.1016/j.jinsphys.2013.04.003
Romero, A., Potter, M. F., Potter, D. A., and Kenneth, F. (2007). Insecticide resistance in the bed bug: a factor in the pest’s sudden resurgence? J. Med. Entomol. 44, 175–178. doi: 10.1603/0022-2585(2007)44[175:IRITBB]2.0.CO;2
Romero, A., and Schal, C. (2014). Blood constituents as phagostimulants for the bed bug Cimex lectularius L. J. Exp. Biol. 217, 552–557. doi: 10.1242/jeb.096727
Romero, A., Potter, M. F., and Haynes, K. F. (2010). Circadian rhythm of spontaneous locomotor activity in the bed bug, Cimex lectularius L. J. Insect Physiol. 56, 1516–1522. doi: 10.1016/j.jinsphys.2010.04.025
Rosén, W. Q., Han, G. B., and Löfstedt, C. (2003). The circadian rhythm of the sex-pheromone-mediated behavioral response in the turnip moth, Agrotis segetum, is not controlled at the peripheral level. J. Biol. Rhythm. 18, 402–408. doi: 10.1177/0748730403256869
Salazar, R., Castillo-Neyra, R., Tustin, A. W., Borrini-Mayorí, K., Náquira, C., and Levy, M. Z. (2014). Bed bugs (Cimex lectularius) as vectors of Trypanosoma cruzi. Am. J. Trop. Med. Hyg. 14:0483. doi: 10.4269/ajtmh.14-0483
Sansom, J. E., Reynolds, N. J., and Peachey, R. D. (1992). Delayed reaction to bed bug bites. Arch. Dermatol. 128, 272–273. doi: 10.1001/archderm.1992.01680120148027
Sato, K., Pellegrino, M., Nakagawa, T., Nakagawa, T., Vosshall, L. B., and Touhara, K. (2008). Insect olfactory receptors are heteromeric ligand-gated ion channels. Nature 452, 1002–1006. doi: 10.1038/nature06850
Saveer, A. M., DeVries, Z. C., Santangelo, R. G., and Schal, C. (2021). Mating and starvation modulate feeding and host-seeking responses in female bed bugs, Cimex lectularius. Sci. Rep. 11, 1–11. doi: 10.1038/s41598-021-81271-y
Scheuermann, E. A., and Smith, D. P. (2019). Odor-specific deactivation defects in a Drosophila odorant-binding protein mutant. Genetics 213, 897–909. doi: 10.1534/genetics.119.302629
Shields, T. L., and Walsh, E. N. (1956). Kissing bug bite. AMA Arch. Derm. Syphilol. 74, 14–21. doi: 10.1001/archderm.1956.01550070016004
Shikanai-Yasuda, M. A., and Carvalho, N. B. (2012). Oral transmission of Chagas disease. Clin. Infect. Dis. 54, 845–852. doi: 10.1093/cid/cir956
Silbering, A. F., and Galizia, C. G. (2007). Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J. Neurosci. 27, 11966–11977. doi: 10.1523/JNEUROSCI.3099-07.2007
Singh, N., Wang, C., and Cooper, R. (2013). Effect of trap design, chemical lure, carbon dioxide release rate, and source of carbon dioxide on efficacy of bed bug monitors. J. Econ. Entomol. 106, 1802–1811. doi: 10.1603/EC13075
Singh, N., Wang, C., Cooper, R., and Liu, C. (2012). Interactions among carbon dioxide, heat, and chemical lures in attracting the bed bug, Cimex lectularius L.(Hemiptera: Cimicidae). Psyche 2012:273613. doi: 10.1155/2012/273613
Singh, R. N., Singh, K., Prakash, S., Mendki, M. J., and Rao, K. M. (1996). Sensory organs on the body parts of the bed-bug Cimex hemipterus fabricius (Hemiptera: Cimicidae) and the anatomy of its central nervous system. Int. J. Insect Morphol. Embryol. 25, 183–204. doi: 10.1016/0020-7322(95)00016-X
Sioli, H. (1937). Thermotaxis und Perzeption von W¨armestrahlen bei der Bettwanze (Cimex lectularius L.). Zool. Jahrb. Physiol. Morphol. 58, 284–296.
Steinbrecht, R. A., and Müller, B. (1976). Fine structure of the antennal receptors of the bed bug, Cimex lectularius L. Tissue Cell 8, 615–636. doi: 10.1016/0040-8166(76)90035-5
Stengl, M., and Funk, N. W. (2013). The role of the coreceptor Orco in insect olfactory transduction. J. Comp. Physiol. A. 199, 897–909. doi: 10.1007/s00359-013-0837-3
Stevens, L., Dorn, P. L., Schmidt, J. O., Klotz, J. H., Lucero, D., and Klotz, S. A. (2011). Kissing bugs. The vectors of Chagas. Adv. Parasitol. 75, 169–192. doi: 10.1016/B978-0-12-385863-4.00008-3
Sun, J. S., Xiao, S., and Carlson, J. R. (2018). The diverse small proteins called odorant-binding proteins. Open Biol. 8:180208. doi: 10.1098/rsob.180208
Taneja, J., and Guerin, P. M. (1997). Ammonia attracts the haematophaguous bug Triatoma infestans: behavioural and neurophysioogical data on nymphs. J. Comp. Pysiol. A 181, 21–34. doi: 10.1007/s003590050089
Ter Poorten, M. C., and Prose, N. S. (2005). The return of the common bedbug. Pediatr. Dermatol. 22, 183–187. doi: 10.1111/j.1525-1470.2005.22301.x
Terrapon, N., Li, C., Robertson, H., Ji, L., Meng, X., Booth, W., et al. (2014). Molecular traces of alternative social organization in a termite genome. Nat. Commun. 5:3636. doi: 10.1038/ncomms4636
Tsitsanou, K. E., Thireou, T., Drakou, C. E., Koussis, K., Keramioti, M. V., Leonidas, D. D., et al. (2012). Anopheles gambiae odorant binding protein crystal complex with the synthetic repellent DEET: implications for structure-based design of novel mosquito repellents. Cell. Mol. Life Sci. 69, 283–297. doi: 10.1007/s00018-011-0745-z
Turner, S. L., Li, N., Guda, T., Githure, J., Cardé, R. T., and Ray, A. (2011). Ultra-prolonged activation of CO2-sensing neurons disorients mosquitoes. Nature 474, 87–91. doi: 10.1038/nature10081
Van der Goes van Naters, W. M., Den Otter, C. J., and Maes, F. W. (1998). Olfactory sensitivity in tsetse flies: a daily rhythm. Chem. Senses 23, 351–357. doi: 10.1093/chemse/23.3.351
Villacís, A. G., Grijalva, M. J., and Catalá, S. S. (2010). Phenotypic variability of Rhodnius ecuadoriensis populations at the Ecuadorian central and southern Andean region. J. Med. Entomol. 47, 1034–1043. doi: 10.1603/ME10053
Villela, M. M., Catalá, S., Juberg, J., Silva, I. G., and Dias, J. C. P. (2005). Patterns of antenal sensilla of Panstrongylus megistus from three Brazilian states. Mem. Inst. Oswaldo Cruz 100, 699–702. doi: 10.1590/S0074-02762005000700002
Wang, G., Carey, A. F., Carlson, J. R., and Zwiebel, L. J. (2010). Molecular basis of odor coding in the malaria vector mosquito Anopheles gambiae. Proc. Natl. Acad. Sci. U. S. A. 107, 4418–4423. doi: 10.1073/pnas.0913392107
Wang, C., Gibb, T. J., Bennett, G. W., and McKnight, S. (2009). Bed bug (Heteroptera: Cimicidae) attraction to pitfall traps baited with carbon dioxide, heat, and chemical lure. J. Econ. Entomol. 102, 1580–1585. doi: 10.1603/029.102.0423
Waris, M. I., Younas, A., Hao, L., Ameen, A., Ali, S., Abdelnabby, H. E., et al. (2018). Silencing of chemosensory protein gene Nlug CSP8 by RNAi induces declining behavioral responses of Nilaparvata lugens. Front. Physiol. 9:379. doi: 10.3389/fphys.2018.00379
Weeks, E. N. I., Logan, J. G., Birkett, M. A., Caulfield, J. C., Gezan, S. A., Welham, S. J., et al. (2020). Electrophysiologically and behaviourally active semiochemicals identified from bed bug refuge substrate. Sci. Rep. 10, 1–14. doi: 10.1038/s41598-020-61368-6
Wiesinger, D. (1956). Die Bedeutung der Umweltfaktoren fiir der Saugakt von Triatoma infestans. Acta Trop. 13, 97–141.
Wigglesworth, V., and Gillett, J. (1934). The function of the antennae in Rhodnius prolixus (Hemiptera) and the mechanism of orientation to the host. J. Exp. Biol. 11, 120–139. doi: 10.1242/jeb.11.2.120
Xiao, S., Sun, J. S., and Carlson, J. R. (2019). Robust olfactory responses in the absence of odorant binding proteins. elife 8:e51040. doi: 10.7554/eLife.51040
Xu, P., Choo, Y. M., De La Rosa, A., and Leal, W. S. (2014). Mosquito odorant receptor for DEET and methyl jasmonate. Proc. Natl. Acad. Sci. U. S. A. 111, 16592–16597. doi: 10.1073/pnas.1417244111
Xu, C., Yang, F., Duan, S., Li, D., Li, L., Wang, M., et al. (2021). Discovery of behaviorally active semiochemicals in Aenasius bambawalei using a reverse chemical ecology approach. Pest Manag. Sci. 77, 2843–2853. doi: 10.1002/ps.6319
Ye, Z., Liu, F., and Liu, N. (2016). Olfactory responses of southern house mosquito, Culex quinquefasciatus, to human odorants. Chem. Senses 41, 441–447. doi: 10.1093/chemse/bjv089
Yoon, K. S., Kwon, D. H., Strycharz, J. P., Hollingsworth, C. S., Lee, S. H., and Clark, J. M. (2008). Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 45, 1092–1101. doi: 10.1603/0022-2585(2008)45[1092:BAMAOD]2.0.CO;2
Zacharias, C. A., Pontes, G. B., Lorenzo, M. G., and Manrique, G. (2010). Flight initiation by male Rhodnius prolixus is promoted by female odors. J. Chem. Ecol. 36, 449–451. doi: 10.1007/s10886-010-9779-1
Zermoglio, P. F., Martin-Herrou, H., Bignon, Y., and Lazzari, C. R. (2015). Rhodnius prolixus smells repellents: behavioural evidence and test of present and potential compounds inducing repellency in Chagas disease vectors. J. Insect Physiol. 81, 137–144. doi: 10.1016/j.jinsphys.2015.07.012
Zhu, F., Gujar, H., Gordon, J. R., Haynes, K. F., Potter, M. F., and Palli, S. R. (2013). Bed bugs evolved unique adaptive strategy to resist pyrethroid insecticides. Sci. Rep. 3:1456. doi: 10.1038/srep01456
Keywords: bed bug, kissing bug, host-seeking behavior, peripheral olfactory system, olfaction, push-pull strategies, reverse chemical ecology
Citation: Liu F, Chen Z, Ye Z and Liu N (2021) The Olfactory Chemosensation of Hematophagous Hemipteran Insects. Front. Physiol. 12:703768. doi: 10.3389/fphys.2021.703768
Edited by:
Peng He, Guizhou University, ChinaReviewed by:
Dingze Mang, Tokyo University of Agriculture and Technology, JapanNai-Yong Liu, Southwest Forestry University, China
Ana Claudia A. Melo, Federal University of Rio de Janeiro, Brazil
Herbert Venthur, University of La Frontera, Chile
Copyright © 2021 Liu, Chen, Ye and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nannan Liu, liunann@auburn.edu
†These authors have contributed equally to this work
 Feng Liu
Feng Liu Zhou Chen
Zhou Chen Zi Ye1,2
Zi Ye1,2 Nannan Liu
Nannan Liu