- 1Center of Medical and Bio-Allied Health Sciences Research, Ajman University, Ajman, United Arab Emirates
- 2Department of Clinical Sciences, College of Pharmacy and Health Sciences, Ajman University, Ajman, United Arab Emirates
- 3Department of Pharmacology and Toxicology, College of Pharmacy, Umm Al-Qura University, Makkah, Saudi Arabia
Background: Globally, the use of amphetamines as therapeutic agents in pediatric medicine is a crucial area of concern, especially given the population’s vulnerability.
Methods: On 6 August 2023, a search was conducted on ClinicalTrials.gov using “amphetamine” as the keyword. Two independent examiners screened trials against set criteria, including a focus on amphetamine, completion status, an interventional approach, and included children. Ongoing or observational studies were excluded. Data extracted from the qualified trials encompassed primary objectives, participant counts, study duration, and outcomes, with the aim of analyzing children disorders treated by amphetamine.
Results: On 6 August 2023, a search of the ClinicalTrials.gov database with the term “amphetamines” identified 179 clinical trials. After extensive exclusion criteria, 19 trials were ultimately selected for analysis. The predominant condition under investigation was attention deficit hyperactivity disorder (ADHD), present in 84.2% of studies. Key study characteristics included: phase 4 trials (36.8%), randomized allocation (63.2%), and the parallel intervention model (42.1%). Masking techniques varied, with no masking in 42.1% of studies, and double and quadruple masking both accounting for 21.1%. Geographically, 78.9% of the studies’ participants were from the United States.
Conclusion: This study highlights the notable therapeutic potential of amphetamines in pediatric ADHD populations and emphasizes the importance of recognizing potential side effects and addiction risks. As pharmacogenomics offers the prospect of personalized treatments, there is potential to increase therapeutic efficacy and decrease adverse reactions. It is vital to balance these benefits against the inherent risks, understanding the need for continued research to optimize the use of amphetamines in medicine.
1 Introduction
Globally, amphetamines play a significant role in pediatric medicine, both as therapeutic agents and subjects of concern. Their dual role necessitates an in-depth analysis, especially when their application targets vulnerable populations such as children (Meyers et al., 2020). As central nervous system (CNS) stimulants, the effects of amphetamines on a developing brain are both beneficial in specific therapeutic scenarios and potentially harmful if misused (Mental Health Services Administration, 1999). The global prevalence of amphetamine use in 2019 was estimated to be 0.5% in children as an antipsychotic medicine and 12% in pregnant mothers (Ochoa et al., 2023). This extensive utilization, predominantly in the medical treatment of conditions like attention deficit hyperactivity disorder (ADHD), underscores the importance of a thorough review of clinical trials focusing on children and amphetamines (Frölich et al., 2012).
Historically, the recognition of amphetamines dates back to the early 20th century. The medical community began to acknowledge its therapeutic potential for ADHD, a prevalent neurodevelopmental disorder in children (Strohl, 2011). However, the propensity for misuse and consequent dependency, especially in this demographic, raised flags. While the most common amphetamines, amphetamine and methamphetamine, dominate discussions, numerous derivatives and formulations cater specifically for children. Dextroamphetamine is frequently prescribed for pediatric ADHD (Clevel Clin, 2023a), while Adderall, which combines AMPH and dextroamphetamine, stands as a staple in ADHD treatment (Faraone and Biederman, 2002). Lisdexamfetamine metabolizes into dextroamphetamine and remains another crucial ADHD management tool for children (Najib et al., 2020). Although some studies have explored the potential therapeutic applications of MDMA for psychiatric conditions in adults, its effects on children remain largely uncharted and controversial.
The spectrum of amphetamines’ influence on children extends beyond ADHD. For instance, their use in pediatric obsessive-compulsive disorder (OCD) patients has yielded mixed results (OCD Kids, 2023). Similarly, interventions using amphetamines for mood dysregulation disorders in children are treated with caution due to the potential side effects (Parsley et al., 2020). One of the primary concerns is the risk of addiction. A developing brain is susceptible, and the introduction of substances that alter its neurochemistry, especially influencing the dopamine system, requires vigilant monitoring (Berman et al., 2009). As children and adolescents engage with these drugs, even for therapeutic reasons, there is controversial evidence regarding the risk of developing a dependency (Clevel Clin, 2023b).
Another emerging area of research revolves around the long-term effects of amphetamines on the developing brain. Preliminary findings indicate potential structural and functional alterations in specific brain regions with prolonged amphetamine use. These changes, although subtle, might have implications for cognitive functions, emotional regulation, and even social behavior in the long term. With an increasing number of children undergoing amphetamine-based treatments, understanding these long-term implications becomes paramount. Continuous longitudinal studies tracking these children over several years can provide crucial insights into these effects (Reynolds et al., 2015a).
Despite the plethora of individual studies on amphetamines, a consolidated, in-depth examination of clinical trials aimed at children remains a conspicuous gap in the literature. ClinicalTrials.gov, a leading clinical trials database, is a treasure trove of information (Hegazi et al., 2023). However, the data it on amphetamines awaits a rigorous and comprehensive analysis. This not only hinders therapeutic advancements but also affect or reduce understanding of amphetamine’s role in modern medicine. To address this gap, this review offers a comprehensive review of clinical trials from ClinicalTrials.gov on amphetamines.
2 Methods
2.1 Search strategy
On 6 August 2023, a comprehensive review of ClinicalTrials.gov was conducted using the keyword “amphetamine”. To ensure objectivity, two independent Examiner evaluated the trials returned in the search, based on pre-established eligibility criteria. Trials qualified for inclusion if they predominantly focused on amphetamine, were completed, adopted an interventional approach, and enrolled children. Studies that were still in progress or of an observational nature were excluded. Relevant data were methodically obtained from ClinicalTrials.gov, highlighting vital components that aided the comprehensive analysis of human disorders treated by amphetamine and related substances. This study detailed the primary objectives of each trial and contained essential details such as the number of participants, the length of the study, and the outcomes.
3 Results
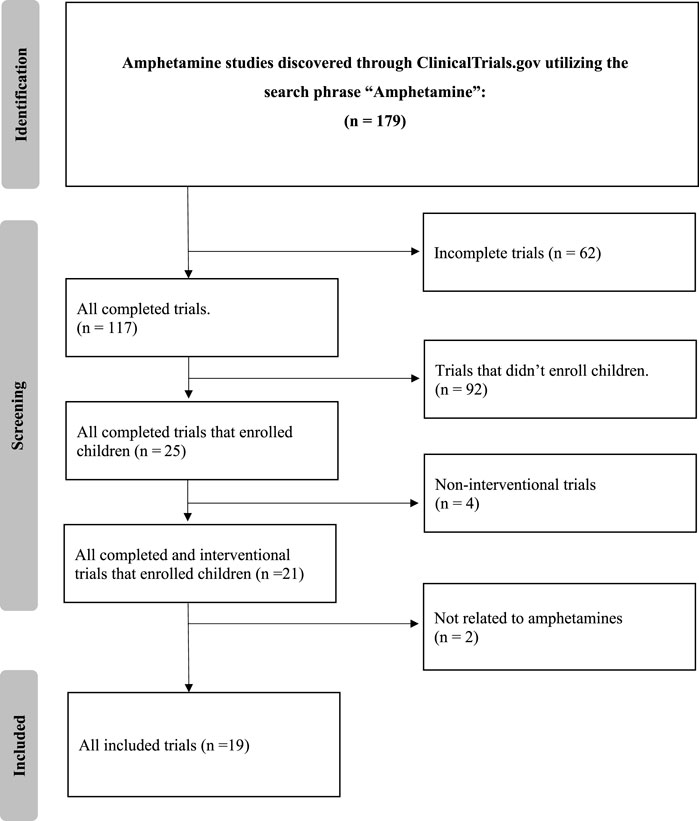
The detailed search of the ClinicalTrials.gov database with the term “amphetamine” yielded 179 clinical trials. After applying strict exclusion criteria–incomplete (n = 62), non-interventional (n = 4), and trials that did not enroll children (n = 92)–21 trials were initially suitable. However, two of these did not fully align with our review’s focus, primarily due to their limited emphasis on amphetamine treatments or a focus on unrelated conditions. Thus, 19 trials were finalized for analysis, offering insights into current amphetamine-associated disorder treatments. The selection methodology is illustrated in Figure 1.
3.1 Characteristics of included studies
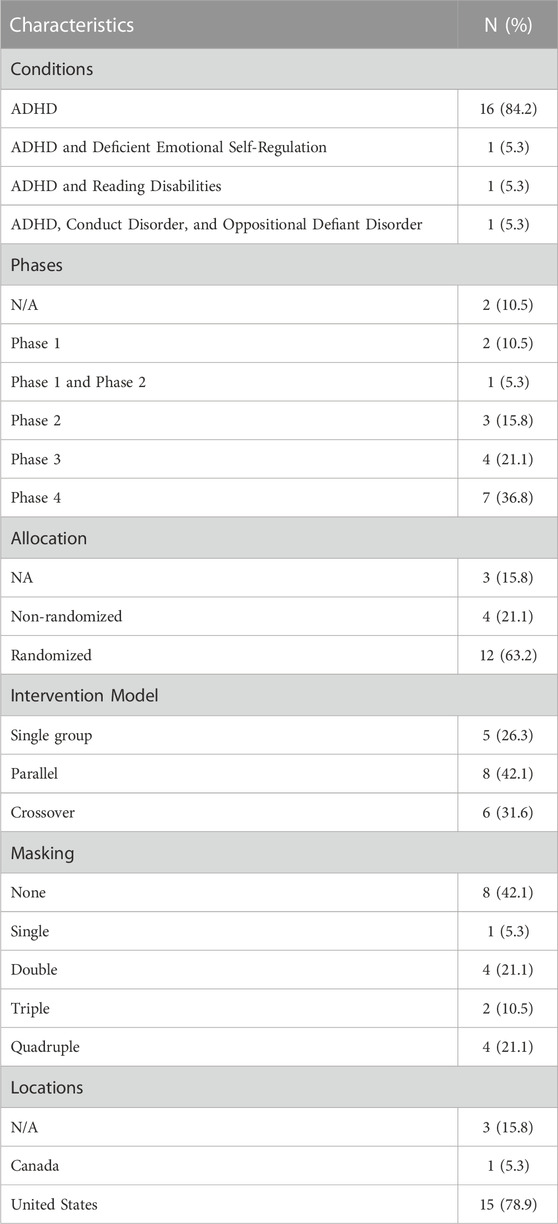
Table 1 provides a detailed breakdown of the various characteristics observed across the 19 trials. The most frequently occurring condition was ADHD, accounting for a significant 84.2%. Three specific sub-groups, each representing 5.3%, were identified: individuals with ADHD coupled with Deficient Emotional Self-Regulation, those with ADHD and Reading Disabilities, and participants with ADHD alongside Conduct Disorder and Oppositional Defiant Disorder.
3.2 The included clinical trials
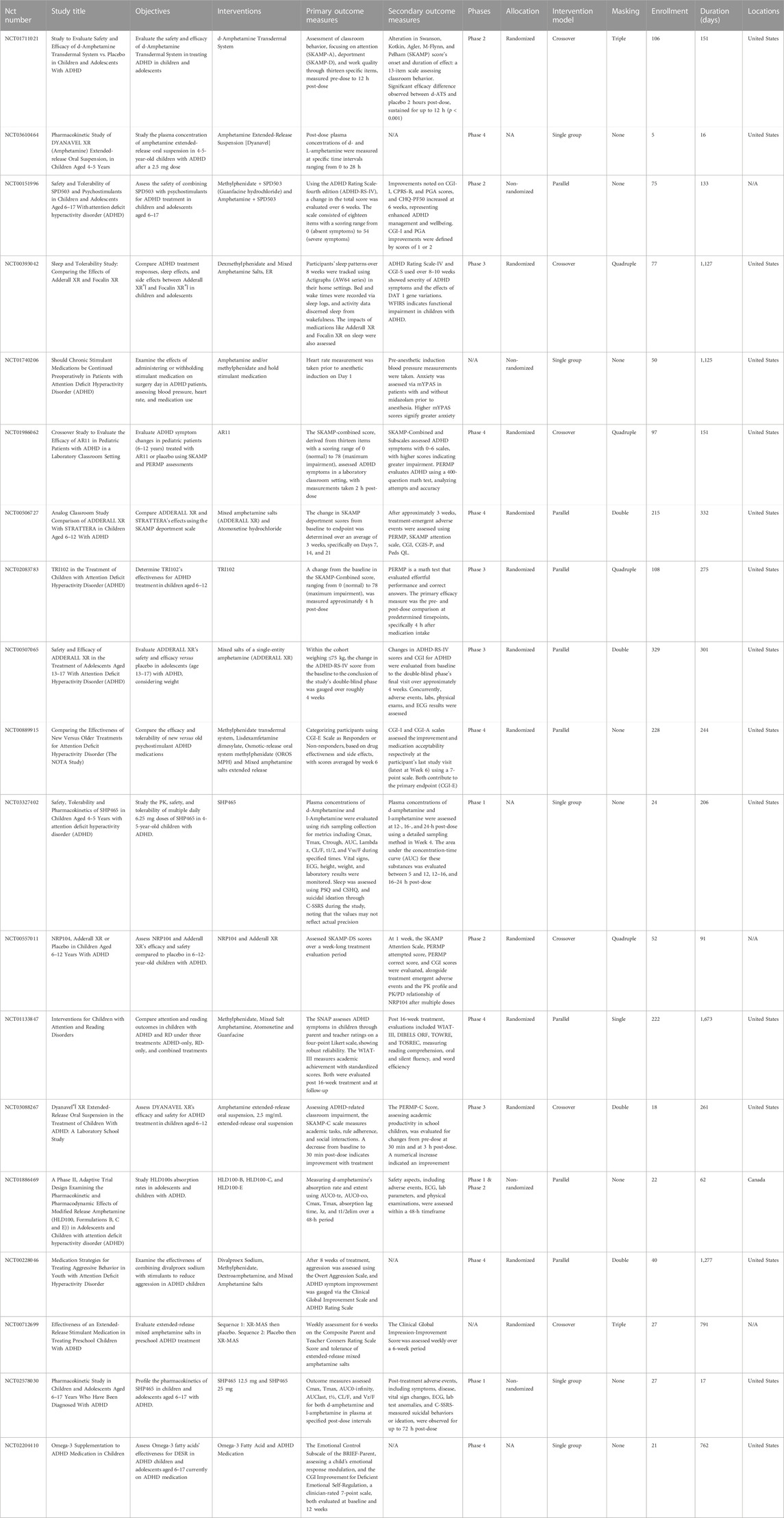
In terms of the trial phases, Phase four stood out with the highest representation, accounting for 36.8%. Regarding allocation methods, a majority of 63.2% of the trials were randomized. When assessing the intervention model, it was observed that the parallel model was predominant with 42.1% of all studies. In the masking category, 42.1% of the studies had no masking, while both double and quadruple masking were equally represented at 21.1% each. As for geographical distribution, a substantial 78.9% of the participants were based in the United States (Table 2).
3.3 General overview, history, and classification
Amphetamines are classified as potent sympathomimetic agents with valuable therapeutic uses. Chemically, they share a great similarity with the body’s innate catecholamines, specifically dopamine and norepinephrine (Heal et al., 2013). First synthesized in 1887 by Lazăr Edeleanu in Germany, their significance in the field of pharmacology was not acknowledged until the 1950s, when they emerged as a potential treatment for certain behavioral disorders (Edeleanu, 1887). The term “amphetamine” designates a particular compound, predominantly present in two enantiomers: levoamphetamine and dextroamphetamine. These mirror-image molecules, while structurally related, vary in terms of their potency and pharmacological effects (Heal et al., 2013). Noteworthy derivatives such as methamphetamine and para-methoxyamphetamine (PMA) also belong to the amphetamine family, and each has a unique set of characteristics (Gough et al., 2002).
3.4 Mechanism of action and medical uses
Amphetamines act by facilitate the release of catecholamines, especially dopamine, from presynaptic neurons while simultaneously blocking their reuptake. This dual action increases their concentration within the synaptic cleft, thereby extending neurotransmission durations (Heal et al., 2013). Elevated dopamine concentrations in the brain’s mesolimbic dopamine system not only lead to feelings of euphoria but also heighten the risk of addiction, further underscoring the potential for amphetamine dependence (Adinoff, 2004; National Institute on Drug Abuse, 2007). On a related note, increased norepinephrine levels within the CNS are correlated with heightened alertness and arousal (Berridge, 2008). When prescribed and monitored by medical professionals, amphetamines serve as effective treatments for conditions such as ADHD, narcolepsy, and, in some cases, treatment-resistant depression. Their therapeutic applications include enhanced wakefulness, improved cognitive control, reduced fatigue, and mood elevation (Stotz et al., 1999; Berman et al., 2009; Ng and O’Brien, 2009).
3.5 Side effects, concerns, and legal implications
Extended and consistent misuse of amphetamines can result in a range of adverse side effects, including inhibited growth, heightened jitteriness, feelings of nausea, and diminished visual clarity (Berman et al., 2009; Craig et al., 2015; Richardson et al., 2017). Long-term abuse escalates the risk of complications such as pronounced dental deterioration commonly referred to as “meth mouth”, significant weight loss, persistent skin lesions, an increased dependency, and a powerful addiction (Craig et al., 2015; Nida, 2019). When an addiction takes hold, users often find themselves developing a tolerance. This can lead to overwhelming cravings, pronounced withdrawal symptoms, and a relentless cycle of consumption, even when faced with detrimental repercussions (Leith and Kuczenski, 1981). In recognition of their substantial potential for abuse, amphetamines are stringently regulated on a global scale. Within the United States, these drugs are categorized as Schedule II controlled substances (Deadiversion, 2023). Due to their elevated risk of abuse in the course of their medical use, their prescription and distribution are heavily restricted. Comparable regulations are implemented internationally, and those found accountable of unauthorized distribution or trafficking can expect severe legal consequences (Marandure et al., 2023).
3.6 Pharmacokinetics
It is crucial to comprehend the pharmacokinetics of amphetamines, not only in order to optimize their therapeutic usage but also to detect their potential for misuse. Once taken orally, these compounds are rapidly absorbed from the gastrointestinal system, reaching their peak concentration in the bloodstream approximately 2, 3 h after ingestion. It is worth noting that the liver plays a central role in their metabolism (Berman et al., 2009; National Institute of Diabetes and Digestive and Kidney Diseases, 2012).
3.7 Detailed compound breakdown
3.7.1 Amphetamine
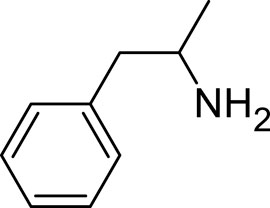
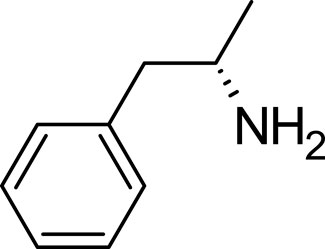
Amphetamine (C9H13N), a derivative of the phenethylamine family, manifests in two distinct enantiomeric forms: levoamphetamine and dextroamphetamine. Notably, dextroamphetamine demonstrates a heightened potency in stimulating the CNS (Berman et al., 2009; Zanda et al., 2017; Losacker et al., 2022). At the neurochemical level, the primary mechanism of action for amphetamine involves the elevation of neurotransmitter levels within the synaptic junctions. This elevation arises from the compound’s disruption of vesicular monoamine transporters, subsequently triggering the release of key neurotransmitters–namely, dopamine, norepinephrine, and serotonin–into the synaptic space (Heal et al., 2013). In addition to this, amphetamine has the capability to delay the reuptake of these neurotransmitters, thereby further amplifying their presence and concentration within the synapse (dela Peña et al., 2015) (Figure 2).
3.7.2 Dextroamphetamine
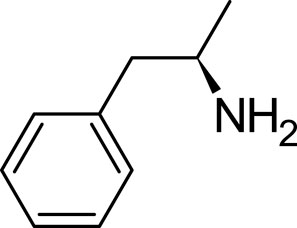
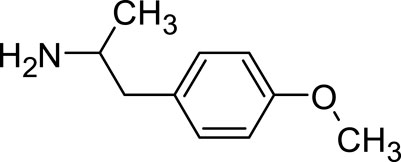
Dextroamphetamine, chemically represented as C9H13N, stands out as one of the two active enantiomers of amphetamine. This prescription medication plays a pivotal role in treating conditions such as ADHD and narcolepsy (Sharbaf Shoar et al., 2023). A notable mention is Adderall, a widely recognized medication for ADHD, which comprises four distinct amphetamine salts. However, dextroamphetamine predominates in its composition (Kolar et al., 2008). Distinguished as (S)-amphetamine, dextroamphetamine is an optically active isomer of the parent compound, amphetamine (Heal et al., 2013). With a chemical structure denoted by C9H13N, its chiral center permits the molecule to manifest in two distinct enantiomeric forms. The “dextro” prefix not only signifies its specific structural configuration but also underscores its capability to rotate plane-polarized light toward the right (Figure 3).
3.7.3 Levoamphetamine
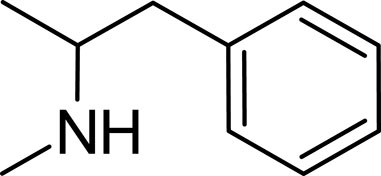
Levoamphetamine, often overshadowed by its more renowned counterpart, dextroamphetamine, is another enantiomer of amphetamine. Unlike dextroamphetamine which primarily has central stimulant effects, levoamphetamine exhibits a stronger peripheral stimulant action. This means that it might lead to more pronounced cardiovascular effects, such as an increased heart rate (Heal et al., 2013; Chen et al., 2023). Levoamphetamine is present in medications such as Adderall, but in reduced quantities compared to dextroamphetamine (Sontheimer and Sontheimer, 2021). Structurally, while both dextroamphetamine and levoamphetamine share the same chemical formula (C9H13N), they differ in the spatial orientation of their atoms (Gough et al., 2002; Heal et al., 2013). This distinction, known as chirality, is reflected in levoamphetamine’s name; the prefix “levo” denotes that it rotates plane-polarized light to the left. Although both compounds share many characteristics, this chiral difference gives levoamphetamine a unique pharmacological profile. Specifically, its interactions with neurotransmitter systems deviate from those of dextroamphetamine due to this structural variation (Goodwin et al., 2009; Heal et al., 2013) (Figure 4).
3.7.4 Methamphetamine
Methamphetamine, a potent derivative of amphetamine (Hall et al., 2008), is known for its heightened effects on the CNS. Although it has legitimate medical uses–available in prescription form as Desoxyn to treat certain cases of ADHD and obesity–it is more notoriously associated with the illicit drug “crystal meth” (Miller et al., 2021). A distinguishing feature of methamphetamine is its elevated lipid solubility, enabling it to penetrate the blood-brain barrier with greater concentration than other amphetamines (Jan et al., 2012; Kirkpatrick et al., 2012). This characteristic significantly escalates its potential for abuse and addiction, particularly when in its crystalline form.
From a chemical perspective, methamphetamine, also known as N-methylamphetamine, stands apart from amphetamine due to the inclusion of a methyl group on its nitrogen atom (Kirkpatrick et al., 2012). Its molecular formula reads as C10H15N. While this modification might seem subtle, it has a profound influence on the compound’s pharmacokinetics. The added methyl group enhances the compound’s lipid solubility, facilitating its rapid movement across the blood-brain barrier (Kirkpatrick et al., 2012). Moreover, methamphetamine exists as two enantiomers: dextromethamphetamine and levomethamphetamine (West et al., 2012) (Figure 5).
3.7.5 Lisdexamfetamine
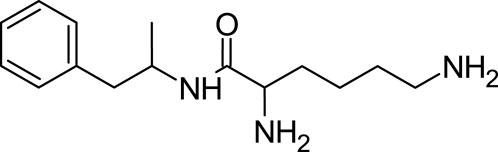
Lisdexamfetamine is a prodrug of dextroamphetamine. It therefore remains inactive upon ingestion, and needs to be activated by metabolic conversion to manifest its active form (Cho and Yoon, 2018). What sets Lisdexamfetamine apart is its intrinsic extended-release mechanism due to its prodrug nature. This not only ensures a more prolonged therapeutic effect but also mitigates its potential for misuse. The reason behind this is its more gradual onset of action, compared to other immediate-release amphetamine formulations. Chemically, Lisdexamfetamine is an amide conjugate, formulated by combining dextroamphetamine with the essential amino acid lysine (Mrazek et al., 2009; Levine and Swanson, 2023). Its chemical formula is C15H25N3O (Figure 6).
3.7.6 Para-methoxyamphetamine
PMA, or para-Methoxyamphetamine, is a synthetic compound that shares a structural resemblance to amphetamines but stands apart due to its unique pharmacological profile (Richter et al., 2019). Although occasionally mistaken for MDMA (commonly known as “Ecstasy”), PMA’s effects can be considerably more toxic. This compound specifically interacts with the brain’s serotonin receptors to produce psychedelic experiences. However, one of its alarming side effects is a potentially hazardous surge in body temperature, making it significantly more perilous than many of its amphetamine counterparts (Freezer et al., 2005). Chemically, while PMA retains the foundational structure of amphetamines, it differentiates itself with an additional methoxy group situated at the para position of the phenyl ring. Its molecular formula is represented as C10H15NO (Figure 7).
3.8 Common conditions managed by amphetamines
3.8.1 Attention deficit hyperactivity disorder
Amphetamines are recognized for their wide-ranging applications, but are particularly notable for their role in treating ADHD (Frölich et al., 2012). ADHD is a complex neurodevelopmental disorder, typified by enduring patterns of inattention, heightened hyperactivity, and pronounced impulsivity (Magnus et al., 2023). The precise origins and causes of ADHD have yet to be definitively ascertained; however, the prevailing scientific consensus shows that imbalances in neurochemicals, especially within dopamine pathways, significantly influence its manifestation (Blum et al., 2008; Wilens and Spencer, 2010a). Medications formulated with amphetamines such as Adderall, which combines amphetamine and dextroamphetamine, and Evekeo, aim to modulate these neurotransmitter concentrations. As a result, they effectively mitigate the predominant symptoms of the disorder (Frölich et al., 2012).
3.8.2 Narcolepsy
Narcolepsy is a long-term sleep disorder marked by an intense and uncontrollable propensity for daytime sleepiness involuntary sleep episodes. People with this condition may find themselves inadvertently dozing off during daily activities, which presents not only an inconvenience but also poses potential safety risks. While the precise mechanisms underpinning narcolepsy remain a topic of ongoing research, many cases have been linked to a deficiency in the neuropeptide hypocretin (Akintomide and Rickards, 2011). Due to their stimulatory effects, amphetamines have demonstrated efficacy in mitigating the debilitating daytime drowsiness symptomatic of narcolepsy. Consequently, medications such as Adderall are frequently incorporated into treatment regimen for the disorder (Turner, 2019; Barker et al., 2020).
3.8.3 Obesity and weight management
Historically, Phentermine, an amphetamine derivative, was prescribed as an anorectic or appetite suppressant to assist with weight reduction (Coulter et al., 2018). Its stimulant properties have the potential to accelerate metabolism and reduce appetite. In the mid-20th century, drugs such as Benzedrine gained prominence for their weight management benefits. However, increasing concerns regarding the potential for misuse, adverse cardiovascular implications, and other side effects precipitated a decrease in their utilization for this objective. It is crucial to underscore that in contemporary medical practice, the prescription of amphetamines solely for weight loss is not commonly endorsed due to these aforementioned concerns (Abenhaim et al., 1996).
3.8.4 Treatment-resistant depression (TRD)
TRD is characterized by major depressive episodes that fail to show sufficient improvement, even after the administration of at least two distinct antidepressant regimens. Recognizing the mood-elevating and energy-boosting properties of amphetamines, researchers have explored their potential as adjunctive treatments for TRD. While certain studies have yielded promising outcomes, the application of amphetamines in this specific scenario is still off-label. It is essential for more comprehensive research to be conducted to firmly determine both their safety and efficacy in treating TRD (Stotz et al., 1999).
3.8.5 Cognitive enhancement and fatigue management
In specific situations, amphetamines have been employed off-label as tools for cognitive augmentation and combating fatigue. The underlying intent is to bolster alertness, sharpen concentration, and enhance overall performance during extended durations of wakefulness or in instances of sleep deprivation. Nonetheless, the repercussions of prolonged use remain inadequately researched, accompanied by legitimate concerns surrounding the potential for misuse and subsequent dependency (Ricci, 2020). With cognitive deterioration being a significant issue in the senior demographic, there has been a growing interest in evaluating the efficacy of stimulants, including amphetamines, in amplifying cognitive abilities in older adults who do not suffer from dementia. Initial research hints at possible advantages in tasks demanding attention and memory, yet the long-term safety and effectiveness for this demographic are still to be conclusively determined (Bagot and Kaminer, 2014).
3.8.6 Traumatic brain injury (TBI)
After experiencing TBI, many patients may face several challenges, such as cognitive impairments, diminished alertness, and delayed processing speeds. Emerging studies have proposed that amphetamines could potentially accelerate recovery and improve cognitive outcomes for these individuals. The postulated mechanism behind this effect is the drug’s capacity to boost synaptic transmission and amplify neural plasticity, which might bolster the brain’s innate healing mechanisms. Nevertheless, the role of amphetamines in the rehabilitation of TBI is a topic under active investigation, and concrete conclusions regarding their effectiveness have yet to be firmly established (Hornstein et al., 1996; Coris et al., 2021).
4 Discussion
It is important to have a comprehensive grasp of the underlying mechanisms, applications, potential adverse effects, and the historical trajectories of drugs is pivotal to ascertaining their suitability and safety for diverse medical conditions. Our meticulous examination of clinical trials centered on amphetamines, strengthened by a profound study of their historical trajectory and present-day status, shows their multifaceted roles, advantages, and associated risks.
From our extensive analysis, it is apparent that the body of clinical trials on ADHD is coherent, reflecting amphetamines’ historical significance and contemporary relevance in managing the disorder. Patients with ADHD, a disorder delineated by its hallmark symptoms of inattention, hyperactivity, and impulsivity, have experienced transformative treatment outcomes with amphetamine-based therapies (Singh et al., 2015). These medications, through their influential role in altering neurotransmitter concentrations, predominantly dopamine, present a compelling strategy for targeting the fundamental symptoms of the disorder (Gough et al., 2002; Heal et al., 2013). Recognized as a neurodevelopmental anomaly that predominantly surfaces during formative years (Wilens and Spencer, 2010b), studies have underscored the pivotal nature of amphetamine-centric interventions for this patient cohort. Notwithstanding their pronounced effectiveness in symptom alleviation, concerns related to side effects like impeded growth demand vigilant scrutiny and periodic oversight, especially among children (Richardson et al., 2017). Moreover, the prospective repercussions on growing neural trajectories and the consequent implications for long-term cognitive capacities call for thorough, sustained investigations (Berman et al., 2009; Reynolds et al., 2015b).
Turning to narcolepsy, a persistent sleep affliction, the therapeutic approach with amphetamines support the fact of determining drug pharmacodynamics in depth. Controlling the stimulative effects of amphetamines, lethargy during the day can be reduced for patients. However, it remains imperative to consistently balance the medicinal gains against any potential adverse outcomes or addiction susceptibility. The assessment of amphetamines across diverse conditions such as obesity, TRD, cognitive augmentation, and TBI unveils the expansive therapeutic potential of these molecules. Yet, as evidenced in the context of obesity management, the evolving landscape of medical protocols and burgeoning knowledge can recalibrate drug adoption trends. The diminished preference for amphetamines in weight management due to concerns over potential misuse and adverse reactions accentuates the necessity for constant evaluation, supervision, and recalibration of clinical directives.
Furthermore, while the therapeutic promise of amphetamines for addressing TRD and enhancing cognition, particularly among the geriatric populace or those with post-traumatic brain injuries, is captivating, it necessitates prudence. The off-label deployment of medications frequently navigates the unclear of clinical practice, making it vital to understand risks against the prospective benefits. A detailed dissection of specific amphetamine derivatives shows the intricate distinctions between them, highlighting that even minor chemical alterations can exert significant effects on pharmacokinetics and pharmacodynamics. This layered understanding can adeptly steer drug choices in specific clinical contexts.
4.1 Future directions
Future investigations into amphetamines should place a high emphasis on pediatric-focused trials, particularly in light of the rising prescriptions for children with ADHD. Alongside this, it is crucial to delve deeper into the intricate mechanisms underpinning addiction, while simultaneously developing robust prevention strategies. Additionally, considering the pharmacogenomics may offer tailored dosing and more predictable patient outcomes. Moreover, there exists a significant opportunity to innovate in the domain of extended-release formulations, aiming to affect an optimal balance between maximizing therapeutic advantages and minimizing the potential for misuse.
5 Conclusion
This study has underscored both the significant therapeutic potential of amphetamines, especially evident in pediatric ADHD populations, and the crucial need for awareness of their potential side effects and addiction risks. As the landscape of medicine expands, innovative formulations and broader therapeutic applications are emerging, with the exciting prospect of pharmacogenomics potentially redefining individualized treatments. This promises to reduce adverse reactions and bolster therapeutic efficacy. Balancing these benefits with the inherent risks remains paramount. Therefore, ongoing research is crucial in order to further understand amphetamines’ broad applications and to navigate the balance between their benefits and risks.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
SOA: Writing–original draft. OEH: Writing–review and editing. MS: Writing–original draft. FSA: Writing–original draft. AMA: Writing–original draft. ASA: Writing–original draft. NMA: Supervision, Writing–original draft.
Funding
The authors declare that no financial support was received for the research, authorship, and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abenhaim, L., Moride, Y., Brenot, F., Rich, S., Benichou, J., Kurz, X., et al. (1996). Appetite-suppressant drugs and the risk of primary pulmonary hypertension. International primary pulmonary hypertension study group. N. Engl. J. Med. 335, 609–616. doi:10.1056/NEJM199608293350901
Adinoff, B. (2004). Neurobiologic processes in drug reward and addiction. Harv Rev. Psychiatry 12, 305–320. doi:10.1080/10673220490910844
Akintomide, G. S., and Rickards, H. (2011). Narcolepsy: A review. Neuropsychiatr. Dis. Treat. 7, 507–518. doi:10.2147/NDT.S23624
Bagot, K. S., and Kaminer, Y. (2014). Efficacy of stimulants for cognitive enhancement in non-attention deficit hyperactivity disorder youth: A systematic review. Addict. Abingdon Engl. 109, 547–557. doi:10.1111/add.12460
Barker, E. C., Flygare, J., Paruthi, S., and Sharkey, K. M. (2020). Living with narcolepsy: Current management strategies, future prospects, and overlooked real-life concerns. Nat. Sci. Sleep. 12, 453–466. doi:10.2147/NSS.S162762
Berman, S. M., Kuczenski, R., McCracken, J. T., and London, E. D. (2009). Potential adverse effects of amphetamine treatment on brain and behavior: A review. Mol. Psychiatry 14, 123–142. doi:10.1038/mp.2008.90
Berridge, C. W. (2008). Noradrenergic modulation of arousal. Brain Res. Rev. 58, 1–17. doi:10.1016/j.brainresrev.2007.10.013
Blum, K., Chen, A. L-C., Braverman, E. R., Comings, D. E., Chen, T. J., Arcuri, V., et al. (2008). Attention-deficit-hyperactivity disorder and reward deficiency syndrome. Neuropsychiatr. Dis. Treat. 4, 893–918. doi:10.2147/ndt.s2627
Chen, G., and Adelman, J. P. (2023). “Chapter 13 - misuse and addiction definitions, demographics, and general concepts,” in Subst. Use addict. Res. Editors A. D. Kaye, R. D. Urman, E. M. Cornett, and A. N. Edinoff (Cambridge, Massachusetts, United States: Academic Press), 147–160. doi:10.1016/B978-0-323-98814-8.00013-5
Cho, S., and Yoon, Y-R. (2018). Understanding the pharmacokinetics of prodrug and metabolite. Transl. Clin. Pharmacol. 26, 1–5. doi:10.12793/tcp.2018.26.1.1
Clevel Clin, Amphetamine: Meaning, uses, side effects and types. 2023b https://my.clevelandclinic.org/health/drugs/23039-amphetamines (Accessed August 19, 2023).
Clevel Clin, (2023a). Dextroamphetamine: Uses and side effects. https://my.clevelandclinic.org/health/drugs/20878-dextroamphetamine-tablets (Accessed August 19, 2023).
Coris, E. E., Moran, B., Sneed, K., Del Rossi, G., Bindas, B., Mehta, S., et al. (2021). Stimulant therapy utilization for neurocognitive deficits in mild traumatic brain injury. Sports Health 14, 538–548. doi:10.1177/19417381211031842
Coulter, A. A., Rebello, C. J., and Greenway, F. L. (2018). Centrally acting agents for obesity: Past, present, and future. Drugs 78, 1113–1132. doi:10.1007/s40265-018-0946-y
Craig, S. G., Davies, G., Schibuk, L., Weiss, M. D., and Hechtman, L. (2015). Long-term effects of stimulant treatment for ADHD: What can we tell our patients? Curr. Dev. Disord. Rep. 2, 1–9. doi:10.1007/s40474-015-0039-5
Deadiversion, (2023). Controlled substance schedules. https://www.deadiversion.usdoj.gov/schedules/ (Accessed August 19, 2023).
dela Peña, I., Gevorkiana, R., and Shi, W-X. (2015). Psychostimulants affect dopamine transmission through both dopamine transporter-dependent and independent mechanisms. Eur. J. Pharmacol. 764, 562–570. doi:10.1016/j.ejphar.2015.07.044
Edeleanu, L. (1887). Über einige Derivate der Phenylmethacrylsäure und der Phenylisobuttersäure. San Diego, California, USA: Preuss.
Faraone, S. V., and Biederman, J. (2002). Efficacy of Adderall for attention-deficit/hyperactivity disorder: A meta-analysis. J. Atten. Disord. 6, 69–75. doi:10.1177/108705470200600203
Freezer, A., Salem, A., and Irvine, R. J. (2005). Effects of 3,4-methylenedioxymethamphetamine (MDMA, “Ecstasy”) and para-methoxyamphetamine on striatal 5-HT when co-administered with moclobemide. Brain Res. 1041, 48–55. doi:10.1016/j.brainres.2005.01.093
Frölich, J., Banaschewski, T., Spanagel, R., Döpfner, M., and Lehmkuhl, G. (2012). The medical treatment of attention deficit hyperactivity disorder (ADHD) with amphetamines in children and adolescents. Z Kinder Jugendpsychiatr Psychother. 40, 287–299. doi:10.1024/1422-4917/a000185
Goodwin, J. S., Larson, G. A., Swant, J., Sen, N., Javitch, J. A., Zahniser, N. R., et al. (2009). Amphetamine and methamphetamine differentially affect dopamine transporters in vitro and in vivo. J. Biol. Chem. 284, 2978–2989. doi:10.1074/jbc.M805298200
Gough, B., Imam, S. Z., Blough, B., Slikker, W., and Ali, S. F. (2002). Comparative effects of substituted amphetamines (PMA, MDMA, and METH) on monoamines in rat caudate: A microdialysis study. Ann. N. Y. Acad. Sci. 965, 410–420. doi:10.1111/j.1749-6632.2002.tb04182.x
Hall, D. A., Stanis, J. J., Avila, H. M., and Gulley, J. M. (2008). A comparison of amphetamine- and methamphetamine-induced locomotor activity in rats: Evidence for qualitative differences in behavior. Psychopharmacol. Berl. 195, 469–478. doi:10.1007/s00213-007-0923-8
Heal, D. J., Smith, S. L., Gosden, J., and Nutt, D. J. (2013). Amphetamine, past and present – A pharmacological and clinical perspective. J. Psychopharmacol. Oxf Engl. 27, 479–496. doi:10.1177/0269881113482532
Hegazi, O. E., Alalalmeh, S. O., Alnuaimi, G. R. H., Shahwan, M., Jairoun, A. A., Alorfi, N. M., et al. (2023). NAFLD and nutraceuticals: A review of completed phase III and IV clinical trials. Front. Med. 10, 10. doi:10.3389/fmed.2023.1227046
Hornstein, A., Lennihan, L., Seliger, G., Lichtman, S., and Schroeder, K. (1996). Amphetamine in recovery from brain injury. Brain Inj. 10, 145–148. doi:10.1080/026990596124647
Jan, R. K., Kydd, R. R., and Russell, B. R. (2012). Functional and structural brain changes associated with methamphetamine abuse. Brain Sci. 2, 434–482. doi:10.3390/brainsci2040434
Kirkpatrick, M. G., Gunderson, E. W., Johanson, C-E., Levin, F. R., Foltin, R. W., and Hart, C. L. (2012). Comparison of intranasal methamphetamine and d-amphetamine self-administration by humans. Addict. Abingdon Engl. 107, 783–791. doi:10.1111/j.1360-0443.2011.03706.x
Kolar, D., Keller, A., Golfinopoulos, M., Cumyn, L., Syer, C., and Hechtman, L. (2008). Treatment of adults with attention-deficit/hyperactivity disorder. Neuropsychiatr. Dis. Treat. 4, 389–403. doi:10.2147/ndt.s6985
Leith, N. J., and Kuczenski, R. (1981). Chronic amphetamine: Tolerance and reverse tolerance reflect different behavioral actions of the drug. Pharmacol. Biochem. Behav. 15, 399–404. doi:10.1016/0091-3057(81)90269-0
Levine, J., and Swanson, H. (2023). The use of lisdexamfetamine to treat ADHD in a patient with stimulant (methamphetamine) use disorder. Case Rep. Psychiatry 2023, e5574677. doi:10.1155/2023/5574677
Losacker, M., Zörntlein, S., Schwarze, B., Staudt, S., Röhrich, J., and Hess, C. (2022). Determination of the enantiomeric composition of amphetamine, methamphetamine and 3,4-methylendioxy-N-methylamphetamine (MDMA) in seized street drug samples from southern Germany. Drug Test. Anal. 14, 557–566. doi:10.1002/dta.3118
Magnus, W., Nazir, S., Anilkumar, A. C., and Shaban, K. (2023). Attention deficit hyperactivity disorder. StatPearls. Tampa, Florida, United States: StatPearls Publishing.
Marandure, B. N., Mhizha, S., Wilson, A., and Nhunzvi, C. (2023). Understanding the nature of substance use in Zimbabwe: State of the art and ways forward: A scoping review protocol. PLOS ONE 18, e0272240. doi:10.1371/journal.pone.0272240
Mental Health Services Administration, (1999). “Chapter 2—how stimulants affect the brain and behavior,” in Treat. Stimul. Use disord. Updat. 2021 (Rockville, Maryland: Substance Abuse and Mental Health Services Administration US).
Meyers, R. S., Thackray, J., Matson, K. L., McPherson, C., Lubsch, L., Hellinga, R. C., et al. (2020). Key potentially inappropriate drugs in pediatrics: The KIDs list. J. Pediatr. Pharmacol. Ther. JPPT 25, 175–191. doi:10.5863/1551-6776-25.3.175
Miller, D. R., Bu, M., Gopinath, A., Martinez, L. R., and Khoshbouei, H. (2021). Methamphetamine dysregulation of the central nervous system and peripheral immunity. J. Pharmacol. Exp. Ther. 379, 372–385. doi:10.1124/jpet.121.000767
Mrazek, D. A., and Schak, K. M. (2009). “Chapter 51 - attention-deficit/hyperactivity disorder,” in Pharmacol. Ther. S. A. Waldman, A. Terzic, L. J. Egan, J-L. Elghozi, A. Jahangir, G. C. Kaneet al. (Philadelphia, PA, USA: W.B. Saunders), 759–768. doi:10.1016/B978-1-4160-3291-5.50055-X
Najib, J., Didenko, E., Meleshkina, D., Yusupov, K., Maw, K., Ramnarain, J., et al. (2020). Review of lisdexamfetamine dimesylate in children and adolescents with attention deficit/hyperactivity disorder. Curr. Med. Res. Opin. 36, 1717–1735. doi:10.1080/03007995.2020.1815002
National Institute of Diabetes and Digestive and Kidney Diseases, (2012).Amphetamines. LiverTox Clin. Res. Inf. Drug-induc. Liver inj Bethesda, MD, USA: National Institute of Diabetes and Digestive and Kidney Diseases.
National Institute on Drug Abuse (2007). Drugs, brains, and behavior: The science of addiction. North Bethesda, MD, United States: National Institute on Drug Abuse, National Institutes of Health, US.
Ng, B., and O’Brien, A. (2009). Beyond ADHD and narcolepsy: Psychostimulants in general psychiatry. Adv. Psychiatr. Treat. 15, 297–305. doi:10.1192/apt.bp.107.004879
Nida, (2019). Methamphetamine DrugFacts. https://nida.nih.gov/download/1076/methamphetamine-drugfacts.pdf?v=04b49969b52aade627329386a3e9d265.
Ocd Kids, (2023). Medication for pediatric OCD. https://kids.iocdf.org/what-is-ocd-kids/how-is-ocd-treated/medication-for-pediatric-ocd/ (Accessed August 19, 2023).
Ochoa, C., Kilgore, P. C. S. R., Korneeva, N., Clifford, E., Conrad, S. A., Trutschl, M., et al. (2023). Trends in drug tests among children: A 22-year retrospective analysis. Pathophysiology 30, 219–232. doi:10.3390/pathophysiology30020019
Parsley, I., Zhang, Z., Hausmann, M., Lerdahl, A., Vaughan, B., Edwards, R., et al. (2020). Effectiveness of stimulant medications on disruptive behavior and mood problems in young children. Clin. Psychopharmacol. Neurosci. 18, 402–411. doi:10.9758/cpn.2020.18.3.402
Reynolds, L. M., Makowski, C. S., Yogendran, S. V., Kiessling, S., Cermakian, N., and Flores, C. (2015a). Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a dcc-dependent manner. Neuropsychopharmacology 40, 1101–1112. doi:10.1038/npp.2014.287
Reynolds, L. M., Makowski, C. S., Yogendran, S. V., Kiessling, S., Cermakian, N., and Flores, C. (2015b). Amphetamine in adolescence disrupts the development of medial prefrontal cortex dopamine connectivity in a dcc-dependent manner. Neuropsychopharmacology 40, 1101–1112. doi:10.1038/npp.2014.287
Ricci, G. (2020). Pharmacological human enhancement: An overview of the looming bioethical and regulatory challenges. Front. Psychiatry 11, 11. doi:10.3389/fpsyt.2020.00053
Richardson, E., Seibert, T., and Uli, N. K. (2017). Growth perturbations from stimulant medications and inhaled corticosteroids. Transl. Pediatr 6, 237–247. doi:10.21037/tp.2017.09.14
Richter, L. H. J., Meyer, M. R., and Maurer, H. H. (2019) “Chapter 19 - overview of common designer drugs” in Crit Issues alcohol drugs abuse test Editor A. Dasgupta 2 (Cambridge, Massachusetts, United States: Academic Press, 237–246. doi:10.1016/B978-0-12-815607-0.00019-8
Sharbaf Shoar, N., Marwaha, R., and Molla, M. (2023). Dextroamphetamine-amphetamine. StatPearls, treasure island (FL). Tampa, Florida, United States: StatPearls Publishing.
Singh, A., Yeh, C. J., Verma, N., and Das, A. K. (2015). Overview of attention deficit hyperactivity disorder in young children. Health Psychol. Res 3, 2115. doi:10.4081/hpr.2015.2115
Sontheimer, H. (2021) “Chapter 15 - drug addiction” in Dis. Nerv. Syst Editor H. Sontheimer 2 (Cambridge, Massachusetts, United States: Academic Press), 357–381. doi:10.1016/B978-0-12-821228-8.00012-3
Stotz, G., Woggon, B., and Angst, J. (1999). Psychostimulants in the therapy of treatment-resistant depression Review of the literature and findings from a retrospective study in 65 depressed patients. Dialogues Clin. Neurosci. 1, 165–174. doi:10.31887/DCNS.1999.1.3/gstotz
Strohl, M. P. (2011). Bradley’s benzedrine studies on children with behavioral disorders. Yale J. Biol. Med. 84, 27–33.
Turner, M. (2019). The treatment of narcolepsy with amphetamine-based stimulant medications: A call for better understanding. J. Clin. Sleep. Med. JCSM Off. Publ. Am. Acad. Sleep. Med. 15, 803–805. doi:10.5664/jcsm.7788
West, R., Pesce, A., Mikel, C., Velasco, J., Gonzales, E., Dizon, Z., et al. (2012). Detection of the d (dextro) and l (levo) methamphetamine enantiomers in a population of those with pain. J. Pain 13, S89. doi:10.1016/j.jpain.2012.01.369
Wilens, T. E., and Spencer, T. J. (2010a). Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad. Med. 122, 97–109. doi:10.3810/pgm.2010.09.2206
Wilens, T. E., and Spencer, T. J. (2010b). Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad. Med. 122, 97–109. doi:10.3810/pgm.2010.09.2206
Zanda, M. T., and Fattore, L. (2017). “Chapter 29 - novel psychoactive substances: A new behavioral and mental Health threat,” in Addict. Subst. Neurol. Dis. Editors R. R. Watson, and S. Zibadi (Cambridge, Massachusetts, United States: Academic Press), 341–353. doi:10.1016/B978-0-12-805373-7.00029-3
Keywords: amphetamines, pediatric medicine, ADHD, dependency, developing brain, clinical trials
Citation: Alalalmeh SO, Hegazi OE, Shahwan M, Alshehri FS, Ashour AM, Algarni AS and Alorfi NM (2023) Amphetamines in child medicine: a review of ClinicalTrials.gov. Front. Pharmacol. 14:1280562. doi: 10.3389/fphar.2023.1280562
Received: 20 August 2023; Accepted: 18 September 2023;
Published: 03 October 2023.
Edited by:
Hanan Farouk Aly, National Research Centre, EgyptReviewed by:
Nemat Ali, King Saud University, Saudi ArabiaAishah Albalawi, University of Tabuk, Saudi Arabia
Copyright © 2023 Alalalmeh, Hegazi, Shahwan, Alshehri, Ashour, Algarni and Alorfi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nasser M. Alorfi, nmorfi@uqu.edu.sa
 Samer O. Alalalmeh
Samer O. Alalalmeh Omar E. Hegazi
Omar E. Hegazi Moyad Shahwan
Moyad Shahwan Fahad S. Alshehri
Fahad S. Alshehri Ahmed M. Ashour
Ahmed M. Ashour Nasser M. Alorfi
Nasser M. Alorfi