- 1Department of Pharmacy, Tongji Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
- 2Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei, China
Objective: This study aims to synthesize evidence on the cost-effectiveness of empagliflozin for heart failure (HF).
Methods: MEDLINE, Embase, the Cochrane Library, EconLit, CNKI, Wanfang Data and Chongqing VIP were searched to identify original articles on cost-effectiveness of empagliflozin for HF, and literature surveillance ended on 20 November 2022. The reporting quality of the included articles was determined using the Consolidated Health Economic Evaluation Reporting Standards statement.
Results: Of 97 articles identified, 11 studies published from 2020 to 2022 met the inclusion criteria, and the overall quality was accepted. The studies were conducted in 8 countries (China, Japan, Korea, Singapore, Thailand, Australia, United States, and United Kingdom). This body of evidence suggested that add-on empagliflozin was cost effective for HF with reduced ejection fraction (HFrEF) patients compared to standard of care alone in all the related studies including China, Japan, Korea, Singapore, Thailand, and Australia. For HF with preserved ejection fraction (HFpEF) patients, add-on empagliflozin was cost effective in China and Australia, but not in United States and Thailand. For HF with diabetes, add-on empagliflozin was cost effective in United Kingdom. Moreover, the incremental cost-effectiveness ratios (ICER) were lower for patients with diabetes than without in subgroup analysis. In the uncertainty analysis of all included studies, the ICERs were most sensitive to the cost of empagliflozin and cardiovascular mortality, followed by the cost of the standard treatment, hazard ratio of HF hospitalization.
Conclusion: add-on empagliflozin for HFrEF might be cost-effective or dominant compared with standard of care alone. However, for HFpEF patients, add-on empagliflozin might be cost-effective in China and Australian, but not cost-effective in United States and Thailand.
Introduction
Heart Failure (HF), a heterogeneous syndrome characterized by significant morbidity and mortality, poor functional capacity and quality of life, and high costs, affects more than 64 million people worldwide (James et al., 2018; Baman and Ahmad, 2020; Savarese et al., 2022). The overall lifetime healthcare costs due to HF per patient was estimated to be USD $126,819 by a systematic review including 16 international studies from 2004 to 2016 (Lesyuk et al., 2018). Due to the raising prevalence of HF, the economic burden of the disease on healthcare expenditures worldwide is even expected to increase. In the US, the total cost for HF was estimated to be USD $30.7 billion in 2012, with projections suggesting a significant increase in costs to USD $69.8 in 2030 (Virani et al., 2021). Therefore, it is imperative to undertake economic evaluation of the therapies for HF to reduce its social and economic burden.
Treatment for HF depends on its cause, symptoms, and ejection fraction, a measure of the heart’s squeezing function. Historically, the standard of care (SoC) for HF is standard heart failure device and drug therapy, which included diuretics, angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, and β-blockers. Recently, several clinical trials have confirmed that sodium-glucose cotransport 2 (SGLT2) inhibitors could reduced the risk of cardiovascular (CV) death or hospitalization for heart failure (Packer et al., 2020; Packer et al., 2021; Santos-Gallego et al., 2021). Empagliflozin, a SGLT2 inhibitor, is the newest medication approved by the US Food and Drug Administration for HF in 2021 and by the Chinese National Medical Products Administration in 2022. The empagliflozin outcome trial in patients with chronic heart failure (EMPEROR) evaluated that empagliflozin reduced CV mortality or HF hospitalization in patients with HFrEF or HFpEF independently of their glycemic status (Anker et al., 2021; Packer et al., 2021). It is currently the only drug that has been proven to improve the outcome of patients with HFpEF by a large randomized controlled trial. Therefore, empagliflozin is recommended not only for HFrEF, but also for HFpEF by 2022 AHA/ACC/HFSA guideline for the management of HF (Heidenreich et al., 2022).
The clinical effects of empagliflozin for patients with HF are demonstrated. Due to the limitation of healthcare resources, the cost effectiveness of empagliflozin for HF must be considered. Several studies from different countries have evaluated the cost effectiveness of empagliflozin for HF, but there were differences in the study methods and results. Therefore, it is necessary to synthesize these studies so that researchers can quickly obtain more comprehensive economic data. This study is the first systematic review to appraise and synthesize the economic evidence of empagliflozin for HF patients. Our results would provide valuable information to administrators and health workers in making the best decisions.
Methods
Literature search
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines (Page et al., 2021). Eligible studies were identified from the following databases: MEDLINE, Embase, the Cochrane Library, and EconLit databases with no language restrictions, and CNKI, Wanfang Data and Chongqing VIP for Chinese-language studies. We restricted the analysis to original articles on cost-effectiveness of empagliflozin for HF, and literature surveillance ended on 20 November 2022. The detailed search strategy was presented in Supplementary Table S1.
Eligibility criteria
Articles meeting the following criteria were included: 1) target population was patients with HF; 2) empagliflozin intervention was included and comparison was not limited; 3) the original economic evaluation, examined costs with their consequences, and reported incremental cost-effectiveness ratios (ICERs) or incremental cost-utility ratios; 4) complete full-text formats were available. Duplicated literature, reviews, commentaries, conference abstracts, expert opinions, and other secondary research were excluded.
Study selection
Titles and abstracts were screened against the eligibility criteria and then full-text formats of all potentially relevant publications were obtained and reviewed to decide whether they met the inclusion criteria by two authors. Another discussion could be conducted to resolve discrepancies.
Reporting quality assessment
The 28-item Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement (Husereau et al., 2022) was used to appraise the reporting quality of studies. Each item was scored as having met the criteria in full (“1”), not at all (“0”), or not applicable (NA). According to the scores, studies were categorized as good (>75%), moderate (50%–75%), and low (<50%).
Data extraction and synthesis
We made standardized forms to extract relevant information such as basic information (i.e., authors’ name, target population, intervention and comparison), methods and the main results. A narrative synthesis was used to evaluate the aims, methods, settings, and results of the included studies. If possible, we undertook horizontal comparison of modeling technique, cost perspective, measures of benefit used, ICERs, and results of uncertainty analysis across the studies. For better comparing the results of economic analysis between different currencies, all reported ICERs are converted in US$ for the common price year 2022 using the “CCEMG-EPPI-Centre Cost Converter” Version 1.6 (CCEMG-EPPI-Centre Cost Converter, 2019).
Result
Studies identified
Of the 97 potential publications retrieved, 25 were excluded for repetitive publications, and 53 were excluded based on title and abstract. The remaining 19 were retrieved for full-text screening, and 8 were excluded for reasons such as review articles (n = 1), not heart failure (n = 1), and meeting abstracts (n = 6). Finally, 11 publications (Reifsnider et al., 2020; Jiang et al., 2021; Liao et al., 2021; Wan et al., 2022a; Jiang and Xie, 2022; Krittayaphong and Persuwan, 2022; Lin et al., 2022; Lou et al., 2022; Tang and Sang, 2022; Zheng et al., 2022; Zhou et al., 2022) were included in this review, and more details about studies identified were reported in Figure 1.
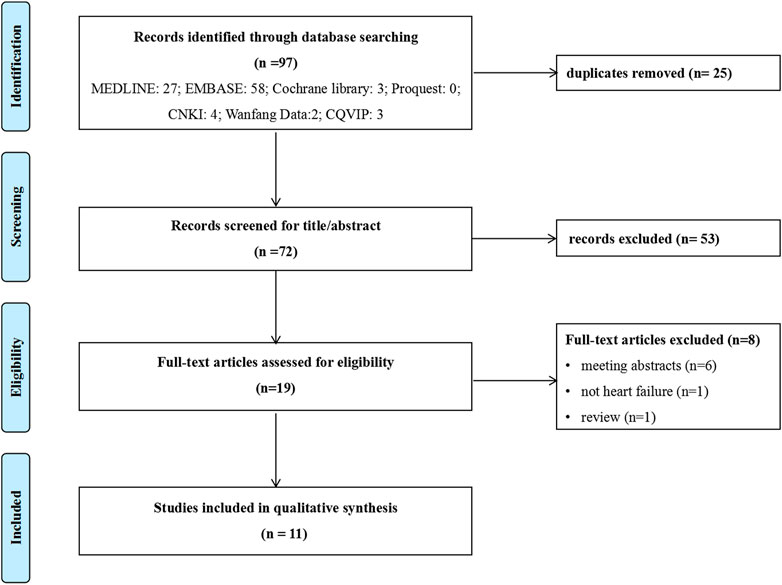
FIGURE 1. Flowchart of literature search. CNKI: China National Knowledge Infrastructure database; CQVIP: Chongqing VIP database.
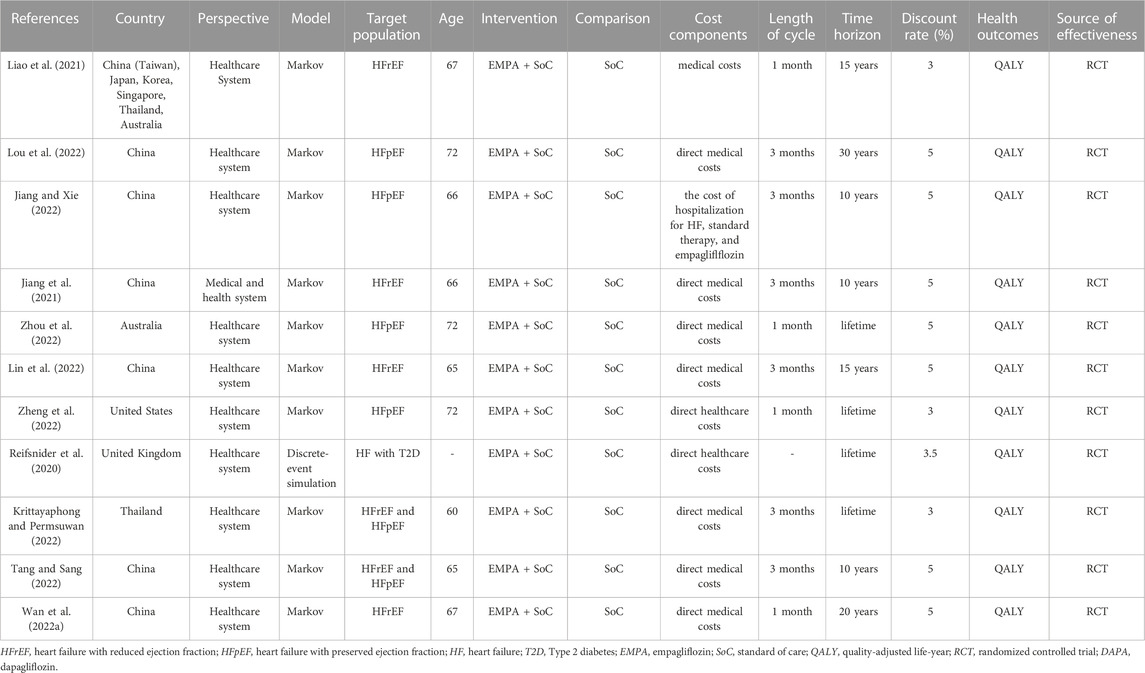
Basic characteristics
The general characteristics of the included studies were reported in Table 1. The included studies were conducted in 6 developed countries (United Kingdom, United States, Australia, Korea, Japan, and Singapore) and 2 developing countries (China and Thailand). The Markov model was used in 10 studies, and discrete-event simulation model was used in 1 study. The populations simulated in all the models were based on the basic characteristics of those in the EMPEROR-Preserved study or the EMPEROR-Reduced study. All the included studies compared empagliflozin plus SoC with SoC alone from the healthcare perspective. The time horizons were applied for 10 years or more. Four studies used 1-month Markov cycles, and 6 studies used 3-month Markov cycles.
One study was funded by award 1K23HL151672-01 from the National Heart, Lung, and Blood Institute of the National Institutes of Health, one by the Natural Science Foundation of Fujian Province, China and the Health Youth Scientific Research Project of Fujian Province, China, one by Boehringer Ingelheim International GmbH, one by the National Heart Foundation of Australia Fellowship, and one by the National Key Research and Development Program of China. The remaining 6 studies were without funding.
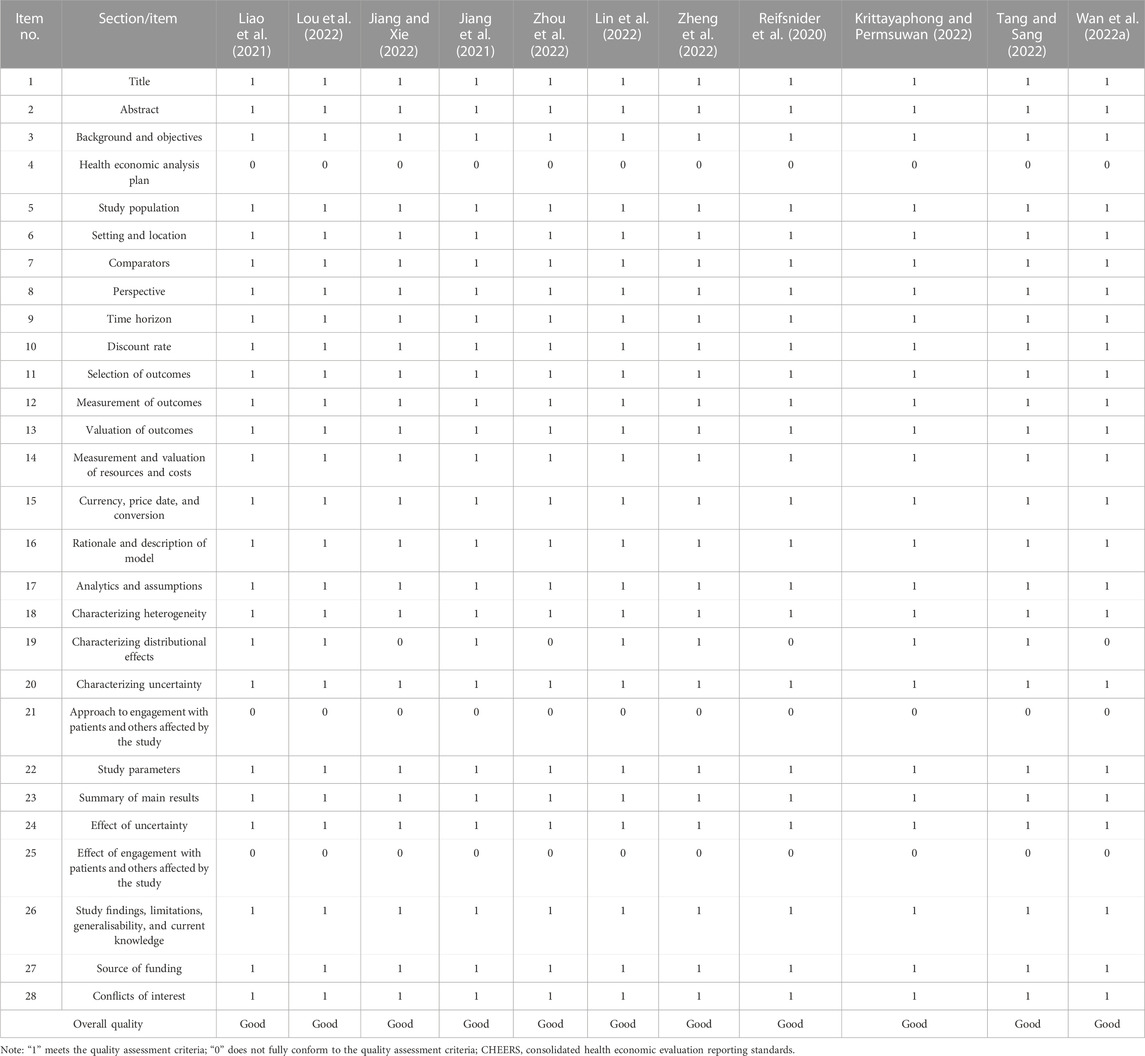
Reporting quality assessment
All studies have not mentioned three items, which were health economic analysis plan, approach to engagement with patients and others affected by the study, and effect of engagement with patients and others affected by the study respectively. Four studies failed to report characterizing distributional effects (Wan YM. et al., 2022; Jiang and Xie, 2022; Zhou et al., 2022; Reifsnider et al., 2020). The four items mentioned above have been added in the CHEERS statement updated in 2022, therefore the studies did not report well. However, the remaining 24 items were reported sufficiently in all of the included studies, and the included studies were all evaluated as of good quality. More details were summarized in Table 2.
Cost-effectiveness analysis
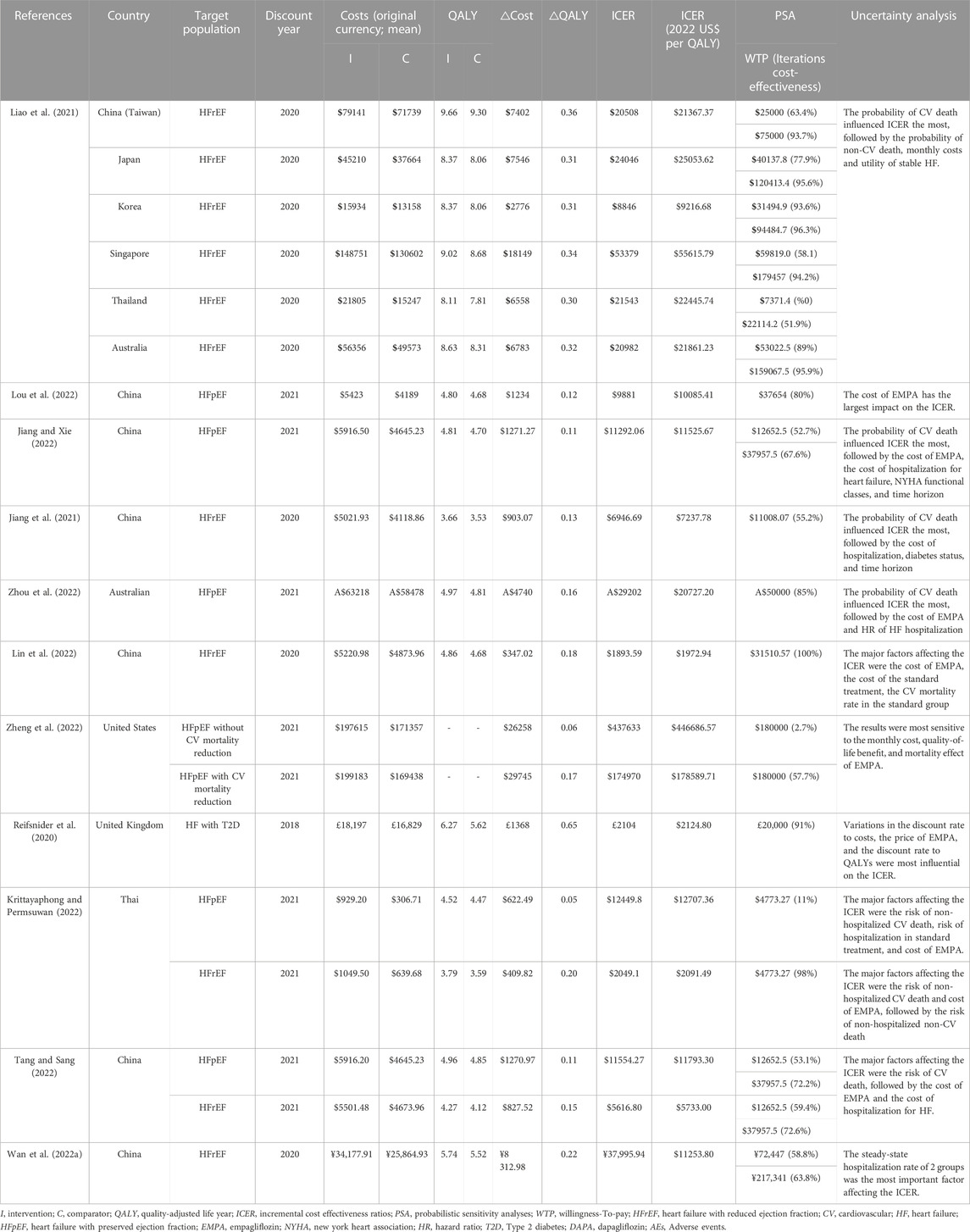
Four studies provided economic evaluation for HFrEF, 4 studies for HFpEF, 2 studies for HFrEF and HFpEF, 1study for HF with type 2 diabetes. The overview of the economic evaluation outcomes are summarized in Table 3.
Six studies provided economic evaluation for HFrEF. Four studies conducted in China indicated that adding empagliflozin to SoC was proven to be more cost-effective for HFrEF from a healthcare system perspective. One study conducted in Thailand have the same results as the above studies in China. One study was conducted in China (Taiwan), Australia, Korea, Singapore, Japan, and Thailand. The results showed that adding empagliflozin to SoC for HFrEF was expected to be a cost-effective option, and the probabilities were highest in Korea, lowest in Thailand.
Six studies provided economic evaluation for HFpEF. Three studies were conducted in China, and suggested that the adding empagliflozin to SoC for HFpEF was cost-effective in healthcare systems. One study in Australian suggested that adding empagliflozin is likely to be cost-effective in the healthcare setting. One study in USA suggested that adding empagliflozin provides low economic value compared with SoC for HFpEF. However, the ICER was lower for HFpEF with CV mortality reduction than without. The last study was conducted in Thailand, and suggested that empaglifozin was not a cost-effective add-on treatment for HFpEF. In total, the ICERs were higher for HFpEF than for HFrEF.
Subgroup analysis
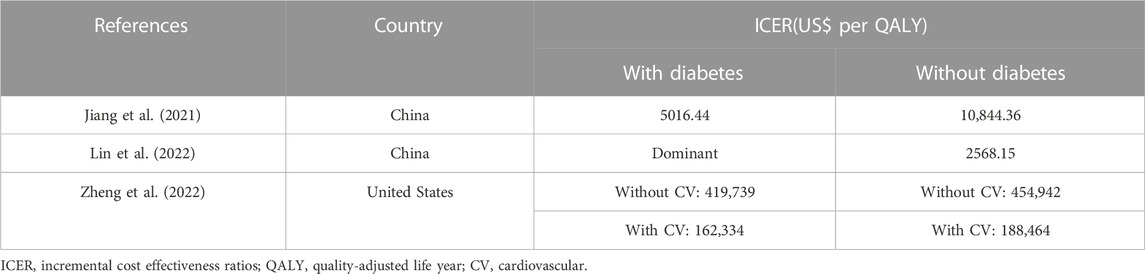
Subgroup analysis was performed according to the different states of diabetes in 3 studies (Jiang et al., 2021; Lin et al., 2022; Zheng et al., 2022), revealing that empagliflozin had similar cost-effectiveness among patients with and without diabetes, and empagliflozin was more cost effective in HErEF patients with diabetes. The details were shown in Table 4. Subgroup analysis was also performed across EF strata and HF-related health status among HErEF patients in 1 study (Zheng et al., 2022), indicating that the ICER was slightly lower for patients with EF less than 50%, and similar for mildly impaired HF and moderately impaired HF.
Uncertainty analysis
One-way sensitivity analysis and probabilistic sensitivity analyses (PSA) were applied in all the included studies. Six studies indicated that the major factor affecting the ICER was the cost of empagliflozin. Three studies (Reifsnider et al., 2020; Lin et al., 2022; Lou et al., 2022) showed when the cost increased to its upper limit, the ICER was still lower than the WTP threshold. One study (Jiang and Xie, 2022) showed when the cost increased to its upper limit, the ICER was higher than one-time GDP but lower than three-time GDP. One study (Zheng et al., 2022) showed that the monthly cost of empagliflozin would need to drop from $326.69 to $153.56 to meet a WTP threshold of $180 000 per quality-adjusted life-year (QALY). One study (Zhou et al., 2022) showed that empagliflozin was no longer cost-effective if its cost exceeded AUD$110 per month.
Six studies displayed the CV mortality to be the most influential parameter. With the decrease of CV mortality in SoC or the increase of CV mortality in adding empagliflozin, the ICERs got higher. One study (Jiang et al., 2021) showed that the CV mortality in adding empagliflozin and SoC had a great impact on the ICER value, which was far more than three-time GDP. Another study (Tang and Sang, 2022) showed the CV mortality in SoC had similar effect. Two studies (Liao et al., 2021; Jiang and Xie, 2022) showed when the CV mortality increased to its upper limit, the ICERs were higher than one-time GDP but lower than three-time GDP. One study (Zhou et al., 2022) showed that adding empagliflozin was no longer cost-effective if the hazard ratio for CV mortality exceeded 0.99. However, there was a study (Krittayaphong and Permsuwan, 2022) showed that the CV mortality did not change the economic outcome.
Discussion
Eleven economic evaluations of empagliflozin for the treatment of HF from 8 countries were identified in our systematic review, where turns out that add-on empagliflozin is cost effective in most countries, especially for HFrEF patients. The results are similar to the economics of dagliflozin for HF (Krittayaphong and Permsuwan, 2021; Cohen et al., 2023). Unfortunately, there is currently few economic comparison between SGLT2 for the treatment of HF, and it is difficult to determine which SGLT2 is more economical.
In this review, all reported ICERs of different regional backgrounds were adjusted to 2022 USD and the results of PSA were summarized in Table 3 for more convenient comparison. According to the results, the ICERs varied greatly in different studies and different countries. For HFrEF patients, add-on empagliflozin was cost effective in all of the included countries. However, the highest ICER was in Singapore, which was $55615.79 per QALY (Liao et al., 2021), and the lowest ICER was in China, which was $1972.94 per QALY (Lin et al., 2022). It was mainly because of the huge cost difference. For HFpEF patients, with the exception of the USA (Zheng et al., 2022) and Thailand (Liao et al., 2021), it was considered that empagliflozin was cost effective in the remaining countries. It means that the economic results of one country cannot be applied to another, and several economic evaluations have already demonstrated the variability of cost-effectiveness estimates for drugs in different countries (Mac et al., 2019; Li et al., 2021).
It is worth mentioning that the results still varied despite the studies coming from the same country. The ICERs ($22445.74 per QALY vs. $2091.49 per QALY) differ by 10-fold in two studies (Liao et al., 2021; Krittayaphong and Permsuwan, 2022) for HFrEF patients from Thailand. Since Liao et al. did not list specific cost data, there was no way to analyze the reasons for the difference. There were similar situations in Chinese studies. The ICERs of five studies for HFrEF patients varied greatly. One came from Taiwan, China, with the ICER of $21367.37 per QALY (Liao et al., 2021), and the other four were from Chinese mainland with the lowest ICER of $1972.94 per QALY (Lin et al., 2022). The possible reasons for heterogeneity were mainly derived from differences in costs as well as time horizon. Wu et al. (Wu et al., 2022) got similar results in an economic systematic review of dapagliflozin for HF. Therefore, we should consider the heterogeneity in different regions of the same country when evaluating the economics of empagliflozin for HF patients.
Uncertainty analysis showed that the cost of empagliflozin was the major factor affecting the ICERs. With the implementation of centralized procurement of drugs in China, a lower price of empagliflozin is negotiable, hence empagliflozin in treatment for HF patients will be more cost effective from a Chinese healthcare system perspective.
Similar to the previous studies, some critical elements of economic evaluation were also found in our studies (Wan Y. et al., 2022; Liu et al., 2022). Firstly, the election of the target population is crucial, and it could lead to different economic outcomes. For instance, add-on empagliflozin was evaluated to be dominant for HFrEF patients, but not cost-effectiveness for HFpEF patients in the same study (Krittayaphong and Permsuwan, 2022). Furthermore, subgroup analysis performed in 3 studies indicated that empagliflozin was more cost-effectiveness in HErEF patients with diabetes or HF with CV mortality reduction. Hence, the selection of target population is one of the most critical structures of economic evaluation. Secondly, the comparator is a very important element. One study (Jiang et al., 2021) from China showed that add-on empagliflozin was more cost-effectiveness compared with SoC, but led to more costs and less QALY compared with dapagliflozin. We have previously reached the similar results in an economic systematic review of elbasvir/grazoprevir for chronic hepatitis C (Liu et al., 2022). Thirdly, the country or region selected is extremely crucial. The included analyses were mainly for a certain country, so the economic outcomes must be markedly affected by the healthcare system, and economic levels of the country. Therefore, the applicability and extrapolation of research is limited. It will be necessary to improve the methods, such as constructing multi-level models and identifying a series of appropriate covariates to enhance the applicability and extrapolation. Furthermore, There are some other elements that need to be considered (Vandepitte et al., 2021; Yang et al., 2021).
Despite scientific and systematic methods used to minimize deviations, several limitations should be acknowledged. First, The CHEERS statement used in the systematic review is a guideline reporting tool that can help determine whether the study is well reported, but it is not a methodological quality assessment tool. Second, it is extremely difficult to synthesize these studies due to the possible divergences in backgrounds and methodology such as length of cycle, time horizons, target populations, healthcare systems, and cost components, etc. For example, although all the studies considered only the direct medical costs, some studies specified the cost details and others did not, which created a limitation to quantitative analysis or horizontal comparison of the studies. Therefore, we summarized the evidence qualitatively and then interpreted the outcomes cautiously. Third, all the included studies have only considered direct medical costs. In fact, HF will cause tremendous social and economic burden if not treated in time. Therefore, it is necessary to carry out further research from the perspective of the whole society. Last but not least, the populations simulated in all the models were based on the EMPEROR study, which means that the reliability of outcomes may be influenced by publication bias.
Conclusion
In conclusion, this study is the first systematic review on the cost-effectiveness of empagliflozin for HF. Based on the available evidence, add-on empagliflozin for HFrEF might be dominant or cost-effective compared with SoC, and add-on empagliflozin for HFpEF might be cost-effective in China and Australian, but not cost-effective in USA and Thailand. The ICERs were most sensitive to the cost of empagliflozin and CV mortality. In further economic evaluations of empagliflozin for HF patients, the country epidemiological real-world data should be taken into account in model building and sensitivity analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
JL and RY: study design and study conduct. JL and AW: data collection. RY, AW, XG, and JL: data interpretation. JL and RY: data analysis and drafting manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1186579/full#supplementary-material
References
Anker, S. D., Butler, J., Filippatos, G., Ferreira, J. P., Bocchi, E., Böhm, M., et al. (2021). Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med. 385 (16), 1451–1461. doi:10.1056/NEJMoa2107038
Baman, J. R., and Ahmad, F. S. (2020). Heart failure. JAMA 324 (10), 1015. doi:10.1001/jama.2020.13310
CCEMG-EPPI-Centre (2019). Cost converter. Available at: http://eppi.ioe.ac.uk/costconversion/default.aspx (Accessed January 6, 2023).
Cohen, L. P., Isaza, N., Hernandez, I., Lewis, G. D., Ho, J. E., Fonarow, G. C., et al. (2023). Cost-effectiveness of sodium-glucose cotransporter-2 inhibitors for the treatment of heart failure with preserved ejection fraction. JAMA Cardiol. 8 (5), 419–428. doi:10.1001/jamacardio.2023.0077
Heidenreich, P. A., Bozkurt, B., Aguilar, D., Allen, L. A., Byun, J. J., Colvin, M. M., et al. (2022). 2022 AHA/ACC/HFSA guideline for the management of heart failure: A report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation 145(18):e895-e1032. doi:10.1161/CIR.0000000000001063
Husereau, D., Drummond, M., Augustovski, F., de Bekker-Grob, E., Briggs, A. H., Carswell, C., et al. (2022). Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: Updated reporting guidance for health economic evaluations. Value Health 25 (1), 3–9. doi:10.1016/j.jval.2021.11.1351
James, S. L., Abate, D., Abate, K. H., et al. (2018). Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet 392, 1789–1858. doi:10.1016/S0140-6736(18)32279-7
Jiang, Y., and Xie, J. (2022). Cost-effectiveness of adding empagliflozin to the standard therapy for Heart Failure with Preserved Ejection Fraction from the perspective of healthcare systems in China. Front. Cardiovasc Med. 9, 946399. doi:10.3389/fcvm.2022.946399
Jiang, Y., Zheng, R., and Sang, H. (2021). Cost-effectiveness of adding SGLT2 inhibitors to standard treatment for heart failure with reduced ejection fraction patients in China. Front. Pharmacol. 12, 733681. doi:10.3389/fphar.2021.733681
Krittayaphong, R., and Permsuwan, U. (2021). Cost-utility analysis of add-on dapagliflozin treatment in heart failure with reduced ejection fraction. Int. J. Cardiol. 322, 183–190. doi:10.1016/j.ijcard.2020.08.017
Krittayaphong, R., and Permsuwan, U. (2022). Cost-utility analysis of combination empagliflozin and standard treatment versus standard treatment alone in Thai heart failure patients with reduced or preserved ejection fraction. Am. J. Cardiovasc Drugs 22 (5), 577–590. doi:10.1007/s40256-022-00542-9
Lesyuk, W., Kriza, C., and Kolominsky-Rabas, P. (2018). Cost-of-illness studies in heart failure: A systematic review 2004-2016. BMC Cardiovasc. Disord. 18, 74. doi:10.1186/s12872-018-0815-3
Li, N., Cornelissen, D., Silverman, S., Pinto, D., Kremer, I., et al. (2021). An updated systematic review of cost-effectiveness analyses of drugs for osteoporosis. Pharmacoeconomics 39 (2), 181–209. doi:10.1007/s40273-020-00965-9
Liao, C. T., Yang, C. T., Kuo, F. H., Lee, M. C., Chang, W. T., Tang, H. J., et al. (2021). Cost-effectiveness evaluation of add-on empagliflozin in patients with heart failure and a reduced ejection fraction from the healthcare system's perspective in the asia-pacific region. Front. Cardiovasc Med. 8, 750381. doi:10.3389/fcvm.2021.750381
Lin, X., Lin, M., Liu, M., Huang, W., Nie, X., Chen, Z., et al. (2022). Cost-effectiveness of empagliflozin as a treatment for heart failure with reduced ejection fraction: An analysis from the Chinese healthcare perspective. J. Thorac. Dis. 14 (5), 1588–1597. doi:10.21037/jtd-22-463
Liu, J., Guo, M., Ke, L., and You, R. (2022). Cost-effectiveness of elbasvir/grazoprevir for the treatment of chronic hepatitis C: A systematic review. Front. Public Health 10, 836986. doi:10.3389/fpubh.2022.836986
Lou, Y., Hu, T., and Huang, J. (2022). Cost-effectiveness of adding empagliflozin to standard treatment for heart failure with preserved ejection fraction patients in China. Am. J. Cardiovasc Drugs 23, 47–57. doi:10.1007/s40256-022-00550-9
Mac, S., Sumner, A., Duchesne-Belanger, S., Stirling, R., Tunis, M., and Sander, B. (2019). Cost-effectiveness of palivizumab for respiratory syncytial virus: A systematic review. Pediatrics 143 (5), e20184064. doi:10.1542/peds.2018-4064
Packer, M., Anker, S. D., Butler, J., Filippatos, G., Pocock, S. J., Carson, P., et al. (2020). Cardiovascular and renal outcomes with empagliflozin in heart failure. N. Engl. J. Med. 383 (15), 1413–1424. doi:10.1056/NEJMoa2022190
Packer, M., Butler, J., Zannad, F., Filippatos, G., Ferreira, J. P., Pocock, S. J., et al. (2021). Effect of empagliflozin on worsening heart failure events in patients with heart failure and preserved ejection fraction: EMPEROR-preserved trial. Circulation 144 (16), 1284–1294. doi:10.1161/CIRCULATIONAHA.121.056824
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 372, 790–799. doi:10.1016/j.rec.2021.07.010
Reifsnider, O. S., Kansal, A. R., Franke, J., Lee, J., George, J. T., Brueckmann, M., et al. (2020). Cost-effectiveness of empagliflozin in the UK in an EMPA-REG OUTCOME subgroup with type 2 diabetes and heart failure. Esc. Heart Fail 7 (6), 3910–3918. doi:10.1002/ehf2.12985
Santos-Gallego, C. G., Vargas-Delgado, A. P., Requena-Ibanez, J. A., Garcia-Ropero, A., Mancini, D., Pinney, S., et al. (2021). Randomized trial of empagliflozin in nondiabetic patients with heart failure and reduced ejection fraction. J. Am. Coll. Cardiol. 77 (3), 243–255. doi:10.1016/j.jacc.2020.11.008
Savarese, G., Becher, P. M., Lund, L. H., Seferovic, P., Rosano, G. M. C., and Coats, A. J. S. (2022). Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc Res. 118, 3272–3287. doi:10.1093/cvr/cvac013
Tang, Y., and Sang, H. (2022). Cost-utility analysis of empagliflozin in heart failure patients with reduced and preserved ejection fraction in China. Front. Pharmacol. 13, 1030642. doi:10.3389/fphar.2022.1030642
Vandepitte, S., Alleman, T., Nopens, I., Baetens, J., Coenen, S., and De Smedt, D. (2021). Cost-effectiveness of COVID-19 policy measures: A systematic review. Value Health 24 (11), 1551–1569. doi:10.1016/j.jval.2021.05.013
Virani, S. S., Alonso, A., Aparicio, H. J., Benjamin, E. J., Bittencourt, M. S., Callaway, C. W., et al. (2021). Heart disease and stroke statistics-2021 update: A report from the American heart association. Circulation 143, e254–e743. doi:10.1161/CIR.0000000000000950
Wan, Y., Zeng, F., Tan, H., Lu, Y., Zhang, Y., Zhao, L., et al. (2022b). Cost-effectiveness analyses of denosumab for osteoporosis: A systematic review. Osteoporos. Int. 33 (5), 979–1015. doi:10.1007/s00198-021-06268-9
Wan, Y. M., Sang, H. Q., Dong, J. Z., et al. (2022a). Pharmacoeconomic evaluation of empagliflozin in the treatment of heart failure with reduced ejection fraction. China Pharm. 3 (1), 74–78.
Wu, M., Qin, S., Wang, L., Tan, C., Peng, Y., Zeng, X., et al. (2022). Economic evaluation of dapagliflozin in the treatment of patients with heart failure: A systematic review. Front. Pharmacol. 13, 860109. doi:10.3389/fphar.2022.860109
Yang, C. Y., Chen, Y. R., Ou, H. T., and Kuo, S. (2021). Cost-effectiveness of GLP-1 receptor agonists versus insulin for the treatment of type 2 diabetes: A real-world study and systematic review. Cardiovasc Diabetol. 20 (1), 21. doi:10.1186/s12933-020-01211-4
Zheng, J., Parizo, J. T., Spertus, J. A., Heidenreich, P. A., and Sandhu, A. T. (2022). Cost-effectiveness of empagliflozin in patients with heart failure with preserved ejection fraction. JAMA Intern Med. 182 (12), 1278–1288. doi:10.1001/jamainternmed.2022.5010
Keywords: empagliflozin, heart failure, economic evaluation, cost-effectiveness, systematic review
Citation: Liu J, Liu D, Gong X, Wei A and You R (2023) Cost-effectiveness of empagliflozin for the treatment of heart failure: a systematic review. Front. Pharmacol. 14:1186579. doi: 10.3389/fphar.2023.1186579
Received: 15 March 2023; Accepted: 20 June 2023;
Published: 30 June 2023.
Edited by:
Tanveer Ahmed Khan, National Institute of Health, PakistanReviewed by:
Daniela Oliveira de Melo, Federal University of São Paulo, BrazilYue Wu, Wuhan University, China
Copyright © 2023 Liu, Liu, Gong, Wei and You. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ruxu You, youruxu2008@163.com
 Jinyu Liu
Jinyu Liu Dong Liu
Dong Liu Xuepeng Gong1
Xuepeng Gong1 Ruxu You
Ruxu You