- Era College of Pharmacy, Era University, Lucknow, Uttar Pradesh, India
Breast cancer is one of the most diagnosed solid cancers globally. Extensive research has been going on for decades to meet the challenges of treating solid tumors with selective compounds. This article aims to summarize the therapeutic agents which are either being used or are currently under approval for use in the treatment or mitigation of breast cancer by the US FDA, to date. A structured search of bibliographic databases for previously published peer-reviewed research papers on registered molecules was explored and data was sorted in terms of various categories of drugs used in first line/adjuvant therapy for different stages of breast cancer. We included more than 300 peer-reviewed papers, including both research and reviews articles, in order to provide readers an useful comprehensive information. A list of 39 drugs are discussed along with their current status, dose protocols, mechanism of action, pharmacokinetics, possible side effects, and marketed formulations. Another interesting aspect of the article included focusing on novel formulations of these drugs which are currently in clinical trials or in the process of approval. This exhaustive review thus shall be a one-stop solution for researchers who are working in the areas of formulation development for these drugs.
1 Introduction
Among all the listed causes of mortalities, Cancer remains one of the leading causes of human death throughout the world every year. As reported by WHO, nearly 10 million deaths have been accounted for Cancer in the year 2020 wherein 2.26 million cases have been reported for Breast Cancer surpassing other leading solid cancers like Lung cancer, Colon and Rectal cancer as well as Prostate cancer.
Breast cancer is the most prevalent cancer among females worldwide. WHO Global Breast Cancer Initiative (GBCI) endeavors to reduce global breast cancer mortality by 2.5% per year thus avoiding 2.5 million breast cancer deaths between 2020 and 2040. The incidence of breast cancer has been rising globally, over the years. According to 2021 estimates breast cancer accounted for about 30% of all the new cancers diagnosed in women in the United States with approx. 15% leading to death. This statistic emphasizes that current research on breast cancer is of utmost importance. Reportedly, incidences of breast cancer have increased to an extent that one new breast cancer is diagnosed every 18 s. Though the mortality rate of breast cancer has changed a little over the years, the survival rate, however, has also increased due to awareness campaigns, early detection programs, and continuous research to develop new drug molecules or new formulations for the treatment of the disease (Roy et al., 2022). Solid Cancers are characterized by the growth of abnormal tissue mass either as sarcomas or carcinomas. Whereas sarcomas arise from the embryonic mesoderm; carcinomas develop from the epithelium or the upper lining of the internal organs. Cancers originating mostly in the breast, stomach and lungs, are carcinomas.
The Breast is made up of glandular tissues which produce and ensure the passage of breast milk, stromal tissues made up of fatty and fibrous connective tissues acting as supporting tissues and lymphatic tissues connected to immune system which draws out the cellular fluids in the form of waste materials. Breast cancer can be classified in different types according to the sites and invasiveness. Most of the tumors in different parts of the breast develop as benign fibrocysts that becomes malignant when it starts to invade different tissues and spreads to the other organs. Breast cancer is characterized by the over-expression of hormone-specific receptors or epidermal growth factor receptors. Further, the classification of Breast cancer includes the consideration of the stage and grade of Breast cancer (Najafi et al., 2021). Considering the amount of ongoing research, there are a variety of drugs and treatments either available or coming into existence. Treatments including chemotherapy and personalized therapy take the help of established drugs including aromatase inhibitors (Miller, 2003), receptor modulators or degraders. The purpose of this article is to review the available information on all FDA-approved drugs for breast cancer with respect to chemical information, drug class, treatments, clinical pharmacology, dosage form, mechanism of action, clinical efficacy for breast cancer and the future direction of ongoing research. An effort has also been made in the review article wherein all registered drug molecules are presented and discussed. We believe that this exhaustive review will become the single stop for reference on all perspectives of an anticancer drug molecule that are present in the market and approved by the FDA to date for breast cancer management.
2 Breast cancer statistics
Cancer survival is typically described in terms of relative survival, which is a measure of life expectancy among cancer patients compared to that among the general population of the same age, race, and sex. The 5-year relative survival rate for all cancers combined has increased substantially since the early 1960s, from 39% to 68% among white people and from 27% to 63% among black people (U.S. Cancer Statistics Data, 2021). American Cancer Society (ACS) estimates the count of new cancer cases as well as deaths in the United States every year. The United States is expected to witness 1, 918, 030 new cancer cases and 6, 09, 360 cancer deaths in the year 2022-2023. As per the predicted statistical analysis of the data obtained, it has been concluded that the progress for disease occurrence and recovery has been stagnant for Prostate cancer in men and Breast cancer in females (Siegel et al., 2022).
The commonest malignancy among women globally is breast cancer and has even surpassed the lung cancer incidences which was supposed to be the most common cancer globally in 2020. Epidemiology estimates indicate that breast cancer will have a global burden of almost 2 million by the end of 2030 (Switon and Hill, 1977). In 2020, 2.3 million women were diagnosed with breast cancer accounting for about 6, 85, 000 deaths, globally (WHO, 2021). The statistics are alarming as it became even worse in 2022. In 2022–2023, the United States will witness invasive breast cancer that will be newly diagnosed in women and men to an estimated count of 287, 850 and 2, 710 respectively. Additionally, 51, 400 cases of ductal carcinoma in situ (DCIS) will be diagnosed in women. An estimated 43, 780 breast cancer deaths (i.e., 43, 250 in women, 530 in men) are likely to occur in 2022.
3 Breast cancer and treatment methodologies
Breast cancer may be classified as Ductal BC, Lobular BC or Connective tissue BC, depending on the type of cell involved. Some cancer cells have overexpressed receptors for few hormones, i.e., Estrogen, Progesteron or HER-2 gene (Orrantia-Borunda et al., 2022) whereas, others may have other molecular markers miRNAs (let-7, miR-155, miR-150, miR-153) and mutations (p53, BRCA 1 and 2 genes). This knowledge help doctors to design more personalized, targeted and effective therapy for the patient (Curigliano and Criscitiello, 2014; Nounou et al., 2015).
Breast Cancer is highly curable if it is detected at an early stage. The choice of medication varies with menopausal status. The nonmetastatic breast cancer has three treatment phases. The preoperative phase uses systemic endocrine or immunotherapies (ER, PR or ERBB2 positive cases). Preoperative chemotherapy may also be used and is the only option when tumors have none of the three receptors. The options for the surgical phase with similar survival rates includes; a lumpectomy with radiation or a mastectomy (Figure 1). The postoperative phase includes radiation, endocrine therapy, immunotherapy, and chemotherapy (Bequet-Romero, 2018; Bhushan et al., 2021; Trayes and Cokenakes, 2021; Cancer, 2023). The metastatic breast cancer cannot be cured but can be treated for longer life expectancy and quality of life. The biggest disadvantage of conventional chemotherapy is its unspecific toxicity, undesirable side effects, and low therapeutic indices with the development of drug resistance (Hart et al., 2021). Chemotherapy uses anti-cancer drugs that can be administered either orally or intravenously. The main function of the drug is to travel through the bloodstream to reach the cancer cells. Adjuvant chemotherapy is considered to ensure the killing of any cancer cell left or prevent the spread of remaining cells after surgery. Whereas, on the other hand, neoadjuvant therapy is to be applied to shrink the tumor to avoid less extensive surgery (Kerr et al., 2022). If after neoadjuvant chemotherapy, malignant tumor cells are still noticed after surgical removal of the cancer cells, more chemotherapy is needed to be offered to reduce cancer recurrence (Orrantia-Borunda et al., 2022).
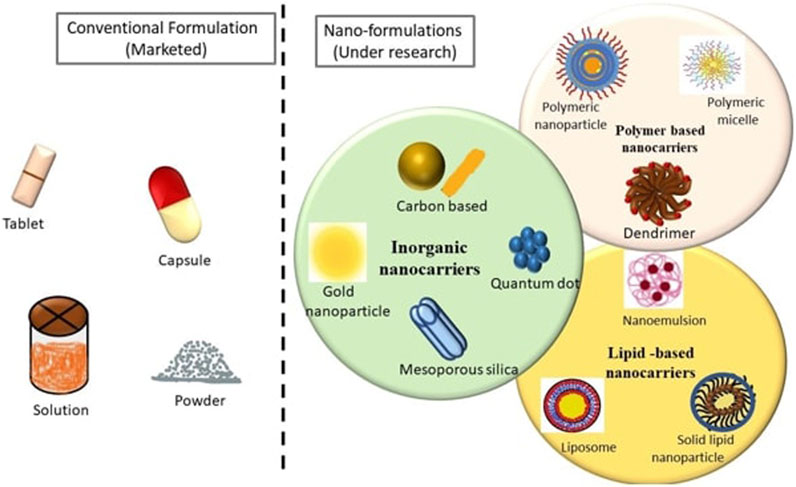
FIGURE 1. Pictorial representation of Conventional and Novel formulations of various FDA approved anticancer Drugs used for Breast Cancer Indications.
Cancer chemotherapeutic drug delivery through the oral route has always been challenging due to first-pass metabolism, gastrointestinal side effects, and low bioavailability, Conversely, however, the oral route is the most accepted route of administration due to patient compliance. There are wide varieties of dosage forms, drug carriers, and drug delivery systems for anticancer drugs, ranging from the conventional mode of treatment to novel drug delivery systems, arising as promising alternative options for breast cancer management.
The common dosage forms available in the market for oral administration include tablets, capsules, suspensions and others. Even when the medicines are not given through the oral route and given through the parenteral route, the formulations which are mostly available in the market are the ones that are considered conventional dosage forms such as solutions, dispersible powders and so on (Nounou et al., 2015). Tremendous research is going on for nanotechnology-based therapeutics in order to overcome the shortcomings of conventional dosage forms (Hartshorn et al., 2019; Orrantia-Borunda et al., 2022; DurgBank, 2023). Figure 2 depicts a pictorial representation of conventional and novel formulations.
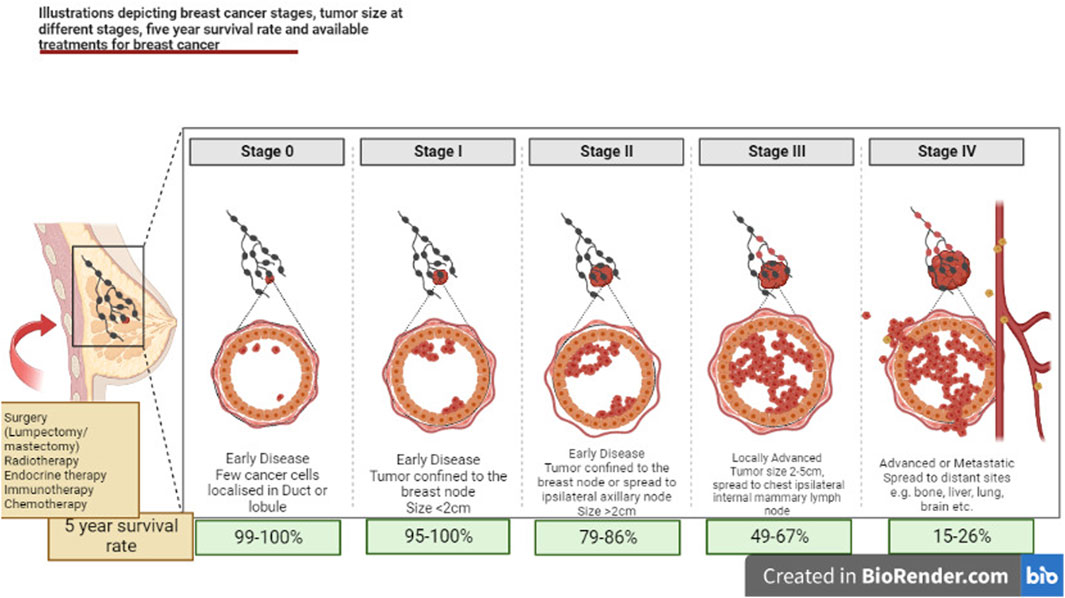
FIGURE 2. Illustrations depicting mechanism for various stages of breast cancer, tumor size at different stages, 5-year survival rate and available treatments for breast cancer.
Though most nanomedicines are the ones which are still in research or in the clinical trial phase and have not reached the market yet, the huge amount of data available proves that the extensive research in the field of nanomedicines will definitely bring about a revolutionary change in the treatment methodologies in near future.
3.1 Nano technology based-drug delivery systems in breast cancer therapy
Nanotechnology has brought about varieties of nanoformulation on the same platform, and which includes nanoparticles, liposomes, nanoemulsion, polymeric micelles dendrimers, etc. Nano formulations are the most emerging drug carriers with promising potential, i.e., controlled release effect of the drug (Byrne et al., 2008; Zhao et al., 2010; Xie et al., 2016), targeting of the pharmaceutical moiety to the desired site (Nobs et al., 2004), bioavailability enhancement (Ahmad et al., 2019) of the therapeutic agent and enhancement of circulation time being the few notable examples. A suitably designed nanoformulation may possess any one or combination of these abilities, though it is also important to study the nature of the drug which will facilitate the synthesis/formation process. A perfectly designed novel drug delivery system can do wonders in treatment by altering the kinetics of the drug in the desired manner, and imparting plenty of advantages to the drug delivery when compared to those delivered through conventional dosage forms. Listed below is some information about the nanotechnology-based formulation which are either in the market or under research for the purpose of breast cancer management (Hussain et al., 2018; Du et al., 2019; Park et al., 2022; Cancer, 2023).
Nano emulsions are thermodynamically stable mixture of oil and water stabilized with appropriate mixture of surfactants and co-surfactants. These are classified as colloidal system in the submicron size range that serve as carriers for both hydrophilic and hydrophobic drug molecules. The droplet size varies from 10 to 1,000 nm (Hart et al., 2021)., mostly carrying a negative charge, except where the formulation is intentionally designed to be positively charged for specific reasons (Kerr et al., 2022). The droplet’s core consists of either water or oil, which gives it the property to act as a super-solvent for molecules that are hydrophobic and hydrophilic. These may be prepared by the high-pressure homogenization, microfuildization as well as ultrasonication (Kumar et al., 2019). The Lipid Nano Emulsions are prepared by the combination of oil and phospholipids with several benefits including high drug loading capacity, long-term stability, reduced irritability or toxicity of the incorporated drugs, reduced drug hydrolysis and no precipitation during administration. The submicron sized droplets of the Nanoemulsion promotes deposition and penetration of the active pharmaceutical ingredient to the target (Talegaonkar and Negi, 2015; Mahato, 2017; Burotto et al., 2019; Hart et al., 2021).
Nanoemulsion has been greatly exploited in drug delivery to breast cancer tumour (Nobs et al., 2004; Ganta et al., 2014). It exhibits better skin penetration due to low surface tension and the large surface area of the emulsion system (Ahmad et al., 2019). In addition, they may be considered as a substitute for liposomes and other vesicles as well (Park et al., 2022). Nanoemulsion are now-a-days considered as one of the promising formulations to achieve safe and effective cancer treatment (Verma et al., 2016; Nirmala et al., 2022). These formulations not only solve the problems related to water solubility issues (Verma et al., 2016; Barradas and de Holanda e Silva, 2021), but also can be customized for targeted cancer treatment (Song et al., 2020; Kaur et al., 2022).
The research in cancer therapy has become more focused on nanoemulsion due to its superficial charge (Migotto et al., 2018; Elena et al., 2019), enhanced half-life in blood circulation (Song et al., 2020; Kashyap et al., 2021), and large surface area (Yin et al., 2009; Gupta et al., 2016). Above all, nanoemulsion can easily accumulate on cancer tissue proving it to be one of the research turning points in cancer treatment through nanotherapeutics (Hussain et al., 2018).
Colloidal Dispersion at submicron range stabilized by surfactants is known as nanosuspensions. Nanosuspension can disperse hydrophilic drugs without any matrix suspended in dispersion enhancing the solubility of drugs. This approach of developing a nanosuspension as the formulation is useful for both poorly permeable and poorly soluble drugs. Above all, this formulation also renders dose reduction as well as enhancement of the physical and chemical stability of the drugs (Singh et al., 2017; Du et al., 2019; Pandey et al., 2020). Reports have proved that nanosuspension is also a well-researched formulation which can be convincingly used for the treatment of different malignant cells, i.e., Glioma and breast cancer respectively.
Liposome is a spherical bi-layered phospholipoidal nano-formulation having the property to encapsulate the fraction of solvent inside the core in which the solvent can easily float or diffuse.
Exclusively, liposome has the ability to carry both hydrophilic as well as lipophilic drug. The hydrophilic drugs are encapsulated in the internal aqueous core whereas; the lipophilic or hydrophobic drugs get embedded in the phospholipid bilayers. Simply by modifying the bi-layered composition of liposomes, the pharmacokinetics and in-vivo biocompatibility of the drugs can be improved. Yet another way of improving the liposomal formulation is by incorporation of PEG for enhancing the retention time of formulation in the systemic circulation. (Hofheinz et al., 2005; Kumar et al., 2019). This variant of the drug delivery system can enhance the duration of action by enhancing the circulation time of the drug for a prolonged period (Talegaonkar and Negi, 2015; Mahato, 2017). Pegylated liposomal Doxorubicin has been used as both combination chemotherapeutics and nanotherapeutics for efficient drug delivery in breast cancer management.
Liposomes are also effective in masking the unwanted toxic effects of various drugs as in the case of anthracyclines. Encapsulation in Liposome has promoted efficient drug cardiotoxicity and prolonged circulation time for effective drug delivery (Ganta et al., 2014).
The nanonization of drugs is getting considered due to a number of focal points which revolve around the conditions like dose reduction, improvisation of solubility, and enhancement of absorbance contrast with the crude form of the drug. The nanoparticles, after optimization usually have a size range of 10–100 nm (Ashfaq et al., 2017; Gavas et al., 2021). Those lying between this size range are considered to be most appropriate for cancer treatment (Smith et al., 2012; Jaiswal and Dudhe, 2015; Han et al., 2021). Nanoparticles can be classified into various types including metallic nanoparticles (Subhan, 2022), polymeric nanoparticles (Sartaj et al., 2021), solid lipid nanoparticles (Ashtari et al., 2020; Ozgenc et al., 2022), and fullerenes. The characterization of nanoparticles for drug release and drug targeting primarily depends on the evaluation of particle size and morphology (Song et al., 2020). Functionalized nanoparticles with ability of targeting complementary receptors are considered very effective in reducing side effects and enhancing efficacy of treatment (Gupta et al., 2016; Migotto et al., 2018; Elena et al., 2019; Kashyap et al., 2021; Kaur et al., 2022).
Nanogel is a modern nano-formulation where the nanoparticle is composed of hydrogel with an extremely crosslinked hydrophilic polymer. Nanogels are also defined as particles of the nanosize range formed by chemically or physically crosslinked networks of polymer that swells in a good solvent (Karthick et al., 2019). Nanogel systems have proved their efficacy in delivering drugs sustainably and in a targetable manner (Ahmad et al., 2019). Luo et al. (2020a) have developed gum Arabic aldehyde gelatin nanogels loaded with Curcumin for the treatment of breast cancer. The nanogel has been reported to improve the bioavailability and therapeutic efficacy of Curcumin in Breast cancer treatment (Luo et al., 2020a). There have been many other research studies that have proved that the use and acceptance of nanogel as a novel nanocarriers for the treatment of breast cancer among the scientific society of researchers (Park, 2002; Gavas et al., 2021). In-situ nanogel has opened a new avenue for long term sustained delivery of anticancer agents into the vicinity of breast tumor (Smith et al., 2012).
Small spherical micro particles, usually made up of biocompatible and biodegradable polymers having a size range of 1–1000 μm have the ability to encapsulate drugs in order to provide stability and enhance the therapeutic sustainability of the drug. Administration of medication through microencapsulation can be highly advantageous in various situations where the drug can be both ingested and injected depending on the need and desire (Subhan, 2022).
A quercetin-loaded PLGA microsphere are developed for the treatment of breast cancer. Similarly, there is a lot of research being conducted where microspheres have also been considered as one of the emerging possible carriers for drugs that can be used for the treatment of breast cancer (Sartaj et al., 2021).
Polymeric micelles have achieved noticeable results in the last few decades as a multifunctional nanotechnology-based delivery system for poorly water-soluble drugs. Hydrophobic part of the polymer forms the core of the polymeric micelle and the hydrophilic part of the polymer forms the corona. As a result the advantages of polymeric micelles as a delivery vehicle are two fold wherein, the hydrophobic core of the micelle assists in solubilization of poorly soluble drugs and the hydrophilic shell provides some protection in minimizing opsonin adsorption, longer blood circulation time to polymeric micelles and better blood stability. Owing to their smaller size polymeric micelles get concealed from scavenging by the mononuclear phagocytic system in the liver and circumvent the filtration of inter-endothelial cells in the spleen, contributing towards longer blood circulation time (Bhatia, 2016; Migotto et al., 2018; Ozgenc et al., 2022).
In addition, dendrimers, lipid nanoparticles (Ashtari et al., 2020), protein nanoparticles (Lin et al., 2009), ceramic nanoparticles, viral nanoparticles, metallic nanoparticles and carbon nanotubes are few more novel drug delivery systems which have been used in cancer therapeutics (Dhman, 2017). The Nanoparticles can be modified/functionalized in many ways to enhance drug localization, reduce opsonization, enhance circulation, enhance stability and bioavailability, reduce toxicity, increase drug efficacy and potentially decrease chances of multidrug resistance (Sarika and Nirmala, 2016; Liu et al., 2020).
3.2 US-FDA-approved drugs available in the market for the treatment of breast cancer to date
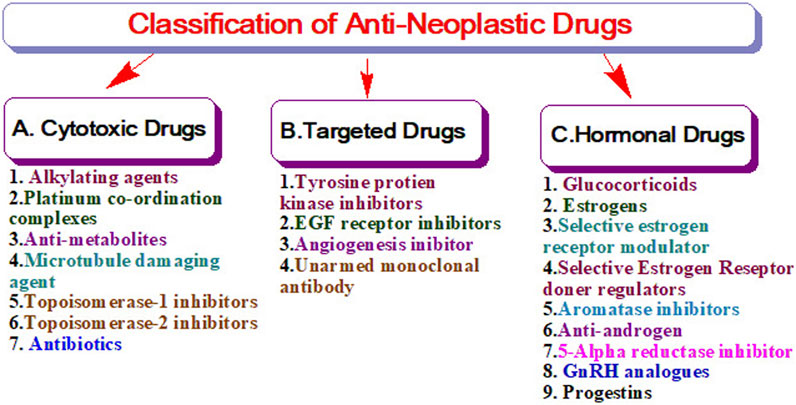
The United States Food and Drug Administration (United States FDA) is a federal agency of the Department of Health and Human Services that regulates the approval process of drugs for various indications in humans and animals, along with various other products. Out of total 207 drugs which have been approved by FDA for Oncology, 39 are specifically approved for either a single or adjuvant treatment of breast cancer. In last 70 years FDA has approved more drugs for breast cancer indications than any other type of cancer (Sarika and Nirmala, 2016). The Figure 3 shows relative fraction of anticancer drugs from various categories which are approved for treatment of breast cancer. Major percentage of these drugs were approved for metastatic breast cancer at first. Later, approximately 31% of drugs received additional adjuvant status. According to a across sectional study published in year 2022 in JAMA Network Open Oncology, 19 drugs have been approved by USFDA between 31 May 2016 to 31 May 2021. However, most of these are for second, third and later line settings (Attama et al., 2022). This section summarizes all the listed and available drugs in the market being used for breast cancer. All the US-FDA-approved anti-neoplastic drugs have been discussed following the classification given in Figure 4. It contains information about the registered molecules, with emphasis on the date of their original approval, molecular targets, formulations and routes of administration and their indications in various forms of breast cancer.
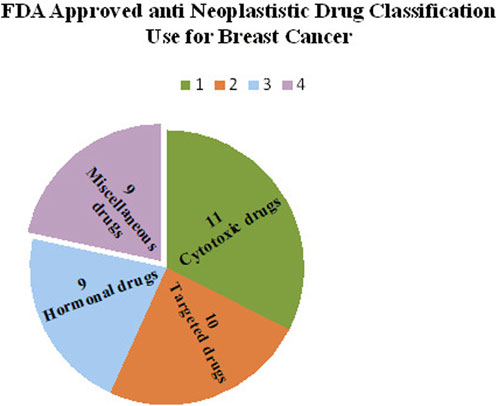
FIGURE 3. Relative Fractions of Drug classes approved by US-FDA for Different Breast cancer Indications.
3.2.1 Cytotoxic drugs
The cytotoxic group of anti-neoplastic drugs consists of both cell cycle specific and non-specific agents which actively act against various cell cycle phases and hinders the growth of cells. These drugs mainly target the metabolic steps of cell division, so as to promote cell apoptosis. The cell cycle non-specific agents are not dependent on a particular phase of the cell cycle rather, it helps in hindering the cellular activity in all phases of mainly slow-growing tumors (Kircik, 2011). As depicted in Figure 4, these drugs are further sub-classified as nitrogen mustards, nitrosoureas and others. The upcoming sections summarize the details of all the drugs considered under the category of cytotoxic drugs used for breast cancer treatment.
3.2.1.1 Cyclophosphamide
Cyclophosphamide is a nitrogen mustard alkylating agent which slows down the cancer cell growth by interfering with functioning of DNA. It was first approved in 1959 for use in malignant diseases including breast carcinoma (Cho and Moniri, 2016; Mirkes, 1985). Cyclophosphamide is a part of either combination or single treatment regime for breast cancer, administered either orally or through infusion, depending upon the need. It has the property of being inactive until metabolized by the liver. The active compounds Acrolein and Phosphoramide, slow cancer cell growth by binding and interfering with the actions of Deoxyribonucleic acid (DNA) within cancerous cells (Singh et al., 2017). Cyclophosphamide is not only available in the form of injection (like intramuscular injection, intra peritoneal injection, intra pleural injection) but also in the form of tablets where the active ingredient is present at a quantity of 25 mg and 50 mg for oral administration (Lin et al., 2009). Cyclophosphamide with methotrexate and fluorouracil has been considered an adjuvant chemotherapeutic regime for breast cancer management (Press, 2012). Being teratogenic in nature it is contraindicated in pregnant women (Yao et al., 2020; Benjamin et al., 2022). Adverse reactions viz. Bone marrow suppression, alopecia, nausea, and hemorrhagic cystitis to cyclophosphamide are due to its cumulative administration. It should be cautiously administered to patients with heart, kidney, and pulmonary diseases. Cyclophosphamide (Cyp) is used in high doses and in combination with other drugs (Pharmacology, 2021). In an attempt to reduce the off-target effects and enhance the therapeutic efficacy (Rao et al., 2016; Tiash and Chowdhury, 2016), Snigdha Tiash and Md Ezharul et al. (2016) prepared pH-sensitive carbonate apatite nanoparticles. The anticancer capacity of Cyclophosphamide is enhanced upon its encapsulation within citric acid dendrimers. Citric acid dendrimers impart solubility and specificity to the drug (Burn, 1961).
3.2.1.2 Tepadina
Thiotepa also known as Tepadina is an organophosphorus alkylating antineoplastic agent approved in 1959 was used to treat a variety of solid and hematologic malignancies. This drug also carries a US Food and Drug Administration (FDA) indication for the treatment of breast adenocarcinoma in the recommended dose of 0.3–0.4 mg/kg intravenously at 1–4 weeks intervals (FDA, 2012c).
This chemical entity causes interference in DNA replication as well as cell division by the formation of cross-linkages between alkylated guanine bases resulting in apoptosis and cell growth inhibition in tumor cells (Zhang et al., 2003). The most common adverse reactions with greater than 10% incidences are neutropenia, anemia, thrombocytopenia, elevated alanine aminotransferase, elevated aspartate aminotransferase, elevated bilirubin, mucositis, cytomegalovirus infection, hemorrhage, diarrhea, hematuria, and rash (Comte et al., 2013). It should be used cautiously with pregnant women and patients with hepatic and renal impairment. Its commercially available as a single dose, lyophilized white powder for reconstitution (FDA, 2012c; FDA, 1959). Novel formulation, i.e., gelatin microparticles with a diameter of approximately 2 μm, and loaded with Thiotepa were prepared through a chilled dehydration procedure. The size of formulation at least doubles the AUC, suggesting that the dose might be halved, thereby reducing side effects associated with this otherwise important drug. Recently, an Iron-Doped Fullerene Cage of Thiotepa has been prepared by Xuan Young to modify drug delivery (Alemrayat et al., 2018; Pandey et al., 2020). C3 N nanotubes (Ge et al., 2012; Torabifard and Fattahi, 2012; Fox et al., 2021). Loaded with Thiotepa has been tried in combination with ifosfamide, etoposide, and rituximab (TIER) for the treatment of PCNSL relapsed or refractory to high-dose methotrexate-based chemotherapy (Kumar et al., 2019).
3.2.1.3 Methotrexate Sodium
Methotrexate is highly exploited antimetabolite antineoplastic agent which acts as a stoichiometric inhibitor of dihydrofolate reductase. It is a structural analog for folic acid due to which it can be used to target the over-expressed folate receptors on the tumor cells. It was approved for breast cancer indication by FDA in 1959. Apart from breast cancer, Methotrexate Sodium is also effective against head and neck cancer, leukemias, lymphomas and carcinomas (Syn et al., 2017). Reditrex, an injection for subcutaneous administration was the first approved formulation which is marketed as prefilled syringes in strengths ranging from 7.5 mg to 25 mg. Patients on MTX therapy should be closely monitored for liver, lung, skin, and kidney toxicities and bone marrow suppression (Mirkes, 1985; Li et al., 2012). Various MTX-loaded nanocarriers have been reported with significance in the treatment of different cancers types including breast cancer. Lipid nanoemulsion (Talegaonkar and Negi, 2015), Nanogel (Mahato, 2017), and (Ganta et al., 2014), hydrogel (Han et al., 2021). Solid lipid nanoparticles (Vuong et al., 2022). Nanosuspension (Accessdata, 2019; Li et al., 2022), niosomes (Moura et al., 2011; Avasatthi et al., 2016), liposomes (Kumar et al., 2004; Agrawal et al., 2020), etc. are few extensively exploited nano-systems. MTX in the nano-formulations indicated high mean residence time in blood circulation that helps them to accumulate at the desired targeted sites.
3.2.1.4 5-Flourouracil
5-Fluoro-1, 3-Diazinane-2, 4-Dione or 5-Flourouracil is the most potent and successful first-line medication treatment for cancer. It is thought to be formed by the covalent binding of the drug, deoxyribonucleotide (FdUMP) and the folate cofactor, N5-10- methylenetetrahydrofolate, to thymidylate synthase (TS) (Battaglia et al., 2017). This inhibits the formation of Thymidylate from Uracil, resulting in the inhibition of DNA and RNA synthesis and causing Thymine less cell death. Fluorouracil can also be incorporated into RNA in place of Uridine Triphosphate (UTP), resulting in a bogus RNA and interfering with RNA processing and protein synthesis (Li et al., 2019). 5-Fluorouracil is widely used for colorectal cancer and was later approved for breast cancer treatment as well. It has been reported to cause fogging and memory impairement, when used for long term in breast cancer (Powar et al., 2021). Similar to Methotrexate nearly all types of novel formulations have been researched for 5-FU. Few recently reported nano-systems are; 5-Fluorouracil formulation in Nanoporous Biogenic Mg-calcite from Blue Crab Shells (Trotta et al., 2004; Horo et al., 2019; Lazar et al., 2021), a photo-responsive chitosan conjugated pro-drug nano-carrier for controlled delivery (Horo et al., 2019), Folate-tagged chitosan-functionalized gold nanoparticles (Longley et al., 2003; Akinyelu and Singh, 2019), 5- fluorouracil-loaded calcium phosphate nanoparticles (Bhadra et al., 2003), Folic acid-navigated and β-cyclodextrin-decorated carbon-encapsulated iron nanoparticles (Wigmore et al., 2010; Kasprzak et al., 2018), folate receptor targeted nanoliposomes (Handali et al., 2019), controlled release PVC/PEG polymeric films, amine functionalised hollow mesoporous silica nanoparticles (HMSN-NH2) and then coated with a biocompatible polydopamine (PDA) (Cheralayikkal et al., 2022), multiple-nano-emulsion etc (Bhadra et al., 2003).
3.2.1.5 Capecitabine
Capecitabine is an oral fluoropyrimidine which is preferentially converted to fluorouracil in tumor tissues in a three-step enzymatic cascade. The final stage of conversion to fluorouracil is catalyzed by thymidine phosphorylase, which is appreciably more active in tumor than in healthy tissues (Radwan et al., 2012), It is a common treatment medicine for HER-2 positive Breast cancer. It is presently the only active treatment regime specifically approved for those cancer patients where the tumor is absolutely resistant to any other treatment regime including Paclitaxel and Anthracyclines. It has been reported that on exposure to Capecitabine cancer cells undergoes apoptosis and cell death (Summary, 2005; Cheralayikkal et al., 2022).
3.2.1.6 Vinorelbine Bitartrate
Vinorelbine Bitartrate is a semi-synthetic derivative of Vinca alkaloid present in the dried leaves of vinca rosea. It is mainly characterized as poisons for spindle formation, hence, known as mitotic spindle poison. It has been reported to interfere in the polymerization of tubulin protein which is involved in the process of cell division. (Ahmad et al., 2022). Vinorelbine has been widely accepted to treat solid tumors, such as non-small cell lung cancer. It was approved for breast cancer indication in 1990 (Radwan et al., 2012). There have been studies which has tested the efficacy of Vinorelbine alone, or in combination with other drugs so as to develop a therapy that is non-cross resistant with Taxanes and Anthracyclines. Vinorelbine alone or in combination is an effective and safe treatment for pre-treated locally advanced, metastatic, or secondary breast cancer patients. When combined with BET inhibitors, metastatic breast cancer in the brain become more sensitive to Vinorelbine (Summary, 2005). with cyclophosphamide, Vinorelbine activate Stem-Like CD8 + T Cells and Improve Anti-PD-1 Efficacy in Triple-Negative Breast Cancer (Walko and Lindley, 2005; Petrelli et al., 2016). It is available in the market as injectable and oral formulations. Few novel formulations, i.e., liposome (Marcucci and María, 2004), transdermal based hydrogel formulations (Fonseca et al., 2021), lipid-based nano-formulation (Cybulska-Stopa et al., 2013; Kanojia et al., 2020). Have also been researched. However, injection is still the only commercially available form.
3.2.1.7 Docetaxel
Docetaxel is the first line of chemotherapeutic among Taxane group of drugs which was approved by the US-FDA in 1996 for the treatment of breast cancer (Falvo et al., 2021). Used at the dosage of 60 mg/m2 to 100 mg/m2 as a single agent and in 75 mg/m2 in combination with Doxorubicin and Cyclophosphamide. Adjustment of dosage is decided by the appearance of the side effects, i.e., febrile neutropenia, neutrophils and lt; 500 cells/mm 3 for more than 1 week, or severe or cumulative cutaneous reactions. It is strictly prohibited for the patient with impaired liver functions and reported hypersensitivity with DTX or polysorbate formulations. Patients who are recommended DTX therapy are pretreated with oral corticosteroid (e.g., Dexamethasone, 16 mg/day) for 3 days to reduce fluid retentions (Falvo et al., 2021). Docetaxel binds to the tubulin-subunit. Tubulin is a building block of microtubules, and Docetaxel binding secures these building blocks. The resulting microtubule/Docetaxel complex is unable to disassemble. This has a negative impact on cell function because the dynamic instability of microtubules is required for their function as a transportation highway for the cell (Marcucci and María, 2004; Ahmad et al., 2014). The biggest disadvantage of Docetaxel is that it leads to chemotherapeutic resistance which can even result in the relapsing of tumors. Challenges with administration during mixing of the docetaxel with the diluent and need of targeting necessitated remodeling of the currently available docetaxel formulation but none has made it to clinical setting as an alternative. A recently published review by Karamot and Oyediran summarized virtually all the novel formulations researched on Docetexal (Fonseca et al., 2021).
3.2.1.8 Doxorubicin Hydrochloride
Doxorubicin Hydrochloride is the cytotoxic antibiotic derivative of Anthracycline obtained from Streptomyces Peucetius micro-organism. (Drug bank, 2005; Bahadori et al., 2014). Its primary mechanism is to intercalate within DNA base pairs by inhibiting the Topoisomerase II enzyme, causing breakage of DNA strands and inhibition of both DNA and RNA synthesis. Cell treated with DOX manifests many morphological changes which leads to apoptosis and is thought to be responsible for therapeutic use. When combined with iron, Doxorubicin also causes free radical-mediated oxidative damage to DNA, further limiting DNA synthesis. Currently, Doxorubicin is used for the treatment of metastatic and orthotropic breast cancer (Luo et al., 2020b). In practice, it is administered in a single dose of 60–75 mg/m2 in intervals of 21 days as i.v. injection which may be reduced to 40–60 mg/m2 when given as a combination. Administration should be slow and in the large vein with saline running in the tubing. Injection should be immediately stopped if any extravasation is suspected (Hanna et al., 2014; Bland et al., 2019). It should not be co-administered with any cardiotoxic drug, and in case of concomitant administration, elimination of previously administered dug is to be ensured. Lipodox, Evaset, Doxil/Caelyx, Myocet are Liposomal formulations of Doxorubicin Hydrochloride. These formulations had market of 1 billion USD in 2020 and is forecasted to have increased to 1.39 billion USD by 2025. The novel formulations are still being tried for DOX. Martina Di Francesco prepared Doxorubicin Hydrochloride-Loaded Nonionic Surfactant Vesicles to Treat Metastatic and Non-Metastatic Breast Cancer (Di Francesco et al., 2021).
3.2.1.9 Paclitaxel
Paclitaxel is a natural product with antitumor activity. TAXOL (paclitaxel) is obtained via a semi-synthetic process from Taxus Baccata. Paclitaxel is a white to off-white crystalline powder that is highly lipophilic, insoluble in water, and melts at around 216–217°C (Oyediran et al., 2022).it is widely used clinically for the treatment of various types of tumors such as breast (Patel, 1996)., pancreatic, cervical, ovarian, etc. Paclitaxel has been utilized in combination therapy with other drugs, or it is also considered as a first line. Paclitaxel emerged as an important agent for breast cancer treatment because of its lack to Anthracycline cross resistance and tolerability towards clinical investigation, though later Paclitaxel was reported with several adverse effects like cardiovascular toxicity, undesirable gastrointestinal effects, bone marrow suppression, etc (Di Francesco et al., 2021). It is marketed as multidose vial packaged in an individual carton. Various strengths available are NDC 0015-3475-30 30 mg/5 mL, NDC 0015-3476-30 100 mg/16.7 mL, and NDC 0015-3479-11 300 mg/50 mL diluted to a final concentration of 0.3–1.2 mg/mL, prior to administration. The treatment may cause Anaphylaxis and severe hypersensitivity reactions. Hence, it should be strictly monitored by experts. Nab-Paclitaxel Nab-Paclitaxel is basically nanoparticle albumin-bound paclitaxel which is a novel marketed nano-formulation showing better anti-neoplastic activity with less toxic effect than the normal solvent-based paclitaxel (Hanna et al., 2014; Alves et al., 2017). This drug is marketed as a 100 mg injection in the name of Abraxane. Lipusu™ and Genexol are other marketed forms of PTX in non-conventional forms (Du et al., 2018). Various reviews published from time to time have listed various novel formulations which have been prepared for Paclitexal to overcome the limitations with present therapy (Marupudi et al., 2007; Yamamoto et al., 2011; Chatterjee et al., 2017).
3.2.1.10 Eribulin Mesylate
Eribulin Mesylate was approved in 2010 by US FDA for metastatic breast cancer (Singla et al., 2002). A patient who received at least 2 chemotherapeutic regimens for metastatic cancer (Of and Information, 2010). (Prior therapy should have included an anthracycline and a Taxane) (Mcbride and Butler, 2012) was treated with an injection of Eribulin Mesylate that is administered as 1.4 mg/min intravenously over 2–5 min on Days 1 and 8 of a 21-day cycle. FDA Reference ID: 2863825. Eribulin inhibits microtubule growth without affecting shortening and sequesters tubulin into nonproductive aggregates. Eribulin acts through a tubulin-based antimitotic mechanism, causing G2/M cell-cycle blockage, mitotic spindle disruption and, eventually, apoptotic cell death after prolonged mitotic blockage (Jain and Vahdat, 2011). Importantly, Eribulin also had an acceptable toxicity profile and therapeutic window in mice across several dosing schedules (Dybdal-Hargreaves et al., 2015).
3.2.1.11 Epirubicin
Epirubicin is the epi-isomer of the anthracycline antibiotic named Doxorubicin (Markes et al., 2006). It is basically a semi-synthetic derivative of Doxorubicin which was first approved in France in 1982. It is used extensively for the management of breast cancer as adjuvant therapy for early breast cancer patients. (Levine, 2000). Epirubicin intercalates into DNA by topoisomerase II inhibition. This ultimately leads to oxygen generation and interferes in the protein synthesis of tumour cells hence the cell growth is stopped. This agent also produces toxic free-radical intermediates and interacts with cell membrane lipids causing lipid peroxidation (Ormrod et al., 1999). It is recommended in doses of 100–120 mg/m2 administered as an intravenous bolus. Administration is recommended in two types of regimens with 5-Fluorouracil and Cyclophosphamide. Therapeutic doses of Epirubicin may cause tissue necrosis, cardiac toxicity, secondary acute myelogenous leukemia, and myelosuppression (Conte et al., 2000). Description. However, in clinical trials, lesser non-hematologic and cardiac toxicities are reported for equimolar doses of Epirubicin than Doxorubicin (Epirubicin, 1992; FDA, 1999). Biocompatible Polymer PLA–PEG–PLA Nanoparticles (Massadeh et al., 2021; Sheydaei, 2021), polymeric nanoparticles (Torchilin, 2007), Polymeric miscelles (FDA, 1998), long-circulating thermo sensitive liposomes are few prominent newer efforts made for better delivery profile of Epirubicin.
3.2.2 Targeted drugs
Targeted drugs mainly work by selectively interacting with the protein receptors that control the growth, differentiation, and migration of cancerous cells or reactivating Programmed cell death which is otherwise compromised in some types of Breast Cancers. Targeted anti-neoplastic drugs are considered to be the foundation for the future of precise oncology. As they are thought to be devoid of unintended side effects on healthy cells, which otherwise is the case with virtually all other classes of anticancer drugs. Most of the targeted drug molecules are either small molecule drugs or monoclonal antibodies. The biggest disadvantage of targeted therapies is the development of drug resistance. The resistance can happen due to the morphological or physiological change in the receptors after being exposed to the same chemical moiety for a longer period of time. As depicted in Figure 5, these drugs are sub-classified as monoclonal antibodies, tyrosine kinase inhibitors as mentioned in other classes.
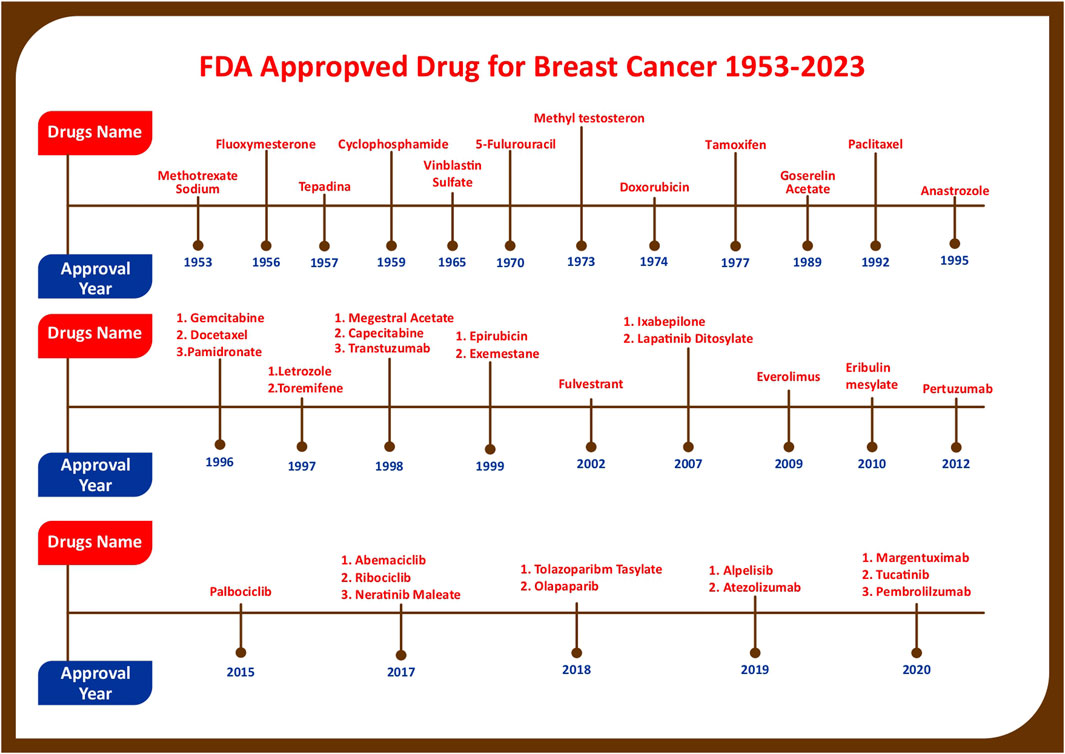
FIGURE 5. Figure showing progress in breast cancer treatment research as year wise account of anticancer drugs which are approved for various Breast Cancer Indications by US-FDA.
3.2.2.1 Lapatinib ditasylate
Lapatinib is a selective and potent reversible Tyrosine Kinase Inhibitor that is considered to inhibit human epidermal growth factor receptors (HER-2) overexpressed in breast cancer cells (Soiza et al., 2018). It targets the intracellular ATP- binding sites of the HER-2 receptors, binds competitively, and hinders cell growth. Lapatinib is administered as a tablet in combination with Capecitabine in patients who have received prior therapy including an Anthracycline (Shantanam, 2018). It can be moderately well tolerated when administered orally once every day in daily doses of 1,250 mg–1,500 mg for 1–21 days. Gastrointestinal complications and skin rashes are the main toxicities observed in more than 20% of patients consuming Lapatinib (Yuan and Xu, 2021). It has been reported that Lapatinib is well accepted in patients who are resistant to Trastuzumab. It has shown improvement in the overall survival period of breast cancer patients (Alemrayat et al., 2019). Clinical use is limited due to its poor aqueous solubility, poor bioavailability, high binding affinity toward blood proteins, and toxicities related to its higher dose, and stability. Various nano-delivery systems, including nanoparticles, polymeric micelle, core-shell nanoparticles, nanochannel, were investigated to overcome these issues (Emens et al., 2021).
3.2.2.2 Transtuzumab
Transtuzumab was the first Monoclonal Antibody approved by US-FDA in 1998 for the treatment of HER2 overexpressing breast cancer and the treatment of HER2-overexpressing metastatic gastric or gastroesophageal junction adenocarcinomas (Guerreiro et al., 2016; Yassemi et al., 2020). Transtuzumab selectively binds with overexpressed HER-2 receptors, inducing an immune-mediated response that causes internalization and recycling of HER-2. It may also upregulate cell cycle inhibitors such as p21 and p27 (Murthy et al., 2020; Khan et al., 2022). It is marketed under the brand name HERCEPTIN® as IV infusion and is administered in two types of regimens, weekly and three weekly regimens and is also on the list of essential drugs by WHO. Though there are several concerns related to cardiotoxicity and the development of resistance that remain in consideration, besides several other therapeutic issues also remain unclear and have been addressed in an inconsistent way. The main reason behind this is that there is still a lot of information to be documented in the scientific literature on pharmaco-dynamics, pharmaco-kinetics, and clinical use of the drug (Kelly and Buzdar, 2010). Transtuzumab is considered to be the golden standard of treatment for this sub-type of breast cancer. Research work is being done on use of trastuzumab in novel formulations (Barradas and de Holanda e Silva, 2021).
3.2.2.3 Margetuximab
US-FDA approved Margetuximab in the year 2020 as a combination chemotherapeutic for use in adult patients suffering from metastatic HER-2 positive breast cancer. This is recommended for only those patients who have already received two anti-HER 2 treatment regimens prior to this (Goss and Tye, 1997). The Margetuximab is a chimeric antibody that binds to the extracellular domain of the human epidermal growth factor receptor 2 protein (HER2) (Markham, 2021; Rugo et al., 2021), inhibits tumor cell proliferation, reduces shedding of the HER2 extracellular domain, and mediates antibody-dependent cellular cytotoxicity (ADCC). Margetuximab shares ERBB2 specificity with Trastuzumab but incorporates an engineered Fc region for increased binding to activating Fcγ receptor IIIA (CD16A) and decreased binding to inhibitory Fcγ receptor IIB (CD32B) relative to Trastuzumab with the aim of improving response rates (Barros-Oliveira et al., 2017). From Phase 3 Randomized Clinical Trial Hope S et al. concluded that Margetuximab plus chemotherapy had an acceptable safety and a statistically significant improvement in PFS compared with Trastuzumab plus single agent chemotherapy in ERBB2-positive ABC after progression on 2 or more prior anti-ERBB2 therapies (Sanford and Plosker, 2008). Unlike other monoclonal antibodies used for treatment of breast cancer, Margetuximab is administered through IV route and is available as 250 mg/10 mL single dose vial.
3.2.2.4 Atezolizumab
Atezolizumab is a humanized, Fc optimized, monoclonal antibody. It has received accelerated approval in March 2019 for Triple-Negative Breast Cancer that has spread or cannot be removed by surgery and tests positive for “PD-L1” (Alyafee et al., 2018; Aleem and Shah, 2023). Atezolizumab kills the cancer cells by blocking the interaction of PD-L1 receptors with PD-1. Hence, preventing the blocking of inhibitory signals to killer cell activation and reactivating programmed cell death (Structures et al., 2023). It is used in combination with the Abraxane (not with Paclitexal). It is manufactured and marketed by Genentech in United States under the name of Tecentriq. It has, however, been reported that in October 2021 the company has withdrawn Tecentriq voluntarily from patients who were under its treatment regimen in the United States, though the withdrawal does not affect the approval for other countries where this drug is used for the treatment of metastatic PD-L1 positive of triple-negative breast cancer (Untch and Jackisch, 2008). The patient may suffer Immune-Mediated Adverse Reactions along with common adverse reactions which may prove fatal (Jayapal and Dhanaraj, 2017).
3.2.3 Tyrosine-protein kinase inhibitors
Until recently, the mainstay of treatment in the majority of hormone receptor (HR)-positive, human epidermal growth factor 2 receptor (HER2)-negative advanced breast cancer (ABC) consisted of single-agent endocrine therapy (ET). However, as the understanding of endocrine resistance has grown, newer targeted agents have come to the fore (Drugbank, 2023).
3.2.3.1 Abemaciclib
Abemaciclib is an anticancer moiety marketed as Verzenio among many others. It has received initial approval in 2017 by US-FDA for the adjuvant treatment of adult patients with HR-positive, HER-negative, and node-positive early Breast Cancer in combination with endocrine therapy (Tamoxifen or an aromatase inhibitor). With aromatase inhibitors and Fulvestrant Abemaciclib is recommended for HR-positive, HER-negative metastatic (McCartney et al., 2018), and advanced breast cancer. Abemaciclib inhibits CDK4 and CDK6 causing inhibition of phosphorylation of the retinoblastoma protein (Rb), and cell cycle progression from G1 to S, and cell proliferation. Ultimately cell death by apoptosis. Currently, it is available as an oral tablet formulation in two strengths 150 mg, and 200 mg.
3.2.3.2 Alpelisib
Phosphoinositol-3- Kinase (PIK-3) is a group of enzymes that are involved in cell growth, cell differentiation, and proliferation. Activation of the Phosphatidylinositol-3-Kinase (PI3K) pathway via PIK3CA mutations occurs in 28%–46% of hormone receptor-positive (HR+), human epidermal growth factor receptor-2-negative (HER2-) advanced breast cancers (ABCs) and is associated with poor prognosis (Cheer et al., 2005; Sullivan et al., 2022). Its inhibition becomes significantly important for the treatment of cancers like advanced or metastatic breast cancer. Alpelisib is an oral Kinase inhibitor that selectively inhibits mutated phosphoinositol-3 kinase (PIK-3). It has been approved by US FDA in 2019. Its indications include post-menopausal women, men, HR-positive and HER-2 negative, and PIK3CA mutated metastatic breast cancer (Cheer et al., 2005; André et al., 2021). The drug is taken orally and administered in combination with Fulvestrant (Papich, 2021). It is marketed under the brand name Piqray among many others. Available in 3 different dose sizes and administered OD. Diarrhea is a common adverse event associated with the use of Alpelisib. However, during post-marketing surveillance, Kathleen and co-workers observed colitis as a new safety signal (Gregory et al., 1985). It is also approved for medical use in Australia and European Union also.
3.2.3.3 Palbociclib
Palbociclib is a reversible inhibitor of Cyclin-Dependent Kinase-4 (CDK-4) and Cyclin-Dependent Kinase-6 (CDK-6) like Abemaciclib and Alpaciclib. Palbociclib received approval from US-FDA in 2015 for the treatment of women who are in their post-menopausal phase suffering from HER-2 negative and ER-positive breast cancer (Sledge et al., 2020). It has shown to improve overall survival in Hormone Receptor–Positive, ERBB2-Negative breast cancer significantly which otherwise progressed on Endocrine Therapy (Bayraktar et al., 2013). In combination with an aromatase inhibitor similar to the other two kinase inhibitors. (Bowles et al., 2015; Houdaihed et al., 2018; Neven et al., 2021). The drug is administered orally and is available in the market in form of capsules. The recommended dose is 125 mg daily for 21 days. However, capsules are available in three different strengths; 75 mg 125 mg as dosage adjustment may be recommended in special cases of dose dependant side effects, kidney and liver disease, and pregnancy state of female patients.
3.2.3.4 Neratinib Maleate
Neratinib is a 4-Anilino-3-Cyano Quinoline derivative, formulated as tablets as Neratinib Maleate (FDA, 2017a). Neratinib is a pan-HER, irreversible TKI with potent preclinical activity against Trastuzumab-resistant breast cancer models (Yin et al., 2016). Neratinib binds irreversibly to different epidermal growth factor receptors like HER2, HER4 and EGFR receptors, reduces the autophosphorylation of the receptors which creates a barrier for the down-streaming of the signal pathway. This exhibits antitumor activity. Neratinib received US-FDA approval in 2017 for the patient with early-stage breast cancer (as single agent adjuvant) and advanced or metastatic HER2-positive breast cancer (in combination with Capacitabin) (Saura et al., 2020). It is available in market as 40 mg film coated tablet for oral use. Most common side effects observed with Neratinib are Diarrhoea, followed by nausea, vomiting, abdominal pain and anorexia. Based on several studies, it is likely that Neratinib-related diarrhea is caused by HER1/EGFR inhibition (Yin et al., 2016).
3.2.3.5 Tucatinib
Tucatinib turned out to be the first chemical moiety which was evaluated under Project Orbis which is an FDA oncology Center of Excellence initiative. Tucatinib was approved by FDA in 2020 for a combination therapy with Trastuzumab and Capecitabine for the treatment of unresectable advanced or metastatic breast cancer with brain metastases (Silvestris et al., 2008). Tucatinib is a Tyrosine Kinase inhibitor of HER-2. Hence, it inhibits the growth of HER2 expressing tumors. The combination of Tucatinib and Trastuzumab showed increased anti-tumor activity in vitro and in vivo compared to either drug alone and had acceptable toxicity (Elith* et al., 2006). It is marketed as tablets, for oral use in two strengths.
3.2.3.6 Pertuzumab
Pertuzumab, a monoclonal antibody against HER2, was approved by the FDA in 2012 for the treatment of patients with HER2-positive MBC who had not previously received anti-HER2 therapy or chemotherapy for metastatic disease. The approval was based on the CLEOPATRA trial, which included patients with HER2-positive MBC who received Trastuzumab and Docetaxel in combination with either placebo or Pertuzumab. The addition of Pertuzumab resulted in a significant improvement in the primary endpoint of PFS. The final OS analysis also revealed a statistically significant benefit for the Pertuzumab arm. This was the first approval after trastuzumab in more than a decade for an antibody targeting HER2 that showed a survival benefit when combined with Transtuzumab.
3.2.4 Hormonal drugs
The breast cancer cells get attracted to hormones like estrogen, progesterone through the specific receptors present on the cells which help them to grow. The treatments to stop these hormones from interacting with the receptors are known as hormone therapy. Hormone therapy mainly revolves around the concept of steroidal hormones and their receptors on the breast cancer with reference to the mechanism of ligand-receptor interaction (Abdulkareem and Zurmi, 2012; US Food and Drug Administration, 2017).
3.2.4.1 Tamoxifen
Tamoxifen (TAM) is a hydrophobic estrogen modulating anticancer agent, approved by the US-FDA for treatment of breast cancer through hormone therapy. Tamoxifen acts as a selective estrogen receptor modulator (SERM) for estrogen receptors. It acts as an anti-estrogen agent for breast cancer cells whereas, performs the function of estrogen agent for normal cells and tissues. Tamoxifen is a specific antagonist for ERα and thus shows an antiproliferative effect (Hortobagyi, 2018; Hong et al., 2020).
3.2.4.2 Toremifene
Toremifene works as a selective non-steroidal triptycene estrogen receptor modifier that is being used for a long time in hormone receptor positive breast cancer of both late and early stage. Toremifene is metabolized in the liver and is excreted out from the body through feces. It is also commonly used in clinical practice as an alternative to Tamoxifen. Both tamoxifen and teromifene are structurally similar drugs which are commonly used for endocrine therapy after breast cancer surgery (Mustonen et al., 2014; FDA, 2017c). It is also teste for neoadjuvant therapy for locally advanced breast cancer in combination with Melatonin or Metformin (Sartaj, AnnuBiswas, Verma, Sahoo, Baboota, et al.). A research work published in International Journal of Cancer (2018) claims that Toremifene, rather than Tamoxifen, might be a better option for the adjuvant endocrine therapy in CYP2D6*10T/T genotype breast cancer patients in (Ahmed et al., 2022; Mazzarino et al., 2013). Another prospective, randomized study of Toremifene vs tamoxifen for the treatment of premenopausal breast cancer shows TOR and TAM have similar side effects on the female genital system and quality of life in premenopausal early breast cancer patients (Fei and Yoosefian, 2021).
3.2.4.3 Fulvestrant
Fulvestrant is a selective degrader of estrogen receptor that first binds with the estrogen receptor and acts as an inhibitor for estrogen signaling which fuel the process of tumor cell growth. Unlike Tamoxifen, Fulvestrant-induced conformational change of estrogen receptors hinders transcriptional activity of proteins. In addition, the unstable complex formed during the interaction of Fulvestrant with estrogen receptor results in accelerated degradation of the cells. Fulvestrant therefore acts as both a competitive antagonist and a selective estrogen receptor degrader (SERD), causing a reduction in cellular estrogen receptor alpha levels. Fulvestrant is drug which is recommended as a monotherapy but there are still multiple trails going on observing its efficacy in combination therapy as a CDK4/6 inhibitor (Bowles et al., 2015; Soiza et al., 2018), (Kabos and Borges, 2010; Shantanam, 2018). Despite its increasing use in the ER + metastatic breast cancer setting, data are available in the literature about its-acquired endocrine resistance (Robson et al., 2017).
3.2.4.4 Letrozole
Diminishing estrogen production by antagonizing the conversion to estrogen from androgens is achieved by aromatase inhibitors. The main function of aromatase inhibitors is to antagonize the activity of aromatase enzyme, in turn resulting in inhibition of estrogen production in breast cancer patients. Letrozole was developed as a highly potent third generation non-steroidal inhibitor of aromatase used for the treatment of breast cancer management. In several preclinical studies, Letrozole has demonstrated greater potency compared with Anastrozole, Exemestane, Formestane and aminoglutethimide. Letrozole has been reported to inhibit the aromatase activity by more than 99% in in-vivo tissues. It has been documented to exhibit its most wide application in recurrent, metastatic and advanced cancer in post-menopausal women. Unlike first and second-generation Aromatase Inhibitors, Letrozole is highly selective for aromatase and does not significantly affect 17a-OH progesterone, cortisol aldosterone, thyroxine, thyroid-stimulating hormone (TSH), luteinizing hormone (LH), follicle-stimulating hormone (FSH) or androstenedione (Holmberg et al., 1997; Clinicaltrials.Gov, 2015; Rugo et al., 2019). It has poor water solubility, rapid metabolism, and a range of side effects. Polymer-based nanoparticles (FDA, 2012a; Alemrayat et al., 2018; Caulfield et al., 2019; Leo et al., 2020), Lipid nanocomplex (Bhatnagar, 2007; Azandaryani et al., 2019; Yassemi et al., 2020), solid lipid nanoparticles (Khan et al., 2022), Pegylated nanoparticles (Kelly and Buzdar, 2010), are few of the novel formulations explored to overcome the limitations and side effects associated with Letrozole.s.
3.2.4.5 Anastrozole
Anastrozole was first approved in United States, EU and in other countries as aromatase inhibitor for adjuvant treatment in postmenopausal women suffering from hormone-positive early-stage breast cancer (Goss and Tye, 1997; Ekedahl et al., 2022). It is generally well-tolerated in patients suffering from early-stage breast cancer. Aromatase inhibitors evolved as an alternative of endocrine therapy for the treatment of hormone sensitive breast cancer. Anastrozole is a potent non-steroidal aromatase inhibitor which selectively blocks estrogen synthesis in women having breast cancer who are in their postmenopausal phase. Though it has been reported in few studies that the pharmacokinetics and pharmacodynamics characteristics of the drug inside a patient’s body is largely effects by inter individual variability, but presently, the drug is used for breast cancers of all configurations (Sanford and Plosker, 2008; USP, 2012). Anastrozole has been associated with a low rate of serum enzyme elevations during therapy and rare instances of clinically apparent liver injury (Shavi et al., 2017; Ekedahl et al., 2022). Like other anticancer drugs targeted and nanoformulations are being researched to overcome ANS, associated serious side effects due to uncontrolled delivery. Low solubility and short plasma half-life (FDA, 2011a; Pearce et al., 2012; Lam et al., 2016; Rahool et al., 2021).
3.2.4.6 Exemestane
Aromatase works on the rate limiting stage of the estrogen biosynthesis process Exemestane (Aromasin) is a novel steroidal aromatase inhibitor (Untch and Jackisch, 2008) which was first approved for the treatment of postmenopausal breast cancer in Japan. Exemestane works on the principle of irreversibly binding with the pseudo-substrate with covalent bond so that it can inhibit the activity of aromatase enzyme It has been reported that the Exemestane has shown anti-tumor activity both conventional as well as testosterone treated- and ovariectomized postmenopausal models (Iirola et al., 2011; Li et al., 2013; Yavuz et al., 2007; Structures et al., 2023). Advanced research is focused on its safe and effective delivery through Novel approaches of drug delivery (Holmberg et al., 1997; Assesse Sophie, 2011; FDA, 2005a; FDA, 2015a; Accessdata, 1999).
3.2.4.7 Goserelin
Goserelin, marketed in the name of Zoladex is a synthetic analogue of gonadotropin-releasing hormone (GnRH) (FDA, 2012d; FDA, 2020b; Gupta et al., 2021). which stimulates gonadotropin and sex hormone release in the short term, and then causes suppression with continued administration. It reduces the estrogen level in plasma/serum for pre- or perimenopausal women who is under Goserelin treatment. Goserelin is an effective alternative to surgery or estrogen therapy in prostate cancer palliation, and possibly to ovariectomy in premenopausal breast cancer (FDA, 2010c; FDA, 2018).
3.2.4.8 MegestroAcetate
Megestrol acetate (MGA) is recognized as one of the first standard progestogen or progestational agent (Gregory et al., 1985; Ah-, 2018) effectively considered for advanced cancer (Schacter et al., 1989; Fjøsne et al., 2008) treatments because of its excellent safety profile. It is also considered as an effective treatment for anorexia-cachexia syndrome in cancer patients. It can penetrate the BBB when given in high doses. The response of this drug is considered comparable with tamoxifen, but megestrol acetate is more beneficial for patients who are suffering from cachexia. There is still a lot of research going on for considering this drug for the treatment of progesterone- and estrogen negative breast cancer (CDSCO, 2019; FDA, 2022).
3.2.4.9 Methyl testosterone
Androgens are testosterone methyltestosterone, fluoxymesterone, and testolactone derivatives that are frequently used for palliative treatment of breast cancer in postmenopausal women who are receiving hormone therapy. The precise mechanism of androgens’ anticancer effect is unknown. However, it is assumed that androgens inhibit cell growth by preventing natural hormone transport into the cell (Vick and Hayton, 2001; Miles and White, 2018; Science direct, 2023a).
3.2.5 Miscellaneous
3.2.5.1 Everolimus
Everolimus is an oral Rapamycin (Natural Macrolide) derivative that selectively inhibits mTOR receptors. mTORC1 is a PI3K pathway signal transducer that becomes activated during human malignancies. Like Rapamycin, it has a binding interaction with FKBP12 and hinders the mTORC1 rather than mTORC2 complex formation. Everolimus has been reported to shunt tumor growth rate rather than promote cell death. It was approved in 2012 for use in postmenopausal women with HER2-negative, hormone-receptor-positive advanced breast cancer patients. It has been observed that Everolimus in advanced breast cancer patients gets quickly absorbed following oral administration, with a median time to peak blood levels. Bonizzi et al. (2019) reported Everolimus nano formulation increases drug responsiveness in resistant and low-responsive Breast Cancer Cell Lines (Bonizzi et al., 2019). Many research works claim that Co-delivery of Paclitaxel and Everolimus at the Optimal Synergistic Ratio may prove to be a Promising Solution for the Treatment of Breast Cancer (Houdaihed et al., 2018; Elena et al., 2019).
3.2.5.2 Pamidronate
Osteolytic bone metastases commonly occur in patients with breast cancer (Evans et al., 2004). Pamidronate is nitrogen-containing bisphosphates used to treat bone metastases in breast cancer. Pamidronate inhibits bone resorption by adsorbing mineralized bone matrix on the surface of hydroxyapatite crystals. By impairing the attachment of osteoclast precursors to the mineralized matrix, pamidronate blocks their maturation into functioning osteoclasts, blocking osteoclast-mediated bone resorption that may lead to the weakening of the bone, fractures, and pain. It is available as an injection and lyophilized powder for reconstitution. Permitted inactive ingredients are mannitol and phosphoric acid. However, delivering drugs inside the diseased bone is a challenge. Nanomedicine which is able to target and deliver therapeutic agents to diseased bone sites could potentially provide an effective treatment option for different types of skeletal cancers. Yin et al. (84) demonstrated the use of pamidronate-functionalized nanoparticles of Polylactide to transport DOX to the bone microenvironment for the targeted treatment of OS. In vivo biodistribution of radiolabeled targeted Pam-NPs demonstrated enhanced bone tumor accumulation and prolonged retention compared with nontargeted NPs (Zhong et al., 2014; Yin and Tang, 2016). Pamidronate has been employed to target drugs inside the bone for the treatment of various other bone diseases. Numerous nano formulations containing Pamidronate as a targeting agent to bone are exploited. However, it is out of the scope of the contents of this paper. The drug may cause renal failure, embryogenic toxicity, electrolyte disorder, and osteonecrosis of the jaw.
3.2.5.3 Gemcitabine (GEM)
GEM is a pyrimidine anti-metabolite that rapidly gets incorporated into DNA as a triphosphate. Gemcitabine (2, 2-difluoro-2-deoxycytidine, GEM) is a deoxycytidine analogue and is one of the most widely used anticancer drugs in the treatment of several types of solid tumours (Silvestris et al., 2008). In combination with paclitaxel, it is for first-line treatment of metastatic breast cancer after failure of prior anthracycline-containing adjuvant chemotherapy, unless anthracyclines were clinically contraindicated (Kushwah et al., 2018; Sung et al., 2021). The recommended dose for BC is 1250 mg/m2 over 30 min on Days 1 and 8 of each 21 days cycle. A study performed by Bijay et al. concludes that a combination of Gemcitabine and Iminoquid as nanoparticles has demonstrated better BC suppression by activation of the immune system. co-delivery of Gemcitabine in novel formulations has been experimented by many scientists (Mozar and Chowdhury, 2017; Kushwah et al., 2018; Lei et al., 2019; García-García et al., 2020; García-García et al., 2020). Various nanometer sized novel formulations carrying GEM individually are also reported (Kennedy, 1957; Mozar and Chowdhury, 2017), which claims to have the capability of improved delivery, lesser toxicity, and side effects.
3.2.5.4 Fluoxymesterone
Fluoxymesterone is a synthetic androgenic anabolic steroid used in men and women to treat hypogonadism, delayed puberty, and breast neoplasms. Fluoxymesterone, has shown to be effective in postmenopausal women with advanced breast cancer (Goldhirsch et al., 1982a; Turner et al., 2023). Its side effects are mostly those associated with the physiologic effects of male hormone, such as virilization with frontal baldness, plethora and acne, hirsutism, fluid retention, and, less frequently, increased libido and clitoral hypertrophy (Kennedy, 1958; Goldhirsch et al., 1982). One case report describes an unusual case of ataxia and unsteadiness of gait caused by Fluoxymesterone therapy (Kennedy, 1958; Science direct, 2023b). It is marketed as oral pill of 10 mg under the brand names Halotestin and Ultandren.
3.2.5.5 Ixabepilone
Ixabepilone is an epothilone analog developed by Bristol-Myers Squibb (FDA, 2005b). It is considered as a new member of anti-neoplastic drugs from 2007 after being approved by US-FDA (Egerton, 2008; Ibrahim, 2021). Ixabepilone is a micro-tubule stabilizer approved as a monotherapy and in combination with Capecitabine for the treatment of metastatic breast cancer in patients with demonstrated resistance to Anthracyclines and Taxanes. Ixabepilone was specially derived for patients who have developed resistance with some other therapies (Huang et al., 2010; Thomas et al., 2015), (Thomas et al., 2022). Epothilones have higher affinity for β-tubulin and are not P-gp substrates. Certain smedications, including (but not limited to) Verapamil, Ketoconazole, Rifampin, Phenytoin, and Phenobarbital, can interfere with this medication (Chuang et al., 2010). CYP3A4 inducers and inhibitors may cause decrease and increase plasma concentration, hence the dose needs to be adjusted accordingly for the patients (Bagegni et al., 2022). It is administered through injection and branded as Ixempra. A pre-formulated liposomal version of Ixabepilone has been reported to have optimal in vivo performance (Hortobagyi, 2018).
3.2.5.6 Pembrolizumab
Pembrolizumab is a humanized anti-programmed cell death monoclonal IgG4 kappa anti-PD1 antibody. Binding of Pembrolizumab to PD1 does not engage FC receptors which is a surface protein mainly found on the surface of cells like B-lymphocytes, natural killer cells, etc. FC receptors plays a significant role in activating immune complexes for healthy cell development. The 50% effective inhibitory concentration in T-cell activation assays ranges from 0.1 to 0.3 nm. This drug has been reported to be used for triple negative breast cancer patients who has active autoimmune disease (March 2017). It is sold in market as injection formulations of 50 mg single dose vial as powder for reconstitution and 25 mg/mL single dose vial as solution (Hortobagyi, 2018; Bagegni et al., 2022).
3.2.5.7 Ribociclib
Ribociclib (RIB) is an oral cyclin-dependent kinase 4 and 6 (CDK4/6) inhibitor that has been recommended in year 2017 as a preferred regimen for the treatment of premenopausal women with HR-positive, HER2-negative Breast cancer. It has been reported to inhibit the phosphorylation of retinoblastoma protein which further arrests cell cycle progression in G1 phase (FDA, 2017; Slamon et al., 2021), (Ahmed and Fatima, 2022; SartaI, 2022). The recommended starting dose of Ribociclib is 600 mg orally (three 200 mg tablets) taken once daily with or without food for 21 consecutive days followed by 7 days off treatment. Ribociclib Nanostructured Lipid Carrier are reported to have overcome the inherent lacuna of limited bioavailability (Fei and Yoosefian, 2021). Ribociclib-Loaded Ethylcellulose-Based Nanosponges Formulation reportedly demonstrated better Cytotoxic Potential against Breast Cancer (Swaminathan et al., 2016; Ji et al., 2020). Ribociclib loaded Polymeric micelles showed anti-cancer potential at much lower doses of Ribociclib (Ji et al., 2020; Robson et al., 2017). s
3.2.5.8 Olaparib
Olaparib represents a rational strong class of drugs called PARP (Poly ADP-ribose polymerase) inhibitors administered orally for metastatic and germline BRCA mutation in breast cancer. Clinical potential of Olaparib monotherapy has been documented in somatic or germline BRCA1/2 metastatic type of breast cancer therapy (Robson et al., 2017; Kuemmel et al., 2020), (Caulfield et al., 2019; Gote et al., 2021). Olaparib is the first treatment approved specifically for BRCA mutation carriers with HER2-negative metastatic breast cancer and previous treatment with chemotherapy in the neoadjuvant, adjuvant, or metastatic setting (Kuemmel et al., 2020; Structures et al., 2023c). It has been reported to enhance chemotherapy without increasing toxicity when administered in nanoparticle form (Migotto et al., 2018). Many novel formulations have been experimented by different independent research groups to enhance oral efficacy, reduced tumor proliferation, to inhibit growth and metastasis (Cortesi et al., 2021).
3.2.5.9 Tolazoparib tasylate
Talazoparib was approved by the FDA for use in germline BRCA mutated, HER2 negative, locally advanced or metastatic breast cancer on. 16 October 2018 (Zhang et al., 2019). It is a poly ADP ribose polymerase (PARP) inhibitor, prevents PARP-mediated DNA repair this enhances the accumulation of DNA stand breaks, promoting genomic instability eventually leading to apoptosis (Zhang et al., 2019). This drug has been recommended for the use for only those patients who have already undergone an ineffective hormonal therapy for breast tumor. Presently, Tolazoparib is available as a capsule with the brand name Talzenna developed by Pfizer.
4 Discussion and conclusion
Drug discovery for Breast Cancer has always been an area of interest and important priority for researchers as even today there is no drug/drug combination which can promise 100% side effects/adverse effects free treatment of Breast Cancer, the most common malignancy in women across the world. In all of 206 anticancer drugs approved by United States-FDA, 39 are for breast cancer treatment alone.
Though the first drug for breast cancer was approved in 1953 the initial research was observed to be quite slow as only 8 drugs could get approval till 1988 for breast cancer indication. However, in later decades the research acquired momentum and offered various treatment choices (Pietrangelo, 2021). Initially several breast cancer approvals granted by FDA were dominated by Cytotoxic drugs with Methoterxate being the first and most widely used. Later in 1970s hormonal drugs, specially Estrogen Receptors Modulators, i.e., Tamixifen eclipsed the market/limelight and marked the inception of precision medicine in cancer. Another milestone in the history of breast cancer therapy was the approval of Trastuzumab, a monoclonal antibody that targets human epidermal growth factor receptor 2 (HER2). Among these hormonal therapy drugs, i.e., aromatase inhibitor (anastrozole, letrozole, or exemestane) in combination with a CDK 4/6 inhibitor, anti-estrogens (fulvestrant and tamoxifen), and Targrted therapy, i.e., Trastuzumab, Pertuzumab, ado-Trastuzumab Emtansine, Trastuzumab Deruxtecan, Tucatinib, Neratinib, Lapatinib, are used as first line treatment for ER/PR positive and HER positive breast cancer respectively. However, the chemotherapy is left as first choice of treatment if the patient is the victim of TNBC (Triple Negative Breast Cancer) (National Cancer Institute, 2017). Generally, these are employed as combination therapy. Most commonly employed combinations are AC (Adriamycin and Cyclophosphamide), AC-T (Adriamycin, Cyclophosphamide and Taxol-Peclitexal), CAF (Cyclophosphamide, Adriamycin and 5-Flurouracil), CMF (Cyclophosphamide, Methotrexate and 5-Flurouracil), FEC (5-Flurouracil, Epirubici Hydrochloride and Cyclophosphamide) and, TAC (Taxol, Adriamycin and Cyclophosphamide) (Álvarez, 2010).
Drugs used to treat breast cancer are considered systemic therapies because they can reach cancer cells almost anywhere in the body. Some can be administered orally, through intramuscular route, or as an intravenous injection or infusion. Depending on the type of breast cancer, different types of drug treatment might be used. In this article we have compiled information about mechanism of action, approval status, limitations and side effects, available commercial formulations, novel and targeted drug delivery systems which have been researched for various types of breast cancer and the formulations which are under clinical trial. This information may considerably help a formulation scientist in deciding the drug on which advanced research is required/possible in terms of formulation development. An idea of side effects and adverse effects of each drug shall further help the scientist for gaining information in designing a drug or a delivery system from a single manuscript which may reduce or eliminate these side effects by targeting to desired sites or by preventing the drug to reach at sites where side effects are observed. Moreover, the information also saves the scientists from the efforts which may be wasted by duplication of research.
A formulator may further decide to work on dose reduction and co-delivery of drugs in single formulation with the acquaintance of frequently used drug combinations in treatments which will surely enhance patient compliance and adherence to the treatment. This article encompasses all that information which may help a scientist to refer to for his further research in the field of breast cancer.
Optimal outcomes for breast cancer therapy are immensely dependent on timely diagnosis followed by effective multidisciplinary approach of cancer treatment. The history of cancer in the medical background started long back in ancient Greek and Egyptian civilizations. There have been a lot of discoveries over the centuries that have helped in the evolution of the therapeutic approach towards breast cancer treatment. The most important fundamental breakthrough in medical oncology took place in the beginning of ’80s when the immergence of specific drugs came intended for molecular targets came into existence. All the chemotherapeutic as well as targeted drugs have improvised the survival and living quality of breast cancer patients (Zardavas and Piccart-Gebhart, 2016; Jerusalem et al., 2018; Waks and Winer, 2019; Junnuthula et al., 2022). The advancement in drug discovery has also led to the further enhancement of clinical oncology with an introduction of monoclonal antibodies into the treatment regime. Likewise, introduction of new drugs for better management will always remain continuous process where it has already been reported that various novel biotechnological drugs have depicted promising preclinical results. Table 1 contains the chronological list of all the drugs till date along with their year of approval, brand name and their formulations which are available in the market, or under clinical trial respectively. Moreover, the novel formulations are getting humungous attentions of researchers and maufacturers as these can provide targeted treatment which is devoid of side/toxic effects and improve quality of life. Table 2 summerizes the novel formulations which are under clinical trials and are promising in better management of breast cancer. Graphical presentation of the data is depicted in Figure 6. Therefore, to conclude, this exhaustive review article will become a one-stop solution for breast oncology scientist and medical professionals to refer to. This aims to reduce to hassle of data searching and enhance the ease of data access about the approved breast cancer drugs for further research.
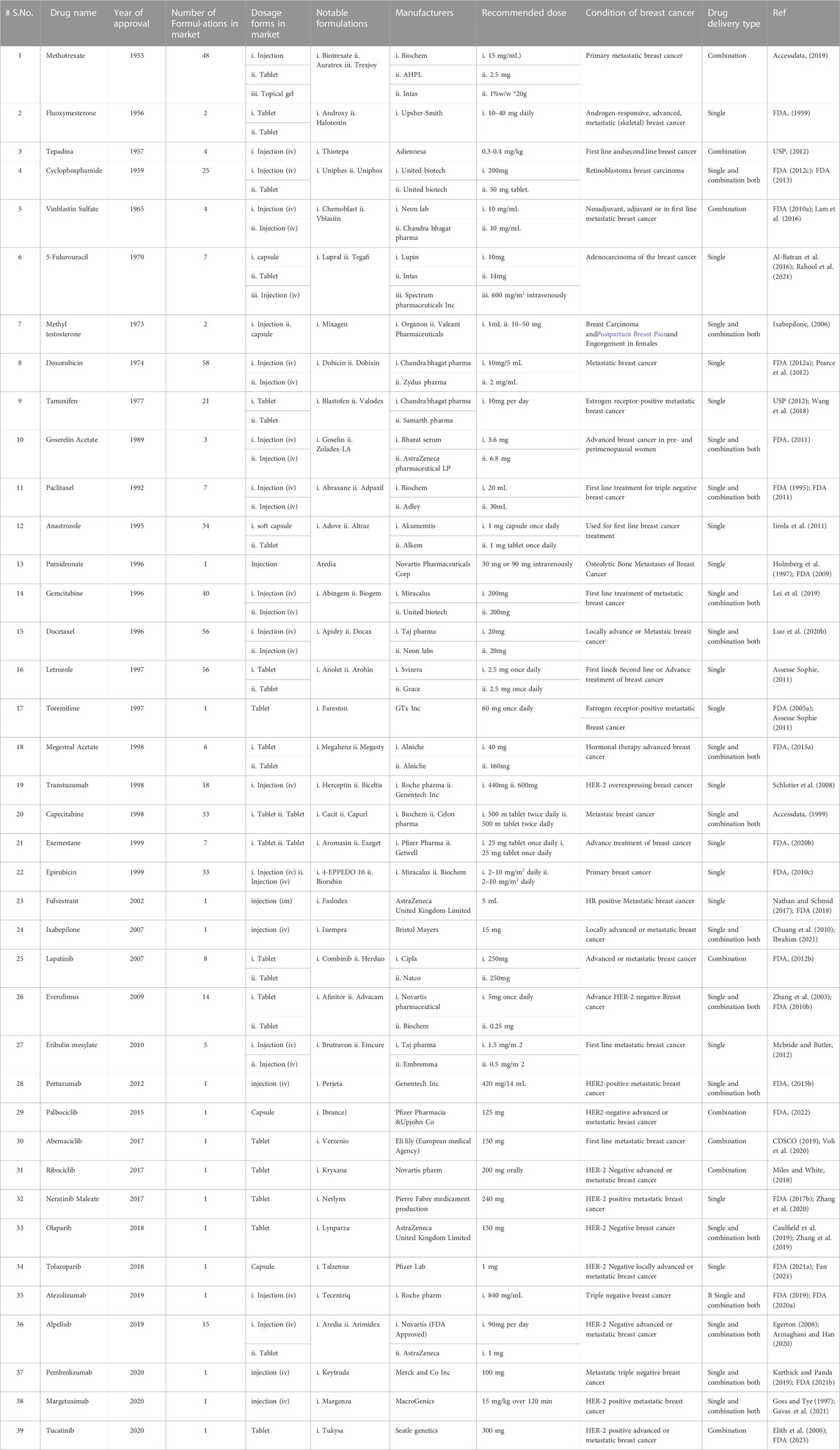
TABLE 1. A Comprehensive Account of Commercial Formulations of Drugs Approved for first line, neoadjuvant, and Adjuvant therapy of various types of Breast Cancers by USFDA [Approval Year wise General information 1953 to 2023].
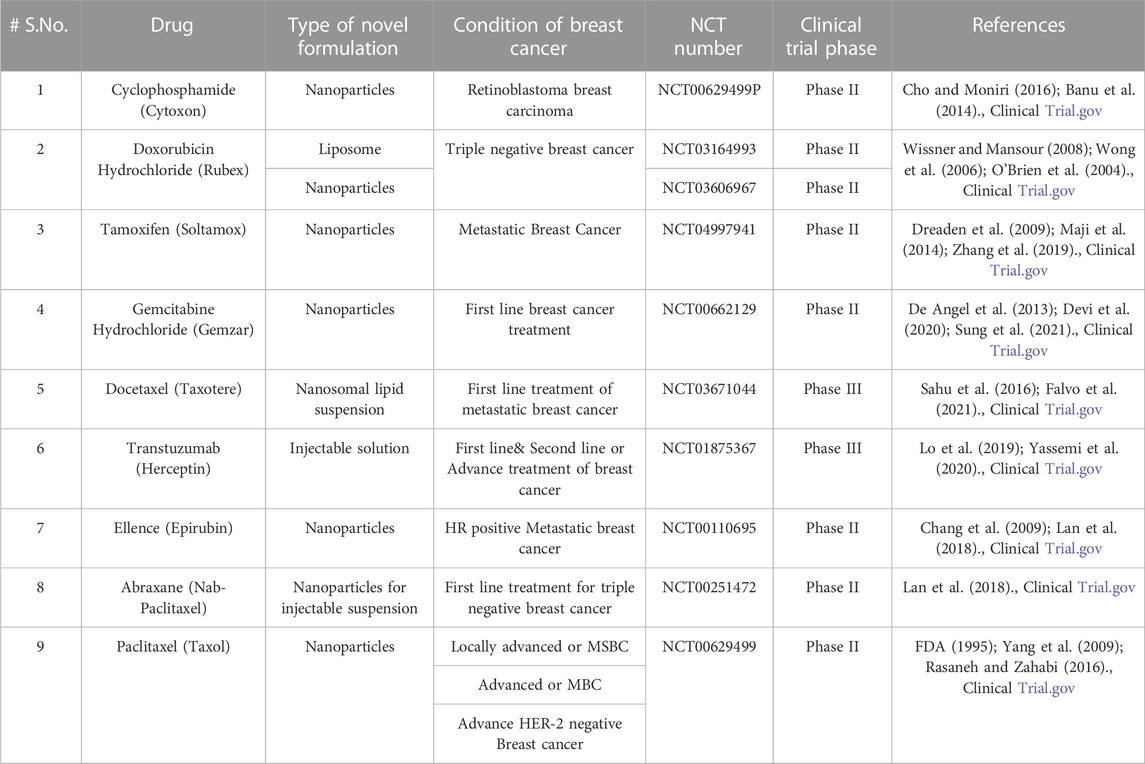
TABLE 2. List of Various novel formulations in clinical trial for various conditions of breast cancer.
Author contributions
MC—original concept, writing of first and second draft, RS—literature survey, figures, tables and referencing, SS—literature survey and first draft preparation. SF—Preparation of final draft, revision and overall supervision. All authors contributed to the article and approved the submitted version.
Acknowledgments
Authors thank Uttar Pradesh Council of Science and Technology Lucknow (UPCST) for financial support in term of a research project involving the assessment of certain formulation development for the treatment of breast cancer.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1184472/full#supplementary-material
References
Abdulkareem, I. H., and Zurmi, I. B. (2012). Review of hormonal treatment of breast cancer. J. Clin. Pract. 15 (1), 9–14. doi:10.4103/1119-3077.94088
Accessdata (1999). Dexmedetomidine hydrochloride injection safely and effectively. see full prescribing information for dexmedetomidine hydrochloride injection. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/206628s000lbl.pdf.
Accessdata (2019). Accessdata. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/210737s000lbl.pdf
Agrawal, Y. O., Mahajan, U. B., Mahajan, H. S., and Ojha, S. (2020). Methotrexate-loaded nanostructured lipid carrier gel alleviates imiquimod-induced psoriasis by moderating inflammation: Formulation, optimization, characterization, in-vitro and in-vivo studies. Int. J. Nanomedicine 15, 4763–4778. doi:10.2147/IJN.S247007
Ah-, M. L. (2018). See, “Time to revisit ‘Megace’ for hot flushes in patients with breast cancer” BMJ Support. Palliat. Care 8 (4), 493. doi:10.1136/bmjspcare-2016-001153
Ahmad, A., Sheikh, S., Taran, R., Srivastav, S. P., Prasad, K., Rajappa, S. J., et al. (2014). Therapeutic efficacy of a novel nanosomal docetaxel lipid suspension compared with taxotere in locally advanced or metastatic breast cancer patients. Clin. breast cancer 14 (3), 177–181. doi:10.1016/j.clbc.2013.09.011
Ahmad, N., Ahmad, R., Alam, M. A., Ahmad, F. J., Amir, M., Pottoo, F. H., et al. (2019). Daunorubicin oral bioavailability enhancement by surface coated natural biodegradable macromolecule chitosan based polymeric nanoparticles. Int. J. Biol. Macromol. 128, 825–838. doi:10.1016/j.ijbiomac.2019.01.142
Ahmad, N., Albassam, A. A., Faiyaz Khan, M., Ullah, Z., Mohammed Buheazah, T., Salman AlHomoud, H., et al. (2022). A novel 5-Fluorocuracil multiple-nanoemulsion used for the enhancement of oral bioavailability in the treatment of colorectal cancer. Saudi J. Biol. Sci. 29 (5), 3704–3716. doi:10.1016/j.sjbs.2022.02.017
Ahmed, M. M., and Fatima, F. (2022). Ribociclib-loaded ethylcellulose-based nanosponges: Formulation, physicochemical characterization, and cytotoxic potential against breast cancer. Adsorpt. Sci. Technol. 2022, 1–11. doi:10.1155/2022/1922263
Ahmed, M. M., Fatima, F., Alali, A., Kalam, M. A., Alhazzani, K., Bhatia, S., et al. (2022). Ribociclib-loaded ethylcellulose-based nanosponges: Formulation, physicochemical characterization, and cytotoxic potential against breast cancer. Adsorpt. Sci. Technol. 2022, 1–11. doi:10.1155/2022/1922263
Akinyelu, J., and Singh, M. (2019). Folate-tagged chitosan-functionalized gold nanoparticles for enhanced delivery of 5-fluorouracil to cancer cells. Appl. Nanosci. 9 (1), 7–17. doi:10.1007/s13204-018-0896-4
Al-Batran, S. E., Hofheinz, R. D., Pauligk, C., Kopp, H. G., Haag, G. M., Luley, K. B., et al. (2016). Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO): Results from the phase 2 part of a multicentre, open-label, randomised phase 2/3 trial. lancet Oncol. 17 (12), 1697–1708. doi:10.1016/s1470-2045(16)30531-9
Aleem, A., and Shah, H. (2023). Atezolizumab. [Updated 2023 jan 9]. In: StatPearls [internet]. Treasure Island (FL): Stat Pearls Publishing.
Alemrayat, B., Elrayess, M. A., Alany, R. G., Elhissi, A., and Younes, H. M. (2018). Preparation and optimization of monodisperse polymeric microparticles using modified vibrating orifice aerosol generator for controlled delivery of letrozole in breast cancer therapy. Drug Dev. Ind. Pharm. 44 (12), 1953–1965. doi:10.1080/03639045.2018.1503298
Alemrayat, B., Elhissi, A., and Younes, H. M. (2019). Preparation and characterization of letrozole-loaded poly(d,l-lactide) nanoparticles for drug delivery in breast cancer therapy. Pharm. Dev. Technol. 24 (2), 235–242. doi:10.1080/10837450.2018.1455698
Álvarez, R. H. (2010). Present and future evolution of advanced breast cancer therapy. Breast Cancer Res. 12 (2), 1–18. doi:10.1186/bcr2572
Alves, R. C., Fernandes, R. P., Eloy, J. O., Salgado, R. N., and Chorilli, M. (2017). Characteristics, properties and analytical methods of paclitaxel: A review. December 8347, 110–118. doi:10.1080/10408347.2017.1416283
Alyafee, Y. A., Alaamery, M., Bawazeer, S., Almutairi, M. S., Alghamdi, B., Alomran, N., et al. (2018). Preparation of anastrozole loaded PEG-PLA nanoparticles: Evaluation of apoptotic response of breast cancer cell lines. Int. J. Nanomedicine 13, 199–208. doi:10.2147/IJN.S151139
André, F., Ciruelos, E. M., Juric, D., Loibl, S., Campone, M., Mayer, I. A., et al. (2021). Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2–negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 32 (2), 208–217. doi:10.1016/j.annonc.2020.11.011
Armaghani, A. J., and Han, H. S. (2020). Alpelisib in the treatment of breast cancer: A short review on the emerging clinical data. Breast Cancer Targets Ther. 12, 251–258. doi:10.2147/BCTT.S219436
Ashfaq, U. A., Riaz, M., Yasmeen, E., and Yousaf, M. (2017). Recent advances in nanoparticle-based targeted drug-delivery systems against cancer and role of tumor microenvironment. Crit. Rev. Ther. Drug Carr. Syst. 34 (4), 317–353. doi:10.1615/CritRevTherDrugCarrierSyst.2017017845
Ashtari, A., Niazvand, F., and Khorsandi, L. (2020). Chemotherapy drugs based on solid lipid nanoparticles for breast cancer treatment. Med. Kaunas. 56, 4–5. doi:10.3390/medicina56120694
Assesse Sophie, H. (2011). “Cardiovascular effects and pattern of use of antineoplastic therapies in female breast cancer patients,”. Doctoral dissertation (University of Ottawa).
Attama, A. A., Nnamani, P. O., Onokala, O. B., Ugwu, A. A., and Onugwu, A. L. (2022). Nanogels as target drug delivery systems in cancer therapy: A review of the last decade. Front. Pharmacol. 13, 874510–874523. doi:10.3389/fphar.2022.874510
Avasatthi, V., Pawar, H., Dora, C. P., Bansod, P., Gill, M. S., and Suresh, S. (2016). A novel nanogel formulation of methotrexate for topical treatment of psoriasis: Optimization, in vitro and in vivo evaluation. Pharm. Dev. Technol. 21 (5), 554–562. doi:10.3109/10837450.2015.1026605
Azandaryani, A. H., Kashanian, S., Shahlaei, M., Derakhshandeh, K., Motiei, M., and Moradi, S. (2019). A comprehensive physicochemical, in vitro and molecular characterization of letrozole incorporated chitosan-lipid nanocomplex. Pharm. Res. 36 (4), 62. doi:10.1007/s11095-019-2597-4
Bagegni, N. A., Davis, A. A., Clifton, K. K., and Ademuyiwa, F. O. (2022). Targeted treatment for high-risk early-stage triple-negative breast cancer: Spotlight on pembrolizumab. Breast Cancer Targets Ther. 14, 113–123. doi:10.2147/BCTT.S293597
Bahadori, F., Topçu, G., Eroʇlu, M. S., and Önyüksel, H. (2014). A new lipid-based nano formulation of vinorelbine. AAPS PharmSciTech 15 (5), 1138–1148. doi:10.1208/s12249-014-0146-3
Banu, H., Stanley, B., Faheem, S. M., Seenivasan, R., Premkumar, K., and Vasanthakumar, G. (2014). Thermal chemosensitization of breast cancer cells to cyclophosphamide treatment using folate receptor targeted gold nanoparticles. Plasmonics 9, 1341–1349. doi:10.1007/s11468-014-9747-7
Barradas, T. N., and de Holanda e Silva, K. G. (2021). Nanoemulsions of essential oils to improve solubility, stability and permeability: A review. Environ. Chem. Lett. 19 (2), 1153–1171. doi:10.1007/s10311-020-01142-2
Barros-Oliveira, M., Costa-Silva, D. R., Andrade, D. B. d., Borges, U. S., Tavares, C. B., Borges, R. S., et al. (2017). Use of anastrozole in the chemoprevention and treatment of breast cancer: A literature review. Rev. Assoc. Med. Bras. 63 (4), 371–378. doi:10.1590/1806-9282.63.04.371
Battaglia, L., Muntoni, E., Chirio, D., Peira, E., Annovazzi, L., Schiffer, D., et al. (2017). Solid lipid nanoparticles by coacervation loaded with a methotrexate prodrug: Preliminary study for glioma treatment. Nanomedicine 12 (6), 639–656. doi:10.2217/nnm-2016-0380
Bayraktar, S., Barrera, A. M., Liu, D., Pusztai, L., Litton, J., Valero, V., et al. (2013). USP-11 as a predictive and prognostic factor following neoadjuvant therapy in women with breast cancer. Cancer J. (Sudbury, Mass.) 19 (1), 10–17. doi:10.1097/PPO.0b013e3182801b3a
Benjamin, D. J., Xu, A., Lythgoe, M. P., and Prasad, V. (2022). Cancer drug approvals that displaced existing standard-of-care therapies. JAMA Netw. Open 5 (3), 2222655–e232021. doi:10.1001/jamanetworkopen.2022.2265
Bequet-Romero, M. (2018). Active immunotherapy with a VEGF targeted vaccine HeberSaVax: The road so far and the future ahead. Ann. Oncol. 29. doi:10.1093/annonc/mdy047
Bhadra, D., Bhadra, S., Jain, S., and Jain, N. K. (2003). A PEGylated dendritic nanoparticulate carrier of fluorouracil. Int. J. Pharm. 257 (1–2), 111–124. doi:10.1016/S0378-5173(03)00132-7
Bhatia, S. (2016). “Nanoparticles types, classification, characterization, fabrication methods and drug delivery applications,” in Natural polymer drug delivery systems (Berlin, Germany: Springer).
Bhatnagar, A. S. (2007). The discovery and mechanism of action of letrozole. Breast Cancer Res. Treat. 105, 7–17. doi:10.1007/s10549-007-9696-3
Bhushan, A., Gonsalves, A., and Menon, J. U. (2021). Current state of breast cancer diagnosis, treatment, and theranostics. Pharmaceutics 13 (5), 723. doi:10.3390/pharmaceutics13050723
Bland, K. A., Kirkham, A. A., Bovard, J., Shenkier, T., Zucker, D., McKenzie, D. C., et al. (2019). Effect of exercise on Taxane chemotherapy–induced peripheral neuropathy in women with breast cancer: A randomized controlled trial. Clin. Breast Cancer 19 (6), 411–422. doi:10.1016/j.clbc.2019.05.013
Bonizzi, A., Truffi, M., Sevieri, M., Allevi, R., Sitia, L., Ottria, R., et al. (2019). Everolimus nanoformulation in biological nanoparticles increases drug responsiveness in resistant and low-responsive breast cancer cell lines. Pharmaceutics 11 (8), 384. doi:10.3390/pharmaceutics11080384
Bowles, H. J., Pharm, D., Clarke, K. L., and Np-Cpalbociclib, (2015). A new option for front-line treatment of metastatic, hormone receptor–positive, HER2-negative breast cancer. J. Adv. Pract. Oncol. 6, 6. doi:10.6004/jadpro.6.6.6
Burotto, M., Wilkerson, J., Stein, W. D., Bates, S. E., and Fojo, T. (2019). Adjuvant and neoadjuvant cancer therapies: A historical review and a rational approach to understand outcomes. Semin. Oncol. 46 (1), 83–99. doi:10.1053/j.seminoncol.2019.01.002
Byrne, J. D., Betancourt, T., and Brannon-Peppas, L. (2008). Active targeting schemes for nanoparticle systems in cancer therapeutics. Drug Deliv. Rev. 60 (15), 1615–1626. doi:10.1016/j.addr.2008.08.005
Cancer (2023). Chemotherapy to treat cancer - NCI. Available at: https://www.cancer.gov/about-cancer/treatment/types/chemotherapy (accessed May 08, 2023).
Caulfield, S. E., Davis, C. C., and Byers, K. F. (2019). Olaparib: A novel therapy for metastatic breast cancer in patients with a BRCA1/2 mutation. J. Adv. Pract. Oncol. 10 (2), 167–174. Epub 2019 Mar 1. PMID: 31538027; PMCID: PMC6750920.
CDSCO (2019). CDSCO. Available at: https://cdsco.gov.in/opencms/resources/UploadCDSCOWeb/2018/UploadPrescribingInfo/3Ribo ciclib%20India%20PI.pdf.
Chang, L. C., Wu, S. C., Tsai, J. W., Yu, T. J., and Tsai, T. R. (2009). Optimization of epirubicin nanoparticles using experimental design for enhanced intravesical drug delivery. Int. J. Pharm. 376 (1-2), 195–203. doi:10.1016/j.ijpharm.2009.04.045
Chatterjee, G., Nimrat Walker, H. K., Lucia Ms, T. J. D. C., et al. (2017). Hyochol ahn 2017, HHS public access. Physiol. Behav. 176 (10), 139–148. Nanoparticle-mediated. doi:10.1016/j.bbcan.2019.04.006
Cheer, S. M., Plosker, G. L., Simpson, D., and Wagstaff, A. J. (2005). Goserelin: A review of its use in the treatment of early breast cancer in premenopausal and perimenopausal women. Drugs 65 (18), 2639–2655. doi:10.2165/00003495-200565180-00011
Cheralayikkal, S., Manoj, K., and Safna Hussan, K. P. (2022). Formulation and evaluation of a smart drug delivery system of 5-fluorouracil for pH-sensitive chemotherapy. Heliyon 8 (7), e09926. doi:10.1016/j.heliyon.2022.e09926
Cho, A., and Moniri, N. H. (2016). HHS public access. Physiol. Behav. 176 (1), 100–106. doi:10.1039/c4nr07102f.Nanoparticle
Chuang, E., Wiener, N., Christos, P., Kessler, R., Cobham, M., Donovan, D., et al. (2010). Phase I trial of ixabepilone plus pegylated liposomal doxorubicin in patients with adenocarcinoma of breast or ovary. Ann. Oncol. 21 (10), 2075–2080. doi:10.1093/annonc/mdq080
Clinicaltrials.Gov (2015). Neoadjuvant Toremifene with Melatonin or Metformin in locally advanced breast cancer. United States: Clinicaltrials.Gov.
Comte, A., Jdid, W., Guilhaume, M. N., Kriegel, I., Piperno-Neumann, S., Dieras, V., et al. (2013). Survival of breast cancer patients with meningeal carcinomatosis treated by intrathecal thiotepa. J. Neurooncol. 115, 445–452. doi:10.1007/s11060-013-1244-x
Conte, P. F., Gennari, A., Landucci, E., and Orlandini, C. (2000). Role of epirubicin in advanced breast cancer. Clin. Breast Cancer 1, S46–S51. doi:10.3816/cbc.2000.s.009
Cortesi, L., Rugo, H. S., and Jackisch, C. (2021). An overview of PARP inhibitors for the treatment of breast cancer. Oncol 16 (3), 255–282. doi:10.1007/s11523-021-00796-4
Curigliano, G., and Criscitiello, C. (2014). Successes and limitations of targeted cancer therapy in breast cancer. Prog. tumor Res. 41, 15–35. doi:10.1159/000355896
Cybulska-Stopa, B., Ziobro, M., Skoczek, M., Kojs-Pasinska, E., Cedrych, I., and Brandys, A. (2013). Evaluation of vinorelbine-based chemotherapy as the second or further-line treatment in patients with metastatic breast cancer. Wspolczesna Onkol. 17 (1), 78–82. doi:10.5114/wo.2013.33779
De Angel, R. E., Blando, J. M., Hogan, M. G., Sandoval, M. A., Lansakara, -P. D. S., Dunlap, S. M., et al. (2013). Stearoyl gemcitabine nanoparticles overcome obesity-induced cancer cell resistance to gemcitabine in a mouse postmenopausal breast cancer model. Cancer Biol. Ther. 14 (4), 357–364. doi:10.4161/cbt.23623
Devi, L., Gupta, R., Jain, S. K., Singh, S., and Kesharwani, P. (2020). Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of Gemcitabine with natural polysaccharides for treatment of breast cancer. J. Drug Deliv. Sci. Technol. 56, 101565. doi:10.1016/j.jddst.2020.101565
Dhman, D. (2017). Nanogel as a pharmaceutical carrier – review article nanogel as a pharmaceutical carrier – review article. Sch. J. Appl. Med. Sci. 2017, 4730–4736. doi:10.21276/sjams.2017.5.11.83
Di Francesco, M., Celia, C., Cristiano, M. C., d'Avanzo, N., Ruozi, B., Mircioiu, C., et al. (2021). Doxorubicin hydrochloride-loaded nonionic surfactant vesicles to treat metastatic and non-metastatic breast cancer. ACS Omega 6 (4), 2973–2989. doi:10.1021/acsomega.0c05350
Dreaden, E. C., Mwakwari, S. C., Sodji, Q. H., Oyelere, A. K., and El-Sayed, M. A. (2009). Tamoxifen− poly (ethylene glycol)−thiol gold nanoparticle conjugates: Enhanced potency and selective delivery for breast cancer treatment. Bioconjugate Chem. 20 (12), 2247–2253. doi:10.1021/bc9002212
Drug bank (2005). Drug Bank. Available at: https://go.drugbank.com/drugs/DB11760.
Drugbank (2023). DrugBank. Available at: https://go.drugbank.com/drugs/DB00014.
Du, X., Khan, A. R., Fu, M., Ji, J., Yu, A., and Zhai, G. (2018). Current development in the formulations of non-injection administration of paclitaxel. Int. J. Pharm. 542 (1-2), 242–252. doi:10.1016/j.ijpharm.2018.03.030
Du, M., Ouyang, Y., Meng, F., Ma, Q., Liu, H., Zhuang, Y., et al. (2019). Nanotargeted agents: An emerging therapeutic strategy for breast cancer. Nanomedicine 14 (13), 1771–1786. doi:10.2217/nnm-2018-0481
DurgBank (2023). Drugs at FDA. Available at: https://go.drugbank.com/drugs/DB11730.
Dybdal-Hargreaves, N. F., Risinger, A. L., and Mooberry, S. L. (2015). Eribulin Mesylate: Mechanism of action of a unique microtubule-Targeting agent. Clin. Cancer Res. 21 (11), 2445–2452. doi:10.1158/1078-0432.CCR-14-3252
Egerton, N. (2007). Ixabepilone (ixempra), a therapeutic option for locally advanced or metastatic breast cancer. Pharm. Ther. 33 (9), 523.
Ekedahl, H., Isaksson, S., Ståhl, O., Bogefors, K., Romerius, P., Eberhard, J., et al. (2022). Low-grade inflammation in survivors of childhood cancer and testicular cancer and its association with hypogonadism and metabolic risk factors. BMC cancer 22 (1), 157. doi:10.1186/s12885-022-09253-5
Elena, S., Guerra, M., Dias-Ferreira, J., Lopez-Machado, A., Ettcheto, M., Cano, A., et al. (2019). Current applications of nanoemulsions in cancer therapeutics. Nanomaterials 9, 821. doi:10.3390/nano9060821
Elith*, J., Graham* C, H., Anderson R, P., Dudík, M., Ferrier, S., Guisan, A., et al. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography 29 (2), 129–151. doi:10.1111/j.2006.0906-7590.04596.x
Emens, L. A., Adams, S., Barrios, C. H., Diéras, V., Iwata, H., Loi, S., et al. (2021). First-line atezolizumab plus nab-paclitaxel for unresectable, locally advanced, or metastatic triple-negative breast cancer: IMpassion130 final overall survival analysis. Ann. Oncol. 32 (8), 983–993. doi:10.1016/j.annonc.2021.05.355
Epirubicin, N. A. (1992). Growth hormone human. React. Wkly. 412, 6. doi:10.2165/00128415-199204120-00021
Evans, A. J., James, J. J., Cornford, E. J., Chan, S. Y., Burrell, H. C., Pinder, S. E., et al. (2004). Brain metastases from breast cancer: Identification of a high-risk group. Clin. Oncol. 16 (5), 345–349. doi:10.1016/j.clon.2004.03.012
Falvo, P., Orecchioni, S., Hillje, R., Raveane, A., Mancuso, P., Camisaschi, C., et al. (2021). Cyclophosphamide and vinorelbine activate stem-like CD8+ T cells and improve anti-PD-1 efficacy in triple-negative breast cancer. Cancer Res. 81 (3), 685–697. doi:10.1158/0008-5472.CAN-20-1818
Fan, Y. (2021). Cancer cell membrane-coated nanosuspensions for enhanced chemotherapeutic treatment of glioma. Molecules 26 (16). doi:10.3390/molecules26165103
FDA (1959). These highlights do not include all the information needed to use CYCLOPHOSPHAMIDE INJECTION safely and effectively. See full prescribing information for CYCLOPHOSPHAMIDE INJECTION. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/212501s000lbl.pdf.
FDA (1995). These highlights do not include all the information needed to use ARIMIDEX safely and effectively. See full prescribing information for ARIMIDEX. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/020541s031lbl.pdf.
FDA (1998). These highlights do not include all the information needed to use Herceptin safely and effectively. See full prescribing information for Herceptin. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/103792s5250lbl.pdf.
FDA (1999). These highlights do not include all the information needed to use ELLENCE safely and effectively. See full prescribing information for ELLENCE. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/050778s021lbl.pdf.
FDA (2005). Assessd date, 200523. Available at:https://www.fda.gov/regulatory-information/search-fda-guidance-documents/initiation-voluntary-recalls-under-21-cfr-part-7-subpart-c.
FDA (2009). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/021113s008lbl.pdf.
FDA (2010a). Bohringer. Available at:https://docs.boehringer.ingelheim.com/Prescribing%20Information/PIs/Ben%20Venue_Bedford%20Labs/55390-091-10%20VIN%2010MG/5539009110.
FDA (2010b). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022334s6lbl.pdf.
FDA (2010c). These highlights do not include all the information needed to use FASLODEX® safely and effectively. See full prescribing information for FASLODEX. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021344s015lbl.pdf.
FDA (2011). Assessed date 200523. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/020262s049lbl.pdf.
FDA (2012a). accessdata.fda. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/062921s022lbl.pdf.
FDA (2012b). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2012/125409orig1s000ltr.pdf.
FDA (2012c). Cyclophosphamide for injection, USP cyclophosphamide tablets, USP. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/012142s109lbl.pdf.
FDA (2012d). Goserelin. Available at: http://www.bccancer.bc.ca/drug-database-site/Drug%20Index/Goserelin_monograph_1Aug2012_formatted.pdf.
FDA (2013). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/012141s090,012142s112lbl.pdf.
FDA (2015a). Assessed date, 200523. Available at:https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/020896s037lbl.pdf.
FDA (2015b). These highlights do not include all the information needed to use IBRANCE safely and effectively. See full prescribing information for IBRANCE. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/207103s008lbl.pdf.
FDA (2017a). Assessed date drugsatfda. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf.
FDA (2017b). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208051s000lbl.pdf.
FDA (2017c). These highlights do not include all the information needed to use KEYTRUDA safely and effectively. See full prescribing information for KEYTRUDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014lbl.pdf.
FDA (2018). These highlights do not include all the information needed to use TYKERB safely and effectively. See full prescribing information for TYKERB. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/022059s007lbl.pdf.
FDA (2019). These highlights do not include all the information needed to use GILOTRIF safely and effectively. See full prescribing information for GILOTRIF. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/201292s015lbl.pdf.
FDA (2020). Highlights of prescribing information. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/761034s028lbl.pdf.
FDA (2020). Assessed date 200523. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/021660s047lbl.pdf
FDA (2021). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/761034s042lbl.pdf.
FDA (2021). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/125514s096lbl.pdf.
FDA (2022). Drugs at FDA. Available at: https://investor.lilly.com/news-releases/news-release-details/us-fda-broadens-indication-verzenior-abemaciclib-hr-her2-node.
FDA (2023). Drugs at FDA. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/213411s004lbl.pdf.
Fei, Z., and Yoosefian, M. (2021). Design and development of polymeric micelles as nanocarriers for anti-cancer Ribociclib drug. J. Mol. Liq. 329, 115574. doi:10.1016/j.molliq.2021.115574
Fjøsne, H. E., Jacobsen, A. B., and Lundgren, S.Norwegian Breast Cancer Group NBCG (2008). Adjuvant cyclic Tamoxifen and Megestrol acetate treatment in postmenopausal breast cancer patients - longterm follow-up. Eur. J. Surg. Oncol. 34 (1), 6–12. doi:10.1016/j.ejso.2007.07.002
Fonseca, A. M., Araújo, C. C. B., da Silva, J. H., Honório, T. S., Nasciutti, L. E., Cabral, L. M., et al. (2021). Development of transdermal based hydrogel formulations of vinorelbine with an evaluation of their in vitro profiles and activity against melanoma cells and in silico prediction of drug absorption. J. Drug Deliv. Sci. Technol. 63, 102449. doi:10.1016/j.jddst.2021.102449
Fox, C. P., Ali, A. S., McIlroy, G., Thust, S., Martinez-Calle, N., Jackson, A. E., et al. (2021). A phase 1/2 study of thiotepa-based immunochemotherapy in relapsed/refractory primary CNS lymphoma: The TIER trial. Blood Adv. 5 (20), 4073–4082. doi:10.1182/bloodadvances.2021004779
Ganta, S., Talekar, M., Singh, A., Coleman, T. P., and Amiji, M. M. (2014). Nanoemulsions in translational research - opportunities and challenges in targeted cancer therapy. AAPS PharmSciTech 15 (3), 694–708. doi:10.1208/s12249-014-0088-9
García-García, G., Fernández-Álvarez, F., Cabeza, L., Delgado, Á. V., Melguizo, C., Prados, J. C., et al. (2020). Gemcitabine-loaded magnetically responsive poly(ε-caprolactone) nanoparticles against breast cancer. Polym. (Basel) 12 (12), 2790–2817. doi:10.3390/polym12122790
García-García etal, G. (2020). Gemcitabine-loaded magnetically responsive poly(ε-caprolactone) nanoparticles against breast cancer. Polym. (Basel) 12 (12), 1–17. doi:10.3390/polym12122790
Gavas, S., Quazi, S., and Karpiński, T. M. (2021). Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Res. Lett. 16 (1), 173. doi:10.1186/s11671-021-03628-6
Ge, Y., Domschke, C., Stoiber, N., Schott, S., Heil, J., Rom, J., et al. (2012). Metronomic cyclophosphamide treatment in metastasized breast cancer patients: Immunological effects and clinical outcome. Cancer Immunol. Immunother. 61 (3), 353–362. doi:10.1007/s00262-011-1106-3
Goldhirsch, A., Leuenberger, U., Ryssel, H. J., Cavalli, F., Sonntag, R. W., Joss, R. A., et al. (1982). Combination hormonotherapy with tamoxifen and fluoxymesterone in patients with advanced breast cancer relapsing on hormonotherapy. Oncol 39 (5), 284–286. doi:10.1159/000225652
Goss, P. E., and Tye, L. M. (1997). Anastrozole: A new selective nonsteroidal aromatase inhibitor. Oncol. Willist. Park. 11 (11), 1697–1703.
Gote, V., Nookala, A. R., Bolla, P. K., and Pal, D. (2021). Drug resistance in metastatic breast cancer: Tumor targeted nanomedicine to the rescue. Int. J. Mol. Sci. 22 (9), 4673. doi:10.3390/ijms22094673
Gregory, E. J., Cohen, S. C., Oines, D. W., and Mims, C. H. (1985). Megestrol acetate therapy for advanced breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 3 (2), 155–160. doi:10.1200/JCO.1985.3.2.155
Guerreiro, M. P., Maximiano, S., Magalha, P., and Morgado, M. (2016). Trastuzumab in the Treatment of Breast Cancer several other therapeutic issues remain unclear and have. Bio Drugs 30, 75. doi:10.1007/s40259-016-0162-9
Gupta, A., Eral, H. B., Hatton, T. A., and Doyle, P. S. (2016). Nanoemulsions: Formation, properties and applications. Soft Matter 12 (11), 2826–2841. doi:10.1039/c5sm02958a
Gupta, A., Bandaru, S., and Manthri, S. (2021). Goserelin ovarian ablation failure in premenopausal women with breast cancer. Breast Cancer 13, e19608–e19617. doi:10.7759/cureus.19608
Han, B., Wang, T., Xue, Z., Wen, T., Lu, L., Meng, J., et al. (2021). Elemene nanoemulsion inhibits metastasis of breast cancer by ROS scavenging. Int. J. Nanomedicine 16, 6035–6048. doi:10.2147/IJN.S327094
Handali, S., Moghimipour, E., Kouchak, M., Ramezani, Z., Amini, M., Angali, K. A., et al. (2019). New folate receptor targeted nano liposomes for delivery of 5-fluorouracil to cancer cells: Strong implication for enhanced potency and safety. Life Sci. 227, 39–50. doi:10.1016/j.lfs.2019.04.030
Hanna, A. D., Lam, A., Tham, S., Dulhunty, A. F., and Beard, N. A. (2014). Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Mol. Pharmacol. 86 (4), 438–449. doi:10.1124/mol.114.093849
Hart, S. E., Brown, D. L., Kim, H. M., Qi, J., Hamill, J. B., and Wilkins, E. G. (2021). Association of clinical complications of chemotherapy and patient-reported outcomes after immediate breast reconstruction. JAMA Surg. 156 (9), 847–855. doi:10.1001/jamasurg.2021.2239
Hartshorn, C. M., Russell, L. M., and Grodzinski, P. (2019). National Cancer Institute Alliance for nanotechnology in cancer-Catalyzing research and translation toward novel cancer diagnostics and therapeutics. Wiley Interdiscip. Rev. Nanomedicine nanobiotechnology. 11 (6), e1570. doi:10.1002/wnan.1570
Hofheinz, R. D., Gnad-Vogt, S. U., Beyer, U., and Hochhaus, A. (2005)., 16. Drugs, 691–707. Liposomal encapsulated anti-cancer drugsAnticancer. Drugs7 doi:10.1097/01.cad.0000167902.53039.5a
Holmberg, L., Ekbom, A., Calle, E., Mokdad, A., and Byers, T. (1997). Breast cancer mortality in relation to self-reported use of breast self-examination. A cohort study of 450,000 women. Breast cancer Res. Treat. 43, 137–140. doi:10.1023/a:1005788729145
Hong, J., Huang, J., Shen, L., Zhu, S., Gao, W., Wu, J., et al. (2020). A prospective, randomized study of Toremifene vs. tamoxifen for the treatment of premenopausal breast cancer: Safety and genital symptom analysis. BMC Cancer 20 (1), 663–710. doi:10.1186/s12885-020-07156-x
Horo, H., Das, S., Mandal, B., and Kundu, L. M. (2019). Development of a photoresponsive chitosan conjugated prodrug nano-carrier for controlled delivery of antitumor drug 5-fluorouracil. Int. J. Biol. Macromol. 121, 1070–1076. doi:10.1016/j.ijbiomac.2018.10.095
Hortobagyi, G. N. (2018). Ribociclib for the first-line treatment of advanced hormone receptor-positive breast cancer: A review of subgroup analyses from the MONALEESA-2 trial. Breast Cancer Res. 20 (1), 123–211. doi:10.1186/s13058-018-1050-7
Houdaihed, L., Evans, J. C., and Allen, C. (2018). Codelivery of paclitaxel and Everolimus at the optimal synergistic Ratio: A promising solution for the treatment of breast cancer. Mol. Pharm. 15 (9), 3672–3681. doi:10.1021/acs.molpharmaceut.8b00217
Huang, H., Menefee, M., Edgerly, M., Zhuang, S., Kotz, H., Poruchynsky, M., et al. (2010). A phase II clinical trial of ixabepilone (Ixempra; BMS-247550; NSC 710428), an epothilone B analog, in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 16 (5), 1634–1641. doi:10.1158/1078-0432.CCR-09-0379
Hussain, Z., Khan, J. A., and Murtaza, S. (2018). Nanotechnology: An emerging therapeutic option for breast cancer. Crit. Rev. Eukaryot. Gene Expr. 28 (2), 163–175. doi:10.1615/CritRevEukaryotGeneExpr.2018022771
Ibrahim, N. K. (2021). Ixabepilone: Overview of effectiveness, safety, and tolerability in metastatic breast cancer. Front. Oncol. 11. doi:10.3389/fonc.2021.617874
Iirola, T., Aantaa, R., Laitio, R., Kentala, E., Lahtinen, M., Wighton, A., et al. (2011). Pharmacokinetics of prolonged infusion of high-dose dexmedetomidine in critically ill patients. Crit. care 15 (5), 2577–R260. doi:10.1186/cc10518
Ixabepilone (2006). PubChem. Available at: https://pubchem.ncbi.nlm.nih.gov/compound/Ixabepilone.
Jain, S., and Vahdat, L. T. (2011). Eribulin mesylate. Clin. Cancer Res. 17 (21), 6615–6622. doi:10.1158/1078-0432.CCR-11-1807
Jaiswal, M., and Dudhe, R. (2015). Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech. 5, 123–127. doi:10.1007/s13205-014-0214-0
Jayapal, J. J., and Dhanaraj, S. (2017). Exemestane loaded alginate nanoparticles for cancer treatment: Formulation and in vitro evaluation. Int. J. Biol. Macromol. 105 (1), 416–421. doi:10.1016/j.ijbiomac.2017.07.064
Jerusalem, G., de Boer, R. H., Hurvitz, S., Yardley, D. A., Kovalenko, E., Ejlertsen, B., et al. (2018). Everolimus plus exemestane vs Everolimus or capecitabine monotherapy for estrogen receptor-positive, HER2-negative advanced breast cancer: The BOLERO-6 randomized clinical trial. JAMA Oncol. 4 (10), 1367–1374. doi:10.1001/jamaoncol.2018.2262
Ji, Y., Liu, X., Li, J., Xie, X., Huang, M., Jiang, J., et al. (2020). Use of ratiometrically designed nanocarrier targeting CDK4/6 and autophagy pathways for effective pancreatic cancer treatment. Nat. Commun. 11 (1), 4249. doi:10.1038/s41467-020-17996-7
Junnuthula, V., Kolimi, P., Nyavanandi, D., and Sampathi, S. (2022). Polymeric micelles for breast cancer therapy: Recent updates, clinical translation and regulatory considerations. Pharmaceutics 14, 1860. doi:10.3390/pharmaceutics14091860
Kabos, P., and Borges, V. F. (2010). Fulvestrant: A unique antiendocrine agent for estrogen-sensitive breast cancer. Expert Opin. Pharmacother. 11 (5), 807–816. doi:10.1517/14656561003641982
Kanojia, D., Panek, W. K., Cordero, A., Fares, J., Xiao, A., Savchuk, S., et al. (2020). BET inhibition increases βIII-tubulin expression and sensitizes metastatic breast cancer in the brain to vinorelbine. Sci. Transl. Med. 12, eaax2879. doi:10.1126/SCITRANSLMED.AAX2879
Karthick, V., and Panda, S. (2019). Quercetin loaded PLGA microspheres induce apoptosis in breast cancer cells. Appl. Surf. Sci. 487, 211–217. doi:10.1016/j.apsusc.2019.05.047
Karthick, V., Panda, S., Kumar, V. G., Kumar, D., Shrestha, L. K., Ariga, K., et al. (2019). Quercetin loaded PLGA microspheres induce apoptosis in breast cancer cells. Appl. Surf. Sci. 487, 211–217. doi:10.1016/j.apsusc.2019.05.047
Kashyap, D., Tuli, H. S., Yerer, M. B., Sharma, A., Sak, K., Srivastava, S., et al. (2021). Natural product-based nanoformulations for cancer therapy: Opportunities and challenges. Semin. Cancer Biol. 69, 5–23. doi:10.1016/J.SEMCANCER.2019.08.014
Kasprzak, A., Gunka, K., Fronczak, M., Bystrzejewski, M., and Poplawska, M. (2018). Folic acid-navigated and β-cyclodextrin-decorated carbon-encapsulated iron nanoparticles as the nanotheranostic platform for controlled release of 5-fluorouracil. ChemistrySelect 3 (38), 10821–10830. doi:10.1002/slct.201802318
Kaur, H., Kaur, K., Singh, A., Bedi, N., Singh, B., Alturki, M. S., et al. (2022). Frankincense oil-loaded nanoemulsion formulation of paclitaxel and erucin: A synergistic combination for ameliorating drug resistance in breast cancer: In vitro and in vivo study. Front. Pharmacol. 13, 1020602–1020614. doi:10.3389/fphar.2022.1020602
Kelly, C. M., and Buzdar, A. U. (2010). Anastrozole. Expert Opin. Drug Saf. 9 (6), 995–1003. doi:10.1517/14740338.2010.515977
Kennedy, B. J. (1958). Fluoxymesterone therapy in advanced breast cancer. N. Engl. J. Med. 259 (14), 673–675. doi:10.1056/NEJM195810022591404
Kerr, A. J., Dodwell, D., McGale, P., Holt, F., Duane, F., Mannu, G., et al. (2022). Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 105, 102375. doi:10.1016/j.ctrv.2022.102375
Khan, M. T., Uddin, Z., Javed, M. A., Shah, N., Bashir, H., Shaikh, A. J., et al. (2022). PEGylated protamine letrozole nanoparticles: A promising strategy to combat human breast cancer via MCF-7 cell lines. Biomed. Res. Int. 2022, 4438518. doi:10.1155/2022/4438518
Kircik, L. H. (2011). Microsphere Technology: Hype or help. J. Clin. Aesthet. Dermatol. 4 (5), 27–31.
Kuemmel, S., Harrach, H., Schmutzler, R. K., Kostara, A., Ziegler-Löhr, K., Dyson, M. H., et al. (2020). Olaparib for metastatic breast cancer in a patient with a germline PALB2 variant. npj Breast Cancer 6 (1), 31–34. doi:10.1038/s41523-020-00174-9
Kumar, B., Sandhu, K., and Kaur, I. (2004). Topical 0.25% methotrexate gel in a hydrogel base for palmoplantar psoriasis. J. Dermatol. 31 (10), 798–801. doi:10.1111/j.1346-8138.2004.tb00602.x
Kumar, M., Bishnoi, R. S., Shukla, A. K., and Jain, C. P. (2019). Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 24 (3), 225–234. doi:10.3746/pnf.2019.24.3.225
Kushwah, V., Katiyar, S. S., Dora, C. P., Kumar Agrawal, A., Lamprou, D. A., Gupta, R. C., et al. (2018). Co-delivery of docetaxel and gemcitabine by anacardic acid modified self-assembled albumin nanoparticles for effective breast cancer management. Acta Biomater. 73, 424–436. doi:10.1016/j.actbio.2018.03.057
Lam, S. W., Guchelaar, H. J., and Boven, E. (2016). The role of pharmacogenetics in capecitabine efficacy and toxicity. Cancer Treat. Rev. 50, 9–22. doi:10.1016/j.ctrv.2016.08.001
Lan, B., Ma, F., Chen, S., Wang, W., Li, Q., Fan, Y., et al. (2018). Toremifene, rather than tamoxifen, might be a better option for the adjuvant endocrine therapy in CYP2D6*10T/T genotype breast cancer patients in China. Int. J. Cancer 143 (10), 2499–2504. doi:10.1002/ijc.31639
Lazar, G., Nekvapil, F., Hirian, R., Glamuzina, B., Tamas, T., Barbu-Tudoran, L., et al. (2021). Novel drug carrier: 5-Fluorouracil formulation in nanoporous biogenic Mg-calcite from Blue Crab shells - proof of concept. ACS Omega 6 (42), 27781–27790. doi:10.1021/acsomega.1c03285
Lei, M., Sha, S., Wang, X., Wang, J., Du, X., Miao, H., et al. (2019). Co-delivery of paclitaxel and gemcitabine via a self-assembling nanoparticle for targeted treatment of breast cancer. RSC Adv. 9 (10), 5512–5520. doi:10.1039/c9ra00276f
Leo, C. P., Leo, C., and Szucs, T. D. (2020). Breast cancer drug approvals by the US FDA from 1949 to 2018. Nat. Rev. Drug Discov. 19 (1), 11. doi:10.1038/d41573-019-00201-w
Levine, M. (2000). Epirubicin in breast cancer: Present and future. Clin. Breast Cancer 1, S62–S67. doi:10.3816/cbc.2000.s.012
Li, J., Malakhova, M., Mottamal, M., Reddy, K., Kurinov, I., Carper, A., et al. (2012). Norathyriol suppresses skin cancers induced by solar ultraviolet radiation by targeting ERK kinases. Cancer Res. 72 (1), 260–270. doi:10.1158/0008-5472.can-11-2596
Li, Z., Liu, K., Sun, P., Mei, L., Hao, T., Tian, Y., et al. (2013). Poly (D, L-lactide-co-glycolide)/montmorillonite nanoparticles for improved oral delivery of exemestane. J. Microencapsul. 30 (5), 432–440. doi:10.3109/02652048.2012.746749
Li, W., Gong, K., Ding, Y., Chaurasiya, B., Ni, Y., Wu, Y., et al. (2019). Effects of triptolide and methotrexate nanosuspensions on left ventricular remodeling in autoimmune myocarditis rats. Int. J. Nanomedicine 14, 851–863. doi:10.2147/IJN.S191267
Li, J. Y., Tang, Y. H., Tang, L., and Chen, L. Y. (2022). Adsorption of thiotepa anticancer drugs on the C (3)N nanotube as promising nanocarriers for drug delivery. J. Mol. Model. 28 (9), 249. doi:10.1007/s00894-022-05248-y
Lin, Y., Maham, A., Tang, Z., Wu, H., Wang, J., and Lin, Y. (2009). Protein-based nanomedicine platforms for drug delivery. Small 15, 1706–1721. doi:10.1002/smll.200801602
Liu, G., Chen, Y., Lu, C., and Lu, A. (2020). pH-responsive fluorescence enhanced nanogel for targeted delivery of AUR and CDDP against breast cancer. Int. J. Nanomedicine 15, 8369–8382.
Lo, Y. W., Sheu, M. T., Chiang, W. H., Chiu, Y. L., Tu, C. M., Wang, W. Y., et al. (2019). In situ chemically crosslinked injectable hydrogels for the subcutaneous delivery of trastuzumab to treat breast cancer. Acta biomater. 86, 280–290. doi:10.1016/j.actbio.2019.01.003
Longley, D. B., Harkin, D. P., and Johnston, P. G. (2003). 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 3 (5), 330–338. doi:10.1038/nrc1074
Luo, D., Jusko, W. J., Carter, K. A., Molins, E. A. G., Straubinger, N. L., Geng, J., et al. (2020). Pharmacokinetics and pharmacodynamics of liposomal chemophototherapy with short drug-light intervals. J. Control Release 297, 39–47. doi:10.1016/j.jconrel.2019.01.030
Luo, L., Xu, F., Peng, H., Luo, Y., Tian, X., Battaglia, G., et al. (2020). Stimuli-responsive polymeric prodrug-based nanomedicine delivering nifuroxazide and doxorubicin against primary breast cancer and pulmonary metastasis. J. Control. Release 318, 124–135. doi:10.1016/j.jconrel.2019.12.017
Mahato, R. (2017). Nanoemulsion as targeted drug delivery system for cancer therapeutics. J. Pharm. Sci. Pharmacol. 3 (2), 83–97. doi:10.1166/jpsp.2017.1082
Maji, R., Dey, N. S., Satapathy, B. S., Mukherjee, B., and Mondal, S. (2014). Preparation and characterization of Tamoxifen citrate loaded nanoparticles for breast cancer therapy. Int. J. nanomedicine 9, 3107–3118. doi:10.2147/IJN.S63535
Marcucci, C., and María, R. (2004). Patent application publication (10) pub. No.: US 2004/0092606A1, United States - pat. Appl. Publ. 1 (1–9), 2002.
Markes, M., Brockow, T., and Resch, K. L. (2006). Exercise for women receiving adjuvant therapy for breast cancer. Cochrane Database Syst. Rev. 2006 (4), CD005001. doi:10.1002/14651858.CD005001
Markham, A. (2021). Margetuximab: First approval. Drugs 81 (5), 599–604. doi:10.1007/s40265-021-01485-2
Marupudi, N. I., Han, J. E., Li, K. W., Renard, V. M., Tyler, B. M., and Brem, H. (2007). Paclitaxel: A review of adverse toxicities and novel delivery strategies. Expert Opin. Drug Saf. 6 (5), 609–621. doi:10.1517/14740338.6.5.609
Massadeh, S., Almohammed, I., Barhoush, E., Omer, M., Aldhawi, N., Almalik, A., et al. (2021). Development of epirubicin-loaded biocompatible polymer pla–peg–pla nanoparticles: Synthesis, characterization, stability, and in vitro anticancerous assessment. Polym. (Basel) 13, 1212–1218. doi:10.3390/polym13081212
Mazzarino, M., Biava, M., De Torre, X., Fiacco, I., and Botrè, F. (2013). Characterization of the biotransformation pathways of clomiphene, tamoxifen and toremifene as assessed by LC-MS/(MS) following in vitro and excretion studies. Anal. Bioanal. Chem. 405, 5467–5487. doi:10.1007/s00216-013-6961-7
Mcbride, A., and Butler, S. K. (2012). Eribulin mesylate: A novel halichondrin B analogue for the treatment of metastatic breast cancer. Am. J. Heal. Pharm. 69 (9), 745–755. doi:10.2146/ajhp110237
McCartney, A., Moretti, E., Sanna, G., Pestrin, M., Risi, E., Malorni, L., et al. (2018). The role of abemaciclib in treatment of advanced breast cancer. Ther. Adv. Med. Oncol. 10, 1758835918776925–14. doi:10.1177/1758835918776925
Migotto, A., Carvalho, V. F. M., Salata, G. C., da Silva, F. W. M., Yan, C. Y. I., Ishida, K., et al. (2018). Multifunctional nanoemulsions for intraductal delivery as a new platform for local treatment of breast cancer. Drug Deliv. 25, 654–667. doi:10.1080/10717544.2018.1440665
Miles, J., and White, Y. (2018). Neratinib for the treatment of early-stage HER2-positive breast cancer. J. Adv. Pract. Oncol. 9 (7), 750–754.
Miller, W. R. (2003). Aromatase inhibitors: Mechanism of action and role in the treatment of breast cancer. Semin. Oncol. 7754 (04), 3–11. doi:10.1016/s0093-7754(03)00302-6
Mirkes, P. E. (1985). Cyclophosphamide teratogenesis: A review. Teratogenesis, Carcinog. Mutagen. 88, 75–88. doi:10.1002/tcm.1770050202
Moura, J. A., Valduga, C. J., Tavares, E. R., Kretzer, I. F., Maria, D. A., and Maranhão, R. C. (2011). Novel formulation of a methotrexate derivative with a lipid nanoemulsion. Int. J. Nanomedicine 6, 2285–2295. doi:10.2147/IJN.S18039
Mozar, F. S., and Chowdhury, E. H. (2017). Surface-modification of carbonate apatite nanoparticles enhances delivery and cytotoxicity of gemcitabine and anastrozole in breast cancer cells. Pharmaceutics 9, 21–2. doi:10.3390/pharmaceutics9020021
Murthy, R. K., Loi, S., Okines, A., Paplomata, E., Hamilton, E., Hurvitz, S. A., et al. (2020). Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N. Engl. J. Med. 382 (7), 597–609. doi:10.1056/nejmoa1914609
Mustonen, M. V., Pyrhönen, S., and Kellokumpu-Lehtinen, P. L. (2014). Toremifene in the treatment of breast cancer. World J. Clin. Oncol. 5 (3), 393–405. doi:10.5306/wjco.v5.i3.393
Najafi, M., Majidpoor, J., Toolee, H., and Mortezaee, K. (2021). The current knowledge concerning solid cancer and therapy. J. Biochem. Mol. Toxicol. 35, e22900. doi:10.1002/jbt.22900
Nathan, M. R., and Schmid, P. (2017). A review of fulvestrant in breast cancer. Ther 5 (1), 17–29. doi:10.1007/s40487-017-0046-2
National Cancer Institute (2017). Drugs approved to treat breast cancer. Maryland: National Cancer Institute.
Neven, P., Rugo, H. S., Tolaney, S. M., Iwata, H., Toi, M., Goetz, M. P., et al. (2021). Abemaciclib plus fulvestrant in hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in premenopausal women: Subgroup analysis from the MONARCH 2 trial. Breast Cancer Res. 23 (1), 87–10. doi:10.1186/s13058-021-01463-2
Nirmala, M. J., Shiny, P. J., Dhas, S. P., Kizhuveetil, U., Raj, U. S., and Nagarajan, R. (2022). “Application of nanoemulsions in breast cancer treatment,” in Handbook of research on nanoemulsion applications in agriculture, food, Health, and biomedical sciences (United States: IGI Global), 277–306.
Nobs, L., Buchegger, F., Gurny, R., and Allémann, E. (2004). Poly(lactic acid) nanoparticles labeled with biologically active Neutravidin for active targeting. Eur. J. Pharm. Biopharm. 58 (3), 483–490. doi:10.1016/j.ejpb.2004.04.006
Nounou, M. I., ElAmrawy, F., Ahmed, N., Abdelraouf, K., Goda, S., and Syed-Sha-Qhattal, H. (2015). Breast cancer: Conventional diagnosis and treatment modalities and recent patents and technologies. Breast Cancer (Auckl). 9 (2), 17–34. doi:10.4137/BCBCR.S29420
O’Brien, M. E., Wigler, N., Inbar, M. C. B. C. S. G., Rosso, R., Grischke, E., Santoro, A., et al. (2004). Reduced cardiotoxicity and comparable efficacy in a phase IIItrial of pegylated liposomal doxorubicin HCl (CAELYX™/Doxil®) versus conventional doxorubicin forfirst-line treatment of metastatic breast cancer. Ann. Oncol. 15 (3), 440–449. doi:10.1093/annonc/mdh097
Of, H., and Information, P. (2010). FULL PRESCRIBING INFORMATION [FINAL] reference ID: 2863825 at a reduced dose and initiate the next cycle no sooner than 2 weeks later.
Ormrod, D., Holm, K., Goa, K., and Spencer, C. (1999). Epirubicin: A review of its efficacy as adjuvant therapy and in the treatment of metastatic disease in breast cancer. Drugs Aging 15 (5), 389–416. doi:10.2165/00002512-199915050-00006
Orrantia-Borunda, E., Anchondo-Nuñez, P., Acuña-Aguilar, L. E., Gómez-Valles, F. O., and Ramírez-Valdespino, C. A. (2022). Subtypes of breast cancer Editor H. N. Mayrovitz (Brisbane (AU).
Oyediran, K. O., Ilomuanya, M. O., Azubuike, C. P., and Nurudeen, L. (2022). A multiscale approach to targeted docetaxel formulations: Combination therapy, nanotechnology, electrospinning and 3D printing—a review. Bull. Natl. Res. Cent. 46, 167. doi:10.1186/s42269-022-00854-5,
Ozgenc, E., Karpuz, M., Arzuk, E., Gonzalez-Alvarez, M., Sanz, M. B., Gundogdu, E., et al. (2022). Radiolabeled trastuzumab solid lipid nanoparticles for breast cancer cell: In vitro and in vivo studies. ACS Omega 7 (34), 30015–30027. doi:10.1021/acsomega.2c03023
Pandey, P., Gulati, N., Makhija, M., Purohit, D., and Dureja, H. (2020). Nanoemulsion: A novel drug delivery approach for enhancement of bioavailability. Recent Pat. Nanotechnol. 14 (4), 276–293. doi:10.2174/1872210514666200604145755
Papich, M. G. (2021). Megestrol acetate. Papich Handb. Vet. Drugs 4, 562–563. doi:10.1016/b978-0-323-70957-6.00327-7, no.
Park, H., Otte, A., and Park, K. (2022). Evolution of drug delivery systems: From 1950 to 2020 and beyond. J. Control. release Off. J. Control. Release Soc. 342, 53–65. doi:10.1016/j.jconrel.2021.12.030
Park, J. W. (2002). Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 4 (3), 95–99. doi:10.1186/bcr432
Patel, J. (1996). Liposomal doxorubicin: Doxil®. J. Oncol. Pharm. Pract. 2 (4), 201–210. doi:10.1177/107815529600200402
Pearce, T. R., Shroff, K., and Kokkoli, E. (2012). Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv. Mater. 24 (28), 3803–3710. doi:10.1002/adma.201200832
Petrelli, F., Di Cosimo, S., Lonati, V., and Barni, S. (2016). Vinorelbine with capecitabine, an evergreen doublet for advanced breast cancer: A systematic literature review and pooled-analysis of phase II-III studies. Clin. Breast Cancer 16, 327–334. doi:10.1016/j.clbc.2016.05.002
Powar, T., Hajare, A., Jarag, R., and Nangare, S. (2021). Development and evaluation of lyophilized methotrexate nanosuspension using quality by design approach. Acta Chim. Slov. 68 (4), 861–881. doi:10.17344/acsi.2021.6858
Press, D. (2012). Nanotechnology-based approaches in anticancer research. Int. J. Nanomedicine 7, 4391–4408. doi:10.2147/IJN.S33838
Radwan, R., Namelo, W. C., Robinson, M., Brewster, A. E., and Williams, G. L. (2012). Case report ileitis secondary to oral capecitabine treatment. Case Rep. Med. 2012, 3–6. doi:10.1155/2012/154981
Rahool, R., Haider, G., Shahid, A., Shaikh, M. R., Memon, P., Pawan, B., et al. (2021). Medical and psychosocial challenges associated with breast cancer survivorship. Cureus 13 (2), e13211. doi:10.7759/cureus.13211
Rao, B. N., Reddy, K. R., and Sekhar, K. B. C. (2016). Withdrawn: Synthesis and functional evaluation of pegylated citric acid dendrimer clusters containing cyclophosphamide for cancer cell targeting. OpenNano 2016. doi:10.1016/j.onano.2016.12.001
Rasaneh, S., and Zahabi, S. S. (2016). The effect of chitosan nanoparticles containing paclitaxel on destruction of breast cancer cells. J. Clin. Res. Paramedical Sci. 2 (4673), 255.
Robson, M., Im, S. A., Senkus, E., Xu, B., Domchek, S. M., Masuda, N., et al. (2017). Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 377 (6), 523–533. doi:10.1056/nejmoa1706450
Roy, P., Sur, S., Das, S., and Tin, W. (2022). Phytochemical - conjugated bio - safe gold nanoparticles in breast cancer: A comprehensive update. Breast Cancer 29, 761–777. doi:10.1007/s12282-022-01368-8
Rugo, H. S., Finn, R. S., Diéras, V., Ettl, J., Lipatov, O., Joy, A. A., et al. (2019). Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 174 (3), 719–729. doi:10.1007/s10549-018-05125-4
Rugo, H. S., Im, S. A., Cardoso, F., Cortés, J., Curigliano, G., Musolino, A., et al. (2021). Efficacy of Margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: A phase 3 randomized clinical trial. JAMA Oncol. 7 (4), 573–584. doi:10.1001/jamaoncol.2020.7932
Sahu, B. P., Hazarika, H., Bharadwaj, R., Loying, P., Baishya, R., Dash, S., et al. (2016). Curcumin-docetaxel co-loaded nanosuspension for enhanced anti-breast cancer activity. Expert Opin. Drug Deliv. 13 (8), 1065–1074. doi:10.1080/17425247.2016.1182486
Sanford, M., and Plosker, G. L. (2008). Anastrozole: A review of its use in postmenopausal women with early-stage breast cancer. Drugs 68 (9), 1319–1340. doi:10.2165/00003495-200868090-00007
Sarika, P. R., and Nirmala, R. J. (2016). Curcumin loaded gum Arabic aldehyde-gelatin nanogels for breast cancer therapy. Mat. Sci. Eng. C 65, 331–337. doi:10.1016/j.msec.2016.04.044
SartaI, A. (2022). Ribociclib nanostructured lipid carrier aimed for breast cancer: Formulation optimization, attenuating in vitro specification, and in vivo scrutinization. Biomed. Res. Int. 2022, 2022. doi:10.1155/2022/6009309
Sartaj, A., Qamar, Z., Qizilbash, F. F., Annu, , Md, S., Alhakamy, N. A., et al. (2021). Polymeric nanoparticles: Exploring the current drug development and therapeutic insight of breast cancer treatment and recommendations. Polym. (Basel) 13, 4400–24. doi:10.3390/polym13244400
Saura, C., Oliveira, M., Feng, Y., Dai, M., Chen, S., Hurvitz, S. A., et al. (2020). Neratinib plus capecitabine versus Lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with ≥ 2 HER2-directed regimens: Phase III NALA trial. J. Clin. Oncol. 38, 3138–3149. doi:10.1200/JCO.20.00147
Schacter, L., Rozencweig, M., Canetta, R., Kelley, S., Nicaise, C., and Smaldone, L. (1989). Megestrol acetate: Clinical experience. Cancer Treat. Rev. 16 (1), 49–63. doi:10.1016/0305-7372(89)90004-2
Schlotter, C. M., Vogt, U., Allgayer, H., and Brandt, B. (2008). Molecular targeted therapies for breast cancer treatment. Breast Cancer Res. 10, 211–4. doi:10.1186/bcr2112
Science direct (2023a). Sciencedircet. Available at: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/methyltestosterone.
Science direct (2023b). Assessed date 200523. Available at: https://www.sciencedirect.com/topics/agricultural-and-biological-sciences/fluoxymesterone.
Shantanam, S. (2018). Mueller, HHS public access. Physiol. Behav. 176 (1), 139–148. doi:10.1038/nature11143.Whole
Shavi, G. V., Nayak, U. Y., Reddy, M. S., Ginjupalli, K., Deshpande, P. B., Averineni, R. K., et al. (2017). A novel long-acting biodegradable depot formulation of anastrozole for breast cancer therapy. Mat. Sci. Eng. C 75, 535–544. doi:10.1016/j.msec.2017.02.063
Sheydaei, M. (2021). Breast cancer and the role of polymer-carriers in treatment. J. Sci. Tech. Res. 34 (5), 27057–27061. doi:10.26717/bjstr.2021.34.005601
Siegel, R. L., Miller, K. D., Fuchs, H. E., and Jemal, A. (2022). Cancer statistics, 2022. Cancer J. Clin. 72 (1), 7–33. doi:10.3322/caac.21708
Silvestris, N., Cinieri, S., La Torre, I., Pezzella, G., Numico, G., Orlando, L., et al. (2008). Role of gemcitabine in metastatic breast cancer patients: A short review. Breast 17 (3), 220–226. doi:10.1016/j.breast.2007.10.009
Singh, S. K., Singh, S., Lillard, J. W., and Singh, R. (2017). Drug delivery approaches for breast cancer. Int. J. Nanomedicine 12, 6205–6218. doi:10.2147/IJN.S140325
Singla, A. K., Garg, A., and Aggarwal, D. (2002). Paclitaxel and its formulations. Int. J. Pharm. X. 235, 179–192. doi:10.1016/s0378-5173(01)00986-3
Slamon, D. J., Neven, P., Chia, S., Jerusalem, G., De Laurentiis, M., Im, S., et al. (2021). Ribociclib plus fulvestrant for postmenopausal women with hormone receptor-positive, human epidermal growth factor receptor 2-negative advanced breast cancer in the phase III randomized MONALEESA-3 trial: Updated overall survival. Ann. Oncol. 32 (8), 1015–1024. doi:10.1016/j.annonc.2021.05.353
Sledge, G. W., Toi, M., Neven, P., Sohn, J., Inoue, K., Pivot, X., et al. (2020). The effect of abemaciclib plus fulvestrant on overall survival in hormone receptor-positive, ERBB2-negative breast cancer that progressed on endocrine therapy - monarch 2: A randomized clinical trial. JAMA Oncol. 6 (1), 116–124. doi:10.1001/jamaoncol.2019.4782
Smith, L., Kuncic, Z., Ostrikov, K., and Kumar, S. (2012). Nanoparticles in cancer imaging and therapy. J. Nanomater. 2012, 1–7. doi:10.1155/2012/891318
Soiza, R. L., Donaldson, A. I. C., and Myint, P. K. (2018). Vaccine against arteriosclerosis: An update. Ther. Adv. Vaccines 9 (6), 259–261. doi:10.1177/2042098618769568
Song, B., Wu, S., Li, W., Chen, D., and Hu, H. (2020). Folate modified long circulating nano-emulsion as a promising approach for improving the efficiency of chemotherapy drugs in cancer treatment. Pharm. Res. 37, 242. doi:10.1007/s11095-020-02811-1
Structures, F. S., Safety, C., Formula, M., and Weight, M. (2023). Exemestane. PubChem 14 (1), 2023. doi:10.5517/cc9cbvt
Structures, F. S., Safety, C., Formula, M., Weight, M., Compound, P., and Compounds, C. (2023). Talazoparib tosylate. United States: National Cancer Institute.
Subhan, M. A. (2022). Advances with metal oxide-based nanoparticles as MDR metastatic breast cancer therapeutics and diagnostics. RSC Adv. 12 (51), 32956–32978. doi:10.1039/d2ra02005j
Sullivan, K. M., Dores, G. M., Nayernama, A., Prowell, T. M., Pradhan, S. M., Osgood, C., et al. (2022). Postmarketing colitis cases associated with Alpelisib use reported to the US food and drug administration. JAMA Oncol. 8 (10), 1503–1505. doi:10.1001/jamaoncol.2022.3249
Sung, H., Ferlay, J., Siegel, R. L., Laversanne, M., Soerjomataram, I., Jemal, A., et al. (2021). Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA a cancer J. Clin. 71 (3), 209–49. doi:10.3322/caac.21660
Swaminathan, S., Cavalli, R., and Trotta, F. (2016). Cyclodextrin-based nanosponges: A versatile platform for cancer nanotherapeutics development. Wiley Interdiscip. Rev. Nanomedicine Nanobiotechnology 8 (4), 579–601. doi:10.1002/wnan.1384
Switon, J., and Hill, G. G. (1977). Clinical oncology. W.B. Saunders Co. 4333 (3). doi:10.5858/2001-125-582b-co
Syn, N. L., Teng, M. W. L., Mok, T. S. K., and Soo, R. A. (2017). De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 18 (12), e731–e741. –e741. doi:10.1016/S1470-2045(17)30607-1
Talegaonkar, S., and Negi, L. M. (2015). “Nanoemulsion in drug targeting,” in Targeted drug delivery: Concepts and design (Berlin Germany: Springer), 433–459. doi:10.1007/978-3-319-11355-5_14
Thomas, E. S., Aubry, W. M., Jacobson, P. D., and Farquhar, C. M. (2015). Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and Taxane treatment randomized clinical trials, clinical use, and Hope: What relationship. Pharmacoeconomic Benefits Capecitabine-Based Chemother. Meta 26–13. doi:10.1200/JCO.2008.16.5019
Thomas, E. S., Aubry, W. M., Jacobson, P. D., and Farquhar, C. M. (2022). Ixabepilone plus capecitabine for metastatic breast cancer progressing after anthracycline and Taxane treatment randomized clinical trials, clinical use, and Hope: What relationship. Pharmacoeconomic Benefits Capecitabine-Based Chemother. Meta 26.
Tiash, S., and Chowdhury, M. E. H. (2016). Passive targeting of cyclophosphamide-loaded carbonate apatite nanoparticles to liver impedes breast tumor growth in a syngeneic model. Curr. Pharm. Des. 22 (37), 5752–5759. doi:10.2174/1381612822666160211141918
Torabifard, H., and Fattahi, A. (2012). Mechanisms and kinetics of thiotepa and tepa hydrolysis: DFT study. J. Mol. Model 18, 3563–3576. doi:10.1007/s00894-012-1354-y
Torchilin, V. P. (2007). Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 24 (1), 1–16. doi:10.1007/s11095-006-9132-0
Trayes, K. P., and Cokenakes, S. E. H. (2021). Breast cancer treatment. Am. Fam. Physician 104 (2), 171–178.
Trotta, M., Peira, E., Carlotti, M. E., and Gallarate, M. (2004). Deformable liposomes for dermal administration of methotrexate. Int. J. Pharm. 270 (1–2), 119–125. doi:10.1016/j.ijpharm.2003.10.006
Turner, N. C., Oliveira, M., Howell, S. J., Dalenc, F., Cortes, J., Gomez Moreno, H. L., et al. (2023). Capivasertib in hormone receptor–positive advanced breast cancer. N. Engl. J. Med. 388 (22), 2058–2070. doi:10.1056/nejmoa2214131
U.S. Cancer Statistics Data (2021). Incidence and relative survival by stage at diagnosis for common cancers the U.S. Cancer statistics data visualizations tool provides cancer statistics by stage at diagnosis. Div. Cancer Prev. Control 25.
Untch, M., and Jackisch, C. (2008). Exemestane in early breast cancer: A review. Ther. Clin. Risk Manag. 4 (6), 1295–1304. doi:10.2147/tcrm.s4007
US Food and Drug Administration (2017). KEYTRUDA (pembrolizumab) for injection, for intravenous use. Available at: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014lbl.pdf.
USP (2012). docs.boehringer ingelheim. Available at: https://docs.boehringer ingelheim.com/Prescribing%20Information/PIs/Ben%20Venue_Bedford%20Labs/55390-091-10%20VIN%2010MG/5539009110#:∼:text=Vials%20of%20Vinblastine%20Sulfate%20for,range%20of%203.5%20to%205.
Verma, P., Meher, J. G., Asthana, S., Pawar, V. K., Chaurasia, M., and Chourasia, M. K. (2016). Perspectives of nanoemulsion assisted oral delivery of docetaxel for improved chemotherapy of cancer. Drug Deliv. 23 (2), 479–488. doi:10.3109/10717544.2014.920430
Vick, A. M., and Hayton, W. L. (2001). Methyltestosterone pharmacokinetics and oral bioavailability in rainbow trout (Oncorhynchus mykiss). Aquat. Toxicol. 52 (3), 177–188. doi:10.1016/S0166-445X(00)00146-6
Voli, L. A., Mamyrbékova, J. A., and Bazureau, J.-P. (2020). Abemaciclib, a recent novel FDA-approved small molecule inhibiting cyclin-dependant kinase 4/6 for the treatment of metastatic breast cancer: A mini-review. Open J. Med. Chem. 10 (03), 128–138. doi:10.4236/ojmc.2020.103007
Vuong, B. X., Hajali, N., Asadi, A., Baqer, A. A., Hachim, S. K., and Canli, G. (2022). Drug delivery assessment of an iron-doped fullerene cage towards thiotepa anticancer drug. Inorg. Chem. Commun. 141, 109558. doi:10.1016/j.inoche.2022.109558
Waks, A. G., and Winer, E. P. (2019). Breast cancer treatment: A review. Jama 321 (3), 288–300. doi:10.1001/jama.2018.19323
Walko, C. M., and Lindley, C. (2005). Capecitabine: A review. Clin. Ther. 27 (1), 23–44. doi:10.1016/j.clinthera.2005.01.005
Wang, Q., Jiang, J., Ying, G., Xie, X. Q., Zhang, X., Xu, W., et al. (2018). Tamoxifen enhances stemness and promotes metastasis of ERα36 + breast cancer by upregulating ALDH1A1 in cancer cells. Cell Res. 28 (3), 336–358. doi:10.1038/cr.2018.15
WHO (2021). Breast cancer. Available at: https://www.who.int/news-room/fact-sheets/detail/breast-cancer#.
Wigmore, P. M., Mustafa, S., El-Beltagy, M., Lyons, L., Umka, J., and Bennett, G. (2010). Effects of 5-FU. Adv. Exp. Med. Biol. 678, 157–164. doi:10.1007/978-1-4419-6306-2_20
Wissner, A., and Mansour, T. S. (2008). The development of HKI-272 and related compounds for the treatment of cancer. Arch. Pharm. Weinh. 341 (8), 465–477. doi:10.1002/ardp.200800009
Wong, H. L., Rauth, A. M., Bendayan, R., Manias, J. L., Ramaswamy, M., Liu, Z., et al. (2006). A new polymer–lipid hybrid nanoparticle system increases cytotoxicity of doxorubicin against multidrug-resistant human breast cancer cells. Pharm. Res. 23, 1574–85. doi:10.1007/s11095-006-0282-x
Xie, J., Yang, Z., Zhou, C., Zhu, J., Lee, R. J., and Teng, L. (2016). Nanotechnology for the delivery of phytochemicals in cancer therapy. Biotechnol. Adv. 34 (4), 343–353. doi:10.1016/j.biotechadv.2016.04.002
Yamamoto, Y., Kawano, I., and Iwase, H. (2011). Nab-paclitaxel for the treatment of breast cancer: Efficacy, safety, and approval. Onco. Targets. Ther. 4, 123–136. doi:10.2147/OTT.S13836
Yang, H., Li, K., Liu, Y., Liu, Z., and Miyoshi, H. (2009). Poly (D, L-lactide-co-glycolide) nanoparticles encapsulated fluorescent isothiocyanate and paclitaxol: Preparation, release kinetics and anticancer effect. J. Nanosci. Nanotechnol. 9 (1), 282–7.
Yao, Y., Zhou, Y., Liu, L., Xu, Y., Chen, Q., Wang, Y., et al. (2020). Nanoparticle-based drug delivery in cancer therapy and its role in overcoming drug resistance. Front. Mol. Biosci. 7, 193–14. doi:10.3389/fmolb.2020.00193
Yassemi, A., Kashanian, S., and Zhaleh, H. (2020). Folic acid receptor-targeted solid lipid nanoparticles to enhance cytotoxicity of letrozole through induction of caspase-3 dependent-apoptosis for breast cancer treatment. Pharm. Dev. Technol. 25 (4), 397–407. doi:10.1080/10837450.2019.1703739
Yavuz, B., Bilensoy, E., and Sumnu, M. (2007). Bioavailability file: Exemestane. FABAD J. Pharm. Sci. 32 (2), 79.
Yin, Q., and Tang, L. (2016). Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Proc. Natl. Acad. Sci. U. S. A. 113 (32), E4601. –E4609. doi:10.1073/pnas.1603316113
Yin, Y.-M., Cui, F. D., Mu, C. F., Choi, M. K., Kim, J. S., Chung, S. J., et al. (2009). Docetaxel microemulsion for enhanced oral bioavailability: Preparation and in vitro and in vivo evaluation. J. Control. release Off. J. Control. Release Soc. 140 (2), 86–94. doi:10.1016/j.jconrel.2009.08.015
Yin, Q., Tang, L., Cai, K., Tong, R., Sternberg, R., Yang, X., et al. (2016). Pamidronate functionalized nanoconjugates for targeted therapy of focal skeletal malignant osteolysis. Proc. Natl. Acad. Sci. U. S. A. 113 (32), E4601–E4609. –E4609. doi:10.1073/pnas.1603316113
Yuan, P., and Xu, B. (2021). Clinical utility of eribulin mesylate in the treatment of breast cancer: A Chinese perspective. Breast Cancer Targets Ther. 13, 135–150. doi:10.2147/BCTT.S231298
Zardavas, D., and Piccart-Gebhart, M. (2016). New generation of breast cancer clinical trials implementing molecular profiling. Cancer Biol. Med. 13 (2), 226–235. doi:10.20892/j.issn.2095-3941.2015.0099
Zhang, Y., Tang, L., and Gonzalez, V. (2003). Selected isothiocyanates rapidly induce growth inhibition of cancer cells. Mol. cancer Ther. 2 (10), 1045–52.
Zhang, D., Baldwin, P., Leal, A. S., Carapellucci, S., Sridhar, S., and Liby, K. T. (2019). A nano-liposome formulation of the PARP inhibitor Talazoparib enhances treatment efficacy and modulates immune cell populations in mammary tumors of BRCA-deficient mice. Theranostics 9 (21), 6224–6238. doi:10.7150/thno.36281
Zhang, L., Jackson, C. B., Mou, H., Ojha, A., Rangarajan, E. S., Izard, T., et al. (2020). The D614G mutation in the SARS-CoV-2 spike protein reduces S1 shedding and increases infectivity. bioRxiv. doi:10.1101/2020.06.12.148726
Zhao, Y., Vivero-Escoto, J. L., Slowing, I. I., Trewyn, B. G., and Lin, V. S. Y. (2010). Capped mesoporous silica nanoparticles as stimuli-responsive controlled release systems for intracellular drug/gene delivery. Expert Opin. Drug Deliv. 7 (9), 1013–1029. doi:10.1517/17425247.2010.498816
Keywords: breast cancer, FDA, formulations, nanoformulation, nanoemulsion, liposomes, cytotoxic drugs, hormonal and targeted drugs
Citation: Chaurasia M, Singh R, Sur S and Flora SJS (2023) A review of FDA approved drugs and their formulations for the treatment of breast cancer. Front. Pharmacol. 14:1184472. doi: 10.3389/fphar.2023.1184472
Received: 11 March 2023; Accepted: 23 June 2023;
Published: 28 July 2023.
Edited by:
Mohd Saeed, University of Hail, Saudi ArabiaReviewed by:
Fahad Khan, Noida Institute of Engineering and Technology (NIET), IndiaSandeep Singhal, University of North Dakota, United States
Prakriti Mishra, Integral University, India
Copyright © 2023 Chaurasia, Singh, Sur and Flora. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: S. J. S. Flora, sjsflora@hotmail.com
†Present address: Srija Sur, Department of Pharmaceutical Technology, School of Medical Sciences, Adamas University, Kolkata, India
 Mohini Chaurasia
Mohini Chaurasia Romi Singh
Romi Singh Srija Sur
Srija Sur S. J. S. Flora
S. J. S. Flora