Erratum: Comparison of bleeding risk and hypofibrinogenemia-associated risk factors between tigecycline with cefoperazone/sulbactam therapy and other tigecycline-based combination therapies
- 1Department of Pharmacy, Shanxi Provincial People’s Hospital, Taiyuan, China
- 2Department of Pharmacy, Shanxi Province Cancer Hospital, Shanxi Hospital Affiliated to Cancer Hospital, Chinese Academy of Medical Sciences, Cancer Hospital Affiliated to Shanxi Medical University, Taiyuan, China
- 3Department of Nephrology, Shanxi Provincial People’s Hospital, Taiyuan, China
- 4Department of Pharmacy, Yuncheng Central Hospital, Yuncheng, China
Background: Tigecycline and cefoperazone/sulbactam can cause coagulation disorders; tigecycline may also lead to hypofibrinogenemia, raising safety concerns. This study aimed to investigate whether tigecycline plus cefoperazone/sulbactam increases the risk of bleeding compared with other tigecycline-based combination therapies and identify risk factors for tigecycline-associated hypofibrinogenemia.
Methods: In this multi-method, multicenter, retrospective study, coagulation and other baseline variables were compared using a cohort study, and risk factors for hypofibrinogenemia using a case-control study.
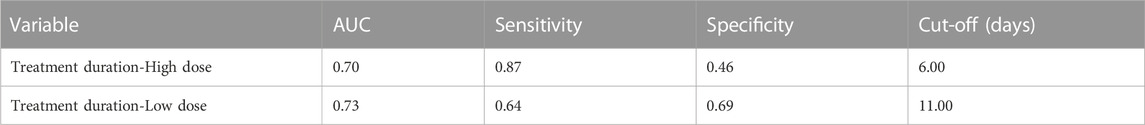
Results: The 451 enrolled participants were divided into three group: tigecycline plus cefoperazone/sulbactam (Group A, 193 patients), tigecycline plus carbapenems (Group B, 200 patients) and tigecycline plus β-lactams without N-methylthio-tetrazole (NMTT) side chains (Group C, 58 patients). Activated partial thromboplastin time and prothrombin time were prolonged, and fibrinogen declined for all patients after tigecycline-based medication (all p < 0.05). Prothrombin time in Group B was significantly longer than in other groups (p < 0.05), but there were no significant differences in bleeding events between the three groups (p = 0.845). Age greater than 80 years (OR: 2.85, 95% CI: 1.07–7.60), treatment duration (OR: 1.29, 95% CI: 1.19–1.41), daily dose (OR: 2.6, 95% CI: 1.29–5.25), total bilirubin (OR: 1.01, 95% CI: 1.01–1.02) and basal fibrinogen (OR: 1.32, 95% CI: 1.14–1.63) were independent risk factors of hypofibrinogenemia. The optimal cut-off for treatment course was 6 days for high-dose and 11 days for low-dose.
Conclusion: Tigecycline plus cefoperazone/sulbactam did not increase the risk of bleeding compared with tigecycline plus carbapenem, or tigecycline plus β-lactam antibiotics without NMTT-side-chains. Coagulation function should be closely monitored in patients receiving tigecycline treatment.
Introduction
Carbapenem-resistant Gram-negative organisms are a serious global challenge for physicians, including carbapenem-resistant Enterobacterales, carbapenem-resistant Acinetobacter baumannii (CRAB) and carbapenem-resistant Pseudomonas aerugnosa. CRAB is one of the main causative pathogens of hospital-acquired infections in China. Due to its extensive antibiotic resistance via complex mechanisms, treatment options are very limited and the patients often have poor prognosis (Group of Infectious Diseases RDBoCMA, 2018). According to the China Bacterial Resistance Surveillance Network, less than 5% of CRAB were resistant to tigecycline and polymyxin, making regimen based on these antibiotics the primary treatment (Paul et al., 2022). Due to the high cost of polymyxin, tigecycline is more widely used in China.
Tigecycline is a minocycline derivative with antimicrobial activity against Gram-positive and Gram-negative bacteria, anaerobes and atypical pathogens. Its unique pharmacological mechanism provides good antibacterial activity against multidrug-resistant bacteria, include CRAB (Giammanco et al., 2017; Rodriguez-Bano et al., 2018). However, accumulating adverse event reports have raised safety concerns regarding tigecycline. Tigecycline may cause increased international normalized ratio (INR), prolonged activated partial thromboplastin time (APTT) and prolonged prothrombin time (PT) according to manufacturer’s label (Tygacil, 2013). Some studies suggest that tigecycline has a more pronounced effect on fibrinogen (FIB) decline (Liu et al., 2021; Leng et al., 2022; Yang et al., 2022).
Cefoperazone/sulbactam is an β-lactam/β-lactamase inhibitor combination, that is, effective for intra-abdominal, urinary tract, and respiratory infections. Sulbactam enhances the activity of cefoperazone and is used in cases of moderate-to-severe infection (Drawz and Bonomo, 2010), and is effective against ESBL-producing E. coli and K. pneumoniae (Su et al., 2018). Cefoperazone/sulbactam is widely used in China to treat hospital-acquired infection caused by CRAB; however, studies suggested that cefoperazone/sulbactam is associated with coagulation disorders (Greenberg et al., 1987; Xin et al., 2013). Case reports have demonstrated that patients receiving cefoperazone/sulbactam have experienced major bleeding, such as upper gastrointestinal bleeding, hematuria and abdominal wall hematoma (Wong et al., 2006; Cai et al., 2016). A possible mechanism for the coagulation abnormalities associated with cefoperazone may be its N-methylthio-tetrazole (NMTT) side chain, which induces vitamin K deficiency and leads to hypoprothrombinemia and bleeding (Lipsky, 1983; Mueller et al., 1987; Strom et al., 1999; Cai et al., 2016). Strom’s retrospective cohort study found that cefoperazone was linked to a higher risk of hypoprothrombinemia compared to the antibiotics without NMTT (Strom et al., 1999). A nested case-control study found that cefoperazone may increase the risk of bleeding compared to other antibiotics (Chen et al., 2016).
Patients with CRAB infection are usually critically ill, making appropriate antibiotic choice particularly important (Garnacho-Montero et al., 2015). As tigecycline is generally not used as a monotherapy due to heterogeneous resistance (Liu et al., 2019; Paul et al., 2022), cefoperazone/sulbactam plus tigecycline is the most used combination regimen for CRAB in China (Garnacho-Montero et al., 2015). However, both cefoperazone/sulbactam and tigecycline could increase the risk of bleeding. There is limited data as to whether the combination of these two agents increases the risk of bleeding compared to other tigecycline-based combination regimens. Therefore, we conducted this retrospective cohort and case-control study to investigate whether tigecycline plus cefoperazone/sulbactam increases the risk of bleeding compared with other tigecycline-based combination regimens, and investigate the risk factors associated with tigecycline-associated hypofibrinogenemia.
Materials and methods
Study design and patient selection
This was a multi-methods, multicenter, retrospective investigation of hospitalized patients receiving tigecycline-based combination therapy at three hospitals, including a cohort study and a case-control study. Each participating center (Shanxi Provincial Peoples Hospital, Shanxi Provincial Cancer Hospital and Yuncheng Central Hospital) was a tertiary hospital with over 2,200 inpatient beds. The study protocol was approved by the Ethics Committee of Shanxi Provincial People’s Hospital (2022-280). Researchers waived the need to obtain written informed consent due to its retrospective nature.
The enrolled population was divided into groups based on adjunct medication. The inclusion criteria were as follows: patients who received tigecycline-based treatment for at least 3 days, older than 18 years, high (loading dose 200 mg followed by 100 mg twice per day) or low tigecycline dose (loading dose 100 mg followed by 50 mg twice per day). The exclusion criteria were as follows: missing data; abnormal coagulation function before treatment; simultaneous use of drugs affecting coagulation, including aspirin, low molecular weight heparin, warfarin, clopidogrel, ticagrelol, and rivaroxaban; sepsis; liver failure; the number of cases in each group is less than 50.
The normal range of coagulation function was as follows in our hospital: APTT 25.1–36.5 s; PT 9.9–12.8 s; FIB 2.38–4.98 g/L. Hypofibrinogenemia was defined as plasma FIB <2.0 g/L (Leng et al., 2022). Naranjo score ≥4 was defined as tigecycline-associated hypofibrinogenemia in this study.
A total of 451 participants who met the inclusion/exclusion criteria between January 2018 and June 2022 at any of the three included hospitals were enrolled in this study.
Cohort study
In this retrospective cohort study, we collected basic data for the enrolled patients and compared the baseline characteristics and coagulation data between patients treated with tigecycline plus cefoperazone/sulbactam, and patients treated with other tigecycline-based combination therapy.
Case-control study
A case-control study was conducted to investigate the risk factors for tigecycline-associated hypofibrinogenemia in patients treated with tigecycline. The enrolled patients were divided into a hypofibrinogenemic and a non-hypofibrinogenemic group. We then assessed the relationship between tigecycline-associated hypofibrinogenemia and patient demographics, comorbidities, medication regimens and baseline laboratory tests.
Data collection
We collected clinical information from the hospital information system including: age, sex, underlying diseases, site of infection, tigecycline dosage, duration of tigecycline treatment, concomitant drugs, PT, APTT, FIB, bleeding events, hemoglobin level, platelet count, transaminase level, albumin level, creatinine level. All laboratory tests performed from 48 h prior to tigecycline administration until 48 h after discontinuation of tigecycline were recorded.
Statistical analysis
A mathematician not involved in the study procedures or patient assessment performed the statistical analyses using IBM-SPSS version 23.0. Shapiro-Wilk test was used to test normality. Statistical descriptions of quantitative variables are expressed as medians and interquartile range (IQR) or mean and standard deviations. Univariate comparisons were performed using the chi-square test for qualitative variables, and Student’s t-test or the Kruskal–Wallis H test for continuous variables, as appropriate. Mixed linear models were used to analyze the effects of different medication regimens and time factors on APTT, PT, and FIB levels. The Kruskal–Wallis H test was used for comparison between groups. The paired samples Wilcoxon test was used to compare the parameters before (T1) and after medication (T2). The risk factors for hypofibrinogenemia were analyzed by multivariate logistic regression. The receiver operating characteristic (ROC) curve was plotted using hypofibrinogenemia and course of treatment to determine the area under the curve (AUC) with 95% confidence interval. The ROC curve was used to determine the optimal cut-off point for course of treatment in all patients taking tigecycline.
Results
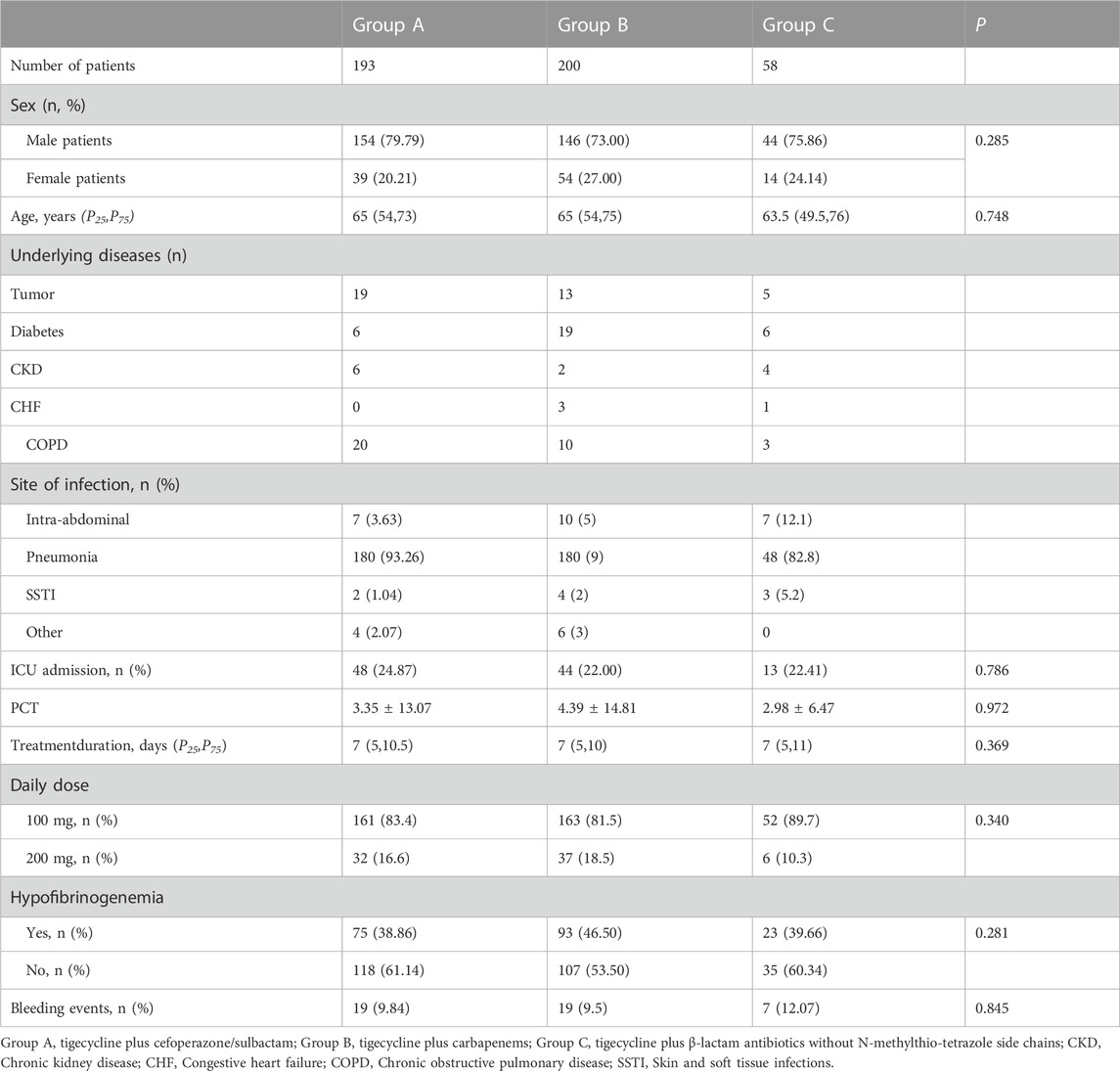
The study cohort comprised 451 patients, divided into three groups: tigecycline plus cefoperazone/sulbactam (Group A), tigecycline plus carbapenems (Group B) and tigecycline plus β-lactam antibiotics without NMTT side chains (Group C). Group A contained 193, Group B contained 200, and Group C contained 58 patients, with no significant differences in age, sex, treatment duration, daily dose, occurrence of hypofibrinogenemia and bleeding events between the three groups (Table 1).
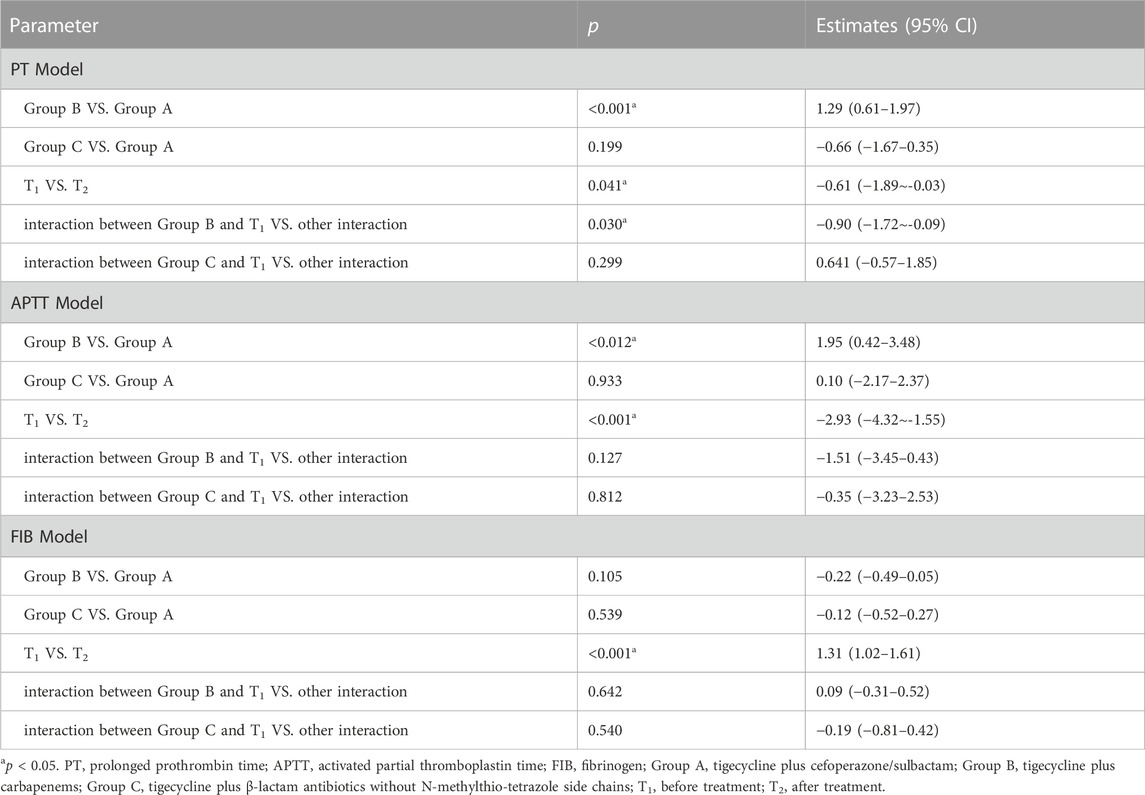
The results of mixed linear models are shown in Table 2. The estimates between group B and group A in PT and APTT were 1.29 (p < 0.01) and 1.95 (p < 0.01) respectively; both PT and APTT were significantly longer in group B than in group A. While estimates between group C and group A in PT and APTT were −0.66 (p > 0.05) and 0.10 (p > 0.05) respectively, there was no significant difference. For both PT and APTT, the difference between T1 versus T2 was <0 (p < 0.05), suggesting PT and APTT were significantly prolonged after treatment for all patients.
In FIB modelling, there was no significant difference in FIB levels between group B and A, or group C and A. But the estimated difference between T1 and T2 was 1.31 (p < 0.01), demonstrating that FIB levels significantly decreased after tigecycline-based combination therapy.
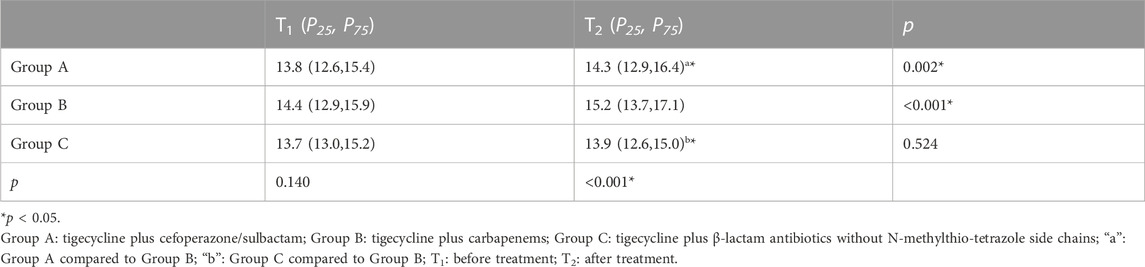
Since the interaction between group and time impacted PT level (p < 0.05), we further analyzed the influence of different time points and medication regimens on PT levels, and the change in PT level at different time points in different groups (Table 3). There were no significant differences in PT levels between the three groups before treatment (p = 0.14), but there were significant differences between the three groups (p < 0.01) after treatment. PT level in group B were significantly higher than that in groups A and C.
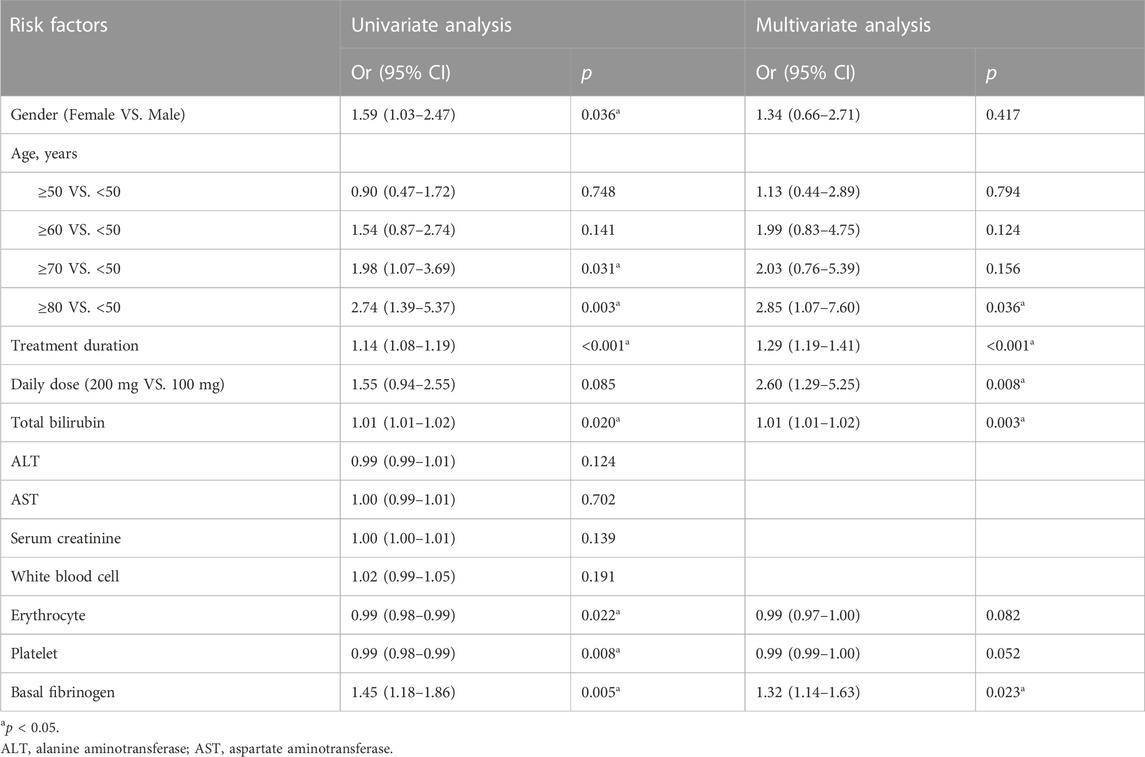
Univariate logistic regression showed that the following variables were significant for the risk of hypofibrinogenemia at p < 0.05: gender, age, long treatment duration, high daily dose, total bilirubin, platelet, erythrocyte, low basal fibrinogen (Table 4). In the multivariate analysis, patients older than 80 years had a higher risk of hypofibrinogenemia compared with those under 50 (OR: 2.85, 95% CI: 1.07–7.60). Furthermore, long treatment duration (OR: 1.29, 95% CI: 1.19–1.41), high daily dose (OR: 2.6, 95% CI: 1.29–5.25), total bilirubin (OR: 1.01, 95% CI: 1.01–1.02) and low basal fibrinogen (OR: 1.32, 95% CI: 1.14–1.63) also were independent risk factors of hypofibrinogenemia.
The ROC curve was used to evaluate the relationship between tigecycline treatment duration and tigecycline-related hypofibrinogenemia. AUC of treatment duration for the high dose was 0.70 (predictive ability of p < 0.05), and for the low dose was 0.73 (p < 0.05). Treatment duration ≥6 days for high-dose regimen and treatment duration ≥11 days for low-dose regimen were selected as the cutoff points for predicting hypofibrinogenemia during tigecycline treatment (Table 5).
Discussion
Previous reports suggested that both tigecycline and cefoperazone sulbactam can cause coagulopathies (Wong et al., 2006; Rossitto et al., 2014; Yilmaz Duran et al., 2018; Wang et al., 2020; Liu et al., 2021; Leng et al., 2022). The widespread use of tigecycline in combination with cefoperazone/sulbactam has increased physicians’ concerns about an increased risk of bleeding associated with this regime. We therefore conducted a multicenter, retrospective, controlled study to assess the coagulopathies associated with tigecycline. To the best of our knowledge, this is the first study to compare the coagulation disorders between tigecycline plus cefoperazone/sulbactam and other tigecycline-based combination therapies, this is also the largest study of tigecycline-induced coagulopathy. In our cohort study, we evaluated the effect of tigecycline with carbapenems, or tigecycline with a β-lactam on coagulation when compared to tigecycline with cefoperazone/sulbactam as a control, given that tigecycline plus cefoperazone/sulbactam is the primary regimen for the treatment of CRAB infections in China.
The results of the mixed linear model showed that APTT and PT were significantly prolonged, and FIB decreased in all enrolled patients after receiving tigecycline-based therapy. It is generally reported that carbapenems have little effect on coagulation (Leng et al., 2022). While among β-lactam antibiotics, only NMTT-side-chain-containing antibiotics (such as cefoperazone and cefmetazole) are known to cause coagulopathies (Chen et al., 2016). The coagulation dysfunction induced by tigecycline has been confirmed by various studies (Rossitto et al., 2014; Liu et al., 2021; Leng et al., 2022). Therefore, we believe that the coagulopathies observed in the patients in this study are caused by tigecycline. However, the underlying mechanism for tigecycline-induced coagulopathies remains unclear.
The known mechanism of antibiotic-associated coagulopathy is through reduced intestinal flora or inhibited vitamin K2 synthesis; however these mechanisms do not influence the production of fibrinogen (Haden, 1957; Shirakawa et al., 1990; Conly and Stein, 1992). The patients did not have symptoms suggestive of overconsumption, such as disseminated intra-vascular coagulation, primary active bleeding, and clotting factors degradation accelerated by acidosis. We therefore concluded that the reduction of FIB has no obvious relationship with clotting factors consumption (Martini, 2009). Tigecycline-induced cytokine inhibition may be responsible for reduced fibrinogen production. Vasse et al. (1996) demonstrated that tigecycline can inhibit IL-6 expression in leucocytes; since IL-6 increases plasma fibrinogen by stimulating gene expression, tigecycline may lead to a decrease in plasma fibrinogen by inhibiting IL-6 synthesis (Fuller et al., 1985).
Both PT and APTT were significantly higher in group B than A, contrary to our initial assumptions. We hypothesize this is due to tigecycline’s greater effect on coagulation than cefoperazone/sulbactam. The proportion of patients receiving high-dose tigecycline in group B (18.5%) was higher than in groups A (16.6%) and C (10.3%); tigecycline induced coagulopathy is usually dose dependent (Cui et al., 2019). PT levels in group B were significantly higher than in groups A and C after medication (Table 3), which supports our hypothesis. There were no differences in APTT and PT between group C and A. Given that carbapenems and β-lactam antibiotics without NMTT side chains had little effect on coagulopathy, and there was no significant difference in bleeding events between the three groups, we did not consider tigecycline with cefoperazone/sulbactam to have an increased risk of bleeding when compared to tigecycline with carbapenem, or tigecycline with a β-lactam. Leng’s retrospective research suggested tigecycline plus cefoperazone/sulbactam did not increase the risk of coagulopathy compared to tigecycline monotherapy, which is consistent with our study (Leng et al., 2019).
Multivariate logistic regression showed that patients >80 years, long treatment duration, high daily dose, high total bilirubin and low basal fibrinogen were independent risk factors for tigecycline-associated hypofibrinogenemia. However, data on the correlation between age and hypofibrinogenemia is inconclusive. Research from China and Austria found that the incidence of hypofibrinogenemia and age are unrelated (Zhang et al., 2015; Campany-Herrero et al., 2020; Hu et al., 2020; Xia and Jiang, 2020; Treml et al., 2021; Leng et al., 2022). However, our study, alongside a few others, suggests that advanced age is a risk factor for tigecycline-associated hypofibrinogenemia (Zhang et al., 2020; Liu et al., 2021). Fibrinogen is synthesized in the liver; we speculate that the hepatic synthetic ability declines in patients with advanced age, which is supported by our finding that total bilirubin levels are also associated with hypofibrinogenemia. Hypofibrinogenemia can be caused by liver disease due to reduced liver synthetic function or dysfibrinogenemia, we suggest this is the reason why hypofibrinogenemia is associated with total bilirubin (May et al., 2021).
Our study showed that a dose of tigecycline higher than 100 mg/day is a risk factor for hypofibrinogenemia, which is consistent previous studies (Campany-Herrero et al., 2020; Xia and Jiang, 2020; Treml et al., 2021). As tigecycline-induced coagulopathy is usually dose dependent, this may explain why hypofibrinogenemia is more likely to occur in the high-dose group.
Long term tigecycline treatment is associated with hypofibrinogenemia (Campany-Herrero et al., 2020; Xia and Jiang, 2020; Liu et al., 2021). Coagulopathy developed at a median of 6 days after tigecycline treatment in one study, which is similar to our research (Hu et al., 2020). However, there are two regimens of tigecycline, high-dose and low-dose, commonly used in clinical practice. Further analysis showed that the cut-off for hypofibrinogenemia in high-dose patients was 6 days, and 11 days for low-dose patients; far below other findings of 4 weeks (Campany-Herrero et al., 2020). However, our study involved a larger cohort and stricter inclusion criteria. In addition, this study was conducted in a Chinese population, and the previous study conducted in a Spanish population. Therefore, it is necessary to closely monitor FIB level when receiving high-dose tigecycline treatment.
Our study has several limitations. First, this is a retrospective observational study, and there may be bias and confounders that we did not control for. Second, Yang et al. (2022) reported serum concentration as a predictor of tigecycline-induced hypofibrinogenemia, but we were unable to include therapeutic drug monitoring for tigecycline. Third, the association between mortality and treatment regimen was not investigate. Despite these limitations, we investigated the effects of tigecycline with cefoperazone/sulbactam on coagulation function, and risk factors for tigecycline-associated hypofibrinogenaemia, which will facilitate further research in this field.
Conclusion
In conclusion, tigecycline with cefoperazone/sulbactam did not increase the risk of bleeding compared to tigecycline with carbapenem, or tigecycline with β-lactam antibiotics without NMTT-side-chains. Patients older than 80 years, on long-term tigecycline treatment, high daily dose, high total bilirubin and low basal fibrinogen were independent risk factors for tigecycline-associated hypofibrinogenemia. Coagulation function should be closely monitored in patients receiving tigecycline treatment.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Committee of Shanxi Provincial People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Conceptualization: LZ, JG, and XC; methodology: JG and ST; Data collection: XW, YL, and FP; formal analysis and investigation: ST and XC; writing—original draft preparation: LZ, JG, and XC; Writing—review and editing: JG and XC. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AUC, area under the curve; APTT, activated partial thromboplastin time; CRAB, carbapenem-resistant Acinetobacter baumannii; IQR, interquartile range; PT, prothrombin time; FIB, fibrinogen; NMTT, N-methylthio-tetrazole; ROC, receiver operating characteristic; T1, before medication; T2, after medication.
References
Cai, Z., Yang, W., He, Y., Chen, Q., Wang, S., Luo, X., et al. (2016). Cefoperazone/sulbactam-induced abdominal wall hematoma and upper gastrointestinal bleeding: A case report and review of the literature. Drug Saf. Case Rep. 3 (1), 2. doi:10.1007/s40800-016-0025-9
Campany-Herrero, D., Larrosa-Garcia, M., Lalueza-Broto, P., Rivera-Sánchez, L., Espinosa-Pereiro, J., Mestre-Torres, J., et al. (2020). Tigecycline-associated hypofibrinogenemia in a real-world setting. Int. J. Clin. Pharm. 42 (4), 1184–1189. doi:10.1007/s11096-020-01072-7
Chen, L. J., Hsiao, F. Y., Shen, L. J., Wu, F. L. L., Tsay, W., Hung, C. C., et al. (2016). Use of hypoprothrombinemia-inducing cephalosporins and the risk of hemorrhagic events: A nationwide nested case-control study. PLoS One 11 (7), e0158407. doi:10.1371/journal.pone.0158407
Conly, J. M., and Stein, K. (1992). The production of menaquinones (vitamin K2) by intestinal bacteria and their role in maintaining coagulation homeostasis. Prog. Food Nutr. Sci. 16 (4), 307–343.
Cui, N., Cai, H., Li, Z., Lu, Y., Wang, G., and Lu, A. (2019). Tigecycline-induced coagulopathy: A literature review. Int. J. Clin. Pharm. 41 (6), 1408–1413. doi:10.1007/s11096-019-00912-5
Drawz, S. M., and Bonomo, R. A. (2010). Three decades of beta-lactamase inhibitors. Clin. Microbiol. Rev. 23 (1), 160–201. doi:10.1128/CMR.00037-09
Fuller, G. M., Otto, J. M., Woloski, B. M., McGary, C. T., and Adams, M. A. (1985). The effects of hepatocyte stimulating factor on fibrinogen biosynthesis in hepatocyte monolayers. J. Cell. Biol. 101 (4), 1481–1486. doi:10.1083/jcb.101.4.1481
Garnacho-Montero, J., Dimopoulos, G., Poulakou, G., Akova, M., Cisneros, J. M., De Waele, J., et al. (2015). Task force on management and prevention of Acinetobacter baumannii infections in the ICU. Intensive Care Med. 41 (12), 2057–2075. doi:10.1007/s00134-015-4079-4
Giammanco, A., Cala, C., Fasciana, T., and Dowzicky, M. J. (2017). Global assessment of the activity of tigecycline against multidrug-resistant gram-negative pathogens between 2004 and 2014 as part of the tigecycline evaluation and surveillance trial. mSphere 2 (1), e00310-e00316. doi:10.1128/mSphere.00310-16
Greenberg, R. N., Reilly, P. M., Weinandt, W. J., Bollinger, M., and Kennedy, D. J. (1987). Cefoperazone-sulbactam combination in the treatment of urinary tract infections: Efficacy, safety, and effects on coagulation. Clin. Ther. 10 (1), 52.
Group of Infectious Diseases RDBoCMA (2018). Guidelines for the diagnosis and treatment of hospital-acquired pneumonia and ventilator-associated pneumonia of adults in China. Chin. J. Tuberc. Respir. Dis. 41 (4), 255∼80.
Haden, H. T. (1957). Vitamin K deficiency associated with prolonged antibiotic administration. AMA Arch. Intern Med. 100 (6), 986–988. doi:10.1001/archinte.1957.00260120130015
Hu, J., Xiao, Y. H., Zheng, Y., Lai, Y. X., Fang, X. L., and Fang, Q. (2020). Clinical characteristics and risk factors of tigecycline-associated hypofibrinogenaemia in critically ill patients. Eur. J. Clin. Pharmacol. 76 (7), 913–922. doi:10.1007/s00228-020-02860-w
Leng, B., Shen, C., Gao, T., Zhao, K., Zhao, X., Guo, Y., et al. (2022). Incidence, characteristics and risk factors of hypofibrinogenemia associated with tigecycline: A multicenter retrospective study in China. Front. Pharmacol. 13, 943674. doi:10.3389/fphar.2022.943674
Leng, B., Xue, Y. C., Zhang, W., Gao, T. T., Yan, G. Q., and Tang, H. (2019). A retrospective analysis of the effect of tigecycline on coagulation function. Chem. Pharm. Bull. (Tokyo) 67 (3), 258–264. doi:10.1248/cpb.c18-00844
Lipsky, J. J. (1983). N-methyl-thio-tetrazole inhibition of the gamma carboxylation of glutamic acid: Possible mechanism for antibiotic-associated hypoprothrombinaemia. Lancet 2 (8343), 192–193. doi:10.1016/s0140-6736(83)90174-5
Liu, H., Jia, X., Zou, H., Sun, S., Li, S., Wang, Y., et al. (2019). Detection and characterization of tigecycline heteroresistance in E. cloacae: Clinical and microbiological findings. Emerg. Microbes Infect. 8 (1), 564–574. doi:10.1080/22221751.2019.1601031
Liu, J., Yan, Y., and Zhang, F. (2021). Risk factors for tigecycline-associated hypofibrinogenemia. Ther. Clin. Risk Manag. 17, 325–332. doi:10.2147/TCRM.S302850
Martini, W. Z. (2009). Fibrinogen metabolic responses to trauma. Scand. J. Trauma Resusc. Emerg. Med. 17, 2. doi:10.1186/1757-7241-17-2
May, J. E., Wolberg, A. S., and Lim, M. Y. (2021). Disorders of fibrinogen and fibrinolysis. Hematol. Oncol. Clin. North Am. 35 (6), 1197–1217. doi:10.1016/j.hoc.2021.07.011
Mueller, R. J., Green, D., and Phair, J. P. (1987). Hypoprothrombinemia associated with cefoperazone therapy. South Med. J. 80 (11), 1360–1362. doi:10.1097/00007611-198711000-00007
Paul, M., Carrara, E., Retamar, P., Tängdén, T., Bitterman, R., Bonomo, R. A., et al. (2022). European Society of Clinical Microbiology and Infectious Diseases (ESCMID) guidelines for the treatment of infections caused by multidrug-resistant Gram-negative bacilli (endorsed by European society of intensive care medicine). Clin. Microbiol. Infect. 28 (4), 521–547. doi:10.1016/j.cmi.2021.11.025
Rodriguez-Bano, J., Gutierrez-Gutierrez, B., Machuca, I., and Pascual, A. (2018). Treatment of infections caused by extended-spectrum-beta-lactamase-AmpC-and carbapenemase-producing enterobacteriaceae. Clin. Microbiol. Rev. 31 (2), 000799-e117. doi:10.1128/CMR.00079-17
Rossitto, G., Piano, S., Rosi, S., Simioni, P., and Angeli, P. (2014). Life-threatening coagulopathy and hypofibrinogenaemia induced by tigecycline in a patient with advanced liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 26 (6), 681–684. doi:10.1097/MEG.0000000000000087
Shirakawa, H., Komai, M., and Kimura, S. (1990). Antibiotic-induced vitamin K deficiency and the role of the presence of intestinal flora. Int. J. Vitam. Nutr. Res. 60 (3), 245–251.
Strom, B. L., Schinnar, R., Gibson, G. A., Brennan, P. J., and Berlin, J. A. (1999). Risk of bleeding and hypoprothrombinaemia associated with NMTT side chain antibiotics: Using cefoperazone as a test case. Pharmacoepidemiol Drug Saf. 8 (2), 81–94. doi:10.1002/(SICI)1099-1557(199903/04)8:2<81::AID-PDS411>3.0.CO;2-G
Su, J., Guo, Q., Li, Y., Wu, S., Hu, F., Xu, S., et al. (2018). Comparison of empirical therapy with cefoperazone/sulbactam or a carbapenem for bloodstream infections due to ESBL-producing Enterobacteriaceae. J. Antimicrob. Chemother. 73 (11), 3176–3180. doi:10.1093/jac/dky323
Treml, B., Rajsic, S., Hell, T., Fries, D., and Bachler, M. (2021). Progression of fibrinogen decrease during high dose tigecycline therapy in critically ill patients: A retrospective analysis. J. Clin. Med. 10 (20), 4702. doi:10.3390/jcm10204702
Vasse, M., Paysant, I., Soria, J., Mirshahi, S. S., Vannier, J. P., and Soria, C. (1996). Down-regulation of fibrinogen biosynthesis by IL-4, IL-10 and IL-13. Br. J. Haematol. 93 (4), 955–961. doi:10.1046/j.1365-2141.1996.d01-1731.x
Wang, W., Liu, Y., Yu, C., Tan, J., Xiong, W., Dong, D., et al. (2020). Cefoperazone-sulbactam and risk of coagulation disorders or bleeding: A retrospective cohort study. Expert Opin. Drug Saf. 19 (3), 339–347. doi:10.1080/14740338.2020.1713090
Wong, R. S., Cheng, G., Chan, N. P., Wong, W. S., and Ng, M. H. (2006). Use of cefoperazone still needs a caution for bleeding from induced vitamin K deficiency. Am. J. Hematol. 81 (1), 76. doi:10.1002/ajh.20449
Xia, G., and Jiang, R. (2020). Clinical study on the safety and efficacy of high-dose tigecycline in the elderly patients with multidrug-resistant bacterial infections: A retrospective analysis. Med. Baltim. 99 (10), e19466. doi:10.1097/MD.0000000000019466
Xin, X., Jian, L., Xia, X., Jia, B., Huang, W., et al. (2013). A multicentre clinical study on the injection of ceftriaxone/sulbactam compared with cefoperazone/sulbactam in the treatment of respiratory and urinary tract infections. Ann. Clin. Microbiol. Antimicrob. 12, 38. doi:10.1186/1476-0711-12-38
Yang, X., Jin, L., Luo, X., Wang, M., Zhu, H., Zhou, Y., et al. (2022). Serum concentration as a predictor of tigecycline-induced hypofibrinogenemia in critically ill patients: A retrospective cohort study. Int. J. Infect. Dis. 123, 136–142. doi:10.1016/j.ijid.2022.08.014
Yilmaz Duran, F., Yildirim, H., and Sen, E. M. (2018). A lesser known side effect of tigecycline: Hypofibrinogenemia. Turk J. Haematol. 35 (1), 83–84. doi:10.4274/tjh.2017.0310
Zhang, Q., Wang, J., Liu, H., Ma, W., Zhou, S., and Zhou, J. (2020). Risk factors for tigecycline-induced hypofibrinogenaemia. J. Clin. Pharm. Ther. 45 (6), 1434–1441. doi:10.1111/jcpt.13250
Keywords: tigecycline, cefoperazone/sulbactam, carbapenems, β-lactam antibiotics, coagulation disorders, hypofibrinogenaemia
Citation: Zhang L, Cai X, Peng F, Tian S, Wu X, Li Y and Guo J (2023) Comparison of bleeding risk and hypofibrinogenemia-associated risk factors between tigecycline with cefoperazone/sulbactam therapy and other tigecycline-based combination therapies. Front. Pharmacol. 14:1182644. doi: 10.3389/fphar.2023.1182644
Received: 09 March 2023; Accepted: 30 May 2023;
Published: 07 June 2023.
Edited by:
Amedeo De Nicolò, University of Turin, ItalyCopyright © 2023 Zhang, Cai, Peng, Tian, Wu, Li and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinlin Guo, taiggll@foxmail.com
†ORCID: Jinlin Guo, orcid.org/0000-0001-8756-1190
‡These authors have contributed equally to this work
 Lei Zhang1‡
Lei Zhang1‡ Jinlin Guo
Jinlin Guo