- 1Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 2Department of Pediatrics and Child Health, School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
- 3Armauer Hansen Research Institute, Addis Ababa, Ethiopia
- 4Department of Human Genetics, Radboud Institute for Health Sciences, Radboud University Medical Center, Nijmegen, Netherlands
Introduction: Genetic variation in the thiopurine S-methyltransferase (TPMT) gene by and large predicts variability in 6-mercaptopurine (6-MP) related toxicities. However, some individuals without genetic variants in TPMT still develop toxicity that necessitates 6-MP dose reduction or interruption. Genetic variants of other genes in the thiopurine pathway have been linked to 6-MP related toxicities previously.
Objective: The aim of this study was to evaluate the effect of genetic variants in ITPA, TPMT, NUDT15, XDH, and ABCB1 on 6-MP related toxicities in patients with acute lymphoblastic leukemia (ALL) from Ethiopia.
Methods: Genotyping of ITPA, and XDH was performed using KASP genotyping assay, while that of TPMT, NUDT15, and ABCB1 with TaqMan® SNP genotyping assays. Clinical profile of the patients was collected for the first 6 months of the maintenance phase treatment. The primary outcome was the incidence of grade 4 neutropenia. Bivariable followed by multivariable cox regression analysis was performed to identify genetic variants associated with the development of grade 4 neutropenia within the first 6 months of maintenance treatment.
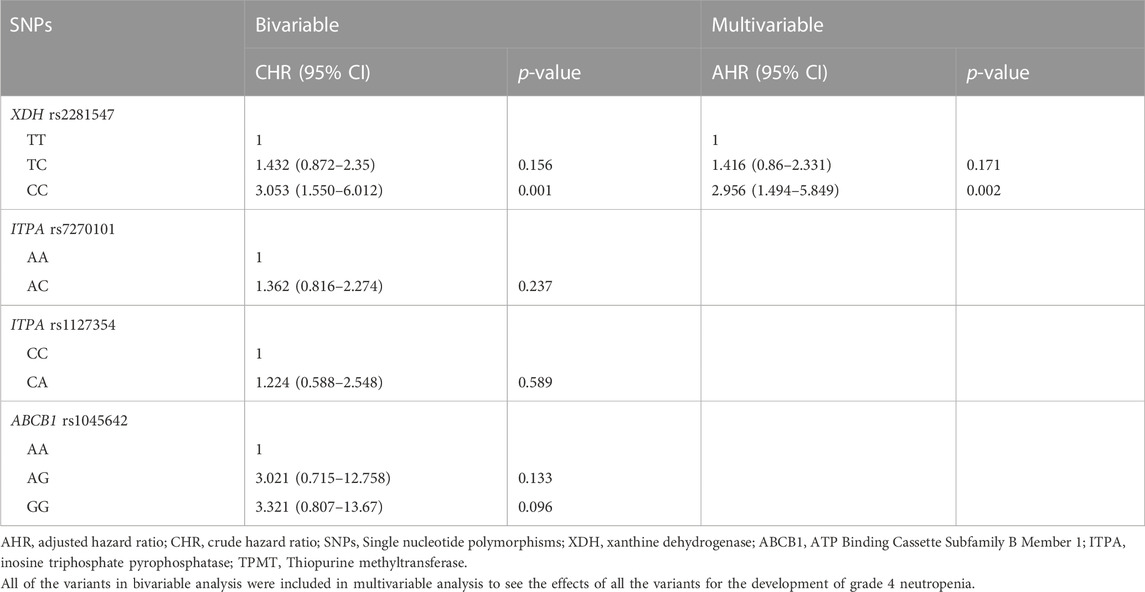
Results: In this study, genetic variants in XDH and ITPA were associated with 6-MP related grade 4 neutropenia and neutropenic fever, respectively. Multivariable analysis revealed that patients who are homozygous (CC) for XDH rs2281547 were 2.956 times (AHR 2.956, 95% CI = 1.494–5.849, p = 0.002) more likely to develop grade 4 neutropenia than those with the TT genotype.
Conclusion: In conclusion, in this cohort, XDH rs2281547 was identified as a genetic risk factor for grade 4 hematologic toxicities in ALL patients treated with 6-MP. Genetic polymorphisms in enzymes other than TPMT involved in the 6-mercaptopurine pathway should be considered during its use to avoid hematological toxicity.
1 Introduction
Globally, acute lymphoblastic leukemia (ALL) is the most common childhood cancer (Johnston et al., 2021). In Ethiopia, the annual incidence of childhood cancers has been estimated between 3,707 and 6,000 cases, with leukemia being the most prevalent cancer (29%) (Aziza et al., 2013; Memirie et al., 2018). Over 90% of patients with childhood ALL in developed countries can be cured as a result of risk-adapted and good systemic chemotherapy. However, treatment outcomes in resource-limited countries are lower, with cure rates ranging from 40% to 70% depending on where the patient is treated (Bahnassy et al., 2020; Tandon, 2020).
In addition to its role in other treatment phases, 6-mercaptopurine (6-MP) is the backbone of the maintenance phase of ALL treatment (Lee et al., 2017). Dose intensity of 6-MP is a key component determining treatment outcome of ALL patients (Relling et al., 1999). Despite good treatment outcomes the downside of 6-MP treatment is that it can induce severe life-threatening hematological and hepatotoxicity. Myelosuppression is particularly complicated when it is accompanied by a severe infection which leads to dose reductions, drug discontinuation, or even treatment interruption and death (Mao et al., 2021). To avoid myelosuppression, 6-MP is titrated to the desired absolute neutrophil count (ANC) value and therefore dose adjustments are routinely made (Karol et al., 2021).
Pharmacogenomics can play a role in increasing the efficacy and reducing toxicity of 6-MP in ALL patients by assisting optimal dose selection for the individual patient. 6-MP is a pro-drug, which requires extensive metabolism to the active metabolite 6-thioguanine. Genetic variants in drug metabolizing enzymes and transporters are known to alter 6-MP treatment outcomes (Jantararoungtong et al., 2021). For instance, it is well established that genetic variants in the thiopurine S-methyltransferase (TPMT) gene are associated with 6-MP treatment intolerance (Wang and Weinshilboum, 2006). To avoid toxicities, 6-MP dose adjustment can be made based on genetic variation in TPMT. TPMT*2, TPMT*3B, and TPMT*3C variants alter the tertiary structure of the TPMT protein, leading to instability and reduced catalytic activity (Chen et al., 2021). Patients that show an intermediate TPMT enzyme activity are advised to start with a reduced dose (30%–50%) and a 10 fold reduction or alternative treatment is recommended for patients with a complete TPMT deficiency (Bertholee et al., 2017). More recently, NUDT15 rs116855232 and rs746071566 variants have been linked to 6-MP intolerance (Chiengthong et al., 2016; Walker et al., 2019) and treatment guidelines have also been developed for this gene (Relling et al., 2019). NUDT15 (rs116855232) mutation adversely affects the protein stability of the enzyme, leading to rapid degradation (Valerie et al., 2016). Whereas, rs746071566 variant reduces NUDT15 activity (Walker et al., 2019). Besides TPMT and NUDT15, other genes in the 6-MP metabolism have also been investigated in relation to development of side effects. For example, genetic variants in ITPA (rs1127354 and rs7270101) reduce enzymatic activity which may increase toxicity risk due to an accumulation of potentially toxic metabolite thioITP (Marinaki et al., 2004; Maeda et al., 2005; Atanasova et al., 2007). A more recent study showed that genetic variants in XDH were associated with thiopurine metabolism and thiopurine related toxicities, including neutropenia, hepatotoxicity, and treatment interruption (Choi et al., 2019). The ATP-binding cassette sub-family B member 1 (ABCB1) gene encodes for P-glycoprotein. ABCB1 overexpression could be accountable for therapy failure or relapse while the reduced activity can lead to more severe 6-MP toxicity (Milosevic et al., 2018).
In the Caucasian population, genetic variants for TPMT have been identified in about 11% (Zeglam et al., 2015) of the population. On the other hand, variants in NUDT15 are rare with frequencies ranging from 0.2% (Coenen, 2019) to 2.4% (Schaeffeler et al., 2019). There is, however, a scarcity of pharmacogenetic studies focusing on other genes in the 6-MP metabolic pathway, especially in non-Caucasian populations. This study therefore aimed to investigate the frequency of variants coding for drug metabolizing enzymes (TPMT, ITPA, NUDT15, and XDH), and transporter (ABCB1) in the thiopurine metabolic pathways in Ethiopian children with ALL and their association with 6-MP-related adverse events.
2 Materials and methods
2.1 Patient recruitment and 6-MP treatment
This study included 160 pediatric patients under the age of 12 years at the time of diagnosis, who were treated from 2019 to 2021 at the pediatric oncology wards of Tikur Anbessa specialized hospital (TASH), which provides organized cancer care services. Patients were treated using a protocol for low- and middle-income countries (Hunger et al., 2009). The maintenance phase of this protocol includes daily oral 6-MP and weekly oral methotrexate (MTX). The patients also received monthly vincristine 1.5 mg/m2, 5 days of dexamethasone per month (6 mg/m2/d for 5 days/mo) and intrathecal MTX (age-adjusted dosing) until 2.5 years from the date of diagnosis. Trimethoprim/sulfamethoxazole (TMP) at a dose of 5 mg/kg/d 3 times per week was given for Pneumocystis jirovecii prophylaxis as co-medication. Patients were stratified into three groups based on a physical examination, age, initial white blood cell count (WBC), central nervous system (CNS) status, and early prednisolone response. Informed consent was obtained from all participants parents or guardians. The study was approved by the Institutional Review Board (IRB) of the College of Health Sciences, Addis Ababa University (021/18), Armauer Hansen Research Institute Ethical Review Committee (P051/18), and National Research Ethics Review Committee of the Federal Democratic Republic of Ethiopia.
2.2 Sample and data collection
EDTA whole blood samples were collected from ALL patients. For all patients, the following information was collected from medical records; demographic data, clinical presentation during diagnosis, complete blood count (CBC) at diagnosis, peripheral morphology, peripheral and bone marrow blast, liver, and kidney function test, and risk group. Clinical data such as CBC, fever, emergency admission, dose reduction, and drug discontinuation were collected for the first 6 months of the maintenance phase treatment. CBC was performed at a 4-week interval unless it was indicated for any clinical reasons.
2.3 DNA isolation and genotyping
Genomic DNA was extracted from 1 mL of whole blood using QIAamp Blood Midi Kit (Qiagen GmbH, Hilden, Germany). DNA quality was checked using gel electrophoresis and NanoDrop™ ND-2000c Spectrophotometer.
Genotyping of SNP rs1127354 and rs7270101 in ITPA and rs2281547 in XDH was performed using a KASPar-On-Demand (KOD) assay (LGC Genomics, Hoddesdon, United Kingdom) as described previously (Vos et al., 2016), with little modification. The final volume for each reaction was 5 µL containing 1 µL of DNA, 2.5 µL of KASP 5000 V4.0 Low ROX, 0.0625 µL of the KASPar assay (40x), and 1.44 µL of MilliQ grade water. The PCR conditions consisted of an initial denaturation at 94°C for 15 min, followed by 10 cycles at 94°C for 20 s and annealing/extension at 61°C for 60 s including a drop of 0.6°C for each cycle. This was followed by 26 cycles of denaturation at 94°C for 10 s and annealing/extension at 55°C for 60 s, followed by 12 cycles of denaturation at 94°C for 20 s and annealing/extension at 57°C for 60 s. TPMT*2, TPMT*3B, TPMT*3C, NUDT15 rs116855232, NUDT15 rs746071566, and ABCB1 rs1045642 variants were genotyped by TaqMan® SNP Genotyping Assays (Assay ID number C__12091552_30 for TPMT*2, C__30634116_20 for TPMT*3B, C_____19567_20 for TPMT*3C, C_154823200_10 for rs116855232, and C___7586657_20 for rs1045642) as described elsewhere (Kumagai et al., 2019). The final reaction volume of 5 µL was prepared by mixing 1 µL of DNA, 2.5 µL of TaqMan® Universal PCR Master Mix (2x; Applied Biosystems by Thermo Fisher scientific, Warrington, United Kingdom), 0.0625 µL of TaqMan® SNP Genotyping Assay (40x; Applied Biosystems by Thermo Fisher Scientific), and 1.44 µL of MilliQ grade water. The PCR conditions included an initial stage at 95°C for 12 min and followed by a stage for 50 cycles step 1 at 92°C for 15 s and step 2 at 60°C for 90 s. For NUDT15 rs746071566 custom designed assay (Id: ANDKGXA) was used. The final reaction volume of 10 µL was prepared by mixing 1 µL of DNA, 5 µL of TaqMan® Universal PCR Master Mix, 0.25 µL of TaqMan® SNP Genotyping Assay, and 3,75 µL of MilliQ grade water. The PCR conditions included an initial stage at 95°C for 10 min and followed by a stage for 50 cycles; step 1 at 95°C for 15 s and step 2 with 60°C for 60 s and post-read stage at 60°C.
2.4 Study outcomes
Myelotoxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE), version 4.0 (CTCAE, 2010). Accordingly, toxicity was classified as grade 4 when WBC <1,000/mm3; ANC <500/mm3; anemia <6.5 g/dL and thrombocytopenia <25,000/mm3. The primary outcome measure was the occurrence of grade 4 neutropenia within the first 6 months of the initiation of maintenance treatment. The secondary outcomes were, drug discontinuation, neutropenic fever, and early-onset grade 4 leukopenia/neutropenia. Early-onset leukopenia/neutropenia was defined as the occurrence of leukopenia/neutropenia during the first 60 days of the maintenance therapy (Zhou et al., 2018). Neutronic fever was defined as a grade 4 neutropenia with temperature of 38°C or greater.
2.5 Statistical analysis
Data were entered and analyzed using SPSS statistical package for Windows SPSS version 26. The Chi-square test was used to assess Hardy–Weinberg equilibrium (HWE). Descriptive statistics were used to examine the demographic characteristics, clinical profiles, and genotype frequencies of participants. Risk factor analysis and hazard ratios for grade 4 neutropenia, early-onset grade 4 leukopenia/neutropenia, treatment interruption, and neutropenic fever were calculated using cox proportional hazard regression analysis. Bivariable followed by multivariable analysis was computed with enter as the variable selection method. Results were expressed as hazard ratios (HRs) and 95% confidence intervals. For all test a p-value of 0.05 was considered significant. All genetic variants except TPMT and NUDT15 were included in multivariable analysis to see the influence of the variants on hematotoxicity.
3 Results
3.1 Demographic, clinical characteristics and incidence of grade 4 hematological toxicity
In this study, 142 patients were included in the final analysis. Due to incomplete data, 18 study participants were not included in the final analysis. The clinical characteristics of the patients are shown in Table 1. The study group consisted of 92 (64.8%) males, and 50 (35.2%) females, and the mean age at diagnosis was 6.2 years old. Sixty-nine (48.6%) participants were categorized as standard risk, while the remaining 73 (51.4%) were high risk. The overall incidence of chemotherapy-induced grade 4 neutropenia was 52.8%. Fifty-six (39.4%) patients received full doses of chemotherapy as scheduled, while therapy was interrupted in 83 (58.5%) patients because of adverse events in the first 6 months of the maintenance treatment. The most frequent adverse event causing discontinuation was myelosuppression.
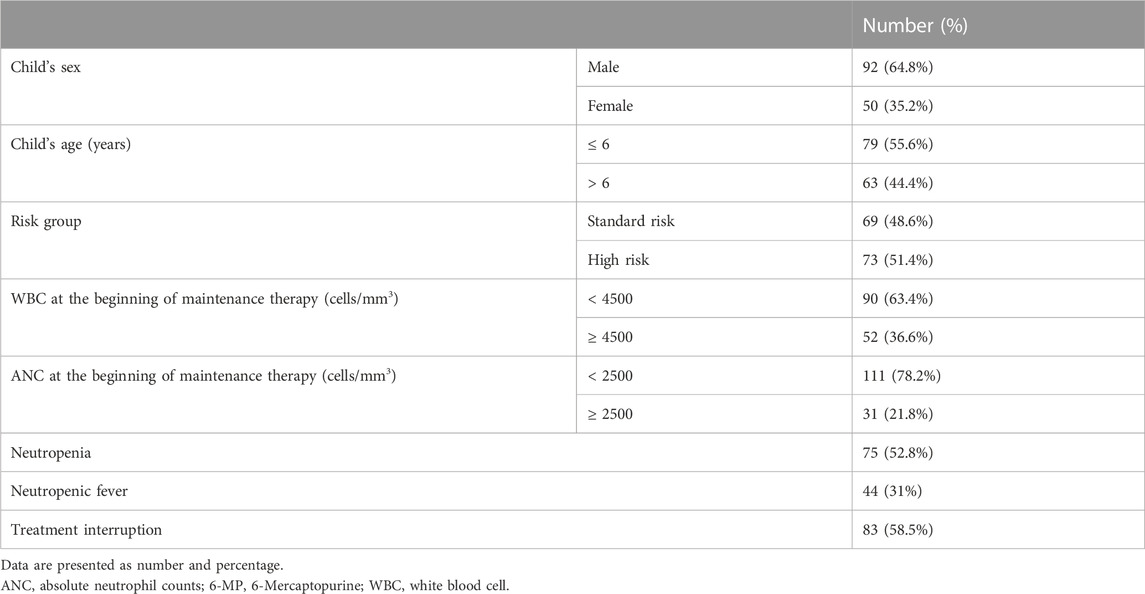
TABLE 1. Characteristics of the study population in the outpatient pediatric oncology department of TASH (n = 142).
3.2 Genotyping and its association with the primary outcome
All genotype frequencies were according to HWE (p > 0.05). The overall allele frequencies of TPMT*3C, ITPA rs1127354, ITPA rs7270101, XDH rs2281547, and ABCB1 rs1045642 variants were 0.35, 4.6, 11.6, 31, and 77.1%, respectively. No variant alleles were identified for TPMT*2, TPMT*3B, NUDT15 rs116855232, and rs746071566. Table 2 shows a comparison of genotype and allele frequencies between patients who developed grade 4 neutropenia versus treatment tolerant patients. Patients carrying the XDH rs2281547 CC/TC genotype had a significantly higher incidence of grade 4 neutropenia (p = 0.01) as compared to TT genotype. Similar results were found for the allele analysis. The incidence of grade 4 neutropenia was higher in patients carrying XDH rs2281547 C allele (38.6%% vs. 22.3%, p = 0.003). The other allele frequencies did not differ significantly between patients with grade 4 neutropenia compared to treatment tolerant patients.
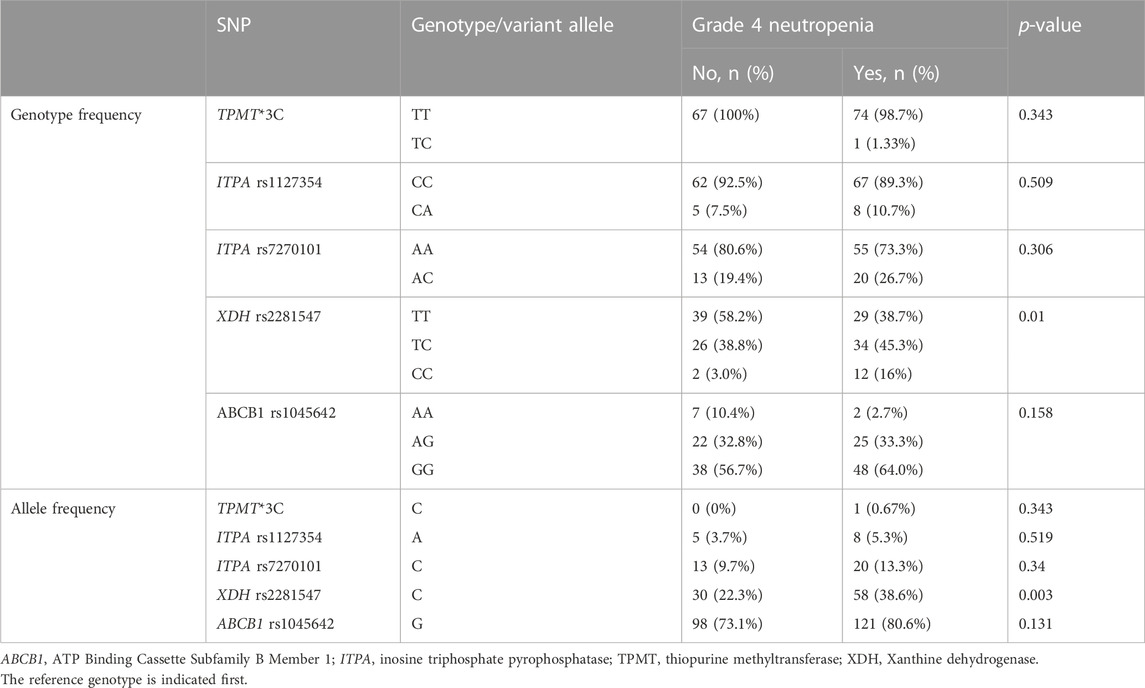
TABLE 2. Genotype and allele frequencies of candidate drug metabolizing enzymes and transporter genes by grade 4 neutropenia.
Detailed data of cox proportional hazard regression analyses for grade 4 neutropenia are depicted in Table 3. Multivariable analysis showed that patients who carry the XDH rs2281547 CC genotype had a higher hazard (AHR 2.956, 95% CI = 1.494–5.849, p = 0.002) to develop grade 4 neutropenia than those with the XDH rs2281547 TT genotype. The result indicated that XDH rs2281547 was an independent genetic predictor of toxicity. Further, Kaplan–Meier hazard curves (Figure 1) show that the risk of developing grade 4 neutropenia increases over time during the first 6 months of maintenance treatment. The cumulative risk of developing grade 4 neutropenia was higher in patients with the CC and TC genotype compared to patients with the TT genotype (p = 0.003).

FIGURE 1. Kaplan-Meier curves to estimate cumulative hazard for the development of Chemotherapy-induced grade 4neutropenia stratified by XDH rs2281547.
Effects of both genetic and clinical factor on developing grade 4 neutropenia is shown in Supplementary Table S1. Patients with low day 1 maintenance WBC counts were 2.093 times more at risk to develop grade 4 neutropenia than those with normal day 1 maintenance WBC counts. Similarly, patients with age ≤6 years were 2.273 times more at risk to develop grade 4 neutropenia than those who were >6 years of age.
3.3 Genotyping and its association with the secondary outcomes
Table 4 shows the results from the bivariable and multivariable cox regression analyses for the secondary outcomes. Multivariable analysis demonstrated that patients with the ITPA rs1127354 AC had a bit more than two-fold (p = 0.029) increased risk of developing neutropenic fever. Furthermore, patients with XDH rs2281547 TC genotype (AHR = 1.61, 95% CI 1.007–2.573, p = 0.047), and CC genotype (AHR 2.704, 95% CI = 1.382–5.289, p = 0.004) were also had to experience more treatment interruption than those carrying the TT variant. Kaplan–Meier hazard curves (Figure 2) show that time to treatment interruption hazard was higher in patients with the XDH rs2281547 CC and TC genotype compared to persons with the TT genotype (p = 0.005). Similarly, the time to neutropenic fever hazard was higher in patients with the ITPA rs1127354 AC genotype compared to persons with the CC genotype (p = 0.041) (Figure 3).
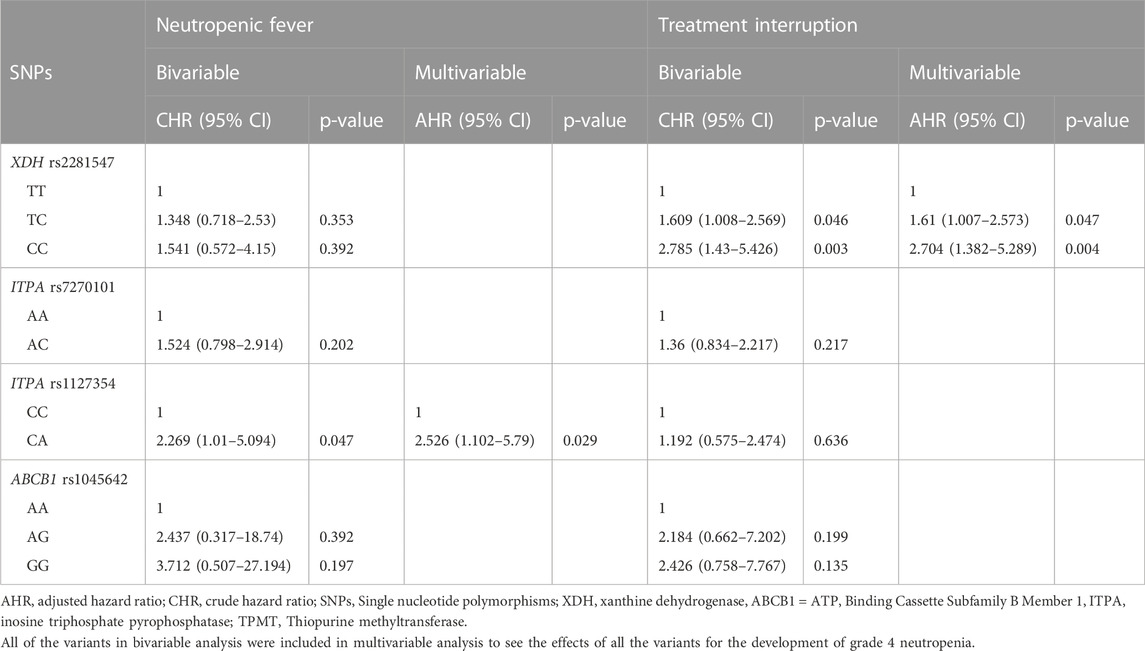
TABLE 4. Cox proportional hazard regression for incidence of neutropenic fever and treatment interruption.
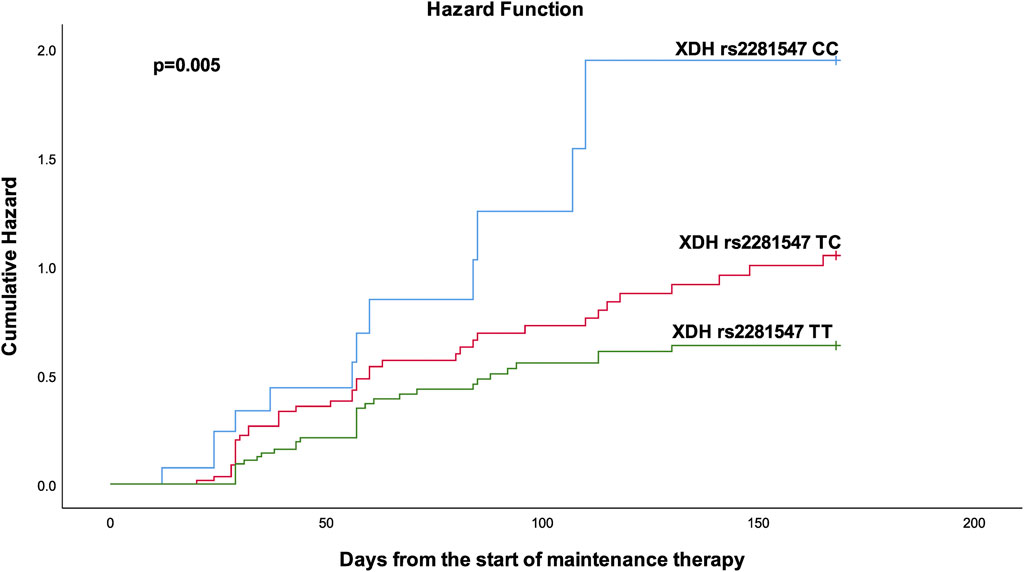
FIGURE 2. Kaplan-Meier curves to estimate cumulative hazard for treatment interruption stratified by XDH rs2281547.
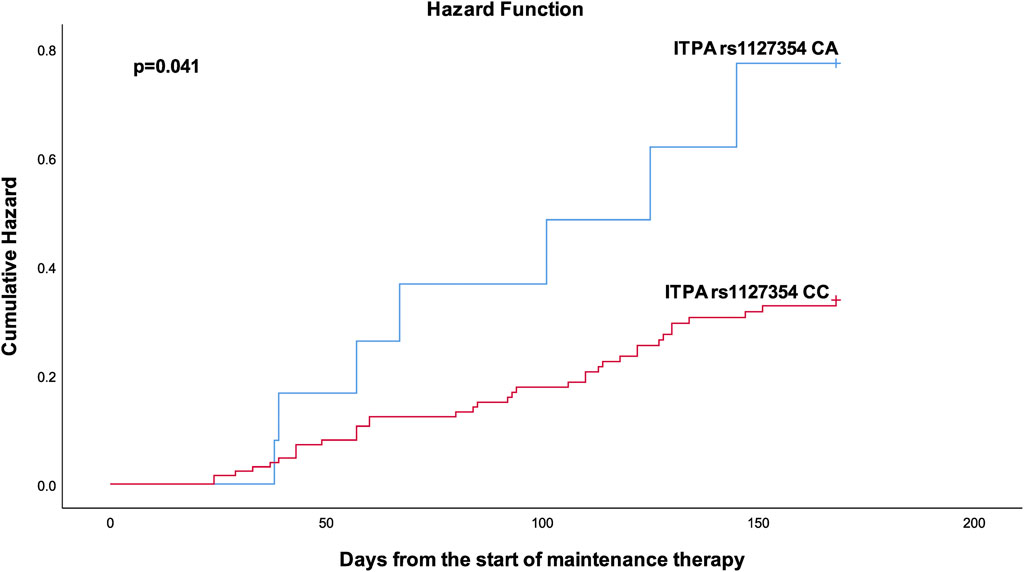
FIGURE 3. Kaplan-Meier curves to estimate cumulative hazard for neutropenic fever stratified by ITPA rs 1127354.
Multivariable analysis showed that none of the genetic variants tested were associated with early-onset grade 4 leukopenia (Supplementary Table S2). On the other hand, patients with the ITPA rs7270101 AC genotype had about two-fold (p = 0.035) increased risk of developing early-onset grade 4 neutropenia.
4 Discussion
Inter-individual genetic variations in drug-metabolizing enzymes and transporters affect the toxicity and efficacy of 6-MP, which poses a challenge to the management of patients. Understanding risk factors linked to individual 6-MP intolerance in different ethnicities is vital to facilitate the development of individualized treatment protocol. This is vital for genetic factors as differences in genotype distribution among different ethnic populations limits the predictive value for toxicities. In this study, the incidence of chemotherapy-induced hematologic toxicity and associated risk factors including genetic variations in drug mobilizing enzymes and transporter relevant for the disposition of 6-MP were investigated in childhood ALL patients from Ethiopia.
NUDT15 rs116855232 and rs746071566, which are considerably lower in frequency in African population, were not detected in this cohort. TPMT*3C is the most prevalent allele among the black population (Ameyaw, 1999; McLeod et al., 1999; Adehin et al., 2017). However, in our cohort, the risk allele frequency of TPMT*3C was 0.35%, about seven times less than described by Ronen et al. (Ofri et al., 2010) in Ethiopian Jews. Besides this, other TPMT variants were not found at all (*2 and *2A). Thus, the value of predicting hematologic toxicity in this population by genotyping TPMT was hindered by the low frequency of well-known variants to be associated with decreased TMPT activity. It is essential to screen a larger group from Ethiopia to determine the exact frequency.
Literature shows that only up to 25% of thiopurine side effects can be explained by TPMT variants (Broekman et al., 2017). Genetic polymorphisms other than TPMT such as ITPA and NUDT15 polymorphisms have been shown to be associated with thiopurine toxicity in patients without TPMT polymorphisms (Chiengthong et al., 2016; Mao et al., 2021). Recently study linked XDH genetic variant with 6-MP induced toxicity. XDH contributes to production of the 6-thioxanthine intermediate from 6-MP and it is also involved in the conversion of the intermediate to the final product (Choughule et al., 2014). This is the first study in Ethiopian ALL patients showing that persons homozygous for the minor allele of XDH rs2281547 have a higher risk on grade 4 neutropenia. rs2281547 is an intronic variant, which could alter gene expression and yet the functionality of this SNP is not known. Hence, confirmation of the association should be sought in future studies. Poor XDH enzyme activity increases the risk for 6-MP related hematological toxicity, whereas rapid XDH metabolizers have an increased risk of thiopurine failure due to low 6-TGTP formation (Dewit et al., 2010). Co-administration of 6-MP and allopurinol or febuxostat (XDH/XO inhibitors) increased production of active as well as toxic compounds that can lead to severe forms of thiopurine-induced toxicity (Chocair et al., 1993; Dewit et al., 2010). Despite the fact that XDH has a role in the metabolism of 6-MP, the potential clinical significance is not yet clear. There are few studies investigating the role of genetic variants in XDH. Molybdenum cofactor sulfurase c.362C > T and c.2107C > A variants alter and reduce the metabolic capacity of XDH, slowing thiopurine metabolism that increases formation of the nucleotide 6-thioguanine, which is hematotoxic (Kurzawski et al., 2012; Stiburkova et al., 2018). A recent pathway genes association study reported the role of XDH in thiopurine-related toxicities; neutropenia, hepatotoxicity, and treatment interruption (Choi et al., 2019). A case report study also identified that hematotoxicity caused by azathioprine is aggravated by xanthine oxidase deficiency (Serre-Debeauvais et al., 1995).
This study also identified associations between ITPA polymorphisms and 6-MP related hematological toxicity. The minor allele frequency of ITPA rs1127354 and ITPA rs7270101 was 4.6%, and 11.6%, respectively, which is similar to other studies (William et al., 2011; Smid et al., 2014; Hareedy et al., 2015). ITPA acts as a house-cleaning enzyme since it hydrolyzes 6-TITP back to 6-TIMP, thus preventing the accumulation of 6-TITP, which is toxic (Zamzami et al., 2013; Barba et al., 2022). A recent meta-analysis suggested that populations with low TPMT and high ITPA variant allele frequencies, such as Asians are more susceptible to the influence of ITPA variants (Barba et al., 2022). In the present study, the TPMT allele frequencies were also low and interestingly ITPA rs1127354 was significantly associated with the incidence of febrile neutropenia. This is in agreement with a previous study (Stocco et al., 2009), showing a significantly higher probability of severe febrile neutropenia in patients with a variant in ITPA among patients who received a TPMT genotype-guided dose of 6-MP. As reported in an Egyptian ALL study, ITPA rs7270101 was associated with the risk of neutropenia and leukopenia (Hareedy et al., 2015). In agreement with these results, we show that ITPA rs7270101 was associated with neutropenia that developed within the initial 60 days of the maintenance therapy. This is suggesting that ITPA rs7270101 could be an important genetic predictor for determining the 6-MP dose intensity with the risk allele carriers requiring a low 6-MP dose. This is the first study in Ethiopian population showing that ITPA alleles are associated with 6-MP related hematological toxicity during maintenance therapy. Nevertheless, there is no consensus on the impact of genetic polymorphisms in ITPA on thiopurine induced hematologic toxicity yet and its application in a clinic setting has not been unified, especially in different ethnic populations (Zhou et al., 2018; Mao et al., 2021).
This study showed that a higher incidence of grade 4 hematologic toxicities (mainly manifested as neutropenic toxicity) caused treatment interruption (Table 1). The finding of this study revealed that XDH rs2281547 are strong genetic predictor of grade 4 hematologic toxicities in this cohort of Ethiopian children. This study provides first information on genetic factors associated with hematologic toxicities in Ethiopian childhood ALL patients. However, the outcome of the current study is limited as only five genes in thiopurine pathway were tested. Other limitations include small sample size, a single institutional study, and lack of validation cohorts.
5 Conclusion
In the current study, the rate of hematological toxicity was high. XDH and ITPA variants were associated with grade 4 hematologic toxicities during maintenance therapy. TPMT*3C variant frequency was very low in this cohort. This study adds knowledge on how to identify pediatric ALL patients at high risk for chemotherapy induced toxicity in a population from Ethiopia. In the future, it is essential to study large groups of patients from different ethnic background to determine which genes are important to predict grade 4 hematological toxicities in different ethnic populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Materials, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by the study protocol was approved by the Institutional Review Board of the College of Health Sciences, Addis Ababa University (021/18/Pharma), the Armauer Hansen Research Institute Ethical Review Committee (PO51/18), and the Ethiopian National Research Ethics Review Committee. Written consent is obtained from guardians of all the study participants. Written informed consent to participate in this study was provided by the participants’ legal guardian/next of kin.
Author contributions
AA, RH, TA, HA, and MC designed the study. AA collected the data. AA and MC did genotyping, analyzed the data and draft the manuscript. AA, HA, DH, EE, RH, TA, and MC involved in the investigation and/or in the discussion of results and critical review of the manuscript.
Funding
This work is funded by the Office of the Graduate Program of Addis Ababa University with grant PV-470931 (www.aau.edu.et). The Armauer Hansen Research Institute (PV-470931, https://ahri.gov.et) and Center for Innovative Drug Development and Therapeutic Trials for Africa (https://www.cdt-africa.org/) also granted a research visit.
Acknowledgments
We thank the study participants and staff members of cancer center of Tikur Anbessa specialized hospital and Armauer Hansen Research Institute for their support during the study period. We would like to extend our sincere appreciation to the staff members of RadboudUMC.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1159307/full#supplementary-material
References
Adehin, A., Bolaji, O. O., Kennedy, M. A., and Adeagbo, B. A. (2017). Allele frequencies of thiopurine S-methyltransferase (TPMT) variants in the Nigerian population. Pol. Ann. Med. 24, 144–147. doi:10.1016/j.poamed.2016.06.007
Ameyaw, M., Collie-Duguid, E. S., Powrie, R. H., Ofori-Adjei, D., and McLeod, H. L. (1999). Thiopurine methyltransferase alleles in British and Ghanaian populations. Hum. Mol. Genet. 8, 367–370. doi:10.1093/hmg/8.2.367
Atanasova, S., Shipkova, M., Svinarov, D., Mladenova, A., Genova, M., Wieland, E., et al. (2007). Analysis of ITPA phenotype-genotype correlation in the Bulgarian population revealed a novel gene variant in exon 6. Ther. Drug Monit. 29, 6–10. doi:10.1097/FTD.0b013e3180308554
Aziza, S., Julia, C., and Mary Louise, C. (2013). Pediatric oncology in Ethiopia: An INCTR-USA and georgetown University hospital twinning initiative with Tikur Anbessa specialized hospital. Cancer control., 108–112.
Bahnassy, A. A., Abdellateif, M. S., and Zekri, A.-R. N. (2020). Cancer in africa: Is it a genetic or environmental Health problem? Front. Oncol. 10, 604214. doi:10.3389/fonc.2020.604214
Barba, E., Kontou, P. I., Michalopoulos, I., Bagos, P. G., and Braliou, G. G. (2022). Association of ITPA gene polymorphisms with adverse effects of AZA/6-MP administration: A systematic review and meta-analysis. Pharmacogenomics J. 22, 39–54. doi:10.1038/s41397-021-00255-3
Bertholee, D., Maring, J. G., and van Kuilenburg, A. B. P. (2017). Genotypes affecting the pharmacokinetics of anticancer drugs. Clin. Pharmacokinet. 56, 317–337. doi:10.1007/s40262-016-0450-z
Broekman, M. M. T. J., Coenen, M. J. H., Wanten, G. J., van Marrewijk, C. J., Klungel, O. H., Verbeek, A. L. M., et al. (2017). Risk factors for thiopurine-induced myelosuppression and infections in inflammatory bowel disease patients with a normal TPMT genotype. Aliment. Pharmacol. Ther. 46, 953–963. doi:10.1111/apt.14323
Chen, Z.-Y., Zhu, Y.-H., Zhou, L.-Y., Shi, W.-Q., Qin, Z., Wu, B., et al. (2021). Association between genetic polymorphisms of metabolic enzymes and azathioprine-induced myelosuppression in 1,419 Chinese patients: A retrospective study. Front. Pharmacol. 12, 672769. doi:10.3389/fphar.2021.672769
Chiengthong, K., Ittiwut, C., Muensri, S., Sophonphan, J., Sosothikul, D., Seksan, P., et al. (2016). NUDT15 c.415C>T increases risk of 6-mercaptopurine induced myelosuppression during maintenance therapy in children with acute lymphoblastic leukemia. Haematologica 101, e24–e26. doi:10.3324/haematol.2015.134775
Chocair, P., Ianhez, L., Arap, S., Sabbaga, E., Duley, J., Simmonds, H. A., et al. (1993). Low-dose allopurinol plus azathioprine/cyclosporin/prednisolone, a novel immunosuppressive regimen. Lancet 342, 83–84. doi:10.1016/0140-6736(93)91287-V
Choi, R., Sohn, I., Kim, M., Woo, H. I., Lee, J. W., Ma, Y., et al. (2019). Pathway genes and metabolites in thiopurine therapy in Korean children with acute lymphoblastic leukaemia. Br. J. Clin. Pharmacol. 85, 1585–1597. doi:10.1111/bcp.13943
Choughule, K. V., Barnaba, C., Joswig-Jones, C. A., and Jones, J. P. (2014). In vitro oxidative metabolism of 6-mercaptopurine in human liver: Insights into the role of the molybdoflavoenzymes aldehyde oxidase, xanthine oxidase, and xanthine dehydrogenase. Drug Metab. Dispos. 42, 1334–1340. doi:10.1124/dmd.114.058107
Coenen, M. J. H. (2019). NUDT15 genotyping in Caucasian patients can help to optimise thiopurine treatment in patients with inflammatory bowel disease. Transl. Gastroenterol. Hepatol. 4, 81. doi:10.21037/tgh.2019.11.09
CTCAE (2010). Common Terminology Criteria for adverse events (CTCAE). Available at: https://ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm.
Dewit, O., Starkel, P., and Roblin, X. (2010). Thiopurine metabolism monitoring: Implications in inflammatory bowel diseases. Eur. J. Clin. Investigation 40, 1037–1047. doi:10.1111/j.1365-2362.2010.02346.x
Hareedy, M. S., El Desoky, E. S., Woillard, J.-B., Thabet, R. H., Ali, A. M., Marquet, P., et al. (2015). Genetic variants in 6-mercaptopurine pathway as potential factors of hematological toxicity in acute lymphoblastic leukemia patients. Pharmacogenomics 16, 1119–1134. doi:10.2217/PGS.15.62
Hunger, S. P., Sung, L., and Howard, S. C. (2009). Treatment strategies and regimens of graduated intensity for childhood acute lymphoblastic leukemia in low-income countries: A proposal. Pediatr. Blood Cancer 52, 559–565. doi:10.1002/pbc.21889
Jantararoungtong, T., Wiwattanakul, S., Tiyasirichokchai, R., Prommas, S., Sukprasong, R., Koomdee, N., et al. (2021). TPMT*3C as a predictor of 6-mercaptopurine-induced myelotoxicity in Thai children with acute lymphoblastic leukemia. JPM 11, 783. doi:10.3390/jpm11080783
Johnston, W. T., Erdmann, F., Newton, R., Steliarova-Foucher, E., Schüz, J., and Roman, E. (2021). Childhood cancer: Estimating regional and global incidence. Cancer Epidemiol. 71, 101662. doi:10.1016/j.canep.2019.101662
Karol, S. E., Pei, D., Smith, C. A., Liu, Y., Yang, W., Kornegay, N. M., et al. (2021). Comprehensive analysis of dose intensity of acute lymphoblastic leukemia chemotherapy. haematol 107, 371–380. doi:10.3324/haematol.2021.278411
Kumagai, H., Miyamoto-Mikami, E., Hirata, K., Kikuchi, N., Kamiya, N., Hoshikawa, S., et al. (2019). ESR1 rs2234693 polymorphism is associated with muscle injury and muscle stiffness. Med. Sci. Sports Exerc. 51, 19–26. doi:10.1249/MSS.0000000000001750
Kurzawski, M., Dziewanowski, K., Safranow, K., and Drozdzik, M. (2012). Polymorphism of genes involved in purine metabolism (XDH, AOX1, MOCOS) in kidney transplant recipients receiving azathioprine. Ther. Drug Monit. 34, 266–274. doi:10.1097/FTD.0b013e31824aa681
Lee, D.-K., Chang, V. Y., Kee, T., Ho, C.-M., and Ho, D. (2017). Optimizing combination therapy for acute lymphoblastic leukemia using a phenotypic personalized medicine digital Health platform: Retrospective optimization individualizes patient regimens to maximize efficacy and safety. SLAS Technol. 22, 276–288. doi:10.1177/2211068216681979
Maeda, T., Sumi, S., Ueta, A., Ohkubo, Y., Ito, T., Marinaki, A. M., et al. (2005). Genetic basis of inosine triphosphate pyrophosphohydrolase deficiency in the Japanese population. Mol. Genet. Metabolism 85, 271–279. doi:10.1016/j.ymgme.2005.03.011
Mao, X., Yin, R., Sun, G., Zhou, Y., Yang, C., Fang, C., et al. (2021). Effects of TPMT, NUDT15, and ITPA genetic variants on 6-mercaptopurine toxicity for pediatric patients with acute lymphoblastic leukemia in yunnan of China. Front. Pediatr. 9, 719803. doi:10.3389/fped.2021.719803
Marinaki, A. M., Ansari, A., Duley, J. A., Arenas, M., Sumi, S., Lewis, C. M., et al. (2004). Adverse drug reactions to azathioprine therapy are associated with polymorphism in the gene encoding inosine triphosphate pyrophosphatase (ITPase). Pharmacogenetics 14, 181–187. doi:10.1097/00008571-200403000-00006
McLeod, H. L., Pritchard, S. C., Githang, J., Indalo, A., Ameyaw, M.-M., Powrie, R. H., et al. (1999). Ethnic differences in thiopurine methyltransferase pharmacogenetics: Evidence for allele specificity in caucasian and Kenyan individuals. Pharmacogenetics 9, 773–776. doi:10.1097/00008571-199912000-00012
Memirie, S. T., Habtemariam, M. K., Asefa, M., Deressa, B. T., Abayneh, G., Tsegaye, B., et al. (2018). Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. JGO 1, 1–11. doi:10.1200/JGO.17.00175
Milosevic, G., Kotur, N., Krstovski, N., Lazic, J., Zukic, B., Stankovic, B., et al. (2018). Variants in TPMT, ITPA, ABCC4 and ABCB1 genes as predictors of 6-mercaptopurine induced toxicity in children with acute lymphoblastic leukemia. J. Med. Biochem. 37, 320–327. doi:10.1515/jomb-2017-0060
Ofri, R., Sara Bar, C., and Deborah, R. (2010). Evaluating Frequencies of thiopurine s-methyl transferase (TPMT) variant alleles in Israeli ethnic subpopulations using DNA analysis. Med. Assoc. J. 12, 721–725.
Relling, M. V., Hancock, M. L., Boyett, J. M., Pui, C.-H., and Evans, W. E. (1999). Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood 93, 2817–2823. doi:10.1182/blood.V93.9.2817
Relling, M. V., Schwab, M., Whirl-Carrillo, M., Suarez-Kurtz, G., Pui, C., Stein, C. M., et al. (2019). Clinical pharmacogenetics implementation consortium guideline for thiopurine dosing based on TPMT and NUDT 15 genotypes: 2018 update. Clin. Pharmacol. Ther. 105, 1095–1105. doi:10.1002/cpt.1304
Schaeffeler, E., Jaeger, S. U., Klumpp, V., Yang, J. J., Igel, S., Hinze, L., et al. (2019). Impact of NUDT15 genetics on severe thiopurine-related hematotoxicity in patients with European ancestry. Genet. Med. 21, 2145–2150. doi:10.1038/s41436-019-0448-7
Serre-Debeauvais, F., Bayle, F., Amirou, M., Bechtel, Y., Boujet, C., Vialtel, P., et al. (1995). Hematotoxicity caused by azathioprine genetically determined and aggravated by xanthine oxidase deficiency in a patient following renal transplantation. Presse Medicale 24, 987–988.
Smid, A., Karas-Kuzelicki, N., Milek, M., Jazbec, J., and Mlinaric-Rascan, I. (2014). Association of ITPA genotype with event-free survival and relapse rates in children with acute lymphoblastic leukemia undergoing maintenance therapy. PLoS ONE 9, e109551. doi:10.1371/journal.pone.0109551
Stiburkova, B., Pavelcova, K., Petru, L., and Krijt, J. (2018). Thiopurine-induced toxicity is associated with dysfunction variant of the human molybdenum cofactor sulfurase gene (xanthinuria type II). Toxicol. Appl. Pharmacol. 353, 102–108. doi:10.1016/j.taap.2018.06.015
Stocco, G., Cheok, M., Crews, K., Dervieux, T., French, D., Pei, D., et al. (2009). Genetic polymorphism of inosine triphosphate pyrophosphatase is a determinant of mercaptopurine metabolism and toxicity during treatment for acute lymphoblastic leukemia. Clin. Pharmacol. Ther. 85, 164–172. doi:10.1038/clpt.2008.154
Tandon, S. (2020). Acute leukemia treatment in low- and middle-income countries: Is it time for tailored therapy? Cancer Res. Stat. Treat. 3, 642. doi:10.4103/CRST.CRST_238_20
Valerie, N. C. K., Hagenkort, A., Page, B. D. G., Masuyer, G., Rehling, D., Carter, M., et al. (2016). NUDT15 hydrolyzes 6-thio-DeoxyGTP to mediate the anticancer efficacy of 6-thioguanine. Cancer Res. 76, 5501–5511. doi:10.1158/0008-5472.CAN-16-0584
Vos, H. I., Guchelaar, H.-J., Gelderblom, H., de Bont, E. S. J. M., Kremer, L. C. M., Naber, A. M., et al. (2016). Replication of a genetic variant in ACYP2 associated with cisplatin-induced hearing loss in patients with osteosarcoma. Pharmacogenetics Genomics 26, 243–247. doi:10.1097/FPC.0000000000000212
Walker, G. J., Harrison, J. W., Heap, G. A., Voskuil, M. D., Andersen, V., Anderson, C. A., et al. (2019). Association of genetic variants in NUDT15 with thiopurine-induced myelosuppression in patients with inflammatory bowel disease. JAMA 321, 773–785. doi:10.1001/jama.2019.0709
Wang, L., and Weinshilboum, R. (2006). Thiopurine S-methyltransferase pharmacogenetics: Insights, challenges and future directions. Oncogene 25, 1629–1638. doi:10.1038/sj.onc.1209372
William, Z.-F., Manuel, B. A., Ana, E., Daniel, C., Aurelio, L., Javier, C., et al. (2011). A pharmacogenetics study of TPMT and ITPA genes detects a relationship with side effects and clinical response in patients with inflammatory bowel disease receiving Azathioprine. J. Gastrointestin Liver Dis. 20, 247–253.
Zamzami, M. A., Duley, J. A., Price, G. R., Venter, D. J., Yarham, J. W., Taylor, R. W., et al. (2013). Inosine Triphosphate Pyrophosphohydrolase (ITPA) polymorphic sequence variants in adult hematological malignancy patients and possible association with mitochondrial DNA defects. J. Hematol. Oncol. 6, 24. doi:10.1186/1756-8722-6-24
Zeglam, H. B., Benhamer, A., Aboud, A., Rtemi, H., Mattardi, M., Saleh, S. S., et al. (2015). Polymorphisms of the thiopurine S -methyltransferase gene among the Libyan population. Libyan J. Med. 10, 27053. doi:10.3402/ljm.v10.27053
Keywords: acute lymphoblastic leukemia, xanthine dehydrogenase, neutropenia, pharmacogenetics, 6-mercactopurine
Citation: Ali AM, Adam H, Hailu D, Engidawork E, Howe R, Abula T and Coenen MJH (2023) Genetic variants of genes involved in thiopurine metabolism pathway are associated with 6-mercaptopurine toxicity in pediatric acute lymphoblastic leukemia patients from Ethiopia. Front. Pharmacol. 14:1159307. doi: 10.3389/fphar.2023.1159307
Received: 05 February 2023; Accepted: 12 April 2023;
Published: 09 May 2023.
Edited by:
Funda Tuzun, Dokuz Eylül University, TürkiyeReviewed by:
Eng Wee Chua, National University of Malaysia, MalaysiaNathalie K. Zgheib, American University of Beirut, Lebanon
Copyright © 2023 Ali, Adam, Hailu, Engidawork, Howe, Abula and Coenen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Awol Mekonnen Ali, awolalim@gmail.com
†Present address: Marieke J. H. Coenen, Erasmus Medical Center, Erasmus University, Rotterdam, Netherlands
 Awol Mekonnen Ali
Awol Mekonnen Ali Haileyesus Adam2
Haileyesus Adam2 Ephrem Engidawork
Ephrem Engidawork Rawleigh Howe
Rawleigh Howe Marieke J. H. Coenen
Marieke J. H. Coenen