- 1Department of Breast Surgery, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 2Guangxi Medical University Cancer Hospital, Nanning, China
- 3Department of Pathology, The First Affiliated Hospital of Hainan Medical University, Haikou, China
- 4Key Laboratory of Molecular Biology, Hainan Medical University, Haikou, China
- 5Department of Biochemistry and Molecular Biology, Hainan Medical University, Haikou, China
- 6Biotechnology and Biochemistry Laboratory, Hainan Medical University, Haikou, China
Primary squamous cell carcinoma of the breast is a rare subtype of carcinoma of chemosis for which there is no effective chemotherapy regimen. Breast squamous cell carcinoma is usually “triple negative”, with poor chemotherapy effects and poor prognosis. Here, we report a successful case of primary breast squamous cell carcinoma treated with apatinib. The patient was treated with 2 cycles of apatinib. The efficacy was evaluated as partial remission, and a sublesion of approximately 4 cm fell off.
Introduction
Primary squamous cell carcinoma (PSCC) of the breast is a very rare subtype of saprophytic carcinoma; it accounts for approximately 0.06%–0.2% of all breast cancers (Chen et al., 2022; Wu et al., 2022). PSCC has large tumors, rapid progression, frequent recurrence, and high mortality (Anne et al., 2019; Shrestha et al., 2021). Surgery and radiotherapy can effectively control low-grade squamous cell carcinoma locally (Qi et al., 2021; Wang et al., 2021). However, the disease recurs at a distant site in approximately 46% of these patients (Wang et al., 2021). Therefore, PSCC requires novel treatment options.
Apatinib is a novel small-molecule tyrosine kinase inhibitor that selectively inhibits VEGFR-2, the primary signaling mediator of VEGF-induced angiogenesis (Wang et al., 2022). Apatinib can inhibit the migration and proliferation of endothelial cells promoted by vascular endothelial growth factor and reduce tumor microvessel density, thereby inhibiting tumor angiogenesis (Feng et al., 2021; Zhang et al., 2022a). Apatinib has been used in the third-line treatment of patients with metastatic gastric cancer; that is, the use of apatinib can effectively improve the progression-free survival and total survival of patients with metastatic gastric cancer who have failed to receive more than two chemotherapy regimens (Yang et al., 2022; Zheng et al., 2022). In addition, Chen et al. demonstrated that the addition of oral apatinib to conventional chemotherapy regimens prolonged progression-free survival in patients with advanced triple-negative breast cancer (Chen et al., 2021). The present report describes the clinical experience with apatinib in a patient with locally advanced PSCC who was insensitive to chemotherapy.
Case report
In October 2017, a 44-year-old premenopausal woman was admitted to the hospital 6 months after self-discovery of a left breast mass. The patient’s left breast mass was approximately 2 × 2 cm in size when the mass was discovered, and the mass gradually increased in size. The patient scratched the skin due to itching 1 month prior, resulting in localized skin breakdown and oozing on the surface of the lump with a dark green exudate. Due to financial constraints, the patient received local herbal medicine treatment (details are unknown) before admission, but it had no effect. The patient had no family history of a malignant tumor and had not undergone genetic testing.
On examination, a mass was found in the central region of the left breast in the direction of 3 o’clock, with a size of approximately 7 × 6 cm. It was hard, with poorly defined borders, poor mobility, and no tenderness (Figure 1A). The central region of the mass was crater-like, with a large internal cavity and a large amount of necrotic tissue. The swelling was accompanied by two satellite foci, approximately 2 and 4 cm in size. Several enlarged lymph nodes could be detected in the left armpit, the largest of which was located in the pectoralis muscle group. It was approximately 3 × 3 cm in size and hard, with indistinct borders, fusion, poor mobility, and no tenderness. The results of the color ultrasound showed multiple structurally abnormal lymph nodes in the bilateral neck, supraclavicular fossa, and axilla.
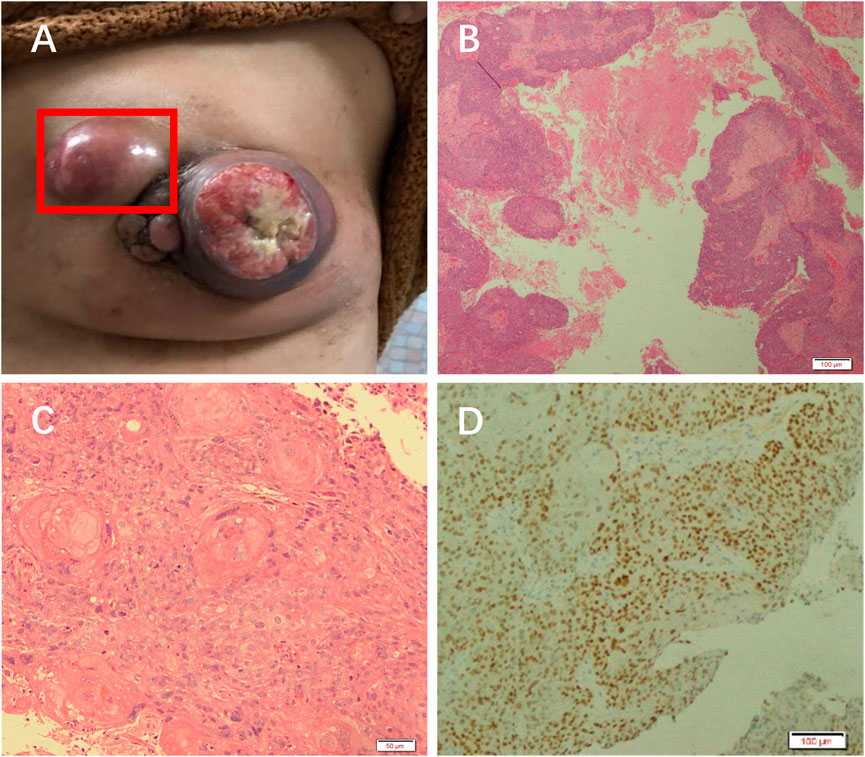
FIGURE 1. Patient was diagnosed with PSCC. (A) A mass in the left breast. H&E staining results at (B) 10× and (C) ×20 objective. (D) Immunohistochemical analysis of GATA.
The necrotic tissue of the tumor was taken for pathological analysis. H&E staining showed squamous cell carcinoma (Figures 1B, C). Immunohistochemical results showed GATA (+) (Figure 1D), ER (weak-moderate +, approximately 5%), PR (−), Her-2 (1+), Ki-67 (50%), P63 (+), CK5/6 (+), Mammaglobin (−), and GCDFP_15 (−). These results indicated that the patient had a chemosquamous carcinoma of the breast.
In addition, abnormal cells were found in the right supraclavicular lymph node by fine needle biopsy. Immunohistochemical results showed ER(+), PR(+), P63(few+), CK5/6(−), and CK7(+). These results indicated that the patient had metastatic cancer of breast origin. The clinical stage was cT4N2M1.
The patient received 2 cycles of neoadjuvant chemotherapy with TEC (docetaxel/epirubicin/cyclophosphamide) with an efficacy evaluation of stable disease. Therefore, the treatment regimen was changed to 2 cycles of chemotherapy with Apatinib + TEC (docetaxel/epirubicin/cyclophosphamide) with an efficacy evaluation of PR, and one of the patient’s approximately 4 cm sublesions fell off (Figure 2A).
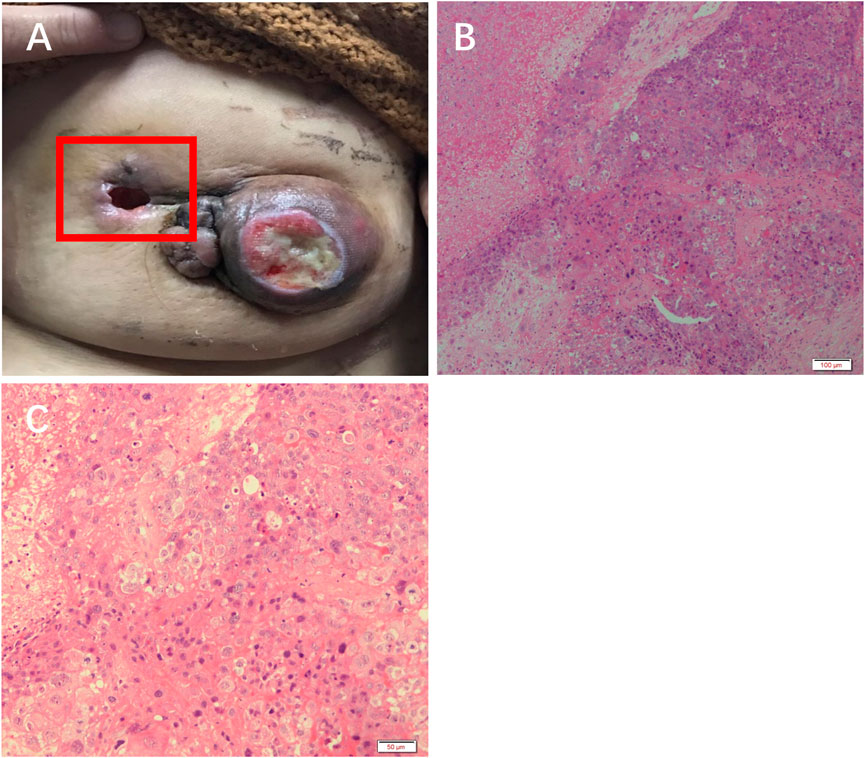
FIGURE 2. Efficacy of apatinib in this patient. (A) The left breast mass fell off. H&E staining results at (B) 10× and (C) ×20 objective.
H&E staining showed massive necrosis of tumor cells, and some residual tumor cells were deeply stained and degenerated after treatment with apatinib (Figure 2B, C).
However, the patient’s poor financial situation prevented her from returning to the hospital on time, and she died after 1 year of treatment.
Discussion
PSCC is highly heterogeneous and is characterized by the predominance of squamous cells, spindle cells, and (or) mesenchymal metaplasia in invasive cancer, while no adenocarcinoma component can be found (Goel et al., 2021). In addition, the diagnosis of PSCC should include the following features: 1) the absence of other tumor components in the tumor tissue and exclusion of adenocarcinoma to squamous carcinoma; 2) the presence of typical squamous cell carcinoma structure, i.e., intercellular bridges and/or keratinization; 3) exclusion of cancerous tissue originating from the skin; and 4) exclusion of the presence of primary squamous cell carcinoma in other organs or tissues of the body (Goel et al., 2021). In this case, the pathology of this patient showed heterogeneous proliferating cells distributed in nested clusters, with visible angular flower beads and intracellular keratinization. Immunohistochemical results showed GATA (+), which suggested squamous cell carcinoma. Systemic physical examination and instrument examination excluded the occurrence of squamous cell carcinomas, such as skin cancer and esophageal cancer. These pathological features indicate that the patient might have had PSCC.
PSCC has no standard treatment (Yoneto et al., 2018; Alan et al., 2019; Cao et al., 2019; Lei and Miao, 2020). Squamous cell carcinoma of the breast is resistant to standard chemotherapy with IDC (cyclophosphamide, methotrexate, 5-FU, and adriamycin) (Chen et al., 2022). Reports suggest that cisplatin-based chemotherapy regimens are effective for PSCC of the breast (Tsung, 2012; Liu et al., 2015). It has been reported that neoadjuvant chemotherapy, including docetaxel, epirubicin, and cyclophosphamide, can achieve complete pathological remission (Seddik et al., 2015; Alan et al., 2019). In this case, the patient was treated with TEC + apatinib, which alleviated the condition even when the mass fell off. Thus, neoadjuvant chemotherapy plus Apatinib is a novel choice for the treatment of PSCC.
Recent studies have demonstrated the antitumor activity of apatinib in several solid tumors (Zhang et al., 2022b; Xiang et al., 2022; Zhu et al., 2022). Tumor angiogenesis plays an important role in tumor development and metastasis, and antiangiogenic drugs may improve the efficacy of conventional chemotherapy in the treatment of breast cancer (Liu et al., 2021; Lv et al., 2021). Apatinib inactivates the NF-κB signaling pathway, which makes TNBC cells sensitive to anthracyclines in vitro and in vivo. This provides a sufficient theoretical basis for the combined use of apatinib and anthracyclines in the chemotherapeutic strategy for breast squamous cell carcinoma (Tang et al., 2020). In addition, apatinib can prevent the multidrug resistance conferred by ABCB1 and ABCG2 proteins (Zhang et al., 2019). Apatinib has been successfully used in a variety of tumors that have experienced failure of standard chemotherapy, showing curable results and significant survival benefits (Zeng et al., 2022). Our patient with PSCC showed no progression after 2 rounds of neoadjuvant therapy and showed good remission with the application of apatinib. Therefore, TEC + apatinib may be considered a conventional PSCC treatment.
Conclusion
This is one of the few reports of the use of apatinib for locally advanced squamous cell carcinoma of the breast with an efficacy assessment of partial remission (PR). Apatinib may be a safe and effective oral targeted agent for patients with locally advanced squamous cell carcinoma of the breast, especially those who have experienced chemotherapy failure or are in poor health.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by the Ethics Committee of the First Affiliated Hospital of Hainan Medical College (2022kyl179). Written informed consent was obtained from the individuals next of kin for the publication of any potentially identifiable images or data included in this article. The patients/participants provided their written informed consent to participate in this study.
Author contributions
Formal analysis: YL funding acquisition: PF investigation: MW methodology: LD project administration: GD resources: FG, JL, and HL writing—original draft: GD, writing—review and editing: GD and PF. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alan, O., Telli, T. A., Ercelep, O., Hasanov, R., Simsek, E. T., Mutis, A., et al. (2019). A case of primary squamous cell carcinoma of the breast with pathologic complete response after neoadjuvant chemotherapy. Curr. Probl. Cancer 43, 308–311. doi:10.1016/j.currproblcancer.2018.04.003
Anne, N., Sulger, E., and Pallapothu, R. (2019). Primary squamous cell carcinoma of the breast: A case report and review of the literature. J. Surg. Case Rep. 2019, rjz182. doi:10.1093/jscr/rjz182
Cao, Y., Yue, Y., Zhou, X., Luo, J., Zeng, X., Dong, J., et al. (2019). Change of HER2 status during disease recurrence predicts good prognosis for primary squamous cell carcinoma of the breast: A case report. Med. Baltim. 98, e14654. doi:10.1097/MD.0000000000014654
Chen, J., Feng, L., Sheng, Q., and Li, L. (2021). Efficacy, safety, and tumor marker inhibition of apatinib combined with conventional chemotherapy regimens for patients with advanced triple-negative breast cancer. Evid. Based Complement. Altern. Med. 2021, 8720679. doi:10.1155/2021/8720679
Chen, Z., An, N., Zhang, L., Cui, H., Jiang, Y., and Zhang, Y. (2022). Clinicopathological and therapeutic analysis of primary breast squamous cell carcinoma. Gland. Surg. 11, 125–135. doi:10.21037/gs-21-810
Feng, H., Jin, Z., Liang, J., Zhao, Q., Zhan, L., Yang, Z., et al. (2021). FOXK2 transcriptionally activating VEGFA induces apatinib resistance in anaplastic thyroid cancer through VEGFA/VEGFR1 pathway. Oncogene 40, 6115–6129. doi:10.1038/s41388-021-01830-5
Goel, D., Rana, C., Babu, S., and Ramakant, P. (2021). Primary squamous cell carcinoma, breast: A challenging diagnosis. Cancer Rep. Hob. 4, e1391. doi:10.1002/cnr2.1391
Lei, R., and Miao, L. (2020). Primary squamous cell carcinoma of the breast: Report of two cases with HER2 overexpression. Cancer Biol. Ther. 21, 1081–1086. doi:10.1080/15384047.2020.1838033
Liu, J., Li, Y., Li, Q., Liang, D., Wang, Q., and Liu, Q. (2021). Biomarkers of response to camrelizumab combined with apatinib: An analysis from a phase II trial in advanced triple-negative breast cancer patients. Breast Cancer Res. Treat. 186, 687–697. doi:10.1007/s10549-021-06128-4
Liu, J., Yu, Y., Sun, J. Y., He, S. S., Wang, X., Yin, J., et al. (2015). Clinicopathologic characteristics and prognosis of primary squamous cell carcinoma of the breast. Breast Cancer Res. Treat. 149, 133–140. doi:10.1007/s10549-014-3224-z
Lv, X., Chen, J., Yi, T., Lu, H., Liu, J., and Yu, D. (2021). The efficacy and safety of low-dose apatinib in the management of stage IV luminal-type breast cancer: A case report and literature review. Anticancer Drugs 32, 773–778. doi:10.1097/CAD.0000000000001102
Qi, W. X., Cao, L., Xu, C., and Chen, J. (2021). Lumpectomy combined with adjuvant radiotherapy could Be a treatment option for primary squamous cell carcinoma of the breast. J. Oncol. 2021, 2497227. doi:10.1155/2021/2497227
Seddik, Y., Brahmi, S. A., and Afqir, S. (2015). Primary squamous cell carcinoma of the breast: A case report and review of literature. Pan Afr. Med. J. 20, 152. doi:10.11604/pamj.2015.20.152.6188
Shrestha, S., Shakya, P., Kharel, S., Dhakal, H. P., Singh, M., and Shrestha, A. K. (2021). Primary squamous cell carcinoma of the breast in a young female- A rare ailment. Clin. Case Rep. 9, e04214. doi:10.1002/ccr3.4214
Tang, D., Ma, J., Chu, Z., Wang, X., Zhao, W., and Zhang, Q. (2020). Apatinib-induced NF-κB inactivation sensitizes triple-negative breast cancer cells to doxorubicin. Am. J. Transl. Res. 12, 3741–3753.
Tsung, S. H. (2012). Primary pure squamous cell carcinoma of the breast might be sensitive to Cisplatin-based chemotherapy. Case Rep. Oncol. 5, 561–565. doi:10.1159/000343745
Wang, H., Su, W., Lowe, S., Zhou, Z., Bentley, R., Zhou, Q., et al. (2022). Association of apatinib and breast cancer: A systematic review and meta-analysis. Surg. Oncol. 44, 101818. doi:10.1016/j.suronc.2022.101818
Wang, X., Zhang, L., Luo, J., Jin, K., Yang, Z., Meng, J., et al. (2021). Postoperative radiotherapy improves overall survival in patients with primary squamous cell carcinoma of the breast. Asia Pac J. Clin. Oncol. 17, 454–461. doi:10.1111/ajco.13466
Wu, Y., Chen, Z., Li, W., Wang, F., and Zhang, Y. (2022). Primary squamous cell carcinoma of the breast: A case report and review of the literature. Front. Oncol. 12, 1033084. doi:10.3389/fonc.2022.1033084
Xiang, J., Gong, W., Wang, C., Sun, P., and Liu, A. (2022). Complete remission of alpha-fetoprotein-producing gastric cancer by combined tislelizumab-apatinib treatment of a patient with proficient mismatch repair: A case report. World J. Surg. Oncol. 20, 289. doi:10.1186/s12957-022-02751-7
Yang, J. R., Zhou, D. Y., Wu, Y., Zhu, Y., Lin, Z. Y., Zhang, T., et al. (2022). The prognostic value of baseline hematological parameters of peripheral blood in metastatic gastric cancer treated with apatinib. J. Cancer 13, 15–20. doi:10.7150/jca.65339
Yoneto, T., Hasumi, K., Yoshimoto, T., Takahashi, N., and Takeda, Y. (2018). Case report: Two cases of extremely rare primary pure squamous cell carcinoma of the breast. Med. Baltim. 97, e12340. doi:10.1097/MD.0000000000012340
Zeng, T., Sun, C., Liang, Y., Yang, F., Yan, X., Bao, S., et al. (2022). A real-world multicentre retrospective study of low-dose apatinib for human epidermal growth factor receptor 2-negative metastatic breast cancer. Cancers (Basel) 14, 4084. doi:10.3390/cancers14174084
Zhang, Y., Chen, Y. X., Zhou, C. G., Liu, J., Liu, S., Shi, H. B., et al. (2022b). Efficacy and safety of the combination of transarterial chemoembolization with camrelizumab plus apatinib for advanced hepatocellular carcinoma: A retrospective study of 38 patients from a single center. Can. J. Gastroenterol. Hepatol. 2022, 7982118. doi:10.1155/2022/7982118
Zhang, Q., Song, Y., Cheng, X., Xu, Z., Matthew, O. A., Wang, J., et al. (2019). Apatinib reverses paclitaxel-resistant lung cancer cells (A549) through blocking the function of ABCB1 transporter. Anticancer Res. 39, 5461–5471. doi:10.21873/anticanres.13739
Zhang, Y., Zhou, L., Xu, Y., Zhou, J., Jiang, T., Wang, J., et al. (2022a). Targeting SMYD2 inhibits angiogenesis and increases the efficiency of apatinib by suppressing EGFL7 in colorectal cancer. Angiogenesis 26, 1–18. doi:10.1007/s10456-022-09839-4
Zheng, Z., Liu, Z., Zhang, H., Guo, X., Jia, X., Wang, J., et al. (2022). Efficacy and safety of apatinib in advanced hepatocellular carcinoma: A multicenter real world retrospective study. Front. Pharmacol. 13, 894016. doi:10.3389/fphar.2022.894016
Keywords: primary squamous cell carcinoma of the breast, apatinib, VEGFR, targeted therapy, neoadjuvant
Citation: Gao F, Li J, Liao H, Fan P, Wang M, Liu Y, Ding L and Du G (2023) Case report: Apatinib combined with neoadjuvant therapy for primary squamous cell carcinoma of the breast: a case report. Front. Pharmacol. 14:1115422. doi: 10.3389/fphar.2023.1115422
Received: 04 December 2022; Accepted: 02 May 2023;
Published: 11 May 2023.
Edited by:
Olivier Feron, Université Catholique de Louvain, BelgiumReviewed by:
Jiang Chen, Zhejiang University, ChinaEleonora Lai, University Hospital and University of Cagliari, Italy
Copyright © 2023 Gao, Li, Liao, Fan, Wang, Liu, Ding and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Pingming Fan, fpmhainan@163.com; Guankui Du, duguankui@163.com
†These authors have contributed equally to this work
 Fangfang Gao1†
Fangfang Gao1† Jingtai Li
Jingtai Li Guankui Du
Guankui Du