- 1Obstetrics and Gynecology Hospital, Fudan University, Shanghai, China
- 2The Shanghai Key Laboratory of Female Reproductive Endocrine-Related Diseases, Shanghai, China
Twin pregnancy was associated with significantly higher rates of adverse neonatal and perinatal outcomes. One of the underlying causes is that twins are prone to preterm birth. Antenatal corticosteroids are widely used for reducing the incidence of neonatal respiratory distress syndrome initially and other neonatal mortality and morbidities subsequently. As it is widely used as a prophylactic treatment for potential premature births, there remain controversies of issues relating to twin gestations, including window for opportunity, timing of use, repeat course, optimal administration-to-delivery intervals, dosage, and type of corticosteroid. Thus, we present a thorough review of antenatal corticosteroids usage in twin gestation, emphasizing the aforementioned issues and attempting to offer direction for future investigation and clinical practice.
1 Introduction
Antenatal corticosteroids (ACS) are used for accelerating fetal lung maturation for women at risk of premature births (Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes, 1995). The initial indication for ACS was reducing the incidence of neonatal respiratory distress syndrome (RDS) (Roberts and Dalziel, 2006), which is a serious complication of preterm birth (PTB) and the primary cause of early neonatal death and disability. And ACS was subsequently extended to generally reduce neonatal mortality and morbidities like serious neurological and abdominal problems including the risk of intraventricular hemorrhage (IVH) and necrotizing enterocolitis (NEC) (Roberts et al., 2017). A course of ACS includes two doses of betamethasone acetate/phosphate 12 mg IM 24 h apart, or two doses of dexamethasone phosphate 12 mg IM 24 h apart and is recommended for women with a high likelihood of preterm birth from 24 to 34 weeks of gestation irrespective of whether single or multiple birth is anticipated by authorities (WHO, 2022; Norman et al., 2021; Stock et al., 2022; American College of and Gynecologists' Committee on Practice Bulletins-Obstetrics SfM-, 2021).
The rates of PTB (28–36 weeks of gestation, 37.1%) and early PTB (28–33 weeks of gestation, 13.0%) among twin pregnancies were substantially higher than those among singletons (7.3% for PTB and 2.4% for early PTB) according to WHO Multicountry Survey on Maternal and Newborn Health (Santana et al., 2018). As twins tend to have an earlier gestational age of delivery, mortality and morbidity following premature birth can carry enormous consequences for societies and economies. Thus, ACS, as one of the tertiary prevention interventions, should be properly provided to overcome the immediate and future health challenges of premature twin newborns.
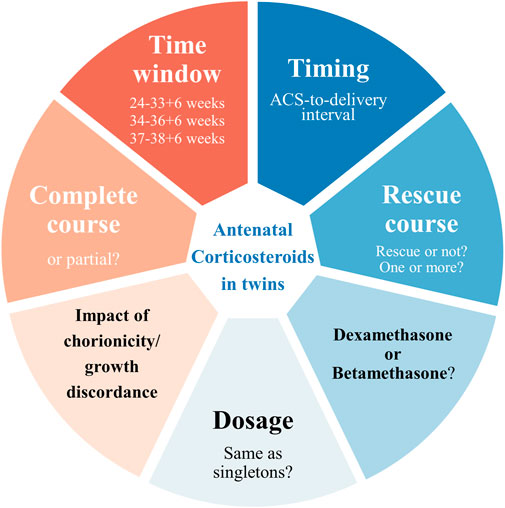
While the efficacy of ACS treatment in singletons is supported by abundant evidence, the current proof of which in twin gestations is relatively limited and less consistent. There remain controversies in various aspects regarding twin gestations, including but not limited to window for opportunity, timing of use, repeat course, optimal ACS-to-delivery intervals, dosage and type of corticosteroid (Figure 1). We here present a comprehensive review of ACS in twin gestation based on the evidence when possible, emphasizing the controversies above and their influence on the efficacy and safety of ACS, and aimed to provide direction for subsequent investigations and recommendations for clinicians.
2 Effects of ACS
2.1 Therapeutic effects of ACS
Corticosteroids are easily transported across the placenta. They act to promote the production and secretion of alveolar surfactant, decrease vascular permeability, and increase compliance and maximal lung volume of fetal lungs according to human and animal studies (Ballard and Ballard, 1995), which can prevent respiratory-related morbidity and mortality affecting preterm newborns. Meanwhile, the increased corticosteroids support other physiologic changes that facilitate neonatal adaption, especially the very preterm infants, including energy substrate metabolism control, increased blood pressure, and enhanced cardiovascular adaptation (Hillman et al., 2012).
In twins, evidence from a meta-analysis of non-randomized studies published in 2022 suggests that ACS are associated with a lower incidence of neonatal mortality and RDS, which was in agreement with the 2020 Cochrane meta-analyses of randomized controlled trials (RCTs) in multiples, but was not convincingly associated with other outcomes including IVH, NEC, bronchopulmonary dysplasia (BPD), periventricular leukomalacia (PVL), and retinopathy of prematurity (ROP) (McGoldrick et al., 2020; Socha et al., 2022) probably due to the paucity of studies focusing on these secondary outcomes in twin pregnancy (Table 1).
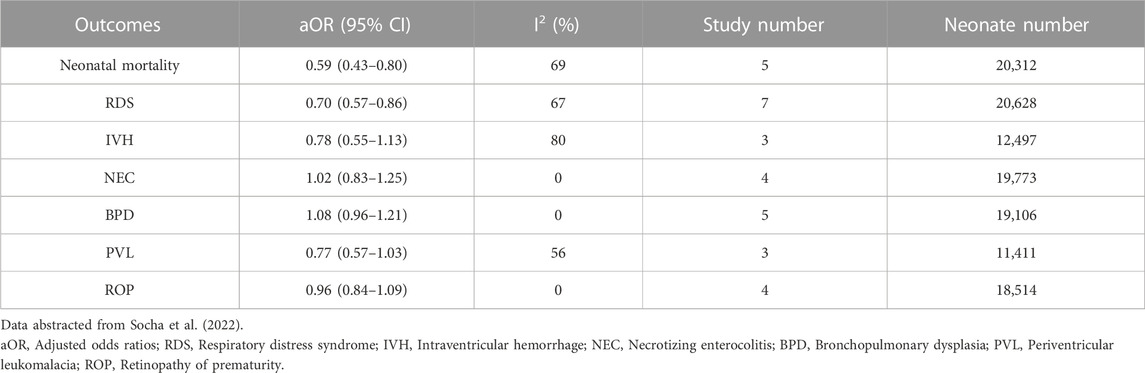
TABLE 1. Overall odds of outcomes among twin neonates who were exposed to antenatal corticosteroids (ACS) vs. those unexposed to ACS.
2.2 Potential adverse effects of ACS
Epidemiological evidence and animal work indicate a possible link between ACS exposure and potential harm.
2.2.1 Short-term risks
Glucocorticoids are recognized to have an adverse effect on growth in animal studies (Braun et al., 2007). In humans, in line with the previous studies (Murphy et al., 2008; Braun et al., 2015), a recent observative study noted that ACS was consistently associated with a reduction in birth size for infants born preterm, near term, or at term (Rodriguez et al., 2019). And it has been suggested that there is a significant reduction in fetal growth velocity including head circumference, abdominal circumference, and estimated fetal weight in pregnancies exposed to ACS (Rizzo et al., 2022). As for twin pregnancies, ACS was found to reduce birth size of preterm neonates in a dose-dependent manner (Braun et al., 2016). Therefore, it is worth attention as impaired growth during pregnancy and infancy is in turn closely associated with neurodevelopmental disorders in the future.
In several studies including a large multi-center randomized controlled trial, hypoglycemia was noted in late preterm infants whose mothers were treated with ACS (Kamath-Rayne et al., 2012; Gyamfi-Bannerman et al., 2016; Ramadan et al., 2016). The finding was the same in twins as two retrospective cohort studies showed (Ben-David et al., 2020; Vieira et al., 2022). Despite appearing self-limiting, hypoglycemia has been connected to poor neurological outcomes in premature newborns (McKinlay et al., 2017), to which thus attention should be paid.
2.2.2 Long-term risks
Negative programming signals may cause long-lasting maladaptive alterations, raising the risk of physical, mental, and developmental impairments in offspring, which has been convinced by numerous animal studies (Quinlivan et al., 2000; Church et al., 2012; Carson et al., 2016). Human evidence is still limited, but it is gradually accumulating and leading to some warning signals. Recent follow-up studies and retrospective cohort studies have shown that exposure to ACS during pregnancy was significantly associated with mental and behavioral disorders from childhood to adolescence (Alexander et al., 2012; Asztalos et al., 2013; Stutchfield et al., 2013; Ilg et al., 2019; Melamed et al., 2019; Raikkonen et al., 2020; Wolford et al., 2020). Although several studies above included twins, no one provided disaggregated estimates in twins, which allows for a great deal of exploration potential.
In animal studies, ACS exposure may result in changes in several aspects like vascular function (Pulgar and Figueroa, 2006), elastin synthesis (Swee et al., 1995), and renin-angiotensin system (Yu et al., 2018), which finally cause long-term cardiovascular disorders. In humans, higher systolic and diastolic blood pressures (Doyle et al., 2000), decreased heart rate variability (Nixon et al., 2017) and changed renin angiotensin aldosterone system (South et al., 2017) were found in 14-year-old children who were exposed to ACS, despite few children having overt hypertension. Decreased aortic distensibility, which is a risk factor for cardiovascular diseases, was also found among ACS-exposed young adults (Kelly et al., 2012).
3 The choice of corticosteroid
Though ACS is frequently used to prevent adverse outcomes in preterm newborns, there is still no agreement on the type of corticosteroid to use. Opinion leaders, local accessibility, and cost are some of the variables influencing the decision. Dexamethasone and betamethasone have been used most widely worldwide. The 2022 Cochrane meta-analyses which included 11 trials found no evidence of a difference between dexamethasone and betamethasone for infant outcomes including RDS, IVH, chronic lung disease, and maternal outcomes like chorioamnionitis (Williams et al., 2022). And as for the long-term child outcomes, there was only a multi-center randomized controlled trial which came up with the conclusion that the incidence of survival without neurosensory disability at age 2 years did not differ between dexamethasone and betamethasone treatment (Crowther et al., 2019a). There were no trials that reported the effects of these medicines beyond 2 years of age.
Despite the fact that there was no difference between these drugs for the majority of maternal, neonatal, and early childhood outcomes, the evidence was ambiguous and left open the possibility of significant benefits or harms for a number of vital outcomes for the mother, infant, child, and adult. Overall, it remains unclear whether there are important differences between dexamethasone and betamethasone.
4 ACS administration patterns in twin pregnancies
Since clinical efficacy and safety of ACS above are closely related to its pattern of use including time window, course of treatment, optimal ACS-to-delivery interval, and dosage, these aspects need to describe in detail.
4.1 Time window
4.1.1 From 24 + 0 to 33 + 6 weeks of gestation
A single course of corticosteroids is recommended for pregnant women between 24 0/7 and 33 6/7 weeks of gestation who are at risk of preterm delivery within 7 days, regardless of fetal number (Committee on Obstetric P. Committee Opinion No, 2017). Although the evidence supporting this recommendation came from RCTs, select observational studies, and extrapolation from studies on singletons, subsequent research on twin pregnancies has also shown that ACS in twins can have the same or comparable efficacy for RDS and neonatal mortality as in singletons (Hacking et al., 2001; Blickstein et al., 2005; Kuk et al., 2013; Melamed et al., 2016; Riskin-Mashiah et al., 2018; Vaz et al., 2018; Kong et al., 2020; Mwita et al., 2022).
4.1.2 Late preterm birth (from 34 + 0 to 36 + 6 weeks of gestation)
After the results of the Antenatal Late Preterm Steroid (ALPS) study published, which advocated the administration of betamethasone to women at risk for late preterm delivery significantly reduced the rate of neonatal respiratory complications in singletons (Gyamfi-Bannerman et al., 2016), change happened in clinical practice, with the American College of Obstetricians and Gynecologists (ACOG) releasing a committee opinion in 2017 recommending treating women at risk of imminent birth in the late preterm period, who had not received prior ACS, with a course of ACS(44). Since then, there has been a continual stream of research on the efficacy and the short-term and long-term adverse effects of ACS in late preterm birth, emphasizing hypoglycemia and adverse neurodevelopmental outcomes (Saccone and Berghella, 2016; Deshmukh and Patole, 2021; Sarid et al., 2022). However, overwhelming emphasis has been placed on singletons. Whether these findings also apply to late-preterm twins is currently unclear. For twins, few studies are focusing on those who received ACS after 34 + 0 weeks of gestation and delivered during the late preterm period. A retrospective cohort study including a total of 290 women with twin pregnancies showed that late preterm ACS administration in twins complicated by late preterm birth did not reduce neonatal respiratory morbidity but was associated with higher rates of neonatal intensive care unit (NICU) admission and hypoglycemia (Ben-David et al., 2020). Another study including 1,032 mother-child pairs in twins showed that exposure to ACS is associated with increased odds of hypoglycemia, but no significant association with respiratory complications (Vieira et al., 2022). So far, the results of ACS for late preterm birth of twins have not been very promising. And there is a study called ACTWIN (Antenatal Corticosteroids in TWIN late preterm neonates) trial which might be the first randomized controlled trial that evaluates the effectiveness of ACS in late-preterm twin neonates (Hong et al., 2019).
4.1.3 Early term birth (from 37 + 0 to 38 + 6 weeks of gestation)
Infants born in the early term period are more likely to suffer respiratory morbidity, particularly if born via caesarean section prior to the onset of labor (Zanardo et al., 2004). Hence, it is meaningful to determine whether ACS could benefit this population with respiratory complications. To date, limited evidence in singletons available suggests that ACS before elective caesarean section at early term does not reduce rates of RDS (Stutchfield et al., 2005; Ahmed et al., 2015), but was associated with a reduction in school performance (Stutchfield et al., 2013). However, no completed or ongoing published studies focusing on ACS for elective caesarean section at early term among twins have been found as yet.
In summary, ACS is recommended for twin pregnancies with a high likelihood of preterm birth from 24 to 34 weeks of gestation based on the current evidence. For those at risk of late preterm birth, ACS administration remains debatable. And there is no reason for advocating ACS at this time when at risk of early term delivery.
4.2 ACS-to-delivery interval
Another contributing factor to unpredictability is the ACS-to-delivery interval. So far, it is advised by guidelines and consensus that the corticosteroids should be given within 7 days before preterm birth (Committee on Obstetric P. Committee Opinion No, 2017; Medicine FWGoGCPiM-F, 2019; Stock et al., 2022). However, the recommendation mentioned above is based on studies in singletons. Recently, a couple of studies based on twins have emerged for the optimal timing of ACS. In twin pregnancies, ACS treatment was associated with a decreased rate of RDS and in-hospital mortality when the ACS-to-delivery interval was ≤7 days (Melamed et al., 2016; Palas et al., 2018; Vaz et al., 2018), even only in the interval between 2 and 7 days (Kuk et al., 2013). In another research, infants in the ACS-to-delivery interval ≥7 days group even suffered from respiratory disorders significantly more often and were hospitalized longer (Kosinska-Kaczynska et al., 2016).
To sum up, it seems that ACS might be most beneficial when administered within 7 days before birth in twins, which is similar to singletons. However, all the studies of twins currently were observational, contained small sample sizes, and thus had weak evidence. It is critical to determine the optimal ACS-to-delivery interval for the reason that the ACS response interval is important for considerations of the timing of deliveries and repeat ACS treatments. As twin pregnancy is an independent risk factor for suboptimal ACS administration (Rottenstreich et al., 2018), targeted measures should be implemented to promote the utilization of ACS in this susceptible population of women.
4.3 Complete course
In 2003, an incomplete course of ACS was proven to be beneficial to perinatal morbidity and mortality (Elimian et al., 2003). Regarding twin births, the results were somewhat different. Blickstein et al. (2005) found that a complete course of ACS significantly reduced the incidence of RDS in twins, whereas partial treatment had the same effect as no treatment. Another study that also considered the gestational age at delivery showed that a complete course of ACS was associated with a reduction in death and RDS in multiple infants born at 28–34 weeks’ gestation, but the incomplete course was not (Herrera et al., 2019). Nevertheless, the administration of even a single dose of ACS to a mother in danger of imminent delivery before 34 weeks of gestation should be strongly considered by clinicians as large sample studies comparing the efficacy of complete and incomplete ACS in twins are still lacking. Where it is deemed safe, tocolytic therapy should be taken into consideration as an intervention to gain time to complete a single course of ACS (WHO, 2022).
4.4 Rescue course
Due to worries about maternal and fetal impairment and the imbalance of benefits and risks, planned multiple courses are not advised. However, given the difficulty to predict preterm birth in twins, a rescue course of ACS is worth considering.
An individual participant data meta-analysis of RCTs including 5,915 infants demonstrated that repeat prenatal corticosteroids given to women at ongoing risk of preterm birth after an initial course reduce the need for respiratory support, although birth weight z-scores were reduced (Crowther et al., 2019b). And there were no significant differences in treatment effects among the subgroups by the number of fetuses in utero. As for twins, a rescue ACS course was associated with a lower rate of RDS, surfactant use, and BPD, while no differences were noted in neonatal birthweight, head circumference, and the rate of neonatal hypoglycemia (Zigron et al., 2022), which was supported by another retrospective study (Bibbo et al., 2013). But the results of these studies above may be limited by retrospective design and small sample size. Although further studies are warranted to determine the effect of rescue corticosteroids in twin pregnancies, in women who are less than 34 weeks of gestation, at risk of preterm delivery within 7 days, and whose previous course of ACS was administered more than 14 days ago, a single repeat course of ACS should be taken into consideration.
4.5 Impact of chorionicity
The effect of chorionicity has not been widely appreciated in population studies and no association was found between chorionicity and the efficacy and ACS in population-based studies. Yet, data from a pharmacokinetic study suggest that the presence of two foetoplacental units in dichorionic (DC) twin pregnancies may increase the betamethasone metabolism by hepatic CYP3A4 and/or placental 11β-HSD2 enzymes (Rodrigues et al., 2022). Therefore, further pharmacological studies are needed to investigate whether these betamethasone pharmacokinetic changes require dose adjustment in DC twin pregnancies. And clinical studies with higher levels of evidence are urgently needed.
4.6 Impact of growth discordance
Growth discordance, another characteristic of twin pregnancy, makes it more challenging to determine the exact gestational age. The question of whether the crown-rump length of the larger or smaller fetus should be used as a standard for establishing gestational age is still up for debate (Salomon et al., 2005; Khalil et al., 2016; DeYoung et al., 2021). To guarantee that all eligible women receive ACS while preventing unnecessary treatment of ineligible women, accurate and standardized gestational age assessment is vital. However, studies examining the impact of ACS in groups with growth discordance are scarce. This would be an indication of ACS in twins if it improves the outcomes for smaller fetuses without raising the danger for larger fetuses. To determine this, however, large sample studies are required.
4.7 Dosage
The optimal corticosteroid dose to use remains unclear. The International Federation of Gynecology and Obstetrics recently recommended the common regimens of two doses of betamethasone 12 mg given intramuscularly 24 h apart and two doses of dexamethasone 12 mg given intramuscularly 24 h apart, although four doses of dexamethasone 6 mg given intramuscularly 12 h apart has also been recommended. There aren’t any differences in the guidelines for administering ACS between multiple pregnancies and singletons, even for the dosage of ACS. A clinical trial from the view of pharmacokinetics published in 2002 pointed out that the shorter half-life of betamethasone in twin pregnancy than in singleton pregnancy may cause the level of betamethasone to be subtherapeutic for lung maturation in twin pregnancy (Ballabh et al., 2002). But in later studies, maternal and umbilical cord blood serum betamethasone concentrations and pharmacokinetics were the same as singletons in twin pregnancies (Della Torre et al., 2010). Thus, if there is no conclusive difference between singletons in twin pregnancies, then the ACS administration of twins may need to continue to follow the guidelines for singletons.
5 Summary
The clinical practice of ACS in twins is almost an extrapolation of that in singletons, but a translation like that could not always be suitable. In the overall population, twin pregnancies are relatively uncommon, thus they are frequently undervalued in clinical studies. Meanwhile, plenty of clinical research only considers singleton pregnancies and excludes twin pregnancies due to the biological differences between them. Nonetheless, future research has the potential to full the gaps in the administration of ACS in twins: whether or not to use it, the actual impact of possible contributing factors, and the long-term outcomes. Firstly, as new trials for fetuses <34 weeks’ gestation were viewed as unethical, it is still reasonable for trials to assess the effect of ACS in late-preterm (34 + 0 to 36 + 6 weeks of gestation) and early-term twins (37 + 0 to 38 + 6 weeks of gestation). Secondly, as timing, the number of courses, dosage, and chorionicity likely affect the association between ACS and outcomes of interest, research containing sufficiently granular data is needed. Thirdly, the follow-up studies spanning childhood, adolescence, and adulthood are required to determine the long-term effects of ACS. We believe that this review is essential for a thorough grasp of the current situation and to determine a direction for future clinical practice and research in ACS of twin pregnancies.
Author contributions
Design: YX Planning and conduct: JZ, SL, and YZ Initial draft of manuscript: JZ Manuscript writing, review and approval: All authors.
Funding
This study was supported by the National Science Fund of Shanghai, China (No. 22ZR1409000) and Medical Innovation Research Program of Shanghai, China (No. 21Y11908000).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, M. R., Sayed Ahmed, W. A., and Mohammed, T. Y. (2015). Antenatal steroids at 37 Weeks, does it reduce neonatal respiratory morbidity? A randomized trial. J. Matern. Fetal Neonatal Med. 28 (12), 1486–1490. Epub 2014/08/29. doi:10.3109/14767058.2014.958461
Alexander, N., Rosenlocher, F., Stalder, T., Linke, J., Distler, W., Morgner, J., et al. (2012). Impact of antenatal synthetic glucocorticoid exposure on endocrine stress reactivity in term-born children. J. Clin. Endocrinol. Metab. 97 (10), 3538–3544. Epub 2012/08/08. doi:10.1210/jc.2012-1970
American College of, O., and Gynecologists' Committee on Practice Bulletins-Obstetrics SfM-, F. M. (2021). Multifetal gestations: Twin, triplet, and higher-order multifetal pregnancies: Acog practice bulletin, number 231. Obstet. Gynecol. 137 (6), e145–e162. Epub 2021/05/21. doi:10.1097/AOG.0000000000004397
Asztalos, E. V., Murphy, K. E., Willan, A. R., Matthews, S. G., Ohlsson, A., Saigal, S., et al. (2013). Multiple courses of antenatal corticosteroids for preterm birth study: Outcomes in children at 5 Years of age (Macs-5). JAMA Pediatr. 167 (12), 1102–1110. Epub 2013/10/16. doi:10.1001/jamapediatrics.2013.2764
Ballabh, P., Lo, E. S., Kumari, J., Cooper, T. B., Zervoudakis, I., Auld, P. A., et al. (2002). Pharmacokinetics of betamethasone in twin and singleton pregnancy. Clin. Pharmacol. Ther. 71 (1), 39–45. Epub 2002/02/02. doi:10.1067/mcp.2002.120250
Ballard, P. L., and Ballard, R. A. (1995). Scientific basis and therapeutic regimens for use of antenatal glucocorticoids. Am. J. Obstet. Gynecol. 173 (1), 254–262. Epub 1995/07/01. doi:10.1016/0002-9378(95)90210-4
Ben-David, A., Zlatkin, R., Bookstein-Peretz, S., Meyer, R., Mazaki-Tovi, S., and Yinon, Y. (2020). Does antenatal steroids treatment in twin pregnancies prior to late preterm birth reduce neonatal morbidity? Evidence from a retrospective cohort study. Arch. Gynecol. Obstet. 302 (5), 1121–1126. Epub 2020/07/31. doi:10.1007/s00404-020-05709-w
Bibbo, C., Deluca, L., Gibbs, K. A., Saltzman, D. H., Rebarber, A., Green, R. S., et al. (2013). Rescue corticosteroids in twin pregnancies and short-term neonatal outcomes. BJOG 120 (1), 58–63. Epub 2012/11/06. doi:10.1111/1471-0528.12021
Blickstein, I., Shinwell, E. S., Lusky, A., Reichman, B., and Israel Neonatal, N. (2005). Plurality-dependent risk of respiratory distress syndrome among very-low-birth-weight infants and antepartum corticosteroid treatment. Am. J. Obstet. Gynecol. 192 (2), 360–364. Epub 2005/02/08. doi:10.1016/j.ajog.2004.10.604
Braun, T., Li, S., Moss, T. J., Newnham, J. P., Challis, J. R., Gluckman, P. D., et al. (2007). Maternal betamethasone administration reduces binucleate cell number and placental lactogen in sheep. J. Endocrinol. 194 (2), 337–347. Epub 2007/07/21. doi:10.1677/JOE-07-0123
Braun, T., Sloboda, D. M., Tutschek, B., Harder, T., Challis, J. R., Dudenhausen, J. W., et al. (2015). Fetal and neonatal outcomes after term and preterm delivery following betamethasone administration. Int. J. Gynaecol. Obstet. 130 (1), 64–69. Epub 2015/04/16. doi:10.1016/j.ijgo.2015.01.013
Braun, T., Weichert, A., Gil, H. C., Sloboda, D. M., Tutschek, B., Harder, T., et al. (2016). Fetal and neonatal outcomes after term and preterm delivery following betamethasone administration in twin pregnancies. Int. J. Gynaecol. Obstet. 134 (3), 329–335. Epub 2016/07/02. doi:10.1016/j.ijgo.2016.02.016
Carson, R., Monaghan-Nichols, A. P., DeFranco, D. B., and Rudine, A. C. (2016). Effects of antenatal glucocorticoids on the developing brain. Steroids 114, 25–32. Epub 2016/06/28. doi:10.1016/j.steroids.2016.05.012
Church, M. W., Adams, B. R., Anumba, J. I., Jackson, D. A., Kruger, M. L., and Jen, K. L. (2012). Repeated antenatal corticosteroid treatments adversely affect neural transmission time and auditory thresholds in laboratory rats. Neurotoxicol Teratol. 34 (1), 196–205. Epub 2011/10/04. doi:10.1016/j.ntt.2011.09.004
Committee on Obstetric P. Committee Opinion No (2017). Committee opinion No. 713: Antenatal corticosteroid therapy for fetal maturation. Obstet. Gynecol. 130 (2), e102–e109. Epub 2017/07/26. doi:10.1097/AOG.0000000000002237
Crowther, C. A., Ashwood, P., Andersen, C. C., Middleton, P. F., Tran, T., Doyle, L. W., et al. (2019). Maternal intramuscular dexamethasone versus betamethasone before preterm birth (asteroid): A multicentre, double-blind, randomised controlled trial. Lancet Child. Adolesc. Health 3 (11), 769–780. Epub 2019/09/17. doi:10.1016/S2352-4642(19)30292-5
Crowther, C. A., Middleton, P. F., Voysey, M., Askie, L., Zhang, S., Martlow, T. K., et al. (2019). Effects of repeat prenatal corticosteroids given to women at risk of preterm birth: An individual participant data meta-analysis. PLoS Med. 16 (4), e1002771. Epub 2019/04/13. doi:10.1371/journal.pmed.1002771
Della Torre, M., Hibbard, J. U., Jeong, H., and Fischer, J. H. (2010). Betamethasone in pregnancy: Influence of maternal body weight and multiple gestation on pharmacokinetics. Am. J. Obstet. Gynecol. 203 (3), 254 e1–e12. Epub 2010/09/08. doi:10.1016/j.ajog.2010.06.029
Deshmukh, M., and Patole, S. (2021). Antenatal corticosteroids for impending late preterm (34-36+6 Weeks) deliveries-a systematic review and meta-analysis of rcts. PLoS One 16 (3), e0248774. Epub 2021/03/23. doi:10.1371/journal.pone.0248774
DeYoung, T. H., Stortz, S. K., Riffenburgh, R. H., Wren, G. M., Spitzer, T. L., Lutgendorf, M. A., et al. (2021). Establishing the most accurate due date in dichorionic twin gestations by first and second trimester ultrasound. J. Ultrasound Med. 40 (11), 2319–2327. Epub 2021/01/12. doi:10.1002/jum.15615
Doyle, L. W., Ford, G. W., Davis, N. M., and Callanan, C. (2000). Antenatal corticosteroid therapy and blood pressure at 14 Years of age in preterm children. Clin. Sci. (Lond) 98 (2), 137–142. Epub 2000/02/05. doi:10.1042/cs19990211
Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes (1995). Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH consensus development panel on the effect of corticosteroids for fetal maturation on perinatal outcomes. JAMA 273 (5), 413–418. Epub 1995/02/01. doi:10.1001/jama.1995.03520290065031
Elimian, A., Figueroa, R., Spitzer, A. R., Ogburn, P. L., Wiencek, V., and Quirk, J. G. (2003). Antenatal corticosteroids: Are incomplete courses beneficial? Obstet. Gynecol. 102 (2), 352–355. Epub 2003/08/09. doi:10.1016/s0029-7844(03)00485-x
Gyamfi-Bannerman, C., Thom, E. A., Blackwell, S. C., Tita, A. T., Reddy, U. M., Saade, G. R., et al. (2016). Antenatal betamethasone for women at risk for late preterm delivery. N. Engl. J. Med. 374 (14), 1311–1320. Epub 2016/02/05. doi:10.1056/NEJMoa1516783
Hacking, D., Watkins, A., Fraser, S., Wolfe, R., and Nolan, T. (2001). Respiratory distress syndrome and antenatal corticosteroid treatment in premature twins. Arch. Dis. Child. Fetal Neonatal Ed. 85 (1), F77–F78. Epub 2001/07/18. doi:10.1136/fn.85.1.f75g
Herrera, T. I., Vaz Ferreira, M. C., Toso, A., Villarroel, L., Silvera, F., Ceriani-Cernadas, J. M., et al. (2019). Neonatal outcomes of antenatal corticosteroids in preterm multiple pregnancies compared to singletons. Early Hum. Dev. 130, 44–50. Epub 2019/01/22. doi:10.1016/j.earlhumdev.2019.01.008
Hillman, N. H., Kallapur, S. G., and Jobe, A. H. (2012). Physiology of transition from intrauterine to extrauterine life. Clin. Perinatol. 39 (4), 769–783. Epub 2012/11/21. doi:10.1016/j.clp.2012.09.009
Hong, S., Lee, S. M., Kwak, D. W., Lee, J., Kim, S. Y., Oh, J. W., et al. (2019). Effects of antenatal corticosteroids in twin neonates with late preterm birth (actwin [antenatal corticosteroids in twin late preterm neonates] trial): Study protocol for a randomized controlled trial. BMC Pregnancy Childbirth 19 (1), 114. Epub 2019/04/05. doi:10.1186/s12884-019-2235-5
Ilg, L., Kirschbaum, C., Li, S. C., Rosenlocher, F., Miller, R., and Alexander, N. (2019). Persistent effects of antenatal synthetic glucocorticoids on endocrine stress reactivity from childhood to adolescence. J. Clin. Endocrinol. Metab. 104 (3), 827–834. Epub 2018/10/05. doi:10.1210/jc.2018-01566
Kamath-Rayne, B. D., DeFranco, E. A., and Marcotte, M. P. (2012). Antenatal steroids for treatment of fetal lung immaturity after 34 Weeks of gestation: An evaluation of neonatal outcomes. Obstet. Gynecol. 119 (5), 909–916. Epub 2012/04/25. doi:10.1097/AOG.0b013e31824ea4b2
Kelly, B. A., Lewandowski, A. J., Worton, S. A., Davis, E. F., Lazdam, M., Francis, J., et al. (2012). Antenatal glucocorticoid exposure and long-term alterations in aortic function and glucose metabolism. Pediatrics 129 (5), e1282–e1290. Epub 2012/04/18. doi:10.1542/peds.2011-3175
Khalil, A., Rodgers, M., Baschat, A., Bhide, A., Gratacos, E., Hecher, K., et al. (2016). Isuog practice guidelines: Role of ultrasound in twin pregnancy. Ultrasound Obstet. Gynecol. 47 (2), 247–263. Epub 2015/11/19. doi:10.1002/uog.15821
Kong, X., Xu, F., Wang, Z., Zhang, S., and Feng, Z. (2020). Antenatal corticosteroids administration on mortality and morbidity in premature twins born at 25∼34 gestational weeks: A retrospective multicenter study. Eur. J. Obstet. Gynecol. Reprod. Biol. 253, 259–265. Epub 2020/09/09. doi:10.1016/j.ejogrb.2020.08.003
Kosinska-Kaczynska, K., Szymusik, I., Urban, P., Zachara, M., and Wielgos, M. (2016). Relation between time interval from antenatal corticosteroids administration to delivery and neonatal outcome in twins. J. Obstet. Gynaecol. Res. 42 (6), 625–631. Epub 2016/03/24. doi:10.1111/jog.12966
Kuk, J. Y., An, J. J., Cha, H. H., Choi, S. J., Vargas, J. E., Oh, S. Y., et al. (2013). Optimal time interval between a single course of antenatal corticosteroids and delivery for reduction of respiratory distress syndrome in preterm twins. Am. J. Obstet. Gynecol. 209 (3), 256 e1–e7. Epub 2013/07/03. doi:10.1016/j.ajog.2013.06.020
McGoldrick, E., Stewart, F., Parker, R., and Dalziel, S. R. (2020). Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 12 (12), CD004454. Epub 2020/12/29. doi:10.1002/14651858.CD004454.pub4
McKinlay, C. J. D., Alsweiler, J. M., Anstice, N. S., Burakevych, N., Chakraborty, A., Chase, J. G., et al. (2017). Association of neonatal glycemia with neurodevelopmental outcomes at 4.5 years. JAMA Pediatr. 171 (10), 972–983. Epub 2017/08/08. doi:10.1001/jamapediatrics.2017.1579
Medicine FwgoGCPiM-F, (2019). Good clinical practice advice: Antenatal corticosteroids for fetal lung maturation. Int. J. Gynaecol. Obstet. 144 (3), 352–355. Epub 2019/02/03. doi:10.1002/ijgo.12746
Melamed, N., Asztalos, E., Murphy, K., Zaltz, A., Redelmeier, D., Shah, B. R., et al. (2019). Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: A population-based study. BMJ Open 9 (9), e031197. Epub 2019/10/03. doi:10.1136/bmjopen-2019-031197
Melamed, N., Shah, J., Yoon, E. W., Pelausa, E., Lee, S. K., Shah, P. S., et al. (2016). The role of antenatal corticosteroids in twin pregnancies complicated by preterm birth. Am. J. Obstet. Gynecol. 215 (4), 482 e1–e9. Epub 2016/06/05. doi:10.1016/j.ajog.2016.05.037
Murphy, K. E., Hannah, M. E., Willan, A. R., Hewson, S. A., Ohlsson, A., Kelly, E. N., et al. (2008). Multiple courses of antenatal corticosteroids for preterm birth (macs): A randomised controlled trial. Lancet 372 (9656), 2143–2151. Epub 2008/12/23. doi:10.1016/S0140-6736(08)61929-7
Mwita, S., Kamala, B. A., Konje, E., Ambrose, E. E., Izina, A., Chibwe, E., et al. (2022). Association between antenatal corticosteroids use and perinatal mortality among preterm singletons and twins in mwanza, Tanzania: An observational study. BMJ Open 12 (4), e059030. Epub 2022/04/09. doi:10.1136/bmjopen-2021-059030
Nixon, P. A., Washburn, L. K., Michael O'Shea, T., Shaltout, H. A., Russell, G. B., Snively, B. M., et al. (2017). Antenatal steroid exposure and heart rate variability in adolescents born with very low birth weight. Pediatr. Res. 81 (1-1), 57–62. Epub 2016/09/16. doi:10.1038/pr.2016.173
Norman, J., Shennan, A., Jacobsson, B., Stock, S. J., and FwgfP, B. (2021). Figo good practice recommendations on the use of prenatal corticosteroids to improve outcomes and minimize harm in babies born preterm. Int. J. Gynaecol. Obstet. 155 (1), 26–30. Epub 2021/09/15. doi:10.1002/ijgo.13836
Palas, D., Ehlinger, V., Alberge, C., Truffert, P., Kayem, G., Goffinet, F., et al. (2018). Efficacy of antenatal corticosteroids in preterm twins: The epipage-2 cohort study. BJOG 125 (9), 1164–1170. Epub 2017/11/10. doi:10.1111/1471-0528.15014
Pulgar, V. M., and Figueroa, J. P. (2006). Antenatal betamethasone administration has a dual effect on adult sheep vascular reactivity. Pediatr. Res. 60 (6), 705–710. Epub 2006/10/27. doi:10.1203/01.pdr.0000246481.05231.17
Quinlivan, J. A., Beazley, L. D., Evans, S. F., Newnham, J. P., and Dunlop, S. A. (2000). Retinal maturation is delayed by repeated, but not single, maternal injections of betamethasone in sheep. Eye (Lond) 14, 93–98. Epub 2001/02/07. doi:10.1038/eye.2000.20
Raikkonen, K., Gissler, M., and Kajantie, E. (2020). Associations between maternal antenatal corticosteroid treatment and mental and behavioral disorders in children. JAMA 323 (19), 1924–1933. Epub 2020/05/20. doi:10.1001/jama.2020.3937
Ramadan, M. K., Hussein, G., Saheb, W., Rajab, M., and Mirza, F. G. (2016). Antenatal corticosteroids in the late preterm period: A prospective cohort study. J. Neonatal Perinat. Med. 9 (1), 15–22. Epub 2016/03/24. doi:10.3233/NPM-16915086
Riskin-Mashiah, S., Reichman, B., Bader, D., Kugelman, A., Boyko, V., Lerner-Geva, L., et al. (2018). Population-based study on antenatal corticosteroid treatment in preterm small for gestational age and non-small for gestational age twin infants. J. Matern. Fetal Neonatal Med. 31 (5), 553–559. Epub 2017/02/09. doi:10.1080/14767058.2017.1292242
Rizzo, G., Mappa, I., Bitsadze, V., Khizroeva, J., Makatsariya, A., and D'Antonio, F. (2022). Administration of antenatal corticosteroid is associated with reduced fetal growth velocity: A longitudinal study. J. Matern. Fetal Neonatal Med. 35 (14), 2775–2780. Epub 2020/07/31. doi:10.1080/14767058.2020.1800634
Roberts, D., Brown, J., Medley, N., and Dalziel, S. R. (2017). Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. 3, CD004454. Epub 2017/03/23. doi:10.1002/14651858.CD004454.pub3
Roberts, D., and Dalziel, S. (2006). Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst. Rev. (3), CD004454. Epub 2006/07/21. doi:10.1002/14651858.CD004454.pub2
Rodrigues, G. F. P., Benzi, J. R. L., Matos, L. H. C., de Freitas, S. F., Marques, M. P., Cavalli, R. C., et al. (2022). Enhanced elimination of betamethasone in dichorionic twin pregnancies. Br. J. Clin. Pharmacol. 88 (4), 1897–1903. Epub 2021/10/20. doi:10.1111/bcp.15111
Rodriguez, A., Wang, Y., Ali Khan, A., Cartwright, R., Gissler, M., and Jarvelin, M. R. (2019). Antenatal corticosteroid therapy (act) and size at birth: A population-based analysis using the Finnish medical birth register. PLoS Med. 16 (2), e1002746. Epub 2019/02/27. doi:10.1371/journal.pmed.1002746
Rottenstreich, A., Levin, G., Kleinstern, G., Haj Yahya, R., Rottenstreich, M., Yagel, S., et al. (2018). Patterns of use and optimal timing of antenatal corticosteroids in twin compared with singleton pregnancies. Acta Obstet. Gynecol. Scand. 97 (12), 1508–1514. Epub 2018/08/12. doi:10.1111/aogs.13439
Saccone, G., and Berghella, V. (2016). Antenatal corticosteroids for maturity of term or near term fetuses: Systematic review and meta-analysis of randomized controlled trials. BMJ 355, i5044. Epub 2016/10/14. doi:10.1136/bmj.i5044
Salomon, L. J., Cavicchioni, O., Bernard, J. P., Duyme, M., and Ville, Y. (2005). Growth discrepancy in twins in the first trimester of pregnancy. Ultrasound Obstet. Gynecol. 26 (5), 512–516. Epub 2005/09/08. doi:10.1002/uog.1966
Santana, D. S., Silveira, C., Costa, M. L., Souza, R. T., Surita, F. G., Souza, J. P., et al. (2018). Perinatal outcomes in twin pregnancies complicated by maternal morbidity: Evidence from the who Multicountry Survey on maternal and newborn health. BMC Pregnancy Childbirth 18 (1), 449. Epub 2018/11/21. doi:10.1186/s12884-018-2082-9
Sarid, E. B., Stoopler, M. L., Morency, A. M., and Garfinkle, J. (2022). Neurological implications of antenatal corticosteroids on late preterm and term infants: A scoping review. Pediatr. Res. 92 (5), 1225–1239. Epub 2022/06/11. doi:10.1038/s41390-022-02135-3
Socha, P., McGee, A., Bhattacharya, S., Young, C., and Wang, R. (2022). Antenatal corticosteroids and neonatal outcomes in twins: A systematic review and meta-analysis. Obstet. Gynecol. 140 (1), 20–30. Epub 2022/07/19. doi:10.1097/AOG.0000000000004835
South, A. M., Nixon, P. A., Chappell, M. C., Diz, D. I., Russell, G. B., Snively, B. M., et al. (2017). Antenatal corticosteroids and the renin-angiotensin-aldosterone system in adolescents born preterm. Pediatr. Res. 81 (1-1), 88–93. Epub 2016/09/17. doi:10.1038/pr.2016.179
Stock, S. J., Thomson, A. J., and Papworth, S.Royal College of O, Gynaecologists (2022). Antenatal corticosteroids to reduce neonatal morbidity and mortality: Green-top guideline No. 74. BJOG 129 (8), e35–e60. Epub 2022/02/17. doi:10.1111/1471-0528.17027
Stutchfield, P., Whitaker, R., and Russell, I.Antenatal Steroids for Term Elective Caesarean Section ASTECS Research Team (2005). Antenatal betamethasone and incidence of neonatal respiratory distress after elective caesarean section: Pragmatic randomised trial. BMJ 331 (7518), 662. Epub 2005/08/24. doi:10.1136/bmj.38547.416493.06
Stutchfield, P. R., Whitaker, R., Gliddon, A. E., Hobson, L., Kotecha, S., and Doull, I. J. (2013). Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (astecs trial). Arch. Dis. Child. Fetal Neonatal Ed. 98 (3), F195–F200. Epub 2013/02/21. doi:10.1136/archdischild-2012-303157
Swee, M. H., Parks, W. C., and Pierce, R. A. (1995). Developmental regulation of elastin production. Expression of tropoelastin pre-mrna persists after down-regulation of steady-state mrna levels. J. Biol. Chem. 270 (25), 14899–14906. Epub 1995/06/23. doi:10.1074/jbc.270.25.14899
Vaz, A., Malheiro, M. F., Severo, M., Rodrigues, T., Guimaraes, H., and Montenegro, N. (2018). Effect of antenatal corticosteroids on morbidity and mortality of preterm singletons and twins. J. Matern. Fetal Neonatal Med. 31 (6), 754–760. Epub 2017/03/10. doi:10.1080/14767058.2017.1297408
Vieira, L. A., Kao, Y. H., Tsevdos, D. S., Lau, Y. K., Wang, Z., Li, S., et al. (2022). Late preterm antenatal corticosteroids in singleton and twin gestations: A retrospective cohort study. BMC Pregnancy Childbirth 22 (1), 904. Epub 2022/12/06. doi:10.1186/s12884-022-05262-1
WHO (2022). "WHO recommendation on tocolytic therapy for improving preterm birth outcomes," in Who guidelines approved by the guidelines review committee. Geneva: WHO.
Williams, M. J., Ramson, J. A., and Brownfoot, F. C. (2022). Different corticosteroids and regimens for accelerating fetal lung maturation for babies at risk of preterm birth. Cochrane Database Syst. Rev. 8, CD006764. Epub 2022/08/10. doi:10.1002/14651858.CD006764.pub4
Wolford, E., Lahti-Pulkkinen, M., Girchenko, P., Lipsanen, J., Tuovinen, S., Lahti, J., et al. (2020). Associations of antenatal glucocorticoid exposure with mental health in children. Psychol. Med. 50 (2), 247–257. Epub 2019/01/29. doi:10.1017/S0033291718004129
Yu, H. R., Tain, Y. L., Tiao, M. M., Chen, C. C., Sheen, J. M., Lin, I. C., et al. (2018). Prenatal dexamethasone and postnatal high-fat diet have a synergistic effect of elevating blood pressure through a distinct programming mechanism of systemic and adipose renin-angiotensin systems. Lipids Health Dis. 17 (1), 50. Epub 2018/03/16. doi:10.1186/s12944-018-0701-0
Zanardo, V., Simbi, A. K., Franzoi, M., Solda, G., Salvadori, A., and Trevisanuto, D. (2004). Neonatal respiratory morbidity risk and mode of delivery at term: Influence of timing of elective caesarean delivery. Acta Paediatr. 93 (5), 643–647. Epub 2004/06/04. doi:10.1111/j.1651-2227.2004.tb02990.x
Keywords: antenatal corticosteroids, twin pregnancies, respiratory distress syndrome, corticosteroids, preterm birth
Citation: Zhu J, Li S, Zhao Y and Xiong Y (2023) The role of antenatal corticosteroids in twin pregnancy. Front. Pharmacol. 14:1072578. doi: 10.3389/fphar.2023.1072578
Received: 17 October 2022; Accepted: 23 January 2023;
Published: 02 February 2023.
Edited by:
Catherine M. T. Sherwin, Wright State University, United StatesReviewed by:
Jiangbo Du, Nanjing Medical University, ChinaDazhi Fan, Foshan Women and Children Hospital, China
Copyright © 2023 Zhu, Li, Zhao and Xiong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yu Xiong, xiongyu1535@163.com
 Jie Zhu
Jie Zhu Shuyue Li1,2
Shuyue Li1,2