- 1Center for Evidence-Based Medicine, Taihe Hospital, Hubei University of Medicine, Shiyan, Hubei, China
- 2Department of Ultrasound, The Third Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan, China
Aims: To evaluate the efficacy of different pharmacologic treatment for severe hypertension during pregnancy.
Methods: Two reviewers searched Ovid MEDLINE, Ovid EMbase, and the Cochrane Library for randomized clinical trials from the establishment of the database to 15 July 2021 that were eligible for inclusion and analyzed the pharmaceuticals used for severe hypertension in pregnancy.
Results: 29 relevant trials with 2,521 participants were involved. Compared with diazoxide in rate of achieving target blood pressure, other pharmaceuticals, including epoprostenol (RR:1.58, 95%CI:1.01–2.47), hydralazine\dihydralazine (RR:1.57, 95%CI:1.07–2.31), ketanserin (RR:1.67, 95%CI:1.09–2.55), labetalol (RR:1.54, 95%CI:1.04–2.28), nifedipine (RR:1.54, 95%CI:1.04–2.29), and urapidil (RR:1.57, 95%CI:1.00–2.47), were statistically significant in the rate of achieving target blood pressure. According to the SUCRA, diazoxide showed the best therapeutic effect, followed by nicardipine, nifedipine, labetalol, and nitroglycerine. The three pharmaceuticals with the worst therapeutic effect were ketanserin, hydralazine, and urapidil. It is worth noting that the high ranking of the top two pharmaceuticals, including diazoxide and nicardipine, comes from extremely low sample sizes. Other outcomes were reported in the main text.
Conclusion: This comprehensive network meta-analysis demonstrated that the nifedipine should be recommended as a strategy for blood pressure management in pregnant women with severe hypertension. Moreover, the conventional pharmaceuticals, including labetalol and hydralazine, showed limited efficacy. However, it was important to note that the instability of hydralazine reducing blood pressure and the high benefit of labetalol with high dosages intakes should also be of concern to clinicians.
Introduction
Hypertension is a common systemic disease that affects multiple organs and increases the risk of other diseases (Ott and Schmieder, 2022). Severe hypertension was classified according to the American College of Obstetricians and Gynecologists (ACOG) in 2020 criteria as systolic blood pressure (SBP) ≥160 mmHg with or without diastolic blood pressure (DBP) ≥110 mmHg (Gestational Hypertension and Preeclampsia, 2020). Hypertensive disorders of pregnancy (HDPs) include chronic hypertension, gestational hypertension, preeclampsia, and chronic hypertension with superimposed preeclampsia (Khedagi and Bello, 2021), and hypertension in pregnancy occurs in 5%–9% of expectant mothers (Reddy and Jim, 2019). During the first 3 weeks of pregnancy, inadequate control of blood pressure can lead to low birth weight and preeclampsia (Lu et al., 2018). Hypertension in pregnancy, simultaneously accompanied with obesity, is associated with cardiovascular complications and increases the mortality of heart disease, diabetes and Alzheimer’s disease for maternal (Papademetriou et al., 2021).
The most commonly used antihypertensive pharmaceuticals in international practice guidelines are methyldopa, labetalol, and nifedipine. There are three pharmaceuticals and methods commonly used in the treatment of severe hypertension during pregnancy: intravenous administration of labetalol, oral administration of nifedipine, intravenous administration of (di)hydralazine (Wertaschnigg et al., 2020). Labetalol and nifedipine are superior to other antihypertensive pharmaceuticals in pregnant women who require long-term medication. Diuretics such as hydrochlorothiazide are the second-line pharmaceuticals for the treatment of hypertension in pregnancy (Roberts et al., 2003). Theoretically, diuretics can have an effect on the fetus, but a systematic review of data did not support this view (Churchill et al., 2007). Other pharmaceuticals such as clonidine and prazosin may be considered under the recommendation of a specialist in maternal-fetal medicine and cardiology (ACOG Practice Bulletin No, 2019). Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers act on renin-dependent vasoconstriction. It can be used as first-line pharmaceuticals to treat non-pregnant patients, but in pregnant patients, it can cause fetal lesions, leading to malformations such as skull dysplasia and growth restriction (Ratnapalan and Koren, 2002).
To date, although many antihypertensive pharmaceuticals are used in women with hypertension during pregnancy, their efficacy and safety are not guaranteed. This study conducted a systematic evaluation of current pharmaceuticals for the treatment of hypertension and compared their antihypertensive effects.
Methods
Search strategy
This network meta-analysis was developed using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Hutton et al., 2015). As of 15 July 2021, we searched Ovid MEDLINE, Ovid EMbase, and the Cochrane Library based on MeSH and keywords to find RCTs suitable for this study (Supplementary Method 1). There were no language restrictions for publications. Some of the available references included in the studies were retrieved as data support.
Inclusion and exclusion criteria
The inclusion criteria were as follows: 1) All study participants were women with severe hypertension during pregnancy, wherein the criteria for severe hypertension (Gestational Hypertension and Preeclampsia, 2020), i.e., SBP ≥160 mmHg with or without DBP ≥110 mmHg should have been strictly followed; 2) Intervention was limited to a single antihypertensive pharmaceutical, including nifedipine, hydralazine\dihydralazine, ketanserin, diazoxide, epoprostenol, labetalol, nicardipine, nitroglycerine, prostaglandin A1, and urapidil. The pharmacological mechanisms and the FDA pregnancy category of all evaluated pharmaceuticals were shown in Supplementary Table S1. 3) The comparison group was the other pharmaceutical; 4) All studies should have included at least one outcome. The primary outcome was the rate of achieving the target blood pressure, defined as the number of patients with target blood pressure and SBP <140 mmHg and DBP <90 mmHg. Secondary outcomes were the dosages and time required to achieve the target blood pressure, and the final SBP and DBP in pregnant women after medication. 5) All studies were RCTs.
Duplicate studies and those that compared different dosages and durations of the same pharmaceuticals were excluded.
Data collection and processing
Three authors (Nian-Jia Deng, Chen-Yang Xian-Yu, and Hui-Jun Li), in consensus with each other, screened the literature independently and extracted data strictly according to inclusion criteria. Any disagreement among the authors was settled by discussions with a fourth author (Chao Zhang). Basic information of the literature including year, study design, and outcomes were extracted from each study.
Quality assessment
Two researchers (Nian-Jia Deng, Chen-Yang Xian-Yu) independently assessed risk of bias using the Revised Cochrane Risk of Bias tool for randomized trials (RoB-2) (Sterne et al., 2019). Risk of bias was evaluated from five domains: bias arising from the randomization process, bias due to deviations from intended interventions, bias due to missing outcome data, bias in outcome measurement, and bias in selection of the reported result. The overall bias from each study could be classified as high risk, some concerns or low risk of bias.
Statistical analysis
Dichotomous and continuous outcomes were expressed as relative risk (RR) with 95% confidence interval (CI) and mean difference (MD) with 95%CI, at a significance level of p < .05, respectively (Higgins and James, 2011). Heterogeneity between studies was assessed using chi-squared tests, in which the significance level was set to p < .10, as well as the I2 statistic (Higgins and James, 2011). I2 values of ≥40% were interpreted as significant heterogeneity and a random-effects model was used to conduct the meta-analysis; for I2 <40%, a fixed-effect model was used (Higgins and James, 2011).
Network meta-analyses can provide reliable evidence for direct and indirect multiple-intervention comparisons (Lu and Ades, 2004). The network plot shows the matching information of different interventions in an outcome and their comparison with each other. The size of the nodes corresponds to the number of trials under study. The larger the node, the larger the number of participants in the study. The results of direct comparisons are connected by a line, the thickness of which corresponds to the sum of the sample sizes compared for each pairwise treatment. The thicker the line, the larger the sample size for comparison. For the network meta-analysis, the design-by-treatment interaction model (Jackson et al., 2014) was employed. Let
Results
Search results
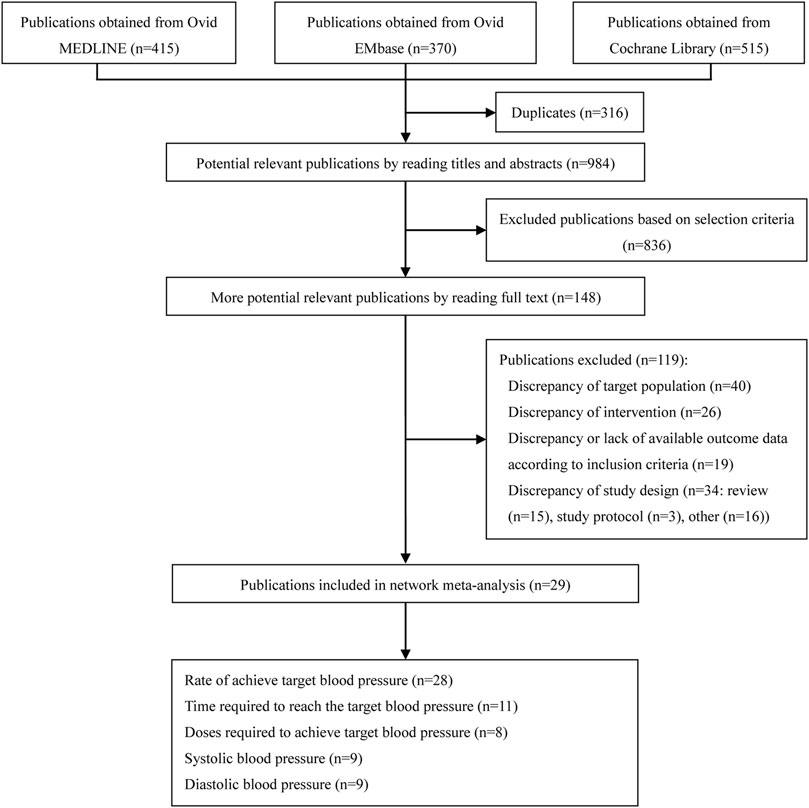
From the initial literature search, 1,300 citations were screened, 316 duplicates were removed, and 984 possible related studies were identified through potentially relevant publications by reading titles and abstracts; 836 studies were excluded. Finally, the full text of 148 potential related publications were read, and 119 of publications, including discrepancy of target population (n = 40), discrepancy of intervention (n = 26), discrepancy or lack of available outcome data according to inclusion criteria (n = 19), review (n = 15), study protocol (n = 3) and other (n = 16) were excluded, and 29 studies (Garden et al., 1982; Mabie et al., 1987; Fenakel et al., 1991; Toppozada et al., 1991; Moodley and Gouws, 1992; Kwawukume and Ghosh, 1995; Jegasothy and Paranthaman, 1996; Steyn and Odendaal, 1997; Bolte et al., 1998; Wacker et al., 1998; Bolte et al., 1999; Aali and Nejad, 2002; Elatrous et al., 2002; Vigil-De Gracia et al., 2006; Hennessy et al., 2007; Manzur-Verástegui et al., 2008; Rezaei et al., 2011; Sathya Lakshmi and Dasari, 2012; Delgado De Pasquale et al., 2014; Bijvank et al., 2015; Morris et al., 2016; Shi et al., 2016; Khan et al., 2017; Sharma et al., 2017; Patel et al., 2018; Zulfeen et al., 2019; Adebayo et al., 2020; Wasim et al., 2020) were included in the meta-analysis. The detailed PRISMA flow chart was depicted in Figure 1.
Basic characteristics and quality assessment
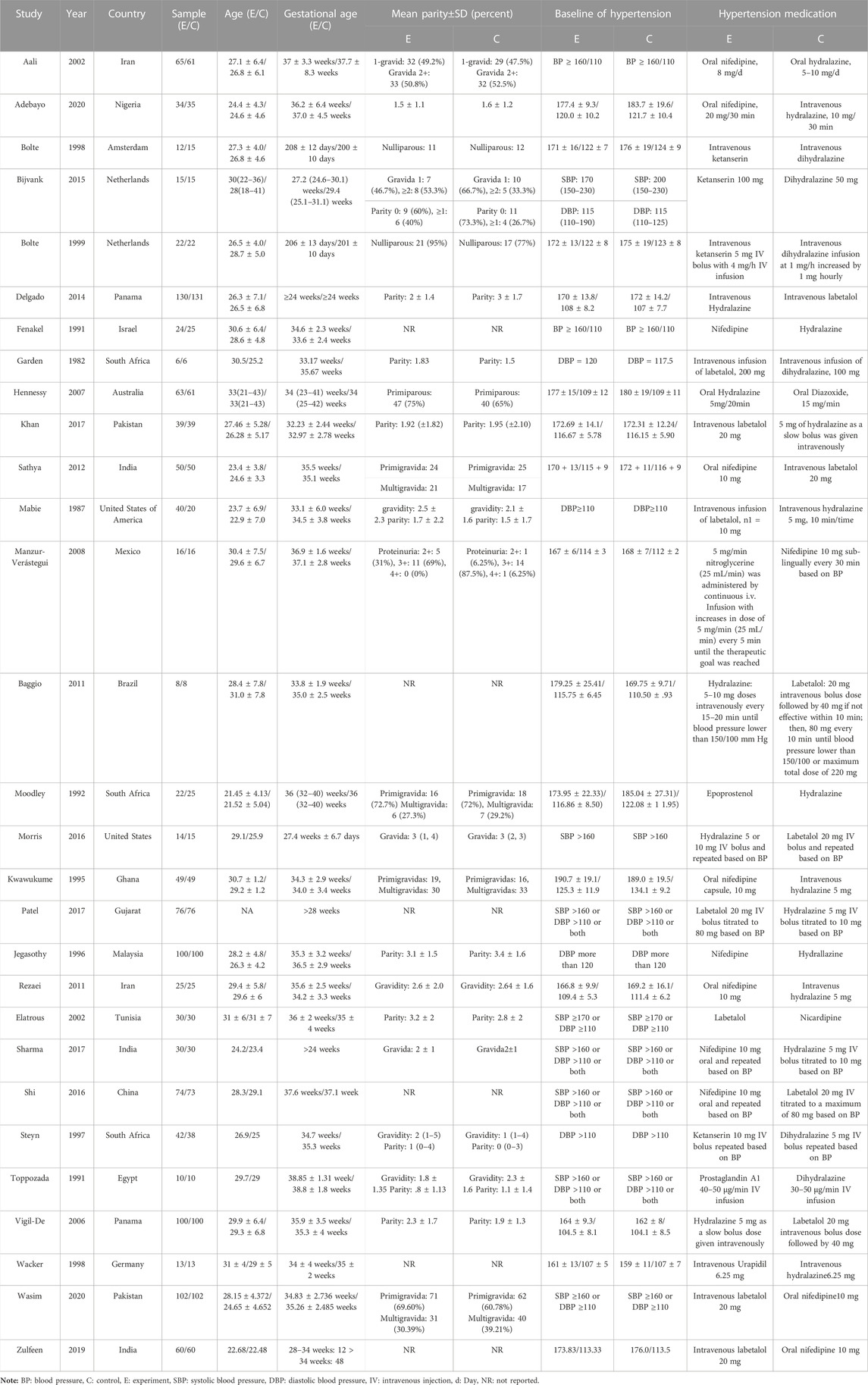
The basic characteristics of the included studies such as the number of subjects and their blood pressure measurements before medication are provided in Table 1. Furthermore, pregnant women were between 21 and 43 years of age and between 23 and 42 weeks of gestation. Ten kinds of interventions, including nifedipine, hydralazine\dihydralazine, ketanserin, diazoxide, epoprostenol, labetalol, nicardipine, nitroglycerine, prostaglandin A1 and urapidil, were included in the study. An assessment was provided for the risk of bias from randomized trials based on the RoB-2 in Supplementary Table S2.
Primary outcome
Rate of achieving target blood pressure
Twenty-eight RCTs (Garden et al., 1982; Mabie et al., 1987; Fenakel et al., 1991; Toppozada et al., 1991; Moodley and Gouws, 1992; Kwawukume and Ghosh, 1995; Jegasothy and Paranthaman, 1996; Steyn and Odendaal, 1997; Bolte et al., 1998; Wacker et al., 1998; Bolte et al., 1999; Aali and Nejad, 2002; Elatrous et al., 2002; Vigil-De Gracia et al., 2006; Hennessy et al., 2007; Manzur-Verástegui et al., 2008; Sathya Lakshmi and Dasari, 2012; Delgado De Pasquale et al., 2014; Bijvank et al., 2015; Morris et al., 2016; Shi et al., 2016; Khan et al., 2017; Sharma et al., 2017; Patel et al., 2018; Zulfeen et al., 2019; Adebayo et al., 2020; Wasim et al., 2020) with 2,471 study participants were included in the primary outcome, presented as the rate of achieved target blood pressure. Figure 2A showed a network plot of primary outcome assessments for eligible antihypertensive agents based on the pharmacological mechanisms of 10 interventions. No inconsistencies were found in the number of patients whose primary outcome was target blood pressure in Supplementary Figure S1A.
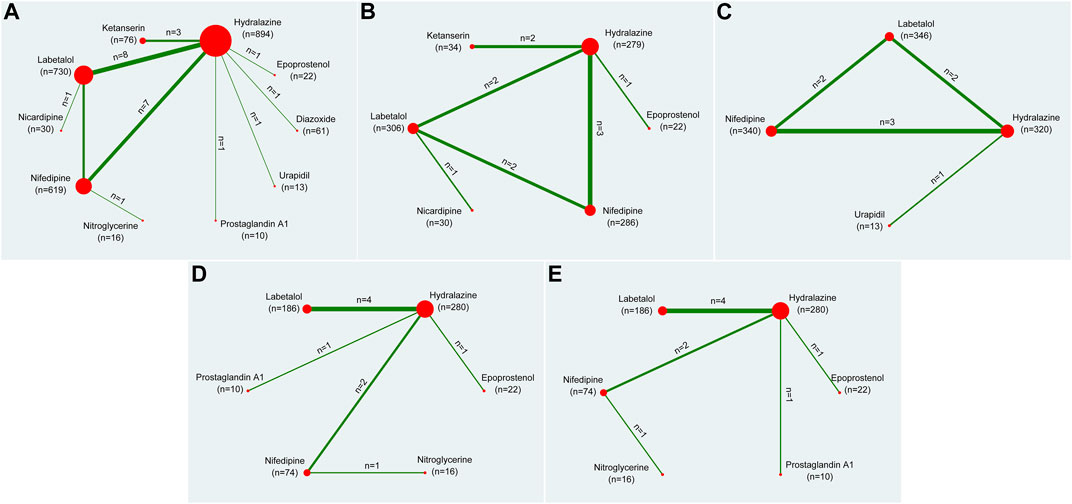
FIGURE 2. Network plots of all outcomes. Note: (A) indicated the rate of achieving target blood pressure, (B) indicated the time required to reach the target blood pressure, (C) indicated the dosage required to reach the target blood pressure, (D) indicated the systolic blood pressure, (E) indicated the diastolic blood pressure. The size of the nodes corresponds to the number of trials under study. The larger the node, the larger the number of participants in the study. The results of direct comparisons are connected by a line, the thickness of which corresponds to the sum of the sample sizes compared for each pairwise treatment. The thicker the line, the larger the sample size for comparison.
Compared with diazoxide in the results of network meta-analysis, other pharmaceuticals, including epoprostenol (RR: 1.58, 95%CI: 1.01–2.47), hydralazine\dihydralazine (RR: 1.57, 95%CI: 1.07–2.31), ketanserin (RR: 1.67, 95%CI: 1.09–2.55), labetalol (RR: 1.54, 95%CI: 1.04–2.28), nifedipine (RR: 1.54, 95%CI: 1.04–2.29), and urapidil (RR: 1.57, 95%CI: 1.00–2.47), were statistically significant in Table 2. However, there were no statistical discrepancies among other pharmaceuticals. The results of direct comparisons showed that only diazoxide versus hydralazine\dihydralazine (RR: 2.73, 95%CI: 1.32–5.68) was statistically significant in Table 2. All pharmaceuticals for this outcome were ranked according to the SUCRA, with diazoxide (96.4%) showing the best therapeutic effect, followed by nicardipine (63.7%), nifedipine (50.4%), labetalol (49.3%), and nitroglycerine (48.2%). The three pharmaceuticals with the worst therapeutic effect were ketanserin (24.5%), hydralazine (40.5%), and urapidil (41.2%) in Figure 3. No publication bias was found in the comparison of primary outcome in Supplementary Figure S2A.
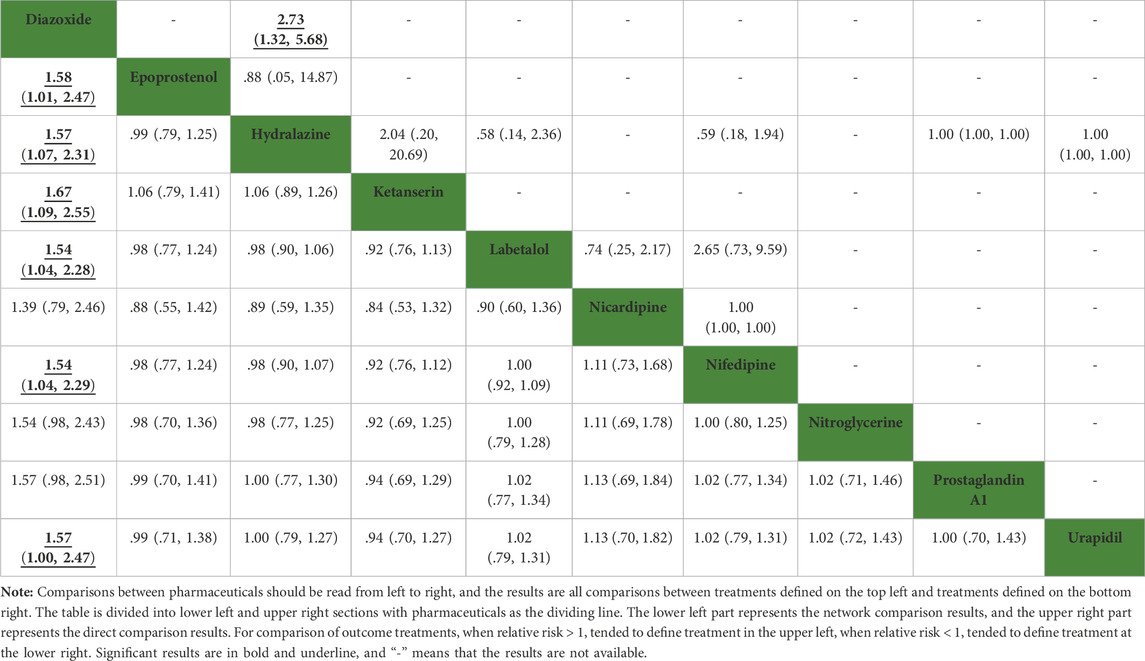
TABLE 2. Network comparison and direct comparison results for the rate of achieving target blood pressure (mmHg).
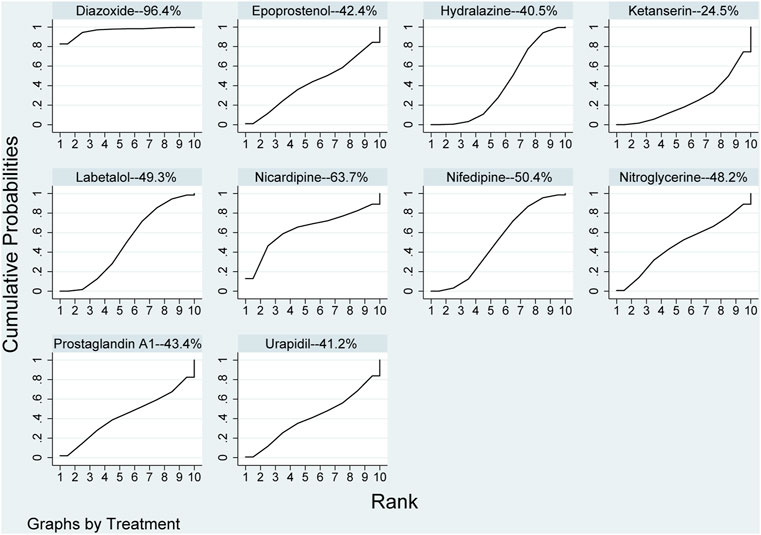
FIGURE 3. Ranking of all pharmaceuticals for achieving target blood pressure. Note: The larger the area under the curve, the more likely it is to be the best intervention. SUCRA, Surface under the cumulative ranking.
Secondary outcomes
Time required to reach the target blood pressure
Eleven RCTs (Mabie et al., 1987; Moodley and Gouws, 1992; Bolte et al., 1998; Bolte et al., 1999; Aali and Nejad, 2002; Elatrous et al., 2002; Rezaei et al., 2011; Patel et al., 2018; Zulfeen et al., 2019; Adebayo et al., 2020; Wasim et al., 2020) with 959 study participants were included in the secondary outcome related to the time required to reach the target blood pressure. It accessed the time requires to reach the aimed blood pressure after the specific pharmaceutical effect through the network plot in Figure 2B. Subsequent inconsistencies were reported in Supplementary Figure S1B.
In the network meta-analysis, the results, including epoprostenol versus hydralazine (MD: −29.31, 95%CI: −56.47 to −2.14), epoprostenol versus ketanserin (MD: 59.38, 95%CI: .50–118.27), hydralazine versus ketanserin (MD: 88.69, 95%CI: 36.40–140.99), ketanserin versus labetalol (MD: −80.51, 95%CI: −133.92 to −27.09), ketanserin versus nicardipine (MD: −79.14, 95%CI: −135.36 to −22.91), and ketanserin versus nifedipine (MD: −82.32, 95%CI: −135.45 to −29.19), were statistically different in Table 3. However, there were no statistical differences among other pharmaceuticals. In direct comparison among pharmaceuticals, including epoprostenol versus hydralazine (MD: −35.70, 95%CI: −60.18 to −11.22), hydralazine versus ketanserin (MD: 88.55, 95%CI: 37.79–139.31), and hydralazine versus labetalol (MD: 13.87, 95%CI: 11.17–16.56), were significant differences in Table 3. All pharmaceuticals were ranked according to the SUCRA, with ketanserin (99.4%), followed by epoprostenol (74.9%); the worst two pharmaceuticals were nifedipine (33.8%) and hydralazine (7.1%) in Figure 4. There was no publication bias of the time required to reach the target blood pressure in Supplementary Figure S2B.
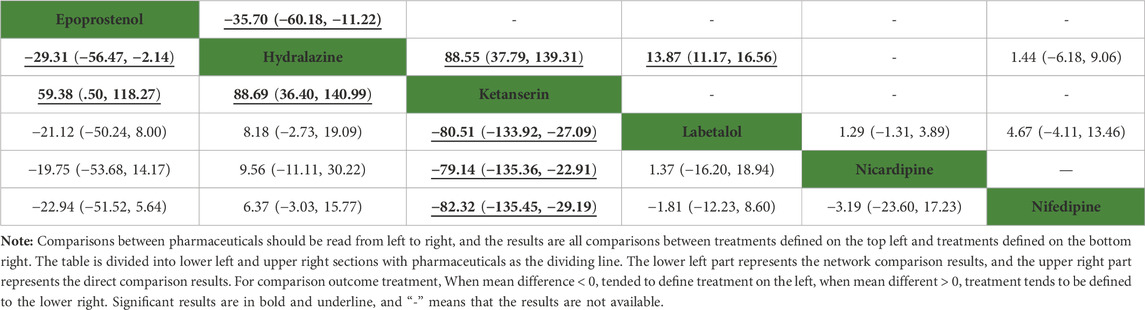
TABLE 3. Network comparison and direct comparison results for time required to reach the target blood pressure (min).
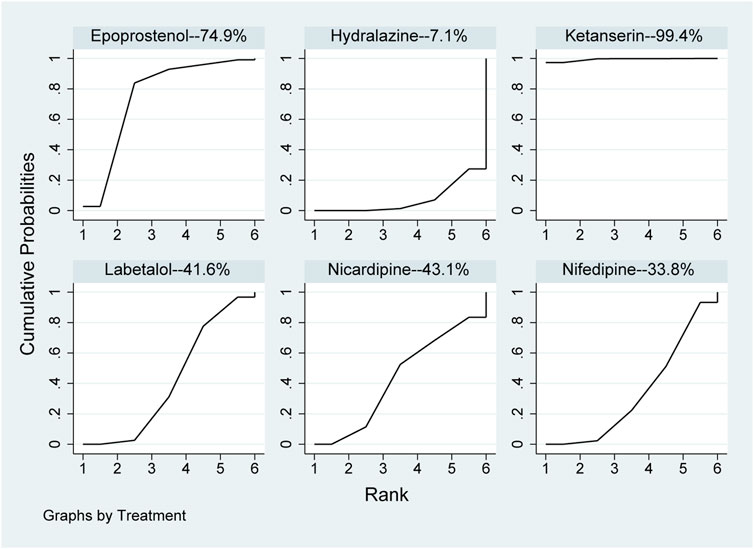
FIGURE 4. Ranking of all pharmaceuticals for the time required to reach the target blood pressure. Note: The larger the area under the curve, the more likely it is to be the best intervention. SUCRA, Surface under the cumulative ranking.
Dosages required to achieve target blood pressure
Seven studies (Mabie et al., 1987; Wacker et al., 1998; Aali and Nejad, 2002; Delgado De Pasquale et al., 2014; Shi et al., 2016; Adebayo et al., 2020; Wasim et al., 2020) including eight RCTs with 1,019 study participants were included in the secondary outcome of the dosages required to reach the target blood pressure. Figure 2C showed the dosages of network plot. No inconsistency was observed in the outcome of the dosages required to achieve the target blood pressure (Supplementary Figure S1C).
In the network meta-analysis, the results of network meta-analysis, including hydralazine versus labetalol (MD: −57.46, 95%CI: −102.04 to −12.89) and labetalol versus nifedipine (MD: 51.56, 95%CI: 6.59–96.54), were statistical difference in Table 4. In direct comparison, compared to urapidil, hydralazine required fewer dosages required to achieve target blood pressure (MD: −10.69, 95%CI: −20.23 to −1.15) in Table 4. All pharmaceuticals were ranked according to the SUCRA, with hydralazine (75.0%) being the most effective one, followed by nifedipine (63.4%), urapidil (55.9%), and labetalol (5.7%) in Figure 5. No publication bias was found in the dosages required to achieve target blood pressure in Supplementary Figure S2C.
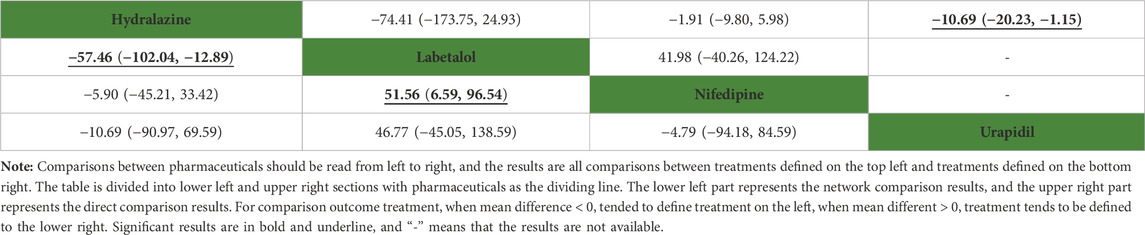
TABLE 4. Network comparison and direct comparison results for dosage required to achieve target blood pressure (mg).
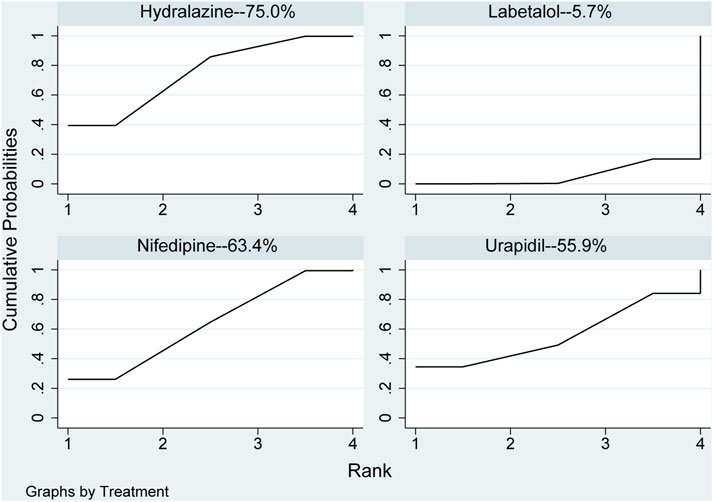
FIGURE 5. Ranking of all pharmaceuticals for the dosage required to reach the target blood pressure. Note: The larger the area under the curve, the more likely it is to be the best intervention. SUCRA, Surface under the cumulative ranking.
Systolic pressure
Eight studies (Fenakel et al., 1991; Toppozada et al., 1991; Moodley and Gouws, 1992; Manzur-Verástegui et al., 2008; Delgado De Pasquale et al., 2014; Khan et al., 2017; Adebayo et al., 2020) including nine RCTs with 588 study participants were included in this secondary outcome. The network plot of the interventions assessed for systolic pressure is shown in Figure 2D.
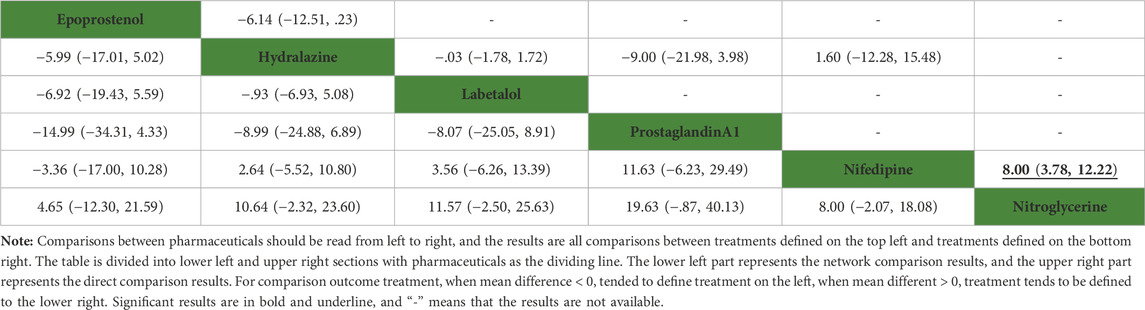
None of the network comparison results showed statistically significant differences. Direct comparison showed statistical differences between nifedipine versus nitroglycerine (MD: 8.00, 95%CI: 3.78–12.22), while other comparisons showed no statistical difference in Table 5. All pharmaceuticals were ranked according to the SUCRA, with nitroglycerine (90.5%) showing the best effect, followed by epoprostenol (73%), and prostaglandin A1 (8.8%) was the least effective in Figure 6. No publication bias was found in systolic pressure in Supplementary Figure S2D.
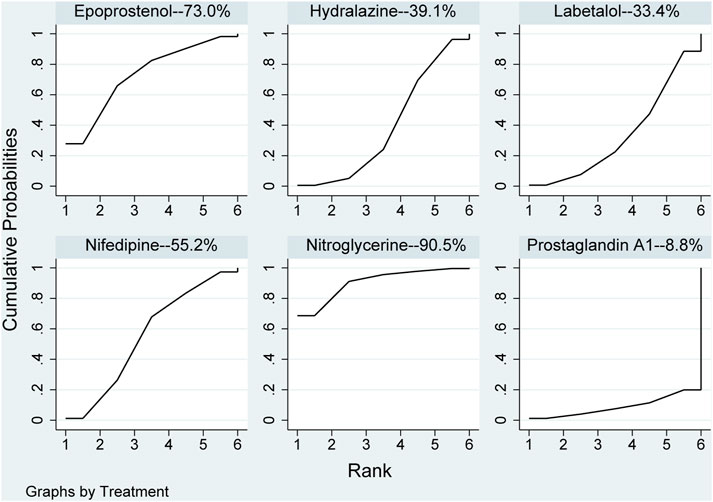
FIGURE 6. Ranking of all pharmaceuticals for systolic blood pressure. Note: The larger the area under the curve, the more likely it is to be the best intervention. SUCRA, Surface under the cumulative ranking.
Diastolic pressure
Eight studies (Fenakel et al., 1991; Toppozada et al., 1991; Moodley and Gouws, 1992; Manzur-Verástegui et al., 2008; Delgado De Pasquale et al., 2014; Khan et al., 2017; Adebayo et al., 2020) including nine RCTs with 588 study participants were included in this secondary outcome. Figure 2E showed the network plot of diastolic pressure.
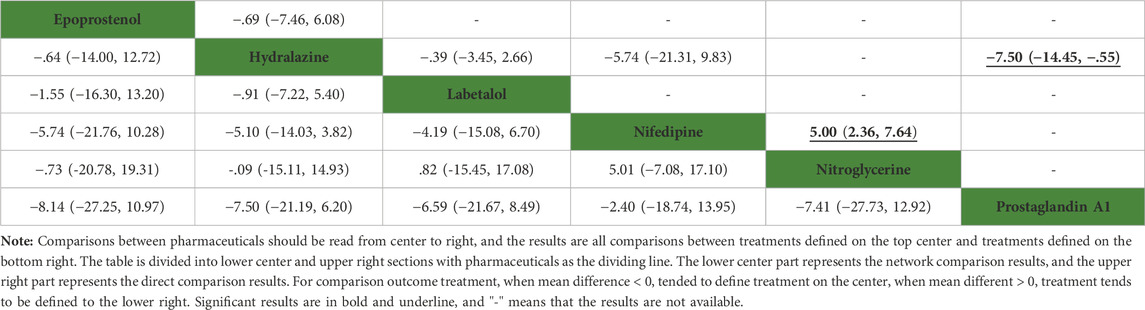
None of the network comparison results were statistically significant. In direct comparison, the results, including hydralazine versus prostaglandin A1 (MD: −7.50, 95%CI: −14.45 to −.55) and nifedipine versus nitroglycerine (MD: 5.00, 95%CI: 2.36–7.64), were statistical difference, while others were not (Table 6). All pharmaceuticals were ranked according to the SUCRA, with hydralazine (65.9%) being the most effective, and prostaglandin A1 (23.5%) being the least in Figure 7. No publication bias was found in diastolic pressure in Supplementary Figure S2E.
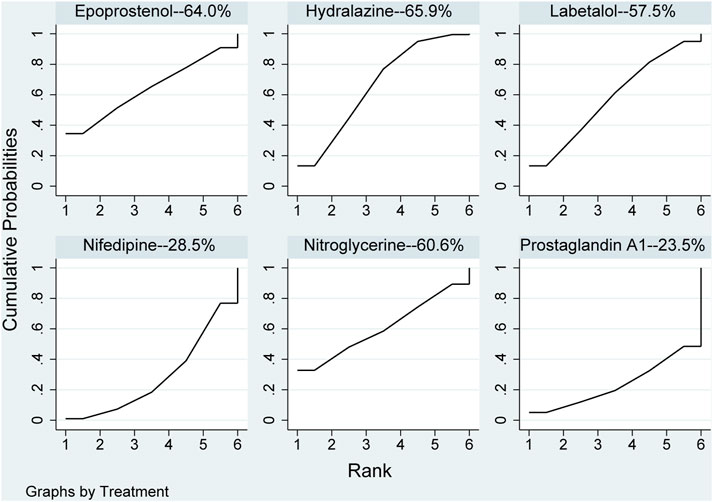
FIGURE 7. Ranking of all pharmaceuticals for diastolic blood pressure. Note: The larger the area under the curve, the more likely it is to be the best intervention. SUCRA, Surface under the cumulative ranking.
Discussion
This network meta-analysis compared the efficacy of different pharmaceuticals in the treatment of severe hypertension during pregnancy. Pharmaceutical therapy was the main clinical treatment for pregnancy hypertension. However, in the process of pharmaceutical treatment, some pharmaceuticals will inevitably cause different degrees of damage to either the fetus or pregnant women or both. The specific pharmaceutical to treat hypertension during pregnancy should be carefully selected. Efficacy of antihypertensive pharmaceuticals was assessed by the risk of persistent severe hypertension (Awaludin et al., 2022).
In this network meta-analysis, we have seen sufficient evidence to conclude that hydralazine, nifedipine, and labetalol have similar and superior efficacy in effective treatment of severe hypertension in pregnancy. We found effective pharmaceuticals including labetalol, nifedipine, and hydralazine to be recommended in the clinical guidelines and classified as first-line pharmaceuticals in pregnancy clinics (Khedagi and Bello, 2021). Non-etheless, oral labetalol is not widely used in lower-income countries (Magee and von Dadelszen, 2018). The results of our network meta-analysis compared the pharmaceuticals with the best efficacy, such as diazoxide and nicardipine, but there were still problems such as small sample size and comparison of single pharmaceuticals, which were not enough to conclude any one antihypertensive pharmaceutical regimen as the most effective. Therefore, these three pharmaceuticals were recommended for the treatment of severe hypertension during pregnancy.
Labetalol is a selective a-1, non-selective β-adrenoceptor blocker that induces peripheral vasodilation and prevented reflex tachycardia that can rapidly reduce peripheral blood resistance and blood pressure, but the variations on heart rate or urine volume were not significant (Chera-Aree et al., 2020; Wu et al., 2020). Hydralazine is an effective direct arteriolar vasodilator that can reduce peripheral blood pressure resistance, mediate the release of adrenalin and norepinephrine receptors, and increase cardiac output and venous return flow (Herman et al., 2022). Furthermore, the pharmaceutical itself could reduce systemic resistance, which has long been used for rapid controlling of severe hypertension during pregnancy (Chera-Aree et al., 2020). Nifedipine is suitable for all kinds of hypertension, especially severe hypertension (Zhao et al., 2020). Moreover, it is essentially a calcium channel blocker that can lower blood pressure, improve hemorheological parameters, expand coronary arteries, increase the blood flow of patients’ coronary arteries, and relax the smooth muscle in the blood vessels (Xiang et al., 2020; Yin and Yang, 2021). Currently, quick-acting nifedipine can only be used in case of urgent hypertension where intravenous fluids are ineffective (Yin et al., 2022). Nifedipine is a cheap oral antihypertensive pharmaceutical that does not require special storage and is more readily available in resource-limited environments (Alavifard et al., 2019).
This multidrug comparison showed a higher rate of achieving target blood pressure in the nifedipine group than in the hydralazine and labetalol groups for severe hypertension during pregnancy. These three pharmaceuticals for this outcome were ranked according to the area of the SUCRA plot nifedipine (50.4%), labetalol (49.3%) and hydralazine (40.5%). A larger area indicates a better effect of medical treatment on this outcome, the higher the effective rate of blood pressure reduction after medication. The analysis results of several previous studies were consistent with our results, for example, two previous studies (Alavifard et al., 2019; Wu et al., 2022) showed that nifedipine compared with labetalol and hydralazine in the treatment of severe hypertension during pregnancy had a higher rate of achieving the target blood pressure. Duley et al. (Duley et al., 2013) conducted a related study that included 35 trials and 3,573 women, and found that nifedipine was more effective than hydralazine in reducing persistent hypertension during pregnancy. Shekhar et al. (Shekhar et al., 2016) in 2016 compared seven trial designs in 363 pregnant women and reported that oral nifedipine was superior to intravenous labetalol in improving severe hypertension during pregnancy. This might be because it is a calcium-channel blocker and can relax the smooth muscle in the blood vessels with a more sustained effect than hydralazine can (Xiang et al., 2020); hydralazine is a direct arteriolar vasodilator with fast but short duration of action (Adebayo et al., 2020). Another study (Lesko et al., 1986) provided evidence that serum nifedipine concentrations were steady when the pharmaceutical was dosed every 8h, indicating that nifedipine remained in the blood for a longer time and had a more obvious effect relative to hydralazine and labetalol.
This study evaluated the outcome of the time required to reach the target blood pressure after medication. These three pharmaceuticals for this outcome were ranked according to the area of the SUCRA plot labetalol (41.6%), nifedipine (33.8%), and hydralazine (7.1%). The larger the area, the shorter the time required for the pharmaceutical to treat severe hypertension in pregnancy. We found that labetalol took less time than nifedipine and hydralazine to obtain the maximum effect. However, another study (Wu et al., 2022) showed that among the three pharmaceuticals hydralazine, nifedipine, and labetalol, hydralazine required a shorter time to reach the target blood pressure; this differed from the results of the current study. Another study (Adebayo et al., 2020) showed that nifedipine and hydralazine showed no difference in the time taken to achieve the target blood pressure. However, the results of this study found that the two pharmaceuticals took different times to reach the target blood pressure, with hydralazine taking longer than nifedipine. Yet another study (Patel et al., 2018) showed that labetalol reached the target blood pressure faster than hydralazine, which was consistent with our results. Labetalol acts as an adrenergic receptor blocker that rapidly lowers peripheral blood resistance and blood pressure, and it had a long-lasting effect on lowering blood pressure. Moreover, the possibility of rebound back to hypertension is small after stopping the pharmaceutical (Zhang et al., 2021).
In addition, it was found that hydralazine required only fewer dosages to lower blood pressure compared to nifedipine and labetalol. These three pharmaceuticals for this outcome were ranked according to the area of the SUCRA plot hydralazine (75.0%), nifedipine (63.4%) and labetalol (5.7%). A larger area indicates that only a smaller therapeutic dose is required for pharmaceutical treatment of severe hypertension in pregnancy. There were studies (Shi et al., 2015; Zulfeen et al., 2019) conducted the RCTs and concluded that oral nifedipine required a less dosages to reach target blood pressure than intravenous labetalol, which was consistent with our viewpoint results. Another study’s (Aali and Nejad, 2002) data analysis indicated that relative to hydralazine, significantly fewer pharmaceutical administrations of nifedipine were required; our results were consistent with this. However, the dose-response of hydralazine was largely unpredictable and might eventually led to an unpredicted response to blood pressure (Cawoski et al., 2021). Another experiment showed that (Shepherd et al., 1984) within the therapeutic dosages range for proper using of the pharmaceutical with the increased of the dosages of hydralazine, maternal blood pressure was found to decrease much more than expected. This could be because a small dosage of hydralazine mediated the released of large amounts of epinephrine and norepinephrine receptors, increasing cardiac output and venous return flow.
Evaluation of the hypotensive effect of each pharmaceutical after the patient took the pharmaceutical revealed that hydralazine could reduce more values of blood pressure, and the blood pressure of patients using nifedipine and labetalol was significantly higher than that of patients using hydralazine. These three pharmaceuticals for this outcome were ranked according to the area of the SUCRA plot. Nifedipine (55.2%), hydralazine (39.1%) and labetalol (33.4%) for Systolic Pressure. These three pharmaceuticals for this outcome were ranked according to the area of the SUCRA plot nifedipine (28.5%), hydralazine (65.9%) and labetalol (57.5%) for Diastolic Pressure. The larger the area, the greater the value of lowering blood pressure by pharmaceutical treatment of severe hypertension in pregnancy. One other study (Fenakel et al., 1991) also arrived at the same results, but they believed that effect of nifedipine was more predictable that of hydralazine. The possible reason is that the dose-response of hydralazine was largely unpredictable and may ultimately lead to an unpredicted response to blood pressure (Cawoski et al., 2021). In addition, the pharmaceutical had long been used for rapid control of severe hypertension during pregnancy (Chera-Aree et al., 2020). In another study (Khan et al., 2017), reduction of blood pressure with labetalol was significant than hydralazine, possibly that the reduction in blood pressure control is related to the dosages of this pharmaceutical used (Zhang et al., 2021) and the maximum permissible dosages was 300 mg/day (Peacock et al., 2012). Two other studies (Magee et al., 2003; Baggio et al., 2011) reported that hydralazine could lower blood pressure more than labetalol, leading to a higher incidence of maternal hypotension. However, lowering blood pressure was not advisable because hypotension can impair uteroplacental circulation and increase the risk of severe nausea, vomiting, threatened abortion, and anemia (Bánhidy et al., 2011; Baggio et al., 2011).
Methyldopa can lower blood pressure by binding to α2-adrenergic receptor as an agonist (Dublin et al., 2022), and it was very popular pharmaceutical of choice in the 70s, but it was replaced by other pharmaceuticals mainly for tolerability (Mah et al., 2009). Diuretics are the mainstay of treatment for non-gestational hypertension because they theoretically act on plasma volume depletion to cause reactive vasoconstriction, but in some earlier studies, diuretics were found to be safe during pregnancy (Garovic et al., 2022). Guidelines from the Multidisciplinary Working Group of the National Organization for Safe Motherhood advocated that reduced the use of magnesium sulfate as a quasi-first-line antihypertensive agent (Liu et al., 2022; Yu and Zhou, 2022). Studies suggested that the exclusive treatment of magnesium sulfate to treat pregnancy-induced hypertension (PIH) will affect fetal development (Pascoal et al., 2019). Li et al. (Li et al., 2021) discussed the use of magnesium sulfate in combination with labetalol, which proved its efficacy in improving delivery outcomes and maternal and fetal outcomes.
Clinical significance
This network meta-analysis has more significant advantages than head-to-head meta-analysis, as the effects of different interventions to treat the disease can be quantified and ranked, according to the efficacy of different outcome measures to help select the best treatment plan. Compared with a previous study (Sridharan and Sequeira, 2018), our study strengthened the selection criteria of the population, applied more stringent restrictions on the inclusion and exclusion criteria according to the ACOG (Gestational Hypertension and Preeclampsia, 2020) in 2020 definition of severe hypertension, unified the study population, and made the conclusions more rigorous. Bridging pharmaceuticals can be used to compare the efficacy of other pharmaceuticals, even without head-to-head clinical studies. Each outcome is analyzed and discussed in terms of type of medication and duration and dosages of medication, thereby providing a ranking of interventions for different outcomes to help clinicians make the best choice. The evidence found in this study adds to the existing relevant clinical guidelines (Shepherd et al., 1984) regarding the efficacy of labetalol, hydralazine, and nifedipine as first-line pharmaceuticals for severe hypertension during pregnancy, further providing and updating new evidence and direction for subsequent research.
Limitations
There are several limitations in this study. Firstly, insufficient information, and no further analysis and discussion of the efficacy of medications for history, urine volume, first or second pregnancy, preeclampsia or eclampsia, may have affected the validity of the results. Moreover, differences in dosages use can lead to heterogeneity when comparing antihypertensive pharmaceuticals. Secondly, the limited sample size of the included studies means that there is a potential difference between the estimated value and the actual effect; this may affect the statistical power of our study. Thirdly, part of the trials in this network meta-analysis had unclear risks of bias, the results may be slightly biased.
Conclusion
This comprehensive network meta-analysis demonstrated that the nifedipine should be recommended as a strategy for blood pressure management in pregnant women with severe hypertension. Moreover, the conventional pharmaceuticals, including labetalol and hydralazine, showed limited efficacy. However, it was important to note that instability of hydralazine reducing blood pressure and high benefit of labetalol with high dosages intakes should also be of concern to clinicians.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CZ designed the research; N-JD, C-YX-Y and C-YH collected the data and verified the accuracy of the data. N-JD, Y-TM and H-JL verified the accuracy of the data; N-JD, XL and C-YX-Y contributed to data interpretation; N-JD, R-ZH, T-YG and CZ performed the statistical analysis and visualization; N-JD wrote the manuscript. The authors read, critically reviewed, and approved the final manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1092501/full#supplementary-material.
References
Aali, B. S., and Nejad, S. S. (2002). Nifedipine or hydralazine as a first-line agent to control hypertension in severe preeclampsia. Acta Obstet. Gynecol. Scand. 81 (1), 25–30. doi:10.1034/j.1600-0412.2002.810105.x
ACOG Practice Bulletin No (2019). ACOG practice Bulletin No. 203: Chronic hypertension in pregnancy. Obstet. Gynecol. 133 (1), e26–e50. doi:10.1097/AOG.0000000000003020
Adebayo, J. A., Nwafor, J. I., Lawani, L. O., Esike, C. O., Olaleye, A. A., and Adiele, N. A. (2020). Efficacy of nifedipine versus hydralazine in the management of severe hypertension in pregnancy: A randomised controlled trial. Niger. Postgrad. Med. J. 27 (4), 317–324. doi:10.4103/npmj.npmj_275_20
Alavifard, S., Chase, R., Janoudi, G., Chaumont, A., Lanes, A., Walker, M., et al. (2019). First-line antihypertensive treatment for severe hypertension in pregnancy: A systematic review and network meta-analysis. Pregnancy Hypertens. 18, 179–187. doi:10.1016/j.preghy.2019.09.019
Awaludin, A., Rahayu, C., Daud, N. A. A., and Zakiyah, N. (2022). Antihypertensive medications for severe hypertension in pregnancy: A systematic review and meta-analysis. Healthc. (Basel). 10 (2), 325. doi:10.3390/healthcare10020325
Baggio, M. R. F., Calderon, A. C. S., Berezowski, A. T., Marcolin, A. C., Duarte, G., et al. (2011). Changes in fetal and maternal Doppler parameters observed during acute severe hypertension treatment with hydralazine or labetalol: A randomized controlled trial. Ultrasound Med. Biol. 37 (1), 53–58. doi:10.1016/j.ultrasmedbio.2010.10.006
Bánhidy, F., Acs, N., Puhó, E. H., and Czeizel, A. E. (2011). Hypotension in pregnant women: A population-based case-control study of pregnancy complications and birth outcomes. Hypertens. Res. 34 (1), 55–61. doi:10.1038/hr.2010.172
Bijvank, S. W., Visser, W., Duvekot, J. J., Steegers, E. A., Edens, M. A., Roofthooft, D. W., et al. (2015). Ketanserin versus dihydralazine for the treatment of severe hypertension in early-onset preeclampsia: A double blind randomized controlled trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 189, 106–111. doi:10.1016/j.ejogrb.2015.02.002
Bolte, A. C., van Eyck, J., Kanhai, H. H., Bruinse, H. W., van Geijn, H. P., and Dekker, G. A. (1999). Ketanserin versus dihydralazine in the management of severe early-onset preeclampsia: Maternal outcome. Am. J. Obstet. Gynecol. 180, 371–377. doi:10.1016/s0002-9378(99)70216-4
Bolte, A. C., van Eyck, J., Strack van Schijndel, R. J., van Geijn, H. P., and Dekker, G. A. (1998). The haemodynamic effects of ketanserin versus dihydralazine in severe early-onset hypertension in pregnancy. Br. J. Obstet. Gynaecol. 105 (7), 723–731. doi:10.1111/j.1471-0528.1998.tb10202.x
Cawoski, J. R., DeBiasio, K. A., Donnachie, S. W., Timanus, E. A., Zimmerman, D. E., Guarascio, A. J., et al. (2021). Safety and efficacy of intravenous hydralazine and labetalol for the treatment of asymptomatic hypertension in hospitalised patients: A systematic review. Int. J. Clin. Pract. 75 (7), e13991. doi:10.1111/ijcp.13991
Chera-Aree, P., Tengtrakulcharoen, P., Leetheeragul, J., Sampaojarean, U., Surasereewong, S., and Wataganara, T. (2020). Clinical experiences of intravenous hydralazine and labetalol for acute treatment of severe hypertension in pregnant Thai women. J. Clin. Pharmacol. 60 (12), 1662–1670. doi:10.1002/jcph.1685
Churchill, D., Beevers, G. D., Meher, S., and Rhodes, C. (2007). Diuretics for preventing pre-eclampsia. Cochrane Database Syst. Rev. 2007 (1), Cd004451. doi:10.1002/14651858.CD004451.pub2
Delgado De Pasquale, S., Velarde, R., Reyes, O., and De La Ossa, K. (2014). Hydralazine vs labetalol for the treatment of severe hypertensive disorders of pregnancy. A randomized, controlled trial. Pregnancy Hypertens. 4 (1), 19–22. doi:10.1016/j.preghy.2013.08.001
Dublin, S., Idu, A., Avalos, L. A., Cheetham, T. C., Easterling, T. R., Chen, L., et al. (2022). Maternal and neonatal outcomes of antihypertensive treatment in pregnancy: A retrospective cohort study. PLoS One 17 (5), e0268284. doi:10.1371/journal.pone.0268284
Duley, L., Meher, S., and Jones, L. (2013). Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst. Rev. 2013 (7), Cd001449. doi:10.1002/14651858.CD001449.pub3
Elatrous, S., Nouira, S., Ouanes Besbes, L., Marghli, S., BoussarssarM., , SakkouhiM., , et al. (2002). Short-term treatment of severe hypertension of pregnancy: Prospective comparison of nicardipine and labetalol. Intensive Care Med. 28 (9), 1281–1286. doi:10.1007/s00134-002-1406-3
Fenakel, K., Fenakel, G., Appelman, Z., Lurie, S., KatZ, Z., and Shoham, Z. (1991). Nifedipine in the treatment of severe preeclampsia. Obstet. Gynecol. 77 (3), 331–337.
Garden, A., Davey, D. A., and Dommisse, J. (1982). Intravenous labetalol and intravenous dihydralazine in severe hypertension in pregnancy. Clin. Exp. Hypertens. B 1 (2-3), 371–383. doi:10.3109/10641958209139860
Garovic, V. D., Dechend, R., Easterling, T., Karumanchi, S. A., McMurtry Baird, S., Magee, L. A., et al. (2022). Hypertension in pregnancy: Diagnosis, blood pressure goals, and pharmacotherapy: A scientific statement from the American heart association. Hypertension 79 (2), e21–e41. doi:10.1161/HYP.0000000000000208
Gestational Hypertension and Preeclampsia. (2020). Gestational hypertension and preeclampsia: ACOG practice Bulletin, number 222. Obstet. Gynecol. 135(6), e237-e260.
Hennessy, A., Thornton, C. E., Makris, A., Ogle, R. F., Henderson-Smart, D. J., Gillin, A. G., et al. (2007). A randomised comparison of hydralazine and mini-bolus diazoxide for hypertensive emergencies in pregnancy: The PIVOT trial. Aust. N. Z. J. Obstet. Gynaecol. 47 (4), 279–285. doi:10.1111/j.1479-828X.2007.00738.x
Herman, L. L., Bruss, Z. S., and Tivakaran, V. S. (2022). Hydralazine. StatPearls. Treasure island (FL). United States: StatPearls Publishing Copyright © 2022. StatPearls Publishing LLC.
Higgins, J, and James, T (2011). Cochrane handbook for systematic reviews of interventions. v.5.1. Available at:http://www.cochrane-handbook.org.
Hutton, B., Salanti, G., Caldwell, D. M., Chaimani, A., Schmid, C. H., Cameron, C., et al. (2015). The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern Med. 162 (11), 777–784. doi:10.7326/M14-2385
Jackson, D., Barrett, J. K., Rice, S., White, I. R., and Higgins, J. P. T. (2014). A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat. Med. 33 (21), 3639–3654. doi:10.1002/sim.6188
Jegasothy, R., and Paranthaman, S. (1996). Sublingual nifedipine compared with intravenous hydrallazine in the acute treatment of severe hypertension in pregnancy: Potential for use in rural practice. J. Obstet. Gynaecol. Res. 22 (1), 21–24. doi:10.1111/j.1447-0756.1996.tb00930.x
Khan, A., Hafeez, S., and Nasrullah, F. D. (2017). Comparison of Hydralazine and Labetalol to lower severe hypertension in pregnancy. Pak J. Med. Sci. 33 (2), 466–470. doi:10.12669/pjms.332.12243
Khedagi, A. M., and Bello, N. A. (2021). Hypertensive disorders of pregnancy. Cardiol. Clin. 39 (1), 77–90. doi:10.1016/j.ccl.2020.09.005
Kwawukume, E. Y., and Ghosh, T. S. (1995). Oral nifedipine therapy in the management of severe preeclampsia. Int. J. Gynaecol. Obstet. 49 (3), 265–269. doi:10.1016/0020-7292(95)02372-j
Lesko, L. J., Hunter, J. R., Burgess, R. C., and Rodgers, G. P. (1986). Accumulation of nifedipine after multiple doses. J. Pharm. Pharmacol. 38 (6), 486–488. doi:10.1111/j.2042-7158.1986.tb04619.x
Li, P., Zhao, J., Gao, P., and Qu, H. (2021). Clinical evaluation of pinggan yiqi yangshen recipe combined with labetalol hydrochloride and magnesium sulfate in the treatment of PIH. Evid. Based Complement. Altern. Med. 2021, 3135043. doi:10.1155/2021/3135043
Liu, C., Wang, F., and Yin, X. (2022). Uterine ultrasound Doppler hemodynamics of magnesium sulfate combined with labetalol in the treatment of pregnancy-induced hypertension using empirical wavelet transform algorithm. Comput. Intell. Neurosci. 2022, 7951342. doi:10.1155/2022/7951342
Lu, G., and Ades, A. E. (2004). Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 23 (20), 3105–3124. doi:10.1002/sim.1875
Lu, Y., Chen, R., Cai, J., Huang, Z., and Yuan, H. (2018). The management of hypertension in women planning for pregnancy. Br. Med. Bull. 128 (1), 75–84. doi:10.1093/bmb/ldy035
Mabie, W. C., Gonzalez, A. R., Sibai, B. M., and Amon, E. (1987). A comparative trial of labetalol and hydralazine in the acute management of severe hypertension complicating pregnancy. Obstet. Gynecol. 70, 328–333.
Magee, L. A., Cham, C., Waterman, E. J., Ohlsson, A., and von Dadelszen, P. (2003). Hydralazine for treatment of severe hypertension in pregnancy: meta-analysis. Bmj 327 (7421), 955–960. doi:10.1136/bmj.327.7421.955
Magee, L. A., and von Dadelszen, P. (2018). State-of-the-Art diagnosis and treatment of hypertension in pregnancy. Mayo Clin. Proc. 93 (11), 1664–1677. doi:10.1016/j.mayocp.2018.04.033
Mah, G. T., Tejani, A. M., and Musini, V. M. (2009). Methyldopa for primary hypertension. Cochrane Database Syst. Rev. 2009 (4), Cd003893. doi:10.1002/14651858.CD003893.pub3
Manzur-Verástegui, S., Mandeville, P. B., Gordillo-Moscoso, A., and Rodriguez-MartinezM., (2008). Efficacy of nitroglycerine infusion versus sublingual nifedipine in severe pre-eclampsia: A randomized, triple-blind, controlled trial. Clin. Exp. Pharmacol. Physiol. 35 (5-6), 580–585. doi:10.1111/j.1440-1681.2007.04838.x
Moodley, J., and Gouws, E. (1992). A comparative study of the use of epoprostenol and dihydralazine in severe hypertension in pregnancy. Br. J. Obstet. Gynaecol. 99 (9), 727–730. doi:10.1111/j.1471-0528.1992.tb13872.x
Morris, R., Sunesara, I., Darby, M., Novotny, S., Kiprono, L., Bautista, L., et al. (2016). Impedance cardiography assessed treatment of acute severe pregnancy hypertension: A randomized trial. J. Matern. Fetal Neonatal Med. 29 (2), 171–176. doi:10.3109/14767058.2014.995081
Ott, C., and Schmieder, R. E. (2022). Diagnosis and treatment of arterial hypertension 2021. Kidney Int. 101 (1), 36–46. doi:10.1016/j.kint.2021.09.026
Papademetriou, V., Stavropoulos, K., Patoulias, D., Papadopoulos, C., Georgios, K., Toumpourleka, M., et al. (2021). Hypertension in pregnancy: Unanswered questions. Curr. Pharm. Des. 27 (36), 3795–3803. doi:10.2174/1381612827666210830091652
Pascoal, A. C. F., Katz, L., Pinto, M. H., Santos, C. A., Braga, L. C. O., Maia, S. B., et al. (2019). Serum magnesium levels during magnesium sulfate infusion at 1 gram/hour versus 2 grams/hour as a maintenance dose to prevent eclampsia in women with severe preeclampsia: A randomized clinical trial. Med. Baltim. 98 (32), e16779. doi:10.1097/MD.0000000000016779
Patel, P., Koli, D., Maitra, N., Sheth, T., and Vaishnav, P. (2018). Comparison of efficacy and safety of intravenous labetalol versus hydralazine for management of severe hypertension in pregnancy. J. Obstet. Gynaecol. India 68 (5), 376–381. doi:10.1007/s13224-017-1053-9
Peacock, W. Ft, Hilleman, D. E., Levy, P. D., Rhoney, D. H., and Varon, J. (2012). A systematic review of nicardipine vs labetalol for the management of hypertensive crises. Am. J. Emerg. Med. 30 (6), 981–993. doi:10.1016/j.ajem.2011.06.040
Ratnapalan, S., and Koren, G. (2002). Taking ACE inhibitors during pregnancy. Is it safe? Can. Fam. Physician 48, 1047–1049.
Reddy, S., and Jim, B. (2019). Hypertension and pregnancy: Management and future risks. Adv. Chronic Kidney Dis. 26 (2), 137–145. doi:10.1053/j.ackd.2019.03.017
Rezaei, Z., Sharbaf, F. R., Pourmojieb, M., Youefzadeh-Fard, Y., Motevalian, M., Khazaeipour, Z., et al. (2011). Comparison of the efficacy of nifedipine and hydralazine in hypertensive crisis in pregnancy. Acta Med. Iran. 49 (11), 701–706.
Roberts, J. M., Pearson, G., Cutler, J., and Lindheimer, M. (2003). Summary of the NHLBI working group on research on hypertension during pregnancy. Hypertension 41 (3), 437–445. doi:10.1161/01.HYP.0000054981.03589.E9
Rücker, G., and Schwarzer, G. (2015). Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 15, 58. doi:10.1186/s12874-015-0060-8
Sathya Lakshmi, B., and Dasari, P. (2012). Oral nifedipine versus intravenous labetalol in hypertensive urgencies and emergencies of pregnancy: A randomized clinical trial. Obstet. Med. 5 (4), 171–175. doi:10.1258/om.2012.120010
Sharma, C., Soni, A., Gupta, A., Verma, A., and Verma, S. (2017). Hydralazine vs nifedipine for acute hypertensive emergency in pregnancy: A randomized controlled trial. Am. J. Obstet. Gynecol. 217 (6), e1–e687. doi:10.1016/j.ajog.2017.08.018
Shekhar, S., Gupta, N., Kirubakaran, R., and Pareek, P. (2016). Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: A systematic review and meta-analysis. Bjog 123 (1), 40–47. doi:10.1111/1471-0528.13463
Shepherd, A. M., Irvine, N. A., Ludden, T. M., Lin, M. S., and McNay, J. L. (1984). Effect of oral dose size on hydralazine kinetics and vasodepressor response. Clin. Pharmacol. Ther. 36 (5), 595–600. doi:10.1038/clpt.1984.227
Shi, D. D., Yang, F. Z., Zhou, L., and WaNgN., (2016). Oral nifedipine vs. intravenous labetalol for treatment of pregnancy-induced severe pre-eclampsia. J. Clin. Pharm. Ther. 41 (6), 657–661. doi:10.1111/jcpt.12439
Shi, Q., Leng, W., Yao, Q., Mi, C., and Xing, A. (2015). Oral nifedipine versus intravenous labetalol for the treatment of severe hypertension in pregnancy. Int. J. Cardiol. 178, 162–164. doi:10.1016/j.ijcard.2014.10.111
Song, F., Xiong, T., Parekh-Bhurke, S., Loke, Y. K., Sutton, A. J., Eastwood, A. J., et al. (2011). Inconsistency between direct and indirect comparisons of competing interventions: meta-epidemiological study. Bmj 343, d4909. doi:10.1136/bmj.d4909
Sridharan, K., and Sequeira, R. P. (2018). Drugs for treating severe hypertension in pregnancy: A network meta-analysis and trial sequential analysis of randomized clinical trials. Br. J. Clin. Pharmacol. 84 (9), 1906–1916. doi:10.1111/bcp.13649
Sterne, J. A. C., Savović, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. Bmj 366, l4898. doi:10.1136/bmj.l4898
Steyn, D. W., and Odendaal, H. J. (1997). Dihydralazine or ketanserin for severe hypertension in pregnancy? Preliminary results. Eur. J. Obstet. Gynecol. Reprod. Biol. 75 (2), 155–159. doi:10.1016/s0301-2115(97)00123-1
Toppozada, M. K., Darwish, E., and Barakat, A. A. (1991). Management of severe preeclampsia detected in early labor by prostaglandin A1 or dihydralazine infusions. Am. J. Obstet. Gynecol. 164, 1229–1232. doi:10.1016/0002-9378(91)90688-n
Vigil-De Gracia, P., Lasso, M., Ruiz, E., Vega-Malek, J. C., de Mena, F. T., Lopez, J. C., et al. (2006). Severe hypertension in pregnancy: Hydralazine or labetalol. A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 128 (1-2), 157–162. doi:10.1016/j.ejogrb.2006.02.015
Wacker, J., Werner, P., Walter-Sack, I., and Bastert, G. (1998). Treatment of hypertension in patients with pre-eclampsia: A prospective parallel-group study comparing dihydralazine with urapidil. Nephrol. Dial. Transpl. 13 (2), 318–325. doi:10.1093/oxfordjournals.ndt.a027825
Wasim, T., Agha, S., Saeed, K., and Riaz, A. (2020). Oral nifidepine versus IV labetalol in severe preeclampsia: A randomized control trial. Pak J. Med. Sci. 36 (6), 1147–1152. doi:10.12669/pjms.36.6.2591
Wertaschnigg, D., Wang, R., Reddy, M., Costa, F. D. S., Mol, B. W. J., and Rolnik, D. L. (2020). Treatment of severe hypertension during pregnancy: We still do not know what the best option is. Hypertens. Pregnancy 39 (1), 25–32. doi:10.1080/10641955.2019.1708383
Wu, H. Z., Cheng, Y., Yu, D., Li, J. B., Jiang, Y. F., and Zhu, Z. N. (2022). Different dosage regimens of nifedipine, labetalol, and hydralazine for the treatment of severe hypertension during pregnancy: A network meta-analysis of randomized controlled trials. Hypertens. Pregnancy 41 (2), 126–138. doi:10.1080/10641955.2022.2056196
Wu, Y., Wang, D. J., Zhang, Y., and Zhang, R. (2020). Regulation of magnesium sulfate combined with nifedipine and labetalol on disease-related molecules in serum and placenta in the treatment of preeclampsia. Eur. Rev. Med. Pharmacol. Sci. 24 (9), 5062–5070. doi:10.26355/eurrev_202005_21199
Xiang, C., Zhou, X., and Zheng, X. (2020). Magnesium sulfate in combination with nifedipine in the treatment of pregnancy-induced hypertension. Pak J. Med. Sci. 36 (2), 21–25. doi:10.12669/pjms.36.2.706
Yin, J., Mei, Z., Shi, S., Du, P., and Qin, S. (2022). Nifedipine or amlodipine? The choice for hypertension during pregnancy: A systematic review and meta-analysis. Arch. Gynecol. Obstet. 306, 1891–1900. doi:10.1007/s00404-022-06504-5
Yin, X., and Yang, Z. (2021). Efficacy of nifedipine tablets plus aspirin in patients with gestational hypertension and the effect on coagulation function and hemorheology. Am. J. Transl. Res. 13 (6), 7059–7064.
Yu, X., and Zhou, Q. (2022). Effects of nifedipine tablets combined with magnesium sulfate on blood coagulation index, oxidative stress, NO and ET-1 levels in patients with pregnancy hypertension. Front. Surg. 9, 862676. doi:10.3389/fsurg.2022.862676
Zhang, F., Duan, B., Liu, Y., and Wang, C. (2021). Efficacy of aspirin combined with labetalol on gestational hypertension and effect on serum PAPP-A, APN and HMGB1. Am. J. Transl. Res. 13 (12), 13750–13758.
Zhao, F., Ai, F., Wu, J., and Dong, X. (2020). Changes and clinical significance of serum inflammatory factors in the treatment of pregnancy hypertension syndrome with magnesium sulfate combined with nifedipine. Exp. Ther. Med. 20 (2), 1796–1802. doi:10.3892/etm.2020.8863
Keywords: hypertension during pregnancy, pharmaceutical administration, target blood pressure, systolic blood pressure, diastolic blood pressure
Citation: Deng N-J, Xian-Yu C-Y, Han R-Z, Huang C-Y, Ma Y-T, Li H-J, Gao T-Y, Liu X and Zhang C (2023) Pharmaceutical administration for severe hypertension during pregnancy: Network meta-analysis. Front. Pharmacol. 13:1092501. doi: 10.3389/fphar.2022.1092501
Received: 08 November 2022; Accepted: 27 December 2022;
Published: 09 January 2023.
Edited by:
Jessica K. Roberts, Cognigen, United StatesReviewed by:
Fernanda Regina Giachini, Federal University of Mato Grosso, BrazilTomohiro Nishimura, Keio University, Japan
Copyright © 2023 Deng, Xian-Yu, Han, Huang, Ma, Li, Gao, Liu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chao Zhang, zhangchao0803@126.com
 Nian-Jia Deng1
Nian-Jia Deng1 Rui-Zheng Han
Rui-Zheng Han Cheng-Yang Huang
Cheng-Yang Huang Teng-Yu Gao
Teng-Yu Gao Chao Zhang
Chao Zhang