- 1Wellcome Trust/CRUK Gurdon Institute and Department Physiology, Development and Neuroscience, University of Cambridge, Cambridge, United Kingdom
- 2Department of Molecular Oncology and Immunology, The Netherlands Cancer Institute, Amsterdam, Netherlands
- 3Cancer Evolution and Genome Instability Laboratory, The Francis Crick Institute, London, United Kingdom
- 4Epithelial Cell Biology in ENT Research (EpiCENTR) Group, Developmental Biology and Cancer Department, Great Ormond Street UCL Institute of Child Health, University College London, London, United Kingdom
- 5CRUK Lung Cancer Centre of Excellence, UCL Cancer Institute, University College London, London, United Kingdom
Organoids have become a prominent model system in pulmonary research. The ability to establish organoid cultures directly from patient tissue has expanded the repertoire of physiologically relevant preclinical model systems. In addition to their derivation from adult lung stem/progenitor cells, lung organoids can be derived from fetal tissue or induced pluripotent stem cells to fill a critical gap in modelling pulmonary development in vitro. Recent years have seen important progress in the characterisation and refinement of organoid culture systems. Here, we address several open questions in the field, including how closely organoids recapitulate the tissue of origin, how well organoids recapitulate patient cohorts, and how well organoids capture diversity within a patient. We advocate deeper characterisation of models using single cell technologies, generation of more diverse organoid biobanks and further standardisation of culture media.
1 Introduction
Organoid cultures are in vitro models derived from stem/progenitor cells, and involve the generation of a heterocellular structure that is reminiscent of the tissue of origin in a three-dimensional cell culture environment (Barkauskas et al., 2017; Liberti and Morrisey, 2021; Sen et al., 2022). In the human respiratory system, nasal (Liu et al., 2020; Rodenburg et al., 2022), tracheobronchial (Rock et al., 2009; Sachs et al., 2019), small airway (Basil et al., 2022) and alveolar (Katsura et al., 2020; Salahudeen et al., 2020; Youk et al., 2020) epithelial organoid models have all been generated from post-natal tissue-resident stem cells. Additionally, organoid cultures derived from developing lung epithelia have been described (Nikolić et al., 2017; Miller et al., 2018), and the stepwise differentiation of pluripotent stem cells has been used to derive mature lung organoids and those resembling developmental intermediates (Jacob et al., 2017; Hawkins et al., 2021; Hein et al., 2022). In addition to providing a platform to study lung stem cell biology, organoids present a platform to investigate respiratory diseases, including developmental disorders such as bronchopulmonary dysplasia (Riccetti et al., 2022), genetic disorders such as cystic fibrosis (Sachs et al., 2019) and ciliary dyskinesias (van der Vaart et al., 2021a), chronic respiratory diseases such as chronic obstructive pulmonary disease (COPD) (Ng-Blichfeldt et al., 2018) and lung cancers (Sachs et al., 2019).
Recent, comprehensive reviews are available on advances in methods to derive lung organoids, and the potential uses of organoids in basic research and translational medicine (Evans and Lee, 2020; van der Vaart et al., 2021b; Liberti and Morrisey, 2021; Sen et al., 2022). While lung organoids can also be derived from mouse lung epithelia, offering an opportunity to address some research questions in vitro and reduce the use of animals used in model species research, we will focus on pertinent open questions within the human lung organoid field with relevance across developmental, post-natal stem cell and cancer studies.
2 How closely do lung organoids recapitulate their tissue of origin?
To increase the likelihood of relevant findings, it is crucial that organoids mimic the tissue of interest as closely as possible. To this end, the development of multiple organoids to capture the diversity of cells within developing lungs, adult homeostatic lungs and tumours has been ongoing. It is important to note that in developmental studies, organoids are typically derived from progenitor cells that are capable of differentiation into different tissue types (e.g., airway and alveolar), while adult lung organoids are typically region-specific with less plasticity.
Developmental studies currently tend to favour the use of induced pluripotent stem cells (iPSCs) due to issues of scalability, availability and the fact that iPSCs represent a better-defined starting population than those available from foetal tissue. Customised media have been developed to expand specific cellular subpopulations from iPSCs; in the airways, basal cells and secretory progenitor cells have all been induced from iPSCs (McCauley et al., 2017; McCauley et al., 2018; Hawkins et al., 2021) and, when cultured at an air-liquid interface, goblet cells and ciliated cells have also been generated from iPSCs (Firth et al., 2014; McCauley et al., 2017). For alveolar modelling, iPSCs and human embryonic stem cells (hESCs) have been used to derive AT2 cells (Jacob et al., 2017; 2019; Tamò et al., 2018; Hurley et al., 2020), which can exhibit relevant morphological characteristics, such as lamellar bodies and microvilli. However, although some iPSC-derived AT2 organoids are stable in long-term culture, there are reported stability issues with cells reverting to non-lung fates.
At the histological level, airway and lung cancer organoids can resemble their in vivo tissue of origin remarkably well, despite the absence of stroma in vitro (Kim et al., 2019; Sachs et al., 2019; Li et al., 2020). Airway organoids contain all of the major airway epithelial cell types, with basal cells, mucosecretory cells and ciliated cells present (Rock et al., 2009; Sachs et al., 2019), although the consistency with which they contain rare populations such as neuroendocrine cells, ionocytes and tuft cells is unclear. Removing extracellular matrix cues can generate organoids with cilia on their outer surface, which has clear advantages for studies of motile cilia (Wijesekara et al., 2022). Media composition appears to be vital in the selection of airway vs. alveolar cells from adult lung tissue, as studies using distal lung resection tissue for organoid generation have led to airway organoid formation but an absence of alveolar organoids (Sachs et al., 2019), in contrast to earlier mouse lung organoid studies in which both organoid types were observed within the same cultures (Lee et al., 2014). The resemblance of alveolar organoids to the alveolus is more limited given the dependence of this tissue on precise architecture. Alveolar organoids typically consist of proliferative AT2-like cells but lack AT1-like cells, though cells resembling AT1 cells can be induced by 2D culture (Youk et al., 2020), suspension culture (Salahudeen et al., 2020) or using inductive culture media (Katsura et al., 2020; Konishi et al., 2022).
Although progenitor differentiation to mature adult cell types is a common goal, the lack of complete lineage characterisation hinders our ability to fully distinguish these differentiated cell types. At present, many iPSC studies accomplish progenitor specification but are not able to fully substantiate claims of mature cells. Marker protein expression remains the most common means to identify differentiated cell types within organoids, which is not in itself sufficient to demonstrate full maturity or functionality. Additional confusion arises when mouse markers are extrapolated to human lung cell types as, particularly in the developing lung, numerous distinct cell types can share marker expression (Miller et al., 2020; He et al., 2022) and marker expression can be transient (McCauley et al., 2018). To overcome this, recent studies have combined marker expression data with spatial information, transcriptomic analysis and organelle characterisation (Dye et al., 2015; Miller et al., 2019; 2020; Sachs et al., 2019; Hurley et al., 2020; Hein et al., 2022). The derivation of organoids that capture the intermediary stages of lung development has also been explored. Bud tip progenitors can be derived from iPSCs, which in turn have the potential to differentiate into all lung epithelial cell types (Hein et al., 2022). iPSCs were also used to culture a progenitor population enriched in basal cells that were reminiscent of those from foetal lungs (Ngan et al., 2021).
The increase in single-cell and bulk RNA sequencing studies of human lung is allowing greater benchmarking of organoid systems and comparisons of iPSC and embryonic tissue-derived cells (Ngan et al., 2021; Alysandratos et al., 2022; He et al., 2022; Murthy et al., 2022). Although cell culture often produces less robust and less mature differentiated cells, iPSC-derived organoids frequently produce more immature cell types than hESC-derived organoids, citing the need for additional validation in these studies, such as through lineage tracing or trajectory analysis (Hurley et al., 2020; Hawkins et al., 2021). In protocols that highlight stepwise differentiation and intermediary stages, the order of differentiation should be carefully analysed, such as by visualising differentiating structures, ensuring proximal-distal cell patterning has been conserved, and/or by matching with corresponding in vivo sequencing data. This level of detail allows for a sequence of key developmental milestones and transitional states to be uncovered, and potentially better recapitulation of human foetal lung development (He et al., 2022). A major limitation across lung organoid studies is the lack of direct comparison to the in vivo tissue, and as yet, the field has not created a standardised protocol for definitively assessing cell maturity and identity. Additionally, there is a large amount of variation between iPSC lines, with some unable to generate mature lung cells.
In contrast to developmental and adult lung organoid studies, the resemblance of cancer organoids to their tumours of origin has been most extensively studied at the genomic level. There is a relatively high concordance (± 80%) of single nucleotide variants (SNVs) and copy number alterations (CNAs). Although whole exome sequencing has been performed in some studies (Kim et al., 2019; 2021; Shi et al., 2020; Hu et al., 2021), others have investigated a limited set of cancer driver genes (Sachs et al., 2019; Chen et al., 2020; Li et al., 2020). Since targeted approaches tend to target clonal mutations (those that are found in every cancer cell), they identify mutations that are less susceptible to loss during organoid culture. Whether lung cancer organoids recapitulate the full spectrum of (subclonal) cancer mutations is not yet clear; while on average the genetic concordance between organoids and tumour tissue is high, for some samples the concordance is considerably lower (Kim et al., 2019; Shi et al., 2020), which most likely reflects the selective outgrowth of minor tumour subclones in those organoid cultures.
The extent to which lung cancer organoids diverge from tumour tissues at the phenotypic level is much less clear. Since organoid culture systems were originally developed to expand epithelial stem cells (Sato et al., 2009), it is unclear if lung cancer organoids can reflect tumour cells with different degrees of differentiation. Organoids established from distinct histological subtypes retain characteristic features, such as a cystic morphology for organoids from acinar adenocarcinoma, and a solid morphology for organoids from the solid subtype (Li et al., 2020). This suggests that tumour histology is at least in part shaped by tumour-intrinsic factors and independent of the tumour microenvironment (TME). The few reports of transcriptomic comparisons of lung cancer organoids and primary tissue are limited to overall concordance rates, which is (as expected) lower than genomic similarity, partially due to the absence of TME pathways (Shi et al., 2020; Hu et al., 2021). The absence of the TME might have profound effects on organoid phenotype and drug responses, as has been shown for pancreatic adenocarcinoma organoids (Raghavan et al., 2021). A more thorough transcriptomic and proteomic comparison of organoids and tumour tissues is needed to identify where divergence occurs. While organoids can converge upon culture specific transcriptional states, these can be modified by adding “missing” paracrine signals from the TME to the media (Raghavan et al., 2021). Alternatively, the TME can be partly reconstituted by adding back cell types of interest, such as lymphocytes or fibroblasts (reviewed in (Fiorini et al., 2020)).
The majority of lung organoid cultures are currently performed as mono (epithelial) cultures, although investigations of epithelial-fibroblast (Tan et al., 2019) or epithelial-macrophage (Iakobachvili et al., 2022) interactions have been possible. Epithelial-mesenchymal organoids have been developed from both embryonic cell lines and iPSCs, which contained basal cells, immature ciliated cells, smooth muscle and myofibroblasts (Dye et al., 2015). Additionally, iPSC differentiation generated organoids that contained bronchi-like structures surrounded by mesenchyme, and cells expressing alveolar markers (Miller et al., 2019). Multi-organ systems have also begun to be developed; iPSCs can be differentiated into cardiac and lung epithelial lineages to establish cardio-pulmonary micro-tissues (Ng et al., 2022).
While we advocate a better phenotypic characterisation of lung organoids to investigate their resemblance to lung tissue, it must be emphasised that organoids will always remain a reductionist model. The generalisation or translational relevance of results obtained in this culture system is best validated using orthogonal model systems (e.g., mouse models, 2D primary cultures, precision-cut lung slices) or datasets (including publicly available omics datasets). Moreover, it is not possible to reproduce the complete in vivo complexity of epithelial-stromal or epithelial-immune interactions, nor systemic variables such as nutrient availability, mechanical forces and circadian rhythms.
3 How well do organoid cultures represent patient cohorts?
A second important aspect of organoid modelling is the extent to which it is possible to capture the biology of the target population. Primary human bronchial epithelial cell cultures can show wide inter-patient variability in differentiation potential and response to stimulation, and so studying sufficient numbers of patient cultures is crucial, particularly when studying phenomena that are likely to vary with biological characteristics of the donor, such as age and/or sex (Peretz et al., 2016; Maughan et al., 2022). At present, organoid studies typically investigate cells isolated from small numbers of human donors and these cultures are rarely common between investigations from independent laboratories, due to limitations around access to human material, restrictions imposed by ethical approvals, and administrative and practical difficulties in sharing tissue or cultures within and between countries.
Organoids have been derived from patients across a wide range of respiratory diseases, including pulmonary fibrosis (Surolia et al., 2019), primary ciliary dyskinesia (van der Vaart et al., 2021a) and cystic fibrosis (Sachs et al., 2019). An extensive organoid collection from 664 cystic fibrosis patients has been reported (Geurts et al., 2020) meaning that models are now available for a wide range of causative mutations; however, these are rectal rather than lung organoids, since airway organoid forskolin-induced swelling is highly variable and limited to well-differentiated organoids. However, in chronic respiratory diseases the use of patient-derived organoids has been limited, likely due to a combination of the difficulty in obtaining material from patients with COPD and pulmonary fibrosis, the invasive nature of procedures to obtain samples, and the intrinsic lower potential of cells from these patients to generate organoid cultures (Ghosh et al., 2018). The extent to which organoid collections capture the diversity within patient cohorts will be disease-specific and depend on factors such as the opportunity to obtain research biopsies (clinically, but also geographically), the stage at which disease is most commonly diagnosed, and/or the diversity of disease phenotypes. iPSC technology might help to improve the range of chronic respiratory disease organoid models available, although pluripotent cell derived-organoids can have immature phenotypes, resembling fetal, rather than adult, transcriptional profiles. Indeed, a recent study of non-alcoholic fatty liver disease pooled iPSC-derived progenitors cells from 24 genotyped donors and derived mixed donor organoid cultures, before inducing a disease-relevant phenotype to investigate phenotype-genotype interactions (Kimura et al., 2022), suggesting that in the future lung organoid models may also have a role in identifying risk factors in addition to studying disease pathogenesis.
To date, lung cancer research has benefited most from organoid-based disease modelling. As many hundreds of organoid lines have been developed by multiple laboratories worldwide, it is possible to assess the extent to which they reflect non-small cell lung cancer (NSCLC) patient cohorts. Surprisingly, organoid establishment rates are similar for lung squamous cell carcinomas or adenocarcinomas (Kim et al., 2019; Shi et al., 2020; Hu et al., 2021), despite their distinct cells of origin, divergent driver gene landscapes, and the use of one medium composition across histological subtypes. Organoids for diverse histological subtypes (e.g., acinar, lepidic, or solid adenocarcinomas) (Kim et al., 2019; Li et al., 2020) have been reported, although a systematic comparison of organoid establishment across subtypes is lacking. It is likely that current approaches are biased towards establishing organoids from certain tumour phenotypes and/or genotypes. Moreover, each of these studies differ in patient cohorts, the tissue digestion protocol used, medium formulation, and criteria of what is considered a successful culture, making it challenging to determine important contributors to successful cultures and resulting in organoid establishment rates of between 15% and 80% (Ma et al., 2022). The field would benefit from standardisation, including systematic comparisons of different protocols. Given the heterogeneity of lung cancer there may not be a “one size fits” medium formulation, and the choice of medium could be guided by driver gene status or features of the tumour TME (Fujii et al., 2016). Larger studies should explore any transcriptional or genomic features that are associated with culture success. Given the heterogeneity of lung cancer, this will likely be best achieved through large-scale collaborative efforts.
To date, most studies have generated lung cancer organoids from surgical resections of primary tumours. To our knowledge, no organoid models have been generated from pre-invasive disease, but pre-cancer organoids would be particularly valuable to study events in early tumourigenesis. Since most lung cancer patients die from metastatic disease, representing these in organoid collections would be valuable. One study established organoids from malignant effusions from patients with advanced lung cancer with a high success rate (Kim et al., 2021), suggesting that establishment rates from metastatic disease could be higher than for early stage disease. Organoid establishment from extrapulmonary metastases can also avoid contamination with normal airway organoids (Sachs et al., 2019; Dijkstra et al., 2020).
An alternative approach to deriving organoids from lung disease patient cohorts, is to use the available model systems to investigate specific hypotheses related to the disease process. In this regard, pluripotent cell-derived organoids have proved useful pulmonary fibrosis models. Introducing Hermansky-Pudlak syndrome (HPS) mutations associated with interstitial pneumonia using CRISPR-Cas9 promotes fibrotic changes in ES-derived lung organoids (Strikoudis et al., 2019) or iPSCs from patients (Korogi et al., 2019), while treatment of iPSC-derived AT2 organoids with bleomycin also promotes fibrotic changes (Suezawa et al., 2021). Moreover, early disease models can be generated by recapitulating aspects of disease development in lung organoids. For example, insights into pulmonary fibrosis have arisen from treating cells with TGFb (Ng-Blichfeldt et al., 2019). These approaches, akin to the sequential introduction of cancer driver mutations into normal organoid cultures to study early events in tumourigenesis (Dost et al., 2020), have the potential to reveal new insights compared to organoids from established disease.
4 To what extent does an organoid culture capture diversity within a patient?
Intra-patient heterogeneity likely represents an understudied source of variation in lung organoid research. At present, authors typically report the diagnosis of the patient from whom organoids were derived and the tissue of origin. However, airway epithelial cell phenotype is known to vary in the proximal-distal axis within normal lungs with cells from the upper airways having distinct transcriptomic profiles (Deprez et al., 2020; Hou et al., 2020), innate immune defences (Mihaylova et al., 2018), and susceptibility to infection (Hou et al., 2020), compared to those from the lower airways. As an example, organoid origins (proximal versus distal, as well as pluripotent versus adult) might partially explain the divergent cell types that have been seen to be infected with SARS-CoV-2 in lung organoid studies (Han et al., 2022). Moreover, patients with chronic respiratory diseases might display additional heterogeneity, particularly as IPF distal airway epithelial cells respond differently in cell culture to proximal epithelial cells from the same patient (Stancil et al., 2021). Since pulmonary fibrosis is characterised by upper lobe emphysema and lower lobe fibrosis, the environment that cells experience even within the same lung varies and might influence their subsequent behaviour. As such, the precise location of a biopsy is likely to be consequential for experimental reproducibility.
Lung cancers are genetically and phenotypically heterogeneous, but the extent to which this is maintained in lung cancer organoid cultures is so far poorly characterised. Given the variable establishment rates of lung cancer organoids, bottlenecks are expected that restrict heterogeneity in vitro. Indeed, one study reported a low correlation of variant allele frequencies (VAFs) between organoids and original tumours in approximately half of the samples analysed (Kim et al., 2019). Evidence for selective subclonal outgrowth has also been observed in bladder (Lee et al., 2018) and colorectal cancer organoids (van de Wetering et al., 2015). This could be due to sampling bias, selective clonal outgrowth, or tumour evolution driven by ongoing genetic instability. While the genetic landscape of organoids is relatively stable during long-term culture (Kim et al., 2019), there is evidence for ongoing genetic instability and clonal selection from single cell sequencing and barcoding studies (Bolhaqueiro et al., 2019; Karlsson et al., 2022; Kester et al., 2022). The establishment of organoids from separate tumour regions, possibly combined with the generation of clonal organoid lines at early passage, could prevent the loss of minor subclones (Fujii et al., 2016; Roerink et al., 2018). Evaluating the extent to which genetic heterogeneity is preserved requires sequencing organoids and tumour tissues at sufficient coverage to detect subclonal mutations.
5 Conclusion
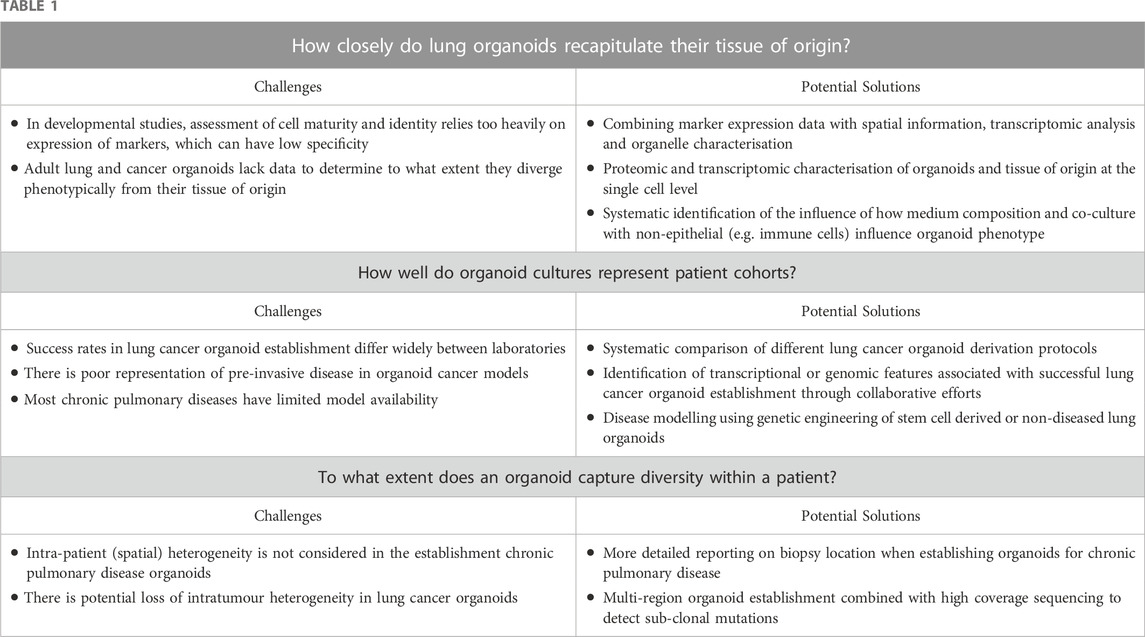
Lung organoids provide a cell culture platform to study lung development, stem/progenitor cell biology and disease pathogenesis. Several open questions remain, however, concerning their ability to recapitulate the tissue of origin and in vivo processes, the ability of organoid collections to accurately reflect population level inter-individual variability and intra-patient heterogeneity (Table 1). We advocate for deeper characterisation of organoids alongside the tissue of origin, for the generation of more diverse organoid biobanks and for greater standardisation of culture media and conditions between laboratories.
Author contributions
TH, KD, and RH planned and wrote the draft manuscript. ER reviewed and edited the manuscript.
Funding
TH is supported by funding from the Biotechnology and Biological Sciences Research Council Doctoral Training Partnership at the University of Cambridge, under award No. 2114230. KD was supported by funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 101024529. ER is supported by the Medical Research Council (MR/P009581/1). RH is an NIHR Great Ormond Street Hospital Biomedical Research Centre Collaborative Catalyst Fellow and receives additional research funding from the CRUK Lung Cancer Centre of Excellence, GOSH Charity and DEBRA International. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. This work was supported by the Francis Crick Institute which receives its core funding from Cancer Research UK (CC2041), the UK Medical Research Council (CC2041), and the Wellcome Trust (CC2041).
Conflict of interest
KD provides consultancy services to Achilles Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Alysandratos, K.-D., de Alba Rivas, C. G., Yao, C., Pessina, P., Villacorta-Martin, C., Huang, J., et al. (2022). Impact of cell culture on the transcriptomic programs of primary and iPSC-derived human alveolar type 2 cells. bioRxiv. doi:10.1101/2022.02.08.479591
Barkauskas, C. E., Chung, M.-I., Fioret, B., Gao, X., Katsura, H., and Hogan, B. L. M. (2017). Lung organoids: Current uses and future promise. Development 144, 986–997. doi:10.1242/dev.140103
Basil, M. C., Cardenas-Diaz, F. L., Kathiriya, J. J., Morley, M. P., Carl, J., Brumwell, A. N., et al. (2022). Human distal airways contain a multipotent secretory cell that can regenerate alveoli. Nature 604, 120–126. doi:10.1038/s41586-022-04552-0
Bolhaqueiro, A. C. F., Ponsioen, B., Bakker, B., Klaasen, S. J., Kucukkose, E., van Jaarsveld, R. H., et al. (2019). Ongoing chromosomal instability and karyotype evolution in human colorectal cancer organoids. Nat. Genet. 51, 824–834. doi:10.1038/s41588-019-0399-6
Chen, J.-H., Chu, X.-P., Zhang, J.-T., Nie, Q., Tang, W.-F., Su, J., et al. (2020). Genomic characteristics and drug screening among organoids derived from non-small cell lung cancer patients. Thorac. Cancer 11, 2279–2290. doi:10.1111/1759-7714.13542
Deprez, M., Zaragosi, L.-E., Truchi, M., Becavin, C., Ruiz García, S., Arguel, M.-J., et al. (2020). A single-cell atlas of the human healthy airways. Am. J. Respir. Crit. Care Med. 202, 1636–1645. doi:10.1164/rccm.201911-2199OC
Dijkstra, K. K., Monkhorst, K., Schipper, L. J., Hartemink, K. J., Smit, E. F., Kaing, S., et al. (2020). Challenges in establishing pure lung cancer organoids limit their utility for personalized medicine. Cell Rep. 31, 107588. doi:10.1016/j.celrep.2020.107588
Dost, A. F. M., Moye, A. L., Vedaie, M., Tran, L. M., Fung, E., Heinze, D., et al. (2020). Organoids model transcriptional hallmarks of oncogenic KRAS activation in lung epithelial progenitor cells. Cell Stem Cell 27, 663–678. e8. doi:10.1016/j.stem.2020.07.022
Dye, B. R., Hill, D. R., Ferguson, M. A. H., Tsai, Y.-H., Nagy, M. S., Dyal, R., et al. (2015). In vitro generation of human pluripotent stem cell derived lung organoids. Elife 4, e05098. doi:10.7554/eLife.05098
Evans, K. V., and Lee, J.-H. (2020). Alveolar wars: The rise of in vitro models to understand human lung alveolar maintenance, regeneration, and disease. Stem Cells Transl. Med. 9, 867–881. doi:10.1002/sctm.19-0433
Fiorini, E., Veghini, L., and Corbo, V. (2020). Modeling cell communication in cancer with organoids: Making the complex simple. Front. Cell Dev. Biol. 8, 166. doi:10.3389/fcell.2020.00166
Firth, A. L., Dargitz, C. T., Qualls, S. J., Menon, T., Wright, R., Singer, O., et al. (2014). Generation of multiciliated cells in functional airway epithelia from human induced pluripotent stem cells. Proc. Natl. Acad. Sci. U. S. A. 111, E1723–E1730. doi:10.1073/pnas.1403470111
Fujii, M., Shimokawa, M., Date, S., Takano, A., Matano, M., Nanki, K., et al. (2016). A colorectal tumor organoid library demonstrates progressive loss of niche factor requirements during tumorigenesis. Cell Stem Cell 18, 827–838. doi:10.1016/j.stem.2016.04.003
Geurts, M. H., de Poel, E., Amatngalim, G. D., Oka, R., Meijers, F. M., Kruisselbrink, E., et al. (2020). CRISPR-based adenine editors correct nonsense mutations in a cystic fibrosis organoid biobank. Cell Stem Cell 26, 503–510. e7. doi:10.1016/j.stem.2020.01.019
Ghosh, M., Miller, Y. E., Nakachi, I., Kwon, J. B., Barón, A. E., Brantley, A. E., et al. (2018). Exhaustion of airway basal progenitor cells in early and established chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 197, 885–896. doi:10.1164/rccm.201704-0667OC
Han, Y., Yang, L., Lacko, L. A., and Chen, S. (2022). Human organoid models to study SARS-CoV-2 infection. Nat. Methods 19, 418–428. doi:10.1038/s41592-022-01453-y
Hawkins, F. J., Suzuki, S., Beermann, M. L., Barillà, C., Wang, R., Villacorta-Martin, C., et al. 2021). Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell 28, 79–95.e8. e8. doi:10.1016/j.stem.2020.09.017
He, P., Lim, K., Sun, D., Pett, J. P., Jeng, Q., Polanski, K., et al. (2022). A human fetal lung cell atlas uncovers proximal-distal gradients of differentiation and key regulators of epithelial fates. bioRxiv, doi:10.1101/2022.01.11.474933
Hein, R. F. C., Conchola, A. S., Fine, A. S., Xiao, Z., Frum, T., Brastrom, L. K., et al. (2022). Stable iPSC-derived NKX2-1+ lung bud tip progenitor organoids give rise to airway and alveolar cell types. Development 149, dev200693. doi:10.1242/dev.200693
Hou, Y. J., Okuda, K., Edwards, C. E., Martinez, D. R., Asakura, T., Dinnon, K. H., et al. (2020). SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell 182, 429–446. e14. doi:10.1016/j.cell.2020.05.042
Hu, Y., Sui, X., Song, F., Li, Y., Li, K., Chen, Z., et al. (2021). Lung cancer organoids analyzed on microwell arrays predict drug responses of patients within a week. Nat. Commun. 12, 2581. doi:10.1038/s41467-021-22676-1
Hurley, K., Ding, J., Villacorta-Martin, C., Herriges, M. J., Jacob, A., Vedaie, M., et al. (2020). Reconstructed single-cell fate trajectories define lineage plasticity windows during differentiation of human PSC-derived distal lung progenitors. Cell Stem Cell 26, 593–608. e8. doi:10.1016/j.stem.2019.12.009
Iakobachvili, N., Leon-Icaza, S. A., Knoops, K., Sachs, N., Mazères, S., Simeone, R., et al. (2022). Mycobacteria-host interactions in human bronchiolar airway organoids. Mol. Microbiol. 117, 682–692. doi:10.1111/mmi.14824
Jacob, A., Morley, M., Hawkins, F., McCauley, K. B., Jean, J. C., Heins, H., et al. (2017). Differentiation of human pluripotent stem cells into functional lung alveolar epithelial cells. Cell Stem Cell 21, 472–488. e10. doi:10.1016/j.stem.2017.08.014
Jacob, A., Vedaie, M., Roberts, D. A., Thomas, D. C., Villacorta-Martin, C., Alysandratos, K.-D., et al. (2019). Derivation of self-renewing lung alveolar epithelial type II cells from human pluripotent stem cells. Nat. Protoc. 14, 3303–3332. doi:10.1038/s41596-019-0220-0
Karlsson, K., Przybilla, M., Xu, H., Kotler, E., Karagyozova, K., Sockell, A., et al. (2022). Experimental evolution in TP53 deficient human gastric organoids recapitulates tumorigenesis. bioRxiv. doi:10.1101/2022.04.09.487529
Katsura, H., Sontake, V., Tata, A., Kobayashi, Y., Edwards, C. E., Heaton, B. E., et al. (2020). Human lung stem cell-based alveolospheres provide insights into SARS-CoV-2-mediated interferon responses and pneumocyte dysfunction. Cell Stem Cell 27, 890–904. e8. doi:10.1016/j.stem.2020.10.005
Kester, L., de Barbanson, B., Lyubimova, A., Chen, L.-T., van der Schrier, V., Alemany, A., et al. (2022). Integration of multiple lineage measurements from the same cell reconstructs parallel tumor evolution. Cell Genomics 2, 100096. doi:10.1016/j.xgen.2022.100096
Kim, M., Mun, H., Sung, C. O., Cho, E. J., Jeon, H.-J., Chun, S.-M., et al. (2019). Patient-derived lung cancer organoids as in vitro cancer models for therapeutic screening. Nat. Commun. 10, 3991. doi:10.1038/s41467-019-11867-6
Kim, S.-Y., Kim, S.-M., Lim, S., Lee, J. Y., Choi, S.-J., Yang, S.-D., et al. (2021). Modeling clinical responses to targeted therapies by patient-derived organoids of advanced lung adenocarcinoma. Clin. Cancer Res. 27, 4397–4409. doi:10.1158/1078-0432.CCR-20-5026
Kimura, M., Iguchi, T., Iwasawa, K., Dunn, A., Thompson, W. L., Yoneyama, Y., et al. (2022). En masse organoid phenotyping informs metabolic-associated genetic susceptibility to NASH. Cell 185, 4216–4232.e16. doi:10.1016/j.cell.2022.09.031
Konishi, S., Tata, A., and Tata, P. R. (2022). Defined conditions for long-term expansion of murine and human alveolar epithelial stem cells in three-dimensional cultures. Star. Protoc. 3, 101447. doi:10.1016/j.xpro.2022.101447
Korogi, Y., Gotoh, S., Ikeo, S., Yamamoto, Y., Sone, N., Tamai, K., et al. (2019). In vitro disease modeling of hermansky-pudlak syndrome type 2 using human induced pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 13, 235. doi:10.1016/j.stemcr.2019.05.022
Lee, J.-H., Bhang, D. H., Beede, A., Huang, T. L., Stripp, B. R., Bloch, K. D., et al. (2014). Lung stem cell differentiation in mice directed by endothelial cells via a BMP4-NFATc1-thrombospondin-1 axis. Cell 156, 440–455. doi:10.1016/j.cell.2013.12.039
Lee, S. H., Hu, W., Matulay, J. T., Silva, M. V., Owczarek, T. B., Kim, K., et al. (2018). Tumor evolution and drug response in patient-derived organoid models of bladder cancer. Cell 173, 515–528. e17. doi:10.1016/j.cell.2018.03.017
Li, Z., Qian, Y., Li, W., Liu, L., Yu, L., Liu, X., et al. (2020). Human lung adenocarcinoma-derived organoid models for drug screening. iScience 23, 101411. doi:10.1016/j.isci.2020.101411
Liberti, D. C., and Morrisey, E. E. (2021). Organoid models: Assessing lung cell fate decisions and disease responses. Trends Mol. Med. 27, 1159–1174. doi:10.1016/j.molmed.2021.09.008
Liu, Z., Anderson, J. D., Deng, L., Mackay, S., Bailey, J., Kersh, L., et al. (2020). Human nasal epithelial organoids for therapeutic development in cystic fibrosis. Genes 11, 603. doi:10.3390/genes11060603
Ma, H.-C., Zhu, Y.-J., Zhou, R., Yu, Y.-Y., Xiao, Z.-Z., and Zhang, H.-B. (2022). Lung cancer organoids, a promising model still with long way to go. Crit. Rev. Oncol. Hematol. 171, 103610. doi:10.1016/j.critrevonc.2022.103610
Maughan, E. F., Hynds, R. E., Pennycuick, A., Nigro, E., Gowers, K. H. C., Denais, C., et al. (2022). Cell-intrinsic differences between human airway epithelial cells from children and adults. iScience 25, 105409. doi:10.1016/j.isci.2022.105409
McCauley, K. B., Alysandratos, K.-D., Jacob, A., Hawkins, F., Caballero, I. S., Vedaie, M., et al. (2018). Single-cell transcriptomic profiling of pluripotent stem cell-derived SCGB3A2+ airway epithelium. Stem Cell Rep. 10, 1579–1595. doi:10.1016/j.stemcr.2018.03.013
McCauley, K. B., Hawkins, F., Serra, M., Thomas, D. C., Jacob, A., and Kotton, D. N. (2017). Efficient derivation of functional human airway epithelium from pluripotent stem cells via temporal regulation of wnt signaling. Cell Stem Cell 20, 844–857. e6. doi:10.1016/j.stem.2017.03.001
Mihaylova, V. T., Kong, Y., Fedorova, O., Sharma, L., Dela Cruz, C. S., Pyle, A. M., et al. (2018). Regional differences in airway epithelial cells reveal tradeoff between defense against oxidative stress and defense against rhinovirus. Cell Rep. 24, 3000–3007. e3. doi:10.1016/j.celrep.2018.08.033
Miller, A. J., Dye, B. R., Ferrer-Torres, D., Hill, D. R., Overeem, A. W., Shea, L. D., et al. (2019). Generation of lung organoids from human pluripotent stem cells in vitro. Nat. Protoc. 14, 518–540. doi:10.1038/s41596-018-0104-8
Miller, A. J., Hill, D. R., Nagy, M. S., Aoki, Y., Dye, B. R., Chin, A. M., et al. (2018). In vitro induction and in vivo engraftment of lung bud tip progenitor cells derived from human pluripotent stem cells. Stem Cell Rep. 10, 101–119. doi:10.1016/j.stemcr.2017.11.012
Miller, A. J., Yu, Q., Czerwinski, M., Tsai, Y.-H., Conway, R. F., Wu, A., et al. (2020). In vitro and in vivo development of the human airway at single-cell resolution. Dev. Cell 53, 117–128. doi:10.1016/j.devcel.2020.01.033
Murthy, P. K. L., Sontake, V., Tata, A., Kobayashi, Y., Macadlo, L., Okuda, K., et al. (2022). Human distal lung maps and lineage hierarchies reveal a bipotent progenitor. Nature 604, 111–119. doi:10.1038/s41586-022-04541-3
Ng-Blichfeldt, J.-P., de Jong, T., Kortekaas, R. K., Wu, X., Lindner, M., Guryev, V., et al. (2019). TGF-β activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am. J. Physiol. Lung Cell. Mol. Physiol. 317, L14–L28. doi:10.1152/ajplung.00400.2018
Ng-Blichfeldt, J.-P., Schrik, A., Kortekaas, R. K., Noordhoek, J. A., Heijink, I. H., Hiemstra, P. S., et al. (2018). Retinoic acid signaling balances adult distal lung epithelial progenitor cell growth and differentiation. EBioMedicine 36, 461–474. doi:10.1016/j.ebiom.2018.09.002
Ng, W. H., Johnston, E. K., Tan, J. J., Bliley, J. M., Feinberg, A. W., Stolz, D. B., et al. (2022). Recapitulating human cardio-pulmonary co-development using simultaneous multilineage differentiation of pluripotent stem cells. Elife 11. doi:10.7554/eLife.67872
Ngan, S. Y., Quach, H., Dierolf, J., Laselva, O., Lee, J.-A., Huang, E., et al. (2021). Modeling lung cell development using human pluripotent stem cells. bioRxiv. doi:10.1101/2021.07.16.452691
Nikolić, M. Z., Caritg, O., Jeng, Q., Johnson, J.-A., Sun, D., Howell, K. J., et al. (2017). Human embryonic lung epithelial tips are multipotent progenitors that can be expanded in vitro as long-term self-renewing organoids. Elife 6, e26575. doi:10.7554/eLife.26575
Peretz, J., Pekosz, A., Lane, A. P., and Klein, S. L. (2016). Estrogenic compounds reduce influenza A virus replication in primary human nasal epithelial cells derived from female, but not male, donors. Am. J. Physiol. Lung Cell. Mol. Physiol. 310, L415–L425. doi:10.1152/ajplung.00398.2015
Raghavan, S., Winter, P. S., Navia, A. W., Williams, H. L., DenAdel, A., Lowder, K. E., et al. (2021). Microenvironment drives cell state, plasticity, and drug response in pancreatic cancer. Cell 184, 6119–6137.e26. e26. doi:10.1016/j.cell.2021.11.017
Riccetti, M. R., Ushakumary, M. G., Waltamath, M., Green, J., Snowball, J., Dautel, S. E., et al. (2022). Maladaptive functional changes in alveolar fibroblasts due to perinatal hyperoxia impair epithelial differentiation. JCI Insight 7, e152404. doi:10.1172/jci.insight.152404
Rock, J. R., Onaitis, M. W., Rawlins, E. L., Lu, Y., Clark, C. P., Xue, Y., et al. (2009). Basal cells as stem cells of the mouse trachea and human airway epithelium. Proc. Natl. Acad. Sci. U. S. A. 106, 12771–12775. doi:10.1073/pnas.0906850106
Rodenburg, L. W., Delpiano, L., Railean, V., Centeio, R., Pinto, M. C., Smits, S. M. A., et al. (2022). Drug repurposing for cystic fibrosis: Identification of drugs that induce CFTR-independent fluid secretion in nasal organoids. Int. J. Mol. Sci. 23, 12657. doi:10.3390/ijms232012657
Roerink, S. F., Sasaki, N., Lee-Six, H., Young, M. D., Alexandrov, L. B., Behjati, S., et al. (2018). Intra-tumour diversification in colorectal cancer at the single-cell level. Nature 556, 457–462. doi:10.1038/s41586-018-0024-3
Sachs, N., Papaspyropoulos, A., Zomer-van Ommen, D. D., Heo, I., Böttinger, L., Klay, D., et al. (2019). Long-term expanding human airway organoids for disease modeling. EMBO J. 38, e100300. doi:10.15252/embj.2018100300
Salahudeen, A. A., Choi, S. S., Rustagi, A., Zhu, J., van Unen, V., de la O, S. M., et al. (2020). Progenitor identification and SARS-CoV-2 infection in human distal lung organoids. Nature 588, 670–675. doi:10.1038/s41586-020-3014-1
Sato, T., Vries, R. G., Snippert, H. J., van de Wetering, M., Barker, N., Stange, D. E., et al. (2009). Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature 459, 262–265. doi:10.1038/nature07935
Sen, C., Freund, D., and Gomperts, B. N. (2022). Three-dimensional models of the lung: Past, present and future: A mini review. Biochem. Soc. Trans. 50, 1045–1056. doi:10.1042/BST20190569
Shi, R., Radulovich, N., Ng, C., Liu, N., Notsuda, H., Cabanero, M., et al. (2020). Organoid cultures as preclinical models of non-small cell lung cancer. Clin. Cancer Res. 26, 1162–1174. doi:10.1158/1078-0432.CCR-19-1376
Stancil, I. T., Michalski, J. E., Davis-Hall, D., Chu, H. W., Park, J.-A., Magin, C. M., et al. (2021). Pulmonary fibrosis distal airway epithelia are dynamically and structurally dysfunctional. Nat. Commun. 12, 4566. doi:10.1038/s41467-021-24853-8
Strikoudis, A., Cieślak, A., Loffredo, L., Chen, Y.-W., Patel, N., Saqi, A., et al. (2019). Modeling of fibrotic lung disease using 3D organoids derived from human pluripotent stem cells. Cell Rep. 27, 3709–3723. e5. doi:10.1016/j.celrep.2019.05.077
Suezawa, T., Kanagaki, S., Moriguchi, K., Masui, A., Nakao, K., Toyomoto, M., et al. (2021). Disease modeling of pulmonary fibrosis using human pluripotent stem cell-derived alveolar organoids. Stem Cell Rep. 16, 2973–2987. doi:10.1016/j.stemcr.2021.10.015
Surolia, R., Li, F. J., Wang, Z., Li, H., Dsouza, K., Thomas, V., et al. (2019). Vimentin intermediate filament assembly regulates fibroblast invasion in fibrogenic lung injury. JCI Insight 4, e123253. doi:10.1172/jci.insight.123253
Tamò, L., Hibaoui, Y., Kallol, S., Alves, M. P., Albrecht, C., Hostettler, K. E., et al. (2018). Generation of an alveolar epithelial type II cell line from induced pluripotent stem cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 315, L921–L932. doi:10.1152/ajplung.00357.2017
Tan, Q., Ma, X. Y., Liu, W., Meridew, J. A., Jones, D. L., Haak, A. J., et al. (2019). Nascent lung organoids reveal epithelium- and bone morphogenetic protein-mediated suppression of fibroblast activation. Am. J. Respir. Cell Mol. Biol. 61, 607–619. doi:10.1165/rcmb.2018-0390OC
van de Wetering, M., Francies, H. E., Francis, J. M., Bounova, G., Iorio, F., Pronk, A., et al. (2015). Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell 161, 933–945. doi:10.1016/j.cell.2015.03.053
van der Vaart, J., Böttinger, L., Geurts, M. H., van de Wetering, W. J., Knoops, K., Sachs, N., et al. (2021a). Modelling of primary ciliary dyskinesia using patient-derived airway organoids. EMBO Rep. 22, e52058. doi:10.15252/embr.202052058
van der Vaart, J., Lamers, M. M., Haagmans, B. L., and Clevers, H. (2021b). Advancing lung organoids for COVID-19 research. Dis. Model. Mech. 14, dmm049060. doi:10.1242/dmm.049060
Wijesekara, P., Yadav, P., Perkins, L. A., Stolz, D. B., Franks, J. M., Watkins, S. C., et al. (2022). Engineering rotating apical-out airway organoid for assessing respiratory cilia motility. iScience 25, 104730. doi:10.1016/j.isci.2022.104730
Keywords: lung stem cells, respiratory biology, 3D cell culture, in vitro models, epithelial cells
Citation: Hughes T, Dijkstra KK, Rawlins EL and Hynds RE (2023) Open questions in human lung organoid research. Front. Pharmacol. 13:1083017. doi: 10.3389/fphar.2022.1083017
Received: 28 October 2022; Accepted: 28 December 2022;
Published: 13 January 2023.
Edited by:
Rebecca L. Heise, Virginia Commonwealth University, United StatesReviewed by:
Nikhil Tanaji Awatade, The University of Newcastle, AustraliaChelsea M. Magin, University of Colorado Denver, United States
Copyright © 2023 Hughes, Dijkstra, Rawlins and Hynds. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Robert E. Hynds, rob.hynds@ucl.ac.uk
†These authors have contributed equally to this work and share first authorship
 Tessa Hughes
Tessa Hughes Krijn K. Dijkstra
Krijn K. Dijkstra Emma L. Rawlins
Emma L. Rawlins Robert E. Hynds
Robert E. Hynds