- 1School of Pharmacy, Jeonbuk National University, Jeonju, Jeonbuk, South Korea
- 2KIURI Research Center, Ajou University, Suwon, South Korea
- 3Department of Pharmacy and Yonsei Institute of Pharmaceutical Sciences, College of Pharmacy, Yonsei University, Incheon, South Korea
- 4Department of Clinical Pharmacy, School of Pharmacy, University of California, San Francisco, San Francisco, CA, United States
- 5Department of Pharmaceutical Medicine and Regulatory Sciences, Colleges of Medicine and Pharmacy, Yonsei University, Incheon, South Korea
- 6College of Pharmacy, Yeungnam University, Gyeongsan, Gyeongsangbuk-do, South Korea
Background: The use of opioid–gabapentinoid combinations has increased, raising several safety concerns. However, meta-analysis studies focusing on this issue are limited.
Objective: To evaluate the risk of central nervous system (CNS) depression, gastrointestinal (GI) adverse events, and mortality of combination therapy compared with those of opioid therapy and to explore the differences in the results according to study design and indications.
Methods: Relevant studies were selected (published before 30 January 2022) by searching the MEDLINE, Embase, and CENTRAL databases. The pooled odds ratios (OR) with 95% confidence intervals (CI) of the outcomes were estimated using the Mantel–Haenszel method. Subgroup and meta-regression analyses were performed according to study characteristics. Quality assessment was conducted using the Risk of Bias 2 tool for randomized controlled trials (RCTs) and Cochrane Collaboration’s Risk of Bias in non-RCTs tool for non-randomized trials.
Results: Adverse events were reported in 26 RCTs and 7 non-RCTs, and mortality was reported in 10 non-RCTs. Compared to opioid therapy, dizziness, cognitive dysfunction, and respiratory depression in combination therapy significantly increased in non-RCTs (OR 3.26, 95% CI 1.82–5.85; OR 3.13, 95% CI 1.51–6.50; OR 1.71, 95% CI 1.31–2.24, respectively), and a similar trend for dizziness and cognitive dysfunction was also identified in the RCT analysis, although the difference was not significant. Combination therapy for cancer pain was associated with the highest risk of sedation in subgroup analysis. Combination therapy significantly decreased the risk of GI adverse events, including nausea, vomiting, and constipation. The mortality risk associated with combination therapy was higher than that associated with opioid therapy (OR 2.76, 95% CI 1.26–6.05).
Conclusion: Opioid-gabapentinoid combination therapy could be associated with an increased risk of CNS depression and mortality, despite tolerable GI adverse events. These data suggest that combination therapy requires close monitoring of CNS depression, especially in cancer patients. Caution is needed in interpreting the clinical meanings owing to the lack of risk difference in respiratory depression in the RCT-only analysis and the absence of RCT or prospective studies investigating mortality.
1 Introduction
Opioid therapy is a major treatment for moderate-to-severe pain associated with surgery, injury, or cancer. However, with the increasing opioid overdoses and opioid-related deaths (Scholl et al., 2018; CDC, 2019), multimodal analgesia involving opioids and non-opioid analgesics with different mechanisms of action has emerged as a strategy to reduce reliance on opioids and effectively control pain (Dowell et al., 2016; Ramirez et al., 2020).
Gabapentin and pregabalin, jointly referred to as gabapentinoids, are commonly used nonopioid analgesics. They are used to treat diabetic neuropathy, fibromyalgia, and postherpetic neuralgia (Goodman and Brett, 2017; Montastruc et al., 2018). In 2017, more than 20% of patients in the United Kingdom who were newly prescribed gabapentinoids were taking opioids concomitantly (Montastruc et al., 2018). In the United States, prescriptions of gabapentinoids increased by about 50% between 2012 and 2016 (Goodman and Brett, 2017).
Gabapentinoids have some safety concerns regarding central nervous system (CNS) depression in that they can cause sedation and dizziness and may lead to cognitive impairment in some patients (Goodman and Brett, 2017). Also, simultaneous use of gabapentinoids with an opioid may change the risk of adverse events associated with opioid use (Kardas et al., 2020). A recent meta-analysis showed that the perioperative therapy of administering a gabapentinoid with an opioid in patients with lower limb arthroplasty reduced the risk of postoperative nausea, vomiting, and pruritus, but not sedation (Campbell et al., 2021). This meta-analysis included only randomized controlled trial (RCT) studies that mostly focused on the short-term use of perioperative analgesics. However, gabapentinoids are prescribed for long-term use for cancer-associated or non-cancer chronic pain, and their medication use could be different from RCT studies in actual clinical settings (Chen et al., 2016; Yu et al., 2021).
The 2019 Beers Criteria recommend avoiding a combination of opioids and gabapentinoids owing to the potential risk of respiratory depression (AGS Beers Criteria Update Expert Panel, 2019). Furthermore, the concurrent use of opioids with gabapentinoids increased mortality risk as demonstrated in an analysis of death registration in the United Kingdom (Chen et al., 2022). However, to the best of our knowledge no meta-analysis has examined the mortality risk associated with the combined use of gabapentinoids and opioids. Therefore, to comprehensively evaluate the safety of gabapentinoids and opioid combinations, a multi-faceted evaluation considering the characteristics of medication use according to indications and real-world evidence is necessary.
This study performed a systematic review and meta-analysis to evaluate the risk of CNS depression, gastrointestinal (GI) adverse events, and mortality when gabapentinoids were used with opioids. Given the difference in study design between RCTs and non-RCTs, we explored the results according to the study design by considering clinical factors such as indications and intervention type.
2 Materials and methods
This study followed the guidelines recommended by the Preferred Reporting Items for Systematic Reviews and Meta-analyses 2020 (PRISMA 2020) (Supplementary Table S1) (Page et al., 2021). The study protocol is available in the PROSPERO database (CRD42022302896). Two investigators (YKJ and SHY) independently performed the literature search, study selection, data extraction, and quality assessment. Discrepancies, if any, were resolved by two other investigators (YMY and YA).
2.1 Search strategy
The MEDLINE, EMBASE, and CENTRAL electronic databases were systematically searched for relevant studies published before 30 January 2022. The search used a combination of medical subject headings and the keywords “opioid analgesics” and “gabapentinoids.” The complete search strategy used in this analysis is listed in Supplementary Table S2.
2.2 Study selection
Studies were considered eligible if they met the following inclusion criteria: 1) population: enrolled adult patients aged 18 years or older undergoing pain management; 2) intervention: a combination of opioid analgesics and gabapentinoids use for more than 24 h; 3) comparison: opioid analgesic use for more than 24 h; 4) outcomes: the risk of adverse events and death; and 5) study design: prospective or retrospective studies. The following studies were excluded: 1) non-human studies, including animal and in vitro studies; 2) reviews, meta-analyses, or ongoing studies; 3) case reports; 4) studies available only in the form of abstracts or posters; and 5) publications not in English.
2.3 Data extraction
Eligible studies were reviewed, and the following data were extracted using a standardized extraction form: first author, publication year, country, study design, database used in the study, number of patients, sex, age, indications, regimens of opioid analgesics and gabapentinoids, duration of treatment, duration of follow-up, and details of adverse events.
2.4 Study outcomes
The primary study outcomes were treatment-related adverse events (TRAEs) such as CNS depression and GI adverse events. CNS depression includes sedation, dizziness, cognitive dysfunction, and respiratory depression. GI adverse events included nausea, vomiting, and constipation. Mortality rate was also evaluated.
2.5 Analysis
In this study, we analyzed the risk of TRAEs and death according to the study design (i.e., RCTs and non-RCTs). The pooled odds ratios (ORs) with 95% confidence intervals (CIs) of TRAEs and deaths associated with the use of opioids and gabapentinoids were computed using the Mantel–Haenszel method. OR and hazard ratio (HR) data for mortality adjusted for confounding factors (such as sex, year, comorbid diseases, and concurrent medications) were weighted and pooled using the generic inverse-variance method. Heterogeneity was assessed using inconsistency statistics (I2), with significance set at I2 > 50% (Higgins and Thompson, 2002). A common-effects model was used in the absence of significant heterogeneity, and a random-effects model was employed when significant heterogeneity was present (Higgins et al., 2019).
We conducted subgroup and meta-regression analyses of RCTs. We evaluated differences in TRAEs between combination therapy and opioid therapy according to indications (perioperative pain, non-cancer chronic pain, and cancer-associated pain), duration of treatment, prescription dosage-morphine milligram equivalents (MME) of oral opioids and defined daily doses (DDDs) of gabapentinoids. Sensitivity analysis was conducted by removing low-quality studies or adding each study in the order of sample size to determine the robustness of the results.
Quality assessment of each included study was conducted using the Risk of Bias 2 (RoB 2) tool for RCTs (J. A. C. Sterne et al., 2019) and the Cochrane Collaboration’s Risk of Bias in non-RCTs (ROBINS-I) tool for non-randomized trials (J. A. Sterne et al., 2016). Publication bias was examined using funnel plots and Egger’s regression test. Statistical significance was defined as p < 0.05. The meta-module in R 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria) was used for statistical analysis.
3 Results
3.1 Study selection
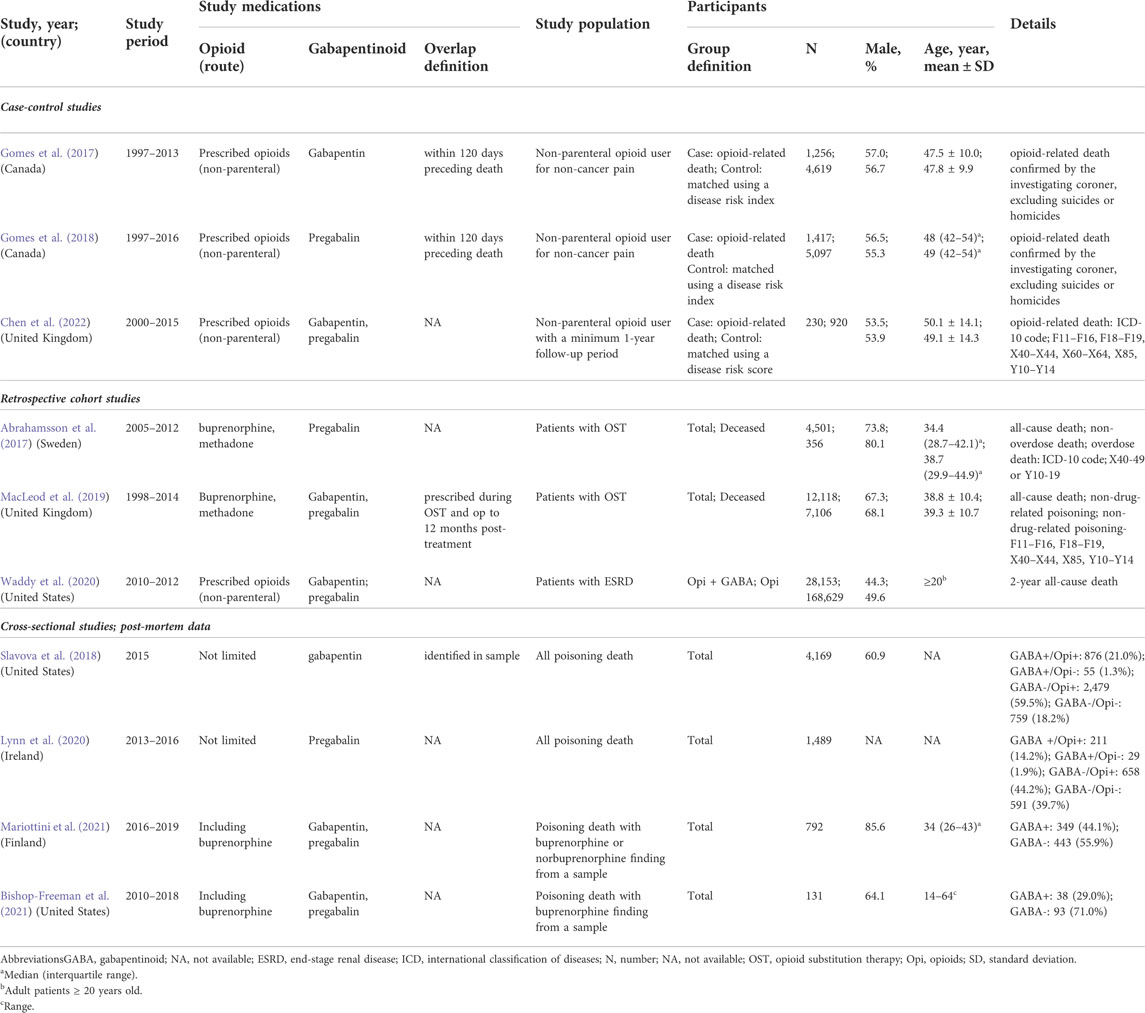
Supplementary Figure 1 shows the process of selecting eligible studies according to the PRISMA 2020 guidelines. After excluding duplicates, 3,699 articles were screened for relevance based on the title and abstract, and 3,520 articles were excluded. After 179 relevant articles were assessed for eligibility through a full-text evaluation, 43 studies with 6,537,444 patients were selected. TRAEs were reported in 26 RCTs (Caraceni et al., 2004; Gilron et al., 2005; Fassoulaki et al., 2006; Turan et al., 2006; Keskinbora et al., 2007; Clarke et al., 2009; Gatti et al., 2009; Clendenen et al., 2010; Rapchuk et al., 2010; Pesonen et al., 2011; Yucel et al., 2011; Chaparro et al., 2012; Jain et al., 2012; Pota et al., 2012; Yadeau et al., 2012; Mercadante et al., 2013; Paul et al., 2013; Clarke et al., 2015; Chen et al., 2016; Dou et al., 2017; Wang et al., 2017; Hah et al., 2018; Jones et al., 2019; Hermann et al., 2020; Jung et al., 2020; Teng et al., 2021) and 7 non-RCTs (Caraceni et al., 1999; Li et al., 2010; Savelloni et al., 2017; Peckham et al., 2018; Bykov et al., 2020; Chae et al., 2021; Dai et al., 2021); mortality was reported in 10 non-RCTs. (Abrahamsson et al., 2017; Gomes et al., 2017; Gomes et al., 2018; Slavova et al., 2018; Macleod et al., 2019; Lynn et al., 2020; Waddy et al., 2020; Bishop-Freeman et al., 2021; Mariottini et al., 2021; Chen et al., 2022).
3.2 Study characteristics
Table 1 summarizes the characteristics of the 26 RCTs (Caraceni et al., 2004; Gilron et al., 2005; Fassoulaki et al., 2006; Turan et al., 2006; Keskinbora et al., 2007; Clarke et al., 2009; Gatti et al., 2009; Clendenen et al., 2010; Rapchuk et al., 2010; Pesonen et al., 2011; Yucel et al., 2011; Chaparro et al., 2012; Jain et al., 2012; Pota et al., 2012; Yadeau et al., 2012; Mercadante et al., 2013; Paul et al., 2013; Clarke et al., 2015; Chen et al., 2016; Dou et al., 2017; Wang et al., 2017; Hah et al., 2018; Jones et al., 2019; Hermann et al., 2020; Jung et al., 2020; Teng et al., 2021) and 7 non-RCTs (Caraceni et al., 1999; Li et al., 2010; Savelloni et al., 2017; Peckham et al., 2018; Bykov et al., 2020; Chae et al., 2021; Dai et al., 2021) reporting TRAE risks. In the RCTs, the number of participants ranged from 29 to 410 per study, totaling 2,335 participants. The mean age of the participants in each study ranged between 34.1 and 79.6 years. The indications included perioperative pain (13 studies), (Fassoulaki et al., 2006; Turan et al., 2006; Clarke et al., 2009; Clendenen et al., 2010; Rapchuk et al., 2010; Pesonen et al., 2011; Yucel et al., 2011; Chaparro et al., 2012; Jain et al., 2012; Yadeau et al., 2012; Paul et al., 2013; Clarke et al., 2015; Hah et al., 2018) cancer-related pain (7 studies), (Caraceni et al., 2004; Keskinbora et al., 2007; Mercadante et al., 2013; Chen et al., 2016; Dou et al., 2017; Hermann et al., 2020; Teng et al., 2021) and non-cancer chronic pain (6 studies) (Gilron et al., 2005; Gatti et al., 2009; Pota et al., 2012; Wang et al., 2017; Jones et al., 2019; Jung et al., 2020).
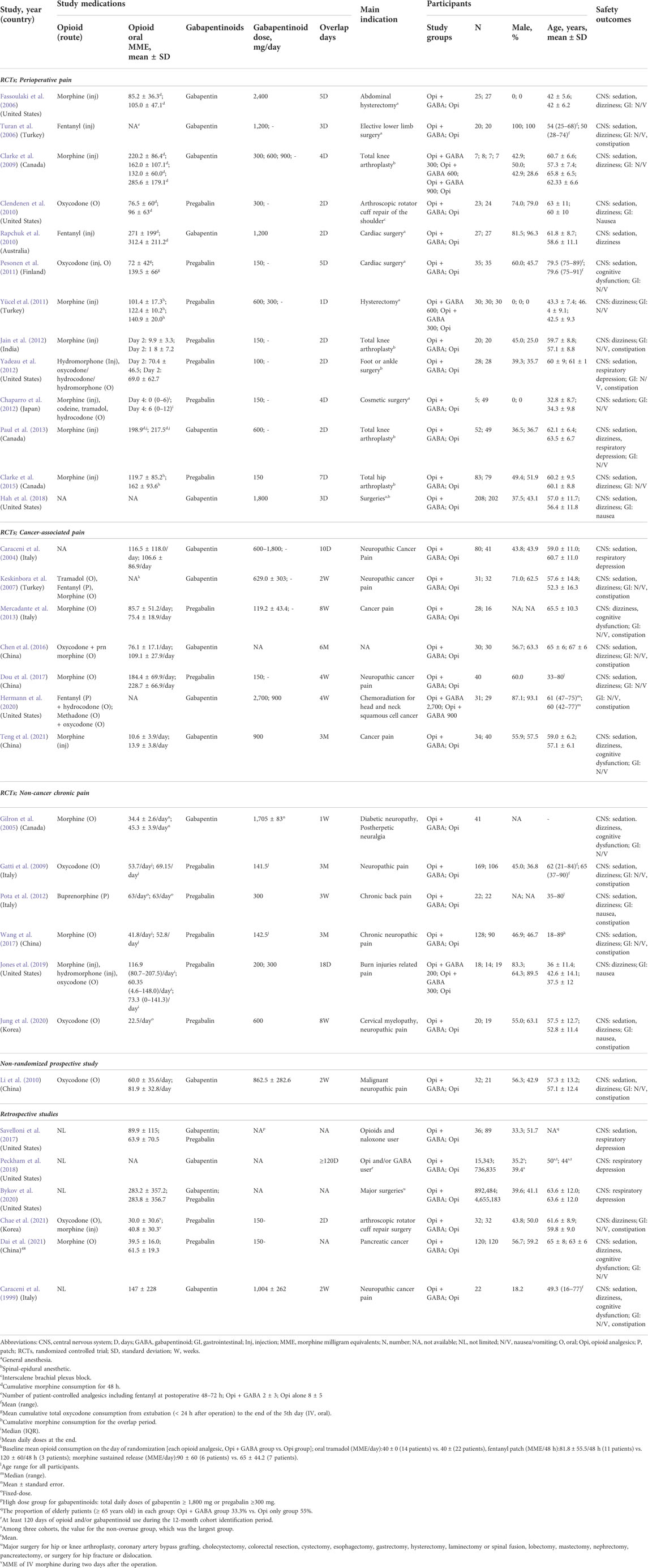
TABLE 1. Characteristics of studies reporting the risk of central nervous system depression and gastrointestinal disorders.
In non-RCTs, the number of participants ranged from 22 to 5,547,667 per study, totaling 6,300,349 participants, with a mean age of 44.4–64.0 years. The indications included perioperative pain (2 studies), (Bykov et al., 2020; Chae et al., 2021) cancer-related pain (3 studies), (Caraceni et al., 1999; Li et al., 2010; Dai et al., 2021) and non-cancer chronic pain (2 studies), (Savelloni et al., 2017; Peckham et al., 2018).
Only ten non-RCTs reported mortality (Table 2) (Abrahamsson et al., 2017; Gomes et al., 2017; Gomes et al., 2018; Slavova et al., 2018; Macleod et al., 2019; Lynn et al., 2020; Waddy et al., 2020; Bishop-Freeman et al., 2021; Mariottini et al., 2021; Chen et al., 2022). Six studies using health databases included 226,940 patients with a mean age of 38.8–49.3 years and a follow-up period of 3–20 years (Abrahamsson et al., 2017; Gomes et al., 2017; Gomes et al., 2018; Macleod et al., 2019; Waddy et al., 2020; Chen et al., 2022). Four other studies using post-mortem databases on poisoning-related deaths involved 6,581 patients (Slavova et al., 2018; Lynn et al., 2020; Bishop-Freeman et al., 2021; Mariottini et al., 2021).
3.3 Treatment-related adverse events
The risks of sedation, dizziness, cognitive dysfunction, and respiratory depression were reported in 16, 18, 2, and 3 RCTs, and 4, 4, 3, and 3 non-RCTs, respectively. The risks of nausea, vomiting, and constipation were reported in 20, 16, and 11 RCTs and 4, 4, and 3 non-RCTs, respectively. In the RCT-only analysis, the risk of sedation, dizziness, and cognitive dysfunction showed an increasing trend for combination therapy compared with that for opioid therapy; however, the differences were not significant (Figure 1). In the non-RCT-only analysis, the use of combination therapy was significantly associated with an increased risk of dizziness, cognitive dysfunction, and respiratory depression (OR 3.26, 95% CI 1.82–5.85; OR 3.13, 95% CI 1.51–6.50; OR 1.71, 95% CI 1.31–2.24, respectively). The risks of nausea, vomiting, and constipation were significantly decreased in combination therapy compared to opioid therapy in the RCT-only analysis (OR 0.73, 95% CI 0.58–0.91; OR 0.72, 95% CI 0.56–0.92; OR 0.63, 95% CI 0.49–0.82, respectively). None of the GI adverse events were significantly different between combination therapy and opioid therapy in the non-RCT-only analysis. Forest plots of individual studies and pooled estimates of the risks of CNS depression and GI adverse events are presented in Supplementary Figures S2, S3, respectively.
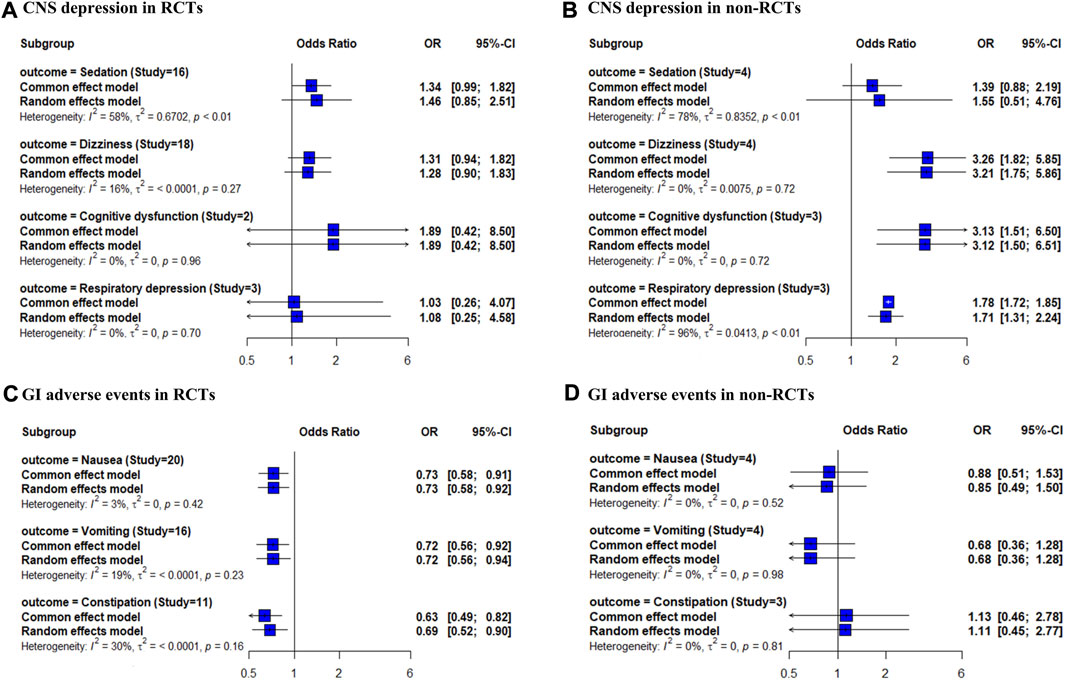
FIGURE 1. Forest plot of the risk of CNS depression and GI adverse events in opioid and gabapentinoid combination therapy compared with opioid therapy. (A) CNS depression in RCTs, (B) CNS depression in non-RCTs, (C) GI adverse events in RCTs, and (D) GI adverse events in non-RCTs. CNS, central nervous system; GI, gastrointestinal; RCTs, randomized controlled trials.
The results of the subgroup and meta-regression analyses in RCTs revealed significant differences among the indications in the risk of sedation and constipation (p < 0.01, Table 3). Combination therapy for cancer pain was associated with the highest risk of sedation (OR 3.45, 95% CI 1.93–6.18) and the lowest risk of constipation (OR 0.04, 95% CI 0.01–0.25). In the subgroup analysis of the risk of nausea and vomiting, perioperative pain, a treatment period of ≤ 7days, and an opioid dose ≥ 50 MME/day showed a significantly decreased risk.
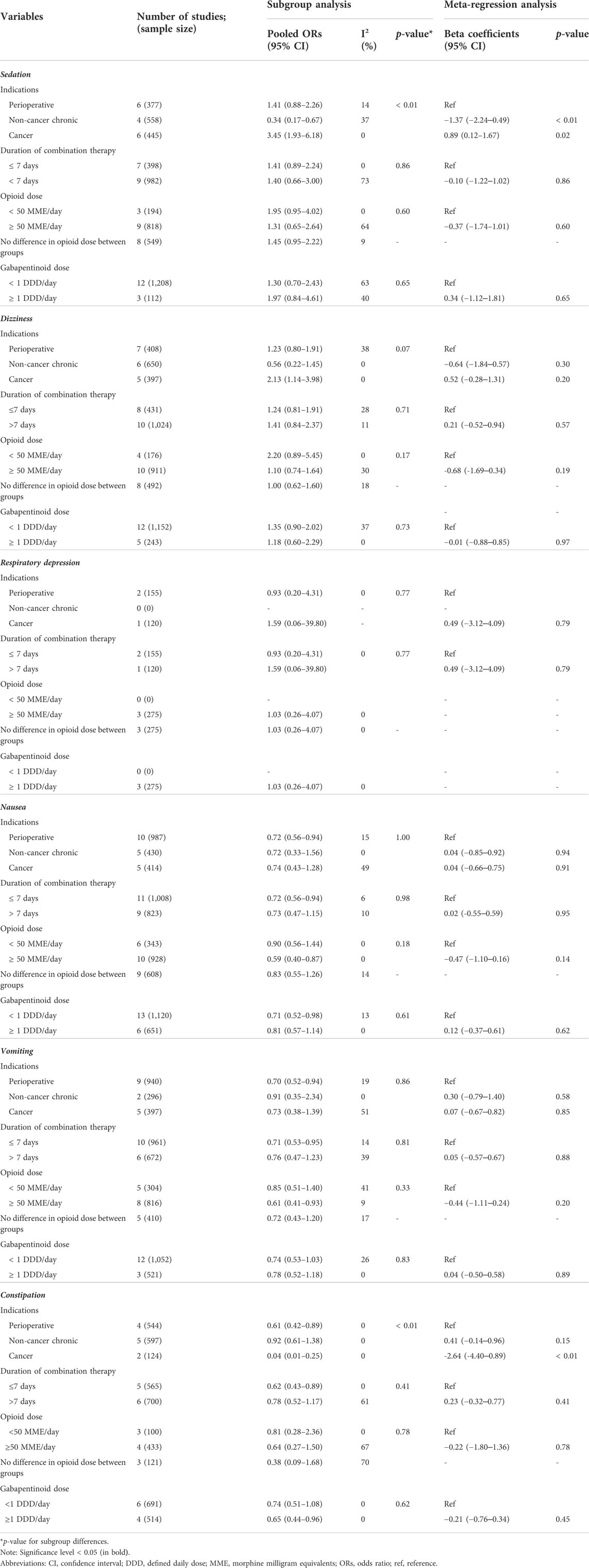
TABLE 3. Subgroup and meta-regression analyses of the risk of central nervous system depression and gastrointestinal disorders in randomized controlled trials.
3.4 Mortality risk
Three case-control studies were analyzed according to gabapentinoid dose, as presented in the included studies (Gomes et al., 2017; Gomes et al., 2018; Chen et al., 2022). For gabapentinoid dose > 1 DDD/day, the adjusted mortality OR was 2.76 with a 95% CI of 1.26–6.05 (Figure 2A), and for gabapentinoid dose ≤ 1 DDD/day, the adjusted mortality OR was 1.56 with a 95% CI of 1.23–1.98 (Figure 2B). The adjusted HR of mortality was also estimated from the data of two retrospective cohort studies (Abrahamsson et al., 2017; Macleod et al., 2019), and showed a similar trend (adjusted HR 1.73, 95% CI 1.38–2.17; data not shown). One cohort study was excluded from the meta-analysis because the reference group did not receive opioid therapy (reference group; without use of either opioid or gabapentinoid). The mortality HR of the combination therapy group was greater than that of the opioid therapy group as follows: gabapentin model, 1.16 (1.12–1.19) and 1.12 (1.09–1.15) and pregabalin model, 1.22 (1.16–1.28) and 1.12 (1.09–1.14) (Waddy et al., 2020).
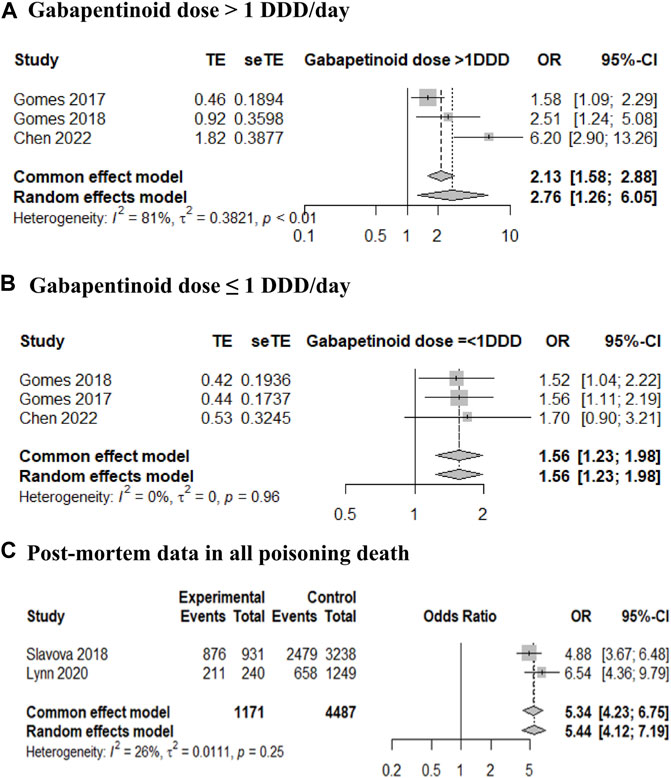
FIGURE 2. Forest plot of the mortality risk in opioid and gabapentinoid combination therapy compared with that in opioid therapy. (A) Gabapentinoid dose > 1 defined daily dose (DDD)/day, (B) gabapentinoid dose ≤ 1 DDD/day, and (C) post-mortem data in all poisoning deaths.
When analyzing two post-mortem cross-sectional studies of all deaths from poisoning (Slavova et al., 2018; Lynn et al., 2020), the OR for gabapentinoid identification in opioid users was 5.34 with a 95% CI of 4.23–6.75 (Figure 2C). In two studies that included deaths due to poisoning with buprenorphine findings (Bishop-Freeman et al., 2021; Mariottini et al., 2021), the prevalence of gabapentinoid combination (29.0% and 44.1%) was similar to that reported in studies of all poisoning deaths (24.3% and 26.1%, respectively).
3.5 Risk of bias, publication bias, and sensitivity analysis
Approximately two-thirds of the 26 RCTs (34.6%) were of some concern or had a high risk of bias (Supplementary Table S3). Among the 13 non-RCTs, over three-quarters had a serious risk of bias (Supplementary Table S4). Visual inspection of the funnel plot and Egger’s test revealed no publication bias (Supplementary Figure S4).
The results of the sensitivity analysis of the RCT study quality are presented in Supplementary Table S5. When analyzing studies without a high or serious risk of bias, the results were similar to the overall findings. Notably, the risk of sedation and dizziness significantly increased with combination therapy only when superior quality RCTs were included. Sensitivity analysis showed no effect of the sample size on the risk of TRAEs (Supplementary Figure S5).
4 Discussion
This meta-analysis evaluated the safety of opioid and gabapentinoid combination therapy compared with that of opioid therapy. In the non-RCT analysis, combination therapy was significantly associated with an increased risk of dizziness, cognitive dysfunction, and respiratory depression. The risk of sedation in combination therapy in cancer patients was greater than that in other indications in the RCT subgroup analysis. The mortality risk associated with combination therapy was also higher than that with opioid therapy. Meanwhile, combination therapy was significantly associated with a decreased risk of GI adverse events in the RCT analysis.
The risk of CNS depression and death has been a major concern when opioid and gabapentinoid combination therapy is used in the elderly population (AGS Beers Criteria Update Expert Panel, 2019). Although it was not possible to conduct subgroup analysis based on age due to the wide range of ages in each study, CNS depression risk and death did not seem to be limited to elderly patients considering the age range in the included studies. This finding agrees with Bykov et al., who reported that the risk of opioid overdose in opioid and gabapentinoid combination therapy did not differ according to age (Bykov et al., 2020).
The increased risk of respiratory depression and mortality with the concurrent use of a gabapentinoid with an opioid could be explained by pharmacokinetic and pharmacodynamic interactions. The bioavailability of gabapentinoids is increased by opioids, which reduce intestinal motility (Eckhardt et al., 2000). Furthermore, gabapentinoids can reduce CO2 responsiveness in the medullary respiratory center in addition to the respiratory depressant effect of opioid analgesics (Henson and Ward, 1994; Becker and Haas, 2011). One animal study reported that a low dose of pregabalin could reverse tolerance to morphine respiratory depression, and a high dose of pregabalin alone could depress respiration (Lyndon et al., 2017). In addition, we could consider the abuse or misuse of gabapentinoids when interpreting mortality risk in combination therapy. Opioid-related and all-cause death is known to be associated with gabapentinoid abuse or misuse in patients undergoing opioid therapy, and opioid use disorder is one of the risk factors for gabapentinoid abuse or misuse (Hägg et al., 2020; Evoy et al., 2021). The results of studies on poisoning deaths included in this study could provide evidence for this aspect. In a similar context, more than two-thirds of deaths due to gabapentinoid poisoning were co-identified with opioids, and the association of gabapentinoid with poisoning-related deaths has been shown to increase (Häkkinen et al., 2014; Elliott et al., 2017; Faryar et al., 2019; Darke et al., 2021). The difference in the risk of respiratory depression between RCTs and non-RCTs might also be associated with the gabapentinoid use patterns in the real world. Therefore, when evaluating gabapentinoid use in patients, especially opioid users, healthcare professionals should consider these factors.
According to the subgroup analysis and meta-regression, the risks of sedation and dizziness with combination therapy were significantly higher in patients with cancer pain than in those with other indications. The risk of dizziness was also significantly increased in patients with cancer pain when the combination therapy was used. This might be because chemotherapy in cancer patients can damage progenitor cells and myelines (Clouston et al., 1992; Meyers, 2008). Close monitoring for sedation and dizziness is necessary for patients with advanced cancer when opioid and gabapentin combination therapy is used.
We confirmed a reduced risk of GI adverse events with combination therapy, especially in short-term (≤ 7 days) therapy, opioid doses of ≥ 50 MME/day, and perioperative pain. This could be explained by the opioid-sparing effects and tolerance development for GI adverse events of opioids (Kim et al., 2017). A short-term addition of gabapentinoids to high doses of opioids after surgery may be recommended to reduce nausea and vomiting.
Most previous systematic reviews and meta-analyses have focused on the perioperative use of gabapentinoids (Liu et al., 2017; Verret et al., 2020; Campbell et al., 2021). We evaluated the risk of opioid and gabapentinoid combination for any type of pain. To the best of our knowledge, ours is the first meta-analysis to evaluate the risk of two common adverse events, CNS depression and GI adverse events, of a combination of opioids and gabapentinoids, and to analyze data using RCTs and non-RCTs. We found that the risks of sedation, dizziness, and GI adverse events were typically assessed with RCTs, whereas the risks of cognitive disorder and respiratory depression were typically assessed with non-RCTs in a large patient population. We evaluated the pooled effect of the combination therapy on mortality in several ways. In RCT studies, CNS depression showed an increasing trend in combination therapy, and in non-RCT studies, although there was a serious risk of bias in over three-quarters of studies, the risk of dizziness, cognitive dysfunction, respiratory depression, and mortality showed a significant increase in combination therapy. The risk of CNS depression and mortality in combination therapy should be interpreted cautiously and confirmed through well-organized non-RCT or long-term RCT studies in the future.
Our study had several limitations. First, the studies included in the meta-analysis were heterogeneous in terms of the baseline characteristics of the population and overlap period, which may have influenced the results of the meta-analysis. To address this limitation, we performed subgroup analyses based on these factors. Second, approximately half of the included RCTs and most non-RCTs had an excessively high risk of bias. However, our sensitivity analyses, which only included studies with a low or moderate risk of bias, support the robustness and validity of our main findings. Third, the number of studies included in the analysis of cognitive dysfunction and respiratory depression is small. Additionally, the validity of findings for respiratory depression could be limited owing to the following factors: 1) the significance and effect size in the non-RCT analysis tended to depend on two retrospective studies, Bykov et al. and Peckham et al.; and 2) no differences in the risk of respiratory depression were identified in the RCT-only analysis. Lastly, the interpretation of mortality risk in combination therapy was limited owing to the absence of RCT or prospective studies with this aim. Therefore, studies providing a high level of evidence such as RCT or prospective studies are needed to confirm the risk of mortality.
In conclusion, combination therapy with opioids and gabapentinoids is associated with an increased risk of CNS depression and mortality, and a reduced risk of GI adverse events. However, caution is needed when interpreting the clinical meanings because no differences in the risk of respiratory depression were identified in the RCT-only analysis, and no RCT or prospective studies investigated mortality. Our data suggest that clinicians should be aware of these potential risks in adults, including the elderly, when combination therapy is initiated. Close monitoring of treatment-related adverse events is required during combination therapy, especially in patients with cancer, owing to an increased risk of CNS depression. Further research on drug safety is needed to establish practical evidence of the tolerability of combination therapies with opioids and gabapentinoids.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
YJ and JH contributed to the study design, data analysis and interpretation, and manuscript writing. Y-MA and YMY contributed to the study conceptualization, interpretation of data, critical revision of the manuscript, and supervision of the study. SHY contributed to data analysis and interpretation. JS contributed to clinical interpretation of the data and critical revision of the manuscript. All authors reviewed, amended, and approved the final manuscript.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. 2020R1G1A1101205). MSIT: Ministry of Science and ICT. This work was supported by the National Research Foundation of Korea (NRF) Grant funded by the Korean government (MSIP; Ministry of Science, ICT, & Future Planning) (No. NRF-2019R1G1A1011055). The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish results.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.1009950/full#supplementary-material
Abbreviations
CI, confidence intervals; CNS, central nervous system; DDs, defined daily doses; GI, gastrointestinal; HR, hazard ratio; MME, morphine milligram equivalents; ORs, odds ratios; RCTs, randomized controlled trials; RoB 2, rish of bias 2; ROBINS-I, Cochrane Collaboration’s Risk of Bias in non-RCTs; TRAEs, treatment-related adverse events.
References
Abrahamsson, T., Berge, J., Öjehagen, A., and Håkansson, A. (2017). Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. 174, 58–64. doi:10.1016/j.drugalcdep.2017.01.013
AGS Beers Criteria Update Expert Panel (2019). American geriatrics society 2019 updated AGS Beers Criteria® for potentially inappropriate medication use in older adults. J. Am. Geriatr. Soc. 67, 674–694. doi:10.1111/jgs.15767
Becker, D. E., and Haas, D. A. (2011). Recognition and management of complications during moderate and deep sedation part 1: Respiratory considerations. Anesth. Prog. 58, 82–92. doi:10.2344/0003-3006-58.2.82
Bishop-Freeman, S. C., Friederich, L. W., Feaster, M. S., and Hudson, J. S. (2021). Buprenorphine-related deaths in North Carolina from 2010 to 2018. J. Anal. Toxicol. 45, 780–791. doi:10.1093/jat/bkab073
Bykov, K., Bateman, B. T., Franklin, J. M., Vine, S. M., and Patorno, E. (2020). Association of gabapentinoids with the risk of opioid-related adverse events in surgical patients in the United States. JAMA Netw. Open 3, e2031647. doi:10.1001/jamanetworkopen.2020.31647
Campbell, R., Khuong, J. N., Liu, Z., Borg, C., Jackson, S., Ramson, D. M., et al. (2021). Perioperative gabapentinoid use lowers short-term opioid consumption following lower limb arthroplasty: Systematic review and meta-analysis. J. Opioid Manag. 17, 251–272. doi:10.5055/jom.2021.0635
Caraceni, A., Zecca, E., Bonezzi, C., Arcuri, E., Tur, R. Y., Maltoni, M., et al. (2004). Gabapentin for neuropathic cancer pain: A randomized controlled trial from the gabapentin cancer pain study group. J. Clin. Oncol. 22, 2909–2917. doi:10.1200/JCO.2004.08.141
Caraceni, A., Zecca, E., Martini, C., and De Conno, F. (1999). Gabapentin as an adjuvant to opioid analgesia for neuropathic cancer pain. J. Pain Symptom Manage. 17, 441–445. doi:10.1016/s0885-3924(99)00033-0
CDC (2019). 2019 annual surveillance report of drug-related risks and outcomes — United States. Surveillance special report 2. Centers for Disease Control and Prevention, U.S. Department of Health and Human Services. Available at: https://www.cdc.gov/drugoverdose/pdf/pubs/2019-cdc-drug-surveillance-report.pdf (Accessed September 3, 2022).
Chae, S. H., Lee, J. E., Kim, M. J., and Yoo, J. C. (2021). Evaluation of analgesic efficacy and opioid sparing effect of pregabalin after arthroscopic rotator cuff repair surgery: A retrospective cohort study. J. Orthop. Sci. 26, 599–603. doi:10.1016/j.jos.2020.07.013
Chaparro, L. E., Clarke, H., Valdes, P. A., Mira, M., Duque, L., and Mitsakakis, N. (2012). Adding pregabalin to a multimodal analgesic regimen does not reduce pain scores following cosmetic surgery: A randomized trial. J. Anesth. 26, 829–835. doi:10.1007/s00540-012-1447-x
Chen, D. L., Li, Y. H., Wang, Z. J., and Zhu, Y. K. (2016). The research on long-term clinical effects and patients' satisfaction of gabapentin combined with oxycontin in treatment of severe cancer pain. Med. Baltim. 95, e5144. doi:10.1097/md.0000000000005144
Chen, T. C., Knaggs, R. D., and Chen, L. C. (2022). Association between opioid-related deaths and persistent opioid prescribing in primary care in england: A nested case-control study. Br. J. Clin. Pharmacol. 88, 798–809. doi:10.1111/bcp.15028
Clarke, H., Pagé, G. M., McCartney, C. J. L., Huang, A., Stratford, P., Andrion, J., et al. (2015). Pregabalin reduces postoperative opioid consumption and pain for 1 week after hospital discharge, but does not affect function at 6 weeks or 3 months after total hip arthroplasty. Br. J. Anaesth. 115, 903–911. doi:10.1093/bja/aev363
Clarke, H., Pereira, S., Kennedy, D., Gilron, I., Katz, J., Gollish, J., et al. (2009). Gabapentin decreases morphine consumption and improves functional recovery following total knee arthroplasty. Pain Res. Manag. 14, 217–222. doi:10.1155/2009/930609
Clendenen, S. R., Rajendran, S., Kopacz, D. J., Greengrass, R. A., Robards, C. B., Weinstein, D. M., et al. (2010). Pregabalin as an adjunct to a multimodal analgesic regimen to achieve opioid sparing in arthroscopic rotator cuff repair. Jurnalul Roman de Anestezie Terapie Intensiva/Romanian J. Anaesth. Intensive Care 17, 5–10. Available at: http://jurnalul-anestezie.ro/2010/2010-1-2.pdf (Accessed September 3, 2022).
Clouston, P. D., DeAngelis, L. M., and Posner, J. B. (1992). The spectrum of neurological disease in patients with systemic cancer. Ann. Neurol. 31, 268–273. doi:10.1002/ana.410310307
Dai, J., Teng, L., Zhao, L., and Zou, H. (2021). The combined analgesic effect of pregabalin and morphine in the treatment of pancreatic cancer pain, a retrospective study. Cancer Med. 10, 1738–1744. doi:10.1002/cam4.3779
Darke, S., Duflou, J., Peacock, A., Farrell, M., and Lappin, J. (2021). Characteristics of fatal gabapentinoid-related poisoning in Australia. Clin. Toxicol. 2021, 2000–2020. doi:10.1080/15563650.2021.1965159
Dou, Z., Jiang, Z., and Zhong, J. (2017). Efficacy and safety of pregabalin in patients with neuropathic cancer pain undergoing morphine therapy. Asia. Pac. J. Clin. Oncol. 13, e57–e64. doi:10.1111/ajco.12311
Dowell, D., Haegerich, T. M., and Chou, R. (2016). CDC guideline for prescribing opioids for chronic pain--United States, 2016. Jama 315, 1624–1645. doi:10.1001/jama.2016.1464
Eckhardt, K., Ammon, S., Hofmann, U., Riebe, A., Gugeler, N., and Mikus, G. (2000). Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesth. Analg. 91, 185–191. doi:10.1097/00000539-200007000-00035
Elliott, S. P., Burke, T., and Smith, C. (2017). Determining the toxicological significance of pregabalin in fatalities. J. Forensic Sci. 62, 169–173. doi:10.1111/1556-4029.13263
Evoy, K. E., Sadrameli, S., Contreras, J., Covvey, J. R., Peckham, A. M., and Morrison, M. D. (2021). Abuse and misuse of pregabalin and gabapentin: A systematic review update. Drugs 81, 125–156. doi:10.1007/s40265-020-01432-7
Faryar, K. A., Webb, A. N., Bhandari, B., Price, T. G., and Bosse, G. M. (2019). Trending gabapentin exposures in Kentucky after legislation requiring use of the state prescription drug monitoring program for all opioid prescriptions(.). Clin. Toxicol. 57, 398–403. doi:10.1080/15563650.2018.1538518
Fassoulaki, A., Stamatakis, E., Petropoulos, G., Siafaka, I., Hassiakos, D., and Sarantopoulos, C. (2006). Gabapentin attenuates late but not acute pain after abdominal hysterectomy. Eur. J. Anaesthesiol. 23, 136–141. doi:10.1017/s0265021505002048
Gatti, A., Sabato, A. F., Occhioni, R., Colini Baldeschi, G., and Reale, C. (2009). Controlled-release oxycodone and pregabalin in the treatment of neuropathic pain: Results of a multicenter Italian study. Eur. Neurol. 61, 129–137. doi:10.1159/000186502
Gilron, I., Bailey, J. M., Tu, D., Holden, R. R., Weaver, D. F., and Houlden, R. L. (2005). Morphine, gabapentin, or their combination for neuropathic pain. N. Engl. J. Med. 352, 1324–1334. doi:10.1056/NEJMoa042580
Gomes, T., Greaves, S., Van Den Brink, W., Antoniou, T., Mamdani, M. M., Paterson, J. M., et al. (2018). Pregabalin and the risk for opioid-related death: A nested case-control study. Ann. Intern. Med. 169, 732–734. doi:10.7326/M18-1136
Gomes, T., Juurlink, D. N., Antoniou, T., Mamdani, M. M., Paterson, J. M., and van den Brink, W. (2017). Gabapentin, opioids, and the risk of opioid-related death: A population-based nested case-control study. PLoS Med. 14, e1002396. doi:10.1371/journal.pmed.1002396
Goodman, C. W., and Brett, A. S. (2017). Gabapentin and pregabalin for pain - is increased prescribing a cause for concern? N. Engl. J. Med. 377, 411–414. doi:10.1056/NEJMp1704633
Hägg, S., Jönsson, A. K., and Ahlner, J. (2020). Current evidence on abuse and misuse of gabapentinoids. Drug Saf. 43, 1235–1254. doi:10.1007/s40264-020-00985-6
Hah, J., Mackey, S. C., Schmidt, P., McCue, R., Humphreys, K., Trafton, J., et al. (2018). Effect of perioperative gabapentin on postoperative pain resolution and opioid cessation in a mixed surgical cohort a randomized clinical trial. JAMA Surg. 153, 303–311. doi:10.1001/jamasurg.2017.4915
Häkkinen, M., Vuori, E., Kalso, E., Gergov, M., and Ojanperä, I. (2014). Profiles of pregabalin and gabapentin abuse by postmortem toxicology. Forensic Sci. Int. 241, 1–6. doi:10.1016/j.forsciint.2014.04.028
Henson, L. C., and Ward, D. S. (1994). Effects of anaesthetics and sedatives on the control of breathing. Ann. Acad. Med. Singap. 23, 125–129.
Hermann, G. M., Iovoli, A. J., Platek, A. J., Wang, C., Miller, A., Attwood, K., et al. (2020). A single-institution, randomized, pilot study evaluating the efficacy of gabapentin and methadone for patients undergoing chemoradiation for head and neck squamous cell cancer. Cancer 126, 1480–1491. doi:10.1002/cncr.32676
Higgins, T. J., Chandler, J., Cumpston, M., Li, T., and Page, M. J. (2019). Cochrane handbook for systematic reviews of interventions. Chichester, United Kingdom: John Wiley & Sons.
Higgins, J. P., and Thompson, S. G. (2002). Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558. doi:10.1002/sim.1186
Jain, P., Jolly, A., Bholla, V., Adatia, S., and Sood, J. (2012). Evaluation of efficacy of oral pregabalin in reducing postoperative pain in patients undergoing total knee arthroplasty. Indian J. Orthop. 46, 646–652. doi:10.4103/0019-5413.104196
Jones, L. M., Uribe, A. A., Coffey, R., Puente, E. G., Abdel-Rasoul, M., Murphy, C. V., et al. (2019). Pregabalin in the reduction of pain and opioid consumption after burn injuries: A preliminary, randomized, double-blind, placebo-controlled study. Med. Baltim. 98, e15343. doi:10.1097/md.0000000000015343
Jung, J. M., Chung, C. K., Kim, C. H., Yang, S. H., and Choi, Y. (2020). Comparison of the use of opioids only and pregabalin add-on for the treatment of neuropathic pain in cervical myelopathy patients: A pilot trial. Sci. Rep. 10, 8120. doi:10.1038/s41598-020-65108-8
Kardas, P., Urbanski, F., Lichwierowicz, A., Chudzynska, E., Czech, M., Makowska, K., et al. (2020). The prevalence of selected potential drug-drug interactions of analgesic drugs and possible methods of preventing them: Lessons learned from the analysis of the real-world national database of 38 million citizens of Poland. Front. Pharmacol. 11, 607852. doi:10.3389/fphar.2020.607852
Keskinbora, K., Pekel, A. F., and Aydinli, I. (2007). Gabapentin and an opioid combination versus opioid alone for the management of neuropathic cancer pain: A randomized open trial. J. Pain Symptom Manage. 34, 183–189. doi:10.1016/j.jpainsymman.2006.11.013
Kim, E. D., Lee, J. Y., Son, J. S., Byeon, G. J., Yeo, J. S., Kim, D. W., et al. (2017). Guidelines for prescribing opioids for chronic non-cancer pain in Korea. Korean J. Pain 30, 18–33. doi:10.3344/kjp.2017.30.1.18
Li, X. M., Liu, D. Q., Wu, H. Y., Yang, C., and Yang, L. (2010). Controlled-release oxycodone alone or combined with gabapentin for management of malignant neuropathic pain. Chin. J. Cancer Res. 22, 80–86. doi:10.1007/s11670-010-0080-1
Liu, B., Liu, R., and Wang, L. (2017). A meta-analysis of the preoperative use of gabapentinoids for the treatment of acute postoperative pain following spinal surgery. Med. Baltim. 96, e8031. doi:10.1097/md.0000000000008031
Lyndon, A., Audrey, S., Wells, C., Burnell, E. S., Ingle, S., Hill, R., et al. (2017). Risk to heroin users of polydrug use of pregabalin or gabapentin. Addiction 112, 1580–1589. doi:10.1111/add.13843
Lynn, E., Cousins, G., Lyons, S., and Bennett, K. E. (2020). A repeated cross-sectional study of factors associated with pregabalin-positive poisoning deaths in Ireland. Drug Alcohol Depend. 206, 107741. doi:10.1016/j.drugalcdep.2019.107741
Macleod, J., Steer, C., Tilling, K., Cornish, R., Marsden, J., Millar, T., et al. (2019). Prescription of benzodiazepines, z-drugs, and gabapentinoids and mortality risk in people receiving opioid agonist treatment: Observational study based on the UK Clinical Practice Research Datalink and Office for National Statistics death records. PLoS Med. 16, e1002965. doi:10.1371/journal.pmed.1002965
Mariottini, C., Kriikku, P., and Ojanperä, I. (2021). Concomitant drugs with buprenorphine user deaths. Drug Alcohol Depend. 218, 108345. doi:10.1016/j.drugalcdep.2020.108345
Mercadante, S., Porzio, G., Aielli, F., Ferrera, P., Codipietro, L., Lo Presti, C., et al. (2013). The effects of low doses of pregabalin on morphine analgesia in advanced cancer patients. Clin. J. Pain 29, 15–19. doi:10.1097/AJP.0b013e318247809a
Meyers, C. A. (2008). How chemotherapy damages the central nervous system. J. Biol. 7, 11. doi:10.1186/jbiol73
Montastruc, F., Loo, S. Y., and Renoux, C. (2018). Trends in first gabapentin and pregabalin prescriptions in primary care in the United Kingdom, 1993-2017. Jama 320, 2149–2151. doi:10.1001/jama.2018.12358
Page, M. J., McKenzie, J. E., Bossuyt, P. M., Boutron, I., Hoffmann, T. C., Mulrow, C. D., et al. (2021). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J. Clin. Epidemiol. 134, 178–189. doi:10.1016/j.jclinepi.2021.03.001
Paul, J. E., Nantha-Aree, M., Buckley, N., Cheng, J., Thabane, L., Tidy, A., et al. (2013). Gabapentin does not improve multimodal analgesia outcomes for total knee arthroplasty: A randomized controlled trial. Can. J. Anaesth. = J. Can. d'anesthesie 60, 423–431. doi:10.1007/s12630-013-9902-1
Peckham, A. M., Fairman, K. A., and Sclar, D. A. (2018). All-cause and drug-related medical events associated with overuse of gabapentin and/or opioid medications: A retrospective cohort analysis of a commercially insured us population. Drug Saf. 41, 213–228. doi:10.1007/s40264-017-0595-1
Pesonen, A., Suojaranta-Ylinen, R., Hammarn, E., Kontinen, V. K., Raivio, P., Tarkkila, P., et al. (2011). Pregabalin has an opioid-sparing effect in elderly patients after cardiac surgery: A randomized placebo-controlled trial. Br. J. Anaesth. 106, 873–881. doi:10.1093/bja/aer083
Pota, V., Barbarisi, M., Sansone, P., Moraci, M., Pace, M. C., Passavanti, M. B., et al. (2012). Combination therapy with transdermal buprenorphine and pregabalin for chronic low back pain. Pain Manag. 2, 23–31. doi:10.2217/pmt.11.71
Ramirez, M. F., Kamdar, B. B., and Cata, J. P. (2020). Optimizing perioperative use of opioids: A multimodal approach. Curr. Anesthesiol. Rep. 10, 404–415. doi:10.1007/s40140-020-00413-6
Rapchuk, I. L., O'Connell, L., Liessmann, C. D., Cornelissen, H. R., and Fraser, J. F. (2010). Effect of gabapentin on pain after cardiac surgery: A randomised, double-blind, placebo-controlled trial. Anaesth. Intensive Care 38, 445–451. doi:10.1177/0310057x1003800306
Savelloni, J., Gunter, H., Lee, K. C., Hsu, C., Yi, C., Edmonds, K. P., et al. (2017). Risk of respiratory depression with opioids and concomitant gabapentinoids. J. Pain Res. 10, 2635–2641. doi:10.2147/jpr.S144963
Scholl, L., Seth, P., Kariisa, M., Wilson, N., and Baldwin, G. (2018). Drug and opioid-involved overdose deaths - United States, 2013-2017. MMWR. Morb. Mortal. Wkly. Rep. 67, 1419–1427. doi:10.15585/mmwr.mm675152e1
Slavova, S., Miller, A., Bunn, T. L., White, J. R., Kirschke, D., Light, T., et al. (2018). Prevalence of gabapentin in drug overdose postmortem toxicology testing results. Drug Alcohol Depend. 186, 80–85. doi:10.1016/j.drugalcdep.2018.01.018
Sterne, J. A. C., Savovic, J., Page, M. J., Elbers, R. G., Blencowe, N. S., Boutron, I., et al. (2019). RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898. doi:10.1136/bmj.l4898
Sterne, J. A., Hernan, M. A., Reeves, B. C., Savovic, J., Berkman, N. D., Viswanathan, M., et al. (2016). ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 355, i4919. doi:10.1136/bmj.i4919
Teng, L., Dai, J., Shao, H., Zhao, L., Lin, S., Zhang, W., et al. (2021). Gabapentin enhances the antinociceptive effect of intrathecal morphine in refractory cancer pain patients. Support. care cancer official J. Multinatl. Assoc. Support. Care Cancer 29, 7611–7616. doi:10.1007/s00520-021-06350-2
Turan, A., Kaya, G., Karamanlioglu, B., Pamukçu, Z., and Apfel, C. C. (2006). Effect of oral gabapentin on postoperative epidural analgesia. Br. J. Anaesth. 96, 242–246. doi:10.1093/bja/aei294
Verret, M., Lauzier, F., Zarychanski, R., Perron, C., Savard, X., Pinard, A.-M., et al. (2020). Perioperative use of gabapentinoids for the management of postoperative acute pain: A systematic review and meta-analysis. Anesthesiology 133, 265–279. doi:10.1097/aln.0000000000003428
Waddy, S. P., Becerra, A. Z., Ward, J. B., Chan, K. E., Fwu, C. W., Eggers, P. W., et al. (2020). Concomitant use of gabapentinoids with opioids is associated with increased mortality and morbidity among dialysis patients. Am. J. Nephrol. 51, 424–432. doi:10.1159/000507725
Wang, Y., Yang, H., Shen, C., and Luo, J. (2017). Morphine and pregabalin in the treatment of neuropathic pain. Exp. Ther. Med. 13, 1393–1397. doi:10.3892/etm.2017.4102
Yadeau, J. T., Paroli, L., Kahn, R. L., Jules-Elysee, K. M., Lasala, V. R., Liu, S. S., et al. (2012). Addition of pregabalin to multimodal analgesic therapy following ankle surgery: A randomized double-blind, placebo-controlled trial. Reg. Anesth. Pain Med. 37, 302–307. doi:10.1097/AAP.0b013e31824c6846
Yu, D., Appleyard, T., Cottrell, E., and Peat, G. (2021). Co-prescription of gabapentinoids and opioids among adults with and without osteoarthritis in the United Kingdom between 1995 and 2017. Rheumatol. Oxf. 60, 1942–1950. doi:10.1093/rheumatology/keaa586
Yucel, A., Ozturk, E., Aydoan, M. S., Durmus, M., Colak, C., and Ersoy, M. O. (2011). Effects of 2 different doses of pregabalin on morphine consumption and pain after abdominal hysterectomy: A randomized, double-blind clinical trial. Curr. Ther. Res. Clin. Exp. 72, 173–183. doi:10.1016/j.curtheres.2011.06.004
Keywords: opioid, gabapentin, pregabalin, safety, mortality
Citation: Hahn J, Jo Y, Yoo SH, Shin J, Yu YM and Ah Y-M (2022) Risk of major adverse events associated with gabapentinoid and opioid combination therapy: A systematic review and meta-analysis. Front. Pharmacol. 13:1009950. doi: 10.3389/fphar.2022.1009950
Received: 02 August 2022; Accepted: 12 September 2022;
Published: 11 October 2022.
Edited by:
Carlos Alves, University of Coimbra, PortugalReviewed by:
Mance E. Buttram, University of Arkansas, United StatesUdo Bonnet, Castrop-Rauxel Evangelical Hospital, Germany
Copyright © 2022 Hahn, Jo, Yoo, Shin, Yu and Ah. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yun Mi Yu, yunmiyu@yonsei.ac.kr; Young-Mi Ah, ymah@ynu.ac.kr
†ORCID: Yun Mi Yu, orcid.org/0000-0003-1607-9828; Young-Mi Ah, orcid.org/0000-0002-8267-9453
‡These authors have contributed equally to this work
 Jongsung Hahn
Jongsung Hahn Youngkwon Jo
Youngkwon Jo So Hee Yoo3
So Hee Yoo3 Jaekyu Shin
Jaekyu Shin Yun Mi Yu
Yun Mi Yu