- 1Department of Orthopedic Surgery, Brigham and Women’s Hospital, Harvard Medical School, Harvard University, Boston, MA, United States
- 2Department of Orthopedics, The First Affiliated Hospital, Guangzhou Medical University, Guangzhou, China
- 3Jing Brand Research Institute, Jing Brand Co., Ltd., Daye, China
- 4Department of Spine Center, Zhongda Hospital, Southeast University Medical School, Nanjing, China
- 5Department of Orthopedic Surgery, Tongji Hospital, School of Medicine, Tongji University, Shanghai, China
- 6Department of Orthopaedics, Qilu Hospital, Shandong University, Jinan, China
- 7Department of Orthopaedics, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 8Department of Neurosurgery, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA, United States
- 9Harvard Stem Cell Institute, Harvard University, Cambridge, MA, United States
Traditional Chinese medicine (TCM) has been practiced in the treatment of bone diseases and alcoholism. Chronic excessive alcohol use results in alcohol-induced bone diseases, including osteopenia and osteoporosis, which increases fracture risk, deficient bone repair, and osteonecrosis. This preclinical study investigated the therapeutic effects of TCM herbal extracts in animal models of chronic excessive alcohol consumption-induced osteopenia. TCM herbal extracts (Jing extracts) were prepared from nine Chinese herbal medicines, a combinative herbal formula for antifatigue and immune regulation, including Astragalus, Cistanche deserticola, Dioscorea polystachya, Lycium barbarum, Epimedium, Cinnamomum cassia, Syzygium aromaticum, Angelica sinensis, and Curculigo orchioides. In this study, Balb/c male mice were orally administrated alcohol (3.2 g/kg/day) with/without TCM herbal extracts (0.125 g/kg, 0.25 g/kg, or 0.5 g/kg) by gavage. Our results showed that after 50 days of oral administration, TCM herbal extracts prevented alcohol-induced osteopenia demonstrated by μ-CT bone morphological analysis in young adults and middle-aged/old Balb/c male mice. Biochemical analysis demonstrated that chronic alcohol consumption inhibits bone formation and has a neutral impact on bone resorption, suggesting that TCM herbal extracts (Jing extracts) mitigate the alcohol-induced abnormal bone metabolism in middle-aged/old male mice. Protocatechuic acid, a natural phenolic acid in Jing extracts, mitigates in vivo alcohol-induced decline of alkaline phosphatase (ALP) gene expression in the bone marrow of Balb/c male mice and in vitro ALP activity in pre-osteoblast MC3T3-E1 cells. Our study suggests that TCM herbal extracts prevent chronic excessive alcohol consumption-induced osteopenia in male mice, implying that traditional medicinal plants have the therapeutic potential of preventing alcohol-induced bone diseases.
Introduction
Traditional Chinese medicine has treated various diseases, including bone diseases and alcoholism. Osteoporosis is the most common bone disease, representing a significant public health problem worldwide (Cosman et al., 2014; Wright et al., 2014; Langdahl and Andersen, 2018; Compston et al., 2019). There has been considerable progress in understanding postmenopausal and senile osteoporosis; however, knowledge gaps still exist in understanding osteoporosis/osteopenia caused by alcoholism. The effects of alcohol on human health are complex. Although it is debatable about the benefits of moderate drinking, epidemiological studies suggest that light-moderate alcohol consumption benefits the heart and circulatory system, protects against diabetes, and is associated with lower risks for mortality and cancer in older adults (Howard et al., 2004; Karlamangla et al., 2009; Mostofsky et al., 2016; Kunzmann et al., 2018). However, heavy drinking is detrimental to many organs and tissues and is a significant cause of preventable stroke, heart failure, and death (Garaycoechea et al., 2018; GBD, 2018; Wood et al., 2018; USDA and HHS, 2020; NIAAA., 2021). Alcohol has a complex effect on the adult skeleton depending on age, drinking pattern, and alcohol consumption. Light to moderate alcohol consumption is generally reported to be beneficial or have a neutral impact on bone health in old adults, while chronic excessive drinking induces bone mass loss and osteoporosis, which increases fracture risk, deficient bone repair and causes alcohol-induced osteonecrosis (Turner, 2000; Chakkalakal, 2005; Maurel et al., 2012; Sommer et al., 2013; Mikosch 2014; Gaddini et al., 2016).
TCM-based herbal therapies often use alcohol as a delivery medium for centuries; one class of Chinese herbal medicines is Chinese herbal Liqueur (Xia, 2013; Cheung et al., 2021). The alcoholic drinks of herbal extracts are also used for a wide range of medicinal purposes documented in classical pharmacy and ethnobotanical studies in Europe (Egea et al., 2015). The Chinese medicinal or herbal Liqueur, an alcoholic beverage, is commonly produced by soaking precious Chinese medicinal materials (Xia, 2013; Cheung et al., 2021). It became prevalent among Chinese people due to its nourishing and tonic functions or as nutraceuticals. A typical TCM formula has multiple ingredients that synergize in treatment for the symptoms of a disease, which have greater efficacy and safety than a single drug in clinical practices, possibly due to the multiple ingredients synergistic interactions and mutual detoxification (Ung et al., 2007; He et al., 2015; Zhang and Cheng, 2019). The TCM herbal extracts (Jing extracts) used in this study were prepared from a herbal combination formula of nine Chinese herbal medicines, which was used in a famous Chinese Herbal Liqueur, Chinese Jing Liqueur (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018; Cai et al., 2020; Hu et al., 2021). Preclinical and clinical studies demonstrated that studied herbal formulation has the properties of improving kidney-yang deficiency in rats and relieving main symptoms of patients with Kidney-Yang Deficiency Syndrome, anti-fatigue, and enhancing immunity in humans and animals (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018; Cai et al., 2020; Hu et al., 2021). The analyses of chemical components by HPLC, LC/MS, and NMR (Liu et al., 2008; Cai et al., 2020; Hu et al., 2021) showed that the extracts of these Chinese herbal medicines (Jing extracts) contain a variety of saponins, flavonoids, active polysaccharides, and other functional factors and nutrients required by the human body. Although preclinical and clinical data show that saponins, flavonoids, and polysaccharides prevent osteoporosis, the therapeutic effects of Jing extracts on alcohol-induced bone diseases were unknown. In this study, we investigated whether TCM herbal extracts prevent chronic alcohol consumption-induced osteopenia in mice.
Materials and Methods
Materials and Experimental Design
The traditional Chinese medicine herbal extracts used in this study is a combinative herbal formula of nine Chinese herbal medicines, including Astragalus mongholicus Bunge (Astragalus) (10–15% of total herbal medicine weight as described in Liu et al., 2008), Cistanche deserticola Y. C. Ma (Cistanche deserticola) (10–15%), Dioscorea polystachya Turcz. (Dioscorea polystachya, Chinese yam) (10–15%), Lycium barbarum L. (Lycium barbarum, Chinese wolfberry or Goji berry) (10–15%), Epimedium brevicornu Maxim (Epimedium, Herba Epimedii) (10–15%), Cinnamomum cassia (L.) J. Presl (Cinnamomum cassia, Chinese Cinnamon) (3–5%), Syzygium aromaticum Merr. and L.M.Perry (Syzygium aromaticum) (3–5%), Angelica sinensis (Oliv.) Diels (Angelica sinensis, Chinese angelica) (10–15%), and Curculigo orchioides Gaertn. (Curculigo orchioides, Rhizoma curculiginis) (10–15%) (Figure 1; Supplementary Figure S1; Table 1; Supplementary Table S1). This herbal formula was originally designed in late China’s Qing Dynasty. After being modified several times by TCM experts in the 1980s, it was approved by China’s National Medical Products Administration (Approval # 1997 No. 728) for anti-fatigue and enhancing immunity as the TCM herbal formula in a famous Chinese Herbal Liqueur, Chinese Jing Liqueur (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018; Cai et al., 2020; Hu et al., 2021). The traditional Chinese medicine herbs were collected by researchers at Jing Brand Research Institute. Voucher specimens (as described in Cai et al., 2020) are deposited at the Herbarium of Jing Brand Research Institute, Daye, Hubei, China. The raw Chinese herbal medicines were washed, dried, and sliced into pieces and superfine pulverization according to the protocols in Chinese Pharmacopoeia. The traditional Chinese medicine herbal extracts (Jing extracts) were prepared with a percolation extraction method with 35% alcohol (50-fold of total herbal medicine weight for 12–15 days) (Liu et al., 2008; Shan et al., 2018; Cai et al., 2020). After percolation, the liquids were separated from insoluble materials with disc centrifuge and membrane separation technology and concentrated via nanofiltration membrane; the concentrated traditional Chinese medicine herbal extracts, referred to as Jing extracts, was obtained for pharmacognostic analysis to discern the effects of Jing extracts on alcohol-induced osteopenia (Figure 1). The extracts of these Chinese herbal medicines (Jing extracts) have been characterized by chemical constituent analysis with LC/MS and NMR (Cai et al., 2020; Hu et al., 2021) as well as high-performance liquid chromatogram-gas chromatography fingerprint analysis (Liu et al., 2008) (Table 2; Supplementary Figure S7).
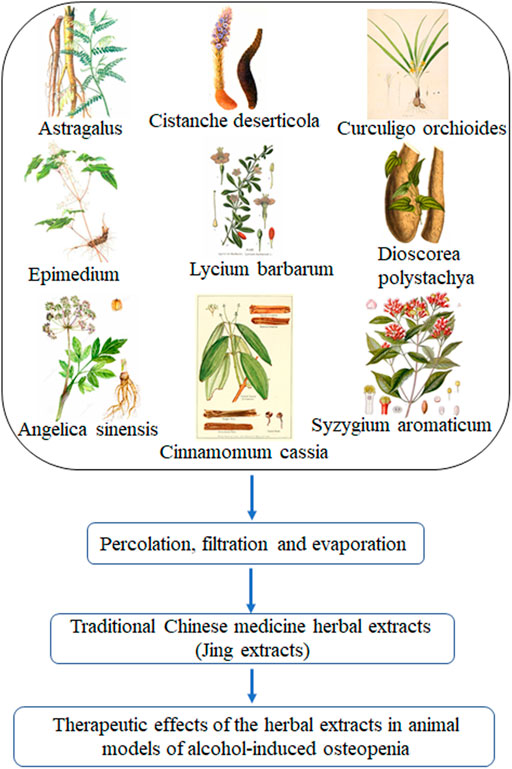
FIGURE 1. Traditional Chinese medicine herbal extracts (Jing extracts) and experimental design. After percolation, filtration, and evaporation, the concentrated extracts of the nine traditional Chinese medicine herbs, referred to as Jing extracts, were used to discern the therapeutic effects of the herbal extracts in animal models of alcohol-induced osteopenia.

TABLE 2. Roles of bioactive compounds in the studied herbs in bone diseases and alcohol use disorder.
Animal Models of Alcohol-Induced Osteopenia
Two-month-old Balb/c male mice were purchased from Jackson lab (Bar Harbor, ME) as the young adult animal in this study (after 50 experimental periods, the mice were 4-month-old). The middle-aged/old Balb/c male mice (10-month-old) (after 50 experimental periods, the mice were about 12-month-old) were purchased from Envigo (Indianapolis, IN). Mice were provided with standard chow and water ad libitum. Animal maintenance and experimental treatments were conducted per the ethical guidelines for animal research established and approved by BWH Institutional Animal Care and Use Committee.
There are several different murine models of alcohol-induced bone loss regarding alcohol administration, including oral alcohol administration by gavage (Iwaniec and Turner, 2013; Martiniakova et al., 2018), alcohol in drinking water (Liu et al., 2016; Okuda et al., 2018), intraperitoneal (i.p.) administration of alcohol by injection (Iwaniec and Turner, 2013), and Liber-DeCarli liquid diet feeding (Dai et al., 2000; Mercer et al., 2012). Although there are advantages and disadvantages among the methods of alcohol administration, oral alcohol administration by gavage in animals is a better model to mimic intermittent drinking in humans. We used the oral alcohol administration by gavage in Balb/c male mice as the murine models of alcohol-induced osteopenia and to study the effects of TCM extracts on alcohol-induced osteopenia. The process of oral alcohol administration by gavage was performed as described in the NIAAA model (Bertola et al., 2013). As shown in Figure 2, oral administration of 3.2 g/kg alcohol (0.2 ml of 40% v/v alcohol for a 20 g body weight mouse) by gavage for 50 days was used to study alcohol-induced osteopenia in male Balb/c mice.
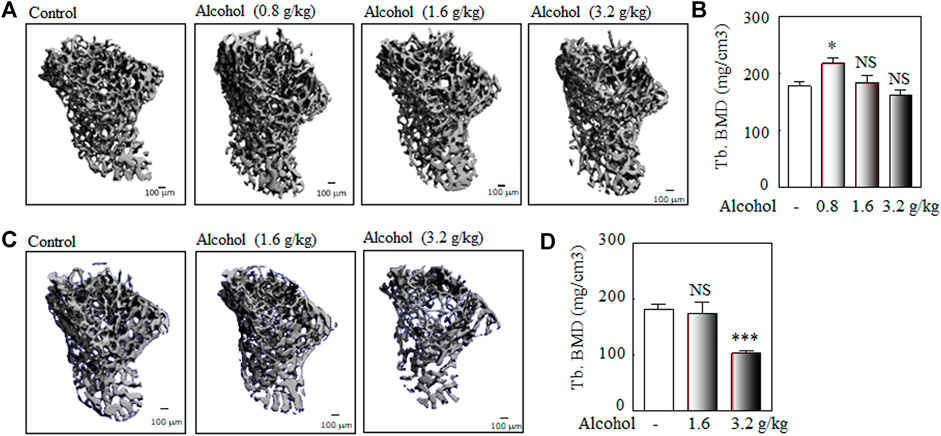
FIGURE 2. The murine models of alcohol-induced osteopenia: the dose and duration. Balb/c male mice (2-month-old) were used to explore the optimal dose and duration of the oral alcohol administration by gavage for alcohol-induced osteopenia. The young adult Balb/c male mice (2-month-old) were used to analyze the optimal dose (0.8, 1.6 g/kg, and 3.2 g/kg body weight) and duration (once per day at 3–4 PM, 5 days per week for 30 or 50 days) of alcohol-induced osteopenia. (A) The representative μ-CT 3-D microstructures of trabecular bone were obtained from male mice with alcohol gavage (Alcohol) or water gavage (Control) for 30 days; bars represent 100 μm. 3-D microstructural properties of the tibia were calculated using software supplied by the manufacturer. (B) The quantitative analysis of trabecular bone mineral density (Tb. BMD); the oral alcohol administration of 0.8 g/kg dose of alcohol (n = 4) for 30 days significantly increased BMD in Balb/c adult male mice (alcohol vs. control group, n = 5, *p < 0.05, Mann-Whitney test); the 1.6 g/kg (n = 7) and 3.2 g/kg (n = 5) doses of alcohol for 30 days did not induce significant bone loss (alcohol vs. control group, n = 5; NS: not significant, Mann-Whitney test). (C) The representative μ-CT 3-D microstructures of trabecular bone were obtained from male mice with alcohol gavage (Alcohol) or water gavage (Control) for 50 days. (D) The quantitative analysis of trabecular bone mineral density (Tb. BMD); a lower dose (1.6 g/kg) of alcohol for a longer duration (50 days) did not induce bone loss (alcohol, n = 7, vs. control, n = 18, NS: not significant, Mann-Whitney test); the oral alcohol administration of 3.2 g/kg alcohol for 50 days induced significant bone loss (alcohol, n = 8 vs. control group, n = 18, ***p < 0.001, t-test).
Traditional Chinese Medicine Herbal Extracts (Jing Extracts) Administration in Mice
The doses of Jing extracts used in the experimental mice were designed based on the recommended human dose (Liu et al., 2008; Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018) and the human-mouse dose conversion (Wojcikowski and Gobe, 2014; Nair and Jacob S., 2016) (details refer to Supplementary Material). Taking into account that the commonly used mouse gavage volume is 10 ml/kg (0.2 ml for a 20 g mouse), we defined the first mouse dose of Chinese herbal extracts (0.2 ml for a 20 g mouse), as the low dose (0.125 g/kg body weight for a 20 g mouse with 0.2 ml oral administration by gavage of 12.5 g/L of Chinese herbal extracts in 40% v/v alcohol); the second and third doses contained 0.25 g/kg (25 g/L of Chinese herbal extracts in 40% v/v alcohol) and 0.50 g/kg (50 g/L of Chinese herbal extracts in 40% v/v alcohol) of Chinese herbal extracts respectively. We tested the effects of these three doses of Chinese herbal extracts on alcohol-induced bone loss in Balb/c male mice. After 50 days of orally administering 3.2 g/kg of alcohol with/without Chinese herbal extracts by gavage, the mice were sacrificed for bone morphological and biochemical analysis.
Bone Morphological Analysis: μ-CT
Bone morphological analysis of proximal tibia was performed with a μ-CT (μ-CT 35, Scanco Medical, Switzerland) as described (Charles et al., 2012). The scanned region proximal to the growth plate and extending 1.4 mm was selected for trabecular bone analysis (indicated with a box Supplementary Figure S2). A second region 0.6 mm in length and centered at the midpoint of the tibia was used to calculate the diaphyseal parameters (shown with a box in Supplementary Figure S2). 3-D microstructural properties of bone, e.g., trabecular bone mineral density (Tb. BMD), tibia trabecular relative bone volume (Tb. BV/TV), trabecular thickness (Tb. Th), trabecular space (Tb. Sp), trabecular number (Tb. N), and cortical bone mineral density (C. BMD), were calculated using software supplied by the manufacturer (Scanco Medical, Switzerland).
Bone Morphological Analysis: Histological Analysis
In addition to μ-CT analysis of skeletal parameters, some murine bones were used for histological analysis. The tibia was removed from the attached muscle tissue, fixed with 10% formalin (at least 48 h), decalcified with EDTA, plastic-embedded (JB-4 Kit, Polysciences, Warrington, PA), and sectioned (10 μm); the sections were stained with 0.5% toluidine blue-O, pH 4.0 (Thermo Fisher Scientific, Waltham, MA, United States).
Biochemical Analysis of Alcohol-Induced Bone Loss
The blood was taken 6 h after fasting on the second day of the last gavage, left for 30–60 min after blood collection, centrifuged at 1,500 g for 15 min, and the serum was separated and kept at −80 C until the biochemical analysis for biomarkers of bone formation, e.g., osteocalcin (OC), and bone resorption, e.g., Type I collagen carboxy-terminal telopeptide (CTX). The serum levels of osteocalcin, a non-collagenous protein formed by osteoblasts, were detected by using the mouse osteocalcin ELISA kit (MyBioSource, San Diego, CA, United States). Type I collagen is the main organic component of bones. When physiological or pathological bone resorption is increased (such as osteoporosis), the degradation of type I collagen is also increased, and the content of decomposed fragments in the blood is correspondingly increased. The main molecular fragment of type I collagen degradation products is the CTX. An increase in the serum CTX level indicates an increase in bone resorption. The serum levels of Type I collagen carboxy-terminal telopeptide (CTX) were detected by using Mouse Cross-Linked C-Telopeptide of Type I Collagen (CTXI) ELISA Kit (MyBioSource, San Diego, CA, United States).
RNA Isolation and RT-PCR
Total RNA was isolated from bone marrow or MC3T3-E1 cells with Trizol reagent (Invitrogen). For RT-PCR, 2 μg of total RNA was reverse-transcribed into cDNA with M-MLV reverse transcriptase (Promega, WI), following the manufacturer’s instructions. One-twentieth of the cDNA was used in each 50 μl PCR reaction with Promega GoTaq Flexi DNA Polymerase (30–35 cycles of 94°C for 1 min, 55–60°C for 1 min, and 72°C for 2 min). Amplification conditions were optimized to reflect the exponential phase of amplification for each gene. Gene-specific primers were used to amplify ALP, TNF-α, IL-1β, BSP, RUNX2, and BMP2 as shown in Supplementary Table S2. PCR products were separated by 2% agarose gel electrophoresis and captured with KODAK Gel Logic 200 Imaging System and KODAK Molecular Imaging Software (Carestream Health, Rochester, NY, United States). The densitometry of inverted ethidium bromide staining PCR bands was quantified by NIH ImageJ. Relative expression levels were calculated by normalizing the densitometric units to GAPDH, the internal control.
Alkaline Phosphatase Enzyme Assay
MC3T3-E1 cells were cultured in quadruplicate in 24-well-plates in a growth medium. Upon confluence, medium was changed to osteoblastogenic medium (phenol red-free α-MEM with 10% FBS-HI, 100 U/ml penicillin, 100 μg/ml streptomycin plus 5 mM β-glycerophosphate and 50 μg/ml ascorbate-2-phosphate) for 6 days. Protein concentrations were quantified using BCA Assay kit, and ALP enzyme activity was measured spectrophotometrically and expressed as μmol/min/g protein (Zhou et al., 2008).
Statistical Analysis
All experiments were performed at a minimum in triplicate; for in vivo experiments, a minimum three mice per experimental group per time were performed; for in vitro ALP activity, quadruplicate wells per experimental group were performed. All test parameters were quantified as continuous data and used standard statistical tests. The Kolmogorov-Smirnov tool was used to test whether data sets were distributed normally. Group data are presented as mean ± SEM. Unless otherwise indicated, quantitative data were analyzed with a non-parametric Mann-Whitney test or if data allowed, a parametric t-test for group comparisons with GraphPad Instat (GraphPad Software, La Jolla, CA, United States). A value of p < 0.05 was considered significant.
Results
The Murine Model of Alcohol-Induced Osteopenia: The Optimal Dose and Duration of Oral Alcohol Administration
The young adult Balb/c male mice (2-month-old) were used to explore the doses (0.8, 1.6, and 3.2 g/kg body weight, once per day at 3–4 PM, 5 days per week) and duration (30 and 50 days) of oral alcohol administration for alcohol-induced osteopenia in male mice. Our data showed that Balb/c male mice (2-month-old) were orally administered alcohol by gavage, the 0.8 g/kg dose of alcohol for 30 days significantly increased BMD in Balb/c adult male mice, and the 1.6 g/kg and 3.2 g/kg alcohol did not induce significant bone loss after 30 days gavage (Figures 2A,B); a low dose of 1.6 g/kg alcohol did not affect bone mineral density after 50 days gavage (Figures 2C,D); after 50 days of oral administered alcohol, 3.2 g/kg of alcohol-induced significant bone loss in Balb/c male mice (about 4-month-old when sacrificed) (Figures 2C,D). Thereafter, we used the dose of 3.2 g/kg alcohol and a duration of 50 days for the effects of TCM extracts on alcohol-induced osteopenia.
Chinese Herbal Extracts Prevent Chronic Alcohol Consumption-Induced Osteopenia in Young Adult Male Mice
We used the dose of 3.2 g/kg of alcohol administered by gavage for 50 days, which induced significant bone loss in young adult male Balb/c mice, as shown in Figure 2, for the effects of herbal extracts on alcohol-induced osteopenia. To explore the optimal doses of traditional Chinese medicine herbal extracts (Jing extracts) that prevent alcohol-induced osteopenia, Balb/c male mice (2-month-old) were orally administered 3.2 g/kg alcohol by gavage with or without 0.125 g/kg, 0.25 g/kg, or 0.5 g/kg of traditional Chinese medicine herbal extracts (Jing extracts) (as described in Figure 1). After 50 days of oral administration of alcohol with/without Chinese herbal extracts by gavage, the mice were sacrificed for bone morphological analysis by μ-CT with the methods described in Supplementary Figure S2 and Figure 3. The 3-D microstructures of trabecular bone (Figure 3A) and quantitative analysis of the microstructural properties of the tibia (Figures 3B–G), including trabecular bone mineral density (Tb. BMD), trabecular relative bone volume (Tb. BV/TV), trabecular number per mm (Tb. N), and trabecular Structure modulus index (Tb. SMI), showed that the effect of Chinese herbal extracts is dose-dependent in preventing alcohol-induced bone loss in young adult male mice. Our data showed that 3.2 g/kg alcohol induced significant bone loss in young adult male Balb/c mice after 50 days of oral administration by gavage (Figure 3); the low, middle, and high doses of Chinese herbal extracts alleviate the chronic alcohol consumption-induced trabecular bone damage; all three doses of Chinese herbal extracts mitigate the alcohol-induced decreases of bone mineral density and relative bone volume or bone volume fraction (Figures 3B,C); the Chinese herbal extracts also alleviate alcohol-reduced trabecular number decrease (Figure 3D) and the corresponding increase of trabecular separation (Figure 3E), but no effects were observed for trabecular thickness (Figure 3F). Although there are controversial in the literature (Salmon et al., 2015), structure model index (SMI), which is widely used to measure rods and plates in trabecular bone, represents the trabecular geometry transitions from being more plate-like (reflected high mechanical stress) to more rod-like (low mechanical stress) as osteoporosis severity increases (Ding et at., 2002; Hildebrand and Rüegsegger, 1997), which inversely correlated with the bone mechanical property (Ding et al., 2002). Our data showed that two doses of 0.25 g/kg and 0.5 g/kg of Chinese herbal medicines significantly mitigate the alcohol-induced damage in the bone mechanical property (Figure 3G).

FIGURE 3. Traditional Chinese medicine herbal extracts (Jing extracts) prevent chronic alcohol consumption-induced osteopenia in young adult male mice. To explore the optimal dose of Jing extracts that prevent alcohol-induced osteopenia in young adult mice, Balb/c male mice (2-month-old) were orally administered 10 ml/kg of 40% alcohol (3.2 g/kg body weight) by gavage for 50 days (gavage once a day, 5 days per week) with or without 0.125 g/kg, 0.25 g/kg or 0.5 g/kg of Jing extracts. (A) The representative μ-CT 3-D microstructures of trabecular bone were obtained from male mice with alcohol gavage (Alcohol) ± Jing extracts or without alcohol (Control); bars represent 100 μm. 3-D microstructural properties of the tibia were calculated using software supplied by the manufacturer. (B) The quantitative analysis of trabecular bone mineral density (Tb. BMD) (3.2 g/kg of Alcohol, n = 9, vs. DDW control, n = 19, ***p < 0.001, t-test; Alcohol + 0.125 g/kg of Jing extracts, n = 4, vs. Alcohol, n = 9, *p < 0.05, Mann-Whitney test; Alcohol + 0.25 g/kg of Jing extracts, n = 11 vs. Alcohol, n = 9, **p < 0.01, t-test; Alcohol + 0.5 g/kg of Jing extracts, n = 5, vs. Alcohol, n = 9, ***p < 0.001, t-test). (C) The quantitative analysis of tibia trabecular relative bone volume (Tb. BV/TV) (***p < 0.001, t-test; *p < 0.05, Mann-Whitney test). (D) The quantitative analysis of tibia trabecular number per mm (Tb. N) (***p < 0.001, **p < 0.01, t-test; *p < 0.05, Mann-Whitney test). (E) The quantitative analysis of tibia trabecular separation (mm) (**p < 0.01, t-test; *p < 0.05, NS: not significant, Mann-Whitney test). (F) The quantitative analysis of trabecular thickness (Tb. Th, μm) (**p < 0.01, t-test; NS: not significant, Mann-Whitney test). (G) The quantitative analysis of tibia trabecular Structure modulus index (Tb. SMI) (***p < 0.001, **p < 0.01, t-test; NS: not significant, Mann-Whitney test).
Traditional Chinese Medicine Herbal Extracts (Jing Extracts) Prevent Chronic Alcohol Consumption-Induced Osteopenia in Middle-Aged/Old Male Mice
Prevention of problematic alcohol use is mainly focused on younger adults, while an epidemiological study showed that heavy drinking in middle-aged and older adults is more frequent with a greater impact on function and health (Veerbeek et al., 2019). There are age-related changes in bone structure and strength in Balb/c mice (Willinghamm et al., 2010). To investigate the preventing effects of Jing extracts on chronic alcohol consumption-induced osteopenia, we used 10-month-old Balb/c male mice (about 12 months old after the experiments of chronic alcohol consumption-induced osteopenia), which have a significantly lower bone mineral density than the young adult mice (about 4 months old after the experiments) (Supplementary Figure S3), but still have enough trabecular bones, as shown by μ-CT and histological staining, to study the chronic alcohol consumption-induced bone loss and the preventive effects of herbal extracts on osteopenia.
To study the effects of traditional Chinese medicine herbal extracts on chronic alcohol consumption-induced osteopenia in middle-aged mice, Balb/c male mice (10-month-old) were orally administered 3.2 g/kg alcohol by gavage with or without 0.25 g/kg of Chinese herbal extracts (as described in Figure 1). After 50 days of oral administration of alcohol by gavage with/without Chinese herbal extracts (the mice were about 12-month-old at the end of the experiments), the mice were sacrificed for bone morphological analysis with μ-CT as the methods described in Supplementary Figure S2, including the 3-D microstructures of trabecular bone (Figure 4A) and quantitative analysis of the microstructural properties of the tibia, including trabecular bone mineral density (Tb. BMD) (Figure 4B) and trabecular relative bone volume (Tb. BV/TV) (Figure 4B). Our data showed that alcohol (3.2 g/kg) induced significant bone loss in middle-aged/old male Balb/c mice after 50 days of oral administration by gavage (Figure 4); the 0.25 g/kg of Chinese herbal extracts alleviate the chronic alcohol consumption-induced trabecular bone damage, which was demonstrated by Chinese herbal extracts mitigating the alcohol-induced decreases of bone mineral density and relative bone volume or bone volume fraction (Figure 4B).
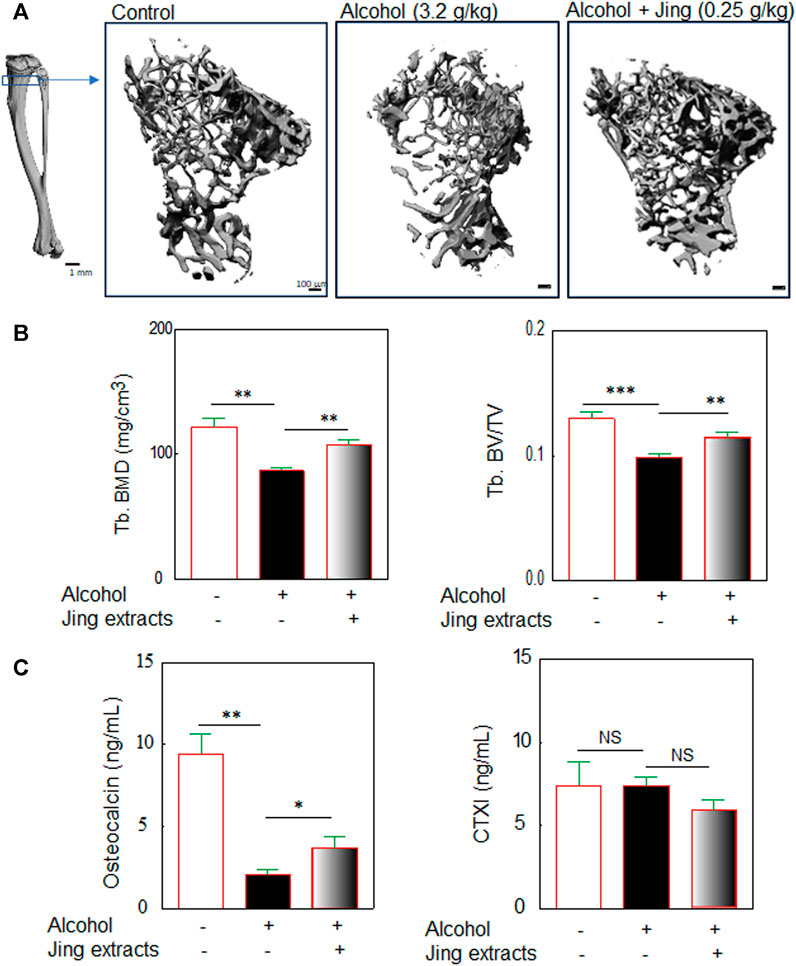
FIGURE 4. Traditional Chinese medicine herbal extracts (Jing extracts) prevent chronic alcohol consumption-induced osteopenia in middle-aged/old male mice. Balb/c male mice (10-month-old) were orally administered 3.2 g/kg body weight of alcohol by gavage for 50 days (gavage once a day, 5 days per week) with or without 0.25 g/kg of Jing extracts. (A) The representative μ-CT 3-D microstructures of trabecular bone were obtained from male mice with alcohol gavage (Alcohol) ± Jing extracts or without alcohol (Control); bars represent 100 μm. 3-D microstructural properties of the tibia were calculated using software supplied by the manufacturer. (B) The quantitative analysis of trabecular bone mineral density (Tb. BMD) (3.2 g/kg of Alcohol, n = 8, vs. DDW control, n = 9, **p < 0.01; Alcohol + 0.25 g/kg of Jing extracts, n = 9, vs. Alcohol, n = 8, **p < 0.01, t-test) and tibia trabecular relative bone volume (Tb. BV/TV) (***p < 0.001, **p < 0.01, t-test). (C) The effects of Jing extracts on biomarkers of bone formation and bone resorption in middle-aged/old Balb/c male mice. Balb/c male mice (10-month-old) were orally administered 3.2 g/kg body weight of alcohol by gavage for 50 days (gavage once a day, 5 days per week) with or without 0.25 g/kg of Jing extracts (about 12-month-old when mice were sacrificed). Biochemical analysis of bone formation biomarker osteocalcin in serum of middle-aged/old mice (alcohol, n = 6, vs. control, n = 9, **p < 0.01, t-test; Jing extracts, n = 7, vs. alcohol, n = 6, *p < 0.05, Mann-Whitney test) and biochemical analysis of bone resorption biomarker cross-linked C-Telopeptide of Type I Collagen (CTXI) (NS: not significant, t-test).
The biochemical analysis for biomarkers of bone formation and bone resorption was performed by using the serum obtained from the middle-aged/old mice. The serum levels of osteocalcin, a bone formation biomarker, were detected with a mouse osteocalcin ELISA kit (MyBioSource, San Diego, CA, United States). An increase in the serum type I collagen carboxy-terminal telopeptide (CTX) level indicates increased bone resorption. The serum levels of Type I collagen carboxy-terminal telopeptide (CTX) were detected by using a Mouse Cross-Linked C-Telopeptide Of Type I Collagen (CTXI) ELISA Kit (MyBioSource, San Diego, CA, United States). Our biochemical analysis (Figure 4C) showed that 3.2 g/kg of alcohol significantly reduced serum levels of osteocalcin (Figure 4C) but did not reduce serum levels of CTXI (Figure 4C) in middle-aged/old Balb/c male mice. Out data suggest that chronic alcohol consumption inhibits bone formation but has no effects on bone resorption, which is consistent with previous reports (summarized in Sampson, 1998), indicated that alcoholic osteoporosis is characterized by decreased bone formation but normal levels of resorption. The Chinese herbal extracts mitigate the alcohol-induced decreases of osteocalcin, a bone formation biomarker (Figure 4C), implying that phytomedicines may prevent alcohol-induced osteopenia via reducing the alcohol-induced damage in osteoblastogenesis.
Protocatechuic Acid Mitigates Alcohol-Induced Decline of Alkaline Phosphatase
Among the 189 compounds identified by HPLC, LC/MS, and NMR (Cai et al., 2020) and 43 bioactive components identified by LC/MS (Hu et al., 2021) in Jing extracts, after a comprehensive literature review, we identify that Acteoside, Daidzin, Echinacoside, Eugenol, Ferulic acid, Hyperoside, Icariin, Oleanolic acid, and Protocatechuic acid have therapeutic potential for either bone or alcohol-related diseases (Table 2). To study the roles of bioactive compounds in the studied herbs in bone diseases, we selected Protocatechuic acid (Figure 5A) as a representative bioactive compound in the Jing extracts to test its effects on alkaline phosphatase and other bone metabolic factors in the bone marrow of Balb/c male mice and in vitro alkaline phosphatase activity in murine pre-osteoblastic cell line MC3T3-E1 cells.
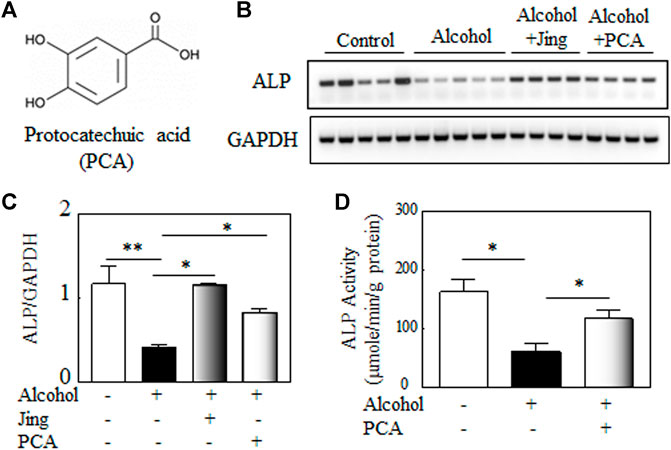
FIGURE 5. Protocatechuic acid (PCA) mitigates alcohol-induced decline of alkaline phosphatase (ALP). (A) The chemical structure of Protocatechuic acid. (B) Representative gel electrophoretogram of RT-PCR products shows ALP and GAPDH, the internal control, gene expression in the bone marrows of Balb/c male mice. (C) The quantitative analysis of ALP gene expression shows that Jing extracts and PCA mitigate the alcohol-induced decline of ALP gene expression in vivo (alcohol, n = 5, vs. control, n = 5, **p < 0.01; alcohol + Jing extracts, n = 4, or alcohol + PCA, n = 4 vs. alcohol, *p < 0.05; Mann-Whitney test). (D) The effects of PCA on ALP activity in murine pre-osteoblastic cell line MC3T3-E1 cells. PCA (100 μM) significantly alleviates the inhibitory effects of alcohol (500 mM) on ALP activity in vitro (osteogenic control or alcohol + PCA vs. alcohol, *p < 0.05; n = 4, Mann-Whitney test).
Balb/c male mice (2-month-old) were orally administered 3.2 g/kg alcohol by gavage with or without 0.25 g/kg of Jing extracts or 50 mg/kg of PCA for 10 days, a time pint that alcohol damaged other organs, e.g., liver (Bertola et al., 2013), but not bones. Our data (Figures 5B,C) showed that as the Jing extracts, PCA significantly mitigates alcohol-induced decline of alkaline phosphatase gene expression in the bone marrow of Balb/c male mice. Jing extracts and PCA reduced the alcohol-induced elevated inflammatory factors, e.g., TNF-α and IL-1β in the bone marrow of Balb/c male mice (Supplementary Figure S6). To study the effects of PCA on alkaline phosphatase (ALP) activity, murine pre-osteoblastic cell line MC3T3-E1 cells were treated with or without alcohol and/or PCA in an osteogenic medium. Our data (Figure 5D) showed that PCA significantly alleviates the inhibitory effects of alcohol on ALP activity in MC3T3-E1 cells.
Discussion
Alcohol is a nonessential diet component, and the overall impact of drinking on bone health, especially at moderate levels, is not well understood (Gaddini et al., 2016). Light to moderate alcohol consumption refers to two drinks or less in a day for men or one drink or less in a day for women per Dietary Guidelines for Americans (USDA and HHS, 2020). Epidemiological studies showed that light to moderate alcohol consumption is generally reported to be beneficial, resulting in higher bone mineral density (BMD) and reduced age-related bone loss, especially in men and postmenopausal women (Felson et al., 1995; Tucker et al., 2009; Sommer et al., 2013). However, heavy alcohol consumption is generally associated with decreased BMD, impaired bone quality, and increased fracture risk (Turner, 2000; Maurel et al., 2012; Mikosch, 2014; Gaddini et al., 2016). Similar to the epidemiological data, our preclinical studies showed that the effects of alcohol on bone health in mice are dose-and time-dependent. Our data showed that oral alcohol administration of a low dose of alcohol (0.8 g/kg body weight) significantly increased BMD in adult male mice (Figure 2); a low dose of oral alcohol administration by gavage (1.6 g/kg for 50 days) or higher doses (3.2 g/kg) for a shorter period of gavage time (30 days) have a neutral effect on bone mineral density in young adult Balb/c male mice (Figure 2). However, a high dose of alcohol administration (3.2 g/kg) with a longer gavage time (50 days) induces significant bone loss in young adult Balb/c male mice (Figure 2 and Figure 3); thus, we used 3.2 g/kg alcohol dose and the duration of 50 days to study the effects of TCM herbal extracts on alcohol-induced osteopenia.
Osteoporosis is the most common bone disease, representing a significant public health problem worldwide (Cosman et al., 2014; Wright et al., 2014; Zhou and Glowacki, 2017; Langdahl and Andersen, 2018; Zhou and Glowacki, 2018; Compston et al., 2019). There has been considerable progress in understanding postmenopausal (Type I) and senile (Type II) osteoporosis, the primary osteoporosis; however, gaps of knowledge still exist in understanding osteoporosis/osteopenia caused by alcoholism, one type of secondary osteoporosis. Alcohol-induced osteopenia/osteoporosis is distinct from primary osteoporosis; particularly, alcohol-induced osteopenia results mainly from decreased bone formation rather than increased bone resorption (Chakkalakal, 2005), which is confirmed in old male mice by our biomarker analysis (Figure 4C). Our biochemical study (Figure 4) showed that alcohol significantly reduced serum levels of bone formation biomarker osteocalcin but not the serum levels of bone resorption biomarker CTXI in the middle-aged/old Balb/c male mice, suggesting that chronic alcohol consumption inhibits bone formation but has no effects on bone resorption. There is a paucity of studies regarding alcohol-induced osteoporosis therapy (Abukhadir et al., 2013). Although the study found that abstinence can stop bone loss and the lost bone can be partially restored when alcohol abuse ends (Alvisa-Negrín et al., 2009), research indicates that the effects of heavy alcohol use on bone cannot be reversed, even if alcohol consumption is terminated (Sampson, 2002). Additional studies should examine whether alcohol-induced osteoporosis is reversible. Nevertheless, severe osteoporosis, especially alcohol-induced osteoporosis in the elders, needs osteoporotic management.
Due to the nature of alcohol-induced osteopenia resulted mainly from decreased bone formation rather than increased bone resorption (Chakkalakal, 2005 and Figure 4C), anabolic treatments for osteoporosis, such as Teriparatide (parathyroid hormone 1–34), Abaloparatide (a parathyroid hormone-related protein analog drug), and Romosozumab (a humanized antibody against sclerostin), may help address these unmet needs. Other anabolic anti-osteoporotic agents, which stimulate bone formation, such as vitamin D and estrogen, should prevent alcohol-induced bone loss and benefit bone loss recovery during alcoholism and abstinence. Our data of oral administration of Vitamin D in Balb/c male mice (Supplementary Figure S5) confirmed a preclinical study that Vitamin D supplementation prevents chronic liquid diet alcohol-induced bone loss in female C57BL/6J mice (Mercer et al., 2012), suggesting that our data of oral alcohol administration by gavage are comparable with the data obtained by Lieber-DeCarli liquid diet feeding.
Traditional Chinese medicine (TCM) formulas have a long history of use in the prevention and treatment of osteoporosis, and phytochemicals from TCM formulas offer great potential for the development of novel antiosteoporotic drugs (Zhang et al., 2016; Lin et al., 2017). One of the molecular mechanisms of TCM antiosteoporotic drugs is the potential of promoting osteoblast-mediated bone formation (An et al., 2016), making TCM a good candidate for alcohol-induced osteoporosis therapy. The Xian-Ling-Gu-Bao capsule (XLGB) is an effective traditional Chinese medicine prescription that is used for the prevention and treatment of osteoporosis in China (Wu et al., 2017; Tang et al., 2020; Ding et al., 2021). We used XLGB as the TCM antiosteoporotic medicine control in our experiments and showed that TCM XLGB might prevent alcohol-induced osteoporosis in young adult and middle-aged/old male mice (Supplementary Figure S5). Traditional Chinese medicines for the treatment of osteoporosis and osteoporotic fractures follow the TCM rule of “Kidneys Govern Bones,” which uses herbs that medicate bone by improving the functions of the kidney (An et al., 2016; Wang et al., 2016). The Bone-kidney axis is critical for bone health. Kidney dysfunction, e.g., Chronic kidney disease (CKD), results in mineral and bone disorder (CKD-MBD), a systemic condition that links disorders of mineral and bone metabolism to either one or all of the following: abnormalities of calcium, phosphorus, PTH, or vitamin D metabolism, abnormalities in bone turnover, mineralization, volume, linear growth or strength, and extraskeletal calcification (Zhou et al., 2013; Zhou and Glowacki, 2017). In this study, the traditional Chinese medicine herbal extracts or Jing extracts (Figure 1) are a combinative herbal prescription of nine Chinese herbal medicines, including Astragalus, Cistanche deserticola, Dioscorea polystachya (Chinese yam), Lycium barbarum, Epimedium, Cinnamomum cassia, Syzygium aromaticum, Angelica sinensis, and Curculigo orchioides. This TCM formula is used in a famous Chinese Herbal Liqueur, Chinese Jing Liqueur (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018; Cai et al., 2020; Hu et al., 2021). According to TCM theory of “kidney is the origin of the congenital constitution”, this empirical formula of the nine TCM herbs was formulated for the purpose of “tonifying kidney.” Among the nine TCM herbs, Cistanche deserticola and Epimedium are used as the herbs to reinforce the kidney-yang, which supplemented by Dioscorea polystachya (Chinese yam) and Lycium barbarum, etc., to replenish kidney-yin (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018). Preclinical and clinical studies demonstrated that studied herbal formulation (Jing extracts) has the properties of improving kidney-yang deficiency in rats and relieving main symptoms of patients with Kidney-Yang Deficiency Syndrome, anti-fatigue, and enhancing immunity in humans and animals (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Shan et al., 2018; Cai et al., 2020; Hu et al., 2021). The assertion of “kidney governing bones” in “Huangdi Neijing—Yellow Emperor Canon of Internal Medicine” has been confirmed by an increasing body of scientific data (Ju et al., 2014; Wang et al., 2016). Our data showed that the Chinese herbal medicine extracts prevent alcohol-induced osteopenia, as demonstrated by bone morphological and biochemical analysis, in both young adult and middle-aged male mice (Figures 3, 4), which may due to its kidney-tonifying property via kidney governing bones. The preventing mechanism of the phytomedicines in Jing extracts on alcohol-induced osteoporosis needs additional study.
The TCM herbs used in this study and the TCM extracts’ bioactive compounds have therapeutic potentials for bone diseases (Tables 1, 2). Astragalus membranaceus (Kang et al., 2013), Astragalus membranaceus and Cinnamomum cassia plus Phellodendron amurense (Huh et al., 2015), Epimedium brevicornum (Zhang et al., 2006) or a combination of Astragalus membranaceus, Angelica sinensis, and Epimedium brevicornum (Xie et al., 2012), Syzygium aromaticum (Karmakar et al., 2012) and Angelica sinensis (Lim and Kim, 2014) reverse bone loss in a rat model of postmenopausal osteoporosis. Cistanche deserticola has antiosteoporotic activity in type I osteoporotic rats (Zhang et al., 2021) and type II osteoporotic mice (Wang et al., 2021), which may result in the stimulation of bone formation (Li et al., 2012). Dioscorea spongiosa prevents glucocorticoid-induced osteoporosis in rats (Han et al., 2016) and aging-induced osteoporosis (Tikhonova et al., 2015). Lycium barbarum ameliorates osteoporosis in OVX mice (Kim et al., 2017). The analyses of chemical components of the Chinese herbal extracts we used in this study by LC/MS and NMR (Hu et al., 2021; Cai et al., 2020) showed that herbal extracts contain a variety of saponins, flavonoids, active polysaccharides, and other functional factors including amino acids, organic acids, trace elements and other nutrients that are required by the human body. Among the 189 compounds detected by LC/MS and NMR (Cai et al., 2020) and 43 bioactive components detected by LC/MS (Hu et al., 2021) from the Chinese herbal extracts used in this study, Acteoside, Calycosin, Daidzin, Echinacoside, Epimedin, Eugenol, Ferulic acid, Hyperoside, Icariside, Icariin, Oleanolic acid, Protocatechuic acid, Sagittatoside, and Ligustilide, etc. are antiosteoporotic compounds and some of these compounds have the therapeutic potential for alcohol use disorder (Table 2; the chemical structures of these bioactive compounds in Supplementary Figure S7). For example, icariin represents a class of flavonoids with bone-promoting activity, which could be used as a potential treatment for postmenopausal osteoporosis (Wang et al., 2018). These antiosteoporotic compounds may prevent alcohol-induced osteopenia by acting alone or in a synergistic manner. Nuclear factor erythroid-derived 2-related factor-2 (Nrf2) is a master regulator of oxidative stress and a critical antiosteoporotic factor (Chen et al., 2021). Cai et al. (2020) reported that Chinese herbal extracts activate Nrf2, implying that Nrf2 may be involved in the preventive effects of Chinese herbal extracts (Jing extracts) on alcohol-induced osteoporosis.
The bone marrow is an alcohol sensitive organ (Wahl et al., 2007); hematopoietic cells and bone cells could be extrinsic factors for each other in the bone marrow niche (Zhou, 2015), which makes bone marrow an ideal organ to study the bone metabolic factors, such as ALP and TNF-α, etc. (Wahl et al., 2007; Zhou, 2015). Our data shows that Protocatechuic acid, a natural phenolic acid in Jing extracts, mitigates in vivo alcohol-induced decline of Alkaline phosphatase (ALP) gene expression (Figure 5) and elevated inflammatory factors (Supplementary Figure S6) in the bone marrow of male Balb/c mice. The murine pre-osteoblastic cell line MC3T3-E1 is a widely used in osteoblast biology (Hwang and Horton, 2019). In this study, MC3T3-E1 cells were treated with or without alcohol and/or PCA to study the effects of PCA on alcohol-induced inhibitory on ALP activity in vitro. Our data (Figure 5D) showed that PCA significantly alleviates the inhibitory effects of alcohol on ALP activity in MC3T3-E1 cells. Protocatechuic acid (PCA) is antioxidant and anti-inflammatory (Kakkar and Bais, 2014). Oxidative stress and inflammation are two common mechanisms involved in alcohol toxicity. PCA may prevent alcohol-induced bone injury via antioxidant and anti-inflammatory pathways. In this study, we used PCA as a representative bioactive compound in the Jing extracts. A typical TCM or any ethnomedicine formula has multiple ingredients that synergize in treatment for the symptoms of a disease, which have greater efficacy and safety than a single drug in clinical practices, possibly due to the multiple ingredients synergistic interactions and mutual detoxification. The other bioactive compounds, e.g., Acteoside, Daidzin, Echinacoside, Eugenol, Ferulic acid, Hyperoside, Icariin, and Oleanolic acid, etc. (Table 2), and their synergizing in treatment for alcohol-induced bone loss need additional investigations.
The TCM herbs used in this study also have therapeutic potentials for alcohol use disorder, especially alcoholic liver damage (Table 1). The chemical composition of TCM herbal extracts includes Acteoside, Daidzin, Echinacoside, Eugenol, Ferulic acid, Hyperoside, Icariin, Oleanolic acid, and Protocatechuic acid have therapeutic potential for alcohol-related diseases (Table 2). Osteoporosis is a common complication of many types of liver disease (Nakchbandi and van der Merwe, 2009). Alcohol consumption is an independent risk factor for the onset of osteoporosis, which is prevalent in patients with alcoholic liver disease (López-Larramona et al., 2013). The roles of TCM herbal extracts and their bioactive compounds in the alcohol-induced dysfunction of the liver-bone axis need additional study.
Conclusion
In summary, our study in pharmacognosy suggests that traditional Chinese medicine herbal extracts prevent chronic excessive alcohol consumption-induced osteopenia in young adult and middle-aged/old male mice, which indicates that TCM might prevent alcohol-induced osteoporosis. Based on our data and previous reports (Liu et al., 2011; Lu et al., 2017; Lu et al., 2018; Cai et al., 2020; Hu et al., 2021), the potential protective mechanism of traditional Chinese medicine herbal extracts in alcohol-induced osteoporosis may involve the kidney-bone axis to prevent alcohol-induced bone loss and the direct antiosteoporotic potential of the bioactive compounds in the Chinese herbal medicines. Protocatechuic acid, a natural phenolic acid in Jing extracts, mitigates in vivo and in vitro alcohol-induced decline of alkaline phosphatase gene expression and activity. Additional analysis of the bioactive compounds in Chinese herbal medicines may identify antiosteoporotic compounds as drug candidates for preventing or treating alcohol-induced osteopenia/osteoporosis.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Ethics Statement
The animal study was reviewed and approved by the BWH Institutional Animal Care and Use Committee (IACUC approval Protocol # 2016N000361).
Author Contributions
SZ, YL, and XW: Conceptualization, methodology, and supervision. SZ, DQ, and HZ: Writing—Original draft preparation. DQ, HZ, PF, TY, YX, YG, LZ: Investigation, data curation, and formal analysis. YL, HZ, ZW, SL, GT, YS, and LW: Conceptualization, resources, and methodology. AP and MO’B: Writing—Review and editing. SZ, HZ, and YL: Project administration and funding acquisition.
Funding
This work was supported by grants from Hubei State Guoqiang Baojianjiu Center for Engineering and Technology Research, Jing Brand Research Institute (SZ and XW).
Conflict of Interest
Authors HZ, ZW, SL, GT, YS, LW, and YL were employed by the company Jing Brand Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank other members of Jing Brand Research Institute for their constructive discussion and suggestions during the design and execution of the experiments as well as their contribution to the extracts of Chinese herbal medicines.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2021.754088/full#supplementary-material
Abbreviations
ALP, Alkaline phosphatase; CTXI, Cross-Linked C-Telopeptide of Type I Collagen; C. BMD, cortical bone mineral density; ELISA, enzyme-linked immunosorbent assay; HPLC, High-performance liquid chromatography; LC/MS, liquid chromatography-mass spectrometry; μ-CT, micro-computed tomography; NMR, nuclear magnetic resonance; OC, osteocalcin; Tb. BMD, trabecular bone mineral density; Tb. BV/TV, tibia trabecular relative bone volume; Tb. Th, trabecular thickness; Tb. Sp, trabecular space; Tb. N, trabecular number; TCM, traditional Chinese medicine.
References
Abukhadir, S. S., Mohamed, N., and Mohamed, N. (2013). Pathogenesis of Alcohol-Induced Osteoporosis and its Treatment: a Review. Curr. Drug Targets. 14, 1601–1610. doi:10.2174/13894501113146660231
Alvisa-Negrín, J., González-Reimers, E., Santolaria-Fernández, F., García-Valdecasas-Campelo, E., Valls, M. R., Pelazas-González, R., et al. (2009). Osteopenia in Alcoholics: Effect of Alcohol Abstinence. Alcohol Alcohol. 44, 468–475. doi:10.1093/alcalc/agp038
An, J., Yang, H., Zhang, Q., Liu, C., Zhao, J., Zhang, L., et al. (2016). Natural Products for Treatment of Osteoporosis: The Effects and Mechanisms on Promoting Osteoblast-Mediated Bone Formation. Life Sci. 147, 46–58. doi:10.1016/j.lfs.2016.01.024
Bertola, A., Mathews, S., Ki, S. H., Wang, H., and Gao, B. (2013). Mouse Model of Chronic and Binge Ethanol Feeding (The NIAAA Model). Nat. Protoc. 8, 627–637. doi:10.1038/nprot.2013.032
Byeon, S., Oh, J., Lim, J. S., Lee, J. S., and Kim, J. S. (2018). Protective Effects of Dioscorea Batatas Flesh and Peel Extracts Against Ethanol-Induced Gastric Ulcer in Mice. Nutrients. 10 (11), 1680. doi:10.3390/nu10111680
Cai, Y.-S., Xu, J., Chen, M., Wang, D., Yang, Y., Manavalan, A., et al. (2020). Compound Analysis of Jing Liqueur and Nrf2 Activation by Jing Liqueur-One of the Most Popular Beverages in China. Beverages. 6, 1. doi:10.3390/beverages6010001
Cao, D. P., Zheng, Y. N., Qin, L. P., Han, T., Zhang, H., Rahman, K., et al. (2008). Curculigo Orchioides, a Traditional Chinese Medicinal Plant, Prevents Bone Loss in Ovariectomized Rats. Maturitas. 59, 373–380. doi:10.1016/j.maturitas.2008.03.010
Cao, S., Dong, X. L., Ho, M. X., Yu, W. X., Wong, K. C., Yao, X. S., et al. (2018). Oleanolic Acid Exerts Osteoprotective Effects and Modulates Vitamin D Metabolism. Nutrients. 10, 247. doi:10.3390/nu10020247
Chakkalakal, D. A. (2005). Alcohol-Induced Bone Loss and Deficient Bone Repair. Alcohol. Clin. Exp. Res. 29, 2077–2090. doi:10.1097/01.alc.0000192039.21305.55
Charles, J. F., Coury, F., Sulyanto, R., Sitara, D., Wu, J., Brady, N., et al. (2012). The Collection of NFATc1-Dependent Transcripts in the Osteoclast Includes Numerous Genes Non-Essential to Physiologic Bone Resorption. Bone. 51, 902–912. doi:10.1016/j.bone.2012.08.113
Chen, X., Zhu, X., Wei, A., Chen, F., Gao, Q., Lu, K., et al. (2021). Nrf2 Epigenetic Derepression Induced by Running Exercise Protects Against Osteoporosis. Bone Res. 9, 15. doi:10.1038/s41413-020-00128-8
Chen, Y., Dai, F., He, Y., Chen, Q., Xia, Q., Cheng, G., et al. (2018). Beneficial Effects of Hyperoside on Bone Metabolism in Ovariectomized Mice. Biomed. Pharmacother. 107, 1175–1182. doi:10.1016/j.biopha.2018.08.069
Cheung, H., Doughty, H., Hinsley, A., Hsu, E., Lee, T. M., Milner‐Gulland, E. J., et al. (2021). Understanding Traditional Chinese Medicine to Strengthen Conservation Outcomes. People Nat. 3, 115–128. doi:10.1002/pan3.10166
Compston, J. E., McClung, M. R., and Leslie, W. D. (2019). Osteoporosis. The Lancet. 393, 364–376. doi:10.1016/S0140-6736(18)32112-3
Cosman, F., de Beur, S. J., LeBoff, M. S., Lewiecki, E. M., Tanner, B., Randall, S., et al. (2014). Clinician's Guide to Prevention and Treatment of Osteoporosis. Osteoporos. Int. 25, 2359–2381. doi:10.1007/s00198-014-2794-2
Dai, J., Lin, D., Zhang, J., Habib, P., Smith, P., Murtha, J., et al. (2000). Chronic Alcohol Ingestion Induces Osteoclastogenesis and Bone Loss Through IL-6 in Mice. J. Clin. Invest. 106, 887–895. doi:10.1172/JCI10483
Ding, M., Odgaard, A., Danielsen, C. C., and Hvid, I. (2002). Mutual Associations Among Microstructural, Physical and Mechanical Properties of Human Cancellous Bone. J. Bone Jt. Surg Br. 84, 900–907. doi:10.1302/0301-620x.84b6.11994
Ding, Y., Ma, H., Xu, Y., Yang, F., Li, Y., Shi, F., et al. (2021). Potentiation of Flutamide-Induced Hepatotoxicity in Mice by Xian-Ling-Gu-Bao through Induction of CYP1A2. J. Ethnopharmacol. 278, 114299. doi:10.1016/j.jep.2021.114299
Egea, T., Signorini, M. A., Bruschi, P., Rivera, D., Obón, C., Alcaraz, F., et al. (2015). Spirits and Liqueurs in European Traditional Medicine: Their History and Ethnobotany in Tuscany and Bologna (Italy). J. Ethnopharmacol. 175, 241–255. doi:10.1016/j.jep.2015.08.053
Fai, Y. M. (2009). A Review of Presence of Oleanolic Acid in Natural Products. Nat. Prod. Med. 2, 64–277.
Felson, D. T., Zhang, Y., Hannan, M. T., Kannel, W. B., and Kiel, D. P. (1995). Alcohol Intake and Bone Mineral Density in Elderly Men and Women. The Framingham Study. Am. J. Epidemiol. 142, 485–492. doi:10.1093/oxfordjournals.aje.a117664
Fu, R., Zhou, J., Wang, R., Sun, R., Feng, D., Wang, Z., et al. (2019). Protocatechuic Acid-Mediated miR-219a-5p Activation Inhibits the P66shc Oxidant Pathway to Alleviate Alcoholic Liver Injury. Oxid Med. Cel Longev. 2019, 3527809. doi:10.1155/2019/3527809
Gaddini, G. W., Turner, R. T., Grant, K. A., and Iwaniec, U. T. (2016). Alcohol: A Simple Nutrient With Complex Actions on Bone in the Adult Skeleton. Alcohol. Clin. Exp. Res. 40, 657–671. doi:10.1111/acer.13000
Garaycoechea, J. I., Crossan, G. P., Langevin, F., Mulderrig, L., Louzada, S., Yang, F., et al. (2018). Alcohol and Endogenous Aldehydes Damage Chromosomes and Mutate Stem Cells. Nature. 553, 171–177. doi:10.1038/nature25154
GBD 2016 Alcohol Collaborators (2018). Alcohol Use and Burden for 195 Countries and Territories, 1990-2016: a Systematic Analysis for the Global Burden of Disease Study 2016. Lancet. 392, 1015–1035. doi:10.1016/S0140-6736(18)31310-2
Guo, Y., Cao, L., Zhao, Q., Zhang, L., Chen, J., Liu, B., et al. (2016). Preliminary Characterizations, Antioxidant and Hepatoprotective Activity of Polysaccharide From Cistanche Deserticola. Int. J. Biol. Macromol. 93 (Pt A), 678–685. doi:10.1016/j.ijbiomac.2016.09.039
Han, N., Xu, J., Xu, F., Liu, Z., and Yin, J. (2016). The In Vivo Effects of a Fraction From Dioscorea Spongiosa on Glucocorticoid-Induced Osteoporosis. J. Ethnopharmacol. 185, 53–59. doi:10.1016/j.jep.2016.03.033
He, B., Lu, C., Wang, M., Zheng, G., Chen, G., Jiang, M., et al. (2015). Drug Discovery in Traditional Chinese Medicine: From Herbal Fufang to Combinatory Drugs. Sponsored Suppl. Sci. 350, S74–S76.
Hildebrand, T., and Rüegsegger, P. (1997). Quantification of Bone Microarchitecture With the Structure Model Index. Comput. Methods Biomech. Biomed. Engin 1, 15–23. doi:10.1080/01495739708936692
Hou, T., Zhang, L., and Yang, X. (2019). Ferulic Acid, a Natural Polyphenol, Protects Against Osteoporosis by Activating SIRT1 and NF-Κb in Neonatal Rats With Glucocorticoid-Induced Osteoporosis. Biomed. Pharmacother. 120, 109205. doi:10.1016/j.biopha.2019.109205
Howard, A. A., Arnsten, J. H., and Gourevitch, M. N. (2004). Effect of Alcohol Consumption on Diabetes Mellitus: a Systematic Review. Ann. Intern. Med. 140, 211–219. doi:10.7326/0003-4819-140-6-200403160-00011
Hu, Y., Wang, Z., Xia, F., Yang, W., Liu, Y. C., and Wan, J. B. (2021). Simultaneous Quantification of Bioactive Components in Chinese Herbal Spirits by Ultra-High Performance Liquid Chromatography Coupled to Triple-Quadrupole Mass Spectrometry (UHPLC-QQQ-MS/MS). Chin. Med. 16, 26. doi:10.1186/s13020-021-00435-0
Huh, J. E., Kim, S. J., Kang, J. W., Nam, D. W., Choi, D. Y., Park, D. S., et al. (2015). The Standardized BHH10 Extract, a Combination of Astragalus Membranaceus, Cinnamomum Cassia, and Phellodendron Amurense, Reverses Bone Mass and Metabolism in a Rat Model of Postmenopausal Osteoporosis. Phytother Res. 29, 30–39. doi:10.1002/ptr.5218
Hwang, P. W., and Horton, J. A. (2019). Variable Osteogenic Performance of MC3T3-E1 Subclones Impacts Their Utility as Models of Osteoblast Biology. Sci. Rep. 9, 8299. doi:10.1038/s41598-019-44575-8
Iwaniec, U. T., and Turner, R. T. (2013). Intraperitoneal Injection of Ethanol Results in Drastic Changes in Bone Metabolism Not Observed When Ethanol Is Administered by Oral Gavage. Alcohol. Clin. Exp. Res. 37, 1271–1277. doi:10.1111/acer.12105
Jacob Jesurun, R. S., and Johan Pandian, J. (2019). Evaluation of Antioxidant and Hepatoprotective Property of Ethanolic Extract of Curculigoorchioides in Albino Rats. Int. J. Basic Clin. Pharmacol. 8, 1685–1692. doi:10.18203/2319-2003.ijbcp20192672
Jang, S. A., Song, H. S., Kwon, J. E., Baek, H. J., Koo, H. J., Sohn, E. H., et al. (2018). Protocatechuic Acid Attenuates Trabecular Bone Loss in Ovariectomized Mice. Oxid Med. Cel Longev. 2018, 7280342. doi:10.1155/2018/7280342
Jin, S. E., Lee, M. Y., Shin, I. S., Jeon, W. Y., and Ha, H. (2016). Syzygium Aromaticum Water Extract Attenuates Ethanol Induced Gastric Injury Through Antioxidant Effects in Rats. Mol. Med. Rep. 14 (1), 361–366. doi:10.3892/mmr.2016.5269
Ju, D., Liu, M., Zhao, H., and Wang, J. (2014). Mechanisms of "Kidney Governing Bones" Theory in Traditional Chinese Medicine. Front. Med. 8, 389–393. doi:10.1007/s11684-014-0362-y
Kakkar, S., and Bais, S. (2014). A Review on Protocatechuic Acid and its Pharmacological Potential. ISRN Pharmacol. 2014, 952943. doi:10.1155/2014/952943
Kang, S. C., Kim, H. J., and Kim, M. H. (2013). Effects of Astragalus Membranaceus With Supplemental Calcium on Bone Mineral Density and Bone Metabolism in Calcium-Deficient Ovariectomized Rats. Biol. Trace Elem. Res. 151, 68–74. doi:10.1007/s12011-012-9527-1
Kanuri, G., Weber, S., Volynets, V., Spruss, A., Bischoff, S. C., and Bergheim, I. (2009). Cinnamon Extract Protects Against Acute Alcohol-Induced Liver Steatosis in Mice. J. Nutr. 139 (3), 482–487. doi:10.3945/jn.108.100495
Karlamangla, A. S., Sarkisian, C. A., Kado, D. M., Dedes, H., Liao, D. H., Kim, S., et al. (2009). Light to Moderate Alcohol Consumption and Disability: Variable Benefits by Health Status. Am. J. Epidemiol. 169, 96–104. doi:10.1093/aje/kwn294
Karmakar, S., Choudhury, M., Das, A. S., Maiti, A., Majumdar, S., and Mitra, C. (2012). Clove (Syzygium Aromaticum Linn) Extract Rich in Eugenol and Eugenol Derivatives Shows Bone-Preserving Efficacy. Nat. Prod. Res. 26, 500–509. doi:10.1080/14786419.2010.511216
Keung, W. M., and Vallee, B. L. (1998). Kudzu Root: an Ancient Chinese Source of Modern Antidipsotropic Agents. Phytochemistry. 47 (4), 499–506. doi:10.1016/s0031-9422(97)00723-1
Khullar, M., Sharma, A., Wani, A., Sharma, N., Sharma, N., Chandan, B. K., et al. (2019). Acteoside Ameliorates Inflammatory Responses Through NFkB Pathway in Alcohol Induced Hepatic Damage. Int. Immunopharmacol. 69, 109–117. doi:10.1016/j.intimp.2019.01.020
Kim, M. H., Lee, J. E., Lee, J. S., and Yang, W. M. (2017). Improvement of Osteoporosis by Lycium Chinense Administration in Ovariectomized Mice. J. Chin. Med. Assoc. 80, 222–226. doi:10.1016/j.jcma.2016.11.006
Kunzmann, A. T., Coleman, H. G., Huang, W. Y., and Berndt, S. I. (2018). The Association of Lifetime Alcohol Use With Mortality and Cancer Risk in Older Adults: A Cohort Study. Plos Med. 15, e1002585. doi:10.1371/journal.pmed.1002585
Langdahl, B. L., and Andersen, J. D. (2018). Treatment of Osteoporosis: Unmet Needs and Emerging Solutions. J. Bone Metab. 25, 133–140. doi:10.11005/jbm.2018.25.3.133
Li, F., Yang, X., Yang, Y., Guo, C., Zhang, C., Yang, Z., et al. (2013). Antiosteoporotic Activity of Echinacoside in Ovariectomized Rats. Phytomedicine. 20, 549–557. doi:10.1016/j.phymed.2013.01.001
Li, N., Tu, Y., Shen, Y., Qin, Y., Lei, C., and Liu, X. (2016). Calycosin Attenuates Osteoporosis and Regulates the Expression of OPG/RANKL in Ovariectomized Rats via MAPK Signaling. Pharmazie. 71, 607–612. doi:10.1691/ph.2016.6627
Li, T. M., Huang, H. C., Su, C. M., Ho, T. Y., Wu, C. M., Chen, W. C., et al. (2012). Cistanche Deserticola Extract Increases Bone Formation in Osteoblasts. J. Pharm. Pharmacol. 64, 897–907. doi:10.1111/j.2042-7158.2012.01483.x
Lim, D. W., and Kim, Y. T. (2014). Anti-osteoporotic Effects of Angelica Sinensis (Oliv.) Diels Extract on Ovariectomized Rats and its Oral Toxicity in Rats. Nutrients. 6, 4362–4372. doi:10.3390/nu6104362
Lin, J., Zhu, J., Wang, Y., Zhang, N., Gober, H. J., Qiu, X., et al. (2017). Chinese Single Herbs and Active Ingredients for Postmenopausal Osteoporosis: From Preclinical Evidence to Action Mechanism. Biosci. Trends. 11, 496–506. doi:10.5582/bst.2017.01216
Liu, J., Wang, X., Liu, R., Liu, Y., Zhang, T., Fu, H., et al. (2014). Oleanolic Acid Co-administration Alleviates Ethanol-Induced Hepatic Injury via Nrf-2 and Ethanol-Metabolizing Modulating in Rats. Chem. Biol. Interact. 221, 88–98. doi:10.1016/j.cbi.2014.07.017
Liu, S., Li, B., Li, X., Tu, P., and Bao, Z. (2008). Method for Checking Quality of Chinese Jing Wine by Using Fingerprint Pattern Technology. China Patent., CN100392399C.
Liu, W., Mao, L., Ji, F., Chen, F., Wang, S., and Xie, Y. (2017). Icariside II Activates EGFR-Akt-Nrf2 Signaling and Protects Osteoblasts from Dexamethasone. Oncotarget. 8, 2594–2603. doi:10.18632/oncotarget.13732
Liu, Y., Bi, Y., Chai, L., Song, L., Huang, J., Wang, Q., et al. (2021). Development of Epimedin A Complex Drugs for Treating the Osteoporosis. J. Mater. Sci. Mater. Med. 32, 17. doi:10.1007/s10856-020-06472-9
Liu, Y., Kou, X., Chen, C., Yu, W., Su, Y., Kim, Y., et al. (2016). Chronic High Dose Alcohol Induces Osteopenia via Activation of mTOR Signaling in Bone Marrow Mesenchymal Stem Cells. Stem Cells. 34, 2157–2168. doi:10.1002/stem.2392
Liu, Y., Yang, Y., Feng, S., Lu, S., Shan, Y., Xia, S., et al. (2011). The Effects of China Jing Wine on the Functions of Hypothalamic-Pituitary-Adrenal axis and Immune System in the Shen-Yang Deficiency Model Rats. Chin. J. Immunol. 27, 1080–1084.
López-Larramona, G., Lucendo, A. J., and González-Delgado, L. (2013). Alcoholic Liver Disease and Changes in Bone mineral Density. Rev. Esp Enferm Dig. 105 (10), 609–621. doi:10.4321/s1130-01082013001000006
Lu, S., Feng, S., LiYin, J. T., Shi, Y., ShiChen, J. Y., and Liu, Y. (2017). The Effects of Chinese JING Liqueur in Relieving the Fatigue of the Patients With Fatigued Sub-Health Status. West. J. Traditional Chin. Med. 30, 82–84.
Lu, S., Li, J., Zhang, Y., Wang, L., Yang, Y., Chen, M., et al. (2018). Clinical Observation of Main Symptoms Relief by Chinese Jing Liqueur in Patients With Kidney Yang Deficiency Syndrome. Chin. Traditional Patent Med. 40, 562–570. wpr-710214.
Martiniakova, M., Sarocka, A., Babosova, R., Grosskopf, B., Kapusta, E., Goc, Z., et al. (2018). Changes in the Microstructure of Compact and Trabecular Bone Tissues of Mice Subchronically Exposed to Alcohol. J. Biol. Res. (Thessalon). 25, 8. doi:10.1186/s40709-018-0079-1
Maurel, D. B., Boisseau, N., Benhamou, C. L., and Jaffre, C. (2012). Alcohol and Bone: Review of Dose Effects and Mechanisms. Osteoporos. Int. 23, 1–16. doi:10.1007/s00198-011-1787-7
Mercer, K. E., Wynne, R. A., Lazarenko, O. P., Lumpkin, C. K., Hogue, W. R., Suva, L. J., et al. (2012). Vitamin D Supplementation Protects Against Bone Loss Associated With Chronic Alcohol Administration in Female Mice. J. Pharmacol. Exp. Ther. 343, 401–412. doi:10.1124/jpet.112.197038
Mikosch, P. (2014). Alcohol and Bone. Wien Med. Wochenschr. 164, 15–24. doi:10.1007/s10354-013-0258-5
Mostofsky, E., Mukamal, K. J., Giovannucci, E. L., Stampfer, M. J., and Rimm, E. B. (2016). Key Findings on Alcohol Consumption and a Variety of Health Outcomes From the Nurses' Health Study. Am. J. Public Health. 106, 1586–1591. doi:10.2105/AJPH.2016.303336
Nair, A. B., and Jacob, S. (2016). A Simple Practice Guide for Dose Conversion Between Animals and Human. J. Basic Clin. Pharm. 7, 27–31. doi:10.4103/0976-0105.177703
Nakchbandi, I. A., and van der Merwe, S. W. (2009). Current Understanding of Osteoporosis Associated with Liver Disease. Nat. Rev. Gastroenterol. Hepatol. 6 (11), 660–670. doi:10.1038/nrgastro.2009.166
NIAAA (2021). Alcohol Facts and Statistics. Available at: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/alcohol-facts-and-statistics.
Okuda, T., Naruo, M., Iijima, O., Igarashi, T., Katsuyama, M., Maruyama, M., et al. (2018). The Contribution of Alcohol Dehydrogenase 3 to the Development of Alcoholic Osteoporosis in Mice. J. Nippon Med. Sch. 85, 322–329. doi:10.1272/jnms.JNMS.2018_85-52
Rukkumani, R., Aruna, K., Suresh Varma, P., and Padmanabhan Menon, V. (2004). Hepatoprotective Role of Ferulic Acid: a Dose-dependent Study. J. Med. Food. 7 (4), 456–461. doi:10.1089/jmf.2004.7.456
Salmon, P. L., Ohlsson, C., Shefelbine, S. J., and Doube, M. (2015). Structure Model Index Does Not Measure Rods and Plates in Trabecular Bone. Front. Endocrinol. (Lausanne). 6, 162. doi:10.3389/fendo.2015.00162
Sampson, H. W. (2002). Alcohol and Other Factors Affecting Osteoporosis Risk in Women. Alcohol. Res. Health. 26, 292–298.
Sampson, H. W. (1998). Alcohol's Harmful Effects on Bone. Alcohol. Health Res. World. 22, 190–194. doi:10.1111/j.1530-0277.1998.tb05912.x
Shan, Y., Zhou, H., Chen, M., Chen, K., Liu, Y., and Wang, L. (2018). Effects of Chinese Jing Liqueur on Fatigue, Immunity, and Sexual Function. Chin. Traditional Patent Med. 40, 1600–1603.
Sommer, I., Erkkilä, A. T., Järvinen, R., Mursu, J., Sirola, J., Jurvelin, J. S., et al. (2013). Alcohol Consumption and Bone mineral Density in Elderly Women. Public Health Nutr. 16, 704–712. doi:10.1017/S136898001200331X
Tang, X. Y., Dai, Z. Q., Wu, Q. C., Zeng, J. X., Gao, M. X., Xiao, H. H., et al. (2020). Simultaneous Determination of Multiple Components in Rat Plasma and Pharmacokinetic Studies at a Pharmacodynamic Dose of Xian-Ling-Gu-Bao Capsule by UPLC-MS/MS. J. Pharm. Biomed. Anal. 177, 112836. doi:10.1016/j.jpba.2019.112836
Tao, Z., Zhang, L., Wu, T., Fang, X., and Zhao, L. (2021). Echinacoside Ameliorates Alcohol-Induced Oxidative Stress and Hepatic Steatosis by Affecting SREBP1c/FASN Pathway via PPARα. Food Chem. Toxicol. 148, 111956. doi:10.1016/j.fct.2020.111956
Tikhonova, M. A., Ting, C. H., Kolosova, N. G., Hsu, C. Y., Chen, J. H., Huang, C. W., et al. (2015). Improving Bone Microarchitecture in Aging With Diosgenin Treatment: A Study in Senescence-Accelerated OXYS Rats. Chin. J. Physiol. 58, 322–331. doi:10.4077/CJP.2015.BAD325
Tucker, K. L., Jugdaohsingh, R., Powell, J. J., Qiao, N., Hannan, M. T., Sripanyakorn, S., et al. (2009). Effects of Beer, Wine, and Liquor Intakes on Bone mineral Density in Older Men and Women. Am. J. Clin. Nutr. 89, 1188–1196. doi:10.3945/ajcn.2008.26765
Turner, R. T. (2000). Skeletal Response to Alcohol. Alcohol. Clin. Exp. Res. 24, 1693–1701. doi:10.1111/j.1530-0277.2000.tb01971.x
Ung, C. Y., Li, H., Cao, Z. W., Li, Y. X., and Chen, Y. Z. (2007). Are Herb-Pairs of Traditional Chinese Medicine Distinguishable From Others? Pattern Analysis and Artificial Intelligence Classification Study of Traditionally Defined Herbal Properties. J. Ethnopharmacol. 111, 371–377. doi:10.1016/j.jep.2006.11.037
USDA and HHS (2020). U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. Available at: DietaryGuidelines.gov.
Veerbeek, M. A., Ten Have, M., van Dorsselaer, S. A., Oude Voshaar, R. C., Rhebergen, D., and Willemse, B. M. (2019). Differences in Alcohol Use Between Younger and Older People: Results From a General Population Study. Drug Alcohol Depend. 202, 18–23. doi:10.1016/j.drugalcdep.2019.04.023
Wahl, E. C., Aronson, J., Liu, L., Liu, Z., Perrien, D. S., Skinner, R. A., et al. (2007). Chronic Ethanol Exposure Inhibits Distraction Osteogenesis in a Mouse Model: Role of the TNF Signaling axis. Toxicol. Appl. Pharmacol. 220, 302–310. doi:10.1016/j.taap.2007.02.011
Wang, F., Tu, P., Zeng, K., and Jiang, Y. (2021). Total Glycosides and Polysaccharides of Cistanche Deserticola Prevent Osteoporosis by Activating Wnt/β-Catenin Signaling Pathway in SAMP6 Mice. J. Ethnopharmacol. 271, 113899. doi:10.1016/j.jep.2021.113899
Wang, H., Li, Y., Liu, J., Di, D., Liu, Y., and Wei, J. (2020a). Hepatoprotective Effect of Crude Polysaccharide Isolated From Lycium Barbarum L. Against Alcohol-Induced Oxidative Damage Involves Nrf2 Signaling. Food Sci. Nutr. 8 (12), 6528–6538. doi:10.1002/fsn3.1942
Wang, K., Xu, J., Liu, Y., Cui, Z., He, Z., Zheng, Z., et al. (2020b). Self-Assembled Angelica Sinensis Polysaccharide Nanoparticles With an Instinctive Liver-Targeting Ability as a Drug Carrier for Acute Alcoholic Liver Damage protection. Int. J. Pharm. 577, 118996. doi:10.1016/j.ijpharm.2019.118996
Wang, X. Q., Zou, X. R., and Zhang, Y. C. (2016). From "Kidneys Govern Bones" to Chronic Kidney Disease, Diabetes Mellitus, and Metabolic Bone Disorder: A Crosstalk between Traditional Chinese Medicine and Modern Science. Evid. Based Complement. Alternat Med. 2016, 4370263. doi:10.1155/2016/4370263
Wang, Z., Wang, D., Yang, D., Zhen, W., Zhang, J., and Peng, S. (2018). The Effect of Icariin on Bone Metabolism and its Potential Clinical Application. Osteoporos. Int. 29, 535–544. doi:10.1007/s00198-017-4255-1
Wei, G., Liang, T., Wei, C., Nong, X., Lu, Q., and Zhao, J. (2019). Daidzin Inhibits RANKL-Induced Osteoclastogenesis In Vitro and Prevents LPS-Induced Bone Loss In Vivo. J. Cel Biochem. 120, 5304–5314. doi:10.1002/jcb.27806
Willinghamm, M. D., Brodt, M. D., Lee, K. L., Stephens, A. L., Ye, J., and Silva, M. J. (2010). Age-Related Changes in Bone Structure and Strength in Female and Male BALB/c Mice. Calcif Tissue Int. 86, 470–483. doi:10.1007/s00223-010-9359-y
Wojcikowski, K., and Gobe, G. (2014). Animal Studies on Medicinal Herbs: Predictability, Dose Conversion and Potential Value. Phytother Res. 28, 22–27. doi:10.1002/ptr.4966
Wood, A. M., Kaptoge, S., Butterworth, A. S., Willeit, P., Warnakula, S., Bolton, T., et al. (2018). Risk Thresholds for Alcohol Consumption: Combined Analysis of Individual-Participant Data for 599 912 Current Drinkers in 83 Prospective Studies. Lancet. 391, 1513–1523. doi:10.1016/S0140-6736(18)30134-X
Wright, N. C., Looker, A. C., Saag, K. G., Curtis, J. R., Delzell, E. S., Randall, S., et al. (2014). The Recent Prevalence of Osteoporosis and Low Bone Mass in the United States Based on Bone Mineral Density at the Femoral Neck or Lumbar Spine. J. Bone Miner Res. 29, 2520–2526. doi:10.1002/jbmr.2269
Wu, J. Z., Liu, P. C., Liu, R., and Cai, M. (2018). Icariin Restores Bone Structure and Strength in a Rat Model of Chronic High-Dose Alcohol-Induced Osteopenia. Cell Physiol Biochem. 46 (4), 1727–1736. doi:10.1159/000489248
Wu, Z. H., Zhu, X., Xu, C. K., Chen, Y. J., Zhang, L., and Zhang, C. L. (2017). Effect of Xianling Gubao Capsules on Bone Mineral Density in Osteoporosis Patients. J. Biol. Regul. Homeost Agents. 31, 359–363.
Xia, X. L. (2013). History of Chinese Medicinal Wine. Chin. J. Integr. Med. 19, 549–555. doi:10.1007/s11655-010-0799-7
Xiao, H., Wignall, N., and Brown, E. S. (2016). An Open-Label Pilot Study of Icariin for Co-Morbid Bipolar and Alcohol Use Disorder. Am. J. Drug Alcohol. Abuse. 42 (2), 162–167. doi:10.3109/00952990.2015.1114118
Xie, Q. F., Xie, J. H., Dong, T. T., Su, J. Y., Cai, D. K., Chen, J. P., et al. (2012). Effect of a Derived Herbal Recipe from an Ancient Chinese Formula, Danggui Buxue Tang, on Ovariectomized Rats. J. Ethnopharmacol. 144, 567–575. doi:10.1016/j.jep.2012.09.041
Yang, F., Lin, Z. W., Huang, T. Y., Chen, T. T., Cui, J., Li, M. Y., et al. (2019a). Ligustilide, a Major Bioactive Component of Angelica Sinensis, Promotes Bone Formation via the GPR30/EGFR Pathway. Sci. Rep. 9, 6991. doi:10.1038/s41598-019-43518-7
Yang, L., Zhang, B., Liu, J., Dong, Y., Li, Y., Li, N., et al. (2019b). Protective Effect of Acteoside on Ovariectomy-Induced Bone Loss in Mice. Int. J. Mol. Sci. 20, 2974. doi:10.3390/ijms20122974
Yildiz, H., and Öztürk, E. (2020). Histopathological and Biochemical Effects of Eugenol on Alcohol-Treated Rat Liver. ADYU J. SCI. 10 (1), 83–99. doi:10.37094/adyujsci.729426
Yimam, M., Jiao, P., Hong, M., and Jia, Q. (2016). A Standardized Composition From Extracts of Myristica Fragrans, Astragalus Membranaceus, and Poria Cocos Protects Liver From Acute Ethanol Insult. J. Med. Food. 19 (8), 780–788. doi:10.1089/jmf.2016.0023
Zeng, X., Zhang, P., Wang, Y., Qin, C., Chen, S., He, W., et al. (2019). CMAUP: a Database of Collective Molecular Activities of Useful Plants. Nucleic Acids Res. 47 (D1), D1118–D1127. doi:10.1093/nar/gky965
Zhang, B., Yang, L. L., Ding, S. Q., Liu, J. J., Dong, Y. H., Li, Y. T., et al. (2021). Anti-Osteoporotic Activity of an Edible Traditional Chinese Medicine Cistanche Deserticola on Bone Metabolism of Ovariectomized Rats Through RANKL/RANK/TRAF6-Mediated Signaling Pathways. Front. Pharmacol. 10, 1412. doi:10.3389/fphar.2019.01412
Zhang, G., Qin, L., Hung, W. Y., Shi, Y. Y., Leung, P. C., Yeung, H. Y., et al. (2006). Flavonoids Derived From Herbal Epimedium Brevicornum Maxim Prevent OVX-Induced Osteoporosis in Rats Independent of its Enhancement in Intestinal Calcium Absorption. Bone. 38, 818–825. doi:10.1016/j.bone.2005.11.019
Zhang, N. D., Han, T., Huang, B. K., Rahman, K., Jiang, Y. P., Xu, H. T., et al. (2016). Traditional Chinese Medicine Formulas for the Treatment of Osteoporosis: Implication for Antiosteoporotic Drug Discovery. J. Ethnopharmacol. 189, 61–80. doi:10.1016/j.jep.2016.05.025
Zhang, Y. W., and Cheng, Y. C. (2019). Challenge and Prospect of Traditional Chinese Medicine in Depression Treatment. Front. Neurosci. 13, 190. doi:10.3389/fnins.2019.00190
Zhao, B. J., Wang, J., Song, J., Wang, C. F., Gu, J. F., Yuan, J. R., et al. (2016). Beneficial Effects of a Flavonoid Fraction of Herba Epimedii on Bone Metabolism in Ovariectomized Rats. Planta Med. 82, 322–329. doi:10.1055/s-0035-1558294
Zhou, S., and Glowacki, J. (2017). Chronic Kidney Disease and Vitamin D Metabolism in Human Bone Marrow-Derived MSCs. Ann. N. Y Acad. Sci. 1402, 43–55. doi:10.1111/nyas.13464
Zhou, S., and Glowacki, J. (2018). Dehydroepiandrosterone and Bone. Vitam Horm. 108, 251–271. doi:10.1016/bs.vh.2018.01.005
Zhou, S., Greenberger, J. S., Epperly, M. W., Goff, J. P., Adler, C., Leboff, M. S., et al. (2008). Age-Related Intrinsic Changes in Human Bone-Marrow-Derived Mesenchymal Stem Cells and Their Differentiation to Osteoblasts. Aging Cell. 7, 335–343. doi:10.1111/j.1474-9726.2008.00377.x
Zhou, S., Leboff, M. S., Waikar, S. S., and Glowacki, J. (2013). Vitamin D Metabolism and Action in Human Marrow Stromal Cells: Effects of Chronic Kidney Disease. J. Steroid Biochem. Mol. Biol. 136, 342–344. doi:10.1016/j.jsbmb.2012.09.009
Zhou, S. (2015). Paracrine Effects of Haematopoietic Cells on Human Mesenchymal Stem Cells. Sci. Rep. 5, 10573. doi:10.1038/srep10573
Keywords: ethnopharmacology, traditional Chinese medicine, alcohol-induced bone diseases, osteoporosis, TCM herbal extracts
Citation: Qian D, Zhou H, Fan P, Yu T, Patel A, O’Brien M, Wang Z, Lu S, Tong G, Shan Y, Wang L, Gao Y, Xiong Y, Zhang L, Wang X, Liu Y and Zhou S (2021) A Traditional Chinese Medicine Plant Extract Prevents Alcohol-Induced Osteopenia. Front. Pharmacol. 12:754088. doi: 10.3389/fphar.2021.754088
Received: 05 August 2021; Accepted: 11 November 2021;
Published: 15 December 2021.
Edited by:
Daohua Xu, Guangdong Medical University, ChinaReviewed by:
Jun Mao, Affiliated Hospital of Nanjing University of Chinese Medicine, ChinaJinghong Zhang, Huaqiao University, China
Wei Yang, Guangdong Pharmaceutical University, China
Copyright © 2021 Qian, Zhou, Fan, Yu, Patel, O’Brien, Wang, Lu, Tong, Shan, Wang, Gao, Xiong, Zhang, Wang, Liu and Zhou. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuanhu Zhou, szhou@bwh.harvard.edu, shuanhuzhou@gmail.com; Yuancai Liu, lyc@jingpai.com
†These authors have equally contributed to this work
 Dongyang Qian
Dongyang Qian Hui Zhou3†
Hui Zhou3† Tao Yu
Tao Yu Anish Patel
Anish Patel Yuan Xiong
Yuan Xiong Lily Zhang
Lily Zhang Xin Wang
Xin Wang Shuanhu Zhou
Shuanhu Zhou